Abstract
Dendritic cell (DC)-based vaccination boosting antigen-specific immunity is being explored for the treatment of cancer and chronic viral infections. Although DC-based immunotherapy can induce immunological responses, its clinical benefit has been limited, indicating that further improvement of DC vaccine potency is essential. In this study, we explored the generation of a clinical-grade applicable DC vaccine with improved immunogenic potential by combining PD-1 ligand siRNA and target antigen mRNA delivery. We demonstrated that PD-L1 and PD-L2 siRNA delivery using DLin-KC2-DMA-containing lipid nanoparticles (LNP) mediated efficient and specific knockdown of PD-L expression on human monocyte-derived DC. The established siRNA-LNP transfection method did not affect DC phenotype or migratory capacity and resulted in acceptable DC viability. Furthermore, we showed that siRNA-LNP transfection can be successfully combined with both target antigen peptide loading and mRNA electroporation. Finally, we demonstrated that these PD-L-silenced DC loaded with antigen mRNA superiorly boost ex vivo antigen-specific CD8+ T cell responses from transplanted cancer patients. Together, these findings indicate that our PD-L siRNA-LNP-modified DC are attractive cells for clinical-grade production and in vivo application to induce and boost immune responses not only in transplanted cancer patients, but likely also in other settings.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1334-1) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cell, PD-1 ligand, siRNA delivery, Lipid nanoparticle, mRNA electroporation, Minor histocompatibility antigen
Introduction
For more than 15 years, the immunotherapeutic potential of ex vivo-generated dendritic cells (DC) has been explored for the treatment of cancer and chronic viral infections [1–4]. DC are the most potent professional antigen-presenting cells (APC) of the immune system and effectively initiate and reactivate T cell-based immune responses. For that reason, DC are considered to be the best means to improve antigen-specific T cell responses in vivo. However, so far clinical effects of DC vaccination therapy have been limited [5–7]. Despite the documented induction of antigen-specific T cell responses in DC-vaccinated patients, the magnitude of the immune response, the functionality of the boosted T cells and the induction of long-lived memory T cells need to be enhanced. Therefore, further improvement of DC vaccine potency is essential to improve the clinical potential of DC-based immunotherapy.
Ex vivo-generated DC vaccines display high expression of peptide-HLA complexes and many accessory molecules involved in cell adhesion, cell migration and co-stimulation [8]. However, DC maturation with pro-inflammatory cytokines and/or toll-like receptor ligands also strongly up-regulates co-inhibitory molecules, including PD-L1 and PD-L2 [8]. The balance in expression levels and interactions between these co-stimulatory and co-inhibitory ligands on APC with their counter-receptors on T cells determines the activation state of the T cells. By favorably modulating this balance toward increased co-stimulation, the potency of DC vaccines could be enhanced. In this regard, we recently showed that by silencing the co-inhibitory molecules programmed death ligand 1 (PD-L1) and PD-L2 using siRNA, DC with strongly improved stimulatory potential could be generated [9]. These PD-L knockdown DC robustly boosted ex vivo expansion of both KLH-specific CD4+ Th1 responses and minor histocompatibility antigen (MiHA)-specific CD8+ effector-memory T cells from transplanted cancer patients. It is generally accepted that these MiHAs, expressed by the patient’s malignant cells, are the key antigens in antitumor immune responses after allogeneic stem cell transplantation (allo-SCT) [10–12]. In order to further increase DC immunogenicity, we exploited MiHA-encoding mRNA electroporation of mature donor-derived DC, which allows natural and long-lasting presentation of antigenic peptides by both MHC class I and II molecules [13]. We and others observed that mRNA-loaded DC efficiently stimulate specific CD8+ T cell responses [13–15]. Furthermore, to generate a superior DC vaccine with high stimulatory potential and capable of eliciting a robust MiHA-specific immune response, it is desirable that PD-L siRNA delivery and MiHA mRNA loading are combined. However, mRNA electroporation is optimal at the mature DC (mDC) stage [13], while PD-L siRNA electroporation must be performed at the immature DC (iDC) stage to efficiently prevent PD-L1 and PD-L2 up-regulation during maturation [9]. Since double electroporation is undesirable due to substantial loss of DC yield, we explored a non-viral clinical-grade applicable siRNA transfection method based on lipid nanoparticles (LNP). Using ionizable cationic lipids, siRNA can be efficiently encapsulated in LNP [16]. Due to the weak basicity of the lipid headgroups, these nanoparticles exhibit a nearly neutral charge at physiologic pH. Additionally, distearoylphosphatidylcholine (DSPC), cholesterol and polyethylene glycol (PEG)-lipid are incorporated for LNP stabilization and size control [17]. Importantly, these LNP formulations have been proven to be well-tolerated and efficient vehicles for low-dose in vivo gene silencing in animals [18–20]. Furthermore, LNP efficiently delivered siRNA into murine and non-human primate APC in both healthy and diseased states [21–23].
In this study, we explored the development of a clinical-grade applicable human DC vaccine with improved immunogenic potential by combining PD-L siRNA and target antigen mRNA delivery. We identified the best siRNA sequence and LNP formulation that efficiently silenced PD-L1 and PD-L2 in monocyte-derived DC with relatively low cell toxicity at the optimal ex vivo dose. Furthermore, we observed that this LNP-mediated siRNA delivery method affected neither DC phenotype nor their migratory capacity. Importantly, we demonstrated that PD-L siRNA-LNP-transfected DC loaded with either MiHA peptide or mRNA are superior APC, strongly augmenting expansion of functional MiHA-specific CD8+ T cells from transplanted cancer patients ex vivo. These findings indicate that the combination of siRNA-LNP and target antigen mRNA transfection could be a major improvement of current DC vaccines to boost effector-memory T cell responses in cancer patients. Importantly, all materials used to deliver siRNA and mRNA have already been approved for use in the clinical setting; therefore, the combination should be easily adopted for human use.
Materials and methods
Patient and donor material
Peripheral blood mononuclear cells (PBMC) were isolated from healthy donor buffy coats (N = 4, Sanquin Blood Bank, Nijmegen, the Netherlands) or aphaeresis material of allogeneic donors (N = 7). Furthermore, we used PBMC from patients who developed MiHA-specific CD8+ T cell responses. These samples were obtained at 12–41 months after allo-SCT (Table 1, N = 5). All cells of healthy donors and patients were obtained after written informed consent.
Table 1.
Characteristics of allo-SCT patients used for MiHA T cell expansion assays
| Pt | Disease | MiHA T cell response | Sample date (months) | DLI prior to sample date | % MiHA-specific CD8+ T cells |
|---|---|---|---|---|---|
| 1 | MM | HA-1 | 20 post-SCT | Yes | 0.03 |
| 2 | NHL | HA-1 | 14 post-SCT | No | 0.02 |
| 3 | AML | LRH-1 | 12 post-SCT | No | 0.04 |
| 4 | CML-AP | LRH-1 | 13 post-SCT | Yes | 0.47 |
| 5 | AML | LRH-1 | 41 post-SCT | Yes | 0.02 |
Characteristics of patients with a hematological malignancy displaying MiHA-specific CD8+ T cell responses post-transplantation
Pt patient, AML acute myeloid leukemia, CML-AP chronic myeloid leukemia-accelerated phase, MM multiple myeloma, NHL non-Hodgkin’s lymphoma, SCT stem cell transplantation, DLI donor lymphocyte infusion, given at 4–10 months after allo-SCT
Synthesis and in vitro screening of siRNAs targeting PD-L1 and PD-L2
In total, 169 and 24 siRNAs with the lowest predicted off-target potentials and 100 % homology with the human PD-L1 or PD-L2 gene sequence NM_014143.2 or NM_025239.3, respectively, were selected for synthesis and screening. Single-strand RNAs were produced and annealed into duplexes at Alnylam Pharmaceuticals as previously described [20]. The siRNA screening for PD-L1 and PD-L2 was done in the Hep3b cell line (i.e., a human hepatocellular carcinoma line) and RKO cell line (i.e., a human colon cancer cell line), respectively. siRNA was transfected using Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols at 0.1 and 10 nM concentrations. mRNA levels for the appropriate transcript were quantified 24 h after transfection by Q-PCR and normalized to GAPDH mRNA. Duplexes showing best knockdown at both concentrations were selected for 12-point dose–response ranging from 10 nM down to 0.01 pM to determine IC50 value for these siRNA duplexes. The best duplex for PD-L1 with the sequence 5′-AGAccuuGAuAcuuucAAAdTsdT-3′ (sense) and 5′- UUUGAAAGuAUcAAGGUCUdTsdT-3′ (antisense) and the best duplex for PD-L2 with the sequence 5′-AuAAcAGccAGuuuGcAAAdTsdT-3′ (sense) and 5′-UUUGcAAACUGGCUGUuAUdTsdT-3′ (antisense) were selected for scaling up, LNP formulation and subsequent ex vivo work. As negative control, a siRNA duplex for luciferase was used, with the sequence 5′-cuuAcGcuGAGuAcuucGAdTsdT-3′ (sense) and 5′-UCGAAGuACUcAGCGuAAGdTsdT-3′ (antisense). Small case represents 2′-O-methyl-modified residues.
Preparation of LNP and siRNA encapsulation
The selected PD-L1 and PD-L2 siRNA duplexes were incorporated in the nanoparticles as published previously [16–18]. In brief, LNPs were prepared with the ionizable cationic lipid DLin-KC2-DMA (KC2) or DLin-MC3-DMA (MC3), DSPC, cholesterol and PEG2000-C-DMG using a spontaneous vesicle formation procedure as previously described [16, 17]. The LNPs had a component molar ratio of ~50/10/39.7/0.3 or ~50/10/38.5/1.5 (cationic lipid/DSPC/cholesterol/PEG2000-C-DMG). The final lipid/siRNA weight ratio was ~10:1. The particle size of LNPs was determined by dynamic light scattering (Zetasizer Nano ZS; Malvern, UK), and the mean diameter was in the range of 60–80 nm for 1.5 % PEG-LNP and ~120 nm for 0.3 % PEG-LNP. siRNA content was determined by HPLC (anion-exchange column, Dionex PA-200) at 260 nm, and siRNA entrapment efficiency was determined by the Quant-iT RiboGreen RNA assay (Invitrogen).
DC culture and LNP-mediated PD-L siRNA delivery
Monocytes were isolated from PBMC via direct magnetic labeling with CD14 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) or via plastic adherence in tissue culture flasks (Greiner Bio-One, Alphen a/d Rijn, the Netherlands). iDC were generated by culturing monocytes in X-VIVO-15 medium (Lonza, Verviers, Belgium) supplemented with 2 % human serum (HS; PAA laboratories, Pasching, Austria), 500 U/ml IL-4 (Immunotools, Friesoythe, Germany) and 800 U/ml GM-CSF (Immunotools). After 3 days, iDC were transferred to 6-wells plates and pre-incubated with 125–500 nM PD-L1, PD-L2 or luciferase control siRNA-LNP (Alnylam) in the absence of HS. After 4 h, X-VIVO-15/4 % HS medium containing 1,000 U/ml IL-4 and 1,600 U/ml GM-CSF was added, and cells were further cultured at 0.5 × 106/ml. At day 7, cells were again incubated for 4 h with 125–500 nM PD-L1, PD-L2 or luciferase control siRNA-LNP, after which maturation was induced in X-VIVO-15/2 % HS containing 500 U/ml IL-4, 800 U/ml GM-CSF, 5 ng/ml IL-1β, 15 ng/ml IL-6, 20 ng/ml TNF-α (all Immunotools) and 1 μg/ml PGE2 (Pharmacia & Upjohn, Bridgewater, NJ, USA). At day 9, mDC were harvested, phenotyped and used in functional assays.
In vitro mRNA transcription and DC loading
IVT mRNA was generated using the mMessage mMachine T7 Ultra Kit according to the manufacturers’ instructions (Ambion-Applied Biosystems, Foster City, CA). Briefly, cDNA encoding the MiHA P2X5 or truncated HMHA1, or the tumor-associated antigen (TAA) MAGE3, was subcloned into the pGEM4Z/A64 vector. The resulting vectors were linearized using restriction enzyme NotI. IVT mRNA was transcribed from the T7 promoter site in the linearized template DNA by incubation at 37 °C for 2 h in a reaction mixture containing T7 RNA polymerase. After purification, the mRNA was quantified by spectrophotometry and checked by agarose gel electrophoresis. mRNA was stored in aliquots at −80 °C. Before mRNA electroporation, DC were washed twice with phenol red-free Optimem buffer (Gibco Invitrogen). Cells were resuspended in 200 μl Optimem buffer and transferred to a 4-mm gene pulser cuvette (Biorad, Hercules, CA) containing 20 μg mRNA. DC were electroporated at 200–300 V, 150 μF using a Biorad Genepulser II (Biorad). Directly after electroporation, cells were resuspended in 4 ml pre-warmed phenol red-free X-VIVO 15/6 % HS and incubated at 37 °C for 1 h before further use. For KC2 washout experiments, DC were resuspended in X-VIVO/2 % HS and washed twice in phenol red-free Optimem buffer according to our standard operating procedure (SOP) used in clinical DC vaccination trials. Subsequently, DC were resuspended in 200 μl Optimem buffer and added to 4 ml pre-warmed phenol red-free X-VIVO 15/6 % HS. Finally, DC were washed twice in PBS/5 % human serum albumin.
LC/MS/MS assay for KC2 quantification
Levels of KC2 in DC, culture media and washouts were quantified by using LC/MS/MS tandem mass spectrometry. Cell pellets were resuspended in 500 μl of PBS and lysed by TissueLyser LT (QIAGEN, Valencia, CA, USA). Subsequently, KC2 was extracted from 50 μl of lysed cell suspension, culture and wash supernatants with acetonitrile/isopropanol (v/v 50:50) containing 50 ng/ml of IS (Internal Standard). After centrifugation at 4000 rpm for 10 min, the supernatant was transferred to a clean 96-well plate for LC/MS/MS analysis using a Phenomenex Luna silica column (2.0 × 50 mm, 5 μm) with an isocratic mobile phase (90 % acetonitrile/isopropanol (v/v 50:50)/10 % 10 mM ammonium acetate) at 40 °C and a flow rate of 0.7 ml/min for 6 min at 10 μl sample volume injection. All MS/MS experiments were performed with an API 5000 (AB Sciex, Foster City, CA, USA) with electrospray ionization source. KC2 was monitored using the MS/MS transition of 642.6–116.1 in positive mode. The calibration curve ranged from 0.5 to 1,000 ng/ml with a lower limit of quantification of 0.5 ng/ml.
Flow cytometry
Phenotype and maturation state of DC were analyzed by staining with the following antibodies: anti-CD14 (clone TÜK4), anti-CD83 (clone HB15a), anti-CD80 (clone MAB104), anti-CD86 (clone HA5.2B7, all from Beckman Coulter, Fullerton, CA, USA), anti-PD-L1 (clone MIH1), anti-PD-L2 (clone MIH18, both from Becton–Dickinson, Franklin Lakes, NJ, USA), anti-CCR7 (clone 150503, R&D Systems, Abingdon, United Kingdom), anti-HLA-ABC (clone W6/32, Dako), anti-HLA-DR (clone Immu357, Beckman Coulter) and isotype controls IgG1 FITC/PE dual-color control (Dako) and IgG2b PE (Beckman Coulter). The mean PD-L fluorescence intensity (MFI) was corrected for the MFI of the isotype control antibody (ΔMFI); subsequently, the relative PD-L knockdown efficiency was calculated as follows: (ΔMFI PD-L LNP-treated DC/ΔMFI Control LNP-treated DC) × 100.
LRH-1- and HA-1-specific T cells were detected by staining cell suspensions with PE-labeled tetramers containing the corresponding MiHA peptide (LRH-1/B7: TPNQRQNVC; HA-1/A2: VLHDDLLEA). Tetramers were kindly provided by Prof. Dr. Frederik Falkenburg (Department of Hematology, Leiden University Medical Center, Leiden, the Netherlands). T cell cultures were incubated with 0.15–0.2 μg tetramer for 15 min at room temperature. Subsequently, cells were labeled with the appropriate concentrations of anti-CD8 (clone LT8, ProImmune, Oxford, United Kingdom) and anti-CD3 (clone UCHT1, Beckman Coulter) for 30 min at 4 °C. After washing with PBS/0.5 % bovine serum albumin (BSA; Sigma, St Louis, MO, USA), cells were resuspended in washing buffer containing 0.1 % 7-amino-actinomycin D (7-AAD; Sigma). Cells were analyzed using the Coulter FC500 flow cytometer (Beckman Coulter).
To determine the expression levels of MAGE-3 upon electroporation, DC were stained with specific antibodies using an indirect labeling approach. DC were washed twice with PBS and fixed for 10 min at room temperature in 4 % cold paraformaldehyde solution. After washing with PBS, cells were resuspended in 0.1 % saponin buffer and incubated for 30 min at 4 °C with mouse-anti-hMAGE3 (clone 57B, kindly provided by Prof. Dr. Giulio Spagnoli, University Hospital Basel, Basel, Switzerland). Then, DC were washed with 0.1 % saponin buffer and, subsequently, incubated with 1:100 diluted goat-antimouse PE antibody (Biosource, Life Technologies, Bleiswijk, the Netherlands) for 30 min at 4 °C. After a final wash, DC were analyzed on the FC500 flow cytometer.
Migration assay
Chemotaxis of DC in response to CCL21 (ligand for CCR7 chemokine receptor) was measured, in triplo, in 24-wells plates containing transwell inserts with 5-μm pores (Corning Costar). IMDM/10 % HS containing 0, 10 or 100 ng/ml CCL21 (Immunotools) was added to the lower compartment in a total volume of 600 μl, and 1 × 105 DC in 100 μl were loaded into the inserts. After 2 h of incubation at 37 °C, DC were harvested from the lower compartment, and migration was quantified using the Coulter FC500 flow cytometer by acquiring events for 90 s.
MiHA-specific T cell expansion and degranulation assays
MiHA-specific CD8+ memory T cells present in PBMC from transplanted patients 1–5 (Table 1) were stimulated for one to two consecutive weeks ex vivo with PD-L-silenced DC loaded with MiHA peptide or mRNA. Mature allogeneic PD-L knockdown or control DC, cultured from apheresis material of a HLA-B7+ LRH-1− or HLA-A2+ HA-1− individual, were either loaded with 5 μM MiHA peptide for 30 min at 37 °C or electroporated with 20 μg MiHA mRNA. PBMC and DC were subsequently co-cultured at a ratio of 1:0.1 in 2 ml Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen) supplemented with 10 % HS in 24-wells plates (Corning Costar). After 5 days, IMDM/10 % HS containing 50 U/ml IL-2 and 5 ng/ml IL-15 (both Immunotools) was added. At day 7, cells were harvested and counted, and the percentage of MiHA-specific CD8+ T cells was determined using flow cytometry.
T cell cultures of patient 5 stimulated for two consecutive weeks with P2X5 mRNA-loaded PD-L knockdown DC were used in a CD107a degranulation assay to determine the cytolytic capacity upon recognition of the LRH-1 peptide. Cells were overnight restimulated with 5 μM cognate peptide in the presence of CD107a antibody (clone H4A3, Becton–Dickinson) and 25 U/ml IL-2. The following day, antigen-specific CD8+ T cell degranulation was determined by analyzing the percentage of CD107a+ cells within the LRH-1-tetramer+ CD8+ T cell population using flow cytometry.
Statistics
To determine statistical differences, a two-sample two-tailed t test assuming independent samples was used. P values <0.05 were considered significant.
Results
LNP containing KC2 most potently deliver siRNA into monocyte-derived DC, mediating efficient PD-L1 and PD-L2 silencing
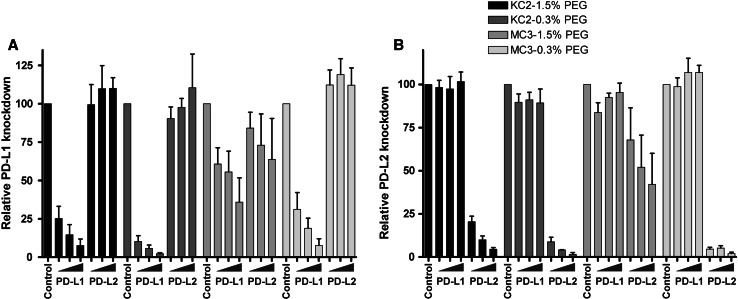
To generate a DC vaccine lacking PD-1 ligand expression in combination with endogenous MiHA peptide presentation, we developed a clinical-grade and versatile application for siRNA transfection of ex vivo-generated DC. In previous work, we showed efficient, specific and long-lasting silencing of PD-L1 and PD-L2 in human monocyte-derived DC using siRNA electroporation of iDC [9]. However, this strategy is incompatible with MiHA mRNA electroporation at the mDC stage. Therefore, we investigated PD-L siRNA delivery using different LNP formulations containing cationic lipids KC2 or MC3 in combination with high (1.5 %) or low (0.3 %) PEG concentrations. These formulations were tested for their knockdown efficiency by adding 125–500 nM of siRNA-LNP at days 3 and 7 of DC culture. Following 48 h of maturation, LNP-treated versus untreated mature DC were analyzed for PD-L1 and PD-L2 expression using flow cytometry. A siRNA concentration-dependent decrease in PD-L1 and PD-L2 expression was observed, with a more pronounced knockdown effect for LNP containing cationic lipid KC2 compared to MC3-LNP (Fig. 1a, b, Supplemental Figure 1, available online). Furthermore, KC2-LNP containing low concentrations of PEG were slightly more efficient in silencing PD-L1 and PD-L2 than 1.5 % PEG-containing KC2-LNP. However, in contrast to the KC2-1.5 % PEG-LNP that retained a particle size of 60–80 nm, the formulation with 0.3 % PEG turned out to be unstable as the particle size (~120 nm) increased 1.6 ± 0.07-fold in 4 months (data not shown). The siRNA encapsulation of KC2-LNP remained >90 %, and no changes were observed between the 0.3 and 1.5 % PEG particles. We chose to continue subsequent experiments with 250 nM of PD-L1 and 125 nM of PD-L2 KC2-1.5 % LNP, referred to as siPDL-LNP from now on, based on the superior combination of efficacy and stability. Collectively, these data clearly show that LNP-mediated siRNA delivery is an effective method for silencing of PD-L1 and PD-L2 on human monocyte-derived DC.
Fig. 1.
LNP-mediated siRNA delivery results in efficient PD-L1 and PD-L2 silencing on monocyte-derived DC. At days 3 and 7 of culture, siRNA-LNP were added at concentrations of 125, 250 or 500 nM for PD-L1 and PD-L2 compared to 500 nM of the control. Subsequently, DC were matured for 2 days, and PD-L expression levels were analyzed using flow cytometry. The relative knockdown of a PD-L1 and b PD-L2 was determined for four different siRNA-containing LNP formulations composed of either cationic lipid KC2 or MC3 in combination with 1.5 or 0.3 % PEG. Data of 3 donors are shown. The relative PD-L knockdown efficiency was calculated as follows: (ΔMFI PD-L LNP-treated DC/ΔMFI Control LNP-treated DC) × 100
siPDL-LNP-treated DC show a mature phenotype and good migratory capacity toward CCR7
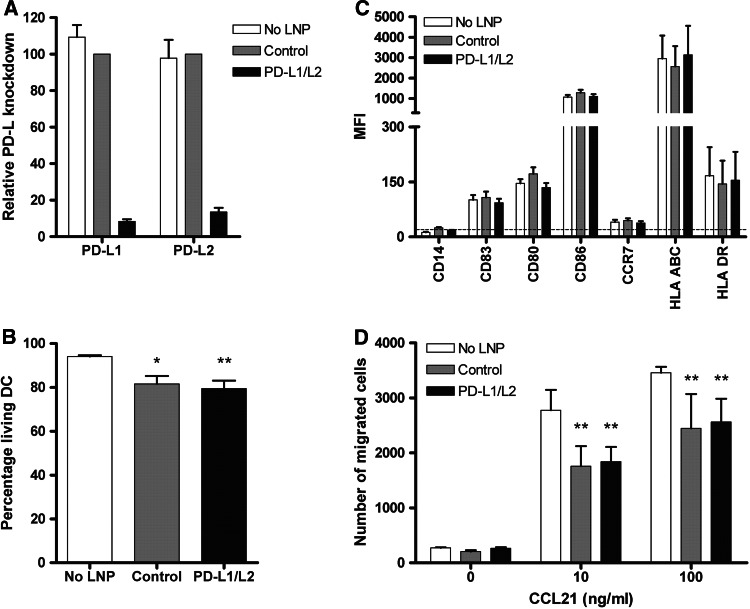
To study the influence of LNP-mediated siRNA delivery on DC characteristics, we studied DC viability, phenotype and migratory capacity following transfection. At days 3 and 7, 250 nM PD-L1 and 125 nM PD-L2 or 375 nM control siRNA-containing KC2-1.5 % PEG-LNP were added to the DC culture. Subsequently, maturation was induced and DC were examined at day 9. Combined delivery of PD-L1 and PD-L2 siRNA resulted in efficient knockdown of both target molecules, that is, 91.7 ± 1.2 % for PD-L1 and 86.4 ± 2.2 % for PD-L2 (Fig. 2a). Although siRNA transfection appears non-toxic to ex vivo-generated DCs relative to other methods, treatment with KC2-1.5 % PEG-LNP resulted in a somewhat reduced DC viability (79.4 ± 3.7 % in case of PD-L1/L2 silencing) compared to untreated DC (94.1 ± 0.6 %) (P < 0.01; Fig. 2b). To exclude negative effects on DC maturation and phenotype, surface expression levels of various maturation and stimulatory molecules were determined. As shown in Fig. 2c, all DC displayed high expression of CD83, CD80, CD86 and HLA class I and II molecules. Neither the siRNAs themselves nor the transfection procedure with the KC2-LNP affected expression levels of the molecules analyzed. Next, we also examined surface expression of lymph node homing receptor CCR7 and migration toward increasing concentrations of its ligand CCL21. siPDL-LNP treatment did not affect CCR7 expression levels (Fig. 2c). Moreover, LNP-treated DC showed substantial expression of CCR7 (>40 %, data not shown), and after 2 h, 20–40 % of the LNP-treated DC had migrated toward CCL21 (Fig. 2d). In some donors, siPDL-LNP-treated DC showed somewhat reduced migration compared to untreated DC, which might most likely be explained by the slightly lower DC viability. Taken together, these data show that DC treated with siRNA-containing KC2-1.5 % PEG-LNP have acceptable viability, show a highly mature and stimulatory phenotype and exhibit good CCR7-mediated migratory capacity.
Fig. 2.
PD-L-silenced DC show a mature phenotype and good migratory capacity toward CCL21. At days 3 and 7 of culture, 250 nM PD-L1 + 125 nM PD-L2 or 375 nM negative control siRNA-containing KC2-1.5 % PEG-LNP was added. Subsequently, DC were matured for 2 days, and DC viability, phenotype and migratory capacity were analyzed. a Relative PD-L1 and PD-L2 knockdown was examined using flow cytometry and calculated as follows: (ΔMFI PD-L LNP-treated DC/ΔMFI Control LNP-treated DC) × 100. b Viability was determined by trypan blue exclusion. Data are expressed as mean + SEM of 9 independent experiments. c Expression of maturation and co-stimulatory molecules by DC was analyzed using flow cytometry. Data are expressed as mean + SEM of 9 donors. The dotted line indicates the MFI of the isotype controls. d CCR7-mediated migratory capacity of DC cultured with or without LNP was determined toward increasing concentrations of chemokine CCL21. Depicted is the mean number of migrated cells + SD from triplo measurements. Data of one representative donor are shown. Statistical analysis was performed using a one-way ANOVA (c) or two-way ANOVA (d) followed by a Bonferroni post hoc test. *P < .05, **P < .01
MiHA-specific T cell expansion is enhanced by siPDL-LNP-treated DC loaded with MiHA peptide
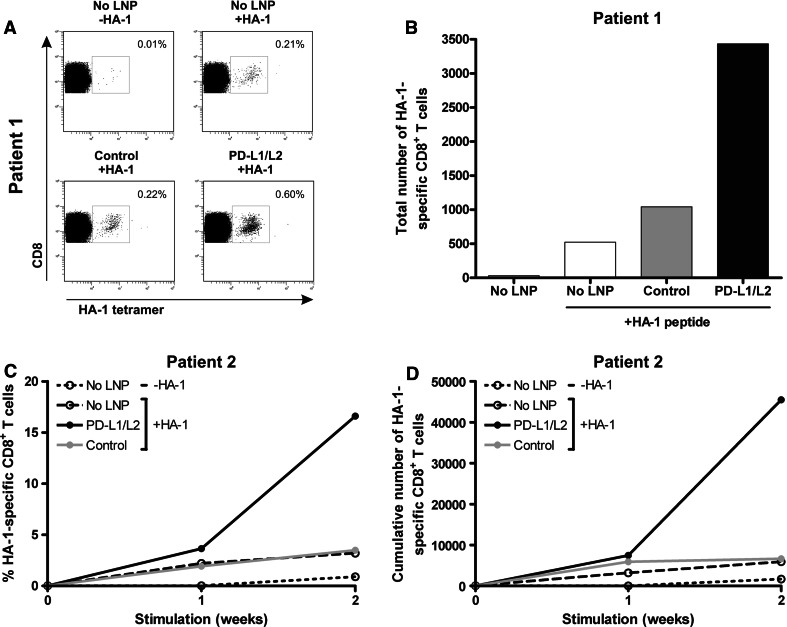
Next, we examined the stimulatory capacity of siPDL-LNP-treated DC on MiHA-specific CD8+ T cell expansion by loading PD-L knockdown DC with 5 μM HA-1 peptide. Subsequently, DC were cultured with PBMC from two allo-SCT patients containing HA-1-specific CD8+ effector-memory T cells (Table 1). At start, PBMC of patient 1 contained 0.03 % HA-1-specific T cells, and 1-week stimulation with PD-L1/L2-silenced DC resulted in a substantial increase in both the percentage and absolute number of HA-1-specific CD8+ T cells as compared to stimulation with control siRNA-LNP or untreated DC (Fig. 3a, b). PBMC of patient 2, containing 0.02 % HA-1-specific CD8+ T cells, were stimulated for two consecutive weeks with HA-1 peptide-loaded DC. In the first week following stimulation with PD-L1/L2 double-knockdown DC, the percentage and absolute number of HA-1-specific CD8+ T cells increased, respectively, 1.9- and 1.3-fold compared to control siRNA-LNP DC stimulation (Fig. 3c, d). Notably, the effects were more pronounced after the second week of stimulation, when siPDL-LNP-silenced DC further boosted HA-1-specific T cell proliferation 6.1-fold compared to 1.1-fold by control siRNA-LNP-treated DC. These data demonstrate that LNP-mediated transfection of PD-L1 and PD-L2 siRNA results in DC with enhanced T cell stimulatory potential.
Fig. 3.
PD-L knockdown DC loaded with MiHA peptide enhance MiHA-specific CD8+ T cell proliferation. At days 3 and 7 of DC culture, 250 nM PD-L1 + 125 nM PD-L2 or 375 nM negative control KC2-1.5 % PEG-LNP was added if indicated. Mature DC were loaded with 5 μM HA-1 peptide and cultured at a ratio of 0.1:1 with patient PBMC containing low numbers of MiHA-specific CD8+ T cells for 1–2 weeks. a After 1 week, cells were analyzed for tetramer-positive CD8+ T cells using flow cytometry. The numbers in the FACS plots represent the percentage of HA-1-specific CD8+ T cells within the total CD3+CD8+ T cell population. Data of one representative patient (patient 1) are shown. b Total number of HA-1-specific CD8+ T cells of patient 1 after 1 week of stimulation with PD-L-silenced DC loaded with or without HA-1 peptide. c and d The percentage (c) and cumulative numbers (d) of HA-1-specific CD8+ T cells of patient 2 obtained after stimulation with PD-L-silenced DC loaded with or without HA-1 peptide
Excess siRNA-LNP is efficiently removed from the DC product upon 2 wash steps
Eventually, we aim to develop a clinical-grade applicable DC vaccine with improved immunogenic potential. Because some toxicities attributable to the lipid formulation were observed in preclinical animal models at doses well above efficacious ones (unpublished observations), it is important to know the amount of lipid taken up by the DC, as well as extracellular residual lipid levels after washing the LNP-treated DC end product. Therefore, we followed the SOP applied in our DC vaccination trials to harvest mDC following maturation and to quantify the amount of lipid remaining in the cells and washout supernatants. We observed that after the first wash already 83 ± 2.0 % of the KC2 lipid was removed from the DC product (Table 2). The second wash resulted in further reduction to 3.0 ± 0.5 % of the original KC2-lipid level present during DC culture. Importantly, about 99 % of the KC2 was removed from the cells with subsequent washing. Furthermore, in the DC on average only 1.1 ± 0.4 % of the added KC2-lipid was detected. Together, this shows that the DC end product, including trace amounts of extracellular residual lipids, contains less than 2 % of the ex vivo-added KC2-lipid, indicating that excess siRNA-LNP are efficiently removed from the DC end product following the established washing procedure.
Table 2.
Removal of excess KC2-lipid from DC product
| Sample | Relative KC2 level (%) |
|---|---|
| Culture supernatant | 100 |
| Wash supernatant 1 | 16.36 ± 1.38 |
| Wash supernatant 2 | 3.00 ± 0.34 |
| Wash supernatant 3 | 0.95 ± 0.09 |
| Wash supernatant 4 | 0.40 ± 0.03 |
Removal of excess KC2-lipid from the DC product was examined using HPLC-VLC. KC2 levels in the wash supernatant was calculated relative to the original KC2 level in the culture supernatant (Mean ± SEM). N = 2
siRNA-LNP-treated DC can be efficiently loaded with target antigen mRNA using electroporation
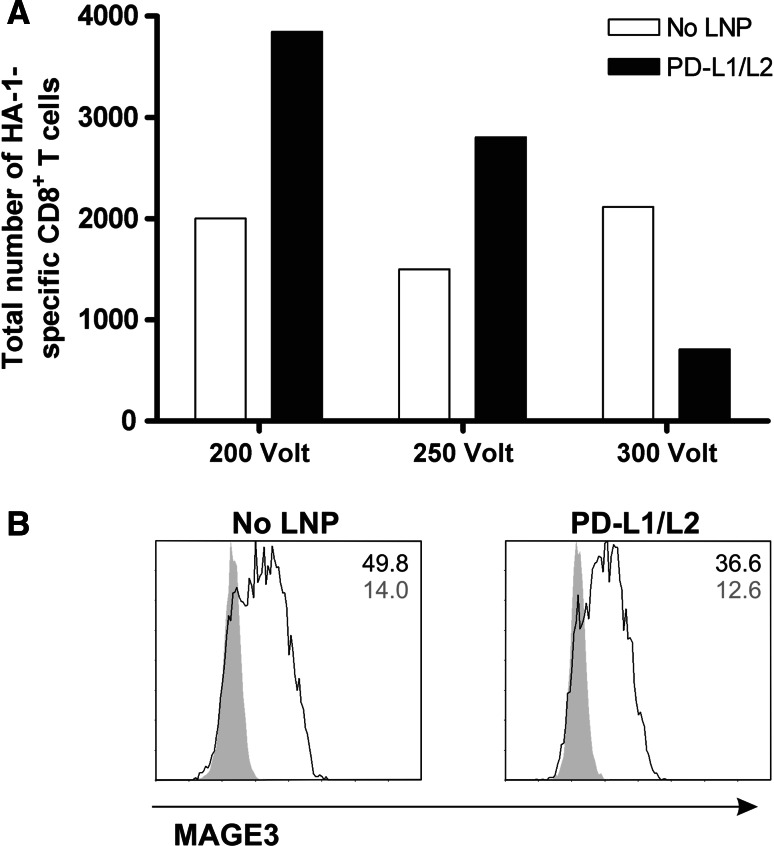
Because of the observed effect on the DC viability following siRNA-LNP treatment (Fig. 2b), we had to optimize the electroporation settings for target antigen mRNA loading to maximally reduce harm to the DC. Hence, siRNA-LNP-treated DC were electroporated with antigen mRNA at different voltages, and T cell stimulatory potential of the DC was examined. Electroporation of target antigen mRNA at 300 V and 150 μF resulted in reduced cell viability and loss of the advantage of PD-L knockdown DC stimulation on T cell expansion (Fig. 4a). Lowering the voltage to 200 V resulted in improved function of the PD-L-silenced DC as compared to untreated DC. Importantly, lowering the voltage to 200 V did not affect mRNA loading efficacy in general, as untreated DC demonstrated similar stimulatory capacity with all voltages tested (Fig. 4a). Notably, further voltage reduction to 100 V was not beneficial for mRNA uptake and processing (data not shown). Because MiHA mRNA loading cannot be visualized by flow cytometry due to the lack of appropriate antibodies, we also electroporated siRNA-LNP-treated DC with the TAA MAGE3 and performed FACS analysis. One hour after electroporation, both siRNA-LNP-treated and untreated DC showed high MAGE3 protein expression (Fig. 4b). Together, these results demonstrate that LNP-mediated siRNA delivery can be efficiently combined with target antigen mRNA electroporation, while preserving appropriate DC numbers and good functionality.
Fig. 4.
siRNA-LNP-treated DC can be efficiently loaded with antigen-specific mRNA using electroporation. At days 3 and 7 of culture, 250 nM PD-L1 + 125 nM PD-L2 siRNA-containing KC2-1.5 % PEG-LNP was added. Mature DC treated with or without siPDL-LNP were electroporated with antigen-specific mRNA. a 1 h after loading with 20 μg HMHA1 mRNA using different electroporation voltages, DC were added at a ratio of 0.1:1 to patient PBMC containing low numbers of HA-1-specific CD8+ T cells. After 1 week, the expansion of HA-1-specific CD8+ T cells was analyzed using flow cytometry. b One hour after electroporation with 10 μg MAGE3 mRNA at 200 V, MAGE3 protein expression (black lines) as compared to isotype control (gray peaks) was analyzed using flow cytometry. Data of one representative donor out of 3 experiments are shown
MiHA mRNA-loaded PD-L knockdown DC effectively boost the proliferative capacity of highly functional MiHA-specific CD8+ effector-memory T cells
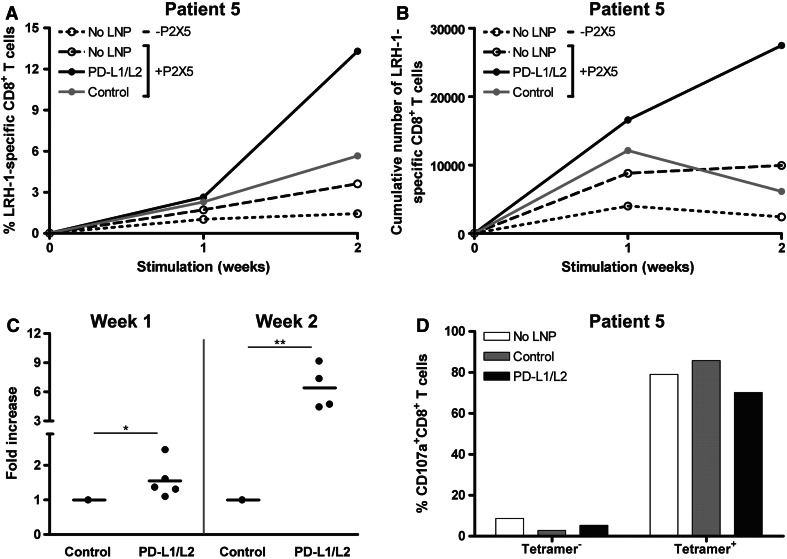
To investigate the stimulatory potential of siPDL-LNP-treated DC electroporated with MiHA-encoding mRNA, we stimulated PBMC of 5 allo-SCT patients containing either HA-1- or LRH-1-specific CD8+ effector-memory T cells for one to two consecutive weeks with these DC. In Fig. 5a, one representative patient is shown. At start, this patient had 0.02 % LRH-1-specific CD8+ T cells, which increased up to 3.6 % following two repetitive stimulations with untreated mRNA-loaded DC. In the first week, stimulation with siPDL-LNP-silenced DC resulted in a moderate increase in the percentage of antigen-specific T cells, compared to control siRNA-LNP DC-stimulated cells (2.6 vs. 2.3 %). But a second stimulation with these PD-L knockdown DC boosted the expansion of the LRH-1-specific CD8+ T cells up to 13.3 % compared to 5.7 % in case of control siRNA-LNP DC. In absolute numbers the advantage of PD-L knockdown DC over control DC in augmenting LRH-1-specific CD8+ T cell expansion was even more pronounced, resulting in a 4.5-fold higher number of antigen-specific T cells after 2 weeks of stimulation (Fig. 5b).
Fig. 5.
The expansion of highly functional MiHA-specific CD8+ T cells is boosted by MiHA mRNA-loaded PD-L-silenced DC. At days 3 and 7 of culture, 250 nM PD-L1 + 125 nM PD-L2 or 375 nM negative control siRNA-containing KC2-1.5 % PEG-LNP was added when indicated. Mature DC were electroporated at 200 Volt with 20 μg MiHA-encoding mRNA and cultured at a ratio of 0.1:1 with patient PBMC containing low numbers of MiHA-specific CD8+ T cells for 1-2 weeks. a One week after each stimulation, cells were analyzed for tetramer-positive CD8+ T cells using flow cytometry. Data of one representative patient (patient 5) having a LRH-1-specific CD8+ T cell response are shown. b Total number of LRH-1-specific CD8+ T cells of patient 5 after 1-2 consecutive weeks of stimulation with PD-L-silenced DC loaded with P2X5 mRNA. c To combine data of all patients, the increase in absolute number of MiHA-specific CD8+ T cells after stimulation with PD-L-silenced DC was calculated relative to control siRNA-LNP DC stimulation. Data of 5 and 4 patients are shown for week 1 and 2, respectively. Lines indicate the mean. d Degranulation of LRH-1-tetramer-negative and positive CD8+ T cells of patient 5, stimulated for 2 weeks with P2X5 mRNA-loaded PD-L knockdown DC, was measured by staining for CD107a during overnight restimulation with 5 μM LRH-1 peptide. Statistical analysis was performed using a two-sample two-tailed t test assuming independent samples. *P < 0.05, **P < 0.01
To combine data of all patients, we calculated for each week the fold increase in the absolute number of MiHA-specific CD8+ T cells following stimulation with PD-L-silenced DC relative to stimulation with control siRNA-LNP DC (Fig. 5c). Importantly, PD-L1/L2 double-knockdown DC loaded with MiHA-encoding mRNA significantly boosted the proliferative capacity of MiHA-specific CD8+ T cells, which becomes even more pronounced after repetitive stimulations. Finally, we examined the functionality of the DC-stimulated MiHA-specific T cells of patient 5, after 2 weeks of expansion (Fig. 5d). These expanded CD8+ T cell cultures were restimulated overnight with LRH-1 peptide in the presence of anti-CD107a to determine the percentage of degranulating CD107a+ LRH-1-tetramer+ CD8+ T cells. Restimulation with the LRH-1 peptide resulted in a specific CD107a staining of >70 % of the tetramer+ CD8+ T cells, whereas less than 10 % of the tetramer− CD8+ T cells were CD107a positive. Together, these results demonstrate that antigen-specific CD8+ effector-memory T cell expansion can be efficiently augmented using siPDL-LNP-silenced DC and that these expanded antigen-specific CD8+ T cells have the capacity to degranulate upon encounter with their target antigen.
Discussion
Although the immune system is capable of eliciting immune responses toward chronic virally infected cells and cancer, the quantity and functionality of these responses is most often insufficient or hampered, contributing to disease progression [24, 25]. To eventually cure these patients, boosting of antigen-specific effector and memory T cell responses in vivo is essential. As DC are the most potent professional APC, they are considered to be an ideal cell-based therapy to enhance immune responses [1, 2]. Especially, in the setting of allo-SCT, DC-based vaccination exploiting hematopoietic-restricted MiHA as tumor target antigens is a promising strategy to selectively boost donor T cell-mediated graft-versus-tumor (GVT) immunity, without evoking severe graft-versus-host-disease (GVHD) [26–28]. During the last decade, clinical DC vaccination strategies have been widely explored. In various hematological and metastatic solid cancers, vaccination with ex vivo-generated DC loaded with tumor antigens has been reported to induce antitumor T cell responses and tumor regression [1, 4–7]. However, overall response rates and durable responses are still limited, implicating that further improvement of DC vaccine potency is crucial to increase clinical benefit.
The efficacy of DC vaccines is dependent on a variety of variables, including DC type, maturation status, antigen-loading strategy, dosing and frequency of the vaccine injections, and the route of administration [4, 29]. The most commonly used cells for generating high numbers of mature DC ex vivo are monocytes [5]. We and others have shown that upon electroporation of monocyte-derived DC with target antigen mRNA, antigen-specific CD8+ T cell responses can be efficiently boosted [13–15]. The advantage of using mRNA instead of peptide loading is that the antigen epitopes are naturally processed, and a variety of different epitopes are long term presented by both HLA class I and class II molecules [30, 31]. In that way, the breadth of the elicited immune response could be enlarged. Besides the recognition of the HLA/antigen complex by the T cell receptor, T cells require co-stimulatory signaling to become activated and exert their effector function. However, antigen-specific T cells can become functionally impaired over time both in chronic viral infections and in cancer [32–35]. It has been shown that these antigen-specific T cells up-regulate various co-inhibitory molecules like programmed death-1 (PD-1) due to chronic antigen exposure. Continued downstream signaling of these molecules eventually results in T cell exhaustion. We previously demonstrated that the PD-1/PD-L pathway is involved in the functional inhibition of MiHA-specific T cell responses post-transplant and that blockade of the PD-1 receptor, using a humanized blocking antibody, in combination with DC stimulation boosted the ex vivo expansion of these MiHA-specific T cells, especially in relapsed allo-SCT patients [35]. Importantly, in two recently published reports on anti-PD-1 (BMS-936,588) and anti-PD-L1 (BMS-936,559) blocking antibody therapy in solid cancers, durable tumor regression was observed with objective clinical responses in 18–28 and 6–17 % of the patients, respectively [36, 37]. However, in vivo treatment of allo-SCT patients with these blocking antibodies might bring high risks of inducing GVHD. Therefore, we developed a more specific approach using siRNA electroporation to silence the co-inhibitory molecules PD-L1 and PD-L2 in DC. In this way, we obtained a DC vaccine with an improved stimulatory phenotype, which strongly enhanced antigen-specific T cell proliferation and cytokine production (IFN-γ, IL-2, TNF-α and IL-5) ex vivo [9]. To eventually generate a superior DC vaccine with high stimulatory potential, and capable of eliciting a robust MiHA-specific immune response, it is desirable to combine PD-L siRNA delivery and MiHA mRNA loading.
Lipid nanoparticles are attractive agents to deliver specific target siRNAs into ex vivo-generated human DC vaccines in a non-viral manner. Importantly, these LNP can be generated in compliance with good manufacturing practice (GMP) guidelines, and all materials required have been approved for use in the clinical setting. Recently, Basha et al. [21] demonstrated that LNP are competent in vivo siRNA delivery reagents for silencing target genes in murine DC. An advantage of using LNP, compared to for instance poly(lactic-co-)glycolic acid particles, is that due to the ionizable cationic lipids and nearly neutral charge, these particles can be efficiently loaded with siRNA [38]. Furthermore, these cationic lipids competently mediate siRNA delivery across the cellular membrane [21, 39]. Moreover, these LNP have been proven safe and efficient vehicles for low-dose in vivo gene silencing in animals [18–20]. Here, we first tested 4 formulations composed of different ionizable cationic lipids (KC2 vs. MC3), containing either high or low PEG concentrations, for their transfection efficiency of human monocyte-derived DC. Treatment with the KC2 siPDL-LNP resulted in specific and efficient silencing of PD-L1 and PD-L2. Furthermore, no effect on DC maturation phenotype was observed, although DC viability and in some donors also migratory capacity were somewhat reduced upon treatment with KC2-1.5 % PEG-LNP. As the effect on DC viability already indicates, the cell integrity may be affected to a minor extent by LNP-mediated siRNA delivery. Especially, for target antigen mRNA loading of siPDL-LNP-treated DC, milder electroporation settings had to be applied due to reduced DC viability and stimulatory potential at the standard voltage of 300, in contrast to untreated DC. Notably, lowering the electroporation voltage did not impair mRNA processing and peptide presentation, while the highly stimulatory function of the PD-L knockdown DC was retained.
In accordance with our previous findings [9], exogenous loading of siPDL-LNP-silenced DC with MiHA peptide strongly augmented the expansion of MiHA-specific CD8+ effector-memory T cell responses in allo-SCT patients. Moreover, we showed that siPDL-LNP-treated DC can also be effectively electroporated with MiHA-encoding mRNA. These improved DC products potently boosted the proliferation of antigen-specific CD8+ T cells. After 1 week of stimulation with PD-L-silenced DC, increased numbers of MiHA-specific CD8+ T cells were observed, as has also been reported by Breton et al. [40]. Interestingly, these PD-L-silenced DC are also superior in boosting MiHA-specific T cell expansion from the naïve repertoire of MiHA− donors (unpublished observations). In addition, we observed that the growth advantage was even further augmented upon repetitive stimulations with PD-L knockdown DC. The amplification of the response upon restimulation with PD-L-silenced DC could be attributed to various mechanisms. During T cell activation, PD-1 expression levels are up-regulated, which would normally mediate dampening of the T cell response. However, since our DC no longer express the PD-1 ligands, the expanded MiHA-specific T cells remain in a highly activated state. Furthermore, Karwacz et al. [41] reported that interference with PD-1/PD-L1 signaling inhibits TCR down-regulation following T cell activation, also rendering T cells in a hyperactivated state. It is likely that because of these mechanisms, the expanded T cells responded better to the restimulation with the highly immunogenic PD-L knockdown DC. Importantly, we demonstrated that these expanded MiHA-specific CD8+ T cells efficiently degranulate upon antigen restimulation. Soon these findings will be implemented in a clinical phase I/II proof-of-concept trial, in which patients treated with HLA-matched partial T cell-depleted allo-SCT will receive DLI in combination with vaccination of PD-L1/L2-silenced donor DC loaded with hematopoietic-restricted MiHA. Due to their restricted expression on the recipient’s normal and malignant hematopoietic cells, T cell responses against these MiHA vaccine targets will likely induce GVT immunity without causing severe GVHD [10–12]. Importantly, our data indicate that the overall dose of the KC2 lipid in the DC end product is less than 2 %, as only a limited part of the LNP are taken up by the DC during ex vivo culturing, and most residual lipids are efficiently removed by washing the cells. This corresponds to a calculated maximum dose of 0.125 μg KC2 lipid per kilogram body weight in our patients, which is far lower than the No Observed Adverse Effect Level (NOAEL) of 1.0 mg/kg in preclinical animal studies (unpublished observation).
In conclusion, we developed a new clinical-grade applicable DC vaccine with improved immunogenic potential by combining PD-1 ligand siRNA and target antigen mRNA delivery. We demonstrated efficient and specific silencing of PD-L1 and PD-L2 on DC using siRNA-LNP, without affecting the phenotype or migratory capacity of the DC. Furthermore, we showed that LNP-mediated siRNA delivery can be successfully combined with target antigen mRNA electroporation. Finally, we demonstrated that these PD-L-silenced DC loaded with MiHA mRNA have superior stimulatory potential and effectively boost ex vivo antigen-specific CD8+ T cell responses in transplanted cancer patients. Together, these findings indicate that our siPDL-LNP-modified DC are attractive cells for clinical-grade production and in vivo application to induce and boost immune responses in the allo-SCT setting, and likely also in other cancers or viral infections.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Anniek van der Waart for her help with flow cytometry, and Frederik Falkenburg and Michel Kester (Department of Hematology, LUMC, Leiden, the Netherlands) for providing us with tetramers. We are also grateful to Brian Bettancourt for designing siRNA sets, Anna Borodovsky as Alnylam contact, Renta Hutabarat for lipid quantification and siRNA and lipid synthesis groups at Alnylam Pharmaceuticals. This work was supported by a grant from the Dutch Cancer Society (KWF 2008-4018).
Conflict of interest
Tatiana I. Novobrantseva, Jamie Wong, Stuart Milstein, Hila Epstein-Barash and Ju Liu are employees of Alnylam Pharmaceuticals. The other authors have no conflicting financial interests.
References
- 1.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 2.Kalinski P, Urban J, Narang R, Berk E, Wieckowski E, Muthuswamy R. Dendritic cell-based therapeutic cancer vaccines: what we have and what we need. Future Oncol. 2009;5:379–390. doi: 10.2217/fon.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia F, Routy JP. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine. 2011;29:6454–6463. doi: 10.1016/j.vaccine.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 6.van de Velde V, Berneman ZN, van Tendeloo V. Immunotherapy of hematological malignancies using dendritic cells. Bull Cancer. 2008;95:320–326. doi: 10.1684/bdc.2008.0602. [DOI] [PubMed] [Google Scholar]
- 7.Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58:1–14. doi: 10.1007/s00262-008-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 9.Hobo W, Maas F, Adisty N, de Witte T, Schaap N, van der Voort R, Dolstra H. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8+ T cells. Blood. 2010;116:4501–4511. doi: 10.1182/blood-2010-04-278739. [DOI] [PubMed] [Google Scholar]
- 10.Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA, van Luxemburg-Heys SA, Hoogeboom M, Mutis T, Drijfhout JW, van Rood JJ, Willemze R, Falkenburg JH. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci USA. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Rijke B, van Horssen-Zoetbrood A, Beekman JM, Otterud B, Maas F, Woestenenk R, Kester M, Leppert M, Schattenberg AV, de Witte T, van de Wiel-van Kemenade E, Dolstra H. A frameshift polymorphism in P2X5 elicits an allogeneic cytotoxic T lymphocyte response associated with remission of chronic myeloid leukemia. J Clin Invest. 2005;115:3506–3516. doi: 10.1172/JCI24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hambach L, Goulmy E. Immunotherapy of cancer through targeting of minor histocompatibility antigens. Curr Opin Immunol. 2005;17:202–210. doi: 10.1016/j.coi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Overes IM, Fredrix H, Kester MG, Falkenburg JH, van der Voort R, de Witte TM, Dolstra H. Efficient activation of LRH-1-specific CD8+ T-cell responses from transplanted leukemia patients by stimulation with P2X5 mRNA-electroporated dendritic cells. J Immunother. 2009;32:539–551. doi: 10.1097/CJI.0b013e3181987c22. [DOI] [PubMed] [Google Scholar]
- 14.van Tendeloo V, Ponsaerts P, Lardon F, Nijs G, Lenjou M, van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.V98.1.49. [DOI] [PubMed] [Google Scholar]
- 15.van Nuffel AM, Corthals J, Neyns B, Heirman C, Thielemans K, Bonehill A. Immunotherapy of cancer with dendritic cells loaded with tumor antigens and activated through mRNA electroporation. Methods Mol Biol. 2010;629:405–452. doi: 10.1007/978-1-60761-657-3_27. [DOI] [PubMed] [Google Scholar]
- 16.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 17.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Rohl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basha G, Novobrantseva TI, Rosin N, Tam YY, Hafez IM, Wong MK, Sugo T, Ruda VM, Qin J, Klebanov B, Ciufolini M, Akinc A, Tam YK, Hope MJ, Cullis PR. Influence of cationic lipid composition on gene silencing properties of lipid nanoparticle formulations of siRNA in antigen-presenting cells. Mol Ther. 2011;19:2186–2200. doi: 10.1038/mt.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novobrantseva TI, Borodovsky A, Wong J, Klebanov B, Zafari M, Yucius K, Querbes W, Ge P, Ruda VM, Milstein S, Speciner L, Duncan R, Barros SA, Basha G, Cullis PR, Akinc A, Donahoe JS, Narayanannair JK, Jayaraman M, Bogorad RL, Love K, Whitehead KA, Levins CG, Manoharan M, Swirski FK, Weissleder R, Langer R, Anderson DG, de Fougerolles A, Nahrendorf M, Koteliansky V. Systemic RNAi-mediated Gene Silencing in Nonhuman Primate and Rodent Myeloid Cells. Mol Ther Nuc Acids. 2012 doi: 10.1038/mtna.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theze J, Chakrabarti LA, Vingert B, Porichis F, Kaufmann DE. HIV controllers: a multifactorial phenotype of spontaneous viral suppression. Clin Immunol. 2011;141:15–30. doi: 10.1016/j.clim.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 26.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Joe G, Zhu J, Carroll R, Levine B, Hexner E, June C, Emerson SG. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 29.Figdor CG, de Vries IJM, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 30.Tavernier G, Andries O, Demeester J, Sanders NN, De Smedt SC, Rejman J. mRNA as gene therapeutic: how to control protein expression. J Control Release. 2011;150:238–247. doi: 10.1016/j.jconrel.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Met O, Eriksen J, Svane IM. Studies on mRNA electroporation of immature and mature dendritic cells: effects on their immunogenic potential. Mol Biotechnol. 2008;40:151–160. doi: 10.1007/s12033-008-9071-6. [DOI] [PubMed] [Google Scholar]
- 32.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 33.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 34.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norde WJ, Maas F, Hobo W, Korman A, Quigley M, Kester MG, Hebeda K, Falkenburg JH, Schaap N, de Witte TM, van der Voort R, Dolstra H. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71:5111–5122. doi: 10.1158/0008-5472.CAN-11-0108. [DOI] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semple SC, Klimuk SK, Harasym TO, Dos SN, Ansell SM, Wong KF, Maurer N, Stark H, Cullis PR, Hope MJ, Scherrer P. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim Biophys Acta. 2001;1510:152–166. doi: 10.1016/S0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 39.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharm. 2009;6:763–771. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breton G, Yassine-Diab B, Cohn L, Boulassel MR, Routy JP, Sekaly RP, Steinman RM. siRNA knockdown of PD-L1 and PD-L2 in monocyte-derived dendritic cells only modestly improves proliferative responses to Gag by CD8(+) T cells from HIV-1-infected individuals. J Clin Immunol. 2009;29:637–645. doi: 10.1007/s10875-009-9313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, Escors D. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med. 2011;3:581–592. doi: 10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.