Abstract
Adoptive immunotherapy leveraging chimeric antigen receptor-modified T (CAR-T) cells holds great promise for the treatment of cancer. However, tumor-associated antigens often have low expression levels in normal tissues, which can cause on-target, off-tumor toxicity. Recently, we reported that GPC3-targeted CAR-T cells could eradicate hepatocellular carcinoma (HCC) xenografts in mice. However, it remains unknown whether on-target, off-tumor toxicity can occur. Therefore, we proposed that dual-targeted CAR-T cells co-expressing glypican-3 (GPC3) and asialoglycoprotein receptor 1 (ASGR1) (a liver tissue-specific protein)-targeted CARs featuring CD3ζ and 28BB (containing both CD28 and 4-1BB signaling domains), respectively, may have reduced on-target, off-tumor toxicity. Our results demonstrated that dual-targeted CAR-T cells caused no cytotoxicity to ASGR1+GPC3− tumor cells, but they exhibited a similar cytotoxicity against GPC3+ASGR1− and GPC3+ASGR1+ HCC cells in vitro. We found that dual-targeted CAR-T cells showed significantly higher cytokine secretion, proliferation and antiapoptosis ability against tumor cells bearing both antigens than single-targeted CAR-T cells in vitro. Furthermore, the dual-targeted CAR-T cells displayed potent growth suppression activity on GPC3+ASGR1+ HCC tumor xenografts, while no obvious growth suppression was seen with single or double antigen-negative tumor xenografts. Additionally, the dual-targeted T cells exerted superior anticancer activity and persistence against single-targeted T cells in two GPC3+ASGR1+ HCC xenograft models. Together, T cells carrying two complementary CARs against GPC3 and ASGR1 may reduce the risk of on-target, off-tumor toxicity while maintaining relatively potent antitumor activities on GPC3+ASGR1+ HCC.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1949-8) contains supplementary material, which is available to authorized users.
Keywords: Hepatocellular carcinoma, Glypican-3, Asialoglycoprotein receptor 1, Chimeric antigen receptors, Dual-targeted T cells
Introduction
Hepatocellular carcinoma is the third leading cause of cancer death worldwide, and it is particularly prevalent in sub-Saharan Africa and Asian countries [2]. Given that the majority of HCC patients are diagnosed at a late stage, conventional curative therapies only benefit a limited number of patients [3]. Sorafenib, the first-line treatment for patients with HCC who have no effective treatment options, only extends overall survival by 2–3 months [4]. Thus, novel treatment strategies are urgently needed.
Engineering T cells with CARs is one of the most promising immunotherapy strategies for cancer treatment. T cells bearing first-generation CARs with only the CD3ζ intracellular signaling domain exhibited poor responses as antitumor therapies, which has been predominantly attributed to limited T cell proliferation and persistence in vivo [5, 6]. It has been speculated that the T cells are not completely activated due to the absence of co-stimulatory signals. To overcome this limitation, signal transduction domains derived from the co-stimulatory receptors CD28, OX40 and/or 4-1BB were incorporated to generate second- and third-generation CARs. These modifications could improve T cell expansion and persistence and the activity of CAR-T cells in preclinical and clinical studies [7–9].
Recently, we demonstrated that third-generation CAR-T cells targeting GPC3 could eradicate established GPC3-positive HCC xenografts in vivo [10]. Thus, these cells might represent a promising treatment strategy for HCC patients. However, we are concerned about their toxicity in humans because we could not completely eliminate GPC3 expression in normal tissues [11]. A patient with metastatic colon cancer died after receiving third-generation HER2-targeted CAR-T cells, and the death was attributed to the CAR-T cells’ attack on the HER2-positive pulmonary parenchyma [12]. This case highlights the importance of reducing on-target, off-tumor toxicity of CAR-T cells.
Dual-targeted CAR-T cells have been proposed as a potential way to reduce on-target, off-tumor toxicity [13, 14]. ASGR1 is a cell surface receptor expressed exclusively on hepatic parenchymal cells that is expressed in 75.2–93.1% of HCC samples [15–17]. However, GPC3 is not expressed on normal hepatocytes [11]. Therefore, we proposed that incorporating an ASGR1 targeting chimeric co-stimulatory receptor, αASGR1-28BB, might increase the antitumor activities of first-generation GPC3-targeted CAR-T cells against the HCC cells expressing both ASGR1 and GPC3. Moreover, as ASGR1 is predominantly expressed on hepatocyte membranes [18], the dual-targeted CAR-T cells would be expected to only have toxicity equivalent to the first-generation GPC3-targeted CAR-T cells, even when GPC3 is expressed in normal tissues. Thus, CAR-T cells carrying αGPC3-CD3ζ (referred to as GZ) for primary signal transduction and αASGR1-28BB (referred to as A28BB) for co-stimulatory signal transduction were prepared and characterized in this study. The results demonstrated that dual-targeted CAR-T cells could potently inhibit GPC3+ASGR1+ but not GPC3+ASGR1− or GPC3−ASGR1+ HCC tumor xenografts in vivo, suggesting that dual-targeted CAR-T cells are a promising immunotherapy for HCC with effective antitumor activities and limited toxicity.
Materials and methods
Generation of vectors and lentivirus production
The fragment of GZ containing anti-GPC3 scFv (based on GC33) linked to the human CD8α hinge, the transmembrane domain and the intracellular signaling domain derived from the CD3ζ molecule was constructed in a previous report [10]. The domain antibody with only the heavy chain specific for ASGR1 was screened by Coulstock and colleagues with phage display [16]. The fragment of A28BB contained the anti-ASGR1 H chain linked to the transmembrane region and the intracellular signaling domain of the human CD28 molecule as well as the intracellular signaling domain of CD137 in tandem. Both constructs were fused with enhanced green fluorescent protein (eGFP) (GZ) or mCherry (A28BB) using a foot-and-mouth disease virus 2A oligopeptide [19]. Finally, all the products were ligated to the third-generation non-self-inactivating EF-1α promoter-based lentiviral expression vector pWPT as reported previously [20].
To construct the pWPT-GPC3 and pWPT-ASGR1 vectors to overexpress human GPC3 (GenBank NM 004484.3) and ASGR1 (GenBank NM 001671.4), the coding sequences were amplified by PCR with the primers GPC3-F/GPC3-R and ASGR1-F/ASGR1-R. The PCR products were ligated into the pWPT vector. All of the primers used are listed in Supplementary Table 1.
Recombinant lentiviral particles were produced by a polyethylenimine linear (MW 25,000) (Polysciences, Warrington, PA, USA) transfection system [21]. Lentiviral particles were concentrated 30-fold by ultracentrifugation (Beckman Coulter, USA) for 2 h at 28,000 rpm and 4 °C.
Cell lines and culture
Human HCC cell lines Huh-7 and HepG2 and the human embryonic kidney (HEK)-293T cell line were purchased from the Chinese Academy of Sciences (Shanghai, China). The HCC cell line MHCC-97L was kindly provided by Dr. Jinjun Li (Shanghai Cancer Institute, China). The empty vector-transduced cells and the GPC3+, ASGR1+ and GPC3+ASGR1+ MHCC-97L cells were established by lentiviral transduction (referred to as MHCC-97L-vec, MHCC-97L-G+, MHCC-97L-A+ and MHCC-97L-G+A+, respectively). All the cell lines mentioned above were cultured in Dulbecco’s modified Eagle medium at 37 °C in a 5% CO2 incubator. The medium was supplemented with 10% fetal bovine serum, 100 μg/mL penicillin and 100 μg/mL streptomycin.
Isolation, transduction and culture of primary T cells
Peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained from the Shanghai Blood Center. Primary human CD4+ and CD8+ T cells were isolated from PBMCs using the negative selection RosetteSep kits (STEMCELL Technologies). Fresh isolated CD4+ and CD8+ T cells were mixed at a 1:1 ratio and cultured in AIM-V® Medium CTS™ (GIBCO) supplemented with 2% human AB serum (Huayueyang Biothecnology, China) and 300 U/mL recombinant human interleukin 2 (rhIL-2) (Huaxin High Biotech, China). These T cells were stimulated for 24 h with anti-CD3/anti-CD28 antibodies immobilized on tosyl-activated paramagnetic beads (Invitrogen) before infection. After stimulation, the T cells were transduced with lentivirus supplemented with polybrene in 24-well plates coated with RetroNectin (TaKaRa) at a multiplicity of infection (MOI) of 15. The transduced T cells were cultured at a concentration of 5 × 105 cells/mL in the presence of rhIL-2 (300 U/mL). Genetically modified T cells were used for functional assays after the end of the first period of proliferation (14 days post-transduction). Engineered T cell populations were adjusted to the same percent of GZ+ T cells by adding untransduced T cells for all the functional assays.
T cell proliferation assays
Tumor cells expressing the desired antigens were treated with 50 Gy before co-culture with 1.0 × 106 T cells at a 1:1 E/T ratio at the start of the experiment. The viable T cell number was then evaluated by trypan blue exclusion every other day, and the cells re-stimulated weekly with irradiated tumor cells. No exogenous cytokines were added during the proliferation assays.
Western blot
To probe the expression of GPC3 and ASGR1 in various HCC cell lines, Western blot analyses were conducted as previously described [10] with the antibodies against GPC3 (Biomosaic, clone 1G12) and ASGR1 (Abcam, #ab49355), both at 1:1000 dilution.
To probe the phosphorylation levels of B cell lymphoma-extra large (Bcl-xL) and extracellular regulated kinase 1 (ERK1) and ERK2 in cellular lysates of control or transduced T cells, T cells were stimulated with MHCC-97L-G+A+ cells for 24 or 2 h, and the extracted proteins were used for immunoblot analysis. The primary antibodies used were an anti-Bcl-xL rabbit mAb (Cell Signaling Technology, clone 54H6), an anti-ERK1/2 rabbit mAb (Cell Signaling Technology, #4695) and an antiphosphorylated ERK1/2 (p-ERK1/2) rabbit mAb (Cell Signaling Technology, #4370). The band intensities were quantified using the ImageJ 1.45 software.
Cytotoxicity assays
Targeting cells were co-cultured with T cells at different E/T ratios of 3:1, 1:1 and 1:3. After 18 h of culture, the supernatant was removed, and the level of released LDH was measured using the CytoTox 96® non-radioactive cytotoxicity kit (Promega) as previously described [10].
The same anti-GPC3 and anti-ASGR1 antibodies used in the Western blot assay were used to determine the antigen expression on human HCC cells. A FITC-conjugated goat antimouse IgG secondary antibody and a FITC-conjugated goat antirabbit IgG secondary antibody (KANGCHENG, China) were used. The level of transduced primary T cells was probed using eGFP or mCherry expression. The central memory phenotype of expanded T cells was examined with a panel of conjugated antibodies, including antibodies directed against human CD45RO (eBioscience, PE-conjugated, clone UCHL1) and CD62L (eBioscience, PerCP-eFluor® 710-conjugated clone DREG56). Additionally, a panel of anti-CD4 (SantaCruz, clone MT310) and anti-CD8 (SantaCruz, clone 32-M4) unconjugated monoclonal antibodies and a goat antimouse PE-conjugated secondary antibody (MultiSciences Biotechnology, China) were used to detect the CD4+ and CD8+ populations. The quantities of circulating human T cells recovered from xenograft-bearing mice treated with engineered T cells were tested using the CD3-PerCP/CD4-FITC/CD8-PE TruCOUNT kit (BD Bioscience) [22]. We counted cells bearing PerCP or/and PE signals, and the difference was regarded as the CD4+ population. All the cells mentioned above were analyzed using a BD FACSCalibur flow cytometer and an LSRII flow cytometer (BD), and results were processed with the Flowjo 7.6.1 software (TreeStar).
Cytokine secretion
The levels of the cytokines IL-2, IFN-γ, TNF-α and IL-4 secreted by various genetically modified T cells co-cultured with different tumor cells at the effector/target ratio of 1:1 for 24 h were measured by an ELISA kit (MultiSciences Biotechnology, China) according to the manufacturer’s instructions. Mouse blood was collected and clotted at 4 °C, and the serum was used for cytokine detection as above.
Xenograft tumor models
Six-week-old NOD/SCID mice obtained from Shanghai SLAC Laboratory Animal Co., Ltd. were housed and treated under specific pathogen-free conditions. To establish subcutaneous (s.c.) Huh-7 or MHCC-97L models, NOD/SCID mice were inoculated with 2 × 106 Huh-7 or MHCC-97L cells in the right flank. When the tumor burdens were approximately 50–100 mm3, mice were randomly separated into four groups (n = 5) and injected intravenously (i.v.) with different CAR-T cells (1 × 107 CAR-T cells/mouse) after lymphocyte depletion with cyclophosphamide. The tumor dimensions were measured with calipers, and the tumor volumes were calculated using the formula V = π/6 × (length × width2), where the length is the greatest longitudinal diameter and the width is the greatest transverse diameter [23]. NOD/SCID mice were housed and treated according to protocols approved by the Shanghai Medical Experimental Animal Care Commission.
Immunohistochemistry
A tissue microarray (TMA) of 75 liver cancer tissue samples (17 stage I, 26 stage II, 22 stage III, 10 stage IV) and the corresponding cancer-adjacent tissue samples was selected for this study (Shanghai Outdo Biotech Co., Ltd., #Hliv-HCC150CS-02). IHC analysis was performed as described previously [24]. The TMAs mentioned above were immune-stained with an anti-GPC3 mAb (1G12) at 1:100 dilution and an anti-ASGR1 rabbit polyclonal antibody at 1:200 dilution. Staining was considered positive when immunoreactivity was present in at least 5% of lesional hepatocytes and located in the cytoplasm, membrane or canaliculi [25]. The levels of GPC3 and ASGR1 expression were evaluated by H-score using the following equation: H-score = (“3+” % cells × 3 + (“2+” % cells) × 2 + (“1+” % cells) × 1 + (“0” % cells) × 0. In the equation, “0” indicates no staining, while “1+”, “2+” and “3+” represent weak, moderate and strong IHC staining, respectively [24]. A higher H-score represents higher expression. The percentage of target-positive cells in each section was counted in five different visual fields [10].
Similarly, the infiltration and persistence of adoptive T cells within the tumor tissues were examined using an anti-CD3e antibody at a 1:150 dilution (Thermo Scientific, clone SP7).
Statistical analysis
For statistical analysis, one-way ANOVA with Bonferroni post-tests for tumor burdens (tumor volume, tumor weight and photon counts) was used. GraphPad Prism 5.0 was used for statistical calculations. P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***) were considered significant.
Results
GPC3 and ASGR1 expression profiles in HCC tissues and cell lines
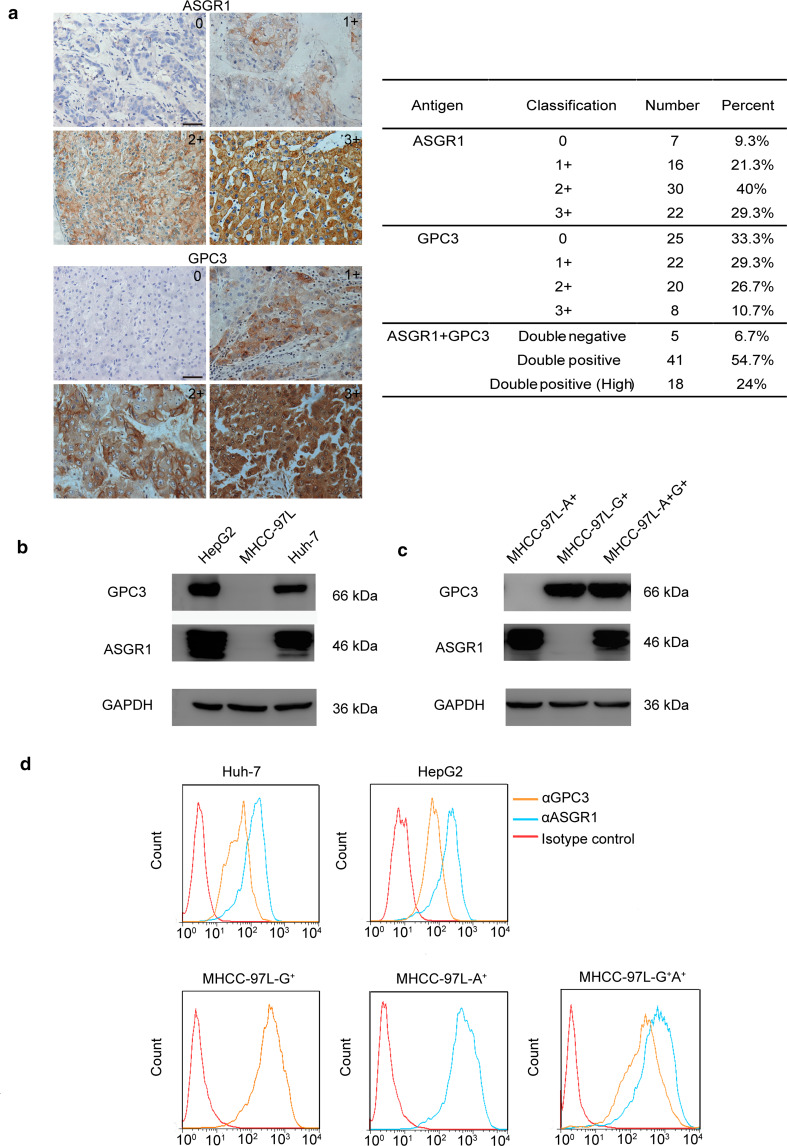
The basis for the use of GPC3 and ASGR1 dual-targeted CAR-T cells is the co-expression of these two antigens on the same cancer cell. Thus, we first examined the expression of GPC3 and ASGR1 in 75 HCC samples and 75 corresponding juxtacancerous liver samples using immunohistochemistry. The cores were evaluated by two experienced pathologists using a 4-point scale of staining intensity. Similar to previous reports [26, 27], the results of the IHC assay showed that GPC3 was overexpressed in most malignant tissue samples (66.7%) (Fig. 1a) but was hardly detected in corresponding juxtacancerous samples (Supplementary Fig. 1a). ASGR1 expression was observed in 90.7% of the malignant tissue samples (Fig. 1a) and all juxtacancerous liver tissues (Supplementary Fig. 1b). Although the IHC results suggested a minor loss of ASGR1 expression in tumor tissues, there was no significant difference between the tumor and adjacent tissues (Supplementary Fig. 1b). Additionally, no significant difference was observed in the H-scores for ASGR1 of various stages of HCC and adjacent normal liver tissues (Supplementary Fig. 1a) [24, 28]. Of the HCC malignant tissue samples, 41 (54.7%) co-expressed GPC3 and ASGR1, and 24% showed a high-level expression of both proteins (Fig. 1a).
Fig. 1.
GPC3 and ASGR1 expression in HCC. a GPC3 and ASGR1 expression in HCC tissues. IHC using the anti-GPC3 and anti-ASGR1 antibodies was performed to determine GPC3 and ASGR1 expression in a tissue microarray of 75 HCC tissues and the corresponding juxtacancerous tissues. b Western blot analysis of the expression of GPC3 and ASGR1 in HepG2, MHCC-97L and Huh-7 cells. c GPC3 and ASGR1 expression in MHCC-97L cells after transduction. The lysates of MHCC-97L-A+, MHCC-97L-G+ and MHCC-97L-G+A+ cells were separated on SDS-PAGE and subjected to Western blot using the indicated antibodies. d Flow cytometric analysis of the levels of GPC3 and ASGR1 expression on the cell surface. The cells were subjected to analysis with the indicated antibodies
To further confirm the co-expression of the two antigens on HCC cells, HepG2, Huh-7 and MHCC-97L cells were used to examine the expression of GPC3 and ASGR1. The results indicated that HepG2 and Huh-7 cells endogenously expressed both antigens, while the MHCC-97L cell line was negative for both antigens (Fig. 1b). Then, MHCC-97L cells were transduced to express GPC3 and ASGR1, either alone or together (Fig. 1c, d).
Expression of the complementary receptors in primary human T cells
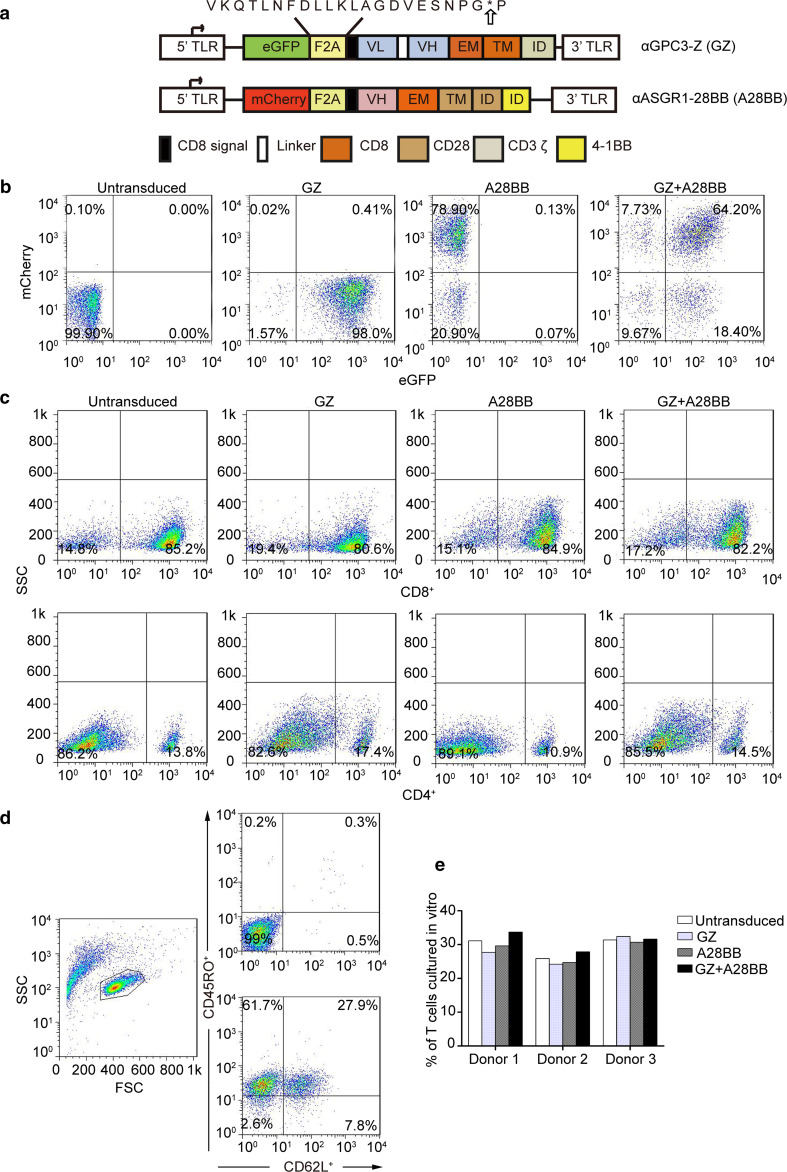
The aim of our work was to develop an effective strategy to establish time- and space-specific optimum dual-targeted T cell activation upon the simultaneous engagement of an HCC-related TAA and a liver-specific molecule, GPC3 and ASGR1, respectively (Supplementary Fig. 2). A first-generation chimeric antigen receptor specific for GPC3 and a chimeric co-stimulatory receptor specific for ASGR1 (A28BB) were constructed. Fourteen days after transduction, T cells expressing both receptors ranged from 40.6 to 64.2% (Fig. 2b and Supplementary Fig. 3). Notably, more than half of the T cells were CD8-positive after a period of expansion in vitro, which occurred independently of the gene transduction of the T cells (Fig. 2c). Previous studies have found that a high CD8/CD4 ratio is an effective indicator for better outcome of adoptive T cell immunotherapy against cancers [29].
Fig. 2.
Construction and characterization of different CAR-T cells. a A schematic representation of the lentiviral vectors expressing GZ or A28BB is shown. b The expression of GZ and A28BB on the surface of T cells was demonstrated by assessing the expression of eGFP and mCherry, respectively. c The flow cytometric analysis of the CD4+ and CD8+ phenotypes of in vitro expanded T cells. d Fourteen days after transduction, the expression levels of CD45RO and CD62L were determined using a FACSCalibur with the indicated antibodies. e The results were consistent in 3 donors
Central memory T cells exist in expanded T cells
Central memory T cells (Tcms) may be the most appropriate type of cells for use in adoptive cell therapy, as they can not only transfer instant antitumor immunity to patients but also endow them with immune memory to prevent cancer from recurring [30]. Therefore, the markers related to Tcms of all the engineered T cells were also analyzed on day 14 after transduction and culture in vitro. The expression of the differentiation markers CD45RO and CD62L on T cells was examined by flow cytometry [31]. The results confirmed that T cells with a Tcm-phenotype existed in all the groups (24.2–33.7%) (Fig. 2d), and this observation was repeated in three representative donors (Fig. 2e).
Dual-targeted T cells exert cytotoxicity against GPC3+ or GPC3+ASGR1+ tumor cells in vitro
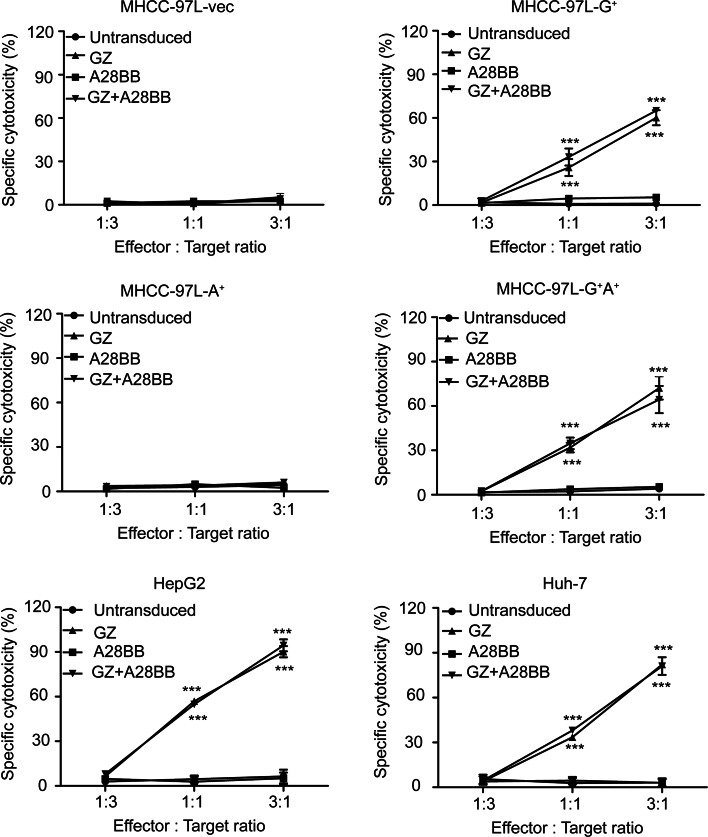
The in vitro cytotoxic activities of the dual-targeted and single-targeted CAR-T cells were first examined using GPC3 or/and ASGR1-transduced MHCC-97L cells. GZ+A28BB T cells and GZ T cells showed obvious cytotoxicity against MHCC-97L-G+ and MHCC-97L-G+A+ cells but not against MHCC-97L-A+ or MHCC-97L-vec cells, as expected (Fig. 3). A28BB T cells and untransduced T cells could not initiate specific cytotoxicity on all the tested tumor cell lines (Fig. 3). Surprisingly, the GZ+A28BB T cells did not exert higher cytotoxicity on all the GPC3-positive HCC cell lines than the GZ T cells.
Fig. 3.
Cytotoxicity of the engineered T cells against tumor cells. Untransduced, GZ, A28BB and GZ+A28BB T cells were used in co-culture with GPC3- or/and ASGR1-transfected MHCC-97L cells, HepG2 cells or Huh-7 cells at the indicated effector/target (E/T) ratios. Each value reflects the mean ± SEM of triplicate
Enhanced cytokine production and the expansion ability of dual-targeted T cells
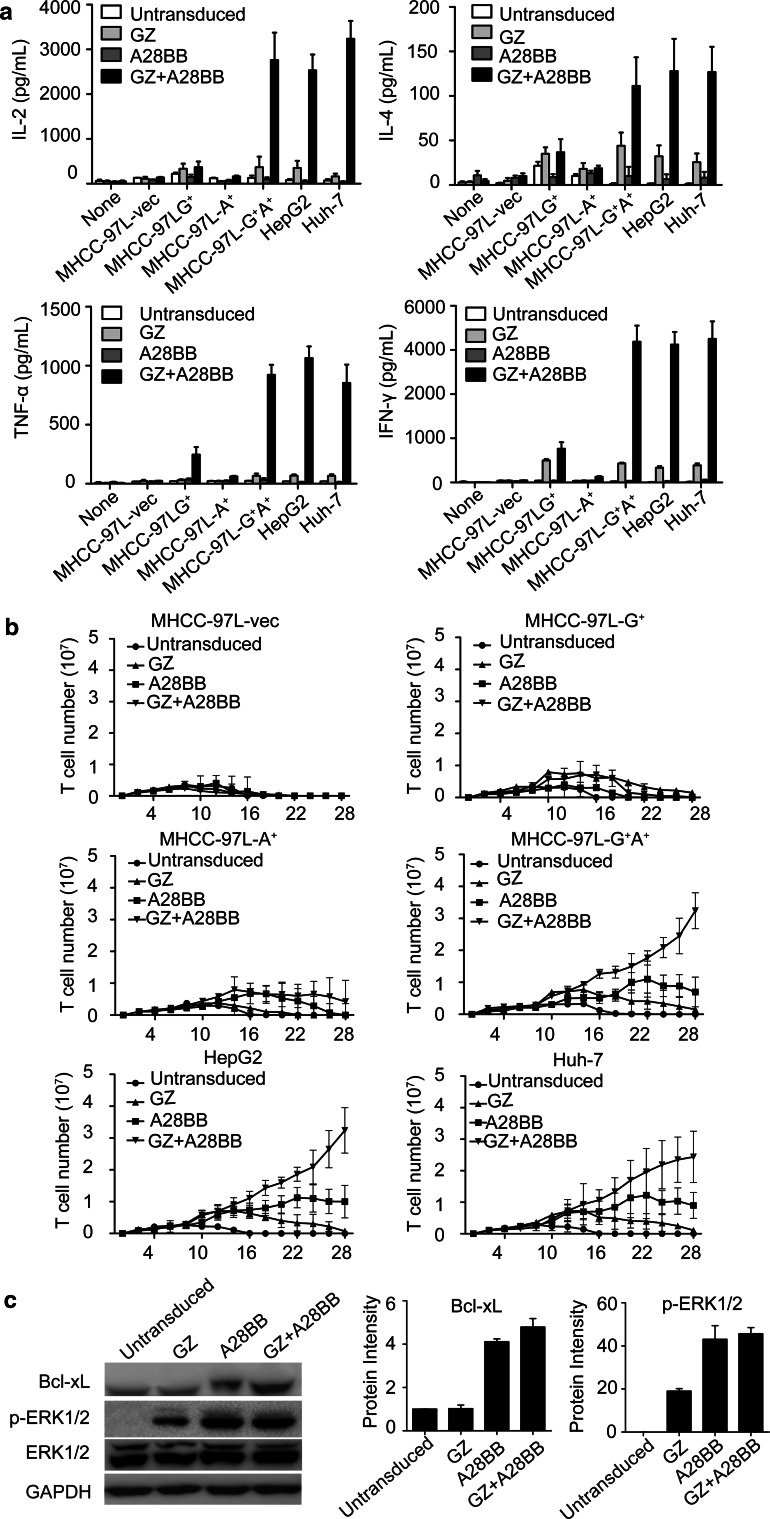
The incorporation of co-stimulatory signals has been demonstrated to enhance CTL response, cytokine production, proliferation ability and the prevention of death [32, 33]. Thus, the integration of CD3ζ and co-stimulatory signals in dual-targeted T cells is thought to synergistically enhance cytokine production. In accordance with previous studies [14], higher levels of IL-2, IFN-γ, TNF-α and IL-4 were produced by dual-targeted T cells than by single-targeted T cells when co-cultured with both GPC3- and ASGR1-positive tumor cells (Fig. 4a).
Fig. 4.
Cytokine release and proliferation capacity of the engineered T cells. a 1 × 106 engineered T cells were co-cultured with 1 × 106 tumor cells for 24 h. The levels of IFN-γ, IL-2, TNF-α and IL-4 in the supernatants were evaluated by ELISA. b 1 × 106 untransduced, GZ, A28BB and GZ+A28BB T cells were co-cultured with irradiated mock, GPC3+ or/and ASGR1+MHCC-97L cell lines with freshly irradiated tumor cells stimulated every week. The viable T cell numbers were measured every other day. c Four groups of T cells were co-cultured with MHCC-97L-G+A+ cells for 24 h. Then, the T cells were separated from tumor cells and subjected to Western blot analysis to measure the level of Bcl-xL and phospho-ERK1/2. The densitometry quantification of the protein levels of Bcl-xL and phospho-ERK1/2 (normalized to untransduced T cells) is shown
We also examined the proliferative ability of modified T cells stimulated weekly by HCC cells in the absence of exogenous cytokines. The results indicated that the GZ+A28BB T cells went through a robust expansion of approximately 25- to 35-fold after 28 days in culture, while untransduced and GZ T cells only went through a modest proliferation over the first 14 days and gradually died off after 2 weeks. Moreover, A28BB T cells could expand for approximately 16–22 days before diminishment (Fig. 4b). Similar results were observed in Huh-7 and HepG2 cells (Fig. 4b). Given that the activation of PI3K/Akt is a key effector downstream of CD28 and CD137, we assessed the expression of the downstream antiapoptosis protein Bcl-xL in different groups of T cells. Furthermore, the ligation of CD137 could activate ERKs and regulate the expression of cyclins [34]. Thus, the ERK1/2 phosphorylation level in T cells after incubation with MHCC-97L-G+A+ cells was tested. Both Bcl-xL expression and the ERK1/2 phosphorylation levels were increased in GZ+A28BB (Bcl-xL 4.8-fold, p-ERK1/2 45.7-fold) and A28BB (Bcl-xL 4.1-fold, p-ERK1/2 44.4-fold) T cells compared to those in untransduced T cells (Fig. 4c). When exposed to the γ-irradiated artificial antigen-presenting cells aK562-64/86, which express the membrane-bound extra-domains of CD64 and CD86, in the presence of immobilized muromonab-CD3 (OKT3) (100 ng/mL), all groups of T cells exhibited a 30- to 40-fold expansion during the 4-week culture (Supplementary Fig. 4).
Dual-targeted T cells exhibit restricted antitumor activity against HCC xenografts with a single antigen
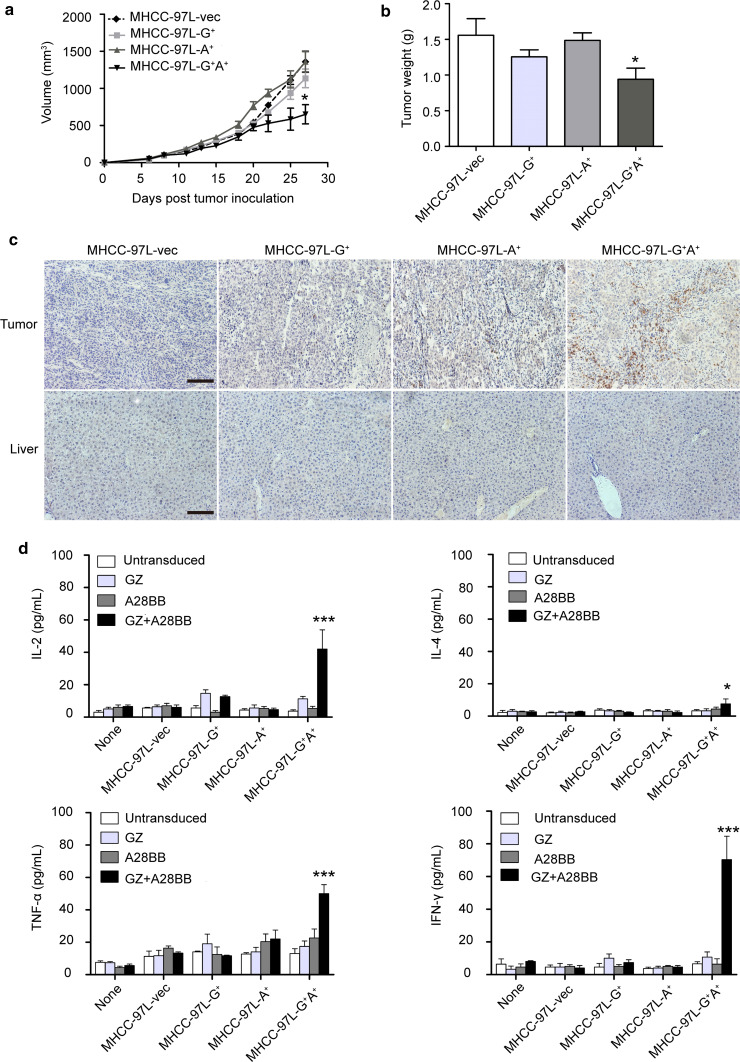
To understand the in vivo cytotoxic activities of the dual-targeted CAR-T cells on target cells with single antigen expression, mouse models bearing MHCC-97L xenografts expressing a single antigen or double antigens were used. The tumors bearing only GPC3 or ASGR1 were considered “normal tissues” to test the on-target, off-tumor cytotoxicity of the CAR-T cells. We first compared the in vivo growth speed of the MHCC-97L cells expressing GPC3 or/and ASGR1 to rule out the effect of tumor growth speed on the antitumor activities of CAR-T cells. The results indicated that the forced expression of the antigens did not noticeably change the growth of MHCC-97L cells in vivo (Supplementary Fig. 5). The potent antitumor effect was observed only in the mice bearing GPC3+ASGR1+ tumors treated with dual-targeted T cells, whereas the other groups of tumors could not be suppressed by GZ+A28BB T cells (Fig. 5a). At the endpoint of the experiment, the weights of the GPC3+ASGR1+ tumors were significantly less than those of the GPC3+, ASGR1+ or MHCC-97L-vec tumors, while no obvious differences were observed among the latter three groups (Fig. 5b). The infiltration of human T cells was confirmed by immunostaining tumor sections and livers. The results revealed that human CD3+ T cells preferentially accumulated and persisted in MHCC-97L-G+A+ residual tumors at approximately 4 weeks after intravenous T cell administration, while fewer or no T cells could be detected in the tumor sections bearing a single antigen or MHCC-97L-vec. There were no T cells observed in the mice livers (Fig. 5c). The levels of IFN-γ, IL-2, TNF-α and IL-4 in the blood of the mouse model were highest in the MHCC-97L-G+A+ group among all the groups (Fig. 5d). Together, these results suggested that powerful T cell activation and persistence occurred only when the GZ+A28BB T cells acquired integrated primary and co-stimulatory signals from the GZ-targeting antigen and the A28BB-targeting antigen, while the effect on the single antigen-bearing tissues was minimal. Thus, we confirmed that the dual-targeted design indeed reduced potential toxicity and improved safety.
Fig. 5.
Significant suppression of tumor growth in vivo by dual-targeted T cells requires both GPC3 and ASGR1 expression in the tumor cells. GZ+A28BB T cells were infused intravenously into NOD/SCID mice bearing established GPC3+ or/and ASGR1+MHCC-97L tumor xenografts. The tumor volumes (a) and weights (b) were quantified. c Formalin-fixed, paraffin-embedded tumor and mouse liver sections were consecutively cut and stained for human CD3e to detect T cell infiltration (brown). The images were taken with a microscope (Axio Scope.A1, Zeiss) under × 200 magnification. The scale bar was 100 μm. d The levels of IFN-γ, IL-2, TNF-α and IL-4 in mouse serum were evaluated by an ELISA
Only dual-targeted T cells caused a significant growth suppression effect on GPC3+ASGR1+ HCC xenografts
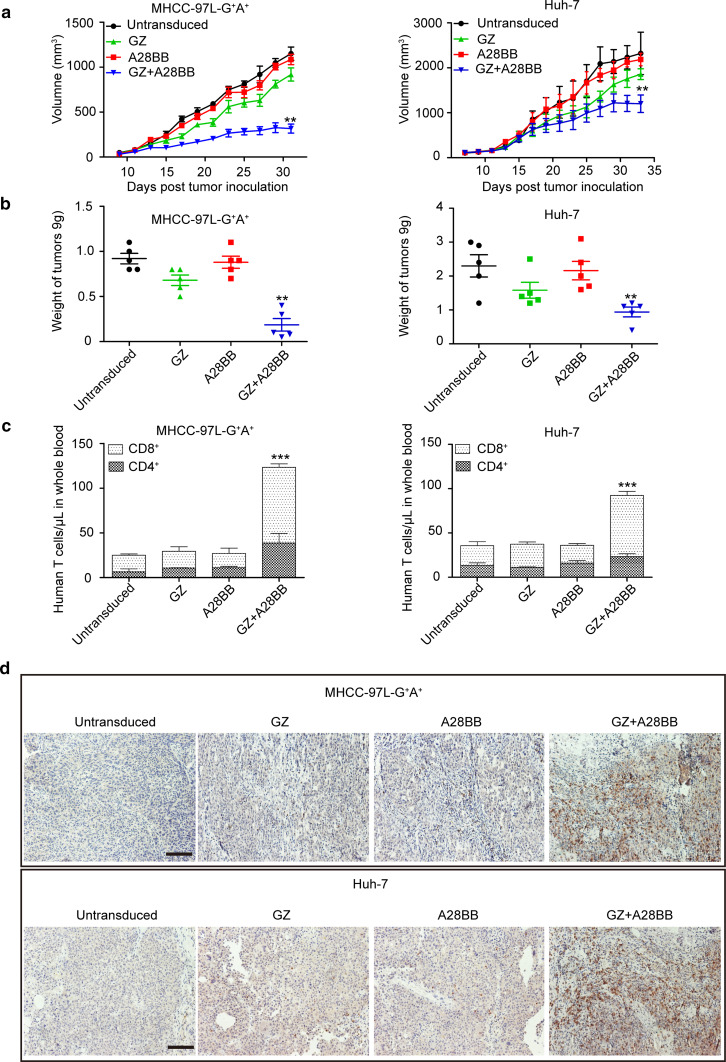
Because we verified that dual-targeted T cell activation requires both antigens using the above experiments, mouse models carrying MHCC-97L-G+A+ and Huh-7 tumor xenografts were used to compare the antitumor activities of the different groups of CAR-T cells. Only the dual-targeted, but not the single-targeted, T cells could significantly inhibit the growth of MHCC-97L-G+A+ and Huh-7 tumor xenografts (Fig. 6a, b). Furthermore, the dual-targeted T cells had better persistence in vivo compared to the other T cell groups (Fig. 6c, d), suggesting that the ASGR1-mediated co-stimulatory signal is important for the in vivo survival of GPC3-targeted CAR-T cells.
Fig. 6.
Antitumor activities of the modified T cells against established GPC3+ASGR1+ HCC xenografts in vivo. a The growth curves of MHCC-97L-G+A+ and Huh-7 xenografts treated with the indicated T cells. b At the endpoint of the experiments, the residual tumors treated with dual-targeted T cells were significantly smaller than those in the other groups (**, P < 0.01). c The quantities of circulating human CD4+ and CD8+ T cells from mice bearing MHCC-97L-G+A+ and Huh-7 xenografts treated with the indicated genetically modified T cells. The mean cell concentration (cells/μL ± SEM) for mice in the untransduced or modified T cell treatment groups and the significant differences (***, P < 0.001) are shown. d The infiltration of adoptive human T cells was detected in subcutaneous xenografts recovered from mice. The images were obtained under ×200 magnification. The scale bar is 100 μm
Discussion
CAR-T cells targeting a series of classical TAAs have been applied in preclinical and clinical studies [35]. However, most TAAs are not absolutely restricted to tumor tissues, which may lead to severe on-target, off-tumor toxicities and sometimes even fatal side effects in clinical trials [12, 36]. Thus, strategies such as using dual-targeted CAR-T cells seem promising to reduce on-target, off-tumor toxicities. For dual-targeted CAR-T cells, the first target for stimulatory signaling should be overexpressed in tumor tissues, but not in normal tissues, while the second target for co-stimulatory signaling should be a tissue-specific protein that has high-level expression in the tumor tissues. GPC3 is frequently expressed in HCC but not in normal liver tissue [11, 27]. ASGR1 is a cell surface receptor expressed exclusively on hepatic parenchymal cells, and it has been exploited in the targeted delivery of therapeutic molecules to the liver [15, 17]. Thus, ASGR1 was used as the co-stimulatory signal to ensure the dual-targeted T cells can be optimally activated upon encountering HCC cells. HCC generally overexpresses both GPC3 and ASGR1 [17], which was confirmed by IHC and FACS analysis in our study. Therefore, GPC3 and ASGR1 are a suitable target combination for dual-targeted CAR-T cells.
Similar to previous studies [14, 37], we delivered two exogenous chimeric receptor genes into primary human T cells using two lentiviruses. Once the appropriate MOI was determined, the transduction efficiency and T cell viability could be guaranteed. Retrovirus- or lentivirus-mediated CAR transduction is the mainstream approach in preclinical or clinical studies, but we were unsure whether it would result in recombinant events that are unwanted after the two vectors were transduced. Using mRNA electroporation instead of viruses in the future could ease concerns about clinical safety.
Our study revealed that GZ+A28BB T cells had similar tumor-killing ability in regard to GPC3+ HCC cells in vitro as GZ T cells. However, GZ+A28BB T cells could proliferate more persistently than single-targeted CAR-T cells when stimulated with GPC3+ASGR1+ tumor cells. Another interesting finding was that A28BB T cells demonstrate more persistent proliferation than GZ T cells in the presence of ASGR1-positive cancer cells. This increased proliferation may be due to the elevated levels of the antiapoptosis protein Bcl-xL expression and ERK1/2 phosphorylation, which can be attributed to the complementary co-stimulatory signals from the chimeric receptor A28BB [32–34]. However, the mechanisms allowing A28BB T cells to proliferate for a longer time without the engagement of signal 1 need to be further explored.
GZ+A28BB T cells exhibited significant tumor suppression capacity against GPC3+ASGR1+ xenografts but not against ASGR1+, GPC3+ or GPC3−ASGR1− tumor xenografts. The histological analysis revealed that abundant dual-targeted T cells could be detected in the GPC3+ASGR1+ xenografts, while GZ+A28BB T cells were rarely detected in GPC3+ tumor xenografts, suggesting that the binding of the complementary chimeric antigen receptors to both GPC3 and ASGR1 are important to the in vivo activation, expansion and persistence of dual-targeted T cells.
Furthermore, in both MHCC-97L-G+A+ and Huh-7 xenograft models, only the GZ+A28BB T cells could significantly inhibit tumor growth, indicating that dual-targeted T cells need to be fully activated to cause significant antitumor effects. After further analyzing the human T cells in peripheral blood from the treated mice, we observed that the engagement of co-stimulatory signals could increase in vivo persistence and antitumor capacity of the adoptively transferred T cells. Curiously, there were more CD8+ T cells than CD4+ T cells in blood samples drawn from the mice; this observation might be due to a more abundant CD8+ population following in vitro culture.
We did not observe any obvious side effects (including rough hair coat, ataxia, weight loss, abnormal body temperature or death) in the mice after the engineered CAR-T cells were transferred. Mouse Asgr1 is expressed abundantly on mouse liver cells [38, 39]. The domain antibody for ASGR1 shows objective but lower cross-reactivity with mice Asgr1 (k D = 4 nM) than with human ASGR1 (k D = 1 nM) [16]. Additionally, it has been reported that the mAb GC33 will not recognize murine Gpc3 [40]. Therefore, we speculated that only dual-targeted or ASGR1-targeted T cells might cause toxicity in the organs, especially the livers of the mice. However, we did not observe infiltration of T cells or any toxicity to all mouse livers. Moreover, since we lacked transgenic mouse models that could mimic human GPC3 and ASGR1 expression, we used subcutaneous xenografts with only GPC3 expression to imitate normal tissues with GPC3 expression. Our study demonstrated that GPC3-targeted or the dual-targeted T cells had no significant growth suppression effect on HCC xenografts with only GPC3 expression, suggesting that the engineered T cells have limited toxicity on normal organs with only GPC3 expression. Unfortunately, because of the differences between normal organs and tumor xenografts, we could not rule out the possibility of toxicity of the dual-targeted T cells on the normal tissues with GPC3 expression. Furthermore, the higher levels of IFN-γ, IL-2, TNF-α and IL-4 in the dual-targeted group of mice indicated cytokine release syndrome might possibly occur in clinical therapy.
Bolhuis reported that the activation of T lymphocytes is co-determined by the expression levels of the chimeric receptors on T lymphocytes and the TAA on tumor cells [41]. T lymphocytes with high expression levels of chimeric receptors kill tumor cells with a wide range of TAA densities [41]. Chimeric receptors containing high-affinity scFv can target tumor cells with various TAA densities on an extremely similar level [42]. According to the FACS data in Fig. 1d, the expression of ASGR1 and GPC3 on MHCC-97L-A+G+ cells was higher than that of Huh-7 and HepG2 cells. However, the dual-targeted CAR-T cells were trigged to exert similar effector functions, including tumor cell lysis, cytokine production and cell proliferation. It may be that the densities of TAAs on HepG2 and Huh-7 cells are sufficient, and we used high-affinity antibodies to construct the chimeric receptors. In our previous study, we found that PLC/PRF/5, an HCC cell line with a significantly lower level of GPC3 expression than Huh-7 cells, displayed less sensitivity to GPC3-targeted CAR-T cells both in vitro and in vivo [10]. Thus, whether the TAA density on the tumor cells is sufficient might be an indicator of the antitumor efficacy of CAR-modified T cells.
Compared to the tumor-killing capacity of third-generation CAR-T cells (GC33-28BBZ) [10] in Huh-7 xenograft models, the dual-targeted CAR-T cells performed worse in terms of their tumor suppression ability. We speculated that the dual-targeted CAR-T construct does not simply separate two kinds of signal domains, but there are many uncertain factors affecting final therapeutic results. A recent report pointed out that the structure of scFv may influence downstream signaling and its ultimate antitumor activity [43]. In our study, we first adopted a domain antibody for ASGR1 [16] as the antigen-binding region, though it has been shown to function as a common scFv in transducing the co-stimulatory signal via binding antigens. However, according to a previous report, the domain antibody is prone to aggregation [44]. Thus, whether the domain antibody for ASGR1 will result in the cross-linking of neighboring receptors and early senescence of T cells is still unknown.
Without available transgenic mouse models that can mimic the human GPC3 and ASGR1 expression patterns, the genuine behaviors of dual-targeted T cells are still hard to delineate. Our mouse models could indicate only that the on-target, off-tumor effect of GPC3- and ASGR1-specific CAR-T cells on GPC3+ tissues was very slight, but the genuine clinical effect in the human body can only be demonstrated in future clinical trials. In summary, our data here indicated that the combination of the chimeric receptors αGPC3-Z and αASGR1-28BB could be potential novel anti-HCC therapeutics with reduced off-tumor toxicities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The study was funded by the Supporting Programs of the National Natural Science Foundation of China (No. 81502672), the “13th Five-Year Plan” for Science and Technology Research of China (2016ZX10002014-009) and the One Hundred Talents Scientific Research Projects of Health System in Shanghai (XBR2013123).
Abbreviations
- ASGR1
Asialoglycoprotein receptor 1
- Bcl-xL
B cell lymphoma-extra large
- CAR-T
Cells chimeric antigen receptor-modified T cells
- eGFP
Enhanced green fluorescent protein
- ERK
Extracellular regulated kinase
- GPC3
Glypican-3
- HCC
Hepatocellular carcinoma
- MOI
Multiplicity of infection
- p-ERK
Phosphorylated ERK
- rhIL-2
Recombinant human interleukin 2
- Tcm
Central memory T cell
- TMA
Tissue microarray
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Hongyang Wang, Email: hywangk@vip.sina.com.
Zonghai Li, Phone: 86-21-64436601, Email: zonghaili@shsmu.edu.cn.
References
- 1.Li ZH, Chen C, Li KS, Jiang H, Song F, Gao HP, Pan XR, Shi BZ, Bi YY, Wang HM, Wang HY. Development of T-cells carrying two complementary chimeric antigen receptors against GPC3 and ASGR1 for the treatment of hepatocellular carcinoma. Mol Ther. 2016;24(supplement 1):S79. doi: 10.1016/S1525-0016(16)33011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer IAfRo . World cancer report 2014. Geneva: WHO; 2014. [Google Scholar]
- 3.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Sola R, Rodes J, Bruix J, Barcelona Liver Cancer G Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, Jensen MC. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 7.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J, Li Z. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20(24):6418–6428. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]
- 11.Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129(6):899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 12.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, Burbridge SE, Box C, Eccles SA, Maher J. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32(5):1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 14.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31(1):71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiume L, Di Stefano G, Busi C, Mattioli A, Bonino F, Torrani-Cerenzia M, Verme G, Rapicetta M, Bertini M, Gervasi GB. Liver targeting of antiviral nucleoside analogues through the asialoglycoprotein receptor. J Viral Hepat. 1997;4(6):363–370. doi: 10.1046/j.1365-2893.1997.00067.x. [DOI] [PubMed] [Google Scholar]
- 16.Coulstock E, Sosabowski J, Ovecka M, Prince R, Goodall L, Mudd C, Sepp A, Davies M, Foster J, Burnet J, Dunlevy G, Walker A. Liver-targeting of interferon-alpha with tissue-specific domain antibodies. PLoS ONE. 2013;8(2):e57263. doi: 10.1371/journal.pone.0057263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu H, Lin KX, Zhao H, Xing S, Li C, Liu F, Lu HZ, Zhang Z, Sun YL, Yan XY, Cai JQ, Zhao XH. Identification of biomarkers for hepatocellular carcinoma by semiquantitative immunocytochemistry. World J Gastroenterol. 2014;20(19):5826–5838. doi: 10.3748/wjg.v20.i19.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzigmann D, Quagliata L, Schenk SH, Quintavalle C, Terracciano LM, Huwyler J. Variable asialoglycoprotein receptor 1 expression in liver disease: implications for therapeutic intervention. Hepatol Res. 2016;46(7):686–696. doi: 10.1111/hepr.12599. [DOI] [PubMed] [Google Scholar]
- 19.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22(5):589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Zhou M, Shi B, Zhang Q, Jiang H, Sun Y, Liu J, Zhou K, Yao M, Gu J, Yang S, Mao Y, Li Z. Identification of an exon 4-deletion variant of epidermal growth factor receptor with increased metastasis-promoting capacity. Neoplasia. 2011;13(5):461–471. doi: 10.1593/neo.101744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroda H, Kutner RH, Bazan NG, Reiser J. Simplified lentivirus vector production in protein-free media using polyethylenimine-mediated transfection. J Virol Methods. 2009;157(2):113–121. doi: 10.1016/j.jviromet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Li K, Pan X, Bi Y, Xu W, Chen C, Gao H, Shi B, Jiang H, Yang S, Jiang L, Li Z. Adoptive immunotherapy using T lymphocytes redirected to glypican-3 for the treatment of lung squamous cell carcinoma. Oncotarget. 2016;7(3):2496–2507. doi: 10.18632/oncotarget.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1(11):1311–1318. [PubMed] [Google Scholar]
- 24.Shi B, Abrams M, Sepp-Lorenzino L. Expression of asialoglycoprotein receptor 1 in human hepatocellular carcinoma. J Histochem Cytochem. 2013;61(12):901–909. doi: 10.1369/0022155413503662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Tommaso L, Destro A, Seok JY, Balladore E, Terracciano L, Sangiovanni A, Iavarone M, Colombo M, Jang JJ, Yu E, Jin SY, Morenghi E, Park YN, Roncalli M. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol. 2009;50(4):746–754. doi: 10.1016/j.jhep.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Gao JZ, Du JL, Wei LX. Prognostic and clinicopathological significance of glypican-3 overexpression in hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20(20):6336–6344. doi: 10.3748/wjg.v20.i20.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hass HG, Jobst J, Scheurlen M, Vogel U, Nehls O. Gene expression analysis for evaluation of potential biomarkers in hepatocellular carcinoma. Anticancer Res. 2015;35(4):2021–2028. [PubMed] [Google Scholar]
- 28.Trere D, Fiume L, De Giorgi LB, Di Stefano G, Migaldi M, Derenzini M. The asialoglycoprotein receptor in human hepatocellular carcinomas: its expression on proliferating cells. Br J Cancer. 1999;81(3):404–408. doi: 10.1038/sj.bjc.6690708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shindo G, Endo T, Onda M, Goto S, Miyamoto Y, Kaneko T. Is the CD4/CD8 ratio an effective indicator for clinical estimation of adoptive immunotherapy for cancer treatment? JCT. 2013;4(8):1382–1390. doi: 10.4236/jct.2013.48164. [DOI] [Google Scholar]
- 30.Yang S, Gattinoni L, Liu F, Ji Y, Yu Z, Restifo NP, Rosenberg SA, Morgan RA. In vitro generated anti-tumor T lymphocytes exhibit distinct subsets mimicking in vivo antigen-experienced cells. Cancer Immunol Immunother. 2011;60(5):739–749. doi: 10.1007/s00262-011-0977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 32.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14(3–4):265–273. doi: 10.1016/S1359-6101(03)00025-X. [DOI] [PubMed] [Google Scholar]
- 33.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3(7):544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 34.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 35.Kohn DB, Dotti G, Brentjens R, Savoldo B, Jensen M, Cooper LJ, June CH, Rosenberg S, Sadelain M, Heslop HE. CARs on track in the clinic. Mol Ther. 2011;19(3):432–438. doi: 10.1038/mt.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stauss HJ, Morris EC. Immunotherapy with gene-modified T cells: limiting side effects provides new challenges. Gene Ther. 2013;20(11):1029–1032. doi: 10.1038/gt.2013.34. [DOI] [PubMed] [Google Scholar]
- 37.Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ., Jr Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1(1):43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takezawa R, Shinzawa K, Watanabe Y, Akaike T. Determination of mouse major asialoglycoprotein receptor cDNA sequence. Biochim Biophys Acta. 1993;1172(1):220–222. doi: 10.1016/0167-4781(93)90300-3. [DOI] [PubMed] [Google Scholar]
- 39.Soukharev S, Berlin WK, Hanover JA, Bethke B, Sauer B. Organization of the mouse ASGR1 gene encoding the major subunit of the hepatic asialoglycoprotein receptor. Gene. 2000;241(2):233–240. doi: 10.1016/S0378-1119(99)00493-X. [DOI] [PubMed] [Google Scholar]
- 40.Nakano K, Yoshino T, Nezu J, Tsunoda H, Igawa T, Konishi H, Tanaka M, Sugo I, Kawai S, Ishiguro T, Kinoshita Y (2007) Anti-glypican 3 antibody. US 2007/0190599 A l [United States Patent Application Publication]
- 41.Weijtens ME, Hart EH, Bolhuis RL. Functional balance between T cell chimeric receptor density and tumor associated antigen density: CTL mediated cytolysis and lymphokine production. Gene Ther. 2000;7(1):35–42. doi: 10.1038/sj.gt.3301051. [DOI] [PubMed] [Google Scholar]
- 42.Caruso HG, Hurton LV, Najjar A, Rushworth D, Ang S, Olivares S, Mi T, Switzer K, Singh H, Huls H, Lee DA, Heimberger AB, Champlin RE, Cooper LJ. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75(17):3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, Kaplan RN, Patterson GH, Fry TJ, Orentas RJ, Mackall CL. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23(9):1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.