Abstract
Introduction
This study aims to reduce potentially inappropriate prescribing (PIP) of statins and foster healthy lifestyle promotion in cardiovascular disease (CVD) primary prevention in low-risk patients. To this end, we will compare the effectiveness and feasibility of several de-implementation strategies developed following the structured design process of the Behaviour Change Wheel targeting key determinants of the clinical decision-making process in CVD prevention.
Methods and analysis
A cluster randomised implementation trial, with an additional control group, will be launched, involving family physicians (FPs) from 13 Integrated Healthcare Organisations (IHOs) of Osakidetza-Basque Health Service with non-zero incidence rates of PIP of statins in 2021. All FPs will be exposed to a non-reflective decision assistance strategy based on reminders and decision support tools. Additionally, FPs from two of the IHOs will be randomly assigned to one of two increasingly intensive de-implementation strategies: adding a decision information strategy based on knowledge dissemination and a reflective decision structure strategy through audit/feedback. The target population comprises women aged 45–74 years and men aged 40–74 years with moderately elevated cholesterol levels but no diagnosed CVD and low cardiovascular risk (REGICOR<7.5%), who attend at least one appointment with any of the participating FPs (May 2022–May 2023), and will be followed until May 2024. We use the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework to evaluate outcomes. The main outcome will be the change in the incidence rate of PIP of statins and healthy lifestyle counselling in the study population 12 and 24 months after FPs’ exposure to the strategies. Moreover, FPs’ perception of their feasibility and acceptability, and patient experience regarding the quality of care received will be evaluated.
Ethics and dissemination
The study was approved by the Basque Country Clinical Research Ethics Committee and was registered in ClinicalTrials.gov (NCT04022850). Results will be disseminated in scientific peer-reviewed journals.
Trial registration number
Keywords: Primary Care, Clinical Decision-Making, Clinical Trial, PREVENTIVE MEDICINE, Implementation Science, Cardiovascular Disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A strength of the De-implementation of low-value pharmacological prescribing (DE-imFAR) study is that it involves an efficient design that combines experimental and non-experimental comparisons through two randomly assigned intervention arms and one non-randomised control arm to test the comparative effectiveness in reducing potentially inappropriate prescribing (PIP) of statins and increasing healthy lifestyle promotion of several de-implementation strategies deployed in real-world settings.
Counting with one non-randomised control arm is a strength because it allows capturing the effect of temporal trends, regression to the mean and the learning curve due to the reference/background strategy to which all targeted family physicians (FPs) are exposed when comparing this reference strategy with the two experimental de-implementation strategies.
Another strength is the use of qualitative methods to better understand, from the perspective of the study participants, the reasons why (why not) the strategies work, to explain the variations in the results achieved and to identify the essential components of the strategy and those that will require to be optimised.
To the best of our knowledge, the DE-imFAR study is one of the first of its kind that specifically uses the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework for the evaluation of the study results in terms of public health impacts.
The main limitation lies in the planned comparisons of the randomised groups with respect to the control arm, likely to differ to some extent at baseline because of the non-random process of generation. To tackle this limitation, in addition to evaluating the change in PIP incidence in all eligible FPs, a matching strategy with the selection of one matched FP from this non-randomised group for each of the randomised FPs will be performed in order to increase comparability and reduce potential bias.
Introduction
Reducing low-value healthcare, that is, clinical practices that have not been shown to be efficient or effective, is becoming a global priority due to the widespread empirical evidence of its high prevalence across healthcare systems, potential harm and its impact on patient safety, resource use and social inefficiency.1 2
Nonetheless, reducing or eliminating low-value practices is a complex matter since drivers that foster or maintain them seem to operate at multiple levels and be context-specific. Therefore, in order to design effective and efficient corrective measures, a careful process of formal analysis of the determinants of the clinical behaviour in question is needed. In this context, behaviour change theory has been extensively applied to understand the factors that may influence clinical behaviour, identify and design possible techniques and interventions that could be used to change it and explain the mechanisms through which such interventions operate.3 4
The DE-imFAR study (‘De-implementation of low-value pharmacological prescribing’ in Spanish) is a two-phase project5 that aims applying behavioural science theory within a structured process involving the main stakeholders (health professionals, patients and researchers) in the design, deployment and evaluation of targeted de-implementation strategies to reduce potentially inappropriate prescribing (PIP). Specifically, in the DE-imFAR study, the target low-value practice is the pharmacological prescription of statins in the primary prevention of cardiovascular disease (CVD) in low-risk patients. In order to prevent CVD, one of the leading causes of morbidity and death worldwide, there is general agreement on the indication of lipid-lowering treatment, mainly with statins, for patients with a 10-year cardiovascular risk (CVR) greater than 10% or in the secondary prevention.6–9 Whereas, in the primary prevention for patients with low CVR (<10%), preventive activities should be focused on the promotion of healthy lifestyles through optimising diet, increasing physical activity and stopping smoking.6–9 Moreover, international guidelines encourage discussion with patients about the benefits of lifestyle modification for the prevention of CVD, as well as other modifiable risk factors, before considering pharmacological treatment.7–9
Within the phase I of the DE-imFAR study, we first conducted a cross-sectional observational study on the incidence of PIP of statins and provision of advice on lifestyle modification in the Basque Health Service-Osakidetza in 2018. The results showed that the prescription of statins had notably increased in the Basque Country (Spain) with an estimated incidence of new PIP of 10.5 per 100 000 persons/year in patients aged 40–75 years, without CVD, with moderately elevated cholesterol levels but with a CVR<5%.10
Second, we applied two of the most successfully used behaviour change theories in the field of Implementation Science, the Theoretical Domains Framework (TDF)3 11 12 and Behaviour Change Wheel (BCW),13 to (a) understand and define the problem (low-value practice) in behavioural terms and select and specify the target behaviours; (b) identify the factors that may influence it and (c) map targeted de-implementation and implementation strategies conducive to reducing the low-value practice in question. Briefly, after having prioritised our specific target behaviour (ie, ‘clinician decision-making on intervention/treatment to be provided based on objective clinical information and subjective schemas and heuristics’), identified the determinants (facilitators of the non-desired behaviour of PIP of statins and barriers to apply the recommended clinical practice behaviour of promoting healthy lifestyles), and mapped specific behaviour change techniques, three types of de-implementation strategies were selected based on being the most potentially effective, feasible and acceptable to influence decision-making through different mechanisms.14 Hence, the three strategies derived from the systematic theory- and evidence-based intervention design process were: (a) a non-reflective decision assistance strategy based on providing family physicians (FPs) with evidence-based information communication technology tools to help and guide decision-making; (b) a decision information strategy based on the dissemination of CVD primary prevention evidence framed in a corporate campaign encouraging FPs to abandon PIP and (c) a reflective decision structure strategy encouraging reflection on actual performance based on an audit/feedback system.14
According to the literature review performed within the phase I of the DE-imFAR project14 regarding the evaluation of effective intervention strategies for the reduction of low-value prescribing,15–24 multicomponent interventions—combining passive dissemination interventions, based on training in or dissemination of clinical practice guidelines (CPGs), with more proactive interventions incorporating decision-making aids or sending audit/feedback—achieve the most positive results. Specifically, in the context of PIP of statins, a positive impact was observed on recording of CVR and prescription adequacy using (a) multicomponent dissemination strategies including informational websites and implementation of electronic CPGs compared with routine practice and training activities and (b) interventions based on sending clinical scenarios/cases and audit/feedback to professionals as well as decision support tools.19–23 All these strategies can be conceived and theoretically differentiated in terms of how they may affect clinicians’ decision-making.25 There is plenty of evidence to support de-implementation of inappropriate medical practices through the lens of clinician cognition using audit/feedback, decision support tools, etc.26–28 In this context, the growing field of choice architecture aims to explore how the structure and framing of decision situations influence the choice of certain behaviours over alternative ones. On the one hand, FPs’ decision-making ability can be influenced by unconscious processes that occur in response to environmental or emotive cues, that is, through Type 1 (or automatic) cognition. On the other, clinicians’ conscious intention to change can be promoted by engaging their reflective cognition to consciously evaluate and correct their inappropriate behaviour, that is, using Type 2 (or reflective) cognition.29 However, further research is needed to determine whether these evidence-based and barrier-specific de-implementation strategies identified in the DE-imFAR phase I are also effective in our context.
Thus, the goal of the present phase II of the DE-imFAR study is to assess the potential effectiveness and feasibility of this set of de-implementation strategies to reduce the PIP of statins in the primary prevention of CVD (low-risk patients, REGICOR30 CVR score <7.5%, with moderately elevated cholesterol levels, low-density lipoprotein (LDL) cholesterol levels between 70 and 189 mg/dL and/or total cholesterol (TC) between 200 and 289 mg/dL, but without ischaemic heart disease/CVD).
Specifically, we aim to answer the following research questions
Observational comparison questions
Compared with a reference non-reflective decision assistance strategy based on reminders and decision support tools integrated into the electronic health record (EHR) to help clinical decision-making, what is the effect on the incidence of PIP of statins and of delivery of healthy lifestyle counselling in CVD primary prevention of (a) a decision information strategy comprising a corporate ‘Stopping Low-Value Prescribing’ campaign and the dissemination of evidence-based CPGs for the primary prevention of CVD; (b) a reflective decision structure strategy based on an audit/feedback system and (c) any intervention based on a reflective de-implementation strategy (a or b)?
Experimental comparison question
Compared with a decision information strategy comprising a corporate ‘Stopping Low-Value Prescribing’ campaign and the dissemination of evidence-based CPGs for the primary prevention of CVD, together with the non-reflective decision assistance intervention based on reminders and decision support tools integrated into the EHR to help clinical decision-making, what is the effect on the incidence of PIP of statins and of delivery of healthy lifestyle counselling in CVD primary prevention of adding a reflective decision structure strategy based on an audit/feedback system?
Methods and analysis
Design
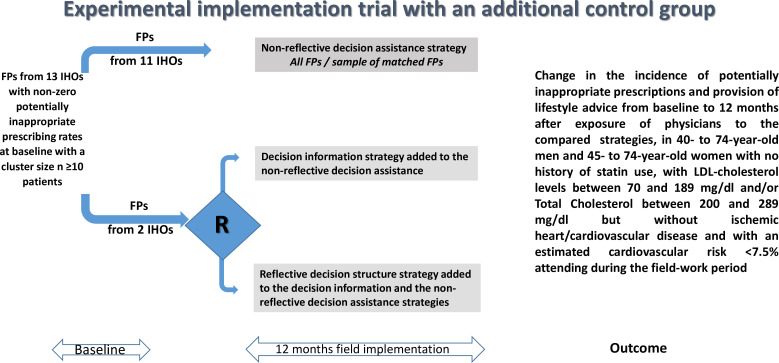
A cluster randomised implementation trial with an additional control group will be conducted to evaluate the potential effectiveness and feasibility of three de-implementation strategies (figure 1). A mixed methods evaluation will be undertaken: quantitative in order to assess the implementation results at professional level (effectiveness outcomes regarding changes in the incidence rates of PIP of statins and provision of healthy lifestyle counselling) and qualitative to assess the feasibility and perceived impact of the de-implementation strategies from the FPs’ perspective and patients’ experience and satisfaction with the clinical care received. The unit of randomisation and intervention will be the primary care (PC) FP, while observation and analysis will be performed at professional and patient levels. The DE-imFAR research protocol was reviewed and approved by the Basque Country Clinical Research Ethics Committee (Reference: EOM2022018, approved on 30 March 2022) and was registered in the US NLM ClinicalTrials.gov database (ClinicalTrials.gov Identifier NCT04022850, Registered 17 July 2019; Last update 5 February 2024).
Figure 1.
Study design diagram. FP, family physician; IHO, Integrated Healthcare Organisation; LDL, low-density lipoprotein; R, randomisation.
Osakidetza-Basque Health Service provides universal coverage and services are free at the point of use, aside from drug copayment, funded through regional general taxation. Primary, specialised and social health-related service provision is organised around 13 Integrated Healthcare Organisations (IHOs) that cover the 3 provinces of the region of the Basque Country: Araba, Bizkaia and Gipuzkoa. Each resident is on the list of one FP or paediatrician who provides comprehensive PC and refers patients to hospitals and specialised services. PC professionals work in full-time teams, which include FPs, paediatricians, nurses and administrative staff, based at local centres that provide users with access to healthcare in a defined geographical area.
We used the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) reporting guidelines and the SPIRIT checklist when writing the present study.31
Participants
Eligibility criteria for the study will be as follows:
Professionals: FPs belonging to any of the 13 IHOs of Osakidetza with a non-zero annual incidence rate of PIP of statins at baseline (2021) with a minimum cluster size of n≥10 patients.
Patients: All men aged 40–74 years and women aged 45–74 years with no history of statin use, LDL cholesterol levels between 70 and 189 mg/dL and/or TC between 200 and 289 mg/dL but without ischaemic heart disease/CVD, and an estimated CVR REGICOR<7.5% who attend at least one appointment with any of the participating FP during the study period (from May 2022 to May 2023).
Clinical interventions
The DE-imFAR study, with regard to the prescription of statins in the primary prevention of CVD, follows the clinical practice recommendations of Osakidetza-Basque Health Service and the Spanish National Health System6 as well as several international guidelines.7–9 Thus, these are the recommendations concerning when to initiate treatment in the primary prevention of CVD6 32:
For individuals aged 40–75 years with an estimated 10-year CVR REGICOR >10%, initiation of statin therapy is recommended.
-
In general, for individuals aged 40–75 years with CVR REGICOR<10% and LDL cholesterol levels<190 mg/dL, it is recommended not to initiate statin therapy, with the following considerations:
With CVR close to 10%, consider the presence of risk-enhancing factors in decision-making.
With CVR<5%, it is recommended not to initiate statin therapy.
For patients with LDL cholesterol levels ≥190 mg/dL, it is recommended to assess the presence of genetic dyslipidaemia and potential cardiovascukar risk-enhancing factors. It is suggested to initiate statin therapy, together with healthy lifestyle recommendations, regardless of cardiovascular risk.
In any case, the indication for treatment should be preceded and/or accompanied by the promotion of healthy lifestyles through healthful diet, regular physical activity and smoking cessation. Moreover, it is recommended that the decision to initiate statin therapy should consider individual baseline risk, absolute risk reduction and whether the risk reduction justifies the potential harms and undesirable consequences of taking a lifelong daily medication.
De-implementation strategies evaluated
Within the present phase II of the DE-imFAR study, the three types of strategies that were derived from the phase I systematic theory- and evidence-based intervention design process will be set up (see online supplemental file 1 for a more detailed description):
bmjopen-2023-078692supp001.pdf (7.6MB, pdf)
A non-reflective decision assistance strategy that targets Type 1 cognitive processes through decision support systems that prompt and remind FPs about the recommended practice in a simplified way, thereby reducing the cognitive burden. In short, pop-up reminders and alerts with associated messages will be integrated into OSABIDE’s (Osakidetza’s EHR system) REGICOR CVR calculator and PRESBIDE (the electronic drug prescribing component). The tools devised include an interactive media-based algorithm with the recommended practice for the primary prevention of CVD in low-risk patients developed by an expert panel, and a patient information sheet that depicts and promotes evidence-based practice to address cholesterol in the primary prevention of CVD in low-risk patients.
A both reflective and non-reflective decision information strategy that targets both Types 1 and 2 cognitive processes, based on the principle of knowledge dissemination and consisting of a ‘Stopping Low-Value Prescribing’ campaign run by the organisation (Osakidetza-Basque Health Service) that also eases access (decreasing the physical effort required) to the evidence-based CPGs for the primary prevention of CVD in low-risk patients.
A reflective decision structure strategy that targets Type 2 cognition through an audit/feedback system that reports data about individual’s and organisational performance indicators with regard to PIP of statins and healthy lifestyle promotion to prompt reflection on their own clinical practice, provided along with intention formation and goal-setting-focused messages.
Allocation of intervention units to compared groups
The DE-imFAR study is a cluster randomised implementation trial conducted under real-world conditions in the primary prevention of CVD in PC where both clinical practices, that is, inappropriate statin prescription and substandard promotion of healthy lifestyles, occur. The aforementioned de-implementation strategies will be cumulatively deployed under routine conditions of healthcare service provision in Osakidetza to reduce the low-value practice and increase the recommended practice by PC healthcare professionals. Specifically, the decision support tools integrated into the EHR (non-reflective decision assistance strategy) will be applied to all FPs from the 13 IHOs of Osakidetza. Further, in addition to this first strategy, eligible FPs belonging to two IHOs (Barakaldo-Sestao and Ezkerraldea-Enkarterri-Cruces) will be randomly assigned to the exposure to either the second (provision of decision information strategy) or the second and third (provision of decision information and reflective decision structure strategies). The allocation sequence within these two groups will be generated using a specific restricted randomisation scheme by one member of the research team. The sequence will be concealed at the coordinating centre. In all cases, FPs will be only allocated to the study groups after they have agreed to participate through an opt-out strategy. The data analyst and the staff in charge of measurements will be blind to FP allocation to study arms. Given that the audit/feedback strategy will involve regular reports privately sent to individuals, the participants in the experimental arms are also expected to be blind to group allocation.
Outcome measures
To evaluate the implementation of the de-implementation strategies in terms of public health impact, we will use the following dimensions of the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework33 :
Reach
Absolute number and percentage of patients in the target population who received the recommended CVD primary prevention clinical intervention 12 months after FP’s exposure to the de-implementation strategies compared; and their representativeness.
Effectiveness
The study’s main outcome will measure both the change in the incidence of the PIP of statins and the change in the incidence of the provision of healthy lifestyle advice in patients in the target population eligible for CVD primary prevention, from baseline to 12 months after exposure of target FPs to the de-implementation strategies.
As a secondary outcome, we will compare the change in the incidence of CVR (REGICOR) recording in the EHR, from baseline to 12 months after exposure of FPs to the de-implementation strategies compared, in men aged 40–74 years and women aged 45–74 years without ischaemic heart disease/CVD.
Adoption
Degree to which the recommended CVD primary prevention clinical intervention is adopted by the FPs 12 months after their exposure to the de-implementation strategies, which will be measured by the percentage of FPs who reduce PIP of statins and/or increase health promotion activities in the target population; and their representativeness.
Implementation
The fidelity of the delivery of each de-implementation strategy under study (ie, the degree to which they were executed as planned) will be evaluated. To this end, a complete record and subsequent description of the execution process, documentation of adaptations made to the planned strategies and process indicators of the delivery of and exposure to the interventions (see online supplemental file 1 for specification of the exposure to each strategy), will be used to assess the following components of fidelity: adherence, dose, quality of delivery, professionals’ responsiveness and programme differentiation.34
Maintenance
Change in the incidence of PIP of statins and provision of healthy lifestyle counselling in eligible patients, 24 months after exposure of FPs to the de-implementation strategies compared with the levels observed at the 12-month assessment.
Other study covariates
In addition, and informed by the cross-sectional observational study performed in the phase I of the DE-imFAR study,10 potential confounders that may bias the estimated effect of the de-implementation strategies on the change in PIP of statins will be measured, both at (a) health professional level: sociodemographic variables (age, sex), baseline incidence rate of PIP of statins and (b) patient level: sociodemographic variables (age, sex, socioeconomic status) and clinical variables (baseline cholesterol level, presence of hypertension, prescribed antihypertensives, tobacco use).
Feasibility evaluation
Professionals’ perception of the feasibility and acceptability of the de-implementation strategies to enhance the provision of the recommended CVD primary prevention clinical practice will be assessed through key informant semi-structured individual interviews. The interviews will be carried out with at least 12 professionals until data saturation is reached: at least 6 (3 from each randomised arm) who reduced their PIP of statins and at least 6 who did not, as informed by the quantitative results. The interview script will contain open-ended questions that will focus on the perceived value of the de-implementation strategies and recommendations for their optimisation.
Patients’ experience and perception of the quality of CVD prevention care received will be also assessed through key informant semi-structured interviews. The interviews will be carried out with at least 10 patients until data saturation is reached: at least 5 patients who were clinically managed according to the recommended practice and 5 who did not. The interview script will contain open-ended questions that will focus on the perceived care received.
Both professional and patient interviews will be conducted by two researchers with experience in qualitative research methods, as well as knowledge of the clinical field and the project. The interviews will be audio recorded, with prior informed consent, and verbatim transcribed. Regarding the analysis of the qualitative study, the responses will be extracted from the interview transcripts. Several members of the research team will participate in the analysis, promoting the exchange of perspectives and consensus, with the aim of triangulating the analysis. Deductive and inductive approaches will be combined. For the deductive approach, the discourse of each professional and patient interviewed will be associated with constructs derived from the behaviour change theories (TDF, BCW, etc).3 11–13 The inductive analysis will be based on the postulates of grounded theory.35 Researchers will use coding techniques, or line-by-line analysis, looking for words and phrases that identify explanatory concepts. Subsequently, thematic connections between the basic theoretical concepts and the data will be developed.
Analysis
Frequencies and proportions along with the corresponding 95% confidence intervals (CIs) will be used to describe the prevalence and cumulative incidence of PIP of statins and healthy lifestyle counselling in the primary prevention of CVD by FPs. The primary effectiveness outcomes will be the changes in the cumulative incidence of PIP of statins and healthy lifestyle counselling in patients from the target population (individuals with no history of statin use, LDL cholesterol levels between 70 and 189 mg/dL and/or TC between 200 and 289 mg/dL, without past or current ischaemic heart disease/CVD, and an estimated CVR REGICOR<7.5% who attend at least one medical appointment with their FP during the study period), from baseline to 12 months after exposure of FPs to the de-implementation strategies. Therefore, to evaluate the impact of the three de-implementation strategies, we will estimate the relative risk reduction of receiving PIP of statins in patients from the target population whose FPs were assigned to the experimental strategies over that in patients from the non-randomised group (non-reflective decision assistance strategy group). With respect to this group and in order to increase comparability and reduce potential bias, in addition to evaluating the change in the incidence rate of PIP of statins in patients from all eligible FPs, we will select two matched FPs from this non-randomised group for each of the randomised FP taking into account both FP’ characteristics (eg, baseline incidence rate of PIP of statins) and characteristics of the patients assigned to the FP (eg, average socioeconomic status). Change in the incidence rates of PIP of statins from baseline to 12 and 24 months after FPs’ exposure to the de-implementation strategies and the relative risk reduction will be estimated with the corresponding 95% CIs. To adjust for potential confounding factors, stratified statistical analyses and logistic models will be used. These models will be extended to generalised mixed effects models to take into account the hierarchical structure of data (patients nested within FPs and FPs within PC teams), with fixed effects (comparison group, effect of time on outcome indicators and time-group interactions) and random effects on the intercept and the time slope (for each patient, FP, centre, etc). These models will be adjusted for potential confounders, following a backward strategy, guided by the stratified analyses. A similar approach will be taken to analyse the secondary outcomes. The analysis will be carried out using SAS (version 9.2, SAS Institute Inc., Cary, NC, US) and R (R Development Core Team, 2014).
Calculation of the required sample size in the worst-case scenario, that is, the comparison between the two randomised de-implementation strategies, was based on (1) a baseline incidence rate of statin PIP of 7.4% estimated among the patients from the target population seen in 2021 by FPs with an incidence rate of statin PIP>0% with a minimum cluster size n≥10 patients, (2) an intraclass correlation coefficient of 0.01, (3) an average cluster size of 39 patients with a coefficient of variation of 0.63, (4) α=0.05 and statistical power of 80% and (5) hypothetical decreases in annual PIP incidence rates of 20% in the decision information strategy group and 50% in the decision structure strategy group. With these assumptions, it was estimated that at least 58 FPs were required for each experimental arm.
Management, quality and safety in data processing
This study will be carried out in accordance with international standards for the conduct of epidemiological studies, included in the International Guidelines for Ethical Review of Epidemiological Studies.36 This is a prospective intervention study mainly focused on the collection of information from data recorded by health professionals in the Osakidetza EHR (OSABIDE) under routine clinical practice conditions. The process indicators related to the professionals’ clinical practice (prescription of statins and record in the EHR of provision of personalised healthy lifestyle advice on the need to increase physical activity, follow a healthy diet and smoking cessation), patients’ sociodemographic and clinical characteristics (age, sex, CVR, active health problems recorded in the EHR, socioeconomic status, etc) and clinical outcomes will be extracted from OSABIDE through the corporate Oracle Business Intelligence platform. In particular, for the provision of healthy lifestyle advice, OSABIDE includes a specific electronic form to check that every single piece of advice (diet, exercise, tobacco quitting) was or was not provided. The Primary Care Research Unit of Bizkaia is formally authorised to extract and use data from the EHR for research purposes by the Healthcare Directorate of Osakidetza. On the other hand, it will be necessary to inform participants (professionals and patients) about the study and obtain their written informed consent concerning the information directly collected from them through the key informant semi-structured interviews (online supplemental files 2 and 3). All the information regarding the study subjects, either expressly extracted for this research from EHRs or collected from the participants, will be protected and treated confidentially for all purposes, in accordance with the provisions of the Spanish Organic Law 3/2018, of 5 December, on Personal Data Protection and digital rights guarantee (LOPD-GDD) and the provisions of Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016, on the protection of natural persons with regard to the processing of personal data and on the free movement of such data (General Data Protection Regulation, GDPR). Specifically, all data will be anonymously documented and de-identified, linked to a unique code that is meaningless without the context of the system. The final resulting database will be exported to a formatted plain text file that then will be compressed and encrypted using a secure algorithm and subsequently will be processed and included in a robust and secure database server.
bmjopen-2023-078692supp002.pdf (248.8KB, pdf)
bmjopen-2023-078692supp003.pdf (240.7KB, pdf)
Patient and public involvement
Patients were involved in the DE-imFAR phase I project as one of the main stakeholders (health professionals, patients and researchers) in the formative process conducted to map and design de-implementation strategies to reduce PIP, which will be evaluated in the DE-imFAR phase II project. Specifically, during the phase I project, a focus group with six patients was conducted to ascertain patients’ experience with the clinical practice of statin prescription and triangulate physicians discourse.14
During the phase II project, semi-structured interviews will be conducted with patients to assess their experience and perception of the clinical care received as a result of their healthcare professionals’ exposure to the different de-implementation strategies. These interviews will help to better understand from the perspective of the study participants the reasons why the strategies work (or do not), to explain the variations in the outcomes and to identify the key strategy components and those that need to be optimised as well as triangulating the analysis.
Discussion
The goal of the present study is to improve CVD primary prevention clinical practice in a real-world setting in PC by putting into practice procedures and methods for the design, deployment and evaluation of implementation/de-implementation strategies informed by behavioural and implementation sciences. Specifically, the phase II of the DE-imFAR study focuses on reducing PIP of statins in CVD primary prevention in patients with moderate hypercholesterolaemia and low CVR and fostering healthy lifestyle promotion as the recommended treatment option. To do so, the study will deploy several de-implementation strategies derived from the phase I formative study that targets key determinants of the decision-making process involved in the provision of CVD primary prevention by FPs. If the results are successful, policy-makers and health managers and professionals will have valid and robust, locally relevant evidence that will support the need to introduce these innovations in methods and procedures informed by implementation science to tackle the hard task of reducing the burden of low-value pharmacological prescription in clinical care services.
Ethics and dissemination
The research protocol (version 1; 170221) was approved by the Basque Country Clinical Research Ethics Committee (Reference: EOM2022018, approved on 30 March 2022) and was registered in the US NLM ClinicalTrials.gov database (ClinicalTrials.gov Identifier NCT04022850, Registered 17 July 2019; Last update 5 February 2024).The Primary Care Research Unit of Bizkaia is explicitly authorised by the Healthcare Directorate of Osakidetza—Basque Health Service to extract and use data from EHRs for research purposes. Since data supporting the present study will mostly concern routine data retrieved from the EHR of the Basque Health Service-Osakidetza, it will be only shared on justified request to the study guarantors. The results of this study will be disseminated via publication in scientific peer-reviewed journals.
Supplementary Material
Acknowledgments
The authors would like to thank the following colleagues in the Basque Health Service (Osakidetza) for their support: Gaspar Lantarón Amas (Subdirectorate of Care Integration) and Nagore Zarraonandia Ayo (Directorate of Care Integration) at Ezkerraldea-Enkarterri-Cruces Integrated Health Organisation, Lourdes Vivanco (Medical Directorate) and Vanesa Martín (Quality Unit) at Barakaldo-Sestao Integrated Health Organisation.
Footnotes
@Alvaro_sanchezp
Contributors: AS, JIP and GG conceived the idea and are the study guarantors. They are primarily responsible for the study design and planning, obtained funding, will be responsible for project coordination and supervision, analysis and interpretation of results and were responsible for manuscript preparation. RSdR, IL, RSV, JAQ, RR, AE, CM, MM-C, MM, CG-R, RS, MOL, SC, NM-I, ML, MGSdT and AG-A are coinvestigators of the projects and collaborated in the study design and/or manuscript preparation; and they will be responsible for study coordination and interpretation of results. AS, JIP and AG-A will be responsible for the analysis of results. All authors read and approved the final version of the manuscript.
Funding: This project was funded by Instituto de Salud Carlos III (ISCIII), cofunded by the European Union (European Regional Development Fund 'A way to make Europe'), through the projects: PI21/00025, RD16/0007/0002, and cofunded by the European Union–NextGenerationEU funds, that finance the actions of the Recovery and Resilience Facility (Mecanismo para la Recuperación y la Resiliencia -MRR), through the project RD21/0016/0003. This project was also funded by the Health Department of the Basque Government (funded projects 2018111085 and 2021111024).
Disclaimer: The funding bodies have had no role in the design of the study, collection, analysis, or interpretation of data or the writing of the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ 2015;351:h4534. 10.1136/bmj.h4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med 2015;13:255. 10.1186/s12916-015-0488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michie S, Johnston M, Abraham C, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26–33. 10.1136/qshc.2004.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eccles M, Grimshaw J, Walker A, et al. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol 2005;58:107–12. 10.1016/j.jclinepi.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 5. Sanchez A, Pijoan JI, Pablo S, et al. Addressing low-value pharmacological prescribing in primary prevention of CVD through a structured evidence-based and theory-informed process for the design and testing of de-implementation strategies: the DE-imFAR study. Implement Sci 2020;15:8. 10.1186/s13012-020-0966-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grupo de Trabajo de la Guía de Práctica Clínica Sobre El Manejo de Los Lípidos Como factor de Riesgo cardiovascular. Guía de Práctica Clínica Sobre El Manejo de Los Lípidos Como factor de Riesgo cardiovascular. Ministerio de Sanidad, Servicios Sociales E Igualdad OSTEBA. Guías de Práctica Clínica en el SNS; 2017. [Google Scholar]
- 7. NICE . National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification, 2014. Available: https://www.nice.org.uk/guidance/cg181
- 8. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Circulation. 2019 Sep 10;140(11):e649-e650] [published correction appears in Circulation. 2020 Jan 28;141(4):e60] [published correction appears in Circulation. 2020 Apr 21;141(16):e774]. Circulation 2019;140:e596–646. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk [published correction appears in Eur Heart J. 2020 Nov 21;41(44):4255]. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 10. Elizondo-Alzola U, Sánchez A, Pijoan JI, et al. Statins in primary prevention of cardiovascular disease: incidence of potentially inappropriate prescriptions in very low risk primary care patients and associated factors. J Gen Prac 2022;10. 10.37421/2329-9126.22 [DOI] [Google Scholar]
- 11. Cane J, O’Connor D, Michie S. Validation of the Theoretical Domains Framework for use in behaviour change and implementation research. Implement Sci 2012;7:37. 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atkins L, Francis J, Islam R, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci 2017;12:77. 10.1186/s13012-017-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanchez A, Elizondo-Alzola U, Pijoan JI, et al. Applying the behavior change wheel to design de-implementation strategies to reduce low-value Statin prescription in primary prevention of cardiovascular disease in primary care. Front Med (Lausanne) 2022;9:967887. 10.3389/fmed.2022.967887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci 2014;9:1. 10.1186/1748-5908-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8:iii–iv, . 10.3310/hta8060 [DOI] [PubMed] [Google Scholar]
- 17. Jamtvedt G, Young JM, Kristoffersen DT, et al. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2006;CD000259. 10.1002/14651858.CD000259.pub2 [DOI] [PubMed] [Google Scholar]
- 18. Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765. 10.1136/bmj.38398.500764.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keller H, Krones T, Becker A, et al. Arriba: effects of an educational intervention on prescribing behaviour in prevention of CVD in general practice. Eur J Prev Cardiol 2012;19:322–9. 10.1177/1741826711404502 [DOI] [PubMed] [Google Scholar]
- 20. Arcoraci V, Santoni L, Ferrara R, et al. Effect of an educational program in primary care: the case of lipid control in cardio-cerebrovascular prevention. Int J Immunopathol Pharmacol 2014;27:351–63. 10.1177/039463201402700305 [DOI] [PubMed] [Google Scholar]
- 21. Dormuth CR, Carney G, Taylor S, et al. A randomized trial assessing the impact of a personal printed feedback portrait on statin prescribing in primary care. J Contin Educ Health Prof 2012;32:153–62. 10.1002/chp.21140 [DOI] [PubMed] [Google Scholar]
- 22. Harris MF, Parker SM, Litt J, et al. Implementing guidelines to routinely prevent chronic vascular disease in primary care: the Preventive Evidence into Practice cluster randomised controlled trial. BMJ Open 2015;5:e009397. 10.1136/bmjopen-2015-009397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liddy C, Hogg W, Singh J, et al. A real-world stepped wedge cluster randomized trial of practice facilitation to improve cardiovascular care. Implement Sci 2015;10:150. 10.1186/s13012-015-0341-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colla CH, Mainor AJ, Hargreaves C, et al. Interventions Aimed at Reducing Use of Low-Value Health Services: A Systematic Review. Med Care Res Rev 2017;74:507–50. 10.1177/1077558716656970 [DOI] [PubMed] [Google Scholar]
- 25. Münscher R, Vetter M, Scheuerle T. A review and taxonomy of choice architecture techniques. J Behav Decis Mak 2016;29:511–24. 10.1002/bdm.1897 [DOI] [Google Scholar]
- 26. Last BS, Buttenheim AM, Timon CE, et al. Systematic review of clinician-directed nudges in healthcare contexts. BMJ Open 2021;11:e048801. 10.1136/bmjopen-2021-048801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoong SL, Hall A, Stacey F, et al. Nudge strategies to improve healthcare providers' implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci 2020;15:50. 10.1186/s13012-020-01011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lamprell K, Tran Y, Arnolda G, et al. Nudging clinicians: A systematic scoping review of the literature. J Eval Clin Pract 2021;27:175–92. 10.1111/jep.13401 [DOI] [PubMed] [Google Scholar]
- 29. Helfrich CD, Rose AJ, Hartmann CW, et al. How the dual process model of human cognition can inform efforts to de-implement ineffective and harmful clinical practices: A preliminary model of unlearning and substitution. J Eval Clin Pract 2018;24:198–205. 10.1111/jep.12855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D’Agostino RB, Grundy S, Sullivan LM, et al. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 2001;286:180–7. 10.1001/jama.286.2.180 [DOI] [PubMed] [Google Scholar]
- 31. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cholesterol and primary prevention of cardiovascular disease: the debate continues. INFAC 2022;30:65–75. [Google Scholar]
- 33. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–7. 10.2105/ajph.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sánchez A, Rogers HL, Pablo S, et al. Fidelity evaluation of the compared procedures for conducting the PVS-PREDIAPS implementation strategy to optimize diabetes prevention in primary care. BMC Fam Pract 2021;22:34. 10.1186/s12875-021-01378-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. Sociology Press: Chicago IL, 1967. [Google Scholar]
- 36. Council for International Organizations of Medical Sciences (CIOMS) in collaboration with the World Health Organization (WHO) . International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva, Switzerland: Council for International Organizations of Medical Sciences (CIOMS), 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078692supp001.pdf (7.6MB, pdf)
bmjopen-2023-078692supp002.pdf (248.8KB, pdf)
bmjopen-2023-078692supp003.pdf (240.7KB, pdf)