Abstract
IL-21 is an immune-enhancing cytokine, which showed promising results in cancer immunotherapy. We previously observed that the administration of anti-CD4 cell-depleting antibody strongly enhanced the anti-tumor effects of an IL-21-engineered neuroblastoma (NB) cell vaccine. Here, we studied the therapeutic effects of a combination of recombinant (r) IL-21 and anti-CD4 monoclonal antibodies (mAb) in a syngeneic model of disseminated NB. Subcutaneous rIL-21 therapy at 0.5 or 1 μg/dose (at days 2, 6, 9, 13 and 15 after NB induction) had a limited effect on NB development. However, coadministration of rIL-21 at the two dose levels and a cell-depleting anti-CD4 mAb cured 28 and 70 % of mice, respectively. Combined immunotherapy was also effective if started 7 days after NB implant, resulting in a 30 % cure rate. Anti-CD4 antibody treatment efficiently depleted CD4+ CD25high Treg cells, but alone had limited impact on NB. Combination immunotherapy by anti-CD4 mAb and rIL-21 induced a CD8+ cytotoxic T lymphocyte response, which resulted in tumor eradication and long-lasting immunity. CD4+ T cells, which re-populated mice after combination immunotherapy, were required for immunity to NB antigens as indicated by CD4+ T cell depletion and re-challenge experiments. In conclusion, these data support a role for regulatory CD4+ T cells in a syngeneic NB model and suggest that rIL-21 combined with CD4+ T cell depletion reprograms CD4+ T cells from immune regulatory to anti-tumor functions. These observations open new perspectives for the use of IL-21-based immunotherapy in conjunction with transient CD4+ T cell depletion, in human metastatic NB.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1536-9) contains supplementary material, which is available to authorized users.
Keywords: Neuroblastoma, Immunotherapy, IL-21, Monoclonal antibody, T cells
Introduction
Neuroblastoma (NB) is a pediatric tumor that arises from the sympathetic nervous system. The clinical presentation of NB is heterogeneous, ranging from localized tumors to metastatic disease requiring intensive multimodal therapies. Stage 4 NB shows metastatic dissemination and represents approximately 50 % of cases at diagnosis. In spite of an overall improvement in survival, conventional therapy for metastatic NB patients, based on chemotherapy, surgery and autologous hematopoietic stem cell transplantation, allows survival rates of approximately 30 % at 5 years [1, 2]. Indeed, current therapeutic regimens frequently induce a minimal residual disease condition, often followed by a fatal outcome [3, 4]. In these patients, immunotherapy (IT) may provide an additional therapeutic strategy [5].
Cytokine administration is a consolidated IT option. One of the first cytokine shown to have anti-tumor activity in preclinical models of NB was IL-2 [6, 7]. IL-2 is currently used in the adjuvant phase of the European protocol for high-risk NB patients (www.siopen.org), where it enhances NK cell numbers [8]. Although clinical studies with IL-2 in melanoma and renal cancer provided evidences that activation of the immune response may lead to sporadic rejection of bulky tumors, complete responses to IL-2 therapy are only ≈7 % [9]. The limited activity of IL-2 may reflect, at least in part, its role in immune regulation [10], as IL-2 mediates immune-suppressive regulatory T (Treg) cell survival and functions [11].
Conversely, IL-21, a recently identified member of the IL-2 family [12], shares some immune-enhancing activities with IL-2, but does not support Treg cell proliferation [13] and counteracts their immune-suppressive effects [14]. In vitro, it costimulates activated T, B and NK cell functions [15], favoring also the generation of CTLs potentially suitable for adoptive IT [16, 17]. IL-21 induces the expansion of high-affinity CTLs also in vivo [18]. Recent data indicate that IL-21 promotes CTL activity through the transcription factor T-bet [19]. Data obtained in different mouse tumor models unveiled anti-tumor effects mediated by recombinant (r) IL-21 or IL-21-secreting tumor cells [20, 21]. These effects were related to the activation of NK and/or CTL responses [18, 22, 23] or, less frequently, to the induction of anti-tumor antibodies [24]. Interestingly, direct in vivo gene delivery of IL-21 potentiated the anti-tumor effects of an antigen-specific vaccine in a NB model [25].
In view of the results from preclinical studies, rIL-21 entered phase I and II clinical trials in patients with metastatic renal cancer or melanoma. In these studies, rIL-21 showed to be safe, well tolerated and able to induce both immune system activation [26] and clinical responses [27, 28]. Indeed, in a recent phase II trial, in metastatic melanoma, rIL-21 showed an overall response rate of 22 % and increased overall survival [29].
We previously showed that IL-21-transduced murine NB cells, administered as a cellular vaccine, are effective in the treatment of disseminated syngeneic NB. In addition, the combination of this vaccine with CD4+ T cell-depleting antibodies has cooperative effects, due to the removal of NB-instructed CD4+ CD25+ FoxP3+ Treg cells endowed with suppressive functions [30]. However, the use of genetically modified NB cell vaccines, already tested in NB clinical trials [31, 32], is costly and not always feasible. In order to more easily translate NB IL-21 treatment to the clinic, we tested rIL-21 alone or in combination with an anti-CD4 mAb in a preclinical model of disseminated NB. These data indicate that subcutaneous (sc) rIL-21, which shows per se limited anti-tumor effects, is highly effective when combined with an anti-CD4 mAb, through the activation of a CTL response. In addition, long-term memory to NB antigens requires re-populating CD4+ T cells, suggesting that combination IT reprograms the CD4+ T cell compartment from immune-suppressive to anti-tumor functions.
Materials and methods
NB cell culture, animal model and treatments
Neuro2a/parental cells (pc) (CCL131, ATCC, Rockville, MD, USA) were grown in DMEM medium as described [22]. Five-week-old female A/J mice were purchased from Harlan (Udine, Italy). The animals were housed in pathogen-free conditions, and experiments were performed according to the National Regulation on Animal Research Resources and approved by the Institutional Review Board. Groups of 5–14 mice were injected in the tail vein with 1 × 106 Neuro2a/pc (>90 % viable) in a volume of 100 μl of serum-free medium. Mice were treated by subcutaneous (sc) injections of murine recombinant (r)IL-21 (R&D systems, Minneapolis, MN) at 0.5–1 μg/mouse at days +2, +6, +9, +13, +15 from challenge. Anti-CD4 (GK 1.5) mAb (ATCC, Rockville, MD) was administered intraperitoneal (ip) at days +1, +5, +8, +11 (100 μg per dose). In the delayed combined IT, rIL-21 was administered at the dosage of 1 μg/dose sc at days +7, +9, +11, +12, +14, +17, and anti-CD4 mAb (100 μg/dose) was given ip at days +6, +8, +10, +14. Mice were monitored for disease symptoms every other day (starting from 2 weeks after tumor challenge) and were euthanized for ethical reasons by CO2 asphyxiation when they showed weight loss (>15 %), presence of tumor masses, abdomen swelling, “sick posture,” paresis of the posterior limbs or other neurological signs. Necropsy, followed by inspection and eventually by histological analysis, confirmed the presence of tumors. Depletion studies were performed by ip injection of anti-CD8 (2.43) mAb at 100 μg/dose, as described [22]. As a control in vivo depletion studies, azide-free IgG2b negative control antibody, clone LO-DNP-11 (GeneTex Inc., San Antonio, TX), was used. All the in vivo experiments were performed at least twice with similar results.
Statistical analyses
Tumor-free survival curves were constructed using the Kaplan–Meier method, and the log-rank (Mantel–Cox) test was used to compare the curves. Mean tumor-free survival times were calculated with 95 % confidence interval. The t test was used to evaluate the differences in cytokine production or lymphocyte surface markers expression, in different groups of mice. All tests were two-sided. Statistical analyses were conducted using the Prism 3.0 software (Microsoft Inc., USA). p values lower than 0.05 were considered as significant.
Immunofluorescence and FACS analysis
Spleen or lymph node cells were stained using anti-NKp46-PE, anti-CD39-APC, anti-CD44-PE, anti-CD103-APC, anti-CD4-FITC, anti-CD4-PE, anti-CD8-FITC, anti-CD8-PE, anti-CD107a-PE and anti-CD25-PE (all from eBioscience, San Diego, CA), at the concentration indicated by manufacturer instructions. Specific mAb isotype-matched control was always used in each analysis. IFN-γ production was detected using MACS cytokine secretion assay (Miltenyi Biotec, Bologna, Italy) after overnight incubation of spleen cells with medium alone or a mixture of MHC-class I-restricted synthetic survivin epitopes (CPTENEPDL, MHC-class I Ld, GWEPDDNPI, MHC-class I Kk from TIB Molbiol, Berlin). Lyophilized peptides were dissolved in sterile water and used at a final concentration of 10 μg/ml each in culture medium. Cells producing IFN-γ were counterstained with anti-CD8-PE. Samples were run on a FACScan or a FACScalibur analyzer (Becton–Dickinson).
Multiplex ELISA
Blood samples were collected from naïve or NB-bearing mice (untreated or receiving rIL-21 or anti-CD4 mAb alone or combined IT) at day +19 from iv tumor challenge. Blood samples were collected from cured mice at day +140 from NB implant. Serum samples were analyzed for cytokine and chemokine concentrations by a magnetic Milliplex Map kit (Millipore, Darmstadt, Germany) using a Luminex MagPix reader with xPONENT software (Millipore).
Treg cell separation and assay for suppressor function
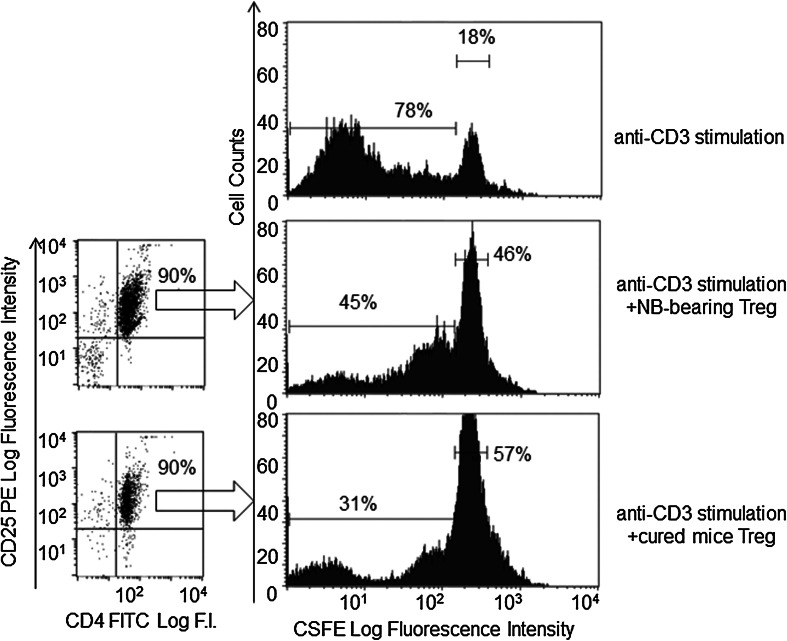
Treg cells from either NB-bearing mice or long-term cured mice were isolated from the spleen of five mice for each group by immunomagnetic cell sorting (Miltenyi Biotec). The suppressive activity of Treg cells was evaluated on a proliferation assay using CFSE (Sigma-Aldrich St Louis, MO)-labeled spleen cells from naïve mice. Briefly, 2 × 105 CFSE-labeled naïve A/J spleen cells were stimulated with soluble anti-CD3 mAb (10 μg/ml, 14.52.C11 clone) and cultured for 5 days in 96-well plates alone or in the presence of 25 × 103 Treg cells from either untreated NB-bearing or long-term cured mice. The assay was performed in triplicate. CFSE-labeled cell proliferation was assessed by FACS analysis using a FACScan (Becton Dickinson).
Results
Anti-CD4 mAb cotreatment potentiates IL-21 immunotherapy of disseminated NB
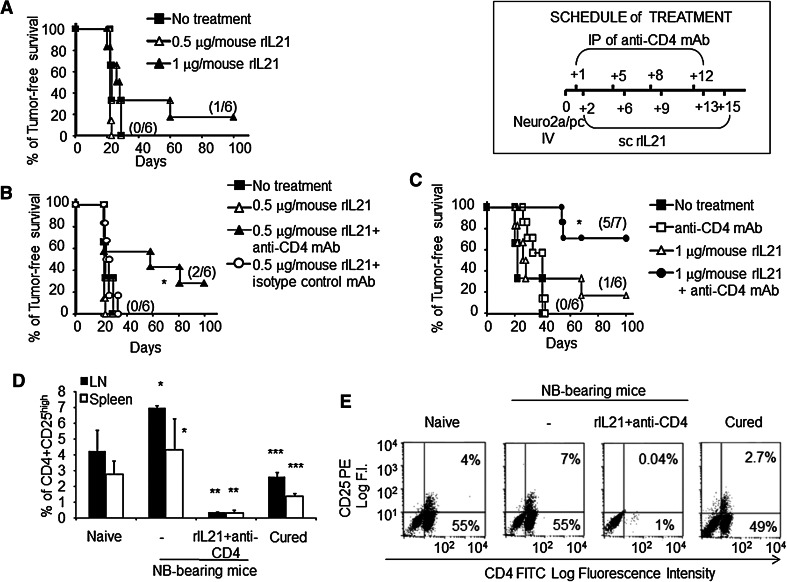
The therapeutic effect of rIL-21 on disseminated NB induced by Neuro2a/pc cell iv challenge in A/J mice was tested at dose levels of 0.5 and 1 μg/mouse sc five times, twice a week, starting at day +2 from NB implant. These dosages were comparable to those used in clinical trials (ranging from 30 to 200 μg/kg). At the 0.5 μg/mouse dose, rIL-21 had no effect, while at 1 μg/mouse, mean tumor-free survival increased from 20.5 ± 16.4 to 37.7 ± 34.2 days and one out of six of NB-challenged mice remained tumor free (p = n.s., by log-rank analysis; Fig. 1a).
Fig. 1.
Anti-CD4 mAb augments rIL-21 immunotherapy. a Kaplan–Meier analysis of A/J mice inoculated iv with a tumorigenic dose of Neuro2a/pc cells on day 0 and treated by sc rIL-21 at two dose levels or with vehicle only. Percentages of tumor-free mice are indicated on the y-axis, and the fraction of tumor-free mice of each group is given in brackets. b, c Combined administration of anti-CD4 mAb and rIL-21 (as described in the treatment schedule) results in a synergistic effect leading to increased cure rate both at the 0.5 (b) and at the 1 μg (c) dose level (*p = 0.03 and **p = 0.027, respectively) relative to a control group of mice receiving rIL-21 and an irrelevant IgG mAb. A representative experiment out of two with consistent results is shown. d Combined treatment leads to a strong decrease in CD4+ CD25high Treg cells in spleen (open bars p < 0.002) and LN cells (black bars p < 0.002) relative to untreated NB-bearing mice, which in turn showed increased proportions of Treg cells relative to naïve mice (p < 0.02 and p < 0.05, for LN and spleen, respectively). Tests were performed at 19 days after tumor cell challenge or at 140 days for cured mice. Treg cell percentages (mean ± SD) were lower in long-term cured mice relative to naïve (p = 0.03 or p = 0.02 for LN and spleen, respectively) and tumor-bearing mice (p < 0.002 or p = 0.047 for LN and spleen, respectively). e Representative two-color dot plot analyses of CD4+CD25+ cells from LN are shown
We further studied possible cooperative effects of rIL-21 and anti-CD4 mAbs in the same model. A significant increase in survival rate at both rIL-21 doses was observed relative to control groups receiving either PBS, anti-CD4 mAb alone or rIL-21 associated with an irrelevant isotype-matched antibody (Fig. 1b, c). These data indicate a therapeutic synergy between rIL-21 and anti-CD4 mAb. This effect is likely related to the removal of CD4+CD25high Treg cells, which increased in untreated tumor-bearing mice relative to naïve mice. Indeed, two-color immunofluorescence analyses of lymph node (LN) and spleen cells showed a striking reduction in Treg cells in mice treated with the combination therapy at day 19 from tumor implant (Fig. 1d and representative dot plots in e).
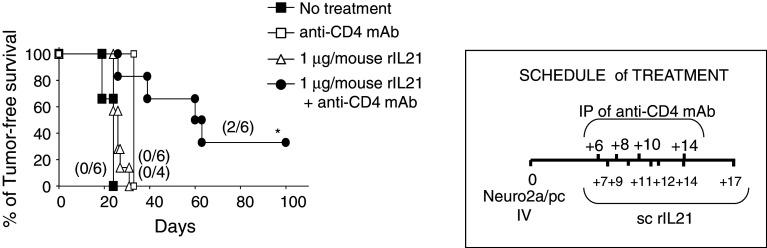
We then examined whether rIL-21-based IT would be effective if started at a later time point. As shown in Fig. 2, rIL-21 alone at 1 μg per dose had no effect on mice survival when administration was begun 7 days after tumor implant. However, rIL-21 combined with anti-CD4 mAb cured 2/6 mice at long term (>120 days) and significantly prolonged mice tumor-free survival (p = 0.001, by log-rank analysis).
Fig. 2.
Delayed combination immunotherapy increased survival of NB-bearing mice. Kaplan–Meier analysis of tumor-free survival of groups of NB-bearing A/J mice either untreated or treated s.c. with 1 μg rIL-21/dose starting at day +7 from NB implant, with anti-CD4 mAb alone or with a combination of the two treatments as detailed in the treatment schedule. Percentages of tumor-free mice are indicated on the y-axis, and the fraction of tumor-free mice of each group is given in brackets. Only combined administration of anti-CD4 mAb and rIL-21 had a significant effect on mice survival (p = 0.001 by log-rank test) relative to untreated mice
CD8+ T cells mediate anti-tumor responses
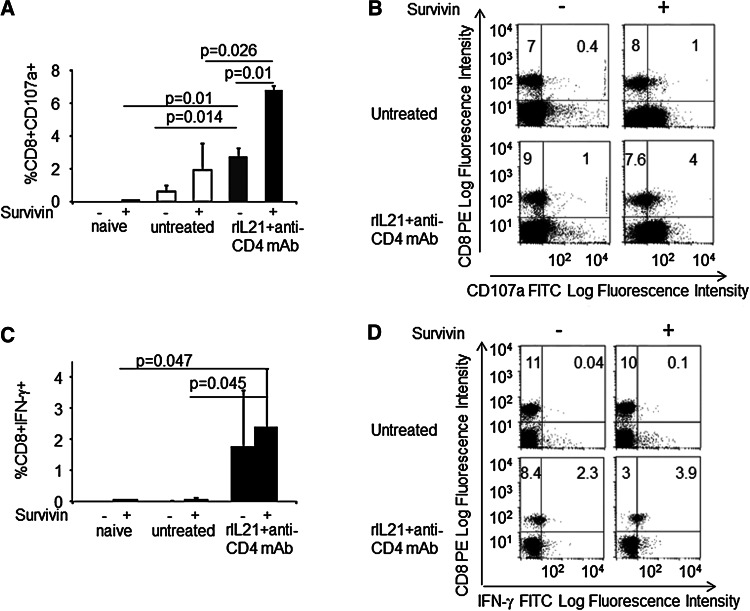
Previous data indicated that IL-21 is an inducer of CD8+ T cell [18, 22, 23] or antibody-mediated anti-tumor immunity [24]. To assess whether CD8+ T lymphocytes mediate the therapeutic effect of rIL-21 and anti-CD4 mAb combination, we studied the expression of CD107a, a marker of CTL degranulation, and IFN-γ secretion. In addition, we tested their response to stimulation by a mixture of two synthetic CTL epitopes of the survivin antigen expressed by Neuro2a/pc cells [22, 33]. These assays were performed 19 days after the first administration of combination IT, a time point at which all treatments had been already withdrawn and untreated mice eventually begun to display signs of disease. In mice receiving combination IT, ex vivo-isolated spleen cells showed greater percentages of CD107a+ CD8+ CTLs relative to untreated NB-bearing mice or naïve mice (Fig. 3a, b). CD107a+ CTLs further increased upon in vitro stimulation with the survivin epitopes (Fig. 3a, b). These findings suggest the existence of a CD8+ T cell population specifically reactive to NB-related tumor antigens. Accordingly, CD8+ cells, spontaneously secreting IFN-γ, increased in the spleen of mice receiving combination IT with respect to untreated mice (Fig. 3c, d). However, stimulation with survivin epitopes could not significantly increase the fraction of CD8+ IFN-γ secreting cells (Fig. 3c, d).
Fig. 3.
Involvement of CD8+ T cells in combined immunotherapy. a CD8+ CD107a+ CTLs are increased in the spleen from mice treated with combination IT relative to naïve or untreated tumor-bearing mice. Overnight stimulation with a mixture of two survivin synthetic epitopes significantly increases the proportion of CD8+ CD107a+ (mean ± SD) in spleen cells from mice receiving combination IT. b Representative dot plots of immunofluorescence analysis for CD107a detection on CD8+ T cells. c The proportion of CD8+ T cells producing IFN-γ (mean ± SD) are increased in mice receiving combination IT, and stimulation with survivin epitopes did not increase the percentage of CD8+IFN-γ+ cells. d Representative dot plots of immunofluorescence analysis for intracellular IFN-γ detection in CD8+ T cells. p values are indicated in panels a and c
Since NK cells may also contribute to combination IT, we studied CD107a and IFN-γ expression on NKP46+ cells. NK cells from mice treated with combination IT showed a partial increase in CD107a or IFN-γ expression, relative to that of untreated mice (Supplemental Figure 1). However, spleen cells from mice receiving combined IT showed no significant changes in cytotoxic activity against the NK-sensitive YAC cells (not shown).
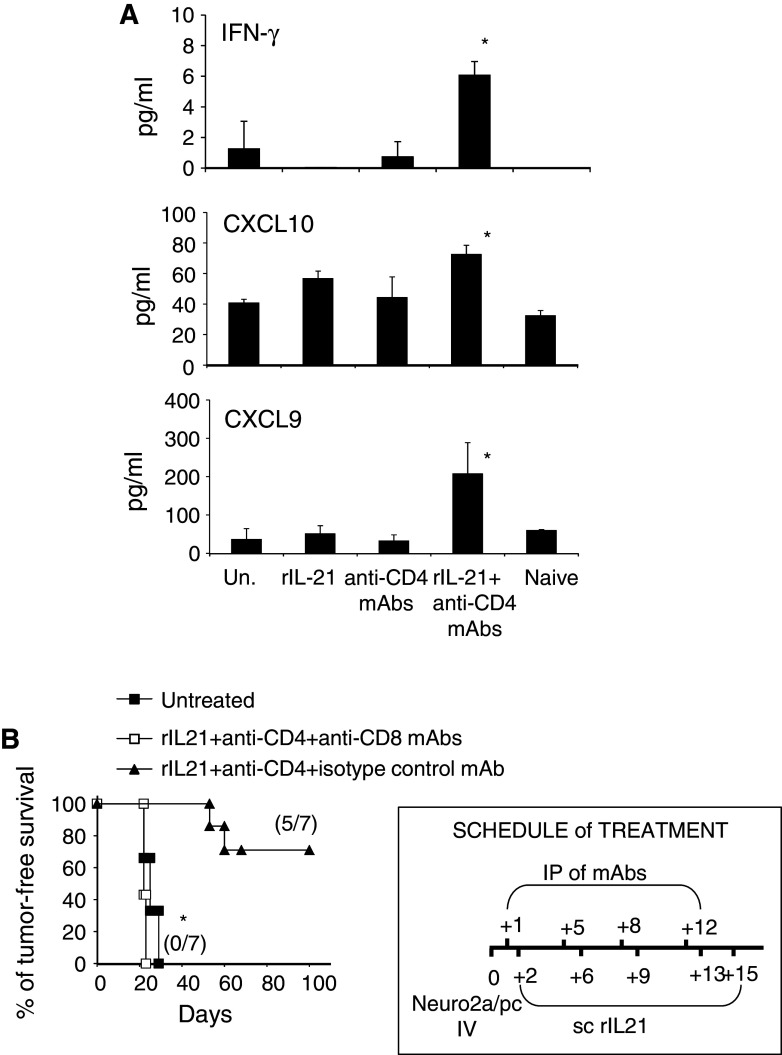
To achieve more information on cytokines potentially involved in the therapeutic effect of combination IT, we tested a panel of cytokines and chemokines by multiplex cytokine ELISA in sera from treated and untreated NB-bearing mice, collected 19 days after the first therapy administration. Increased concentrations of IFN-γ, as well as the IFN-γ-dependent anti-angiogenic chemokines CXCL9 (MIG) and CXCL10 (IP10), were observed in mice treated with combination IT with respect to untreated mice (Fig. 4a). IL-2 appeared also increased, although not significantly (data not shown).
Fig. 4.
In vivo role of cytokines and CTLs during combination IT. a Serum IFN-γ, CXCL10 and CXCL9 levels, measured at 19 days after tumor induction, in naive mice, untreated tumor-bearing mice and in mice treated with single agents or combined immunotherapy. Data are shown as mean values + SD in groups of five mice each, *p < 0.03. b Effect of CD8+ T cell depletion on combination immunotherapy. In mice depleted of CD8+ T cells by an anti-CD8 mAb, combination IT is ineffective as compared to a control group receiving combination IT and an irrelevant IgG mAb (*p = 0.0003 log-rank test). A representative experiment out of two with consistent results is shown
Finally, CD8+ T cell depletion experiments were carried out. As shown in Fig. 4b, all mice receiving combination IT associated with a depleting anti-CD8 mAb developed disseminated NB in a similar fashion as untreated mice. On the opposite, coadministration of an irrelevant mAb did not hamper the effect of the combination IT. Altogether these data support the concept that combination IT induces tumor rejection through a CD8+ T cell response and skews the immune response toward a type 1 polarization.
CD4+ T cell depletion is followed by reconstitution
In order to verify whether antibody-mediated CD4+ T cell depletion was a transient event, we analyzed total CD4+ and Treg cell reconstitution in the spleen and LN of mice receiving combination IT. At 14 and 28 days from iv tumor cell challenge, CD4+ T cell values decreased by >90 %, while, at day +40, CD4+ T cell number recovered to approximately 50 % of the pretreatment levels both in the spleen and in the LN (Supplemental Figure 2A). In long-term cured mice, the CD4+ T cell pool was almost completely restored 140 days after Neuro2a/pc challenge (Supplemental Figure 2A). During reconstitution, CD4+ CD25high Treg cells showed a parallel recovery (Supplemental Figure 2B). However, at 140 days, the percentages of CD4+CD25high Treg cells found in spleen and LN were significantly lower than those found in naïve or untreated tumor-bearing mice (Supplemental Figure 2B and Figure 1D and E). After combination IT, the percentages of CD8+ and NKp46+ cells transiently increased and returned to baseline levels after complete reconstitution (Supplemental Figure 2C and 2D, respectively).
We then tested whether combination IT would also alter the phenotypic and suppressive functions of reconstituting Treg cells. Immunofluorescence analyses of CD4+ CD25high Treg cells from long-term cured mice showed no significant differences in the expression of CD39 and CD103, two markers of Treg suppressor activity [34, 35], relative to the Treg cells from tumor-bearing or naïve mice (Supplemental Figure 3). Accordingly, Treg cells isolated by immune magnetic cell sorting from the spleen of untreated NB-bearing mice or long-term cured mice showed similar suppressive functions on naïve responder T cells stimulated with anti-CD3 mAb ( Fig. 5). Altogether these findings indicate that, after combined IT, functionally active Treg cells re-populate lymphoid organs, although at lower proportions than those found in tumor-bearing and naïve mice.
Fig. 5.
Functional suppressor activity of CD4+ CD25high Treg cells isolated from the spleen of untreated tumor-bearing (right middle panel) or long-term cured mice (right lower panel) on naïve responder T cells. Spleen Treg cells isolated by immunomagnetic cell sorting (dot plot analysis indicating purity of sorted cells is shown in the left) were tested at a 1:8 (Treg:responder T cell) ratio in an anti-CD3 mAb proliferation assay. CFSE-labeled spleen cells from naïve mice were used as responder cells. The right upper panel shows proliferation of naïve T cells in the absence of added Treg cells. Experiments are representative of three replicates with similar results. Inhibition of CSFE dilution was significant for both untreated NB-bearing and long-term cured mice Treg cells (p < 0.05)
Long-term immunity by combination immunotherapy requires both CD8+ and CD4+ T cells
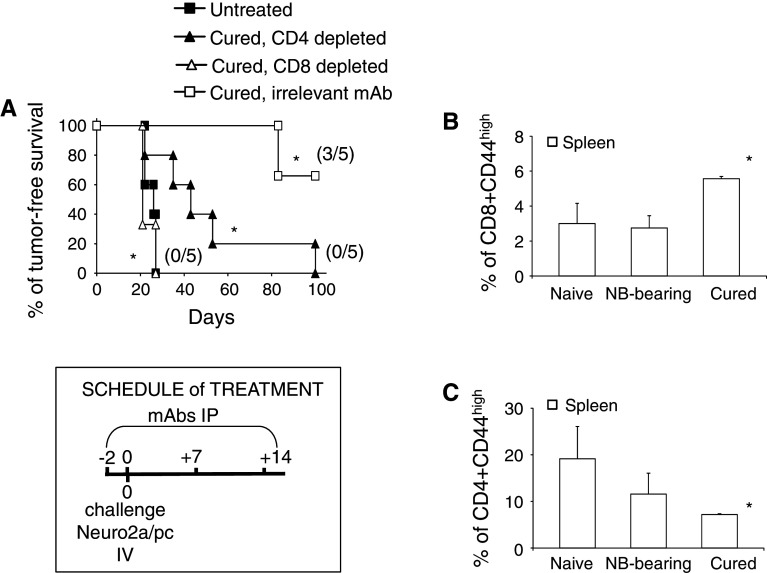
To assess whether long-term cured animals treated by combination IT developed persistent immunity to NB, they were re-challenged with tumor cells 150 days after the first Neuro2a/pc cell injection. Indeed, 60 % of mice remained tumor free after a re-challenge, while 100 % of naïve mice, simultaneously injected with Neuro2a/pc cells, developed tumor (Fig. 6a). A group of cured mice receiving an anti-CD8-depleting mAb, concomitantly with Neuro2a/pc re-challenge, developed NB growth in a similar fashion as naïve mice (Fig. 6a). In addition, the percentage of CD8+ CD44high memory T cells increased in the spleen of cured mice relative to naïve or tumor-bearing mice (Fig. 6b). This finding further supports the essential role of CD8+ T cells in the memory response to NB antigens.
Fig. 6.
Both CD4+ and CD8+ T cells are required for long-term immunity to NB in mice treated by combination immunotherapy. a Mice surviving after combination IT were re-challenged iv with a tumorigenic dose of Neuro2a/pc cells, 150 days after the first NB implant (*p = 0.0031 vs challenge in naïve untreated mice). Mice receiving anti-CD8 or anti-CD4-cell-depleting antibodies showed no immunity to NB cell re-challenge compared to mice receiving irrelevant mAb (*p = 0.0023 and p = 0.015, respectively). Percentages of tumor-free mice are indicated on the y-axis, and the fraction of tumor-free mice of each group is given in brackets. b Percentages of CD8+ CD44high memory T cells in spleen cells from naïve, untreated NB-bearing and long-term cured mice assessed by immunofluorescence analysis. Data (mean and SD) refer to groups of three mice (p < 0.05 between cured mice and the other groups). c Percentages of CD4+ CD44high memory T cells (Mean ± SD) in spleen cells from naive, untreated NB-bearing and long-term cured mice assessed by immunofluorescence analysis (p < 0.05 between cured mice and the other groups)
We then investigated the role of CD4+ T cells, which re-populate mice after successful combination IT, in long-lasting immunity. Administration of anti-CD4 mAb to long-term cured mice, re-challenged with NB cells, reduced the anti-tumor response, and all mice finally developed tumors. However, the kinetics of tumor growth differed between mice depleted of CD8+ or CD4+ T cells, being slower in the latter group of mice (Fig. 6a). In spite of their role in the memory response to NB, after reconstitution, the percentage of CD4+ CD44high memory cells were lower in mice receiving combined IT than in naïve or tumor-bearing mice (Fig. 6c). The decrease in the number of memory T cells may depend on the reconstitution of the T cell pool by a new population of naïve CD4+ cells, of which only a subset had already been stimulated by NB antigens. These data indicate that although transient CD4+ T cell removal augments rIL-21 efficacy against disseminated NB, CD4+ T helper (Th) cells, which re-populate long-term cured mice, are necessary to maintain an efficient CTL memory response.
Discussion
In this report, we show that sc rIL-21 treatment mediates a limited anti-tumor effect in a syngeneic model of systemic NB, as only a minority of mice showed prolonged survival at the highest dose used. Similarly, recent data of clinical trials in metastatic renal carcinoma or melanoma showed that rIL-21 IT has limited toxicity and induces clinical responses in about 20 % of patients [27–29] and increased survival [29]. Thus, the search of combinatorial treatments capable of augmenting rIL-21 therapeutic effects seemed a relevant goal. In the present study, the combined administration of an anti-CD4 mAb strongly potentiated rIL-21 IT in a preclinical NB model, resulting in a significant increase in the efficacy of treatment, and supporting clinical applications. This treatment was maximally active if started at early time points after NB implant, although it was partially active in mice with larger tumor burden. Nonetheless, a potential limitation of this work is related to the use of a single mouse model of syngeneic NB, and further investigation in transgenic mice, which develop spontaneous NB [36], might be needed to corroborate these data, before undertaking clinical studies.
The synergistic effect of anti-CD4 mAbs combined with rIL-21 may relate to the depletion of NB-instructed CD4+ Treg cells. Indeed, CD4+ CD25high cells endowed with immune-suppressive properties increase in different human and experimental tumors [37–39], including syngeneic NB [30]. Anti-CD4 mAb was effective at eliminating CD4+ CD25high Treg cells, but we cannot formally exclude that other CD4+ cell subsets, such as Tr1 [40], or type II NK-T cells [41] could play a role in immune suppression, in murine NB.
Previous studies suggested that CD4+ T cell depletion skews the immune response toward type 1 polarization [42]. However, in the present study, IFN-γ and the IFN-γ-dependent Th1 chemokines CXCL9 and CXCL10 increased in mouse serum during combination IT, but not after anti-CD4 mAb or rIL-21 treatment as single agents. It is likely that the Th1 polarizing effect of CD4 depletion cannot be detected at the level of circulating cytokines, but this effect is synergistically boosted by IL-21. Indeed, this cytokine potentiates CTL responses and induces production of IFN-γ and IFN-γ-dependent CXC chemokines endowed with anti-angiogenic properties in vivo [23]. In fact, the cooperative effect of anti-CD4 mAb and rIL-21 is strictly dependent on the induction of a CD8 response as CD8+ T cell depletion resulted in a complete inefficacy of this combination therapy. In addition, IFN-γ-producing and CD107a+ CTLs increased in mice receiving combined treatment relative to untreated tumor-bearing mice. These data suggest that CD8+ CTLs that recognize NB cells increase and are functionally active in vivo in response to combination IT. This possibility is also supported by the ability of synthetic epitopes of the survivin antigen to further activate CD8+ T cells from treated mice as detected by degranulation assays. Other reports had shown that IL-21 also increases the cytotoxic functions of NK cells [15]. At the doses used herein, we found only a minor increase in IFN-γ+ or CD107a+ NKp46+ cells without significant changes in NK activity, although we cannot exclude that NK cells may also contribute to the early anti-tumor response during combined IT.
It is well known that CD8+ T cell responses require the support of Th1-derived cytokines, which mediate CTL differentiation and proliferation. In the combination IT setting, the requirement of a Th response in CD4-depleted mice was bypassed by the supply of exogenous rIL-21, which is a Th-derived cytokine and an inducer of CTL activity [15–23]. In fact, the administration of anti-CD4 mAb in the absence of rIL-21 did not result in the cure of NB-bearing mice. Similar findings were previously observed with the use of an IL-21-secreting NB cell vaccine, whose anti-tumor activity cooperated with anti-CD4 mAb treatment [30]. Moreover, CD4+ cell depletion augmented another combinational NB therapy, based on syngeneic hematopoietic stem cell transplantation, adoptive transfer of NB-sensitized T cells and post-transplantation tumor vaccination [43]. Although this combined treatment mediated an early increase in tumor-reactive CD8+ T cells with an effector phenotype, the induction of long-lasting immunity to NB was compromised in mice cotreated with anti-CD4 mAb as evidenced by tumor re-challenge experiments. This finding is in agreement with the accepted view that the concomitant induction of CD4+ Th cell responses is essential for a long-lasting effect of cancer IT [44]. Importantly, in the present study, the use of rIL-21 IT combined with a short treatment with anti-CD4 mAb mediated a transient CD4+ T cell depletion, followed by a progressive reconstitution of the CD4+ T cell pool and the development of long-lasting immunity to NB. Notably, this memory response occurs in the absence of exogenously added Th cytokines, such as IL-21. Indeed, full immunity to NB in cured mice required both CD8+ and CD4+ T cell subsets, as the depletion of either subset, respectively, abolished or reduced the resistance to NB cell re-challenge. However, CD8-depleted mice showed a more rapid mortality after NB re-challenge than the CD4-depleted ones, indicating a crucial role of CD8+ cells in the memory response to NB; indeed, CD8+ CD44high cells increased in long-term cured mice. This finding indicates that CD8+ T cells may be partially active without CD4+ T lymphocytes, which are required for a full CTL response and the complete eradication of re-challenged NB cells. Although total CD4+ CD44high memory T cells decreased in mice cured by combined IT, it is likely that memory CD4+ T cells reactive to NB may develop during CD4+ T cell re-population of the host, when NB antigens or residual NB cells may still be present. In addition, these data suggest that rIL-21 and transient T cell depletion are followed by a switch from a CD4+ T cell repertoire characterized by immune-suppressive properties to the re-constitution of a “reprogrammed” CD4+ T cell compartment, which contains cells capable of providing an effective help to CD8+ effector T cells. IL-21 may play a role in this reprogramming as it does not support Treg cell expansion [13] and may inhibit the functions of Treg cells [14]. However, functionally active CD4+ CD25highCD39+ Treg cells re-populate cured mice, but their proportion was lower than in naïve and untreated NB-bearing mice.
The availability of cell-depleting anti-CD4 mAbs for clinical use in humans [45, 46], together with the very encouraging results shown by rIL-21 therapy in other metastatic cancers [29], suggests that a similar combination treatment may be attempted in NB patients. Notably, in cutaneous and non-cutaneous T cell lymphomas, anti-CD4 mAbs were effective in depleting CD4+ T cells, without significantly increasing the risk of concomitant infections [45, 46]. In conclusion, these data suggest that combination rIL-21 immunotherapy and CD4+ lymphocyte depletion may represent a new therapeutic strategy, which could integrate conventional therapies for refractory or relapsing NB.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Funding organizations are as follows: Associazione Italiana per la Ricerca sul Cancro-IG13518 (Silvano Ferrini), Fondazione Italiana per la lotta al Neuroblastoma (Michela Croce and Maria Valeria Corrias), Compagnia di San Paolo (Silvano Ferrini, Gilberto Filaci) and Italian Ministry of Health RC and 5 per mille (Silvano Ferrini).
Conflict of interest
The authors state that they have no conflict of interest to declare.
Abbreviations
- APC
Allophycocyanin
- CFSE
5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester
- CTL
Cytotoxic T lymphocyte
- FITC
Fluorescein isothiocyanate
- IFN-γ
Interferon-gamma
- IL
Interleukin
- ip
Intraperitoneal
- IP-10
IFN-inducible protein-10
- IT
Immunotherapy
- iv
Intravenous
- LN
Lymph node
- mAb
Monoclonal antibody
- MHC
Major histocompatibility complex
- MIG
Monokine induced by IFN-γ
- NB
Neuroblastoma
- NK
Natural killer
- PE
Phycoerythrin
- pc
Parental cells
- sc
Subcutaneous
- Th
T helper
- Tr-1
Type-1 regulatory T
- rIL-21
Recombinant interleukin-21
- SD
Standard deviation
- Treg
Regulatory T
Footnotes
Michela Croce and Silvano Ferrini have contributed equally to this work.
Contributor Information
Michela Croce, Phone: +39-10-5558372, FAX: +39-10-5558374, Email: michela.croce@hsanmartino.it.
Silvano Ferrini, Phone: +39-10-5558372, FAX: +39-10-5558374, Email: silvanodomenico.ferrini@hsanmartino.it, Email: silvano.ferrini@libero.it.
References
- 1.Haupt R, Garaventa A, Gambini C, et al. Improved survival of children with neuroblastoma between 1979 and 2005: a report of the Italian neuroblastoma registry. J Clin Oncol. 2010;28:2331–2338. doi: 10.1200/JCO.2009.24.8351. [DOI] [PubMed] [Google Scholar]
- 2.Cohn SL, Pearson AD, London WB, et al. The international neuroblastoma risk group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse: a retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45:2835–2842. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 4.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. 2011;29:3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeger RC. Immunology and immunotherapy of neuroblastoma. Semin Cancer Biol. 2011;21:229–237. doi: 10.1016/j.semcancer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 7.Corrias MV, Basso S, Meazza R, Musiani P, Santi L, Bocca P, Occhino M, Ferrini S, Pistoia V. Characterization and tumorigenicity of human neuroblastoma cells transfected with the IL-2 gene. Cancer Gene Ther. 1998;5:38–44. [PubMed] [Google Scholar]
- 8.Ladenstein R, Pötschger U, Siabalis D, et al. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after mega therapy and autologous stem-cell reinfusion. J Clin Oncol. 2011;29:441–448. doi: 10.1200/JCO.2009.23.5465. [DOI] [PubMed] [Google Scholar]
- 9.Coventry BJ, Ashdown ML. The 20th anniversary of interleukin-2 therapy: bimodal role explaining longstanding random induction of complete clinical responses. Cancer Manag Res. 2012;4:215–221. doi: 10.2147/CMAR.S33979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+) CD25(+) T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comes A, Rosso O, Orengo AM, et al. CD25+ regulatory T cell depletion augments immunotherapy of micrometastasis by an IL-21-secreting cellular vaccine. J Immunol. 2006;176:1750–1758. doi: 10.4049/jimmunol.176.3.1750. [DOI] [PubMed] [Google Scholar]
- 14.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, Macdonald TT, Pallone F, Monteleone G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 15.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht J, Frey M, Teschner D, Carbol A, Theobald M, Herr W, Distler E. IL-21-treated naive CD45RA+ CD8+ T cells represent a reliable source for producing leukemia-reactive cytotoxic T lymphocytes with high proliferative potential and early differentiation phenotype. Cancer Immunol Immunother. 2011;60:235–248. doi: 10.1007/s00262-010-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wölfl M, Merker K, Morbach H, Van Gool SW, Eyrich M, Greenberg PD, Schlegel PG. Primed tumor-reactive multifunctional CD62L+ human CD8+ T cells for immunotherapy. Cancer Immunol Immunother. 2011;60:173–186. doi: 10.1007/s00262-010-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland AP, Joller N, Michaud M, Liu SM, Kuchroo VK, Grusby MJ. IL-21 promotes CD8+ CTL activity via the transcription factor T-bet. J Immunol. 2013;190:3977–3984. doi: 10.4049/jimmunol.1201730. [DOI] [PubMed] [Google Scholar]
- 20.Di Carlo E, de Totero D, Piazza T, Fabbi M, Ferrini S. Role of IL-21 in immune-regulation and tumor immunotherapy. Cancer Immunol Immunother. 2007;56:1323–1334. doi: 10.1007/s00262-007-0326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andorsky DJ, Timmerman JM. Interleukin-21: biology and application to cancer therapy. Expert Opin Biol Ther. 2008;8:1295–1307. doi: 10.1517/14712598.8.9.1295. [DOI] [PubMed] [Google Scholar]
- 22.Croce M, Meazza R, Orengo AM, et al. Immunotherapy of neuroblastoma by an Interleukin-21-secreting cell vaccine involves survivin as antigen. Cancer Immunol Immunother. 2008;57:1625–1634. doi: 10.1007/s00262-008-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 24.Daga A, Orengo AM, Gangemi RM, Marubbi D, Perera M, Comes A, Ferrini S, Corte G. Glioma immunotherapy by IL-21 gene-modified cells or by recombinant IL-21 involves antibody responses. Int J Cancer. 2007;121:1756–1763. doi: 10.1002/ijc.22901. [DOI] [PubMed] [Google Scholar]
- 25.Kowalczyk A, Wierzbicki A, Gil M, et al. Induction of protective immune responses against NXS2 neuroblastoma challenge in mice by immunotherapy with GD2 mimotope vaccine and IL-15 and IL-21 gene delivery. Cancer Immunol Immunother. 2007;56:1443–1458. doi: 10.1007/s00262-007-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodds MG, Frederiksen KS, Skak K, Hansen LT, Lundsgaard D, Thompson JA, Hughes SD. Immune activation in advanced cancer patients treated with recombinant IL-21: multianalyte profiling of serum proteins. Cancer Immunol Immunother. 2009;58:843–854. doi: 10.1007/s00262-008-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, DeVries TA, Hausman DF. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 28.Davis ID, Brady B, Kefford RF, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 29.Petrella TM, Tozer R, Belanger K, et al. Interleukin-21 has activity in patients with metastatic melanoma: a phase II study. J Clin Oncol. 2012;30:3396–3401. doi: 10.1200/JCO.2011.40.0655. [DOI] [PubMed] [Google Scholar]
- 30.Croce M, Corrias MV, Orengo AM, Brizzolara A, Carlini B, Borghi M, Rigo V, Pistoia V, Ferrini S. Transient depletion of CD4(+) T cells augments IL-21-based immunotherapy of disseminated neuroblastoma in syngeneic mice. Int J Cancer. 2010;127:1141–1150. doi: 10.1002/ijc.25140. [DOI] [PubMed] [Google Scholar]
- 31.Russell HV, Strother D, Mei Z, Rill D, Popek E, Biagi E, Yvon E, Brenner M, Rousseau R. A phase 1/2 study of autologous neuroblastoma tumor cells genetically modified to secrete IL-2 in patients with high-risk neuroblastoma. J Immunother. 2008;31:812–819. doi: 10.1097/CJI.0b013e3181869893. [DOI] [PubMed] [Google Scholar]
- 32.Rousseau RF, Haight AE, Hirschmann-Jax C, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotoxin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–1726. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 33.Fest S, Huebener N, Bleeke M, et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–114. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 34.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 35.Annacker O, Coombes JL, Malmstrom V, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesler L, Goldenberg DD, Seales IT, et al. Malignant progression and blockade of angiogenesis in a murine transgenic model of neuroblastoma. Cancer Res. 2007;67:9435–9442. doi: 10.1158/0008-5472.CAN-07-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 38.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 39.Frumento G, Piazza T, Di Carlo E, Ferrini S. Targeting tumor-related immunosuppression for cancer immunotherapy. Endocr Metab Immune Disord Drug Targets. 2006;6:233–237. doi: 10.2174/187153006778250019. [DOI] [PubMed] [Google Scholar]
- 40.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings M. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 41.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinzel FP, Rerko RM. Cure of progressive murine leishmaniasis: interleukin 4 dominance is abolished by transient CD4(+) T cell depletion and T helper cell type 1-selective cytokine therapy. J Exp Med. 1999;189:1895–1906. doi: 10.1084/jem.189.12.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing W, Gershan JA, Johnson BD. Depletion of CD4 T cells enhances immunotherapy for neuroblastoma after syngeneic HSCT but compromises development of antitumor immune memory. Blood. 2009;113:4449–4457. doi: 10.1182/blood-2008-11-190827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YH, Duvic M, Obitz E, et al. Clinical efficacy of zanolimumab (HuMax-CD4), two Phase 2 studies in refractory cutaneous T cell lymphoma. Blood. 2007;109:4655–4662. doi: 10.1182/blood-2006-12-062877. [DOI] [PubMed] [Google Scholar]
- 46.D’Amore F, Radford J, Relander T, et al. Phase II trial of zanolimumab (HuMax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma. Br J Haematol. 2010;150:565–573. doi: 10.1111/j.1365-2141.2010.08298.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.