Abstract
Purpose
In spite of increased rates of complete response to initial chemotherapy, most patients with advanced ovarian cancer relapse and succumb to progressive disease. Immunotherapy may have potential for consolidation therapy.
Experimental design
This randomized open-label phase I/II trial evaluated responses of patients with advanced ovarian cancer in remission for vaccination with monocyte-derived dendritic cells (DC) loaded with Her2/neu, hTERT, and PADRE peptides, with or without low-dose intravenous cyclophosphamide. All patients also received pneumococcal vaccine and were randomized to cyclophosphamide 2 days prior to first vaccination. Blood samples were analyzed by ELISPOT and flow cytometry.
Results
Of 11 patients, 2 recurred during vaccination. Nine received all 4 doses: 3 patients recurred at 6, 17, and 26 months, respectively, and 6 have no evidence of disease at 36 months. No grade 3/4 vaccine-related toxicities were noted. The 3-year overall survival was 90%. Patients receiving cyclophosphamide showed a non-significant improvement in survival over controls. Patients receiving cyclophosphamide had a transient reduction in neutrophils, but no change in total lymphocytes or regulatory T cells. Modest T-cell responses to Her2/neu and hTERT were seen post-vaccine by IFN-γ ELISPOT. Patients demonstrated below normal responses to the diphtheria conjugate protein CRM197, a component of the pneumococcal vaccine.
Conclusions
In this setting, peptide-loaded DC vaccination elicits modest immune responses, but survival is promising. Pneumococcal vaccination revealed substantial immune suppression, even in patients in remission. Rational design of consolidative strategies for ovarian cancer will need to overcome tolerance and immunosuppression.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1081-8) contains supplementary material, which is available to authorized users.
Keywords: Ovarian cancer, Dendritic cell, Vaccine, Clinical trial, Consolidation therapy
Introduction
In 2010, it is estimated that 21,880 new cases of ovarian cancer will be diagnosed in the United States, accounting for 13,850 deaths, making ovarian cancer the most common cause of death from a gynecologic malignancy [1]. Unfortunately, 60–70% of patients present with stages III and IV disease and have a 5-year survival of <25% [2, 3]. Improvements have been made in recent years in the treatment of advanced epithelial ovarian cancer, but the single most common outcome is still initial tumor shrinkage after primary chemotherapy and surgery, only to be followed by relapse and eventual death from progressive disease. Even among those achieving a complete clinical response after initial therapy, the majority will harbor gross or microscopic disease, detectable only at second look surgery. Furthermore, half of patients achieving a pathologic complete response (negative second look surgery) will still relapse due to the presence of occult, undetectable disease [4]. Clearly, new approaches are needed to improve the outcome of these women.
Ovarian cancer is an immune responsive disease [5–8]. Patients with advanced ovarian carcinoma in remission offer unique opportunities for immunotherapy following primary standard therapy with debulking surgery and platinum-based combination chemotherapy, as their tumor burden has been reduced to microscopic or minimal volume gross disease and thus may not have the significant immunosuppression that is found in patients with advanced, recurrent ovarian carcinoma [8]. Though there is limited evidence that immune suppression in cancer patients is related to the stage of disease, evidence suggests that chemotherapy may have a role in persistent immunosuppression at the T-cell level, particularly in association with taxane-based therapy, which is standard for patients with advanced ovarian cancer [9, 10]. Despite favorable responses to initial chemotherapy, most patients experience high rates of recurrence. Small disease burden after initial therapy combined with high risk for recurrence makes these patients optimal candidates for vaccine therapy.
Preclinical and early clinical data have confirmed the ability of dendritic cell (DC) vaccines to induce potent immune responses that in some instances can lead to measurable clinical responses [11, 12]. The first significant association between a specific immune response and clinical improvement related to a DC vaccine was demonstrated in a group of patients with colon and lung cancer [13]. Most recently, others have demonstrated that dendritic cell vaccination leads to a survival benefit in men with hormone-resistant metastatic prostate cancer [14].
In the present phase I/II study, 11 patients in clinical remission after primary surgery and chemotherapy for advanced disease or secondary debulking for good prognosis first recurrence underwent vaccination with autologous DCs loaded with Her2/neu, human telomerase reverse transcriptase (hTERT), and pan-DR epitope (PADRE) peptides. Peptides were selected based upon their rates of expression in ovarian cancers. Her2/neu, the product of the proto-oncogene, is over-expressed in about 10% of primary ovarian cancers and in over 90% of recurrent ovarian cancers [15, 16]. Similarly, hTERT is essential for maintaining the transformed state of many tumor cells and is expressed in approximately 90% of ovarian cancers, but is only found in low levels in normal cells [17, 18]. PADRE is a non-natural epitope optimized for HLA-DR binding that binds to 15 of 16 most common HLA-DR types [19]. Both preclinical and clinical studies have indicated the ability of PADRE to augment the cytotoxic T-lymphocyte (CTL) response [20, 21], and its addition may enhance the induction of a durable humoral immune response [22].
In the 1980s, North and Berd demonstrated that cyclophosphamide reduced the immune-suppressive functions of T cells in mouse tumor models and improved immune function in cancer patients [23, 24]. The therapeutic benefit of cyclophosphamide in animal studies has been linked to a systemic reduction in Treg and enhancement of an anti-tumor T-cell response [25]. Because patients with advanced ovarian cancer have increased regulatory T cells (Tregs) in the blood and tumor microenvironment [8, 26], and because these are associated with poor prognosis [27, 28], we designed a trial to determine whether cyclophosphamide would improve tumor vaccine responses in ovarian cancer. Patients were randomized to receive a single infusion of low-dose intravenous cyclophosphamide prior to vaccination, to evaluate its potential to suppress Tregs and enhance vaccine potency.
Patients, materials, and methods
Study design and patients
The phase I/II study protocol was approved by the University of Pennsylvania Institutional Review Board and conducted with Food and Drug Administration approval of an investigator-sponsored investigational new drug application. The study was conducted as an open-label, single-institution study of HLA-A2-positive patients with advanced epithelial ovarian or primary peritoneal cancer in remission.
The primary study endpoints were dual: (1) to assess the effect of cyclophosphamide on the immunogenicity of the vaccine and to evaluate immunogenicity by determining the frequency of peptide-specific HLA-A2-restricted T cells following vaccination with Her-2/neu, hTERT, PADRE-loaded DCs as indicated by ELISPOT and tetramer analysis to measure the frequency of HLA-A2-restricted T cells and (2) to assess safety of the vaccine.
Signed written informed consent was obtained from each patient. To be eligible, patients had to be ≥18 years of age with a baseline Eastern Cooperative Oncology Group clinical performance status of 0–2. Eligible patients included either that with stages II–IV disease with no clinical evidence of disease after primary debulking surgery and chemotherapy, or those with stages I–IV disease with no clinical evidence of disease after secondary surgical treatment for a first recurrence diagnosed after a progression-free interval of at least 2 years. Patients demonstrated adequate hematologic function (hemoglobin ≥10 g/dl, white blood cell count ≥3,000 cells/mm3, platelets ≥100,000/mm3), renal function (serum creatinine ≤2.0 mg/dl), hepatic function (aspartate aminotransferase within double of normal range and total bilirubin ≤2.0 mg/dl), and cardiac function (asymptomatic or symptomatic with ejection fraction at rest ≥50%). Patients were required to demonstrate no radiologic evidence of disease by contrast computed tomography (CT) or magnetic resonance imaging (MRI) scans, as well as a normal serum CA125 level of ≤35 IU/ml. Vaccination was required to commence within 6 months of completion of prior chemotherapy. Four weeks were required to elapse between completion of prior chemotherapy, biologic therapy, and radiation before enrollment.
Patients were excluded for a history of prior invasive malignancy (except basal cell or squamous cell skin cancer) within the past 5 years; evidence of active central nervous system disease; serious systemic disease; active viral, fungal, or bacterial infection; seropositivity for HIV, HTLV-1, or HTLV-2; or active autoimmunity or immunosuppression. Pregnant or breastfeeding subjects were excluded. Concomitant use of radiation, chemotherapy, biologic therapy, immunosuppressive drugs, and non-steroidal anti-inflammatory medications was not allowed.
Peptides
The telomerase and Her2/neu peptides chosen in this trial were all previously described as MHC class I-binding peptides with specificity for HLA-A2; therefore, for eligibility, patients were required to be HLA-A2 positive. The PADRE peptides are presented via MHC class II, and because they are degenerate, particular MHC class II genotypes were not a requirement for study entry.
hTERT.988Y
This is a peptide with the sequence: YLQVNSLQTV. This peptide is a heteroclitic peptide derived from the natural sequence in hTERT beginning at peptide #988: DLQVNSLQTV. This binds with high affinity to HLA-A201 [29].
Her2/neu.369V2V9
This peptide is a heteroclitic fixed-anchor analog peptide derived from the natural sequence in her2/neu peptide beginning at peptide #369: KIFGSLAFL. By increasing supertype binding through substitution of both MHC anchor residues, the V2V9 analog of HER-2/neu.369 is expected to demonstrate even broader immunogenicity in HLA-A2 supertype individuals [30–32].
Her2/neu.689 is a wild-type peptide having the sequence RLLQETELV. This peptide is an immunodominant epitope that binds to HLA A201 [33].
Vaccine production
Study subjects underwent up to two large-volume apheresis procedures to collect peripheral blood mononuclear cells (PBMCs) using Baxter Fenwal CS-3000 Plus Blood Cell Separator or COBE Spectra apheresis systems according to established procedures. Collected PBMCs were shipped to an Immuno-Designed Molecules, Inc. (IDM) manufacturing facility (Irvine, CA) for DC generation and vaccine production. Autologous PMBCs were cultured in serum-free, non-adherent conditions in the presence of GM-CSF and IL-13 for 7 days in vitro to generate DCs. The cells underwent elutriation for DC enrichment, followed by maturation by culture with membrane fractions from Klebsiella pneumoniae and IFN-γ [34]. Mature DCs were then pulsed with HLA-A2-restricted hTERT 988Y, Her2/neu 369V2V9, Her2/neu 689, and PADRE peptides (PolyPeptide Laboratories, San Diego, CA). Single doses of the peptide-loaded DC cell product were cryopreserved in 2 ml of 5% human serum albumin (HSA) containing 10% dimethylsulfoxide (DMSO) and individually shipped to the site for administration.
Treatment schedule
Patients were randomized to receive a single dose of cyclophosphamide 2 days prior to vaccination (Suppl Fig. 1a). Four weeks were required for vaccine manufacture, testing, and release. Therefore, approximately 4 weeks after PBMC collection, patients commenced vaccination. Patients in Arm 1 received autologous vaccine without cyclophosphamide, and those on Arm 2 received 300 mg/m2 of cyclophosphamide in 500 ml of normal saline intravenously over 1 h, followed 2 days later by the first vaccine. Four doses were given at 3-week intervals. For each dose, a single cryovial containing 35 × 106 cells (before cryopreservation) was thawed, resuspended in a total of 5 ml of sterile saline, and injected into the medial thighs at 24 intradermal sites (approximately 0.2 ml each) following application of lidocaine 2.5%/prilocaine 2.5% cream. Injections were administered in a 3 × 4 grid with 12 injections on each thigh, each injection separated by at least 1 cm from other sites. All eligible patients also received a single dose of Prevnar™ heptavalent pneumococcal vaccine (i.m.) with the first dose of DCs.
Patients were monitored for 3 h following the completion of the vaccination procedure and then discharged. Toxicities were recorded and graded using NCI Common Toxicity Criteria (CTC) version 3.0. Vaccination was held for evidence of grade 2 allergic reaction, but could be resumed if patients responded to supportive care. Patients were removed from the study for any grade 4 vaccine-related toxicity, postponement of vaccination beyond 30 days from the originally scheduled administration date, or disease recurrence. Patients were evaluated for disease recurrence at 10 weeks and 6 months after initial vaccination by CT or MRI, and monitored clinically by physical exam and CA125 for 24 months. Only patients receiving 2 or more DC vaccinations were deemed evaluable for vaccine safety endpoints.
Samples for immune assessment
Peripheral blood was drawn for immune assessment at the time of second and fourth vaccines, as well as at 4, 5, 6, 9, and 12 months after initial vaccination. A 2–3 l apheresis procedure was performed 10 weeks after initial vaccination to collect PBMCs for immune response endpoint testing. PBMCs were isolated by Ficoll centrifugation, washed with HBSS, and cryopreserved for later analysis.
Enzyme-linked immunosorbent spot (ELISPOT) analysis
ELISPOT assays using interferon gamma (IFN-γ) reagents and pre-coated plates (MabTech, Sweden) were performed according to the manufacturer’s instructions. In brief, cryopreserved PBMCs were thawed and rested overnight in RPMI supplemented with 10% fetal calf serum. Cells were plated 200,000/well, in triplicate, then incubated overnight either with 62.5 μg/ml peptide (her2/neu 369V2V9, 689, PADRE or hTERT 988Y), 20 μg/ml CRM197 protein (Sigma), or media alone. PMA/ionomycin (Sigma) was used as a positive control. Plates were scanned, and spots were counted using ImmunoSpot v 4 (CTL, Cleveland). Background reactivity was determined using cells plus media alone and was subsequently subtracted in calculating mean effector cells.
Analysis of T-regulatory cells
Cryopreserved PBMCs were thawed and rested overnight. Cells were stained using the Human Regulatory T Cell Staining Kit (eBioscience) as per manufacturer’s instruction augmented with anti-CD3 (BD). Flow cytometry was acquired using an LSR II (BD) and analyzed with FlowJo v 8 (TreeStar). Events were gated on singlets (FSC-A vs. FSC-H), then CD3+CD4+. The percentage of Treg cells were reported as the percentage of CD25+FoxP3+ minus the percentage of CD25+Isotype+. Absolute numbers of lymphocytes and Tregs were calculated by using a concurrent lymphocyte count obtained from a complete blood count.
Statistical analysis
Patient characteristics were compared among the two treatment arms with a pooled sample variance t test for the continuous variable age and Fisher’s exact test for the categorical variables. Kaplan–Meier curves were plotted for each of the two outcomes, Time to Recurrence and Time to Last Follow-Up (SAS, Version 9.2 and Sigma plot Version 11). Time to Recurrence and Time to Last Follow-Up were determined from vaccination 1 (day 0). Due to the small sample size and few events recorded, exact P values were calculated for each outcome by comparing the two treatment arms (StatXact, Version 8). The study was initially powered for a total of 24 patients, with 12 on each arm. Approval was obtained from the Institutional Review Board to enroll up to 32 subjects in order to allow for subject dropout.
Results
Clinical characteristics and toxicity
Between August 2005 and September 2007, 14 patients were enrolled with 7 patients randomized to each arm. Suppl Fig. 1 shows the trial design and CONSORT statement. All subjects underwent apheresis; however, 3 were removed from study prior to vaccination (one experienced disease progression during the vaccine production interval, one developed an active lung infection, and another developed dermatomyositis). There were 10 evaluable patients including 9 patients who received all 4 vaccinations and one patient who recurred after 2 vaccinations. One additional patient recurred after only one vaccine and was therefore not evaluable by protocol specifications for both safety endpoints as well as immunogenicity (098-120) (Table 1). The age of evaluable patients ranged from 18 to 61, with mean age of 48.1. The arms were similar with respect to age, stage, histology, rate of optimal cytoreduction, receipt of intraperitoneal chemotherapy, and tumor grade. Protocol enrollment was terminated early due to lack of vaccine supply as a result of financial limitations on the part of the manufacturing sponsor.
Table 1.
Characteristics of evaluable patients
| Variable | Arm 1 | Arm 2 | All | P value* |
|---|---|---|---|---|
| (n = 5) | (n = 5) | (n = 10) | ||
| Age on study (years) | ||||
| Mean (std) | 50.0 (6.44) | 46.2 (17.22) | 48.1 (12.42) | 0.6564** |
| Median (min–max) | 49 (41–58) | 51 (18–61) | 50 (18–61) | |
| Stage | ||||
| III | 4 | 4 | 8 | 1.00 |
| IV | 1 | 0 | 1 | |
| Recurrent | 0 | 1 | 1 | |
| Histology | ||||
| Serous | 3 | 3 | 6 | 1.00 |
| Clear cell | 0 | 1 | 1 | |
| Poorly differentiated | 1 | 0 | 1 | |
| Endometrioid | 1 | 0 | 1 | |
| Mixed | 0 | 1 | 1 | |
| Optimal cytoreduction | 5 | 5 | 10 | N/A |
| Intraperitoneal chemotherapy | ||||
| Yes | 3 | 3 | 6 | 1.00 |
| No | 2 | 2 | 4 | |
| Grade | ||||
| 1 | 1 | 1 | 2 | 1.00 |
| 2 | 1 | 2 | 3 | |
| 3 | 3 | 3 | 5 | |
* Fisher’s exact test P value
** The pooled variance t test P value
A total of 39 vaccines were administered to 11 subjects. All tolerated the vaccines well; no grade 3 or 4 vaccine-related toxicities were reported. The most common study-related toxicities included reactogenicity as indicated by erythema, induration, pruritus, and pain at the site of injection, fever and fatigue. Only one patient experienced a grade 3 toxicity consisting of worsening of pre-existing glaucoma, deemed to be unrelated to vaccination, which occurred over 1 year after vaccination and was successfully treated with laser surgery.
Of the 11 patients receiving vaccine (Table 2), only 1 patient (098-050) has died of disease within 36 months after initial vaccination. This patient recurred 3 months after completing protocol vaccinations and died 20 months later of disease progression. Of the remaining 10 evaluable patients, 3 experienced disease recurrence (098-060, 098-100, 098-110), but all achieved a second remission after additional chemotherapy treatment. An additional patient (098-130) was diagnosed with an unrespectable brainstem meningioma and was successfully treated with radiation and chemotherapy, without evidence of recurrence of ovarian cancer. One patient recurring after receiving only one dose of vaccine (098-012) was not evaluable/protocol. The remaining 5 patients have no evidence of disease.
Table 2.
Study subjects and responses
| ID # | Age on study (years) | Stage | Initial treatment | Arm | Number of vaccines received | Disease status |
|---|---|---|---|---|---|---|
| 098-010 | 61 | IC recurrent | P/CB × 4 then D/CB × 4 | 2 | 4 | NED |
| 098-030 | 43 | IIIB | P/CB × 6 | 2 | 4 | NED |
| 098-040 | 18 | IIIC | IV/IP P/CDDP × 6 | 2 | 4 | NED |
| 098-050 | 54 | IV | P/CB × 8 | 1 | 4 | Recurrence at 6 months, DOD at 26 months |
| 098-060 | 58 | IIIC | IV/IP P/CDDP × 6 | 2 | 4 | Recurrence at 26 months, NED in second remission |
| 098-080 | 49 | IIIB | P/CB × 8 | 1 | 4 | NED |
| 098-100 | 58 | IIIB | IP P/CB × 6 | 1 | 2 | Recurrence after V2, NED in second remission |
| 098-110 | 48 | IIIC | IV/IP P/CDDP × 6 | 1 | 4 | Recurrence at 17 months, NED in second remission |
| 098-120 | 62 | IIIC | P/CB × 6 | 2 | 1 | Recurrence after V1, AWD |
| 098-130 | 41 | IIIC | IV/IP P/CDDP × 6 | 1 | 4 | NED; unresectable meningioma detected 7/09 treated with radiation/chemo. NED |
| 098-140 | 51 | IIIC | IV/IP P/CDDP × 6 | 2 | 4 | NED |
P paclitaxel, CB carboplatin, D docetaxel, IV intravenous, IP intraperitoneal, CDDP cisplatin, NED no evidence of disease, DOD died of disease, AWD alive with disease, V vaccine
Progression-free survival (PFS) and overall survival (OS) curves are depicted in Fig. 1. Although the survival rates for both curves were higher for Arm 2 (cyclophosphamide), there were no significant differences between the survival curves for the two groups. The estimated 3-year PFS is 40% versus 80% for Arms 1 and 2, respectively; the estimated 3-year OS is 80 and 100%, respectively.
Fig. 1.
Kaplan–Meier survival plots. Progression-free survival (a) and overall survival (b) of vaccinated patients receiving no cyclophosphamide IDD-6 (Arm 1) or cyclophosphamide (Arm 2). There is no difference in PFS (exact P = 0.17) or OS (exact P = 1.00); 0 indicates censored
Induction of vaccine-specific T-cell responses
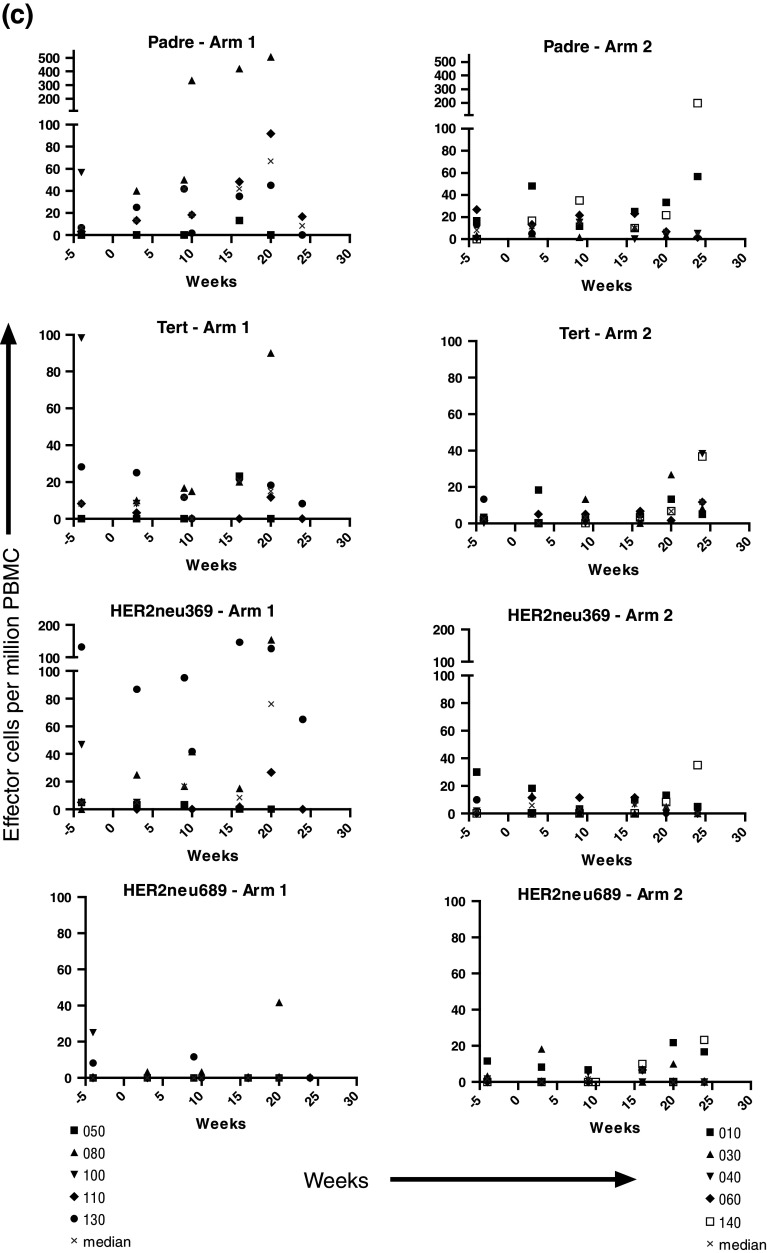
Samples for immune assessment were collected at initial apheresis, at the time of second and fourth vaccines, week 10 apheresis, as well as at 4, 5, 6, 9, and 12 months after initial vaccination. Initial studies using tetramer analysis with flow cytometry did not detect responses at the level of sensitivity of the assay (0.05% CD8+ tetramer+ T cells among total CD8+ T cells), and therefore, ELISPOT analysis was used as a more sensitive measure of the responses to vaccination. With rare exceptions, at baseline IFN-γ ELISPOT responses to hTERT 988Y, Her2/neu 369V2V9, 689, and PADRE peptides were undetectable (Fig. 2a, b). Low-level responses were detected in a subset of patients after vaccination, with few patients achieving >50 specific effector cells/106 PBMC. There was no difference between the responses of Arm 1 and Arm 2 patients as assessed by the individual patterns of response (Fig. 2a, b), and as expected, the responses to pharmacologic stimulation of T cells with PMA and ionomycin were much higher, and also similar between Arms 1 and 2. The patterns of responses to PADRE and to hTERT 988Y, Her2/neu 369V2V9, 689 were similar in Arm 1 and Arm 2 patients (Fig. 2c). Patient 098-080 on Arm 1 developed the most robust response to all 4 peptides, along the same temporal course, with responses rising approximately week 16 after initial vaccination. Patient 098-140 on Arm 2 also developed a strong response to PADRE. When examined for fold increase from baseline, there was no difference between Arms 1 and 2 in the fraction of patients achieving a threefold or a tenfold increase in reactive cells.
Fig. 2.
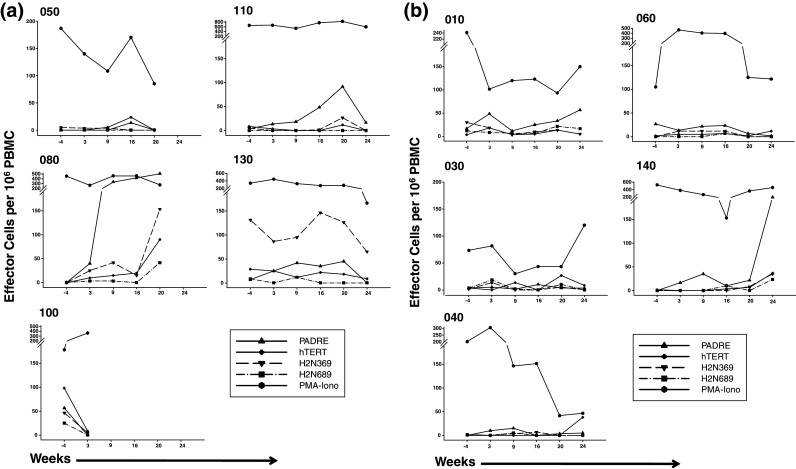
ELISPOT analysis was performed using PBMCs obtained at the indicated time points post-vaccination against hTERT 988Y, her2/neu 369V2V9, 689, and PADRE for patients on Arm 1 (a), and Arm 2 or a single low dose of cyclophosphamide prior to IDD-6 (b). Responses to PMA and ionomycin from 5 × 103 cells are also shown; note scale difference. Longitudinal analysis was performed at baseline (−4 weeks) and post-vaccination. Composite plots for each peptide are also depicted with median values denoted by times symbol at each time point (c)
Of note, the two patients who recurred during the course of vaccination (098-100 and 098-120) were the only patients to experience an actual decrease in IFN-γ-releasing cells to all 4 peptides (Fig. 2a, b), consistent with tumor-associated suppression of T-cell function or trafficking of effector cells from blood. Similarly, the only patient on study to die of disease (098-050) demonstrated no functional IFN-γ-secreting effector cells against any of the 4 peptides by the 5-month time point post-initial vaccine. She was noted to have disease recurrence 1 month later.
Induction of CRM197 responses
In addition to DC vaccines, eligible study subjects received a single dose of the Prevnar pneumococcal conjugate vaccine in order to assess baseline responses to the diphtheria conjugate protein CRM197 [35]. Unexpectedly, most patients also displayed inability to mount a robust response to the highly immunogenic carrier protein (Suppl Fig. 2). Though two individuals on Arm 1 developed a transient response to CRM197 (098-050 and 098-080), the only subjects with sustained responses (098-030 and 098-060) were treated on Arm 2. Overall, responses were well below that of healthy individuals [35] and even below those of patients with myeloma following high-dose chemotherapy and T-cell reconstitution [36].
Analysis of circulating leukocytes, lymphocytes, and Treg cells
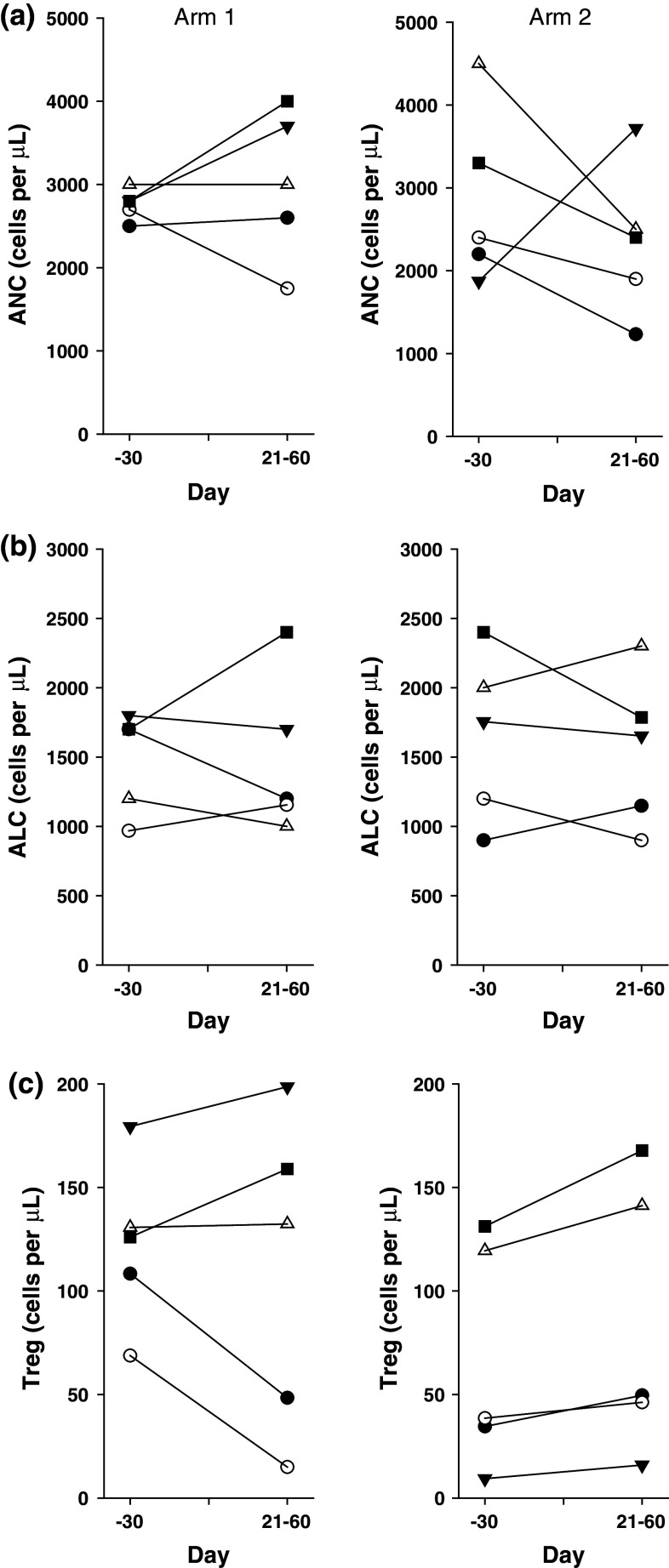
We monitored the effects of the cyclophosphamide administration by determining the neutrophils, lymphocytes, and Tregs in peripheral blood. As expected, patients receiving a single dose of cyclophosphamide (Arm 2) showed a transient decrease in neutrophils that was not observed in Arm 1 patients (Fig. 3a). In contrast, there was no consistent change in circulating lymphocytes or Tregs in Arm 1 or 2 patients. The neutrophil counts returned to normal levels in Arm 2 patients, and longitudinal follow-up revealed no differences in neutrophils, total lymphocytes, or Tregs between Arms 1 and 2 (Fig. 4).
Fig. 3.
(a) Absolute neutrophil count (ANC), (b) absolute lymphocyte count (ALC), and (c) Treg count in peripheral blood for Arm 1 and Arm 2 patients at baseline and at days 21–60. The ANC and ALC were determined from CBC measurements. The Tregs were determined by flow cytometry performed on PBMC to quantify CD3+CD4+CD25+FoxP3+ cells. The first value post-treatment in the time window of day +21 to +60 is plotted
Fig. 4.

Longitudinal analysis of (a) absolute neutrophil count (ANC), (b) absolute lymphocyte count (ALC), (c) Treg count in peripheral blood for Arm 1 and Arm 2 patients, and (d) longitudinal plots of individual Treg levels plotted as change from baseline in Arm 1 and Arm 2 patients. The individual patients are plotted as well as the mean values
Discussion
Here we demonstrate the safety and feasibility of autologous DC vaccination as consolidation therapy for advanced ovarian and primary peritoneal cancers in remission. The use of a randomized trial design has permitted the identification analysis of vaccine efficacy in a limited number of patients with advanced ovarian cancer. The three most notable observations are as follows (1) clinical outcomes are promising with 90% overall survival at 36 months of observation; (2) a single dose of cyclophosphamide does not appear to deplete Tregs or increase vaccine immunogenicity; and (3) considerable immunosuppression, as evidenced by a profound inability to respond to pneumococcal vaccination, is present in advanced ovarian cancer even in patients who are in clinical remission.
The DC vaccine was found to be well tolerated. Despite concerns that hTERT and her2/neu are present in certain normal cells, no significant toxicity was seen; however, the modest responses may not have uncovered potential toxicities. No patients discontinued therapy due to side effects. In terms of clinical efficacy, only 1 patient has died of her disease, and though 4 others have recurred, all have undergone successful salvage therapy and are currently in a second remission. Two of those patients recurred during their course of vaccination, likely indicating the ongoing progression of disease during the vaccine production period, as opposed to experiencing failure of vaccine. It is intriguing to note that the results with the dendritic cell vaccine for prostate cancer (sipuleucel-T) indicate that despite the apparently weak immunogenicity of the vaccine, a clinically evident survival benefit has been demonstrated in 3 randomized trials [14].
The primary objective of this study was to evaluate the effects of a single dose of cyclophosphamide on the immunogenicity of a panel of self-peptides. Primary epithelial ovarian cancer expresses Her2/neu at low levels, and indeed treatment of recurrent ovarian cancer with traztuzumab demonstrates low response rates [37]. However, the peptides chosen in this study were selected primarily for their ability to have measureable responses, for the existence of previous safety data that would allow the pooling of peptides used for dendritic cell loading, and for their availability in clinical grade format. Therefore, testing of patients’ archived tissue for the target peptides was not required for study entry.
Cyclophosphamide has been used extensively for patients with ovarian cancer and was considered first-line therapy before the use of platinum-based compounds. In mouse models, North presented evidence that cyclophosphamide enhanced tumor immunotherapy by the elimination of CD8+ tumor suppressor cells [23] and that the effect was independent of cytotoxic reductions in tumor burden [38]. In patients with advanced colorectal carcinoma and melanoma, cyclophosphamide was shown to increase DTH responses to a KLH vaccine in adjuvant [24]. Our study is the only randomized study to evaluate the effects of cyclophosphamide on Tregs, and our findings indicate that a single intravenous dose (300 mg/m2) has no effect on the number of circulating Tregs. The effects of cyclophosphamide are known to be dose and schedule dependent. In 2007, a single-arm study reported on the use of low-dose “metronomic” cyclophosphamide given orally (50 mg twice daily 1 week on and 1 week off for a month or more), which was shown to decrease Tregs in patients with advanced cancer [39]. However, this study has not been confirmed with randomized trial design. Given that our study was designed in 2003 and initiated in 2004 prior to this report describing metronomic administration, we did not utilize this schedule in our study. Audia tested a single i.v. infusion of cyclophosphamide (250, 500, and 750 mg/m2) in a non-randomized trial design and did not observe a decrease in circulating Tregs [40]. A single dose of cyclophosphamide (250 mg/m2) given prior to an allogeneic tumor vaccine may augment vaccine responses in pancreatic cancer patients; however, the effects of cyclophosphamide on Tregs were not measured in this trial [41]. In patients with metastatic breast cancer, cyclophosphamide was tested with an allogeneic tumor cell vaccine given 3 times, and humoral responses were augmented at doses <200 mg/m2 [42]; the effects of cyclophosphamide on Tregs were not reported in this trial. Based on our results and the literature, we have concluded that cyclophosphamide may improve patient outcome by a mechanism that may not involve the depletion of Tregs from the blood. Whether cyclophosphamide alters the function of Tregs in the tumor microenvironment remains unknown. As pointed out by Columbo, it may be more important to inhibit the function of Tregs, rather than deplete them from the microenvironment [43]. Consistent with this, a recent study in patients with hepatocellular carcinoma suggests that cyclophosphamide at 150 mg/m2 can impair the function of Tregs isolated from peripheral blood, while higher doses do not reverse the suppressive effects of Tregs [44].
Our patient population demonstrated unexpected immunosuppression on multiple levels despite having no clinical evidence of malignancy and having completed active chemotherapy many weeks prior to enrollment. The response to the peptide-loaded DC vaccine as assessed by IFN-γ-secreting T cells was modest, which may reflect a weak peptide vaccine or tumor-induced tolerance and other mechanisms. However, it was unexpected that the response to the diphtheria carrier protein component of the Prevnar™ vaccine was essentially undetectable. Virtually, all healthy adults mount vigorous responses to CRM197 carrier protein [35], and CRM197 has been administered to advanced cancer patients and found to elicit inflammatory responses [45]. We have found in two previous trials that myeloma patients have robust T-cell responses to CRM197 [36, 46] so that the near absence in ovarian cancer suggests a more profound level of immunosuppression in ovarian cancer.
Despite the modest immunologic responses, DC vaccination was well tolerated and associated with a promising progression-free and overall survival in this small study. Though our data suggest that cyclophosphamide has beneficial effects in patients receiving anti-cancer vaccines, a single dose, while not promising in its ability to improve vaccine immunogenicity, may have other effects to prolong patient survival. This effect may be independent of vaccine-elicited T-cell responses as measured by ELISPOT assays. Strategies to specifically target Tregs will be crucial to allow the field of tumor vaccines to progress, as well as approaches to improve vaccine immunogenicity, perhaps through improved tumor targeting via selection of tumor-associated antigens. In current studies, our group is targeting cancer testis antigens as one candidate for future vaccination, due to emerging data indicating that their expression appears limited to tumors, as well as due to the higher avidity of the T-cell repertoire with specificity for these antigens that can be elicited. Overall, future study is warranted in trials utilizing DC vaccination for consolidation of complete responses after primary surgery and chemotherapy of advanced ovarian cancer, and our results further highlight the importance of identifying effective strategies to inhibit Treg function in cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful for the support from Bonnie Mills at IDM, Inc. This study is dedicated to the memories of Cynthia R. June and Richard G. Carroll. In addition, we thank members of the Human Immunology Core (Abramson Cancer Center) for analysis of patient samples, and patients in the Ovarian Cancer Research Center for participating in this research protocol. This study is financially supported by NIH grants R21CA115049 (CC), 5P50CA083638 SPORE in Ovarian Cancer (GC PAG and CHJ), Cancer Biostatistics Training Grant T32CA093283 and the Ovarian Cancer Research Fund (GC and CHJ).
Conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF. Recurrent ovarian cancer: evidence-based treatment. J Clin Oncol. 2002;20:1161–1163. doi: 10.1200/JCO.2002.20.5.1161. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Ozols RF. Chemotherapy of advanced ovarian cancer. Semin Oncol. 1998;25:340–348. [PubMed] [Google Scholar]
- 4.Rubin SC, Randall TC, Armstrong KA, Chi DS, Hoskins WJ. Ten-year follow-up of ovarian cancer patients after second-look laparotomy with negative findings. Obstet Gynecol. 1999;93:21–24. doi: 10.1016/S0029-7844(98)00334-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 6.Sabbatini P, Odunsi K. Immunologic approaches to ovarian cancer treatment. J Clin Oncol. 2007;25:2884–2893. doi: 10.1200/JCO.2007.11.0775. [DOI] [PubMed] [Google Scholar]
- 7.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. doi: 10.1111/j.1600-065X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 8.Woo EY, Chu CS, Goletz TJ, Schlienger K, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 9.Tange S, Scherer MN, Graeb C, Weiss T, Justl M, Frank E, Andrassy J, Jauch KW, Geissler EK. The antineoplastic drug paclitaxel has immunosuppressive properties that can effectively promote allograft survival in a rat heart transplant model1. Transplantation. 2002;73:216. doi: 10.1097/00007890-200201270-00011. [DOI] [PubMed] [Google Scholar]
- 10.Si MS, Imagawa DK, Ji P, Wei X, Holm B, Kwok J, Lee M, Reitz BA, Borie DC. Immunomodulatory effects of docetaxel on human lymphocytes. Investig New Drugs. 2003;21:281–290. doi: 10.1023/A:1025408425660. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 12.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 13.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 15.Hellstrom I, Goodman G, Pullman J, Yang Y, Hellstrom KE. Overexpression of HER-2 in ovarian carcinomas. Cancer Res. 2001;61:2420–2423. [PubMed] [Google Scholar]
- 16.Ross JS, Fletcher JA. The HER-2/neu oncogene: prognostic factor, predictive factor and target for therapy. Semin Cancer Biol. 1999;9:125–138. doi: 10.1006/scbi.1998.0083. [DOI] [PubMed] [Google Scholar]
- 17.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 18.Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674–679. doi: 10.1038/sj.onc.1205074. [DOI] [PubMed] [Google Scholar]
- 19.Alexander J, Sidney J, Southwood S, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/S1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 20.Wierecky J, Muller MR, Wirths S, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]
- 21.Hung CF, Tsai YC, He L, Wu TC. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther. 2007;15:1211–1219. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander J, del Guercio M, Maewal A, et al. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J Immunol. 2000;164:1625. doi: 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- 23.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berd D, Mastrangelo MJ, Engstrom PF, Paul A, Maguire H. Augmentation of the human immune response by cyclophosphamide. Cancer Res. 1982;42:4862–4866. [PubMed] [Google Scholar]
- 25.Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008;109:215–219. doi: 10.1016/j.ygyno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 28.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scardino A, Gross DA, Alves P, et al. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- 30.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58:4902–4908. [PubMed] [Google Scholar]
- 31.Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8:1014–1018. [PubMed] [Google Scholar]
- 32.Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394–3400. [PubMed] [Google Scholar]
- 33.Rongcun Y, Salazar-Onfray F, Charo J, et al. Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol. 1999;163:1037–1044. [PubMed] [Google Scholar]
- 34.Kavanagh B, Ko A, Venook A, et al. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007;30:762–772. doi: 10.1097/CJI.0b013e318133451c. [DOI] [PubMed] [Google Scholar]
- 35.Kamboj K, Kirchner H, Kimmel R, Greenspan N, Schreiber J. Significant variation in serotype-specific immunogenicity of the seven-valent Streptococcus pneumoniae capsular polysaccharide-CRM_197 conjugate vaccine occurs despite vigorous T cell help induced by the carrier protein. J Infect Dis. 2003;187:1629–1638. doi: 10.1086/374785. [DOI] [PubMed] [Google Scholar]
- 36.Rapoport A, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 37.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the gynecologic oncology group. J Clin Oncol. 2003;21:283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 38.Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- 39.Ghiringhelli F, Menard C, Puig P, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+CD25+T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor–secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emens L, Asquith J, Leatherman J, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor–secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colombo M, Piconese S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 44.Greten T, Ormandy L, Fikuart A, Höchst B, Henschen S, Hörning M, Manns M, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211–218. doi: 10.1097/CJI.0b013e3181bb499f. [DOI] [PubMed] [Google Scholar]
- 45.Buzzi S, Rubboli D, Buzzi G, Buzzi A, Morisi C, Pironi F. CRM197 (nontoxic diphtheria toxin): effects on advanced cancer patients. Cancer Immunol Immunother. 2004;53:1041–1048. doi: 10.1007/s00262-004-0546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination based on hTERT and survivin following ASCT for myeloma. Blood. 2011;117:788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.