Abstract
Glucocorticoid (GC) hormones have been introduced as therapeutic agents in blood cancers six decades ago. The effectiveness of GC treatment stems from its ability to induce apoptotic death of hemopoietic cells. A major impediment in GC therapy is the acquisition of resistance to the drug upon repeated treatment. In addition, some blood cancers are a priori resistant to GC therapy. Usually, resistance to GC correlates with poor prognosis. Albeit the wide use of GC in clinical practice, their mode of action is not fully understood. The cellular response to GC is initiated by its binding to the cytosolic GC receptor (GR) that translocates to the nucleus and modulates gene expression. However, nuclear activities of GR occur in both apoptosis-sensitive and apoptosis-resistant cells. These apparent controversies can be resolved by deciphering non-genomic effects of GCs and the mode by which they modulate the apoptotic response. We suggest that non-genomic consequences of GC stimulation determine the cell fate toward survival or death. Understanding the cellular mechanisms of GC apoptotic sensitivity contributes to the development of new modalities for overcoming GC resistance.
Keywords: Glucocorticoids, Hemopoietic tumors, Mitochondria, BIM, Glycogen synthase kinase 3, CITIM 2013
Introduction
Glucocorticoid (GC) hormones are commonly used in the treatment for hemopoietic malignancies, due to their ability to induce apoptosis of malignant lymphoid cells. However, a major obstacle to GC-based therapy is the emergence of resistant cells that no longer respond to GC with apoptotic death. Even though GCs have been used in the treatment for leukemias and lymphomas for more than half a century, the mechanisms underlying their apoptogenic activity remain obscure.
So far, most studies on cellular responses to GCs have focused on their genomic effects. The reason for that lies in the documented ability of GC to translocate the GC receptor (GR) to the nucleus, where it modulates transcription of some 300 genes through transactivation or transrepression [1–3]. However, only few of the GC-affected genes, e.g., GR and BIM, have been ascribed to apoptosis, and the mere expression of these gene products is insufficient to induce apoptotic death [4]. It is thus conceivable that additional postgenomic effects operating in lymphoid cells that are sensitive to GC-induced apoptosis might be accountable for the apoptogenic activities of these hormones. In the present article, we shall review some of the non-genomic functions of GC that are associated with the progression of the death response and their relevance in the context of GC-based therapy.
GC-induced apoptosis in lymphoid cells: role of the mitochondria
Almost all cells of our body express GR and are therefore affected by GC in various ways. However, GC-induced apoptosis is restricted to lymphoid cells, whether normal or malignant. What is the molecular basis for this selectivity? The answer begins to unfold by understanding the consequences of GC binding to its receptor. Normally, the GR is sequestered in the cytosol by a multi-subunit complex of heat shock proteins [5]. Upon GC binding the complex dissociates, setting the GR free to undergo dimerization and phosphorylation before its translocation to the nucleus. These molecular events occur in both apoptosis-sensitive and apoptosis-resistant cells and, thus, cannot be the sole basis for GC-induced apoptosis. We therefore hypothesized that non-genomic effects restricted to lymphoid cells are involved in determining the selectivity of the apoptotic response to GCs. The first validation of our hypothesis emerged when we followed the intracellular trafficking of the GR following stimulation by the synthetic GC dexamethasone (DEX). We detected a mitochondrial localization signal (MLS) within the ligand-binding domain of the GR, which enables its trafficking to the mitochondria in sensitive lymphoid cells only [6, 7]. Mitochondrial translocation of the GR reduces the outer membrane potential, thereby allowing release of cytochrome c and Smac/DIABLO [7]. This is a crucial step in activating the intrinsic mitochondrial apoptosis pathway, which occurs in lymphoid cells only, making them sensitive to GC-induced apoptosis.
In this regard, it should be stressed that translocation of GR to the mitochondria is necessary but not sufficient for GC-induced apoptosis. The reason for this is the expression of mitochondrial anti-apoptotic proteins, such as the BCL2 family proteins and the voltage-dependent anion channel (VDAC), that stabilize the outer membrane and offset the pro-apoptotic activity of GR [8]. These proteins have to be deactivated before the GR can advance the intrinsic apoptotic pathway. This step of apoptotic sensitization is regulated through phosphorylation enabled by the cellular kinome as outlined below.
GC-induced apoptosis in lymphoid cells: role of the kinome
Studies in our laboratory have indicated that glycogen synthase kinase 3 (GSK3) and the pro-apoptotic BH3‐only BCL2-like protein (BIM) are key protein kinases involved in the GC-induced apoptotic pathway. The role of GSK3 has been demonstrated using specific inhibitors of GSK3, which blocked DEX-mediated apoptosis in sensitive cells [9]. Likewise, a kinase-inactive, dominant negative GSK3 inhibited DEX-induced apoptosis [9]. GSK3 is active in GC-sensitive cells but not in GC-resistant ones as its phosphorylation level is low in the former and high in the latter (unpublished data). GSK3 is further activated upon treatment with DEX. Our investigation indicated that GSK3α is part of the cytosolic multi-subunit complex of GR in unstimulated cells [9]. Once GC binds to the GR, GSK3α dissociates from the complex and exerts its kinase activity alongside GSK3β [9].
The other protein kinase involved is BIM, whose expression is upregulated by GR translocation to the nucleus [10, 11]. Albeit BIM does not pose GC response elements (GREs) in the promoter [10, 12], its expression is upregulated by FOXO3a transcription factor (TF) [13, 14] that responds to GC with upregulated synthesis [15, 16]. Upregulation of BIM is crucial for conferring GC-induced apoptosis in cells that display low basal levels of this protein [17, 18]. However, some GC-sensitive cells express a sufficient amount of BIM that is not further upregulated by DEX [8, 19, 20]. Moreover, upregulation of BIM per se is insufficient to trigger apoptosis as it has to be posttranslationally activated [20]. Indeed, shortly after exposure to DEX, GSK3α and β transiently interact with BIM and trigger its apoptotic activity [9]. BIM mediates BAX and BAK oligomerization, either directly by interaction with BAK and BAX or indirectly by neutralization of BCL2 family proteins that inhibit oligomerization of BAK and BAX [21]. In unstimulated cells, the inactive pro-apoptotic BAK is located in the outer mitochondrial membrane, forming complexes with anti-apoptotic proteins (such as BCL2 and BCL-XL), while BAX is detected in the cytosol [21]. BAX and BAK are crucial pro-apoptotic proteins involved in the formation of the mitochondrial permeability transition pore that initiates the irreversible step of the apoptotic process by enabling release of cytochrome c and other pro-apoptotic proteins [22–24]. Hence, activation of BIM by GSK3 relieves the anti-apoptotic effect of BCL2 on GC-induced apoptosis. Furthermore, GSK3β directly phosphorylates BAX at Ser163 and promotes its mitochondrial localization [25], thereby advancing the apoptotic response. In addition, GSK3β phosphorylates VDAC, which prevents its binding to hexokinase II (HKII) [26]. HKII is abundantly expressed in tumor cells since they are highly glycolytic. Avoiding HKII from binding to VDAC potentiates chemotherapy-induced cytotoxicity [26]. Indeed, overexpression of HKII inhibits DEX-induced apoptosis [27] and interferes with the ability of BAX to bind to mitochondria and induce the release of cytochrome c [28]. Thus, GC-induced phosphorylation of VDAC and BAX by GSK3 and its interaction with BIM fine-tunes the apoptotic threshold. GSK3 contributes to the activation of the intrinsic apoptotic pathway by deactivating BCL2, VDAC, and HKII while activating BIM, BAX, and BAK, thus allowing mitochondrial GR to advance cellular death.
The model
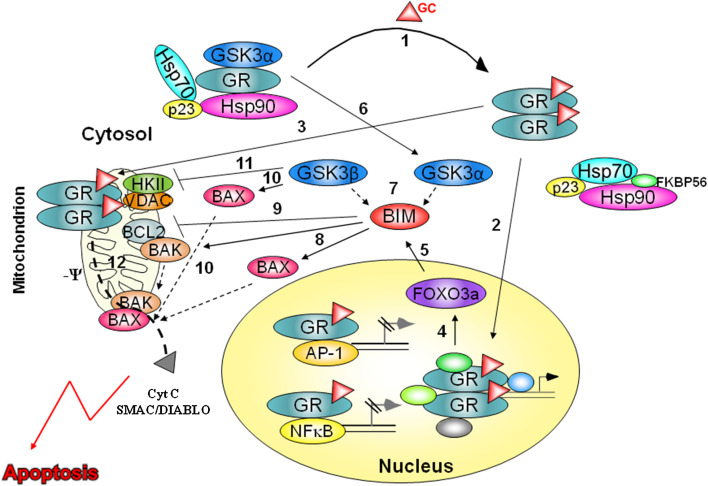
Based on these studies, we propose the following model: In unstimulated cells, GSK3α associates with the GR multi-subunit complex [9] (Fig. 1). When a ligand is bound to the GR, GSK3α dissociates and GR dimers are trafficking to the nucleus and mitochondria (Fig. 1). At the same time, the two isoforms of GSK3 interact with BIM [9], which is upregulated by nuclear GR through FOXO3a TF [13–16] (Fig. 1). BIM inhibits BCL2 and activates BAX and BAK [21]. Furthermore, GSK3 directly activates BAX by promoting its mitochondrial localization [25] and inhibits VDAC association with HKII [26]. These interactions enable mitochondrial GR to reduce the outer membrane potential followed by the release of cytochrome c and Smac/DIABLO which initiates the apoptotic process [7] (Fig. 1).
Fig. 1.
The consequences of GC stimulation in GC-sensitive cells. In the absence of GC, GR and GSK3α associate in a large heteromeric complex. Upon GC binding, GR dissociates from the complex and undergoes dimerization and phosphorylation (1). Dimerized GR translocates to both nucleus (2) and mitochondria (3). In the nucleus, GR transactivates and transrepresses multiple genes. Of special note is FOXO3a TF (4) which upregulates BIM expression (5). In addition to GR, GSK3α also dissociates from the heteromeric complex (6) and, following immediate interaction with GSK3β, binds BIM to promote its pro-apoptotic activity (7). Upregulated and activated BIM induces BAX and BAK oligomerization (8) and inhibits BCL2 (9). GSK3 phosphorylates BAX and promotes its mitochondrial translocation (10). Concomitantly, it phosphorylates VDAC and inhibits its subsequent association with HKII, thus enabling reduction in mitochondrial membrane potential (11) by GR (12). Reduction in mitochondrial membrane potential along with oligomerization of BAX and BAX results with release of cytochrome c and Smac/DIABLO that initiates the apoptotic process
Relevance to therapy
Given this knowledge, we could ask several questions related to therapy of leukemias and lymphomas by GC hormones, such as prednisone and DEX. Why some of these cancers are resistant a priori to hormonal treatment? And why sensitive leukemias and lymphomas gradually develop resistance to the GC treatment? The reason can be attributed to the dysfunction of GSK3 and BIM. Activity of GSK3 is regulated by the PI3K–AKT axis, which inactivates GSK3 by phosphorylation at Ser21 and Ser9 on GSK3α and GSK3β, respectively [29, 30]. One mode by which a resistant phenotype is attained is through hyperactivity of AKT. GC-resistant cells display highly activated AKT that leads to GSK3 inactivation [9]. In these cells, the GR does not translocate to the mitochondria [6]. AKT antagonizes GC-induced apoptosis in follicular lymphoma [31], multiple myeloma [32], peripheral T cells [33], thymoma [9], T-acute lymphoblastic leukemia [9], and Burkitt’s lymphoma (unpublished data). Therefore, GC resistance may be reversed by specific AKT inhibitors, such as wortmanin and staurosporine [8, 9]. These drugs, when given together with DEX, retain the sensitivity of leukemia cells to apoptosis and prolong the response of the cells to GC [8, 9]. Indeed, apoptosis conferred by wortmanin or staurosporine, when combined with DEX, is prevented by inhibition of GSK3 [9] (unpublished data). In addition to their effect on GSK3, PI3K/AKT inhibitors also mediate BIM upregulation through activation of FOXO3a [13, 34]. AKT phosphorylates FOXO3a on Thr32, Ser253, and Ser315, a process that causes its binding to the 14-3-3 proteins. This interaction leads to their immediate export from the nucleus followed by inhibition of FOXO3a-dependent transcription [34]. Indeed, FOXO family proteins regulate cell survival by transcriptionally modulating the expression of death receptor ligands and BIM [13]. Hence, inhibition of PI3K/AKT signaling relieves the inhibitory phosphorylation from FOXO3a, which upregulates expression of BIM.
We have also found that inhibition of PI3K/AKT mediates Nur77 activation [8, 35] through inhibition of its proteasomal degradation [8]. Nur77 is a transcription factor controlled by external stimuli, but it is not expressed in resting cells. Nur77 mRNA is upregulated in both B [36] and T [37, 38] lymphocytes following activation by antigen–receptor ligation. It has been implicated in negative selection of T cells [39], whose pro-apoptotic action cannot be offset by BCL2 [40, 41]. Nuclear Nur77 may cause apoptosis by upregulating pro-apoptotic proteins, such as FasL and TRAIL [41, 42]. Nur77 also acts at the mitochondria by binding to BCL2 and converting it into a pro-apoptotic protein [40, 41]. AKT directly phosphorylates Nur77 in its DNA-binding domain, resulting in reduced Nur77 DNA binding and transcriptional activity [35]. In addition, AKT stimulates Nur77 association with 14-3-3 protein by phosphorylating Nur77, a process that inhibits Nur77 activity [43]. Furthermore, AKT phosphorylation of Nur77 blocks its mitochondrial targeting and association with BCL2 [44]. Indeed, AKT mediates reduction in activation-induced cell death of T cell hybridomas by inhibition of Nur77 [43]. We demonstrated that in low-BCL2-expressing cells, inhibition of PI3K/AKT causes Nur77 to act mainly at the nucleus as a promoter of GC-induced apoptosis. However, in high-BCL2-expressing cells, when PI3K/AKT is inhibited, Nur77 acts at both the nucleus and mitochondria, since BCL2 entraps some Nur77 particles and avoids them from translocating to the nucleus (unpublished data). Inhibition of Nur77 in BCL2-positive or BCL2-negative GC-resistant cells avoids PI3K/AKT inhibitors from sensitizing these cells to GC-induced apoptosis [8] (unpublished data). Nur77 is not affected by DEX treatment in both GC-sensitive and GC-resistant cells; thus, Nur77 upregulation is not directly involved in advancing GC-induced apoptosis but acts to overcome GC resistance. Finally, the PI3K/AKT inhibitor staurosporine slightly induces GR translocation to the mitochondria upon DEX treatment of GC-resistant cells [8]. However, the level of mitochondrial GR in such cells is far below that observed in GC-sensitive ones. Hence, it is unlikely that this is the major mode by which staurosporine sensitizes cells to GC-induced apoptosis.
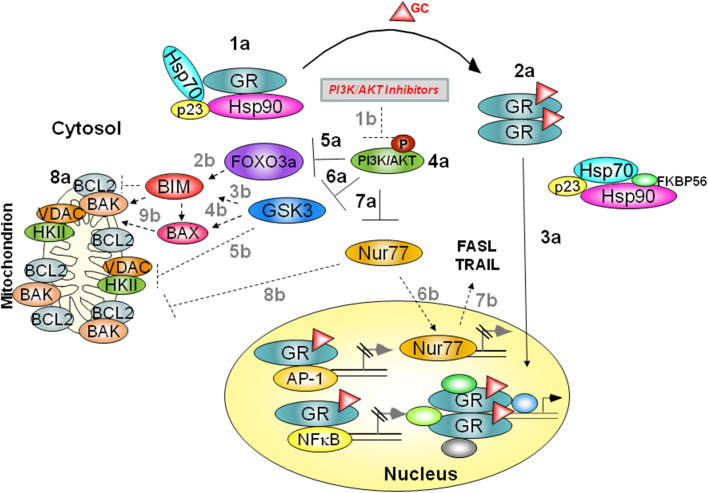
In summary, GC-resistant cells are distinguished from sensitive ones by three parameters as follows: (1) GSK3α is not bound to their cytosolic unstimulated GR, (2) upon stimulation with GC, their GR does not translocate to the mitochondria, and (3) their PI3K/AKT signaling pathway is highly activated. They express high level of anti-apoptotic proteins belonging to the BCL2 superfamily (Fig. 2). AKT inactivates FOXO3a by phosphorylation on Thr32, Ser253, and Ser315 [34], and as a consequence, BIM upregulation is inhibited (Fig. 2). AKT also inactivates GSK3 and Nur77 by inhibitory phosphorylation on Ser21/9 and Ser350, respectively [30, 35] (Fig. 2). BIM and GSK3 are essential for GC-induced apoptosis, and therefore, their inhibition by AKT confers GC resistance. In addition, resistant cells usually express high levels of BCL2 superfamily proteins. Such proteins further antagonize GC-induced apoptosis by inactivating BAX and BAK, a process that can be overcome by hyperexpression of BIM [45] (Fig. 2). Forced inhibition of PI3K/AKT signaling relieves the inhibitory phosphorylation from FOXO3a, GSK3, and Nur77 but rarely induces mitochondrial GR translocation (Fig. 2). As a consequence, GC-mediated FOXO3a upregulation is not inhibited by AKT, and therefore, it is free to induce BIM transcription. Active GSK3 further activates BIM and BAX and inhibits many survival signaling pathways, such as β-catenin, VDAC, and HKII. Finally, activated Nur77 translocates either to the nucleus, where it upregulates transcription of pro-apoptotic genes such as FasL and TRAIL [41, 42], or to the mitochondria, where it converts anti-apoptotic BCL2 to a pro-apoptotic protein [40]. Upregulated and activated BIM, along with inhibition of BCL2 and VDAC, positively regulates BAK and BAX, thereby enabling the advancement of the apoptotic pathway.
Fig. 2.
a The consequences of GC stimulation in GC-resistant cells. In the absence of GC, GR is sequestered in a large heteromeric complex (1a). Upon GC exposure, GR dissociates from the complex and undergoes dimerization and phosphorylation (2a). Dimerized GR translocates to nucleus, but not to mitochondria (3a). In addition, BIM does not respond with upregulation as AKT is highly activated (4a). Activated AKT phosphorylates and inactivates FOXO3a (5a). AKT also inactivates GSK3 and Nur77 by inhibitory phosphorylations (6a–7a). Moreover, resistant cells express high levels of anti-apoptotic BCL2 superfamily proteins (8a), conferring a resistant phenotype on the cells. b Overcoming GC resistance by PI3K/AKT inhibitors: Inhibition of PI3K/AKT signaling by wortmanin or staurosporine (1b) relieves the inhibitory phosphorylation from FOXO3a, GSK3, and Nur77. GC-mediated FOXO3a upregulation is not inhibited by AKT, which is free to induce BIM transcription (2b). Non-phosphorylated GSK3 further activates BIM (3b) and BAX (4b) and inhibits VDAC association with HKII (5b). Finally, Nur77 is upregulated and translocates to the nucleus (6b), where it upregulates pro-apoptotic genes, such as FasL and TRAIL (7b). In addition, Nur77 translocates to mitochondria and converts anti-apoptotic BCL proteins to pro-apoptotic ones (8b). Activated BIM, accompanied with pro-apoptotic BCL2, activates BAX and BAK (9b), thus enabling the advancement in the apoptotic pathway
Conclusions
We have defined two levels at which GC-induced apoptosis of leukemia and lymphoma cells is regulated. Receptor translocation to the mitochondria distinguishes between sensitive lymphoid cells and other cells expressing GR but inherently resistant to GC-induced apoptosis. Mitochondrial GR is mandatory but not sufficient to induce apoptosis. Concomitantly, the cell kinome is playing a crucial role in advancing the intrinsic apoptotic pathway initiated by mitochondrial GR. In this regard, activation of the protein kinases GSK3 and BIM is essential for the GC-mediated apoptotic death response.
Each level can become a subject for intervention to prolong the sensitivity of hemopoietic malignant cells to GC therapy or even resensitize fully resistant cells to respond. We have shown earlier that staurosporine induces GSK3 activation, as well as upregulation of Nur77 and BIM. By these virtues, staurosporine can turn resistant cells into sensitive ones. Specific inhibitors of PI3K and AKT, such as wortmanin, help in keeping GSK3 active and defer the emergence of cells that become apoptotic resistant in response to GCs, thus adding a new opportunity for retaining the apoptotic response to CG hormones. Further studies may identify additional protein kinase-specific inhibitors that can effectively sensitize hematopoietic cancer cells to GC-induced apoptosis.
Glucocorticoid-based therapy has been practiced for more than half a century and seems to remain as a principal tool in the management of blood malignancies. It is thus highly desirable to search for treatment modalities that maintain and prolong the apoptotic response to the hormonal treatment. To this end, combining GC therapy with protein kinase inhibitors that offset GC resistance may be a promising approach.
Acknowledgments
The authors wish to thank Dr. Ronit Vogt-Sionov, Hali Spokoini, and Prof. Ingrid Herr for their contribution. The study described in this review was supported in part by the German-Israel Foundation (GIF) and Concern Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Third International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2013), held in Krakow, Poland, 22nd–25th April 2013. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Zhang G, Zhang L, Duff GW. A negative regulatory region containing a glucocorticosteroid response element (nGRE) in the human interleukin-1beta gene. DNA Cell Biol. 1997;16(2):145–152. doi: 10.1089/dna.1997.16.145. [DOI] [PubMed] [Google Scholar]
- 2.Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10(23):2807–2816. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- 3.Kfir-Erenfeld S, Sionov RV, Spokoini R, Cohen O, Yefenof E. Protein kinase networks regulating glucocorticoid-induced apoptosis of hematopoietic cancer cells: fundamental aspects and practical considerations. Leuk Lymphoma. 2010;51(11):1968–2005. doi: 10.3109/10428194.2010.506570. [DOI] [PubMed] [Google Scholar]
- 4.Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E. Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv Cancer Res. 2008;101:127–248. doi: 10.1016/S0065-230X(08)00406-5. [DOI] [PubMed] [Google Scholar]
- 5.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/er.18.3.306. [DOI] [PubMed] [Google Scholar]
- 6.Sionov RV, Cohen O, Kfir S, Zilberman Y, Yefenof E. Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis. J Exp Med. 2006;203(1):189–201. doi: 10.1084/jem.20050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talaber G, Boldizsar F, Bartis D, Palinkas L, Szabo M, Berta G, Setalo G, Jr, Nemeth P, Berki T. Mitochondrial translocation of the glucocorticoid receptor in double-positive thymocytes correlates with their sensitivity to glucocorticoid-induced apoptosis. Int Immunol. 2009;21(11):1269–1276. doi: 10.1093/intimm/dxp093. [DOI] [PubMed] [Google Scholar]
- 8.Kfir S, Sionov RV, Zafrir E, Zilberman Y, Yefenof E. Staurosporine sensitizes T lymphoma cells to glucocorticoid-induced apoptosis: role of Nur77 and Bcl-2. Cell Cycle. 2007;6(24):3086–3096. doi: 10.4161/cc.6.24.5023. [DOI] [PubMed] [Google Scholar]
- 9.Spokoini R, Kfir-Erenfeld S, Yefenof E, Sionov RV. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol Endocrinol. 2010;24(6):1136–1150. doi: 10.1210/me.2009-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Malone MH, He H, McColl KS, Distelhorst CW. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem. 2003;278(26):23861–23867. doi: 10.1074/jbc.M301843200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem. 2004;279(20):20858–20865. doi: 10.1074/jbc.M310643200. [DOI] [PubMed] [Google Scholar]
- 12.Bouillet P, Zhang LC, Huang DC, Webb GC, Bottema CD, Shore P, Eyre HJ, Sutherland GR, Adams JM. Gene structure alternative splicing, and chromosomal localization of pro-apoptotic Bcl-2 relative Bim. Mamm Genome. 2001;12(2):163–168. doi: 10.1007/s003350010242. [DOI] [PubMed] [Google Scholar]
- 13.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162(4):613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168(10):5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Xie Y, Shi Y, Qin W, Zhao B, Jin Y. Glucocorticoid-induced apoptosis requires FOXO3A activity. Biochem Biophys Res Commun. 2008;377(3):894–898. doi: 10.1016/j.bbrc.2008.10.097. [DOI] [PubMed] [Google Scholar]
- 16.Urbich C, Knau A, Fichtlscherer S, Walter DH, Bruhl T, Potente M, Hofmann WK, de Vos S, Zeiher AM, Dimmeler S. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005;19(8):974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 17.Abrams MT, Robertson NM, Yoon K, Wickstrom E. Inhibition of glucocorticoid-induced apoptosis by targeting the major splice variants of BIM mRNA with small interfering RNA and short hairpin RNA. J Biol Chem. 2004;279(53):55809–55817. doi: 10.1074/jbc.M411767200. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Quearry B, Harada H. p38-MAP kinase activation followed by BIM induction is essential for glucocorticoid-induced apoptosis in lymphoblastic leukemia cells. FEBS Lett. 2006;580(14):3539–3544. doi: 10.1016/j.febslet.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 19.O’Reilly LA, Cullen L, Visvader J, Lindeman GJ, Print C, Bath ML, Huang DC, Strasser A. The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am J Pathol. 2000;157(2):449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9(5):505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 21.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27(Suppl 1):S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3(10):932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 23.Rambal AA, Panaguiton ZL, Kramer L, Grant S, Harada H. MEK inhibitors potentiate dexamethasone lethality in acute lymphoblastic leukemia cells through the pro-apoptotic molecule BIM. Leukemia. 2009;23(10):1744–1754. doi: 10.1038/leu.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laane E, Panaretakis T, Pokrovskaja K, Buentke E, Corcoran M, Soderhall S, Heyman M, Mazur J, Zhivotovsky B, Porwit A, Grander D. Dexamethasone-induced apoptosis in acute lymphoblastic leukemia involves differential regulation of Bcl-2 family members. Haematologica. 2007;92(11):1460–1469. doi: 10.3324/haematol.10543. [DOI] [PubMed] [Google Scholar]
- 25.Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24(44):9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65(22):10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 27.Sade H, Khandre NS, Mathew MK, Sarin A. The mitochondrial phase of the glucocorticoid-induced apoptotic response in thymocytes comprises sequential activation of adenine nucleotide transporter (ANT)-independent and ANT-dependent events. Eur J Immunol. 2004;34(1):119–125. doi: 10.1002/eji.200324650. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277(9):7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 29.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 30.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79(4):173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuutinen U, Postila V, Matto M, Eeva J, Ropponen A, Eray M, Riikonen P, Pelkonen J. Inhibition of PI3-kinase-Akt pathway enhances dexamethasone-induced apoptosis in a human follicular lymphoma cell line. Exp Cell Res. 2006;312(3):322–330. doi: 10.1016/j.yexcr.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa M, Nishiura T, Oritani K, Yoshida H, Yoshimura M, Okajima Y, Ishikawa J, Hashimoto K, Matsumura I, Tomiyama Y, Matsuzawa Y. Cytokines prevent dexamethasone-induced apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in a new multiple myeloma cell line. Cancer Res. 2000;60(15):4262–4269. [PubMed] [Google Scholar]
- 33.Sade H, Sarin A. IL-7 inhibits dexamethasone-induced apoptosis via Akt/PKB in mature, peripheral T cells. Eur J Immunol. 2003;33(4):913–919. doi: 10.1002/eji.200323782. [DOI] [PubMed] [Google Scholar]
- 34.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 35.Pekarsky Y, Hallas C, Palamarchuk A, Koval A, Bullrich F, Hirata Y, Bichi R, Letofsky J, Croce CM. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc Natl Acad Sci USA. 2001;98(7):3690–3694. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittelstadt PR, DeFranco AL. Induction of early response genes by cross-linking membrane Ig on B lymphocytes. J Immunol. 1993;150(11):4822–4832. [PubMed] [Google Scholar]
- 37.Liu ZG, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367(6460):281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- 38.Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367(6460):277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 39.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109(Suppl):S57–S66. doi: 10.1016/S0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 40.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116(4):527–540. doi: 10.1016/S0092-8674(04)00162-X. [DOI] [PubMed] [Google Scholar]
- 41.Li QX, Ke N, Sundaram R, Wong-Staal F. NR4A1, 2, 3 an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol. 2006;21(5):533–540. doi: 10.14670/HH-21.533. [DOI] [PubMed] [Google Scholar]
- 42.Rajpal A, Cho YA, Yelent B, Koza-Taylor PH, Li D, Chen E, Whang M, Kang C, Turi TG, Winoto A. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 2003;22(24):6526–6536. doi: 10.1093/emboj/cdg620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuyama N, Oishi K, Mori Y, Ueno T, Takahama Y, Gotoh Y. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J Biol Chem. 2001;276(35):32799–32805. doi: 10.1074/jbc.M105431200. [DOI] [PubMed] [Google Scholar]
- 44.Chen HZ, Zhao BX, Zhao WX, Li L, Zhang B, Wu Q. Akt phosphorylates the TR3 orphan receptor and blocks its targeting to the mitochondria. Carcinogenesis. 2008;29(11):2078–2088. doi: 10.1093/carcin/bgn197. [DOI] [PubMed] [Google Scholar]
- 45.Sionov RV, Kfir S, Zafrir E, Cohen O, Zilberman Y, Yefenof E. Glucocorticoid-induced apoptosis revisited: a novel role for glucocorticoid receptor translocation to the mitochondria. Cell Cycle. 2006;5(10):1017–1026. doi: 10.4161/cc.5.10.2738. [DOI] [PubMed] [Google Scholar]