Abstract
We previously demonstrated that autologous dendritic cells that have endocytosed apoptotic bodies of chronic lymphocytic leukemia (CLL) cells (Apo-DC) can stimulate antileukemic T cell responses in vitro. In this phase I study, we vaccinated 15 asymptomatic CLL patients at five time points with Apo-DC administered intradermally either alone (cohort I), or in combination with subcutaneous granulocyte–macrophage-colony-stimulating-factor (GM-CSF) (cohort II) or with GM-CSF and intravenous low-dose cyclophosphamide (cohort III). Aim of the study was to evaluate the safety and immunogenicity of Apo-DC alone or in combination with GM-CSF and low-dose cyclophosphamide in CLL patients. All patients completed the vaccination schedule without dose-limiting toxicity. No objective clinical responses were seen. Vaccine-induced leukemia-specific immune responses were evaluated by IFN-γ ELISpot and proliferation assays over a 52 weeks observation period and immune response criteria were defined. According to these criteria, 10/15 patients were defined as immune responders. The frequency of immune-responding patients was higher in cohorts II (3/5) and III (5/5) than in cohort I (2/5). In order to further characterize the induced immune response, estimation of secreted cytokines and CD107-degranulation assay were performed. Clustering of T and CLL cells was observed in CD107-degranulation assay and visualized by confocal microscopy. Additionally, assessment of regulatory T cells (Tregs) revealed their significantly lower frequencies in immune responders versus non-responders (P < 0.0001). Cyclophosphamide did not reduce Tregs frequency. In conclusion, vaccination with Apo-DC + GM-CSF and cyclophosphamide was safe and elicited anti-CLL immune responses that correlated inversely with Tregs levels. Lack of clinical responses highlights the necessity to develop more potent vaccine strategies in B cell malignancies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1149-5) contains supplementary material, which is available to authorized users.
Keywords: CLL, Dendritic cells, Immunotherapy, Cyclophosphamide, Regulatory T cells, GM-CSF
Introduction
Chronic lymphocytic leukemia (CLL) is a B cell malignancy characterized by the progressive accumulation of B lymphocytes in blood, bone marrow, and lymphoid organs. Typically, patients are treated only upon disease progression. First-line combination chemoimmunotherapy produces complete remission in approximately 40% of patients, which is associated with a prolonged overall survival [1, 2]. However, patients may have a reduced tolerability to intensive combination treatments with advanced age or comorbid conditions [3]. There is no established therapy to prevent or delay disease progression, or for maintenance therapy in the response/plateau phase following chemotherapy. There is a great unmet medical need to develop such therapies in CLL.
Mature dendritic cells (DC) derived from cancer patients can process tumor-associated antigens (TAAs) correctly and display them on their surface [4]. In previous studies, [5, 6] we showed that apoptotic bodies of leukemic cells were superior to other approaches (tumor lysate, tumor RNA, cell fusion hybrids) for loading of whole tumor cells into autologous DC and selected as a vaccine for this explorative clinical trial.
The aims of the present study were to elucidate whether apoptotic bodies of autologous leukemic cells loaded onto DC (Apo-DC) could induce anti-tumor immunity in vivo and if granulocyte–macrophage-colony-stimulating-factor (GM-CSF) and low-dose cyclophosphamide (CTX) could enhance vaccine-induced immune responses. We defined criteria for immune response assessment, which might be applied in forthcoming leukemia vaccination studies.
Patients and methods
Study population and eligibility criteria
Patients with diagnosis of CLL [7], aged 18–80 years, asymptomatic, with no expected need for anti-tumor treatment within the next 6 months and able to undergo a leukapheresis were eligible. Patients must not have evidence of hepatic or renal disease or cardiovascular disease ≥ grade I according to American Heart Association criteria. Patients on concurrent NSAID therapy or corticosteroid therapy other than a maintenance dose of prednisone ≤10 mg/day were also excluded from the study.
The study was performed in keeping with the Helsinki declaration on research with human subjects and approved by the regional ethics committee. All patients provided written informed consent.
Preparation of the vaccine
The vaccine was produced at a certified GMP laboratory from a leukapheresis product in adherence to a previously validated protocol [8]. Briefly, CD14+ and CD19+ cells were isolated by immunomagnetic separation using the CliniMACS® affinity-based technology (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). B cells were cultured without cytokines and irradiated (5 Gy). CD14+ cells were cultured in CellGro serum-free DC-medium (CellGenix, Freiburg, Germany) supplemented with GM-CSF (100 ng/ml) (Leukine, BERLEX®, Seattle, WA) and IL-4 (20 ng/ml) (CellGenix, Freiburg, Germany). On day four, immature DC (imDC) and apoptotic B cells were mixed at a ratio of 2:1. Tumor necrosis factor alpha (TNF-α) (20 ng/ml) (CellGenix, Freiburg, Germany) was added on day 5 and the cells cultured for an additional 48 h. The cells (Apo-DC) were suspended in autologous heat-inactivated plasma with 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO), aliquoted and cryopreserved at −150°C until used for vaccination. The Apo-DC vaccines were tested for sterility and endotoxins using the Limulus test. All the vaccines produced (n = 17) were free from contaminants and detectable endotoxins.
Vaccination schedule
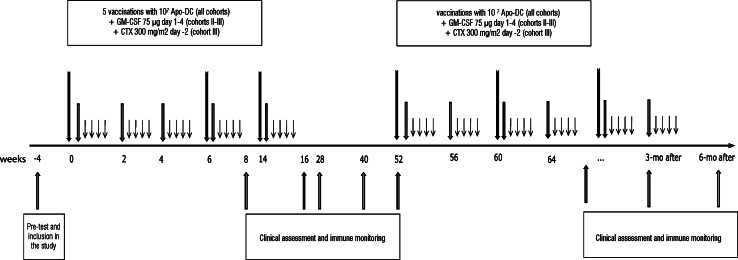
The patients were accrued in three consecutive cohorts, each consisting of 5 patients. Patients withdrawn from the study due to progression of disease before completion of the vaccination schedule (5 injections) were replaced. A schematic view of the study protocol is shown in Fig. 1. Cohort I received the Apo-DC vaccine alone as intradermal injection at day 1, weeks 0, 2, 4, 6, and 14. At each vaccination, a minimum of 107 viable Apo-DC suspended in 1 ml of sterile saline were administered in the upper left arm. The same site was used for all subsequent injections. Cohort II received the vaccine as above together with 75 μg/day of GM-CSF subcutaneously at the same site as the vaccine [9], for four consecutive days (1–4). Cohort III received the same treatment as cohort II but with the addition of CTX 300 mg/m2 intravenously at day −2 at week 0, 6, and 14 [10].
Fig. 1.
Schematic view of the study protocol. Patients received the Apo-DC vaccine as intradermal injection at day 1, weeks 0, 2, 4, 6, and 14 (middle arrows). Cohort II patients received the vaccine as above together with 75 μg/day of GM-CSF subcutaneously at the same site as the vaccine, for four consecutive days (1–4) (short arrows). Cohort III received the same treatment as cohort II but with the addition of CTX 300 mg/m2 intravenously at day −2 at week 0, 6, and 14 (long arrows)
The optimization of the vaccine production platform achieved during the study allowed producing a greater amount of vaccine for each individual patient. For this reason, patients who had no need of anti-tumor treatment at week 52 were offered to receive additional immunizations every fourth week as long as vaccine was available for a maximum of 1 year (“maintenance vaccination”). Maintenance vaccination was performed according to the same schedule the patients had followed in the first part of the study. Patients belonging to cohort II received therefore subcutaneous GM-CSF day 1–4 and patients belonging to cohort III received subcutaneous GM-CSF day 1–4 and CTX 300 mg/m2 intravenously at day −2 before every other vaccine administration.
Efficacy assessment and clinical response criteria
The patients were evaluated for clinical effects by physical examination and blood counts at weeks 8, 16, 28, 40, and 52. A CT scan of the thorax/abdomen was performed before vaccination and repeated at week 40 at the discretion of the physician. Clinical responses were assessed by the IWCLL response criteria [7].
Immune function testing
Isolation and enrichment of mononuclear cells for immune assays
Venous blood was collected in heparinized tubes at each immune test time point. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation on a Ficoll-Hypaque gradient (GE Healthcare, Uppsala, Sweden). PBMC were washed three times with phosphate-buffered saline (PBS), placed on a nylon wool column (Biotest, Breiech, Germany), and CD19neg cells were eluted from the column [11]. Effluent cells (CD19neg cells) were collected to be used as effector cells in the immune tests. In case the purity of this cell population was below 90% as determined by flow cytometry, further immunomagnetic depletion with anti-CD19 Microbeads (Miltenyi Biotec) was performed. The viability check of the effector cell population was carried out prior to each test occasion and found to be above 95%.
Activated CLL cells were generated before vaccination start by coculturing autologous CLL cells on hCD40L-transfected NIH-3T3 fibroblasts [12] for 48 h. Activated CLL cells were cryopreserved at a concentration of 25 × 106 cells/ml in RPMI medium with 10% human AB+ serum and 10% DMSO at −150°C until thawed to be used as targets for immune tests. Mean viability after thawing was 90%. Prior to use in the assays, targets were subject to 50 Gy irradiation using a GAMMA CELL 2000 device (Molsgaard Medical, Horsholm, Denmark) to get them into apoptosis.
Lymphocyte proliferation assay
2 × 104 activated CLL cells were added to 1 × 105 autologous freshly isolated effector cells in a 96-well U-bottom plate in quadruplicates in RPMI with 10% human AB+ serum, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin and incubated at 37°C for 6 days in humidified air with 5% CO2 atmosphere. Effector cells stimulated with phytohemagglutinin (PHA) (10 μg/ml) served as a positive control. Effectors cells cultured in the absence of the targets served as negative control.
One μCi methyl-3H-thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) was added to each well for the final 18 h. On day 6, cells were harvested by an automatic cell harvester (Skatron, Lier, Norway). The incorporated radioactivity was measured in a beta-scintillation counter (Wallac, Turku, Finland). The results were expressed as counts per minute (CPM)/105 effector cells. The coefficient of variation (CV) for the assay performance was calculated to be 34%. The values outranging the mean ± CV were considered as outliers and excluded from the analysis. The results are expressed as the mean of quadruplicates excluding the outliers. Counts were corrected for background incorporation of radioactivity by effector cells in the absence of any stimulation (netCPM). For all the assays performed (n = 138), the median background was 108 CPM/105 effector cells. The background incorporation of target cells cultured alone was insignificant (data not shown). PBMC from a healthy donor were included in each experiment as a control.
Cytokine secretion assay
After 5 days of culture, 75 μl of the supernatant from each well of the proliferation assay was collected and stored at −70°C until analyzed. The supernatants were assayed for 7 different cytokines (IL-2, IL-4, IL-5, IL-10, GM-CSF, IFN-γ, and TNF-α) using the Bio-Plex Pro Tm Cytokine Reagent Kit and the Exp Hu Cyto Grp 1, 7-plex (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions.
ELISPOT assay
Nitrocellulose membrane bottomed-plates (Millipore, Bedford, MA) were coated at 4°C overnight with a mouse anti-human IFN-γ mAb, clone 1-D1 K (10 μg/ml; Mabtech AB, Stockholm, Sweden). After removal of the coating solution, the plates were washed 3 times in Tris-buffered saline (TBS) and 1 × 105 autologous freshly isolated purified effector cells were added and incubated at 37°C for 20 h with 2 × 104 CD40L-stimulated autologous B cells in RPMI with 10% FBS, 2 mM l-glutamine, 100 IU/ml penicillin and 100 μg/ml streptomycin. T cells stimulated with PHA (10 μg/ml) served as a positive control.
The cells were then washed away with PBS and the plates incubated for 2 h at room temperature with 1 μg/ml biotin-conjugated anti-human IFN-γ 7B6-1 mAb (Mabtech).
After washing, streptavidin-ALP (Mabtech) was added, and the plates were incubated for 1 h at room temperature, washed and developed with BCIP/NBT Plus (Mabtech). The number of spots was determined using the AID ELISPOT reader (Autoimmun Diagnostika, Strasburg, Germany). In every experiment, PBMC from a healthy donor were run in parallel. The results are expressed as spot-forming cells (SFC)/105 effector cells. Background spot counts from effector cells without any stimulation and of target cells cultured alone were subtracted from experimental values. The results are expressed as the mean of quadruplicates. For all the assays performed (n = 131), the median background was 0,75 SFC/105 effector cells. With the exception of one patient (Pt III-05) for whom a high background of target cells cultured alone was observed in all the tests, the background of target cells cultured alone was insignificant (data not shown).
Tregs analysis
The percentage of regulatory T cells (Tregs) was evaluated by intracellular hFOXP3 staining of purified T cells using a staining kit including PE-labeled anti-human FOXP3 and a APC-labeled anti-hCD25/FITC-labeled anti-hCD4 antibody cocktail (eBioscience, San Diego, CA) following manufacturer’s instructions.
CD107 degranulation assay
1 × 105 autologous freshly isolated purified effector cells were added and incubated at 37°C 5% CO2 atmosphere with 2 × 105 CD40L-stimulated autologous B cells (effector/target cell ratio = 1:2) in 200 μl RPMI with 10% FBS. Spontaneous degranulation was determined using effector cells alone.
Ten μl of a mix of FITC–conjugated CD107a/CD107b mAb (BD Bioscience) was added to each well containing the B/effector cell mixture. Monesin was added to a final concentration of 0.1%. After 5 h coculture, cells were washed in PBS and stained with mAbs for flow cytometry (CD3, CD19, and either a CD4/CD8 mix or a CD16/CD56 mix, respectively, as well as AF700 and PE isotype controls).
T cells were characterized by staining with PE-labeled anti-CD4, AF700-labeled anti-CD8a, APC-labeled anti-CD3, and PE-labeled anti-CD19 (BioLegend, San Diego, CA).
Natural killer (NK) cells were characterized by staining samples with PE-labeled anti-hCD56, AF700-labeled anti-hCD16, APC-labeled anti-hCD3, and PE-labeled anti-CD19 (all from BioLegend). The frequency of CD107 positivity in the CD8+, CD4+, and CD16+CD56dim populations, respectively, were normalized for the proportion of cells in the parent populations.
Immunofluorescence and confocal microscopy image acquisition
CD40L-stimulated autologous B cells and freshly isolated effector cells were cocultured at an E/T cell ratio of 1:2 after staining with Cell Tracker CM-Dil (Invitrogen Molecular Probes, Eugene, OR) and carboxyfluorescein diacetate succinimidyl ester (CFSE) (Sigma-Aldrich, St. Louis, MO), respectively. Briefly, purified B cells were resuspended in RPMI with 5% FBS and incubated with CM-Dil for 5 min at room temperature and subsequently for 15 min at +4°C. Cells were then washed twice with RPMI with 5% FBS. For labeling of effector cells, the cells were resuspended in PBS with 5% FBS and incubated with 2 μM CFSE as described [13]. After labeling, effector and target cells were coincubated for 5 h at 37°C, harvested, washed and seeded on poly-l-lysine microscope slides (Polyscience, Warrington, PA). The slides were incubated for 30 min at +4°C, washed with PBS and fixed with 4% PFA for 15 min at room temperature. Fixed cells were incubated for 5 min at +4°C with 1 μM of TO-PRO (Invitrogen Molecular Probes, Eugene, OR) for staining of the nuclei. The specimens were then mounted on Vectashield H-1000 reagent (Vector Laboratories, Burlingame, CA) and covered with coverslips (Polyscience, Warrington, PA). Medial optical section images were captured by laser scanning using a Leica TCS SP5 confocal microscope.
Statistical methods
Statistical analyses were conducted using the JMP software 5.1.2 and the StatView software 5.0.1 (SAS Institute, Cary, NC, USA). Comparisons of numerical variables between two groups of patients were done with the non-parametric Mann–Whitney’s U test. The non-parametric Kruskal–Wallis test was used for cohort analyses. Comparison of numerical variables measured at ≥2 time points in the same individual was done with paired t test. Simple regression analysis was used to examine the relationship between the frequency of Tregs and proliferation netCPM values.
Univariate survival curves were generated using the Kaplan–Meier method. A P value <0.05 was considered statistically significant; all tests were 2-sided.
Immune monitoring
Tumor-specific immune responses were measured before vaccination (mean of two pre-tests) and at weeks 8, 16, 28, 40, and 52 in all patients. During maintenance vaccination, tumor-specific immune responses were measured every 3 months up to 6 months after the last vaccination.
Definition of tumor-specific and vaccine-induced tumor-specific immune response
For the proliferation assay, CPM in experimental wells was compared with that of control wells (effector cells alone). A P value <0.05 (Mann–Whitney's U test) was considered a tumor-specific immune response. For the ELISPOT assay, the same statistics were applied. A vaccine-induced immune response was defined if all of the following criteria were met: (a) presence of a tumor-specific immune response in either proliferation or ELISPOT assay as defined above; (b) statistically significant increase in cpm (proliferation) or number of spots (ELISPOT) in experimental wells at the follow-up time point vs pre-vaccination values (Mann–Whitney’s U test); (c) ≥2-fold increase in immune response compared with pre-vaccination values at ≥1 time points. Such criteria were defined prior to the initiation of the study.
Results
Patient characteristics
Between June 2005 and October 2009, 16 CLL patients were included in the study. Of these, 15 completed the vaccination schedule receiving 5 vaccine doses. Patient III-02 was withdrawn after the first four injections due to disease progression. One additional patient (I-06) was initially enrolled in the study and vaccine prepared when routine screening detected hepatitis C virus (HCV) positivity. Following consultation with infectious disease specialists, the decision was made to vaccinate the patient off-protocol as the patient wanted to remain in the study. Patient I-06 was vaccinated according to a separate schedule (details in Fig. S3, available on-line). The patient never received antiviral therapy and the HCV viral load remained unchanged at a 4-year follow-up.
Patient demographics, baseline characteristics and clinical outcome of the 15 evaluable patients and of patient I-06 are presented in Table 1.
Table 1.
Patient characteristics and clinical effects during and after Apo-DC vaccination
| Cohort-patient | Age, years/sex | Modified Rai stage | IgHV mutational status | Previous treatment, (response/response duration) | Time since last Cht (months) | Lymph count at start of vaccination × 109/l | Clinical response at week 28 (lymph count × 109/l) | Time-to-progression (months) | Time to next anti-CLL treatment (months) | Post-vaccination therapy | Status at last follow-up, OS after first vaccination (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Immune-responding patients | |||||||||||
| I-01 | 64/M | Intermediate risk | Mutated | 3 months chlorambucil (PR/18 months), 2 months chlorambucil (PR/22 months) | 22 | 51.7 | SD (54.6) | 14 | 14 | Cladrabine (rediagnosed as HCL 2006), Fludarabine + CTX | NA, 68+ |
| I-05 | 65/F | Intermediate risk | Mutated | None | 22.5 | SD (25.1) | 30 | 34 | Fludarabine + CTX | CR, 60+ | |
| I-06* | 65/M | Low risk | Mutated | None | 12.4 | SD (9.8) | Not progressed | Not reached | No additional tx | Dead, 47 (hepatitis) | |
| II-01 | 72/M | Intermediate risk | Mutated | None | 42.4 | SD (51.1) | 12 | Not reached | No additional tx | PD (asympt.), 52+ | |
| II-02 | 51/M | Intermediate risk | Mutated | None | 65.8 | SD (95.8) | 12 | 33 | COP, Rituximab, Splenectomy, Hyper C-VAD Ara-C | PR, 50+ | |
| II-05 | 66/F | Intermediate risk | Mutated | None | 20.5 | SD (23.9) | 10 | Not reached | Maintenance vaccination started at month 13 and continued for 10 months | PD (asympt.), 47+ | |
| III-01 | 43/F | Intermediate risk | Mutated | None | 77.5 | SD (82.5) | 30 | Not reached | Maintenance vaccination started at month 13 and continued for 10 months | SD, 30+ | |
| III-03 | 66/M | Low risk | Mutated | None | 28.3 | SD (24.7) | Not progressed | Not reached | Maintenance vaccination started at month 13 and continued for 7 months | SD, 28+ | |
| III-04 | 62/F | Intermediate risk | Mutated | None | 36.8 | SD (27.9) | Not progressed | Not reached | Maintenance vaccination started at month 13 and continued for 6 months | SD, 28+ | |
| III-05 | 51/F | Low risk | Unmutated | None | 19.8 | PD (40) | 7 | Not reached | Maintenance vaccination started at month 13 and continued for 6 months | PD (asympt.), 23+ | |
| III-06 | 64/M | Intermediate risk | Mutated | None | 26.9 | SD (25.1) | Not progressed | Not reached | Maintenance vaccination (after week 52) ongoing | SD, 17+ | |
| Non-immune-responding patients | |||||||||||
| I-02 | 79/M | Intermediate risk | Mutated | 6 months chlorambucil/prednisone (PR/12 months) | 6 | 96.1 | PD (105) | 2 | 21 | Fludarabine + CTX, alemtuzumab | Dead, 53 |
| I-03 | 73/M | High risk | Mutated | None | 15.1 | PD (29) | 4 | 9 | Chlorambucil, Imatinib (CML 2006), Rituximab (WM 2008) | NA, 63+ | |
| I-04 | 64/F | Intermediate risk | Unmutated | None | 26.6 | SD (29.6) | 27 | Not reached | No additional tx | PD (asympt.), 61+ | |
| II-03 | 76/M | Intermediate risk | Mutated | 3 months chlorambucil (PR/12 months), 2 months chlorambucil (PR/15 months), 2 months chlorambucil (PR/10 months) | 15 | 9.0 | SD (11.9) | 12 | Not reached | No additional tx | Dead, 34 (Sarcoma diagnosed June-08) |
| II-04 | 67/M | Intermediate risk | Mutated | 6 months chlorambucil (CR/5 years) | 58 | 3.6 | SD (4.8) | 22 | Not reached | Maintenance vaccination started at month 15 and continued for 7 months | PD (asympt.), 48+ |
Cht chemotherapy, OS overall survival, CTX Cyclophosphamide, VCR vincristine, COP cyclophosphamide, vincristine, prednisone, Hyper C-VAD Ara-C Cyclophosphamide, Vincristine, Doxorubicin, Ara-C, SD stable disease, PD progressive disease, CR complete response, PR partial response, tx therapy, RT radiotherapy, CML chronic myeloid leukemia, WM Waldenström’s macroglobulinemia, NA not applicable, asympt asymptomatic, NE not evaluable
* Vaccinated on individual schedule, see “Patients and methods”
Pt I-01 was re-classified as atypical hairy cell leukemia after completion of the trial. In total, seven patients received maintenance vaccination, two in cohort II and five in cohort III (details in Table 1).
Quality control of vaccine preparations
Details of the cellular vaccine production from the first ten patients (cohorts I and II) have been reported in detail elsewhere [8]. Briefly, CD14+ selection resulted in a highly enriched cell population 93 ± 1.7% (mean ± SEM) (n = 16). Flow cytometric analysis of the Apo-DC showed a mature DC phenotype with the expression of CD80 (94.5 ± 2%), CD86 (91.9 ± 1.9%), CD83 (71.5 ± 4.9%), DC-SIGN (87.1 ± 4.3%), and CD1a (44 ± 6.7%). The expression of other markers was as follows: ILT-3 (31.1 ± 5.5%), HLA-DR (94.8 ± 1.7%), CD14/CD45 (19.6 ± 6.9%), CD20 (1 ± 0.2%), and CCR-7 (18 ± 6%).
Cell viability and recovery of thawed Apo-DC was 96 ± 0.2% and 98 ± 4.7% (mean ± SEM) (n = 101), respectively, with no significant differences between the three cohorts. The median total number of Apo-DC received by each patient in cohort I was 67 × 106 cells (range: 35–89) (n = 5); in cohort II 88 × 106 (range: 76–120) (n = 5), and 83 × 106 (range: 57–97) in cohort III (n = 6). The mean number of Apo-DC administered during the induction phase at each vaccination was 13 ± 0.3 × 106 (n = 25) in cohort I, 18 ± 0.4 × 106 (n = 26) in cohort II, and 17 ± 0.9 × 106 (n = 28) in cohort III; during maintenance, it was 16 ± 0.5 × 106 (n = 34). Patient I-06 received a total of 195 × 106 Apo-DC.
Safety
In cohort I, only one patient had a grade 1 injection-site reaction after the first vaccination. In cohort II and III, grade 2 injection-site reactions occurred in all patients following GM-CSF administration. Swelling and erythema were seen in all patients, pruritus in 8/11, and local pain in 2/11 patients. All reactions were transient, did not require medication and resolved within 48 h. One patient (II-03) had grade 2 fevers and grade 1 chills after the first vaccination. The dose of GM-CSF was reduced to one–third, and at the second vaccination, grade 1 fever occurred. The six patients who received maintenance vaccination had more intense injection-site reactions ≤ grade 2. Pt I-06 had grade 2 erythema when GM-CSF was added. No cumulative toxicities were observed during the induction or the maintenance phase. Pt I-03 was diagnosed with chronic myeloid leukemia (CML) 1 year after inclusion in the trial.
Vaccine-induced immune responses
All patients had antileukemic immune reactivity before vaccination detectable either in the proliferation (14/15) or in the ELISPOT assay (12/15) or in both (11/15). Such leukemia-specific immune response persisted in the majority of the patients at the majority of the time points after vaccination.
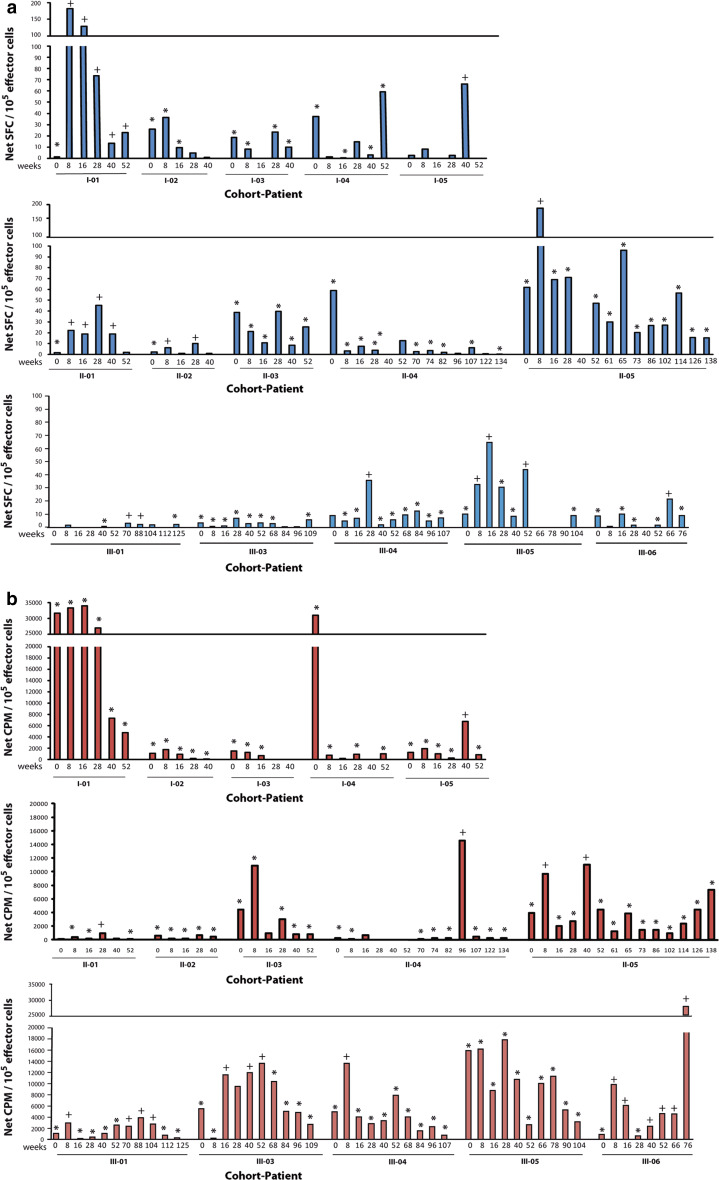
The vaccine-induced immune responses during the induction and the maintenance vaccination and follow-up are shown in Fig. 2. Collectively, 10/15 patients demonstrated increased antileukemic immune reactivity post-vaccination, 2/5 in cohort I, 3/5 in cohort II, and 5/5 in cohort III. Patients I-04 and II-04 experienced a significant decrease in tumor-specific immune response during follow-up. Higher concentrations of IL-2, IL-5, IL-10, IFN-γ, GM-CSF were noted in immune responders while higher IL-4 and TNF-α were in immune non-responders (Luminex assays). Significantly, higher through values (all cytokine secretion values over time) were seen in immune responders for IL-2 and IFN-γ (P = 0.003 and 0.0009, respectively, Mann–Whitney’s U test), while marginally significant values for TNF-α were observed in immune non-responders (Fig. S1, available on-line).
Fig. 2.
CLL-specific immune responses in cohort I, II, and III. a IFN-γ ELISPOT and b lymphocyte proliferation assay in the individual patients comparing pre-vaccination and follow-up. * indicates leukemia-specific immune response; (+) indicates response meeting the criteria for a vaccine-induced immune response (see “Patients and methods” for definition). SFC spot-forming cells, CPM count per minute
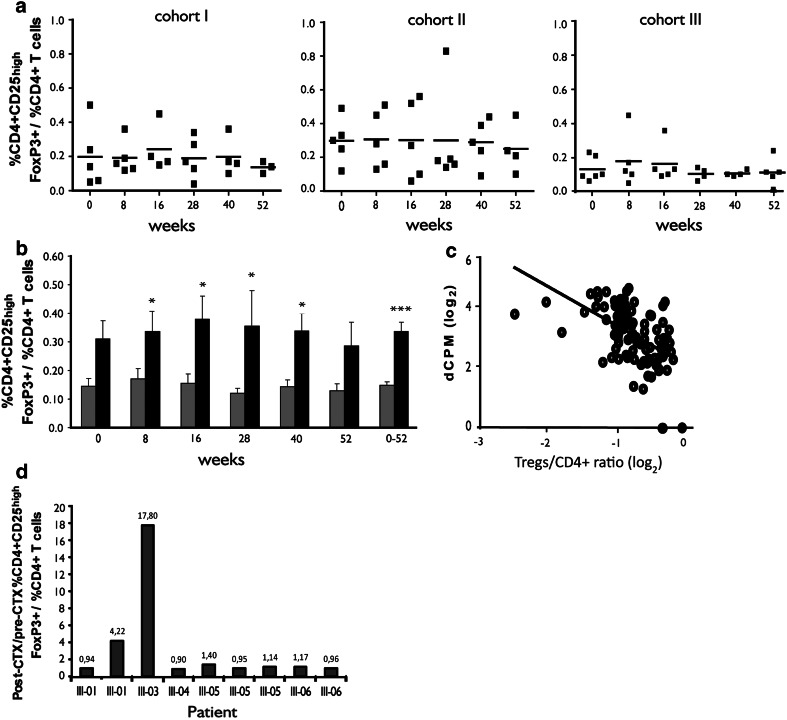
The frequency of CD4+CD25highFOXP3+ T cells (Tregs) over time in the different cohorts is shown in Fig. 3a. No statistically significant difference between the three cohorts at baseline or during the follow-up was noted (Kruskal–Wallis test). However, significantly lower levels of Tregs during the first year of follow-up (P < 0.0001, Mann–Whitney’s U test) were noted in immune responders compared with immune non-responders (Fig. 3b). Tregs levels correlated inversely to the proliferative response (Pearson’s r = −0.51, P < 0.0001; Fig. 3c). Vaccine-induced immune responses in patient I-06 are showed in Fig. S3 (available on-line). The frequency of Tregs in this patient was low during the whole follow-up.
Fig. 3.
CD4+CD25highFOXP3+ T cells. a Frequency of Tregs in cohorts I, II, and III (lines represent mean values). b Immune-responding (gray columns) and immune non-responding patients (black columns) (mean ± SEM) patients, (*P < 0.05; *** P < 0.0001). c Correlation between frequency of CD4+CD25highFOXP3+ T cells and proliferation netCPM values in all the 15 vaccinated patients tested at all time points during both the 52 weeks follow-up and the maintenance vaccination follow-up (n = 109) (Pearson's r = −0.51; P < 0.0001). d. Ratio between the frequency of CD4+CD25high FOXP3+ T cells at day 10 after a CTX administration (post-CTX) and the frequency of CD4+CD25highFOXP3+ T cells prior to CTX administration (pre-CTX). Columns represent individual patients in cohort III tested at different time points (n = 9)
We also tested the effect of CTX administration on Tregs by comparing Tregs levels before CTX administration and 10 days later. At 7/9 testing times, the levels remained stable and increased in 2/9 (Fig. 3d).
CD8+ and CD4+ T cells degranulation was detected in 11/11 patients in whom the test was performed at ≥ 2 time points. All the patients (n = 7) in whom baseline values were available had an increased frequency of CD8+CD107+ and CD4+CD107+ cells after vaccination; 5/7 had an increased frequency of CD16+CD56dimCD107+. Such increase did not reach statistical significance (paired t test). Individual patient data are reported in Fig. S2 (available on-line).
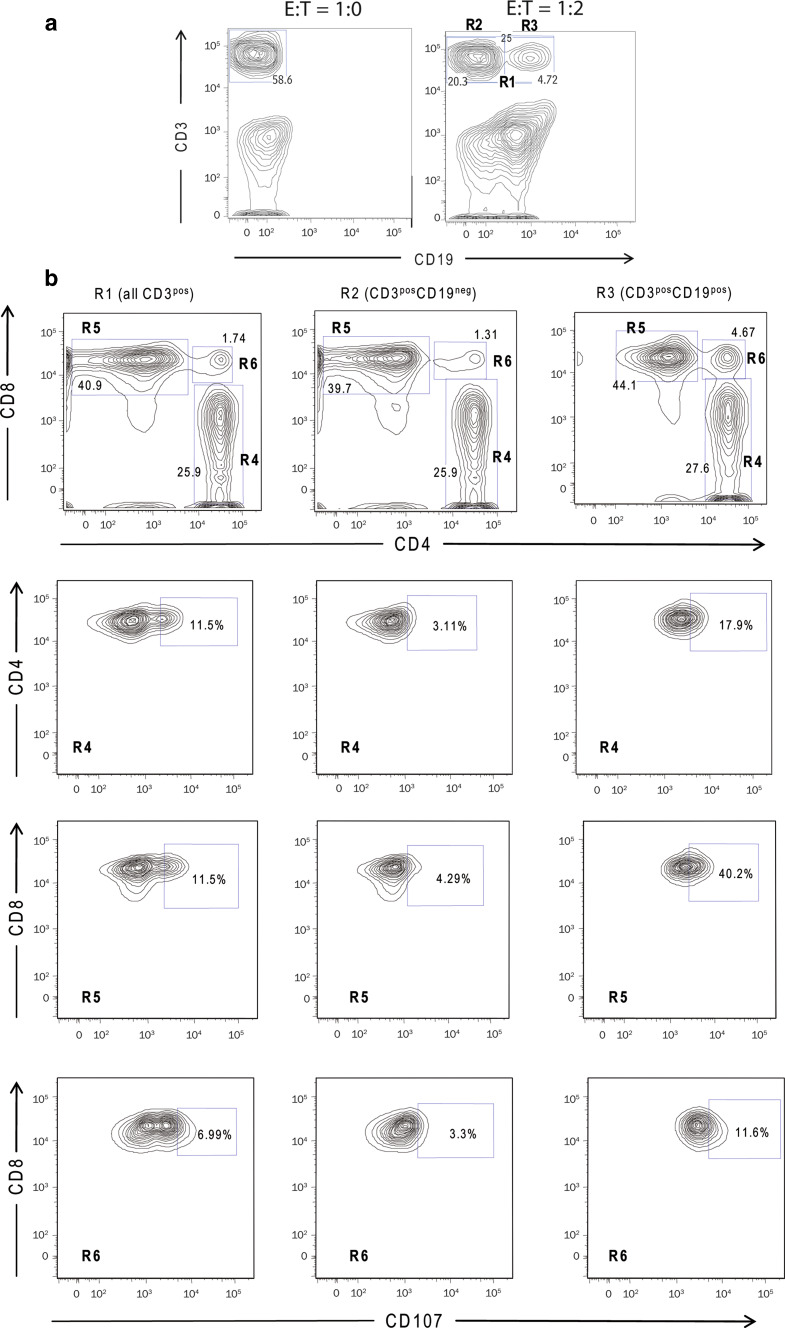
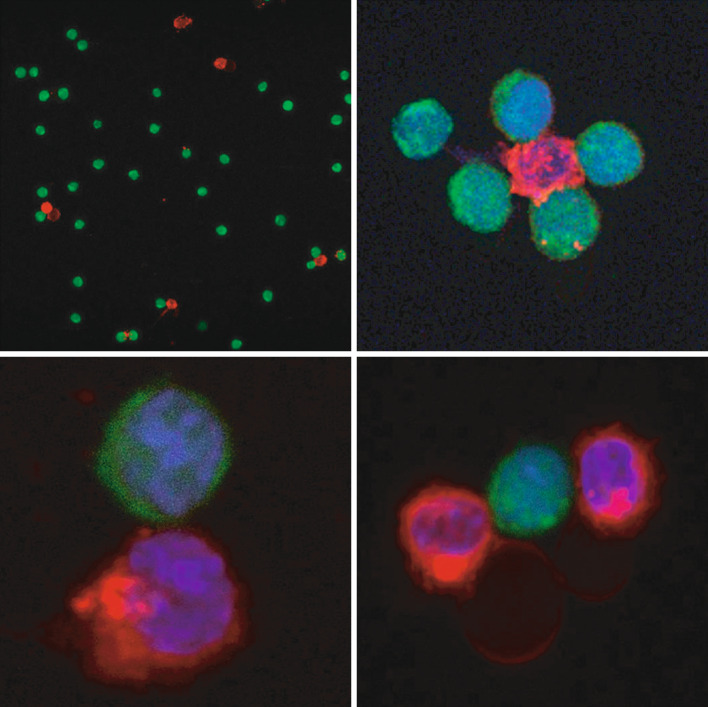
In all patients, a double-positive CD3+CD19+ cell fraction was noted in all in vitro tests at an E/T cell ratio of 1:2 (Fig. 4a). In this fraction, the frequency of degranulating T cells was high, indicating that the double positivity may represent T/B cell aggregates, including T cells with a cytotoxic capability (Fig. 4b). The presence of such aggregates and destruction of the leukemic targets was confirmed by confocal microscopy (Fig. 5).
Fig. 4.
A representative experiment of CD107 degranulation assay in immune-responding patient III-04 (week 52). a Cell populations detected in E/T cell ratio 1:2 versus E/T cell ratio 1:0. b Frequency of degranulating CD4+, CD8+, and CD4+CD8+ T cells in the CD3pos (gate R1), CD3posCD19neg (gate R2), and CD3posCD19pos (gate R3) populations
Fig. 5.
CLL cells (red)–effector T cells (green) aggregates after 5 h of coculture as assessed by immunofluorescence and confocal microscopy. The staining was performed in parallel with the CD107 degranulation assay shown in Fig. 4
Efficacy
The clinical outcome of the patients is reported in Table 1. The leukemic cell count remained stable in most patients during the study and no patient (except Pt I-06, see below) fulfilled the IWCLL criteria for partial response [7]. The lymphocyte doubling time was not affected in evaluable patients (data not shown). The median time-to-progression (TTP) in immune responders and immune non-responders was similar (14 and 12 months, respectively). Patient I-06 had a gradual reduction in the lymphocyte count during the vaccination period (Fig. S3, available on-line) and achieved a nodular partial remission in the bone marrow.
Discussion
Anecdotal reports of spontaneous remissions of CLL [14] as well as established “graft-versus-leukemia” effect after allogeneic hematopoietic stem cells transplant or donor lymphocyte infusion [15–18] may indicate that CLL is responsive to immune effector functions. Furthermore, natural occurring T cells specifically recognizing CLL cells have been reported [19–24].
Non-progressive CLL should present an optimum disease setting for testing active (i.e., vaccine) immunotherapy approaches. The tumor burden is low and the indolent course may allow sufficient time for the induction of an effective immune response. Moreover, immune functions may be better preserved in this setting than during the progressive phase or following initiation of immunosuppressive anti-tumor therapy.
The use of whole tumor cells as a vaccine has the potential advantage of targeting the complete repertoire of TAAs. In CLL, such cells are easily accessible facilitating the production of a personalized cell-based vaccine.
The induction of an effective immune response in CLL might be hampered by the underlying immune dysfunction related to the disease [25] and aggravated by anti-cancer treatments [26]. A successful vaccination should therefore combine measures to improve antigen presentation and strategies to restore immune functions.
During the last decades, a large number of clinical trials have explored the possibility to induce an immune response against cancer cells by vaccination. Collectively, these trials showed that immunotherapy is safe with low toxicity, but most of them have failed to show clinical anti-tumor effects. The majority of the trials recruited patients with advanced disease, who had essentially exhausted every other therapeutic option [27]. Encouraging results have been obtained in some patients with limited disease or in the adjuvant setting, indicating that patients with a low tumor burden and not heavily pre-treated may be more prone to develop a functional anti-tumor immune response [28–30].
The few cell-based immunotherapy trials conducted in CLL used vaccines based on either allogeneic DC loaded with tumor lysates or apoptotic bodies (n = 9) [31], or autologous DC pulsed with tumor lysates (n = 12) [32]. No vaccine adjuvants were used. Patients in these trials seldom achieved objective clinical responses as defined by standard criteria such as IWCLL [7], which is in line with the lack of objective clinical remissions in our trial.
In a recently reported study in multiple myeloma [33], autologous DC fused with patient-derived tumor plasma cells were used to vaccinate patients. In this trial, GM-CSF was used as adjuvant and expansion of circulating tumor-reactive lymphocytes was observed in 11/15 evaluable patients. GM-CSF was used also in a recent peptide-based vaccination study in CLL resulting in specific T cell responses but again no objective clinical partial remissions were obtained [34]. The only clinical responding patient in our trial was Pt I-06, who had a complete response (CR) in blood and a nodular partial response (PR) in bone marrow. This has to be viewed with great caution, due to the concomitant chronic/stable HCV infection with unknown effects on the immune system in general. A spontaneous remission cannot be fully excluded, even though spontaneous CLL remissions are rare [35]. Finally, Pt I-06 was vaccinated according to an individual “maintenance” schedule every 4 weeks from week 10 to week 52. Whether this may have contributed to the overall effect is unknown [36]. The lack of objective clinical remissions in most CLL vaccine trials highlights the necessity to identify new strategies which can induce immune responses that ultimately mediate a clinical effect. Disease stabilization itself at the cost of minimal toxicity would be an important therapeutic goal in CLL, as no established maintenance therapy exists. An interesting approach that has shown promising results is represented by T lymphocytes with chimeric antigen receptors (CARs) targeting CLL-specific antigens. In a recently reported pilot study, CAR T cells targeting CD19 were infused in three patients with advanced CLL. These engineered T cells expanded in vivo, induced a leukemia-specific immune response and induced a complete remission in two out of three patients [37].
In our study, patients were vaccinated with DC loaded with apoptotic bodies of autologous leukemic cells. The vaccine proved to be safe and well tolerated with only mild injection-site reactions. Beyond the safety evaluation, a primary aim of the study was to elucidate whether Apo-DC could induce anti-tumor immunity in vivo and the additive effect of adjuvant GM-CSF and low-dose CTX to modulate the immune response.
A secondary goal was to establish criteria for leukemia-specific vaccine-induced immune responses, to be applied in forthcoming leukemia vaccination studies. In the majority of active immunotherapy trials, numerical criteria (e.g., x-fold increase in T cell subpopulations frequencies) are applied for the definition of vaccine-induced immune responses. In our trial, we tried to make immune response criteria more stringent by applying extensive statistical analysis. Based on these criteria systematically applied for both proliferation and ELISpot assay, 10/15 patients were defined as immune responders. We then tried to further characterize the immune effector cell populations both phenotipically and functionally by CD107 degranulation assay and confocal microscopy imaging. We could show that the frequency of CD8+ and CD4+ cells degranulating in the presence of the leukemic targets increased after vaccination in all evaluable patients. It was of special interest the formation in vitro of cell aggregates between CLL cells and cytotoxic T cells further suggesting that T cells recognized leukemic cells. The formation of T cell conjugates between autologous T cells and CLL cells has also been shown by Ramsay et al. [38].
Regarding the additive effect to the immunogenicity of the vaccine of the two adjuvants used, GM-CSF and low-dose CTX, the observation that the frequency of the immune responses was higher in cohort III compared with cohorts I and II could possibly indicate the existence of such additive effect. It must nevertheless be taken into account that a proper statistical comparison between the three cohorts cannot be done due to the low number of patients accrued and that other factors might have contributed to the higher frequency of immune responses in patients in cohort III. Differences in the biological characteristics of the disease as well as in the previous treatment history of the individual patients (2/5 patients in cohorts I and II were previously treated, while all the patients in cohort III were untreated) might indeed have played a role in determining the patients′ ability to mount a vaccine-induced immune response. Moreover, it could be argued that the different frequencies of immune responses observed in the three cohorts could be due to the different number of Apo-DC that the patients received. However, despite the fact that patients in cohorts II and III received approximately the same amount of vaccine, 5/5 patients in cohort III mounted a CLL-specific vaccine-induced T cell response compared with 3/5 in cohort II. This observation may suggest that the combination of the two vaccine adjuvants had an additive effect. Tregs levels were significantly lower in immune-responding patients indicating a relation between Tregs and the capability to mount a specific cellular response. Unexpectedly, CTX did in our study not reduce the number of blood Tregs, which is in agreement with at least one other study in humans [39]. An immune enhancing effect of CTX might be nevertheless mediated through different mechanisms of action and discriminating which is most relevant might be difficult [40]. One of these mechanisms is the induction of lymphopenia which in turn allows homeostasis-driven expansion of T cells. Furthermore, phenotypic characteristics of Tregs as assessed in this study may not relate to the functional capability of the population [41].
In conclusion, our pilot study suggests that immunization of CLL patients with Apo-DC may induce significant immune responses and indicates that the combination with adjuvants such as GM-CSF and low-dose CTX might have an additive effect on the immunogenicity of the vaccine. Our study also shows how criteria for the evaluation of vaccine-induced immune responses in leukemia can be established that can be applied in forthcoming studies on potent vaccination strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Supported by grants from The Swedish Cancer Society, The Cancer Society in Stockholm, King Gustav V Jubilee Fund, The Cancer and Allergy Foundation, The Karolinska Institutet Foundations, the EU-grant LSHB-CT-2004-512074; DC-THERA, The Stockholm County Council; IMTAC and Miltenyi Biotec GmbH. For excellent secretarial help, we thank Ms. Leila Relander.
Conflict of interest
The authors have no conflict of interest to disclose.
Footnotes
Marzia Palma and Lotta Hansson contributed equally.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Wierda WG, Kipps TJ, Dürig J et al. (2009) Ofatumumab combined with fludarabine and cyclophosphamide (O-FC) Shows high activity in patients with previously untreated chronic lymphocytic leukemia (CLL): Results from a randomized, multicenter, international, two-dose, parallel group, phase II trial. Blood (ASH annual meeting abstracts) 114 (abs. 207)
- 3.Badoux X, Keating MJ, O′Brien SM et al. (2009) Long term results of chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab (FCR) for patients with relapsed and refractory chronic lymphocytic leukemia. Haematologica 94 (suppl. 3):abs. 10.32
- 4.Fiammenghi L, Ancarani V, Rosales T, et al. FRET microscopy autologous tumor lysate processing in mature dendritic cell vaccine therapy. J Transl Med. 2010;8:52. doi: 10.1186/1479-5876-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokhaei P, Choudhury A, Mahdian R, et al. Apoptotic tumor cells are superior to tumor cell lysate, and tumor cell RNA in induction of autologous T cell response in B-CLL. Leukemia. 2004;18:1810–1815. doi: 10.1038/sj.leu.2403517. [DOI] [PubMed] [Google Scholar]
- 6.Kokhaei P, Rezvany MR, Virving L, et al. Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell-tumour hybrids in B-CLL. Leukemia. 2003;17:894–899. doi: 10.1038/sj.leu.2402913. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamson L, Palma M, Choudhury A, et al. Generation of a dendritic cell-based vaccine in chronic lymphocytic leukaemia using CliniMACS platform for large-scale production. Scand J Immunol. 2009;69:529–536. doi: 10.1111/j.1365-3083.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- 9.Ullenhag GJ, Frodin JE, Mosolits S, et al. Immunization of colorectal carcinoma patients with a recombinant canarypox virus expressing the tumor antigen Ep-CAM/KSA (ALVAC-KSA) and granulocyte macrophage colony- stimulating factor induced a tumor-specific cellular immune response. Clin Cancer Res. 2003;9:2447–2456. [PubMed] [Google Scholar]
- 10.Mitchell MS. Combinations of anticancer drugs and immunotherapy. Cancer Immunol Immunother. 2003;52:686–692. doi: 10.1007/s00262-003-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyum A. Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology. 1977;10:71–76. [PubMed] [Google Scholar]
- 12.Gitelson E, Hammond C, Mena J, et al. Chronic lymphocytic leukemia-reactive T cells during disease progression and after autologous tumor cell vaccines. Clin Cancer Res. 2003;9:1656–1665. [PubMed] [Google Scholar]
- 13.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 14.Ribera JM, Vinolas N, Urbano-Ispizua A, et al. “Spontaneous” complete remissions in chronic lymphocytic leukemia: report of three cases and review of the literature. Blood Cells. 1987;12:471–483. [PubMed] [Google Scholar]
- 15.Dreger P, Brand R, Milligan D, et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia. 2005;19:1029–1033. doi: 10.1038/sj.leu.2403745. [DOI] [PubMed] [Google Scholar]
- 16.Gribben JG, Zahrieh D, Stephans K, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–4396. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollgaard T, Petersen SL, Hadrup SR, et al. Evidence for involvement of clonally expanded CD8+ T cells in anticancer immune responses in CLL patients following nonmyeloablative conditioning and hematopoietic cell transplantation. Leukemia. 2005;19:2273–2280. doi: 10.1038/sj.leu.2403972. [DOI] [PubMed] [Google Scholar]
- 18.Marks DI, Lush R, Cavenagh J, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100:3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 19.Giannopoulos K, Li L, Bojarska-Junak A, et al. Expression of RHAMM/CD168 and other tumor-associated antigens in patients with B-cell chronic lymphocytic leukemia. Int J Oncol. 2006;29:95–103. [PubMed] [Google Scholar]
- 20.Giannopoulos K, Schmitt M. Targets and strategies for T-cell based vaccines in patients with B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47(10):2028–2036. doi: 10.1080/10428190600709721. [DOI] [PubMed] [Google Scholar]
- 21.Kokhaei P, Palma M, Hansson L, et al. Telomerase (hTERT 611–626) serves as a tumor antigen in B-cell chronic lymphocytic leukemia and generates spontaneously antileukemic, cytotoxic T cells. Exp Hematol. 2007;35:297–304. doi: 10.1016/j.exphem.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Rezvany MR, Jeddi-Tehrani M, Rabbani H, et al. Autologous T lymphocytes recognize the tumour-derived immunoglobulin VH-CDR3 region in patients with B-cell chronic lymphocytic leukaemia. Br J Haematol. 2000;111:230–238. doi: 10.1046/j.1365-2141.2000.02307.x. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt SM, Schag K, Muller MR, et al. Induction of adipophilin-specific cytotoxic T lymphocytes using a novel HLA-A2-binding peptide that mediates tumor cell lysis. Cancer Res. 2004;64:1164–1170. doi: 10.1158/0008-5472.CAN-03-2538. [DOI] [PubMed] [Google Scholar]
- 24.Siegel S, Wagner A, Kabelitz D, et al. Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood. 2003;102:4416–4423. doi: 10.1182/blood-2003-01-0198. [DOI] [PubMed] [Google Scholar]
- 25.Zenz T, Mertens D, Kuppers R, et al. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10:37–50. doi: 10.1038/nrc2764. [DOI] [PubMed] [Google Scholar]
- 26.Lundin J, Porwit-MacDonald A, Rossmann ED, et al. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukaemia. Leukemia. 2004;18:484–490. doi: 10.1038/sj.leu.2403258. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmichael MG, Benavides LC, Holmes JP, et al. Results of the first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine in disease-free breast cancer patients: United States Military Cancer Institute Clinical Trials Group Study I-04. Cancer. 2010;116:292–301. doi: 10.1002/cncr.24756. [DOI] [PubMed] [Google Scholar]
- 29.Lacy MQ, Mandrekar S, Dispenzieri A, et al. Idiotype-pulsed antigen-presenting cells following autologous transplantation for multiple myeloma may be associated with prolonged survival. Am J Hematol. 2009;84:799–802. doi: 10.1002/ajh.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeel DG, Dunphy EJ, Davies JG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hus I, Rolinski J, Tabarkiewicz J, et al. Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1621–1627. doi: 10.1038/sj.leu.2403860. [DOI] [PubMed] [Google Scholar]
- 32.Hus I, Schmitt M, Tabarkiewicz J, et al. Vaccination of B-CLL patients with autologous dendritic cells can change the frequency of leukemia antigen-specific CD8+ T cells as well as CD4+ CD25+ FoxP3+ regulatory T cells toward an antileukemia response. Leukemia. 2008;22:1007–1017. doi: 10.1038/leu.2008.29. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt J, Vasir B, Uhl L, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 2011;117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannopoulos K, Dmoszynska A, Kowal M, et al. Peptide vaccination elicits leukemia-associated antigen-specific cytotoxic CD8+ T-cell responses in patients with chronic lymphocytic leukemia. Leukemia. 2010;24:798–805. doi: 10.1038/leu.2010.29. [DOI] [PubMed] [Google Scholar]
- 35.Del Giudice I, Chiaretti S, Tavolaro S, et al. Spontaneous regression of chronic lymphocytic leukemia: clinical and biologic features of 9 cases. Blood. 2009;114:638–646. doi: 10.1182/blood-2008-12-196568. [DOI] [PubMed] [Google Scholar]
- 36.Spaner DE, Hammond C, Mena J, et al. A phase I/II trial of oxidized autologous tumor vaccines during the “watch and wait” phase of chronic lymphocytic leukemia. Cancer Immunol Immunother. 2005;54:635–646. doi: 10.1007/s00262-004-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalos M, Levine BL, Porter DL et al (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3:95ra73. doi:3/95/95ra73 [DOI] [PMC free article] [PubMed]
- 38.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrausch U, Poehlein CH, Jensen SM, et al. Cancer immunotherapy: the role regulatory T cells play and what can be done to overcome their inhibitory effects. Curr Mol Med. 2009;9:673–682. doi: 10.2174/156652409788970670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.