Abstract
Preeclampsia is a common multifactorial disease of pregnancy. Dysregulation of the complement activation is among emerging candidates responsible for disease pathogenesis. In a targeted exomic sequencing study we identified 14 variants within nine genes coding for components of the membrane attack complex (MAC, C5b-9) that are associated with preeclampsia. We found two rare missense variants in the C5 gene that predispose to preeclampsia (rs200674959: I1296V, OR (CI95) = 24.13 (1.25–467.43), p-value = 0.01 and rs147430470: I330T, OR (CI95) = 22.75 (1.17–440.78), p-value = 0.01). In addition, one predisposing rare variant and one protective rare variant were discovered in C6 (rs41271067: D396G, OR (CI95) = 2.93 (1.18–7.10), p-value = 0.01 and rs114609505: T190I, 0.02 OR (CI95) = 0.47 (0.22–0.92), p-value = 0.02). The results suggest that variants in terminal complement pathway predispose to preeclampsia.
Introduction
Preeclampsia (PE) is a common pregnancy-specific vascular disorder that affects approximately 3% of pregnancies (1, 2). It accounts for over 50 000 maternal and 900 000 perinatal deaths annually (3, 4). The clinical characteristics are diverse and the course of the disease is unpredictable. The cornerstones of management are observation and delivery. Even when high quality antenatal healthcare is available, maternal morbidity is considerable and indicated preterm deliveries may result in newborn complications. There is a familial predisposition to PE and strong epidemiological evidence points to PE being partially inherited (5, 6). Furthermore, loci harbouring genes that have inflammatory or immune regulatory function were recently found to link PE and hypertension in pregnancy (7, 8).
Complement system is at the frontline of innate immunity with a capacity to cause cell death and tissue destruction as well as to trigger adaptive immune responses. In particular, the complement system has a unique capacity to discriminate between self and non-self structures and to recognize nonviable cells (9). Inadequate regulation may result in poor placentation and predispose to the development of PE (10–12). Activation of any of the three pathways of the complement system, the classical (CP), lectin (LP) or alternative pathway (AP), can lead to a common end point: terminal pathway activation featuring a membrane attack complex (MAC, C5b-9) formation. Insertion of the MAC in the plasma membrane is initiated by the C5b-8 complex composed of the C5b, C6, C7 and C8 (13). After cleavage of C5, the generated soluble C5b binds C6, which then recruits C7 and C5b67 can attach to a membrane. Further binding of C8 leads to insertion of the C5b-8 complex into a membrane. This step enables recruitment of multiple C9 molecules to complete the cylindrical MAC structure (14). In the MAC pore, C9 proteins constitute a polymeric ring with an inner diameter of about 10 nm (15). Because of a risk of membrane damage during complement activation, human cells are protected from damage by protectin (CD59). This GPI anchored protein inhibits MAC formation by binding to C5b-8 and C5b-9 to prevent further C9 from attaching to these complexes (16, 17). Therefore, the balanced regulatory capacity of CD59 and MAC activation is crucial for integrity and function of endothelial surfaces and placental trophoblast cells (CD59 is highly expressed in both of these cell types) (18).
The major activators of the CP are antibody-antigen complexes and C-reactive protein (CRP). Subsequent proteolytic activation steps by C1 lead to cleavage of C4 and C2 and formation of the CP C3 convertase (C4b2a). This bimolecular enzymatic complex is similar to the AP C3 convertase (C3bBb) and converts C3 into C3a and C3b. Contained within the AP is an efficient feedback or amplification loop for the generation of large amounts of C3b to opsonize a pathogen. Due to the feedback loop, AP activation accounts for most of the activity of the complement system (~ 80%) even if CP or LP was initially activated. Because the complement system provides a rapidly activated and potent surveillance mechanism for the host, strict control is required to avoid damage to self. Thus, inhibition of complement activation is mediated by host regulators in plasma as well as on cells. Control is aimed at each of the major steps in the pathway: initiation, amplification (leading to C3 and C5 cleavage), and formation of the MAC or C5b-9. The membrane-associated regulators are membrane cofactor protein (MCP; CD46), decay-accelerating factor (DAF; CD55) and complement receptor 1 (CR1; CD35). The fluid-phase regulators are factor H (FH) and C4b-binding protein (C4BP). During pregnancy, complement assists in the clearance of placental fragments that enter the maternal circulation as a result of syncytiotrophoblast turn-over (19). One prevailing hypothesis is that improper clearance of such components, driven by an inadequately regulated complement cascade, may lead to deposition of debris in tissues and vascular walls leading to an overly exuberant inflammatory response (11). Such unwarranted complement activation at the maternal-fetal border may lead to inadequate spiral artery remodelling, lack of maternal-fetal tolerance and poor placentation, all of which are potential key mechanisms in the pathogenesis of PE (12, 20). Genetic variants resulting in aberrant protein function in complement receptors CR3 and CR4 have been linked to increased risk of PE (19). We have recently identified and published rare genetic variants in Factor H that predispose to PE, due to excessive complement activation (21).
We designed a targeted exome sequencing protocol to screen the exomes and splicing areas of selected genes within the complement system in PE patients and controls.
Materials and Methods
In this targeted exomic sequencing study, we combined data from exomic sequencing of 487 (body mass index < 30 kg/m2) PE mothers and 187 lean non-PE control mothers from the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC)(22) and 122 women with a history of PE. The diagnosis of PE was based on a clinician’s diagnosis obtained through the comprehensive national Hospital Discharge Registry. Controls included 1905 parous women with no such history from the national FINRISK study (THL Biobank permit no BB2016_8). From the FINNPEC cohort, nulliparous or multiparous women with a singleton pregnancy and no history of chronic hypertension, diabetes or renal disease were included. All patients were of European ancestry. A jury consisting of a midwife and an obstetrician independently confirmed the diagnosis of PE. For the diagnosis, newly-onset hypertension and proteinuria after 20 weeks of gestation were required. Hypertension was defined as a systolic blood pressure of ≥ 140 mm Hg and/or a diastolic blood pressure of ≥ 90 mm Hg after 20 weeks of gestation. Proteinuria was defined as the urinary excretion of ≥ 0.3 g protein in a 24-hour specimen or > 0.3 g/l of protein in urine, or two positive dipstick readings in the absence of a urinary tract infection.
In a previously described custom-made targeted exomic sequencing protocol, we combined Illumina sequencing libraries for capture and sequencing with Nimblegen sequence capture (23, 24). The subjects and methods of this study have been described in detail previously (19, 21, 24, 25).
Statistics
Sequence data were analyzed in PLINK/Seq, Plink (26)and R programs. Quality control before meta-analysis included removal of singleton and monomorphic variants, removal of sites with > 10% missing data in the targeted sequencing or a significant departure from Hardy-Weinberg equilibrium in controls (p < 0.001). Analyses of the significant associations were performed by the Fisher’s exact test. P-values < 0.05 were considered to indicate a significant difference. The tail of the p-value distribution of benign variants was as expected, suggesting that the overall study design and quality control were successful without multiple test adjustments. In addition to an appropriate statistical probability test, odds ratios (OR) with 95% confidence intervals (CI95) were calculated for all variants.
Study approval
All subjects provided a written informed consent for the study. The FINNPEC study protocol was approved by the coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (permit number 149/E0/07). National FINRISK study description and ethical approvals are available online: https://www.thl./documents/10531/1921702/2015+FINRISK+description_for_researchers_nal.pdf/fc952cba-86f6-4ef5-8ef2-fa13c23173c3.
Results
To analyze if variants in genes coding for 40 components of the complement system are associated with PE, we performed association testing on Finnish preeclamptic mothers (609) and controls (2,092). The studied genes are listed in Supplementary table 1 and results of these genetic association analyses are listed in Table 1, Of the 15 associating variants, 10 were rare (MAF < 0.1) and, of these, 7 were missense variants. The lack of inflated p-values in comparison of benign and putatively functional variants indicates that confounders such as stratification are not causing false positives.
Table 1.
Genetic variants within genes coding for components of the complement system that are associated with preeclampsia (p ≤ 0.05).
| RSID | Gene name | P-value | OR (95% confidence interval) | MAF cases/MAF controls (%) | Consequence (distance from exon, base pairs) | ACMG/ClinVar | Role of gene |
|---|---|---|---|---|---|---|---|
| rs45532534 | C3 | 0.01 | 13.80 (1.36 – 677.10) | 0.33/0.02 | intron variant (+39) | VUS/NA | AP activation |
| rs2230201 | C3 | 0.04 | 1.17 (1.01 – 1.37) | 30.61/26.08 | synonymous variant R304=; TF binding site | Benign/Benign | AP activation |
| rs41258244 | CD46 | 0.05 | 1.37 (0.995 – 1.863) | 5.15/3.82 | intron variant (−41) | VUS/Benign | CP and AP inhibition, surface-bound |
| rs1800947 | CRP | 0.02 | 1.36 (1.06 – 1.74) | 7.66/5.69 | synonymous variant, L184= | VUS/NA | CP activation, AP inhibition |
| rs3828032 | CFHR2 | 0.020 | 0.825 (0.703 – 0.971) | 26.93/22.22 | intron variant (−20) | VUS/Benign | AP regulation |
| rs114727460 | CFHR4 | 0.026 | 6.855 (0.981 – 75.743) | 0.33/0.05 | intron variant (−34) | VUS/NA | AP regulation |
| rs7417769 | CFHR4 | 0.012 | 0.811 (0.688 – 0.956) | 25.62/20.77 | missense variant, N209S | VUS/Benign | AP regulation |
| rs35662416 | CFHR5 | 0.033 | 1.819 (1.030 – 3.131) | 1.81/1.00 | missense variant, R359H | VUS/Likely-Benign | AP regulation |
| rs200674959 | C5 | 0.01 | 24.13 (1.25 – 467.43) | 0.25/0 | missense variant, I1296V | VUS/VUS | TP activation |
| rs147430470 | C5 | 0.01 | 22.75 (1.17 – 440.78) | 0.25/0 | missense variant, I330T | VUS/Likely-Benign | TP activation |
| rs41271067 | C6 | 0.01 | 2.93 (1.18 – 7.10) | 0.90/0.31 | missense variant, D396G | VUS/Benign | TP activation |
| rs114609505 | C6 | 0.02 | 0.47 (0.22 – 0.92) | 0.82/1.73 | missense variant, T190I | VUS/Likely-Benign | TP activation |
| rs605648 | C8B | 0.01 | 1.34 (1.08 – 1.66) | 11.56/8.62 | intron variant (−20) | VUS/Benign | TP activation |
| rs72670361 | C8B | 0.02 | 1.46 (1.056 – 2.007) | 4.94/3.43 | intron variant (+40) | VUS/NA | TP activation |
RSID, SNP identifier; OR, odds ratio; MAF, minor allele frequency in total sample; VUS, variant of uncertain significance
NA, not available; C, complement; CP, classical pathway; TP, terminal pathway; AP, alternative pathway
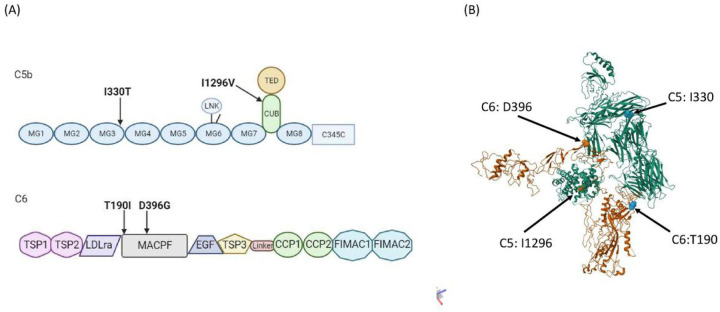
Interestingly, two rare missense variants in both C5 and C6 were found to associate with PE (Fig. 1A). Of these, the rs200674959 (I1296V), located in conserved complement-activating CUB (complement C1r/C1s, Uegf, Bmp1) domain and rs147430470 (I330T) located in Macroglobulin-like domain 3 (MG3) in C5, were predisposing variants (OR = 24.13, CI95 = 1.25–467.43; p = 0.01 and OR = 22.75, CI95 = 1.17–440.78; p = 0.01, respectively). Also, the rs41271067 (D396G) variant in C6 was predisposing (OR = 2.93, CI95 = 1.18–7.10; p = 0.01), while rs114609505 (T190I) had a protective effect (OR = 0.47, CI95 = 0.22–0.92; p = 0.02). Both of the associating C6 variants were located in the membrane attack complex/perforin domain (MACPF). No associating variants were discovered in the MAC inhibitor protectin (CD59).
Figure 1.
(A) The domain structure of C5b (top) and C6 (bottom) with PE associating variants indicated. The C5 variants are located in exon 31/41 (I330) coding the Immunoglobulin-like domain 3 (MG3) and in exon 9/41 (I1296) coding the conserved complement-activating CUB (complement C1r/C1s, Uegf, Bmp1) domain Both C6 variants in exons 6/16 (T190) and 12/16 (D396) are located in the membrane attack complex/perforin domain (MACPF). (B) The protein structure of C6 (orange) in complex with C5b (green) with the PE-associating amino acids’ positions indicated. The D396 is located directly in the interaction site between the two molecules. The structural model is based on construct 4A5W (37).
Other potential associations were found in rare predisposing variants in genes coding for CRP (rs1800947, p = 0.02), CFI (rs200040240, p = 0.003), C3 (rs45532534, p = 0.011) and C5 (rs200674959, p = 0.011, discussed above). The strongest protective associations were found in common variants in genes coding for CFHR4 (rs7417769, p = 0.012) and C8B (rs605648, p = 0.01). To summarize, associating variants were found in genes coding for components of the classical and alternative pathways, but not in the activating components of the lectin pathway. Importantly, six variants were found in the common terminal pathway.
Discussion
In this study we identified that rare missense variants in genes coding for C5 and C6 of the terminal pathway of complement system activation are associated with PE. We have previously described genetic associations of PE to C3, complement receptors CR3 and CR4 and to the key regulator factor H. Taken together our findings suggest that abnormalities in the alternative and terminal pathway are of particular importance to PE pathogenesis. Potential or known disease associations of the PE-associated variants discovered in this study are scarce in literature.
The terminal pathway of complement activation has received less attention in PE studies than the classical or alternative pathways. CD59 is the only membrane-bound regulator of the terminal pathway, which is attached to the surface of the endothelium and trophoblast cells by a glycophosphatidylinositol (GPI) anchor. The plasma levels of CD59 are increased in PE and associated with end-organ injury related laboratory measures often observed in severe PE, such as elevated liver enzymes and lactase dehydrogenase and decreased platelet count (27). We have previously shown that CD59 is abundant in the placental trophoblasts, where it is exposed to damage by turbulent intervillous maternal circulation, a characteristic of the PE placenta caused by inadequate uterine arterial remodulation associated with the disease etiology (18, 28). It is possible that shear damage caused to the syncytiotrophoblast releases CD59 to maternal circulation thereby leaving the villous tissue vulnerable to aberrant complement attack. Terminal complement complex deposits have been localized in the fibrinoid material of the decidua of the basal plate, in the stroma of the chorionic villi and in the vessel walls, and their levels are increased in preeclamptic placentas (29). This may be exacerbated in patients with MAC-coding genetic variants. Both associating variants in C6 are located in the membrane attack complex/perforin domain, which is responsible the MAC function of C6. The D396G is directly in the interaction site between C6 and C5b (Fig. 1B).
We have previously shown that genetic variants of complement component 3 (C3) are associated with susceptibility to PE (25). The protective variant rs2230201 in PE is known to relate to levels of C3 in serum as well as to associate with dense deposit disease (DDD) and systemic lupus erythematosus (SLE) (30–32). Furthermore, the rs2230201 observed in this study is in linkage disequilibrium (D’=1; Finnish population) with the rare variant rs190390034 in C3, which was previously found to be independently, and in a C3-haplotype, associated with increased risk for severe PE (25). Thus, the association of rs2230201 corroborates our previous finding.
CRP is an inflammation marker and trigger of the CP of complement activation. In addition, CRP controls the AP to promote iC3b-mediated clearance of debris by recruiting factor H (33). The minor PE-associated allele of rs1800947 in CRP has been found to be associated with a reduced plasma level of CRP in an elderly Finnish population (34). We found that the rs1800947 predisposes to PE (p-value = 0.021; OR 1.356 CI95 = 1.044–1.748). While the variant, also known as CRP2 is synonymous, it has been suggested to have a possible regulatory function. Studies in complex diseases have shown an association of rs1800947, for example, with systemic lupus erythematosus, the severity of prostate cancer and protection from cardiovascular disease (35–37).
This study adds to previous evidence by us and others indicating that in discordant patients, rare complement variants may play an important role in risk for PE. Variants of uncertain significance in genes coding for components of the terminal pathway of complement system may, in the context of physiological changes typical to PE become pathogenic. This is in support of the multiple hit theory of PE aetiology, where multiple factors, one being a rare complement variant, together create the perfect storm of disease-causing conditions.
Acknowledgements:
We thank Elisha D.O. Roberson for assistance with the data analysis (P30-AR073752).
Funding:
The analysis and writing of this paper was supported by Jane and Aatos Erkko Foundation (HL), Sakari and Päivikki Sohlberg Foundation (HL), The Academy of Finland (121196 and 278941, HL), Sigrid Jusélius Foundation (SM), Alfred Kordelin Foundation (AIL), and laboratory work and analysis was supported by National Institutes of Health grants U54 HL112303 (JPA) and R01 GM099111 (JPA). Finnish Medical Foundation, University of Helsinki Funds, Special State Subsidy for Health Research (VTR funding TYH2022315), Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, and Foundation for Pediatric Research contributed to FINNPEC sample collection.
Funding Statement
The analysis and writing of this paper was supported by Jane and Aatos Erkko Foundation (HL), Sakari and Päivikki Sohlberg Foundation (HL), The Academy of Finland (121196 and 278941, HL), Sigrid Jusélius Foundation (SM), Alfred Kordelin Foundation (AIL), and laboratory work and analysis was supported by National Institutes of Health grants U54 HL112303 (JPA) and R01 GM099111 (JPA). Finnish Medical Foundation, University of Helsinki Funds, Special State Subsidy for Health Research (VTR funding TYH2022315), Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, and Foundation for Pediatric Research contributed to FINNPEC sample collection.
Footnotes
Competing interests:
AJ serves on the Scientific advisory boards of Alexion, AstraZeneca Rare Disease, and Novartis International AG, and serves as a consultant for Dianthus Therapeutics and Aurinia Pharmaceuticals. She has been a principal investigator for Apellis Pharmaceuticals and Novartis International AG. She also received royalty from UptoDate. HL received honoraria from Orion Corporation. JPA is part of the Scientific Advisory Board of Complement Corporation and Kypha, Inc., Scientific Advisory Board. Furthermore, he served as a consultant in Celldex Therapeutics, formerly Avant Immunotherapeutics, Inc., Biothera and Clinical Pharmacy Services, CDMI. SM received honoraria from Alexion, AstraZeneca Rare Disease, Biogen, Merck, Pfizer, and UCB, and research funding from Alexion.
Supplementary Files
Data availability:
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality but are available from Professor Hannele Laivuori (hannele.laivuori@helsinki.fi) on reasonable request.
References
- 1.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544.e1–544.e12. [DOI] [PubMed] [Google Scholar]
- 2.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2013;170(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3.Van Lerberghe W, Manuel A, Matthews Z, Cathy W. The World Health Report 2005 - make every mother and child count. World Health Organization; 2005. [Google Scholar]
- 4.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–7. [DOI] [PubMed] [Google Scholar]
- 5.Salonen Ros H, Lichtenstein P, Lipworth L, Cnattingius S. Genetic effects on the liability of developing pre-eclampsia and gestational hypertension. Am J Med Genet. 2000;91(4):256–60. [PubMed] [Google Scholar]
- 6.Cnattingius S, Reilly M, Pawitan Y, Lichtenstein P. Maternal and fetal genetic factors account for most of familial aggregation of preeclampsia: A population-based Swedish cohort study. Am J Med Genet A. 2004;130A(4):365–71. [DOI] [PubMed] [Google Scholar]
- 7.Tyrmi JS, Kaartokallio T, Lokki AI, Jääskeläinen T, Kortelainen E, Ruotsalainen S, et al. Genetic Risk Factors Associated With Preeclampsia and Hypertensive Disorders of Pregnancy. JAMA Cardiol. 2023;8(7):674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honigberg MC, Truong B, Khan RR, Xiao B, Bhatta L, Vy HMT, et al. Polygenic prediction of preeclampsia and gestational hypertension. Nature Medicine 2023;29(6):1540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meri S. Self-nonself discrimination by the complement system. FEBS Lett. 2016;590(15):2418–34. [DOI] [PubMed] [Google Scholar]
- 10.Girardi G, Lingo JJ, Fleming SD, Regal JF. Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond. Front Immunol. 2020;11:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teirilä L, Heikkinen-Eloranta J, Kotimaa J, Meri S, Lokki AI. Regulation of the complement system and immunological tolerance in pregnancy. Semin Immunol. 2019;45:101337. [DOI] [PubMed] [Google Scholar]
- 12.Regal JF, Burwick RM, Fleming SD. The Complement System and Preeclampsia. Current Hypertension Reports. 2017;19:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie CB, Jane-Wit D, Pober JS. Complement Membrane Attack Complex: New Roles, Mechanisms of Action, and Therapeutic Targets. Am J Pathol. 2020;190(6):1138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller-Eberhard HJ. The Killer Molecule of Complement. Journal of Investigative Dermatology. 1985;85(1):S47–52. [DOI] [PubMed] [Google Scholar]
- 15.Tschopp J, Engel A, Podack ER. Molecular weight of poly(C9). 12 to 18 C9 molecules form the transmembrane channel of complement. Journal of Biological Chemistry. 1984;259(3):1922–8. [PubMed] [Google Scholar]
- 16.Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, et al. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144(9):3478–83. [PubMed] [Google Scholar]
- 18.Meri S, Waldmann H, Lachmann PJ. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991;65(5):532–537. [PubMed] [Google Scholar]
- 19.Lokki AI, Teirilä L, Triebwasser M, Daly E, Bhattacharjee A, Uotila L, et al. Dysfunction of complement receptors CR3 (CD11b/18) and CR4 (CD11c/18) in preeclampsia: a genetic and functional study. BJOG. 2021;128(8):1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuyama T, Tomimatsu T, Mimura K, Yagi K, Kawanishi Y, Kakigano A, et al. Complement activation by an angiogenic imbalance leads to systemic vascular endothelial dysfunction: A new proposal for the pathophysiology of preeclampsia. J Reprod Immunol. 2021;145:103322. [DOI] [PubMed] [Google Scholar]
- 21.Lokki AI, Ren Z, Triebwasser M, Daly E, Perola M, Auro K, et al. Identification of complement factor H variants that predispose to pre-eclampsia: A genetic and functional study. BJOG. 2023;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jääskeläinen T, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, et al. Cohort profile: The Finnish Genetics of Pre-eclampsia Consortium (FINNPEC). BMJ Open. 2016;6(11):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triebwasser M. Excessive Complement Activation Due to Genetic Haploinsufficiency of Regulators in Multiple Human Diseases. Washington University in St.Louis, Arts & Sciences Electronic Theses and Dissertations. 2015. http://openscholarship.wustl.edu/art_sci_etds/427 Accessed Jan 15, 2024. [Google Scholar]
- 24.Lokki AI, Daly E, Triebwasser M, Kurki MI, Roberson EDO, Häppölä P, et al. Protective Low-Frequency Variants for Preeclampsia in the Fms Related Tyrosine Kinase 1 Gene in the Finnish Population. Hypertension. 2017;70(2):365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokki AI, Kaartokallio T, Holmberg V, Onkamo P, Koskinen LLE, Saavalainen P, et al. Analysis of Complement C3 Gene Reveals Susceptibility to Severe Preeclampsia. Front Immunol. 2017;8:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velásquez JA, Burwick RM, Hersh AR, Silva JL, Lenis V, Bernal Y, et al. Plasma CD59 concentrations are increased in preeclampsia with severe features and correlate with laboratory measures of end-organ injury. Pregnancy Hypertens. 2020;22:204–209. [DOI] [PubMed] [Google Scholar]
- 28.Lokki AI, Heikkinen-Eloranta J, Jarva H, Saisto T, Lokki ML, Laivuori H, et al. Complement activation and regulation in preeclamptic placenta. Front Immunol. 2014;5:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedesco F, Radillo O, Candussi G, Nazzaro A, Mollnes TE, Pecorari D. Immunohistochemical detection of terminal complement complex and S protein in normal and pre-eclamptic placentae. Clin Exp Immunol. 1990;80(2):236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyagawa H, Yamai M, Sakaguchi D, Kiyohara C, Tsukamoto H, Kimoto Y, et al. Association of polymorphisms in complement component C3 gene with susceptibility to systemic lupus erythematosus. Rheumatology. 2008;47(2):158–64. [DOI] [PubMed] [Google Scholar]
- 31.Abrera-Abeleda MA, Nishimura C, Frees K, Jones M, Maga T, Katz LM, et al. Allelic variants of complement genes associated with dense deposit disease. J Am Soc Nephrol. 2011;22(8):1551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury SJ, Karra VK, Gumma PK, Bharali R, Kar P. rs2230201 polymorphism may dictate complement C3 levels and response to treatment in chronic hepatitis C patients. J Viral Hepat. 2015;22(2):184–91. [DOI] [PubMed] [Google Scholar]
- 33.Laine M, Jarva H, Seitsonen S, Haapasalo K, Lehtinen MJ, Lindeman N, et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol. 2007;178(6):3831–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kettunen T, Eklund C, Kahonen M, Jula A, Paiva H, Lyytikainen LP, et al. Polymorphism in the C-reactive protein (CRP) gene affects CRP levels in plasma and one early marker of atherosclerosis in men: The Health 2000 Survey. Scand J Clin Lab Invest. 2011;71(5):353–61. [DOI] [PubMed] [Google Scholar]
- 35.Hernández-Díaz Y, Tovilla-Zárate CA, Juárez-Rojop I, Baños-González MA, Torres-Hernández ME, López-Narváez ML, et al. The role of gene variants of the inflammatory markers CRP and TNF-α in cardiovascular heart disease: Systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(8):11958–11984. [PMC free article] [PubMed] [Google Scholar]
- 36.Markt SC, Rider JR, Penney KL, Schumacher FR, Epstein MM, Fall K, et al. Genetic variation across C-reactive protein and risk of prostate cancer. Prostate. 2014;74(10):1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edberg JC, Wu J, Langefeld CD, Brown EE, Marion MC, Mcgwin G, et al. Genetic variation in the CRP promoter: association with systemic lupus erythematosus. Hum Mol Genet. 2008;17(8):1147–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality but are available from Professor Hannele Laivuori (hannele.laivuori@helsinki.fi) on reasonable request.