Abstract
Purpose
Survivin is a member of the inhibitor-of-apoptosis family. Essential for tumor cell survival and overexpressed in most cancers, survivin is a promising target for anti-cancer immunotherapy. Immunogenicity has been demonstrated in multiple cancers. Nonetheless, few clinical trials have demonstrated survivin-vaccine-induced immune responses.
Experimental design
This phase I trial was conducted to test whether vaccine EMD640744, a cocktail of five HLA class I-binding survivin peptides in Montanide® ISA 51 VG, promotes anti-survivin T-cell responses in patients with solid cancers. The primary objective was to compare immunologic efficacy of EMD640744 at doses of 30, 100, and 300 μg. Secondary objectives included safety, tolerability, and clinical efficacy.
Results
In total, 49 patients who received ≥2 EMD640744 injections with available baseline- and ≥1 post-vaccination samples [immunologic-diagnostic (ID)-intention-to-treat] were analyzed by ELISpot- and peptide/MHC-multimer staining, revealing vaccine-activated peptide-specific T-cell responses in 31 patients (63 %). This cohort included the per study protocol relevant ID population for the primary objective, i.e., T-cell responses by ELISpot in 17 weeks following first vaccination, as well as subjects who discontinued the study before week 17 but showed responses to the treatment. No dose-dependent effects were observed. In the majority of patients (61 %), anti-survivin responses were detected only after vaccination, providing evidence for de novo induction. Best overall tumor response was stable disease (28 %). EMD640744 was well tolerated; local injection-site reactions constituted the most frequent adverse event.
Conclusions
Vaccination with EMD640744 elicited T-cell responses against survivin peptides in the majority of patients, demonstrating the immunologic efficacy of EMD640744.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1516-5) contains supplementary material, which is available to authorized users.
Keywords: Survivin, EMD640744, Cancer vaccines, Cancer immunotherapy
Introduction
Survivin fulfills major criteria to be considered a prime target antigen for anti-cancer vaccination with broad applicability: (1) tumor cells depend on its actions, (2) expression is strong in multiple tumors but rarely detectable in normal tissues, (3) immunogenicity has been demonstrated in patients with different cancers, and (4) peptides restricted by different HLA molecules are known. Survivin is expressed in the majority of tumor cells at all stages, from premalignant to metastatic lesions, solid tumors, and hematopoietic malignancies, and is the fourth most abundantly expressed gene in melanomas and cancers of the colon, lung, brain, and breast [1–4]. It is involved in apoptosis evasion and molecular pathways driving unrestricted proliferation and angiogenesis in tumor cells [5, 6]. Survivin expression is a marker of poor prognosis and/or resistance to therapy in multiple cancers [7, 8].
In patients with a variety of cancers, adaptive cellular as well as humoral survivin-specific responses have been shown, demonstrating its immunogenicity [9–15]. Preclinical and clinical experiences of vaccination against survivin suggest that survivin vaccines can induce immune responses and do not raise substantial safety concerns [16–29].
Recently, multiple HLA class I-binding peptides and one HLA class II-binding peptide of survivin have been identified. Some have been shown to induce immune responses in clinical trials, but these trials tested only single survivin peptides associated with a restricted number of common HLA alleles [13, 30–32].
We report a multicenter, open-label, parallel-group, randomized first-in-man phase I study with EMD640744, a cocktail of survivin-derived, partially modified HLA class I-restricted peptides in Montanide® ISA 51 VG. The aim was to compare three dosages of EMD640744 with respect to immunologic efficacy (using ELISpot and pHLA-multimer staining), safety, tolerability, and clinical activity in patients with different types of metastatic or locally advanced solid tumors (Clinical trials.gov identifier NCT01012102).
Materials and methods
The reporting of the methods has been aligned with the MIATA guidelines (please see the Supplementary MIATA information) [33].
Study design and treatments
This was a multicenter, parallel-group, open-label, randomized phase I trial to determine the immunologic activity, safety and tolerability, and clinical activity of EMD640744 in Montanide® ISA 51 VG in subjects with advanced solid tumors, conducted in five centers in Switzerland (Clinical trials.gov identifier NCT01012102).
EMD640744 is a cocktail of equal quantities by weight of five short peptides based on the amino acid sequence of different regions of the survivin protein, which were previously shown to bind HLA-A1, HLA-A2, HLA-A3, HLA-A24, or HLA-B7 (Supplementary Table 1). These five peptides were chosen based on the following criteria: (1) T-cell stimulatory activity, (2) ability to formulate a stable lyophilizate, and (3) distribution of HLA types (with the aim of maximizing the number of patients that could benefit). EMD640744 was reconstituted and emulsified with an equal volume of Montanide® ISA 51 VG, and 1 ml administered subcutaneously into the left or right thigh (alternating).
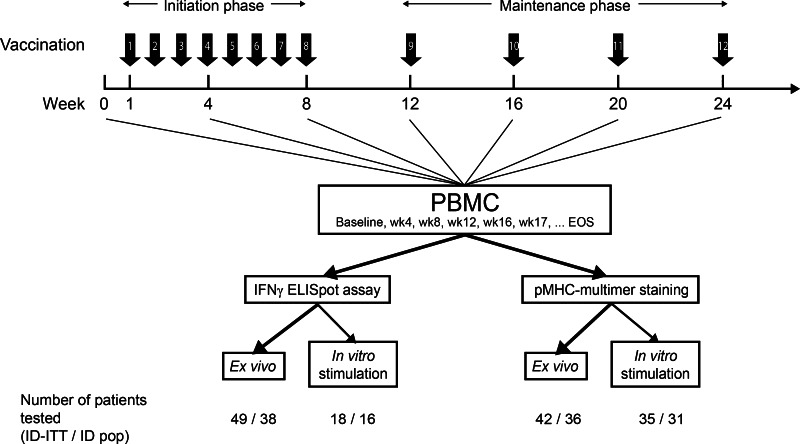
The trial consisted of a preliminary safety evaluation phase in two subjects treated sequentially at the lowest planned dose (30 μg), followed by a randomized phase contingent on adequate safety observations in the first two subjects. In the randomized phase, subjects were allocated to one of the 3 doses of the treatment (1:1:1; 30, 100, or 300 μg peptide) using a central randomization procedure. Treatment was planned for 11-week initiation therapy (8 treatments), followed by 13-week maintenance therapy (4 weekly treatments) (Fig. 1). Patients with clinical benefit (complete response, partial response, or stable disease) could continue until tumor progression or unacceptable toxicity.
Fig. 1.
Vaccination and immunomonitoring schedule. Strength of arrows indicates priorities of analyses. EOS, end-of-study sample; ID-ITT, immunologic-diagnostic intent-to-treat population; ID population, immunologic diagnostic population
Main eligibility criteria
Eligible patients (Supplementary Table 2) expressed at least one of the alleles HLA-A1, HLA-A2, HLA-A3, HLA-A24, or HLA-B7 according to local typing procedures. They were ≥18-year old, with an ECOG performance status ≤1 and histologically or cytologically documented metastatic or locally advanced survivin-expressing solid tumors, for which no established therapy existed. Main exclusion criteria were previous radiotherapy, chemotherapy, surgery (excluding diagnostic biopsy), immunotherapy, or any investigational drug within 30 days before start of the study treatment and any previous treatment with an investigational anticancer vaccine. Patients were not required to have progressive disease at the time of enrollment. Patients with rapidly progressive disease (tumor lysis syndrome) were excluded.
Populations analyzed
The safety/intention-to-treat (ITT) population consisted of all subjects who received ≥ 1 dose study medication (Supplementary Figure 1). The immunologic-diagnostic-ITT analysis set (ID-ITT) consisted of all subjects of the safety/ITT population with available blood samples for ELISpot at baseline and ≥1 blood sample after first vaccination. The ID analysis set consisted of all subjects of the safety population who were not replaced according to the following criteria:
subjects received <6 vaccinations during the initiation phase
subjects omitted the vaccinations in weeks 7 and 8
subjects without available blood samples for ELISpot at baseline and subjects without ≥1 sample in weeks 12, 16, or 17.
Study objectives
The primary objective of this trial was to compare three subcutaneous doses of EMD640744 in Montanide® ISA 51 VG with regard to immunologic efficacy. The primary endpoint was immune response as assessed by ELISpot before and until week 17 after the beginning of the study treatment. Secondary objectives comprised assessment of safety and tolerability and clinical efficacy.
Immunologic analysis
For each patient, not all five single peptides were analyzed, just those that matched that particular patient’s HLA group.
Peptide-specific T-cell responses were analyzed directly ex vivo (evELISpot and evMultimer staining; evMMS) and following short-term in vitro stimulation of peripheral blood mononuclear cells (PBMC) with EMD640744 or specific peptides (ivsELISpot and ivsMultimer staining; ivsMMS). Combination of these approaches facilitated detection of peptide-specific T cells at frequencies ranging from 10−4 to ~10−6.
PBMC sample collection and preparation
PBMC samples were prepared in laboratories located at five study sites. PBMC from blood or leukapheresis were prepared by Ficoll density gradient centrifugation. Cells were frozen in 90 % FCS with 10 % DMSO.
Peptides
Control peptides used for immunomonitoring were >90 % pure (control CE peptide mix, JPT Peptide Technologies, Berlin, Germany). Single survivin peptides were purchased from Bachem (Bubendorf, Switzerland) and were >95 % pure.
IFNγ ELISpot assays
evELISpot assays were performed according to guidelines established in international proficiency panels [34]. After thawing and overnight resting, the number and quality of PBMC were determined by trypan blue staining and flow cytometry. After standard preparation of the ELISpot plates, 5 × 105 PBMC per well of the 96-well ELISpot plates were seeded and pulsed with 2 μM of single survivin peptides or 10 μM of the EMD640744 peptide cocktail. Controls were unstimulated PBMC, PHA (Murex Biotech)—stimulated PBMC and PBMC challenged with a cocktail of 11 hCMV—and EBV-B-derived peptides (CE-Mix, JPT; 5 μM). The assay medium was AIM V (Invitrogen) w/o serum. Reactions were tested in triplicates. After 48-h incubation, assays were developed as described by Britten et al. [35] and analyzed using the KS ELISpot Automated Reader System and analysis software KS ELISpot 4.9 (Carl Zeiss, Goettingen, Germany).
For evELISpot assays, a positive response was defined by a spot number ≥10 and twofold higher than background (PBMC only) and twofold higher than the standard deviation of all combined negative values. For significance testing, the Student’s t test was applied to calculate p values.
ivsELISpot was developed to facilitate detection of peptide-specific T cells at frequencies below the evELISpot detection limit. Thawed PBMC were seeded in 96-well plates after overnight resting and counting. Based on the previous flow cytometry analyses, PBMC numbers were adjusted to give 1x104 CD8+ T cells per well in AIM V supplemented with 10 % pooled human serum from healthy donors. They were stimulated on days 0 and 7 with EMD640744 (10 μM) and tested on day 12 for peptide-specific T-cell responses. Target cells were COS-7 cells transiently transfected with the HLA alleles of interest and pulsed with single survivin peptides and the peptide cocktail. Control cells were HLA transfectants without peptides.
For ivsELISpot assays, responses of microcultures were scored positive when spot numbers were greater than or equal to twofold higher than the average values of the background control plates (COS-7/HLA transfectants).
See Supplementary Figure 2 for representative example control data for evELISpot and ivsELISpot.
Peptide/HLA-multimer staining
For ex vivo peptide/HLA-multimer stainings (evMultimer), 3.4–9 million thawed and washed PBMC were used. Cells were stained with the dead-cell stain Live/Dead aqua (Invitrogen) and then stained with PE-labeled peptide-HLA-tetramers for HLA-A1, HLA-A2, or HLA-A3 (Beckman Coulter) or PE-labeled peptide-HLA-dextramers for HLA-A24 and HLA-B7 (Immudex). After 20 min at room temperature, surface-staining antibodies (CD8, CD45RA, CD3, CD4, CCR7, and CD14) were added for an additional 20 min. The cells were washed, fixed, and permeabilized. Intracellular staining was performed with GranzymeB-antibody for 25 min, after which the cells were washed again and resuspended in PBS.
To control the antibody panel and staining conditions, control cells as external reference samples were used for each round of staining. To determine basic fitting of gates, FMO (fluorescence minus one) controls were used. Supplementary Figure 3 presents examples of stainings and the gating strategy.
The standard cut-off criteria for a positive response by pHLA-multimer staining were detection of ≥50 cells in the multimer gate and a minimum percentage of 0.03 % of the CD8+ T cells. However, in 8 patients, smaller numbers of pHLA-multimer positive cells were seen in evMultimer analysis, just below the detection threshold. In these cases, when looking at the distribution of cells within the FACS-dot plots, the number of cells needed to represent a trustable result was set lower, provided that the pHLA-multimer positive cells formed a population clearly separate from the pHLA-multimer negative population. In 7 of these 8 patients, the existence of survivin-specific T cells below the regular detection limit was also confirmed by detection after in vitro expansion.
For the in vitro stimulated peptide/HLA-multimer stainings (ivsMultimer), PBMC were thawed, washed, and seeded in a 12-well plate (2–4 million cells/ml) in MLPC Medium (RPMI1640 with 10 % human pooled serum (Lonza), gentamycine, pyruvate, and nonessential amino acids) and stimulated with the corresponding peptide or EMD640744 peptide mix (10 μg/ml). The next day IL2 (5 U/ml, Roche, Mannheim, Germany) and IL7 (10 ng/ml, TEBU-bio, Offenbach, Germany) were added. Over the next 12–14 days, half the medium was replaced every 3–4 days with fresh MLPC medium containing IL2 (5 U/ml). After the in vitro stimulation, cells were harvested, washed, and stained with peptide/HLA-multimers as described above.
Assessment of safety and clinical efficacy
Physical examination (including vital signs) was performed weekly during the first 8 weeks, thereafter 4 weekly corresponding to the vaccination visits, and at the end-of-study visit 28 days after last vaccination. Injection-site reactions and extent of exposure to EMD640744 in Montanide® ISA 51 VG were assessed at the vaccination visits. ECG and fundoscopy were performed at screening, weeks 8 and 16 (ECG) or week 12 (fundoscopy), week 24, every 12 weeks thereafter, and at the end-of-study visit.
Adverse events (AEs) were graded according to the National Cancer Institute common criteria (3.0). Treatment-emergent AEs (TEAEs) were defined as AEs that either emerged or worsened within the treatment period, up to 28 days after the last dose of study drug, relative to the pre-treatment state.
For those cases in which disease was not measurable by RECIST [36, 37], tumor response was assessed by imaging, physical examination, nuclear scanning, and/or serum tumor markers established for the given tumor entity. Assessments were listed at screening and in week 11 (with the exception of tumor markers, assessed in week 12), in week 24, every 12 weeks thereafter, and at the end-of-study visit.
Statistics
It was planned that 24–36 subjects were recruited into the study to have 8 evaluable subjects per treatment group. Assuming a true T-cell response rate of ≥50 % of subjects in this population, 3 or more responders of 8 treated subjects were to be seen with a probability >80 % based on binomial distribution assumption. The sample size was not based on test power considerations.
Safety analyses were performed on the safety/ITT population and primary immunologic efficacy analyses on the ID population. Secondary immunologic efficacy analyses were performed on both the ID and ID-ITT populations. No analysis on a per-protocol population was foreseen, and the study was not designed to examine differences between the ITT, ID-ITT, and ID populations.
All efficacy and safety data were reported in a descriptive manner. No formal inferential statistical analyses were planned for efficacy or safety analyses. For tumor response, best overall response was presented by subject and for treatment groups. Progression-free survival time and overall survival time were listed by subject. No summary analysis was performed. A formal analysis to correlate clinical and immunologic efficacy was not planned.
Ethical considerations
This trial was conducted in accordance with the protocol and protocol amendments, the International Conference on Harmonization guideline for good clinical practice, applicable local regulations, and the declaration of Helsinki, and was approved by independent ethics committees and by Swiss Medic. Written informed consent was received from participants.
Results
Study population and treatment
A total of 104 cancer patients were pre-screened for inclusion between December 2007 and July 2009. Sixty-six subjects expressing ≥1 of the alleles HLA-A1, HLA-A2, HLA-A3, HLA-A24, or HLA-B7 underwent screening, and of these, 53 received ≥1 dose trial medication (51 randomized to three treatment groups plus 2 subjects in the preliminary safety phase who received 30 μg; Supplementary Figure 1 and Supplementary Table 3). The ID population comprised 38 subjects, the ID-ITT population 49 subjects, and the safety population 53 subjects. The cutoff for clinical and safety data analyses was October 2009, 17 weeks after randomization of the last subject.
All subjects were of Caucasian origin, 59 % were male, and the median age was 57.7 years. Baseline demographic characteristics and HLA genotypes were well-balanced between treatment groups (Supplementary Tables 4, 5). The most common HLA allele observed was HLA-A2 in 34 (64 %) subjects overall.
Patients had a wide range of advanced solid tumors, including cancers of the ovary, colon, kidney, rectum, breast, testicle, and lung, as well as melanoma and mesothelioma. The most frequent tumor entities were colorectal carcinoma (19 %), ovarian carcinoma (17 %), and melanoma (17 %). Detailed listings of patient demographics, HLA type, dose group, and tumor entity are provided in Supplementary Table 6.
The mean treatment duration in the safety population was 13.1 weeks (30 μg group), 11.7 weeks (100 μg group), and 13.8 weeks (300 μg group), and the median treatment duration was 8.0 weeks in each group. The median number of vaccinations was 8.0 in each group. The number of vaccinations received ranged from 2 to 19. The most common reason for trial discontinuation was progressive disease: 12 subjects (63 %) with 30 μg study treatment, 9 (53 %) with 100 μg, and 11 (65 %) with 300 μg. Four discontinued due to death [3 (15.8 %), 30 μg group; 1 (5.9 %), 100 μg group], and one subject per group discontinued due to AE (5.3, 5.9, and 5.9 %, respectively). The longest treatment duration until data cutoff was 53 weeks.
Primary endpoint analysis
Analysis of the primary endpoint was performed in the ID population (N = 38; Table 1). Overall, 14 patients (37 %) displayed detectable survivin-specific T-cell responses in the 17 weeks following vaccination according to evELISpot and/or ivsELISpot: 4 subjects (33 %, 30 μg), 4 subjects (31 %, 100 μg), and 6 subjects (46 %, 300 μg). The majority of observed responses were directed against the A2 and A3 peptides; in the ID population, no responses to the A1 or B7 peptides were observed (although 4 responses to the A1 peptide were observed in the broader ID-ITT population, discussed below).
Table 1.
Summary of T-cell responders according to ELISpot until week 17 (primary analysis) combining evELISpot and ivsELISpot data—ID analysis set
| Dose groups | Response type | |||
|---|---|---|---|---|
| 30 μg | 100 μg | 300 μg | ||
| evELISpots | 0001–0009 | Sur96-104/M2 | ||
| 0001–0013 | EMD640744 | |||
| 0003–0009 | Sur96-104/M2 | |||
| 0003–0019 | Sur96-104/M2 | |||
| 0004–0015 | Sur96-104/M2 + EMD640744 | |||
| 0005–0011 | Sur20-28 + EMD640744 | |||
| 0005–0014 | Sur96-104/M2 + EMD640744 | |||
| ivsELISpots | 0001–0007 | Sur18-27/K10 + Sur18-27 + EMD640744 | ||
| 0001–0012 | Sur96-104/M2 + Sur96-104 + EMD640744 | |||
| 0001–0016 | Sur96-104/M2 + EMD640744 | |||
| 0001–0035 | Sur96-104/M2 + Sur96-104 + EMD640744 | |||
| 0002–0004 | Sur18-27/K10 + EMD640744 | |||
| 0003–0005 | Sur96-104/M2 | |||
| 0003–0014 | Sur96-104/M2 + Sur96-104 + EMD640744 | |||
| N | 4/12 | 4/13 | 6/13 | |
The denominators indicate the total number of subjects assessed within each dose group at any of weeks 4, 8, 12, 16, and 17. The numerators provide the number of subjects with a positive response. Responses against the cocktail but not to any of the single (expected) peptides within it (e.g., patient 0001–0013) can be explained by responses to other, unexpected peptides presented by one of the patient’s HLA alleles. Conversely, a response to a single peptide in the absence of a response against the cocktail can be explained by competition among cocktail peptides for HLA binding (0001–0009)
Overall immune responses to the vaccine
The immunomonitoring data reported here are derived from the ID-ITT population, which provided a more comprehensive data set than the ID population.
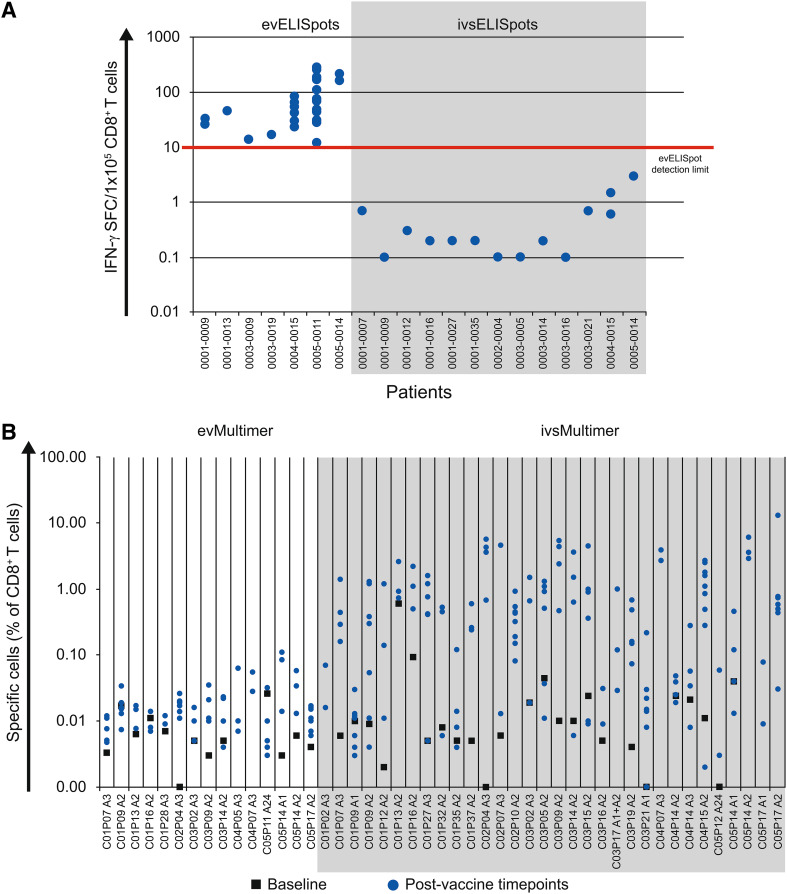
Ex vivo ELISpot assays were performed for all 49 patients of the ID-ITT group. They revealed a pre-existing response (to Sur96-104) at baseline in just one patient, while responses in post-vaccination samples were detectable in 7 of the 49 patients (14 %). In vitro stimulation assays increased the detection sensitivity and showed treatment-related responses in 13 of 18 patients tested (72 %, Fig. 2a).
Fig. 2.
a Frequencies of spot-forming cells (SFC) per 105 CD8+ T cells as determined by ex vivo ELISpot assays (evELISpots) and ELISpots after short-term in vitro stimulation (ivsELISpots) for all patients who showed responses to survivin peptides. b Frequencies of pHLA-multimer-stained cells (as % of vital CD8-positive T cells) analyzed ex vivo (evMultimer) or after in vitro stimulation (ivsMultimer) for all patients with available PBMC samples
Vaccination-induced survivin-specific T cells were also detected ex vivo by pHLA-multimer staining in 15 of 42 patients (36 %) and in 28 of 35 patients (80 %) after short in vitro stimulation with specific peptides, confirming ex vivo responses in 12 of 14 patients (Fig. 2b).
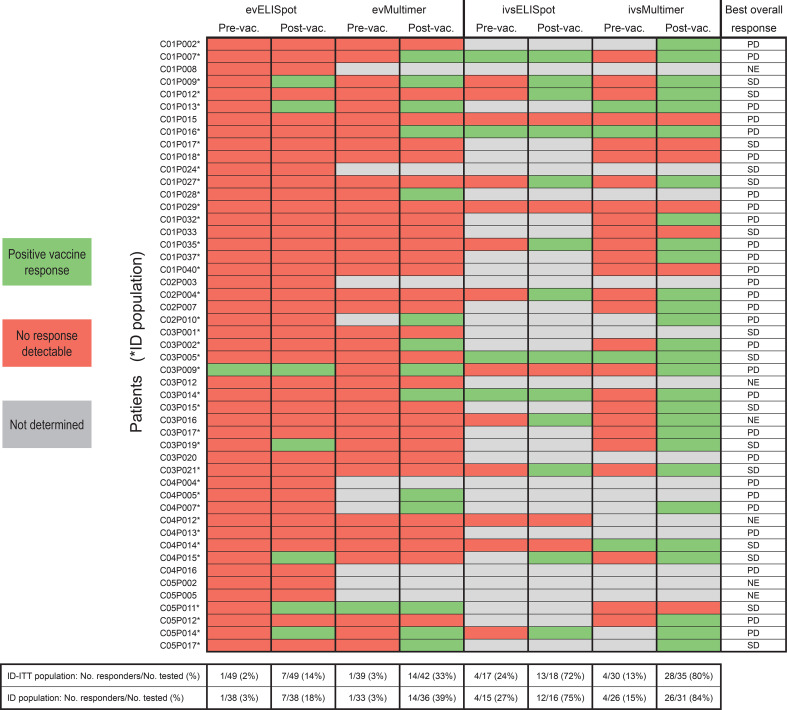
Overall, 31 of 49 patients (63 %) displayed detectable ex vivo and/or in vitro stimulated survivin-specific T-cell responses post-vaccination, as detected by ≥1 assays. Just 8 of these 31 responding patients showed detectable pre-existing immune responses, which, however, significantly increased upon vaccination. In 19 of the 31 responders (61 %), specific responses were detected only after vaccination. In four responders, corresponding baseline samples were only available for ex vivo ELISpot assay but not for the more sensitive in vitro stimulation assays. Since post-vaccination responses in those patients were detected after in vitro stimulation, the presence of pre-existing responses cannot be excluded (Fig. 3).
Fig. 3.
Summary of all T-cell responses detected by ELISpot and pMHC-multimer staining
In the ID population, 29 of 38 patients (76 %) displayed detectable survivin-specific T-cell responses post-vaccination (Fig. 3).
Time course of promotion and duration of immune responses
In 23 patients with a survivin-specific T-cell response, PBMCs from at least time points 4, 8, and 12 weeks after first vaccination were analyzed by pHLA-multimer stainings. Of those, four patients had a pre-existent survivin-specific T-cell response. Of the remaining 19 patients, seven showed a first vaccine-induced T-cell response at week 4, eight patients responded first at week 8, and four patients at week 12. During the course of vaccination, the T-cell frequencies further increased, and from the first detectable response to the peak, there was a mean 12-fold increase (median = 4.6). No correlation of the onset of vaccine-induced responses with the dose, patient HLA type, the number of HLA-matching peptides, or tumor entity was observed.
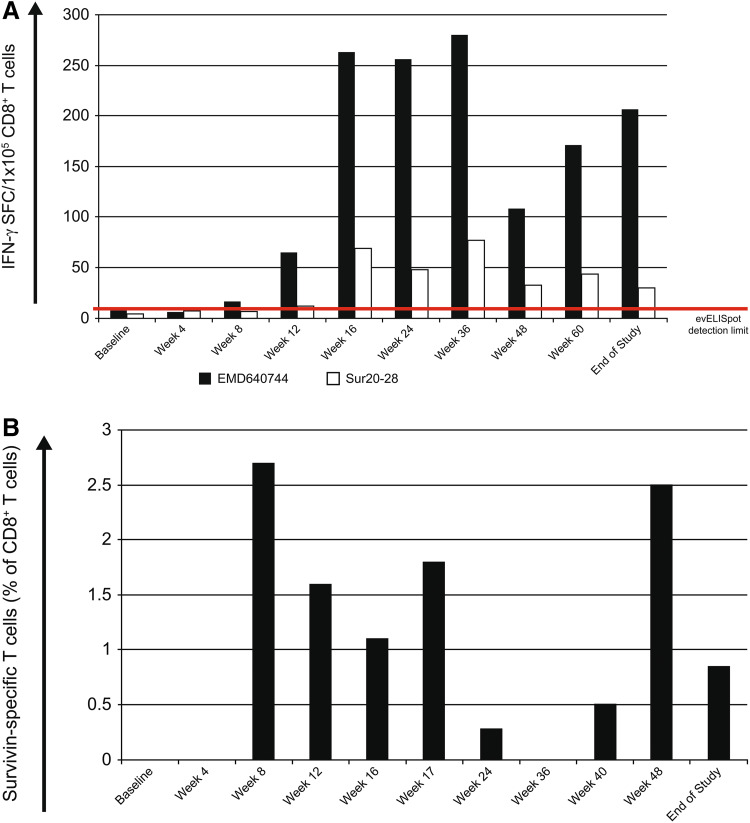
Figure 4 presents representative time courses of promotion and duration of vaccine-specific immune responses measured with two of the four assays employed (4A: evELISpot and 4B: ivsMMS).
Fig. 4.
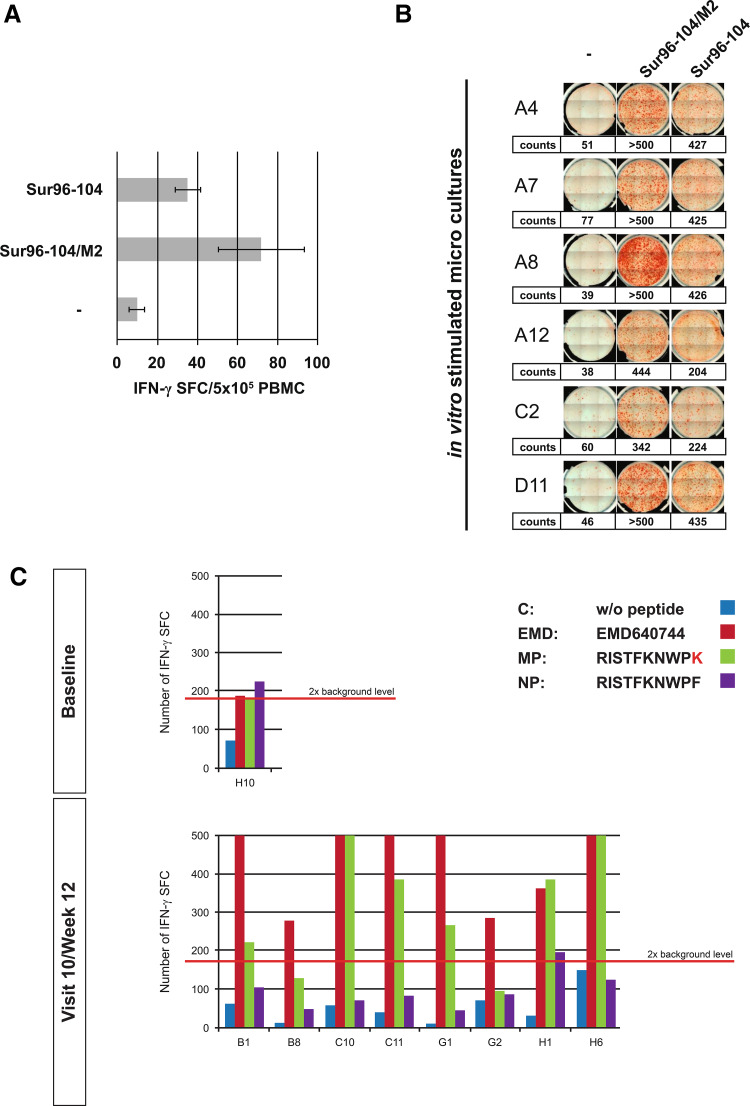
a Response course of patient 0005-0011 determined by ex vivo ELISpot analysis. The HLA-A24-positive patient showed responses to the vaccine cocktail (EMD640744) and to the HLA-A24-restricted peptide Sur20-28. b Response course of patient 0004–0015 to the HLA-A2-binding peptide Sur96-104/M2 by pHLA-multimer staining after in vitro stimulation with the corresponding peptide
Immunogenicity of the different peptides and dose dependency
Immune responses were detected against the vaccination peptide mix EMD640744 and against the single candidate peptide(s) corresponding to the patients’ HLA. In some cases (e.g., patient 0005–0011, Fig. 4a), responses against EMD640744 were even stronger than against the respective single peptide, suggesting peptide binding and immune responses beyond the expected peptide/HLA combinations. Similarly, in some cases (e.g., patient 0001–0013), there was a response against the cocktail but not to the tested single peptides matching the patients’ HLA (Table 1). This might be due to responses to peptides within the cocktail not matching patients’ HLA and therefore not tested, since unexpected. In some patients, for example, patient 0001–0009, there was a response against a single peptide but not against the cocktail. This may be attributed to competition among cocktail peptides for HLA binding.
Survivin-specific T cells were best activated by HLA-A2-binding peptides (Sur96-104/M2, 66 %; 19 of 29 HLA-A2 positive tested patients) and HLA-A3-binding peptides (Sur18-27/K10, 77 %; 10 of 13 tested patients), whereas only 24 % (4 of 17) of the A1-positive patients and 33 % (2 of 6) of the A24-positive patients showed vaccine-specific responses to their cognate HLA-binding epitopes (Sur93-101/T2 and Sur20-28, respectively; note that, these results pertain to the broader ID-ITT population: No response to the HLA-A1-binding peptide Sur93-101/T2 was observed in the ID set). No responses against the B7-binding peptide (Sur6-14) were detected (9 patients tested). Even the lowest dose of EMD640744 (30 μg) promoted good responses, and no evidence of a dose-dependent effect of EMD640744 with regards to immunologic efficacy was found.
Recognition of native peptides by modified peptide-activated T cells
Three peptides contained in EMD640744 were modified to carry anchor amino acids facilitating binding to the respective HLA proteins (Sur93-101/T2, Sur96-104/M2, and Sur18-27/K10; Supplementary Table 1). The modified peptides were previously shown to activate T cells capable of recognizing their respective native counterparts [13, 38].
Herein, responses against modified peptides were accompanied by native peptide recognition in 1 of 8 responders tested by evELISpots, and 7 of 13 responders tested by the more sensitive ivsELISpot assays (see examples in Fig. 5). Responses against native peptides were lower than those against modified peptides.
Fig. 5.
a ELISpot in patient 0004–0015. Responses to the HLA-A2-associated peptides Sur96-104 (native epitope) and Sur96-104/M2 (modified epitope) were detected by evELISpots in weeks 16, 24, and 60 but not at time points in between (not shown). b ivsELISpots on week 36- and end-of-study samples in the same patient (0004–0015) confirmed these responses by showing specific T cells at frequencies below the evELISpot detection limit. c Patient 0001–0007 showed a response to the A3-modified peptide with concurrent reactivity to the native peptide. (Counts were set to >500 when a significant proportion of spots were confluent, and the reader system was unable to count them as separate spots. **p < 0.001; SFC, spot-forming cells)
Assessment of clinical efficacy
Since objective clinical response rates are generally low after cancer vaccination, clinical efficacy can only be reasonably assessed in an appropriately designed trial with a time-to-event endpoint. Therefore, a formal analysis to correlate clinical efficacy with immunologic efficacy was not planned. The best overall response per patient is presented in Fig. 3, and the best overall response within dose groups is presented in Supplementary Table 7. It is important to note that, as progressive disease was not an inclusion criterion, stable disease as best response cannot be interpreted as clinical benefit.
Safety assessments
All subjects experienced ≥1 TEAE, as expected in this population with advanced cancers. The most commonly reported TEAEs (Supplementary Tables 8 and 9) were general disorders and administration site conditions (79, 77, and 88 % of subjects, respectively) and gastrointestinal disorders (58, 53, and 59 %).
The only TEAEs reported in ≥20 % of subjects in any treatment group were injection-site induration (29 % of subjects in the 300-μg dose group) and injection-site reaction, in 21 and 29 % of subjects in the 30 and 100 μg treatment groups respectively (Supplementary Table 8). Typically, these TEAEs slowly resolved over several months. Two subjects experienced Grade 3 TEAEs: One report of thrombosis in the 100 μg group and a granuloma with concomitant Grade 3 injection-site induration in the 300 μg group. Additionally, two related serious AEs were reported: an episode of enthesitis in the 100 μg group and renal failure in the 300 μg group.
The frequencies of TEAEs by systemic organ class were similar between the three treatment groups (Supplementary Table 8). Overall, the trial treatment was well tolerated with local injection-site reactions being the most frequent AE.
Discussion
The literature provides a small number of active immunotherapy trials that use survivin peptides as target antigens and show the induction of specific T cells in restricted numbers of patients [17, 21, 29]. Very recently, a phase-II vaccination trial in melanoma patients reported prolonged overall survival in the subgroup of patients (13/41) who mounted a survivin-specific T-cell response as shown by ex vivo ELISpot analysis [16]. Patients were vaccinated with modified survivin peptides binding to HLA-A1, HLA-A2, and HLA-B35, also partially contained in EMD640744.
All these trials tested particular survivin-peptide/HLA combinations in single tumor entities. In contrast, the present study analyzed a set of five different peptides restricted by five of the most common HLA alleles in the Caucasian population in a heterogeneous cancer patient cohort. This strategy allowed us to test the immunogenicity of different doses of survivin peptides in different tumor settings with coverage of about 80 % of Caucasian patients eligible for the treatment.
Application of two immunologic assays (ELISpot and pHLA-multimer staining) has yielded convincing results that vaccination with the survivin-multipeptide vaccine EMD640744 promotes CD8+ responses in a high proportion of patients, with no dose effect observed. Even the lowest dose (30 μg per single peptide, 150 μg combined) activated T cells as efficiently as the highest dose (300 μg, 1.5 mg), and on the other hand, the latter proved to be safe as well. Taking the results of ELISpot- and multimer assays together, 63 % (31 of 49) of vaccinated patients showed survivin-specific T-cell responses ex vivo and/or following in vitro stimulation. Importantly, every post-vaccination response detected by ELISpot was confirmed with pHLA-multimer staining, suggesting that both assays detected the same T cells. The fact that multimer stainings found higher frequencies of responders (76 %) than ELISpots (37 %) is probably due to the higher material requirements of the ELISpot assays: After having performed the evELISpot assays in 49 patients, there were only sufficient PBMC left to conduct ivsELISpots in 18 patients (Fig. 3). Availability of more PBMC for the more sensitive ivsELISpot in further patients would most likely have increased the number of responders as detected by this assay as well. In addition to frequency analyses, it would have been important to test whether different vaccine doses caused qualitatively different T-cell responses. Here again, the unique and restricted patient material was not sufficient for avidity- or affinity analyses of survivin-specific T cells or to test the lymphocytes against target cells endogenously expressing survivin (autologous tumor cells were not available). Nonetheless, detection of positive responses with both functional and multimer-staining assays provides robust evidence that these individuals were true responders.
In 61 % of the responding patients (19 of 31, Fig. 3), we found evidence for de novo induction of survivin-specific CD8+ T cells by vaccination. T cells responding to modified peptides were able to recognize the corresponding native peptides in 1 of 8 samples identified by evELISpot and in 7 of 13 samples identified by ivsELISpot assays. Consistent with previous reports [31] showing this cross-reactivity for the HLA-A2-binding Sur96-104/M2 and its native counterpart, to the best of our knowledge, this study is the first to confirm this phenomenon for Sur93-101/T2 (HLA-A1)- and Sur18-27/K10 (HLA-A3)-responsive T cells as well. Overall, responses to the native epitopes were generally weaker when compared to modified peptide responses. However, we are unable to draw any conclusions from this finding with respect to clinical implications.
Two lines of evidence argue against survivin as appropriate target antigen for immunotherapy: (1) Schendel et al. have reported that endogenous survivin expression in activated T cells can affect survivin-specific T cells due to fratricide [39]. However, our data clearly show that vaccination in vivo and stimulation in vitro expanded survivin-specific T cells. (2) While most normal, terminally differentiated adult tissue cell types do not express survivin, some specialized normal cells do; these include thymocytes, CD34+ bone marrow-derived hematopoietic progenitor cells, basal colonic epithelial cells, and activated epithelial cells. Pisarev et al. [40] induced survivin multiepitope-specific CTLs that recognized tumor cells but did not affect the function of hematopoietic progenitor cells. In our study, the induction of survivin-specific T-cell responses neither led to clinically apparent autoimmune side effects nor did it recognizably affect the hematopoietic or vascular system in vaccinated patients. EMD640744 was generally well tolerated, with injection-site reactions constituting the bulk of reported TEAEs.
Hailemichael et al. recently reported that oil emulsion adjuvants may promote persistence of antigen at the vaccination site with T-cell sequestration and dysfunction, findings that call for caution to be exercised with montanide-formulated vaccines. The survivac vaccine elicited high response rates associated with a favorable safety profile. Nonetheless, investigation of injection-site-infiltrating lymphocytes would be desirable in future studies [41].
Due to the limitations of this phase I study design—a small study population precluding formal statistical comparison between the three doses, a formal analysis to correlate clinical efficacy with immunologic efficacy was not planned [42]. Future studies may examine combinations with standard therapy or maintenance vaccination of patients who responded to the standard treatment. The recently reported correlation of prolonged survival in melanoma patients and development of immune responses to modified survivin peptides [16], partially contained in EMD640744, argue for a clinically relevant immunopotency of the vaccine and warrant further study.
In aggregate, the presence of antigen-specific T cells in 63 % of patients—and in particular the supportive evidence for de novo induction in 61 % of responders—is remarkable for a tumor vaccination trial and clearly supports the concept of using survivin as a relevant target for cancer immunotherapy in tumor entities expressing survivin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The study was sponsored by Merck KGaA Darmstadt, Germany. The authors take full responsibility for the content of this publication. The authors would like to thank the patients, the investigators, co-investigators, and study teams at each of the participating centers and at Merck KGaA, Darmstadt, Germany. The contributions of Annett Hamann, the team of the cell sorting and immunomonitoring core unit in Erlangen and Daniela Eberts (Mainz), for their excellent technical assistance, are also gratefully acknowledged. Editorial assistance in the preparation of this manuscript was provided by Paola Accalai, International Medical Press, funded by Merck KGaA, Darmstadt, Germany.
Conflict of interest
Juergen Zieschang is a Merck employee. Ulf Forssmann was a Merck employee until the end of March 2013. Ulrike Gnad-Vogt was a Merck employee from 2005 to 2009 and received consultancy fees and a travel grant from Merck from 2009 to 2011 and has been a CureVac GmbH employee since 2011. All other authors declare that they have no competing interests.
Abbreviations
- AE
Adverse event
- evELISpot
Ex vivo ELISpot
- evMMS
Ex vivo multimer staining
- FMO
Fluorescence minus one
- ID
Immunologic-diagnostic population
- ID-ITT
Immunologic-diagnostic intention-to-treat population
- ITT
Safety/intention-to-treat population
- ivsELISpot
ELISpot following in vitro stimulation of PBMC
- ivsMMS
Multimer staining following in vitro stimulation of PBMC
- PBMC
Peripheral blood mononuclear cells
- TEAE
Treatment-emergent adverse event
Footnotes
Volker Lennerz, Stefanie Gross, Ulrike Gnad-Vogt, Ulf Forssmann, Thomas Woelfel, and Eckhart Kaempgen have contributed equally to this work.
Study number/Clinicaltrials.gov reference: EMR 200032-001/NCT01012102.
The authors of this paper report on their T cell assays transparently and comprehensively as per field-wide consensus, allowing the community a full understanding and interpretation of presented data as well as a comparison of data between groups. The electronic supplementary materials of this publication include a MIATA checklist.
References
- 1.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 2.Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, Lal A, Wang CJ, Beaudry GA, Ciriello KM, Cook BP, Dufault MR, Ferguson AT, Gao Y, He TC, Hermeking H, Hiraldo SK, Hwang PM, Lopez MA, Luderer HF, Mathews B, Petroziello JM, Polyak K, Zawel L, Kinzler KW, et al. Analysis of human transcriptomes. Nat Genet. 1999;23(4):387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 3.Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92(2):271–278. doi: 10.1002/1097-0142(20010715)92:2<271::AID-CNCR1319>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Grossman D, McNiff JM, Li F, Altieri DC. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J Invest Dermatol. 1999;113(6):1076–1081. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 5.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22(53):8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 6.Emens LA. Survivin’ cancer. Cancer Biol Ther. 2004;3(2):180–183. doi: 10.4161/cbt.3.2.751. [DOI] [PubMed] [Google Scholar]
- 7.Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, Ohira M, Hashizume K, Kobayashi H, Kaneko Y, Nakagawara A. High expression of survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19(5):617–623. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 8.Swana HS, Grossman D, Anthony JN, Weiss RM, Altieri DC. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med. 1999;341(6):452–453. doi: 10.1056/NEJM199908053410614. [DOI] [PubMed] [Google Scholar]
- 9.Rohayem J, Diestelkoetter P, Weigle B, Oehmichen A, Schmitz M, Mehlhorn J, Conrad K, Rieber EP. Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res. 2000;60(7):1815–1817. [PubMed] [Google Scholar]
- 10.Schmidt SM, Schag K, Müller MR, Weck MM, Appel S, Kanz L, Grünebach F, Brossart P. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102(2):571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz M, Diestelkoetter P, Weigle B, Schmachtenberg F, Stevanovic S, Ockert D, Rammensee HG, Rieber EP. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res. 2000;60(17):4845–4849. [PubMed] [Google Scholar]
- 12.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61(16):5964–5968. [PubMed] [Google Scholar]
- 13.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61(3):869–872. [PubMed] [Google Scholar]
- 14.Casati C, Dalerba P, Rivoltini L, Gallino G, Deho P, Rini F, Belli F, Mezzanzanica D, Costa A, Andreola S, Leo E, Parmiani G, Castelli C. The apoptosis inhibitor protein survivin induces tumor-specific CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res. 2003;63(15):4507–4515. [PubMed] [Google Scholar]
- 15.Tanaka M, Butler MO, Ansén S, Imataki O, Berezovskaya A, Nadler LM, Hirano N. Induction of HLA-DP4-restricted anti-survivin Th1 and Th2 responses using an artificial antigen-presenting cell. Clin Cancer Res. 2011;17(16):5392–5401. doi: 10.1158/1078-0432.CCR-10-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker JC, Andersen MH, Hofmeister-Müller V, Wobser M, Frey L, Sandig C, Walter S, Singh-Jasuja H, Kämpgen E, Opitz A, Zapatka M, Bröcker EB, Thor Straten P, Schrama D, Ugurel S. Survivin-specific T-cell reactivity correlates with tumor response and patient survival: a phase-II peptide vaccination trial in metastatic melanoma. Cancer Immunol Immunother. 2012;61(11):2091–2103. doi: 10.1007/s00262-012-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Fang HB, Cai L, Janofsky S, Chew A, Storek J, Akpek G, Badros A, Yanovich S, Tan MT, Veloso E, Pasetti MF, Cross A, Philip S, Murphy H, Bhagat R, Zheng Z, Milliron T, Cotte J, Cannon A, Levine BL, Vonderheide RH, June CH. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117(3):788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honma I, Kitamura H, Torigoe T, Takahashi A, Tanaka T, Sato E, Hirohashi Y, Masumori N, Tsukamoto T, Sato N. Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother. 2009;58(11):1801–1807. doi: 10.1007/s00262-009-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55(10):1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuruma T, Iwayama Y, Ohmura T, Katsuramaki T, Hata F, Furuhata T, Yamaguchi K, Kimura Y, Torigoe T, Toyota N, Yagihashi A, Hirohashi Y, Asanuma H, Shimozawa K, Okazaki M, Mizushima Y, Nomura N, Sato N, Hirata K. Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med. 2008;6:24. doi: 10.1186/1479-5876-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki A, Kobayashi J, Torigoe T, Hirohashi Y, Yamamoto T, Yamaguchi A, Asanuma H, Takahashi A, Michifuri Y, Nakamori K, Nagai I, Sato N, Hiratsuka H. Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer Sci. 2011;102(2):324–329. doi: 10.1111/j.1349-7006.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 22.Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, thor Straten P, Andersen MH, Pedersen AE, Claesson MH, Lorentzen T, Johansen JS, Svane IM. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother. 2008;31(8):771–780. doi: 10.1097/CJI.0b013e3181833818. [DOI] [PubMed] [Google Scholar]
- 23.Hadrup SR, Gehl J, Sorensen RB, Geertsen PF, Straten PT, Andersen MH. Persistence of survivin specific T cells for seven years in a melanoma patient during complete remission. Cancer Biol Ther. 2006;5(5):480–482. doi: 10.4161/cbt.5.5.2652. [DOI] [PubMed] [Google Scholar]
- 24.Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol. 2004;22(14):2808–2815. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 25.Ellebaek E, Engell-Noerregaard L, Iversen TZ, Froesig TM, Munir S, Hadrup SR, Andersen MH, Svane IM. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: results from a phase II trial. Cancer Immunol Immunother. 2012;61(10):1791–1804. doi: 10.1007/s00262-012-1242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kameshima H, Tsuruma T, Torigoe T, Takahashi A, Hirohashi Y, Tamura Y, Tsukahara T, Ichimiya S, Kanaseki T, Iwayama Y, Sato N, Hirata K. Immunogenic enhancement and clinical effect by type-I interferon of anti-apoptotic protein, survivin-derived peptide vaccine, in advanced colorectal cancer patients. Cancer Sci. 2011;102(6):1181–1187. doi: 10.1111/j.1349-7006.2011.01918.x. [DOI] [PubMed] [Google Scholar]
- 27.Widenmeyer M, Griesemann H, Stevanović S, Feyerabend S, Klein R, Attig S, Hennenlotter J, Wernet D, Kuprash DV, Sazykin AY, Pascolo S, Stenzl A, Gouttefangeas C, Rammensee HG. Promiscuous survivin peptide induces robust CD4+ T-cell responses in the majority of vaccinated cancer patients. Int J Cancer. 2012;131(1):140–149. doi: 10.1002/ijc.26365. [DOI] [PubMed] [Google Scholar]
- 28.Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen LØ, Rath JC, Böck M, Bröcker EB, Straten PT, Kämpgen E, Becker JC. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23(7):884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, Kurotaki T, Yamamoto M, Yagihashi A, Ohmura T, Yamaguchi K, Katsuramaki T, Yasoshima T, Sasaki K, Mizushima Y, Minamida H, Kimura H, Akiyama M, Hirohashi Y, Asanuma H, Tamura Y, Shimozawa K, Sato N, Hirata K. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedrichs B, Siegel S, Andersen MH, Schmitz N, Zeis M. Survivin-derived peptide epitopes and their role for induction of antitumor immunity in hematological malignancies. Leuk Lymphoma. 2006;47(6):978–985. doi: 10.1080/10428190500464062. [DOI] [PubMed] [Google Scholar]
- 31.Reker S, Meier A, Holten-Andersen L, Svane IM, Becker JC, thor Straten P, Andersen MH. Identification of novel survivin-derived CTL epitopes. Cancer Biol Ther. 2004;3(2):173–179. doi: 10.4161/cbt.3.2.611. [DOI] [PubMed] [Google Scholar]
- 32.Karanikas V, Soukou F, Kalala F, Kerenidi T, Grammoustianou ES, Gourgoulianis KI, Germenis AE. Baseline levels of CD8+ T cells against survivin and survivin-2B in the blood of lung cancer patients and cancer-free individuals. Clin Immunol. 2008;129(2):230–240. doi: 10.1016/j.clim.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O’Donnell-Tormey J, Odunsi K, Old LJ, Ottenhoff TH, Ottensmeier C, Pawelec G, Roederer M, Roep BO, Romero P, van der Burg SH, Walter S, Hoos A, Davis MM. T cell assays and MIATA: the essential minimum for maximum impact. Immunity. 2012;37(1):1–2. doi: 10.1016/j.immuni.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, Kast WM, Hoos A. Elispot proficiency panel of the CVC immune assay working group. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol Immunother. 2008;57(3):303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Britten CM, Meyer RG, Frankenberg N, Huber C, Wolfel T. The use of clonal mRNA as an antigenic format for the detection of antigen-specific T lymphocytes in IFN-gamma ELISPOT assays. J Immunol Methods. 2004;287(1–2):125–136. doi: 10.1016/j.jim.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Bernatchez C, Zhu K, Li Y, Andersson H, Ionnides C, Fernandez-Vina M, Cano P, Cooper L, Abbruzzese J, Hwu P, Chang DZ, Radvanyi LG. Altered decamer and nonamer from an HLA-A0201-restricted epitope of survivin differentially stimulate T-cell responses in different individuals. Vaccine. 2011;29(16):3021–3030. doi: 10.1016/j.vaccine.2011.01.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W, Schendel DJ. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest. 2010;120(11):3869–3877. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pisarev V, Yu B, Salup R, Sherman S, Altieri DC, Gabrilovich DI. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9(17):6523–6533. [PubMed] [Google Scholar]
- 41.Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoos A, Britten CM, Huber C, O’Donnell-Tormey J. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol. 2011;29(10):867–870. doi: 10.1038/nbt.2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.