Abstract
The effectiveness of attenuated Salmonella in inhibiting tumor growth has been demonstrated in many therapeutic models, but the precise mechanisms remain incompletely understood. In this study, we show that the anti-tumor capacity of Salmonella depends on a functional MyD88-TLR pathway and is independent of adaptive immune responses. Since myeloid suppressor cells play a critical role in tumor growth, we investigated the consequences of Salmonella treatment on myeloid cell recruitment, phenotypic characteristics, and functional activation in spleen and tumor tissue of B16.F1 melanoma-bearing mice. Salmonella treatment led to increased accumulation of splenic and intratumoral CD11b+Gr-1+ myeloid cells, exhibiting significantly increased expression of various activation markers such as MHC class II, costimulatory molecules, and Sca-1/Ly6A proteins. Gene expression analysis showed that Salmonella treatment induced expression of iNOS, arginase-1 (ARG1), and IFN-γ in the spleen, but down-regulated IL-4 and TGF-β. Within the tumor, expression of iNOS, IFN-γ, and S100A9 was markedly increased, but ARG1, IL-4, TGF-β, and VEGF were inhibited. Functionally, splenic CD11b+ cells maintained their suppressive capacity following Salmonella treatment, but intratumoral myeloid cells had significantly reduced suppressive capacity. Our findings demonstrate that administration of attenuated Salmonella leads to phenotypic and functional maturation of intratumoral myeloid cells making them less suppressive and hence enhancing the host’s anti-tumor immune response. Modalities that inhibit myeloid suppressor cells may be useful adjuncts in cancer immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1543-x) contains supplementary material, which is available to authorized users.
Keywords: Myeloid suppressor cells, Salmonella, Tumor immunity, Macrophages
Introduction
Tumor development is accompanied by a peculiar alteration of hematopoiesis that selectively expands myelomonocytic cells and leads to their progressive accumulation at the tumor site, bone marrow, blood, and peripheral lymphoid organs [1, 2]. The accrual of myeloid cells, including tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), within tumors leads to the suppression of anti-tumor activity of CTLs and NK cells, promotes angiogenesis and tumor cell metastasis and correlates with poor prognosis [1, 3–7]. These findings highlight the central role played by macrophages in tumor growth and metastasis and in regulating the host’s anti-tumor responses, providing new targets for cancer immunotherapy. Interestingly, MDSCs cells share similar phenotypic and functional characteristics with myeloid suppressor cells first shown to be induced by Salmonella infection [8, 9] and subsequently observed in a variety of bacterial, fungal, and parasitic infections [10, 11].
Different subpopulations of myeloid cells are involved in tumor initiation, progression, and metastasis. Myeloid cells with a phenotype resembling classically activated, or M1, macrophages are associated with cancer promotion at the initial phases of development [12]. As tumors become gradually more established and progressively malignant, macrophage phenotype is changed to M2 trophic, or “alternatively activated” macrophages, a characteristic of TAMs. M2 macrophages have reduced inflammatory signature, which includes decreased iNOS and TNF-α expression, due to the dominant inhibition of NFκB [13]. Functionally, M2 macrophages play a central role in promoting angiogenesis and supporting the progressive growth and malignant transformation of tumors [14]. TAMs, which are recruited to tumors via hypoxia and growth factors such as CSF, are a major source of angiogenic molecules, including VEGF, and their depletion leads to retardation of tumor growth [15, 16]. Moreover, these macrophages are critical for the establishment of an immunoregulatory microenvironment that further protects against host anti-tumor immune attack.
MDSCs represent another immunosuppressive myeloid population within the tumor milieu. Phenotypically, murine MDSCs are characterized by expression of CD11b and Gr-1 surface proteins, relative immaturity, and reduced expression of mature myeloid markers. They include precursors of macrophages and granulocytes, and hence, the two recognized subpopulations of MDSCs are known as monocytic or granulocytic MDSCs. MDSCs secrete high levels of reactive oxygen and nitrogen species and suppress immune responses through the production of arginase-1 (ARG1), IL-10, and TGF-β [3]. Given their heterogeneity, many factors are known to influence the recruitment and activation of MDSCs, including products of activated Th1 and NK cells (IL-2 and IFN-γ), Th2 cells (IL-4 and IL-13), and tumor cells, such as IL-1β, IL-6, CSF, VEGF, TGF-β, and prostaglandin E2 (recently reviewed in [1] ). Tumor-derived factors maintain the MDSC population in an undifferentiated state. Knockdown of STAT1 in breast tumors or enforced expression of a specific microRNA, miR-142-3p, in bone marrow impaired the generation of MDSCs and attenuated tumor progression [17, 18], thus validating the targeting of MDSCs as a viable strategy for immunotherapy.
Increased understanding of the tumor microenvironment and growth properties of facultative anaerobes, such as Salmonella species, has renewed interest in their potential use as anti-cancer agents [19, 20]. Administration of Salmonella typhimurium to tumor-bearing animals results in bacterial concentrations within tumors exceeding 109 CFUs per gram of tissue and leads to tumor regression [21, 22]. The chaotic vascularization and hypoxic conditions within tumors provide an ideal environment for the growth of anaerobic bacteria. Previously, we demonstrated the efficacy of a recombinant Salmonella strain in retarding the growth of B16.F1 tumors and enhancing host survival [23, 24]. Although the mechanism(s) by which Salmonella exert their effect remain incompletely understood, the treatment inhibited tumor angiogenesis and increased apoptosis [23].
Salmonella organisms utilize host macrophages as their primary niche for survival [25, 26]. Given the central role of macrophages in Salmonella infections and the demonstrated efficacy of Salmonella in inhibiting tumor growth, we hypothesized that this may be due to the targeting of myeloid cells within the tumors. In this report, we provide evidence that Salmonella-induced tumor retardation occurs concurrently with alterations in myeloid cells consistent with maturation to macrophage effectors and a reduction in their suppressive capacity.
Materials and methods
Cell lines, bacterial strains and mice
The melanoma cell line B16.F1 (H-2b) was maintained in RPMI with 10 % FCS and supplements, as described [27]. BRD509E, an auxotrophic mutant of S. typhimurium, was prepared as described [28]. C57BL/6 mice (Harlan Olac, Bicester, UK) were bred in the animal facility of the College of Medicine and Health Sciences. MyD88-deficient [29] and CD154-deficient mice [30] have been described [31, 32]. Athymic NMRI/nudenu/nu mice were purchased from Charles River Laboratories (Sulzfeld, Germany) and housed in filtered-air laminar flow cabinets. Male mice were used at 8–12 weeks of age for the present studies, which were carried out in accordance with, and after approval of, the Animal Research Ethics Committee of the College of Medicine and Health Sciences, UAE University.
Tumor implantation and treatment with live Salmonella
C57BL/6 or immune-deficient mice (8–10 mice per group unless otherwise indicated) were inoculated s.c. in the right flank with 2 × 105 B16.F1 cells and staged to day 12–14, as described [23]. Tumor growth was followed by quantitative determination of tumor volume, measured as the product of the perpendicular diameters using digital calipers, according to the formula: volume = LxW2/2. Once tumors were established, mice were inoculated i.p. with 1–5 × 105 CFUs of BRD509E strain and followed for the subsequent 4 weeks. In some experiments, the bacterial load in spleen and tumor tissue of treated mice was quantitated as detailed previously.
Isolation of spleen and tumor cells
Tumor and spleen cells were extracted 7 days post-Salmonella treatment. Spleen cell suspensions were prepared as detailed [32]. For tumor cell preparation, excised tumors were subjected to two 15-min enzymatic digestion cycles in HBSS containing collagenase type1 (50 μg/ml), hyaluronidase (25 μg/ml), DNase1 (10 μg/ml), and Soybean trypsin inhibitor (0.2 TIU/ml) at 37 °C and filtered through a 40 μm mesh. Myeloid cells were subsequently purified from spleen or tumor cell suspensions using CD11b+ microbeads on the autoMACS separator, according to manufacturer’s protocol (Miltenyi Biotec, Germany). The purity of cell populations was evaluated by flow cytometry and exceeded 80 % (for tumor) and 90 % (for spleen).
Antibodies and flow cytometry
Analysis of total spleen or tumor cells, or CD11b+ myeloid cells was carried out using a 6-color FACS following a standard procedure [33]. Washed cells in staining buffer were incubated with anti-CD16/CD32-specific mAb for 30 min on ice to block FcγIII/II receptor sites. Cells were stained with a combination of directly conjugated mAbs, washed and analyzed using FACSCanto II (BD Biosciences, Mountain View, CA). The antibodies used were CD11b-APC-Cy7, MHC class II (I-A/I-E)-APC, and Sca1-PE-Cy7 (eBioscience, San Diego, CA), Gr1-PE, CD80-FITC, CD86-APC (BD Biosciences), and CD40-FITC (SouthernBiotech, Birmingham, AL). Non-viable cells staining positive with 7AAD dye (eBioscience) were excluded from the analysis. Data collected on 50,000 cells were analyzed using FlowJo software (TreeStar Inc, Ashland, OR).
Quantitative RT-PCR analysis
RNA was extracted by TRIzol method and repurified on Qiagen columns (RNA easy mini kit, Qiagen, Valencia, CA). The quality and quantity of the RNA was determined using the Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA). cDNA was synthesized using Taqman reverse transcription reagents (Applied Biosystems, Foster City, CA) using manufacturer’s protocol. Premade TaqMan primers and probes (Applied Biosystems) were used to study the expression of a set of genes (ARG1, iNOS, IFN-γ, IL-4, S100A9, TGF-β, IL-10, VEGF) associated with myeloid and tumor cell functions. Transcript levels of target genes were normalized according to the ΔCq method to respective mRNA levels of the housekeeping gene HPRT. The expression of the target gene is reported as the level of expression relative to HPRT.
In vitro T cell suppression assay
CD11b+ myeloid cells were isolated from spleen and tumor tissues of tumor-bearing mice, with or without treatment with the attenuated Salmonella BRD509 strain and from the spleens of non-tumor-bearing mice (control). CD4+ splenic T cells were isolated from normal spleens by magnetic sorting using anti-CD4 microbeads, and their purity confirmed to be >93 %. For the co-culture assay, CD4+ T cells (1 × 105 cells/well) were stimulated with plate-bound anti-CD3 (1 μg/ml) plus anti-CD28 (10 μg/ml) mAbs in 96-well round-bottom plate. T cells were co-cultured in the absence or presence of different CD11b+ populations (either 1 × 104 or 1 × 103 per well) for a total of 24 h. Control cultures with myeloid cells cultured alone in the presence or absence of immobilized mAbs, and purified T cells cultured in the absence of mAbs were also setup. Culture supernatants were collected at 24 h and analyzed for IFN-γ using a specific ELISA (BD Biosciences) following manufacturer’s instructions. Data are expressed as percentage suppression calculated as follows: % suppression = [1 − (IFNγ concentration of test cells/IFNγ concentration of control cells)] × 100.
Statistical analysis
Statistical significance between control and treated groups was analyzed using the unpaired, two-tailed Student’s t test, using the statistical program of GraphPad Prism software (San Diego, CA). Survival analysis was performed by Kaplan–Meier survival curves and log-rank test, using the GraphPad Prism program. Differences between experimental groups were considered significant when p values were <0.05.
Results
Treatment with attenuated Salmonella inhibits the growth of B16.F1 tumors
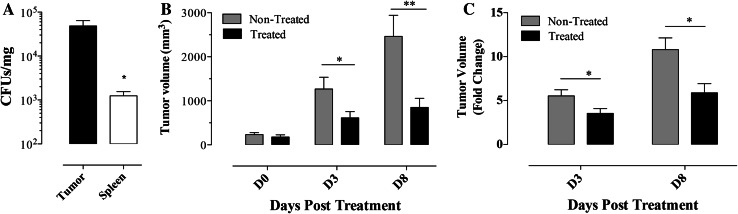
B16.F1-preimplanted C57BL/6 mice with mean tumor volume of 200–300 mm3 were injected with Salmonella strain BRD509E (“Treated” group), or with saline (“non-treated” group) and followed for 4 weeks. In agreement with previous findings [23, 24], Salmonella organisms reach significantly (p = 0.023) higher growth densities in tumors compared with their usual target organs, including spleen (Fig. 1a). Salmonella treatment resulted in a significant inhibition of tumor growth with mean tumor volumes in treated mice reaching 48.3 (p = 0.044) and 34.6 % (p = 0.007) of corresponding means of non-treated groups on days 3 and 8 post-treatment, respectively (Fig. 1b). On average, tumors in non-treated mice grew 5.5- and 10.8-fold by days 3 and 8, respectively, while the corresponding growth in BRD509E-treated animals was approximately 3.5- and 5.9-fold (p values of 0.042 and 0.013 between treated and non-treated groups on days 3 and 8, respectively) (Fig. 1c).
Fig. 1.
Inhibition of tumor growth following treatment with attenuated Salmonella organisms. a Tumor-bearing C57BL/6 mice were inoculated with Salmonella and 8 days later spleen and tumor bacterial loads were determined. Each data point represents the mean ± SEM of 4 mice per group. b Changes in tumor volumes at the indicated days post-treatment with Salmonella (Treated group) or saline (non-treated group). c The data in graph b is represented as fold change in volumes in comparison with the means for each group prior to start of treatment. Results are representative of four independent experiments. Asterisks denote statistically significant differences between tumor and spleen CFUs (**p < 0.01; *p < 0.05)
Salmonella-mediated inhibition of tumor growth and enhanced host survival are independent of adaptive immune responses
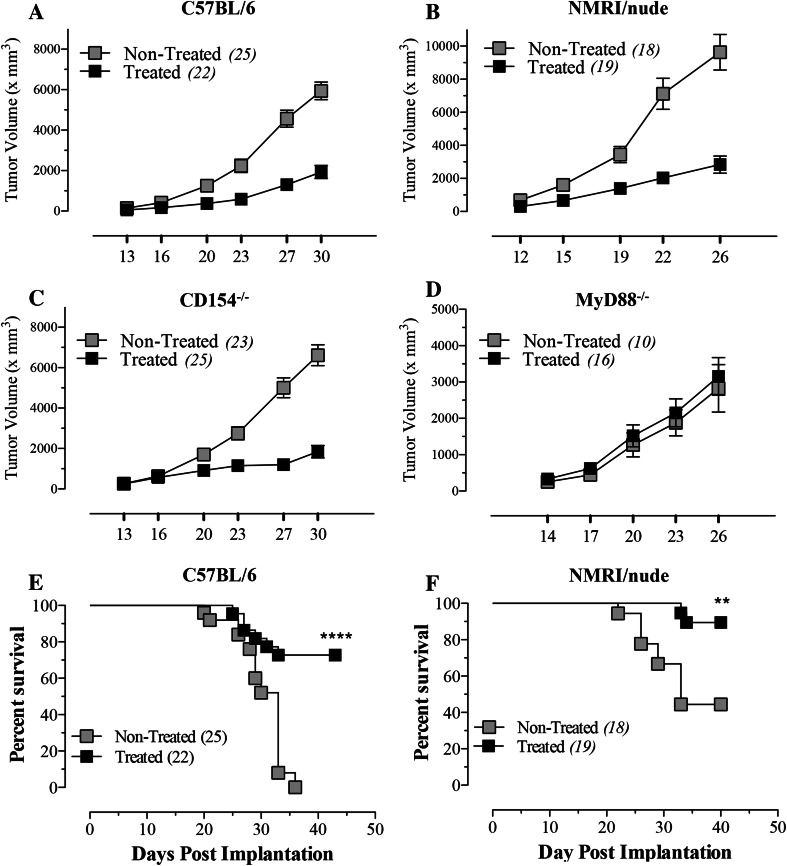
To map the immune system requirements for the observed Salmonella-mediated tumor suppression, we used three different mouse strains with varying genetic deficiencies. Athymic NMRI/nude mice are deficient in T lymphocytes. CD154−/− mice have a disrupted costimulatory pathway required for T-B cell interactions and immunoglobulin isotype switching and MyD88−/− mice lack a critical adaptor molecule required for most TLR signaling pathways. As shown in Fig. 2a–d, identical tumor inhibition was observed in C57BL/6 (Fig. 2a), NMRI/nude (Fig. 2b) and CD154−/− (Fig. 2c) mice, suggesting that Salmonella-mediated tumor inhibition is independent of T lymphocytes and T-dependent antibody responses. In contrast, tumor growth was unimpeded in MyD88−/− mice (Fig. 2d), indicating that Salmonella-induced inhibition is dependent on TLR signaling pathways, most likely in the myeloid cell compartment. Animal survival was also assessed in tumor-bearing wild-type and NMRI/nude mice following Salmonella inoculation. The data presented in Fig. 2e, f demonstrate that significant improvement in survival was equally evident in wild-type C57BL/6 (p < 0.0001) and NMRI/nude mice (p = 0.002), further confirming that this response is independent of T lymphocytes.
Fig. 2.
Salmonella-mediated inhibition of tumor growth and enhanced host survival are independent of adaptive immune responses. B16.F1 tumor growth was followed in C57BL/6 (a), athymic NMRInu/nu (b), CD154−/− (c), or MyD88−/− (d) mice either non-treated or treated with Salmonella. e, f Animal survival was also followed in Salmonella-treated C57BL/6 (e) and NMRInu/nu (f) mice for up to 6 weeks. Numbers in parenthesis denote the number of mice per group and each data point represents the mean ± SEM in the respective group, pooled from 2 to 3 individual experiments. Asterisks denote statistically significant differences between indicated groups (****p < 0.0001; **p < 0.01)
Accumulation of myeloid cells in the spleens of tumor-bearing mice following Salmonella treatment
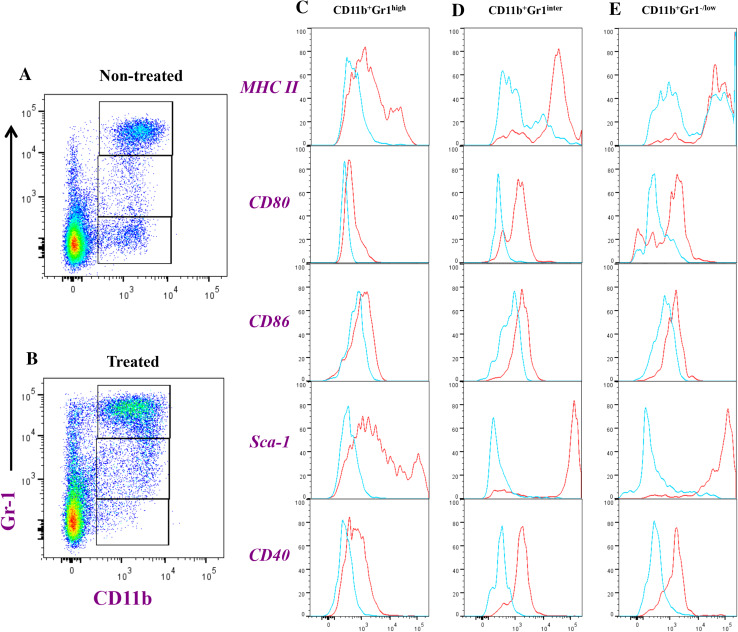
The phenotypic and functional alterations in myeloid spleen populations of tumor-bearing mice were analyzed 8 days after treatment with Salmonella. Based on staining with mAbs specific to CD11b and Gr-1, three subpopulations of myeloid cells were identified, CD11b+Gr-1H, CD11b+Gr-1Int and CD11b+Gr-1−/L (Fig. 3). The co-expression of CD11b and Gr-1 proteins defines the population of MDSCs within spleen and tumor tissues. In the spleens of non-treated animals, the mean percentages of each of these populations were 16.3 ± 1.8, 2.6 ± 0.1, and 6.2 ± 0.1, respectively. In the spleens of treated mice, the respective percentages were 29.3 ± 0.3, 6.7 ± 0.2 and 3.2 ± 0.2, which represents ~2-fold increase in the two Gr-1H and Gr-1Int myeloid populations (Supplementary Fig. S1A-C). In non-tumor-bearing animals, the corresponding percentages of the three myeloid populations were 3.3 ± 0.1, 1.4 ± 0.3, and 4.3 ± 0.3, respectively (control mice). The differences between non-treated and Salmonella-treated mice were also evident when the groups were compared in terms of absolute cell numbers. As shown in Supplementary Fig. S1D-F, the fold increase in cell number of each myeloid subpopulation in the treated group was sevenfold, tenfold, and 1.9-fold for CD11b+Gr-1H, CD11b+Gr-1Int and CD11b+Gr-1−/L cells, respectively. The Gr-1H and Gr-1Int subpopulations correspond to granulocytic and monocytic MDSCs, respectively [34].
Fig. 3.
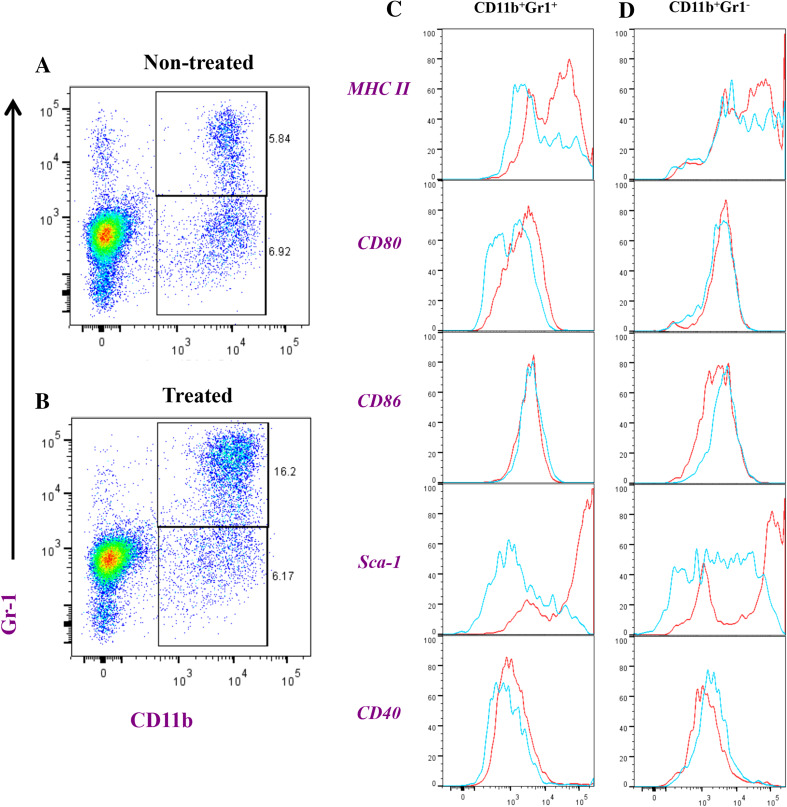
Flowcytometric analysis of splenic myeloid cells in tumor-bearing mice. Splenocytes were prepared from saline (non-treated; plot a) or Salmonella-injected mice (treated; plot b) and analyzed for CD11b and Gr-1 positivity. CD11b+Gr-1high (panel c), CD11b+Gr-1Inter (d), and CD11b+Gr-1−/low (e) were further analyzed for expression of MHC class II, CD80, CD86, Sca-1 and CD40, as indicated. The histograms depict extent of staining in cells of non-treated (blue line) or Salmonella-treated mice (red line). Each experimental group included 3 mice. Results of individual mice are shown and are representative of three independent experiments
Enhanced expression of costimulatory molecules and activation markers on splenic macrophages following Salmonella treatment
Analysis of the expression of activation/maturations markers on CD11b+Gr-1H and CD11b+Gr-1Int cells from non-treated and Salmonella-treated animals revealed a significant upregulation in MHC class II, CD80, CD86, CD40 and Sca-1 proteins in treated mice (Fig. 3 and Supplementary Fig. S2A-E). Although the increased expression of these proteins was evident in both CD11b+Gr-1H and CD11b+Gr-1Int populations, the extent of this upregulation was greater in CD11b+Gr-1Int cells. In CD11b+Gr-1H cells, Salmonella treatment led to increased expression of MHC class II (6.6-fold), CD80 (2.6-fold), CD86 (1.7-fold), CD40 (3.2-fold) and Sca-1 (61.1-fold). By contrast, the corresponding increases in CD11b+Gr-1Int cells were 9.2, 5.0, 2.2, 5.9, and 140.3-fold, respectively (Supplementary Fig. S2).
Expression of inflammatory genes is upregulated in splenic macrophages following Salmonella treatment
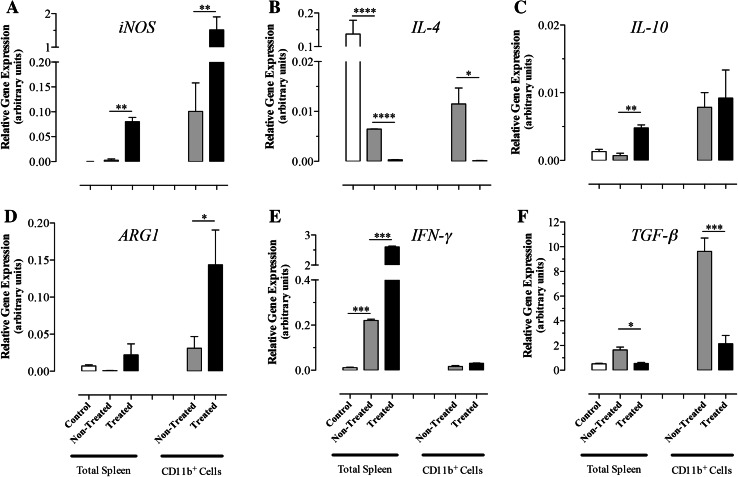
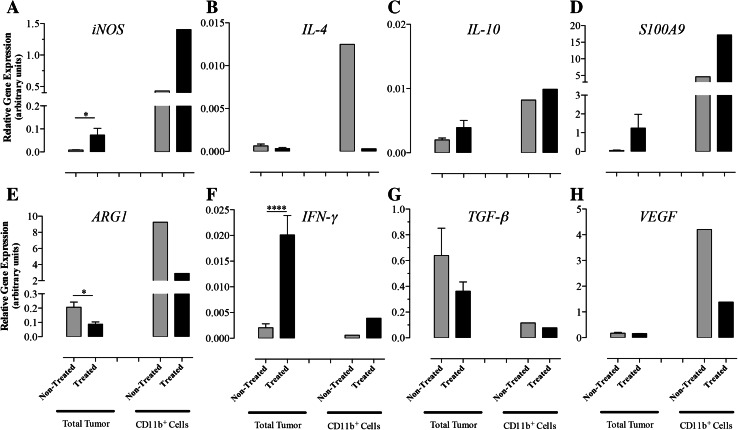
RNA was extracted from total splenocytes or CD11b+ splenic myeloid cells (>90 % purity) of non-treated or Salmonella-treated mice to assess the expression levels of iNOS, IL-4, IL-10, ARG1, IFN-γ, and TGF-β genes by qRT-PCR (Fig. 4a–f). Salmonella treatment led to a significant upregulation in iNOS (15-fold; p = 0.005) and ARG1 (4.8-fold; p = 0.017) in CD11b+ cells. In contrast, the expression of IL-4 and TGF-β was markedly reduced following Salmonella treatment (p values 0.018 and 0.0008 between non-treated and treated groups, respectively) but no significant change in IL-10 levels was observed. At the level of the whole spleen, the expression level of IL-4 was reduced by 21-fold in tumor-bearing mice compared with wild-type controls (p ≤ 0.0001) and was further inhibited (by 22-fold) after Salmonella treatment (p ≤ 0.0001). In sharp contrast, expression of IFN-γ increased by 19-fold in tumor-bearing mice compared with controls (p = 0.0008) and was further increased (by ~12-fold) upon Salmonella treatment (p = 0.0002). The induced changes in gene expression are consistent with the induction of an inflammatory response involving activated macrophages in the spleen of Salmonella-inoculated animals.
Fig. 4.
Relative expression levels of iNOS (a), IL-4 (b), IL-10 (c), ARG1 (d), IFN-γ (e), and TGF-β (f) mRNA isolated from whole spleen or splenic myeloid cells. Cells were obtained from normal (control group) or B16.F1-bearing mice at day 8 post-injection with Salmonella (treated group) or saline (non-treated group). Each data point represents the mean ± SEM of 6 (total spleens) or 3 (CD11b+ cells) mice per group. Asterisks denote statistically significant differences between the indicated groups (***p < 0.001; **p < 0.01; *p < 0.05). The results are representative of three independent experiments
Salmonella treatment induces myeloid cell recruitment and activation within the tumor tissue
The next series of experiments were focused on studying the phenotypic and functional characteristics of tumor-infiltrating cells. Staining with mAbs specific to CD11b and Gr-1 identified two myeloid populations, CD11b+Gr-1+ and CD11b+Gr-1− cells (Fig. 5a). Treatment with Salmonella resulted in >3-fold expansion of the CD11b+Gr-1+ cells but not CD11b+Gr-1− population (Fig. 5a, b and Supplementary Fig. S3A-B). Analysis of the relative expression of activation markers on intratumoral cell populations demonstrated that Salmonella treatment led to an enhancement in the expression of several proteins, particularly on CD11b+Gr-1+ cells, including MHC class II (4.8-fold increase), CD80 (twofold) and Sca-1 (53.6-fold) (Fig. 5c, d and Supplementary Fig. S3C-E).
Fig. 5.
Flowcytometric analysis of intratumoral myeloid cells in B16.F1-bearing mice. Tumor-infiltrating cells were prepared 8 days post-inoculation of Salmonella (treated; plot b) or saline (non-treated; plot a) and analyzed for CD11b and Gr-1 positivity. CD11b+Gr-1+ (c) and CD11b+Gr-1− (d) were further analyzed as described in the legend of Fig. 3. Results are representative of two independent experiments
Intratumoral myeloid cells up-regulate inflammatory genes and downregulate expression of suppressive phenotype following bacterial treatment
Gene expression analysis was carried out in whole tumor cells and intratumoral CD11b+ myeloid cells (Fig. 6a–h). Treatment with Salmonella led to a significant increase in the expression of iNOS, both at the level of the whole tumor (8.9-fold; p = 0.034) and, more importantly, in CD11b+ cells (3.3-fold) (Fig. 6a). In contrast, expression of ARG1 was reduced by 2.4- and 3.2-fold in whole tumor (p = 0.031) and myeloid cells, respectively (Fig. 6e). These alterations were accompanied by a pronounced inhibition (41.7-fold) in IL-4 expression in myeloid cells and an increase (tenfold; p < 0.0001) in IFN-γ expression in tumors of Salmonella-treated animals (Fig. 6b, f). The expression of S100A9, a proinflammatory product of macrophages [35], was greatly enhanced in intratumoral CD11b+ cells of Salmonella-treated mice (Fig. 6d). Interestingly, expression of VEGF by myeloid cells was reduced by threefold following Salmonella treatment (Fig. 6h), consistent with the previously reported decrease in angiogenesis that was observed in tumors of treated animals [23]. Finally, analysis of the expression of IL-10 and TGF-β, two immunomodulatory mediators of macrophages, revealed that while IL-10 was not altered in intratumoral myeloid cells from Salmonella-treated mice (Fig. 6c), expression of TGF-β was reduced by approximately twofold, though not reaching statistical significance (Fig. 6g). This is perhaps a reflection of the complex nature of gene regulation seen in tumor tissue in the presence of viable Salmonella organisms.
Fig. 6.
Relative expression levels of iNOS (a), IL-4 (b), IL-10 (c), S100A9 (d), ARG1 (e), IFN-γ (f), and TGF-β (g) and VEGF (h) mRNA isolated from tumor-infiltrating cells or purified intratumoral myeloid cells. Tumor cells were isolated at day 8 post-inoculation with Salmonella (treated group) or saline (non-treated group). For total tumor groups, each data point represents the mean ± SEM of 3 mice. For the purified CD11b+ groups, mRNA was isolated from a pool of cells obtained from 5 to 6 tumors per group. Asterisks denote statistically significant differences between the indicated groups (***p < 0.001; *p < 0.05). The results are representative of two independent experiments
Reduced T cell suppressive capacity of intratumoral myeloid cells, but not splenic macrophages, following treatment with Salmonella
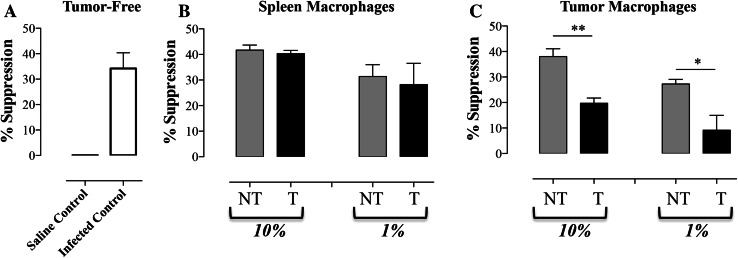
Purified myeloid cells from tumors or spleens of non-treated or Salmonella-treated mice were co-cultured at various ratios with normal splenic CD4+ T cells and stimulated with anti-CD3/CD28 mAbs. T cell responsiveness was assessed by determining the level of IFN-γ released into the culture supernatant 24 h later. As shown in Fig. 7a, addition of CD11b+ cells isolated from 7 day-infected spleens to normal T cells (at 1:10 ratio, respectively) suppressed IFN-γ production (mean of 34.2 ± 6.1 %; “infected control” group), which is in agreement with earlier studies [8, 36, 37]. As expected, no suppression of the response was observed when splenic CD11b+ cells from non-infected animals were used (Fig. 7a; “saline control” group). Next, splenic CD11b+ cells isolated from tumor-bearing mice 7 days following inoculation with Salmonella (treated or “T” groups) or saline (non-treated or “NT” groups) were used (Fig. 7b). Co-culture with splenic myeloid cells from non-treated, tumor-bearing mice at a final ratio of 10 or 1 % resulted in significant inhibition of IFN-γ secretion (41.7 ± 2.0 and 31.3 ± 4.6 %, respectively; Fig. 7b, “NT” groups). Equivalent suppression of the T cell response was observed in co-cultures with splenic CD11b+ cells isolated from Salmonella-treated, tumor-bearing animals (40.3 ± 1.3 and 28.2 ± 8.3 %, respectively; Fig. 7b, “T” groups). Finally, CD11b+ cells were isolated from the tumors of animals with or without treatment with Salmonella organisms (Fig. 7c). Co-culture with CD11b+ cells from non-treated tumors resulted in marked inhibition of T cell responsiveness (38.1 ± 3.0 and 27.3 ± 1.8 % at 10 and 1 % ratio, respectively; “NT” groups). Interestingly, however, significant loss of suppressive capacity was observed when T cells were co-cultured with intratumoral CD11b+ cells of Salmonella-treated mice (Fig. 7c; “T” groups). The degree of T cell suppression observed in these co-cultures was reduced to 19.7 ± 2.0 and 9.2 ± 5.8 % at final 10 and 1 % cell ratios, respectively. This represents a significant reduction in suppressive capacity (48.3 %; p = 0.002 and 66.3 %; p = 0.041, respectively) compared with myeloid cells of non-treated tumors (Fig. 7c, compares corresponding “NT” and “T” groups).
Fig. 7.
Reduced suppressive capacity of intratumoral myeloid cells following Salmonella treatment. a CD11b+ cells were isolated from the spleens of normal or Salmonella-infected mice and added at a final concentration of 10 % to cultures of CD4+ T cells. Alternatively, CD11b+ cells were isolated from the spleens (b) or tumors (c) of tumor-bearing mice, with or without Salmonella treatment (“NT”: non-treated, “T”: treated), and added at a final concentration of 1 or 10 % to CD4 T cells. All cultures were set up in triplicates and IFN-γ production was assayed in supernatants collected after 24 h. The data are expressed as percentage suppression in IFN-γ production compared with cultures containing stimulated T cells alone. Asterisks denote statistically significant differences between the indicated groups (**p < 0.01; *p < 0.05)
Discussion
Systemic administration of facultative anaerobic bacteria, such as S. typhimurium, to tumor-bearing mice can effectively retard tumor growth. The capacity of Salmonella strains to achieve this appears to be dependent on their ability to stimulate the host’s innate immune system. Bacterial strains with mutated lipid A or defective purine synthesis are unable to inhibit tumor growth due to poor tissue colonization [38]. The preferential targeting of Salmonella organisms to tumors is facilitated by TNF-α-mediated increase in intratumoral blood vessel permeability and the ability of Salmonella to respond to chemoattractants released by dying tumor cells [39, 40]. Being motile, Salmonella organisms invade tumor tissue efficiently and are able to reach deep into poorly vascularized regions. Moreover, the hypoxic conditions, which represent a major hindrance to conventional radiotherapy and chemotherapy, provide ample environment for the growth and proliferation of Salmonella organisms.
Once in the tumor, Salmonella organisms were shown to be inherently cytotoxic to a variety of tumor cell types, causing the tumors to regress [19]. The capacity of Salmonella to induce the host’s innate immune responses, such as infiltration of tumor tissue by inflammatory cells and secretion of proinflammatory cytokines, is partly responsible for their anti-cancer properties [41, 42]. Salmonella could also increase accessibility of tumor tissue to innate immune cells, including DCs, by upregulating the expression of connexin 43 by tumor cells, a key cellular protein involved in the formation of gap junctions [43]. The increase in gap junction formation leads to enhanced cross-presentation of tumor antigens and hence more effective anti-tumor T cell responses. Thus, cancer treatment with oncolytic Salmonella organisms relies not only on the inherent anti-tumor properties of the bacteria but also is greatly aided by cooperation with the host’s immune system. Our findings with a number of different immune-deficient mouse strains suggest that the effectiveness of Salmonella anti-tumor treatment relies mostly on the induction of innate immune responses through the TLR-MyD88 signaling pathway. This is consistent with a previous report demonstrating that Salmonella treatment of tumor-bearing mice is rather ineffective in the absence of TLR4, a receptor for the innate recognition of Gram-negative bacterial LPS by macrophages and neutrophils [42].
Since immune responses within the tumor microenvironment are largely regulated by myeloid suppressor cells, we reasoned that Salmonella treatment may also target these cells. In the present study, we evaluated the capacity of attenuated Salmonella to influence the phenotype and functional activity of myeloid suppressor cells in mice with established melanoma. Our findings demonstrate an apparent dichotomy in the effect of treatment on myeloid cells in the spleen and tumor tissue. Thus, while the systemic treatment of tumor-bearing hosts with Salmonella leads to increased frequency of classically activated, proinflammatory, CD11b+/Gr-1+ myeloid cells in both spleen and tumor, reversal of the suppressive functional capacity was only observed in intratumoral myeloid cells. This suggests that splenic and intratumoral myeloid cells are differentially regulated by Salmonella organisms.
Infection with attenuated Salmonella induces a transient state of splenomegaly that normalizes over a period of 4 weeks as the infection is resolved by the host’s immune system [32, 44]. Splenomegaly is associated with a large influx of inflammatory cells, largely made up of neutrophils and macrophages, into the spleen of infected animals. Systemic administration of attenuated Salmonella is also associated with the induction of IL-12, IL-18, IFN-γ, TNF-α, iNOS and reactive oxygen intermediates within 24–48 h of inoculation, a process that helps to limit intracellular bacterial proliferation and ultimately leads to the development of a protective Th1-mediated immune response [32, 45]. Curiously, however, Salmonella infection also leads to the emergence of nitric oxide-secreting myeloid cells with suppressive capacity [8, 36, 37]. Given these effects on the immune system, the influence of Salmonella organisms on anti-tumor immune responses is likely to be quite complex.
Treatment with attenuated Salmonella was associated with increased cell ratios of both granulocytic (CD11b+/Gr-1H) and monocytic (CD11b+/Gr-1Int) MDSCs within the spleen as well as an increase in CD11b+/Gr-1+ myeloid cells within the tumor tissue. Salmonella treatment led to an increase in the expression of several IFNγ-regulated proteins, such as MHC class II and costimulatory molecules, which are known to enhance antigen-presentation of myeloid cells. These alterations correlated with a significant enhancement in IFN-γ expression in both spleen and tumor tissue. By contrast, expression of IL-4 was inhibited in both splenic and intratumoral myeloid cells following treatment. A notable exception to this co-regulation was the differential effect of Salmonella treatment on ARG1 expression. While the expression of ARG1 was substantially decreased in intratumoral myeloid cells after Salmonella treatment, it was increased in splenic myeloid cells (compare Figs. 4d, 6e). ARG1 acts to hydrolyze l-arginine to urea and ornithine, ultimately leading to the production of reactive nitrogen intermediates, such as peroxynitrites, which is a potent inhibitor of T cell responses [46]. Given the fact that, within the tumor microenvironment, ARG1 expression is coordinated by IL-4/IL-13 [47], it is perhaps not surprising that Salmonella treatment also resulted in a dramatic loss of IL-4 expression in intratumoral myeloid cells. In contrast, expression of iNOS in intratumoral myeloid cells was increased after Salmonella treatment. Therefore, our current findings suggest that Salmonella-mediated inhibition of ARG1 expression in tumor myeloid cells appears to be mainly responsible for the loss of their T cell suppressive capacity. This is consistent with the view that ARG1 expression is the prototypical marker for defining tumor-associated suppressor macrophages [4]. Importantly, our findings also highlight the IL-4-IL-13/ARG1 axis as a target of Salmonella treatment in cancer immunotherapy. A recent report demonstrated that intratumoral injection of attenuated Salmonella bacteria also results in increased percentage of CD11b+Gr-1+ myeloid cells [48]. These cells were shown to secrete TNF-α, but their functional suppressive activity was not directly assessed.
A previous report demonstrated that treatment with attenuated Salmonella choleraesuis led to an inhibition of tumor growth that was associated with an upregulation in the expression of IFN-γ and IFN-γ-dependent chemokines CXCL9 and CXCL10 [42]. This response was dependent on the expression of a functional TLR4 protein, suggesting that the host immune response to LPS plays a critical role in this process. Moreover, inhibition of tumor growth correlated with decreased angiogenesis and increased level of apoptosis within the tumor tissue. Importantly, CXCL9 and CXCL10 chemokines are known to induce cell recruitment into tumor tissue and to exert potent anti-angiogenic activity [49, 50]. The previously reported reduction in microvessel density [23] and the current findings of decreased VEGF expression in macrophages of Salmonella-treated animals suggest an inhibition in the activity of tumor-associated angiogenic macrophages. Taken together, our data identify MDSCs and TAMs as targets of treatment with Salmonella, thereby providing another mechanism by which Salmonella organisms mediate their anti-tumor effects. The data also highlight the potential of utilizing this pathway in boosting immune responses to tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Toby Eisenstein (Temple University School of Medicine, Philadelphia, USA) for critical review of the manuscript. We are grateful to Dr. Samir Attoub (Department of Pharmacology, College of Medicine & Health Sciences, United Arab Emirates University) for providing us with the NMRI/nude mice. We also thank Arshad Khan for animal care and husbandry. This work was funded by grants from the Terry Fox Fund for Cancer Research and the UAE University-NRF (to B. K. al-Ramadi).
Conflict of interest
The authors declare no competing interests.
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 3.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144(3):250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, Rosol TJ, Fernandez S, Huang K, Leone G, Ostrowski MC. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70(4):1323–1333. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.al-Ramadi BK, Brodkin MA, Mosser DM, Eisenstein TK. Immunosuppression induced by attenuated Salmonella. Evidence for mediation by macrophage precursors. J Immunol. 1991;146(8):2737–2746. [PubMed] [Google Scholar]
- 9.al-Ramadi BK, Chen YW, Meissler JJ, Jr, Eisenstein TK. Immunosuppression induced by attenuated Salmonella. Reversal by IL-4. J Immunol. 1991;147(6):1954–1961. [PubMed] [Google Scholar]
- 10.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207(7):1453–1464. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Ginderachter JA, Beschin A, De Baetselier P, Raes G. Myeloid-derived suppressor cells in parasitic infections. Eur J Immunol. 2010;40(11):2976–2985. doi: 10.1002/eji.201040911. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66(23):11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 14.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hix LM, Karavitis J, Khan MW, Shi YH, Khazaie K, Zhang M. Tumor STAT1 transcription factor activity enhances breast tumor growth and immune suppression mediated by myeloid-derived suppressor cells. J Biol Chem. 2013;288(17):11676–11688. doi: 10.1074/jbc.M112.441402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonda N, Simonato F, Peranzoni E, Cali B, Bortoluzzi S, Bisognin A, Wang E, Marincola FM, Naldini L, Gentner B, Trautwein C, Sackett SD, Zanovello P, Molon B, Bronte V. miR-142-3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity. 2013;38(6):1236–1249. doi: 10.1016/j.immuni.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10(11):785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CH. Engineering bacteria toward tumor targeting for cancer treatment: current state and perspectives. Appl Microbiol Biotechnol. 2012;93(2):517–523. doi: 10.1007/s00253-011-3695-3. [DOI] [PubMed] [Google Scholar]
- 21.Eisenstein TK, Bushnell B, Meissler JJ, Jr, Dalal N, Schafer R, Havas HF. Immunotherapy of a plasmacytoma with attenuated Salmonella. Med Oncol. 1995;12(2):103–108. doi: 10.1007/BF01676710. [DOI] [PubMed] [Google Scholar]
- 22.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 23.al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H, Al-Salam S, Bashir G, Chouaib S. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin Immunol. 2009;130(1):89–97. doi: 10.1016/j.clim.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 24.al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H, Al-Salam S, Attoub S, Xu D, Chouaib S. Attenuated bacteria as effectors in cancer immunotherapy. Ann N Y Acad Sci. 2008;1138:351–357. doi: 10.1196/annals.1414.036. [DOI] [PubMed] [Google Scholar]
- 25.Richter-Dahlfors A, Buchan AMJ, Finlay BB. Murine Salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.al-Ramadi BK, Adeghate E, Mustafa N, Ponery AS, Fernandez-Cabezudo MJ. Cytokine expression by attenuated intracellular bacteria regulates the immune response to infection: the Salmonella model. Mol Immunol. 2002;38(12–13):931–940. doi: 10.1016/S0161-5890(02)00020-2. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Cabezudo MJ, El-Kharrag R, Torab F, Bashir G, George JA, El-Taji H, al-Ramadi BK. Intravenous administration of manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS One. 2013;8(2):e55993. doi: 10.1371/journal.pone.0055993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.al-Ramadi BK, Bashir G, Rizvi TA, Fernandez-Cabezudo MJ. Poor survival but high immunogenicity of IL-2-expressing Salmonella typhimurium in inherently resistant mice. Microbes Infect. 2004;6(4):350–359. doi: 10.1016/j.micinf.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/S1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 31.Issac JM, Sarawathiamma D, Al-Ketbi MI, Azimullah S, Al-Ojali SM, Mohamed YA, Flavell RA, Fernandez-Cabezudo MJ, al-Ramadi BK. Differential outcome of infection with attenuated Salmonella in MyD88-deficient mice is dependent on the route of administration. Immunobiology. 2013;218(1):52–63. doi: 10.1016/j.imbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 32.al-Ramadi BK, Fernandez-Cabezudo MJ, Ullah A, El-Hasasna H, Flavell RA. CD154 is essential for protective immunity in experimental salmonella infection: evidence for a dual role in innate and adaptive immune responses. J Immunol. 2006;176(1):496–506. doi: 10.4049/jimmunol.176.1.496. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Cabezudo MJ, Mechkarska M, Azimullah S, al-Ramadi BK. Modulation of macrophage proinflammatory functions by cytokine-expressing Salmonella vectors. Clin Immunol. 2009;130(1):51–60. doi: 10.1016/j.clim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 35.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86(3):557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 36.al-Ramadi BK, Greene JM, Meissler JJ, Jr, Eisenstein TK. Immunosuppression induced by attenuated Salmonella: effect of LPS responsiveness on development of suppression. Microb Pathog. 1992;12(4):267–278. doi: 10.1016/0882-4010(92)90045-P. [DOI] [PubMed] [Google Scholar]
- 37.al-Ramadi BK, Meissler JJ, Jr, Huang D, Eisenstein TK. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur J Immunol. 1992;22(9):2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]
- 38.Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, Lin SL, Luo X, Miller SI, Zheng L, King I, Pawelek JM, Bermudes D. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17(1):37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 39.Kasinskas RW, Forbes NS. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007;67(7):3201–3209. doi: 10.1158/0008-5472.CAN-06-2618. [DOI] [PubMed] [Google Scholar]
- 40.Leschner S, Westphal K, Dietrich N, Viegas N, Jablonska J, Lyszkiewicz M, Lienenklaus S, Falk W, Gekara N, Loessner H, Weiss S. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS One. 2009;4(8):e6692. doi: 10.1371/journal.pone.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganai S, Arenas RB, Sauer JP, Bentley B, Forbes NS. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther. 2011;18(7):457–466. doi: 10.1038/cgt.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis . Clin Cancer Res. 2008;14(6):1905–1912. doi: 10.1158/1078-0432.CCR-07-2050. [DOI] [PubMed] [Google Scholar]
- 43.Saccheri F, Pozzi C, Avogadri F, Barozzi S, Faretta M, Fusi P, Rescigno M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med. 2010;2(44):44ra57. doi: 10.1126/scitranslmed.3000739. [DOI] [PubMed] [Google Scholar]
- 44.al-Ramadi BK, Al-Dhaheri MH, Mustafa N, Abouhaidar M, Xu D, Liew FY, Lukic ML, Fernandez-Cabezudo MJ. Influence of vector-encoded cytokines on anti-Salmonella immunity: divergent effects of interleukin-2 and tumor necrosis factor alpha. Infect Immun. 2001;69(6):3980–3988. doi: 10.1128/IAI.69.6.3980-3988.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dougan G, John V, Palmer S, Mastroeni P. Immunity to salmonellosis. Immunol Rev. 2011;240(1):196–210. doi: 10.1111/j.1600-065X.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 46.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 47.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116(10):2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong EH, Chang SY, Lee BR, Pyun AR, Kim JW, Kweon MN, Ko HJ. Intratumoral injection of attenuated Salmonella vaccine can induce tumor microenvironmental shift from immune suppressive to immunogenic. Vaccine. 2013;31(10):1377–1384. doi: 10.1016/j.vaccine.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182(1):155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR, Yao L, Gupta G, Kanegane C, Tosato G. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood. 1997;89(8):2635–2643. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.