Abstract
Here we show that low-dose cyclophosphamide (CY), that depends for its therapeutic effectiveness on the immunopotentiating activity of the drug for T cell-mediated tumor-eradicating immunity, is curative for ~80% of wild-type (WT) mice bearing a large s.c. MOPC-315 tumor, but only for ~10% of IFN-α/βR−/− mice bearing a large s.c. MOPC-315 tumor. Histopathological examination of the s.c. tumors of such mice on day 4 after the chemotherapy revealed that the low dose of CY led to accumulation of T lymphocytes in both the WT and the IFN-α/βR−/− mice. However, in the CY treated tumor bearing WT mice the T lymphocytes were present throughout the tumor mass and in direct contact with tumor cells, but in the CY treated tumor bearing IFN-α/βR−/− mice most of the T lymphocytes remained in blood vessels. In addition to being important for CY-induced transendothelial migration of T lymphocytes into the tumor mass, we show here that signaling via the IFN-α/βR is also important for CY-induced control of metastatic tumor progression in the spleen and liver of the tumor bearing mice. Finally, CY cured tumor bearing WT mice were resistant to a subsequent challenge with MOPC-315 tumor cells, but the few CY cured tumor bearing IFN-α/βR−/− mice were not. Thus, signaling via the IFN-α/βR on host cells in MOPC-315 tumor bearers is important for CY-induced: (a) transendothelial migration of T lymphocytes into the tumor mass and the eradication of the primary tumor, (b) control of metastatic tumor progression, and (c) resistance to a subsequent tumor challenge.
Keywords: Immunomodulation by anticancer drugs, Cancer therapy, IFN-α/β
Introduction
The ability of anticancer drugs to facilitate the acquisition of T cell-dependent antitumor immunity in tumor bearers has been demonstrated in both tumor bearing animals and in patients with malignancies [1, 2, 4, 14, 32, 36, 38, 43]. Studies into the mechanisms through which anticancer drugs mediate this effect revealed that the anticancer drugs lead to a shift in the cytokine profile from cytokines such as TGF-β and IL-10, with inhibitory activity for the development of cell-mediated antitumor immunity, toward cytokines such as TNF-α and IFN-γ that favor the development of cell-mediated antitumor immunity [12, 16, 19, 23, 24, 41, 43]. This chemotherapy-induced shift in cytokine profile was first evident at the mRNA level 24 h or longer after the chemotherapy, but until recently there was no indication regarding earlier events after the chemotherapy that may be contributing to the shift in cytokine profile and the ensuing acquisition of T cell-mediated tumor-eradicating immunity.
Recently, Schiavoni et al. [37] have shown that administration of cyclophosphamide (CY) to normal mice leads within 6 h to elevated IFN-α and IFN-β (IFN-α/β) mRNA expression, which is followed by the production of biologically active IFN-α/β. In addition, this group of investigators in collaboration with Tough’s group [20, 25] generated data that are consistent with the idea that IFN-α/β is important for CY-induced up-regulation of IFN-γ expression. Specifically, Matti et al. [25] have shown that exposure of splenic dendritic cells from normal mice to exogenous IFN-α/β results in upregulated expression of IL-15, and exogenous IL-15 in turn was shown to induce the production of markedly increased levels of IFN-γ. In addition, Le Bon et al. [20] have shown that signaling via the IFN-α/βR is essential for the induction of IFN-γ production by CD4+ T cells in response to the in vivo administration of poly I:C to normal mice.
In light of the above reports [20, 25, 37], we carried out studies to determine if IFN-α/β is important for the realization of the therapeutic effectiveness of low-dose CY for MOPC-315 tumor bearing mice, under conditions that depend on the immunopotentiating activity of the chemotherapy for the acquisition of cell-mediated tumor-eradicating immunity [11, 12, 14, 27, 38]. Here we show that a low dose of CY, which is curative for most wild-type (WT) mice bearing a large MOPC-315 tumor, was curative only for a few IFN-α/βR−/− mice bearing a large MOPC-315 tumor. In addition, we demonstrate that signaling via the IFN-α/βR is important for facilitating the CY-induced transendothelial migration of T lymphocytes from the blood vessels into the s.c. tumor mass, as well as for the CY-induced control of metastatic progression and the development of long-lasting tumor-eradicating immunity.
Materials and methods
Mice
Mice deficient in signaling via the IFN-α/βR (IFN-α/βR−/−) on 129 Ev/Sv background were kindly provided to us by Drs. H. Virgin and R. Schreiber (Washington University, St. Louis, MO, USA) [21, 31], and kept under pathogen-free conditions in our Barrier Animal Facility. The IFN-α/βR−/− mice were backcrossed to BALB/cAnNCrlBR mice (Charles Rivers Breeding Laboratories, Wilmington, MA, USA) for more than ten generations before they were employed in the current studies. WT mice on 129 Ev/Sv background were similarly backcrossed to BALB/cAnNCrlBR mice for use in studies assessing the therapeutic effectiveness of CY, and in studies in which tissue sections were processed for histopathological examination. Procedures and care of the animals were in accordance with the University of Illinois guidelines provided by the Institutional Animal Care Committee.
Tumor cells
The MOPC-315 plasmacytoma was maintained in vivo, as previously described [13, 14], in BALB/cAnNCrlBR mice. Briefly, the mice were inoculated s.c. with 1.5–2.0×106 MOPC-315 tumor cells, a dose that is at least 300-fold greater than the minimal lethal tumor dose and kills the mice in approximately 18 days. On day 9–11 after tumor inoculation, when the mice bore a large s.c. tumor (i.e., ~20 mm in diameter), the tumors were removed and single cell suspensions were prepared by mechanical disruption between glass slides.
Chemotherapy and follow-up of mice
A single injection of 15–30 mg CY (Cytoxan, Bristol-Myers Squibb, Princeton, NJ, USA) per kg body weight (low-dose) was administered i.p. to WT mice and to IFN-α/βR−/− mice bearing a large s.c. MOPC-315 tumor. This dose of CY, in cooperation with host T cell-dependent antitumor immunity that emerges after the chemotherapy, was previously shown to be curative for 80–90% of WT mice bearing a large MOPC-315 tumor [13, 14]. In the studies reported herein the mice were monitored for tumor progression/regression for a period of 6 weeks. Mice in which the s.c. tumor grew to ≥25 mm in diameter and interfered with their ability to ambulate, eat and drink were sacrificed. In addition, we sacrificed mice whose s.c. tumor regressed by more than 30%, if the mice had lost more than 20% of their body weight (minus tumor), were lethargic and did not eat and drink. Such mice were evaluated, however, for metastatic foci in the spleen and liver to confirm tumor progression as the cause of their health condition.
Anti-proliferative activity of IFN-β for MOPC-315 tumor cells
MOPC-315 tumor cells were cultured at a concentration of 1×104 cells/well with graded concentrations of mouse recombinant IFN-β (PBL Biomedical Laboratories, New Brunswick, NJ, USA) in 96-well flat-bottom plates (Falcon-BD Biosciences Discovery Labware). The culture medium consisted of DMEM supplemented with 10% FBS, 0.1 mM nonessential amino acids, 50 U/ml penicillin, 50 μg/ml streptomycin, 15 nM HEPES buffer (Invitrogen Life Technologies), and 2×10−5 M 2-ME (Sigma-Aldrich Corporation). After 48 h in culture in the presence of IFN-β, we determined the number of viable tumor cells in these cultures relative to control cultures, which did not contain exogenous IFN-β. This was done with the aid of a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) colorimetric assay (Promega Corporation, Madison, WI, USA).
Histology
Primary s.c. tumor masses were harvested from untreated MOPC-315 tumor bearing IFN-α/βR−/− mice, and from MOPC-315 tumor bearing IFN-α/βR−/− mice that were treated with low-dose CY 4 days earlier. Concurrently, s.c. tumor masses were also collected from untreated MOPC-315 tumor bearing WT mice, and from MOPC-315 tumor bearing WT mice that were treated with low-dose CY 4 days earlier. The tumor tissues were placed in buffered 10% formalin (Allegiance, McGaw Park, IL, USA) for fixation and trimmed into plastic cassettes. Processing for histological examination consisted of dehydration through graded alcohols to xylene and infiltration with paraffin. Subsequently, the tumors were sectioned at 5 μm and stained with H&E. For immunohistological identification of T cell lineage, sections were incubated with rabbit anti-mouse CD3 Ab (AO452; DAKO Corporation, Carpinteria, CA, USA) and the specific binding detected with secondary antibody (LSAB2; DAKO corporation). Finally, the sections were counterstained with hematoxylin.
Statistical analysis
To determine the significance of the difference in the fraction of tumor bearing WT mice and tumor bearing IFN-α/βR−/− mice surviving as a consequence of low-dose CY therapy, the generalized Savage (Mantel-Cox) test was performed. To determine the significance of the difference in the number of MOPC-315 tumor cells surviving when cultured with IFN-β relative to the number surviving in control cultures, the Student’s t test was performed. A P value of 0.05 or lower was considered significant in both tests.
Results
Assessment of the importance of IFN-α/β for the realization of the therapeutic benefits of low-dose CY for mice bearing a large MOPC-315 tumor
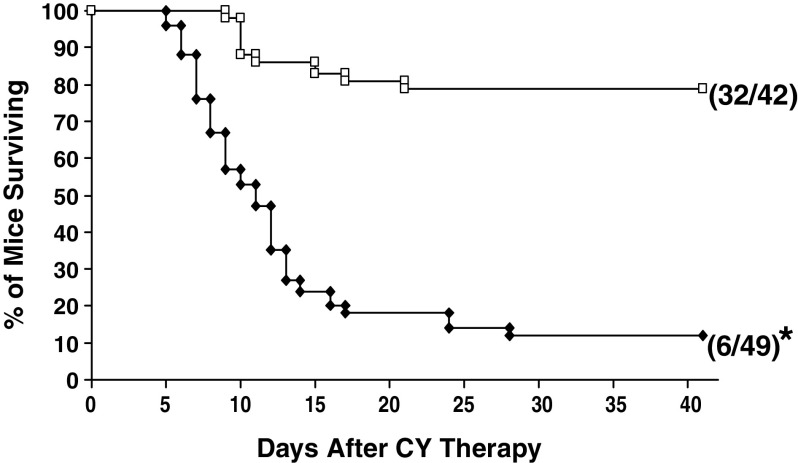
Experiments were performed to determine if IFN-α/β is important for the curative effectiveness of low-dose CY, under conditions in which the therapeutic outcome of CY depends on T cell-mediated tumor-eradicating immunity that is acquired as a consequence of the chemotherapy [14, 27, 38]. Specifically, we carried out experiments to determine if a single i.p. injection of 15–30 mg/kg CY is curative only for WT mice bearing a large MOPC-315 tumor or also for IFN-α/βR−/− mice bearing a large MOPC-315 tumor. A total of five experiments were carried out. In one experiment low-dose CY was administered to the mice when their s.c. tumors reached ~15 mm in diameter, while in the other four experiments low-dose CY was administered when the s.c. tumors reached ~20 mm in diameter. Similar results were obtained in all experiments. The cumulative results of all five experiments are provided in Fig. 1. As seen in Fig. 1, and in confirmation of our previous observations [13, 14], the low dose of CY was curative for most (32/42; i.e., ~80%) tumor bearing WT mice. At the same time, the low dose of CY was curative for very few (6/49; i.e., ~10%) tumor bearing IFN-α/βR−/− mice. Thus, signaling via the IFN-α/βR on host cells is important for the realization of the therapeutic benefits of low-dose CY for mice bearing a large MOPC-315 tumor.
Fig. 1.
Importance of signaling via the IFN-α/βR for the realization of the curative effectiveness of low-dose CY for mice bearing a large MOPC-315 tumor. Wild-type (WT) mice (open square) or IFN-α/βR−/− mice (filled diamond) bearing a large (15–20 mm) s.c. tumor were given a single i.p. injection of a low dose of CY (15–30 mg/kg). The mice were monitored for tumor progression/regression for a period of 6 weeks. Mice whose s.c. tumor reached ≥25 mm in diameter as well as mice that lost >20% of their body weight and showed signs of distress were sacrificed (please see Materials and Methods). In all mice that were alive on day 42 after CY administration, the s.c. tumor regressed completely and the mice were clinically healthy. Number in parentheses indicate number of mice alive out of total mice treated with CY. Asterisk indicates significantly lower (P<0.0001) survival time relative to WT mice treated with CY
Assessment of the anti-proliferative activity of IFN-α/β for MOPC-315 tumor cells
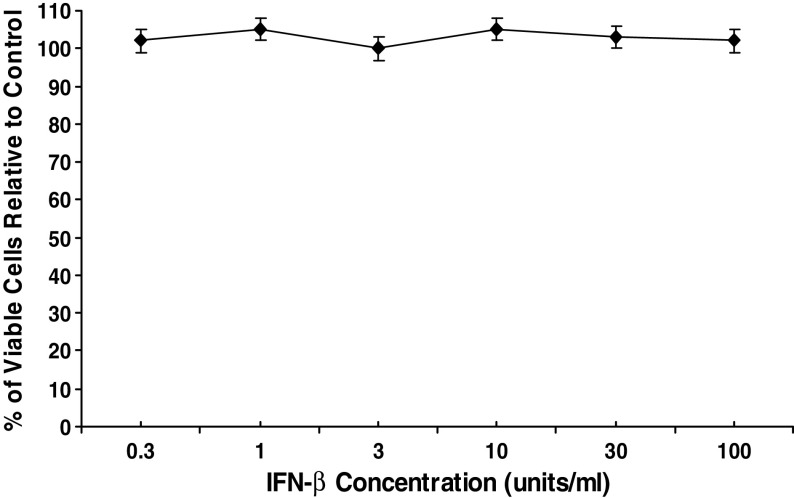
We first considered the possibility that the much greater therapeutic effectiveness of low-dose CY for tumor bearing WT mice than for tumor bearing IFN-α/βR−/− mice was the result of greater anti-proliferative activity of IFN-α/β for MOPC-315 tumor cells in the WT mice than in the IFN-α/βR−/− mice. Such a possibility was considered in light of reports that: (a) CY induces IFN-α/β production [37], and (b) signaling via the IFN-α/βR amplifies the production of IFN-α/β [22, 39], which together imply that higher concentrations of IFN-α/β may be present in CY treated WT mice than in CY treated IFN-α/βR−/− mice. To this end, we carried out studies to determine if MOPC-315 tumor cells are sensitive to the anti-proliferative effects of IFN-α/β. For this purpose, we cultured MOPC-315 tumor cells for 48 h with various concentrations of IFN-β, and subsequently determined the number of viable tumor cells present in such cultures relative to cultures of MOPC-315 tumor cells that were not exposed to exogenous IFN-β (control). IFN-β was chosen for these studies because IFN-β and IFN-α signal via the same single receptor (i.e., the IFN-α/βR) and elicit a similar range of biological activities [40], but in many cases IFN-β was shown to exert a more potent anti-proliferative effect than IFN-α [5, 10, 35]. The concentrations of IFN-β employed in the current experiments ranged between 0.3 U/ml and 100 U/ml because: (a) the combined concentrations of IFN-α and IFN-β reached in the blood of CY treated WT mice ranged between <4 U/ml and 32 U/ml [37], and (b) IFN-β at a concentration of 25 U/ml caused a massive reduction in the proliferation of tumor cell lines sensitive to the anti-proliferative activity of this cytokine [10, 35]. A total of four experiments were performed with similar results. As seen in Fig. 2, which depicts the results of a representative experiment, no reduction in the number of viable MOPC-315 tumor cells (relative to control) was observed when MOPC-315 tumor cells were cultured in the presence of any of the IFN-β concentrations employed, including 30 U/ml or 100 U/ml. These results indicate that the much greater therapeutic effectiveness of low-dose CY for WT mice, than for IFN-α/βR−/− mice, bearing a large MOPC-315 tumor is not the result of greater anti-proliferative activity of IFN-α/β for MOPC-315 tumor cells in the CY treated WT mice.
Fig. 2.
Assessment of the anti-proliferative effect of IFN-β for MOPC-315 tumor cells. MOPC-315 tumor cells were cultured with various concentrations of IFN-β for 48 h. The anti-proliferative effect was determined by the MTS tetrazolium colorimetric assay. The results are presented as the mean percentage of viable cells relative to control (MOPC-315 tumor cells cultured in the absence of exogenous IFN-β)±SE. No significant reduction in the percentage of viable tumor cells was observed with any of the IFN-β concentrations used
Histopathological changes induced by low-dose CY therapy in the s.c. tumor masses of WT mice, and IFN-α/βR−/− mice, bearing a large MOPC-315 tumor
In light of the above observations (Figs. 1, 2), and because: (a) administration of low-dose CY to WT mice bearing a large MOPC-315 tumor was previously shown to lead to accumulation of T lymphocytes in their s.c. tumors, and (b) T lymphocytes were found to be important for the curative effectiveness of the low-dose chemotherapy for MOPC-315 tumor bearing WT mice [14], experiments were performed to determine if signaling via the IFN-α/βR is important for CY-induced accumulation of T lymphocytes in the s.c. tumors of MOPC-315 tumor bearers. Specifically, we carried out studies to determine if CY therapy leads to accumulation of T lymphocytes only in the s.c. tumors of WT mice or also in the s.c. tumors of IFN-α/βR−/− mice. Initially, we examined H&E stained sections from the s.c. tumors of untreated IFN-α/βR−/− mice bearing a large MOPC-315 tumor (Fig. 3b) as well as H&E stained sections from the s.c. tumors of IFN-α/βR−/− mice that were treated with CY 4 days earlier when the mice bore a large MOPC-315 tumor (Fig. 4c, d). As a reference point, we examined simultaneously H&E stained sections from tumors of untreated WT mice bearing a large MOPC-315 tumor (Fig. 3a) as well as H&E stained sections from the tumors of WT mice that were treated with CY 4 days earlier when the mice bore a large MOPC-315 tumor (Fig. 4a, b).
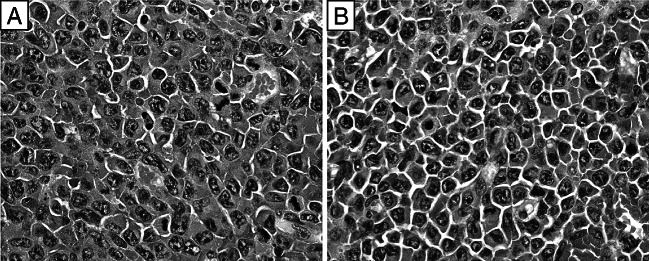
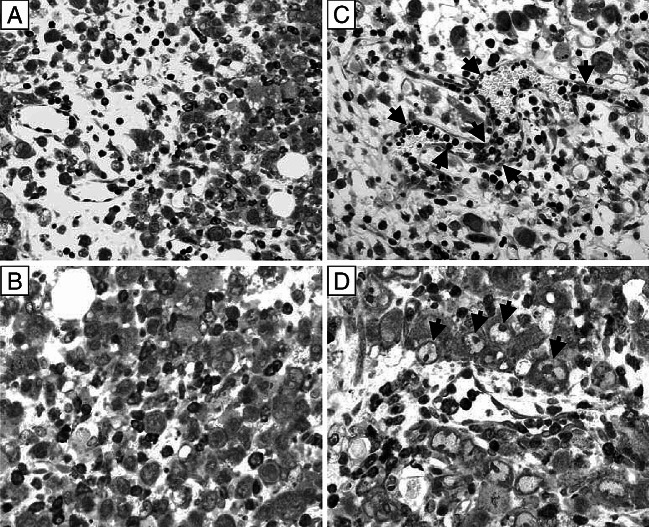
Fig. 3.
Neoplastic cells with large irregularly shaped nuclei, prominent nucleoli and high mitotic rate in H&E stained sections of the s.c. tumors of untreated WT mice (a) or untreated IFN-α/βR−/− mice (b) bearing a large (~20 mm in diameter) s.c. MOPC-315 tumor. Original magnification ×500
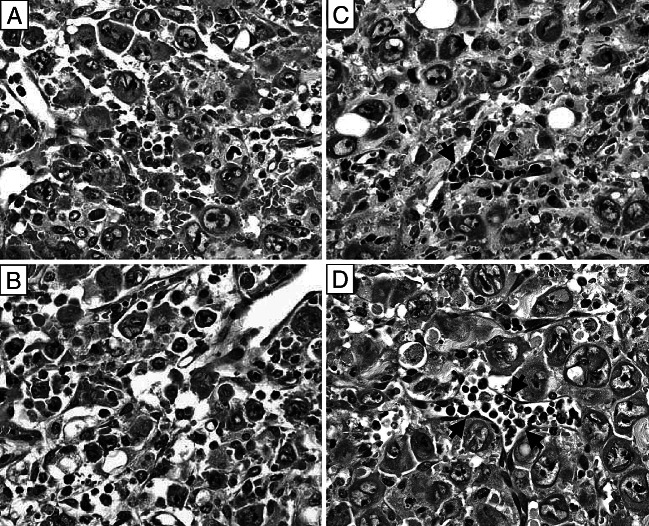
Fig. 4.
Diffuse lymphocytic infiltration throughout the tumor mass of CY treated tumor bearing WT mice and lymphocytic presence predominantly in blood vessels of the tumor mass of CY treated tumor bearing IFN-α/βR−/− mice. a, b represent two different fields of the same H&E stained section on day 4 after low-dose CY administration to WT mice bearing a large MOPC-315 tumor. c, d represent two different fields of the same H&E stained section on day 4 after low-dose CY administration to IFN-α/βR−/− mice bearing a large MOPC-315 tumor. Arrows in c and d point to lymphocytes localized within blood vessels. Original magnification ×500
As seen in Fig. 3, sections of s.c. tumors from untreated WT mice as well as of s.c. tumors from untreated IFN-α/βR−/− mice had anaplastic plasmacytoid tumor cells with high mitotic rate, prominent nucleoli and coarse chromatin, and very little (if any) lymphocytic infiltration. However, on day 4 after the chemotherapy, prominent lymphocytic infiltration was evident throughout the tumor masses of MOPC-315 tumor bearing WT mice (Fig. 4a, b). At the same time, although lymphocytes also accumulated in the tumors of MOPC-315 tumor bearing IFN-α/βR−/− mice, most of the lymphocytes remained within the blood vessels, with only a few lymphocytes apparent in the tumor mass (Fig. 4c, d). To determine if the cells identified as intratumoral lymphocytes in the H&E staining were actually T cells, we used anti-CD3 Ab to immunohistochemically stain the T cells in sections from tumor bearing WT mice and from tumor bearing IFN-α/βR−/− mice treated with CY 4 days earlier. Figure 5a, b illustrates that a massive infiltrate of T lymphocytes was present throughout the tumor mass of CY treated MOPC-315 tumor bearing WT mice. In fact, the T lymphocytes were in direct contact with viable tumor cells and appeared to be engaged in tumor cell killing (Fig. 6), consistent with their previously described role in CY-induced T-cell-mediated tumor eradication [13, 28]. At the same time, most of the T lymphocytes that were detected in the s.c. tumor of CY treated MOPC-315 tumor bearing IFN-α/βR−/− mice were localized within blood vessels with only a few T lymphocytes apparent within the tumor mass, but not in direct contact with tumor cells (Fig. 5c, d). In fact, clusters of viable tumor cells were seen that did not have T lymphocytes in their immediate vicinity. Thus, signaling via the IFN-α/βR on host cells is important for facilitating the transendothelial migration of T lymphocytes into the tumor mass of CY treated MOPC-315 tumor bearers.
Fig. 5.
T lymphocyte infiltration throughout the tumor mass of CY treated tumor bearing WT mice and T lymphocyte presence predominantly in blood vessels of the tumor mass of CY treated tumor bearing IFN-α/βR−/− mice. T lymphocytes were identified immunohistochemically by CD3 expression. a, b represent two different fields of the same section on day 4 after low-dose CY administration to WT mice bearing a large MOPC-315 tumor, and c, d represent two different fields of the same section on day 4 after low-dose CY administration to IFN-α/βR−/− mice bearing a large MOPC-315 tumor. Arrows in c point to T lymphocytes localized within blood vessels and arrows in d point to tumor cells with large vesicular nuclei and prominent nucleoli that indicate continued tumor growth. Original magnification for a and c ×300 and for b and d ×500
Fig. 6.
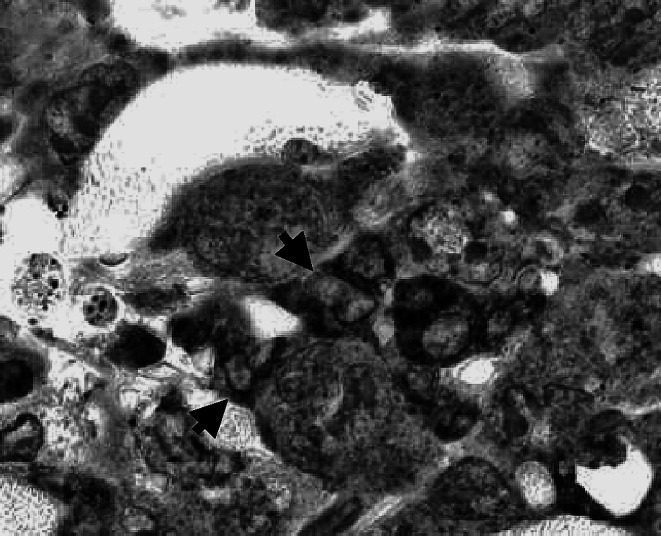
Tumor infiltrating T lymphocytes in close contact with tumor cells. Immunoperoxidase staining of cells expressing CD3 in a section from the s.c. tumor mass on day 4 after CY administration to WT mice bearing a large MOPC-315 tumor. Arrows are pointing to T lymphocytes that are in close contact with tumor cells. Original magnification ×1,200
Follow-up of MOPC-315 tumor bearing WT mice and MOPC-315 tumor bearing IFN-α/βR−/− mice in which low-dose CY therapy was not curative
As part of the survival studies depicted in Fig. 1, we followed the low-dose CY treated MOPC-315 tumor bearing mice for progression/regression of the primary s.c. tumors (Table 1). All tumor bearing WT mice that were not cured by low-dose CY (10/42; Fig. 1) either died with a progressively growing primary s.c. tumor or were sacrificed when the primary s.c. tumor grew and reached ≥25 mm in diameter and the mice showed signs of distress. At the same time, among the 43/49 tumor bearing IFN-α/βR−/− mice that were not cured by the low dose of CY (Fig. 1), only 22 (i.e., ~50%) died with a progressively growing primary s.c. tumor or were sacrificed when the primary s.c. tumor grew and reached ≥25 mm in diameter and the mice showed signs of distress (Table 1). The remaining 21 tumor bearing IFN-α/βR−/− mice that were not cured by the low dose of CY were sacrificed when the primary s.c tumor regressed by more than 30% in response to the chemotherapy, but the mice lost more than 20% of their body weight (minus tumor) and were very lethargic and did not eat and drink (Table 1). Three of these mice had a large s.c. tumor at a distant site from the primary MOPC-315 tumor (Table 1). Histopathological examination of a new s.c. tumor from one of these mice revealed broad sheets of neoplastic cells that were dividing rapidly, similar to the appearance of the original s.c. tumor nodule (Fig. 3). Finally, we evaluated the remaining tumor bearing IFN-α/βR−/− mice in which the primary s.c. tumor regressed by more than 30%, but the mice showed signs of distress for the presence of metastatic foci in their spleen and liver. Metastatic foci were grossly apparent in the spleen from such mice (Fig. 7). In addition, histopathological examination of sections from the liver of such mice revealed several foci of neoplastic cell colonies, with the colonization being primarily juxtavenular (Fig. 8). Thus, even in IFN-α/βR−/− mice in which a significant regression of the primary s.c. MOPC-315 tumor occurred as a consequence of low-dose CY therapy, neoplastic cells proliferated and established multiple foci in the spleen and liver. These results indicate that signaling via the IFN-α/βR is important for CY-induced control of metastatic progression in MOPC-315 tumor bearing mice.
Table 1.
Incidence of endpoint criteria for MOPC-315 tumor bearing mice that were not cured as a consequence of low-dose CY administration
| Fraction of WT mice with | Fraction of IFN-α/βR−/− mice with | ||
|---|---|---|---|
| A progressively growing primary s.c. tumor that died or were sacrificed when the primary s.c tumor reached at least 25 mma | A progressively growing primary s.c. tumor that died or were sacrificed when the primary s.c tumor reached at least 25 mma | A large secondary s.c tumora | Metastases in internal organsb |
| 10/10 | 22/43 | 3/43 | 18/43 |
For mice used in the survival studies depicted in Fig. 1
aThese mice were not examined for metastasis to internal organs
bMice in which the s.c. tumor nodule regressed by ≥30%,but the mice lost more than 20% of their body weight (minus tumor) and had difficulties ambulating. These mice were examined for metastasis to the spleen and liver
Fig. 7.
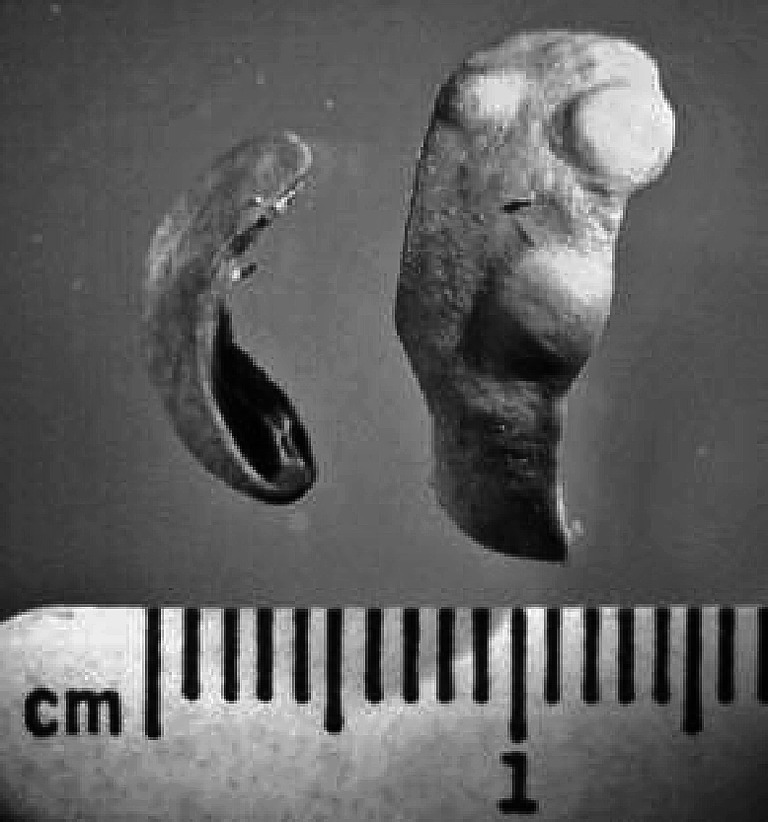
Metastatic foci in the spleen from a representative IFN-α/βR−/− mouse in which the large primary s.c MOPC-315 tumor regressed by more than 30% as a consequence of low-dose CY therapy, but the mouse lost more than 20% body weight and showed signs of distress. The spleen was fixed in Bouins’ fixative. As a reference point a normal spleen similarly fixed was placed next to it
Fig. 8.
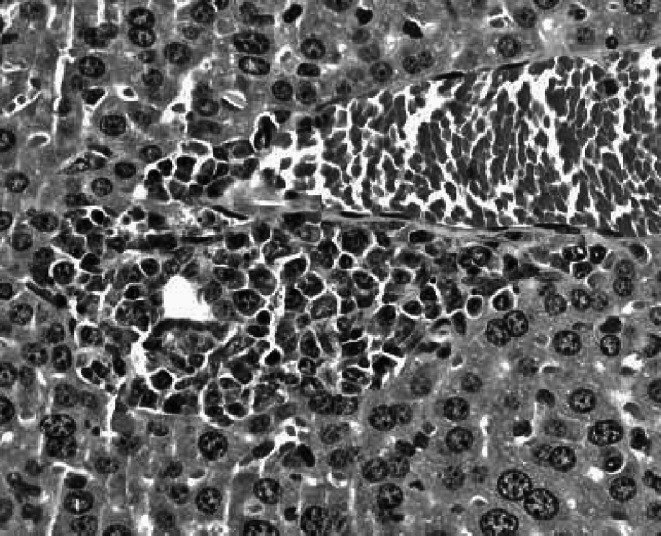
Metastatic foci in the liver of a representative IFN-α/βR−/− mouse in which the s.c. tumor regressed by more than 30% as a consequence of CY therapy, but the mouse lost more than 20% of its body weight and showed signs of distress. H&E stained section, original magnification ×500
Assessment of the resistance to tumor challenge of CY cured MOPC-315 tumor bearing mice
We have previously shown that WT mice cured of a large s.c. MOPC-315 tumor as a consequence of low-dose CY are resistant to a subsequent tumor challenge [13, 28]. Experiments were performed to determine if IFN-α/βR−/− mice in which the primary tumor regressed completely following low-dose CY therapy and the mice appeared clinically normal at the end of the 6 week observation period (these mice will be referred to as cured mice) were also resistant to a subsequent tumor challenge. Accordingly, we challenged four CY cured IFN-α/βR−/− mice with 2×106 MOPC-315 tumor cells, with CY cured WT mice serving as control. None of the IFN-α/βR−/− mice that were cured of a large MOPC-315 tumor as a consequence of low-dose CY therapy resisted the tumor challenge, with all four mice succumbing to tumor progression within 10 days. In contrast, in confirmation of our previous observations [13, 28], all of the WT mice that were cured of a large MOPC-315 tumor as a consequence of low-dose CY therapy were resistant to the tumor challenge. Thus, signaling via the IFN-α/βR on host cells is important for rendering CY cured MOPC-315 tumor bearing mice resistant to a subsequent tumor challenge, indicating that signaling via the IFN-α/βR on host cells is important for establishing long-lasting antitumor immunity in low-dose CY cured MOPC-315 tumor bearers.
Discussion
Schiavoni et al. [37] have previously shown that CY administration to normal mice leads within 6 h to up-regulation of IFN-α/β mRNA expression, which is followed by up-regulation of IFN-α/β at the protein level. Here we extend these observations by demonstrating that IFN-α/β is actually important for the realization of the therapeutic benefits of CY for mice bearing a large MOPC-315 tumor, under conditions in which the therapeutic outcome of the chemotherapy depends on T cell-mediated tumor-eradicating immunity that emerges in the hitherto immunosuppressed tumor bearers as a consequence of the chemotherapy [14, 27, 38]. Specifically, we show that low-dose CY is much less effective in the therapy of IFN-α/βR−/− mice bearing a large MOPC-315 tumor than in the therapy of WT mice bearing a large MOPC-315 tumor. We would like to point out, however, that although signaling via the IFN-α/βR is important for the realization of the therapeutic benefits of low-dose chemotherapy, other chemotherapy-induced effects, which are independent of signaling via the IFN-α/βR (e.g., up-regulation of B7-1 expression) are also important [8].
Low-dose chemotherapy was previously shown to be effective in up-regulating IFN-β mRNA expression not only in spleens of WT mice but also in spleens of IFN-α/βR−/− mice [18]. Still, since signaling via the IFN-α/βR can lead to a subsequent increase in IFN-α/β production [22, 39], it is reasonable to assume that as a consequence of the positive feedback loop, higher concentrations of IFN-α/β were achieved in CY treated WT mice than in CY treated IFN-α/βR−/− mice. It follows, that MOPC-315 tumor cells may have been exposed to a higher concentration of IFN-α/β in CY treated tumor bearing WT mice than in CY treated tumor bearing IFN-α/βR−/− mice. Here we show that exposure of MOPC-315 tumor cells to IFN-β, which is known to exert a much more potent anti-proliferative effect than IFN-α [5, 17, 35], was not inhibitory for MOPC-315 tumor cell proliferation even at a concentration of 100 U/ml. This concentration of IFN-β is much higher than the concentrations of IFN-β that were reported by other investigators to inhibit drastically the proliferation of tumor cell lines sensitive to the anti-proliferative activity of IFN-β (e.g., 25 U/ml [10, 35]). Moreover, this concentration of IFN-β is much higher than the combined concentrations of IFN-β and IFN-α that were reached in the blood of CY treated WT mice, which ranged between <4 U/ml and 32 U/ml [37]. Thus, it is unlikely that the much greater therapeutic efficacy of low-dose CY for MOPC-315 tumor bearing WT mice than for MOPC-315 tumor bearing IFN-α/βR−/− mice was the result of greater anti-proliferative activity of IFN-α/β for MOPC-315 tumor cells in the WT mice. These observations are not surprising in light of our previous observations with T-cell-depleted MOPC-315 tumor bearing WT mice, which revealed that tumor-eradicating immunity that emerges after the chemotherapy, and not the tumoricidal/tumoristatic activity of the low dose of CY, is responsible for the eradication of the vast majority of the tumor burden [14, 29]. Moreover, in the absence of the contribution of T-cell-mediated tumor-eradicating immunity, the low dose of CY is not sufficient even for the cure of WT mice bearing a barely palpable (≤2 mm) s.c. MOPC-315 tumor [13, 29].
We have previously shown that the curative effectiveness of low-dose CY for WT mice bearing a large MOPC-315 tumor depends on the accumulation of T lymphocytes with acquired CTL activity in the s.c. tumors [30, 38]. Consistent with these observations, we show here that CY, which was much less effective in the therapy of tumor bearing IFN-α/βR−/− mice than tumor bearing WT mice, led to a different pattern of accumulation of T lymphocytes in the s.c. tumors of the IFN-α/βR−/− mice than of the WT mice. Specifically, while on day 4 after low-dose CY administration, a massive T lymphocytic infiltrate was evident throughout the tumor mass in the WT mice and the T lymphocytes were in direct contact with tumor cells, most of the T lymphocytes that were present in the s.c. tumors of the CY treated IFN-α/βR−/− mice remained within the blood vessels with very few T lymphocytes present within the tumor mass. Thus, even if T lymphocytes from tumor bearing IFN-α/βR−/− mice, like T lymphocytes from tumor bearing WT mice [14, 27, 30, 38], acquire CTL activity as a consequence of low-dose chemotherapy (which is not at all clear that they do), the location of the T lymphocytes in the s.c. tumors of CY treated IFN-α/βR−/− mice would hamper their ability to kill MOPC-315 tumor cells.
It is not known at present why on day 4 after the chemotherapy T lymphocytes that accumulated in the s.c. tumors of CY treated IFN-α/βR−/− mice remained in the blood vessels while T lymphocytes that accumulated in the s.c. tumors of CY treated WT mice dispersed throughout the tumor mass and came in direct contact with tumor cells. One possibility is that the production/activity of a factor(s) needed for transendothelial migration of the T lymphocytes from the blood vessels into the tumor mass depends on (or is facilitated by) signaling via the IFN-α/βR. In this regard it should be pointed out that binding of IFN-β to the IFN-α/βR on human fibroblasts was shown to result in activation of the β-R1 gene that encodes for the interferon-inducible T cell alpha chemoattractant (I-TAC; CXCL11) [33, 34], and I-TAC, in turn, was shown to be a very potent chemoattractant for activated T cells [6], as well as a potent inducer of T cell transendothelial migration [26]. It follows that while I-TAC was probably induced via this pathway in CY treated WT mice, it was not induced in CY treated IFN-α/βR−/− mice. However, although IFN-β is an important inducer of I-TAC expression [33, 34], IFN-γ alone or in combination with TNF-α was also shown to induce I-TAC expression [26]. Still, since signaling via the IFN-α/βR is important for chemotherapy-induced upregulation of IFN-γ and TNF-α expression [7, 18, 20], it is conceivable that low-dose CY therapy up-regulated IFN-γ and TNF-α expression to a lesser extent (if at all) in IFN-α/βR−/− mice. It follows that the induction of I-TAC production via the IFN-γ pathway was probably also much less efficient (if at all) in the CY treated IFN-α/βR−/− mice than the CY treated WT mice.
Here we show that IFN-α/βR−/− mice in which low-dose CY therapy caused a significant regression of the primary s.c. tumor, but the mice showed signs of distress, had extensive splenic metastases. Since low-dose chemotherapy was previously found to lead to the appearance of antitumor immunity in spleens of MOPC-315 tumor bearing WT mice [13, 38, 42], our current observations suggest that low-dose CY therapy of MOPC-315 tumor bearing IFN-α/βR−/− mice did not lead to the acquisition of sufficient antitumor immunity in their spleens to control metastatic tumor progression. Such an explanation is consistent with reports that IFN-α/β can potentiate the generation of CTL responses [9, 15], which implies that weaker CTL activity would develop in the absence of signaling via the IFN-α/βR. In addition, such an explanation is consistent with our recent unpublished observations illustrating the development of weaker CTL activity in stimulation cultures of spleen cells from CY treated MOPC-315 tumor bearing WT mice as a consequence of IFN-α/β neutralization.
Similar to our previous observations [13, 28], we show here that WT mice cured of a large MOPC-315 tumor as a consequence of low-dose CY therapy were resistant to a subsequent challenge with 2×106 viable MOPC-315 tumor cells. In contrast, none of four IFN-α/βR−/− mice cured of a large MOPC-315 tumor as a consequence of low-dose CY therapy resisted the tumor challenge, and all succumbed to tumor progression within 10 days. These observations indicate that signaling via the IFN-α/βR is important for the acquisition of long-lasting tumor-eradicating immunity by low-dose CY cured MOPC-315 tumor bearers. In this regard it should be pointed out that IFN-α/β was previously shown to selectively increase the proliferation of memory phenotype (CD44high) CD8+ T cells, and CD8+ T cells that proliferated following injection of mice with tumor cells engineered to secrete IFN-α persisted for at least 70 days [3].
Finally, we have previously shown that low-dose melphalan [18], like low-dose CY [37], also leads to a rapid upregulation of IFN-α/β expression. In addition, the realization of the therapeutic benefits of low-dose melphalan [27, 30, 38], like the realization of the therapeutic benefits of low-dose CY [14], for MOPC-315 tumor bearers was shown to depend on T cell-mediated tumor-eradicating immunity that is acquired as a consequence of the chemotherapy. Given these observations, it is conceivable that our current observations regarding the role of IFN-α/β in the realization of the therapeutic benefits of low-dose CY are not limited to this anticancer drug.
Abbreviations
- CY
Cyclophosphamide
- WT mice
Wild-type mice
- I-TAC
Interferon-inducible T cell alpha chemoattractant
Footnotes
This work was supported by Research Grant 03-19 from the American Cancer Society-Illinois Division.
References
- 1.Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649. [PubMed] [Google Scholar]
- 2.Bass KK, Mastrangelo MJ. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol Immunother. 1998;47:1. doi: 10.1007/s002620050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belardelli F, Ferrantini M, Santini SM, Baccarini S, Proietti E, Colombo MP, Sprent J, Tough DF. The induction of in vivo proliferation of long-lived CD44hi CD8+ T cells after the injection of tumor cells expressing IFN-alpha1 into syngeneic mice. Cancer Res. 1998;58:5795. [PubMed] [Google Scholar]
- 4.Berd D, Mastrangelo MJ. Active immunotherapy of human melanoma exploiting the immunopotentiating effects of cyclophosphamide. Cancer Invest. 1988;6:337. doi: 10.3109/07357908809080657. [DOI] [PubMed] [Google Scholar]
- 5.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7:1821. [PubMed] [Google Scholar]
- 6.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deonarain R, Verma A, Porter AC, Gewert DR, Platanias LC, Fish EN. Critical roles for IFN-beta in lymphoid development, myelopoiesis, and tumor development: links to tumor necrosis factor alpha. Proc Natl Acad Sci USA. 2003;100:13453. doi: 10.1073/pnas.2230460100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donepudi M, Raychaudhuri P, Bluestone JA, Mokyr MB. Mechanism of melphalan-induced B7-1 gene expression in P815 tumor cells. J Immunol. 2001;166:6491. doi: 10.4049/jimmunol.166.11.6491. [DOI] [PubMed] [Google Scholar]
- 9.Gehring S, Gregory SH, Kuzushita N, Wands JR. Type 1 interferon augments DNA-based vaccination against hepatitis C virus core protein. J Med Virol. 2005;75:249. doi: 10.1002/jmv.20264. [DOI] [PubMed] [Google Scholar]
- 10.Giandomenico V, Vaccari G, Fiorucci G, Percario Z, Vannuchi S, Matarrese P, Malorni W, Romeo G, Affabris GR. Apoptosis and growth inhibition of squamous carcinoma cells treated with interferon-alpha, IFN-beta and retinoic acid are associated with induction of the cyclin-dependent kinase inhibitor p21. Eur Cytokine Netw. 1998;9:619. [PubMed] [Google Scholar]
- 11.Gorelik L, Mokyr MB. Low-dose-melphalan-induced up-regulation of type-1 cytokine expression in the s.c. tumor nodule of MOPC-315 tumor bearers and the role of interferon gamma in the therapeutic outcome. Cancer Immunol Immunother. 1995;41:363. doi: 10.1007/BF01526556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelik L, Prokhorova A, Mokyr MB. Low-dose melphalan-induced shift in the production of a Th2-type cytokine to a Th1-type cytokine in mice bearing a large MOPC-315 tumor. Cancer Immunol Immunother. 1994;39:117. doi: 10.1007/BF01525317. [DOI] [PubMed] [Google Scholar]
- 13.Hengst JC, Mokyr MB, Dray S. Importance of timing in cyclophosphamide therapy of MOPC-315 tumor-bearing mice. Cancer Res. 1980;40:2135. [PubMed] [Google Scholar]
- 14.Hengst JC, Mokyr MB, Dray S. Cooperation between cyclophosphamide tumoricidal activity and host antitumor immunity in the cure of mice bearing large MOPC-315 tumors. Cancer Res. 1981;41:2163. [PubMed] [Google Scholar]
- 15.Hiroishi K, Tuting T, Lotze MT. IFN-alpha-expressing tumor cells enhance generation and promote survival of tumor-specific CTLs. J Immunol. 2000;164:567. doi: 10.4049/jimmunol.164.2.567. [DOI] [PubMed] [Google Scholar]
- 16.Inagawa H, Nishizawa T, Honda T, Nakamoto T, Takagi K, Soma G. Mechanisms by which chemotherapeutic agents augment the antitumor effects of tumor necrosis factor: involvement of the pattern shift of cytokines from Th2 to Th1 in tumor lesions. Anticancer Res. 1998;18:3957. [PubMed] [Google Scholar]
- 17.Johns TG, Mackay IR, Callister KA, Hertzog PJ, Devenish RJ, Linnane AW. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon beta. J Natl Cancer Inst. 1992;84:1185. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- 18.Jovasevic VM, Mokyr MB. Melphalan-induced expression of IFN-beta in MOPC-315 tumor-bearing mice and its importance for the up-regulation of TNF-alpha expression. J Immunol. 2001;167:4895. doi: 10.4049/jimmunol.167.9.4895. [DOI] [PubMed] [Google Scholar]
- 19.Lattime EC, Mastrangelo MJ, Bagasra O, Li W, Berd D. Expression of cytokine mRNA in human melanoma tissues. Cancer Immunol Immunother. 1995;41:151. doi: 10.1007/BF01521340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461. doi: 10.1016/S1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 21.Leib DA, Machalek MA, Williams BR, Silverman RH, Virgin HW. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA. 2000;97:6097. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matar P, Rozados VR, Gervasoni SI, Scharovsky GO. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol Immunother. 2002;50:588. doi: 10.1007/s00262-001-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matar P, Rozados VR, Gonzalez AD, Dlugovitzky DG, Bonfil RD, Scharovsky OG. Mechanism of antimetastatic immunopotentiation by low-dose cyclophosphamide. Eur J Cancer. 2000;36:1060. doi: 10.1016/S0959-8049(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 25.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 26.Mohan K, Ding Z, Hanly J, Issekutz TB. IFN-gamma-inducible T cell alpha chemoattractant is a potent stimulator of normal human blood T lymphocyte transendothelial migration: differential regulation by IFN-gamma and TNF-alpha. J Immunol. 2002;168:6420. doi: 10.4049/jimmunol.168.12.6420. [DOI] [PubMed] [Google Scholar]
- 27.Mokyr MB, Barker E, Weiskirch LM, Takesue BY, Pyle JM. Importance of Lyt 2+ T-cells in the curative effectiveness of a low dose of melphalan for mice bearing a large MOPC-315 tumor. Cancer Res. 1989;49:4597. [PubMed] [Google Scholar]
- 28.Mokyr MB, Dray S. Some advantages of curing mice bearing a large subcutaneous MOPC-315 tumor with a low dose rather than a high dose of cyclophosphamide. Cancer Res. 1983;43:3112. [PubMed] [Google Scholar]
- 29.Mokyr MB, Hengst JC, Dray S. Role of antitumor immunity in cyclophosphamide-induced rejection of subcutaneous nonpalpable MOPC-315 tumors. Cancer Res. 1982;42:974. [PubMed] [Google Scholar]
- 30.Mokyr MB, Rubin M, Newell KA, Prokhorova A, Bluestone JA. Involvement of TCR-V beta 8.3+ cells in the cure of mice bearing a large MOPC-315 tumor by low dose melphalan. J Immunol. 1993;151:4838. [PubMed] [Google Scholar]
- 31.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 32.Nagarkatti M, Toney DM, Nagarkatti PS. Immunomodulation by various nitrosoureas and its effect on the survival of the murine host bearing a syngeneic tumor. Cancer Res. 1989;49:6587. [PubMed] [Google Scholar]
- 33.Rani MR, Foster GR, Leung S, Leaman D, Stark GR, Ransohoff RM. Characterization of beta-R1, a gene that is selectively induced by interferon beta (IFN-beta) compared with IFN-alpha. J Biol Chem. 1996;271:22878. doi: 10.1074/jbc.271.37.22878. [DOI] [PubMed] [Google Scholar]
- 34.Rani MR, Gauzzi C, Pellegrini S, Fish EN, Wei T, Ransohoff RM. Induction of beta-R1/I-TAC by interferon-beta requires catalytically active TYK2. J Biol Chem. 1999;274:1891. doi: 10.1074/jbc.274.4.1891. [DOI] [PubMed] [Google Scholar]
- 35.Rosenblum MG, Yung WK, Kelleher PJ, Ruzicka F, Steck PA, Borden EC. Growth inhibitory effects of interferon-beta but not interferon-alpha on human glioma cells: correlation of receptor binding, 2′,5′-oligoadenylate synthetase and protein kinase activity. J Interferon Res. 1990;10:141. doi: 10.1089/jir.1990.10.141. [DOI] [PubMed] [Google Scholar]
- 36.Sahasrabudhe DM, deKernion JB, Pontes JE, Ryan DM, O’Donnell RW, Marquis DM, Mudholkar GS, McCune CS. Specific immunotherapy with suppressor function inhibition for metastatic renal cell carcinoma. J Biol Response Modif. 1986;5:581. [PubMed] [Google Scholar]
- 37.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024. [PubMed] [Google Scholar]
- 38.Takesue BY, Pyle JM, Mokyr MB. Importance of tumor-specific cytotoxic CD8+ T-cells in eradication of a large subcutaneous MOPC-315 tumor following low-dose melphalan therapy. Cancer Res. 1990;50:7641. [PubMed] [Google Scholar]
- 39.Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14:111. doi: 10.1016/S0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 40.Uze G, Lutfalla G, Mogensen KE. Alpha and beta interferons and their receptor and their friends and relations. J Interferon Cytokine Res. 1995;15:3. doi: 10.1089/jir.1995.15.3. [DOI] [PubMed] [Google Scholar]
- 41.Weiskirch LM, Bar-Dagan Y, Mokyr MB. Transforming growth factor-beta-mediated down-regulation of antitumor cytotoxicity of spleen cells from MOPC-315 tumor-bearing mice engaged in tumor eradication following low-dose melphalan therapy. Cancer Immunol Immunother. 1994;38:215. doi: 10.1007/BF01533512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiskirch LM, Baumgartel BA, Barker E, Mokyr MB. Phorbol ester-induced enhancement in lytic activity of CD8+ splenic T cells from low-dose melphalan-treated MOPC-315-tumor bearers. Cancer Immunol Immunother. 1991;32:353. doi: 10.1007/BF01741330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan L, Kuramitsu Y, Li Y, Kobayashi M, Hosokawa M. Restoration of interleukin-2 production in tumor-bearing rats through reducing tumor-derived transforming growth factor beta by treatment with bleomycin. Cancer Immunol Immunother. 1995;41:355. doi: 10.1007/BF01526555. [DOI] [PMC free article] [PubMed] [Google Scholar]