Abstract
Cell membrane microfragments called microvesicles (MV) originating from different cells are circulating in the blood of healthy subjects and their elevated numbers are found in different diseases, including cancer. This study was designed to characterise MV present in plasma of gastric cancer patients. Since majority of MV in blood are platelets-derived (PMV), plasma samples deprived of PMV were used. In comparison to control, the number of MV in patients was significantly elevated in all stages, higher in more advanced disease. Patients’ MV showed an increased membrane expression of CCR6 and HER-2/neu. The proportion of MV carrying some leucocyte determinants was low and similar in patients and control. Transmission electron microscopy showed their substantial heterogeneity in size and shape. The size determined by dynamic light scattering analysis confirmed this heterogeneity. The MV size distribution in patients was broader within the range of 10–800 nm, while in control MV showed 3-mode distribution within the range of 10–400 nm. Atomic force microscopy confirmed MV size heterogeneity with implication that larger objects represented aggregates of smaller microparticles. Patients’ MV exhibited increased absolute values of zeta potential, indicating a higher surface charge. Tumour markers HER-2/neu, MAGE-1, c-MET and EMMPRIN were detected both in control and patients’ samples with stronger expression in the latter. Significantly higher expression of MAGE-1 and HER-2/neu mRNA was observed in individual patients. All together, it suggests that at least some MV in plasma of gastric cancer patients are tumour-derived. However, their role in cancer requires further studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-009-0808-2) contains supplementary material, which is available to authorized users.
Keywords: Microvesicles, Gastric cancer, Chemokine receptors, Tumour markers
Introduction
Microvesicles (MV) are membrane microfragments secreted from cytoplasmic membrane compartments by normal and malignant cells in vitro and in vivo. MV are defined as spherical proteolipids and divided by size into two groups: smaller (30–100 nm), more homogeneous in size, released by endosomal compartment (called exosomes) and larger (0.1–1 μm), released from the surface membranes during membrane blebbing through calcium-dependent mode [1]. Although biological activity of these two types of MV appears to be similar [2], exosomes have a more homogeneous protein composition than surface membrane MV and are viewed as antigen carrying vehicles [1, 3, 4]. Surface membrane MV are characterised by the presence of procoagulant phospholipids, which confer possible prothrombotic potential, and antigens similar to those present in the membranes of the cells from which MV originate [5, 6].
Tumour cells release MV (tumour-derived microvesicles, TMV) continuously, and the rate of their shedding is increased in more malignant tumours [7, 8]. Our and others results showed that TMV released by different cell lines in vitro expressed CD44, β1 integrins, EMMPRIN, CEA, tetraspanin, HLA class I and II, TRAIL, FasL, ADAM 10, L1 and CX3CR1 [3, 9, 10] and carry mRNA for some of them [5, 9].
In in vitro cultures TMV stimulate secretion of cytokines [11] and chemokines by monocytes (Baj-Krzyworzeka, unpublished). TMV also induce secretion of several proangiogenic factors by stromal fibroblasts, chemoattract endothelial cells and enhance their proliferation [12, 13]. An increasing attention has recently been focused on MV as they are present in plasma and other body fluids of cancer patients [14–16].
Plasma level of MV is independent of donor gender or age [17], but depends on the clinical status of the patient [14, 18, 19]. In plasma of normal subjects, platelet-derived microvesicles (PMV) constitute the largest proportion of MV (~80%) [20]. The remaining 20% is composed of leucocyte, erythrocyte and endothelial cell-origin MV [13, 20]. The number of circulating MV changes during inflammation or malignant processes. The elevated counts of MV, mainly PMV, were observed in plasma of gastric cancer patients, and their level may be a predictive marker for metastasis formation [14]. An increasing number of PMV and monocyte-derived MV was found in patients with lung cancer and may be associated with vascular complications [21]. The number of endothelial and hepatic origin MV, but not total MV, correlates with the tumour size in hepatocellular carcinoma [22]. FasL-bearing MV, described in sera of patients with oral squamous [12], ovarian [2], colorectal [23] carcinomas and melanoma [24] induce apoptosis of activated T cells. TMV may also impair monocyte differentiation to dendritic cells in vitro [25].
The TMV may be involved in tumour escape (relocation of determinants) [26], induction of immunotolerance or immunosuppression [25, 27], chemoresistance of tumours [28] and promotion of angiogenesis [12]. On the other hand, MV isolated from plasma and dendritic cells from cancer patients may carry tumour-associated antigens (TAA) and induce antitumour response [29]. It may suggest that circulating MV/TMV are important factors in affecting immune cells at a distance from the tumour microenvironment [30]. To the best of our knowledge, there is no complete characterisation of circulating TMV in patients with cancer.
Here, we present, for the first time, the broad characterisation of MV found in PMV-depleted plasma of patients with gastric cancer. The analysis comprises their quantity, immunophenotype, size and physical characteristics at single particle level.
Materials and methods
Patients
Thirty-seven patients (median age 66 years, range 40–76) with biopsy proven gastric cancer at different clinical stages were studied. The postsurgical clinicopathological staging system of the UICC TNM Classification of Malignant Tumours (sixth edition) was used [31]. The control group consisted of ten healthy subjects (median age 41 years, range 33–61). All study participants signed informed consent form, and the Bioethical Committee of the Jagiellonian University approved the protocol.
Blood samples and CD61− plasma from donors and patients with gastric cancer
Peripheral blood was drawn into polypropylene tubes containing EDTA (Vacutainer System, BD Biosciences, San Jose, CA, USA). The patients’ blood was taken during routine laboratory tests. The whole blood samples were centrifuged for 15 min at 1,500×g; within 60 min of drawing plasma was collected and spun down for 30 min at 3,000×g to remove platelets. Next, plasma was carefully aspirated from the pellet, centrifuged at 15,000×g for 30 min, aliquoted and kept frozen at −80°C until use. In some analysis plasma samples were additionally centrifuged at 50,000×g for 60 min to obtain purified MV (called isolated MV).
Immunophenotyping of plasma MV
The following monoclonal antibodies (mAbs) were used to characterise the phenotype of MV: FITC-conjugated against CD3, CD11a, CD61, CD64 (all from BD Pharmingen, San Diego, CA, USA); PE-conjugated against CD29, CD34, CD41, HLA-class I, HLA-DR, CCR6, CCR7 (all from BD Pharmingen), CCR1, CCR2, CCR3, CXCR1, CXCR4 (all from R&D Systems Minneapolis, MN, USA), and APC-conjugated anti-CD14 and anti-HER-2/neu (both from BD Biosciences). Plasma samples (20 μl) were incubated with mAbs or appropriate isotype control for 30 min at 4°C, and after washing resuspended in 0.5 ml of PBS. Samples were analysed in FACSCanto flow cytometer (BD Biosciences, Immunocytometry Systems, San Jose, CA, USA) after gating on a forward (FSC) and side scatter (SSC) parameters set in log scale. Data from 500,000 events were acquired and analysed using FACS DiVa software. To create histogram overlays, data were saved in FCS 2.0 format and exported to CellQuest software (BD Biosciences).
Determination of absolute number of MV
To determine absolute counts of MV, flow cytometry with the use of BD TruCOUNT™ Tubes (BD Biosciences) was used. MV gating was accomplished by preliminary standardisation experiments using beads of different size. Samples (0.5 ml) of plasma (diluted 1:100) in TruCOUNT™ Tubes containing known quantity of 4.2-μm beads were analysed in FACSCanto flow cytometer on FSC, and SSC set in log scale. By this approach, we were able to use FSC as a trigger and analyse beads and MV on the same cytogram without using fluorochrome conjugated mAb to label MV. Samples were run at the “low flow” mode, and acquisition was stopped after 1,000 events were counted in the beads gate. Absolute numbers of MV were calculated as previously described [32], according to the formula:
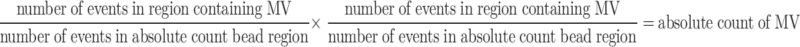 |
Transmission electron microscopy (TEM) of MV
Purified MV obtained from pooled plasma samples of ten stage IV gastric cancer patients were mixed with 2% of uranyl acetate and applied to carbon coated copper grid. The microphotographs were taken with Philips EM 300 electron microscope (Philips, Eindhoven, The Netherlands) operating at 80 kV.
Determination of MV size distribution
For particle size determination a dynamic light scattering method was used. Samples were diluted with 0.15 M NaCl to the level giving an appropriate optical signal (usually 1:100) and measured in Zetasizer Nano ZS apparatus (Malvern Instruments, Malvern, UK) equipped with a laser of λ = 633 nm. Particle size distributions were obtained from measured diffusion coefficients assuming spherical shape of particles. The obtained values represent the radius of spherical particles which move in viscous media with the same velocity as studied particles.
Zeta potential determination
Particle electrophoretic mobility was measured in samples fourfold diluted with distilled water to keep sample conductivity at the level of about 4 mS/cm assuring measurable signal. Electrophoretic mobility was recalculated to zeta potential using Smoluchowski equation valid under conditions of thin electric double layer, in comparison to particle size. Measurements were performed using Zeta Pals apparatus (Brookhaven Instruments Corporation, New York, NY, USA).
Atomic force microscopy (AFM)
Samples for AFM were prepared from isolated MV diluted 1:10,000 to 1:100,000 with 0.15 M NaCl by adsorbing onto HOPG (ZYH—mosaic spread 3.5–5.0°) highly ordered pyrolytic graphite supports (NT-MDT Co., Zelenograd, Russia). 5 μl of diluted sample was placed onto freshly exposed HOPG surface and kept there for the time necessary to get an appropriate coverage (usually from 0.5 to 5 min). After adsorption, the sample was carefully washed with distilled water to remove any traces of NaCl which could crystallise at the support surface. Next, the dry sample was placed under 7–10 nm AFM tip. The measurements were performed using Ntegra Vita and Solver P47-PRO microscopes (NT-MDT). The AFM images were recorded in semicontact mode. Experiments were performed using a commercial silicon tip with polysilicon lever (NT-MDT) with resonance frequency within a range of 140–240 kHz and spring constants k = 3.4 N/m or k = 5.8 N/m.
Western blot analysis
Plasma samples were treated for 4 min at 95°C in a sodium dodecyl sulphate (SDS) reducing buffer containing β-mercaptoethanol, electrophoresed on SDS-polyacrylamide gel (12% polyacrylamide) and transferred onto polyvinylidene difluoride membrane (BioRad, Hercules, CA, USA). The membranes were blocked for 1 h at room temperature in Tris buffered saline buffer (TBS) with 0.1% Tween and 1% bovine serum albumin (BSA, Sigma, St. Louis, MO, USA). The membranes were then incubated overnight at 4°C in TBS-Tween-BSA with various antibodies: goat anti-EMMPRIN (N-19), mouse anti-HER-2/neu (F-11), rabbit anti-MAGE-1 (FL-309) and rabbit anti-c-Met (C-12). The membranes were washed in TBS-Tween-BSA and incubated with donkey anti-goat, goat anti-mouse or goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (dilution 1:2,000). All antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). The protein bands were visualised with the SuperSignal West Pico Chemiluminescence Substrate kit as recommended by the manufacturer (Pierce, Rockforld, IL, USA) and analysed with KODAK GEL LOGIC 1500 Digital Imaging System (Kodak, Rochester, NY, USA).
Expression of MAGE-1 and HER-2/neu mRNA
Total RNA from isolated MV of individual plasma samples was obtained using the P.A.L.M. RNA Isolation Kit (PALM, Berniard, Germany) according to the manufacturer’s protocol. Reverse transcription (RT) of the obtained RNA as well as MAGE-1 “real-time” PCR were performed as previously described [33]. HER-2/neu “real-time” PCR was performed in the LightCycler (Roche, Mannheim, Germany) using the protocol and set of primers described previously [34]. The quantification of relative mRNA gene expression (fold increase), normalised to an endogenous control β-actin mRNA, was analysed using the 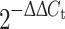 method, where ΔΔC
t = (C
t target − C
t actin)patient − (C
t target − C
t actin)donor. To verify the amplified product, melting curve analysis using the LightCycler software was performed for each sample.
method, where ΔΔC
t = (C
t target − C
t actin)patient − (C
t target − C
t actin)donor. To verify the amplified product, melting curve analysis using the LightCycler software was performed for each sample.
Statistical analysis
Statistical analysis was performed by nonparametric Mann–Whitney test in all experiments using GraphPad Prism 4 Software. Differences were considered significant at P < 0.05.
Results
The number of MV present in platelet-free plasma of gastric cancer patients
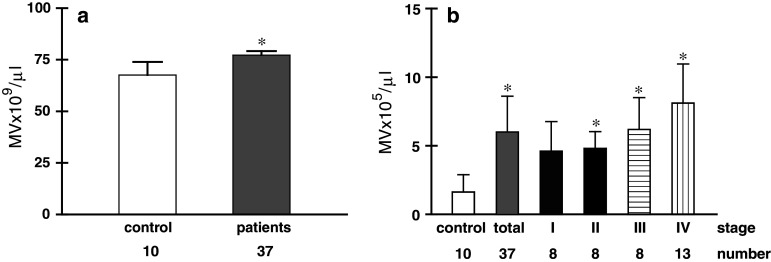
In the initial observations, the number of MV in individual plasma of patients and healthy subjects was determined by flow cytometry. Significantly elevated level of MV was observed in patients’ plasma (Fig. 1a). However, FACS analysis revealed that these MV consist of two different populations, which also differ in the expression of CD61 (Fig. 2a). Over 90% of MV located in R2 were CD61+ indicating their platelet origin (Fig. 2a, right). As in the present study we wished to analyse MV of none platelet origin, plasma samples were centrifuged for 30 min at 15,000×g [35]. The occurrence of PMV was determined by staining with anti-CD61 mAb. Figure 2b shows data from a representative experiment, which indicates that following centrifugation at 15,000×g CD61+ MV in plasma were substantially decreased and the number of MV in plasma dropped by 4 logs (Fig. 1b). The majority of CD61+ MV was seen in the pellet (Fig. 2b, right dot plot). For simplicity such samples, platelet and PMV depleted will be called thereafter platelet-free plasma (PFP). All data presented below is based on PFP samples. Next, we determined the number of MV in PFP of patients with different stages of gastric cancer (Fig. 1b). In comparison to control, the number of CD61− MV was significantly increased in the whole group of patients, as well as in stage II–IV disease. There were no differences between stages, but the highest level was observed in the most advanced disease.
Fig. 1.
The number of MV (mean ± SD) in a whole plasma of control and patients’ samples. b PFP of control and patients with different stages of gastric cancer. Significance in comparison to control; *P < 0.001
Fig. 2.
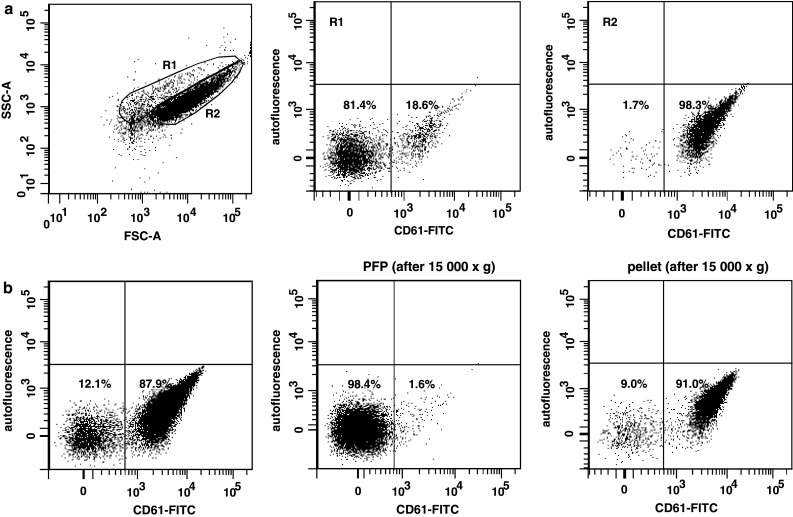
FACS analysis of morphology and CD61 expression in different preparations of plasma. Acquisition parameters were set-up to exclude remaining platelets. a Events from plasma samples were gated into two populations according to FSC and SSC parameters (R1 and R2) (left dot plot) and analysed for CD61 expression. Data from R1 (middle) and R2 (right) are shown. b Plasma samples were centrifuged (15,000×g) and both supernatants and pellets were stained for CD61 expression. Dot-plots show CD61 staining in whole plasma (left dot plot), supernatant (middle) and pellet (right). Data from representative experiment are shown
Phenotype of MV
Determination of the expression of various leucocyte surface determinants by flow cytometry revealed low level of MV expressing CD45 (mean 0.8%; range 0.2–1.2%), CD64 (1.7%; range 1–2.2%), CD11a (1.9%; range 0.9–2.6%) and CD18 (1.4%; range 0.8–2.0%) both in normal and patients’ plasma and a lack of expression of CD3, CD19, CD1a, CD14, CD34 and HLA-DR. Approximately up to 2% MV were HLA-class I positive (range 1.5–2.6%). Interestingly enough, expression of several chemokine receptors (CR) was observed (Table 1). Patients’ MV were characterised by a higher expression of CCR6 and lower expression of CXCR4. CCR1 was increased only in patients with stage I cancer.
Table 1.
Expression of chemokine receptors (%) on MV from gastric cancer patients (different stage) or healthy donors
| Marker | Control | Patients | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|---|---|
| CCR1 | 1.3 ± 0.9 | 0.7 ± 0.8 | 0.3 ± 0.3* | 0.8 ± 0.7 | 0.6 ± 0.7 | 0.9 ± 0.9 |
| CCR2 | 3.5 ± 2.0 | 2.5 ± 2.6 | 2.9 ± 3.2 | 3.7 ± 3.8 | 2.1 ± 1.9 | 2.1 ± 1.6 |
| CCR3 | 4.0 ± 3.0 | 4.5 ± 2.7 | 6.1 ± 0.7 | 6.6 ± 1.6 | 1.9 ± 0.8 | 4.2 ± 1.0 |
| CCR6 | 0.8 ± 1.2 | 4.5 ± 2.7* | 6.4 ± 0.6*** | 4.2 ± 0.3* | 2.8 ± 0.5 | 4.5 ± 0.5** |
| CCR7 | 2.4 ± 2.8 | 2.7 ± 2.4 | 2.8 ± 2.0 | 2.0 ± 1.7 | 1.1 ± 0.7 | 3.6 ± 2.7 |
| CXCR1 | 0.8 ± 0.8 | 0.7 ± 0.5 | 0.8 ± 2.3 | 0.4 ± 3.3 | 0.7 ± 2.1 | 0.7 ± 2.6 |
| CXCR4 | 4.8 ± 4.3 | 1.1 ± 1.0* | 0.8 ± 2.7* | 1.6 ± 2.0 | 0.9 ± 1.4* | 1.2 ± 2.4** |
Data are presented as mean ± SD from eight different experiments
* P < 0.05 comparing to control
** P < 0.005 comparing to control
*** P < 0.0001 comparing to control
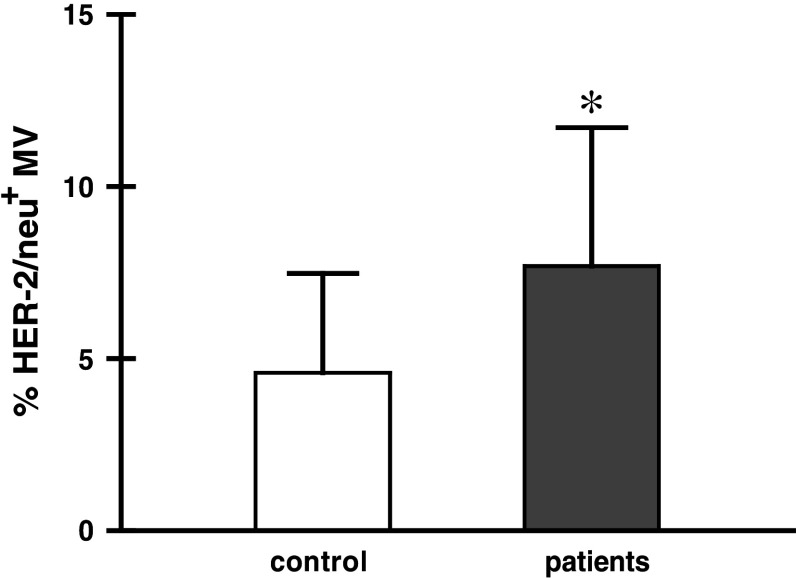
Then, we looked for the presence of HER-2/neu on MV from both patients (stage IV) and control (Fig. 3). The patients showed significantly higher proportion of HER-2/neu+ MV when compared to control. Expression of MUC-1 was observed on approximately 1–2% MV (data not shown).
Fig. 3.
Membrane expression of HER-2/neu on MV of stage IV gastric cancer patients (n = 13) and control (n = 10); *P < 0.05
Characteristics of MV
This has been performed on isolated MV from two different pools of PFP from both patients with stage IV of gastric cancer and control, each containing ten individual samples.
Morphology and the size
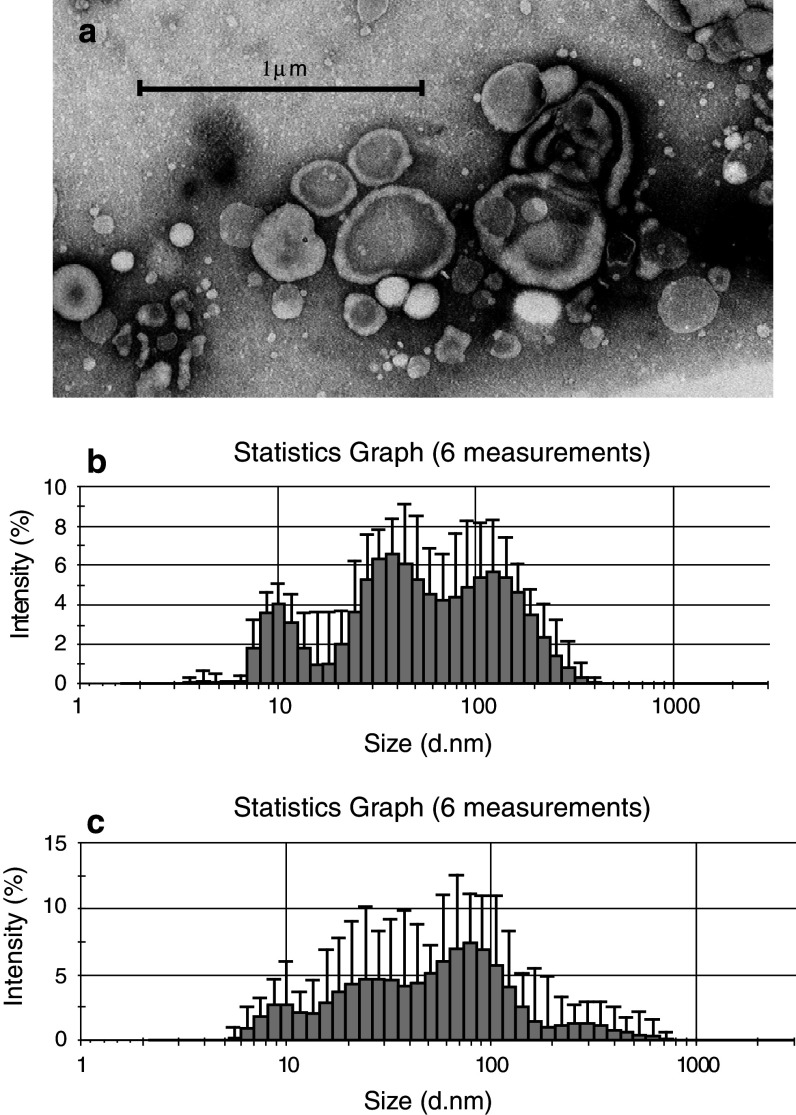
Transmission electron microscopy revealed substantial heterogeneity of isolated MV both in size and shape (Fig. 4a), seen in patients’ and control plasma samples. The dynamic light scattering analysis was used to determine the size distribution of MV present in PFP plasma of donors and patients. The size range of MV in control was approximately 10–400 nm and showed 3-mode distribution with sizes of approximately 10, 30–50 and 140–200 nm (Fig. 4b). The most frequent events in patients and control were around 10 nm (data not shown). In plasma from patients’ MV size distribution was very dispersed showing the whole range of sizes from around 10 to 800 nm, the most common being also around 10 nm (Fig. 4c). This indicated that population of MV in patients is more heterogeneous in size.
Fig. 4.
Morphology and size of isolated MV from PFP. a Transmission electron microscopy of purified MV from plasma of gastric cancer patients (magnification 60,000×) and size distribution of MV in PFP from b control and c patients analysed by dynamic light scattering
Zeta potential analysis
Zeta potential characterises the effective charge of any objects, including particles, membranes, etc. Using PALS Zeta Potential Analyzer it was found that control samples had −11.9 ± 1.8 mV and patients’ −16.5 ± 1.13 mV (significantly different at P < 0.05), indicating a higher electrokinetic charge of patients’ MV.
Atomic force microscopy
Purified MV isolated from control samples were analysed in atomic force microscopy to identify precisely the shape of the objects seen. Single particles were detected only when adsorption was performed on samples 100,000 times diluted. Pictures taken by AFM characterise well object shapes and sizes in two dimensions (X and Y) whereas Z value cannot be directly correlated with the height of the real object. Figure 5a shows that majority of MV formed aggregates of the sizes corresponded to those found by light scattering analysis (Fig. 5b). AFM pictures of single particles indicated that they are spheroid in shape with diameters of about: 8.0–9.5 nm × 13.0–16.5 nm. These values corresponded well with the most frequent fraction of objects measured by light scattering (first peak of maximum size of about 10 nm) (Fig. 5c). Such small objects tend to form larger aggregates, and the first step of this process can be already noticed when analysing the cross-section of them (Fig. 5d). The object shown is formed from a few subunits.
Fig. 5.
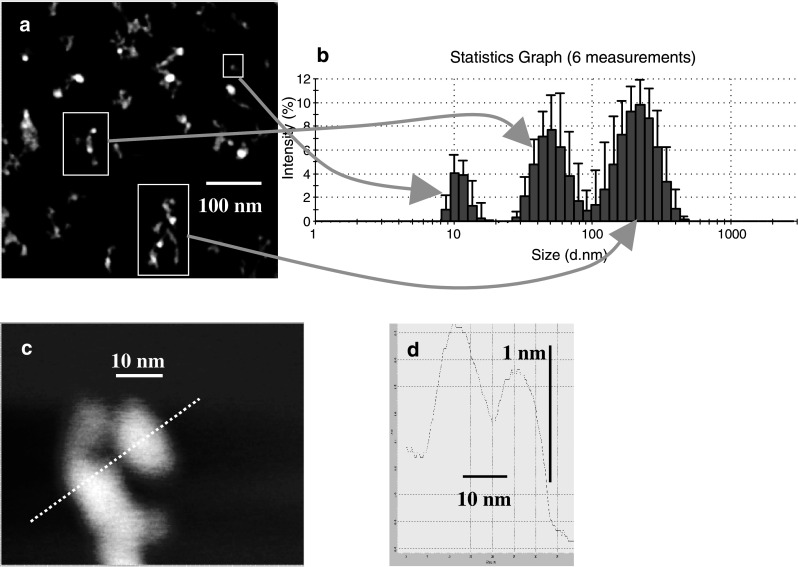
AFM analysis of MV. a Scan of MV taken at scan area 500 nm × 500 nm. b Marked three representative MV sizes detected by dynamic light scattering. c Scan of MV with quantified cross-section. d Complex structure of MV after cross-section
Expression of tumour-associated proteins
Western blot was used for detection of HER-2/neu, c-MET, MAGE-1 and EMMPRIN, which are known to be overexpressed by cancer cells. In patients’ samples, the presence of all of them was found (supplementary data). However, weaker bands of these markers were also found in control samples (data not shown). These proteins were not detected in plasma samples after centrifugation at 50,000×g, i.e. following removal of MV.
Expression of MAGE-1 and HER-2/neu mRNA
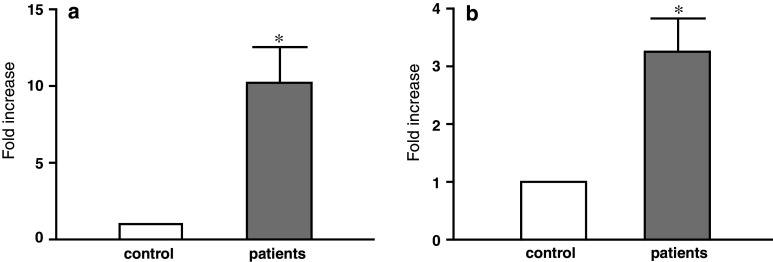
Isolated MV from individual plasma samples of five different patients with stage IV gastric cancer and healthy donors were analysed for MAGE-1 and HER-2/neu mRNA expression. The mRNA for each of the two genes was found both in control and patients’ MV; however, it was substantially higher in the latter. The MAGE-1 mRNA expression was about 210 higher in the patients’ samples than in control. HER-2/neu-mRNA expression was 23 higher in the patients’ samples (Fig. 6).
Fig. 6.
Fold increase in a MAGE-1 and b HER-2/neu mRNA expression in isolated MV of patients with stage IV gastric cancer (n = 5) as compared to control subjects (n = 5); *P < 0.05
Discussion
In this study, the attempts were made to characterise MV present in plasma of gastric cancer patients. Since it is known that the vast majority (over 80%) of MV present in plasma are of platelet origin [20], initial centrifugation at 3,000×g to remove platelets was followed by centrifugation at 15,000×g. This was effective in depletion of CD61+ PMV, which were detected in the pellet (up to 91% PMV). By this approach, the number of MV in PFP dropped substantially. Although the number of MV in the initial plasma of patients was higher than in control, the use of PFP revealed much more pronounced differences. The levels of MV did not correlate significantly with different stages of cancer, but they were more numerous in more advanced cancer. However, as AFM revealed that MV form aggregates, it may be that the numbers detected are underestimated. Determination of immunophenotype by flow cytometry indicated a very small proportion of MV expressing CD45, CD64, CD11a, CD18 and HLA-class I, but no other T and B lymphocytes, monocytes and probably no endothelial/stem cells determinants, as judged by the absence of CD34. This may indicate that the vast majority of MV is not of leucocyte origin. However, it is also possible that mAbs may not be able to bind to very small particles, which are the most frequent. On the other hand, expression of surface determinants on MV often does not reflect that on the membrane of cells they are originating from [5, 6]. However, when the small percentages of MV with leucocyte determinants are recalculated to absolute numbers, e.g. CD45+ cells in normal plasma samples, they are similar to those found in the other studies [36]. MV expressed several chemokine receptors, but only CCR6 was elevated on MV from the patients. We have previously shown that micrometastatic tumour cells which have a high expression of CCR6 are present in the blood, bone marrow and lymph nodes from patients with gastric cancer [37]. Also, several cell lines established from gastric cancer and MV released by them expressed CCR6 [our unpublished data]. It may suggest that tumour cells are a source of CCR6+ MV. CCR6 have recently been shown to be involved in prostate cancer progression [38] and metastasis formation in colorectal cancer [39]. Another possible source of CCR6+ MV might be CD3+ T cells infiltrating gastric mucosa as they have a significantly increased CCR6 expression. However, it is not the case here as no CD3 on MV was detected. We also observed the elevated level of CCR1 positive MV in plasma of stage I patients, which is in keeping with an increased expression of CCR1 on gastric cancer tissue described by Sugasawa [40]. Surprisingly, no CCR7 which is associated with gastric cancer metastasis to lymph nodes [41] was detected, but there are no data on other metastatic lesions. Furthermore, none of gastric cancer cell lines established by us expressed CCR7 (data not shown). CXCR4 and its ligands influence the dissemination, immune rejection and neoangiogenesis of human gastrointestinal cancers [42]. Strong expression of CXCR4 on gastric cancer was described, which correlated with lymph node metastases [43]. We observed lower level of CXCR4 positive MV in all patients, and the reason for this downregulation is unclear. Finally, MV showed membrane expression of HER-2/neu. Although HER-2/neu+ MV were seen in both control and patients, their proportion was substantially higher in the latter. The presence of this antigen in normal plasma is not surprising as it is present on some epithelial cells but overexpressed on cancer cells, which may explain the increased HER-2/neu+ MV in patients samples. This suggests that at least some HER-2/neu+ MV in patients are of tumour cells origin. To our knowledge, this is the first demonstration of the expression of this TAA on plasma MV.
TEM pictures showed heterogeneity and large differences both in size and shape with the most common round shaped particles. Differences in size were confirmed by dynamic light scattering technique which measures temporary fluctuation in intensity of light dispersed by particles and provides precise measure of particle size, even as small as 10 nm. Although flow cytometry is recommended as a reference method for MV analysis [36, 44], it should be noted that this method is unable to determine particles smaller than 200 nm. There were some differences in MV sizes between patients and control, and their distribution was also different. Size distribution was more dispersed in patients, while three clear cut peaks were seen in control. However, in both cases the most frequent MV size was around 10 nm. This corresponded well to AFM scans of control sample in which MV of three sizes were identified. The larger size MV represented aggregates of small particles. However, AFM pictures indicated that even small size particles are composed of a few subunits. It should be stressed that in contrast to TEM objects observed in AMF are much closer to those existing in solutions.
Patients’ MV were characterised by significantly higher absolute values of zeta potential, which provides information about electrokinetic charge of the particle. Increased values of zeta potential may indicate higher stability of suspension containing similar type particles [45]. The small particles adsorbed by local interactions at various surfaces produce or modify their heterogeneity [46]. It may be suggested that higher zeta potential facilitate known role of small particles in adherence to other particles, surfaces, phospholipid membranes, etc., as shown for MV in patients with gastrointestinal diseases [47].
Western blot analysis showed the presence of HER-2/neu, c-MET, MAGE-1, EMMPRIN, seen both in patients’ and control samples, though were less expressed in the latter (supplementary data). However, it should be treated with caution as we have no direct proof that they are MV-associated. However, since no such proteins were detected in plasma samples following centrifugation at 50,000×g, typically used to obtain MV in the pellet, it may suggest indirectly that these found in PFP are MV-associated.
Expression of MAGE-1 and HER-2/neu mRNA was found in isolated MV of individual plasma samples both from patients and control; however, the mRNA expression was substantially higher in the former. This corresponded well to these proteins detected by Western blot. The indirect evidence that these mRNAs were MV-associated was obtained by finding that they were also present in the pellet of plasma samples obtained by centrifugation at 50,000×g, i.e. containing purified MV. This is also in keeping with our previous observations that MV from cancer cell lines carry mRNA for IL-8, VEGF and HGF mRNA [5]. Therefore, it could be stated that circulating MV present in plasma also carry mRNA for some TAA.
In summary, this study shows that plasma level of MV of non-platelet origin is elevated in gastric cancer patients and describes their characteristics, including immunophenotype, morphology, size, structure at the single particle level, expression of some TAA proteins and their mRNA. However, further studies are required on their biological role in cancer as TMV may be involved in induction of immunosuppression, dysfunction or death of immune cells and metastasis formation [30]. On the other hand, they may play a role as a cell-free antigen source for anticancer vaccines [26].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by the State Committee for Scientific Research (grant no. 2 PO5A 049 29) and by Jagiellonian University Medical College (grant no: CRSW K/ZBW/000092). We thank Dr. Barbara Urbanowicz for TEM analysis and Mrs. Irena Ruggiero for skilful technical assistance.
Abbreviations
- AFM
Atomic force microscopy
- MV
Microvesicles
- PFP
Platelet-free plasma
- PMV
Platelet-derived microvesicles
- TEM
Transmission electron microscopy
- TMV
Tumour-derived microvesicles
References
- 1.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DD, Gerçel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM, Kunzelmann C. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69:785–793. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 4.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3798. [PubMed] [Google Scholar]
- 5.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor DD, Black PH. Shedding of plasma membrane fragments. Neoplastic and developmental importance. Dev Biol. 1986;3:33–57. doi: 10.1007/978-1-4684-5050-7_3. [DOI] [PubMed] [Google Scholar]
- 7.Ginestra A, Miceli D, Dolo V, Romano FM, Vittorelli ML. Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res. 1999;19:3439–3445. [PubMed] [Google Scholar]
- 8.Poste G, Nicolson GL. Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci USA. 1980;77:399–403. doi: 10.1073/pnas.77.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi DS, Lee JM, Park GW, Lim HW, Bang JY, Kim YK, Kwon KH, Kwon HJ, Kim KP, Gho YS. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res. 2007;6:4646–4655. doi: 10.1021/pr070192y. [DOI] [PubMed] [Google Scholar]
- 10.Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36:247–254. doi: 10.1385/IR:36:1:247. [DOI] [PubMed] [Google Scholar]
- 11.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Zembala M. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol Lett. 2007;113:76–82. doi: 10.1016/j.imlet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 13.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 14.Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR, Kim HK, Ryu KW, Bae JM, Kim S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastatic predictor. Eur J Cancer. 2003;39:184–191. doi: 10.1016/S0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 16.Taylor DD, Gercel-Taylor C, Gall SA. Expression and shedding of CD44 variant isoforms in patients with gynecologic malignancies. J Soc Gynecol Invest. 1996;3:289–294. doi: 10.1016/S1071-5576(96)00022-6. [DOI] [PubMed] [Google Scholar]
- 17.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 18.Fujimi S, Ogura H, Tanaka H, Koh T, Hosotsubo H, Nakamori Y, Kuwagata Y, Shimazu T, Sugimoto H. Activated polymorphonuclear leukocytes enhance production of leukocyte microparticles with increased adhesion molecules in patients with sepsis. J Trauma. 2002;52:443–448. doi: 10.1097/00005373-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–1135. doi: 10.2741/1270. [DOI] [PubMed] [Google Scholar]
- 20.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.CIR.0000036596.59665.C6. [DOI] [PubMed] [Google Scholar]
- 21.Kanazawa S, Nomura S, Kuwana M, Muramatsu M, Yamaguchi K, Fukuhara S. Monocyte-derived microparticles may be a sign of vascular complication in patients with lung cancer. Lung Cancer. 2003;39:145–149. doi: 10.1016/S0169-5002(02)00441-5. [DOI] [PubMed] [Google Scholar]
- 22.Brodsky SV, Facciuto ME, Heydt D, Chen J, Islam HK, Kajstura M, Ramaswamy G, Aguero-Rosenfeld M. Dynamics of circulating microparticles in liver transplant patients. J Gastrointestin Liver Dis. 2008;17:261–268. [PubMed] [Google Scholar]
- 23.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Lorenzo MJ, Anel A, Alava MA, Piñeiro A, Naval J, Lasierra P, Larrad L. The human melanoma cell line MelJuSo secretes bioactive FasL and APO2L/TRAIL on the surface of microvesicles. Possible contribution to tumor counterattack. Exp Cell Res. 2004;295:315–329. doi: 10.1016/j.yexcr.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 26.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 27.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 28.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 29.Chaput N, Schartz NE, Andre F, Zitvogel L. Exosomes for immunotherapy of cancer. Adv Exp Med Biol. 2003;532:215–221. doi: 10.1007/978-1-4615-0081-0_17. [DOI] [PubMed] [Google Scholar]
- 30.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Sobin LH, Wittekind C. TNM classification of malignant tumours, 6th edn. Hoboken: Wiley; 2002. [Google Scholar]
- 32.Hong F, Hansen RD, Yan J, Allendorf DJ, Baran JT, Ostroff GR, Ross GD. β-Glucan functions as a adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 2003;63:9023–9031. [PubMed] [Google Scholar]
- 33.Szatanek R, Drabik G, Baran J, Kolodziejczyk P, Kulig J, Stachura J, Zembala M. Detection of isolated tumour cells in the blood and bone marrow of patients with gastric cancer by combined sorting, isolation and determination of MAGE-1, -2 mRNA expression. Oncol Rep. 2008;19:1055–1060. [PubMed] [Google Scholar]
- 34.Goebel SU, Iwamoto M, Raffeld M, Gibril F, Hou W, Serrano J, Jensen RT. HER-2/neu expression and gene amplification in gastrinomas: correlations with tumor biology, growth, and aggressiveness. Cancer Res. 2002;62:3702–3710. [PubMed] [Google Scholar]
- 35.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Pituch-Noworolska A, Drabik G, Szatanek R, Białas M, Kołodziejczyk P, Szczepanik A, Stachura J, Zembala M. Immunophenotype of isolated tumour cells in the blood, bone marrow and lymph nodes of patients with gastric cancer. Pol J Pathol. 2007;58:93–97. [PubMed] [Google Scholar]
- 38.Ghadjar P, Loddenkemper C, Coupland SE, Stroux A, Noutsias M, Thiel E, Christoph F, Miller K, Scheibenbogen C, Keilholz U. Chemokine receptor CCR6 expression level and aggressiveness of prostate cancer. J Cancer Res Clin Oncol. 2008;134:1181–1189. doi: 10.1007/s00432-008-0403-5. [DOI] [PubMed] [Google Scholar]
- 39.Rubie C, Oliveira-Frick V, Rau B, Schilling M, Wagner M. Chemokine receptor CCR6 expression in colorectal liver metastasis. J Clin Oncol. 2006;24:5173–5174. doi: 10.1200/JCO.2006.07.9095. [DOI] [PubMed] [Google Scholar]
- 40.Sugasawa H, Ichikura T, Tsujimoto H, Kinoshita M, Morita D, Ono S, Chochi K, Tsuda H, Seki S, Mochizuki H. Prognostic significance of expression of CCL5/RANTES receptors in patients with gastric cancer. J Surg Oncol. 2008;97:445–450. doi: 10.1002/jso.20984. [DOI] [PubMed] [Google Scholar]
- 41.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 42.Schimanski CC, Galle PR, Moehler M. Chemokine receptor CXCR4-prognostic factor for gastrointestinal tumors. World J Gastroenterol. 2008;14:4721–4728. doi: 10.3748/wjg.14.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HJ, Kim SW, Kim HY, Li S, Yun HJ, Song KS, Kim S, Jo DY. Chemokine receptor CXCR4 expression, function, and clinical implications in gastric cancer. Int J Oncol. 2009;34:473–480. [PubMed] [Google Scholar]
- 44.Gelderman MP, Simak J. Flow cytometric analysis of cell membrane microparticles. Methods Mol Biol. 2008;484:79–93. doi: 10.1007/978-1-59745-398-1_6. [DOI] [PubMed] [Google Scholar]
- 45.Tadros TF. General principles of colloid stability and the role of surface forces. In: Tadros TF, editor. Colloid stability part I. Weinheim: Viley-VCH Verlag GmbH; 2007. [Google Scholar]
- 46.Adamczyk Z, Zembala M, Michna A. Polyelectrolyte adsorption layers studied by streaming potential and particle deposition. J Colloid Interface Sci. 2006;303:353–364. doi: 10.1016/j.jcis.2006.07.083. [DOI] [PubMed] [Google Scholar]
- 47.Jansa R, Sustar V, Frank M, Susanj P, Bester J, Mancek-Keber M, Krzan M, Iglic A. Number of microvesicles in peripheral blood and ability of plasma to induce adhesion between phospholipid membranes in 19 patients with gastrointestinal diseases. Blood Cells Mol Dis. 2008;41:124–132. doi: 10.1016/j.bcmd.2008.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.