Abstract
The expression of programmed cell death 1 ligand 1 (PD-L1) and interferon-γ (IFN-γ) is of great interest for the development of chemoradiotherapy and immune checkpoint inhibitor treatments. Patients with nodal metastasis (pN+) tend to have a poor prognosis, even after neoadjuvant chemoradiotherapy (neoCRT) and surgical treatment. In this study, we examined the roles of tumor PD-L1 and IFN-γ before and after neoCRT in locally advanced rectal cancer (LARC) patients. Our results demonstrate that patients with high PD-L1 expression in post-neoCRT tissues exhibit improved 5-year disease-free survival (DFS) and overall survival (OS) compared with those with low PD-L1 expression (p < 0.001). Furthermore, in the pN+ population, patients with high PD-L1 expression in post-neoCRT tissues exhibit improved 5-year DFS and OS. PD-L1 and IFN-γ upregulation increased in tumor tissues after neoCRT, and patients with high PD-L1 and high IFN-γ exhibit improved 5-year DFS and OS (p = 0.04 and p = 0.001, respectively). To the best of our knowledge, this study is the first to demonstrate that PD-L1 upregulation in a pN+ cohort correlates with improved prognosis, which is similar to that in patients without nodal metastasis. Moreover, this study verified that PD-L1 and IFN-γ were upregulated by neoCRT treatment in LARC patients and demonstrated that neoCRT may be useful not only for immune checkpoint inhibitor treatment but also for reinvigorating preexisting anti-cancer immunity.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2275-0) contains supplementary material, which is available to authorized users.
Keywords: Programmed death ligand 1, Interferon-γ, Locally advanced rectal cancer, Lymph node metastasis, Neoadjuvant chemoradiotherapy
Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide [1] and rectal cancer cases comprise 27% up to 58%of CRCs [2]. However, these two distinct diseases require different treatment strategies. Preoperative (neoadjuvant) chemoradiotherapy (neoCRT) has been considered the most effective strategy for down-staging and improving local control in patients with locally advanced rectal cancer [3, 4]. Approximately 40–60% of LARC patients treated with neoCRT achieved some degree of pathological response. But only 15–20% of LARC patients achieve a complete response after neoCRT treatment [5].
NeoCRT causes both direct damage to tumor cells and immunogenic cell death (ICD) via the release of danger-associated molecular patterns (DAMPs) from damaged tumor cells that boost immune response. This process triggers the dispersion of numerous cytokines—especially interferon-γ (IFN-γ)—released from tumor and immune cells to create an inflammatory and immunogenic microenvironment where immune cells can recognize, activate and eliminate tumor cells [6, 7]. Yet IFN-γ is a multifunctional cytokine which not only has a pivotal role in anti-tumor immunity via immunomodulation of inflammation of the innate and acquired immune responses, but participates in pro-tumor activity by suppressing cell-mediated adaptive immunity [8].
Programmed cell death 1 ligand 1 (PD-L1) is a 40-kDa immune checkpoint protein that negatively regulates T lymphocytes to cause lymphocyte “exhaustion” through the PD-1 receptor [9]. The upregulation of PD-L1 in tumor cells leads to adaptive immune resistance by suppressing cytotoxic T lymphocyte activity [10, 11]. Immune checkpoint blockade (ICB) therapy such as PD-1/PD-L1 has been proposed as an option to activate the host immune system to eradicate tumors. Numerous studies showed that blockade of PD-1/PD-L1 signaling pathway demonstrated impressive therapeutic responses in patients with melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and bladder cancer [12]. Hence, tumor PD-L1 expression status is considered to be a predictive marker for the success of anti-PD-1/PD-L1 immunotherapy [13].
Recent studies have demonstrated that IFN-γ may drive the upregulation of PD-L1 in tumor cells to mediate the immune adaptive resistance mechanism [14, 15], and the combination of local radiotherapy and PD-1/PD-L1 blockade improved both local and systemic tumor control in animal models [16–18]. However, the prognostic value of PD-L1 and IFN-γ expression in CRC remains controversial; specifically, prolonged IFN-γ activation leads to increased resistance to ICB and radiation treatment in melanoma mouse models [19–21]. Therefore, the role of IFN-γ and PD-L1 expression in LARC patients receiving neoCRT remains unclear. In this study, we aim to evaluate tumor PD-L1 and IFN-γ expression levels prior to and after neoCRT treatment, and to determine independent prognostic factors for LARC patients.
Materials and methods
Patients
In total, 104 patients [33 male, 71 female; mean age 59.3 ± 12.5 years (age range 31–90 years)] with locally advanced rectal cancer were enrolled in the study from 2006 to 2013 at China Medical University Hospital. The study cohorts were composed of patients with biopsy-proven, locally advanced (cT3-4 or cN+) rectal cancer and treated with preoperative chemoradiotherapy followed by radical resection at China Medical University Hospital.
All Patients were treated with neoCRT with a median radiotherapy dose of 50.4 Gy in 30 fraction and concurrent fluoropyrimidine-based chemotherapy (mainly single-agent infusional capecitabine, 500 mg/m2/day). Patients were assessed for their clinical response 6–8 weeks after the completion of neoCRT according to rigorous criteria of clinical, endoscopic, and radiologic findings. The three criteria for complete clinical response (cCR) were (a) the absence of a residual ulceration, mass, or mucosal irregularity upon clinical/endoscopic assessment; (b) whitening of the mucosa and the presence of neovasculature; and (c) radiologic imaging, such as CT, RUS, or MRI, without the evidence of extrarectal residual disease.
After the chemoradiotherapy regime was completed, surgery was performed 6–8 weeks later. Low anterior resection, proctectomy with coloanal reconstruction, abdominoperineal resection, or multivisceral rectal resection were included according to total mesorectal excision (TME) principles. Resected specimen pathologic staging was performed after resection in accordance with the guidelines of the College of American Pathologists. Adjuvant chemotherapy was recommended for patients with metastatic lymph node(s) in surgical specimens and consisted of fluorouracil infusion or capecitabine for a period of 4–6 months.
Clinical staging and pathological evaluation
Clinical and pathological stages were assessed as previously described [22], and was performed by gastrointestinal cancer pathologists in accordance with the guidelines of the College of American Pathologists. Pathologic complete response (pCR) was defined as previously described [22]. Tumor regression grade (TRG) was scored as follows: TRG 0, no regression; TRG 1, dominant tumor mass with obvious fibrosis in ≤ 25% tumor mass; TRG 2, dominant tumor mass with obvious fibrosis in 26–50%; TRG 3, dominant fibrosis outgrowing the tumor mass (greater than 50% tumor mass regression); TRG 4, no visible tumor cells, only fibrotic mass (is defined “NA” group in post-neoCRT tissue).
Construction of tissue microarrays (TMAs) and immunohistochemistry
Tissue microarrays were constructed from 104 pair-matched pre-neoCRT biopsies and post-neoCRT surgical tissue from rectal cancer patients as previously described [22]. Immunohistochemistry (IHC) was performed using 3-µm thick histological TMA sections as previously described [22]. The following antibodies were used in this study: anti-PD-L1 (1:100, ab205921, Abcam, Cambridge, UK), anti-IFN-γ (1:500, ab205921, Abcam, Cambridge, UK), anti-TGF-β (1:500, ab205921, Abcam, Cambridge, UK), anti-MSH2 (1:100, ab92372, Abcam, Cambridge, UK), anti-MLH1 (1:100, ab92312, Abcam, Cambridge, UK), anti-MSH6 (1:100, ab92471, Abcam, Cambridge, UK), and anti-PMS2 (1:100, ab110638, Abcam, Cambridge, UK). The stained tissue sections were scored separately by two pathologists blinded to the clinicopathological parameters.
IFN-γ and TGF-β levels in tumor cells were scored on the intensity of the staining as follows: 0, absent; 1+, weak; 2+, moderate; 3+, strong. Tumor PD-L1 immunostaining was scored in accordance with the intensity and extent of the staining on a semiquantitative scale (0–3+) as follows: 0, absent; 1+, weak; 2+, moderate; 3+, strong. The percentage of membranous PD-L1 on tumor cells was recorded as follows: a score of 0 was assigned when no staining was evident or < 5% of the tumor cells were positive, and a score of 1 was assigned when membranous staining was present in > 5% of the positive cell proportion. If membranous staining was present, distinct membrane staining above the cytoplasmic staining level was observed. The 5% threshold was based on a previous phase I trial of anti-PD-1 agents and studies of other malignancies [10, 23].
Cell culture, treatment, western blot analysis, flow cytometry and enzyme-linked immunosorbent assay (ELISA)
Cells were cultured and maintained in RPMI1640 medium supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, New York, USA), 2 mM glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 1 mM pyruvate at 37 °C in a humidified, 5% CO2 atmosphere.
SW480 cells were seeded onto a 6-cm dish at ~ 80% confluence in RPMI1640 supplemented with 10% FBS the day before radiation treatment. After exposure to radiation, cells were harvested for western blot analysis at indicated times. SW480 cells were treated with oxaliplatin (10 µM) and CPT-11 (5 µM) for 24 h. The cell lysates and medium were then analyzed by immunoblotting, and the results were quantified. Cells were incubated with medium containing different recombinant proteins (IFN-γ, TGF-β, TNF-α and LPS, ProSpec-Tany TechnoGene Ltd, Rehovot, Israel) for the indicated times. Total lysates (30 µg) were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 6–12% resolving gel) and were electrophoretically transferred onto a PVDF membrane (GE, Amersham, UK). The membranes were blocked with 5% non-fat milk and probed with specific antibodies overnight at 4 °C. Then, horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000, GE Healthcare, Amersham, UK) were applied to the membranes followed by detection using the Immobilon Western Chemiluminescent HRP Substrate (Millipore, MA, USA). The densitometric analysis of western blots was performed using the AlphaImager2200 digital imaging system (Digital Imaging System, CA, USA). Digital images were processed with Adobe Photoshop 7.0 (Adobe Systems, CA, USA). Each blot was stripped using Restore Western Blot Stripping Buffer (Pierce, IO, USA) and incubated with the other antibodies. The results were assessed using ImageJ software (NIH, MD, USA) [24, 25].
To detect IFN-γ release, the conditioned medium was collected 24 h after irradiation and analyzed by LEGEND MAX™ Human IFN-γ ELISA Kit (Biolegend, San Diego, CA, USA) according to the manufacturer’s manual. Surface PD-L1 expression on the tumor cells was analyzed by flow cytometry. Cells were harvested and washed with PBS. The samples were washed with incubation buffer (PBS containing 2% bovine serum albumin) twice and incubated with APC conjugated anti-PD-L1 IgG (1:100, Biolegend, San Diego, CA, USA) for 1 h at room temperature. The samples were finally washed and resuspended in PBS for analysis by flow cytometry (BD FACSCanto, San Jose, CA, USA).
Statistical analysis
Statistical analysis was performed using the SAS statistical software, version PC 9.4 (SAS Institute, Cary, NC, USA), as previously described. Influential factors affecting rectal cancer patient survival rates were assessed using Cox models; these factors included age (< 65 versus ≥ 65 years), TRG (3 + 4 vs. 1 + 2), clinical response (CR + PR vs. SD + PD), cN stage (positive vs. negative), and pN stage (positive vs. negative). The Kaplan–Meier estimation method was used to assess the 5-year overall survival and disease-free survival. The survival time was defined as the time from surgery to death.
Results
Clinical characteristics and tumor IFN-γ and PD-L1 expression in LARC patients
Pre- and post-neoCRT tissue specimens were retrospectively collected from 104 LARC patients and constructed into tissue microarrays (TMAs) [26, 27]. All the patients underwent total or tumor-specific mesorectal excisions depending on the extent and location of the tumor. Most patients were male (68%), and most tumors were stage II (46%) or III (50%). The median radiation dose was 50.4 Gy in 28 fractions. The concurrent chemotherapy was capecitabine (Xeloda) in 48% of patients and fluorouracil in 37% of patients. After neoCRT treatment, 15 patients exhibited a complete response (pathological tumor regression grade 4, TRG 4, “NA” group in post-neoCRT tissues), and 15 patients also exhibited a clinical complete response (cCR). Thirty patients (29%) presented with lymph node metastases after surgery, and 20 patients (19%) presented with distant metastasis within 5 years (Table 1). Only two patients (2%) were MSI-deficient (Table 1).
Table 1.
Clinicopathologic characteristics and PD-L1 expression status of enrolled patients (n = 104)
| Clinicopathological characteristics | Total cases, n (%) | Tumor PD-L1 (pre-neoCRT) | Tumor PD-L1 (post-neoCRT) | |||||
|---|---|---|---|---|---|---|---|---|
| High, n (%) | Low, n (%) | p value | High, n (%) | Low, n (%) | p value | NA, n (%) | ||
| 104 (100%) | 53 (51%) | 51 (49%) | 57 (64%) | 32 (36%) | 15 (100%) | |||
| Age | 0.88 | 0.3 | ||||||
| < 65 | 66 (63%) | 34 (64%) | 32 (63%) | 40 (70%) | 19 (59%) | 7 (47%) | ||
| ≥ 65 | 38 (37%) | 19 (36%) | 19 (37%) | 17 (30%) | 13 (41%) | 8 (53%) | ||
| Sex | 0.24 | 0.97 | ||||||
| Female | 33 (32%) | 14 (26%) | 19 (37%) | 18 (32%) | 10 (31%) | 5 (33%) | ||
| Male | 71 (68%) | 39 (74%) | 32 (63%) | 39 (68%) | 22 (69%) | 10 (67%) | ||
| cN stage | 0.56 | 0.42 | ||||||
| Negative | 52 (50%) | 28 (53%) | 24 (47%) | 30 (53%) | 14 (44%) | 8 (53%) | ||
| Positive | 52 (50%) | 25 (47%) | 27 (53%) | 27 (47%) | 18 (56%) | 7 (47%) | ||
| Clinical TNM stage (7th AJCC) | 0.04* | 0.04* | ||||||
| I | 4 (4%) | 3 (6%) | 1 (2%) | 2 (4%) | 2 (6%) | 0 (0%) | ||
| II | 48 (46%) | 25 (47%) | 23 (45%) | 28 (49%) | 12 (38%) | 8 (53%) | ||
| III | 52 (50%) | 25 (47%) | 27 (53%) | 27 (47%) | 18 (56%) | 7 (47%) | ||
| pN stage | 0.32 | 0.57 | ||||||
| Negative | 74 (71%) | 40 (75%) | 34 (67%) | 39 (68%) | 20 (63%) | 15 (100%) | ||
| Positive | 30 (29%) | 13 (25%) | 17 (33%) | 18 (32%) | 12 (38%) | 0 (0%) | ||
| TRG | 0.004* | 0.4 | ||||||
| 4 | 15 (14%) | 6 (11%) | 9 (18%) | 0 (0%) | 0 (0%) | 15 (100%) | ||
| 3 | 57 (55%) | 32 (60%) | 25 (49%) | 37 (65%) | 20 (63%) | 0 (0%) | ||
| 2 | 23 (22%) | 12 (23%) | 11 (22%) | 16 (28%) | 7 (22%) | 0 (0%) | ||
| 1 | 9 (9%) | 3 (6%) | 6 (12%) | 4 (7%) | 5 (16%) | 0 (0%) | ||
| Clinical response | 0.002* | 0.04* | ||||||
| CR | 15 (14%) | 5 (9%) | 10 (20%) | 1 (2%) | 0 (0%) | 14 (93%) | ||
| PR | 39 (38%) | 22 (42%) | 17 (33%) | 24 (42%) | 14 (44%) | 1 (7%) | ||
| SD | 45 (43%) | 22 (42%) | 23 (45%) | 29 (51%) | 16 (50%) | 0 (0%) | ||
| PD | 5 (5%) | 4 (8%) | 1 (2%) | 3 (5%) | 2 (6%) | 0 (0%) | ||
| Chemotherapy | 0.002* | 0.02* | ||||||
| Capecitabine | 50 (48%) | 30 (57%) | 20 (39%) | 29 (51%) | 15 (47%) | 6 (40%) | ||
| UFT | 38 (37%) | 15 (28%) | 23 (45%) | 20 (35%) | 12 (38%) | 6 (40%) | ||
| 5-FU | 11 (11%) | 6 (11%) | 5 (10%) | 6 (11%) | 4 (13%) | 1 (7%) | ||
| Others | 5 (5%) | 2 (4%) | 3 (6%) | 2 (4%) | 1 (3%) | 2 (13%) | ||
| Local recurrence | 0.1 | 0.13 | ||||||
| Negative | 93 (89%) | 50 (94%) | 43 (84%) | 53 (93%) | 27 (84%) | 13 (87%) | ||
| Positive | 11 (11%) | 3 (6%) | 8 (16%) | 4 (7%) | 5 (16%) | 2 (13%) | ||
| Distant metastasis | 0.04* | 0.62 | ||||||
| Negative | 84 (81%) | 47 (89%) | 37 (73%) | 47 (82%) | 25 (78%) | 12 (80%) | ||
| Positive | 20 (19%) | 6 (11%) | 14 (27%) | 10 (18%) | 7 (22%) | 3 (20%) | ||
| Tumor IFN-γ (pre-neoCRT) | 0.0001* | |||||||
| High | 44 (42%) | 32 (60%) | 12 (24%) | – | – | – | ||
| Low | 60 (58%) | 21 (40%) | 39 (76%) | – | – | – | ||
| Tumor IFN-γ (post-neoCRT) | 0.26 | |||||||
| High | – | – | – | 50 (88%) | 28 (88%) | 0 (0%) | ||
| Low | – | – | – | 7 (12%) | 4 (13%) | 0 (0%) | ||
| NA | – | – | – | 15 (100%) | ||||
| Tumor TGF-β (pre-neoCRT) | 0.3 | |||||||
| High | 62 (70%) | 35 (74%) | 27 (62%) | – | – | – | ||
| Low | 27 (30%) | 12 (26%) | 15 (36%) | – | – | – | ||
| Tumor TGF-β (post-neoCRT) | 0.1 | |||||||
| High | – | – | – | 52 (91%) | 26 (81%) | 0 (0%) | ||
| Low | – | – | – | 5 (9%) | 6 (19%) | 0 (0%) | ||
| NA | – | – | – | 15 (100%) | ||||
| Microsatellite instability (MSI) status | 1.0 | 1.0 | ||||||
| Proficient | 102 (98%) | 52 (98%) | 50 (98%) | 56 (98%) | 31 (97%) | 15 (100%) | ||
| Deficient | 2 (2%) | 1 (2%) | 1 (2%) | 1 (2%) | 1 (3%) | 0 (0%) | ||
Chi-squared test was used. Fisher’s exact test was used when > 25% of the cells had expected counts < 5
The contrast test did not include the “NA” group
*p < 0.05
cN stage positive (stage 1 + 2) vs. negative (stage 0), pN stage positive (stage 1a + 1b + 2) vs. negative (stage 0 + X), CR complete response, PR partial response, SD stable disease, PD progressive disease, tumor PD-L1 high (grade 2 + 3) vs. low (grade 0 + 1), tumor IFN-γ high (grade 2 + 3) vs. low (grade 0 + 1), tumor TGF-β high (grade 2 + 3) vs. low (grade 0 + 1), NA surgical specimen is TRG4 with no residue tumor tissue after neoCRT treatment
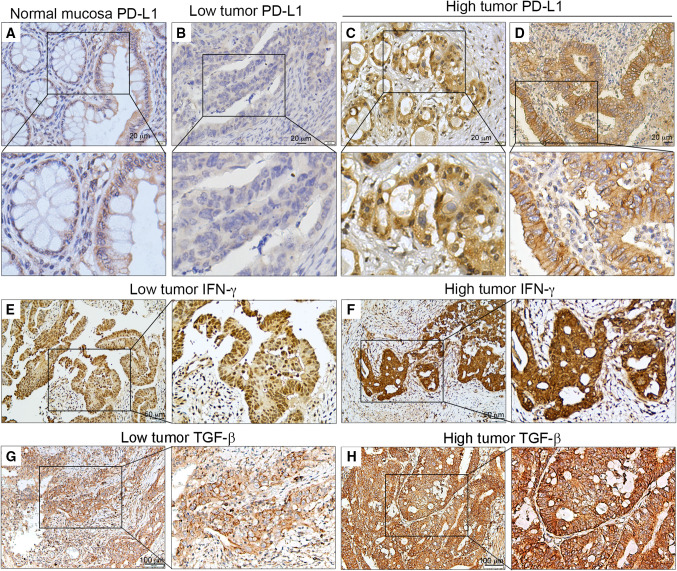
Tumor PD-L1 expression was examined in the cancer tissues and adjacent normal mucosae. PD-L1 was detectable in epithelial cells from normal colonic mucosae and cancer cells (Fig. 1a–d). The correlation between PD-L1 expression and the clinicopathologic characteristics of patients with pre-neoCRT biopsies and post-neoCRT tissues is presented in Table 1. No correlation was observed between PD-L1 expression and age, sex, pN stage, nodal metastasis or MSI status. Tumor PD-L1 expression was correlated with TNM stage, chemotherapy, TRG, and clinical response. PD-L1 levels were increased by neoCRT [Table 1; pre-neoCRT: 53/104 (51%) vs. post-neoCRT: 57/89 (64%)]. Moreover, patients with high tumor PD-L1 expression in pre-neoCRT biopsies exhibited a reduced incidence of distant metastasis [6/53 (11%)] compared with those with low tumor PD-L1 [14/51 (27%), p < 0.05]. However, patients with high tumor PD-L1 did not exhibited any noticeable changes in local recurrence rate compared with those with low tumor PD-L1.
Fig. 1.
Representative PD-L1, IFN-γ and TGF-β immunohistochemical images from TMA patients with LARC. a PD-L1 expression in normal mucosa. b–d Low and high tumor PD-L1 expression in pre-neoCRT biopsies. e, f Low and high tumor IFN-γ expression in post-neoCRT tissues. g, h Low and high tumor TGF-β expression in post-neoCRT tissues
High tumor PD-L1 expression is associated with improved 5-year DFS and 5-year OS
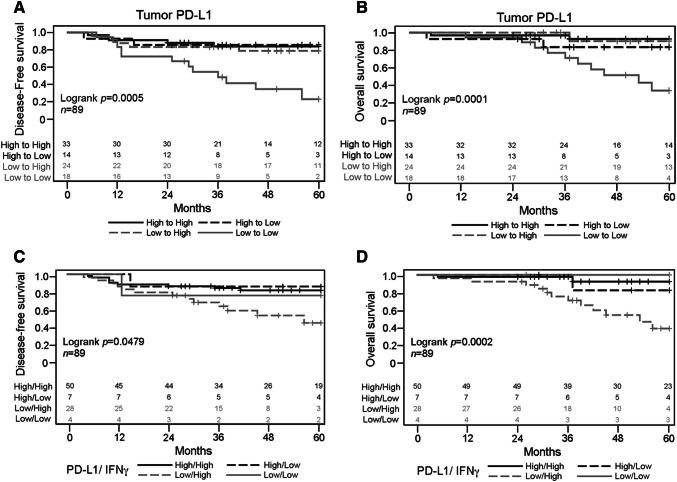
We found that 26 LARC patients (25%) died within the 5-year follow-up period (median follow-up time was 3.8 year). The estimated 5-year disease-free survival (DFS) and overall survival (OS) rates were 69% and 75%, respectively (Table 2). In the 5-year DFS analysis, patients with negative pN stage, good clinical response, and high tumor PD-L1 expression in pre-neoCRT biopsies and post-neoCRT tissues exhibited significantly better DFS compared with the other subgroups (76 vs. 53%, 81 vs. 58%, 84 vs. 56%, and 81 vs. 48%). Furthermore, patients with high tumor PD-L1 expression in post-neoCRT tissues exhibited significantly better OS (91 vs. 51%; Table 2). Kaplan–Meier survival analysis revealed that low PD-L1 expression in both pre-neoCRT biopsies and post-neoCRT tissues was associated with a worsened DFS (Log rank p = 0.0005, Fig. 2a) and OS (Log rank p = 0.0001, Fig. 2b) compared with other subgroups. These results suggest that neoCRT-induced upregulation of tumor PD-L1 is indicative of improved prognosis in LARC patients.
Table 2.
Clinicopathologic parameters, 5-year disease-free survival, and 5-year overall survival
| Variable | Total cases | 5-y DFS | 5-y OS | ||
|---|---|---|---|---|---|
| % | p value | % | p value | ||
| 104 | 69% | 75% | |||
| cN stage | 0.48 | 0.32 | |||
| Negative | 52 | 74% | 82% | ||
| Positive | 52 | 65% | 70% | ||
| pN stage | 0.007* | 0.12 | |||
| Negative | 74 | 76% | 78% | ||
| Positive | 30 | 53% | 69% | ||
| Clinical response | 0.02* | 0.18 | |||
| Good response | 54 | 81% | 78% | ||
| Poor response | 50 | 58% | 72% | ||
| TRG | 0.12 | 0.15 | |||
| Good response | 72 | 74% | 79% | ||
| Poor response | 32 | 60% | 68% | ||
| Pre-neoCRT | 104 | ||||
| Tumor PD-L1 | 0.01* | 0.05 | |||
| High | 53 | 84% | 88% | ||
| Low | 51 | 56% | 65% | ||
| Tumor IFN-γ | 0.26 | 0.41 | |||
| High | 44 | 74% | 80% | ||
| Low | 60 | 67% | 71% | ||
| Post-neoCRT | 89 | ||||
| Tumor PD-L1 | 0.008* | 0.0002* | |||
| High | 57 | 81% | 91% | ||
| Low | 32 | 48% | 51% | ||
| NA | 15 | 70% | 67% | ||
| Tumor IFN-γ | 0.47 | 0.36 | |||
| High | 78 | 67% | 75% | ||
| Low | 11 | 82% | 89% | ||
| NA | 15 | 70% | 67% | ||
| pN stage: positive | 30 | ||||
| Tumor PD-L1 (post-neoCRT) | 0.02* | 0.02* | |||
| High | 18 | 69% | 85% | ||
| Low | 12 | 31% | 45% | ||
| pN stage: negative | 74 | ||||
| Tumor PD-L1 (post-neoCRT) | 0.13 | 0.006* | |||
| High | 39 | 87% | 94% | ||
| Low | 20 | 57% | 53% | ||
| NA | 15 | 70% | 67% | ||
Kaplan–Meier method was used for survival analysis. The contrast test did not include the “NA” group. p value was obtained from log-rank test
*p < 0.05
cN stage positive (stage 1 + 2) vs. negative (stage 0), pN stage positive (stage 1a + 1b + 2) vs. negative (stage 0 + X), clinical response good response (complete response and partial response) vs. poor response (stable disease and progressive disease), TRG good response (TRG 3–4) vs. poor response (TRG 1–2), tumor PD-L1 high (grade 2 + 3) vs. low (grade 0 + 1), tumor IFN-γ high (grade 2 + 3) vs. low (grade 0 + 1), SE standard error
Fig. 2.
Association of DFS and OS with tumor PD-L1 and IFN-γ expression in LARC patients. Kaplan–Meier curves presenting a DFS and b OS in pre- and post-neoCRT samples based on tumor PD-L1 expression status. Kaplan–Meier curves are also presented for c DFS and d OS based on tumor PD-L1 and IFN-γ expression status
Tumor PD-L1 expression is associated with clinical outcomes of nodal metastasis LARC patients
We found that high tumor PD-L1 expression in the pre-neoCRT biopsies was associated with a lower incidence of distant metastasis and a longer DFS and OS in post-neoCRT tissues. However, patients with lymph node metastasis exhibited a high incidence of distant metastasis. We then stratified the patients by lymph node metastasis into pN+ (positive nodal metastasis; stage 1a + 1b + 2) and pN- (negative nodal metastasis; stage 0 + X) subgroups to examine the role of tumor PD-L1 expression. In pN+ and pN− patients, the estimated 5-year DFS was 58% and 76%, respectively, and the estimated OS was 69% and 78%, respectively. These results suggest that patients with lymph node metastasis following neoCRT treatment typically exhibit poorer outcomes (Table 2). However, high PD-L1 expression in the post-neoCRT tissue was strongly correlated with improved 5-year DFS and OS in lymph node metastasis patients (69% vs. 31%, p = 0.02; 85% vs. 45%, p = 0.02, respectively; Table 2). Even in pN− patients, we found that high PD-L1 expression in the post-neoCRT tissues was associated with improved 5-year OS (93% vs. 52%, p = 0.03; Table 2). Taken together, these results indicate that PD-L1 expression has prognostic value for patients with nodal metastasis and that upregulation of PD-L1 is correlated with improved survival after neoCRT treatment in LARC patients.
Univariate and multivariate analysis of PD-L1 expression
As shown in Table 3, we used univariate and multivariate analyses to assess 5-year DFS and 5-year OS risk factors in LARC patients. Univariate analysis demonstrated that pN stage, clinical response, and PD-L1 expression were significantly associated with 5-year DFS in both the pre-neoCRT biopsies and post-neoCRT tissues (Table 3). pN stage [hazard ratio (HR) 2.55, 95% confidence interval (CI) 1.03–6.32] and low tumor PD-L1 expression (HR 2.55, 95% CI 1.05–6.20) were identified as independent risk factors for 5-year DFS in pre-neoCRT biopsies using multivariate analysis. pN stage (HR 4.35, 95% CI 1.69–11.22), TRG (HR 2.68, 95% CI 1.07–6.72), and low tumor PD-L1 expression (HR 4.27, 95% CI 1.62–11.26) were identified as independent risk factors for 5-year DFS in the post-neoCRT tissues by multivariate analysis.
Table 3.
Univariate and multivariate analyses for 5-year disease-free survival and overall survival
| Variables | 5-year disease-free survival | 5-year overall survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate-I | Multivariate-II | Univariate | Multivariate-I | Multivariate-II | |||||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Pre-neoCRT (n = 104) | ||||||||||||
| Age (≥ 65 vs < 65) | 1.23 (0.58–2.63) | 0.59 | 1.26 (0.53–3.01) | 0.61 | 1.36 (0.58–3.19) | 0.48 | 1.42 (0.57–3.53) | 0.45 | 1.63 (0.59–4.53) | 0.35 | 1.81 (0.67–4.88) | 0.24 |
| cN stage (positive vs. negative) | 1.31 (0.62–2.76) | 0.48 | 0.9 (0.38–2.13) | 0.81 | 0.99 (0.43–2.28) | 0.97 | 1.61 (0.63–4.09) | 0.32 | 1.41 (0.50–3.99) | 0.51 | 1.51 (0.54–4.20) | 0.43 |
| pN stage (positive vs. negative) | 2.67 (1.27–5.62) | 0.01* | 2.55 (1.03–6.32) | 0.04* | 2.37 (0.99–5.67) | 0.05* | 2.05 (0.83–5.11) | 0.12 | 1.95 (0.64–5.92) | 0.24 | 1.87 (0.64–5.52) | 0.26 |
| Clinical response (poor vs. good) | 2.56 (1.16–5.66) | 0.02* | 1.55 (0.60–4.00) | 0.37 | 1.55 (0.60–4.01) | 0.36 | 1.9 (0.75–4.84) | 0.18 | 1.05 (0.33–3.32) | 0.94 | 1.06 (0.33–3.37) | 0.92 |
| TRG (poor vs. good) | 1.76 (0.83–3.73) | 0.14 | 1.76 (0.73–4.23) | 0.21 | 1.72 (0.72–4.12) | 0.22 | 1.88 (0.76–4.62) | 0.17 | 1.94 (0.68–5.57) | 0.22 | 1.93 (0.67–5.53) | 0.22 |
| Tumor PD-L1 (low vs. high) | 2.65 (1.17–6.02) | 0.02* | 2.55 (1.05–6.20) | 0.04* | – | – | 2.59 (0.93–7.21) | 0.07 | 2.37 (0.76–7.38) | 0.14 | – | – |
| IFN-γ/ tumor PD-L1 (L∥L vs. H&H) | 1.76 (0.72–4.35) | 0.22 | – | – | 1.55 (0.62–3.84) | 0.35 | 1.84 (0.61–5.55) | 0.28 | – | – | 1.57 (0.51–4.80) | 0.43 |
| Post-neoCRT (n = 89) | ||||||||||||
| Age (≥ 65 vs < 65) | 1.57 (0.70–3.54) | 0.28 | 1.14 (0.47–2.75) | 0.78 | 1.07 (0.41–2.80) | 0.88 | 1.66 (0.62–4.45) | 0.32 | 0.99 (0.34–2.92) | 0.99 | 1.09 (0.35–3.41) | 0.88 |
| cN stage (POSITIVE vs. negative) | 0.93 (0.42–2.08) | 0.87 | 0.41 (0.16–1.10) | 0.08 | 0.48 (0.19–1.26) | 0.14 | 1.24 (0.46–3.32) | 0.67 | 0.38 (0.10–1.46) | 0.16 | 0.54 (0.15–1.89) | 0.33 |
| pN stage (positive vs. negative) | 2.87 (1.28–6.42) | 0.01* | 4.35 (1.69–11.22) | 0.002* | 3.04 (1.18–7.84) | 0.02* | 2.21 (0.83–5.91) | 0.11 | 3.96 (1.17–13.42) | 0.03* | 2.37 (0.69–8.11) | 0.17 |
| Clinical response (poor vs. good) | 3.3 (1.23–8.85) | 0.02* | 2.48 (0.76–8.10) | 0.13 | 2.18 (0.66–7.18) | 0.2 | 2.37 (0.77–7.36) | 0.13 | 1.66 (0.35–7.99) | 0.53 | 1.4 (0.30–6.55) | 0.67 |
| TRG (poor vs. good) | 1.85 (0.83–4.12) | 0.13 | 2.68 (1.07–6.72) | 0.04* | 2.13 (0.84–5.38) | 0.11 | 2.09 (0.78–5.62) | 0.14 | 3.2 (1.01–10.12) | 0.048* | 2.44 (0.76–7.84) | 0.13 |
| Tumor PD-L1 (low vs. high) | 2.86 (1.27–6.45) | 0.01* | 4.27 (1.62–11.26) | 0.003* | – | – | 6.43 (2.07–19.97) | 0.001* | 10.71 (2.61–43.87) | 0.001* | – | – |
| IFN-γ/ tumor PD-L1 (L∥L vs. H&H) | 2.32 (1.02–5.32) | 0.046* | – | – | 2.51 (1.02–6.19) | 0.046* | 6.25 (1.78–21.96) | 0.004* | – | – | 6.61 (1.72–25.42) | 0.006* |
*p < 0.05
pN stage positive (stage 1a + 1b + 2) vs. negative (stage 0 + X), clinical response good response (complete response and partial response) vs. poor response (stable disease and progressive disease), TRG good response (TRG 3–4) vs. poor response (TRG 1–2), tumor PD-L1 high (grade 2 + 3) vs. low (grade 0 + 1), tumor IFN-γ high (grade 2 + 3) vs. low (grade 0 + 1), IFN-γ/tumor PD-L1 (L∥L vs. H&H) low IFN-γ or tumor PD-L1 vs. high IFN-γ and high PD-L1
Furthermore, univariate analysis demonstrated that low tumor PD-L1 expression in the post-neoCRT tissues was the only factor significantly associated with 5-year OS (Table 3). pN stage (HR 3.96, 95% CI 1.17–13.42), TRG (HR 3.20, 95% CI 1.01–10.12), and low tumor PD-L1 expression (HR 10.71, 95% CI 2.61–43.87) were identified as independent risk factors for 5-year OS in the post-neoCRT tissues by multivariate analysis.
Radiation upregulates IFN-γ and PD-L1 expression in CRC cells
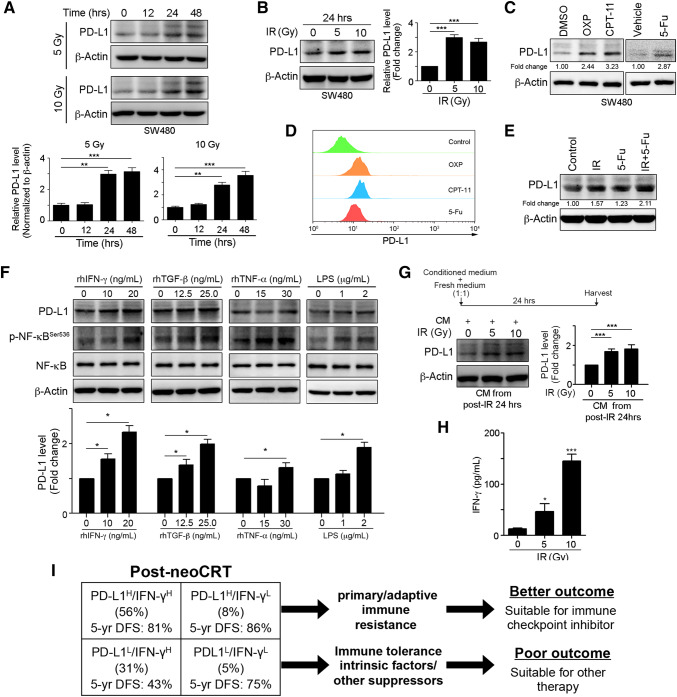
To determine whether upregulation of tumor PD-L1 in the post-neoCRT tissues was induced by neoCRT, we treated CRC cell lines with radiation or chemotherapeutic agents. As shown in Fig. 3a, b, immunoblotting results demonstrated that PD-L1 expression was significantly upregulated by radiation in a time- and concentration-dependent manner. Treatment with the anti-neoplastic chemotherapeutic agents oxaliplatin (OXP), irinotecan (CPT-11) and 5-fluorouracil (5-Fu) clearly triggered the upregulation and membranous expression of PD-L1 (Fig. 3c, d). Moreover, combinational treatment of 5-Fu and irradiation synergic increased PD-L1 expression (Fig. 3e). These results indicate that tumor PD-L1 is upregulated by both radiation and chemotherapeutic agents.
Fig. 3.
Radiation-triggered IFN-γ release upregulates PD-L1 expression. a, b SW480 cells were exposed to radiation and then harvested. Cell lysates were analyzed via immunoblotting, and the quantification is presented (n = 3). c SW480 cells were treated with oxaliplatin (10 µM), CPT-11 (5 µM) or 5-Fu (10 mg/kg) for 24 h. Cell lysates were analyzed via immunoblotting, and the quantification is presented (n = 3). d SW480 cells were treated with oxaliplatin (10 µM), CPT-11 (5 µM) or 5-Fu (10 mg/kg) for 24 h. Surface PD-L1 expression on tumor cells was analyzed via flow cytometry (n = 3). e SW480 cells were exposed to radiation and then treated with 5-Fu (10 mg/kg) for 24 h. Cell lysates were analyzed via immunoblotting, and the quantification is presented (n = 3). f SW480 cells were treated with cytokines and LPS for 24 h. Cell lysates were analyzed via immunoblotting, and the quantification is shown presented. g SW480 cells were exposed to radiation, and conditioned medium was harvested. Thereafter, a mixture of conditioned medium and fresh medium (1:1) was used to culture SW480 cells for 24 h. Cell lysates were analyzed via immunoblotting, and the quantification is presented (n = 3). h The conditioned medium from SW480 cells after 24 h of radiation was analyzed via an enzyme-linked immunosorbant assay, and the IFN-γ concentration is presented (n = 3). i Changes in PD-L1 and IFN-γ expression ratios correlate with 5-y DFS and neoCRT treatment outcomes in patients with locally advanced rectal cancer. **p < 0.01 and ***p < 0.001
In addition to radiation and chemotherapeutic agents, the inflammatory milieu and inflammatory cytokines, such as IFN-γ and tumor necrosis factor-α (TNF-α), can also upregulate PD-L1 expression [28]. Hence, we incubated CRC cells with lipopolysaccharides (LPS), TNF-α, transforming growth factor-β (TGF-β), and IFN-γ and observed PD-L1 expression. As shown in Fig. 3f, we found that these cytokines induced PD-L1 expression in the CRC cells after 48 h of incubation, and the highest expression was observed in cells exposed to IFN-γ. Therefore, we collected the post-irradiation conditioned medium after 24 h for cytokine detection and added this medium to cancer cells. The immunoblotting results demonstrated that conditioned media from irradiated CRC cells stimulated PD-L1 expression. We also found that IFN-γ was released from irradiated cancer cells based on ELISA analysis (Fig. 3g, h). These results indicate that radiation can trigger IFN-γ release to induce PD-L1 expression.
neoCRT upregulates tumor IFN-γ and PD-L1 in LARC
Next, we analyzed IFN-γ and TGF-β expression by immunohistochemistry (IHC) in tumor tissues from LARC TMAs (Fig. 1e–h). The number of patients with high IFN-γ expression increased following neoCRT treatment [44/104 (42%) vs. 78/89 (88%)], and the number of patients with high IFN-γ expression and high PD-L1 expression [32/104 (31%) vs. 50/89 (56%)] also increased (Table 1). These data strongly suggest that tumor PD-L1 upregulation is correlated with IFN-γ expression and that neoCRT indeed induced tumor PD-L1 and IFN-γ upregulation. However, IFN-γ expression alone did not significantly affect the 5-year DFS and OS (Table 2). Kaplan–Meier survival analysis demonstrated that low PD-L1 and IFN-γ expression in the post-neoCRT tissues was associated with a worsened DFS (Log rank p = 0.0497, Fig. 2c) and OS (Log rank p = 0.0002, Fig. 2d) compared with the other subgroups. Moreover, univariate and multivariate analyses revealed that low PD-L1 or IFN-γ expression was not significantly associated with 5-year DFS or OS in the pre-neoCRT biopsies. However, low PD-L1 and IFN-γ expression was identified as an independent risk factor for 5-year DFS (HR 2.51, 95% CI 1.02–6.19) and 5-year OS (HR 6.61, 95% CI 1.72–25.42) in the post-neoCRT tissues based on multivariate analysis (Table 3). However, TGF-β expression does not correlate with PD-L1 expression.
Our clinical data also demonstrated that upregulation of PD-L1 is associated with IFN-γ overexpression and is a positive prognostic factor for clinical outcome after neoCRT treatment for LARC patients.
Discussion
Our study showed that tumor PD-L1 expression has prognostic value for neoCRT-treated LARC patients with or without nodal metastases. In our in vitro study, we found that radiation and chemotherapeutic drugs could trigger tumor cells to produce IFN-γ and induce PD-L1 upregulation. Our clinical data also demonstrated that upregulation of PD-L1 is associated with IFN-γ overexpression and is a positive prognostic factor for clinical outcome after neoCRT treatment for LARC patients. The controversy potentially stemmed from the observation that in a naïve tumor environment, PD-L1 expression may lead to tumor growth by escaping immune surveillance; however, surgical removal of the tumor after neoCRT treatment removes this confounding element. Instead, we found that tumors, even in the event of nodal spread, expressing PD-L1 via upregulation of IFN-γ exhibited viable adaptive immune response capabilities and, therefore, do better. They likely reinvigorate the anti-tumor immune response after neoCRT against those tumor cells metastasized to other organs. Finally, even in nodal metastatic LARC patients, PD-L1 upregulation in post-neoCRT tissues was a positive prognostic factor for DFS and OS. Our data confirmed that this group of patients did as good as those without nodal metastasis.
The PD-1/PD-L1 axis, an immune checkpoint, is typically upregulated to create an immunosuppressive microenvironment and to help cancer cells escape immune-mediated cell death [13]. PD-1/PD-L1 checkpoint blockade is considered an effective immunotherapy to reinvigorate the host immune system to attract tumor cell and cause tumor destruction. Tumor PD-L1 expression is considered favorable for PD-1/PD-L1 blockade treatment, but the clinical outcome of PD-L1 upregulation remains controversial. Many studies have revealed that PD-L1 expression is associated with a poor prognosis in a variety of human cancers, such as malignant melanoma [29], lung cancer [30], RCC [31], gastric cancer [32, 33], breast cancer [34], and CRC [35, 36]. These data suggest that PD-L1 expression by tumor cells can contribute to impaired tumor-infiltrating lymphocyte (TIL) function, thus impeding antitumor immunity. In contrast, recent reports have demonstrated that PD-L1 expression in tumor cells was strongly associated with improved outcomes in breast cancer [37–39], NSCLC [40], malignant melanomas [41], and CRC [21]. These studies found that PD-L1 expression is the result of a feedback mechanism caused by induction of IFN-γ, which is secreted from tumor cells and TILs [21]. Hence, our data demonstrate that PD-L1 is upregulated by the host immune response and causes adaptive immune resistance to promote primary tumor growth. However, subsequent surgical operations and treatment could reinvigorate the preexisting anti-tumor immune response, leading to better responses.
Consistent with our findings, Dovedi et al. indicated that radiotherapy-mediated gradually increases in tumor PD-L1 expression after 72 h and vanish after 7 days following fractionated radiotherapy [17]. Hecht et al. also demonstrated that neoCRT induced long-lasting PD-L1 overexpression at least 7 weeks before surgery, which was associated with a favorable prognosis in rectal adenocarcinoma patients [42]. Interestingly, neoCRT seems to induce long term overexpression of PD-L1, which may persist for a therapy-free period of at least 6–8 weeks following the last radiotherapy treatment and prior to surgery.
Our results demonstrate several potential benefits for immune checkpoint inhibitor treatment. First, we observed a significant increase in the proportion of PD-L1-positive cells (51–64%) in 104 pre-neoCRT biopsies and 89 corresponding post-neoCRT surgical specimens from LARC patients. Multivariate analysis also revealed that PD-L1 expression in post-neoCRT surgical specimens was a significant independent prognostic factor for DFS and OS. In vitro data demonstrated that PD-L1 expression was induced by radiation and chemotherapy. These results imply that radiation not only affects the tumor itself but also causes upregulation of PD-L1 in tumor cells. Furthermore, we observed that the proportion of tumor cells co-expressing PD-L1 and IFN-γ significantly increased (from 31 to 56%) in pre- and post-neoCRT LARC patients and resulted in improved 5-year DFS and OS. In vitro data also demonstrated that IFN-γ induced PD-L1 expression in tumor cells after radiation treatment. Hence, radiation could induce IFN-γ production, leading to increased PD-L1 expression in tumor cells.
In addition, it is worth mentioning that approximately one-third of patients exhibited low PD-L1 expression despite high IFN-γ expression and did worse (Fig. 3g). From our study, we caught sight of two faces of IFN-γ: one with intact neoCRT-induced PD-L1 machinery and another impaired immune functions. Patients who expressed PD-L1 after neoCRT (with the majority via upregulation of IFN-γ) were candidates for ICB. On the other hand, if neoCRT failed to increase PD-L1 expression, this cohort may not be suitable for ICB after neoCRT and should consider other adjuvant therapy (Fig. 3g).
A combination of PD-1/PD-L1 pathway blockade and ionizing radiation synergistically inhibits tumor growth in animal models of various tumor types and also improves tumor control in contralateral non-irradiated secondary tumors in mice [16, 17, 43]. Several retrospective clinical outcome analyses in esophageal cancer, bladder cancer, and rectal cancer also support the synergistic effects of neoCRT and PD-1/PD-L1 immunotherapy [42, 44–47]. Hence, neoCRT treatment may be an effective strategy to stimulate adaptive immune resistance to improve tumor control for immune checkpoint inhibitor treatment. Understanding the mechanisms responsible for inducing the upregulation of PD-L1 along with determining when to observe this effect are pivotal for the development of immune checkpoint inhibitor treatments. Taken together, this study demonstrated that increased PD-L1 expression in both pre- and post-neoCRT tissues correlated with improved prognosis for patients with or without nodal metastasis. PD-L1 expression may be a useful biomarker to predict outcomes in patients receiving neoCRT treatment for LARC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CRC
Colorectal cancer
- DAMPs
Danger-associated molecular patterns
- DFS
Disease-free survival
- ICB
Immune checkpoint blockade
- ICD
Immunogenic cell death
- IFN-γ
Interferon-γ
- LARC
Locally advanced rectal cancer
- MSI
Microsatellite instability
- neoCRT
Neoadjuvant chemoradiotherapy
- OS
Overall survival
- pCR
Pathologic complete response
- PD-1
Programmed cell death 1 receptor
- PD-L1
Programmed cell death 1 ligand 1
- pN stage
Pathologic lymph node stage
- TILs
Tumor-infiltrating lymphocytes
- TMA
Tissue microarray
- TRG
Tumor regression grade
Author contributions
C-YH and S-FC conducted and performed the experiments; WT-LC, T-WK and T-WC enrolled the LARC patients and performed IHC evaluation; Y-SY and Y-CL performed the statistical analysis; S-FC and KSCC supervised this study; C-YH, S-FC, and KSCC analyzed the data and wrote the manuscript. All authors read and approved the final manuscript version.
Funding
This study was supported by grants from China Medical University Hospital [DMR-107-103 (Taiwan) and DMR-CELL-17022 (Taiwan)], Ministry of Science and Technology (MOST 107-2314-B-039 -027 -MY3, Taiwan), Ministry of Health, and Welfare (MOHW107-TDU-B-212-123004, Taiwan), and Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW107-TDU-B-212-114024, Taiwan).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
William Tzu-Liang Chen and K. S. Clifford Chao contributed equally to this work.
Contributor Information
William Tzu-Liang Chen, Phone: 886-4-22052121, Email: golfoma22@gmail.com.
K. S. Clifford Chao, Phone: 886-4-22052121, Email: chao_ks@yahoo.com.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Conde-Muino R, Cuadros M, Zambudio N, Segura-Jimenez I, Cano C, Palma P (2015) Predictive biomarkers to chemoradiation in locally advanced rectal cancer. Biomed Res Int 2015:921435. 10.1155/2015/921435 [DOI] [PMC free article] [PubMed]
- 3.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rodel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 4.Yoon WH, Kim HJ, Kim CH, Joo JK, Kim YJ, Kim HR. Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer. Ann Surg Treat Res. 2015;88(1):15–20. doi: 10.4174/astr.2015.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balko JM, Black EP. A gene expression predictor of response to EGFR-targeted therapy stratifies progression-free survival to cetuximab in KRAS wild-type metastatic colorectal cancer. BMC Cancer. 2009;9:145. doi: 10.1186/1471-2407-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wennerberg E, Vanpouille-Box C, Bornstein S, Yamazaki T, Demaria S, Galluzzi L. Immune recognition of irradiated cancer cells. Immunol Rev. 2017;280(1):220–230. doi: 10.1111/imr.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Showalter A, Limaye A, Oyer JL, Igarashi R, Kittipatarin C, Copik AJ, Khaled AR. Cytokines in immunogenic cell death: applications for cancer immunotherapy. Cytokine. 2017;97:123–132. doi: 10.1016/j.cyto.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi MR, Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17(19):6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- 12.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–5039. doi: 10.2147/OTT.S105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 14.Teng MWL, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Can Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.can-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol. 2015;51(3):221–228. doi: 10.1016/j.oraloncology.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Investig. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 18.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, Li Q, Cai S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. doi: 10.1186/s12943-016-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167(6):1540–1554 e1512. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49(9):2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Huang CY, Chiang SF, Ke TW, Chen TW, Lan YC, You YS, Shiau AC, Chen WT, Chao KSC. Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1 + TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol Immunother. 2018;67(4):551–562. doi: 10.1007/s00262-017-2109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 24.Liu LC, Su CH, Wang HC, Chang WS, Tsai CW, Maa MC, Tsai CH, Tsai FJ, Bau DT (2014) Contribution of personalized Cyclin D1 genotype to triple negative breast cancer risk. BioMedicine 4 (1). 10.7603/s40681-014-0003-4 [DOI] [PMC free article] [PubMed]
- 25.Wang X, Sheu JJC, Lai MT, Yin-Yi Chang C, Sheng X, Wei L, Gao Y, Wang X, Liu N, Xie W, Chen C-M, Ding WY, Sun L. RSF-1 overexpression determines cancer progression and drug resistance in cervical cancer. BioMedicine. 2018;8(1):4. doi: 10.1051/bmdcn/2018080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao KSC, Chen WTL, Ke TW, Chiang SF, Chen TW, Huang CY, You YS, Kuo YC. Acquired immunity trumps ypN + and TRG as the sole prognostic biomarker for locally advanced rectal cancer (LARC) treated with neoadjuvant chemoradiation therapy (NeoCRT). ASTRO annual meeting 2016. Int J Radiat Oncol Biol Phys. 2016;96(2):S100. doi: 10.1016/j.ijrobp.2016.06.265. [DOI] [Google Scholar]
- 27.Huang CY, Chiang SF, Chen TLW, Ke TW, Chen TW, You YS, Chao KSC. Upregulation of tumor PD-L1 by neoCRT may hold the key to successes in patients with pN + locally advanced rectal cancer. ASTRO annual meeting 2017. Int J Radiat Oncol Biol Phys. 2017;99(2):S65. doi: 10.1016/j.ijrobp.2017.06.160. [DOI] [Google Scholar]
- 28.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 30.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 31.Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, Go H. Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol. 2016;23(2):694–702. doi: 10.1245/s10434-015-4903-7. [DOI] [PubMed] [Google Scholar]
- 32.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10(6):e0131403. doi: 10.1371/journal.pone.0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eto S, Yoshikawa K, Nishi M, Higashijima J, Tokunaga T, Nakao T, Kashihara H, Takasu C, Iwata T, Shimada M. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer. 2016;19(2):466–471. doi: 10.1007/s10120-015-0519-7. [DOI] [PubMed] [Google Scholar]
- 34.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146(1):15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu H, Qin H, Huang Z, Li S, Zhu X, He J, Yang J, Yu X, Yi X. Clinical significance of programmed death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int J Clin Exp Pathol. 2015;8(8):9351–9359. [PMC free article] [PubMed] [Google Scholar]
- 36.Koganemaru S, Inoshita N, Miura Y, Miyama Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, Tanabe Y, Moriyama J, Fujii T, Matoba S, Kuroyanagi H, Takano T. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108(5):853–858. doi: 10.1111/cas.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20(10):2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 38.Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D, Bertucci F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6(7):5449–5464. doi: 10.18632/oncotarget.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47(1):78–84. doi: 10.1016/j.humpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Investig. 2014;94(1):107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hecht M, Buttner-Herold M, Erlenbach-Wunsch K, Haderlein M, Croner R, Grutzmann R, Hartmann A, Fietkau R, Distel LV. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer. 2016;65:52–60. doi: 10.1016/j.ejca.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu CT, Chen WC, Chang YH, Lin WY, Chen MF. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci Rep. 2016;6:19740. doi: 10.1038/srep19740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saigusa S, Toiyama Y, Tanaka K, Inoue Y, Mori K, Ide S, Imaoka H, Kawamura M, Mohri Y, Kusunoki M. Implication of programmed cell death ligand 1 expression in tumor recurrence and prognosis in rectal cancer with neoadjuvant chemoradiotherapy. Int J Clin Oncol. 2016;21(5):946–952. doi: 10.1007/s10147-016-0962-4. [DOI] [PubMed] [Google Scholar]
- 46.Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh D, Ahn YC, Jung SH, Ahn MJ, Park K, Zo JI, Shim YM, Sun JM. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer. 2016;52:1–9. doi: 10.1016/j.ejca.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Jomrich G, Silberhumer GR, Marian B, Beer A, Mullauer L. Programmed death-ligand 1 expression in rectal cancer. Eur Surg. 2016;48(6):352–356. doi: 10.1007/s10353-016-0447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.