Abstract
Eosinophils are a subset of granulocytes mostly known for their ability to combat parasites and induce allergy. Although they were described to be related to cancer more than 100 years ago, their role in tumors is still undefined. Recent observations revealed that they display regulatory functions towards other immune cell subsets in the tumor microenvironment or direct cytotoxic functions against tumor cells, leading to either antitumor or protumor effects. This paradoxical role of eosinophils was suggested to be dependent on the different factors in the TME. In addition, the clinical relevance of these cells has been recently addressed. In most cases, the accumulation of eosinophils both in the tumor tissue, called tumor-associated tissue eosinophilia, and in the peripheral blood were reported to be prognostic markers for a better outcome of cancer patients. In immunotherapy of cancer, particularly in therapy with immune checkpoint inhibitors, eosinophils were even shown to be a potential predictive marker for a beneficial clinical response. A better understanding of their role in cancer progression will help to establish them as prognostic and predictive markers and to design strategies for targeting eosinophils.
Keywords: Eosinophils, Cancer, Prognostic marker, Immunotherapy, PIVAC 17
Introduction
In recent decades, cancer research has widened from the analysis of tumor cells and their genetic alterations towards a broader investigation including the TME. Tumor growth is not only dependent on malignant cells, but also on chronic inflammation maintained by surrounding cells, including leukocytes, fibroblasts and endothelial cells. Tumor-infiltrating host cells can either conduct protumor or antitumor functions by secreting soluble factors such as cytokines and chemokines [1]. Eosinophils represent one subset of the leukocytes infiltrating tumors. Compared to other immune cells in the TME, such as T cells, macrophages, neutrophils, MDSC and DC, the function of eosinophils is poorly investigated. There is an undisputable need for a more detailed analysis of their impact on tumor development since eosinophils have been described to act as regulatory and effector cells within the TME [2–4]. Moreover, they are known to produce a wide range of factors influencing other leukocytes. The classical view on eosinophils only combatting parasites and inducing allergies must be revisited [5].
In this review, we discuss the biology of eosinophils, their modulation of tumor growth and the mechanisms of such modulation. Furthermore, the potential properties of eosinophils as a marker for survival or therapy response in cancer patients as well as strategies of targeting eosinophils will be analyzed.
Biology of eosinophils
Eosinophils represent a subpopulation of granulocytes and are phenotypically characterized by their bilobed nuclei, large specific granules, and their capability to be stained by acidophilic dyes. They mature in the bone marrow upon specific stimuli and are then released into the peripheral bloodstream. Eosinophils can further migrate into various organs such as lung, thymus, adipose tissue and especially the gastrointestinal (GI) tract [6]. Tissue-resident eosinophils execute different functions, often based on their cytokine release [7] via exocytotic degranulation. However, even under homeostatic conditions, the distinct role of eosinophils in different tissues remains uncertain and needs to be further explored.
Mature eosinophils express a wide range of surface receptors involved in adhesion, activation, migration and pattern recognition [5]. Sialic acid-binding Ig-like lectins 8 (Siglec)-8 in human [8] and Siglec-F in mice [9] are predominantly expressed on eosinophils. Although Siglec-8 was detected also on basophils [8] and Siglec-F was found on alveolar macrophages [10], both surface molecules are considered as eosinophil markers [8, 9]. In addition, eosinophils express the IL-5 receptor alpha subunit and the CC-chemokine receptor (CCR) 3, also known as CD125 and eotaxin receptor, respectively. Although not uniquely expressed on eosinophils, these two receptors define the eosinophil phenotype and can serve as markers in combination [5]. Both receptors play a pivotal role in the activation process.
Next to their surface receptor repertoire, eosinophils can be characterized by their intracellular content and, in particular, by their specific granules that contain major basic protein (MBP) and eosinophil peroxidase, as well as the ribonucleases eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) [11]. Furthermore, eosinophils store lipid bodies containing lipid mediators such as leukotrienes, prostaglandins and platelet-activating factor [5].
It has been demonstrated that IL-5, which is largely produced by type 2 Th cells (Th2) [12] and type 2 innate lymphoid cells [13], plays a crucial role in eosinophil proliferation, activation and survival. The expansion and trafficking of human eosinophils were shown to be critically dependent on IL-5 [14]. The importance of this cytokine was demonstrated by applying anti-IL-5 mAbs in patients with severe asthma and eosinophilia, leading to a reduction of eosinophil numbers in the peripheral blood [15]. Eosinophil migration was found to be supported by eotaxins, a group of chemokines that can recruit eosinophils synergistically with IL-5 [16]. The effect of eotaxins is based on their binding to CCR3 expressed on eosinophils [17].
Human eosinophils can also be activated via the signaling through pattern-recognition receptors such as TLR and nucleotide-binding oligomerization domain-like receptors [18].
Functional properties of eosinophils
After activation and migration to the target site, eosinophils implement their functions through different mechanisms. The most prominent one is degranulation, which is performed mainly as a controlled transport of specific vesicles to the cell surface, and therefore, called “piecemeal degranulation” [19]. Through the release of cationic proteins and ribonucleases, activated eosinophils have direct cytotoxic effects especially on pathogens like viruses and bacteria in vitro [20, 21]. The cationic proteins were found to be components of extracellular traps [22] and to exert antibacterial effects in vivo, resembling the function of neutrophil extracellular traps [23].
Furthermore, eosinophils release cytokines by degranulation to modulate other leukocyte subpopulations [5]. Eosinophils were shown to produce Th2-type chemoattractants to orchestrate Th2 cells into the lung under allergic conditions in mice [24]. Moreover, they were reported to support the survival of plasma cells in the bone marrow by secreting a proliferation-inducing ligand and IL-6 [25]. The role of eosinophils as immune regulatory leukocytes may even be executed by direct cell-to-cell contact as shown by in vitro ultrastructural studies of mast cell–eosinophil interaction [26]. In addition, eosinophils are able to exert antigen presenting functions, resulting in T cell activation [27]. Although they are considered “non-professional” APCs, they express MHC class II and the costimulatory molecules CD80 and CD86 [28].
Eosinophils participate in innate immunity by recognizing pathogen- and damage-associated molecular patterns (PAMPs/DAMPs) leading to degranulation [18]. In adaptive immunity, they play a role in the recruitment of other leukocytes, direct interaction with them, and antigen presentation to T cells [27]. Their capability of combatting parasites is among their most prominent properties and was confirmed in several studies in vivo [29]. In addition, they also execute antibacterial and antiviral functions, which was shown in Pseudomonas aeruginosa infections in mice [30], respiratory syncytial virus infections [31] and HIV infections [32].
Under homeostatic conditions, the major part of the eosinophil population is resident in the GI tract. However, their function within this tissue remains unclear. It was shown that eosinophils contribute to immune homeostasis in the GI tract by fostering IgA-producing plasma cells and promoting T cell-independent IgA class switching [33]. In the thymus, eosinophils were found to be localized in proximity to immature double-negative thymocytes in the corticomedullary region, suggesting that they play a role in negative T cell selection [34]. Eosinophils were reported to contribute to the metabolic processes in the adipose tissue by promoting alternatively activated macrophages [35].
Eosinophils in various diseases
Besides the impact in infections, eosinophils are associated with atopic disorders such as asthma. In the pathogenesis of asthma, they contribute by releasing MBP, which triggers mast cell and basophil degranulation. Furthermore, they were found to release functional exosomes promoting the progression of asthma [36]. In asthmatic patients, targeting eosinophils can be achieved by humanized anti-IL-5 mAbs, although a clinical improvement is only seen in a subset of patients displaying a persistent sputum eosinophilia before the treatment [37].
Furthermore, there is a link between eosinophils and GI disorders, in particular, eosinophilic esophagitis. This disease is characterized by eosinophilic infiltration of the esophageal mucosa and chronic inflammation mediated by Th2 cells [38].
Eosinophil-related diseases also include hypereosinophilic syndromes defined by eosinophilia and associated organ damage; the accumulation of eosinophils is based on activated eosinopoiesis or an overproduction of eosinophilopoietic cytokines [39]. Another reason for eosinophilia could be primary immunodeficiency, which represents a group of very rare genetic diseases [40].
Eosinophil recruitment in cancer
Although eosinophils were described in relation to cancer more than 100 years ago [41], the mechanisms underlying their accumulation in the peripheral blood or tumor tissue are still not completely understood. The most prominent attractor of eosinophils, IL-5, was demonstrated to be produced by human cancer cells [42] and to recruit eosinophils to the peripheral bloodstream [43]. Furthermore, other factors such as GM-CSF were shown to be produced by tumor cells as well as to recruit and activate eosinophils [44]. Additionally, the Th2 cytokine IL-4 displayed antitumor function in vivo through the induction of eosinophil migration to the tumor site and local eotaxin expression [45, 46]. Moreover, in vivo studies revealed that CC-chemokine ligand 11 (CCL11), also called eotaxin-1, was largely responsible for orchestrating eosinophils within the tumor [47]. This role of eotaxins in cancer can be underlined by their expression in human cancer tissue [48]. In addition to the CCR3-dependent recruiting, eosinophil migration was found to be regulated by CCR1 [49].
Interestingly, it has been demonstrated in a mouse melanoma model that eosinophil recruitment to the tumor belongs to an early inflammation reaction [50]. In this paper, the authors reported that this early migration was rather due to chemotactic factors produced by dying tumor cells than induced inflammatory mediators secreted by CD4+ T cells. Indeed, stress signals such as alarmins or DAMPs are known to attract eosinophils [5, 6]. These DAMPs include the high-mobility group box 1 protein (HMGB1) released by stressed or dying cells, which led to recruitment, survival and activation of eosinophils [51]. Another alarmin, IL-33, was found to have the ability to recruit eosinophils based on the IL-33/ST2-axis in vivo [3]. Furthermore, ATP, acting as a DAMP, was able to attract and activate eosinophils through binding to the P2Y purinergic receptor [52].
Modulation of tumor growth by eosinophils
Numerous studies have addressed the role of eosinophils in fighting cancer, but the experimental studies have revealed conflicting results. Some mechanisms of eosinophil functions in tumors are illustrated in Fig. 1.
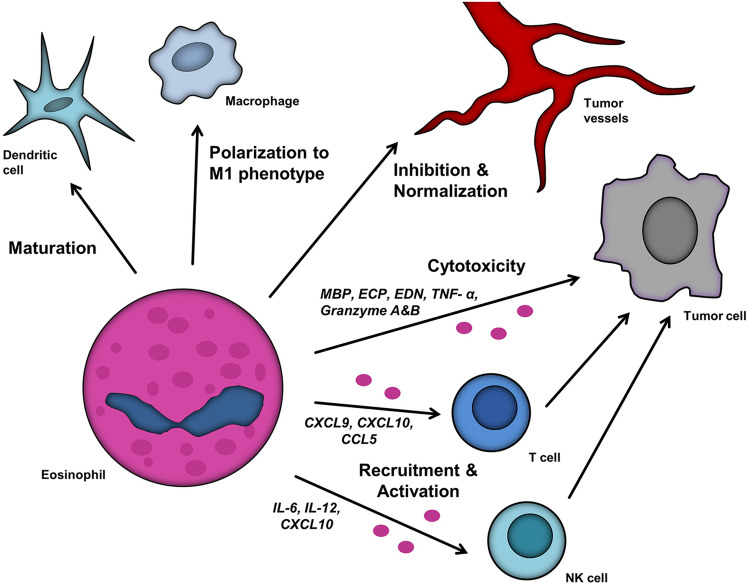
Fig. 1.
Impact of eosinophils within the TME. Eosinophils influence other leukocytes, including T cells, NK cells, DC and macrophages, and alter their effect on tumor growth. T cell recruitment and activation is based on the chemokines C-X-C-chemokine ligand (CXCL) 9, CXCL10 and CC-chemokine ligand (CCL) 5, whereas NK cells are attracted by IL-6, IL-12 and CXCL10. Eosinophils modulate tumor vessels and exert direct cytotoxic functions towards tumor cells via major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), TNF-α, granzyme A and granzyme B
Antitumor functions were demonstrated by the injection of IL-4 secreting tumor cells into mice which resulted in tumor regression mediated mainly by eosinophils [45]. In a melanoma mouse model, it was found that elimination of metastasis was based on eosinophil degranulation [46]. The eosinophils were attracted and activated by tumor-specific CD4+ T cells. Furthermore, Simson et al. [47] demonstrated an increased tumor incidence and growth of methylcholanthrene-induced fibrosarcomas in mice deficient for either CCL11 or CCL11 and IL-5, with reduced or deficient eosinophil levels, respectively. The tumor-protective effect of IL-5 was histologically proven to be dependent on enhanced eosinophilic infiltration [47].
In contrast, some experimental studies indicated a pro-tumorigenic role for eosinophils. It was demonstrated that the incidence of squamous cell carcinoma induced by 4-nitroquinoline-1-oxide was enhanced in wild-type mice compared to eosinophil-deficient mice [53], indicating that eosinophils might be involved in the promotion of carcinogenesis. In a carcinogen-induced hamster oral squamous cell carcinoma (OSCC) model characterized by tumor-associated tissue eosinophilia (TATE), animals were treated with anti-IL-5 mAbs, resulting in attenuated tumor growth [54], emphasizing the protumoral role of eosinophils. These observations led to the hypothesis that eosinophils may be able to execute both, pro- and antitumor functions, depending on different stimuli in the TME. Such polarized functions in tumor-bearing hosts were also observed for other myeloid cells such as macrophages and neutrophils.
A recent study of Carretero et al. [2] elucidated the role of eosinophils in tumor rejection. The authors observed a tumor regression upon Treg cell depletion associated with tumor eosinophilia in a melanoma mouse model. Further investigation revealed that eosinophils produced chemokines like CCL5, CXCL9 and CXCL10, which recruited CD8+ T cells into the TME. Additionally, the authors observed an induction of macrophage polarization towards the M1-like cells which were previously described to exert anti-tumor effects [1]. Another publication presented evidence of tumor growth delay after injection of IL-33 and attributed this effect to the intratumoral accumulation of eosinophils and CD8+ T cells [3]. Moreover, eosinophils migrated to the lungs, preventing the formation of metastases. Upon depletion of eosinophils with a Siglec-F mAb, CD8+ T cell infiltration was diminished. Furthermore, the authors showed that eosinophils exerted direct cytotoxic effects on tumor cells in vitro [3]. In addition, eosinophils were demonstrated to interact with other immune cells such as NK cells [55] and DC [56].
Interestingly, eosinophils were demonstrated to also affect angiogenesis, which is an essential process for tumor growth. Eosinophils were able to induce normalization of tumor vessels, secreting less angiogenic factors, such as VEGF, in the TME [2]. Furthermore, Xing et al. [57] found that CCL11-induced eosinophils inhibit angiogenesis in a fibrosarcoma mouse model.
Importantly, it was observed that eosinophils can exert direct cytotoxic effects towards tumor cells by releasing granular content. Eosinophil lysate was reported to kill B16 melanoma cells in vitro [3]. Furthermore, eosinophils were shown to release MBP into the tumor tissue in vivo, suggesting that MBP was responsible for the cytotoxic effect [46]. Indeed, in vitro studies demonstrated that eosinophilic MBP has cytotoxic function against tumor cell lines [58]. Additionally, the tumoricidal effect of MBP was found to be enhanced by the combination with TNF-α in multicellular tumor spheroids [59]. The release of TNF-α together with ECP and EDN was also observed after the co-culture of eosinophils together with colon cancer cells, enhancing cytotoxic activity [4]. In addition, eosinophils exerted cytotoxicity via the release of the apoptosis inducing protease granzyme B [60]. Besides the release of cytotoxic factors, eosinophils were found to build close contacts with viable tumor cells [61]. This cross-talk could be at least partly based on the binding of ICAM-1 on eosinophils to the junctional adhesion molecule A on tumor cells, which was shown for colon cancer cells [62]. Interestingly, the binding was found to be dependent on IL-18, which upregulated adhesion molecules on eosinophils and tumor cells.
On the other side, some publications reported protumor functions of eosinophils. They were shown to recruit Treg cells via production of CCL22 that facilitated pulmonary metastasis in mice [63]. Furthermore, Kratochvill et al. [64] found that eosinophils produce IL-13, thereby driving macrophage polarization towards the M2-like immunosuppressive phenotype. Another immunosuppressive effect of eosinophils was mediated by the production of indoleamine 2,3-dioxygenase, which is capable of inhibiting T and NK cell function [65] as described in NSCLC patients [66]. However, the tumor-promoting role of eosinophils not only involves other immune cells. It was also demonstrated that MBP in a sub-cytotoxic subcytotoxic dose induced angiogenesis by promoting endothelial cell proliferation and enhancing the effect of VEGF [67].
Eosinophils as a prognostic marker for clinical outcome
In the past, various studies have highlighted the involvement of eosinophils in numerous tumor entities and their increasingly important potential utilization as a prognostic marker. They were shown to be related to a beneficial prognosis in most cases, although evidence of association to a non-beneficial prognosis was described [68]. This connection towards the clinical prognosis was either reported for TATE or peripheral eosinophil counts.
A prognostic impact of TATE for OSCC patients has already been described for many years [69]. Several studies revealed a positive correlation between eosinophil infiltration and a favorable prognosis. Dorta et al. [70] showed an association between an increased disease-free survival (DFS) as well as overall survival (OS) and the intensity of TATE. Furthermore, the OS rate was found to be particularly elevated in the case of intratumoral TATE as compared to other regions [71]. Recently, other authors tried to simplify the evaluation of TATE and found a cut-off at four eosinophils per high power field (HPF) with patients having more than four eosinophils per HPF displaying a better survival [72]. However, other publications described eosinophilic tumor infiltration as a marker for an unfavorable prognosis in OSCC patients. TATE was found to predict lymph node metastasis [73] and was demonstrated to correlate with locoregional recurrence [74]. In addition, Alrawi et al. [75] described an impaired survival of patients with high numbers of tumor-infiltrating eosinophils. Interestingly, another publication revealed no significant impact of TATE on the 5- and 10-year survival of OSCC patients [76].
In colorectal cancer (CRC), an infiltration of eosinophils was first described by Moezzi et al. [77]. A link between decreasing eosinophilic infiltration and increasing malignancy of CRC was confirmed by other studies, including in total around 1000 tissue samples [78, 79]. Furthermore, a correlation between high eosinophilic infiltration of the tumor and a beneficial 5-year survival rate in CRC patients was reported [80]. Moreover, the occurrence of CRC was found to be inversely correlated with circulating eosinophils, suggesting a protective role for eosinophils in CRC development [81]. In addition, a retrospective study of CRC patients with stage I-III documented a better OS and DFS in case of a higher eosinophil blood count [82].
The prognostic value of eosinophils has been demonstrated for breast cancer as well. A reduced risk of disease recurrence was described in primary breast cancer patients displaying a higher peripheral eosinophil count [83]. In contrast, it has been reported that high numbers of eosinophils correlate with an impaired DFS in patients treated with the antibody trastuzumab [84].
The association of eosinophils with an unfavorable prognosis has been particularly described for cervical cancer. This tumor entity is characterized by the development from intraepithelial neoplastic lesions. In contrast to CRC, a higher density of eosinophilic infiltration was associated with accelerated tumor progression [85]. Furthermore, TATE was found to be correlated with enhanced tumor depth and size leading to an impaired survival [86]. Interestingly, the authors failed to observe any impact of the peripheral eosinophil count on the survival rate. In contrast, Bethwaite et al. [87] described intensive TATE as a beneficial factor for the 5-year survival rate in cervical cancer patients.
The link between eosinophil counts or eosinophilic tumor infiltration and survival was also studied in patients with other tumor entities. In gastric cancer, high levels of TATE correlated with an improved survival rate [88, 89]. A similar association of TATE with metastasis, recurrence and prognosis can be seen in esophageal cancer [90, 91]. Furthermore, high peripheral eosinophil counts have been shown to be related to an enhanced OS in hepatobiliary cancer [92]. In addition, the eosinophil count was found to be elevated in patients with renal cell carcinoma that responded to the therapy with tyrosine kinase inhibitor sorafenib [93].
Eventually, eosinophils were found to be associated with a better prognosis in most cases, although further studies should be performed, especially in OSCC, breast cancer and cervical cancer. Nevertheless, the controversial prognostic value reported for eosinophils might be based upon different measures for the intensity of eosinophilia and non-sufficient statistical power.
Eosinophils as a predictive marker in cancer immunotherapy
Eosinophilia is a frequently observed side effect of immunotherapy and has been described for administration of IL-2 [94], IL-4 [95], GM-CSF [96], anti-PD-1- [97] and anti-CTLA-4-antibodies [98]. An enhanced degranulation of eosinophils is shown during treatment with IL-2 [94] and IL-4 [95], supporting a possible involvement of these cells in tumor control.
Recently, the role of eosinophils as a predictive marker has been intensively studied in malignant melanoma (MM) patients treated with immune checkpoint inhibitors (ICI). It was found that a high baseline eosinophil count is correlated with an improved OS in patients treated with the anti-PD1 antibody pembrolizumab [97]. In contrast, our study on MM patients treated with the ICI ipilimumab revealed that not the baseline eosinophil count but rather an early increase in the eosinophil count is linked to an improved clinical response to this immunotherapy [98]. Moreover, we found that the concentration of eotaxin-1 in serum from non-responding MM patients was significantly lower than before therapy, leading to diminished eosinophil count observed in the peripheral blood of non-responders [98]. These results suggest that eosinophils could potentially be used as a predictive marker for clinical response to ICI therapy. Another paper described a trend towards an association of eosinophilia with a better survival rate independent of the treatment [99], emphasizing, however, a prolonged OS in patients treated with an ICI and experiencing eosinophilia. Interestingly, the significance of eosinophils as a predictive marker was also confirmed for uveal MM patients by demonstration of increased OS in patients with higher eosinophil levels during ICI therapy [100].
Similar to MM patients, eosinophils were also described as a potential predictive marker in lung cancer patients treated with ICI. A multivariate analysis of patients with NSCLC and treated with ICI revealed a significant positive correlation between increased baseline eosinophil count and improved OS [101]. However, Shelton et al. [102] found a correlation between a high peripheral eosinophil count and a favorable OS in lung cancer (including SCLC and NSCLC) independent of the treatment.
In addition to MM and lung cancer, a correlation between elevated eosinophil counts during immunotherapy with sipuleucel-T and improved OS was shown in prostate cancer patients [103].
Taken together, eosinophils were shown to be a predictive marker in immunotherapy in different tumor entities. However, further studies on greater patient cohorts would be necessary for the implementation of this parameter into the clinical use. Table 1 summarizes the data on eosinophils as a biomarker.
Table 1.
Evidence on eosinophils as prognostic and predictive markers
| Tumor entity | n | Conclusion | Ref. |
|---|---|---|---|
| OSCC | 125 |
Intense TATE is associated with prolonged DFS and OS TATE is an independent prognostic marker |
[70] |
| OSCC | 87 |
Intense intratumoral TATE is associated with prolonged OS Intratumoral TATE is an independent prognostic marker |
[71] |
| OSCC | 99 | Medium and high TATE is associated with prolonged OS | [72] |
| OSCC | 71 |
Intense TATE is associated with occult lymph node metastasis Intense TATE is associated with impaired DFS |
[73] |
| OSCC | 14 | Intense TATE is associated with locoregional recurrence | [74] |
| OSCC |
87 20 |
TATE predicts invasiveness of the tumor Intense TATE is associated with impaired cumulative survival |
[75] |
| OSCC | 43 | No significant association between intensity of TATE and outcome | [76] |
| CRC | 441 |
Strong stromal eosinophilic infiltration is associated with prolonged 5-year survival TATE is an independent prognostic marker |
[80] |
| CRC | 242 | AEC is inversely associated with CRC incidence | [81] |
| CRC | 569 |
Low AEC is associated with impaired OS and DFS AEC is an independent prognostic marker |
[82] |
| Breast cancer | 419 | High AEC is associated with a prolonged DFS | [84] |
| Breast cancer | 62 |
Low eosinophil count is associated with prolonged DFS in patients treated with trastuzumab AEC is an independent prognostic marker |
[83] |
| Cervical cancer | 61 |
Moderate and extensive TATE is associated with impaired OS TATE is an independent prognostic marker |
[86] |
| Cervical cancer | 73 | Moderate and intense TATE is associated with a prolonged 5-year survival | [87] |
| Gastric cancer | 308 | Moderate and marked TATE is associated with a prolonged 5-year survival | [88] |
| Gastric cancer | 324 |
Numerous TATE is associated with a prolonged 5-year survival TATE is an independent prognostic marker |
[89] |
| Eso-phageal cancer | 36 |
High TATE is associated with a prolonged OS TATE is an independent prognostic marker |
[90] |
| Eso-phageal cancer | 97 | Large number of eosinophils is associated with a prolonged 2- and 5-year survival in patients with lymph node metastasis | [91] |
| Hepato-biliary cancer | 206 | High peripheral eosinophils counts are associated with a prolonged cumulative survival | [92] |
| Renal cell cancer | 282 |
Increase of REC during therapy with sorafenib is associated with a prolonged OS and PFS REC is an independent prognostic marker |
[93] |
| MM |
177 346 |
High AEC and REC associated with prolonged OS of patients treated with pembrolizumab High REC is an independent prognostic marker for a better OS of patients treated with pembrolizumab |
[97] |
| MM | 59 |
Increase in AEC associated with improved clinical response to treatment with ipilimumab Increase in AEC is an independent predictive marker for the clinical response to ipilimumab |
[98] |
| MM |
86 40 |
High REC is associated with a prolonged OS in patients treated with ICI High REC is associated with a better OS in immunotherapy-naïve patients who lived at least 12 months |
[99] |
| uveal MM | 86 |
High REC is associated with prolonged OS of patients treated with ICI REC is an independent prognostic marker |
[100] |
| NSCLC | 134 |
High AEC is associated with a prolonged OS and PFS in patients treated with nivolumab AEC is an independent prognostic marker |
[101] |
| NSCLC & SCLC | 358 |
High eosinophil count associated with prolonged cumulative survival in NSCLC and SCLC Eosinophil count is an independent prognostic marker |
[102] |
| Prostate cancer | 377 | Increase of eosinophil count during therapy with sipuleucel-T is associated with an induced immune response, cancer-specific survival and prolonged OS | [103] |
AEC absolute eosinophil count, CRC colorectal cancer, DFS disease-free survival, ICI immune checkpoint inhibitor, MM malignant melanoma, n number of patients, OS overall survival, OSCC oral squamous cell carcinoma, PFS progression-free survival, REC relative eosinophil count, Ref. references, TATE tumor-associated tissue eosinophilia
Eosinophils as a therapeutic target
Several studies on pre-clinical mouse tumor models described the possibility of eosinophil targeting [104]. However, none of these drugs were approved for the application in cancer patients. Interestingly, it has been reported that inhibitors of dipeptidylpeptidase 4 (DPP4) impaired eosinophil trafficking within tumors by blocking posttranslational modifications of chemokines [105]. In hepatocellular carcinoma mouse model, the treatment with the DPP4-inhibitor sitagliptin resulted in an enhanced tumor immunity associated with the regression of tumors. These effects were diminished upon eosinophil depletion or inhibition of their degranulation. Therefore, the authors started a phase Ib clinical study in which patients with hepatocellular carcinoma are treated with sitagliptin prior to surgery [105].
Conclusion
Taken together, eosinophils participate in the regulation of various physiological and pathological processes, including cancer. The accumulation of eosinophils in the TME or in the peripheral blood of cancer patients was already observed a long time ago but has been mainly neglected so far. However, during the last decade the attention to this topic has been constantly increasing due to the challenging recent results showing the role of eosinophils in the tumor defense in vivo (Fig. 1) [2, 3]. Some authors even suggested a possibility of eosinophil polarization into distinct subsets having either immunostimulatory or immunoinhibitory functions [2, 3, 106]. However, more studies would be needed to investigate this possibility in tumor-bearing hosts in vivo. Furthermore, numerous observations have appeared, indicating the possible application of eosinophils as a prognostic biomarker. The intensity of TATE has been correlated with a better clinical outcome for many solid tumors. Furthermore, a high eosinophil blood count was found to be associated with patients’ responsiveness to immunotherapy, in particular to ICI [97, 98]. Nevertheless, further investigation in greater cohorts is needed for the definite use of eosinophils as a prognostic or predictive marker. In addition, due to a lot of outstanding questions, an attempt to target eosinophils in cancer therapeutically has not been made yet. This approach should be reconsidered when there are more clinical data and evidence about functional mechanisms.
Acknowledgements
The authors wish to thank Elisabeth Cordell for editing the manuscript, Xiaoying Hu for designing figure elements, Rebekka Weber and Zeno Riester for helpful advice. The literature search was assisted by Maurizio Grilli from the library for the Medical Faculty of Mannheim, Ruprecht-Karl University of Heidelberg.
Abbreviations
- AEC
Absolute eosinophil count
- CCL
CC-chemokine ligand
- CCR
CC-chemokine receptor
- CRC
Colorectal cancer
- CXCL
C-X-C-chemokine ligand
- DAMPs
Damage-associated molecular patterns
- DFS
Disease-free survival
- DPP4
Dipeptidylpeptidase 4
- ECP
Eosinophil cationic protein
- EDN
Eosinophil-derived neurotoxin
- GI
Gastrointestinal
- HMGB1
High-mobility group box 1 protein
- HPF
High power fields
- ICI
Immune checkpoint inhibitor
- MBP
Major basic protein
- MM
Malignant melanoma
- OSCC
Oral squamous cell carcinoma
- PAMP
Pathogen-associated molecular patterns
- PFS
Progression free survival
- REC
Relative eosinophil count
- Siglec
Sialic acid-binding immunoglobulin-like lectin
- TATE
Tumor-associated tissue eosinophilia
- Th2
Type 2 Th cells
Author contributions
SCSS: writing, review and revision of the manuscript, preparation and revision of the figure and table. JU: review and revision of the manuscript. VU: writing, review and revision of the manuscript and revision of the table and figure.
Funding
This work was supported by grants from the German Research Council (RTG2099) to J. Utikal and V. Umansky and the Cooperation between German Cancer Research Center (DKFZ) and Ministry of Science, Technology and Space of Israel (MOST) in Cancer Research (CA181 to V. Umansky). This work was kindly backed by the COST Action BM1404 Mye-EUNITER (http://www.mye-euniter.eu). COST is supported by the EU Framework Program Horizon 2020.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4(8):641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 2.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16(6):609–609+. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 3.Lucarini V, Ziccheddu G, Macchia I, La Sorsa V, Peschiaroli F, Buccione C, Sistigu A, Sanchez M, Andreone S, D’Urso MT, Spada M, Macchia D, Afferni C, Mattei F, Schiavoni G. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology. 2017 doi: 10.1080/2162402x.2017.1317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, Capron M. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol (Baltim Md 1950) 2010;185(12):7443–7451. doi: 10.4049/jimmunol.1000446. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 7.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17(12):746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, Crocker PR. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275(2):861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 9.Dyer KD, Garcia-Crespo KE, Killoran KE, Rosenberg HF. Antigen profiles for the quantitative assessment of eosinophils in mouse tissues by flow cytometry. J Immunol Methods. 2011;369(1–2):91–97. doi: 10.1016/j.jim.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327(1–2):63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 12.Upadhyaya B, Yin Y, Hill BJ, Douek DC, Prussin C. Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J Immunol (Baltim Md 1950) 2011;187(6):3111–3120. doi: 10.4049/jimmunol.1101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989;73(6):1504–1512. [PubMed] [Google Scholar]
- 15.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182(4):1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183(5):2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136(1):11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dvorak AM, Estrella P, Ishizaka T. Vesicular transport of peroxidase in human eosinophilic myelocytes. Clin Exp Allergy. 1994;24(1):10–18. doi: 10.1111/j.1365-2222.1994.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 20.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177(6):1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 21.Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol (Baltim Md 1950) 1989;142(12):4428–4434. [PubMed] [Google Scholar]
- 22.von Kockritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med (Berl) 2009;87(8):775–783. doi: 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205(3):699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12(2):151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 26.Minai-Fleminger Y, Elishmereni M, Vita F, Soranzo MR, Mankuta D, Zabucchi G, Levi-Schaffer F. Ultrastructural evidence for human mast cell-eosinophil interactions in vitro. Cell Tissue Res. 2010;341(3):405–415. doi: 10.1007/s00441-010-1010-8. [DOI] [PubMed] [Google Scholar]
- 27.Wen T, Rothenberg ME. The regulatory function of eosinophils. Microbiol Spectr. 2016 doi: 10.1128/microbiolspec.MCHD-0020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105(7):945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Appleton JA. Eosinophils in Helminth Infection: Defenders and Dupes. Trends Parasitol. 2016;32(10):798–807. doi: 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linch SN, Kelly AM, Danielson ET, Pero R, Lee JJ, Gold JA. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77(11):4976–4982. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110(5):1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 32.Bedoya VI, Boasso A, Hardy AW, Rybak S, Shearer GM, Rugeles MT. Ribonucleases in HIV type 1 inhibition: effect of recombinant RNases on infection of primary T cells and immune activation-induced RNase gene and protein expression. AIDS Res Hum Retrovir. 2006;22(9):897–907. doi: 10.1089/aid.2006.22.897. [DOI] [PubMed] [Google Scholar]
- 33.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, Kruglov A, Berek C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40(4):582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c + eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol (Baltim Md 1950) 2000;165(4):1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzeo C, Canas JA, Zafra MP, Rojas Marco A, Fernandez-Nieto M, Sanz V, Mittelbrunn M, Izquierdo M, Baixaulli F, Sastre J, Del Pozo V. Exosome secretion by eosinophils: a possible role in asthma pathogenesis. J Allergy Clin Immunol. 2015;135(6):1603–1613. doi: 10.1016/j.jaci.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 38.Gorriz Gil C, Matallana Royo V, Alvarez Montero O, Rodriguez Valiente A, Fernandez Manzano C, Conde Garcia B, Garcia-Berrocal JR. Eosinophilic esophagitis: an underdiagnosed cause of dysphagia and food impaction to be recognized by otolaryngologists. HNO. 2018 doi: 10.1007/s00106-018-0516-3. [DOI] [PubMed] [Google Scholar]
- 39.Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92(11):1243–1259. doi: 10.1002/ajh.24880. [DOI] [PubMed] [Google Scholar]
- 40.Navabi B, Upton JE. Primary immunodeficiencies associated with eosinophilia. Allergy Asthma Clin Immunol. 2016;12:27. doi: 10.1186/s13223-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldbausch F. The occurrence of eosinophilic leukocytes in tumours. Archiv Fur Pathologische Anatomie Physiologie Fur Klinische Medicin. 1900;161(1):1–18. [Google Scholar]
- 42.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55(17):3847–3853. [PubMed] [Google Scholar]
- 43.Pandit R, Scholnik A, Wulfekuhler L, Dimitrov N. Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. Am J Hematol. 2007;82(3):234–237. doi: 10.1002/ajh.200789. [DOI] [PubMed] [Google Scholar]
- 44.Curran CS, Evans MD, Bertics PJ. GM-CSF production by glioblastoma cells has a functional role in eosinophil survival, activation, and growth factor production for enhanced tumor cell proliferation. J Immunol. 2011;187(3):1254–1263. doi: 10.4049/jimmunol.1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257(5069):548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 46.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197(3):387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR. Regulation of carcinogenesis by IL-5 and CCL11: A potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178(7):4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 48.Lorena SCM, Oliveira DT, Dorta RG, Landman G, Kowalski LP. Eotaxin expression in oral squamous cell carcinomas with and without tumour associated tissue eosinophilia. Oral Dis. 2003;9(6):279–283. doi: 10.1034/j.1601-0825.2003.00958.x. [DOI] [PubMed] [Google Scholar]
- 49.Hirai H, Fujishita T, Kurimoto K, Miyachi H, Kitano S, Inamoto S, Itatani Y, Saitou M, Maekawa T, Taketo MM. CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin Exp Metastasis. 2014;31(8):977–989. doi: 10.1007/s10585-014-9684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O’Neill K, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal advance: Eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79(6):1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol (Baltim Md 1950) 2009;183(8):5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi T, Soma T, Noguchi T, Nakagome K, Nakamoto H, Kita H, Nagata M. ATP drives eosinophil effector responses through P2 purinergic receptors. Allergol Int. 2015;64(Suppl):S30–S36. doi: 10.1016/j.alit.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.da Silva JM, Queiroz-Junior CM, Batista AC, Rachid MA, Teixeira MM, da Silva TA. Eosinophil depletion protects mice from tongue squamous cell carcinoma induced by 4-nitroquinoline-1-oxide. Histol Histopathol. 2014;29(3):387–396. doi: 10.14670/hh-29.387. [DOI] [PubMed] [Google Scholar]
- 54.Wong DTW, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncol. 1999;35(5):496–501. doi: 10.1016/s1368-8375(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 55.O’Flaherty SM, Sutummaporn K, Haggtoft WL, Worrall AP, Rizzo M, Braniste V, Hoglund P, Kadri N, Chambers BJ. TLR-stimulated eosinophils mediate recruitment and activation of NK cells in vivo. Scand J Immunol. 2017;85(6):417–424. doi: 10.1111/sji.12554. [DOI] [PubMed] [Google Scholar]
- 56.Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83(3):456–460. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 57.Xing Y, Tian Y, Kurosawa T, Matsui S, Touma M, Yanai T, Wu Q, Sugimoto K. CCL11-induced eosinophils inhibit the formation of blood vessels and cause tumor necrosis. Genes Cells. 2016;21(6):624–638. doi: 10.1111/gtc.12371. [DOI] [PubMed] [Google Scholar]
- 58.Kubo H, Loegering DA, Adolphson CR, Gleich GJ. Cytotoxic properties of eosinophil granule major basic protein for tumor cells. Int Arch Allergy Immunol. 1999;118(2–4):426–428. doi: 10.1159/000024154. [DOI] [PubMed] [Google Scholar]
- 59.Furbert-Harris PM, Smith MA, Law MP, Currie E, Young JM, Gause DP, Vaughn TR, Harris VL, Oredipe OA. Eosinophil granular protein major basic protein combines with tumor necrosis factor alpha to kill prostate multicellular spheroids in vitro. J Immunother. 2005;28(6):619–619. doi: 10.1097/01.cji.0000190967.73030.72. [DOI] [Google Scholar]
- 60.Costain DJ, Guha AK, Liwski RS, Lee TDG. Murine hypodense eosinophils induce tumour cell apoptosis by a granzyme B-dependent mechanism. Cancer Immunol Immunother. 2001;50(6):293–299. doi: 10.1007/pl00006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caruso RA, Parisi A, Quattrocchi E, Scardigno M, Branca G, Parisi C, Luciano R, Paparo D, Fedele F. Ultrastructural descriptions of heterotypic aggregation between eosinophils and tumor cells in human gastric carcinomas. Ultrastruct Pathol. 2011;35(4):145–149. doi: 10.3109/01913123.2011.578233. [DOI] [PubMed] [Google Scholar]
- 62.Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, Lefevre G, Capron M. IL-18 Is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J Immunol. 2015;195(5):2483–2492. doi: 10.4049/jimmunol.1402914. [DOI] [PubMed] [Google Scholar]
- 63.Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, Connelly L, Dulek D, Peebles RS, Jr, Fingleton B, Yull FE, Stathopoulos GT, Blackwell TS. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 2015;75(8):1624–1634. doi: 10.1158/0008-5472.CAN-14-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, McEvoy J, Roussel MF, Dyer MA, Qualls JE, Murray PJ. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015;12(11):1902–1914. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196(4):459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Astigiano S, Morandi B, Costa R, Mastracci L, D’Agostino A, Ratto GB, Melioli G, Frumento G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. 2005;7(4):390–396. doi: 10.1593/neo.04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puxeddu I, Berkman N, Nissim Ben Efraim AH, Davies DE, Ribatti D, Gleich GJ, Levi-Schaffer F. The role of eosinophil major basic protein in angiogenesis. Allergy. 2009;64(3):368–374. doi: 10.1111/j.1398-9995.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 68.Varricchi G, Galdiero MR, Loffredo S, Lucarini V, Marone G, Mattei F, Marone G, Schiavoni G. Eosinophils: the unsung heroes in cancer? Oncoimmunology. 2018;7(2):e1393134. doi: 10.1080/2162402X.2017.1393134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Looi LM. Tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. A pathologic study of 422 primary and 138 metastatic tumors. Cancer. 1987;59(3):466–470. doi: 10.1002/1097-0142(19870201)59:3<466::AID-CNCR2820590319>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 70.Dorta RG, Landman G, Kowalski LP, Lauris JRP, Latorre M, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41(2):152–157. doi: 10.1046/j.1365-2559.2002.01437.x. [DOI] [PubMed] [Google Scholar]
- 71.Jain D, Tikku G, Bhadana P, Dravid C, Grover RK. The impact of peritumoral retraction clefting & intratumoral eosinophils on overall survival in oral squamous carcinoma patients. Pathol Oncol Res. 2017 doi: 10.1007/s12253-017-0328-x. [DOI] [PubMed] [Google Scholar]
- 72.Peurala E, Tuominen M, Loyttyniemi E, Syrjanen S, Rautava J. Eosinophilia is a favorable prognostic marker for oral cavity and lip squamous cell carcinoma. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2018;126(3):201–207. doi: 10.1111/apm.12809. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira DT, Biassi TP, Faustino SE, Carvalho AL, Landman G, Kowalski LP. Eosinophils may predict occult lymph node metastasis in early oral cancer. Clin Oral Investig. 2012;16(6):1523–1528. doi: 10.1007/s00784-011-0651-7. [DOI] [PubMed] [Google Scholar]
- 74.Rakesh N, Devi Y, Majumdar K, Reddy SS, Agarwal K. Tumour associated tissue eosinophilia as a predictor of locoregional recurrence in oral squamous cell carcinoma. J Clin Exp Dent. 2015;7(1):e1–e6. doi: 10.4317/jced.51610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alrawi SJ, Tan D, Stoler DL, Dayton M, Anderson GR, Mojica P, Douglas W, Hicks W, Jr, Rigual N, Loree T. Tissue eosinophilic infiltration: a useful marker for assessing stromal invasion, survival and locoregional recurrence in head and neck squamous neoplasia. Cancer J. 2005;11(3):217–225. doi: 10.1097/00130404-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Tostes Oliveira D, Tjioe KC, Assao A, Sita Faustino SE, Lopes Carvalho A, Landman G, Kowalski LP. Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol. 2009;17(3):244–249. doi: 10.1177/1066896909333778. [DOI] [PubMed] [Google Scholar]
- 77.Moezzi J, Gopalswamy N, Haas RJ, Markert RJ, Suryaprasad S, Bhutani MS. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol. 2000;95(2):520–523. doi: 10.1111/j.1572-0241.2000.01778.x. [DOI] [PubMed] [Google Scholar]
- 78.Kiziltas S, Sezgin Ramadan S, Topuzoglu A, Kullu S. Does the severity of tissue eosinophilia of colonic neoplasms reflect their malignancy potential? Turk J Gastroenterol. 2008;19(4):239–244. [PubMed] [Google Scholar]
- 79.Cho H, Lim SJ, Won KY, Bae GE, Kim GY, Min JW, Noh BJ. Eosinophils in colorectal neoplasms associated with expression of CCL11 and CCL24. J Pathol transl Med. 2016;50(1):45–51. doi: 10.4132/jptm.2015.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prizment AE, Vierkant RA, Smyrk TC, Tillmans LS, Lee JJ, Sriramarao P, Nelson HH, Lynch CF, Thibodeau SN, Church TR, Cerhan JR, Anderson KE, Limburg PJ. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod Pathol. 2016;29(5):516–527. doi: 10.1038/modpathol.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Inverse association of eosinophil count with colorectal cancer incidence: atherosclerosis risk in communities study. Cancer Epidemiol Biomark Prev. 2011;20(9):1861–1864. doi: 10.1158/1055-9965.Epi-11-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei Y, Zhang X, Wang G, Zhou Y, Luo M, Wang S, Hong C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage - colorectal cancer. Asia Pacific J Clin Oncol. 2018 doi: 10.1111/ajco.12871. [DOI] [PubMed] [Google Scholar]
- 83.Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast-cancer. Cancer. 1983;52(1):126–130. doi: 10.1002/1097-0142(19830701)52:1<126::Aid-cncr2820520123>3.0.Co;2-y. [DOI] [PubMed] [Google Scholar]
- 84.Gunduz S, Goksu SS, Arslan D, Tatli AM, Uysal M, Gunduz UR, Sevinc MM, Coskun HS, Bozcuk H, Mutlu H, Savas B. Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab. Mol Clin Oncol. 2015;3(5):1109–1112. doi: 10.3892/mco.2015.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie F, Liu LB, Shang WQ, Chang KK, Meng YH, Mei J, Yu JJ, Li DJ, Li MQ. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364(2):106–117. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 86.vanDriel WJ, Hogendoorn PCW, Jansen FW, Zwinderman AH, Trimbos JP, Fleuren GJ. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for ex less effective immune response. Hum Pathol. 1996;27(9):904–911. doi: 10.1016/s0046-8177(96)90216-6. [DOI] [PubMed] [Google Scholar]
- 87.Bethwaite PB, Holloway LJ, Yeong ML, Thornton A. Effect of tumor-assoicated tissue eosinophilia on survival of women with stage-IB carcinoma of the uterine cervix. J Clin Pathol. 1993;46(11):1016–1020. doi: 10.1136/jcp.46.11.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwasaki K, Torisu M, Fujimura T. Malignant-tumor and eosinophils. 1. Prognostic-significance in gastric-cancer. Cancer. 1986;58(6):1321–1327. doi: 10.1002/1097-0142(19860915)58:6<1321::Aid-cncr2820580623>3.0.Co;2-o. [DOI] [PubMed] [Google Scholar]
- 89.Cuschieri A, Talbot I, Weeden S. Influence of pathological tumour variables on long-term survival in resectable gastric cancer. Br J Cancer. 2002;86(5):674–679. doi: 10.1038/sj.bjc.6600161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Ren H, Wang L, Ning Z, Zhuang Y, Gan J, Chen S, Zhou D, Zhu H, Tan D, Zhang H. Clinical impact of tumor-infiltrating inflammatory cells in primary small cell esophageal carcinoma. Int J Mol Sci. 2014;15(6):9718–9734. doi: 10.3390/ijms15069718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishibashi S, Ohashi Y, Suzuki T, Miyazaki S, Moriya T, Satomi S, Sasano H. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26(2B):1419–1424. [PubMed] [Google Scholar]
- 92.Steel JL, Kim KH, Dew MA, Unruh ML, Antoni MH, Olek MC, Geller DA, Carr BI, Butterfield LH, Gamblin TC. Cancer-related symptom clusters, eosinophils, and survival in hepatobiliary cancer: an exploratory study. J Pain Symptom Manag. 2010;39(5):859–871. doi: 10.1016/j.jpainsymman.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang HK, Wan FN, Gu WJ, Zhu Y, Dai B, Shi GH, Zhang HL, Ye DW. Eosinophil percentage elevation as a prognostic factor for overall survival in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitor. Oncotarget. 2016;7(42):68943–68953. doi: 10.18632/oncotarget.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huland E, Huland H. Tumor-associated eosinophilia in interleukin-2-treated patients: evidence of toxic eosinophil degranulation on bladder cancer cells. J Cancer Res Clin Oncol. 1992;118(6):463–467. doi: 10.1007/BF01629431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sosman JA, Bartemes K, Offord KP, Kita H, Fisher SG, Kefer C, Ellis TA, Fisher RI, Higgins TJ, Gerald GJ. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: Effects of interleukin-4 alone and following interleukin-2 administration. Clin Cancer Res. 1995;1(8):805–812. [PubMed] [Google Scholar]
- 96.Bristol JA, Zhu M, Ji H, Mina M, Xie Y, Clarke L, Forry-Schaudies S, Ennist DL. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Mol Ther. 2003;7(6):755–764. doi: 10.1016/S1525-0016(03)00103-5. [DOI] [PubMed] [Google Scholar]
- 97.Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, Simeone E, Mangana J, Schilling B, Di Giacomo AM, Brenner N, Kahler K, Heinzerling L, Gutzmer R, Bender A, Gebhardt C, Romano E, Meier F, Martus P, Maio M, Blank C, Schadendorf D, Dummer R, Ascierto PA, Hospers G, Garbe C, Wolchok JD. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A, Schadendorf D, Utikal J, Umansky V. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. 2015;21(24):5453–5459. doi: 10.1158/1078-0432.CCR-15-0676. [DOI] [PubMed] [Google Scholar]
- 99.Moreira A, Leisgang W, Schuler G, Heinzerling L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy. 2017;9(2):115–121. doi: 10.2217/imt-2016-0138. [DOI] [PubMed] [Google Scholar]
- 100.Heppt MV, Heinzerling L, Kahler KC, Forschner A, Kirchberger MC, Loquai C, Meissner M, Meier F, Terheyden P, Schell B, Herbst R, Goppner D, Kiecker F, Rafei-Shamsabadi D, Haferkamp S, Huber MA, Utikal J, Ziemer M, Bumeder I, Pfeiffer C, Schad SG, Schmid-Tannwald C, Tietze JK, Eigentler TK, Berking C. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer (Oxf Engl 1990) 2017;82:56–65. doi: 10.1016/j.ejca.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 101.Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, Yonesaka K, Kudo K, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Ito A, Nakagawa K. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13(1):97–105. doi: 10.1016/j.jtho.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 102.Shelton A, Green RH, Bradding P, Free CM. Peripheral blood eosinophil count correlates with survival in lung cancer. Lung Cancer (Amst Neth) 2010;67:S40–S41. doi: 10.1016/s0169-5002(10)70123-9. [DOI] [Google Scholar]
- 103.McNeel DG, Gardner TA, Higano CS, Kantoff PW, Small EJ, Wener MH, Sims RB, DeVries T, Sheikh NA, Dreicer R. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer Immunol Res. 2014;2(10):988–999. doi: 10.1158/2326-6066.Cir-14-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract. 2015;3(2):167–174. doi: 10.1016/j.jaip.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hollande C, da Silva RB, Bondet V, Llibre A, Duffy D, Mallet V, Pol S, Albert M. DPP4 inhibition reveals interleukin-33-dependent eosinophil-mediated tumor immunity in hepatocellular carcinoma. J Hepatol. 2017;66(1):S225–S225. doi: 10.1016/s0168-8278(17)30748-1. [DOI] [Google Scholar]
- 106.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol (Baltim Md 1950) 2007;179(7):4840–4848. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]