Abstract
Tumor progression in the colon moves from aberrant crypt foci to adenomatous polyps to invasive carcinomas. The composition of the tumor-infiltrating leukocyte population affects the ability of the immune system to fight the tumor. T cell infiltration into colorectal adenocarcinomas, particularly T helper 1 (Th1) type T cells as well as increased regulatory T cell (Treg) frequencies, is correlated with improved prognosis. However, whether Th1 cells and Tregs are already present at the adenoma stage is not known. In this study, the APCMin/+ mouse model of intestinal adenomatous polyposis was used to investigate tumor-associated lymphocyte subsets and the mechanisms of their accumulation into gastrointestinal adenomas. Compared to unaffected tissue, adenomas accumulated CD4+FoxP3+ putative Treg in parallel with lower frequencies of conventional T cells and B cells. The accumulation of Treg was also observed in human adenomatous polyps. Despite high Treg numbers, the function of conventional T cells present in the APCMin/+ adenomas was not different from those in the unaffected tissue. Adenomas displayed an altered chemokine balance, with higher CCL17 and lower CXCL11 and CCL25 expression than in the unaffected tissue. In parallel, CXCR3+ Tregs were largely absent from adenomas. The data indicate that already in early stages of tumor development, the balance of lymphocyte-recruiting chemokines is altered possibly contributing to the observed shift toward higher frequencies of Treg.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1555-6) contains supplementary material, which is available to authorized users.
Keywords: Regulatory T cells, Tumor-infiltrating lymphocytes, Colorectal cancer, APCMin/+, Anti-tumor immunity, Chemokines
Introduction
Colorectal adenocarcinoma is one of the most prevalent cancer forms, with an annual incidence of nearly 1 million cases worldwide and an annual mortality of more than 500,000 individuals [1]. The development of colorectal cancer (CRC) begins with formation of aberrant crypt foci, which are the earliest recognized lesions. At this stage, genetic alterations such as adenomatous polyposis coli (apc) gene silencing may occur, which in turn lead to adenomatous polyp formation, and as several other mutations accumulate, this will drive the progression to invasive adenocarcinoma [2]. Colorectal tumors often display mutations that affect the wnt-signaling pathway, the tumor suppressor TP53, or the RAS family of oncogenes [3] and life style, diet and environmental factors have a strong impact on CRC risk [4].
The immune system contributes to the control of CRC progression, as evidenced by the observations that frequencies of tumor-infiltrating memory T cells, Th1 type CD4+ T cells, cytotoxic T cells and local production of Th1-recruiting chemokines correlate with disease-free and overall survival of the patient [5–7]. To be effective, these cells must leave the circulation through the blood vessel endothelium, guided by adhesion molecules and chemokines, into the tumor tissue. However, the migration of effector lymphocytes into the tumor may be inhibited by the action of regulatory T cells (Tregs) [8]. Tregs are defined as CD4+ T cells expressing the transcription factor FoxP3, which is considered to be the “master regulator” of Treg development and function [9–11]. Treg can inhibit the function of effector T helper cells and cytotoxic T cells, and also act on antigen-presenting cells to reduce their capacity to activate naive T cells [10]. In several types of solid tumors, the presence of Treg has been associated with a poor patient prognosis [12–15]. However, in CRC, the function of tumor-associated Treg and the prognostic impact of their presence are unclear. Two independent studies recently showed that Treg infiltration into colon adenocarcinomas actually correlates with a better prognosis [16, 17]. In contrast, recent findings indicate that Tregs present in colon adenocarcinomas to a large extent co-express the Th17-associated transcription factor ROR-γT and are pro-inflammatory and may promote tumor progression [18]. It is clear that FoxP3+ putative Tregs accumulate in colorectal tumors, compared to unaffected colon tissue from the same patients [19–22], and the complexity of Treg phenotype and function may explain the conflicting findings [23]. In human adenomatous polyps, it is still not known whether Tregs accumulate, but Lan et al. [24] have shown elevated FoxP3 mRNA levels in human intestinal polyps, albeit not to the same extent as in CRC.
In this study, we used the APCMin/+ mouse model to study immune cell infiltration during intestinal tumor progression. The APCMin/+ mouse is a relevant model for human CRC, as it harbors a mutation in the apc gene, resulting in a truncated protein lacking the C terminal domain, which will lead to dysregulation of the wnt-signaling pathway through increased β-catenin translocation to the nucleus [25, 26]. Similar or even identical mutations are found in individuals with familial adenomatous polyposis and in 70 % of all sporadic CRC cases [3]. However, the wnt-signaling pathway is also involved in many immunological processes, both during lymphocyte development and in responses to antigen [27, 28]. In this study, basic cellular immune parameters in APCMin/+ and wild-type (WT) mice were compared, focussing on accumulation of lymphocytes and chemokine production in intestinal adenomas to define early events in the immune response and the mechanisms for local recruitment of Treg. Our results show that basic systemic and mucosal immune components are similar in young APCMin/+ and WT mice, but within the adenoma there are substantial local changes in lymphocyte composition and chemokine production. In particular, a profound accumulation of Tregs was detected in the adenomas of APCMin/+ mice.
Materials and methods
Volunteers and tissue collection
Unaffected colon tissue and tissue from adenomatous polyps were collected from eight patients (two males and six females, aged 59–77 years) undergoing colonoscopy as part of investigations of suspected colon cancer. All polyps displayed a tubulovillous morphology with high-grade (n = 3) or low-grade dysplasia (n = 4), and one case with undetermined dysplasia rate. Tissue was embedded in OCT medium and immediately frozen. The study was approved by the research ethics committee of west Sweden, and volunteers gave informed consent to participate.
Mouse strains and breeding
APCMin/+ mice on a C57BL/6 background were obtained from Prof Sven Pettersson, Karolinska Institute, Stockholm. Male APCMin/+ mice were bred with female C57BL/6 mice to generate APCMin/+ mice at the Department of Experimental Biomedicine, University of Gothenburg, and kept under specific pathogen-free conditions in filter-top cages. Genotyping was performed at week 4 by PCR [29]. Age- and sex-matched WT littermates were used as controls. The study was approved by the animal ethics committee at University of Gothenburg.
Lymphocyte preparation
Blood, spleen, peripheral lymph nodes (PLN), mesenteric lymph nodes (MLN), small intestine (SI) and colon were collected from APCMin/+ and WT mice. Single-cell suspensions were prepared from spleen, PLN and MLN by forcing the organs through nylon nets using a syringe plunger. Red cell lysis was performed on splenic cells with 0.07 M NH4Cl, pH 7.3 37° for 5 min. Lamina propria lymphocytes (LPL) from the small and large intestine, and from adenomas were isolated essentially as described before [30] but with the use of collagenase VIII (Sigma-Aldrich) for colon digestion.
Flow cytometry
Single-cell suspensions were stained with CD4-PerCp, CD8-Pacific Blue, CD25-PE, NKp46-PE, CD19-APC-H7, CXCR3-PE, α4β7-PE and CD69-PE-Cy7 (BD Biosciences) and CCR6-PE-Cy7, CCR4-PE-Cy7 and CCR9-PE (Biolegend). For intracellular detection, permeabilization was performed with FOXP3 staining buffer set (eBioscience) followed by staining with FoxP3-APC, Ki67-Alexa 700 and granzyme B-PE (eBioscience). Samples were acquired on an LSR-II flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree Star Inc).
PMA/ionomycin stimulation
Lymphocytes from MLN, lamina propria and adenomas were incubated with phorbol myristate acetate (PMA; 50 ng/ml, Sigma-Aldrich) and ionomycin (500 ng/ml; Sigma-Aldrich) for 4 h at 37 °C, and brefeldin A (10 μg/ml; Sigma-Aldrich) was added after 2 h. Cells were stained with CD4-PerCp and CD8-Pacific blue, and intracellular staining of IL-17-PE (BD Biosciences) and IFN-γ-Alexa700 (BD Biosciences) was performed with Fix&Perm kit (ADG).
Immunofluorescence
The colon and the distal third of the small intestine were collected from APCMin/+ mice and WT controls, and immediately frozen in OCT (Tissue-Tek). Eight micrometer tissue sections were cut and fixed in ice-cold acetone. Sections used for staining of FoxP3 were rehydrated, fixed in 4 % paraformaldehyde and permeabilized with 0.5 % Triton ×-100 (Sigma-Aldrich). Sections from murine tissue were stained with rat anti-mouse antibodies to FoxP3 (eBioscience), CD4 (BD Biosciences), CD8 (BD Biosciences), mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) (eBioscience) and biotinylated B220 (BD Biosciences), and appropriate isotype controls were used. Sections stained for B220 were blocked with biotin/streptavidin blocking kit (Invitrogen). Binding was visualized with goat anti-rat antibodies (Alexa Fluor 568, Alexa Fluor 488) or streptavidin-Alexa Fluor 594 (all from Invitrogen). Human tissues were stained for FOXP3 and CD4 as previously described [22]. Slides were mounted using Gold-antifade mounting medium containing DAPI (Invitrogen) and analyzed using a Zeiss Axioscope fluorescence microscope. The frequency of lymphocytes in unaffected, adenoma-free lamina propria was assessed by counting in seven random fields of vision along the intestine and relating cell numbers to tissue area. In addition, all adenoma tissues in a section were examined. Conventional CD4+ T cells were defined as CD4+FoxP3− cells. Expression of MAdCAM-1 was quantified by measuring the percentage of the total tissue area in unaffected and adenoma tissue, respectively, stained for MAdCAM-1. Tissue area was calculated using the Biopix iQ 2.0 software.
RNA isolation and quantitative PCR
Tissue was incubated overnight in RNAlater (Ambion) at 4 °C, with subsequent storage at −80 °C. Tissues were lysed and homogenized (TissuelyserII, Qiagen) before total RNA was isolated using the RNeasy mini kit (Qiagen), including DNAse digestion. RNA concentration was determined spectrophotometrically (Nano drop ND-100). The Omniscript kit (Qiagen) was used for cDNA synthesis, using 2,000 ng RNA as template in a total reaction volume of 20 µl. Each real-time PCR mixture contained 40 ng cDNA, Power SYBR Green Master Mix (Applied Biosystems) and oligonucleotide primers to detect MAdCAM-1, vascular cell adhesion molecule-1 (VCAM-1), CCR9, CCL17, CCL20, CCL22, CCL25, CXCL9, CXCL10, CXCL11, IL-10, IL-17, IFN-γ, FoxP3 and β-actin as housekeeping gene (supplementary table 1). Primers were designed in Primer Express software (Applied Biosystems) and ordered from Eurofins MWG. Assays were run at standard thermal cycling conditions described for the 7500 real-time PCR system (Applied Biosystems). Relative expression was determined by the ΔΔCT method using β-actin as endogenous control [31].
Statistical analysis
Calculation of statistical significance was performed using Mann–Whitney test for unpaired observations and Wilcoxon matched-pairs signed-rank test when comparing paired unaffected and adenoma tissue. p values of <0.05 were considered significant. Statistical analyses were performed in PRISM GraphPad 5.
Results
Systemic T cell frequencies are similar in APCMin/+ and WT mice
To determine whether the apc mutation alone, or increasing adenoma burden in the APCMin/+ mice, affects lymphocyte infiltration, the frequencies of CD8+ and CD4+ T cells, recently activated CD69+ cells, CD25+ cells and FoxP3+ cells within total T cells from spleen, MLN, PLN and small intestinal lamina propria were analyzed using flow cytometry. Macroscopically visible adenomas were detectable at week 14, and by week 18, multiple adenomas were present in all mice. By week 22, adenomas were larger and more numerous, and some mice were euthanized because of colonic obstruction. Up to 18 weeks of age, in the organs examined, the frequencies of CD4+ and CD8+ T cells were similar in APCMin/+ and WT mice, and also, the frequencies of CD69+, CD25+ and FoxP3+ cells among CD4+ T cells did not differ significantly between APCMin/+ and WT (Fig. 1a, b). However, at 22 weeks of age frequencies of CD4+ and CD8+ T cells in the spleen of APCMin/+, mice were decreased compared with WT mice, as previously reported [32, 33]. Furthermore, the frequencies of CD4+CD25+ and CD4+FoxP3+ putative Tregs in the intestine of APCMin/+ mice showed a trend of increasing with age, with a significant difference in frequencies of CD4+CD25+ cells between 10 and 22 weeks of age (p < 0.05, Fig. 1b). Flow cytometric analysis of polyclonally activated MLN cell suspensions showed no difference between APCMin/+ mice and WT littermates with regard to IFN-γ and IL-17A production from CD4+ and CD8+ T cells (supplementary fig. 1). Taken together, these studies indicate that there are no major differences in the compositions of conventional T cells in WT and APCMin/+ mice in our colony, up to 18 weeks of age, but by 22 weeks of age splenic lymphopenia and intestinal putative Treg accumulation occur. However, increased lymphocyte infiltration into gut lamina propria, as previously reported [33], was not observed.
Fig. 1.
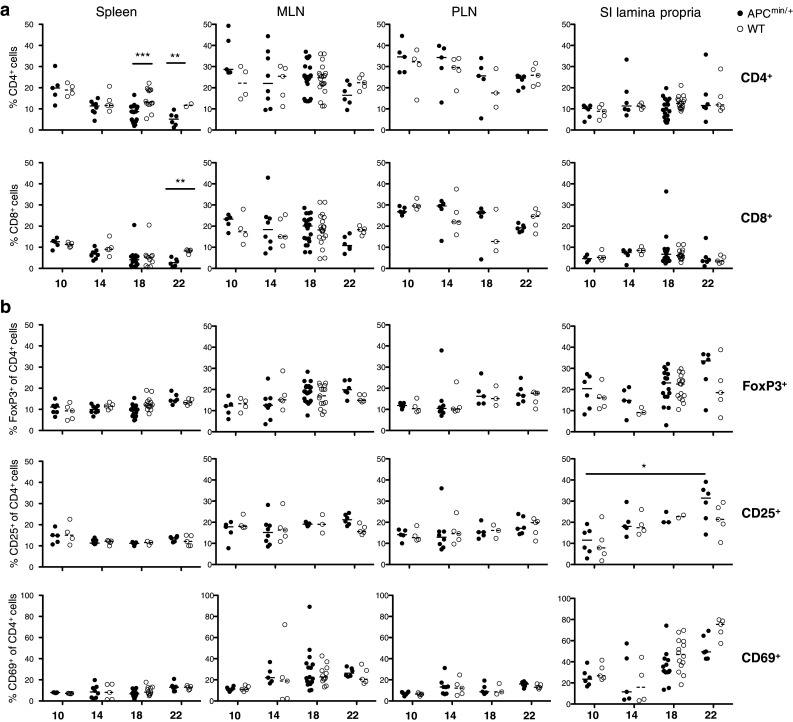
Lymphocytes frequencies in spleen, MLN, PLN and small intestine in both APCMin/+ and WT mice. The frequencies of CD8+ and CD4+ T cells (a), as well as CD4+CD69+, CD4+CD25+ and CD4+FoxP3+ (b) cells from spleen, MLN, PLN and small intestinal lamina propria were analyzed by flow cytometry at 10, 14, 18 and 22 weeks of age. Symbols represent individual mice (black APCMin/+, white WT), and lines represent median. Mann–Whitney test was used for statistical analysis *p < 0.05; **p < 0.01; ***p < 0.001
Intestinal adenomas harbor decreased frequencies of conventional T and B cells
Immunofluorescence was used to evaluate the presence of conventional T and B cells and FoxP3+ putative Treg in the adenoma tissue compared to unaffected intestinal tissue of APCMin/+ mice. In small intestinal adenomas, the numbers of both CD4+FoxP3− and CD8+ conventional T cells were substantially lower than in the surrounding unaffected tissue. In colon adenomas, on the other hand, there was no marked decrease in either of the T cell subsets compared to surrounding normal lamina propria (Fig. 2a, b). In the unaffected mucosa, conventional CD4+ T cells were evenly distributed throughout the lamina propria, but within adenomas usually luminally clustered and observed exclusively in the lamina propria (Fig. 2d). CD8+ T cells were present in the lamina propria and epithelium in the unaffected mucosa from APCMin/+ and WT small intestine, but were virtually absent from adenoma tissue. B220+ B cells were also similarly distributed in the WT and unaffected lamina propria of APCMin/+ mice. In contrast, B cell numbers were significantly decreased in adenoma tissue (Fig. 2c).
Fig. 2.
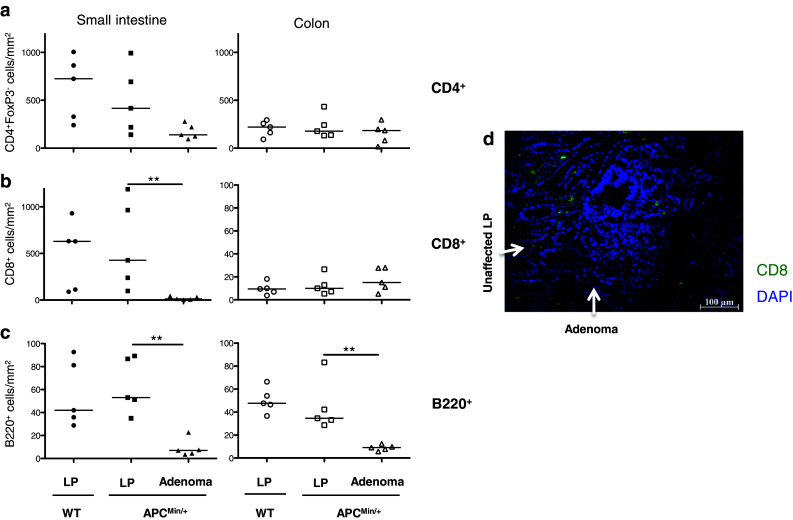
Lymphocyte distribution in unaffected intestinal lamina propria (LP) and adenomas. The frequencies of CD4+, CD8+ and B220+ lymphocytes were determined by immunofluorescence in small intestinal and colon sections from APCMin/+ and WT mice, and in APCMin/+ intestinal adenomas. Frequencies of conventional CD4+ T cells (a), CD8+ T cells (b) and B220+ B cells (c) are expressed as the number of cells per mm2. d Representative picture showing immunofluorescence staining of CD8+ T cells (green) in small intestinal section, displaying both adenoma and unaffected lamina propria. Symbols represent individual mice, and lines represent median. Mann–Whitney test was used for statistical analysis, **p < 0.01
To further investigate the differences in lymphocyte infiltration between adenomas and unaffected lamina propria, lymphocyte populations from WT and APCMin/+ mice at 18 weeks of age were evaluated by flow cytometry (Fig. 3). Firstly, lymphocyte subsets in the intestinal lamina propria from WT mice and from the adenoma-free regions of APCMin/+ mouse intestine were compared. These analyses revealed similar frequencies of CD4+FoxP3− and CD8+ conventional T cells, NKp46+ NK cells, CD19+ B cells, activated CD69+ T cells, proliferating Ki67+ T cells and cytotoxic Granzyme B+ CD8+ T cells within LPL (Fig. 3a–c). When comparing adenoma tissue with unaffected lamina propria, there was no significant difference in conventional T cell or NK cell frequencies, except for an increase in CD4+FoxP3− cells in colon adenomas. Furthermore, CD19+ B cell frequencies were significantly lower in adenoma tissue (Fig. 3a).
Fig. 3.
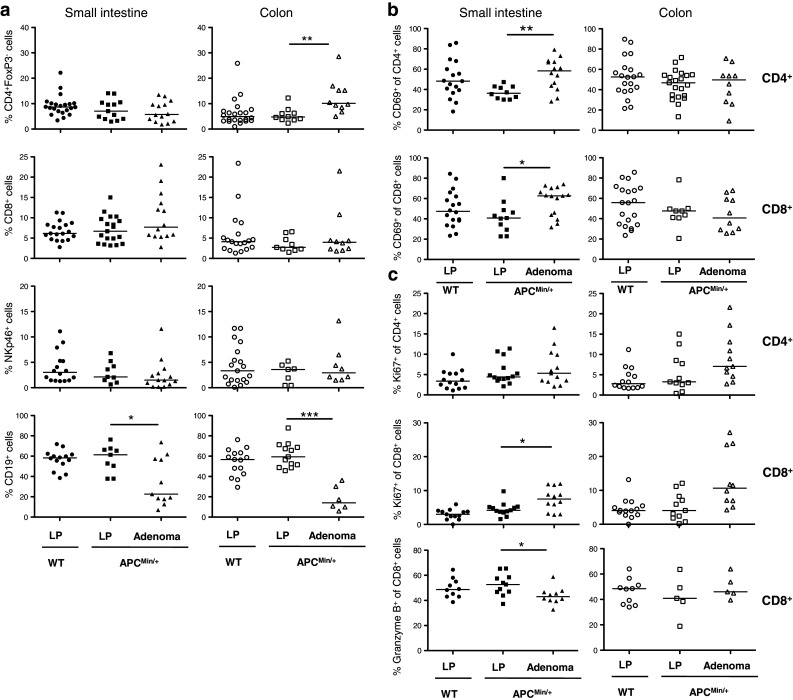
Lymphocyte compositions in unaffected intestinal lamina propria (LP) and adenomas. Conventional, proliferating and activated T cells as well as B cells and NK cells were examined by flow cytometry in small intestine and colon. The frequencies of CD4+, CD8+, NKp46+, CD19+ (a), among all lymphocytes and CD69+ (b), Ki67+, Granzyme B+ (c), among CD4+ and CD8+ T cells were analyzed in LP of 18 weeks old APCMin/+ and WT mice, and in APCMin/+ intestinal adenomas. Symbols represent individual mice, and lines represent median. Mann–Whitney test was used as statistical analysis, *p < 0.05; **p < 0.01; ***p < 0.001
CD4+FoxP3+ Tregs accumulate in intestinal adenomas
Tregs, identified using immunofluorescence as CD4+FoxP3+ lymphocytes, were present in similar densities in the lamina propria of WT mice and the unaffected tissue in APCMin/+ mice in both the small intestine and the colon. In the adenoma tissue, there was a substantial accumulation of Treg compared with unaffected tissue, particularly in the colon (Fig. 4a). The frequency of Treg within CD4+ T cells was significantly increased in both small intestinal and colonic adenomas compared to the respective unaffected tissue (Fig. 4b). This was also confirmed by flow cytometric analyses of small intestinal LPL from WT and APCMin/+ mice (Fig. 4c) and by real-time PCR analysis of FoxP3 mRNA (data not shown). Tregs in the adenomas were usually localized toward the gut lumen and close to the transition zone between adenoma and adenoma-free mucosa. A close association between Treg and conventional CD4+ T cells was occasionally observed (Fig. 4d). In human adenomatous polyps, a similar significant accumulation of FOXP3+ putative Treg was also documented (Fig. 4e).
Fig. 4.
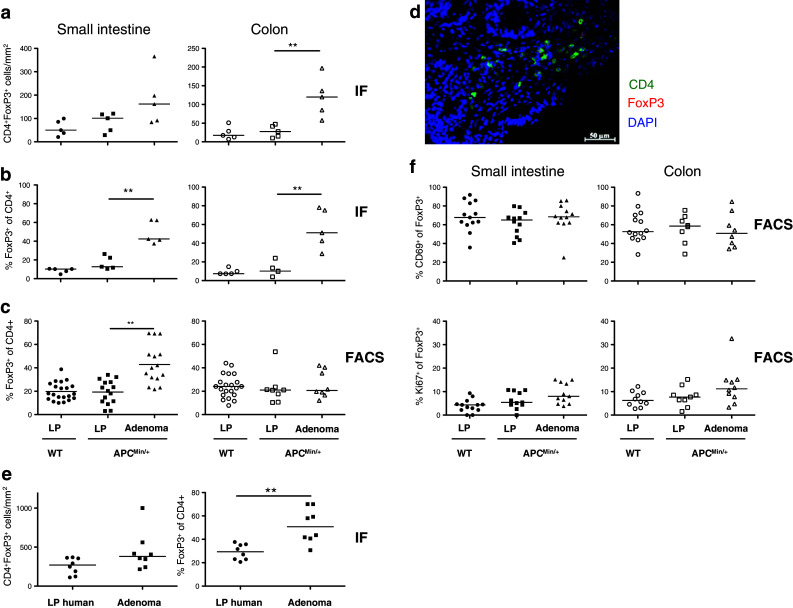
Regulatory T cells in murine and human unaffected intestinal tissue and adenomas. The frequencies of CD4+FoxP3+ Treg were determined by immunofluorescence (IF) and FACS in small intestinal and colon lamina propria (LP) from APCMin/+ and WT mice and in APCMin/+ adenomas, and by IF in human colon adenomatous polyps and unaffected tissue. Frequencies of CD4+FoxP3+ T cells are expressed as the number of cells per mm2 (a) or as percentage of FoxP3+ Treg among all CD4+ cells (b). c FACS analysis of CD4+FoxP3+ Treg in unaffected LP from WT mice and LP and adenoma from APCMin/+ mice. d Representative picture showing immunofluorescence staining of CD4 (green) and FoxP3 (red) in murine small intestinal adenoma section. Nuclei are stained blue with DAPI. e Frequencies of CD4+FOXP3+ T cells in human adenomatous polyps patients. f Frequencies of CD69+ and Ki67+ among CD4+FoxP3+ T cells. Symbols represent individual mice or patients, and lines represent median. Mann–Whitney test was used for statistical analysis **p < 0.01
To further characterize FoxP3+ putative Tregs in APCMin/+ adenomas, their expression of the early activation marker CD69 and the proliferation marker Ki67 was analyzed. The majority of Treg in the adenomas expressed CD69, but there was no difference in expression of CD69 between Tregs in adenomas and unaffected tissue (Fig. 4f). Notably, CD69 expression levels were higher (p < 0.05) on Tregs than on conventional CD4+ T cells present in the adenomas (Fig. 3b). The frequency of Ki67+ Treg was similar in adenomas and unaffected tissue (Fig. 4f). Taken together, these observations suggest that activated Treg accumulate in the adenomas of APCMin/+ mice.
MAdCAM-1 and VCAM-1 expression is similar in adenomas and unaffected tissue
Because the lymphocyte composition was changed in the adenomas, endothelial adhesion molecules involved in lymphocyte recruitment were investigated to determine whether differential expression in adenomas may explain the differences in lymphocyte recruitment. Firstly, endothelial expression of the mucosal adhesion molecule MAdCAM-1 was analyzed by immunofluorescence histochemistry. MAdCAM-1 expression was similar in adenomas and unaffected mucosa in the APCMin/+ mice. This result was also confirmed by real-time PCR analyses (supplementary fig. 2a, b). Furthermore, there was no significant difference in frequencies of cells expressing the mucosal homing receptor α4β7 when comparing unaffected and adenoma tissue in APCMin/+ mice (supplementary fig. 2c). VCAM-1 expression was also investigated by real-time PCR analysis, but these experiments showed no difference between adenomas and unaffected tissue (data not shown).
Altered chemokine balance in the adenomas of APCMin/+mice
To further investigate lymphocyte recruitment mechanisms in the intestinal adenomas, real-time PCR was used to determine the expression of chemokines with potential importance for recruitment of Treg. CCL17, which attracts CCR4+ Treg and effector T cells [34], was significantly upregulated in adenoma tissue compared with unaffected tissue (p < 0.05, Fig. 5a). There was also a trend for higher levels of CCL22, another CCR4 ligand, in the adenomas although this was not significant (supplementary fig. 3). The expression of CCR4 by Tregs recruited to the adenomas was, however, not different from expression by Tregs in unaffected tissue (Fig. 5a). Another chemokine that has been reported to contribute to Treg recruitment to adenomas is CCL20 [35]. Its only receptor, CCR6, is associated with Th17-type T cells but is also expressed on many lymphocytes homing to the gut [36, 37]. No changes in CCL20 levels were detected in the small intestinal adenomas compared to unaffected tissue, but there was actually a small, but significant, increase in the frequency of CCR6+ Treg in the adenomas compared with the unaffected mucosa (Fig. 5b). Furthermore, substantially decreased levels of the CCR9 ligand CCL25, which is crucial for organ specific homing of effector T cells to the small intestine [38], were revealed in adenomas. However, the low levels of CCL25 in the adenomas did not result in changes in frequencies of Treg expression of CCR9 (Fig. 5c). Finally, the chemokines CXCL9, CXCL10 and CXCL11 are induced by IFN-γ and important for recruitment of Th1 type cells, cytotoxic T cells and NK cells [39], but there is also a subpopulation of Treg that express CXCR3, the receptor for these chemokines [40, 41]. There was a significantly lower expression of CXCL11 in the adenomas (Fig. 5d), while the other two CXCR3 ligands, CXCL9 and CXCL10, were expressed at low levels and no difference between unaffected and adenoma tissue could be observed (supplementary fig. 3). The decreased expression of CXCL11 was accompanied by a decreased frequency of CXCR3+ Tregs in the adenomas (Fig. 5d). Taken together, these results show that the Tregs accumulating in adenomas have a different phenotype compared to Treg in unaffected mucosa, expressing more CCR6, but largely lacking expression of CXCR3. This phenotype may be partly explained by decreased production of CXCL11 in the adenomas.
Fig. 5.

Expression of chemokines and corresponding chemokine receptors by Treg in unaffected small intestine (SI) and adenomas. The expression of CCL17 (a), CCL20 (b), CCL25 (c) and CXCL11 (d) mRNA was examined in WT and APCMin/+ SI tissue and adenomas from 18-week-old mice by real-time PCR and normalized against β-actin. The frequencies of CCR4+ (a), CCR6+ (b), CCR9+ (c) and CXCR3+ (d) cells among CD4+FoxP3+ cells were analyzed by flow cytometry in WT small intestinal lamina propria (LP) and APCMin/+ LP and adenomas from 18-week-old mice. Symbols represent individual mice, and lines represent median. Mann–Whitney test was used as statistical analysis *p < 0.05; **p < 0.01
The lymphocyte effector profile is similar in adenomas and unaffected intestinal tissue
Th1 cells are typically recruited by CXCR3 ligands, for example CXCL11 [39], and to further understand the consequence of altered expression of chemokines and increased presence of Tregs in adenoma tissue, we investigated potential effector functions in conventional T cells from adenomas and unaffected tissue. IL-17A and IFN-γ production was detected among CD4+ and CD8+ cells from both adenomas and unaffected lamina propria following polyclonal stimulation. Generally, the levels of IFN-γ producing CD8+ T cells in both APCMin/+ and WT were higher in colon than small intestine. However, no difference between wild-type, unaffected APCMin/+ tissue and adenomas was observed within T cells producing either IL-17A or IFN-γ (Fig. 6a, b). These results were also confirmed by real-time PCR analysis of mRNA expression (Fig. 6c). FoxP3+ Tregs have previously been reported to produce IL-17 in adenomas from mice with apc mutations [42], but we detected only very few FoxP3+ IL-17A+ T cells in cell suspensions from adenomas. Likewise, few, if any, Treg produced IFN-γ following stimulation (data not shown). Furthermore, real-time PCR analyses demonstrated that there was no difference in IL-10 expression between the small intestinal adenomas and unaffected lamina propria (supplementary fig. 3).
Fig. 6.

Cytokine producing intestinal T cells in unaffected intestinal tissue and adenomas. Lymphocytes were isolated from intestinal lamina propria (LP) from both WT and APCMin/+ mice and from adenoma tissue. The frequencies of IL-17A+ (a) and IFN-γ+ cells (b) among CD4+ or CD8+ T cells were determined by flow cytometry following PMA/ionomycin stimulation. IL-17A and IFN-γ mRNA expression (c) was determined in small intestine from both WT and APCMin/+ mice, by real-time PCR and normalized against β-actin. Symbol represents individual mice, and bar represents median
In addition to cytokine responses, the activation status and proliferation of CD4+ and CD8+ conventional T cells were determined. Significant accumulations of CD69+ recently activated T cells in the adenomas from small intestine (Fig. 3b) were observed. CD8+ T cells in the small intestinal adenomas more actively participated in cell division (Ki67+) than corresponding cells in unaffected lamina propria. Finally, there was a somewhat lower cytotoxic capacity (Granzyme B+) in CD8+ T cells from the small intestinal adenomas (Fig. 3c). Taken together, these results indicate a generally preserved functional capacity of conventional T cells in the adenomas, despite increased Treg accumulation.
Discussion
This study used the APCMin/+ spontaneous intestinal adenoma mouse model to investigate the mechanisms of lymphocytes infiltration into intestinal adenomas. The continuous wnt-signaling characteristic of APCMin/+ mice may affect lymphocyte development [28, 32, 43, 44], and with this in mind, T cell frequency and function in APCMin/+ and WT mice were first evaluated. No major differences were found at 18 weeks of age, but with increasing age and adenoma burden lymphopenia and splenomegaly which have previously been described became evident [32, 33].
Subsequently, local lymphocyte accumulation in adenomas was examined and compared to surrounding unaffected mucosa. There were some differences in lymphocyte composition between small and large intestinal adenomas, which may depend on the different morphology of adenomas in the two locations. However, a decrease in conventional T cells in adenomas compared with adjacent unaffected mucosa was the norm. In addition, B cells were significantly decreased in both small and large intestinal adenomas, indicating a shift in lymphocyte composition in the adenomas. We have recently demonstrated a similar change in human CRC, in which IgA-producing B cells were almost excluded from colon tumor, due to low CCL28 production from tumors [45, 46]. B cells have been recently demonstrated to be part of a protective network of adaptive immune cells improving disease-free survival in CRC [47]. It is possible, then, that a reduction in IgA+ B cells may result in low levels of secretory IgA in the tumor, resulting in defective barrier function, increased bacterial colonization and concomitant Th17 responses, in turn driving tumor progression [7, 48]. It is interesting to note that IgA-mediated immune exclusion may be impaired already at the adenoma stage, and this may contribute to conversion from adenoma to invasive adenocarcinoma.
An additional major change in lymphocyte composition was a local accumulation of CD4+FoxP3+ Tregs in APCMin/+ small intestinal and colon adenomas, as previously shown by others [42]. A corresponding accumulation has also been reported in human colon tumors by ourselves and others [20–22]. The present study showed, for the first time, an increased frequency of FOXP3+ putative Treg within the CD4+ cell population infiltrating human adenomatous polyps. This validates our findings in the APCMin/+ model and suggests that the Treg accumulation seen in invasive human colon tumors is evident already at the stage of adenomatous polyps and may be the result of early changes in chemokine production. In contrast to most other solid tumors, high intra-tumor Treg frequencies correlate with improved patient outcome in CRC [16, 17]. The high bacterial load of the colon together with reduced barrier function in colorectal tumors [48] may explain the protective role of Tregs in CRC; it is possible that Tregs suppress microbial-induced inflammation and thus remove signals such as IL-17-inducing factors that would otherwise have promoted tumor growth [33, 48, 49]. However, a functional change in tumor-associated Treg in both human CRC and APCMin/+ mice, from immunosuppressive IL-10-secreting cells to a more pro-inflammatory, IL-17-secreting phenotype, has also been reported [18, 42]. Adoptive transfer of WT Treg to APCMin/+ mice leads to regression of adenomas [49], thus supporting a protective role for IL-10-competent WT Treg. However, the present study did not detect an accumulation of IL-17-producing T cells in adenomas, and there was also no difference with regard to IL-17 production between Treg in adenomas and Treg in unaffected tissue. One possible explanation for these discrepancies could be differences in the intestinal microbiota between mouse colonies, or the use of mouse strains with different apc mutations. In particular, we did not observe any intestinal inflammation in our mouse colony, which is in contrast to previous studies [33]. Microbially driven inflammation reactions in the gut may be important for tumor progression [50], and differences between animal colonies may be viewed as correlated with differences between individuals. Thus, it is possible that differences in the intestinal flora contribute to the risk of developing colon cancer. If so, there would be possibilities to treat patients with adenomatous polyps with anti-inflammatory drugs targeting the signaling pathways activated by microbial components.
Tregs present in adenomas appear to be activated as they express CD69, and a proportion of the adenoma-associated Tregs also proliferate. However, as the conventional T cells in the adenomas are activated, proliferate and secrete cytokines to the same extent as T cells in the unaffected lamina propria, our data would appear to suggest that local Treg do not have an additional suppressive effect on conventional T cells in the adenoma compartment. However, the cytotoxic capacity of CD8+ T cells was lower in adenoma tissue, and this may result from local immune regulation by Treg. This notion is supported by a recent study in a mouse model of lung adenocarcinoma in which granzyme B-expressing T cells were inhibited by Treg, thus diminishing antitumor immunity [51]. Furthermore, it is not possible to determine the extent of effector cell activation required to reduce tumor progression, and the increased presence of Treg may well render antitumor effector functions less effective or ineffective. Furthermore, the possibility that tumor-induced Tregs act in another location, for example MLN, or on other cell types, for example dendritic cells [52], or endothelial cells cannot be excluded. Related to this, we have previously reported that Tregs may contribute to suppression of tumor-specific immunity by limiting recruitment of effector T cells. Thus, Tregs from colon cancer patients potently inhibited transendothelial migration of effector T cells, while Tregs from healthy individuals had no such effect [8].
To determine whether Tregs, or other factors in the adenoma microenvironment, modulate the signals mediating T cell entry into tissues, local expression of endothelial adhesion molecules and chemokines was assayed. While endothelial adhesion molecules were not changed in the adenomas, an increase in CCL17 and decreases in CCL25 and CXCL11 were observed. However, increased CCL17 expression was not accompanied by an increased influx of CCR4+ Treg in adenomas, as previously observed in human CRC [22].
The intestinal chemokine CCL25 is produced by epithelial cells [53] and is crucial for effector T cell recruitment to the intestinal lamina propria. The present study reports for the first time that intestinal adenomas produce less CCL25 than the unaffected tissue. This is analogous to another epithelial mucosal chemokine, CCL28, which is also produced in lower amounts in colon adenocarcinomas than unaffected tissue [46]. Furthermore, adenomas produced less of the Th1-associated chemokine CXCL11 than the surrounding tissue. A lack of CCL25 and CXCL11 may benefit tumors by reducing recruitment of effector lymphocytes, but also by allowing extraintestinal metastasis of CCR9+ tumor cells, as CCR9-CCL25 interactions prevent the spread of colon cancer cells from the intestine [54]. In addition, high levels of CCR6+ Tregs in both unaffected and adenoma tissue in the APCMin/+ mouse were detected. Interestingly, Liu et al. [35] have shown that CCR6 is necessary for accumulation of Treg in an induced CRC mouse model. Thus, the Tregs accumulating in adenomas have a distinct chemokine receptor profile, largely failing to express CXCR3 together with CCR6, unlike Treg from unaffected tissue. This difference in Treg populations may be beneficial for antitumor immunity already at the adenoma stage as Treg expressing CXCR3 are most efficient in down-regulating Th1-type immune responses [40, 41]. If these Treg cannot gain access to the adenoma, they will not regulate effector T cell responses within the adenoma.
In conclusion, we show that the altered lymphocyte composition previously observed in invasive colorectal tumors is manifest already in intestinal adenomatous polyps, with increased frequencies of Treg and decreased B cell frequencies. The observed altered chemokine production, with higher CCL17 and lower CCL25 and CXCL11 expression in the adenoma may account for these changes. These findings indicate that once immunomodulatory interventions that reduce CRC progression have been identified, they will probably be beneficial already in patients with intestinal polyps and may prevent conversion from adenomas to invasive tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank all patients who participated in the study. The study was supported by grants from the Swedish Research Council, the Swedish Cancer Foundation, the Sahlgrenska University Hospital, the Swedish Society of Medicine, the Ruth and Richard Julin foundation, Assar Gabrielssons foundation, Wilhelm and Martina Lundgren’s foundation, Sigurd and Elsa Goljes foundation, Olle Engkvist´s foundation and Hvitfeldska foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- APC
Adenomatous polyposis coli
- CRC
Colorectal cancer
- LPL
Lamina propria lymphocytes
- MAdCAM-1
Mucosal addressin cellular adhesion molecule-1
- MLN
Mesenteric lymph node
- PLN
Peripheral lymph node
- PMA
Phorbol myristate acetate
- SI
Small intestine
- Treg
Regulatory T cell
- VCAM-1
Vascular cell adhesion molecule-1
- WT
Wild-type
Footnotes
Paulina Akeus and Veronica Langenes have contributed equally to this work.
References
- 1.Weitz J, Moritz K, Jurgen D, Thomas H, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Schulmann K, Reiser M, Schmiegel W. Colonic cancer and polyps. Best Pract Res Clin Gastroenterol. 2002;16:91–114. doi: 10.1053/bega.2002.0268. [DOI] [PubMed] [Google Scholar]
- 3.Choong MK, Tsafnat G. Genetic and epigenetic biomarkers of colorectal cancer. Clin Gastroenterol Hepatol. 2012;10:9–15. doi: 10.1016/j.cgh.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 4.de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769–780. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- 5.Fridman WH, Mlecnik B, Bindea G, Pagès F, Galon J. Immunosurveillance in human non-viral cancers. Curr Opin Immunol. 2011;23:272–278. doi: 10.1016/j.coi.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Mlecnik B, Sanchez-Cabo F, Charoentong P, Bindea G, Pagès F, Berger A, Galon J, Trajanoski Z. Data integration and exploration for the identification of molecular mechanisms in tumor-immune cells interaction. BMC Genomics. 2010;11:S7. doi: 10.1186/1471-2164-11-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 8.Enarsson K, Lundin BS, Johnsson E, Brezicka T, Quiding-Järbrink M. CD4+CD25 high regulatory T cells reduce T cell transendothelial migration in cancer patients. Eur J Immunol. 2007;37:282–291. doi: 10.1002/eji.200636183. [DOI] [PubMed] [Google Scholar]
- 9.Raghavan S, Quiding-Järbrink M. Regulatory T cells in gastrointestinal tumors. Expert Rev Gastroenterol Hepatol. 2011;5:489–501. doi: 10.1586/egh.11.44. [DOI] [PubMed] [Google Scholar]
- 10.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2009;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 11.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi M-B, Harpole DH, Patz EF. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 15.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu Y-X, Wang F-S. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 16.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 17.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 18.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, Warren BF, Piris J, Roncador G, Fox SB, Banham AH, Cerundolo V. Increased frequency of regulatory T cells in peripheral blood and tumor infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007;7:7. [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette M-T, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, Farcet J-P, Sobhani I. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57:772–779. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 21.Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008;99:1867–1873. doi: 10.1038/sj.bjc.6604756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson H, Olofsson V, Lundin S, Yakkala C, Björck S, Börjesson L, Gustavsson B, Quiding-Järbrink M. Accumulation of CCR4+CTLA-4hi FOXP3+CD25hi regulatory T Cells in colon adenocarcinomas correlate to reduced activation of conventional T cells. PLoS One. 2012;7:e30695. doi: 10.1371/journal.pone.0030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteside TL. Regulatory T cell subsets in human cancer: Are they regulating for or against tumor progression? Cancer Immunol Immunother. 2013;63:67–72. doi: 10.1007/s00262-013-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan F, Zhang L, Wu J, Zhang J, Zhang S, Li K, Qi Y, Lin P. IL-23/IL-23R: potential mediator of intestinal tumor progression from adenomatous polyps to colorectal carcinoma. Int J Colorectal Dis. 2011;26:1511–1518. doi: 10.1007/s00384-011-1232-6. [DOI] [PubMed] [Google Scholar]
- 25.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 26.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 27.Staal FJT, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 28.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietrich WF, Lander ES, Smith JS, Moser AR, Gould KA, Luongo C, Borenstein N, Dove W. Genetic identification of Mom-l, a major modifier locus affecting min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 30.Westlund J, Livingston M, Fahlén-Yrlid L, Oldenborg P-A, Yrlid U. CD47-deficient mice have decreased production of intestinal IgA following oral immunization but a maintained capacity to induce oral tolerance. Immunology. 2012;135:236–244. doi: 10.1111/j.1365-2567.2011.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.You S, Ohmori M, Peña MMO, Nassri B, Quiton J, Al-Assad ZA, Liu L, Wood PA, Berger SH, Liu Z, Wyatt MD, Price RL, Berger FG, Hrushesky WJM. Developmental abnormalities in multiple proliferative tissues of ApcMin/+ mice. Int J Exp Pathol. 2006;87:227–236. doi: 10.1111/j.1365-2613.2006.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell ALM. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci. 2010;107:5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotheraphy of cancer. Cancer Sci. 2006;97:1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J, Wang H. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6:e19495. doi: 10.1371/journal.pone.0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 38.Kunkel EJ, Cambell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–323. doi: 10.1080/mic.10.3-4.313.323. [DOI] [PubMed] [Google Scholar]
- 39.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno EDT, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. The cytokines interleukin 27 and interferon-γ promote distinct treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 2012;72:4351–4360. doi: 10.1158/0008-5472.CAN-12-0579. [DOI] [PubMed] [Google Scholar]
- 42.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staal FJT, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coletta PL. Lymphodepletion in the ApcMin/+ mouse model of intestinal tumorigenesis. Blood. 2004;103:1050–1058. doi: 10.1182/blood-2003-03-0707. [DOI] [PubMed] [Google Scholar]
- 45.Muthuswamy RV, Sundström P, Börjesson L, Gustavsson B, Quiding-Järbrink M. Impaired migration of IgA-secreting cells to colon adenocarcinomas. Cancer Immunol Immunother. 2013;62:989–997. doi: 10.1007/s00262-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dimberg J, Hugander A, Wågsäter D. Protein expression of the chemokine, CCL28, in human colorectal cancer. Int J Oncol. 2006;28:315–319. [PubMed] [Google Scholar]
- 47.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu G-Y, Österreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumor growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Hu L-L, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, Herdman S, Varki N, Corr M, Lee J, Raz E. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16:665–670. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganesan AP, Johansson M, Ruffell B, Beltran A, Lau J, Jablons DM, Coussens LM. Tumor-infiltrating regulatory t cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. J Immunol. 2013;191:2009–2017. doi: 10.4049/jimmunol.1301317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hubert P, Jacobs N, Caberg J-H, Boniver J, Delvenne P. The cross-talk between dendritic and regulatory T cells: Good or evil? J Leukoc Biol. 2007;82:781–794. doi: 10.1189/jlb.1106694. [DOI] [PubMed] [Google Scholar]
- 53.Wurbel M-A, McIntire MG, Dwyer P, Fiebiger E. CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS One. 2011;6:e16442. doi: 10.1371/journal.pone.0016442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen HJ, Edwards R, Tucci S, Bu P, Milsom J, Lee S, Edelmann W, Gümüs ZH, Shen X, Lipkin S. Chemokine 25-induced signaling suppresses colon cancer invasion and metastasis. J Clin Invest. 2012;122:3184–3196. doi: 10.1172/JCI62110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


