Abstract
Tumors are highly heterogeneous tissues where malignant cells are surrounded by and interact with a complex tumor microenvironment (TME), notably composed of a wide variety of immune cells, as well as vessels and fibroblasts. As the dialectical influence between tumor cells and their TME is known to be clinically crucial, we need tools that allow us to study the cellular composition of the microenvironment. In this focused research review, we report MCP-counter, a methodology based on transcriptomic markers that assesses the proportion of several immune and stromal cell populations in the TME from transcriptomic data, and we highlight how it can provide a way to decipher the complex mechanisms at play in tumors. In several malignancies, MCP-counter scores have been used to show various prognostic impacts of the TME, which we also show to be linked with the mutational burden of tumors. We also compared established molecular classifications of colorectal cancer and clear-cell renal cell carcinoma with the output of MCP-counter, and show that molecular subgroups have different TME profiles, and that these profiles are consistent within a given subgroup. Finally, we provide insights as to how knowing the TME composition may shape patient care in the near future.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-2058-z) contains supplementary material, which is available to authorized users.
Keywords: Tumor microenvironment, Immune cells, Transcriptome, Gene expression, MCP-counter, NIBIT 2016
Introduction
Tumors are living and dynamic tissues where malignant cells are in an intricate relationship with host components recruited and modified by tumor cells. Either through direct interactions or through the chemokines and cytokines they produce, host cells influence tumor growth, migration, and metastasis, and, therefore, patient clinical outcome [1, 2]. The composition of the tumor microenvironment (TME) is diverse, containing cancer cell-supporting partners such as matrix fibroblasts, feeding and routing elements such as blood and lymphatic vessels, and hematopoietic cells such as lymphocytes, granulocytes, macrophages, mast cells, dendritic cells (DC), and myeloid-derived suppressor cells (MDSC) [3].
As a general rule, high densities of T and B lymphocytes, cytotoxic T and NK cells, M1 macrophages, and mature DC correlate with the patient’s favorable prognosis, while high densities of M2 macrophages, granulocytes, mast cells, MDSC, immature DC, Treg, and TH17 lymphocytes are associated with poor prognosis [1, 2, 4]. Quantification of these cell populations in tumors provides a major insight into patient outcome. The composition of the TME, at each stage of a cancer, untreated or even after chemo-, radio-, or immunotherapy often represents the strongest predictor for disease control [5, 6]. Moreover, it has recently been established and continuously updated that the TME provides targets and tools for efficient immunotherapies such as checkpoint blockade [7] and bi-specific antibodies [8], tumor-infiltrating lymphocytes, or chimeric antigen receptor-expressing T cells [9]. It is, therefore, essential to quantify the numbers and the types of TME cells at diagnosis, and if possible following treatment, to guide efficient patient care. Different methodologies have been successfully developed, including cell quantification by immunohistochemistry (IHC) or immunofluorescence (IF) [10–12]. These methods have the advantage of taking into account the architecture of the cancerous tissue. However, they are complex to interpret and time consuming. Transcriptomic analyses require small amounts of RNA, allowing sequential analyses and the study of both malignant cells and TME characteristics in large series of cancers. In addition, they enable the simultaneous analysis of a very large number of markers and, by measuring gene expression of chemokines, cytokines, and inflammatory molecules, they inform about the functional orientation of tumor–host interactions. Moreover, transcriptomic studies allow for the use of Next-Generation Sequencing (NGS) technologies, with a high throughput. Finally, they can easily be integrated with other molecular analyses, such as the calling of molecular subgroups. We will review here several transcriptomic methodologies and provide examples of integration of TME composition analysis with the molecular subgroups of tumors.
Analysis of whole-tumor transcriptomic data to study the TME
Many efforts have been devoted to analyzing tumor transcriptomes and estimating the composition of the TME. The difficulty lies in the extremely high cellular heterogeneity within and/or around tumors, creating several sources of variability in the transcriptomic signal [13], notably the frequency of the cell populations and the plasticity of their phenotypes. All these aspects contribute to the transcriptomic measure for a given gene, which is an average of the single-cell signals for all cells present in the sample.
Several approaches have been used to solve this problem. In pioneering work [14], a mathematical framework has been established to estimate the participation of different cell types in gene expression of heterogeneous samples. Another study [15] focused on immune cells, using four immune-originated transformed cell lines to establish distinct expression profiles of B cells, monocytes, and T cells, hence allowing deconvolution of micro-array transcriptomic data for these three cell types. Gene expression signatures have been described for 28 subclasses of immune cells [16], including effector, memory, and helper T lymphocytes. These signatures have been used, for instance, with Gene Set Enrichment Analysis (GSEA) to derive scores for each immune cell subset [17]. GSEA has also been used to define the “immunophenoscore” [18], which interestingly associates with the response to anti-checkpoint therapies.
Recent methods aim at providing highly precise quantitative information about the cell content of heterogeneous samples using deconvolution techniques. CIBERSORT, a method using support vector regression [19], allows us to estimate the relative proportions of 22 immune cell subtypes within heterogeneous tumor samples. Our team has established extremely stringent and robust gene signatures for 8 immune cell types, as well as fibroblasts and vessels [20], and used them in a method called MCP-counter, in which scores are proportional to the amounts of cells within the samples. It has been validated against controlled RNA mixtures and correlates well with IHC measures, demonstrating that it can provide an interesting alternative. ISOpure [21] software allows for the identification of cancer profiles in tumor samples, and quantification of the contribution of malignant and healthy cells to the transcriptomic signal from tumor data coupled with reference profiles. MCP-counter does not allow for the quantification of malignant cells, but does not need any reference “normal” profile. In some cases, such profiles may be hard to obtain, for instance when working with biopsies.
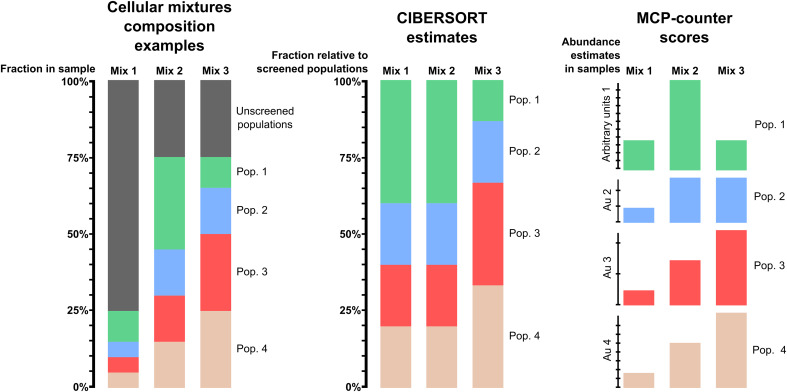
In the context of the analysis of the TME, it is important to highlight that these methods are not intended for the same use. CIBERSORT allows comparison of cell populations within a sample, whereas MCP-counter allows inter-sample comparison of immune and stromal cell populations. This is illustrated in Fig. 1. CIBERSORT estimates each screened population as a proportion within the total of the screened populations, but ignores unscreened populations. Therefore, it is a powerful tool to compare populations with one another, but is limited when used to compare different samples. In contrast, MCP-counter scores are expressed in arbitrary units specific for each population, and are proportional to the proportion of cells of this population within the whole sample, thus allowing comparisons between samples in a cohort.
Fig. 1.
Comparison of CIBERSORT estimates and MCP-counter scores for different configurations of mixture compositions. The left panel is a schematic representation of three possible cell mixtures, while the middle and right panels represent, respectively, the estimates that would be suggested by CIBERSORT and MCP-counter. We notice that the estimates of CIBERSORT for the first two mixes are similar, as they are expressed as percentages of cells among the screened populations only, regardless of the total infiltration in the sample. Conversely, MCP-counter scores are proportional to the amount of each cell population in the total sample, which allows inter-sample comparison for each population. However, these scores are expressed in a different arbitrary unit for each population, which prevents intra-sample comparison between populations. CIBERSORT allows this type of comparison
We summarize in Table 1 the availability, methodology, requirements, and outputs provided by the MCP-counter, CIBERSORT, and ISOpure.
Table 1.
Comparison of MCP-counter, CIBERSORT, and ISOpure with regards to the algorithm involved, the input necessary, and the output provided
| MCP-counter | CIBERSORT | ISOpure | ||
|---|---|---|---|---|
| Availability | R package | Online and R code | R and Matlab packages | |
| Algorithmic method | Identification of transcriptomic markers | Support vector regression | Bayesian network inference | |
| Input | Tumor transcriptome | Preprocessed | Preprocessed | Preprocessed |
| Reference profiles | None needed | None needed | Necessary | |
| Output | Tumor purity | No | No | Yes, and separation of tumor and normal profiles |
| Cell populations quantified | 8 Immune populations, endothelial cells and fibroblasts | 22 hematopoietic populations | Malignant and normal | |
| Absolute quantification | Scores proportional to each cell population’s abundance within the whole sample | Proportion of each population within the analyzed populations | Estimation of the proportion of malignant and normal cells in the whole sample |
One of the major hurdles of transcriptomic analyses of cell quantification is the potential discrepancies between quantification of cell population by gene signatures expression and the density of the corresponding cell type in a tissue, particularly in a tumor. We addressed this question recently. We have shown using in vitro mRNA multi-populations spike-in experiments that MCP-counter scores strongly correlate with the abundance of the corresponding population (Pearson’s r between 0.92 and 0.99). Moreover, although not as perfect, the metagene expression of T cells, cytotoxic cells, and monocytes correlated (r ≥ 0.67) with the intra-tumoral density of CD3+, CD8+, and CD68+ cells quantified by IHC [20].
Applying MCP-counter to 25 different cancers, we confirmed that the inferred densities of lymphoid subsets, particularly T, B, NK, and cytotoxic cells, correlate with favorable prognosis in most cases, whereas those of fibroblasts are markers of poor prognosis [20]. Endothelial cell metagene expression is variably correlated with prognosis [20], renal cell cancer being an interesting example. In this disease, a high density of T cells, including CD8+ lymphocytes [11], as well as the expression of their corresponding metagenes correlate with shorter disease-free survival, while the metagene expression of endothelial cells correlates with longer survival [20]. We have recently shown that the deleterious impact of T cells on clear cell renal cell cancer (ccRCC) patients’ clinical outcome correlates with a strong infiltration by T regulatory cells [22] which, in addition to PD-L1 and PD-L2 expression in malignant cells [11], potentially inhibit the anti-tumor immune reaction [22]. In addition, it is surprising that a high endothelial cell density and subsequent angiogenesis positively correlates with the patient’s survival [20]. Whether this may be due to a good response rate of these patients to anti-angiogenic therapies [23] remains an open question [24].
It is of interest that, depending on the cancer type, combinations of different metagene expression correlate better with patient survival. Thus, a combination of high T and B lymphocyte metagene expression, probably reflecting the density of tertiary lymphoid structures [25, 26], is the strongest favorable parameter for lung adenocarcinoma, whereas a combination of high T cells and low fibroblast gene signatures expression predict best survival in colorectal cancer; a combination of high cytotoxic lymphocyte and low monocytic lineage signatures sign best prognosis in breast cancer [20]. It is, therefore, easily conceivable that the phenotypes of the malignant cells influence the composition and functional orientation of the TME. To address this question, we investigated the relationships between molecularly defined subgroups of cancers and TME composition.
Associations between TME composition and mutational load of tumors
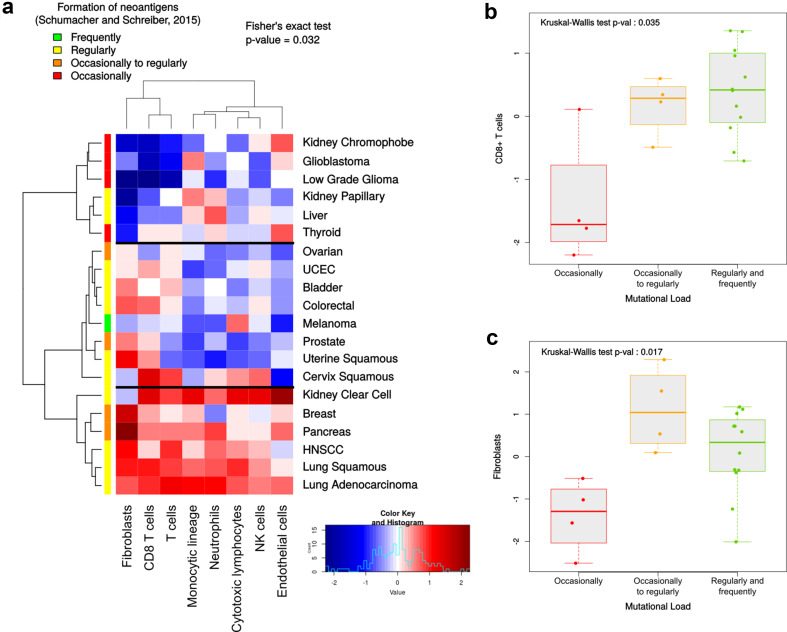
In parallel with the appearance and accumulation of somatic mutations, tumors tend to express neoantigens that may trigger anti-tumoral immune responses via activation of lymphocytes directed against them [27]. We, therefore, hypothesized that the immune infiltrate, and more generally the whole TME, may vary according to the mutational load of the tumors. Following Schumacher and Schreiber’s classification of malignancies according to their propensity to generate neoantigens (as defined by the somatic mutation prevalence) [27], we analyzed the cohort-wide TME of 20 cancers from the TCGA PANCAN cohort, using MCP-counter (Fig. 2). Malignancies are classified into four groups, referring to the formation of neoantigens: frequently, regularly, occasionally to regularly, and occasionally. We grouped them according to the TME composition in terms of T cells, CD8+ T cells, cytotoxic lymphocytes, NK cells, monocytic lineage, neutrophils, endothelial cells, and fibroblasts (Fig. 2a). Interestingly, this analysis grouped the cancers having the least amount of somatic mutations (thyroid, low grade glioma, glioblastoma, and kidney chromophobe) in the same cluster characterized by low lymphocytic and fibroblastic infiltrates associated with heterogeneous myeloid and vascularization contents. In particular, when comparing CD8+ T cells (Fig. 2b) and fibroblasts (Fig. 2c) with the mutational burden of malignancies, we found that the infiltration in the “occasionally mutated” tumors was significantly lower. However, of all the other screened populations, only T cells exhibited a variation of their infiltrate between the cancer groups (Supplementary Fig. 1).
Fig. 2.
a Classification of 20 malignancies based on the TME composition assessed by MCP-counter, along with the estimation of the frequency of neoantigens proposed by Schumacher and Schreiber [27]. b, c Infiltration by CD8+ T cells (b) and fibroblasts (c) is significantly lower in tumors in which mutations occur only occasionally
Integration of molecular subgroups and TME composition
Whole transcriptome analyses have been already used to classify different malignancies with prognostic-impacting molecular subgroups. The first reports were made in lymphomas [28, 29] and breast cancers [30, 31]. Many others have been published and are being refined (reviewed in [1]). We have applied MCP-counter to characterize tumors that were previously classified into molecular subgroups: colorectal cancer (CRC) and ccRCC. Efforts associating six laboratories have converged into a consensus classification of CRC in four molecular subgroups named “Consensus Molecular Subgroups” 1–4 [32]. CMS1 corresponds to highly mutated tumors, encompassing most microsatellite unstable (MSI) tumors lacking DNA-repair genes; these are known to be highly infiltrated by lymphocytes. CMS2 contains canonical tumors with WNT/β-Catenin/MYC activation. CMS3 is rich in metabolically deregulated tumors, with some prevalence of KRAS-mutated cancers. CMS4 has markers of epithelial–mesenchymal transition, with a TGF-β signature and high fibroblast content. Using MCP-counter, we confirmed and extended the analysis of Guinney et al., validating our approach, defining CMS1 as a subgroup highly enriched in cytotoxic T cells and moderate fibroblastic abundance, and we also showed that this subgroup had high immune checkpoint expression, IFNγ signature, high class 1 major histocompatibility complex (MHC1) antigen expression, moderate inflammation, and angiogenesis [33]. It is striking that it is, indeed, the best prognostic group for disease-free survival [32] and that most if not all MSI patients from this group respond to anti-PD1 antibodies [34], which is consistent with the findings of Guiney et al. showing PD-1 activation for CMS1 tumors. CMS2 and CMS3 are immune “deserts” with low MHC1 expression, no signs of immune, and/or inflammatory cells. CMS4 has a high lymphocytic infiltration, with checkpoint inhibitors and high MHC1 expression but lacking IFNγ gene expression, together with high inflammatory, angiogenic, and fibroblastic invasion. Strikingly, despite its high infiltration by lymphocytes, this subgroup exhibits the worst clinical outcome. It illustrates the putative overcoming of a potential anti-tumor reaction by inflammatory, angiogenic, and immunosuppressive (via TGFβ for instance) mechanisms. Therefore, the integration of TME and malignant cell classifications paves the way for different immunotherapeutics, combined and/or sequential, to control CRC. These ways are being explored [33, 35].
Metastatic ccRCC has also been recently stratified into four molecular subgroups [36] that we have subjected to MCP-counter analysis [37]. ccRCC1 is a poor prognosis group, with a low response rate to tyrosine kinase inhibitor (TKI) Sunitinib, with little to no immune infiltration; it also exhibits low MHC1 expression. Patients from ccRCC2 and ccRCC3 subgroups respond to sunitinib and have a good prognosis; their tumors are heterogeneous in terms of TME composition. ccRCC4 is the worst prognostic group, very poorly responding to Sunitinib; it is highly enriched in T and NK lymphocytes in the context of high myeloid and fibroblastic infiltration. It probably corresponds to the subgroup recently being defined as being rich in Treg [22]. This subgroup, which also exhibits high MHC1 expression, checkpoints inhibitors on T cells and their ligands on malignant cells certainly might benefit from therapies associating immune checkpoint inhibition with other approaches.
Conclusions
The dialectic interactions of TME components with malignant cells depend on the organ where they happen, the oncogenic processes involved, and their modification by treatments. The recent availability of high-throughput methodologies to quantify the different elements of the TME and understand their functionality opens the way for generalization of these approaches and the subsequent application of precision tailored therapies based on these landscapes rather than on cancer subtypes only. Associating cancer-targeted therapies with TME targeted agents is still in its infancy (Fig. 3). The main issue in the years to come will be to bring these powerful tools from the bench to the bedside. To use these high-throughput methodologies in clinical routine, two pre-requisites will be needed: first, we will have to prove and validate their predictive value of response to tailored therapies rather than their overall prognostic value; second, we will have to validate these approaches using widely available tissues such as formalin-fixed and paraffin-embedded tumor tissues. Once these major steps have been achieved, we will be ready to enter in a new era of real precision and tailored medicine and there can be no doubt that we will see more patients cured.
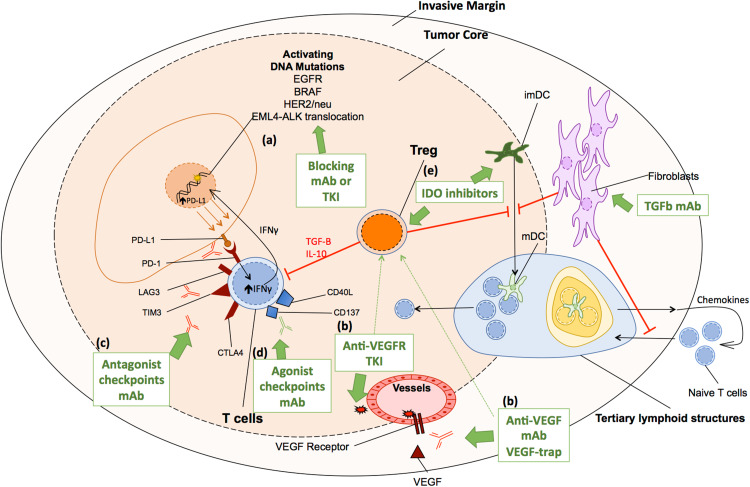
Fig. 3.
Mainly targeted therapies currently approved or at a late stage of development for human solid tumors. Targeted therapies act directly on tumor cells or on populations of the tumor microenvironment (TME). a Tumor cells harbor activating DNA mutations or translocations which can be efficiently targeted by monoclonal antibodies (mAb) or TKI. b Endothelial cells involved in neoangiogenesis are efficiently targeted either by a VEGF or a VEGFR blocking mAb, a VEGF-trap, or a VEGFR-TKI. Effector function of CD8 T cells can be restored by c antagonist mAb of co-inhibition signals such as PD-1, Tim-3, Lag-3, or CTLA-4, or by d agonist mAb of co-stimulation signals such as CD137, CD40L. Regulatory T cells (Treg) impede the anti-tumor immune response by a direct effect on CD8 T cells and by the blockade of the maturation of dendritic cells. Treg functions, as well as Myeloid-Derived Suppressor Cells (MDSC), may be suppressed by e IDO inhibitors or VEGF–VEGFR axis inhibitors. Fibroblasts control the trafficking of T cells from the invasive margin (IM) to the tumor stroma, hamper DC maturation, inhibit T-cell proliferation, and sustain angiogenesis. Fibroblasts might be targeted by TGFb blocking mAb
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We wish to thank Benoît Beuselinck, Laetitia Lacroix, Pierre Laurent-Puig, Stéphane Oudard, Jessica Zucman-Rossi, and all other members of the teams who contributed to the data.
Abbreviations
- ccRCC
Clear-cell renal cell carcinoma
- CIBERSORT
Cell-type identification by estimating relative subsets of RNA transcripts
- CMS
Consensus molecular subgroups
- CRC
Colorectal cancer
- GSEA
Gene set enrichment analysis
- MCP-counter
Microenvironment cell population-counter
- MSI
Microsatellite instable
- NGS
Next-generation sequencing
- NIBIT
Network Italiano per la Bioterapia dei Tumori (the Italian network for cancer biotherapy)
- TCGA PANCAN
The cancer genome atlas pan-cancer cohort
- Th17
T helper lymphocyte producing interleukin 17
- TME
Tumor microenvironment
- Treg
Regulatory T cell
Compliance with ethical standards
Funding
This work was supported by the Institut National de la santé et de la Recherche Medicale (INSERM), University Paris-Descartes, University Pierre and Marie Curie, the Site de Recherche Integrée sur le Cancer (SIRIC) Cancer Research for Personalized Medicine (CARPEM) program, the LabEx Immuno-Oncology (LAXE62_9UMRS972 FRIDMAN), the Institut National Du Cancer (INCa), and the Cancéropôle Ile-de-France, O. Lecomte. Florent Petitprez is recipient of a CARPEM fellowship.
Conflict of interest
Yann A. Vano has received speaker honoraria from Novartis, Pfizer, Bristol-Myers-Squibb, and Astellas Sanofi, and has received financial support for attending symposia from Bristol-Myers-Squibb and Novartis. Aurélien de Reyniès holds intellectual property rights for patents related to immune cell population abundance estimation through transcriptomic analysis. Wolf H. Fridman is a consultant for Pierre Fabre Medicament, Sanofi, Bristol-Myers-Squibb, Novartis, Curetech, Servier, Efranet, Efralys, and Adaptimmune. All other authors declare no conflicts of interest.
Footnotes
Florent Petitprez and Yann A. Vano contributed equally.
References
- 1.Becht E, Giraldo NA, Germain C, et al. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv Immunol. 2016;130:95–190. doi: 10.1016/bs.ai.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Becht E, Giraldo NA, Dieu-Nosjean M-C, et al. Cancer immune contexture and immunotherapy. Curr Opin Immunol. 2016;39:7–13. doi: 10.1016/j.coi.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 6.Remark R, Alifano M, Cremer I, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 7.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Wang Y, Han W. Chimeric antigen receptor-modified T cells for solid tumors: challenges and prospects. J Immunol Res. 2016;2016:3850839. doi: 10.1155/2016/3850839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 11.Giraldo NA, Becht E, Pagès F, et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res. 2015;21:3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 12.Granier C, Dariane C, Combe P, et al. Tim-3 Expression on tumor-infiltrating PD-1(+)CD8(+) T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2017;77:1075–1082. doi: 10.1158/0008-5472.CAN-16-0274. [DOI] [PubMed] [Google Scholar]
- 13.Shen-Orr SS, Gaujoux R. Computational deconvolution: extracting cell type-specific information from heterogeneous samples. Curr Opin Immunol. 2013;25:571–578. doi: 10.1016/j.coi.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venet D, Pecasse F, Maenhaut C, Bersini H. Separation of samples into their constituents using gene expression data. Bioinformatics. 2001;17:S279–S287. doi: 10.1093/bioinformatics/17.suppl_1.S279. [DOI] [PubMed] [Google Scholar]
- 15.Abbas AR, Wolslegel K, Seshasayee D, et al. Deconvolution of blood microarray data identifies cellular activation patterns in systemic lupus erythematosus. PLoS One. 2009;4:e6098. doi: 10.1371/journal.pone.0006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Şenbabaoğlu Y, Gejman RS, Winer AG, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 2016;17:231. doi: 10.1186/s13059-016-1092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charoentong P, Finotello F, Angelova M, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17:218. doi: 10.1186/s13059-016-1070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quon G, Haider S, Deshwar AG, et al. Computational purification of individual tumor gene expression profiles leads to significant improvements in prognostic prediction. Genome Med. 2013;5:29. doi: 10.1186/gm433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giraldo NA, Becht E, Vano Y, et al. Tumor-infiltrating and peripheral blood T cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2848. [DOI] [PubMed] [Google Scholar]
- 23.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 24.Bauman TM, Huang W, Lee MH, Abel EJ. Neovascularity as a prognostic marker in renal cell carcinoma. Hum Pathol. 2016;57:98–105. doi: 10.1016/j.humpath.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Dieu-Nosjean M-C, Goc J, Giraldo NA, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Dieu-Nosjean M-C, Giraldo NA, Kaplon H, et al. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271:260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 27.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 28.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 29.Ando T, Suguro M, Kobayashi T, et al. Multiple fuzzy neural network system for outcome prediction and classification of 220 lymphoma patients on the basis of molecular profiling. Cancer Sci. 2003;94:906–913. doi: 10.1111/j.1349-7006.2003.tb01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guedj M, Marisa L, de Reynies A, et al. A refined molecular taxonomy of breast cancer. Oncogene. 2012;31:1196–1206. doi: 10.1038/onc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 32.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becht E, de Reyniès A, Giraldo NA, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22:4057–4066. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 34.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dienstmann R, Vermeulen L, Guinney J, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 36.Beuselinck B, Job S, Becht E, et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin Cancer Res. 2015;21:1329–1339. doi: 10.1158/1078-0432.CCR-14-1128. [DOI] [PubMed] [Google Scholar]
- 37.Becht E, Giraldo NA, Beuselinck B, et al. Prognostic and theranostic impact of molecular subtypes and immune classifications in renal cell cancer (RCC) and colorectal cancer (CRC) Oncoimmunology. 2015;4:e1049804. doi: 10.1080/2162402X.2015.1049804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.