Abstract
Aging immune deterioration and Epstein–Barr (EBV) intrinsic mechanisms play an essential role in EBV-positive diffuse large B-cell lymphoma (DLBCL) of the elderly (EBV + DLBCLe) pathogenesis, through the expression of viral proteins, interaction with host molecules and epigenetic regulation, such as miR-155, required for induction of M1 phenotype of macrophages. This study aims to evaluate the relationship between macrophage polarization pattern in the tumor microenvironment and relative expression of miR-155 in EBV + DLBCLe and EBV-negative DLBCL patients. We studied 28 EBV + DLBCLe and 65 EBV-negative DLBCL patients. Tumor-associated macrophages (TAM) were evaluated by expression of CD68, CD163 and CD163/CD68 ratio (degree of M2 polarization), using tissue microarray. RNA was extracted from paraffin-embedded tumor samples for miR-155 relative expression study. We found a significantly higher CD163/CD68 ratio in EBV + DLBCLe compared to EBV-negative DLBCL. In EBV-negative DLBCL, CD163/CD68 ratio was higher among advanced-staged/high-tumor burden disease and overexpression of miR-155 was associated with decreased polarization to the M2 phenotype of macrophages. The opposite was observed in EBV + DLBCLe patients: we found a positive association between miR-155 relative expression and CD163/CD68 ratio, which was not significant after outlier exclusion. We believe that the higher CD163/CD68 ratio in this group is probably due to the presence of the EBV since it directly affects macrophage polarization towards M2 phenotype through cytokine secretion in the tumor microenvironment. Therapeutic strategies modulating miR-155 expression or preventing immuno-regulatory and pro-tumor macrophage polarization could be adjuvants in EBV + DLBCLe therapy since this entity has a rich infiltration of M2 macrophages in its tumor microenvironment.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2273-2) contains supplementary material, which is available to authorized users.
Keywords: Non-Hodgkin lymphoma, EBV, Immunohistochemistry, Tumor-associated macrophages, microRNA
Introduction
The World Health Organization (WHO) classification of hematological malignancies included, in the 4th edition, a provisional entity described as EBV (Epstein–Barr)-positive diffuse large B-cell lymphoma of the elderly (EBV + DLBCLe) [1]. Mechanisms such as the imbalance between the effector B- and T-cell responses, in addition to depletion of naïve T-cell diversity inherent to aging immune deterioration, were proposed as the likely cause of this neoplasm [2, 3]. Epstein–Barr virus intrinsic mechanisms may also play an essential role in this entity pathogenesis by expression of specific sets of viral proteins, interaction with host molecules and epigenetic gene regulation, including microRNA-mediated mechanisms [4].
The WHO classification described two histological patterns of EBV + DLBCLe; the large B-cell lymphoma (monomorphic) and polymorphic variants [5]. The latter was sub-classified into three subtypes: canonical large B cell, DLBCL with polymorphic lymphoproliferative disease features, and DLBCL with Hodgkin’s lymphoma features; depending on the presence of Hodgkin/Reed–Sternberg (HRS)-like cells and the population of transformed B lymphocytes [6, 7]. Besides, these subtypes present a rich inflammatory infiltrate among neoplastic cells composed mainly of plasma cells, lymphocytes, centroblasts, immunoblasts, and epithelioid and non-epithelioid histiocytes [5–7]. The monomorphic variant of EBV + DLBCLe, in turn, is composed of a homogeneous population of transformed B cells [5–7].
Within its spectrum of presentations, the polymorphic variant of EBV + DLBCLe is distinguished by a tumor microenvironment rich in inflammatory cells when compared to the monomorphic variant. Our hypothesis is that this morphology reflects a set of changes in gene expression and functional profile of transformed cells and tumor microenvironment, raised by interactions of viral proteins with host molecules and, particularly, epigenetic gene regulation mechanisms mediated by microRNAs.
A microRNA signature profile for EBV + DLBCLe was proposed by our group [8] in a multicenter study comparing the differential expression of microRNAs in patients diagnosed with this entity to those with EBV-negative DLBCL. Among the differentially expressed microRNAs, miR-155 is particularly important due to its ability to interfere with functionality and differentiation pattern of tumor microenvironment-associated cells [9]. Therefore, we would like to complement previous study focusing on miR-155. MiR-155 is required for induction of M1 macrophages, recruiting inflammatory cells and antitumor response at the beginning of carcinogenesis [9]. Recent reports suggest that miR-155 depletion in myeloid lineage cells such as macrophages and dendritic cells is associated with an increased tumor growth, probably due to a protumoral effect [9].
The tumor environment plays an important role in the biology of DLBCL. The tumor tissues are infiltrated by variable immune cells, including CD4 or CD8 T cells, regulatory T cells, natural killers, macrophages, dendritic cells and myeloid cells [10]. Inside the tumor, myeloid cells show a varied range of phenotypes; M1 macrophages have an antigen-presenting function, mounting an antitumor response against neoplastic cells. On the other hand, tumor-associated macrophages (TAM) (M2 macrophages) have a predominantly immunosuppressive role, remodel the extracellular matrix and, therefore, favor cancer growth and progression by supporting neoangiogenesis, tissue invasion and metastasis [11, 12]. The influence of macrophage polarization in tumor development is a consensus in the literature; however, individualizing M1 and M2 phenotypes is still difficult with available markers [13, 14]: CD68 is frequently used to estimate the total population of macrophages in the tumor microenvironment; TAM (M2 phenotype) are usually assessed from CD163 antigen detection [15, 16]. Patients with DLBCL have distinct clinical outcomes based on microenvironment components [17] and low CD68/CD163 is associated with unfavorable clinical prognosis [18–21]. However, there is no information regarding EBV + DLBCLe patients.
In this study, we evaluated the relationship between macrophage polarization pattern in the tumor microenvironment and relative expression of miR-155, Ann Arbor staging, International Prognostic Index (IPI) and histopathological characterization proposed by Nakamura [5] and Montes-Moreno [6] in EBV + DLBCLe patients comparing them with features of EBV-negative diffuse large B-cell lymphoma (DLBCL).
Materials and methods
Patients
DLBCL patients aged 50 years or older, with no history of congenital/acquired immunodeficiency (all patients were HIV negative) or any previous lymphoproliferative disease treated at Hospital Sao Paulo between 2000 and 2010, were enrolled in this study, excluding patients of primary central nervous system lymphoma. Formalin-fixed paraffin-embedded (FFPE) tumor tissue from these patients was obtained. 69 patients were considered DLBCL not otherwise specified (NOS) and 2 were T-cell/histiocyte-rich large B-cell lymphoma [8]. Among them, six (8.5%) patients were considered EBV + DLBCLe (see “In situ hybridization” method). The remaining 65 DLBCL were considered “controls” for this study (EBV-negative DLBCL). Clinical information such as sex, age, Ann Arbor staging, IPI, and histological classification of Montes-Moreno were obtained from databanks and patients’ medical records. To increase the number of EBV + DLBCLe cases, six EBV + patients from Hospital Sao Paulo were supplemented with 22 additional EBV + cases from the other centers at Sao Paulo state, which provided samples already tested for EBER-1 positivity and that fulfilled the criteria described above. Therefore, we compared a total of 93 patients, comprising 28 cases of EBV + DLBCLe and 65 cases of EBV-negative DLBCL (Supplementary Fig. 1). Groups are comparable regarding median age, gender, Ann Arbor staging (early-stage I/II versus advanced-stage III/IV disease) and IPI according to Andrade et al [8]. IPI [22] is a tool capable of stratifying the risk of patients with DLBCL and is based on the presence or not of five risk factors: age > 60 years, high DHL, performance status ≥ 2, Ann Arbor staging ≥ 3 and the presence of disease in two or more extra-nodal sites.
Tissue microarray (TMA)
During the Andrade et al. [8] study, formalin-fixed paraffin-embedded tissue (FFPET) (lymph node biopsy or tumor mass) was used to construct a TMA with Beecher Instruments equipment (Estigen, Tartu, Estonia). Each case of the study was evaluated by an experienced pathologist (Antonio C. Alves) using a hematoxylin–eosin-stained slide for histological confirmation and demarcation of the area to be drilled for the construction of the TMA. All slides had more than 70% of tumor area; that is, they were representative of tumor and did not showed significant tissue necrosis. Precise cuts in a cylinder shape, with a diameter of 1 mm, were obtained from each case and were inserted into the receptor block, in duplicates. The TMA block was cut at different levels, comprising initial, intermediate and final portions of the samples to obtain slides to be evaluated by immunohistochemistry (i.e., six samples of each case). The morphological classification of the EBV + DLBCLe group was performed according to the Montes-Moreno criteria [6].
In situ hybridization
Epstein–Barr virus detection was performed by in situ hybridization (EBV-encoded RNA probe, ZytoVision, Bremerhaven, Germany) using the Dako hybridizer (Carpinteria, CA, USA) during the Andrade et al. [8] study. We classified patients with at least 50% cell positivity as EBV + DLBCLe.
RNA extraction
RNA extraction was performed using the Recover All Total Nucleic Acid Isolation kit for FFPE (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. Samples containing the isolated RNA were stored at − 80 °C. Quantification and qualitative analyses of total RNA were performed during this study using DeNovix DS-11 spectrophotometer (Denovix, Wilmington, Delaware, USA).
cDNA synthesis
RNA isolated from tumor samples was thawed and diluted for cDNA preparation following TaqMan Small RNA Assay protocol (Applied Biosystems, Foster City, CA, USA) for miR-155 TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and for the manufacturer-suggested endogenous control RNU6B.
Real-time PCR (qPCR)
qPCR was performed using 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Amplifications were performed using TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA) in triplicates. The relative expression of miR-155 was normalized to the endogenous control RNU6B (Applied Biosystems, Foster City, CA). Relative expression was calculated through the 2− ΔCT equation [23]. miR-155 was considered differentially expressed using the 1.5 cutoff [8, 23, 24]. We could not use a normal tissue to perform this comparison since our palatine tonsil samples, which are reactive lymphoid tissue used for this purpose, were mostly EBV positive.
Immunohistochemistry
We evaluated the expression of CD68 (universal macrophage marker) (clone KP1, Dako, Carpinteria, USA), CD163 (TAM M2 marker) (clone 10D6, Novocastra, Newcastle, UK) and CD163/CD68 ratio (to estimate the proportion of macrophages polarized to M2 phenotype) to assess macrophage polarization pattern in tumor microenvironment. The slides were scanned using Scan Scope® Aperio CS2 (Aperio Technologies, Vista, CA, USA) (Supplementary Fig. 2). Positivity was estimated from the following formula:
Parameters were obtained from the image analysis software—ImageJ® v1.51n (NIH, USA)—by two independent observers (Wagner A. Poles and Erika E. Nishi). Determination of the staining detection threshold was guided by an experienced pathologist (Antonio C. Alves) to diminish background interference in staining area. Tissues with total area less than 0.5 mm2 were excluded from the analysis. We also took into consideration quality of the material and correlation between the same patient sample in two different slides.
Statistical analysis
We used chi-square test to evaluate possible differences between the clinical variables of EBV + DLBCLe and EBV-negative DLBCL. Mann–Whitney test was performed to estimate the significance of the difference between the medians. Spearman’s correlation was used to evaluate possible correlations between variables (non-parametric). For all statistical analysis, p value < 0.05 was considered statistically significant. Statistical analysis and gene expression graphs were created with Graphpad Prism software version 6.0.
Results
Patients
Patients’ clinical data grouped by age, gender, Ann Arbor staging and International Prognosis Index (IPI) are shown in Supplementary Table 1. In EBV-negative DLBCL group (65 patients), age varied from 50 to 85 years (median age of 67 years). 61.5% had advanced Ann Arbor stage disease (III or IV) at diagnosis and 53.3% already had IPI > 2. In the EBV + DLBCL group (28 patients), the median age was 68 years and ranged from 51 to 88 years. 61.6% were diagnosed with advanced-disease (Ann Arbor III or IV) and 42.9% had International Prognostic Index > 2. There was no statistically significant difference in clinical variables between the two groups (Supplementary Table 1). In EBV + DLBCLe group, 11 (39.3%) were classified as monomorphic and 17 (60.7%) were polymorphic, according to Montes-Moreno [6] classification. Of these, two patients were sub-classified as Hodgkin lymphoma-like type, seven patients as canonical large B-cell-type, and eight as polymorphic lymphoproliferative disorder-like type, described in our group previous publication [8]. In the EBV + DLBCLe group, 28% of patients had germinal center B cell (GCB) and 72% non-GCB; in the EBV–negative DLBCL group, 52% of patients had GCB and 48% non-GCB (p = 0.0472, chi-square test) [8].
Expression of miR-155
We found increased miR-155 expression (above 1.5 cutoff) in 78.5% of EBV-negative DLBCL and in 67.9% of EBV + DLBCLe. There was no statistically significant difference between the relative expression of this microRNA (miRNA) when comparing the medians of EBV-negative and EBV + DLBCLe groups (p = 0.5064, Mann–Whitney test) (Supplementary Fig. 3).
Macrophage marker expression in EBV + DLBCLe and EBV-negative DLBCL
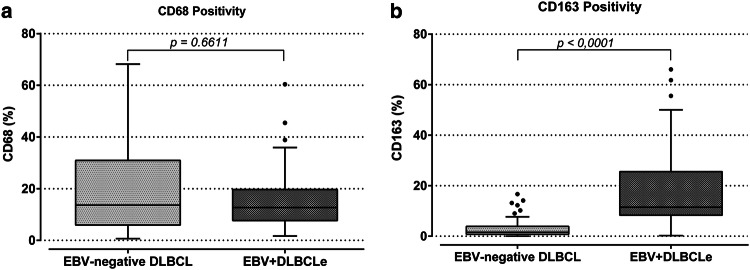
In EBV + DLBCLe, CD163 marker positivity (M2 macrophages) was significantly higher (median 11.51%) than in EBV-negative DLBCL (median of 1.58%) (p < 0, 0001, Mann–Whitney test). There was no statistically significant difference in CD68 marker expression between EBV + DLBCLe (median value of 12.71%) and EBV-negative DLBCL (median value of 13.67%) (p = 0.6611, Mann–Whitney) (Fig. 1).
Fig. 1.
Macrophage marker positivity in EBV + DLBCLe and EBV-negative DLBCL. a There was no difference in CD68 marker positivity between the studied groups. b The figure shows CD163 marker positivity (%) in EBV-negative DLBCL and EBV + DLBCLe. The p values were obtained by the Mann–Whitney test. A significant higher infiltration of CD163-positive cells was detected in EBV + DLBCLe patients. (p < 0.0001, Mann–Whitney)
Macrophage polarization in EBV + DLBCLe and EBV-negative DLBCL
In EBV-positive patients, CD163/CD68 ratio was significantly higher (median value 1.24) than in EBV-negative DLBCL group (median value 0.14) (p < 0.0001, Mann–Whitney test) (Fig. 2). Thus, EBV + DLBCLe cases showed a significant increase in M2 polarization.
Fig. 2.
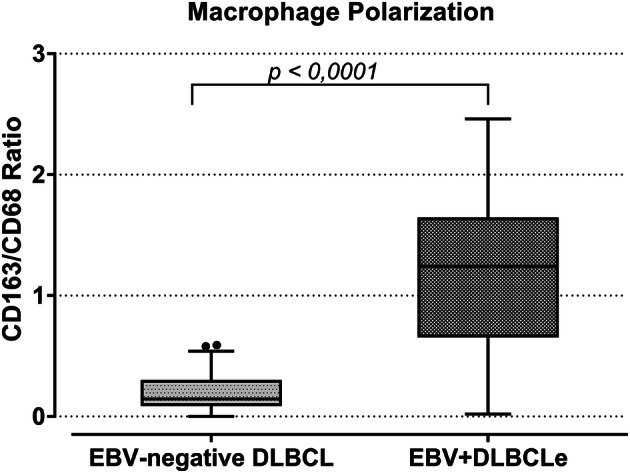
Macrophage polarization in EBV + DLBCLe and EBV-negative DLBCL. The figure shows the CD163/CD68 ratio in patients of EBV-negative DLBCL and EBV + DLBCLe. The p value was obtained by the Mann–Whitney test. A significantly higher CD163/CD68 ratio was found in EBV + DLBCLe (p < 0.0001, Mann–Whitney)
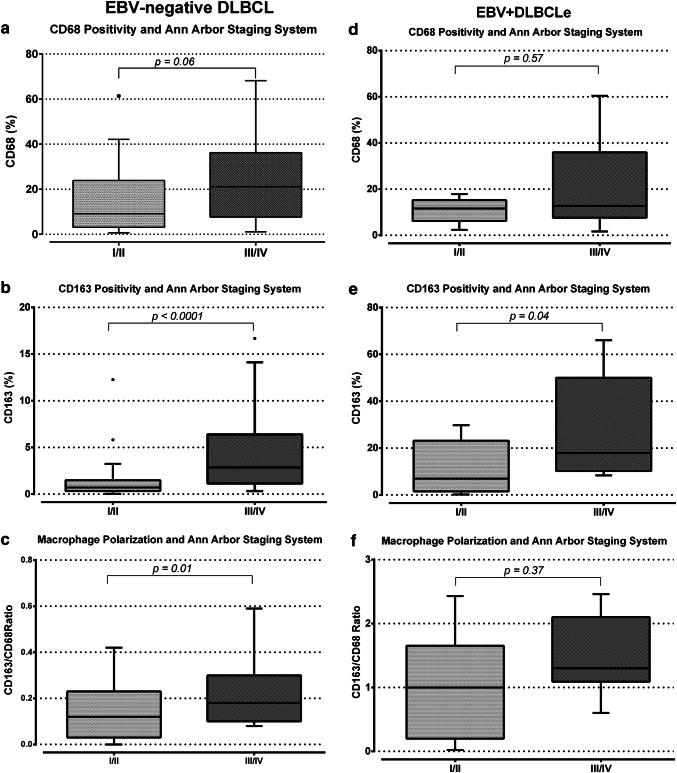
Macrophage polarization and Ann Arbor staging
In EBV-negative DLBCL, there was no statistically significant difference between the medians of stages I/II and III/IV when we compared CD68 marker alone (Fig. 3a). In the same group of patients, CD163 marker positivity was also higher among stages III/IV (p < 0.0001, Mann–Whitney test) (Fig. 3b). Also, CD163/CD68 ratio was significantly higher in advanced-stage disease (p = 0.01, Mann–Whitney test) (Fig. 3c). In EBV + DLBCLe, CD163 marker positivity was significantly higher (median value 17.88%) among patients with advanced-disease (III/IV) than in early-stage (I/II) EBV + DLBCLe (median value 6.97%) (p = 0.04, Mann–Whitney test) (Fig. 3e). However, there was no significant difference between stages I/II and III/IV regarding CD68 marker positivity and CD163/CD68 ratio (Fig. 3d, f).
Fig. 3.
Macrophage polarization and Ann Arbor staging. The figure shows CD68, CD163 and CD163/CD68 ratio as a function of Ann Arbor staging in EBV-negative DLBCL (a–c) and EBV + DLBCLe (d–f). p values were obtained by Mann–Whitney test. An association between advanced-stage disease (III/IV) and increased CD163/CD68 ratio was found, as well as CD163 positivity in EBV-negative DLBCL. The same phenomenon was observed for CD163 positivity in EBV + DLBCLe
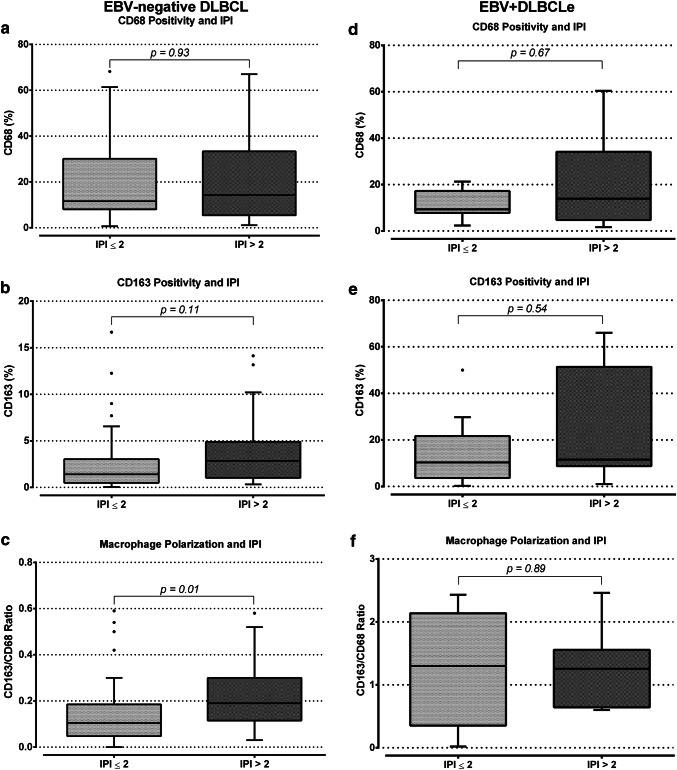
Macrophage polarization and International Prognostic Index
There was no statistically significant difference between EBV-negative DLBCL patients classified as IPI ≤ 2 or > 2 regarding CD68 and CD163 markers alone (Fig. 4a, b). However, the CD163/CD68 ratio was higher (median value 0.19) among patients classified as IPI > 2 compared to patients with IPI ≤ 2 (median value 0.10) (p = 0.01, Mann–Whitney test) (Fig. 4c) in the EBV-negative DLBCL cases. In EBV + DLBCLe group, there was no significant difference in CD68, CD163 and CD163/CD68 ratio regarding the IPI (Fig. 4d–f).
Fig. 4.
Macrophage polarization and International Prognostic Index. The figure shows CD68, CD163 and CD163/CD68 ratio, and the International Prognosis Index in EBV-negative DLBCL (a–c) and EBV + DLBCLe (d–f). p values were obtained by the Mann–Whitney test. An association between IPI > 2 and higher CD163/CD68 ratio was found in EBV-negative DLBCL
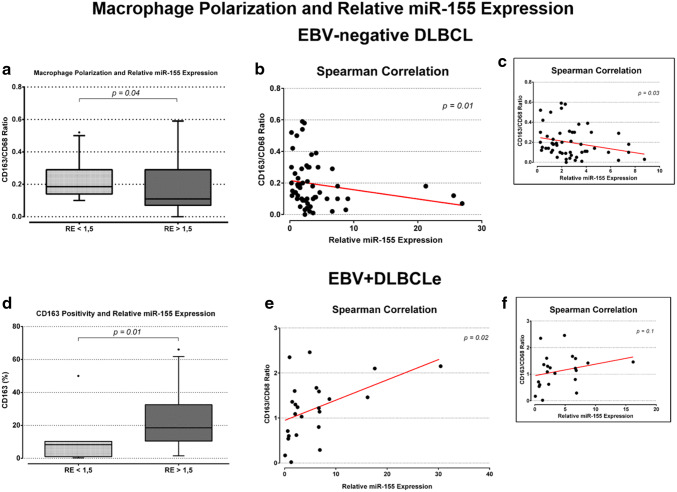
Macrophage polarization and relative miR-155 expression
In EBV-negative DLBCL, there was no correlation between CD68 and CD163 positivity and relative expression of miR-155 (Supplementary Fig. 4b and d). However, EBV-negative DLBCL samples showed a significant decrease in the CD163/CD68 ratio (median value 0.1) among patients with miR-155 overexpression when compared to those with normal/under expression (median value 0.18) (p = 0.04, Mann–Whitney test) (Fig. 5a). Figure 5b shows a low negative correlation [25] (Spearman’s correlation coefficient = − 0.32, p = 0.01) between CD163/CD68 ratio and miR-155 expression. CD68 and CD163 markers positivity alone did not show a statistically significant difference between groups with overexpression and normal/under expression of miR-155. In EBV + DLBCLe, CD163 positivity was significantly higher (median value 18.56%) among patients with overexpression of miR-155 than in patients with normal/under expression (median value 9.12%) (p = 0.03, Mann–Whitney test) (Fig. 5d). Although there was no statistically significant difference between CD68 positivity and CD163/CD68 ratio regarding the expression of miR-155 (Supplementary Fig. 5a/c), Fig. 5e shows a low positive correlation [25] between CD163/CD68 ratio and miR-155 expression (Spearman’s correlation coefficient = 0.46, p = 0.02). No significant correlation between CD163/CD68 ratio and miR-155 expression was found in the EBV + DLBCLe group after exclusion of two outliers (Fig. 5f).
Fig. 5.
a–c Macrophage polarization, and relative expression of miR-155 in EBV-negative DLBCL. a The figure shows the CD163/CD68 ratio as a function of the relative expression of miR-155, p values were obtained with the Mann–Whitney test. b Spearman correlation between CD163/CD68 ratio and miR-155 relative expression. c Spearman correlation after exclusion of four outliers. An association between overexpression of the studied miRNA (RE > 1.5) and a lower CD163/CD68 ratio was found (a), as well as a low negative correlation (rs = − 0.32) between these variables (b). A low negative correlation (rs = − 0.30; p = 0.03) is still observed after outlier exclusion (c). d–f CD163 positivity, and relative expression of miR-155 in EBV + DLBCLe. d CD163 as a function of the relative expression of miR-155, p values were obtained with the Mann–Whitney test. e Spearman correlation between CD163/CD68 ratio and miR-155 relative expression. f Spearman correlation after exclusion of two outliers. An association between overexpression of the studied miRNA (RE > 1.5) and higher CD163 marker positivity was found (d). A low positive correlation (rs = 0.46) between the CD163/CD68 ratio and relative expression of miR-155 was observed (e). No significant correlation between these variables was found in the EBV + DLBCLe group after excluding the outliers (f). RE relative expression, rs Spearman’s correlation coefficient
Macrophage marker positivity, macrophage polarization and miR-155 expression in the histological subtypes of EBV + DLBCLe
We found no statistically significant difference between CD68, CD163 or CD163/CD68 ratio and relative expression of miR-155 when comparing monomorphic and polymorphic subtypes of EBV + DLBCLe (Supplementary Fig. 6).
Discussion
In the present study, we found an increased expression of miR-155 in both EBV-positive and EBV-negative DLBCL patients. However, relative expression of this miRNA was not statistically different between the two groups. These findings are consistent with those reported in the literature since miR-155 is often found to be overexpressed in B-cell lymphoproliferative disorders [26]. The exact mechanism of action of miR-155 in malignant transformation is still not fully understood [27]. Several mechanisms have already been identified as capable of altering or modulating miRNA expression levels [28–31]. In addition, EBV infection may also contribute to altering miRNA levels in B-cell lymphomas. EBV was the first virus in which miRNAs expression were detected [32]. These miRNAs can use cell’s miRNAs to control levels of other miRNAs and, consequently, the expression of target genes that contribute to the process of neoplastic transformation [33].
In the present study, we observed a shift in macrophage polarization towards M2 phenotype in EBV + DLBCLe, when compared to EBV-negative DLBCL (Fig. 2). Morscio et al. [34] obtained similar results, in which there was evidence of increased infiltration by CD163-positive M2 macrophages in EBV-positive post-transplant DLBCL when compared to EBV-negative. Sato et al. [35] demonstrated, in a co-culture of human monocytic cell line (THP-1) with EBV-positive or EBV-negative Burkitt lymphoma cell line (Akata cell line), that EBV directly affects the amount of M2 macrophages in the tumor microenvironment. In the same study, macrophage depletion has been shown to cause almost total depletion of EBV + B lymphocytes, suggesting that such macrophages are crucial for the survival of infected neoplastic cells. Therefore, these studies highlight the importance of understanding the role of macrophages in lymphoproliferative disorders because they may contribute to prognosis in patients with DLBCL [18]. Our results also demonstrated that advanced-stage EBV-negative DLBCL and especially EBV + DLBCLe show a significant deviation towards the immuno-regulatory and pro-tumor phenotype of macrophages (M2) in the tumor microenvironment. The same phenomenon was observed in EBV-negative DLBCL. It can be explained by the theory of inflammation associated with cancer [36]. In advanced stages, with the absolute increase of neoplastic cells, the presence of a local and systemic immunosuppressive environment, secretion of cytokines which do not perform significant antitumor activity and hypoxia factors, contribute to mounting a proper microenvironment for immuno-regulatory and pro-tumor macrophages (M2 phenotype).
In the pre-rituximab era, EBV-positive DLBCL, including EBV + DLBCLe, had a worse prognosis when compared to patients with EBV-negative DLBCL, regardless of IPI [37]. The mechanism involved in such difference has not yet been clarified. One possible explanation would be the role of the tumor microenvironment in EBV-related lymphomas, which present a large infiltration by non-tumor cells. From our data, the increased infiltration by M2 macrophages could contribute to the worse outcome, since these cells act by repairing and remodeling tumor structure, favoring its progression by contributing to the processes of neoangiogenesis, tissue invasion and metastasis [11, 12].
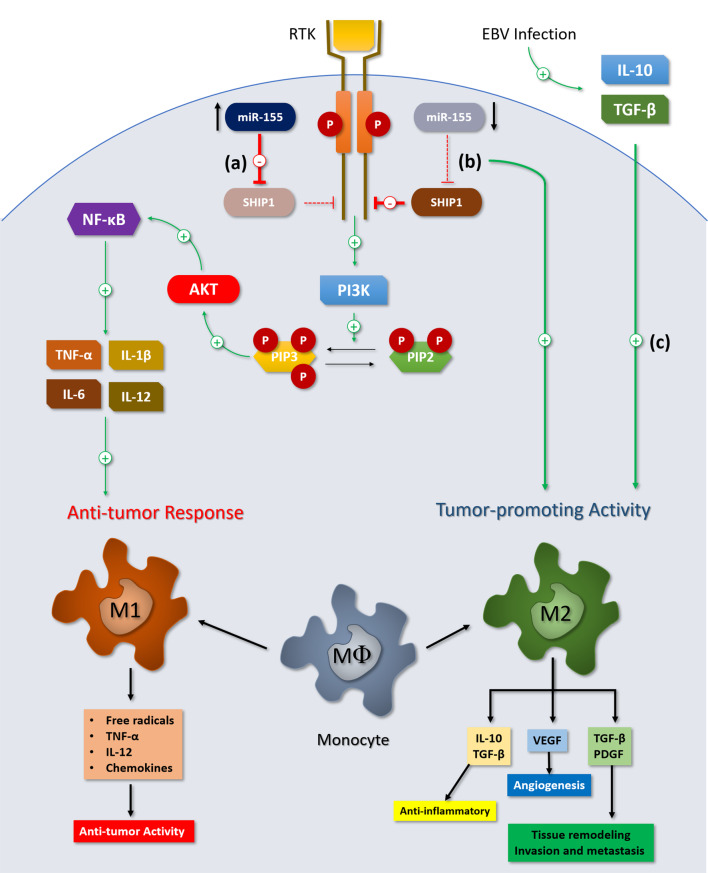
High CD68 expression was associated with favorable prognosis in patients treated with R-CHOP regimen [10, 38] and those who were chemo-resistant to this therapy [39]. However, other studies [40–42] did not find significant associations between CD68 expression and clinical outcome. Besides, no statistically significant difference in CD68 expression was reported regarding IPI score and Ann Arbor staging in the literature [10, 38, 41], and these findings are consistent with those observed in our cohort. On the other hand, the assessment of TAMs based on CD163, which is considered more specific for M2 phenotype, showed more consistent results. The study by Nam et al. [10] reported that increased CD163-positive cells and higher CD163/CD68 ratio significantly affected clinical outcomes in R-CHOP-treated patients and that the ratio was a strong predictor of short survival. Two studies [43, 44] reported that double staining for CD163 and CD68 (M2 macrophages) was also associated with poor prognosis in these patients. Wang et al. [18] reported the same adverse effect on prognosis in patients with high CD163-positive cells in the tumor microenvironment. The unfavorable clinical and biological characteristics associated with increased M2 macrophages in DLBCL were also reported [43, 45] and are concordant with our findings. Our data also showed that EBV-negative DLBCL group has a significant negative association between relative expression of miR-155 and CD163/CD68 ratio: the higher expression of mir-155, the lower CD163/CD68 ratio. A possible explanation for this finding involves the inositol phosphatase SHIP1 which is downregulated by miR-155 [46]. This protein can negatively modulate the PI3-kinase/Akt (PI3K/Akt) signaling pathway [47, 48]. With increased cytoplasmic concentration of miR-155, PI3K/Akt pathway is indirectly upregulated by the decrease in SHIP1 concentration, stimulating the release of pro-inflammatory cytokines and macrophagic activation [49]. NF-κB is a key transcription factor in M1 macrophage polarization during the early stages of carcinogenesis by upregulating the expression of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, and IL-12, resulting in cytotoxic and inflammatory functions [50, 51] (Fig. 6). Zonari et al. [9] showed that insufficiency of miR-155 in macrophages resulted in an impaired classical activation for M1 phenotype and a tendency for differentiation into M2 phenotype, classically associated with Th2 lymphocytes, which secretory cytokine pattern does not exhibit significant antitumor activity. In our study, we evaluated different levels of miR-155 expression and the CD163/CD68 ratio in patients with DLBCL and found an association between overexpression of this miRNA with a lower CD163/CD68 ratio in the EBV-negative DLBCL, which corroborates their findings, despite the low negative correlation, which we believe is due to interference of other variables on macrophage polarization, since there is no mir-155 level described in the literature that can be considered as the cutoff from which macrophage polarization would be strongly affected.
Fig. 6.
Macrophage polarization and miR-155 expression. miR-155 targets the inositol phosphatase SHIP1: this protein can negatively modulate the PI3K/Akt signaling pathway. a Overexpression of miR-155 indirectly upregulates PI3K/Akt pathway by the decrease in SHIP1 concentration, stimulating the release of pro-inflammatory cytokines and macrophagic activation, inducing M1 phenotype polarization. b Decreased levels of miR-155 suppress the secretion of pro-inflammatory cytokines and are associated with impaired macrophage classical activation and a tendency to differentiate to M2 phenotype. c EBV may also affect macrophage polarization towards M2 phenotype by inducing B cells to secrete immunosuppressive cytokines (IL-10, TGF-β) in the tumor microenvironment. RTK receptor tyrosine-kinase, SHIP1 Src homology 2-containing inositol 5′ phosphatase-1, PTEN phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase, PI3K/Akt phosphatidylinositol-3-kinase/Akt, PIP2 phosphatidylinositol (4,5)-bisphosphate, PIP3 phosphatidylinositol (3,4,5)-trisphosphate, PDGF platelet-derived growth factor, VEGF vascular endothelial growth factor, TGF-β transforming growth factor-β, TNF-α tumor necrosis factor α
On the other hand, among EBV + DLBCLe patients, we found an opposite relation to that identified in EBV-negative DLBCL. The results suggest higher infiltration by M2 macrophages among patients with miR-155 overexpression (RE > 1.5) when compared to those with normal/under expression of this microRNA. Although a low positive correlation between CD163/CD68 ratio and miR-155 expression was found, it was not significant after outlier exclusion. We believe that the increased M2 macrophage infiltration in this group is probably due to the presence of EBV. EBV directly affects macrophage polarization towards the M2 phenotype by inducing infected B cells to secrete immunosuppressive cytokines, including IL-10 and TGF-β in the tumor microenvironment [35, 52]. Besides, although it does not encode a miRNA with homology to miR-155 itself, EBV infection is notorious for miR-155 cellular overexpression [53–55]. The mechanism by which this phenomenon is installed is related to the expression of viral latent membrane protein-1 (LMP-1) and its positive regulation of the NF-κB pathway, which activates the expression of this miRNA at the transcription level [55].
Assigning macrophages to an alternative activation state (M2) solely based on CD163 expression may be reductionist. Our first attempt was to identify both markers in the tissue using immunofluorescence. However, we detected that paraffin-embedded tissue has auto-fluorescence and false positives would be generated. After 1 year trying to solve this problem without success, we moved to conventional immunohistochemistry. In fact, if our CD163/CD68 ratio was a good source of information, it could be used in clinical practice with more reproducibility than more sophisticated techniques.
To our knowledge, this is the first study that compared the differences in M2 macrophage population between EBV + and EBV-negative DLBCL patients without immunosuppression. Similar findings were reported by Morscio et al. [34] but in immunosuppressed patients who developed post-transplant lymphoproliferative disease (PTLD) and also in cell culture by Sato et al. [35]. Moreover, we believe that this is the first study that showed, in EBV-negative DLBCL, an association between miR-155 overexpression and lower M2 polarization in real patients, that is consistent with the findings in disease models reported in the literature [56–58]. One limitation of this study relies on the associations between macrophage polarization and miR-155 expression. We did not individualize expression of miR-155 among the cells in tumor microenvironment using in situ hybridization or even laser microdissection. Therefore, we cannot assume if miR-155 is most likely coming from tumor cells or reactive cells. Another point to be addressed in EBV + DLBCLe is the CD163/CD68 ratio above 1 in some patients using staining area to evaluate the quantity of antigen expression (and not positive cell count, as it is usually done in other studies). We found stronger background interference in CD68 staining in EBV + group; thus, we had to apply a higher threshold to avoid considering background as positive cell staining. Also, CD163 was highly expressed in EBV + DLBCLe. For both reasons, the CD163/CD68 ratio is above 1 in some patients in this group. Despite this limitation, we believe that the ratio still reflects a strong deviation towards M2 polarized macrophages in the EBV + group. Besides, a study from Barros et al [13] evaluated immunohistochemistry signature for identifying M1 and M2 macrophages in different disease models and reported a higher number of CD163-labeled cells than CD68-positive cells, showing no perfect overlap between CD163 and CD68. Last, after the conclusion of this work, a revision of the WHO classification of hematological malignancies [59] was published in 2016 and has replaced the provisional entity EBV + DLBCL of the elderly by the EBV + DLBCL not otherwise specified (NOS), once it may occur in younger patients as well. Therefore, we believe that our results could be extrapolated to the younger group.
Therapeutic strategies approaching to modulate the expression of miR-155 or prevent and reprogram immuno-regulatory and pro-tumor macrophage polarization could be evaluated as adjuvants in the EBV + DLBCLe therapy, since this entity has a rich infiltration of this macrophage phenotype in its tumor microenvironment compared to similar EBV-negative patients; in addition, recent evidence suggests that survival of transformed B lymphocytes infected by EBV depends directly on the presence of M2 macrophages [35]. Use of bisphosphonates, such as zoledronic acid, have proven to reverse the TAM polarization to M1 phenotype [60, 61]. A new drug (IPI-549) that selectively inhibits PI3-kinase-gamma (PI3K-γ) is being tested [62]. Preclinical studies have demonstrated that IPI-549 shifts the polarization of immuno-regulatory and pro-tumor macrophages (M2) to the antitumor phenotype (M1) in the tumor microenvironment, in addition to increasing the number and activity of T lymphocytes that attack the tumor and produce pro-inflammatory cytokines [63, 64].
Conclusions
This is a study of macrophage infiltration and miR-155 expression in diffuse large B-cell lymphomas (DLBCL) patients over 50 years old and without immunosuppression, comparing a cohort of 65 patients with EBV-negative DLBCL to 28 patients with EBV + DLBCLe. We found a variable, yet substantial infiltration of DLBCL with CD68 + macrophages whereby CD163 + macrophages dominated in EBV + DLBCLe. Moreover, the degree of infiltration by CD163 + macrophages appeared to correlate with advanced stage in both cohorts, consistent with previously reported data. Higher miR-155 expression was associated with a lower CD163/CD68 ratio in EBV-negative DLBCL, while the opposite was found in EBV + DLBCL. Stimulation of T cells to tumors at old age is difficult as a result of age-related deficiencies in the immune system; therefore, targeting the polarization towards protumoral macrophages in these elderly EBV + DLBCLe and modulating miR-155 expression might be a new approach to treat EBV + DLBCLe.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- DLBCL
Diffuse large B-cell lymphoma
- DLBCLe
Diffuse large B-cell lymphoma of the elderly
- EBV
Epstein-Barr virus
- FFPE
Formalin-fixed paraffin embedded
- IPI
International Prognostic Index
- miRNA
microRNA
- qPCR
Quantitative real-time polymerase chain reaction
- R-CHOP
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- TAM
Tumor-associated macrophages
- TMA
Tissue microarray
Author contributions
Wagner A. Poles and Gisele W. B. Colleoni designed the work, acquisition, analysis, and interpretation of data, wrote the manuscript; Erika E. Nishi, Tathiana A. de Andrade, and Ruy R. de Campos Jr. analyzed data and critically reviewed the manuscript for important intellectual content; Mariana B. de Oliveira and Angela I. P. Eugênio wrote and critically reviewed the manuscript; Antonio Hugo F. M. Campos, Gilles Landman, José Vassallo, Cristovam Scapulatempo Neto, Roberto Antonio Pinto Paes, and Antonio C. Alves reviewed samples, analyzed data and critically reviewed the manuscript; Maria Cláudia N. Zerbini contributed substantially to the conception, analyzed data, and critically reviewed the work.
Funding
Wagner A. Poles was partially supported by Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP), Brazil. This work was supported by Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP), Brazil 2010/17668-6.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Ethical approval
This study was approved by the ethical committees of all collaborating centers: Department of Clinical and Experimental Oncology, Universidade Federal de São Paulo, Brazil; Department of Physiology, Universidade Federal de São Paulo, Brazil; Department of Pathology, AC Camargo Cancer Center, Brazil; Department of Pathology, Universidade Federal de São Paulo, Brazil; Molecular Oncology Research Center, Barretos Cancer Hospital, Brazil; Faculty of Medical Sciences of Santa Casa of São Paulo; Department of Pathology, Universidade de São Paulo, Brazil, and was in accordance with the ethical standards of the institutional committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Swerdlow SH, World Health Organization (2008) Who Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. edn, World Health Organization Classification of Tumours. Lyon: International Agency for Research on Cancer (IARC)
- 2.Oyama T, Ichimura K, Suzuki R, Suzumiya J, Ohshima K, Yatabe Y, et al. Senile EBV + B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27(1):16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8(7):512–22. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo JJ, Beltran BE, Miranda RN, Paydas S, Winer ES, Butera JN. Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16(1):87–96. doi: 10.1634/theoncologist.2010-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura S, Jaffe ES, Swerdlow SH (2008) EBV positive diffuse large B-cell lymphoma of the elderly. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (eds) WHO classification of tumours of haematopoietic and lymphoid tissues. 4th Lyon: International Agency for Research on Cancer (IARC), pp 243–244
- 6.Montes-Moreno S, Odqvist L, Diaz-Perez JA, Lopez AB, de Villambrosia SG, Mazorra F, et al. EBV-positive diffuse large B-cell lymphoma of the elderly is an aggressive post-germinal center B-cell neoplasm characterized by prominent nuclear factor-kB activation. Mod Pathol. 2012;25(7):968–982. doi: 10.1038/modpathol.2012.52. [DOI] [PubMed] [Google Scholar]
- 7.Ok CY, Papathomas TG, Medeiros LJ, Young KH. EBV-positive diffuse large B-cell lymphoma of the elderly. Blood. 2013;122:328–340. doi: 10.1182/blood-2013-03-489708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade TA, Evangelista AF, Campos AH, Poles WA, Borges NM, Camillo CM, et al. A microRNA signature profile in EBV + diffuse large B-cell lymphoma of the elderly. Oncotarget. 2014;5(23):11813–11826. doi: 10.18632/oncotarget.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zonari E, Pucci F, Saini M, Mazzieri R, Politi LS, Gentner B, et al. A role for miR-155 in enabling tumor-infiltrating innate immune cells to mount effective antitumor responses in mice. Blood. 2013;122(2):243–252. doi: 10.1182/blood-2012-08-449306. [DOI] [PubMed] [Google Scholar]
- 10.Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW, et al. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2014;55(11):2466–2476. doi: 10.3109/10428194.2013.879713. [DOI] [PubMed] [Google Scholar]
- 11.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8(11):e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int. 2009;59(5):300–5. doi: 10.1111/j.1440-1827.2009.02369.x. [DOI] [PubMed] [Google Scholar]
- 16.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: preliminary results. J Neuroinflamm. 2012;9:222. doi: 10.1186/1742-2094-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Gelvez JC, Salama ME, Perkins SL, Leavitt M, Inamdar KV. Prognostic impact of tumor microenvironment in diffuse large B-cell lymphoma uniformly treated with R-CHOP chemotherapy. Am J Clin Pathol. 2016;145(4):514–523. doi: 10.1093/ajcp/aqw034. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Gao K, Lei W, Dong L, Xuan Q, Feng M, et al. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget. 2017;8(3):5414–5425. doi: 10.18632/oncotarget.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, et al. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12(3):259–267. doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- 20.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber M, Buttner-Herold M, Hyckel P, Moebius P, Distel L, Ries J, et al. Small oral squamous cell carcinomas with nodal lymphogenic metastasis show increased infiltration of M2 polarized macrophages–an immunohistochemical analysis. J Craniomaxillofac Surg. 2014;42(7):1087–1094. doi: 10.1016/j.jcms.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 22.International Non-Hodgkin. ’. s Lymphoma Prognostic Factors P A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Goni R, García P, Foissac S. The qPCR data statistical analysis. Integromics W P. 2012;9:1–9. [Google Scholar]
- 25.Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5. Boston: Houghton Mifflin; 2003. [Google Scholar]
- 26.Sandhu SK, Croce CM, Garzon R. Micro-RNA expression and function in lymphomas. Adv Hematol. 2011;2011:347137. doi: 10.1155/2011/347137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrie CH. MicroRNAs and lymphomagenesis: a functional review. Br J Haematol. 2013;160(5):571–581. doi: 10.1111/bjh.12157. [DOI] [PubMed] [Google Scholar]
- 28.Wada M, Okamura T, Okada M, Teramura M, Masuda M, Motoji T, et al. Frequent chromosome arm 13q deletion in aggressive non-Hodgkin’s lymphoma. Leukemia. 1999;13(5):792–798. doi: 10.1038/sj.leu.2401395. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Kim SW, Rai D, Bolla AR, Adhvaryu S, Kinney MC, et al. Copy number abnormalities, MYC activity, and the genetic fingerprint of normal B cells mechanistically define the microRNA profile of diffuse large B-cell lymphoma. Blood. 2009;113(26):6681–6690. doi: 10.1182/blood-2009-01-202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KY, Yim RL, Kwong YL, Leung CY, Hui PK, Cheung F, et al. Epigenetic inactivation of the MIR129-2 in hematological malignancies. J Hematol Oncol. 2013;6:16. doi: 10.1186/1756-8722-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertus JL, Kluiver J, Weggemans C, Harms G, Reijmers RM, Swart Y, et al. MiRNA profiling in B non-Hodgkin lymphoma: a MYC-related miRNA profile characterizes Burkitt lymphoma. Br J Haematol. 2010;149(6):896–899. doi: 10.1111/j.1365-2141.2010.08111.x. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, et al. Identification of virus-encoded microRNAs. Science. 2004;304(5671):734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 33.Barth S, Meister G, Grasser FA. EBV-encoded miRNAs. Biochim Biophys Acta. 2011;1809(11–12):631–640. doi: 10.1016/j.bbagrm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Morscio J, Finalet Ferreiro J, Vander Borght S, Bittoun E, Gheysens O, Dierickx D, et al. Identification of distinct subgroups of EBV-positive post-transplant diffuse large B-cell lymphoma. Mod Pathol. 2017;30(3):370–381. doi: 10.1038/modpathol.2016.199. [DOI] [PubMed] [Google Scholar]
- 35.Sato A, Yamakawa N, Okuyama K, Kotani A, Nakamura N, Ando K. The critical interaction between Epstein-Barr virus (EBV) positive B-cells and tumor associated macrophages (TAMs) Blood. 2014;124:2989. [Google Scholar]
- 36.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 37.Park S, Lee J, Ko YH, Han A, Jun HJ, Lee SC et al (2007) The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood 110(3):972–978. 10.1182/blood-2007-01-067769 [DOI] [PubMed]
- 38.Riihijarvi S, Fiskvik I, Taskinen M, Vajavaara H, Tikkala M, Yri O, et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: a correlative study from a Nordic phase II trial. Haematologica. 2015;100(2):238–245. doi: 10.3324/haematol.2014.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marinaccio C, Ingravallo G, Gaudio F, Perrone T, Nico B, Maoirano E, et al. Microvascular density, CD68 and tryptase expression in human diffuse large B-cell lymphoma. Leuk Res. 2014;38(11):1374–1377. doi: 10.1016/j.leukres.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Meyer PN, Fu K, Greiner T, Smith L, Delabie J, Gascoyne R, et al. The stromal cell marker SPARC predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am J Clin Pathol. 2011;135(1):54–61. doi: 10.1309/AJCPJX4BJV9NLQHY. [DOI] [PubMed] [Google Scholar]
- 41.Hasselblom S, Hansson U, Sigurdardottir M, Nilsson-Ehle H, Ridell B, Andersson PO. Expression of CD68 + tumor-associated macrophages in patients with diffuse large B-cell lymphoma and its relation to prognosis. Pathol Int. 2008;58(8):529–532. doi: 10.1111/j.1440-1827.02268.x. [DOI] [PubMed] [Google Scholar]
- 42.Coutinho R, Clear AJ, Mazzola E, Owen A, Greaves P, Wilson A, et al. Revisiting the immune microenvironment of diffuse large B-cell lymphoma using a tissue microarray and immunohistochemistry: robust semi-automated analysis reveals CD3 and FoxP3 as potential predictors of response to R-CHOP. Haematologica. 2015;100(3):363–369. doi: 10.3324/haematol.2014.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchesi F, Cirillo M, Bianchi A, Gately M, Olimpieri OM, Cerchiara E, et al. High density of CD68+/CD163 + tumour-associated macrophages (M2-TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B-cell lymphoma. Hematol Oncol. 2015;33(2):110–112. doi: 10.1002/hon.2142. [DOI] [PubMed] [Google Scholar]
- 44.Wada N, Zaki MA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y, et al. Tumour-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology. 2012;60(2):313–319. doi: 10.1111/j.1365-2559.2011.04096.x. [DOI] [PubMed] [Google Scholar]
- 45.Jeong J, Oh EJ, Yang WI, Kim SJ, Yoon SO. Implications of infiltrating immune cells within bone marrow of patients with diffuse large B-cell lymphoma. Hum Pathol. 2017;64:222–231. doi: 10.1016/j.humpath.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 46.O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106(17):7113–7118. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB. The inositol 5′-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem. 2003;278(40):38628–38636. doi: 10.1074/jbc.M305021200. [DOI] [PubMed] [Google Scholar]
- 48.Mondal S, Subramanian KK, Sakai J, Bajrami B, Luo HR. Phosphoinositide lipid phosphatase SHIP1 and PTEN coordinate to regulate cell migration and adhesion. Mol Biol Cell. 2012;23(7):1219–1230. doi: 10.1091/mbc.E11-10-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajaram MV, Butchar JP, Parsa KV, Cremer TJ, Amer A, Schlesinger LS, et al. Akt and SHIP modulate Francisella escape from the phagosome and induction of the Fas-mediated death pathway. PLoS One. 2009;4(11):e7919. doi: 10.1371/journal.pone.0007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Tan G, Visser L, Tan L, Berg A, Diepstra A. The microenvironment in Epstein–Barr virus-associated malignancies. Pathogens. 2018;7:2. doi: 10.3390/pathogens7020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus. J Virol. 2010;84(22):11670–11678. doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin Q, McBride J, Fewell C, Lacey M, Wang X, Lin Z, et al. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J Virol. 2008;82(11):5295–5306. doi: 10.1128/JVI.02380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gatto G, Rossi A, Rossi D, Kroening S, Bonatti S, Mallardo M. Epstein-Barr virus latent membrane protein 1 trans-activates miR-155 transcription through the NF-kappaB pathway. Nucleic Acids Res. 2008;36(20):6608–6619. doi: 10.1093/nar/gkn666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Essandoh K, Li Y, Huo J, Fan GC. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122–131. doi: 10.1097/SHK.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu L, McCurdy S, Huang S, Zhu X, Peplowska K, Tiirikainen M, et al. Time series miRNA-mRNA integrated analysis reveals critical miRNAs and targets in macrophage polarization. Sci Rep. 2016;6:37446. doi: 10.1038/srep37446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P, Wang H, Luo X, Liu H, Lu B, Li T, et al. MicroRNA-155 inhibits polarization of macrophages to M2-type and suppresses choroidal neovascularization. Inflammation. 2018;41(1):143–153. doi: 10.1007/s10753-017-0672-8. [DOI] [PubMed] [Google Scholar]
- 59.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miselis NR, Wu ZJ, Van Rooijen N, Kane AB. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7(4):788–799. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 62.Clinical Trials NCT02637531 (2017) https://clinicaltrials.gov/ct2/show/NCT02637531>. Accessed 18 Jul 2017
- 63.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016;539(7629):437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539(7629):443–447. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.