Abstract
Combinatory strategies are becoming of increasing interest in cancer immunotherapy. Costimulation by individual members of the immunoglobulin-like (Ig)- and TNF superfamily have already shown promising antitumor potential, thus prompting the exploration of their synergistic abilities in combinatorial approaches. Here, we pursued a targeted strategy with antibody-fusion proteins composed of a tumor-directed antibody and the extracellular domain of the costimulatory ligand B7.1, 4-1BBL, OX40L, GITRL or LIGHT, respectively. Costimulatory activity was assessed in an experimental setting where initial T cell activation was induced by a bispecific antibody (tumor-related antigen × CD3). Advantage of combined targeted costimulation was shown for either B7.1 or 4-1BBL with OX40L, GITRL, LIGHT and 4-1BBL in terms of T cell proliferation and IFN-γ release. Since encouraging results were obtained by the combination of B7.1 and 4-1BBL, we adapted the model system for a time-shift setting. Here, enhanced proliferation and granzyme B expression as well as reduced PD-1 expression on the T cell population demonstrated the benefit of costimulation-assisted restimulation. Finally, the antitumor potential of this combinatorial setting was confirmed in vivo in a lung metastasis mouse model. Thus, combinatorial approaches with costimulatory antibody–ligand fusion proteins seem a promising strategy to be further investigated for cancer immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1441-7) contains supplementary material, which is available to authorized users.
Keywords: Antibody-fusion proteins, Costimulation, TNFSF ligands, Cancer immunotherapy
Introduction
Interfering with the costimulatory/inhibitory ligand-receptor network that regulates the immune response has become a promising approach in cancer immunotherapy. Thus, antagonistic antibodies directed against inhibitory receptors of the immunoglobulin-like (Ig) superfamily (e.g., CTLA-4, PD-1) and agonistic antibodies directed against costimulatory receptors of the tumor necrosis factor receptor (TNFR) superfamily (e.g., 4-1BB, OX40, GITR, CD127, CD40) are being evaluated in clinical trials [1, 2]. In addition, it is becoming apparent that strongest antitumor effects might be achieved by combinatorial treatments [3, 4]. In preclinical models, synergistic actions have been described for the combined activation of different costimulatory receptors (e.g., 4-1BB with OX40 or CD40) [5–7] as well as for combined activation and blockade of costimulatory and coinhibitory receptors, respectively (e.g., anti-4-1BB mAb with either anti-CTLA-4 or anti-PD-1) [8–10]. The evaluation of indicated combinations is complicated since the expression pattern of these receptors and corresponding ligands orchestrating the immune response is cell-type specific, time-dependent and cell context-related [11]. Thus, seeking maximal antitumor potential of costimulatory/inhibitory reagents in combinatorial settings constitutes a major challenge in the field.
For immunotherapeutic approaches, costimulation has been provided either by agonistic antibodies for particular receptors or respective ligands [12, 13]. Studies with ligands involved mainly multimeric recombinant proteins composed of the extracellular domain of the ligand fused to antibody Fc or multimerization domains (e.g., surfactant protein D (SP-D), modified core streptavidin (SA), tenascin (TNC)) [14–17]. Although effective costimulatory activity was achieved, undirected costimulation bears the risk of unexpected adverse events and autoimmunity (e.g., liver toxicity observed after anti-4-1BB mAb treatment in preclinical and clinical studies [18]). Thus, targeted approaches are being developed, creating tumor-specific antibody-fusion proteins with costimulatory ligands in order to direct, confine and improve the immune response at the tumor site [14, 19–22]. Enhanced antitumor effects could be shown by target-directed costimulation with B7.1 or 4-1BBL in diverse tumor mouse models [19, 20, 23]. Furthermore, combination therapies with regulatory T cell (Treg) depletion [19] and a bispecific antibody [20] resulted in improved therapeutic effects.
We have reported previously a combinatorial approach of two costimulatory antibody–ligand fusion proteins, targeting B7.2 and 4-1BBL to the tumor cell via two independent antigens. In this model system, T cells were retargeted by a bispecific antibody and stimulation was enhanced by target-mediated costimulation of B7.2 and 4-1BBL [22]. Here we report further combinations of antibody–ligand fusion proteins focusing on the combination of B7.1 and 4-1BBL with the costimulatory members OX40L, LIGHT and GITRL of the TNF superfamily analyzing cytokine release, proliferation and the cytotoxic potential of T cells. Since encouraging results were obtained by the combination of B7.1 and 4-1BBL, we adapted the model system for a time-shift setting, where the benefit of costimulation-assisted restimulation was shown. Finally, the antitumor potential of this combinatorial setting was confirmed in vivo in a lung metastasis mouse model.
Materials and methods
Materials
Antibodies were purchased from Biolegend (Uithoorn, The Netherlands), Immunotools (Friesoythe, Germany), Miltenyi Biotec (Bergisch Gladbach, Germany) and Santa Cruz (Santa Cruz, USA). DuoSet enzyme-linked immunosorbent assay (ELISA) kit for human IFN-γ was obtained from R&D Systems (Minneapolis, USA). Further materials include CellTrace™ CFSE cell proliferation kit (Life Technologies, Darmstadt, Germany) and NuPAGER 4–12 % Bis–Tris Gel (Bio-Rad Laboratories, München, Germany). Mouse endoglin was produced in our laboratory [24]. B16-FAP, HT1080-FAP (transfectants with human fibroblast activation protein) and HT1080 wild-type (Klaus Pfizenmaier, IZI) were cultured in RPMI 1640, 5 % fetal bovine serum (FBS), supplemented with 200 μg/ml G418 in the case of HT1080-FAP and 200 µg/ml zeocin in the case of B16-FAP cells. HEK293 were cultured in RPMI 1640, 5 % FBS. Human PBMC were isolated from buffy coat of healthy donors (blood bank, Klinikum Stuttgart (Katharinenhospital), Germany) and cultivated in RPMI 1640, 10 % FBS. C57BL/6Jrj mice were purchased from Elevage Janvier (France). Animal care and experiments carried out were in accordance with federal guidelines and had been approved by university and state authorities.
Generation of costimulatory antibody–ligand fusion proteins
Cloning of scDbFAPCD3, B7.2-Db and scFv-4-1BBL into pSecTagA backbone vector (Life Technologies, Darmstadt, Germany) has been described previously [22]. B7.1-Db was cloned by ligand (35–242 aa) replacement (SfiI/NotI) in the B7.2-Db construct. Based on the scFv-4-1BBL comprising plasmid, scFv-OX40L, scFv-LIGHT and scFv-TNC-GITRL were cloned by the combination of the respective scFv (SfiI/NotI) and ligand (BamHI/XbaI) cassettes. They are composed of the scFv A5 directed against human endoglin [25] and the extracellular domain of human OX40L (51–183 aa), LIGHT (85–240 aa) and GITRL (72–199 aa), the latter fused N-terminally to the chicken tenascin domain (110–139 aa) described elsewhere [26]. The scFv-4-1BBL(m) was generated by introducing the mouse-specific scFvmEDG [24] and mouse ligand 4-1BBL (104–309 aa). Ligand-DNA was either synthesized (Life Technologies) or obtained by PCR from template plasmids provided by H. Wajant (University Hospital Würzburg). scDbFAPCD3(m) was obtained by replacing the anti-human CD3 antibody moiety by the anti-mouse CD3 specific (2C11) [27] one. The recombinant proteins were produced in stable transfected HEK293 cells and purified by immobilized metal ion affinity chromatography (IMAC) as described previously [22].
Binding analysis
Binding specificity of the scFv–ligand fusion proteins to endoglin was analyzed by flow cytometry. 2 × 105 HT1080-FAP (EDG+) or HEK293 (EDG−) cells were incubated with 300 nM fusion protein for 2 h at 4 °C. After washing, cells were incubated for 1 h at 4 °C with R-phycoerythrin (PE)-conjugated mouse anti-hexahistidyl-tag monoclonal antibody. Washing and incubation steps were carried out in PBS, 2 % FBS, 0.02 % sodium azide. Cell analysis was performed in an EPICS FC500 (Beckman Coulter, Krefeld, Germany) and data was analyzed using FlowJo (Tree Star, Ashland, USA).
Ligand activity in soluble and cross-linked form
ELISA plates were coated over night with scDbFAPCD3 (3 and 10 nM) at 4 °C. Ligand activity in cross-linked form was analyzed by coating the respective fusion protein (50 nM) together with the scDbFAPCD3. Subsequently, plates were washed, and 2 × 105 CFSE-labeled PBMC/well was added. Therefore, PBMC were stained before with carboxyfluorescein diacetate succinimidyl ester (CFSE) at a concentration of 625 nM/1 × 106 cells/ml, following the instructions of the manufacturer. Ligand activity in soluble form was assessed on the ELISA plates coated with scDbFAPCD3 only. Here, after blocking with medium, the scFv-ligand (50 nM) was added in solution altogether with the PBMC. After 4 days proliferation was measured by flow cytometry as indicated above.
Isochronic combinatorial setting
2 × 104 HT1080-FAP cells/well were seeded in 96-well-plates. The next day, cells were incubated for 1 h at RT with scDbFAPCD3 (16 pM) in combination with one (10 nM or titration) or two of the antibody–ligand fusion proteins (10 nM of each one), before the addition of 2 × 105 PBMC/well. After 48 h, cell-free supernatant was removed, and IFN-γ concentration was determined in a sandwich-ELISA, following the instructions of the manufacturer’s protocol. Alternatively after 7 days, proliferation of T cells (CFSE-labeled PBMCs/anti-CD3-PerCP) and T cell expression of granzyme B (anti-CD3-FITC, anti-granzyme B-PE) was assessed by flow cytometry. Block shift correction was applied to the data [28].
Time-shift combinatorial setting
2 × 104 HT1080-FAP cells/well were seeded in 96-well-plates. The next day, cells were incubated for 1 h at RT with scDbFAPCD3 (33 pM), before washing and the addition of 2 × 105 PBMC/well. After 3 days of coculture, stimulated PBMCs were transferred for restimulation to a fresh plate with HT1080-FAP cells previously incubated with scDbFAPCD3 as indicated above. After 4 additional days of coculture, PBMCs were harvested and analyzed by flow cytometry. Costimulation was provided by the addition of B7.1-Db (10 nM) and scFv-4-1BBL (10 nM) during coculture. They were added together either in combination with scDb-mediated initial or restimulation. Alternatively, B7.1-Db was administered together with scDb-mediated initial stimulation, followed by the administration of scFv-4-1BBL together with scDb-mediated restimulation. T cell proliferation and granzyme B expression was analyzed as indicated previously. Furthermore, T cell expression of PD-1 and CTLA-4 was measured by corresponding antibody staining. Block shift correction was applied to the data [28].
Animal experiments
Pharmacokinetics were determined for B7.2-Db and scFv-4-1BBL(m). C57BL/6 mice (n = 3 or 4) were injected intravenously (i.v.) with 0.5 nmol fusion protein/animal. Blood samples were taken at different time points (3 min, 0.5, 1, 2, 6 and 24 h) and serum concentration of the fusion protein determined via ELISA. Therefore, anti-CD86 monoclonal antibody or mouse endoglin were coated and bound B7.2-Db or scFv-4-1BBL(m), respectively, detected via the anti-His-Tag-HRP antibody. Data was normalized considering the first time point (3 min) as 100 %. Pharmacokinetic parameters [(t 1/2α, t 1/2β, area under the curve (AUC)] were calculated via Excel using the first 3 time points to calculate t 1/2α and the last 3 time points to calculate t 1/2β. AUC was determined for the time interval of 3 min-24 h.
Therapeutic efficacy of the combinatorial approach with the recombinant proteins was assessed in a B16-FAP lung metastasis model. C57BL/6JRj mice (3 month) were injected i.v. with 1 × 106 B16-FAP cells/mouse on day 0. Treatment was administrated i.p. to groups of 5–6 mice as follows: (1) PBS (day 1, 2, 3, 10, 11, 12); (2) scDb(m) (day 1, 2, 3, 10, 11, 12); (3) scDb(m) + B7.1-Db (day 1, 2, 3) and scDb(m) + scFv-4-1BBL(m) (day 10, 11, 12); (4) scDb(m) (day 1, 2, 3) and scDb(m) + B7.1-Db + scFv-4-1BBL(m) (day 10, 11, 12); (5) B7.1-Db (day 1, 2, 3) and scFv-4-1BBL(m) (day 10, 11, 12); (6) B7.1-Db + scFv-4-1BBL(m) (day 10, 11, 12). Dosage: 4 pmol scDb(m) and 0.2 nmol (B7.2-Db and scFv-4-1BBL(m)). Mice were sacrificed on day 21. Lungs were removed, fixed in formaldehyde and metastases counted.
Statistical analysis
For comparison between multiple groups, the one-way analysis of variance (ANOVA) followed by the Tukey post test was applied, using the GraphPad Prism software (GraphPad Software, La Jolla, USA). P values of <0.05 were considered to be significant.
Results
Generation of costimulatory antibody-fusion proteins with OX40L, LIGHT and GITRL
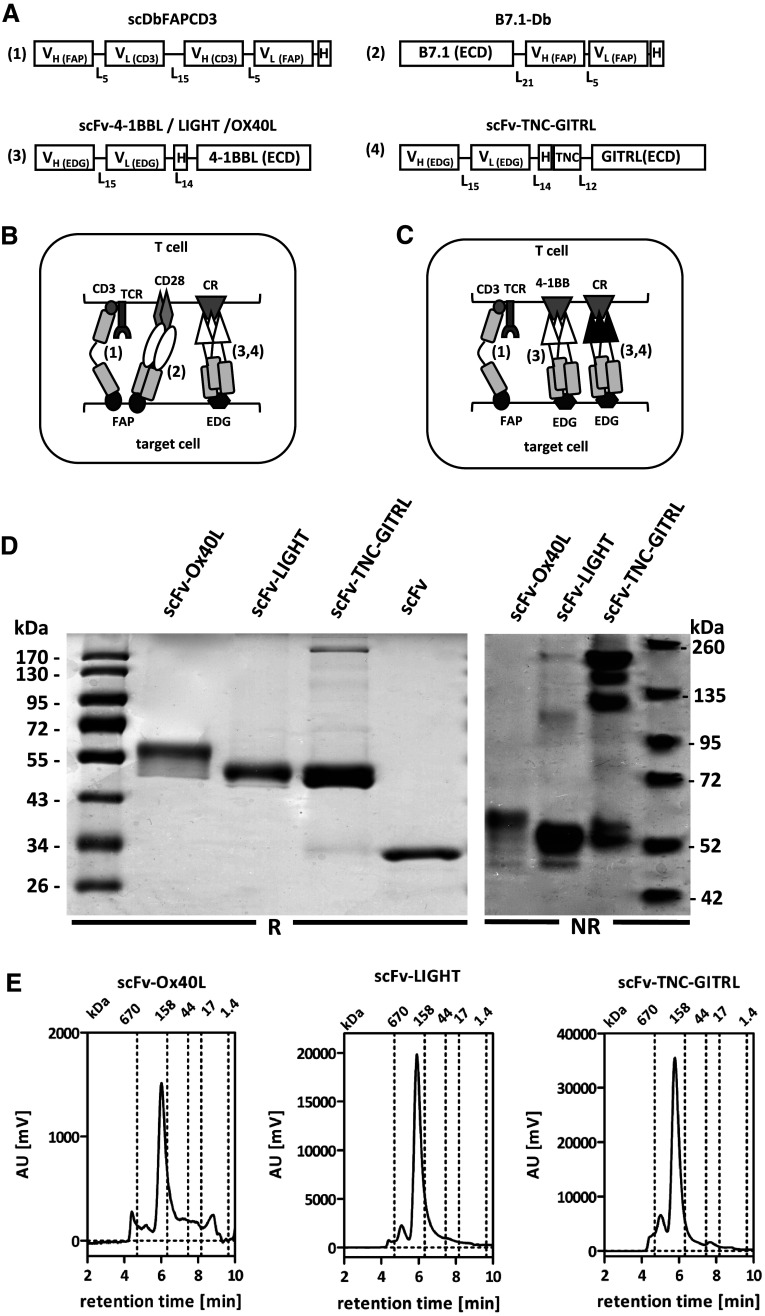
The bispecific single-chain diabody scDbFAPCD3 and costimulatory antibody-fusion proteins with B7 and 4-1BBL had been described previously [22]. In brief, in our model system, scDbFAPCD3 is directed against the fibroblast activation protein (FAP) on the target cells and CD3 on T cells. Thus, simultaneous binding of the scDb to both antigens leads to target-mediated T cell activation. The stimulation of T cells can be further enhanced by costimulation with the antibody-fusion proteins B7-Db and scFv-4-1BBL. These fusion proteins are composed of the extracellular domain of the ligands B7 and 4-1BBL fused to a FAP-specific antibody in the diabody format and an endoglin (EDG) specific antibody in the scFv format, respectively (Fig. 1a). Thus, by antibody binding, these costimulatory ligands are also targeted to the tumor cell and presented there in a membrane-bound and therefore active form (Fig. 1b). To further evaluate this concept we decided to extend it to a broader range of ligand combinations, generating also antibody-fusion proteins with OX40L, LIGHT and GITRL. These costimulatory ligands were chosen based on their high antitumor potential documented in the literature [11]. In order to avoid FAP-targeting competition with the scDbFAPCD3 and B7-Db and taking into consideration their affiliation to the TNF-family, the extracellular domain of these costimulatory ligands (OX40L, LIGHT, GITRL) was fused to the endoglin-specific antibody A5 in the scFv format, creating fusion proteins analogue to the scFv-4-1BBL (Fig. 1a, c). In the case of scFv-GITRL, trimerization was enforced by introducing a tenascin domain into the linker (scFv-TNC-GITRL). Thus, a covalently intermolecular connected homotrimer was created. Stable transfected HEK293 producer cell lines were generated and fusion proteins purified from the supernatant by immobilized metal ion affinity chromatography (IMAC) with a yield of 2.4 mg/l (scFv-OX40L), 17.4 mg/l (scFv-LIGHT) and 8 mg/l (scFv-TNC-GITRL), respectively. SDS-PAGE analysis (Fig. 1d) under reducing conditions revealed single bands of 58 kDa (scFv-OX40L) and 51 kDa (scFv-LIGHT, scFv-TNC-GITRL) that could be further reduced after deglycosylation, correlating to the calculated molecular mass of 42 kDa (scFv-OX40L), 44 kDa (scFv-LIGHT) and 46 kDa (scFv-TNC-GITRL) (data not shown). Under non-reducing conditions, the multimeric format of the scFv-TNC-GITRL was confirmed (Fig. 1d). Furthermore, size exclusion chromatography (SEC) of the fusion proteins indicated the presence of homogeneous populations in accordance with a native trimeric disposition (Fig. 1e). Binding specificity to endoglin-expressing target cells was shown by flow cytometry (Fig. 2a). Ligand activity was demonstrated by artificial cross-linking through coating of the fusion proteins on an ELISA plate together with the scDbFAPCD3 as first stimulus. Here, proliferation of PBMCs was enhanced clearly by the presence of the costimulatory ligands in immobilized form, while their soluble form remained mainly inactive (Fig. 2b). Similar results have been previously reported by us for the scFv-4-1BBL [22]. In addition, costimulatory activity of antibody-fusion proteins on target cells was confirmed for all three antibody–TNFSF ligand fusion proteins, enhancing approximately twofold the scDb-induced proliferation of PBMCs (Fig. 2c). The costimulatory nature of these signals was verified by the lack of activation observed in absence of the first signal (scDb). Thus, these antibody–TNFSF ligand fusion proteins perfectly met the conditions to be incorporated in the combinatorial setting established previously.
Fig. 1.
a Modular schema of the bispecific antibody and antibody–ligand fusion proteins. V H/L variable region of the heavy/light antibody chain, FAP fibroblast activation protein, EDG endoglin, scDb single-chain diabody, Db diabody, ECD extracellular domain, H Histidine tag, TNF tenascin, L n linker of n amino acids. Schema of the combinatorial settings: b targeting scDb/B7-Db to FAP and the scFv-ligand to endoglin; c Targeting scDb to FAP and scFv-4-1BBL plus a second scFv-ligand to EDG. CR costimulatory receptor. d SDS-PAGE analysis of the scFv–ligand fusion proteins (3 μg/lane) under reducing (R) and non-reducing (NR) conditions on a 12 % SDS-PAGE and 4–12 % Bis–Tris Gel, respectively; Coomassie staining. e HPLC analysis on a TSK-GEL G3000SWXL column (Tosoh Bioscience) of scFv-OX40L (2 μg), scFv-LIGHT (15 μg) and scFv-TNC-GITRL (15 μg). Mobile phase was PBS at a flow rate of 0.5 ml/min
Fig. 2.

Functional properties of scFv-OX40L, scFv-LIGHT and scFv-TNC-GITRL. a Antibody binding by flow cytometry. HT1080-FAP (EDG+) and HEK293 (EDG−) cells were incubated with 300 nM scFv-ligand. Bound construct were detected by an anti-hexahistidyl-tag-PE conjugated antibody. Gray filled: cells only; gray line: detection antibody only; black line: scFv-ligand followed by detection antibody. b Ligand activity of the fusion proteins in coated and soluble form. scDbFAPCD3 was coated and incubated with CFSE-labled PBMC together with scFv-ligand (50nM) presented either coated or in solution. Proliferation was determined after 4 days by flow cytometry. [n = 3. Mean ± SD, One-way ANOVA, Tukey post test, *p < 0.05, **p < 0.01, ***p < 0.0001]. c Costimulatory properties of the fusion proteins in a cellular assay. Target cells (HT1080-FAP) were incubated with scDb in combination with the scFv-ligands for 1 h before the addition of CFSE-labeled PBMC. Proliferation of PBMC was measured after 4 days by flow cytometry
Combinations of costimulatory antibody-fusion proteins that modulate scDb-mediated T cell stimulation
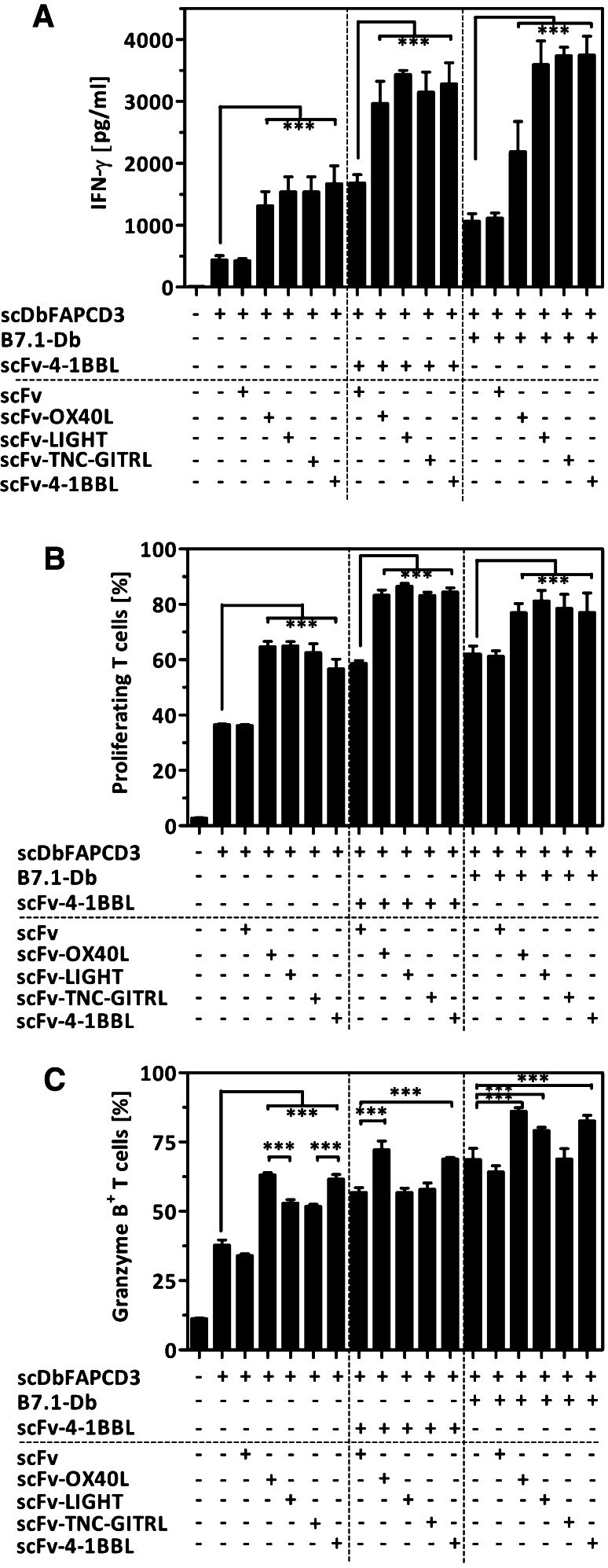
Two combinatorial strategies were explored. In the first approach, the scDbFAPCD3 as first signal was applied together with B7.1-Db in combination with each of the scFv-TNFSF–ligand fusion proteins (scFv-4-1BBL, scFv-OX40L, scFv-LIGHT, scFv-TNC-GITRL) (Fig. 1b). In the second approach, scDbFAPCD3 as first signal was applied together with scFv-4-1BBL in combination with each of the other scFv-TNFSF–ligand fusion proteins (scFv-OX40L, scFv-LIGHT, scFv-TNC-GITRL) (Fig. 1c). For the latter, scFv-4-1BBL was applied at a concentration where the addition of equal concentration of endoglin-specific scFv was not interfering with the costimulatory signal and doubling the concentration of scFv-4-1BBL led to a further signal enhancement. Thus, masking of individual costimulatory effects by competition of two fusion proteins for the same target cell antigen (endoglin) was avoided. In both settings, target cells (HT1080-FAP+EDG+) were cocultured with PBMCs in presence of the respective combination of bispecific antibody/antibody–ligand fusion proteins and T cell stimulation monitored by cytokine release (IFN-γ), proliferation and granzyme B expression. Analysis of IFN-γ release revealed that all scFv-TNFSF–ligand fusion proteins and B7.1-Db were individually able to enhance the scDb-mediated signal (3 to 3.8-fold and 2.4-fold, respectively) (Fig. 3a). The combination of either B7.1-Db or scFv-4-1BBL with the other scFv-TNFSF–ligands could further improve this effect, indicating the feasibility and clear benefit of a combined application. Here, the combination of scFv-4-1BBL with each other of the scFv-TNFSF–ligands was highly effective (6.7- to 7.8-fold increase). On the other hand, B7.1-Db in combination with either of the scFv-4-1BBL, scFv-LIGHT or scFv-TNC-GITRL showed also strong signal enhancements (8.1- to 8.5-fold) and were clearly more effective than the combination of B7.1-Db with scFv-OX40L (5-fold increase). As expected, in terms of proliferation, all costimulatory fusion proteins were able to enhance the scDb-mediated T cell activation (1.5- to 1.8-fold) (Fig. 3b). Also in this case, either the combination of B7.1-Db or scFv-4-1BBL with the scFv-TNFSF–ligand fusion proteins could further enhance T cell proliferation (2.1- to 2.4-fold). Thus, emphasizing proliferation as a hallmark of T cell activation, a maximal effect was achieved to a similar extent by all combinations of costimulatory fusion proteins. In contrast, differential impact was observed in the acquirement of cytotoxic potential as indicated by the percentage of granzyme B expressing T cells (Fig. 3c). Although all antibody-fusion proteins were able to enhance the population of granzyme B positive T cells, B7.1-Db, scFv-4-1BBL and scFvOX40L were more effective (1.6- to 1.8-fold) than scFv-LIGHT and scFv-TNC-GITRL (1.4-fold). In combination, strongest effects were obtained by B7.1-Db together with scFv-4-1BBL, scFvOX40L and scFv-LIGHT (2.1- to 2.3-fold increase). No further improvement was observed by addition of scFv-TNC-GITRL. In regard to the combinatorial setting with scFv-4-1BBL, only scFv-OX40L could further increase the cytotoxic potential (1.9-fold), while scFv-LIGHT and scFv-TNC-GITRL did not. Thus, according to these results all costimulatory antibody-fusion proteins showed to be suitable and promising for combinatorial approaches. Especially the combined applications of B7.1-Db with either scFv-LIGHT or scFv-4-1BBL were highly effective in all aspects analyzed. However, for subsequent time-delayed studies we decided to focus at first on the combination of a single antibody-fusion protein pair. Considering that according to the receptor expression profile costimulation by B7/CD28 and LIGHT/HVEM are related to the initial phase and 4-1BBL/4-1BB to a later phase in the immune response [29], the combination of B7.1-Db and scFv-4-1BBL, covering different time frames, seemed most appropriate for further studies.
Fig. 3.
Costimulatory effects mediated by the combined application of the antibody-fusion protein panel. Target cells (HT1080-FAP) were cocultured with PBMCs in presence of scDb (16 pM) in combination with one or two costimulatory antibody-fusion proteins (10 nM). a IFN-γ-release was measured after 4 days by Sandwich-ELISA. b Proliferation of T cells was determined (CFSE/anti-CD3-PerCP) after 7 days by flow cytometry. c Cytotoxic potential of T cells was measured after 7 days via granzyme B staining. Therefore, PBMC were fixed, permeabilized and granzyme B expressing T cells identified (anti-CD3-FITC/anti-granzyme B-PE) by flow cytometry. [n = 3. Mean ± SD, One-way ANOVA, Tukey post test, *p < 0.05, **p < 0.01, ***p < 0.0001]
Time-delayed, combined costimulation of scDb-activated T cells
Based on this model system, we approached the question of the influence of costimulation on T cell response in a restimulation context, establishing a time-shift setting. Therefore, T cells were stimulated with scDb on day 0 for initial stimulation and on day three for restimulation, followed by T cell analysis of proliferation, cytotoxic potential and inhibitory receptor expression (PD-1, CTLA-4) on day 7. Costimulation by B7.1-Db and scFv-4-1BBL was provided either with initial stimulation or restimulation. Alternatively, following the costimulatory receptor expression pattern, B7.1-Db was provided in combination with initial scDb-stimulation and scFv-4-1BBL with scDb-restimulation. In terms of proliferation, early combined costimulation enhanced scDb-mediated T cell proliferation to a similar degree than scDb-mediated restimulation (Fig. 4a). Successive costimulation or late combined costimulation could further enhance the proliferation to a maximum. Early combined costimulation was less effective than scDb-mediated restimulation in enhancing the percentage of granzyme B expressing T cells (Fig. 4b). Nevertheless, also in this case successive costimulation or late combined costimulation could further enhance the signal to a maximal degree. Analyzing the PD-1 receptor expression on T cells revealed that initial scDb-mediated stimulation enhanced the percentage of PD-1 expressing T cells, whereas early combined costimulation did not further increase it (Fig. 4c). Moreover, while scDb-mediated restimulation even further enhanced this population, successive costimulation and mainly late combined costimulation significantly decreased the level of PD-1-positive T cells. Here, the costimulatory effect was especially prominent on CD8+T cells (data not shown). The same pattern was observed for CTLA-4 expression on T cells, although at much lower expression level (Fig. 4d). Thus, in our in vitro setting the combined costimulation with B7.1-Db and scFv-4-1BBL during restimulation seemed advantageous over an initial single term application.
Fig. 4.
Costimulatory effects of the combination of B7.1-Db and scFv-4-1BBL in a time-shift setting. HT1080-FAP cells were incubated with scDbFAPCD3 (33 pM) for 1 h, followed by washing and the addition of PBMC. After 3 days, PBMC were transferred to a well with freshly seeded HT1080-FAP and cocultured for another 7 days. For restimulation purpose, HT108-FAP had been previously incubated with the scDb (33 pM), followed by washing. Costimulation was applied by the addition of B7.1-Db (10 nM) and scFv-4-1BBL (10 nM) either simultaneously during initial stimulation or during restimulation with the scDb. Alternatively, B7.1-Db was applied together with initial scDb-mediated stimulation followed by scDb-mediated restimulation in presence of scFv-4-1BBL. T cell proliferation (a) and expression of granzyme B (b), PD-1 (c) and CTLA-4 (d) was measured after 7 days via flow cytometry. [n = 3. Mean ± SD, One-way ANOVA, Tukey post test, *p < 0.05, **p < 0.01, ***p < 0.0001]
Therapeutic animal experiment
Next, the antitumor potential of the combinatorial approach with costimulatory fusion proteins and the bispecific antibody was demonstrated in vivo in a syngeneic tumor mouse model. Therefore, the bispecific antibody and the scFv-4-1BBL fusion protein had to be adjusted for compatibility with the mouse model by introducing mouse-specific CD3 and EDG directed antibodies and mouse 4-1BBL, respectively. The FAP-specific antibodies and the B7 ligand which are known to be cross-reactive (human/mouse) were retained. Specific binding to target cells was confirmed by flow cytometry (Fig. S1). Analysis of the pharmacokinetic properties revealed similar half-lives for B7.2-Db (t 1/2α 24.5 ± 9.2 min; t 1/2β 6.6 ± 1 h) and scFv-4-1BBL(m) (t 1/2α 23.9 ± 3.5 min; t 1/2β 6.0 ± 0.6 h). Also similar bioavailability was measured for B7.2-Db and scFv-4-1BBL(m) with an area under the curve (AUC0–24 h) of 148.8 ± 76.4 and 130.7 ± 63 h* %, respectively.
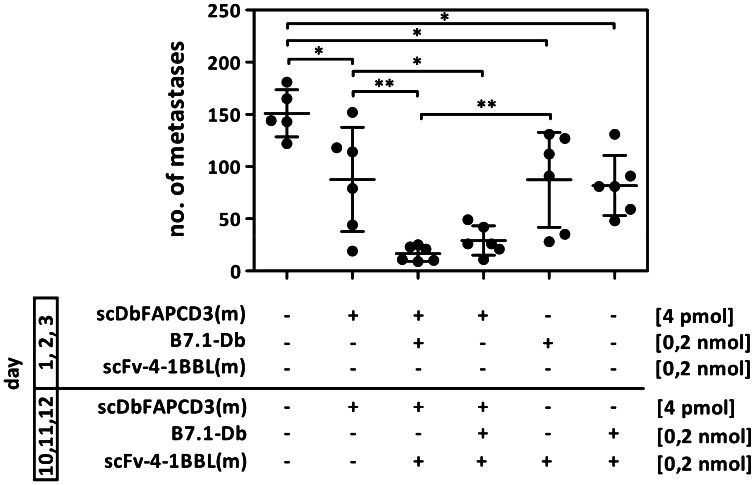
Mice received i.v. injections of B16-FAP cells and were treated with recombinant proteins applied during an early (day 1, 2, 3) and a later (day 10, 11, 12) stage. After 21 days, lungs were removed and tumor foci counted. Strongest reduction in the metastasis formation was observed after the treatment with the bispecific antibody in combination with the costimulatory fusion proteins either applied consecutively (86 % average reduction) or together at the later time stage (77 % average reduction). Neither treatment with the scDb(m) alone (29 % average reduction) nor the costimulatory fusion proteins alone (30 and 34 % average reduction) were able to reach this effect (Fig. 5). Thus, combined costimulation by the fusion proteins B7.1-Db and scFv-4-1BBL(m) improved considerably the anti-tumor effect of the bispecific antibody scDbFAPCD3(m) in vivo.
Fig. 5.
Antitumor effect of the combined treatment with scDb(m), B7.1-Db and scFv-4-1BBL(m) in a metastatic tumor mouse model. C57BL/6 mice were injected i.v. with B16-FAP cells on day 0. Treatment with the scDb(m) (4 pmol) and the antibody–ligand fusion proteins (0.2 nmol) in the indicated combinations were performed once a day on day 1, 2, 3, 10, 11 and 12. Lungs were removed on day 21 and metastasis counted. [n = 5–6 mice/group, One-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001]
Discussion
Ligands of the TNF superfamily are defined by the TNF-homology domain (THD) that mediates non-covalent self-association into homotrimers [30]. Their activity can differ considerably according to the presentation on the cell surface (expression as type II membrane protein) or in soluble form (generated by ectodomain shedding or alternative splicing) [31]. Taking advantage of this property, antibody-fusion proteins with ligands that are less active in solution can be used to recover their activity by target-directed immobilization on the cell surface [32]. We and others have shown previously that this is the case for human 4-1BBL in scFv-4-1BBL formats. Thus, costimulatory activity was almost absent in untargeted form and restored after antibody-mediated binding to target cells [17, 33]. Here, we observed that strong costimulatory activity was also displayed by scFv-OX40L, scFv-LIGHT and scFv-TNC-GITRL on target cells and in coated, that is, cross-linked form, while their activity was almost absent in solution. Likewise, a 200-fold increase in activity from non-targeted to targeted status was described for a scFv-Flag-OX40L fusion protein [33]. In the case of human GITRL, it has been described as a rather unstable trimer, due to a smaller intersubunit interface than other TNF ligands [34]. Accordingly, studies with an scFv-Flag-GITRL indicated a mixed population of monomers and trimers or tetramers and targeted immobilization-mediated activity was rather low in comparison with other ligands like 4-1BBL or OX40L [17, 33]. By introducing a tenascin domain that forces trimerization of soluble TNF ligands by formation of disulfide bonds [17], we could further stabilize the antibody-fusion protein format (scFv-TNC-GITRL). Although the formation of a minor fraction of molecular subspecies could not be prevented, a mainly homogeneous population was generated that showed strong antibody binding and costimulatory properties catching up with those observed for scFv-4-1BBL and scFvOX40L in terms of proliferation and IFN-γ release. For LIGHT, antibody-mediated ligand cell surface presentation was here shown for the first time. Thus, the underlying concept of antibody-mediated TNFSF-ligand presentation was confirmed for OX40L and shown to profit by GITRL stabilization and the incorporation of the family member LIGHT.
To further investigate combinatorial approaches considering ligand composition- and time-related aspects, the generation of antibody-fusion proteins with stable ligand presentation and receptor activation properties was crucial. In our model system, we confirmed these properties by setting up equal amounts of antibody-fusion proteins together with a suboptimal bispecific antibody concentration retrieving similar T cell costimulation in terms of proliferation and IFN-γ release. In addition, further signal enhancement could be achieved by the combination of B7.1-Db and scFv-4-1BBL with either of scFv-4-1BBL, scFv-OX40L, scFv-LIGHT and scFv-TNC-GITRL, respectively. Thus, it was corroborated that in both targeting-defined experimental settings (Fig. 1b, c) the combined application of antibody–ligand fusion proteins was perfectly suitable, so that each of these ligands could effectively contribute to modulate the T cell response. In regard to proliferation, different costimulatory pattern have been observed in a setting providing costimulation with the ligands transfected into cells expressing a membrane-bound anti-CD3 antibody [35]. Here, stronger T cell proliferation was attributed to B7.1, 4-1BBL and OX40L than GITRL, while LIGHT remained practically inactive. Since ligand expression was monitored and adjusted to similar levels, ligand presentation and the strength of CD3-mediated T cell activation are most likely to account for this differential outcome, in which the application of antibody-fusion proteins seems advantageous.
In terms of promoting the cytotoxic potential of T cells, we observed that scFv-TNC-GITRL and scFv-LIGHT were less effective than the other antibody-fusion proteins, either alone or in combination with scFv4-1BBL and B7.1-Db. Nevertheless they are known to be involved in other physiological functions of therapeutic importance. For GITR, besides activation of effector T cells, the influence to overcome Treg suppressor functions seems to be of particular interest [2]. In the case of LIGHT, ligand-mediated upregulation of chemokine and adhesion molecules expression in the tumor environment might be of special relevance, since it was shown to correlate with infiltration and activation of naive T cells leading to tumor rejection [36].
Our results are consistent with some of the expected relations of costimulatory ligands/receptors during the immune response. For the combination of B7 and 4-1BBL, strongly enhanced effects on T cell response and consecutive improved antitumor effects have been reported in different tumor mouse models [37, 38]. Also, synergistic or additive effects were demonstrated for B7.1 with OX40L or OX40L with 4-1BBL on antiviral CD8+ T cell response [5]. Collaborative receptor expression might be one reason for combinatorial success. It seems reasonable for CD28 with 4-1BB and OX40, since CD28 costimulation is known to participate in the initial T cell activation process and 4-1BB and OX40 expression is subsequently induced on these activated cells [29]. In addition, it has been shown in vitro that costimulation by 4-1BB is able to restore CD28 expression [39], while GITR was described to lower the threshold for CD28 costimulation in mouse CD8+ T cells [40]. On the other hand, LIGHT was shown to participate in T cell activation in a CD28-independent manner [41] whereat up-regulation of 4-1BB could be expected. Indeed, an improved antitumor effect was described by the combined application of LIGHT and 4-1BBL in a vaccination approach with ligand-coated tumor cells, supporting the importance of co-display on the same cell surface for maximum efficacy [16].
Thus, for comprehensive costimulatory ligand combination assessment, spatiotemporal considerations need to be taken into account. Our data from the time-shift setting indicate that costimulation by B7.1-Db and scFv-4-1BBL is most effective when applied during scDb-mediated restimulation. Here, costimulation showed not only the ability to further raise the proliferation and cytotoxic potential of T cells in support of repeated CD3-induced T cell stimulation, it also showed to counteract the scDb-driven enhancement of T cells expressing the inhibitory receptors PD-1, commonly associated with immune restrain. PD-1 is expressed on antigen-experienced T cells in the periphery to limit their activity during inflammatory response and autoimmunity [42]. Up-regulation of its ligand PD-L1 in tumors has been identified as a mechanism of immune evasion [43]. The importance of this checkpoint receptor in the context of cancer immunotherapy is reflected by the fact that several antagonistic antibodies are in clinical trials [44].
We showed here in a B16 lung metastasis tumor mouse model that the application of the costimulatory antibody-fusion proteins could enhance the antitumor effect of the bispecific antibody in vivo, providing evidence for the benefit of such a combinatorial approach. This suggests that even powerful tumor-directed polyclonal T cell activation can benefit from additional costimulation. On the other hand, metastases reduction, although to a much lesser extent was observed for the treatment with the costimulatory fusion proteins only, indicating also an enhancement of the endogenous tumor-specific immune response in this rather poorly immunogenic model. Thus, the potential to enforce a broad spectrum of cancer immunotherapeutic strategies could be expected. However, extensive in vivo studies will be required to understand in detail the underlying immunological mechanisms.
By now, the antitumor potential of individual costimulation has been widely acknowledged. Nevertheless, it has also become apparent that it might take combinatorial approaches to fully exploit this strategy. As we could corroborate in this study, the evaluation of configurations and spatiotemporal considerations are crucial. Thus, addressing these issues by establishing experimental settings with costimulatory antibody–ligand fusion proteins constitutes a valuable contribution to the field.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a Grant from the Deutsche Krebshilfe (108743). We would like to thank Robert Lindner for technical support on the high‐performance liquid chromatography analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- GITRL
Glucocorticoid-induced TNF receptor ligand
- LIGHT
Homologous to lymphotoxins, shows inducible expression and competes with herpes simplex virus glycoprotein D for herpesvirus entry mediator (HVEM), a receptor expressed by T lymphocytes
- TNFSF
Tumor necrosis factor superfamily
- mAb
Monoclonal antibody
- FAP
Fibroblast activation protein
References
- 1.Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med. 2012;209(2):201–209. doi: 10.1084/jem.20112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol. 2012;24(2):217–224. doi: 10.1016/j.coi.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melero I, Martinez-Forero I, Dubrot J, Suarez N, Palazón A, Chen L. Palettes of vaccines and immunostimulatory monoclonal antibodies for combination. Clin Cancer Res. 2009;15(5):1507–1509. doi: 10.1158/1078-0432.CCR-08-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda K, Kojima Y, Uno T, Hayakawa Y, Teng MW, Yoshizawa H, Yagita H, Gejyo F, Okumura K, Smyth MJ. Combination therapy of established tumors by antibodies targeting immune activating and suppressing molecules. J Immunol. 2010;184(10):5493–5501. doi: 10.4049/jimmunol.0903033. [DOI] [PubMed] [Google Scholar]
- 5.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, Sekaly RP, Ostrowski M, Bernard NF, Watts TH. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4–1BBL. J Immunol. 2005;175(10):6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 6.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6(4):528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 7.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12(6):693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 8.Kocak E, Lute K, Chang X, May KF, Jr, Exten KR, Zhang H, Abdessalam SF, Lehman AM, Jarjoura D, Zheng P, Liu Y. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66(14):7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 9.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4–1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS ONE. 2011;6(4):e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7–H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- 11.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229(1):126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7(2):95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 13.Murphy KA, Lechner MG, Popescu FE, Bedi J, Decker SA, Hu P, Erickson JR, O’Sullivan MG, Swier L, Salazar AM, Olin MR, Epstein AL, Ohlfest JR. An in vivo immunotherapy screen of costimulatory molecules identifies Fc-OX40L as a potent reagent for the treatment of established murine gliomas. Clin Cancer Res. 2012;18(17):4657–4668. doi: 10.1158/1078-0432.CCR-12-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechner MG, Russell SM, Bass RS, Epstein AL. Chemokines, costimulatory molecules and fusion proteins for the immunotherapy of solid tumors. Immunotherapy. 2011;3(11):1317–1340. doi: 10.2217/imt.11.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanagavelu SK, Snarsky V, Termini JM, Gupta S, Barzee S, Wright JA, Khan WN, Kornbluth RS, Stone GW (2012) Vaccine. 30(4):691–702. doi:10.1016/j.vaccine.2011.11.088 [DOI] [PMC free article] [PubMed]
- 16.Sharma RK, Schabowsky RH, Srivastava AK, Elpek KG, Madireddi S, Zhao H, Zhong Z, Miller RW, Macleod KJ, Yolcu ES, Shirwan H. 4–1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res. 2010;70(10):3945–3954. doi: 10.1158/0008-5472.CAN-09-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyzgol A, Müller N, Fick A, Munkel S, Grigoleit GU, Pfizenmaier K, Wajant H. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J Immunol. 2009;183(3):1851–1861. doi: 10.4049/jimmunol.0802597. [DOI] [PubMed] [Google Scholar]
- 18.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37(5):508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu A, Hu P, Khawli LA, Epstein AL. B7.1/NHS76: a new costimulator fusion protein for the immunotherapy of solid tumors. J Immunother. 2006;29(4):425–435. doi: 10.1097/01.cji.0000208260.80791.3d. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, Jiang W, Yang M, Guo H, Zhang Y, Wang J, Zhu H, Shi R, Fan D, Yang C, Zhu Z, Xie Y, Xiong D. Efficient inhibition of human B-cell lymphoma in SCID mice by synergistic antitumor effect of human 4–1BB ligand/anti-CD20 fusion proteins and anti-CD3/anti-CD20 diabodies. J Immunother. 2010;33(5):500–509. doi: 10.1097/CJI.0b013e3181d75c20. [DOI] [PubMed] [Google Scholar]
- 21.Burckhart T, Thiel M, Nishikawa H, Wüest T, Müller D, Zippelius A, Ritter G, Old L, Shiku H, Renner C. Tumor-specific crosslinking of GITR as costimulation for immunotherapy. J Immunother. 2010;33(9):925–934. doi: 10.1097/CJI.0b013e3181f3cc87. [DOI] [PubMed] [Google Scholar]
- 22.Hornig N, Kermer V, Frey K, Diebolder P, Kontermann RE, Müller D. Combination of a bispecific antibody and costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. J Immunother. 2012;35(5):418–429. doi: 10.1097/CJI.0b013e3182594387. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N, Sadun RE, Arias RS, Flanagan ML, Sachsman SM, Nien YC, Khawli LA, Hu P, Epstein AL. Targeted and untargeted CD137L fusion proteins for the immunotherapy of experimental solid tumors. Clin Cancer Res. 2007;13(9):2758–2767. doi: 10.1158/1078-0432.CCR-06-2343. [DOI] [PubMed] [Google Scholar]
- 24.Müller D, Trunk G, Sichelstiel A, Zettlitz K, Quintanilla M, Kontermann RE. Murine endoglin-specific single-chain Fv fragments for the analysis of vascular targeting strategies in mice. J Immunol Methods. 2008;339(1):90–98. doi: 10.1016/j.jim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Völkel T, Müller R, Kontermann RE. Isolation of endothelial cell-specific human antibodies from a novel fully synthetic scFv library. Biochem Biophys Res Commun. 2004;317(2):515–521. doi: 10.1016/j.bbrc.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 26.Gerspach J, Muller D, Munkel S, Selchow O, Nemeth J, Noack M, Petrul H, Menrad A, Wajant H, Pfizenmaier K. Restoration of membrane TNF-like activity by cell surface targeting and matrix metalloproteinase-mediated processing of a TNF prodrug. Cell Death Differ. 2006;13(2):273–284. doi: 10.1038/sj.cdd.4401735. [DOI] [PubMed] [Google Scholar]
- 27.Liao KW, Lo YC, Roffler SR. Activation of lymphocytes by anti-CD3 single-chain antibody dimers expressed on the plasma membrane of tumor cells. Gene Ther. 2000;7(4):339–347. doi: 10.1038/sj.gt.3301080. [DOI] [PubMed] [Google Scholar]
- 28.Cochran WG, Cox GM. Experimental designs. 2. Oxford: Wiley; 1992. [Google Scholar]
- 29.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 30.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27(1):19–26. doi: 10.1016/S0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 31.Bremer E, de Bruyn M, Wajant H, Helfrich W. Targeted cancer immunotherapy using ligands of the tumor necrosis factor super-family. Curr Drug Targets. 2009;10(2):94–103. doi: 10.2174/138945009787354593. [DOI] [PubMed] [Google Scholar]
- 32.Wajant H, Gerspach J, Pfizenmaier K. Tumor therapeutics by design: targeting and activation of death receptors. Cytokine Growth Factor Rev. 2005;16(1):55–76. doi: 10.1016/j.cytogfr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Müller N, Wyzgol A, Münkel S, Pfizenmaier K, Wajant H. Activity of soluble OX40 ligand is enhanced by oligomerization and cell surface immobilization. FEBS J. 2008;275(9):2296–2304. doi: 10.1111/j.1742-4658.2008.06382.x. [DOI] [PubMed] [Google Scholar]
- 34.Chattopadhyay K, Lazar-Molnar E, Yan Q, Rubinstein R, Zhan C, Vigdorovich V, Ramagopal UA, Bonanno J, Nathenson SG, Almo SC. Sequence, structure, function, immunity: structural genomics of costimulation. Immunol Rev. 2009;229(1):356–386. doi: 10.1111/j.1600-065X.2009.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stöckl J, Herndler-Brandstetter D, Grubeck-Loebenstein B, Reipert BM, Pickl WF, Pfistershammer K, Steinberger P. The capacity of the TNF family members 4–1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38(10):2678–2688. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 37.Melero I, Bach N, Hellström KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4–1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28(3):1116–1121. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Wu X, Zhang F, Li X, Sun B, Yu Y, Yin A, Deng L, Yin J, Wang X. Triple expression of B7–1, B7–2 and 4–1BBL enhanced antitumor immune response against mouse H22 hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;137(4):695–703. doi: 10.1007/s00432-010-0905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habib-Agahi M, Jaberipour M, Searle PF. 4–1BBL costimulation retrieves CD28 expression in activated T cells. Cell Immunol. 2009;256(1–2):39–46. doi: 10.1016/j.cellimm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C. Glucocorticoid-induced TNFR-related protein lowers the threshold of CD28 costimulation in CD8 + T cells. J Immunol. 2007;179(9):5916–5926. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- 41.Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D, Boone T, Hsu H, Fu YX, Nagata S, Ni J, Chen L. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6(3):283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 42.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 43.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 44.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.