Abstract
Tumor-mediated immunosuppression via regulatory T-cells is a key player among the various immune-escape mechanisms in multiple myeloma. We analyzed the generation, distribution, function and immunophenotype of CD8+CD28− regulatory T-cells in patients with multiple myeloma. Functionality of CD8+CD28− T-cells was assessed by immunological assays using ex vivo generated antigen-specific T-cells from patients with plasma cell dyscrasias and healthy donors. Detailed analysis of distribution, immunophenotype and cytotoxic potential of CD8+CD28− T-cells was performed by flow cytometry and ELISA. We found that the amount of CD8+CD28− T-cells was directly correlated with the suppression of antigen-specific T-cell responses in patients with plasma cell dyscrasia. Analyzing the CD8+CD28− T-cells in detail, increased numbers of these cells were observed in the bone marrow (i.e., tumor microenvironment) of patients with plasma cell dyscrasia. Furthermore, we identified the expression of lymphocyte function-associated antigen 1 (LFA-1) as a marker of immunosuppression and defined the CD8+CD28−CD57+LFA-1high population as the relevant immunosuppressive compartment. These regulatory T-cells act as immunosuppressors via soluble factors and incubation with IL-10 augmented their immunosuppressive capacity. The immunosuppressive regulatory network of IL-10 and the CD8+CD28−CD57+LFA-1high regulatory T-cells show unique characteristics and contribute to the tumor immune escape mechanism in patients with multiple myeloma.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2230-0) contains supplementary material, which is available to authorized users.
Keywords: Multiple myeloma, IL-10, LFA-1, CD57+
Introduction
For years, the accumulation of CD8+CD28− T-cells in peripheral blood (PB) during the human life span was considered a normal physiological process throughout aging [1]. It was assumed that these T-cells lost CD28 as sign of senescence due to multiple rounds of stimulation by a specific antigen and that those T-cells were exhausted and lost their capacity to appropriately respond to antigens [2]. Multiple findings in recent years show that a subpopulation of CD8+ T-cells, namely CD8+CD28− T-cells, also show regulatory characteristics. These CD8+ regulatory T-cells seem to play a pivotal role in modulating the immune system in various cancer entities and also in autoimmune diseases [3–5]. The CD8+CD28− T-cell compartment has been described as a heterogeneous population. At the same time, different functional studies have been performed without clearly defining any (sub-) populations within this compartment. Therefore, a more detailed analysis of phenotype and function of CD8+CD28− subpopulations is important to clarify their role in regulatory activity in cancer patients.
While some studies showed that the CD8+CD28− T-cell compartment had gone through many rounds of antigen-specific activation and shortening of telomeres [6, 7], others found that loss of CD28 can be induced or accelerated by a variety of cytokines [8, 9]. While these CD8+CD28− T-cells were antigen-specific, Filaci et al. described antigen-non-specific CD8+CD28− T-cells with immunosuppressive capacity via soluble factors [10, 11]. Of special interest, the CD8+CD28− T-cells found in the PB of healthy donors (HD) showed no immunosuppressive capacity, in contrast to CD8+CD28− T-cells isolated from the tumor microenvironment of cancer patients [4]. These suppressive CD8+CD28− T-cells were shown to inhibit tumor infiltrating cytotoxic T-cells [4] and to originate from non-suppressive CD8+CD28− T-cells via incubation with IL-10 [11]. While retrospective studies demonstrated an increased number of CD8+CD28− T-cells [12] in cancer patients, little is known about the phenotypical and functional alterations of CD8+CD28− T-cells in these patients.
In contrast, in autoimmune diseases such as systemic lupus erythematosus (SLE), CD8+CD28− T-cells are unable to inhibit CD3-triggered T-cell responses, likely due to their altered secretion of IL-6 and IL-12 [3]. In patients with other autoimmune diseases, in vitro-generated CD8+CD28− T-cells showed reduced suppressive capacity and increased CD127 expression as well as decreased CD39 expression [13]. The authors claimed that the CD8+CD28− T-cell compartment is composed of several subgroups with different effector or suppressor functions, and they concluded that it is thus far not possible to distinguish between CD8+CD28− T-cells with or without suppressive capacity [13].
Few published studies addressed the role of immunoregulatory CD8+ T-cells in multiple myeloma (MM), mainly analyzing different subpopulations of CD8+ T-cells. Raja et al. found an increase of antigen-specific CD8+CD25+FoxP3+ regulatory T-cells in MM patients compared to HD [14]. They also showed in 16 MM patients that treatment with lenalidomide and dexamethasone leads to an expansion of the CD8+CD25+FoxP3+ T-cell compartment [14]. Recently Zelle-Rieser et al. showed that the percentage of CD8+CD28−CD57+ T-cells was lower in treated MM patients compared to untreated ones [15].
In this manuscript, we focused on the antigen-non-specific CD8+CD28− regulatory T-cells mainly described by Filaci et al. We analyzed these cells in patients with plasma cell dyscrasia (PCD) and in HD. In addition to non-specific stimulation of T-cells by anti-CD3/CD28 beads, we also used a MART-1 antigen-specific model, since MART-1aa26–35*A27L peptide (ELAGIGILTV) is cross-reactive with HM1.24, an antigen that is expressed on the surface of plasma cells in all PCD patients [16].
Materials and methods
Blood samples and ethics statement
PB/buffy coats from HD (Institute for Immunology/IKTZ, University Heidelberg, Germany) and PB/BM from patients with PCD were used. Patients were diagnosed, staged, and response to treatment was assessed according to standard criteria. They were subdivided into early plasma-cell diseases (EPD): monoclonal gammopathy of undetermined significance (MGUS), and MM stage I, and advanced plasma-cell diseases (APD): MM stage II and III.
Cell culture medium and reagents
Cells were maintained in RPMI 1640 cell culture medium with penicillin/streptomycin and 2 mM L-glutamine (all from PAA Laboratories, Pasching, Austria), either with 10% heat inactivated fetal calf serum for cell lines or 5% heat inactivated human serum (both from PAA Laboratories) for primary cells. Unless otherwise specified, reagents were purchased from Sigma (Sigma-Aldrich, Steinheim, Germany).
Peptides
The MART-1aa26–35*A27L peptide (ELAGIGILTV) and HLA-A2 restricted irrelevant control peptide (LLIIVILGV; as a control for non-specific T-cell activation) were synthesized as previously described [17].
Isolation of mononuclear cells and T-cells
Mononuclear cells (MNC) from PB or BM were purified using ficoll-hypaque density gradient centrifugation (Biochrom, Berlin, Germany). Subpopulations of T-cells were enriched from MNC by immunomagnetic cell sorting (MACS-System, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s protocol.
Generation of CD8+CD28−LFAhigh regulatory T-cells by IL-10
To induce CD8+CD28−LFAhigh regulatory T-cells by IL-10, PBMC, CD8+ T-cells or CD8+CD28− T-cells from HD were incubated for 7 days with IL-2 (50 IE/ml) and IL-10 (40 ng/ml). After 7 days, the CD8+CD28−LFAhigh T-cells were isolated by FACS.
Fluorescence-activated cell sorting
Isolation of CD8+CD28− and CD8+CD28−LFAhigh regulatory T-cells from the PB was performed by FACS according to standard procedure.
In vitro generation of dendritic cells and expansion of MART-1aa26–35*A27L specific T-cells
Immature DC were obtained by culturing plastic adherent MNC from HLA-A*02+ patients. Maturation of DC was then induced as previously published [16]. During this incubation, MART-1aa26–35*A27L/control peptide (10 µg/ml) was added to load DC.
Expansion of antigen-specific T-cells was performed by incubating autologous MNC with peptide-loaded DC for 7 days in the presence of 5% human serum and IL-2 (50 IE/ml, Proleukin, Chiron GmbH, Munich, Germany). HLA-A typing was performed by flow cytometry analysis as previously published [18].
In another approach (Fig. 1d), we expanded antigen-specific T-cells from HD as described above. During the 7-day incubation of MNC with autologous DC, we co-cultured the peptide-pulsed DC + T cells (lower compartment of a trans-well plate) and MNC from the BM of PCD patients with different CD8+CD28− T-cell concentrations (upper compartment) in a transwell plate (BD Biosciences, Heidelberg, Germany), which separated HD cells and patients’ cells by a pored membrane. The MNCs from PCD patients were sorted using magnetic beads to achieve higher concentrations of CD8+CD28− T-cells as described above. In a further set of experiments, we expanded antigen-specific T-cells from MNC from HD in a transwell plate co-cultured with autologous CD8+CD28−LFAhigh regulatory T-cells, which were generated and purified beforehand as described above, in a MNC : regulatory T-cell ratio of 1:5 (Fig. 3a). Additionally, we repeated the above-described experiment, expanding antigen-specific T-cells from MNCs of HD with autologous DC, but co-culturing them with CD8+CD28−LFAhigh T-cells isolated from the PB of MM patients without prior IL-10 incubation. Subsequently, antigen-specific responses of expanded T-cells were analyzed using IFN-γ ELISPOT-assay.
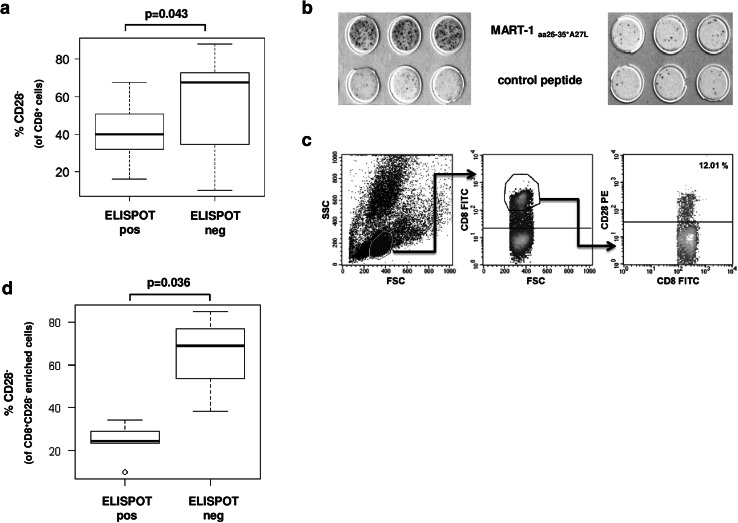
Fig. 1.
A high percentage of CD8+CD28− T-cells in the bone marrow of patients with plasma cell disease is associated with impaired antigen-specific T-cell response. a Correlation of present or absent antigen-specific T-cell responses (positive or negative ELISPOT) and the frequency of CD8+CD28− T-cells in the BM CD8+ T-cell compartment in patients with PCD (n = 33). b Representative ELISPOT-assays from two patients with PCD with (left) or without (right) a specific T-cell response against the MART-1aa26–35*A27L peptide. c Representative flow cytometry analysis and gating strategy for the analysis in A. d Effect of MNCs from BM of PCD patients (n = 8) on the expansion of MART-1aa26–35*A27L-specific T-cells from HD (n = 6). Correlation between expansion of HD T-cells and the amount of CD8+CD28− from PCD patients present during expansion
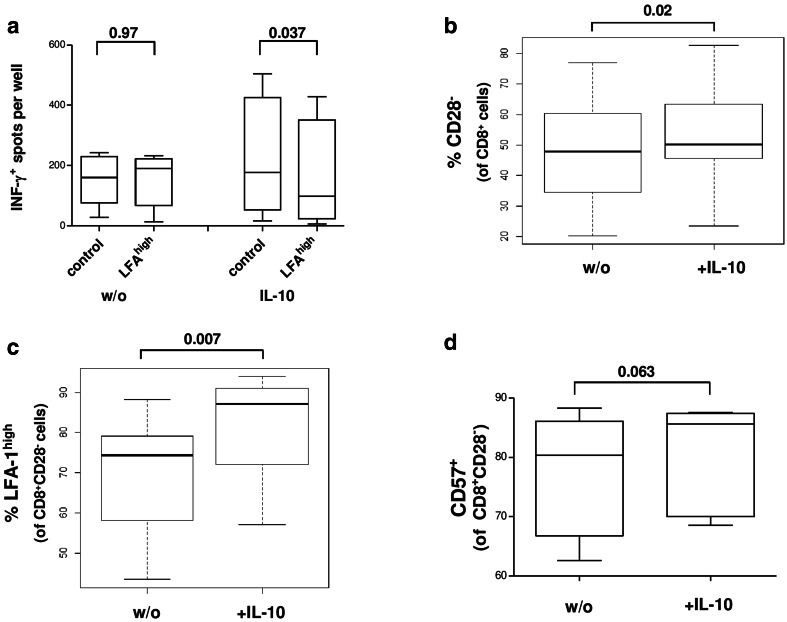
Fig. 3.
a Effect of CD8+CD28−LFAhigh T-cells from HD with/without prior IL-10 incubation (n1 = 5; n2 = 4) on the expansion of MART-1 specific T-cells with antigen-pulsed DC. b, c Flow cytometric analysis of IL-10 dependent frequency of CD8+CD28− and CD8+CD28−LFA-1high T-cells in CD8+ T-cells from HD (n = 7). d Flow cytometric analysis of IL-10 dependent frequency of CD8+CD28−CD57+ T-cells in PBMCs from HD (n = 6)
IFN-γ ELISPOT-assay
To assess the efficiency of T-cell expansion, IFN-γ ELISPOT-assay were performed as previously described [18, 19].
To analyze MART-1aa26–35*A27L specific IFN-γ synthesis by CD8+ cells, an effector: target ratio of 1:5 was used.
Flow cytometry analysis
Expression of surface antigens on T-cells was analyzed by flow cytometry. If not otherwise specified, all antibodies were purchased from BD Biosciences, Heidelberg, Germany. The following antibodies (conjugated fluorochromes) were used: CD8 (APC), CD8 (APC-Cy7), CD8 (FITC, AbD Serotech, Oxford, United Kingdom), CD28 (PE), CD3 (PerCP), CCR7 (FITC), CD45-RA (APC), CD27 (FITC), CD57 (FITC) and LFA-1 (APC) CD107 (FITC), Granzyme B (FITC), Perforin (PacBlue), CD11 + CD18/mAb24 (FITC, Abcam, Cambridge, United Kingdom), PI, Annexin V (FITC). Cells were stained according to the manufacturer’s instructions. To detect the expression of CD107a on the surface of CD8+ T-cells, the samples were incubated for 12 h with monensin (Biolegend, Fell, Germany) in a concentration of 2 µM and the CD107a antibody (clone H4A3, BD Bioscience, Heidelberg, Germany, dilution 1/20). The conformation-specific analysis of LFA-1 was performed as described by Paccani et al. [20]. For the intracellular staining of Granzyme B and Perforin, samples were treated with Brefeldin A in a concentration of 10 µg/ml 12 h prior to staining and stained as previously published [17]. The determination of apoptotic cells was performed with the Annexin V Apoptosis Detection Kit I (BD Bioscience, Heidelberg, Germany) according to the manufacturer’s instructions.
Non-specific activation of T-cells and IFN-γ /Granzyme B ELISA
To analyze non-specific activation of T-cells, cells were activated with anti-CD3/CD28 microbeads (Dynabeads, Invitrogen Dynal, Oslo, Norway) for 24 h with a cell:bead ratio of 5:1 (Fig. 6a, b). Afterwards, the concentration of IFN-γ in the supernatants of T-cell cultures was determined as previously published [21].
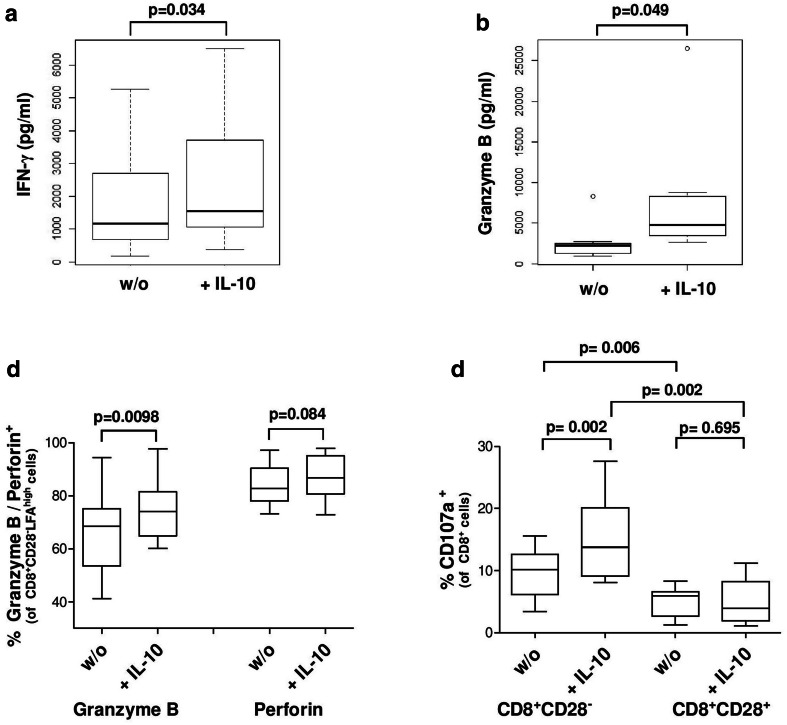
Fig. 6.
Impact of IL-10 on the secretion of cytokines. a, b Cytokine secretion of CD3/CD28 activated CD8+ T-cells of HD (n = 7). c Granzyme B+ and Perforin+ T-cells in CD8+CD28−LFAhigh T-cells after IL-10 incubation as determined by flow cytometry (n = 10). d Secretion of cytotoxic granules assessed by measuring CD107a expression on CD8+ T-cells by flow cytometry
Isolation and retroviral T-cell receptor transduction of PBMCs
For the experiments analyzing the effect of CD8+CD28− regulatory T-cells on TCR-transducted MDM2(81–88)-specific T-cells [22, 23], PBMCs were isolated as described above and then stimulated for 48 h with 600 IU/ml human IL-2 (Proleukin® S, Novartis, Basel, Switzerland) and 30 ng/ml Okt-3 [Muromonab-CD3 (Orthoclone Okt-3®, Janssen-Cilag GmbH, Frankfurt, Germany)] at 2 × 106 cells/well in a 24-well plate with 1 ml/well medium used for T-cell culture: RPMI 1640 containing 10% heat inactivated AB-serum, 1% l-glutamine, 1% Pen/Strep and 2% HEPES buffer. After 48 h, retroviral transduction and peptide-specific expansion of TCR-engineered T-cells using K562_A2 CD80+ cells as APC loaded with 10− 4 M peptide was performed as described in [24]. TCR-engineered T-cells were used in functional tests 4 days post transduction.
Carboxyfluorescein succinimidyl ester analysis
MDM2(81–88)TCR-engineered T-cells were labeled with 5 µM CFSE for 5 min at room temperature then washed 3x with PBS. Then, T-cells (0.2 × 106 cells per well) were co-cultured in the above-described transwell model with autologous regulatory CD8+CD28−LFAhigh T-cells (0.3 × 106 cells per well), generated as described above. Wells with TCR-engineered T-cells only were used as controls. Experiments were performed in a transwell plate, which separated TCR-engineered T-cells and regulatory CD8+CD28−LFAhigh T-cells by a porous membrane. After 3 days incubation, proliferation was assessed by analyzing CFSE dilution, and apoptosis was assessed by analyzing Annexin-V FITC/PI staining via flow cytometry.
Morphological analysis of CD8+CD28− T-cells
T-cells with the immunophenotype CD8+CD28−LFA+ and CD8+CD28−LFAhigh were isolated by FACS and stained (Wright Stain, Modified, Sigma-Aldrich, Munich, Germany) after a cytospin was performed, according to manufacturer’s instructions. Morphological analysis was performed by microscopy with 1000× magnification (supplementary Fig. 1).
Statistical analysis
Differences in the number of spots per well in the IFN-γ ELISPOT-assay experiments between T2 cells loaded with MART-1aa26–35*A27L compared to T2 cells loaded with an irrelevant peptide were calculated by student’s T test using the Statistica for Windows software (StatSoft, Tulsa OK, USA). ELISPOT-assays were defined as positive if MART-1aa26–35*A27L peptide activation achieved at least 10 spots more than the control peptide and if the difference was significant (p < 0.05). p values in Figs. 1, 2, 3 and Figs. 5 and 6 were calculated by the Wilcoxon rank sum test/Wilcoxon signed rank test (paired) or using Student’s T Test. Wilcoxon rank sum test, Wilcoxon signed rank test (paired) and Student’s T Test were calculated with the freeware “R” (The R Foundation for Statistical Computing, http://www.r-project.org/) and GraphPad Prism (v5.01), which were also used to generate boxplots. p values describing the impact of CD8+CD28−LFAhigh T-cells on the antigen-specific expansion of autologous T-cells were calculated using Student’s T test.
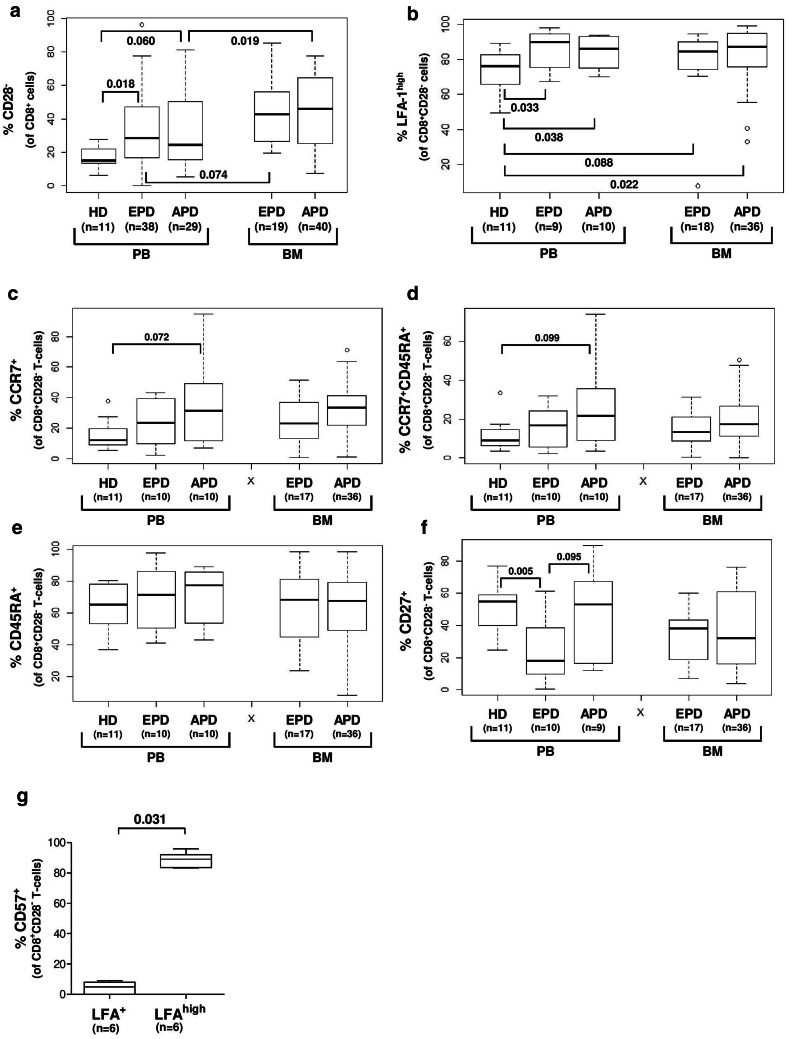
Fig. 2.
a–f Surface markers in the CD8+CD28− T-cells compartment of patients with plasma cell disease (EPD = MGUS and MM stage I, APD = MM stage II and III) in PB and BM. g Percentage of CD57+ T-cells in the CD8+CD28− T-cell compartment depending on expression of LFA-1 on T-cells from HD (n = 6)
Fig. 5.
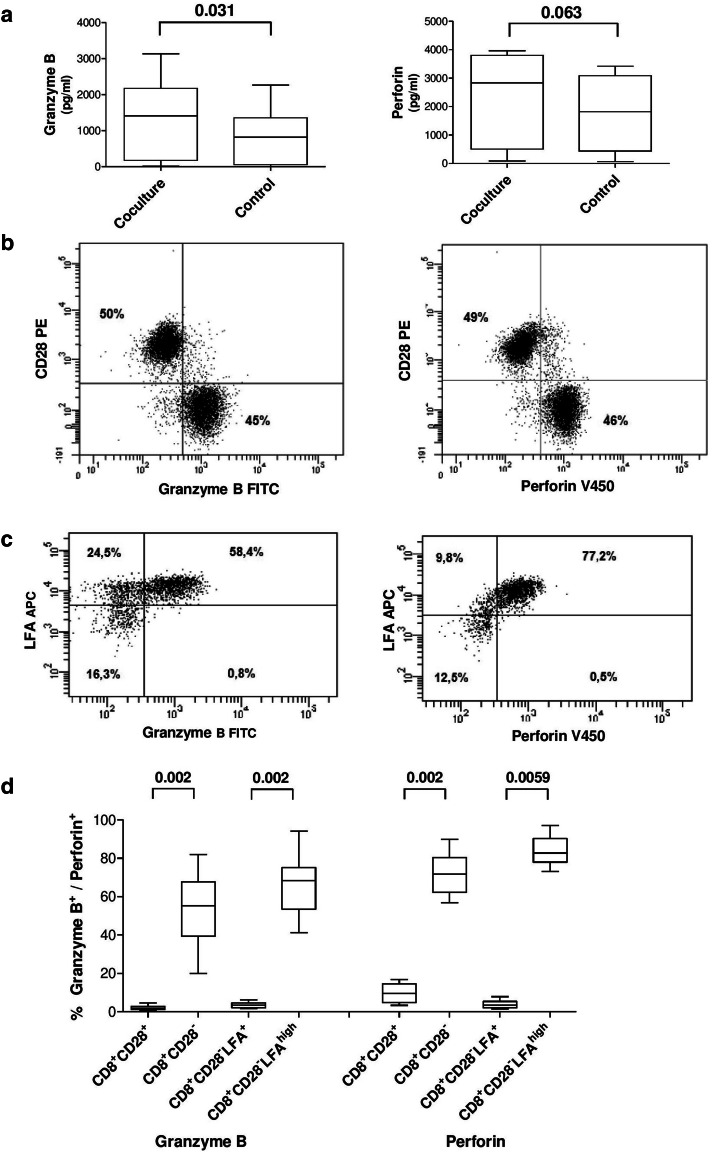
Secretion of cytokines. a Concentration of Granzyme B and Perforin after expansion of MART-1aa26–35*A27L specific T-cells in the presence (coculture) and absence (control) of IL-10 pretreated CD8+CD28−LFAhigh T-cells (HD). b Flow cytometric analysis of Granzyme B and Perforin expression in CD8+ T-cells from HD, gated on CD8+ T-cells. c Gated on CD8+CD28− T-cells. d Cumulative data from 10 HD
Results
CD8+CD28− T-cells and antigen specific T-cell responses in patients with plasma cell dyscrasia
We used the MART-1aa26–35*A27L model as described above to analyze antigen-specific T-cell responses in MM patients. MNC were isolated from the tumor microenvironment (bone marrow) of patients with PCD. After incubation of MNC for 7 days with MART-1aa26–35*A27L peptide-loaded autologous DC, specific T-cell responses were analyzed by IFN-γ ELISPOT-assay. Figure 1a highlights the role of CD8+CD28− T-cells in these assays. We correlated the percentage of CD28− T-cells in the BM CD8+ T-cell compartment of PCD patients with the MART-1aa26–35*A27L specific T-cell response in these patients. A negative (non-specific) ELISPOT-assay, displaying decreased specific T-cell response, was associated with higher amounts of CD8+CD28− T-cells in the BM of individual PCD patients (p = 0.043). A representative ELISPOT-assay is shown in Fig. 1b, and the flow cytometry gating strategy for determination of CD8+CD28− T-cells is explained in Fig. 1c.
To explore the immunosuppressive capacity of the CD8+CD28− T-cell compartment, we used isolated MNC from the BM of PCD patients with different CD8+CD28− T-cell concentrations. After enumeration by flow cytometry (demonstrated on the Y-axis), we determined the impact of these cells on MART-1aa26–35*A27L specific T-cell responses of HD in the above described transwell-plate model. In assays with a high amount of CD8+CD28− T-cells from PCD patients in the transwell cell culture insert, a significant inhibition of T-cell responses was found (p = 0.036, Fig. 1d).
CD8+CD28− T-cells in healthy donors and patients with plasma cell dyscrasia
PBMC from HD and patients with PCD and BM MNC from PCD patients were analyzed by flow cytometry to determine the amount of CD28− T-cells in the CD8+ T-cell compartment. We found a higher amount of CD8+CD28− T-cells in the PB from PCD patients compared to HD (Fig. 2a). Regarding the BM, i.e., the MM tumor microenvironment, an increased CD28− fraction within the BM CD8+ T-cell compartment was determined compared to the PB of PCD patients with APD (Fig. 2a). To evaluate possible characteristic surface markers in the CD8+CD28− T-cells compartment, we analyzed the maturation markers and adhesion molecules CCR7 (CD197), CD27, CD45RA and LFA-1 (CD11a) on T-cells from HD and patients with PCD. Regarding CCR7, CD27 and CD45RA, we found no clear correlation between HD compared to PCD patients, or between PB and BM of PCD patients (Fig. 2c–f). Nevertheless, we found an increased amount of CD8+CD28− T-cells with high expression of LFA-1 (LFA-1high) in BM and PB from patients with PCD compared to CD8+CD28− T-cells from the PB of HD (Fig. 2b). Furthermore, based on the finding that CD57 is expressed on regulatory CD8+ T-cells [25], we analyzed the expression of CD57 on CD8+CD28− T-cells in HD and observed a coexpression of CD57 on LFA-1high T-cells (Fig. 2g). We concluded that the CD8+CD28−LFA-1high and previously described [26, 27] regulatory CD8+CD57+ population are identical. For visualization, we sorted CD8+CD28−LFA-1high T-cells and CD8+CD28−LFA-1+ T-cells by FACS and stained the sorted cells after cytospin preparation according to the Wright staining protocol (supplementary Fig. 1a). The morphology between the 2 populations is clearly differed; the CD8+CD28−LFA-1high T-cells show a wide cytoplasm and a bright nucleus, while the CD8+CD28−LFA-1+ T-cells have a tightened cytoplasm and a condensed nucleus. Appropriately, CD8+CD28−CD57+ show a similar morphology to the CD8+CD28−LFA-1high T-cells (supplementary Fig. 1b).
Impact of CD8+CD28− LFAhigh T-cells on antigen-specific T-cells
In analogy to the above-described correlation of the amount of CD8+CD28− T-cells and the diminished antigen-specific immune response (Fig. 1), we analyzed the impact of CD8+CD28−LFAhigh T-cells from HD on the expansion of autologous MART-1aa26–35*A27L specific T-cells with or without IL-10 pre-incubation for 7 days. By IFN-γ ELISPOT-assay, we found only a minor immunosuppression using untreated CD8+CD28−LFAhigh T-cells, but a clear and significant immunosuppression using IL-10 pre-incubated CD8+CD28−LFAhigh T-cells (Fig. 3a).
To analyze if CD8+CD28−LFAhigh T-cells from MM patients also depend on IL-10 preincubation to gain their immunosuppressive capacity, we repeated the experiment, this time using CD8+CD28−LFAhigh T-cells from MM patients without any prior IL-10 incubation. Here, we also saw an immunosuppression in every patient tested (n = 3, supplementary Fig. 2).
Induction of CD8+CD28−CD57+LFA-1high T-cells by IL-10
Based on our findings and the data of Filaci et al. [11], who described that CD8+CD28− T-cells from HD can gain immunosuppressive capacity after incubation with IL-10, we speculated that IL-10 might be a possible regulator of LFA-1 and CD57 expression. We, therefore, analyzed the immunophenotype of CD8+ T-cells after incubation with IL-10.
After incubation of CD8+ T-cells from HD with IL-10 and IL-2 for 7 days, we analyzed the expression of CD28 and LFA-1 on CD8+ T-cells by flow cytometry. We found a slight increase of CD8+CD28− T-cells in the CD8+ compartment upon stimulation with IL-10 (Fig. 3b) and a distinct augmentation of the CD8+CD28−LFAhigh T-cell subset (Fig. 3c). Next, we excluded the possibility that a change of conformation (active/inactive) of the LFA-1 molecule [28] might play a role in the IL-10 induced effects. We used a conformation-specific antibody and performed flow cytometry analysis of CD8+CD28− T-cells in HD, but found no significant effect of IL-10 on the conformation of LFA-1 (data not shown). In line with the described co-expression of CD57 in the CD8+CD28−LFAhigh T-cell compartment, the CD8+CD28−CD57+ T-cell compartment was also enlarged after IL-10 incubation (Fig. 3d).
Of special interest, we analyzed the FACS separated CD8+CD28−LFA+ and CD8+CD28−LFAhigh T-cells after 7 days of coculture with DC and PBMC and found that CD8+CD28−LFA+ T-cells were able to regain CD28 expression, while the CD8+CD28−LFAhigh T-cells remained with an unaltered immunophenotype (supplementary Fig. 3). This finding shows the transient character of the CD8+CD28−LFA+ T-cells and emphasizes the different characteristics of the two CD8+CD28− T-cell populations. Furthermore, the expression of the maturation marker CCR7 was clearly decreased in the CD8+CD28−LFAhigh T-cells compared to CD8+CD28−LFA+ T-cells (supplementary Fig. 3), demonstrating that these 2 compartments are unequal not only for LFA expression but also for their maturation stage.
MDM2 specific T-cells
To further clarify the mode of action of IL-10 induced immunosuppression via CD8+CD28−LFAhigh T-cells and the role of their Granzyme B secretion, we analyzed the impact of FACS purified CD8+CD28−LFAhigh T-cells on the viability and proliferation of T-cell receptor (TCR)-transfected CD8+ T-cells. TCR-transfected, MDM2-specific CD8+ T-cells were stimulated with specific peptide in the presence or absence of CD8+CD28−LFAhigh T-cells, which had been stimulated with IL-10 and IL-2 for 7 days. We found no significant effect of CD8+CD28−LFAhigh T-cells on the expansion of MDM2-specific T-cells regarding the viability (Fig. 4a) or proliferation rate (Fig. 4b).
Fig. 4.
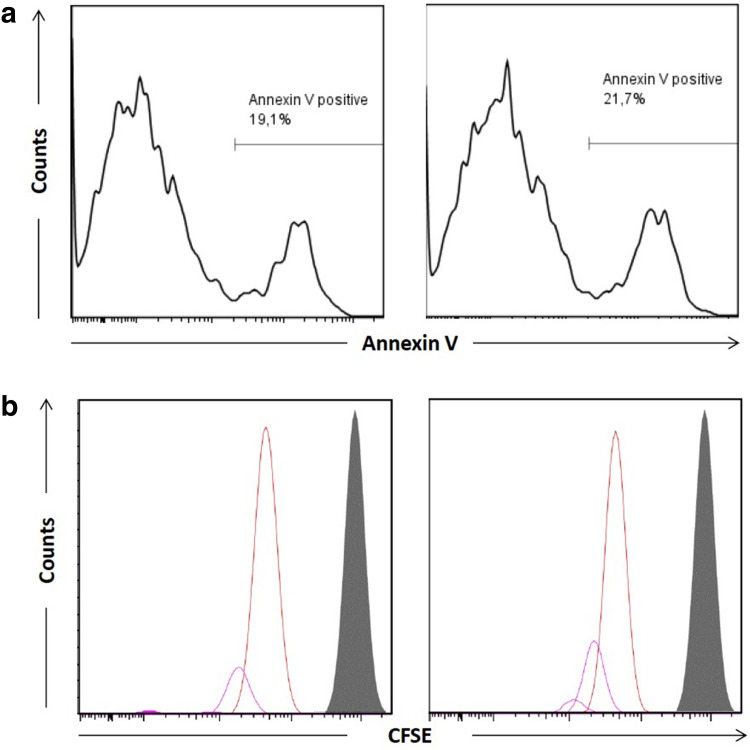
Impact of CD8+CD28−LFAhigh T-cells incubated with IL-10 for 7 days on the proliferation of TCR-transfected MDM-2-specific CD8+ T-cells upon K562 mediated antigen-stimulation. a Cell viability of MDM-2 specific CD8+ T-cells after co-culture with autologous regulatory CD8+CD28−LFAhigh T-cells for 3 days analyzed by Annexin/PI staining (left) MDM-2 specific CD8+ T-cells only (right) MDM-2 specific CD8+ T-cells + CD8+CD28−LFAhigh T-cells. b Proliferation of MDM-2 specific T-cells after co-culture with CD8+CD28−LFAhigh T-cells for 3 days analyzed by CFSE Staining. (gray) day 0 (left plot) MDM-2 specific CD8+ T-cells only (right plot) MDM-2 specific CD8+ T-cells + CD8+CD28− LFAhigh T-cells
Cytokine secretion of CD8+CD28− T-cells
We hypothesized that CD8+CD28−LFAhigh T-cells might have an inhibitory impact on the secretion of cytotoxic cytokines from effector T-cells. We analyzed the supernatant after 7 days of coculture of DC and PBMC in the absence/presence of CD8+CD28−LFAhigh T-cells. Unexpectedly, we found higher levels of Granzyme B and Perforin in the presence of CD8+CD28−LFAhigh T-cells (Fig. 5a).
Furthermore, high amounts of intracellular Granzyme B and Perforin were detected in the CD8+CD28− T-cell compartment without previous stimulation, whereas unstimulated CD8+CD28+ T-cells showed only small amounts of these proteins. Analyzing the CD8+CD28− T-cell compartment more closely, we found that the CD8+CD28−LFAhigh T-cells exhibited high amounts of Granzyme B and Perforin without prior stimulation, whereas CD8+CD28−LFA+ T-cells showed no significant expression of these proteins (Fig. 5b–d). While the secretion of Granzyme B and Perforin by CD57+ T-cells is well described [29], Filaci et al. found no secretion of Granzyme B and Perforin in the regulatory T-cells they characterized [11].
Cytokine secretion of CD8+CD28−LFAhigh T-cells is modulated by IL-10
To characterize the effect of IL-10 on the functionality of CD8+CD28−LFAhigh T-cells, we analyzed the cytokine secretion of IFN-γ, Granzyme B and Perforin after incubation with IL-10. CD8+ T-cells were isolated and incubated with IL-10 and IL-2 for 7 days and subsequently activated by CD3/C28 and analyzed for the secretion of IFN-γ and Granzyme B by ELISA (Fig. 6a, b). Furthermore, the intracellular amount of Granzyme B and Perforin in CD8+CD28−LFAhigh T-cells was analyzed by flow cytometry-based intracellular staining (Fig. 6c) after incubating PBMC with IL-10 and IL-2 for 7 days. We found an increase of all 3 cytokines after incubation with IL-10. Additionally, the level of CD107a expression on the cell surface, reflecting the amount of secreted Granzyme B, was increased on CD8+CD28− T-cells but not on CD8+CD28+ T-cells (Fig. 6d).
Discussion
In agreement with the reported immunosuppressive properties of CD8+CD28− T-cells in patients with solid tumors [4], we found that in patients with PCD the presence of CD8+CD28− T-cells in the BM is associated with an inhibition of antigen-specific T-cell responses in our in vitro model (Fig. 1a). Clearly, a higher percentage of CD8+CD28− T-cells is also associated with the inhibition of expansion of antigen-specific T-cells from HD (Fig. 1d), although we did not see a significant correlation (R = 0.7) between the number of CD8+CD28− T-cells and the number of activated T-cells in the ELISPOT-assay (data not shown).
While we observed an enrichment of CD8+CD28− T-cells in the BM of PCD patients (Fig. 2), there was no difference in the frequencies of these cells between EPD and APD patients (Fig. 2). We therefore conclude that the reported difference in immunosuppression between EPD and APD patients cannot be solely accounted for by differences in the quantity of CD8+CD28− T-cells in the periphery or the BM, i.e., the MM tumor microenvironment. While CD8+ T-cell senescence and subsequent loss of CD28 on T-cells due to significant differences in age between EPD and APD patients (EPD vs. APD: p = 0.059, supplementary Table 1) might influence the analysis [30], we found no difference between these two groups regarding the amount of CD8+CD28− T-cells (Fig. 2). Regarding the CD28 expression in T-cells from HD, it was not possible to determine the age of the HD in our setting, so we could not rule out that age difference might play a role in the frequency of CD28− T-cells in HD.
Surprisingly, we found an increased amount of CD8+CD28− T-cells from BM and PB with high expression of LFA-1 in patients with PCD compared to HD. The role of the ß-integrin LFA-1 as adhesion molecule is extensively described, and it is well known that LFA-1 mediates the interaction between T-cells and antigen-presenting cells [31]. Recent data show that LFA-1 is also important for the promotion of activation [32, 33] and differentiation of T-cells [34] and that activation of T-cells via LFA-1 increases the frequency of IFN-γ producing Th1 cells [34]. Ligation of LFA-1 enhances T-cell signaling due to a rearrangement of the immunological synapse [35] and mediates cytolytic procedures [36]. In this context, Filaci et al. described a moderately decreased expression of LFA-1 in patients with SLE and hypothesized a diminished regulatory function of the CD8+CD28− T-cells in autoimmune diseases [3].
Instead of LFA, CD57 is a well-described marker of senescence in T-cells, and there are few reports that show the immunosuppressive capacity of CD8+CD57+ T-cells in MM [25] via soluble factors. Due to this data, we correlated the expression of CD57 with the expression of LFA and found that all T-cells from the CD8+CD28−LFAhigh T-cells express CD57; therefore, CD57 and LFAhigh might describe the same T-cell population.
Another important finding of Filaci et al. was that CD8+CD28− T-cells from HD obtained suppressive function only after incubation with IL-10. Regarding the change of immunophenotype upon incubation with IL-10, Filaci et al. described a downregulation of CD127 on CD8+CD28− T-cells, paralleled by the loss of effector functions. By combining the published data with our findings, we speculated that IL-10 might be a regulator of LFA-1 expression on CD8+CD28− T-cells, which is clearly the case (Fig. 3). While Guan et al. showed that IL-10 upregulates the expression of LFA-1 in adult neural stem cells in mice [37], to the best of our knowledge LFA-1 modulation on T-cells by IL-10 has not been described so far and might be related to the described phenomenon that IL-10 from CD8+CD28− T-cells inhibits proliferation of autologous lymphocytes [38]. Taken together, we showed that the IL-10 induced immunosuppression of CD8+CD28− T-cells is attended by an enlargement of the CD8+CD28−LFAhighCD57+ T-cell compartment.
An unexpected finding is the secretion of Granzyme B and Perforin by CD8+CD28−LFAhigh T-cells. While it is clearly shown that the Granzyme B/Perforin pathway is a well-understood mechanism of T-cells to improve tumor clearance [39], data also exist highlighting the possibility of regulatory T-cells to kill effector T-cells by these cytokines [40]. We evaluate the possibility that IL-10 triggered Granzyme B secretion of CD8+CD28−LFAhigh T-cells results in killing of tumor-specific T-cells. For that we used a Granzyme B inhibitor in the above described co-culture of DC and PBMC, but found no effect regarding the generation of antigen-specific T-cells or the apoptosis rate of T-cells or DC (data not shown).
We observed immunosuppressive effects of CD8+CD28−LFAhigh T-cells only in a DC based coculture model (Fig. 3a). In a coculture model using K562_A2 CD80+ cells as APCs, we did not see any immunosuppressive effect of CD8+CD28−LFAhigh T-cells on T-cell expansion (Fig. 4). It seems likely that CD8+CD28−LFAhigh T-cells impact the DC/T-cell interaction.
Taken together, these data indicate that the immunosuppression of CD8+CD28−CD57+LFAhigh T-cells is induced by IL-10, mediated by a soluble factor, and influences the interaction of antigen-presenting cells with T-cells during the expansion of antigen-specific T-cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- APD
Advanced plasma-cell disease
- EPD
Early plasma-cell disease
- HD
Healthy donor
- LFA-1
Lymphocyte function-associated antigen 1
- MGUS
Monoclonal gammopathy of undetermined significance
- MM
Multiple myeloma
- MNC
Mononuclear cells
- PB
Peripheral blood
- PCD
Plasma cell dyscrasia
- SLE
Systemic lupus erythematosus
Author contributions
Study conception and design: Julian Plaumann, Melanie Engelhardt, Michael Hundemer. Acquisition of data: Julian Plaumann, Melanie Engelhardt, Brigitte Neuber, Eva Amman. Analysis and interpretation of data: Julian Plaumann, Melanie Engelhardt, Eva Amman, Hakim Echchannaoui, Mohamed H. S. Awwad, Michael Hundemer. Drafting of manuscript: Julian Plaumann, Michael Hundemer. Critical revision: Mohamed H. S. Awwad, Marc S. Raab, Jens Hillengass, Niels Halama, Carsten Müller-Tidow, Hartmut Goldschmidt.
Funding
No relevant funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All human studies were performed after obtaining written informed consent in accordance with the Declaration of Helsinki and were approved by the ethics committee of the Medical Faculty, University of Heidelberg according to the institutional guidelines. Data safety management was performed according to the data safety regulations of the University Hospital Heidelberg.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Julian Plaumann and Melanie Engelhardt contributed equally to this paper.
References
- 1.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28 T cells in healthy ageing people, including centenarians. Immunology. 1996;88(4):501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28 and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, Setti M, Puppo F, Indiveri F. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol. 2001;166(10):6452–6457. doi: 10.4049/jimmunol.166.10.6452. [DOI] [PubMed] [Google Scholar]
- 4.Filaci G, Fenoglio D, Fravega M, Ansaldo G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi M, Ferrera F, Balestra P, Ghio M, Contini P, Setti M, Olive D, Azzarone B, Carmignani G, Ravetti JL, Torre G, Indiveri F. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179(7):4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 5.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8+ T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J Clin Invest. 1995;95(6):2711–2719. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105(2):117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+ CD8+ counterparts. J Immunol. 1996;156(10):3587–3590. [PubMed] [Google Scholar]
- 8.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12(7):1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 9.Chiu WK, Fann M, Weng NP. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006;177(11):7802–7810. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filaci G, Rizzi M, Setti M, Fenoglio D, Fravega M, Basso M, Ansaldo G, Ceppa P, Borgonovo G, Murdaca G, Ferrera F, Picciotto A, Fiocca R, Torre G, Indiveri F. Non-antigen-specific CD8(+) T suppressor lymphocytes in diseases characterized by chronic immune responses and inflammation. Ann N Y Acad Sci. 2005;1050:115–123. doi: 10.1196/annals.1313.013. [DOI] [PubMed] [Google Scholar]
- 11.Filaci G, Fravega M, Negrini S, Procopio F, Fenoglio D, Rizzi M, Brenci S, Contini P, Olive D, Ghio M, Setti M, Accolla RS, Puppo F, Indiveri F. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+ CD28 T cells and inhibit both T-cell proliferation and CTL function. Hum Immunol. 2004;65(2):142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, Ferrari M, Cosentino M, Marino F, Pozzi E, Fietta AM. Foxp3 expressing CD4+ CD25+ and CD8+ CD28 T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67(1–2):1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Bernuzzi F, Fenoglio D, Battaglia F, Fravega M, Gershwin ME, Indiveri F, Ansari AA, Podda M, Invernizzi P, Filaci G. Phenotypical and functional alterations of CD8 regulatory T cells in primary biliary cirrhosis. J Autoimmun. 2010;35(3):176–180. doi: 10.1016/j.jaut.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja KR, Plasil M, Rihova L, Pelcova J, Adam Z, Hajek R. Flow cytometry based enumeration and functional characterization of CD8 T regulatory cells in patients with multiple myeloma before and after lenalidomide plus dexamethasone treatment. Cytometry B Clin Cytom. 2013 doi: 10.1002/cytob.21109. [DOI] [PubMed] [Google Scholar]
- 15.Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, Greil R, Johrer K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol. 2016;9(1):116. doi: 10.1186/s13045-016-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen O, Lupu A, Schmidt S, Condomines M, Belle S, Maier A, Hose D, Neuber B, Moos M, Kleist C, Terness P, Ho AD, Goldschmidt H, Klein B, Hundemer M. Melan-A/MART1 analog peptide triggers anti-myeloma T-cells through crossreactivity with HM1.24. J Immunother. 2009;32(6):613–621. doi: 10.1097/CJI.0b013e3181a95198. [DOI] [PubMed] [Google Scholar]
- 17.Neuber B, Herth I, Tolliver C, Schoenland S, Hegenbart U, Hose D, Witzens-Harig M, Ho AD, Goldschmidt H, Klein B, Hundemer M. Lenalidomide enhances antigen-specific activity and decreases CD45RA expression of T cells from patients with multiple myeloma. J Immunol. 2011;187(2):1047–1056. doi: 10.4049/jimmunol.1002460. [DOI] [PubMed] [Google Scholar]
- 18.Hundemer M, Schmidt S, Condomines M, Lupu A, Hose D, Moos M, Cremer F, Kleist C, Terness P, Belle S, Ho AD, Goldschmidt H, Klein B, Christensen O. Identification of a new HLA-A2-restricted T-cell epitope within HM1.24 as immunotherapy target for multiple myeloma. Exp Hematol. 2006;34(4):486–496. doi: 10.1016/j.exphem.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munder M, Engelhardt M, Knies D, Medenhoff S, Wabnitz G, Luckner-Minden C, Feldmeyer N, Voss RH, Kropf P, Muller I, Conradi R, Samstag Y, Theobald M, Ho AD, Goldschmidt H, Hundemer M. Cytotoxicity of tumor antigen specific human T cells is unimpaired by arginine depletion. PLoS One. 2013;8(5):e63521. doi: 10.1371/journal.pone.0063521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paccani SR, Finetti F, Davi M, Patrussi L, D’Elios MM, Ladant D, Baldari CT. The Bordetella pertussis adenylate cyclase toxin binds to T cells via LFA-1 and induces its disengagement from the immune synapse. J Exp Med. 2011;208(6):1317–1330. doi: 10.1084/jem.20101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, Ho AD. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108(5):1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 22.Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, Ruppert T, Bolhuis RL, Melief CJ, Huber C, Stauss HJ, Theobald M. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2(10):962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 23.Voss RH, Kuball J, Engel R, Guillaume P, Romero P, Huber C, Theobald M. Redirection of T cells by delivering a transgenic mouse-derived MDM2 tumor antigen-specific TCR and its humanized derivative is governed by the CD8 coreceptor and affects natural human TCR expression. Immunol Res. 2006;34(1):67–87. doi: 10.1385/IR:34:1:67. [DOI] [PubMed] [Google Scholar]
- 24.Knies D, Klobuch S, Xue SA, Birtel M, Echchannaoui H, Yildiz O, Omokoko T, Guillaume P, Romero P, Stauss H, Sahin U, Herr W, Theobald M, Thomas S, Voss RH. An optimized single chain TCR scaffold relying on the assembly with the native CD3-complex prevents residual mispairing with endogenous TCRs in human T-cells. Oncotarget. 2016;7(16):21199–21221. doi: 10.18632/oncotarget.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frassanito MA, Silvestris F, Cafforio P, Dammacco F. CD8+/CD57 cells and apoptosis suppress T-cell functions in multiple myeloma. Br J Haematol. 1998;100(3):469–477. doi: 10.1046/j.1365-2141.1998.00589.x. [DOI] [PubMed] [Google Scholar]
- 26.Focosi D, Marco T, Kast RE, Maggi F, Ceccherini-Nelli L, Petrini M. Progressive multifocal leukoencephalopathy: what’s new? Neuroscientist. 2010;16(3):308–323. doi: 10.1177/1073858409356594. [DOI] [PubMed] [Google Scholar]
- 27.Frassanito MA, Silvestris F, Silvestris N, Cafforio P, Camarda G, Iodice G, Dammacco F. Fas/Fas ligand (FasL)-deregulated apoptosis and IL-6 insensitivity in highly malignant myeloma cells. Clin Exp Immunol. 1998;114(2):179–188. doi: 10.1046/j.1365-2249.1998.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma NK, Kelleher D. Adaptor regulation of LFA-1 signaling in T lymphocyte migration: potential druggable targets for immunotherapies? Eur J Immunol. 2014;44(12):3484–3499. doi: 10.1002/eji.201344428. [DOI] [PubMed] [Google Scholar]
- 29.Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, De Rosa SC. The cytolytic enzymes granzyme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85(1):88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng NP, Akbar AN, Goronzy J. CD28() T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395(6697):82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 32.Kandula S, Abraham C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J Immunol. 2004;173(7):4443–4451. doi: 10.4049/jimmunol.173.7.4443. [DOI] [PubMed] [Google Scholar]
- 33.Shier P, Otulakowski G, Ngo K, Panakos J, Chourmouzis E, Christjansen L, Lau CY, Fung-Leung WP. Impaired immune responses toward alloantigens and tumor cells but normal thymic selection in mice deficient in the beta2 integrin leukocyte function-associated antigen-1. J Immunol. 1996;157(12):5375–5386. [PubMed] [Google Scholar]
- 34.Perez OD, Mitchell D, Jager GC, South S, Murriel C, McBride J, Herzenberg LA, Kinoshita S, Nolan GP. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat Immunol. 2003;4(11):1083–1092. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- 35.Graf B, Bushnell T, Miller J. LFA-1-mediated T cell costimulation through increased localization of TCR/class II complexes to the central supramolecular activation cluster and exclusion of CD45 from the immunological synapse. J Immunol. 2007;179(3):1616–1624. doi: 10.4049/jimmunol.179.3.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 2005;102(18):6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan Y, Jiang Z, Ciric B, Rostami AM, Zhang GX. Upregulation of chemokine receptor expression by IL-10/IL-4 in adult neural stem cells. Exp Mol Pathol. 2008;85(3):232–236. doi: 10.1016/j.yexmp.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Fenoglio D, Ferrera F, Fravega M, Balestra P, Battaglia F, Proietti M, Andrei C, Olive D, Antonio LC, Indiveri F, Filaci G. Advancements on phenotypic and functional characterization of non-antigen-specific CD8+ CD28 regulatory T cells. Hum Immunol. 2008;69(11):745–750. doi: 10.1016/j.humimm.2008.08.282. [DOI] [PubMed] [Google Scholar]
- 39.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Efimova OV, Kelley TW. Induction of granzyme B expression in T-cell receptor/CD28-stimulated human regulatory T cells is suppressed by inhibitors of the PI3K-mTOR pathway. BMC Immunol. 2009;10:59. doi: 10.1186/1471-2172-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.