Abstract
Objective
Dendritic cell (DC)-based cancer vaccines may have a significant benefit to patients with advanced pancreatic cancer. However, variations among clinical studies make it difficult to compare clinical outcomes. Here, we identified factors that determined the clinical benefits by analyzing data obtained at seven Japanese institutions that employed the same DC preparation and treatment regimens.
Methods
Of 354 patients who met the inclusion criteria, 255 patients who received standard chemotherapy combined with peptide-pulsed DC vaccines were analyzed.
Results
The mean survival time from diagnosis was 16.5 months (95 % CI 14.4–18.5) and that from the first vaccination was 9.9 months (95 % CI 8.0–12.9). Known prognostic baseline factors related to advanced pancreatic cancer, namely ECOG-PS, peritoneal metastasis, liver metastasis, and the prognostic nutrition index, were also representative. Importantly, we found that erythema reaction after vaccination was an independent and treatment-related prognostic factor for better survival and that OK-432 might be a good adjuvant enhancing the antitumor immunity during DC vaccination.
Conclusions
This is the first report of a multicenter clinical study suggesting the feasibility and possible clinical benefit of an add-on DC vaccine in patients with advanced pancreatic cancer who are undergoing chemotherapy. These findings need to be addressed in well-controlled prospective randomized trials.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1554-7) contains supplementary material, which is available to authorized users.
Keywords: Advanced pancreatic cancer, Chemotherapy, Dendritic cell vaccine, OK-432, Erythema
Introduction
Pancreatic cancer was the fourth leading cause of death from cancer in the United States in 2010 [1] and the fifth leading cause of death from cancer in Japan, and its incidence is still increasing [2]. Although a complete surgical resection is the only way to offer potentially curative therapy to patients with pancreatic cancer, only 5–25 % of patients with pancreatic cancer can be treated surgically [3]. Thus, the majority of pancreatic cancer cases are usually advanced and inoperable, and they are highly intractable because of the limited number of chemotherapeutic agents that can be applied.
Gemcitabine (GEM) has been a standard and first-line chemotherapeutic agent against advanced pancreatic cancers worldwide, and it was the first agent to demonstrate significant survival and clinical benefits over fluorouracil (5-FU) in a randomized trial [4]. The results of a randomized phase III study (the GEST Study) were reported as Asian race-specific definitive evidence of the efficacy of GEM and the chemotherapeutic agent S-1 and their combination against advanced pancreatic cancer in Japan and Taiwan [5]. S-1 (Taiho Pharmaceutical Co., Tokyo) is an oral drug containing tegafur (a prodrug of 5-FU), with 5-chloro-2,4-dihydropyrimidine (CDHP), and potassium oxonate in a molar ratio of 1:0.4:1 [6].
CDHP reversibly antagonizes the activity of dihydropyrimidine dehydrogenase, the rate-limiting enzyme for the degradation of 5-FU. The GEST study demonstrated the noninferiority of S-1 to GEM [mean survival time (MST): 8.8 months in the GEM group, 9.7 months in the S-1 group, and 10.1 months in the combination group] [5]. However, these prognostic improvements of patients with pancreatic cancer are still unsatisfactory, and novel agents and approaches are much desired.
Dendritic cells (DCs) are well known as potent antigen-presenting cells in humans [7], and since the first promising clinical study using DC-based vaccines [8, 9], a number of clinical trials using DC-based vaccines against advanced malignancies, including pancreatic cancers [10–15], have been conducted worldwide. All of these were early clinical studies with limited numbers of patients, and the results demonstrated that the DC vaccines elicited antitumor immune responses without any serious toxicity. However, very limited information regarding the survival benefits achieved with these vaccines is available, and therefore, randomized control studies with large patient series are thus needed to clarify the exact benefits of DC-based vaccines for advanced pancreatic cancers.
Preconceptions regarding the myelosuppressive effect of chemotherapeutic agents have made researchers reluctant to combine chemotherapy and DC vaccines in clinical settings. However, some valuable experimental studies indicated that GEM and fluorouracil could augment antitumor immune responses in vitro and in vivo [16, 17], suggesting that the add-on use of DC vaccines with standard chemotherapy may have a synergistic benefit for patients with advanced pancreatic cancers.
Therefore, here, we retrospectively analyzed the clinical data of 255 patients under standard chemotherapy for inoperable pancreatic cancer, vaccinated with synthetic tumor antigen peptide-pulsed DCs at seven individual medical institutions in Japan. Importantly, these institutions: (1) used a unified Standard Operating Procedure (SOP) to generate DC vaccines based on previous clinical studies with minor modifications [15, 18–21] by tella Inc. (Tokyo), (2) used the same synthetic peptides of Wilms’ tumor gene 1 (WT1) and/or Mucin 1 (MUC1) as tumor antigens under an ascertained rule, and (3) performed a similar treatment regimen for the vaccinations. The identification of independent factors related to the survival of the patients in this study will definitely contribute to the design of future larger-sized randomized prospective studies of DC vaccines against pancreatic malignancies.
Patients and methods
Patients
This study was a retrospective analysis of the cases that were institutional review board (IRB) approved compassionate treatments, but not prospectively planned clinical trials, among seven medical centers in Japan. A total of 354 Japanese patients with inoperable pancreatic cancers treated between June 2007 and July 2012 who met the following inclusion criteria were eligible for the present analyses: (1) they were clinically diagnosed as having inoperable pancreatic cancer due to locally advanced or metastatic pancreatic cancer; (2) the expected prognosis was over 4 months, and they had (3) a white blood cell (WBC) count of 2,500 cells/μL or more, (4) hemoglobin (Hb) of 7.0 g/dL or more, (5) a platelet count of 70,000 counts/μL or more, and they were (6) without serious dysfunction of vital organs.
The patients were enrolled at seven medical centers in Japan (Shinshu University Hospital, Nagasaki University Hospital, Sapporo Hokuyu Hospital, Seren Clinic Tokyo, Seren Clinic Nagoya, Seren Clinic Kobe, and Seren Clinic Fukuoka). Each patient had received the DC vaccine more than five times as described below. Treatment was done according to the Declaration of Helsinki, and all participants signed informed consent forms. This patient treatment was approved by the IRB of each institution (Approval numbers: #1199 for Shinshu University Hospital, #10100133 for Nagasaki University Hospital, #15 for Sapporo Hokuyu Hospital, and Medicine 24-4 for Seren Clinic Tokyo, Nagoya, Seren Kobe, and Fukuoka).
DC vaccines
Preparation of DCs
DCs were prepared as described [15, 18–21]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from the leukapheresis products by Ficoll-Hypaque gradient density centrifugation. These PBMCs were placed on tissue culture plates, and the adherent cells were cultured in medium containing human recombinant granulocyte–macrophage colony-stimulating factor (500 ng/mL; Primmune Inc., Kobe, Japan) and human recombinant interleukin-4 (250 ng/mL; R&D Systems Inc., Minneapolis, MN) to generate immature DCs. Five days later, the DCs were stimulated with OK-432 (10 μg/mL) and prostaglandin E2 (50 ng/mL) for 24 h. The DCs were then pulsed with peptide antigens according to the HLA-A pattern. WT1 was pulsed to the DCs 24 h after treatment with OK-432 and prostaglandin E2. MUC1 was added to the DCs’ culture media at the same time as OK-432 and prostaglandin E2. The DCs were cryopreserved and kept until the day of administration. The phenotype CD14−/low/HLA-DR+/HLA-ABC+/CD80+/CD83+/CD86+/CD40+/CCR7+ was taken to define mature DCs. Cells were prepared by well-trained technical staff in each institutional cell processing center under the SOP provided by tella Inc. (http://www.tella.jp/en/). Regarding release criteria, testing for sterility, mycoplasma (PCR method), and endotoxin (Endospecy™, Seikagaku Corp., Tokyo) was done using the supernatant or cell suspension just before the tube filling.
Peptide antigens
WT1 and/or MUC1 peptide antigens were pulsed to DCs, and WT1 was used according to patient’s HLA-A type; CYTWNQMNL (mutant WT1 peptide, Neo-MPS, San Diego, CA) for HLA-A*24:02 or RMFPNAPYL (WT1 peptide, Neo-MPS) for HLA- A*02:01/02:06. MUC1 long peptide TRPAPGSTAPPAHGVTSAPDTRPAPGSTAP (Greiner Japan, Tokyo) was used for any HLA-A type. We did not include immunohistochemistry to select these peptides, because previous studies showed the overexpression of the WT1 and MUC1 with pancreatic cancer: WT1 gene was detected by immunohistochemistry in 75 % of patients with pancreatic cancer [22], and MUC1 mRNA was also detected in 83 % [23].
Patient treatment and clinical assessments
All of the patients were injected five or more times intradermally with DCs in close proximity to axial and/or inguinal lymph nodes, biweekly. At the vaccination, 0.1 mL of intradermal DC vaccine at the forearm was used for the assessment of erythema response. When the patient wanted it, OK-432 (a lyophilized preparation of Streptococcus pyogenes to enhance the Th1 response) [24, 25] was administered at appropriate doses (0.5 KE [Klinische Einheit clinical unit] as the initial dose, increased gradually until the patient’s temperature reached 38 °C, that should be less than 5.0 KE/dose) simultaneously with the DC vaccine as an immunological adjuvant. The clinical parameters studied were gender, age, Eastern Cooperative Oncology Group performance status (ECOG-PS), clinical stage, laboratory data at leukapheresis, and combined chemotherapy.
The maximum diameter of erythema was measured after 24–48 h had passed, and patients who exhibited erythema ≥30 mm in diameter at least once were categorized as showing a positive; the others were negative. Adverse events were graded and documented according to the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0). The prognostic nutritional index (PNI) [26, 27] was used as an index assessing the patients’ nutritional condition. The PNI was calculated using the following equation: PNI = 10 × serum albumin (g/dL) + 0.005 × total lymphocyte counts.
Statistical analyses
Survival curves were plotted using the Kaplan–Meier method, and survival curve comparisons were conducted with the log-rank test as well as Wilcoxon tests. We conducted multivariate analyses of the impacts of the factors using Cox’s proportional hazards regression model, and we used the laboratory data at leukapheresis in this analysis. The differences between the two groups in category data were analyzed by means of the Mann–Whitney U test or Pearson’s chi-square test. The significance of results was accepted at P values <0.05. Analyses were conducted using JMP version 9.0 (SAS Institute Japan, Tokyo).
Results
Patient characteristics
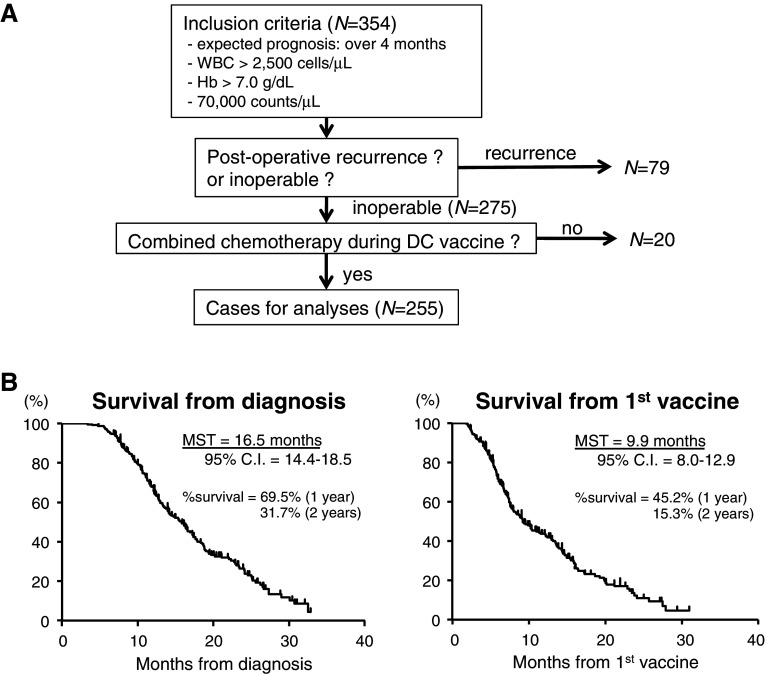
A total of 354 patients with advanced pancreatic cancer were enrolled. The flow diagram of patient selection for the analyses is shown in Fig. 1. Of these patients, 79 patients with postoperative recurrence were excluded to avoid bias in the overall survival. Twenty patients who did not receive chemotherapy during their DC vaccine period were also excluded. Finally, 255 patients were eligible for analyses. Only 12 patients received DC vaccines simultaneously with first-line chemotherapy; the other 243 patients began receiving DC vaccines after first- or second-line chemotherapy.
Fig. 1.
Recruited patients and their overall survivals. a Diagram of the selection of patients for statistical analyses. Data collection was done with patients who met the inclusion criteria (n = 354). After the exclusion of patients with postoperative recurrence and DC vaccines without chemotherapy, the cases of the remaining 255 patients who received combined chemotherapy and an add-on DC vaccine (with previous chemotherapy: n = 243, 95.3 %; without previous chemotherapy: n = 12, 4.7 %) were used for the analyses. b Kaplan–Meier plots for the overall survival of the 255 patients from diagnosis (left graph, MST = 16.5 months) and that from the first DC vaccine (right graph, MST = 9.9 months)
The clinical characteristics of all patients are summarized in the left column of Table 1. Among the 255 patients, 78 (31 %) patients had locally invasive pancreatic cancer, and the other 177 (69 %) had metastatic disease including liver, lung, peritoneum, and lymph nodes. WT1 peptide-pulsed DCs were used for 207 patients (WT1 only: n = 27; WT1 and MUC1: n = 180), and the other 48 patients were administered DCs pulsed with MUC1 only. All patients were simultaneously treated with standard chemotherapy: GEM only = 135 (53 %), GEM + S-1 = 63 (25 %), and S-1 only = 44 (17 %). Twelve (5 %) patients received radiotherapy during their DC vaccine treatment.
Table 1.
Baseline characteristics of the 255 patients with inoperable pancreatic cancer
| All cases (n = 255)a | Erythema | P value | ||
|---|---|---|---|---|
| negative (n = 145) | positive (n = 107) | |||
| Age (year) | ||||
| Median (range) | 63 (27–84) | 62 (27–81) | 63 (38–84) | 0.42 |
| Gender—no. (%) | ||||
| Male | 135 (53) | 78 (54) | 55 (51) | 0.71 |
| Female | 120 (47) | 67 (46) | 52 (49) | |
| ECOG performance status score—no. (%) | ||||
| 0 | 59 (23) | 30 (21) | 27 (25) | 0.58 |
| 1 | 159 (62) | 94 (65) | 64 (60) | |
| 2 | 31 (12) | 17 (12) | 14 (13) | |
| 3 | 4 (1.6) | 3 (2) | 1 (1) | |
| 4 | 1 (0.4) | 0 (0) | 1 (1) | |
| NA | 1 (0.4) | 1 (1) | 0 (0) | |
| Clinical stage—no. (%) | ||||
| locally invasive | 78 (31) | 37 (26) | 39 (36) | 0.06 |
| metastasis | 177 (69) | 108 (74) | 68 (64) | |
| Laboratory data at leukapheresis (mean ± S.D.) | ||||
| WBC (/mL) | 5,447 (±2,140) | 5,556 (±2,082) | 5,306 (±2,174) | 0.10 |
| No. of lymphocytes (/mL) | 1,305 (±550) | 1,353 (±595) | 1,245 (±481) | 0.20 |
| Hemoglobin (g/dL) | 11.4 (±1.8) | 11.4 (±1.6) | 11.4 (±1.6) | 0.98 |
| Albumin (g/dL) | 3.9 (±0.5) | 3.9 (±0.6) | 4.0 (±0.4) | 0.24 |
| CRP (mg/dL) | 0.9 (±1.7) | 1.04 (±2.1) | 0.59 (±0.9) | 0.24 |
| WT1 A*2,402/*0,201/*0206 (%) | 207 (81) | 114 (79) | 90 (84) | 0.27 |
| MUC1 (%) | 226 (89) | 129 (89) | 95 (89) | 0.96 |
| OK432 | 184 | 88 | 96 | <0.01* |
| Time to start DC vaccination from diagnosis (months) | ||||
| Median (range) | 4.0 (1–36) | 3.9 (1–36) | 4.2 (1–31) | 0.70 |
| Number of DC vaccines (/leukapheresis) | ||||
| Median (range) | 8 (5–55) | 7 (5–38) | 8 (5–55) | 0.16 |
| Viability of DC vaccines (%) | ||||
| Median (range) | 84.4 (42.0–97.5) | 85.0 (45.3–95.1) | 83.2 (42.0–97.5) | 0.27 |
| Standard therapy combined with DC vaccine—no. (%) | ||||
| Chemotherapy | ||||
| GEM | 135 (53) | 78 (54) | 56 (52) | 0.63 |
| GEM+S-1 | 63 (25) | 38 (26) | 24 (22) | |
| S-1 | 44 (17) | 23 (16) | 21 (20) | |
| others | 13 (5) | 6 (4) | 6 (6) | |
| Radiotherapy | 12 (5) | 4 (3) | 8 (7) | 0.18 |
aincluding no record of erythema (n = 3)
* Statistically significant
As shown in Fig. 1b, the MST of these patients from diagnosis was 16.5 months [left graph, 95 % confidence interval (CI) = 14.4–18.5] and that from the first vaccination was 9.9 months (right graph, 95 % CI = 8.0–12.9). The 1-year survival rates from diagnosis and the first vaccination were 69.5 and 45.2 %, respectively, and the 2-year survival rate from each was 31.7 and 15.3 %, respectively.
Safety
The treatments were well tolerated by all of the patients. The common adverse events in this study were injection site reaction (42 %, Grade 1 or 2) and fever (25 %, Grade 1 or 2) within a few days after vaccination. There were no serious adverse events due to the DC vaccinations.
Prognostic factors related to the survival from first DC vaccination
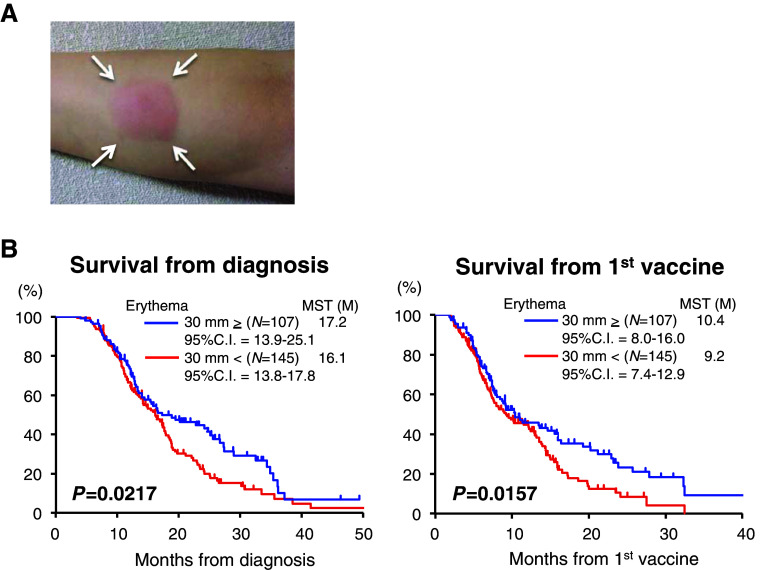
The univariate analyses with log-rank tests demonstrated that several previously known prognostic baseline factors related to advanced pancreatic cancer, namely ECOG-PS [28, 29], peritoneal metastasis [29–31], liver metastasis [29], and PNI, were associated with the survival from the first DC vaccine (Suppl. Fig. S1). Among the treatment-related factors, erythema at the injected site (30 mm in longitudinal diameter or more, Fig. 2a) was the only significant factor correlated with survival not only from the first vaccination (Fig. 2b, right graph, P = 0.0157) but also with that from overall survival from diagnosis (Fig. 2b, left graph, P = 0.0217), exhibiting a typical ‘delayed separation’ curve [32]. These findings regarding the treatment-related factors were also confirmed by the multivariate analysis (Cox’s proportional hazards regression model), as shown in Table 2, indicating that erythema at the injected site might be an independent prognostic marker predicting patient survival.
Fig. 2.
Erythema at the injected site as a treatment-related prognostic factor affecting overall survival. a Typical and representative findings of the erythema (white arrows) at the monitoring injection site of the forearm. For each patient, 0.1 μL of vaccine solution (containing approx. 1 × 106 cells) was injected intradermally, and the longitudinal axis of erythema was measured on the same day, the next day, and the day after. B. Kaplan–Meier plots for the overall survival of all 255 patients showing erythema (≥30 mm in dia.) or not from diagnosis (left graph) and that from the first DC vaccine (right graph)
Table 2.
Uni- and multivariate analyses: treatment factors related to DC vaccination
| Cases | MST | Log-rank | Wilcoxon | Cox’s hazard regression | |||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95 % CI | P value | |||||
| A. Overall survival | |||||||
| WT1 | |||||||
| Y | 207 | 16.1 | 0.682 | 0.444 | 1.179 | 0.795–1.808 | 0.422 |
| N | 48 | 18.3 | |||||
| MUC1 | |||||||
| Y | 227 | 16.3 | 0.208 | 0.400 | 1.394 | 0.175–2.374 | 0.175 |
| N | 28 | 18.8 | |||||
| Erythema | |||||||
| ≥3 cm | 107 | 17.2 | 0.022* | 0.120 | 0.685 | 0.497–0.938 | 0.018* |
| <3 cm | 145 | 16.1 | |||||
| Radiation during vaccine | |||||||
| Y | 12 | 16.3 | 0.543 | 0.676 | 0.928 | 0.412–1.808 | 0.840 |
| N | 243 | 16.5 | |||||
| B. Survival from first vaccine | |||||||
| WT1 | |||||||
| Y | 207 | 9.2 | 0.987 | 0.654 | 1.082 | 0.731–1.658 | 0.701 |
| N | 48 | 13.8 | |||||
| MUC1 | |||||||
| Y | 227 | 9.2 | 0.159 | 0.259 | 1.467 | 0.914–2.504 | 0.116 |
| N | 28 | 13.9 | |||||
| Erythema | |||||||
| >3 cm | 107 | 10.4 | 0.016* | 0.120 | 0.659 | 0.478–0.901 | 0.009* |
| <3 cm | 145 | 9.2 | |||||
| Radiation during vaccine | |||||||
| Y | 12 | 7.4 | 0.595 | 0.529 | 1.445 | 0.645–2.793 | 0.343 |
| N | 243 | 10.2 | |||||
* Statistically significant
Predicting factors correlated with the erythema
The patients’ baseline characteristics according to the erythema (positive: n = 107 and negative: n = 145) are given in Table 1, right columns. Almost all factors, except for the use of OK-432 (P < 0.01), were not significant between these two groups; however, neither the use of OK-432 (P = 0.313) nor the amount of OK-432 used (P = 0.476) showed a significant correlation to the Kaplan–Meier curves and log-rank tests (data not shown), indicating that the use of OK-432 itself did not contribute to the survival of the patients.
We therefore next hypothesized that not only the use of OK-432 but also other multiple factors might be related to the erythema reaction, and we analyzed the clinical factors by Pearson’s chi-square test. As shown in Table 3, in addition to the use of OK-432 (P = 0.005), fever after vaccination (P < 0.0001), serum albumin at the time of leukapheresis (P = 0.027), and PNI (P = 0.023) were significantly correlated with the appearance of erythema, suggesting that the overlap of some of these factors may be important to prolong patient survival.
Table 3.
Factors correlated with the erythema
| Erythema | Odds ratio | 95 % CI | P value | ||
|---|---|---|---|---|---|
| <30 mm | >30 mm | ||||
| Ascites | |||||
| N | 130 | 99 | 0.700 | 0.286–1.717 | 0.435 |
| Y | 15 | 8 | |||
| Liver | |||||
| N | 5 | 69 | 0.780 | 0.466–1.307 | 0.345 |
| Y | 60 | 38 | |||
| Peritoneum | |||||
| N | 120 | 93 | 0.723 | 0.356–1.467 | 0.367 |
| Y | 25 | 14 | |||
| Lung | |||||
| N | 125 | 94 | 0.864 | 0.409–1.826 | 0.702 |
| Y | 20 | 13 | |||
| Lymph node | |||||
| N | 123 | 90 | 1.106 | 0.552–2.216 | 0.776 |
| Y | 21 | 17 | |||
| Stage | |||||
| Local | 37 | 39 | 0.597 | 0.347–1.023 | 0.062 |
| Metastasis | 108 | 68 | |||
| WT1 | |||||
| N | 31 | 17 | 1.440 | 0.750–2.766 | 0.273 |
| Y | 114 | 90 | |||
| MUC1 | |||||
| N | 16 | 12 | 0.982 | 0.444–2.172 | 0.964 |
| Y | 129 | 95 | |||
| Fever | |||||
| N | 125 | 63 | 4.365 | 2.373–8.027 | <0.0001* |
| Y | 20 | 44 | |||
| OK432 | |||||
| N | 49 | 19 | 2.364 | 1.293–4.323 | 0.005* |
| Y | 96 | 88 | |||
| Previous radiation | |||||
| N | 106 | 76 | 1.523 | 0.744–3.116 | 0.237 |
| Y | 39 | 31 | |||
| Combined radiation | |||||
| N | 141 | 99 | 2.848 | 0.835–9.720 | 0.082 |
| Y | 4 | 8 | |||
| N/L ratio | |||||
| <4 | 95 | 78 | 0.706 | 0.409–1.220 | 0.212 |
| ≥4 | 50 | 29 | |||
| CRP | |||||
| <0.5 | 87 | 74 | 0.641 | 0.373–1.103 | 0.107 |
| ≥0.5 | 55 | 30 | |||
| Albumin | |||||
| <3.5 | 31 | 12 | 2.228 | 1.083–4.582 | 0.027* |
| ≥3.5 | 109 | 94 | |||
| Hb | |||||
| <12 | 90 | 70 | 0.865 | 0.514–1.456 | 0.585 |
| ≥12 | 55 | 37 | |||
| PNI | |||||
| <40 | 28 | 10 | 2.400 | 1.109–5.193 | 0.023* |
| ≥40 | 112 | 96 | |||
N/L neutrophils/lymphocytes Pearson’s χ2-test
* Statistically significant
To test this hypothesis, we statistically analyzed the possible link between the use of OK-432 and fever increase after vaccination. As shown in Supplementary Table S1 and Table 4, although OK-432 was given to patients without discrimination according to their baseline characteristics including serum albumin level and PNI, the adjuvant use of OK-432 was significantly correlated with fever increase after vaccination.
Table 4.
Correlation analysis of the use of OK432 and fever increase after vaccination
| OK432 | P value | |||
|---|---|---|---|---|
| − | + | |||
| Albumin | <3.5 | 12 | 31 | 0.716 |
| >3.5 | 52 | 154 | ||
| PNI | <40 | 8 | 30 | 0.476 |
| >40 | 56 | 155 | ||
| Fever | − | 58 | 132 | 0.017* |
| + | 10 | 55 | ||
Pearson’s χ2 test
* Statistically significant
Together these findings suggest that: (1) patients demonstrating higher serum albumin levels (≥3.5) and good PNI values (≥40) might be better responders for erythema reaction; (2) patients showing an increased fever might be better responders for erythema reaction; in other words, erythema might predict a better prognosis for an advanced pancreatic cancer patient treated with DC vaccine, and (3) erythema reaction would be seen more frequently in patients treated with OK-432, suggesting that OK-432 might be a good adjuvant enhancing the antitumor immunity during DC vaccination.
Discussion
The main aim of this study was to identify essential factors that are related to the survival of patients with inoperative pancreatic cancers treated not only with chemotherapy but also an add-on DC vaccine. To do this, we performed an exploratory analysis of 255 Japanese patients with inoperable pancreatic cancer under standard chemotherapy and vaccinated with synthetic WT1 and/or MUC1 peptide-pulsed DCs in seven individual medical institutions. The key findings obtained in this study were as follows: (1) no treatment-related serious adverse event was observed during the study period, (2) previously known prognostic baseline factors related to advanced pancreatic cancer, i.e., ECOG-PS, peritoneal metastasis, liver metastasis, and PNI were also representative in this study, and (3) erythema at the forearm injection site after vaccination as a monitoring parameter was an independent prognostic factor for the survival of patients. To our best of knowledge, this is the first report of a multicenter clinical study using intradermal DC vaccines for advanced pancreatic cancer patients with well-organized and well-controlled autologous cell preparation and a similar treatment regimen.
It has been thought that chemotherapeutic agents might not be appropriate for add-on cancer vaccines, because it is possible that their toxicity to blood cells and immune cells might reduce the vaccine-originated antitumor immune responses. However, we here confirmed that a DC vaccine add-on to standard chemotherapy was safe, feasible, and well tolerated by patients under treatment with GEM and/or S-1 and that the outcomes showed a typical ‘delayed separation’ survival curve in view of the ‘skin erythema reaction at the DC injected site’ that suggested the possible effect of the cancer vaccine, as noted previously [32]. Together these findings and previous reports indicating the beneficial effect of GEM and 5-FU on antitumor immune response in experimental conditions [16, 17] suggest that the use of GEM and 5-FU and its related compounds (i.e., S-1) may be a good option when prospective cancer vaccine trials are planned.
Secondly, our present findings confirmed that previously known prognostic baseline factors related to advanced pancreatic cancer, namely ECOG-PS [28, 29], peritoneal metastasis [29–31], and liver metastasis [29], were also representative in this study; therefore, we primarily consider that these factors related to the chemotherapeutic responses were still shared by patients who received the add-on DC vaccine. Further prospective studies, however, are still necessary to determine whether a DC vaccine can significantly improve the survival of advanced pancreatic cancer patients whose cases present with these factors.
Thirdly, the most important finding of the present study was the identification of a treatment-related factor—erythema at the forearm injected site after vaccination for monitoring the local reaction—as a significant and independent prognostic factor demonstrating better survival of patients, by univariate and multivariate analyses. Importantly, divergences of survival rates accompanying the delayed separation curve were observed in two groups according to the presence of erythema, implying a possible vaccine effect [32]. It was shown previously that delayed-type hypersensitivity (DTH)-based local responses represent an important source of information concerning in vivo T cell function and tumor antigen-specific T cells in the DTH reaction [33] and that DTH was also correlated with clinical responses [34, 35]. Precisely speaking, the erythema reaction observed in the present study was not equal to DTH in these earlier studies; however, it may be reasonable to propose that these two methods reflect similar responses. Further studies are needed to clarify whether erythema after DC vaccines definitively reflects a therapeutic effect and whether monitoring erythema might be one approach to evaluate the reaction to a vaccine.
Important questions remain: (1) which baseline factor(s) is (are) essential to detect the possible responders to DC vaccines and (2) whether adjuvant treatment is required to augment the effect of a DC vaccine. In the present study, although serum albumin and PNI at leukapheresis were not significant and independent factors directly affecting patient survival, they were significantly correlated with the frequency of erythema reaction (Table 3).
These findings suggest that these two baseline factors may be used to identify better responders for erythema reaction. Regarding the second factor, the use of OK-432 was significantly correlated with increased fever after vaccination (Table 4). We should examine the possible antitumor effect of OK-432 itself on these study populations; however, such an effect seems unlikely because there has been no clinical trial demonstrating definitive efficacy; for example, a multicenter prospective randomized study of advanced gastric cancer patients that used doses and frequencies of OK-432 administration similar to those used in the present study demonstrated negative results [36]. The hypothesis that OK-432 might be a good adjuvant enhancing antitumor immunity during DC vaccination should be tested in well-controlled prospective clinical trials.
In summary, in the present retrospective study, we identified erythema after DC vaccination as a good prognostic factor of advanced pancreatic cancer patients, and we observed that erythema reactions are seen more frequently in patients treated with OK-432, suggesting that OK-432 might be a good adjuvant enhancing the antitumor immunity during DC vaccination. These findings seem reasonable and encouraging; however, because of the retrospective and exploratory nature of the present study, these findings need to be addressed in well-controlled prospective randomized trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This report is dedicated to the patients who participated in our studies and to their primary oncology doctors. We also thank the present and former staff of each participating institution. Data collection from each institution was supported by the Research and Development Division of tella Inc., and data analyses were supported and checked by Dr. J. Kishimoto, Kyushu University, as an independent specialist from the company.
Conflict of interest
Prof. Y. Yonemitsu is also a member of the Board of Directors on Science and Medicine at tella Inc., and Drs. S. Yusa and T. Ishidao are the current and former chiefs of the Research and Development Division of tella Inc., respectively. Dr. Okamoto, who was excluded from the data analyses, is a stockholder of tella Inc. All remaining authors have declared no conflicts of interest.
Contributor Information
Masaki Nagaya, Phone: +81-3-3449-6095, Email: m2nagaya@marianna-u.ac.jp.
Yoshikazu Yonemitsu, Phone: +81-92-642-4777, FAX: +81-92-642-4777, Email: yonemitu@med.kyushu-u.ac.jp.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Wada K, Takaori K, Traverso LW, Hruban RH, Furukawa T, Brentnall TA, et al. Clinical importance of familial pancreatic cancer registry in Japan: a report from kick-off meeting at international symposium on pancreas cancer 2012. J Hepatobiliary Pancreat Sci. 2013;20:557–566. doi: 10.1007/s00534-013-0611-5. [DOI] [PubMed] [Google Scholar]
- 3.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: gEST Study. J Clin Oncol. 2013;31:1640–1648. doi: 10.1200/JCO.2012.43.3680. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima M, Satake H, Uchida J, Shimamoto Y, Kato T, Takechi T, et al. Preclinical antitumor efficacy of S-1: a new oral formulation of 5-fluorouracil on human tumor xenografts. Int J Oncol. 1998;13:693–698. doi: 10.3892/ijo.13.4.693. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 9.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 10.Pecher G, Häring A, Kaiser L, Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother. 2002;51:669–673. doi: 10.1007/s00262-002-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo H, Hazama S, Kawaoka T, Yoshino S, Yoshida S, Tokuno K, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28:379–387. [PubMed] [Google Scholar]
- 12.Suso EM, Dueland S, Rasmussen AM, Vetrhus T, Aamdal S, Kvalheim G, et al. hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol Immunother. 2011;60:809–818. doi: 10.1007/s00262-011-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer C, Dauer M, Saraj S, Schnurr M, Bauernfeind F, Sterzik A, et al. Dendritic cell-based vaccination of patients with advanced pancreatic carcinoma: results of a pilot study. Cancer Immunol Immunother. 2011;60:1097–1107. doi: 10.1007/s00262-011-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rong Y, Qin X, Jin D, Lou W, Wu L, Wang D, et al. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin Exp Med. 2011;12:173–180. doi: 10.1007/s10238-011-0159-0. [DOI] [PubMed] [Google Scholar]
- 15.Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195–205. doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 16.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 17.Bauer C, Bauernfeind F, Sterzik A, Orban M, Schnurr M, Lehr HA, et al. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut. 2007;56:1275–1282. doi: 10.1136/gut.2006.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagayama H, Sato K, Morishita M, Uchimaru K, Oyaizu N, Inazawa T, et al. Results of a phase I clinical study using autologous tumour lysate-pulsed monocyte-derived mature dendritic cell vaccinations for stage IV malignant melanoma patients combined with low dose interleukin-2. Melanoma Res. 2003;13:521–530. doi: 10.1097/00008390-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Kuwabara K, Nishishita T, Morishita M, Oyaizu N, Yamashita S, Kanematsu T, et al. Results of a phase I clinical study using dendritic cell vaccinations for thyroid cancer. Thyroid. 2007;17:53–58. doi: 10.1089/thy.2006.0178. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi H, Okamoto M, Shimodaira S, Tsujitani S, Nagaya M, Ishidao T, DC-vaccine study group at the Japan Society of Innovative Cell Therapy (J-SICT) et al. Impact of dendritic cell vaccines pulsed with Wilms’ tumour-1 peptide antigen on the survival of patients with advanced non-small cell lung cancers. Eur J Cancer. 2013;49:852–859. doi: 10.1016/j.ejca.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, et al. DC-vaccine study group at the Japan society of innovative cell therapy (J-SICT) dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–1617. doi: 10.1007/s11605-013-2286-2. [DOI] [PubMed] [Google Scholar]
- 22.Oji Y, Nakamori S, Fujikawa M, Nakatsuka S, Yokota A, Tatsumi N, et al. Overexpression of the Wilms’ tumor gene WT1 in pancreatic ductal adenocarcinoma. Cancer Sci. 2004;95:583–587. doi: 10.1111/j.1349-7006.2004.tb02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingsworth MA, Strawhecker JM, Caffrey TC, Mack DR. Expression of MUC1, MUC2, MUC3 and MUC4 mucin mRNAs in human pancreatic and intestinal tumor cell lines. Int J Cancer. 1994;57:198–203. doi: 10.1002/ijc.2910570212. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto M, Oshikawa T, Tano T, Ohe G, Furuichi S, Nishikawa H, et al. Involvement of Toll-like receptor 4 signaling in interferon-gamma production and antitumor effect by streptococcal agent OK-432. J Natl Cancer Inst. 2003;95:316–326. doi: 10.1093/jnci/95.4.316. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, et al. Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation. Cancer Res. 2004;64:5461–5470. doi: 10.1158/0008-5472.CAN-03-4005. [DOI] [PubMed] [Google Scholar]
- 26.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI) Br J Cancer. 2012;106:1439–1445. doi: 10.1038/bjc.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Chung MJ, Park JY, Chung JB, Bang S, Park SW, et al. Phase II trial of erlotinib plus gemcitabine chemotherapy in Korean patients with advanced pancreatic cancer and prognostic factors for chemotherapeutic response. Gut Liver. 2013;7:611–615. doi: 10.5009/gnl.2013.7.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers MM, Powell ED, Asmis TR, Jonker DJ, Hilton JF, O’Callaghan CJ, et al. Comorbidity, age and overall survival in patients with advanced pancreatic cancer - results from NCIC CTG PA.3: a phase III trial of gemcitabine plus erlotinib or placebo. Eur J Cancer. 2012;48:1434–1442. doi: 10.1016/j.ejca.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Yi JH, Lee J, Park SH, Lee KT, Lee JK, Lee KH, et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology. 2011;80:175–180. doi: 10.1159/000328449. [DOI] [PubMed] [Google Scholar]
- 31.Inal A, Ciltas A, Yildiz R, Berk V, Kos FT, Dane F, et al. Long term survivors with metastatic pancreatic cancer treated with gemcitabine alone or plus cisplatin: a retrospective analysis of an Anatolian Society of Medical Oncology multicenter study. Asian Pac J Cancer Prev. 2012;13:1841–1844. doi: 10.7314/APJCP.2012.13.5.1841. [DOI] [PubMed] [Google Scholar]
- 32.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, et al. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 34.Hersey P, Menzies SW, Halliday GM, Nguyen T, Farrelly ML, DeSilva C, et al. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol Immunother. 2004;53:125–134. doi: 10.1007/s00262-003-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida S, Koido S, Takeda Y, Homma S, Komita H, Takehara A, et al. Wilms tumor gene (WT1) peptide-based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother. 2014;37:105–114. doi: 10.1097/CJI.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimachi K, Maehara Y, Akazawa K, Kondo Y, Kunii Y, Kitamura M, et al. Postoperative chemotherapy including intraperitoneal and intradermal administration of the streptococcal preparation OK-432 for patients with gastric cancer and peritoneal dissemination: a prospective randomized study. Cancer Chemother Pharmacol. 1994;33:366–370. doi: 10.1007/BF00686264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.