Abstract
Recombinant interleukin-2 (rIL-2) is associated with objective responses in 15–20 % of patients with metastatic melanoma and renal cell carcinoma. More recently, rIL-2 has also demonstrated improved clinical activity in patients with melanoma. Given the toxicity of high-dose rIL-2 and the availability of many new immunotherapy agents, it has been suggested that lower doses of rIL-2 may be preferred for combination clinical studies. In order to determine the impact of low doses of rIL-2 on anti-tumor immunity and therapeutic effectiveness, we challenged C57BL/6 mice with poorly immunogenic B16-F10 melanoma and treated them with varying doses of rIL-2 (range 103–105 IU). Tumor growth at day 14 was significantly reduced when rIL-2 was administered at 10,000 (P < 0.02) and 100,000 (P < 0.02) IU doses, but tumor growth was significantly increased when mice were treated at 1000 IU rIL-2 (P < 0.02), as compared to placebo treatment. While the proportions of CD8+ and CD4+ T cells in the tumor were similar at all doses tested, the proportion of NK cells was decreased and the proportion of Tregs was increased in tumors exposed to low-dose rIL-2. The ratio of gp100-specific CD8+ to CD4+ regulatory T cells was increased in tumors treated at 10,000 and 100,000 IU of rIL-2 but was decreased at the 1000 IU dose compared to placebo-treated mice. These findings suggest that low-dose rIL-2 may impair host anti-tumor immunity and promote tumor growth. Early-phase adjuvant and combination clinical studies should include patient cohorts with higher doses of rIL-2.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1916-4) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, Interleukin-2, Cancer vaccines, CD8+ T cell, Regulatory T cell, Natural killer cell
Introduction
Immunotherapy is a rapidly advancing field in the treatment of cancer that relies on activating the patient’s immune system against the offending tumor. Cancer vaccines are a promising area of research in immunotherapy and have the potential to induce strong anti-tumor responses within the tumor microenvironment [1]. Cancer vaccines can be divided into two subtypes: preventative cancer vaccines and cancer treatment vaccines. As their names imply, preventative vaccines work to prevent cancer in healthy individuals, while treatment vaccines treat tumors in afflicted individuals [1]. Currently, only three preventative cancer vaccines have been approved by the US Food and Drug Administration (FDA). Two vaccines prevent specific strains of human papillomaviruses (HPV 16 and 18), which can cause vulvar, vaginal, anal, penile, and oropharyngeal cancers [2–5]. The third approved preventative vaccine targets hepatitis B virus, which can cause liver cancer [6–8]. Preventative vaccines against other oncogenic infectious agents are being tested in order to vaccinate against additional cancers [9]. On the other hand, cancer treatment vaccines induce the immune system to treat existing tumors. The FDA has approved one treatment vaccine for castration-resistant prostate cancer: sipuleucel-T [10].
In order to induce a greater immunologic response to cancer vaccines, adjuvants are often added to the vaccination regimen. Adjuvants strengthen the immune response by recruiting additional antigen-presenting cells, initiating non-specific immune responses, and promoting the innate immune system. Interleukins (ILs), and more specifically rIL-2, have been promising candidates for use as adjuvants for cancer vaccines. The therapeutic action of rIL-2 is facilitated by the expansion of CD8+ T lymphocytes and NK cells. When combined with cancer vaccines, it is believed that rIL-2 enhances the activation of tumor-specific T cells that are more efficient in destroying tumors [1, 11]. However, T regulatory cells (Tregs) express high levels of the IL-2 receptor, CD25, and may expand with rIL-2 treatment [12, 13]. Therefore, it is important to understand how the levels of IL-2 achieved in vaccination or supplemented with vaccination augment therapeutic activity based on the pleiotropic effects of rIL-2 on T cells [14].
Results from preclinical and clinical trials of combinatorial approaches of cancer vaccines and rIL-2 suggest an immunomodulatory role for rIL-2 in cancer treatment vaccines. However, the amount of rIL-2 needed to promote therapeutic activity as opposed to increasing immune suppression remains a pertinent question [15–20]. In this study, we explored the importance of rIL-2 in cancer treatment by determining the impact of rIL-2 doses on tumor growth and immune T cell responses.
Materials and methods
Animals
Six- to eight-week-old C57BL/6 J (B6) and B6.Cg-Thy1a/CyTg(TcraTcrb)8Rest/J (pmel) male mice (4–10 per group per experiment, Jackson Laboratory, Ben Harbor ME, USA) were housed under specific pathogen-free conditions at Rush University Medical Center. All animal procedures were performed in accordance with Rush University Medical Center Institutional Animal Care and Use Committee guidelines.
Cell culture and adoptive cell transfer
B16-F10 melanoma cells were cultured in RPMI supplemented with 10 % heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA, USA), 2 mM L-glutamine (Mediatech, Manassas, VA, USA), and 1 % penicillin/streptomycin (Mediatech). Pmel CD8+ T cells (100,000; specific for gp10025–33) were purified by negative magnetic bead selection (Miltenyi Biotec Inc., San Diego, CA, USA) and adoptively transferred to B6 mice via retro-orbital venous injection in 100 µl PBS.
Tumor challenge and monitoring
B6 mice were anesthetized with isoflurane and challenged in the shaved right flank with B16-F10 melanoma cells (via intradermal injection of 100,000 cells) in a 50/50 v/v mixture with BD Matrigel basement membrane matrix (BD Biosciences, Bedford, MA, USA). The tumor area (length x width) was measured on days 9, 12, and 14, and mice were sacrificed on day 14. Primary outcomes included tumor size and T cell characterization of cells obtained from tumors and tumor-draining inguinal lymph nodes. Four to ten mice were utilized in each group per study, as described in the figure legends.
Interleukin (IL)-2 treatment
B6 mice bearing tumors were treated on days 5–9 via intraperitoneal (i.p.) injection with rIL-2 (aldesleukin) at 100,000, 10,000, or 1,000 IU (Prometheus Laboratories Inc., San Diego, CA, USA) in 100 µl PBS or left untreated (placebo; 100 µl PBS injection).
Flow cytometry analysis
Cells obtained from B6 mice were analyzed by flow cytometric analysis using a Canto II flow cytometer (BD, Franklin Lakes, NJ, USA) and FlowJo software (Tree Star, Ashland, OR, USA). Gating on live, singlet, non-debris lymphocytes was performed, as previously described (22). All antibodies were purchased from eBiosciences (San Diego, CA, USA).
Statistical analysis
Student’s t test (two-tailed) was used for comparisons of data using GraphPad Prism software (v4.0, GraphPad Software, Inc., La Jolla, CA, USA). Statistically significant comparisons were denoted by a P < 0.05.
Results
Effect of rIL-2 dose selection on B16 melanoma tumor growth
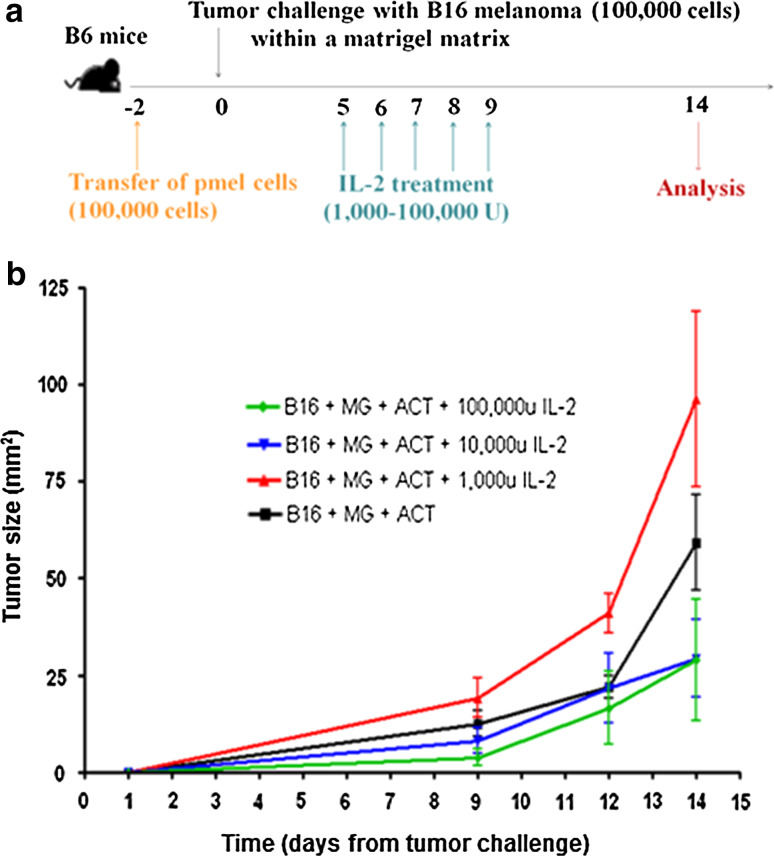
To determine the effect of different rIL-2 doses on tumor growth, three groups of C57BL/6 (B6) mice were challenged with B16 melanoma within a Matrigel matrix solution (for easy monitoring of tumor growth) (Fig. 1a). To track anti-tumor CD8+ T cell responses, pmel CD8+ T cells (specific to gp10025–33 expressed by melanocytes and melanoma) were transferred (at day-2) to all mice via retro-orbital injection. Transfer of the tumor-specific CD8+ T cells (pmels) had no significant effect on tumor growth (Fig. 1b). Tumor-bearing mice were treated with clinically relevant rIL-2 doses at 100,000 international units (IU) corresponding to standard high-dose IL-2 treatment in patients, 10,000 IU, or 1,000 IU or left untreated (placebo group; PBS) on days 5–9 (Fig. 2a). At day 14, tumor growth was significantly reduced with 10,000 and 100,000 IU of rIL-2 (31 and 38 mm2, respectively) compared to the placebo (61 mm2). However, tumor growth was significantly increased in mice treated with 1,000 IU of rIL-2 (96 mm2) compared to placebo (P < 0.02 for all doses versus placebo) (Fig. 2b).
Fig. 1.
Validation of a tumor growth model utilizing adoptive cell transfer and Matrigel tumor challenge. a Schematic of the experimental design. b Tumor growth curve after challenge of B6 mice (n = 10 per group) with B16 melanoma (100,000 cells) alone or in the context of Matrigel (MG) and/or in the context of CD8+ pmel adoptive cell transfer (ACT). P > 0.05 (not statistically significant) for comparison of all lines with the black (B16 + ACT + MG) line. Results are representative of two experiments with similar results
Fig. 2.
Effect of IL-2 dose selection on B16 melanoma growth. a Schematic of the experimental design. b Tumor growth curve after challenge of B6 mice (n = 10 per group) with B16 melanoma and IL-2 immunotherapy at varying doses. All mice received the tumor challenge in the context of Matrigel (MG) and CD8+ pmel adoptive cell transfer (ACT). P < 0.02 for comparison of red and black lines to all other lines. P > 0.05 (not statistically significant) for comparison of blue line to green line. Results are representative of two experiments with similar results
Impact of rIL-2 dosing on host immune cells within tumor-draining lymph nodes
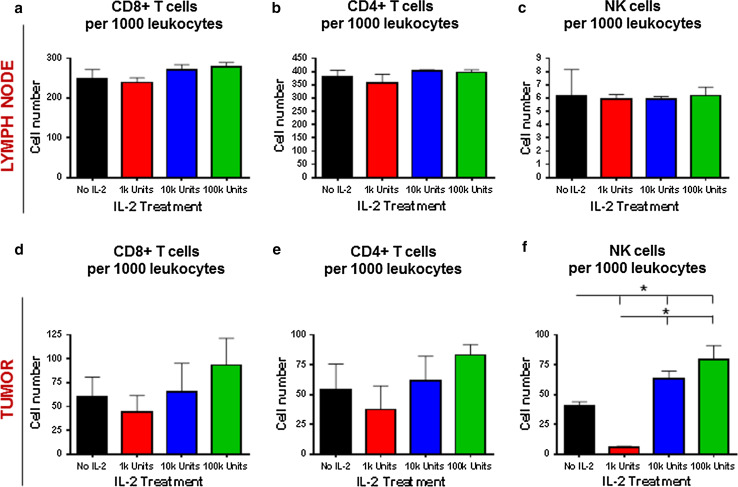
Recombinant IL-2 has been shown to trigger anti-tumor effector responses (via CD8+ T cells and NK cells) and immunosuppressive (pro-tumor) responses (via CD4+ FoxP3+ Tregs [Tregs]). To determine how the varying doses of rIL-2 impact tumor growth, we assessed the infiltrating immune cells within the tumor-draining lymph nodes of tumor-bearing mice treated at various doses of rIL-2 (Supplementary Figure S1). The proportion of CD8+ T cells, CD4+ T cells, and NK cells within the tumor-draining lymph nodes were similar in all groups tested and did not correlate with tumor growth (Fig. 3a–c). However, when these cell populations were evaluated in the tumor microenvironment, there was a slight trend toward increased CD8+ T cells (Fig. 3d) and CD4+ T cells (Fig. 3e) in mice treated with high doses of rIL-2. There was a significant expansion of tumor NK cells at high doses of rIL-2; however, this effect was absent when mice were treated with low doses of rIL-2 (Fig. 3f).
Fig. 3.
Correlation of CD8+ T cells, CD4+ T cells, and NK cells with IL-2 treatment dose outcomes. Ratios of total a CD8+ T cells, b CD4+ T cells, and c NK cells obtained from tumor-draining lymph nodes of mice (n = 4 per group) in Fig. 2 on day 14. Ratios of d CD8+ T cells, e CD4+ T cells, and f NK cells obtained from tumors of mice (n = 4 per group) in Fig. 2 on day 14. *Comparisons for which P < 0.05. Results are representative of two experiments with similar results
rIL-2 treatment outcome correlates with a high CD8+ T cell/Treg ratio
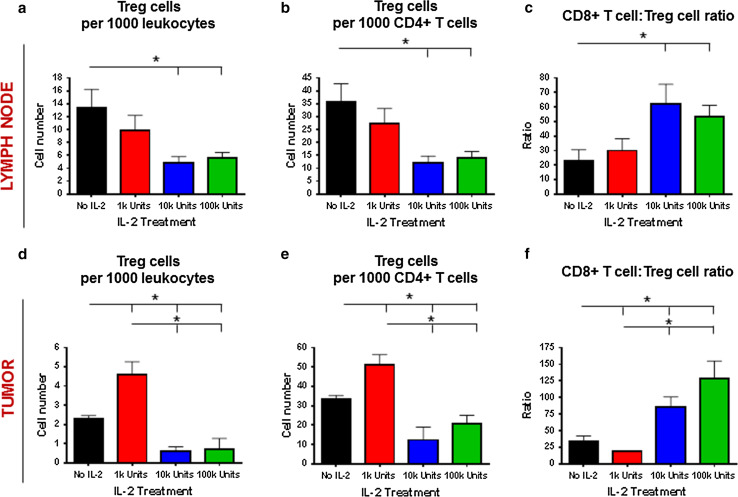
We analyzed the Tregs in tumor-draining lymph nodes and found a significant decrease in Tregs at high doses of IL-2 (Fig. 4a–b), which was also associated with an increase in the CD8+ T cell/Treg ratio in the lymph nodes (Fig. 4c). In the tumor microenvironment, a significant Treg expansion was observed in mice treated with low doses of rIL-2 (Fig. 4d–e), but a paradoxical decrease in Tregs was seen when mice were exposed to higher doses of rIL-2. The ratio of CD8+ T cells/Tregs was, thus, increased at high doses of rIL-2 (Fig. 4f).
Fig. 4.
Correlation of Tregs with IL-2 treatment dose outcomes. Number of Tregs per a 1000 leukocytes and b 1000 CD4+ T cells, as well as the c CD8+ T cell-to-CD4+ Treg cell ratio obtained from tumor-draining lymph nodes of mice (n = 4 per group) from Fig. 2 on day 14. Number of Tregs per d 1000 leukocytes and e 1000 CD4+ T cells, as well as the f CD8+ T cell-to-CD4+ Treg cell ratio obtained from tumor-draining lymph nodes of mice (n = 4 per group) from Fig. 2 on day 14. *Comparisons for which P < 0.05. Results are representative of two experiments with similar results
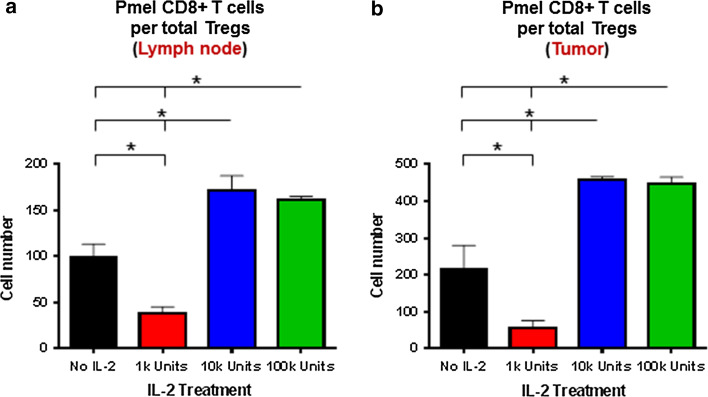
Previous studies have demonstrated that a high CD8+/CD4+ regulatory T cells ratio correlates with decreased tumor growth rate. Thus, we determined whether the dose of rIL-2 affects the ratio of tumor-specific CD8+ T cells to Tregs in the tumor-draining lymph nodes and within the tumor microenvironment following adoptive transfer of pmel CD8+ T cells. At higher doses of rIL-2, 10,000 and 100,000 IU, the pmel CD8+ T cell/Treg ratio was increased, while with the lower dose, 1,000 IU, the pmel CD8+ T cell/Treg ratio was decreased when compared to the placebo group (no rIL-2) (Fig. 5a). The correlation between this ratio and tumor growth after rIL2 therapy was verified in the tumor microenvironment (Fig. 5b).
Fig. 5.
Correlation of tumor-specific (pmel) CD8+ T cells numbers among per total Tregs with IL-2 treatment dose outcomes. Number of pmel CD8+ T cells per total Tregs obtained from the a tumor-draining lymph nodes and b tumor microenvironment of mice (n = 4 per group) from Fig. 2 on day 14. *Comparisons for which P < 0.05. Results are representative of two experiments with similar results
Discussion
The efficacy of cancer vaccines depends on the ability to stimulate host anti-tumor immunity and this often requires additional adjuvant strategies to generate tumor-specific T cells to low-affinity antigens. IL-2 is important for CD8+ T cell responses with IL-2 orchestrating CD8+ T cell activation, expansion, exhaustion, and anergy [21]. In addition, IL-2 may promote expansion of both effector T lymphocytes and suppressive regulatory T cells making the immunologic and physiologic outcomes of IL-2 therapy challenging to predict. Our data show that high-dose rIL-2 (100,000 IU) in mice results in a decrease in B16 melanoma tumor growth. Interestingly, we found that a moderate dose of rIL-2 (10,000 IU) decreases tumor growth in a comparable manner to high-dose rIL-2. The moderate dose may be a better since it provides the same benefit as the higher dose, but potentially limits adverse effects that are common with high-dose rIL-2. Furthermore, lower doses of rIL-2 may promote tumor growth as seen with our results, showing that 1000 IU of rIL-2 actually increased B16 tumor growth. Thus, a threshold for IL-2 concentration may exist in which the anti-tumor effects of high doses may reverse to pro-tumor effects at lower doses. Understanding the mechanisms by which rIL-2 mediates immune responses, especially in combination with vaccines and other tumor immunotherapy agents, will be critical for further clinical development of rIL-2 combination strategies.
In this study, we explored the impact of varied rIL-2 doses on the immune cell infiltration within the tumor microenvironment and in tumor-draining lymph nodes. The proportions of CD8+ T cells, CD4+ T cells, and NK cells were similar in all rIL-2 dosing groups when compared to untreated controls. On the other hand, rIL-2 did have a meaningful impact on tumor-specific CD8+ T cells and Tregs. Our results show that higher doses of rIL-2 were associated with increased therapeutic activity and correlated with a higher tumor-specific CD8+ T cell/Treg ratio. While we observed a significant expansion of NK cells in the tumor when mice were treated with high doses of IL-2 (Fig. 3f), there was only a modest trend in CD4+ and CD8+ T cell expansion, which may relate to the intra-tumoral location or the collection of cells on day 14, which was selected for analysis because this is when the therapeutic differences were greatest (Fig. 1b). This is consistent with other work in which T cells were evaluated on day 14 of tumor challenge [22].
On the contrary, lower doses of rIL-2 promoted tumor growth and correlated with a lower CD8+/regulatory T cell ratio. In particular, Tregs expanded at low doses of IL-2 within the tumor microenvironment (Fig. 4d, e), but this effect was much less pronounced at higher IL-2 doses. The expansion of both effector and regulatory T cells is consistent with previous reports and reviews of IL-2 in chronic virus infection models [23–26]. The impact on tumor growth may, thus, relate to the concentration of CD8+ effector T cells, which may preferentially expand above the Treg subsets at higher IL-2 doses, and was reflected in the changes in CD8+ T cell/Treg ratio as shown in Fig. 4c for tumor-draining lymph nodes and Fig. 4f for the tumor microenvironment. In our model, we also observed a similar pattern when adoptively transferred cells were included in the therapeutic regimen (Fig. 5). These data support the use of high-dose IL-2 in adoptive T cell transfer studies. Another possibility could be that the high NK cell expansion observed within the tumor at high rIL-2 doses might deplete Tregs, as previously reported [27, 28]. Another potential mechanism would be indirect release of other immunoregulatory cytokines by IL-2 exposure. This possibility, however, seems unlikely as human studies have shown no appreciable changes in serum cytokine levels during high-dose IL-2 therapy [29].
There have been contradictory models proposed for how IL-2 mediates host immune responses due to the mixed influence of IL-2 on effector T versus Treg populations [30, 31]. Feinerman et al. [30] evaluated the impact of increasing IL-2 exposure in bulk T cells containing both effector CD8+ and regulatory CD4+ FoxP3+ T cells and reported that the population outcome (immunity vs. suppression) was based on the number of IL-2 receptors, which was highly variable across all cells, and the activation status of the effector T cells. In this model, an equal number of effector and Treg cells resulted in effector T cell expansion in response to higher doses of IL-2 only when the cells were in an activated state but resulted in suppression when the CD8+ T cells were naïve or only weakly activated. In contrast, the model reported by Busse et al. [31] suggests that IL-2 binding and internalization were dependent on the number of IL-2 receptors and, in an autocrine system, bound to Tregs before effector T cells. These authors concluded that receptor internalization and turnover of IL-2 was responsible for the preferential expansion of Tregs at low doses [31]. This model, however, does not consider the potential paracrine effects of exogenous or systemic IL-2 exposure. Our data support the general concept that IL-2 dosing may influence the host immune response in the therapeutic setting, and further investigation is needed to more completely understand how IL-2 expands individual T cells and, subsequently, contributes to the population-based physiologic outcome in the host.
While lower doses of rIL-2 may be appealing to include in combination regimens, the regulatory role of IL-2 in maintaining T cell homeostasis may be associated with a more suppressive immune response and could be counterproductive. Further studies of vaccines and other immunotherapy agents with high-dose rIL-2 might be more important in early-phase drug development of potential combination immunotherapy regimens to avoid missing a physiologically meaningful response. Although not part of current clinical practice, it may also be of interest to more accurately measure serum IL-2 concentrations during therapeutic treatment with rIL-2 containing regimens.
Because only 10–15 % of patients treated with rIL-2 achieve responses, it may be important to monitor CD8+ T cell/regulatory T cell ratio when choosing a rIL-2 dose for treatment or in combination immunotherapy clinical trials, to provide the optimal immune potentiating dose. Further studies of rIL-2 might benefit from consideration of IL-2 dosing since immune responses, and possibly therapeutic effectiveness, may depend on dosing, and serum IL-2 concentrations have not shown correlation with clinical outcomes [29]. The data also support caution in using low doses of rIL-2, and possible other IL-2-related cytokines, in tumor immunotherapy regimens.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported, in part, by National Cancer Institute (NCI) grant UM1 CA186716-01 (to Howard L. Kaufman) and utilized shared resources at Rutgers Cancer Institute of New Jersey supported by NCI P30CA72720.
Author contributions
Andrew Zloza and Howard L. Kaufman conceived and designed the experiments; Erica J. Huelsmann, Joseph R. Broucek, and Tasha Hughes performed the experiments; Andrew Zloza, Neal D. Dharmadhikaril, and Frederick J. Kohlhapp interpreted the data; Andrew Zloza, Neal D. Dharmadhikaril, Frederick J. Kohlhapp, and Howard L. Kaufman wrote the manuscript and all authors edited the manuscript.
Abbreviations
- FDA
Food and Drug Administration
- rIL-2
Recombinant interleukin-2
- Tregs
T regulatory cells
Compliance with ethical standards
Conflict of interest
Howard L. Kaufman has served as a consultant for Alkermes, Amgen, EMD Serono, Merck, Prometheus, and Sanofi and receives research funding from Bristol-Myers-Squibb, Merck, and Viralytics. All other authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Footnotes
Data in part were previously presented as an abstract [J Immunother Cancer. 2013; 1(Suppl 1): P266] and poster at the 28th Annual Scientific Meeting of the Society for Immunotherapy of Cancer (SITC), National Harbor, MD, USA, November 8–10, 2013.
References
- 1.Pazdur MP, Jones JL. Vaccines: an innovative approach to treating cancer. J Infus Nurs. 2007;30(3):173–178. doi: 10.1097/01.NAN.0000270677.66804.40. [DOI] [PubMed] [Google Scholar]
- 2.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116(5):1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA, Females United to Unilaterally Reduce Endo/Ectocervical Disease II Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 4.Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum Vaccin. 2011;7(2):161–169. doi: 10.4161/hv.7.2.13690. [DOI] [PubMed] [Google Scholar]
- 5.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, Group HPS Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 6.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336(26):1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 7.Chang MH, Chen TH, Hsu HM, Wu TC, Kong MS, Liang DC, Ni YH, Chen CJ, Chen DS, Taiwan Childhood HCCSG Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res. 2005;11(21):7953–7957. doi: 10.1158/1078-0432.CCR-05-1095. [DOI] [PubMed] [Google Scholar]
- 8.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ, Advisory Committee on Immunization P (2005) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 54 (RR-16):1–31 [PubMed]
- 9.Frazer IH, Lowy DR, Schiller JT. Prevention of cancer through immunization: prospects and challenges for the 21st century. Eur J Immunol. 2007;37(Suppl 1):S148–S155. doi: 10.1002/eji.200737820. [DOI] [PubMed] [Google Scholar]
- 10.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117(5):1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32(1–3):155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 14.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overwijk WW, Theoret MR, Restifo NP. The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am. 2000;6(Suppl 1):S76–S80. [PMC free article] [PubMed] [Google Scholar]
- 16.Bronte V, Tsung K, Rao JB, Chen PW, Wang M, Rosenberg SA, Restifo NP. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995;154(10):5282–5292. [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek S, Kim CS, Kim SB, Kim YM, Kwon SW, Kim Y, Kim H, Lee H. Combination therapy of renal cell carcinoma or breast cancer patients with dendritic cell vaccine and IL-2: results from a phase I/II trial. J Transl Med. 2011;9:178. doi: 10.1186/1479-5876-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek S, Kim YM, Kim SB, Kim CS, Kwon SW, Kim Y, Kim H, Lee H. Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: a phase I/II trial. Cell Mol Immunol. 2015;12(1):87–95. doi: 10.1038/cmi.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123(6):2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206(4):751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9(5):540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 24.Cousens LP, Orange JS, Biron CA. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155(12):5690–5699. [PubMed] [Google Scholar]
- 25.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 26.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 27.Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109(4):1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481(7381):394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabatino M, Kim-Schulze S, Panelli MC, Stroncek D, Wang E, Taback B, Kim DW, Deraffele G, Pos Z, Marincola FM, Kaufman HL. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27(16):2645–2652. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinerman O, Jentsch G, Tkach KE, Coward JW, Hathorn MM, Sneddon MW, Emonet T, Smith KA, Altan-Bonnet G. Single-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune response. Mol Syst Biol. 2010;6:437. doi: 10.1038/msb.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A, Hofer T. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci USA. 2010;107(7):3058–3063. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.