Abstract
Background
The status of antitumor immunity represented by the expression of programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) and immune cell (IC) infiltration is unknown in HIV-infected patients with non-small cell lung cancer (NSCLC).
Methods
Fifteen HIV-infected patients with NSCLC were compared with 29 non-HIV-infected patients with NSCLC. Analysis of 13 propensity-score-matched patients in the two groups was also compared. The expression of PD-1/PD-L1 and tumor infiltration by CD4+, CD8+, and CD56+ immune cells were examined by immunohistochemistry; score of ≥ 2 was defined as positive.
Results
Although high PD-L1 expression in tumor cells was observed in HIV and non-HIV cohorts, the association of PD-1/PD-L1 was significant only in the HIV cohort. In overall as well as the propensity-matched analyses, HIV-infected patients with high PD-L1 expression showed shorter survival than HIV-infected patients with low PD-L1 expression; no significant difference was observed in this respect in the non-HIV cohort.
Conclusion
High PD-L1 expression in tumor tissue was associated with poor prognosis in HIV-infected NSCLC patients but not in non-HIV-infected NSCLC patients. These results suggest that antitumor immunity by PD-1/PD-L1 axis might be suppressed more in HIV-infected NSCLC patients as compared to their non-HIV-infected counterparts.
Electronic supplementary material
The online version of this article (10.1007/s00262-017-2103-y) contains supplementary material, which is available to authorized users.
Keywords: HIV, Non-small cell lung cancer (NSCLC), Immunohistochemistry, PD-1, PD-L1, Immune cell infiltration
Introduction
Recent advances in highly active antiretroviral therapy have helped to achieve stronger inhibition of HIV and restoration of immune function, which has made survival of HIV-infected patients similar to that of non-HIV-infected populations [1]. However, a recent increase in mortality from non-AIDS defining cancers (NADCs) in HIV-infected patients has been reported. Of these, lung cancer is the most common NADC and accounts for 5% of all deaths among HIV-infected patients [2]. Immunodeficiency is a significant risk factor for malignancy and the high incidence of lung cancer in HIV-infected patients seems to be related with HIV-induced immunodeficiency. In a previous study, microsatellite alterations that generate genetic instability were observed more frequently in lung cancer patients infected with HIV, as compared to their non-HIV-infected counterparts [3]. However, a retrospective analysis found no direct association of low CD4+ T cell counts or elevated HIV viral load on the incidence of lung cancer in HIV-infected patients [4]. The etiopathogenesis of lung cancer in HIV-infected patients is not well understood owing to a paucity of investigations on the molecular mechanism of tumor development in these patients.
The advent of the immune checkpoint blockade therapy with mAbs targeting immune co-inhibitory molecules has brought about a paradigm shift in the treatment of malignant tumors. The blockade of interaction between PD-1 on the immune effector cells and the PD-L1 on TC has been shown to reverse the suppression of antitumor immunity and to show a significant antitumor effect in the context of different malignancies including advanced non-small cell lung cancer (NSCLC) [5]. Currently, different types of mAb therapies are available and these include: nivolumab for tumors with squamous cell histology [6] and non-squamous cell histology [7] in the second-line setting, pembrolizumab for tumors with high PD-L1 expression (≥ 50%) in the first-line setting [8], or in the second-line setting for tumors with 1–49% PD-L1 expression [9], and atezolizumab for all subtypes of NSCLC in the second-line setting [10].
The efficacy of immune checkpoint blockade therapy with anti-PD-1 mAbs against NSCLC in HIV-infected patients is not known. Exhaustion of CD8+ T cells owing to persistent immune stimulation is well documented in HIV- infected patients on antiretroviral therapy [11]. Moreover, the status of antitumor immune response in HIV-infected NSCLC patients, which is an essential prerequisite for successful immune checkpoint blockade therapy, is not well characterized. In the present study, PD-1/PD-L1 expression and immune cell infiltration in lung cancer tissues were analyzed and correlations with clinical characteristics examined in HIV-infected and non-HIV-infected patients with NSCLC.
Patients and methods
Patients and tumor specimens
All consecutive HIV-infected NSCLC patients (n = 15; all males; median age at diagnosis: 63 years) diagnosed between June 1996 and August 2015 at the Tokyo Metropolitan Cancer and Infectious disease Center Komagome Hospital were included in the study. Data pertaining to the following clinical and biological characteristics were compared: age, gender, smoking status; Eastern Cooperative Oncology Group (ECOG) performance status (PS) at diagnosis; tumor histology (2004 World Health Organization classification); clinical stage (7th TNM classification), and treatment received for lung cancer. HIV viral load and median CD4+ lymphocyte counts at diagnosis of NSCLC were also examined. The patients were observed until August 2015. Thirty clinically-matched non-HIV-infected patients with NSCLC, diagnosed between 2007 and 2015 at our institution were included in the control group.
Immunohistochemistry
The expression of PD-1/PD-L1 and infiltration of CD4+, CD8+, and CD56+ immune cells (ICs) in NSCLC tissue from patients were examined by immunohistochemistry (IHC). Five micrometer-thick sections were prepared from formalin-fixed, paraffin-embedded tissue specimen blocks. Immunostaining was automatically performed using HISTSTAINER (Nichirei Biosciences, Tokyo, Japan). Sections were treated with primary antibodies: CD8 (C8/144B; 1:100, Nichirei Bioscience, Tokyo, Japan); CD4 (NCL-CD4-IF6; 1:100, Novocastra Laboratories, Newcastle, UK); CD56 (clone CD56; 1:50, Novocastra Laboratories, Newcastle, UK); PD-1 (NAT105, 1:500, Abcam, Cambridge, UK); PD-L1 (E1L3N, 1:800, Cell Signaling Technology, Danvers, MA). Secondary antibodies used were SKJ-4100 (Vector Laboratories, Burlingame, CA) and DAB peroxidase substrate kit SK-4100 (Vector Laboratories).
Stained slides were examined independently by two pathologists, who were blind to the clinical information. Whole areas of tumor cells (TC) were examined for PD-L1 expression under light microscope, at 100× magnification, in at least five fields of randomly selected tumor areas. PD-L1 expression in the ICs was excluded from this analysis. Counts of CD4+, CD8+, CD56+, and PD-1 ICs in the stromal areas were performed at 400× magnification in at least five randomly selected fields (Supplementary figures; 1, 2, 3, and 4).
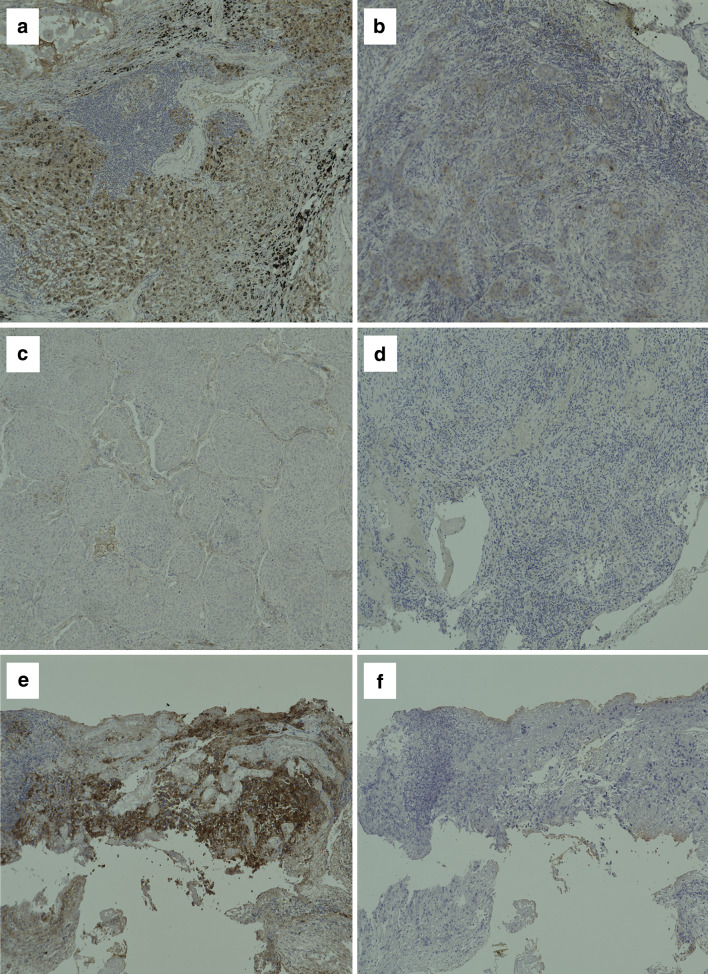
The intensity scoring criteria were adopted from the criteria used in OAK studies [10] and POPLAR [12]. The expression of PD-L1 was scored as percentage of observed area: TC3 ≥ 50% (Fig. 1a); TC2 ≥ 5% and < 50% (Fig. 1b); TC1 ≥ 1 and < 5% (Fig. 1c); and TC0 < 1% (Fig. 1d). The infiltration of ICs was scored as a percentage of the observed area: IC3 ≥ 10% (Fig. 1e); IC2 ≥ 5 and < 10%; IC1 ≥ 1 and < 5%; and IC0 < 1% (Fig. 1f).
Fig. 1.
Immunohistochemistry of PD-L1 expression and infiltration of ICs in tumor tissues from patients. Representative images of few examples of PD-L1+ non-small cell lung cancer tissue sections, stained for PD-L1 by Immunohistochemistry. The expression of PD-L1 was scored as percentage of observed area, as described in “Patients and methods”. a 3 +, b 2 +, c 1 +, d 0. Infiltration of CD8+ T cells in tumor microenvironment. The infiltration of ICs was scored as a percentage of the observed area. e 3 + and f 0
This study was approved by the institutional review board at The Jikei University School of Medicine (26-010 7515) and the Tokyo Metropolitan Cancer and Infectious disease Center Komagome Hospital (#1468).
Statistical analysis
We performed matched analyses to minimize the effect of selection bias and potential confounding. We adjusted for differences in the baseline characteristics of patients by propensity-score matching [13, 14]. The following five variables were used to calculate the propensity scores: age, epidermal growth factor receptor (EGFR) mutation sites, PS, cigarette smoking, recurrence, and advanced stage. The statistic was calculated to evaluate the goodness of fit. Patients in the HIV and the non-HIV groups were matched with the closest estimated propensity score using the following algorithm: 1:1 optimal match with a ± 0.02 caliper and no replacement [15].
The association between the intensity of PD-L1 expression or the infiltration of ICs and clinicopathological variables was analyzed statistically using Pearson’s Chi-square test. The correlation between the intensity of PD-L1 expression and IC infiltration was assessed using Spearman’s rank correlation. In addition, between-group differences with respect to PD-L1 expression and categorical variables were assessed with the Mann–Whitney U test.
Survival time was measured from the date of diagnosis to the date of death or lost follow-up and survival curves were generated by Kaplan–Meier method. Between–group difference in survival was assessed using the log-rank test. Univariate analyses were performed using the Mann–Whitney U test for continuous variables, and Fisher’s exact test for categorical variables.
A two-sided p value < 0.05 on univariate analysis was regarded as statistically significant. Each variable (HIV status, staging, EGFR mutation status, and PD-L1 expression) was quantified using Cox proportional hazard model with 95% confidence interval (CI) (a two-sided p value < 0.05 was considered as statistically significant). Analyses were performed for the overall cohort as well as for the propensity-matched cohorts to confirm reproducibility.
All statistical analyses were performed with JMP 11.2.1 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Clinical characteristics of the NSCLC patients in HIV or non-HIV cohort of all the patients or the propensity-matched cohorts are shown in Table 1. Fifteen HIV and twenty-nine non-HIV patients were included for the unmatched analysis, and for the propensity-matched analysis, thirteen patients from each propensity-matched cohort were included. NSCLC was diagnosed by histopathological examination of surgical specimen in 53.3% (n = 8) of the HIV cohort and 50.0% (n = 15) of the non-HIV cohort, and by that of bronchoscopic biopsy specimen in the remaining patients. Treatment details and prognosis of the lung cancer patients are shown in Table 2. Although patient profiles were comparable between the HIV and non-HIV cohorts, 1-year survival rate in the HIV cohort (66.7%) was significantly lower than that in the non-HIV cohort (100%) (Table 2).
Table 1.
Baseline demographics and clinical characteristics of lung cancer patients living with or without HIV infection
| Patient characteristics | HIV cohort | Non-HIV cohort | Propensity score matched | |||||
|---|---|---|---|---|---|---|---|---|
| HIV cohort | Non-HIV cohort | |||||||
| No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % | |
| Number of patients | 15 | – | 29 | – | 13 | 13 | ||
| Median age, years (range) | 63 (39–77) | – | 62 (39–76) | 63 (50–77) | 64 (43–76) | |||
| Tobacco use | ||||||||
| Heavy smoker | 12 | 80.0 | 23 | 79.3 | 10 | 76.9 | 8 | 61.5 |
| Never smoked | 3 | 20.0 | 6 | 20.7 | 3 | 23.1 | 5 | 38.5 |
| Performance status (ECOG) | ||||||||
| 0, 1 | 12 | 80.0 | 24 | 82.7 | 10 | 76.9 | 11 | 84.6 |
| 2 | 3 | 20.0 | 4 | 13.8 | 0 | 0 | 1 | 7.7 |
| 3, 4 | – | 1 | 3.5 | 3 | 23.1 | 1 | 7.7 | |
| Median CD4 count (cells/μL) [range] | 415 [118–635] | – | – | – | 425 [118–635] | – | – | – |
| HIV viral load (copies/μL) | ||||||||
| ≥ 50 | 6 | 40.0 | – | – | 5 | 38.5 | – | – |
| Undetectable | 9 | 60.0 | 8 | 61.5 | ||||
| Lung cancer characteristics | ||||||||
| Histology | ||||||||
| Adenocarcinoma | 11 | 73.3 | 19 | 65.5 | 9 | 69.2 | 9 | 69.2 |
| Squamous cell carcinoma | 4 | 26.7 | 10 | 34.5 | 4 | 30.8 | 4 | 30.8 |
| Driver mutation | ||||||||
| EGFR mutation status | ||||||||
| Mutation (exon 19/exon 21) | 3 (2/1) | 20.0 | 6 (3/4) | 20.7 | 3 | 23.1 | 1 | 7.7 |
| Wild type | 8 | 53.3 | 12 | 41.4 | 7 | 53.8 | 8 | 61.5 |
| Unknown | 4 | 26.7 | 9 | 31.0 | 3 | 23.1 | 4 | 30.8 |
| ALK rearrangement | – | – | 2 | 6.9 | – | – | – | – |
| Stage | ||||||||
| I (A/B) | 6 (4/2) | 40.0 | 8 (3/5) | 27.6 | 4 (2/2) | 30.7 | 4 (2/2) | 30.7 |
| II (A/B) | 0 (0/0) | 0.0 | 1 (1/0) | 3.4 | 0 (0/0) | 0 | 0 (0/0) | 0 |
| III (A/B) | 6 (3/3) | 40.0 | 11 (5/6) | 37.9 | 6 (3/3) | 46.2 | 6 (2/4) | 46.2 |
| IV | 3 | 20.0 | 9 | 31.0 | 3 | 23.1 | 3 | 23.1 |
Heavy smoker is defined as Brinkman Index or BI ≥ 400
EGFR epidermal growth factor receptor, ALK anaplastic lymphoma kinase.
Table 2.
Lung cancer treatment modalities for the patients infected with HIV
| HIV cohort (n = 15) | % | Non-HIV cohort (n = 29) | % | Propensity score matched | ||||
|---|---|---|---|---|---|---|---|---|
| HIV cohort (n = 13) | % | Non-HIV cohort (n = 13) | % | |||||
| Cancer therapy in the initial setting | ||||||||
| Surgery alone | 8 | 53.3 | 15 | 51.7 | 6 | 46.2 | 7 | 53.8 |
| Radiotherapy alone | 1 | 6.7 | 1 | 3.4 | 1 | 7.7 | 0 | 0 |
| Chemoradiotherapy (concurrent) | 2 | 13.3 | 4 | 13.8 | 2 | 15.4 | 4 | 30.8 |
| Chemotherapy | 4 | 26.7 | 9 | 31.0 | 4 | 30.7 | 2 | 15.4 |
| Survival time | ||||||||
| Patients in all stages (95% CI) | 45.1 months (21.3–not reached) | – |
57.5 months (21.4–102.6) |
– | 45.1 months (21.3–not reached) | – | 102.6 months (12.9–102.6) | – |
| Advanced stage (Stage IV), (95% CI) | 21.3 months (2.4–46.1) | – | 21.4 months (14.1–57.7) | – | 21.3 months (2.4–46.1) | – | 21.4 months (14.1–57.5) | – |
| 1-year survival rate in advanced stages | 66.7% | – | 100.0% | – | 66.7% | – | 100.0% | – |
| 2-year survival rate in advanced stages | 33.3% | – | 35.7% | – | 33.3% | – | 35.7% | – |
CI confidence interval
Immunohistochemical analyses of PD-1/PD-L1 expression and IC infiltration of NSCLC tissue in HIV and non-HIV cohort
Representative IHC images of stained sections are shown in Fig. 1. Overall, PD-L1 expression in NSCLC tissues was positive (IHC 2 + and 3 +) in 33.3% of HIV patients and 27.6% of non-HIV patients (Fig. 1). Positive PD-1 expression was found in 13.3% of HIV patients but only in 6.9% of non-HIV patients. Infiltration and accumulation of CD4+ T cells in tumor specimens from HIV patients (69.6%) were less intense than that in the non-HIV patients (93.1%). No significant difference was seen in the infiltration of CD8+ T cells and CD56+ cells between HIV and non-HIV (Fig. 2).
Fig. 2.
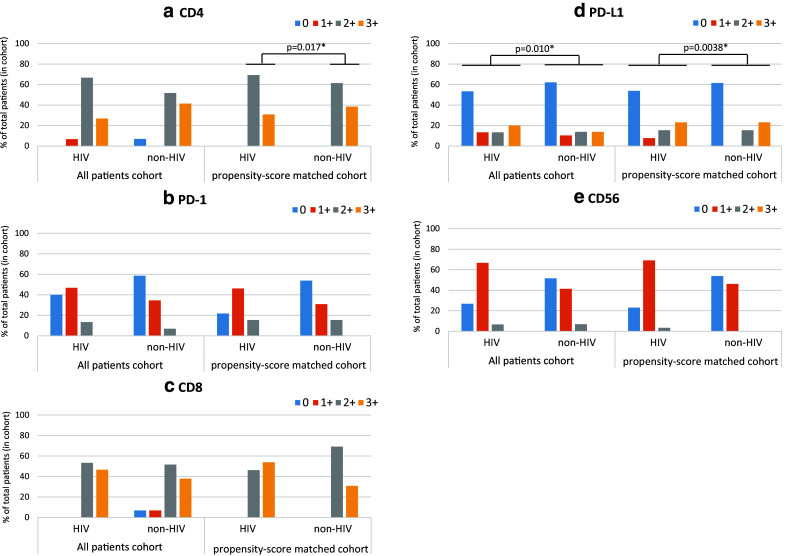
Percent of tumor-infiltrating cells (IC) expressing CD4 (a), PD-1 (b), CD8 (c), or CD56 (e), and percent of tumor cells (TC) expressing PD-L1 (d) in all patients and in propensity-score matched patients. Blue bars, red bars, gray bars, and yellow bars indicate the intensity of staining and correspond to: 0 (< 1%), 1 + (≥ 1% and < 5%), 2 + (≥ 5% and < 10%), 3 + (≥ 10%) of the stained population, respectively for figures (a–c, e), and 0 (< 1%), 1 + (≥ 1% and < 5%), 2 + (≥ 5 and < 50%), 3 + (≥ 50%) of the stained population, respectively for figure (d)
In the total study population (HIV plus non-HIV cohort), no correlation of the PD-L1 score with CD8 (p = 0.86), CD4 (p = 0.080), CD56 (p = 0.73), and PD-1 scores (p = 0.56) was observed. However, in the HIV cohort, a significant correlation between PD-L1 and PD-1 scores was observed (p = 0.021), while no such correlation was seen in the non-HIV cohort. In the propensity-matched patients, PD-L1 expression showed a strong correlation with PD-1 (p = 0.0081) and a weak correlation with CD8 (p = 0.035) scores; no other correlations were observed in the propensity-matched patients.
Association of patient survival with PD-1/PD-L1 expression in NSCLC tissues in HIV and non-HIV cohort
Overall survival results are shown in Fig. 3 for all (Fig. 3a–j) and propensity-score-matched (Fig. 3k–t) HIV-infected and non-HIV-infected NSCLC patients, according to the IHC-based expression status of CD4, CD8, CD56, PD1, and PD-L1 in the tumors. These results are further analyzed by univariate and multivariate analyses (Table 3). On univariate analysis, no significant difference in survival was observed between the high and low PD-L1 expression subgroups in the non-HIV cohort (p = 0.80). However, the high PD-L1 expression subgroup in the HIV cohort showed significantly shorter survival, compared to the low PD-L1 expression subgroup (p = 0.0003) (Fig. 3i; Table 3). In the propensity-score matched cohort, survival of high PD-L1 expression subgroup in the HIV cohort was significantly shorter than in the low PD-L1 expression subgroup (p = 0.0010) (Fig. 3s). No significant difference in survival was observed between the high and low PD-L1 expression subgroups in the non-HIV cohort (p = 0.67) (Fig. 3t). Low level of infiltration of CD56+ cells in the tumor tissue was significantly associated with poor prognosis in both the HIV (p = 0.0002) (Fig. 3e) and the non-HIV cohorts (p = 0.002) (Fig. 3f).
Fig. 3.
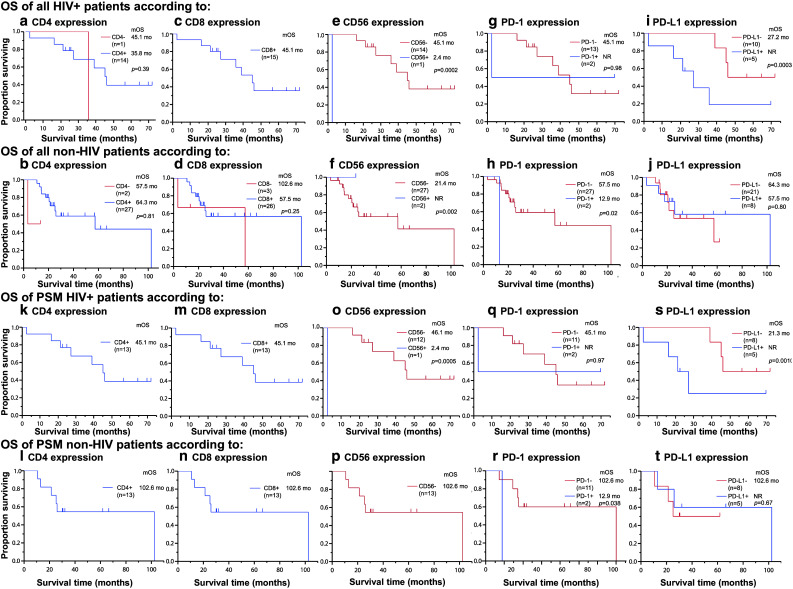
Kaplan–Meier curves showing overall survival (OS) for HIV patients and non-HIV patients having lung cancer. OS of all patients was shown according to expression status in IHC of CD4 (a, b), CD8 (c, d), CD56 (e, f), PD-1 (g, h), and PD-L1 (i, j). OS of the propensity-score-matched HIV patients and non-HIV patients having lung cancer was shown according to expression status in IHC of CD4 (k, l), CD8 (m, n), CD56 (o, p), PD-1 (q, r), and PD-L1 (s, t). OS overall survival, mOS median overall survival, mo month, NR not reached, PSM propensity-score matched, IHC Immunohistochemistry
Table 3.
Univariate and multivariate analyses of overall survival in lung cancer patients with and without HIV
| Univariate analysis | ||||||
|---|---|---|---|---|---|---|
| Variants | HIV | Non-HIV | ||||
| n | Median OS (months) [95% CI] | p value | n | Median OS (months) [95% CI] | p value | |
| Immunological status | ||||||
| PD-L1 | ||||||
| High | 5 | NR [35.8–NR] | 0.0003* | 8 | 64.3 [3.4–102.6] | 0.80 |
| Low | 10 | 27.2 [2.4–27.2] | 21 | 57.5 [20.5–NR] | ||
| PD-1 | ||||||
| High | 2 | NR [2.4–NR] | 0.98 | 3 | 12.9 [NR] | 0.002 |
| Low | 13 | 45.1 [35.8–NR] | 26 | 57.5 [21.4–102.6] | ||
| CD4 | ||||||
| High | 14 | 45.1 [21.3–NR] | 0.039 | 27 | 57.5 [21.4–102.6] | 0.023* |
| Low | 1 | 35.8 [NR] | 2 | NR [3.4–NR] | ||
| CD8 | ||||||
| High | 15 | 45.1 [21.3–NR] | – | 26 | 102.6 [21.4–102.6] | 0.25 |
| Low | – | 3 | 57.5 [3.4–57.5] | |||
| CD56 | ||||||
| High | 1 | 2.4 [2.4–NR] | 0.0002* | 2 | NR [21.4–NR] | 0.002* |
| Low | 14 | 45.1 [16.2–46.1] | 27 | 21.4 [12.9–57.5] | ||
| Multivariate analysis | ||||||
|---|---|---|---|---|---|---|
| Variants | HIV | Non-HIV | ||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| PD-L1 | 3.90 | 3.8–23.9 | 0.002* | 0.55 | 0.05–4.1 | 0.56 |
| CD56 | 0.46 | 0.08–2.5 | 0.36 | 10.7 | 1.39–231.7 | 0.02* |
CI confidence interval, HR hazard ratio, NR not reached, OS overall survival
*p < 0.05
On multivariate analysis, high expression of PD-L1 in the HIV cohort and low infiltration of CD56+ cell in the non-HIV cohort showed a strong correlation with shorter survival (Table 3).
Discussion
In this study, we compared the immune- and cancer-related antigens in NSCLC tissue specimens of patients with and without HIV infection. PD-L1 expressions in TCs and ICs in stroma of NSCLC were not significantly different between the HIV and non-HIV cohorts and between the propensity-score-matched cohorts. However, prognosis of NSCLC patients with high PD-L1 expression was significantly poorer in the HIV cohort as compared to that in the non-HIV cohort, despite a comparable expression of PD-L1. These results were reproduced in the propensity-matched cohort.
As reported in earlier studies [16, 17], the risk of lung cancer incidence is higher, the age of lung cancer incidence younger, and prognosis poorer in HIV-infected cohort than in the non-HIV cohort. However, a recent analysis of surveillance data pertaining to the post-antiretroviral era showed a comparable prognosis for HIV- and non-HIV-infected population [18]. Although several factors that confer poor prognosis of lung cancer in HIV-infected patients have been proposed, definitive evidence in this respect is yet to be obtained. From the standpoint of antitumor immunity, suppression of T cell-mediated antitumor immune response is likely to underlie the association between HIV infection and poor prognosis for lung cancer. Indeed, lung cancer development was found to be more frequent when CD4+ counts were lower [2]. In HIV-infected patients, antigen presenting cells such as dendritic cells and B cells efficiently express PD-L1, which results in the suppression of T cell-mediated antitumor immune activity [19]. HIV infection also promotes PD-L1 expression in neutrophils in the blood of HIV-infected patients with lung cancer, leading to the suppression of T cell-mediated antitumor immunity through a PD-1/PD-L1 mediated pathway [20]. Furthermore, antiretroviral therapy may modulate immune status of HIV-infected lung cancer patients, and possibly induce an immune environment favorable for tumor development [21]. In spite of these assumptions, their contribution to poor prognosis of HIV-infected lung cancer patients is still obscure.
On the other hand, the immune checkpoint mechanism through PD-1/PD-L1 pathway plays a pivotal role in the suppression of antitumor immunity and evasion of tumors from immunological attack [22]. Recent seminal work has demonstrated spontaneous induction of neo-antigen-targeting antitumor immune response, which is sometimes potent enough to eradicate the tumor from the body. However, this potent antitumor immune activity is suppressed by the immune checkpoint mechanism in which PD-1/PD-L1 axis plays an important role [23]. Blockade of PD-1/PD-L1 pathway by treatment with the anti-PD-1/anti-PD-L1 mAb showed significant antitumor effect in patients with NSCLC [6–10]. For efficient antitumor effect by PD-1/PD-L1 blockade, an active T cell response to tumor antigens, presumably neo-antigens generated by gene mutation in the tumor tissue, is an essential prerequisite [24]. Interestingly, microsatellite instability in tumor tissue occurs more frequently in HIV-infected patients with lung cancer as compared to that in non-HIV-infected patients with lung cancer [3], which suggests that more immunogenic neo-antigens might be generated in the lung cancer tissues of HIV-infected patients. In the present study, infiltration of immune effecter cells, CD4+ and CD8+ T cells, and CD56+ cells in tumor tissues was comparable in antiretroviral therapy-administered HIV-infected NSCLC patients and in non-HIV-infected NSCLC patients. These results suggest that with effective antiretroviral therapy, antitumor immune response could efficiently work even in HIV-infected NSCLC patients.
Unexpectedly, in the present study, the prognosis of HIV-infected NSCLC patients with high PD-L1 expression was significantly worse than that in patients with low PD-L1 expression, while no significant difference in prognosis was seen between patients with high and low PD-L1 expression in the non-HIV-infected cohort. In some previous reports, PD-L1 expression in malignant tumors (including lung cancer) was shown to be associated with poor prognosis [25–27]. In the present study, high PD-L1 expression in NSCLC tissue was more strongly associated with poor prognosis in HIV-infected patients as compared to non-HIV-infected counterparts.
It is also noteworthy that expression of PD-1 and PD-L1 showed correlation only in the HIV-infected cohort. These results suggest that T cell immune response in NSCLC tissue might be suppressed, at least in part, via the PD-1/PD-L1 pathway, and that the suppression might be more potent in HIV-infected patients than in the non-HIV-infected patients. The disordered modulation of immune system in HIV-infected NSCLC patients may be involved in the enhanced suppression of antitumor T cell response via the PD-1/PD-L1 axis [28–30]. Although antiretroviral therapy improves long-term survival of HIV-infected patients, it does not eliminate the virus completely. Consequently, persistent viral antigenic stimulation continues even in antiretroviral therapy-receiving patients, and this results in continuous activation and exhaustion of HIV-specific CD8+ T cells [31]. Overproduction of cytokines or other biological modulators produced by activated HIV-specific CD8+ T cells may influence the activation status of non-HIV-specific CD8+ T cells, including that of tumor-specific cytotoxic T cells. If tumor-specific CD8+ cytotoxic T cells undergo exhaustion and become PD-1+ cells under such circumstances, antitumor activity mediated by these CTLs may be suppressed more strongly in NSCLC patients with high PD-L1 expression.
The results of the present study do not necessarily support the effectiveness of PD-1/PD-L1 blockade therapy against NSCLC in HIV-infected patients. Efficacy of PD-1/PD-L1 blockade therapy will likely depend on the potency of cytotoxic effect of CTLs in HIV-infected NSCLC patients. On severe exhaustion of CD8+CTLs due to persistent immune stimulation by HIV, antitumor activity of CTLs is likely to be weak even after blockade of the PD-1/PD-L1 pathway. Nonetheless, it seems plausible that in a subset of HIV-infected patients with NSCLC, PD-1/PD-L1 blockade therapy may be able to inhibit tumor activity as long as tumor-specific CTLs retain considerable cytotoxic activity.
We found that low NK cell infiltration in lung cancer tissue was closely associated with poor prognosis in the non-HIV-infected NSCLC patients but not in the HIV-infected NSCLC patients. Although NK cells play an important role in the prevention of tumor development in lung cancer, they are known to elicit promotive function of tumors [30]. The reason for the lack of association of low NK cell infiltration with poor prognosis in HIV-infected NSCLC patients is known. Due to the intimate cross talk between NK cells and T cells via OX40 [32], modulation of T cell function due to HIV infection may have influenced the NK cell response in tumor tissue. Interestingly, it was reported that CD56neg CD16+ NK cells are induced during HIV infection and that they show impaired effecter functions as NK cells [33]. Implications of the NK cell-mediated antitumor activity and tumor promoting activity in HIV-infected NSCLC patients are not clear; however, our findings suggest a considerable influence of NK cells on the incidence and development of NSCLC in HIV-infected patients.
The key limitation of the present study is the small number of patients in a single institution in which the data were collected over a long period of time, and this may have had an influence in the treatment effect. Small sample of patients expressing PD-L1 did not show significant differences in survival between HIV and non-HIV patients. However, this is a common limitation of retrospective studies of rare fraction of cancers. By utilizing propensity scores, patient backgrounds were widely equalized. Therefore, the under power of the test is avoided. Second, we cannot exclude selection bias on the choice of the patients in our institutional characteristics. In addition, the assessment criteria of PD-L1 and ICs (PD-1, CD4, CD8, and CD56) are well validated. We have adopted the similar criteria from recent clinical trials.
In conclusion, PD-L1 expression status in NSCLC tissues was similar between HIV- and non-HIV-infected cohorts; PD-L1 expression in NSCLC tissues was associated with poor prognosis in patients with HIV infection. These results suggest more potent suppression of antitumor immune response, mediated via PD-1/PD-L1 axis, in NSCLC patients with HIV than in those not infected by HIV.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Dr. Makoto Saito, the Senior Biostatistician in the Office for Clinical Research Support in Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital, for advice on statistical analyses. They also thank Masumi Ogawa, the Technical Staff of Department of Pathology in Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital, for pathological procedures. The authors would like to acknowledge Enago (http://www.enago.jp) for English language editing services.
Abbreviations
- CI
Confidence interval
- ECOG
Eastern Cooperative Oncology Group
- EGFR
Epidermal growth factor receptor
- FFPE
Formalin-fixed paraffin-embedded
- ICs
Immune cells
- IHC
Immunohistochemistry
- NADCs
Non-AIDS defining cancers
- NSCLC
Non-small cell lung cancer
- PS
Performance status
- TC
Tumor cells
Compliance with ethical standards
Conflict of interest
The author(s) declare that they have no competing interests.
References
- 1.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT, Centers for Disease C, Prevention Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged < 18 months and for HIV infection and AIDS among children aged 18 months to < 13 years—United States, 2008. MMWR Recomm Rep. 2008;57(RR-10):112. [PubMed] [Google Scholar]
- 2.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24(9):1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 3.Wistuba II, Behrens C, Milchgrub S, Virmani AK, Jagirdar J, Thomas B, Ioachim HL, Litzky LA, Brambilla EM, Minna JD, Gazdar AF. Comparison of molecular changes in lung cancers in HIV-positive and HIV-indeterminate subjects. JAMA. 1998;279(19):1554–1559. doi: 10.1001/jama.279.19.1554. [DOI] [PubMed] [Google Scholar]
- 4.Lavole A, Chouaid C, Baudrin L, Wislez M, Raguin G, Pialoux G, Girard PM, Milleron B, Cadranel J. Effect of highly active antiretroviral therapy on survival of HIV infected patients with non-small-cell lung cancer. Lung Cancer. 2009;65(3):345–350. doi: 10.1016/j.lungcan.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19(5):1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K- Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 10.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR, Group OAKS. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203(10):2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A, Group PS. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 15.Connors AF, Jr, Speroff T, Dawson NV, Thomas C, Harrell FE, Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Jr, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276(11):889–897. doi: 10.1001/jama.1996.03540110043030. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen ML, Farrell KJ, Gunthel CJ. Non-AIDS-defining malignancies in patients with HIV in the HAART era. Curr Infect Dis Rep. 2010;12(1):46–55. doi: 10.1007/s11908-009-0075-6. [DOI] [PubMed] [Google Scholar]
- 17.Hakimian R, Fang H, Thomas L, Edelman MJ. Lung cancer in HIV-infected patients in the era of highly active antiretroviral therapy. J Thorac Oncol. 2007;2(4):268–272. doi: 10.1097/01.JTO.0000263707.31202.d7. [DOI] [PubMed] [Google Scholar]
- 18.Rengan R, Mitra N, Liao K, Armstrong K, Vachani A. Effect of HIV on survival in patients with non-small-cell lung cancer in the era of highly active antiretroviral therapy: a population-based study. Lancet Oncol. 2012;13(12):1203–1209. doi: 10.1016/S1470-2045(12)70466-7. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182(10):5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog. 2014;10(3):e1003993. doi: 10.1371/journal.ppat.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitsin S, Tustin NB, Riedel E, 3rd Tustin R, Murray JB, Peck LM, Khan M, Quinn J, Douglas SD. Programmed death 1 receptor changes ex vivo in HIV-infected adults following initiation of highly active antiretroviral therapy. Clin Vaccine Immunol. 2012;19(5):752–756. doi: 10.1128/CVI.00093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, Li H, Luo X, Ye T, Sun Y, Chen H. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567–573. doi: 10.2147/OTT.S59959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Investig. 2014;94(1):107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 28.Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P, Dong H, Maserati R, Shearer GM, Chen L, Clerici M. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101(7):2514–2520. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 29.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 30.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, Sautes-Fridman C, Cremer I. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 31.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 32.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40–OX40 ligand interactions. J Immunol. 2004;173(6):3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 33.Frias M, Rivero-Juarez A, Gordon A, Camacho A, Cantisan S, Cuenca-Lopez F, Torre-Cisneros J, Pena J, Rivero A. Persistence of pathological distribution of NK cells in HIV-infected patients with prolonged use of HAART and a sustained immune response. PLoS One. 2015;10(3):e0121019. doi: 10.1371/journal.pone.0121019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.