Abstract
Background
While surgical resection of pancreatic adenocarcinoma provides the only chance of cure, long-term survival remains poor. Immunotherapy may improve outcomes, especially as adjuvant to local therapies. Gene-mediated cytotoxic immunotherapy (GMCI) generates a systemic anti-tumor response through local delivery of an adenoviral vector expressing the HSV-tk gene (aglatimagene besadenovec, AdV-tk) followed by anti-herpetic prodrug. GMCI has demonstrated synergy with standard of care (SOC) in other tumor types. This is the first application in pancreatic cancer.
Methods
Four dose levels (3 × 1010 to 1 × 1012 vector particles) were evaluated as adjuvant to surgery for resectable disease (Arm A) or to 5-FU chemoradiation for locally advanced disease (Arm B). Each patient received two cycles of AdV-tk + prodrug.
Results
Twenty-four patients completed therapy, 12 per arm, with no dose-limiting toxicities. All Arm A patients were explored, eight were resected, one was locally advanced and three had distant metastases. CD8+ T cell infiltration increased an average of 22-fold (range sixfold to 75-fold) compared with baseline (p = 0.0021). PD-L1 expression increased in 5/7 samples analyzed. One node-positive resected patient is alive >66 months without recurrence. Arm B RECIST response rate was 25 % with a median OS of 12 months and 1-year survival of 50 %. Patient-reported quality of life showed no evidence of deterioration.
Conclusions
AdV-tk can be safely combined with pancreatic cancer SOC without added toxicity. Response and survival compare favorably to expected outcomes and immune activity increased. These results support further evaluation of GMCI with more modern chemoradiation and surgery as well as PD-1/PD-L1 inhibitors in pancreatic cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1679-3) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic adenocarcinoma, Immuno-oncology, Gene therapy, PD-L1, Adenovirus, AdV-tk
Introduction
More effective therapies for pancreatic adenocarcinoma are desperately needed. Pancreatic cancer (PanCa) has a 5-year survival rate <5 %, the lowest of all major cancers, with more than 37,000 deaths in the USA each year [1]. Although surgical resection remains the only potential curative therapy, only 10–20 % of pancreatic tumors are resectable and even resectable patients have a median survival of only 20–24 months. Patients with unresectable disease fare much worse. Combinations such as FOLFIRINOX and gemcitabine/nab-paclitaxel are considered current standards with median survival of <1 year and evidence of significant toxicity [2, 3]. Unfortunately, for most PanCa patients, micrometastatic disease is likely present at diagnosis. As such, immunotherapies that augment systemic disease control without added toxicity are a logical addition to standard of care (SOC) therapies for PanCa.
Gene-mediated cytotoxic immunotherapy (GMCI™) is a multi-pronged viral-based approach to generating a systemic anti-tumor response through local delivery of an adenoviral vector expressing the herpes simplex virus thymidine kinase gene (aglatimagene besadenovec, AdV-tk) followed by an anti-herpetic prodrug, such as valacyclovir [4]. PanCa cells are susceptible to adenoviral transduction [5]. The expressed HSV-TK (TK) protein has two principal functions: (1) enzymatically, it phosphorylates the prodrug, generating nucleotide analogs that kill tumor cells undergoing DNA replication or repair; (2) physically, it is a super-antigen-like molecule that stimulates a potent immune reaction. The consequent tumor cell death via necrosis and apoptosis, which releases tumor antigens and elicits danger signals, combined with the immunostimulatory milieu generated by the super-antigen effect, leads to the in vivo generation of a tumor-specific multivalent immune response. In animal models, the systemic effect protects against tumor re-challenge, is present in immunocompetent but not immunodeficient mice and is transferrable via CD8+ T cells [6–12].
In animal models, GMCI has been shown to synergize with surgery, radiation therapy (RT) and various chemotherapies. For example, in mammary and prostate cancer models, AdV-tk delivered to the tumor bed following surgical resection delayed local recurrence and had systemic anti-tumor effects [13]. Positive immune effects after cytoreductive surgery included decreased myeloid-derived suppressor cells (MDSCs) and increased CD8+ T cell stimulation [14]. Radiation plus AdV-tk not only had increased local tumor effects but also had synergistic systemic effects on lung metastases [15]. Mechanistically, synergy with RT is likely due to increased incorporation of nucleotide analogs during DNA repair and innate immune stimulation including recruitment of antigen-presenting cells (APCs) [16]. Cytotoxic and immunologic synergy has also been seen with chemotherapies, including PanCa SOC gemcitabine and 5-FU [17, 18]. Mechanistically, this likely reflects chemotherapy-induced inhibition of regulatory T cells (T-regs) and MDSCs [19–21]. In PanCa, chemoradiation has been shown to improve dendritic cell function [22, 23]. These data provide further rationale for evaluating immunotherapy in combination with chemoradiation and surgery in this disease.
Safety and signs of efficacy for GMCI have been demonstrated in phase 1 and 2 clinical trials in various tumor types, including prostate, malignant glioma, retinoblastoma, mesothelioma and ovarian cancer, but a definitive efficacy study has not yet been completed in any tumor type [24–31]. In prostate cancer, single-agent activity was demonstrated with PSA responses, including repeated responses after re-administration, increased necrosis, apoptosis and CD8+ T cell infiltration in tumor samples, and ultimately increased long-term disease control [24, 25, 31]. Tumor shrinkage and immune responses were seen in malignant glioma, retinoblastoma and mesothelioma [26–28]. A phase 2 trial in newly diagnosed prostate cancer with RT demonstrated safety and a threefold to fivefold decrease in tumor recurrence [32]. This study constitutes the first evaluation of GMCI in the treatment of PanCa. The primary objective was to evaluate GMCI safety and secondarily to evaluate its potential clinical benefit in the treatment of PanCa as an adjuvant to standard of care.
Patients and methods
Study design
This dose escalation study evaluated two courses of AdV-tk + valacyclovir as an adjunct to surgery in potentially resectable patients (Arm A) or in combination with chemoradiation in patients with locally advanced pancreatic cancer (LAPC-Arm B). Institutional review boards in all participating institutions approved the protocol and informed consent documents. Specific written informed consent was obtained from each patient before enrollment.
The AdV-tk vector has been previously described [33]. Four dose levels of AdV-tk were evaluated: 3 × 1010 vector particles (vp), 1 × 1011 vp, 3 × 1011 vp and 1 × 1012 vp. Valacyclovir was administered at 2000 mg by mouth three times per day for 14 days starting 1–3 days after each AdV-tk injection. Intravenous acyclovir at a dose of 10 mg/kg was substituted when patients were unable to take oral medication. Valacyclovir doses were adjusted for renal impairment based on calculated creatinine clearance. The first AdV-tk dose was delivered intratumorally via either endoscopic ultrasonography (EUS) or CT guidance for both Arm A and Arm B [34]. For Arm A, the second AdV-tk dose was delivered intraoperatively 2–3 weeks later, either into the tumor bed after resection or into the primary tumor, if not resectable. For Arm B, RT was initiated within 3–7 days after the first AdV-tk injection at 1.8 Gy per day for approximately 6 weeks to a total of 50.4 Gy. Continuous infusion of 5-FU at 200 mg/m2/day for 5 days per week began during the first week and continued until completion of RT. The second AdV-tk dose for Arm B was delivered 2–4 weeks after the first via EUS- or CT-guided injection during chemoradiation. After surgery or chemoradiation completion, patients received standard of care chemotherapy at the discretion of their physician.
Patients
Inclusion criteria included age ≥18 years, ECOG performance status of 0–2, presumed diagnosis of resectable (Arm A) or LAPC (Arm B) without evidence of metastatic disease and no prior therapy for PanCa. Pathologic confirmation of diagnosis was required prior to initiating AdV-tk injections. Laboratory inclusion criteria included AST ≤ 3× upper limit of normal, platelets > 100,000/mm3, WBC > 3000/mm3, ANC > 1500/mm3, serum creatinine <2 mg/dl and calculated creatinine clearance >10 ml/min.
Assessments
Toxicity was assessed using the National Cancer Institute common terminology criteria for adverse events (CTCAE) version 3.0. Dose-limiting toxicities (DLTs) were defined as any grade 4 toxicity or a grade 3 toxicity requiring interruption in therapy for more than 7 days.
Tumor response was determined according to response evaluation criteria in solid tumors (RECIST) [2]. Partial response (PR) was defined as at least a 30 % decrease in image size compared with baseline, and progressive disease (PD) was an increase of at least 20 % or the appearance of new lesions. Stable disease (SD) was either decrease insufficient to qualify as PR or increase insufficient to qualify as PD. CA 19-9 response was the maximum percentage decline relative to baseline calculated as follows: [(baseline CA 19-9 − lowest post-treatment CA 19-9)/baseline CA 19-9] × 100. Patients without CA 19-9 elevation at baseline or without post-treatment CA 19-9 measurement were not evaluable.
The Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) version 4 was used to assess patient-reported outcomes at baseline and at follow-up visits. The FACT-Hep instrument is a 45-item questionnaire with physical, social, emotional, functional and hepatobiliary domains validated for use in PanCa patients [35].
Immunohistochemistry
Paraffin-embedded tumor sections were stained with antibodies specific for PD-L1 (programmed death ligand 1, Novus Biologicals, Littleton, CO), CD4 (Novocastra/Leica Biosystems, Buffalo Grove, IL) and CD8 (Dako, Carpinteria, CA) using standard techniques.
Statistical analysis
The primary method of data analysis for this phase I study was descriptive. Progression-free survival (PFS) and overall survival (OS) were calculated from the time of first AdV-tk injection until progression or death using the Kaplan–Meier method. Density of T cell infiltration before and after treatment was compared with the Mann–Whitney test using GraphPad Prism version 5.0.
Results
Patients and treatment
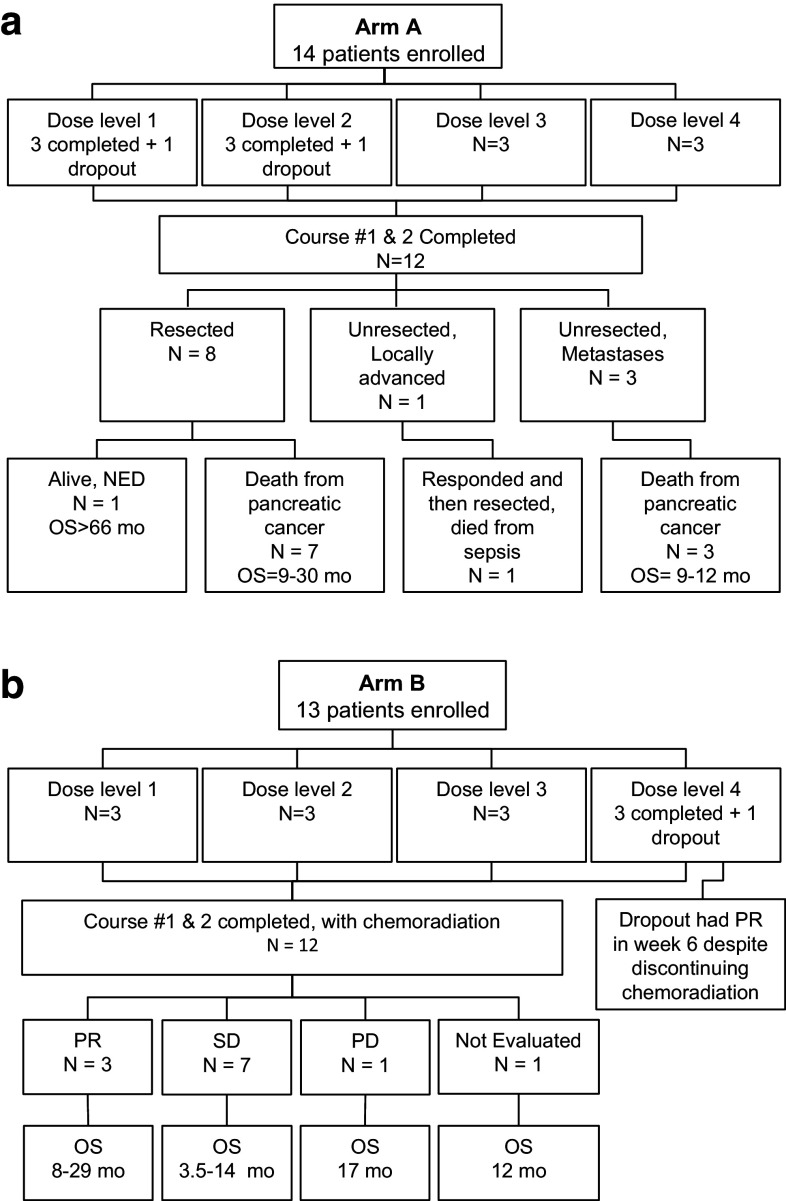
Three institutions collaborated on this project: James Cancer Hospital at The Ohio State University Wexner Medical Center (Columbus, OH), City of Hope National Medical Center (Duarte, CA) and Scripps Green Hospital (La Jolla, CA). A total of 27 patients were enrolled: 14 patients in Arm A (Fig. 1a) and 13 patients in Arm B (Fig. 1b). Twenty-four of 27 patients completed both AdV-tk plus prodrug courses with three at each dose level in each arm (Tables 1, 2). The three dropouts included: one patient in Arm A that completed one course and subsequently died due to an unrelated myocardial infarction; a second patient in Arm A withdrew after metastases were discovered on operative exploration; the third was a patient in Arm B who chose to withdraw due to valacyclovir intolerance. All 12 patients completing therapy in Arm A had operative exploration. Eight underwent tumor resection and four were not resected: three due to distant metastases and one due to LAPC found at the time of surgery. Patient characteristics at baseline and TNM surgical stage are shown in Table 1. For Arm B, all 12 patients completed chemoradiation without significant delays or interruptions. Baseline characteristics for Arm B are shown in Table 2.
Fig. 1.
CONSORT diagrams for Arm A (a) and Arm B (b)
Table 1.
Patient demographics and outcomes for Arm A
| Case # | Dose (vp) | Age (years) | Baseline ECOG | Baseline CA 19-9 | Resected | Tumor location* | Stage | Injection method | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1A02 | 3 × 1010 | 40 | 0 | 250 | No | Head | M1 | EUS/open | 9 |
| 1A03 | 3 × 1010 | 70 | 0 | 69 | Yes | Head | pT3N1, 1/15 LN+ | EUS/open | >66 |
| 1A04 | 3 × 1010 | 57 | 0 | 1384 | No | Head | M1 | EUS/open | 11.7 |
| 2A02 | 1 × 1011 | 53 | 1 | 21,660 | Yes | Head | pT3N1, 7/21 LN+ | EUS/open | 10 |
| 2A03 | 1 × 1011 | 59 | 0 | 893 | No | Head | T4 | EUS/open | 9.4 |
| 2A04 | 1 × 1011 | 68 | 0 | 40 | Yes | Head | pT3N1, 5/24 LN+ | EUS/open | 30.4 |
| 3A01 | 3 × 1011 | 66 | 1 | 1223 | Yes | Head | pT3N1, 6/27 LN+ | EUS/open | 10.4 |
| 3A02 | 3 × 1011 | 78 | 1 | 15,136 | Yes | Head | pT3N1, 6/24 LN+ | EUS/open | 10.2 |
| 3A03 | 3 × 1011 | 71 | 0 | 451 | Yes | Head | T2N0, 0/15 LN+ | EUS/open | 20.6 |
| 4A01 | 1 × 1012 | 68 | 1 | 1721 | Yes | Head | pT3N1, 2/13 LN+ | EUS/open | 11.5 |
| 4A02 | 1 × 1012 | 65 | 0 | 15 | Yes | Tail | pT3N0, 0/16 LN+ | EUS/open | 9.4 |
| 4A03 | 1 × 1012 | 70 | 1 | 127 | No | Head | M1 | EUS/open | 12.4 |
| Median | 67 | 0 | 672 | 11 |
vp vector particles; ECOG ECOG performance status; LN lymph nodes; EUS endoscopic ultrasound; open injection during surgery; n/a not available
aRegion of the pancreas in which the tumor was located
Table 2.
Patient demographics and outcomes for Arm B
| Case # | Dose (vp) | Age (years) | Baseline ECOG | Baseline CA 19-9 | Baseline tumor (cm)a | Tumor locationb | Injection method | Tumor response (RECIST) | CA 19-9 response | PFS (mo) | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1B01 | 3 × 1010 | 81 | 1 | 79 | 4.5 | Head | EUS | SD | n/a | 3.5 | 3.5 |
| 1B02 | 3 × 1010 | 65 | 1 | 1995 | n/a | Head | EUS | SD | 72 % | 5.8 | 5.8 |
| 1B03 | 3 × 1010 | 62 | 0 | 1252 | 13.3 | Tail | CT | PR | 82 % | 11.5 | 21.5 |
| 2B01 | 1 × 1011 | 76 | 0 | 2424 | 4.2 | Body | CT | n/a | n/a | 12.2 | 12.2 |
| 2B02 | 1 × 1011 | 55 | 1 | 298 | 7.4 | Head | CT | PR | 100 % | 18.3 | 28.7 |
| 2B03 | 1 × 1011 | 62 | 2 | 29 | 5.9 | Head/body | CT | SD | n/a | 4.7 | 11.7 |
| 3B01 | 3 × 1011 | 75 | 0 | 16,364 | 3.0 | Head | EUS | PD | 96 % | 2.1 | 16.9 |
| 3B02 | 3 × 1011 | 67 | 1 | 9835 | 3.5 | Head | CT | SD | n/a | 4.5 | 10 |
| 3B03 | 3 × 1011 | 56 | 0 | 5817 | 7.3 | Body | EUS | PR | 91 % | 3 | 8.4 |
| 4B01 | 1 × 1012 | 73 | 1 | 61 | 9.0 | Head | EUS | SD | 72 % | 8.3 | 14.2 |
| 4B03 | 1 × 1012 | 55 | 0 | 10 | 6.79 | Head | CT | SD | n/a | 9.3 | 13.4 |
| 4B04 | 1 × 1012 | 62 | 0 | 10,913 | 2.8 | Head | EUS | SD | 96 % | 5.8 | 5.8 |
| Median | 64 | 1 | 1624 | 5.9 | 5.8 | 12 |
aSum of target lesion diameters; vp vector particles; ECOG ECOG performance status; EUS endoscopic ultrasound; CT CT-guided percutaneous; SD stable disease; PR partial response; PD progressive disease; n/a not available
bRegion of the pancreas in which the tumor was located
Safety
No DLTs were observed. The majority of clinical abnormalities observed were grade 1 or 2 and designated by the investigators as unrelated or unlikely related to GMCI. Grade 2–4 clinical abnormalities designated as possibly or probably related are shown in Table 3. Fatigue, anorexia, nausea, vomiting and abdominal pain were the most frequent adverse events (AEs) and are common symptoms in patients with PanCa.
Table 3.
Adverse events after first AdV-tk injection until 3 weeks after second AdV-tk injection
| Arm A | Arm B | |||||
|---|---|---|---|---|---|---|
| CTC grade | CTC grade | |||||
| 2 | 3 | 4 | 2 | 3 | 4 | |
| Possibly or probably related clinical abnormalities | ||||||
| Abdominal pain | 1 | 2 | 0 | 2 | 0 | 0 |
| Anorexia | 0 | 0 | 0 | 1 | 0 | 0 |
| Dehydration | 0 | 1 | 0 | 0 | 1 | 0 |
| Fatigue | 0 | 0 | 0 | 3 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 2 | 0 | 0 |
| Laboratory abnormalities | ||||||
| Elevated alkaline phosphatase | 1 | 3 | 0 | 0 | 1 | 0 |
| Elevated amylase | 0 | 1 | 0 | 0 | 0 | 0 |
| Elevated AST/ALT | 3 | 6 | 0 | 2 | 0 | 0 |
| Elevated bilirubin | 3 | 1 | 3 | 1 | 1 | 0 |
| Elevated creatinine | 3 | 0 | 0 | 0 | 1 | 0 |
| Elevated lipase | 1 | 1 | 1 | 0 | 0 | 0 |
| Low calcium | 4 | 1 | 0 | 1 | 0 | 0 |
| Low hemoglobin | 5 | 2 | 2 | 1 | 1 | 0 |
| Low Lymphocytes | 2 | 1 | 1 | 0 | 7 | 6 |
| Low potassium | 0 | 0 | 0 | 0 | 1 | 0 |
| Low sodium | 0 | 1 | 0 | 0 | 3 | 0 |
| Low WBC | 0 | 0 | 0 | 1 | 0 | 0 |
CTC Common terminology criteria for adverse events version 3.0
Arm A Three patients experienced grade 3 AEs deemed possibly related to the experimental intervention after the first injection prior to the second injection. Two experienced worsening of abdominal pain soon after injection and one developed dehydration with renal insufficiency that resolved with hydration. There were no grade 3 or 4 possibly related AEs after the second injection. The most common laboratory abnormalities occurring after the first injection, some of which were abnormal at baseline but worsened, were AST/ALT and bilirubin elevation. Most of these patients had biliary obstruction at baseline but did not have stents placed since resection was planned and yet, still tolerated the first course of AdV-tk/prodrug prior to surgery. The most common laboratory abnormalities occurring after the surgery and second injection were anemia, AST/ALT and alkaline phosphatase elevation. None of the laboratory abnormalities were clinically significant.
Arm B Only one grade 3 and no grade 4 clinical events occurred that were deemed possibly or probably related. The grade 3 event was transient dehydration with creatinine elevation probably related to valacyclovir. The most common grade 3–4 laboratory abnormality occurring after the first and second injections during chemoradiation was transient lymphopenia.
Clinical outcome
Arm A All eight resected patients had R0 resection: four were not resected, one due to LAPC and three due to metastases found at the time of surgery (Table 1). One of the resected patients that had lymph node involvement is alive without recurrence more than 66 months after starting treatment. The patient with LAPC (superior mesenteric artery involvement) initially opted to forego additional therapy. Restaging after 3 months showed no progression. Subsequently, gemcitabine/erlotinib was given for 2 months followed by 2 months of 5-FU/RT. Response was adequate for resection but was complicated by finding erosion of the biliary stent into the portal vein, which likely led to postoperative sepsis from which the patient subsequently died. The three patients with metastatic disease survived from 9 to 12.4 months.
Arm B All 12 patients had LAPC and received the study-specified 5-FU/RT, after which other standard chemotherapy, gemcitabine with or without erlotinib, was given to most patients. Three patients achieved a confirmed partial response (PR), seven patients had stable disease (SD), and one patient had progression based on metastases identified at 2.1 months (Table 2). One patient refused further evaluations or additional treatment after 5-FU/RT. This patient reported preserved quality of life until death at 12 months. The one patient that withdrew in Arm B after the first injection (dose level 4) received approximately half of the first valacyclovir course and a shortened course of chemoradiation and yet had a partial response. All seven patients with evaluable serum CA 19-9 levels had a drop of at least 50 % with a range of 72–100 % (Table 2).
The RECIST objective response rate for Arm B was 25 % and disease control rate (PR + SD) was 83 %. In the three PR patients, response duration was 7 and 7.9 months in two of them; in the third, response was sufficient to attempt resection; however, due to metastases observed at the time of laparotomy, resection was not performed. Median progression-free survival (PFS) and overall survival (OS) for Arm B were 5.8 and 12 months, respectively, with 1-year survival of 50 %.
Health-related quality of life assessed using the patient-reported FACT-Hep questionnaire was stable after treatment compared with baseline for Arm A and Arm B (Supplementary Materials Fig. 1).
Immunologic effects
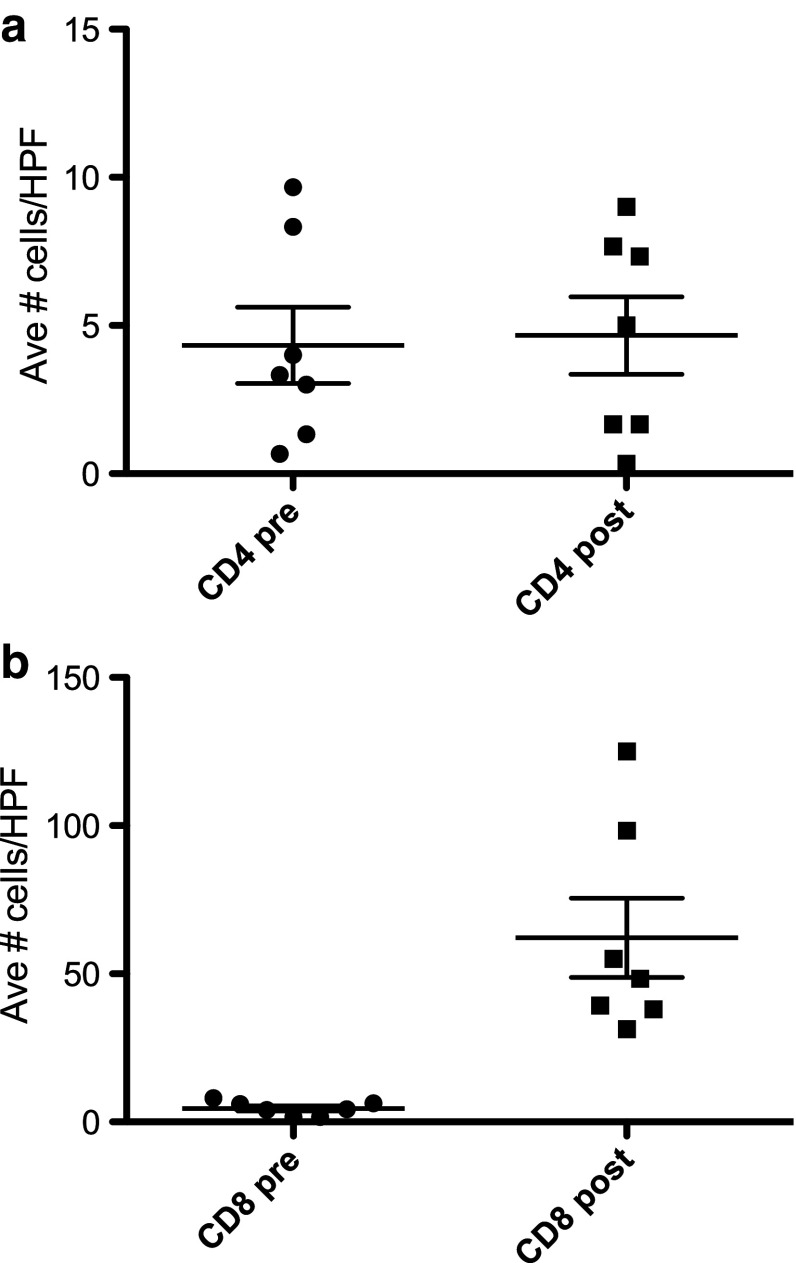
Immune cell infiltration was characterized in resected tumors after AdV-tk/prodrug compared with pre-treatment biopsy samples in seven patients (Table 4). All patients had an increase in CD8+ T cell infiltrate (Fig. 2b), with an average fold increase of 21.66 (range 6.00–74.85, p = 0.0021). CD4+ infiltrates were not significantly changed (Fig. 2a). Programmed death ligand 1 (PD-L1) levels were increased in five of seven samples analyzed (Table 4 and Supplementary Materials Fig. 2).
Table 4.
PD-L1 expression and T cell infiltration in tumors before and after AdV-tk + prodrug
| Dose level | Case # | PD-L1 | CD4 | CD8a | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Prea | Posta | Fold change | Prea | Posta | Fold change | ||
| 1 | 1A03 | − | ++ | 0.67 | 1.67 | 2.49 | 4 | 31.33 | 7.83 |
| 2 | 2A02 | − | ++ | 4 | 9 | 2.25 | 1.67 | 55 | 32.93 |
| 3 | 3A01 | + | + | 1.33 | 0.33 | 0.25 | 1.67 | 125 | 74.85 |
| 3 | 3A02 | + | +++ | 3 | 1.67 | 0.56 | 4.33 | 48.33 | 11.16 |
| 3 | 3A03 | + | +++ | 9.67 | 7.33 | 0.76 | 8 | 98.33 | 12.29 |
| 4 | 4A01 | + | + | 8.33 | 5 | 0.60 | 6 | 39.33 | 6.56 |
| 4 | 4A02 | − | +++ | 3.33 | 7.67 | 2.30 | 6.33 | 38 | 6.00 |
| Average CD4 change: 1.32 | Average CD8 change: 21.66 | ||||||||
aAverage number of positive cells in three high-powered fields before (pre) or after (post) AdV-tk + prodrug
Fig. 2.
Significant increase in CD8+ but not CD4+ T cell infiltration in tumors after AdV-tk injection and prodrug course. Paraffin sections from pre-treatment biopsy or post-treatment surgical resection for seven patients with available samples were stained with anti-CD4 (a) or anti-CD8 (b) antibodies and the number of positive cells per high-power field (hpf) counted. The average number of positive cells in three high-powered fields is displayed in the scattergrams. The before (pre) and after (post) treatment values were compared using the Mann–Whitney test
Discussion
In this study, GMCI was well tolerated and generated favorable responses compared with historical controls. Adding GMCI to 5-FU/RT or surgery did not increase toxicity and showed preliminary evidence of efficacy with survival and response data comparing favorably to standard of care alone. The clinical activity and immune stimulation did not seem to be dose-related as it was seen in multiple dose levels. This was also seen in prostate cancer, where beyond a threshold dose, PSA responses occurred in each of three higher dose levels [25]. A broad therapeutic window is consistent with the unique mechanism of action for tumor vaccines. There were no DLTs observed, and only 4/24 patients had transient grade-3 clinical abnormalities that were deemed possibly related by the investigators (Table 3). Immune-related adverse events were not observed. This phase 1 study documents the safety and potential efficacy of GMCI in patients with PanCa.
For the resectable arm (Arm A), patients tolerated receiving one course of GMCI prior to surgery and a second AdV-tk injection at the time of surgery. This allowed assessment of immune biomarkers in tumor samples from the eight patients that underwent resection. Of those eight, six had positive lymph nodes, a poor prognostic factor. One of the six with positive lymph nodes is recurrence free after >66 months of follow-up. Response rates were not evaluated for Arm A since surgical resection was part of the treatment plan. The three patients with metastatic disease discovered during surgery survived from 9 to 12.4 months after receiving gemcitabine-based chemotherapy. The expected median survival for this population is ~6 months [36]. In one of these patients, necrosis in a non-injected metastatic lesion in the liver was observed (data not shown). This may indicate a systemic response to the tumor.
The median OS for patients with LAPC (Arm B) was 12 months, with a 1-year survival rate of 50 %. This compares favorably to median OS of 9 months and 1-year survival of 28 % reported for 5-FU/RT [37]. It also compares favorably with the improvements to 11.1 and 42 % with gemcitabine/RT, but GMCI did not show toxicity above that seen with 5-FU/RT alone which is less than the toxicity of gemcitabine/RT [37, 38]. Similarly, RECIST objective response rate for Arm B was 25 % and disease control rate (PR + SD) was 83 %, which also compares well to the gemcitabine/RT rates of 6 and 74 %, respectively [38]. While conclusions cannot be drawn from comparison of individual studies conducted separately, these data support further evaluation of this approach in randomized comparison studies.
Pancreatic cancer is one of the most recalcitrant of human malignancies. Complete resection is the only potentially curative option. Unfortunately, PanCA is most often a systemic disease at diagnosis. For most patients following resection, metastases appear soon after surgery, indicating preexisting micro-metastasis and persistent residual disease. Non-resectable LAPC and metastatic disease have an even more dismal prognosis. Recently, nab-paclitaxel received FDA approval in combination with gemcitabine for metastatic disease based on a randomized trial with a median survival of 8.5 months for the combination compared with 6.7 months for gemcitabine alone [3]. In another recent report, FOLFIRINOX improved median survival to 11.1 months compared with 6.8 months with gemcitabine [2]. While these more aggressive regimens show improved survival, at least in the metastatic setting, toxicities threaten quality of life relative to gemcitabine alone [39, 40]. Nonetheless, use of these more aggressive regimens at earlier stages of the disease may improve outcomes and increase the number of patients that can be resected. In a recent report using FOLFIRINOX followed by chemoradiation in LAPC, overall response rate was 27 % leading to RO resections in 5/22 (23 %) patients [41]. Unfortunately, three of five resected patients developed distant metastases within 5 months, indicating the presence of persistent residual disease.
Immunotherapy may provide an approach to attacking minimal residual disease. The results from the current study and previous studies demonstrate that pancreatic tumors are susceptible to immune therapy, especially after debulking. Although PanCa has been reported to elicit immune inhibitory factors including MDSCs, Tregs and inhibitory cytokines, these are suppressed by surgery, radiation and chemotherapy [22, 23, 42, 43]. PanCa patients undergoing surgery and/or chemoradiation have been shown to be capable of mounting humoral or cellular immune responses [44–46], and antigen-specific tumor vaccine studies with K-ras, CEA and MUC1 have shown that PanCa patients can mount long-term T cell reactivity [47, 48]. However, in single-antigen approaches, tumor escape can be a problem [49]. In a multivalent study using GM-CSF-modified allogeneic tumor cell vaccine (GVAX), which presents a large number of tumor antigens, patients with longer disease-free survival had an expanded repertoire of T cells responding to a number of mesothelin epitopes [50]. However, antigens from allogeneic cell lines may not represent the dominant tumor antigens of the individual patient. The GMCI approach presented here provides both a multivalent and a patient-specific systemic anti-tumor immune response [4].
This study demonstrated the feasibility, safety and preliminary activity of AdV-tk administration to pancreatic cancer patients via EUS- or CT-guided injection as well as intraoperative injections. It showed no overlapping toxicity when combined with SOC 5-FU/XRT chemoradiation or surgery. It also showed encouraging efficacy signs. Although modern, more aggressive chemotherapeutic regimens have also shown improved outcomes, their toxicity profile approaches the limit of tolerability.
It is hard to imagine adding more aggressive, more toxic reagents to the present approaches. However, immunotherapy, that does not add further toxicity, may provide a good option to further combat systemic disease and immunotherapy may have the highest likelihood of success in the setting of minimal residual disease after aggressive primary debulking. T cell infiltration in melanoma and some other tumor types is considered a good prognostic factor [51]. However, pancreatic adenocarcinoma is considered to be “immunologically inert” without immune cell infiltration. Another phenomenon seen in melanoma is adaptive immune resistance in which immune responses lead to up-regulation of immune inhibitory factors such as the programmed death 1 (PD-1) pathway [51]. GMCI elicited a potent induction of CD8+ T cell infiltration in the tumors after treatment compared to before treatment. Immune stimulation with GMCI also led to increased expression of PD-L1 in pancreatic tumor cells. This has also been observed with other immune therapies. For example, increased PD-L1 expression was recently reported in pancreatic tumors in a GVAX study [52]. The induction of PD-L1 expression is another sign of the biologic activity of GMCI, but this may dampen the effector T cell response. Combining GMCI with PD-1/PD-L1 inhibitors would be a logical next step, initiating an anti-tumor immune response with GMCI and preventing down-regulation of the effector cells with checkpoint inhibitors, thus potentiating the overall systemic immunity.
The potential for added efficacy without added toxicity from combination of the most efficient SOC with one or more immunotherapies is tantalizing. The data presented here provide strong rationale for further evaluation of GMCI in newly diagnosed PanCa in combination with more modern chemotherapeutic regimens and the rationale for possibly combining with checkpoint inhibitors. A randomized phase 2 study combining GMCI with neoadjuvant modified FOLFIRINOX and gemcitabine-based chemoradiation is being launched building on recently published results with this regimen [53].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported in part by the National Cancer Institute at the National Institute of Health [Grant Number R43CA119847] and from The Ohio State University Comprehensive Cancer Center Core Grant from the National Institute of Health [P30CA016058]. The trial is listed in the ClinicalTrials.gov registry, clinical trials identification number NCT00638612.
Conflict of interest
L. K. Aguilar, D. Sanchez, A. G. Manzanera and E. Aguilar-Cordova are employees of Advantagene, Inc. All remaining authors have declared no conflicts of interest related to this study.
Abbreviations
- AdV-tk
Adenoviral vector expressing the HSV-thymidine kinase gene
- AEs
Adverse events
- APCs
Antigen-presenting cells
- CTCAE
Common Terminology Criteria for Adverse Events
- DLTs
Dose-limiting toxicities
- EUS
Endoscopic ultrasonography
- FACT-Hep
Functional Assessment of Cancer Therapy-Hepatobiliary
- GMCI
Gene-mediated cytotoxic immunotherapy
- LAPC
Locally advanced pancreatic cancer
- MDSCs
Myeloid-derived suppressor cells
- OS
Overall survival
- PanCa
Pancreatic cancer
- PD-1
Programmed death 1
- PD-L1
Programmed death ligand 1
- PFS
Progression-free survival
- PR
Partial response
- PD
Progressive disease
- RECIST
Response Evaluation Criteria in Solid Tumors
- RT
Radiation therapy
- SD
Stable disease
- SOC
Standard of care
- TK
Thymidine kinase protein from the HSV-thymidine kinase gene
- T-regs
Regulatory T cells
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul J-L, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet J-B, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Von-Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112:1969–1977. doi: 10.1002/jcb.23126. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld ME, Vickers SM, Raben D, Wang M, Sampson L, Feng M, Jaffee E, Curiel DT. Pancreatic carcinoma cell killing via adenoviral mediated delivery of the herpes simplex virus thymidine kinase gene. Ann Surg. 1997;225:609–618. doi: 10.1097/00000658-199705000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall SJ, Mutchnik SE, Chen SH, Woo SL, Thompson TC. Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer. 1997;70:183–187. doi: 10.1002/(SICI)1097-0215(19970117)70:2<183::AID-IJC8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Cruet MJ, Trask TW, Chen SH, Goodman JC, Woo SL, Grossman RG, Shine HD. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994;39:506–511. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- 8.Vile RG, Nelson JA, Castleden S, Chong H, Hart IR. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 9.Agard C, Ligeza C, Dupas B, Izembart A, El Kouri C, Moullier P, Ferry N. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001;8:128–136. doi: 10.1038/sj.cgt.7700281. [DOI] [PubMed] [Google Scholar]
- 10.Predina JD, Judy B, Aliperti LA, Fridlender ZG, Blouin A, Kapoor V, Laguna B, Nakagawa H, Rustgi AK, Aguilar L, Aguilar-Cordova E, Albelda SM, Singhal S. Neoadjuvant in situ gene-mediated cytotoxic immunotherapy improves postoperative outcomes in novel syngeneic esophageal carcinoma models. Cancer Gene Ther. 2011;18:871–883. doi: 10.1038/cgt.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagandeep S, Brew R, Green B, Christmas SE, Klatzmann D, Poston GJ, Kinsella AR. Prodrug-activated gene therapy: involvement of an immunological component in the “bystander effect”. Cancer Gene Ther. 1996;3:83–88. [PubMed] [Google Scholar]
- 12.Kuriyama S, Kikukawa M, Masui K, Okuda H, Nakatani T, Akahane T, Mitoro A, Tominaga K, Tsujinoue H, Yoshiji H, Okamoto S, Fukui H, Ikenaka K. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int J Cancer. 1999;83:374–380. doi: 10.1002/(SICI)1097-0215(19991029)83:3<374::AID-IJC13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Sukin SW, Chhikara M, Zhu X, Ayala G, Aguilar LK, O’Brian Smith E, Miles BJ, Thompson TC, Kadmon D, Aguilar-Cordova E. In vivo surgical resection plus adjuvant gene therapy in the treatment of mammary and prostate cancer. Mol Ther. 2001;3:500–506. doi: 10.1006/mthe.2001.0285. [DOI] [PubMed] [Google Scholar]
- 14.Predina JD, Kapoor V, Judy BF, Cheng G, Fridlender ZG, Albelda SM, Singhal S. Cytoreduction surgery reduces systemic myeloid suppressor cell populations and restores intratumoral immunotherapy effectiveness. J Hematol Oncol. 2012;5:34. doi: 10.1186/1756-8722-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhikara M, Huang H, Vlachaki MT, Zhu X, Teh B, Chiu KJ, Woo S, Berner B, Smith EO, Oberg KC, Aguilar LK, Thompson TC, Butler EB, Aguilar-Cordova E. Enhanced therapeutic effect of HSV-tk+ GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3:536–542. doi: 10.1006/mthe.2001.0298. [DOI] [PubMed] [Google Scholar]
- 16.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucher PD, Shewach DS. In vitro and in vivo enhancement of ganciclovir-mediated bystander cytotoxicity with gemcitabine. Mol Ther. 2005;12:1064–1071. doi: 10.1016/j.ymthe.2005.07.643. [DOI] [PubMed] [Google Scholar]
- 18.Wildner O, Blaese RM, Candotti F. Enzyme prodrug gene therapy: synergistic use of the herpes simplex virus-cellular thymidine kinase/ganciclovir system and thymidylate synthase inhibitors for the treatment of colon cancer. Cancer Res. 1999;59:5233–5238. [PubMed] [Google Scholar]
- 19.Fridlender ZG, Sun J, Singhal S, Kapoor V, Cheng G, Suzuki E, Albelda SM. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther. 2010;18:1947–1959. doi: 10.1038/mt.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 21.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133:98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 22.Yanagimoto H, Takai S, Satoi S, Toyokawa H, Takahashi K, Terakawa N, Kwon A-H, Kamiyama Y. Impaired function of circulating dendritic cells in patients with pancreatic cancer. Clin Immunol. 2005;114:52–60. doi: 10.1016/j.clim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Liyanage UK, Moore TT, Joo H-G, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 24.Ayala G, Satoh T, Li R, Shalev M, Gdor Y, Aguilar-Cordova E, Frolov A, Wheeler TM, Miles BJ, Rauen K, Teh BS, Butler EB, Thompson TC, Kadmon D. Biological response determinants in HSV-tk+ ganciclovir gene therapy for prostate cancer. Mol Ther. 2006;13:716–728. doi: 10.1016/j.ymthe.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Miles BJ, Shalev M, Aguilar-Cordova E, Timme TL, Lee HM, Yang G, Adler HL, Kernen K, Pramudji CK, Satoh T, Gdor Y, Ren C, Ayala G, Wheeler TM, Butler EB, Kadmon D, Thompson TC. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum Gene Ther. 2001;12:1955–1967. doi: 10.1089/104303401753204535. [DOI] [PubMed] [Google Scholar]
- 26.Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, Hamilton WJ, Rojas-Martinez A, Chen SH, Woo SL, Grossman RG. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 27.Chévez-Barrios P, Chintagumpala M, Mieler W, Paysse E, Boniuk M, Kozinetz C, Hurwitz MY, Hurwitz RL. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol. 2005;23:7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- 28.Sterman DH, Recio A, Vachani A, Sun J, Cheung L, DeLong P, Amin KM, Litzky LA, Wilson JM, Kaiser LR, Albelda SM. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res. 2005;11:7444–7453. doi: 10.1158/1078-0432.CCR-05-0405. [DOI] [PubMed] [Google Scholar]
- 29.Hasenburg A, Tong XW, Fischer DC, Rojas-Martinez A, Nyberg-Hoffman C, Kaplan AL, Kaufman RH, Ramzy I, Aguilar-Cordova E, Kieback DG. Adenovirus-mediated thymidine kinase gene therapy in combination with topotecan for patients with recurrent ovarian cancer: 2.5-year follow-up. Gynecol Oncol. 2001;83:549–554. doi: 10.1006/gyno.2001.6442. [DOI] [PubMed] [Google Scholar]
- 30.Chiocca EA, Aguilar LK, Bell SD, Kaur B, Hardcastle J, Cavaliere R, McGregor J, Lo S, Ray-Chaudhuri A, Chakravarti A, Grecula J, Newton H, Harris KS, Grossman RG, Trask TW, Baskin DS, Monterroso C, Manzanera AG, Aguilar-Cordova E, New PZ. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rojas-Martínez A, Manzanera AG, Sukin SW, Esteban-María J, González-Guerrero JF, Gomez-Guerra L, Garza-Guajardo R, Flores-Gutiérrez JP, Elizondo Riojas G, Delgado-Enciso I, Ortiz-López R, Aguilar LK, Butler EB, Barrera-Saldaña HA, Aguilar-Cordova E. Intraprostatic distribution and long-term follow-up after AdV-tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther. 2013;20:642–649. doi: 10.1038/cgt.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teh BS, Ayala G, Aguilar L, Mai W-Y, Timme TL, Vlachaki MT, Miles B, Kadmon D, Wheeler T, Caillouet J, Davis M, Carpenter LS, Lu HH, Chiu JK, Woo SY, Thompson T, Aguilar-Cordova E, Butler EB. Phase I-II trial evaluating combined intensity-modulated radiotherapy and in situ gene therapy with or without hormonal therapy in treatment of prostate cancer-interim report on PSA response and biopsy data. Int J Radiat Oncol Biol Phys. 2004;58:1520–1529. doi: 10.1016/j.ijrobp.2003.09.083. [DOI] [PubMed] [Google Scholar]
- 33.Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci USA. 1994;91:3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirley LA, Aguilar LK, Aguilar-Cordova E, Bloomston M, Walker JP. Therapeutic endoscopic ultrasonography: intratumoral injection for pancreatic adenocarcinoma. Gastroenterol Res Pract. 2013;2013:207129. doi: 10.1155/2013/207129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, Bookbinder M, Fong Y, Jarnagin W, Blumgart L. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20:2229–2239. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- 36.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 37.Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters PWT, Lee JE, Lenzi R, Vauthey JN, Wong ABS, Phan T, Nguyen Q, Janjan NA. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293–1302. doi: 10.1016/S0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 38.Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR, Benson AB. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz MHG, Fleming JB, Lee JE, Pisters PWT. Current status of adjuvant therapy for pancreatic cancer. Oncologist. 2010;15:1205–1213. doi: 10.1634/theoncologist.2010-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bekaii-Saab T, Goldberg RM. FOLFIRINOX in locally advanced pancreas adenocarcinoma: back to the future? Oncologist. 2013;18:487–489. doi: 10.1634/theoncologist.2013-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faris JE, Blaszkowsky LS, McDermott S, Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen JN, Dias LE, Kwak EL, Lillemoe KD, Thayer SP, Murphy JE, Zhu AX, Sahani DV, Wo JY, Clark JW, Fernandez-del Castillo C, Ryan DP, Hong TS. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543–548. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 43.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng JF, Willett CG, Fernandez-del Castillo C, Ryan DP, Clark JW, Zhu AX, Rattner DW, Winkelmann JL, Warshaw AL. Patients undergoing treatment for pancreatic adenocarcinoma can mount an effective immune response to vaccinations. Pancreatology. 2005;5:67–74. doi: 10.1159/000084492. [DOI] [PubMed] [Google Scholar]
- 45.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen Y-C, Huang L-Q, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wedén S, Klemp M, Gladhaug IP, Møller M, Eriksen JA, Gaudernack G, Buanes T. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer. 2011;128:1120–1128. doi: 10.1002/ijc.25449. [DOI] [PubMed] [Google Scholar]
- 49.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, Onners B, Tartakovsky I, Choi M, Sharma R, Illei PB, Hruban RH, Abrams RA, Le D, Jaffee E, Laheru D. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P, Wuthrick E, Williams TM, Reardon J, Christopher Ellison E, Bloomston M, Bekaii-Saab T. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.