Abstract
Several types of vaccine-delivering tumor-associated antigens (TAAs) have been developed in basic and clinical research. Wilms’ tumor 1 (WT1), identified as a gene responsible for pediatric renal neoplasm, is one of the most promising TAA for cancer immunotherapy. Peptide and dendritic cell-based WT1 cancer vaccines showed some therapeutic efficacy in clinical and pre-clinical studies but as yet no oral WT1 vaccine can be administrated in a simple and easy way. In the present study, we constructed a novel oral cancer vaccine using a recombinant Bifidobacterium longum displaying WT1 protein. B. longum 420 was orally administered into mice inoculated with WT1-expressing tumor cells for 4 weeks to examine anti-tumor effects. To analyze the WT1-specific cellular immune responses to oral B. longum 420, mice splenocytes were isolated and cytokine production and cytotoxic activities were determined. Oral administrations of B. longum 420 significantly inhibited WT1-expressing tumor growth and prolonged survival in mice. Immunohistochemical study and immunological assays revealed that B. longum 420 substantially induced tumor infiltration of CD4+T and CD8+T cells, systemic WT1-specific cytokine production, and cytotoxic activity mediated by WT1-epitope specific cytotoxic T lymphocytes, with no apparent adverse effects. Our novel oral cancer vaccine safely induced WT1-specific cellular immunity via activation of the gut mucosal immune system and achieved therapeutic efficacy with several practical advantages over existing non-oral vaccines.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-1984-0) contains supplementary material, which is available to authorized users.
Keywords: Bifidobacterium, WT1, Cancer vaccine, Oral vaccine, Immunotherapy
Introduction
Several significant breakthroughs in cancer immunotherapy have been made, including immune checkpoint inhibitors and CAR-T cells [1, 2]. The major principle of cancer immunotherapy involves the induction or activation of CTL-recognizing tumor-associated antigens (TAAs) [3]. TAAs are encoded by mutated oncogenes or genes that are overexpressed in tumor cells but either minimally or not expressed in normal cells. The first human TAA gene, melanoma antigen (MAGEA1), was cloned in 1991 [4]. Since then, several cancer vaccines containing TAAs have been developed in basic and clinical research.
One common type of TAA cancer vaccines is peptide-based vaccine containing HLA-restricted epitopes on TAAs. Peptide-based vaccines generally contain MHC class I epitopes to activate TAA-specific CTLs, or longer peptides including multiple MHC class I and II epitopes to activate both CD8+T and CD4+T cells, usually administered with immunologic adjuvants [5]. A possible drawback of peptide-based vaccines is HLA restriction, which may be suitable only for patients with HLA subtypes matched to TAA epitopes in the vaccine. Another common type of TAA cancer vaccine is dendritic cell (DC)-based vaccine [6]. Currently, sipuleucel-T, a DC-based vaccine loaded with a prostate cancer antigen, prostatic acid phosphatase, was approved by the US Food and Drug Administration for castration-resistant prostate cancer patients as the first licensed tumor vaccine [7].
TAAs can be delivered by vectors such as viruses, bacteria, and other means [8]. Adenovirus and poxvirus vectors are the most frequently used [9], and administered only by injection into veins, muscles, or tumors. A few oral cancer vaccines employ lactic acid bacteria. Lactobacillus casei can display HPV E7 protein against cervical intraepithelial neoplasia [10], and Lactobacillus plantarum can display cancer testis antigen NY-ESO-1 against NY-ESO-1-expressing cancers [11].
We developed an oral vaccine platform using Bifidobacterium longum and tested two experimental vaccines: B. longum displaying Salmonella-flagellin protein against typhoid fever [12] and B. longum displaying HCV nonstructural protein 3 multi-epitopes against chronic hepatitis C [13]. B. longum adheres strongly to human intestinal epithelial cells [14] and induces high activation of T helper type 1 (Th1) cell-mediated immune responses [15]. B. longum is a probiotic bacteria generally recognized as safe for humans [12] which may make it an ideal vehicle for an oral vaccine platform. In this study we developed a novel oral cancer vaccine using B. longum displaying Wilms’ tumor 1 (WT1) protein.
The WT1 gene encodes a zinc finger transcription factor important for the normal development of urogenital organs, and specifically expressed in normal tissues including the kidney, uterus, testis, ovary, and mesothelium [16, 17]. WT1 gene is reportedly overexpressing in various tumors, including leukemia, breast cancer, and most pediatric kidney tumors (Wilms’ tumor), suggesting that WT1 plays an oncogenic role in tumorigenesis [18, 19]. WT1 was a highly ranked TAA in a National Cancer Institute pilot project developing a priority list of tumor vaccine target antigens [20].
Various peptide-, DC-, and adenovirus-based WT1 vaccines have been thoroughly investigated both in clinical and pre-clinical studies [21, 22]. Phase I and II clinical trials using peptide- or DC-based WT1 vaccines have been widely conducted to treat leukemia and solid tumors. Although results demonstrated that WT1 vaccines could induce adaptive tumor immunity, clinical efficacy was limited and highly variable across patients, and adverse effects such as local injection site erythema were reported [21, 23]. Thus, we sought to develop a novel way to efficiently and safely deliver WT1, a TAA considered to have great potential as a vaccine target. Our previous studies demonstrated that the Bifidobacterium vaccine platform for antigens could induce efficient cellular immunity with the advantages of an oral vaccine [12, 13], and we developed a vaccine using Bifidobacterium displaying WT1 protein to investigate its feasibility for cancer treatment.
Materials and methods
Strains and media
B. longum 105-A was obtained from the Japan Collection of Microorganisms, RIKEN Bioresource Center. B. longum was grown anaerobically in Gifu Anaerobic Medium (GAM) broth (Nissui, Tokyo, Japan) at 37 °C. C1498-WT1, a C57BL/6 origin recombinant murine leukemia cell line stably expressing murine WT1 protein, was generated by transfection of the full length of murine-WT1 cDNA cloned from C57BL/6 mouse kidney with the pcDNA3.1(+) vector containing CMV promoter. C1498-mock was generated by transfection of the empty pcDNA3.1(+) vector [24]. Both cell lines were kindly provided by Dr. Sugiyama (Osaka University Graduate School of Medicine, Japan) and maintained in RPMI-1640 medium supplemented with 10% FBS, 50 μM 2-mercaptoethanol, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.5 mg/ml G418.
Construction of recombinant B. longum expressing GLBP-WT1 protein
A partial murine-WT1 gene (117–419 amino acid residues, GenBank: M55512.1; UniProtKB: P22561) was synthesized by GensScript (NJ, USA). The partial protein contains three known CD8+T cell epitopes (126–134, 187–195, and 235–243 aa), and one known CD4+T cell epitope (332–347 aa), already tested for immunogenicity in human and murine models [24–26]. The synthesized WT1 gene was fused to galacto-N-biose/lacto-N-biose I binding protein (GLBP) coding gene, and then corresponding gene was ligated with the Escherichia coli-B. longum shuttle vector, pJW241, as described previously [12, 13]. GLBP is a membrane protein in the ATP-binding cassette transporter on the wild-type B. longum cell wall, which was used as an anchor to display antigen on the bacterial cell surface. The resulting plasmid carrying GLBP-WT1 was introduced into B. longum 105-A by electroporation to generate the recombinant B. longum 420 strain. The B. longum 2012 strain with a plasmid carrying GLBP only, constructed in our previous study, was used as a control [13]. Both strains were grown anaerobically in GAM broth with 50 µg/ml spectinomycin at 37 °C. Figure 1a shows a schematic drawing of the antigen surface displaying system of B. longum 420 and the corresponding protein sequence of WT1.
Fig. 1.
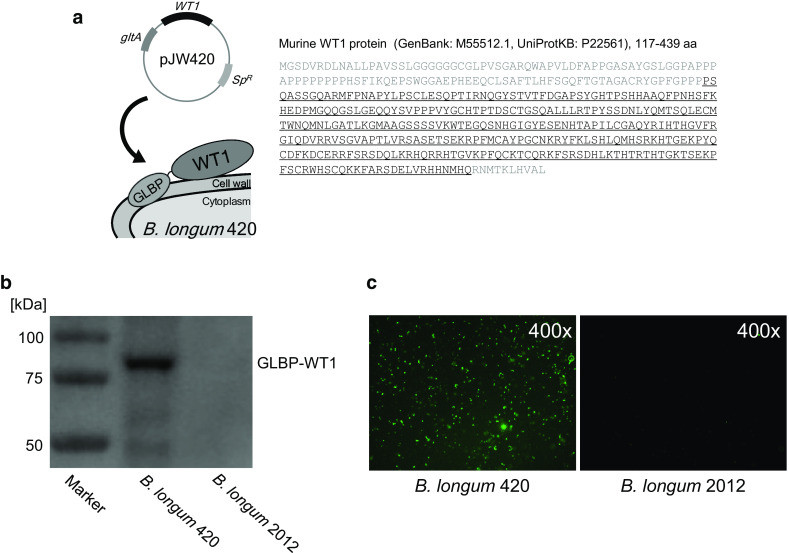
WT1 protein expression on B. longum 420. a Schematic drawing of recombinant B. longum 420. The recombinant WT1 protein including one CD4+T and three CD8+T cell epitopes is fused to the C-terminal of the GLBP protein. The underlined amino acid sequences of WT1 protein were transduced into B. longum 420. SP R: spectinomycin resistance gene. The expression of GLBP-WT1 fusion protein on B. longum 420 was detected by b Western blotting analysis and c immunofluorescence staining
Western blotting
B. longum 420 and B. longum 2012 were cultured overnight, collected, lysed with the sample buffer and heated for 5 min at 95 °C. The samples were separated by SDS–PAGE and transferred to a polyvinylidene difluoride membrane. After blocking and washing, the membrane was incubated for 1 h at room temperature (RT) with rabbit anti-WT1 antibody (Santa Cruz Biotechnology, Dallas, TX), 1:500 and then incubated for 1 h at RT with HRP-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology), 1:1000. Antibodies binding to proteins were detected by LAS 3000 mini, using the ECL Western Blotting Analysis System (GE Healthcare Japan, Tokyo, Japan).
Immunofluorescence staining of WT1 protein
Overnight-cultured B. longum 420 and B. longum 2012 were collected and blocked with Blocking One Histo (Nacalai Tesque, Kyoto, Japan) for 30 min at 37 °C. Then bacterial cells were washed and incubated with rabbit anti-WT1 antibody, 1:100 for 1 h at RT. After incubation the cells were washed in PBS twice and incubated with Alexa Fluor™ 488 goat anti-rabbit IgG (Life Technologies, Carlsbad, CA), 1:100 for 1 h at 37 °C.
Immunization and sampling for evaluation of immune responses
Fifteen female C57BL/6 N mice (H-2Db), 6–8 weeks of age, were purchased from CLEA Japan (Tokyo, Japan). Mice were randomly assigned to three oral vaccination groups (5 mice/group): B. longum 420; B. longum 2012; or PBS control. B. longum 420 or B. longum 2012 (1.0 × 109 colony forming units/100 µl of PBS), or 100 µl of PBS, was orally administered directly into the stomach using a feeding needle 5 days a week for 4 weeks (days 0–4, 7–11, 14–18, 21–25; total of 20 times), using a previously determined dose and immunization period [27]. Food and water were freely accessible. On day 27, mice were killed and spleens were aseptically removed and prepared for in vitro assays. All aspects of the experimental design and procedure were reviewed and approved by the institutional ethics and animal welfare committees of the Kobe University Graduate School of Medicine.
Anti-tumor effect of B. longum 420 vaccination against C1498-WT1 tumor
On day 0, C1498-WT1 cells (1 × 106 cells) were injected subcutaneously into the right flanks of female C57BL/6 N mice as described previously [28]. Following tumor injection, mice were randomly assigned to three treatment groups as described above (5 mice/group). Treatment was performed for 0–25 days and tumor development was monitored every fourth day after tumor injection. Tumor volume was expressed by the following formula: (longest diameter) × (shortest diameter)2 × 0.5. Another ten mice were also inoculated with C1498-mock cells (1 × 106 cells) as controls. At day 25, mice were sacrificed and tumors were resected for immunohistochemistry. Tumors were fixed with 10% formalin neutral buffer solution and embedded in paraffin. To test the survival rate, another 18 mice were inoculated with C1498-WT1 tumor cells (1 × 106 cells) and treated as described above (n = 6). Tumor growth was monitored from day 0 to 35 after tumor inoculation. Mice were euthanized when tumor diameter was >20 mm.
Immunohistochemical study
Three-µm thick paraffin embedded tumor tissue sections were deparaffinized and rehydrated. Antigen retrieval was performed in Bond epitope retrieval buffer (pH6.0 for CD4, pH9.0 for CD8a; Leica Microsystems, Wetzlar, Germany) at 98 °C for 20 min. Immunohistochemical staining was performed in an automatic tissue processor (Leica Microsystems Bond-Max) by the manufacturer’s standard protocol. Briefly, tissue sections were incubated at RT for 30 min with rabbit monoclonal anti-mouse CD4 antibody (1:500, Abcam, Cambridge, UK) or rat monoclonal anti-mouse CD8a antibody (1:100, Affymetrix Japan, Tokyo, Japan). After washing, sections were incubated for 30 min with biotinylated goat anti-rabbit IgG (1:400, Vector Laboratories, Burlingame, CA) or biotinylated goat anti-rat IgG (1:400, Vector Laboratories). After incubation, sections were washed and incubated with R.T.U. Vectastain Elite ABC reagent (Vector Laboratories) at RT for 30 min. After washing, sections were incubated with 3,3′-diaminobenzidine at RT for 10 min and counterstained with hematoxylin for 8 min.
ELISA expression levels of IFN-γ, IL-2, and TNF-α in splenocytes
In vitro splenocyte stimulation was performed as described previously [13] with slight modifications. Briefly, isolated splenocytes were strained, hemolyzed, and suspended in RPMI-1640 medium supplemented with 10% FBS, 10 mM HEPES, 1 mM nonessential amino acids, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 100 IU/ml penicillin, and 100 μg/ml streptomycin (complete RPMI-1640). The splenocytes were plated (4 × 105 cells/well) with a total volume of 200 µl/well into 96-well microplates and stimulated with mitomycin C-treated C1498-WT1 cells or C1498-mock cells (4 × 104 cells/well). Splenocyte cultures were incubated for 72 h at 37 °C in 5% CO2, and the supernatants were collected for ELISA and frozen at −80 °C until use. The production of IFN-γ, IL-2 and TNF-α in the supernatants was determined using a Mouse IFN-gamma Quantikine ELISA Kit (R&D Systems, Minneapolis, MN), Mouse IL-2 ELISA Kit (Thermo Scientific, Waltham, MA), and Mouse TNF-alpha Quantikine Kit (R&D Systems), respectively, with measurement according to each manufacturer’s instructions.
Quantification of CD4+T and CD8+T cells producing IFN-γ, IL-2, and TNF-α by intracellular cytokine staining
Intracellular cytokine staining (ICCS) was performed as described elsewhere [13]. ICCS was performed by BD Cytofix/Cytoperm™ Plus Fixation/Permeabilization Kit (BD Biosciences, San Diego, CA). Briefly, isolated splenocytes (2 × 106 cells) were re-stimulated with C1498-WT1 cells (2 × 105 cells) in complete RPMI-1640 on 24-well culture plates for 42 h. GolgiStop or GolgiPlug was added to each well followed by incubation for an additional 6 h. The splenocytes were collected and washed, then blocked with 10 µg/ml purified anti-mouse CD16/32 antibody (BioLegend, San Diego, CA) in staining buffer (1% FBS, 0.09% sodium azide) in PBS, for 20 min on ice. After washing, the cells were stained with 2 µg/ml FITC-conjugated anti-mouse CD4 antibody, PerCP-conjugated anti-mouse CD3 antibody, and Alexa Fluor™ 647-conjugated anti-mouse CD8a antibody (BD Biosciences) for 30 min on ice in the dark. The cells were washed twice and incubated with Fixation/Permeabilization Solution for 20 min on ice in the dark. After washing twice, the cells were stained with 5 µg/ml PE-conjugated anti-mouse IFN-γ antibody, PE-conjugated anti-mouse IL-2 antibody, or PE-conjugated anti-mouse TNF-α antibody (BD Biosciences) for 30 min on ice in the dark. After staining, the cells were washed three times and resuspended in the staining buffer. Detection of fluorescence staining was assessed using FACSVerse (BD Biosciences) and analyzed using FACSuite software (BD Biosciences).
H-2Db WT1-tetramer assay for WT1-specific CTLs
We performed the tetramer assay to determine the frequency of CD8+T cells recognizing the WT1 CTL-epitope using a H-2Db WT1 Tetramer-RMFPNAPYL (MBL Co., Ltd, Nagoya, Japan) according to the manufacturer’s instructions. Briefly, isolated splenocytes were re-stimulated in the same way as in ICCS for 7 days. Then, 20 IU/ml of mouse IL-2 (Wako, Osaka, Japan) were added to the cultures on days 1 and 3. The splenocytes were collected and washed, then blocked with 10 µg/ml purified anti-mouse CD16/32 antibody in flow cytometry (FCM) buffer (2% FBS, 0.05% sodium azide in PBS) for 15 min on ice. After washing with FCM buffer, the cells were stained with 4 µg/ml PE-conjugated H-2Db WT1 Tetramer-RMFPNAPYL for 30 min at RT in the dark. The cells were washed once and incubated with 4 µg/ml FITC-conjugated anti-mouse CD8 antibody and PerCP-conjugated anti-mouse CD3 antibody for 20 min on ice in the dark. After staining, the cells were washed two times and resuspended in the FCM buffer. Detection of fluorescence staining was assessed using a FACSVerse with analysis by FACSuite software.
Assay for WT1-specific CTL activity
Isolated splenocytes (3 × 107 cells) were co-cultured on 6-well culture plates for 6 days with mitomycin C-treated C1498-WT1 cells (3 × 106 cells) and 20 IU/ml mouse IL-2 to generate effector cells [29]. The effector splenocytes and target C1498-WT1 or C1498-mock cells (1 × 104 cells, respectively) were co-cultured in 96-well plates for 8 h at 37 °C in 5% CO2, at ratios of 20:1, 10:1, and 5:1. Specific CTL activity was measured using a LDH Cytotoxicity Assay Kit (CytoTox 96 Non-Radioactive Cytotoxicity Assay; Promega, Fitchburg, WI) according to the manufacturer’s instructions. The percentage of specific killing was calculated by the following formula: % specific killing = (experimental release − effector spontaneous release − target spontaneous release)/(target maximum release − target spontaneous release) × 100.
Statistical analysis
Comparisons between multiple groups were performed by one-way ANOVA followed by the Tukey–Kramer method. Survival between groups was analyzed by the log-rank test on Kaplan–Meier curves. Differences among experimental groups were considered significant when p < 0.05.
Results
GLBP-WT1 fusion protein expression
We performed western blotting to determine the expression of GLBP-WT1 fusion protein on the recombinant B. longum. B. longum 420 expressed GLBP-WT1 protein with molecular masses similar to the theoretical molecular masses of 83 kDa (Fig. 1b). In immunofluorescence staining, fluorescence was observed on the recombinant B. longum 420 cell surface but not on B. longum 2012, which transduced only GLBP (Fig. 1c). These results showed that B. longum 420 successfully displayed WT1 protein on the cell surface via GLBP.
B. longum 420 oral administration did not cause body weight change in mice
Since WT1 is reported to be expressed in normal cells or tissues, we investigated the possibility of autoaggression by immunization against self-WT1. To evaluate this potential adverse effect, the body weights of the mice were measured throughout the immunization period and the mean body weights showed no significant differences among the different groups (Suppl. Figure 1). There were no obvious signs of toxicity, such as rough hair, hunched posture, or lethargy.
B. longum 420 oral vaccination induced significant in vivo anti-tumor effect on WT1-expressing tumors
To investigate the anti-tumor effect of B. longum 420, we challenged WT1-expressing tumors in mice. After subcutaneous injection with C1498-WT1 or C1498-mock cells, the mice received oral B. longum 420 for 4 weeks. The tumor growth of C1498-WT1 with B. longum 420 was markedly reduced compared with the other groups (Fig. 2a). At day 25 after inoculation, the mean tumor volume in the B. longum 420 group was significantly smaller than the other groups (p < 0.05). B. longum 420 also significantly prolonged the survival of mice bearing C1498-WT1 tumors compared with other treatment groups (p < 0.05) (Fig. 2b). In contrast, B. longum 420 did not elicit an anti-tumor effect against C1498-mock, which was not expressing WT1 protein (Fig. 2c). These results indicated that B. longum 420 oral vaccinations could induce WT1-specific cellular immunity and elicit a highly functional anti-tumor effect against WT1 in vivo.
Fig. 2.
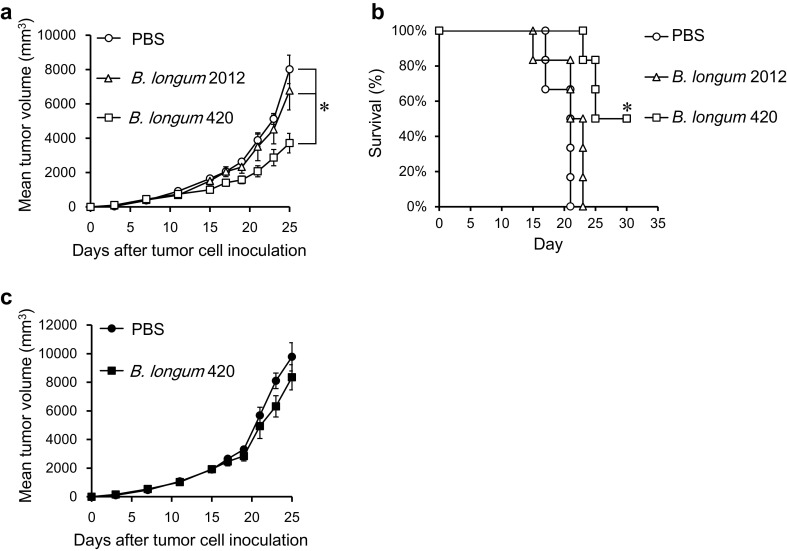
Anti-tumor effect of B. longum 420 oral administrations. Mice (n = 5 per group) received treatment with B. longum 420, B. longum 2012, or PBS 5 times a week for 4 weeks after subcutaneous injection with C1498-WT1 or C1498-mock cells. a Tumor growth curve and b Kaplan–Meier survival curve (n = 6) of mice with C1498-WT1 cells, and c tumor growth curve of C1498-mock cells, respectively (*p < 0.05). Each data point represents the average of each group (bars, ±SE)
B. longum 420 induced tumor-infiltrating CD4+T and CD8+T cells in mice
In the immunohistochemical study, we found that CD4 positive and CD8 positive lymphocytes were remarkably infiltrated into C1498-WT1 tumor tissues in the B. longum 420 group (Fig. 3a, d), while infiltration was barely observed in B. longum 2012 (Fig. 3b, e) and PBS group (Fig. 3c, f). Oral administration of B. longum 420 appeared to induce tumor-infiltrating T cells in local tumor tissues and elicited strong cytotoxic activity in vivo.
Fig. 3.
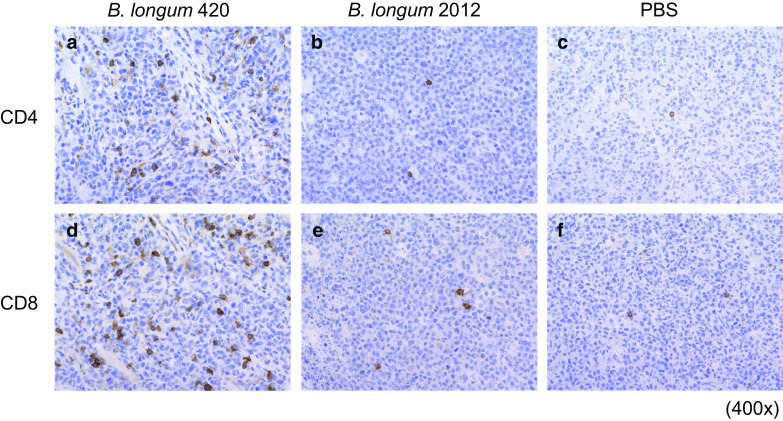
Immunohistochemical staining for T cell infiltration in mouse tumor. Subcutaneous C1498-WT1 tumors were resected and sections were stained with anti-CD4 and anti-CD8a antibodies after oral vaccination. Representative pictures of anti-CD4 staining are shown in a B. longum 420, b B. longum 2012, c PBS, respectively (400×). Representative pictures of anti-CD8a staining are shown in d B. longum 420, e B. longum 2012, and f PBS, respectively (400×)
The highest WT1-specific cytokine productions in splenocytes were induced by B. longum 420 oral vaccination
To determine the systemic cellular immune response induced by B. longum 420, mice splenocytes were isolated after immunization and re-stimulated with C1498-WT1 (pulsed) or C1498-mock (non-pulsed) tumor cells in vitro for 72 h. The levels of IFN-γ, IL-2, and TNF-α secretion in the culture supernatants were measured by ELISA, which showed that the splenocytes from the B. longum 420 group secreted significantly higher IFN-γ, IL-2, and TNF-α following pulsation of C1498-WT1 compared with the other groups (Fig. 4a–c, respectively. p < 0.05).
Fig. 4.
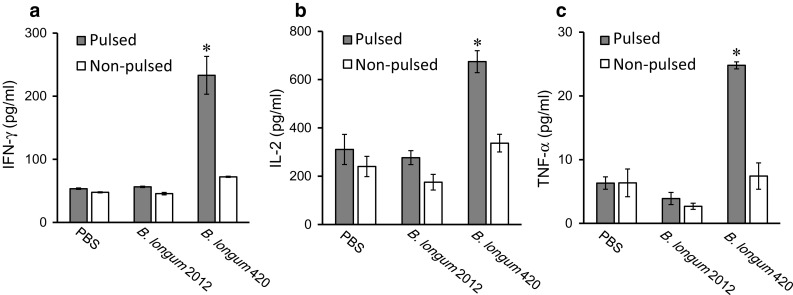
Cytokine production in mouse splenocytes induced by B. longum 420. After the last vaccination, splenocytes in the three vaccination groups (B. longum 420, B. longum 2012, and PBS; n = 5 per group) were isolated and re-stimulated with mitomycin C-treated C1498-WT1 (pulsed) or C1498-mock (non-pulsed) for 72 h in vitro. a IFN-γ, b IL-2, and c TNF-α production were determined by ELISA (*p < 0.05). Each data point represents the average of each group (bars, ±SE)
B. longum 420 vaccination induced the highest number of CD4+T and CD8+T cells expressing cytokines by ICCS
Using ICCS to detect cytokine-producing T cells, the frequency of CD4+T and CD8+T cells producing IFN-γ, IL-2, and TNF-α in the B. longum 420 group was significantly higher than that in the other groups (Fig. 5a–f. p < 0.05). These results showed that B. longum 420 oral administration could induce cytokine-producing CD4+T and CD8+T cells that were responsible for WT1-specific T cell-mediated cellular immune responses.
Fig. 5.

Intracellular cytokine staining (ICCS) for detection of cytokine-producing CD4+T and CD8+T cells. After the last vaccination, splenocytes in the three vaccination groups (B. longum 420, B. longum 2012, and PBS; n = 5 per group) were isolated and re-stimulated with mitomycin C-treated-C1498-WT1 cells for 48 h in vitro. The frequencies of a IFN-γ-producing CD4+T, b IL-2-producing CD4+T, c TNF-α-producing CD4+T cells, d IFN-γ-producing CD8+T, e IL-2-producing CD8+T, and f TNF-α-producing CD8+T were detected by ICCS (*p < 0.05). Each data point represents the average of each group (bars, ±SE)
B. longum 420 oral vaccination induced WT1 T cell epitope-specific CD8+T cells
We investigated the frequency of WT1 CD8+T epitope-specific CTLs responsible for WT1-specific anti-tumor immunity using a tetramer assay. Figure 6a shows that the frequency of CD8+T cells responding to the H-2Db-restricted WT1 peptide (RMFPNAPYL) was significantly higher in B. longum 420-immunized mouse splenocytes compared with the other groups (p < 0.05). B. longum 420 induced WT1-specific CD8+T cells which responded in a MHC-class I-restricted manner, even though this oral vaccine carries a nearly full-length WT1 protein.
Fig. 6.
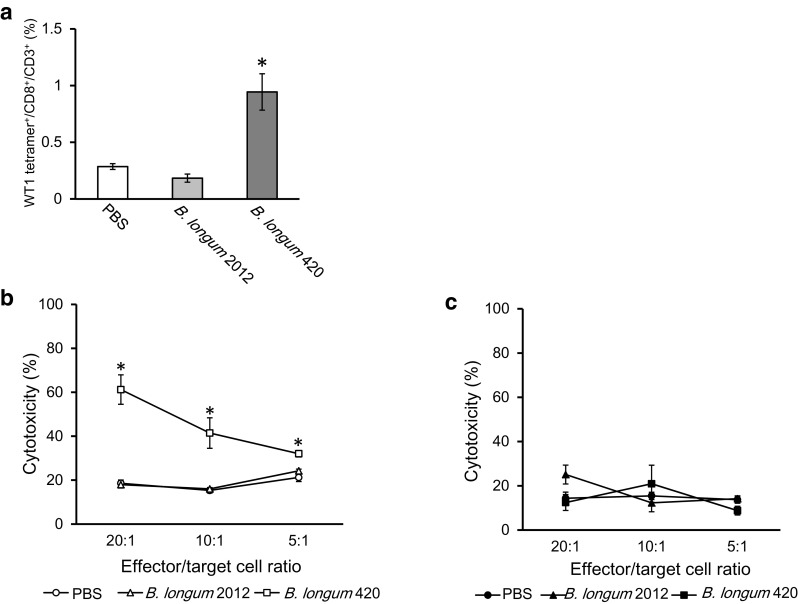
Detection of WT1-specific CTLs by tetramer assay and CTL assay. After the last vaccination, splenocytes in the three vaccination groups (B. longum 420, B. longum 2012, and PBS; n = 5 per group) were isolated and re-stumulated with mitomycin C-treated C1498-WT1 cells for 7 days in vitro. a The frequency of WT1 T-cell epitope-specific CD8+T cells was determined by H-2Db tetramer (*p < 0.05). For CTL assay, 6 days re-stimulated splenocytes (n = 5 per group) were co-cultured with target cells (C1498-WT1 or C1498-mock) for 8 h at ratios of 20:1, 10:1, and 5:1. Cytotoxicity of effector cells against b C1498-WT1 and c C1498-mock cells, respectively (*p < 0.05). Each data point represents the average of each group (bars, ±SE)
B. longum 420 oral vaccination induced the highest WT1-specific CTL activity in vitro
To examine WT1-specific CTL activity, isolated splenocytes from immunized mice were cultured in vitro to generate effector cells, followed by a CTL assay against WT1-expressing tumor cells (C1498-WT1). The WT1-specific CTL activities of each effector/target ratio were significantly stronger in the B. longum 420 group after 8 h of co-culturing the effector splenocytes and the target C1498-WT1 cells (Fig. 6b. p < 0.05). In contrast, CTL activities were at background level against the C1498-mock cells (Fig. 6c). These results indicated that multiple oral administrations of B. longum 420 induced functional WT1-specific CTL activity.
Discussion
The future trend in cancer immunotherapy is towards combined approaches using multiple types of immunotherapies targeting different intervention points. This includes tumor-specific CTLs induced by tumor vaccines or transduced by adaptive T cell therapy, enhanced by combination with immune checkpoint inhibitors [2]. The development of novel cancer vaccines is highly desirable to establish an efficient combination immunotherapy with immune checkpoint blockades, such as PD-1 and CTLA4 antibodies. We recently developed an oral vaccine platform using Bifidobacterium [12, 13], which can efficiently deliver TAAs to mucosa-associated lymphoid tissue (MALT) through microfold cells of the intestinal epithelium. Bifidobacterium also has a self-adjuvant effect via TLR-9 stimulation by unmethylated CpG, producing proinflammatory cytokines and promoting the Th1 response [30].
Currently, Lactobacillus-based oral vaccines are being studied since previous studies reported that oral vaccination with recombinant Lactobacillus casei expressing HPV-E7 protein activated antigen-specific mucosal T cell immunity in mice [31] and had some clinical responses in cervical intraepithelial neoplasia patients [10]. Both lactobacilli and bifidobacteria are Gram-positive bacilli producing lactic acid, and have similar immunomodulatory properties in the mammalian mucosal immune system [32]. Orally administered Lactobacillus or Bifidobacterium expressing TAAs is digested by mucosal DCs, which can sample and process the TAAs and interact with T and B cells in the Peyer’s patches to induce TAA-specific immunity [13, 33]. The TAA-specific immunity induced by an oral mucosal vaccine may systemically reach tumors located at mucosal sites (lung, colorectal, genital, head and neck, etc.), which are exposed to mucosal immunity in the MALT network [34]. Reportedly, Bifidobacterium alone enhanced the anti-tumor effect of PD-L1-specific antibody therapy [35]. These findings support our hypothesis that oral vaccination of B. longum displaying WT1 protein induces WT1 specific cellular immunity in humans, and could be a crucial component of novel combination immunotherapies.
In the present study, we generated recombinant B. longum 420 displaying murine-WT1 protein containing known CD4+T and CD8+T cell epitopes [24–26] as an oral cancer vaccine, and confirmed the expression of WT1 protein (Fig. 1). Although we used murine WT1 gene to generate the B. longum 420, the homology with human WT1 is quite high (96% at the amino acid level) and WT1 has similar tissue distribution and function [25]. Several previous studies have used murine models to demonstrate high cross-reactivity between human and murine-WT1 T cell-epitopes as an immunogen [26, 36]. Our oral cancer vaccine also contains a major component of the WT1 protein and so can be sampled and processed predominantly by DCs, which can then present the peptides from the WT1 protein as immune epitopes according to the HLA subtype of individual patients. Theoretically, this oral cancer vaccine may have the advantage of non-HLA restriction and be capable of activating both CD8+T and CD4+T cells specific to WT1 protein.
Our animal study demonstrated that the oral administration of B. longum 420 significantly inhibited the growth of C1498-WT1 tumor (Fig. 2a) and significantly prolonged survival compared with controls (Fig. 2b), while B. longum 420 had no antitumor effect on C1498-mock tumor not expressing WT1 (Fig. 2c). In addition, B. longum 420 showed no severe adverse effects or toxicity. Immunohistochemistry revealed that B. longum 420 remarkably induced CD4+T and CD8+T cell recruitment and infiltration to the tumor tissues, where these T cells had important anti-tumor effects (Fig. 3). Most cancer vaccines, including peptide- and DC-based vaccines, require immune adjuvants or immune augmentation by cytokines such as interleukin and interferon to induce a significant anti-tumor effect [28, 37], and such injection vaccines generally cause erythema at the injection site [21], but our oral WT1 vaccine alone achieved significant tumor-inhibitory effects in WT1-expressing tumors without the need for adjuvants or cytokines, and with fewer side effects.
We also demonstrated the induction of strong WT1-specific cellular immune responses by our novel oral cancer vaccine. B. longum 420 significantly increased WT1-specific production of IFN-γ, IL-2, and TNF-α in the immunized mouse splenocytes, compared with control treatments (Fig. 4). ICCS also showed that our oral vaccine significantly increased the frequency with which CD4+T and CD8+T cells produced these cytokines, compared with controls (Fig. 5). IFN-γ and IL-2 are representative Th1 cytokines, and TNF-α which is especially secreted by CTLs is known to play an important role in the direct anti-tumor activity of cellular immunity [38, 39]. These results support the idea that B. longum 420 activates WT1-specific cellular immunity in vivo.
We also demonstrated that B. longum 420 significantly induced WT1 murine-CD8+T cell epitope (126–134 aa; RMFPNAPYL) specific CTLs, the representative CTL epitope of WT1. (Fig. 6a). This finding strongly indicated that the WT1 protein in our oral vaccine was sampled and processed to a certain epitope by DCs in MALT, and that the DCs presenting the processed WT1 peptide induced WT1-epitope-specific CTLs. Importantly, since RMFPNAPYL-peptide is a common epitope and highly cross-reactive in both murine H-2Db and human HLA-A *0201 [24, 25], it is widely used in current WT1 peptide-/DC-based vaccinations for cancer treatment. A number of clinical studies revealed that induction of RMFPNAPYL-specific CTLs significantly correlates with tumor regression in patients [23, 37]. We also found that splenocytes from mice vaccinated with B. longum 420 showed significantly higher WT1-spefic cytotoxicity against C1498-WT1 cells compared with the other treatment groups in vitro (Fig. 6b, c). Taken together, these results indicated that our oral cancer vaccine could induce WT1-specific CTLs and that these CTLs had high cytotoxicity against WT1-expressing tumor cells.
In conclusion, we successfully developed a novel oral cancer vaccine displaying WT1 protein. Our oral vaccine induced WT1-specific cellular immunity and a strong anti-tumor effect against WT1-expressing tumors with no severe side effects in a mouse experimental model. Our novel oral cancer vaccine has the practical advantages of an oral preparation, and represents a valuable step towards a new generation of combinational cancer immunotherapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 26461503.
Abbreviations
- CAR
Chimeric antigen receptor
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- FCM
Flow cytometry
- GAM
Gifu Anaerobic Medium
- GLBP
Galacto-N-biose/lacto-N-biose I binding protein
- HLA
Human leukocyte antigen
- ICCS
Intracellular cytokine staining
- IFN
Interferon
- IL
Interleukin
- MALT
Mucosa-associated lymphoid tissue
- MHC
Major histocompatibility complex
- RT
Room temperature
- TAA
Tumor associated antigen
- Th1
T helper type 1
- TNF
Tumor necrosis factor
- WT1
Wilms’ tumor 1
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All aspects of the experimental design and procedures involving animals were reviewed and approved by the institutional ethics and animal welfare committees of the Kobe University Graduate School of Medicine.
Footnotes
Note on previous publication:
American Society of Gene & Cell Therapy 19th Annual Meeting (ASGCT), May 5, 2016, Washington DC, USA, Abstract #395: Molecular Therapy (2016); 24 Supplement 1, S157. doi:10.1038/mt.2016.78.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaux P, Luiten R, Demotte N, Vantomme V, Stroobant V, Traversari C, Russo V, Schultz E, Cornelis GR, Boon T, van der Bruggen P. Identification of five MAGE-A1 epitopes recognized by cytolytic T lymphocytes obtained by in vitro stimulation with dendritic cells transduced with MAGE-A1. J Immunol. 1999;163:2928–2936. [PubMed] [Google Scholar]
- 5.Butterfield LH (2015) Cancer vaccines. BMJ 350:h988 [DOI] [PMC free article] [PubMed]
- 6.Anguille S, Smits EL, Bryant C, Van Acker HH, Goossens H, Lion E, Fromm PD, Hart DN, Van Tendeloo VF, Berneman ZN. Dendritic cells as pharmacological tools for cancer immunotherapy. Pharmacol Rev. 2015;67:731–753. doi: 10.1124/pr.114.009456. [DOI] [PubMed] [Google Scholar]
- 7.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 8.Bloy N, Buqué A, Aranda F, Castoldi F, Eggermont A, Cremer I, Sautès-Fridman C, Fucikova J, Galon J, Spisek R, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Naked and vectored DNA-based anticancer vaccines. Oncoimmunology. 2015;4:e1026531. doi: 10.1080/2162402X.2015.1026531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallel H, Kamen AA. Large-scale adenovirus and poxvirus-vectored vaccine manufacturing to enable clinical trials. Biotechnol J. 2015;10:741–747. doi: 10.1002/biot.201400390. [DOI] [PubMed] [Google Scholar]
- 10.Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, Nishida H, Nagasaka K, Arimoto T, Yokoyama T, Wada-Hiraike O, Oda K, Sewaki T, Osuga Y, Fujii T. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine. 2014;32:6233–6239. doi: 10.1016/j.vaccine.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Mobergslien A, Vasovic V, Mathiesen G, Fredriksen L, Westby P, Eijsink VG, Peng Q, Sioud M. Recombinant Lactobacillus plantarum induces immune responses to cancer testis antigen NY-ESO-1 and maturation of dendritic cells. Hum Vaccin Immunother. 2015;11:2664–2673. doi: 10.1080/21645515.2015.1056952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto S, Wada J, Katayama T, Jikimoto T, Nakamura M, Kinoshita S, Lee KM, Kawabata M, Shirakawa T. Genetically modified Bifidobacterium displaying Salmonella-antigen protects mice from lethal challenge of Salmonella Typhimurium in a murine typhoid fever model. Vaccine. 2010;28:6684–6691. doi: 10.1016/j.vaccine.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Takei S, Omoto C, Kitagawa K, Morishita N, Katayama T, Shigemura K, Fujisawa M, Kawabata M, Hotta H, Shirakawa T. Oral administration of genetically modified Bifidobacterium displaying HCV-NS3 multi-epitope fusion protein could induce an HCV-NS3-specific systemic immune response in mice. Vaccine. 2014;32:3066–3074. doi: 10.1016/j.vaccine.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol. 2008;125:286–292. doi: 10.1016/j.ijfoodmicro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 15.López P, Gueimonde M, Margolles A, Suárez A. Distinct Bifidobacterium strains drive different immune responses in vitro. Int J Food Microbiol. 2010;138:157–165. doi: 10.1016/j.ijfoodmicro.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell. 1990;60:509–552. doi: 10.1016/0092-8674(90)90601-A. [DOI] [PubMed] [Google Scholar]
- 17.Scharnhorst V, van der Eb AJ, Jochemsen AG. WT1 proteins: functions in growth and differentiation. Gene. 2001;273:141–161. doi: 10.1016/S0378-1119(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21:868–876. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Sugiyama H, Ogawa H, Nakagawa M, Yamagami T, Miwa H, Kita K, Hiraoka A, Masaoka T, Nasu K, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84:3071–3079. [PubMed] [Google Scholar]
- 20.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashii Y, Sato E, Ohta H, Oka Y, Sugiyama H, Ozono K. WT1 peptide immunotherapy for cancer in children and young adults. Pediatr Blood Cancer. 2010;55:352–355. doi: 10.1002/pbc.22522. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, Homma S, Okamoto M. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195–205. doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 23.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E, Scheibenbogen C. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113:6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 24.Oka Y, Udaka K, Tsuboi A, Elisseeva OA, Ogawa H, Aozasa K, Kishimoto T, Sugiyama H. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol. 2000;164:1873–1880. doi: 10.4049/jimmunol.164.4.1873. [DOI] [PubMed] [Google Scholar]
- 25.Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96:1480–1489. [PubMed] [Google Scholar]
- 26.Kobayashi H, Nagato T, Aoki N, Sato K, Kimura S, Tateno M, Celis E. Defining MHC class II T helper epitopes for WT1 tumor antigen. Cancer Immunol Immunother. 2006;55:850–860. doi: 10.1007/s00262-005-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poo H, Pyo HM, Lee TY, Yoon SW, Lee JS, Kim CJ, Sung MH, Lee SH. Oral administration of human papillomavirus type 16 E7 displayed on Lactobacillus casei induces E7-specific antitumor effects in C57/BL6 mice. Int J Cancer. 2006;119:1702–1709. doi: 10.1002/ijc.22035. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima H, Oka Y, Tsuboi A, Tatsumi N, Yamamoto Y, Fujiki F, Li Z, Murao A, Morimoto S, Hosen N, Shirakata T, Nishida S, Kawase I, Isaka Y, Oji Y, Sugiyama H. Enhanced tumor immunity of WT1 peptide vaccination by interferon-β administration. Vaccine. 2012;30:722–729. doi: 10.1016/j.vaccine.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 29.Frelin L, Ahlén G, Alheim M, Weiland O, Barnfield C, Liljeström P, Sällberg M. Codon optimization and mRNA amplification effectively enhances the immunogenicity of the hepatitis C virus nonstructural 3/4 A gene. Gene Ther. 2004;11:522–533. doi: 10.1038/sj.gt.3302184. [DOI] [PubMed] [Google Scholar]
- 30.Jiang W, Pisetsky DS. Enhancing immunogenicity by CpG DNA. Curr Opin Mol Ther. 2003;5:180–185. [PubMed] [Google Scholar]
- 31.Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, Tomio K, Kojima S, Oda K, Sewaki T, Yasugi T, Kozuma S, Taketani Y. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine. 2010;28:2810–2817. doi: 10.1016/j.vaccine.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Vlasova AN, Kandasamy S, Chattha KS, Rajashekara G, Saif LJ. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol. 2016;172:72–84. doi: 10.1016/j.vetimm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hervouet C, Luci C, Bekri S, Juhel T, Bihl F, Braud VM, Czerkinsky C, Anjuère F. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal Immunol. 2014;7:280–291. doi: 10.1038/mi.2013.45. [DOI] [PubMed] [Google Scholar]
- 35.Sivan A, Corrales L, Hubert N, Williams JB, Michaels KA, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osada T, Woo CY, McKinney M, Yang XY, Lei G, Labreche HG, Hartman ZC, Niedzwiecki D, Chao N, Amalfitano A, Morse MA, Lyerly HK, Clay TM. Induction of Wilms’ tumor protein (WT1)-specific antitumor immunity using a truncated WT1-expressing adenovirus vaccine. Clin Cancer Res. 2009;15:2789–2796. doi: 10.1158/1078-0432.CCR-08-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oka Y, Tsuboi A, Oji Y, Kawase I, Sugiyama H. WT1 peptide vaccine for the treatment of cancer. Curr Opin Immunol. 2008;20:211–220. doi: 10.1016/j.coi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, White DE, Steinberg SM, Rosenberg SA. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


