Abstract
Fusion proteins consisting of the ligand-binding domain of CTLA4 covalently attached to an antigen (Ag) are potent immunogens. This fusion strategy effectively induces Ag-specific immunity both when introduced as a DNA-based vaccine and as a recombinant protein. CTLA4 is a ligand for B7 molecules expressed on the surface of antigen-presenting cells (APCs), and this interaction is critical for the fusion protein to stimulate Ag-specific immunity. We show that interaction of the fusion protein with either B7-1 or B7-2 is sufficient to stimulate immune activity, and that T cells are essential for the development of IgG responses. In addition, we demonstrate that human dendritic cells (DCs) pulsed with CTLA4–Ag fusion proteins can efficiently present Ag to T cells and induce an Ag-specific immune response in vitro. These studies provide further mechanistic understanding of the process by which CTLA4–Ag fusion proteins stimulate the immune system, and represent an efficient means of generating Ag-specific T cells for immunotherapy.
Keywords: CTLA4–PSMA fusion proteins, Dendritic cell targeting, Antigen presentation
Introduction
In the last decade, there has been great interest in using dendritic cells (DCs) to boost the immune response. Most of the studies used whole-cell DC vaccines pulsed with peptides, cell lysates, RNA, or genetically modified to express one or more antigens (Ag), followed by injection into the animal or human host [1–5]. An alternative and promising method of directed Ag presentation is immunotargeting, in which ligands to receptors present on antigen-presenting cells (APCs) are fused either chemically or physically to an Ag and used for immunizations. Most immunotargeting studies have used antibodies against the class II major histocompatability complex (MHC) [6, 7], the Fcγ receptor (FcγR) [8], or surface immunoglobulins [9] to target Ag to APCs. Some studies have used antibodies against cell surface molecules specific to DCs such as CD11c and DC SIGN for Ag targeting, and have reported increases in Ag-specific serum antibody titers relative to immunization with nontargeted Ag [10, 11]. Vaccination with fusion proteins between a single-chain idiotype and chemokine was also reported to induce protective immune responses against a large tumor challenge [12].
Recently, impressive results have been obtained in the use of DNA vaccines encoding fusion proteins of human IgG [13], influenza hemagglutinin-protein [14], the HIV-1 envelope glycoprotein gp120 [15] or the cytotoxic T lymphocyte antigen-4 (CTLA4) as priming immunogens capable of eliciting both cell-mediated and antibody responses in mice. A potential disadvantage of this approach is that it requires DNA transfer either directly into hosts or transduction of cells using viral or nonviral vectors. Moreover, there are a number of rate-limiting steps in the pathway of immune induction mediated by DNA vaccines, for example, limited transgene expression and lack of easy access to APCs, especially DCs. In addition, many of the DNA vaccine studies are inherently inadequate in providing the precise mechanisms of immune induction, largely because of the complexity of DC in various tissue sites [16–18], as well as the multitude of different antigens (xenogeneic) being tested for vaccine efficacy in mice [13, 15].
Some studies have demonstrated that direct injection of purified fusion protein causes potent immune stimulation in the absence of adjuvants [13, 19, 20]. Furthermore, these studies have shown that CTLA4–Ag fusion proteins are transported to lymph nodes, are capable of generating Ag-specific antibody and T cell responses, and anti-tumor immunity. CTLA4 is a high affinity ligand for B7-1 and B7-2 molecules expressed by APC, A point mutation in the CTLA4 moiety, which prevents its interaction with the B7-1 and B7-2 molecules, abrogates the immunostimulatory capacity of the fusion molecules [19], thus demonstrating the specificity of these receptor-ligand interactions in the targeting process.
The purpose of this study was to further our understanding of the mechanism behind the immunogenicity of CTLA4–Ag fusion proteins and to determine whether we could generate human T cell responses ex vivo using autologous DCs pulsed with CTLA4–Ig–Ag fusion proteins. For DC targeting purposes fragments of human prostate specific membrane antigen protein (PSMA) containing two antigenic epitopes known to be presented by HLA-A2 were chosen as model antigens. The results from mouse models demonstrate that T cells are critical to the efficient development of antibody responses to the CTLA4–Ag fusion proteins. Also, binding of the CTLA4–Ag fusion protein to either B7-1 or B7-2 is sufficient to generate a humoral response against the fusion protein; however, neither of them is individually necessary. Finally, we show that when fused to the ligand-binding domain of CTLA4, a tumor-associated antigen (TAA) could be efficiently targeted to DCs and presented to T cells ex vivo, and could generate large numbers of Ag-specific T cells using minimal amounts of fusion protein. We believe that this method may represent a simple and efficient approach to generate Ag-specific T cells for adoptive immunotherapy applications.
Materials and methods
CTLA4–Ig fusion proteins
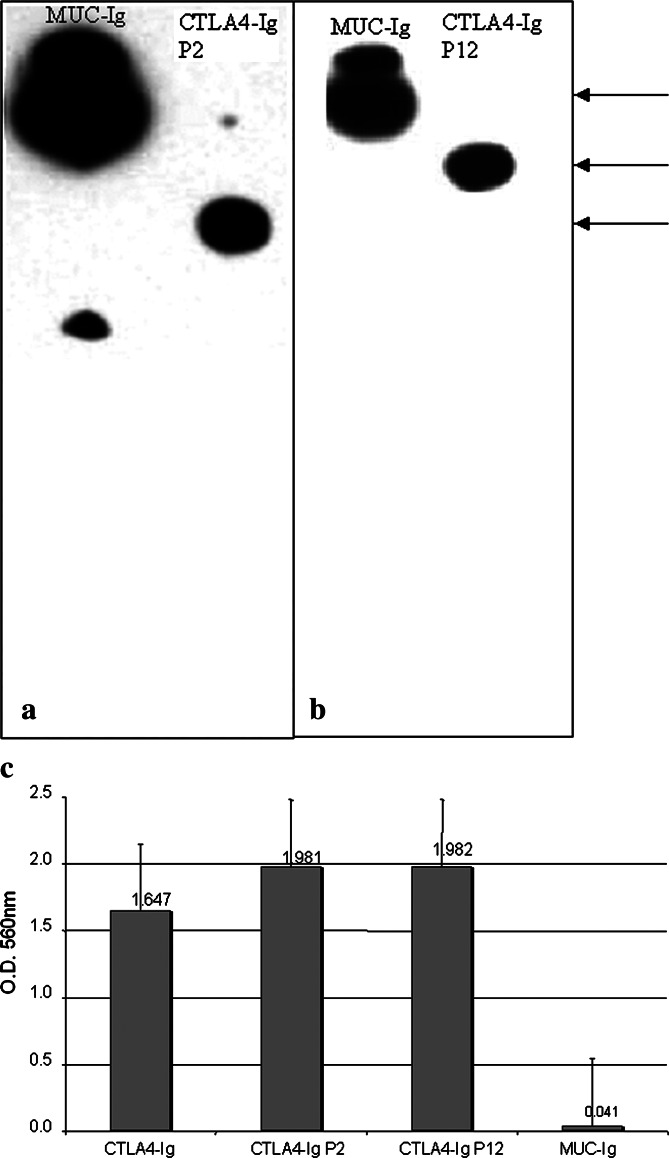
Mouse CTLA4 fused to human IgG1 Fc was purchased from R&D systems (Minneapolis, MN, USA). The Muc–Ig vector was purchased from Novagen, (Madison, WI, USA). All other fusion proteins were expressed and purified as previously described [21]. The extracellular domain of human CTLA4 (amino acids 2–116) was cloned into and expressed from the signal-pIg vector (Novagen, Madison, WI, USA). Both the CTLA4–Ig–P2 and CTLA4–Ig–P12 constructs contained the human CTLA4–Ig protein as the amino terminal portion and a fragment of the PSMA protein as the C-terminus. The P2 fragment contained amino acids SHNKYAG... and the remaining 45 amino acids through the C-terminus of PSMA. The P12 fragment contained amino acids SERLQDF... and the remaining 97 amino acids through the C-terminus of PSMA. Specific cloning details of the constructs encoding P2 and P12 CTLA4–Ig–PSMA fusion proteins are available on request. These constructs were cloned into the pIRES neo3 vector (Clontech, Palo Alto, CA, USA), and the fusion proteins were expressed in Chinese hamster ovary (CHO) cells essentially as described by Oaks et al. [19] and purified from the culture supernatants of transfected CHO cells grown in protein-free medium (HyQ PF CHO medium, Hyclone, Logan, UT, USA) by protein A chromatography. The homogeneity of the purified protein preparations was evaluated by SDS-PAGE and coomassie blue staining. Greater than 95% of the fusion proteins migrated as a single band in the expected size range, and expressed epitopes for CTLA4, human Ig, and human PSMA as determined by Western blot (Fig. 1a, b). Binding of CTLA4–Ig–PSMA fusion proteins to human B7 was verified in an ELISA (Fig. 1c).
Fig. 1.
Expression of human CTLA4 immunoglobulin PSMA fusion proteins. Western blots of fusion proteins of CTLA4–Ig–PSMA P2 (a) and PSMA P12 (b) when probed with anti-human IgG. Upper arrow shows Muc18-Ig control fusion protein of approximate molecular mass of 118 kDa. Middle arrow shows the P12 protein of 65 kDa, and bottom arrow indicates P2 fusion of approximately 60 kDa. Similar results were obtained by probing with anti-human PSMA polyclonal antiserum (Zymed, South San Francisco, CA, USA). c Shows ELISA binding of PSMA fusion proteins to human CD80. CTLA4–Ig and Muc18-Ig were used as positive and negative controls, respectively. The data represent the mean of triplicate values and experiments were done in duplicate with similar results
Mouse strains and immunizations
B7-1 knockout mice B6.129S4-CD86 (tm1shr)/J [22], B7-2 knockouts B6.129S4-CD80 (tm1shr)/J [23] and nude mice CbyJ.Cg-Foxn1(nu)/J were purchased from Jackson labs (Bar Harbor, ME, USA). Controls were heterozygous littermates. Protein immunizations were performed by intramuscular route. One microgram of purified fusion proteins in PBS was injected into the anterior tibialis muscles of groups of four to six mice on days 0, 14, and 28. Serum was collected from each mouse on days 0, 14, 28, and 35 by tail-vein bleeds and tested for the presence of humoral responses against human IgG. In select cases, sera were tested for IgM responses to human IgG.
ELISA
Antibody titers to human IgG1 were determined by ELISA. Briefly, Nunc Maxisorp wells were coated overnight with 200 ng/well of purified human IgG1 (Chemicon International, Temecula, CA, USA), and then blocked by the addition of 5% dry milk, 1% bovine serum albumin (BSA), 5% sucrose in phosphate buffered saline (PBS). Mouse sera were diluted twofold serially in PBS and then incubated for 1 h at room temperature on shaking platform. The wells were washed four times with PBS containing 0.05% Tween, and then a horseradish peroxidase conjugated anti-mouse IgG or anti-mouse IgM (both from Pierce Immunochemicals, Rockford, IL, USA) was added and incubated for 1 h at room temperature. After washing as above, a two-part TMB (3,3′,5,5′-tetramethylbenzidine) peroxidase substrate (Pierce Immunochemical) was added and the reactions were terminated after 15 min by the addition of 0.5 M H2SO4. Absorbance at 450 nm was determined, and titers were expressed as 1/dilution that was greater than three times the absorbance of wells that did not receive mouse serum.
Isolation of monocytes from peripheral blood buffy coats
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats obtained from healthy donors (Blood Center of Wisconsin, Milwaukee, WI, USA) by density gradient centrifugation over Ficoll-PaqueTM Plus (Amersham Biosciences AB, Uppsala, Sweden). Aliquots of PBMCs were cryopreserved in liquid nitrogen until use. To obtain CD14+ monocytes, the cryopreserved PBMCs were thawed and plated in 75 cm2 tissue culture flask (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ, USA) at 5×106 cells/ml in a total volume of 10 ml OptiMEM-1 (GibcoTM, Invitrogen Corp., Grand Island, NY, USA) containing 5% human AB serum (HS; BioWhittaker, Walkersville, MD, USA) and 10 U/ml of DNase-I (Sigma, St Louis, MO, USA), and incubated at 37°C with 5% CO2 for 2–3 h to allow cell adhesion. The adherent monocytes were collected after removing the nonadherent cells by extensive washings with Calcium (Ca) and Magnesium (Mg)-free PBS (Invitrogen Corp., Franklin Lakes, NJ, USA). Adherent cells were further enriched for CD14+ monocytes using Monocyte Negative Isolation kit (Dynal Biotech Inc., Lake Success, NY, USA) according to the manufacturer’s instructions. The purity of the monocytes was ∼98% as determined by the expression of monocyte cell surface marker CD14 (data not shown).
Generation of DC
To generate immature DCs (iDCs) purified monocytes were plated at 1×106 cells/well in six-well plate (Costar®, Corning Inc., Corning, NY, USA) containing 2 ml/well DC culture medium [OptiMEM-1 supplemented with 5% HS, 800 U/ml of recombinant human (rh) granulocyte macrophage colony stimulating factor (GM-CSF) and 500 U/ml of interleukin (IL)-4 (both from R&D Systems, Minneapolis, MN, USA)]. After 5-days of culture, the cells were harvested and used as iDCs. At this stage approximately 80% of the cells had typical iDC morphology and displayed cell surface markers: CD14−, CD11c+, CD80+, CD86++, HLA-A, HLA-B, HLA-C+, HLA-DR+, and CD83−.
Flow cytometry
Monoclonal antibodies (mAb) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or peridinin chlorophyll protein (PerCP) were purchased from BD-PharMingen (San Diego, CA, USA). The purity of monocytes was determined by staining them with mAbs against the human blood cell lineage specific markers CD3, CD19, CD14, and CD56, following the manufacturer’s instruction. To determine the immunophenotype before and after exposure to protein Ags and/or maturation agents, DCs were stained with mouse mAbs against human CD14, CD11c, CD80 (B7.1), CD86 (B7-2), HLA-A, HLA-B, HLA-C, HLA-DR, and CD83. Isotype control antibodies (IsoAb) were used to assess the specificity of immunostaining. Five to ten thousand events were acquired and analyzed with a FACScan flow cytometer and CellQuestTM software (Becton Dickinson, San Jose, CA, USA).
To detect the reactivity of CTLA4–Ig or CTLA4–Ig–PSMA fusion proteins to DCs, 5×105 cells were incubated with 2 μg/ml of each protein at 4°C for 30 min. The bound proteins were detected with FITC-labeled goat anti-human IgG (ICN Biomedical, Costa Mesa, CA, USA). In some experiments DCs were pretreated with mAbs against B7 molecules for 30 min prior to the analysis of fusion proteins binding to DCs.
Pulsing of DCs with protein Ags
Pulsing of autologous human DC with proteins was performed by simple coincubation with DCs. In brief, iDCs (1×106) were plated in 2 ml of fresh DC culture medium containing GM-CSF and IL-4 in six-well plate and pulsed with 1 μg/ml of one of the following recombinant proteins: rPSMA (Northwest Biotherapeutics, Inc. Bothell, WA, USA), CTLA4–Ig, CTLA4–Ig–P2, CTLA4–Ig–P12, CTLA4–Ig + rPSMA or recombinant carcinoembryonic antigen (rCEA, Sigma, St Louis, MO, USA). After 16 h of pulsing, DC cultures were exposed to 10 ng/ml of tumor necrosis factor (TNF)-α (PeproTech, Rocky Hill, NJ, USA) and 500 ng/ml of soluble CD40 ligand (sCD40L, Serotec Inc., Raleigh, NC, USA) for 48 h to induce maturation of DCs. The cells obtained expressed high levels of CD80, CD86, HLA-A, HLA-B, HLA-C, HLA-DR, and CD83, consistent with the features of mature DCs (data not shown) and used in subsequent experiments. Protein pulsed DCs were used as stimulators for CTL and as cellular targets in cytotoxicity assays.
Generation of Ag-specific effector T cells
To generate and expand Ag-specific effector T cells ex vivo, the nonadherent fraction of PBMCs were cocultured with autologous DCs unpulsed or pulsed with various recombinant protein Ags at a stimulator: responder ratio of 1:10. T cells were cultured at a density of 1×106 cells/mL R5 media (RPMI medium containing 5% HS and 2 mM l-glutamine) in six-well plate. Three days after the initial stimulation, IL-2 (20 IU/ml) and IL-7 (10 ng/ml) (both from PeproTech, Rocky Hill, NJ, USA) were added to the cultures. T cells were restimulated at weekly intervals with respective stimulator DCs at a stimulator to effector ratio of 1:10. Cultures were fed every 3 days with fresh media containing IL-2 and IL-7. At the end of three stimulations, effector T cells were harvested, washed, and cultured in cytokine-free R5 media for 24 h and then tested for Ag-specific proliferation, interferon (IFN)-γ secretion, and cytotoxic function.
Proliferation assay
The effector T cells generated ex vivo were cultured in triplicate with autologous stimulator DCs (unpulsed or pulsed with various protein antigens) at a ratio of 10:1 in 96-well microtiter plates in triplicate wells in 200 μl of R5 media. Cells were pulsed with 0.3 μCi [3H] thymidine (Amersham Biosciences Corp, Piscataway, NJ, USA) for the last 16–18 h of a 3-day culture, harvested using a FilterMateTM cell harvester (Packard, Meriden, CT, USA), and [3H] thymidine incorporation was measured using a Matrix 9600 gas-phase beta counter (Packard, Meriden, CT, USA) to determine the proliferation of T cells. Data are expressed as mean counts per minute (cpm) ± SEM of triplicate wells. Wells containing only stimulators or T cells always incorporated less than 100 cpm [3H] thymidine.
Intracellular cytokine staining to detect IFN-γ
Ag-specific T cells were generated as described before and cocultured for 72 h with various stimulator DCs at a stimulator: T cell ratio of 10:1. Brefeldin A was added at 10 μg/ml during the last 5–8 h of culture. IFN-γ secretion in cultured T cell lines was determined by intracellular cytokine staining and flow cytometry. Aliquots of cells were costained with PerCp and PE-conjugated CD3 and CD8 mAb, respectively. Subsequently cells were washed, fixed, permiabilized, and stained with FITC-labeled mAb specific for IFN-γ (BD-Pharmingen, San Diego, CA, USA). Stained cells were analyzed using a FACScan flow cytometer. Specificity of staining was demonstrated by lack of labeling with respective fluorochrome-conjugated IsoAb.
Cytotoxicity assay
Ag-specific effector T cells were generated as described before and tested for cytotoxicity in a standard chromium (51Cr) release assay. Target cells included protein pulsed or unpulsed autologous DCs as indicated in Results. Target cells were labeled with sodium chromate (51Cr) for 1–2 h and plated in a 96-well U-bottom plate at 2×104 cells/well and mixed with effector T cells at different effector/target ratios in a total volume of 200 μl of culture media. After 6-h incubation at 37°C, 150 ml of supernatant from each well was removed and the amount of chromium released by cell lysis was determined using a gamma counter. The percent specific 51Cr release was calculated as: [(mean experimental cpm-mean spontaneous cpm)/(mean maximum cpm-mean spontaneous cpm)]×100%, in which spontaneous release represents cpm in supernatants from wells containing target cells with medium only and maximum release represents cpm in supernatants from wells containing target cells in medium with 2% Triton X-100 cpm. Assay wells were run in triplicate and average values are presented. Spontaneous release was less than 15% of the total release by detergent in all assays. SD of the means of triplicate wells was less than 5%.
Results
CTLA4–Ig fusions can interact with either B7-1 or B7-2 to initiate a humoral response
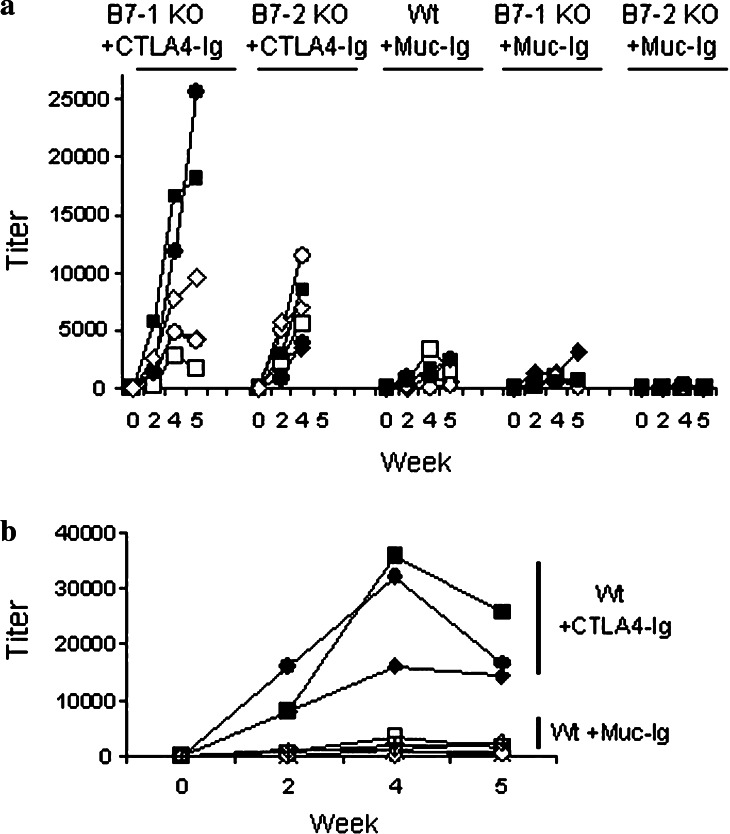
The extracellular domain of CTLA4 contains a motif, MYPPPY (amino acids 99–104) that is essential for interaction of B7-1 and B7-2. Mutation of Y104 to an alanine (MYPPPA) completely disrupts the interaction of CTLA4 with B7-1 and B7-2 [8]. Chimeric proteins of antigen and mutated CTLA4Y104A are not effective vaccines [19]. Although this clearly demonstrates a need for interaction of CTLA4 with the B7 receptors to initiate humoral responses, it does not identify the individual role of each B7 molecule in the process. This issue was addressed by injecting B7-1 or B7-2 knockout mice with the mouse CTLA4–human IgG1 fusion protein as immunogen. As shown in Fig. 2a, mice received multiple injections of fusion protein and were tested for the presence of humoral responses against the fusion protein several times. Although lower when compared to wild-type mice (Fig. 2b), detectable humoral responses against human IgG1 occurred in the majority of B7-1 and B7-2 knockouts. As expected no antibody response to mouse CTLA4 was detected in any of the vaccinated animals studied (data not shown). The reduced humoral response to hIgG1 portion of the fusion proteins in single knockouts for B7-1 or B7-2 may reflect a general immune deficiency in these animals or may indicate that the CTLA4 fusion protein is most effective at stimulating immune responses when both B7 molecules are expressed.
Fig. 2.
B7.1 and B7.2 are the targets for CTLA4-antigen immunization strategies. a Six B7-1 KO mice and six B7-2 KO mice were injected with murine CTLA4 and human IgG1 fusion protein (CTLA4–Ig) as detailed in Materials and methods. Four B7-1 KO mice and four B7-2 KO mice were injected with Muc–Ig. Titers of mouse IgG specific for human IgG were tested on indicated days. b Comparison of immunization of wild-type mice with either CTLA4–Ig or Muc–Ig fusion proteins. Immunizations were done as described above and mouse IgG specific for human IgG was tested on indicated days. Data shown are from individual mice (each symbol) treated the same way and experiments were repeated in duplicate with similar results
T lymphocytes are essential to the development of humoral responses against CTLA4–Ig fusion proteins
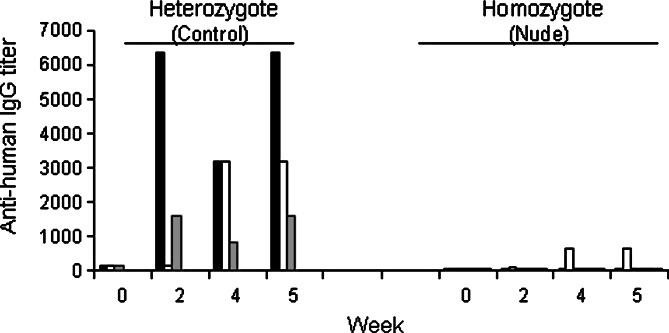
B lymphocytes express three cell surface receptors that could bind the dimeric CTLA4–Ig fusion protein; namely, the B7 molecules on B cells themselves, Fc receptors via the IgG1 constant region, and through the B cell receptor in the form of surface Ig. Consequently, it is possible that the CTLA4–Ig fusion protein can activate multiple signaling pathways in B lymphocytes and that lead to class switching independent of T cell help. In order to evaluate this possibility, we injected the fusion proteins into nude mice and periodically tested their sera for the presence of IgG molecules specific for the antigen (human IgG1 Fc portion of the fusion protein). Antigen-specific humoral responses were markedly reduced in nude mice (Fig. 3); thus, functional T cells are essential in the induction of the humoral immune response against CTLA4–Ig fusion proteins. In addition, neither homozygous nude mice nor heterozygous littermates showed appreciable IgM responses to CTLA4–Ig.
Fig. 3.
Nude mice do not elicit a humoral response against the CTLA4–Ig fusion protein. Six nude mice (homozygote) and three unaffected littermates (heterozygote) were injected with CTLA4–Ig fusion protein and serum was collected to determine titers of IgG specific for human Ig
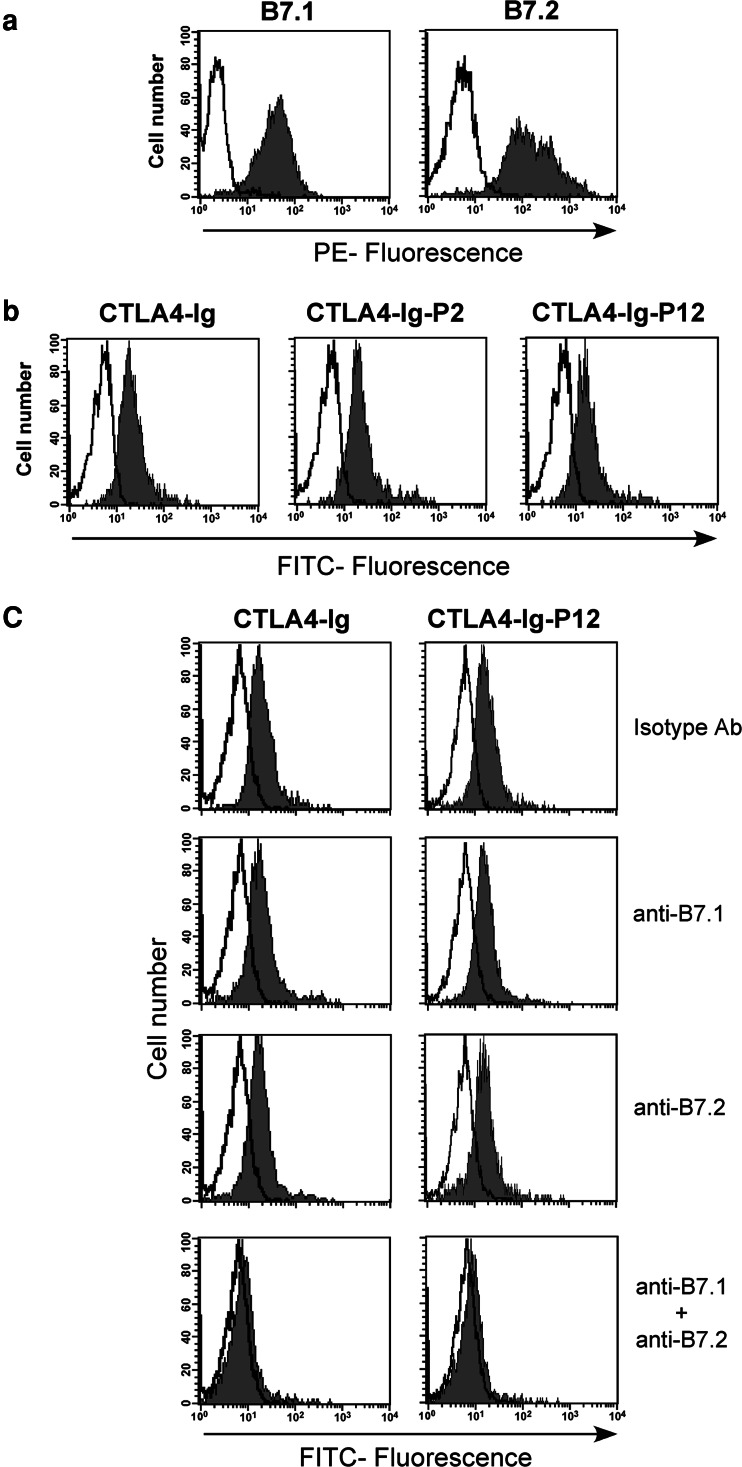
Reactivity of CTLA4–Ig and CTLA4–Ig–PSMA fusion proteins with B7-1 and B7-2 molecules on DCs
To determine the functional activity of CTLA4–Ig–Ag fusion proteins, we first tested their ability to bind to B7 molecules on human MoDCs. The human MoDCs express high levels of B7 molecules on cell surface (Fig. 4a) and the CTLA4–Ig, CTLA4–Ig–Ag (CTLA4–Ig–P2 and CTLA4–Ig–P12) fusion proteins efficiently bind to DCs (Fig. 4b). Further the results shown in Fig. 4c indicate that both the B7 molecules were involved in targeting the CTLA4–Ig or CTLA4–Ig–P12 fusion proteins to DCs as blocking the cells with one of the B7 molecules on DCs could not inhibit their binding (Fig. 4c). However, it was significantly reduced if the cells were blocked simultaneously with antibodies against both the B7 molecules prior to CTLA4–Ig or CTLA4–Ig–Ag fusion protein binding assay (Fig. 4c). The data from blocking experiments to confirm specific targeting of CTLA4 to B7 molecules shows that blocking of one of the B7 molecules did not reduce the binding of CTLA4–Ig fusion proteins while reduced binding was achieved with double-blockade. This may be due to the subsaturating amount of CTLA4–Ig proteins used in these experiments. Irrespective of this issue, our data clearly indicate that B7 molecules are exclusively involved in CTLA4 binding and ruled out the possible involvement of Fcγ receptors or other unknown molecules and further suggest that presence of one of the B7 molecules is sufficient for the binding of CTLA4–Ig fusion proteins.
Fig. 4.
CTLA4–Ig–Ag fusion proteins specifically bind to B7-1 and B7-2 molecules on human MoDCs. a Expression of B7 molecules on the human MoDCs. Cells were stained with either PE-conjugated B7.1 or B7.2 (closed histograms in gray color) or isotype control Ab (open histogram). b Human MoDCs were stained with CTLA4–Ig, CTLA4–Ig–P2, or CTLA4–Ig–P12. Binding of CTLA4–Ig fusion proteins to B7 molecules on DCs was detected using FITC-conjugated goat anti-human IgG. c Human MoDCs were treated with mAb(s) against either B7.1 or B7.2 or both to block either one or both of the B7 molecules. Under identical conditions aliquots of cells were treated with isotype control Abs to determine the specificity of blocking of B7 molecules. Cells were then stained with CTLA4–Ig or CTLA4–Ig–P12. Binding of CTLA4–Ig fusion proteins to B7 molecules on DCs was detected using FITC-conjugated goat anti-human IgG. b, c The closed histograms represent fluorescence from cells stained with either CTLA4–Ig or CTLA4–Ig–P12 and FITC-conjugated goat antihuman IgG and the open histograms represent fluorescence from cells stained with FITC-conjugated goat antihuman IgG alone
The human DCs pulsed with CTLA4–Ig–Ag fusion proteins elicit cellular immune responses in vitro and specific interaction between the Ag and CTLA4–Ig is required for an enhanced T cell response
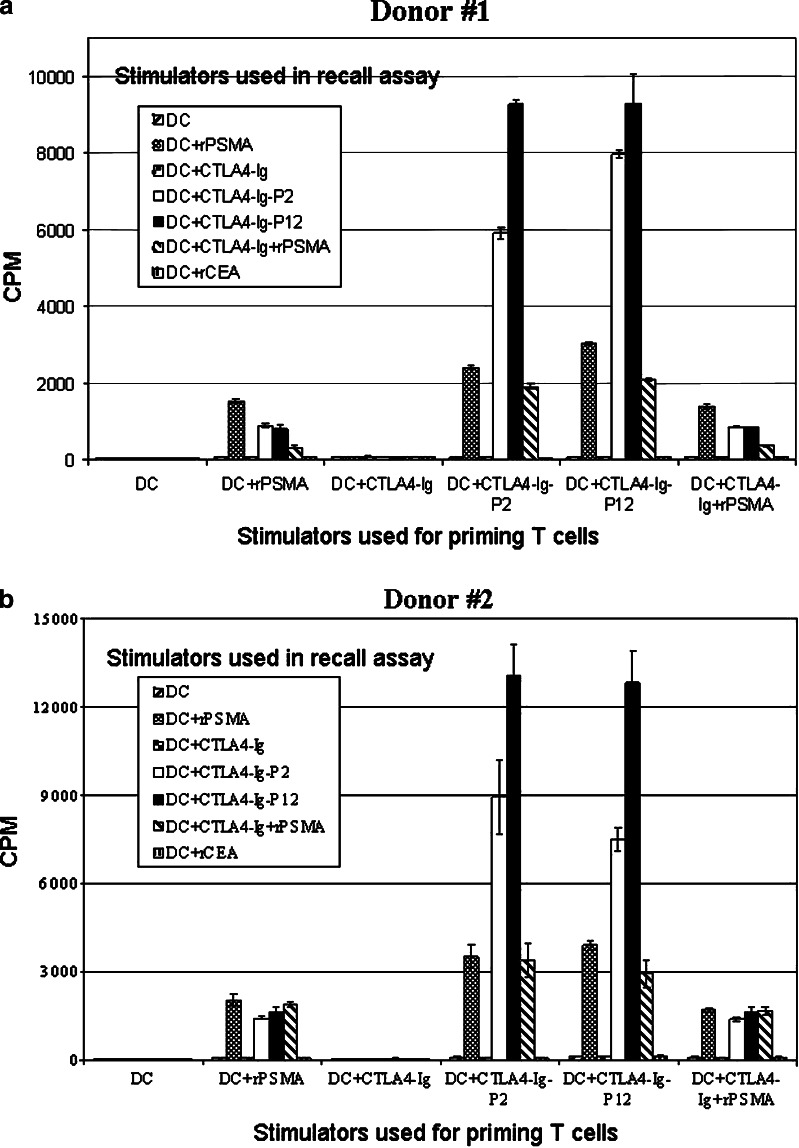
Though the mechanism is unknown, CTLA4–Ig protein that is injected into muscle rapidly accesses lymph nodes [14]. Efficient trafficking of Ag to lymph nodes typically occurs when DCs engulf Ag in the periphery and then travel to a draining lymph node for efficient presentation of Ags to circulating lymphocytes [14]. In order to directly test the significance of delivery of Ags to DCs as CTLA4–Ig fusion proteins and the ability of DCs to ingest and present antigens that are part of the CTLA4–Ig fusion proteins, we constructed fusion proteins in which CTLA4–Ig was fused to the fragments of PSMA containing known HLA-A2-specific epitopes as described in Materials and methods. DCs generated ex vivo from PB monocytes of two HLA-A2+ normal human donors were pulsed with various protein formulations recombinant PSMA (rPSMA), carcinoembryonic antigen (rCEA), or CTLA4–Ig–PSMA fusion proteins or unlinked CTLA4–Ig plus rPSMA and evaluated for their ability to prime naïve autologous T cells against the target antigen PSMA in vitro using T cell proliferation assays as described in Materials and methods.
As shown in Fig. 5, CTLA4–Ig–PSMA fusion proteins inhibited neither the activation of naïve T cells nor the stimulation of previously activated T cells. Rather, they behaved as efficient vehicles for targeting Ag to DCs. The CTLA4–Ig–PSMA fusion proteins enabled efficient presentation of PSMA by DCs (Fig. 5) as determined by severalfold increase in T cell proliferation in Ag recall assays. Moreover, T cell proliferation was PSMA-specific because they did not proliferate when exposed to autologous DCs pulsed with either the CTLA4–Ig portion of the fusion protein or an irrelevant Ag, CEA (Fig. 5).
Fig. 5.
Dendritic cell targeting enhances specific T cell response to PSMA. Monocyte-derived DCs were generated from two HLA-A2+ donors and pulsed with various forms of protein antigens and used as stimulators to prime autologous T cells ex vivo as described in Materials and methods. Effector T cells were collected at the end of three weekly stimulations (21 days) and challenged with various stimulator DCs as shown and tested for Ag-specific proliferation using [3H] thymidine incorporation assay as described in Materials and methods. Results are shown as mean cpm ± SEM of triplicate wells
To eliminate the possibility that the amplified autologous T cell response seen in vitro using the DC pulsed with CTLA4–Ig–PSMA fusion proteins was caused by nonspecific stimulation by CTLA4–Ig, DCs were pulsed with a mixture of CTLA4–Ig and rPSMA protein and used to stimulate the T cell lines that were previously primed with CTLA4–Ig–PSMA fusion proteins in vitro. As shown in Fig. 5 during Ag recall, the DCs pulsed with CTLA4–Ig alone or the mixture of unlinked CTLA4–Ig plus PSMA protein did not amplify the proliferation of these T cells more than that obtained with rPSMA alone, indicating that covalent attachment of the antigen to CTLA4–Ig is required for its effective targeting of Ag to DCs and its presentation to T cells. Further these results demonstrate that co-incubation of unlinked CTLA4–Ig and rPSMA with DCs does not block the immune response against PSMA as we could detect similar level of T cell proliferative response against rPSMA pulsed DCs in the presence or absence of unlinked CTLA4–Ig (Fig. 5).
CTLA4–Ig–Ag fusion protein pulsed DCs are efficient in inducing Ag-specific CTL response
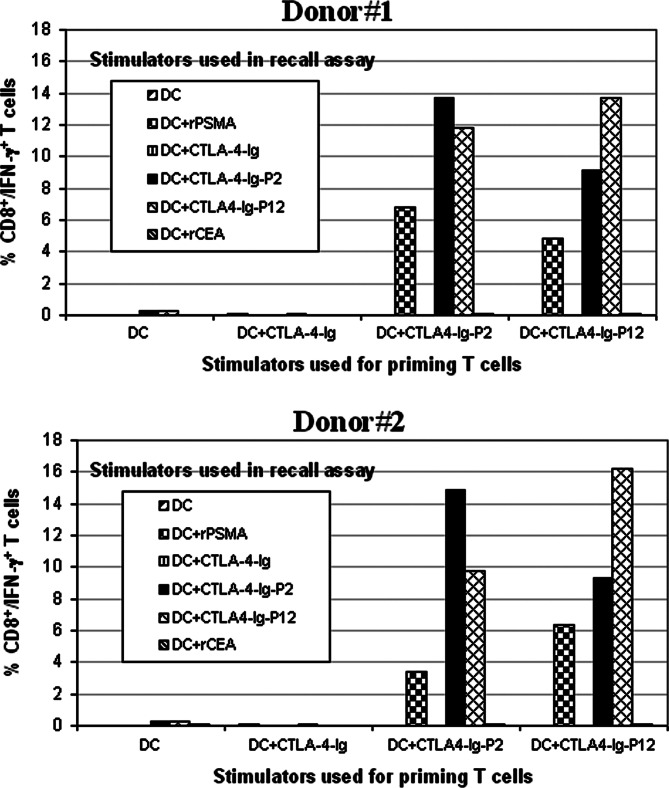
To examine the specificity and nature of the immune responses induced by DC pulsed with CTLA4–Ig–Ag fusion proteins, the T cell lines generated ex vivo using autologous DCs pulsed with various fusion proteins were tested for Ag-specific secretion of Th-1 cytokine interferon (IFN)-γ in recall assays. Autologous DCs either unpulsed or pulsed with CTLA4–Ig, CTLA4–Ig–P2, CTLA4–Ig–P12, rPSMA, or rCEA protein were used as stimulators. Activated CD3+/CD8+ T cells were identified by flow cytometry after co-staining of cells with mAbs detecting CD3, CD8, and IFN-γ. Mean values of the absolute numbers of CD3+/CD8+/IFN-γ+ cells from two different donors are shown in Fig. 6. The results show that effector T cells generated using CTLA4–Ig–P2 or CTLA4–Ig–P12 protein pulsed DCs contained CD8+ T cells that displayed markedly increased IFN-γ production upon restimulation with relevant fusion protein or rPSMA protein pulsed autologous DCs. However, none of the T cell lines were responsive to CTLA4–Ig or an irrelevant CEA protein pulsed or unpulsed control DC stimulators.
Fig. 6.
Effector T cells generated using DC pulsed with CTLA4–Ig–Ag fusion proteins secrete IFN-γ upon Ag recall. Effector T cells were generated as described in Fig. 5 and challenged with various stimulator DCs as indicated. Three days later the absolute numbers of CD3+/CD8+ IFN-γ+ T cells were quantified by intracellular cytokine staining and flow cytometry as described in Materials and methods. Data are presented as percent CD8+ T cells that were positive for intracellular IFN-γ
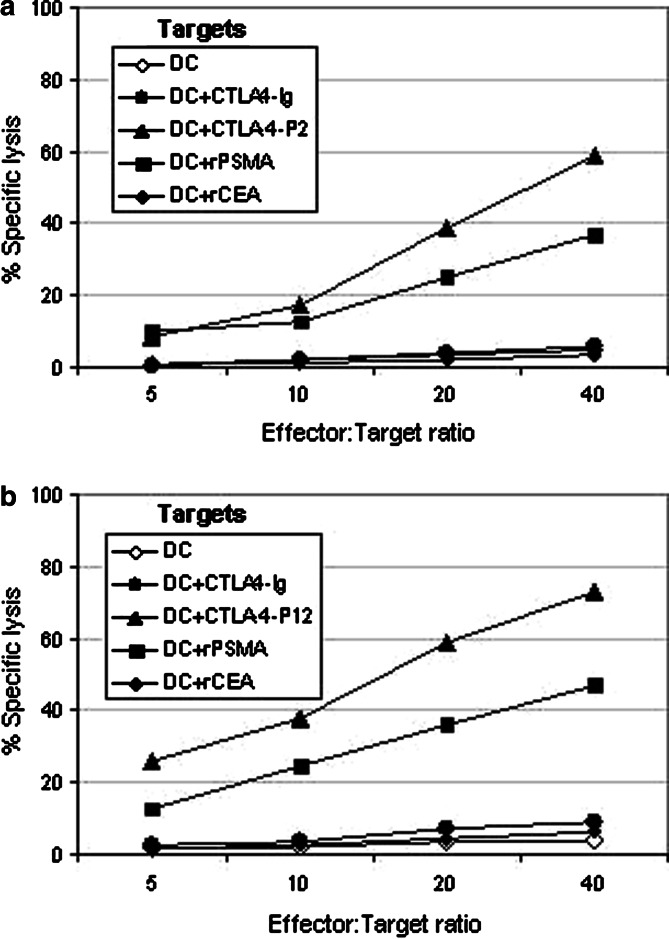
To further test the functionality and Ag-specificity of the T cell lines, cytotoxicity assay was performed using autologous DCs pulsed with CTLA4–Ig, CTLA4–Ig-P2, CTLA4–Ig–P12, rPSMA or rCEA protein or unpulsed control DCs as targets (Fig. 7). Similar to previous experiments shown in Figs. 5 and 6, the effector T cells generated using CTLA4–Ig–P2 (Fig. 7a) or CTLA4–Ig–P12 (Fig. 7b) fusion protein pulsed DCs showed specific lysis of autologous DC targets pulsed with relevant fusion protein or rPSMA, but not those pulsed with CTLA4–Ig or rCEA or unpulsed control DCs. In contrast, T cell lines generated using either CTLA4–Ig pulsed DC or control DC failed to lyse any of the target cells tested (data not shown). Collectively, results of these experiments confirm that CTLA4-mediated targeting of Ag to DC may be an efficient method of generating Ag-specific CTLs in vitro.
Fig. 7.
CTLA4-mediated targeting of antigens to DCs induces Ag-specific CTL responses in vitro. T cells from two HLA-A2+ donors were stimulated with autologous DCs pulsed with various protein Ags as described in Fig. 5. At the end of three weekly stimulations effector T cells were collected and tested for cytotoxicity against various 51Cr-labeled autologous DC targets indicated in figure using standard chromium release assay as described in Materials and methods. a Effector cells generated using DC pulsed with CTLA4–Ig–P2. b Effector cells generated using DC pulsed with CTLA4–Ig–P12. Assay wells were run in triplicate and average values are presented. A representative of two experiments performed using samples from two different donors is shown
Discussion
Antigen targeting to sites of immune induction is an efficient means of enhancing immune responses to DNA vaccines. Particularly, Ag targeting to cell subsets specialized in Ag presentation, such as DCs, may be more effective for immune stimulation. Notwithstanding, however, is the fact that DCs can also capture proteins nonspecifically for eventual presentation to T cells. Evidence has accumulated in recent years to suggest that DCs are pivotal to the generation of T cell responses. They can be customized in vitro to meet different goals of harnessing the immune repertoire. The rationale behind specific targeting of Ags to DCs, therefore, appears to be centered on the relative efficiency of Ag processing and presentation in terms of both qualitative and quantitative immune responses. One means to facilitate the uptake and processing of Ag has been to target them to APCs.
CTLA4 is a glycoprotein expressed on activated T cells that has a strong binding affinity to both B7-1 (CD80) and B7-2 (CD86) molecules, which are primarily expressed on APCs. Directing Ags to B7 molecules expressed on APCs using their ligand CTLA4 may have utility in modulating the immune response. Although initial studies demonstrated that CTLA4–Ig can inhibit immune responses to third party Ags in vivo [24, 25], DNA-based vaccines, using constructs that encoded CTLA4–Ag fusion protein, has been shown to substantially promote the development of immune responses against the fusion Ag [13–15, 26]. Injection of purified CTLA4 fusion protein also leads to an Ag-specific immune response [19, 20]. Our results extend the observations of others regarding the ability of CTLA4 fusion proteins to stimulate antigen-specific immune responses in vivo.
Although the injection of CTLA4 fusions and/or DNA vaccines has been clearly shown to activate the immune system in vivo, the mechanism by which this happens has not been formally demonstrated. Our experiments were designed to gain a greater understanding of this mechanism. The first issue we addressed was to examine the role of the two known CTLA4 ligands, B7-1 and B7-2, in the stimulation of immune responses by CTLA4–Ig. Using knockout mice that fail to express either B7-1 or B7-2, we showed that both B7 molecules are sufficient to allow the CTLA4-Ag fusion proteins to initiate a humoral response. Notably, small quantities, 1 μg or less, of fusion protein were sufficient to stimulate antigen-specific responses when injected into animals (unpublished data from Martin Oaks and reference [19, 20]).
Because we were injecting a fusion protein of CTLA4 and the Fc portion of Ig, it was possible that the fusion protein stimulated B lymphocytes by simultaneously stimulating B7 molecules and Fc receptors expressed by the B cells. To determine if these two stimuli allowed production of Ag-specific antibodies independent of T cell help, we evaluated the humoral response against the fusion protein when injected into nude mice that lacked T cells. These experiments demonstrated that T cell activity is essential to humoral responses against CTLA4–Ig–Ag fusion proteins. Our observation is consistent with previous studies based on the CTLA4 fusion approach [13, 14, 20].
Further we extended our studies to examine the ability of CTLA4–Ig to target Ags to DCs and to generate/stimulate Ag-specific T cell responses in vitro. Our experiments have demonstrated that fragments of PSMA physically linked to CTLA4 have retained the ability to bind B7.1 and B7.2 on human DCs in vitro, and were able to induce the production of high numbers of PSMA-specific CTLs that secreted INF-gamma and had cytotoxic function. In addition, we have shown here that the modulation of immune responses by the fusion protein is dependent on the physical linkage between the Ag and CTLA4–Ig, which confirms that the presence of CTLA4 moiety ensure its targeting to APCs and, therefore, leads to more efficient uptake and processing of the target Ag. Importantly, the presence of CTLA4–Ig in the assay did not inhibit activation of either naïve or primed lymphocytes and cellular immunity against the CTLA4–Ig molecule itself was undetectable. In summary, we demonstrated that fusion of a weak Ag to CTLA4–Ig dramatically increases its immunogenicity and substantially promotes specific Ab and cell-mediated immune responses without the requirements for immunologic adjuvants. It is noteworthy that CTLA4–Ig-fused Ag fusion proteins are able to stimulate immune responses in vitro at relatively low doses (1 μg). Overall, the use of CTLA4–Ig as vaccine carrier provides a new and simple strategy to develop vaccines against cancer and infectious diseases that have several advantages over current vaccination strategies.
Because of the simplicity of these fusion proteins in terms of their preparation and storage, it is an attractive strategy for the induction of primary T cell and antibody (B cell) responses in animals and patients. In addition, the tolerance of the human immune system to the human CTLA4 and human Ig portions of the fusion proteins may make this an ideal vehicle for repeated use in vaccination protocols. Importantly, it could be used as priming immunogens that would tremendously help achieve the highly effective means of boosting the immune response by DC vaccines that need multiple prime-boost regimens. Also, because the fusion protein stimulates immunity when directly injected into animals, it may be particularly useful as a vaccine in patients with weakened immune systems. Several current vaccines exist that use attenuated microorganisms to develop immunity. Occasionally, some patients develop the disease that the vaccine was intended to prevent, and this is an even greater concern in patients with weakened immune systems. Using a fusion protein as the immunostimulatory component of a vaccine should eliminate this problem. Finally, our in vitro experiments show that human lymphocytes do not respond to the human-derived CTLA4–Ig fusion protein, which makes this as an ideal strategy for vaccination protocols that require multiple exposures of the Ag with minimal risk of inducing immunity directly against the carrier protein. This is an advantage over current methods using viral vectors for immunization because pre-existing immunity to the virus may limit the efficacy of the vaccines by eliminating them before they have had an opportunity to stimulate the immune system against the antigen of interest.
Footnotes
Dhanalakshmi Chinnasamy and Matt Tector contributed equally to this work
References
- 1.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley R, Tozer R, Wan Y. Genetically modified dendritic cells in cancer therapy: implications for transfusion medicine. Transfus Med Rev. 2001;15:292. doi: 10.1053/tm.2001.26960. [DOI] [PubMed] [Google Scholar]
- 3.Holtl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777. doi: 10.1016/S0022-5347(01)61767-1. [DOI] [PubMed] [Google Scholar]
- 4.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 5.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 6.Berg SF, Mjaaland S, Fossum S. Comparing macrophages and dendritic leukocytes as antigen-presenting cells for humoral responses in vivo by antigen targeting. Eur J Immunol. 1994;24:1262. doi: 10.1002/eji.1830240604. [DOI] [PubMed] [Google Scholar]
- 7.Carayanniotis G, Barber BH. Adjuvant-free IgG responses induced with antigen coupled to antibodies against class II MHC. Nature. 1987;327:59. doi: 10.1038/327059a0. [DOI] [PubMed] [Google Scholar]
- 8.Guyre PM, Graziano RF, Goldstein J, Wallace PK, Morganelli PM, Wardwell K, Howell AL. Increased potency of Fc-receptor-targeted antigens. Cancer Immunol Immunother. 1997;45:146. doi: 10.1007/s002620050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lees A, Morris SC, Thyphronitis G, Holmes JM, Inman JK, Finkelman FD. Rapid stimulation of large specific antibody responses with conjugates of antigen and anti-IgD antibody. J Immunol. 1990;145:3594. [PubMed] [Google Scholar]
- 10.Wang H, Griffiths MN, Burton DR, Ghazal P. Rapid antibody responses by low-dose, single-step, dendritic cell-targeted immunization. Proc Natl Acad Sci USA. 2000;97:847. doi: 10.1073/pnas.97.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schjetne KW, Thompson KM, Aarvak T, Fleckenstein B, Sollid LM, Bogen B. A mouse C kappa-specific T cell clone indicates that DC-SIGN is an efficient target for antibody-mediated delivery of T cell epitopes for MHC class II presentation. Int Immunol. 2002;14:1423. doi: 10.1093/intimm/dxf110. [DOI] [PubMed] [Google Scholar]
- 12.Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- 13.Boyle JS, Brady JL, Lew AM. Enhanced responses to a DNA vaccine encoding a fusion antigen that is directed to sites of immune induction. Nature. 1998;392:408. doi: 10.1038/32932. [DOI] [PubMed] [Google Scholar]
- 14.Deliyannis G, Boyle JS, Brady JL, Brown LE, Lew AM. A fusion DNA vaccine that targets antigen-presenting cells increases protection from viral challenge. Proc Natl Acad Sci USA. 2000;97:6676. doi: 10.1073/pnas.120162497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayak BP, Sailaja G, Jabbar AM. Enhancement of gp120-specific immune responses by genetic vaccination with the human immunodeficiency virus type 1 envelope gene fused to the gene coding for soluble CTLA4. J Virol. 2003;77:10850. doi: 10.1128/JVI.77.20.10850-10861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 17.Pulendran B, Banchereau J, Maraskovsky E, Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41. doi: 10.1016/S1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang TH, Wu PY, Lee CN, Huang HI, Hsieh SL, Kung J, Tao MH. Enhanced antitumor immunity by fusion of CTLA4 to a self tumor antigen. Blood. 2000;96:3663. [PubMed] [Google Scholar]
- 20.Rohrbach F, Weth R, Kursar M, Sloots A, Mittrucker HW, Wels WS. Targeted delivery of the ErbB2/HER2 tumor antigen to professional APCs results in effective antitumor immunity. J Immunol. 2005;174:5481. doi: 10.4049/jimmunol.174.9.5481. [DOI] [PubMed] [Google Scholar]
- 21.Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA4. Cell Immunol. 2000;201:144. doi: 10.1006/cimm.2000.1649. [DOI] [PubMed] [Google Scholar]
- 22.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303. doi: 10.1016/S1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 23.Freeman GJ, Borriello F, Hodes RJ, Reiser H, Hathcock KS, Laszlo G, McKnight AJ, Kim J, Du L, Lombard DB. Uncovering of functional alternative CTLA4 counter-receptor in B7-deficient mice. Science. 1993;262:907. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- 24.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 25.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4–Ig. Transplantation. 1994;57:1701. [PubMed] [Google Scholar]
- 26.Chaplin PJ, De Rose R, Boyle JS, McWaters P, Kelly J, Tennent JM, Lew AM, Scheerlinck JP. Targeting improves the efficacy of a DNA vaccine against Corynebacterium pseudotuberculosis in sheep. Infect Immun. 1999;67:6434. doi: 10.1128/iai.67.12.6434-6438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]