Abstract
Hyperthermia (HT), in combination with other conventional therapeutic modalities, has become a promising approach in cancer therapy. In addition to heat-induced apoptosis, an augmented immunological effect is considered to be a benefit of hyperthermic treatment over chemo- or radiotherapy. Here, we investigated the effect of regional HT targeting the liver on immune cells, especially T cells and antigen-presenting cells, which are important in recognizing and eliminating tumor cells and pathogens such as viruses. In healthy volunteers exposed to such regional HT, both CD4+ and CD8+ T cells that express an activation marker CD69 increased transiently at 1 h post-treatment, with a subsequent decrease to base levels at 6 h after the treatment. At 24 h post-treatment, the percentage of CD69-positive cells significantly increased again but only among CD8+ T cells. IFN-γ production from PHA-stimulated peripheral blood mononuclear cells was gradually and significantly increased in the 2 days following the heating procedure, peaking at 36 h post-treatment. Furthermore, we found marked increases in plasma levels of IL-1β and IL-6 starting at 24 h post-treatment. With regard to the number of each leukocyte subpopulation, a transient and dramatic decrease in the number of a subset of monocytes, CD14+ CD16− cells, was observed at 1 h after the hyperthermic treatment, suggesting that the regional HT aimed at the liver may have influenced the extravasation of blood monocytes. No significant changes in T-cell activities or monocyte counts were observed in the volunteers exposed to heating of the lungs or the legs. These results suggest that heating of the liver may efficiently induce cellular immune responses to liver cancers.
Keywords: T cell, Monocyte, Cytokines, Cellular immunity, Hyperthermia, Liver cancer
Introduction
Hyperthermia (HT) has been used in combination with chemotherapy and/or radiation therapy of human malignant disorders and is considered to be a promising adjuvant therapeutic strategy for the treatment of certain types of tumors [9, 31, 33]. In parallel to the encouraging clinical observations, a large number of investigations have been performed to evaluate the ability of heat in modulating cell death, tumor blood flow, and actions of radiation therapy and antineoplastic drugs (reviewed in [12]). Furthermore, increased body temperature has been shown to stimulate the immune system through augmentation of (a) activities of T cells and NK cells, (b) production of cytokines, (c) immune responses to viral infection, and (d) mobility of leukocytes [4, 8, 12, 21, 24, 27].
The number of patients with malignant liver tumors is increasing in Japan, the vast majority of which are associated with Hepatitis C virus (HCV)/Hepatitis B virus (HBV) infection. Regional HT aimed at the liver in combination with other modalities has produced promising results in the treatment of hepatocellular carcinomas [16, 29, 35]. We have recently demonstrated that, in patients with hepatocellular carcinoma, their CD4+/CD8+ ratio of T cells and the NK-cell activity increased following the regional HT treatment [20]. Stimulation of host immune responses is considered to be a possible mechanism by which regional HT treatment, as well as systemic HT, exerts its antitumor and antiviral activities, although clear evidence is still lacking. To maximize the potential clinical benefits of the HT treatment, it is now necessary to understand thoroughly the presumed immunological effects of the regional HT. Therefore, we have focused our attention here on the effects of heating of the liver on T cells and monocytes that are involved in cellular immune responses. Our findings include a drastic decrease in blood CD14+ CD16− inflammatory monocytes soon after the HT treatment, along with the activation of T cells. This is the first report indicating the effect of regional HT on changes in the populations of peripheral monocytes.
Materials and methods
Volunteer blood donors
Eleven healthy volunteers, five males and six females aged between 29 and 65 (average: 34.2±3.4) were enrolled in the regional HT treatment or the heating of the legs and feet. All of the volunteers had no signs or symptoms of fever, infectious diseases, or immune disorders, and their leukocyte counts in peripheral blood ranged from 4,000 to 8,000 per microliter of blood. Among our subjects, one had undergone splenectomy 22 years prior to the enrollment. Some volunteers were treated with both the regional HT procedures aimed at the liver and at the lungs with an adequate interval in between. All the volunteers were given thorough explanation of the purpose, procedures, and possible risks of the experiment through written information, and have given their consent. The entire experimental procedure has been examined and approved by the Research Evaluation Committee of the Wakayama Medical College.
Hyperthermic treatment
A radio-frequency capacitive heating device Thermotron RF-8 (Yamamoto Vinita, Yao, Japan) was used to achieve regional capacitive heating of the right upper abdominal region across the liver or of the chest across the lungs as described [20]. The device has been approved as a therapeutic instrument for non-invasive HT treatment of deep-seated malignant tumors by the Ministry of Health, Labor and Welfare of Japan (Medical Device Approval No. 59B1728). Applications of this device are covered by the government’s health insurance system, being regarded as a routine clinical modality [28]. Each volunteer’s body was wrapped with a cooling jacket and placed between two electrodes. The effective diameter of the electrodes was 18 cm, and each enrollee received an average continuous radio-frequency irradiation of 750 W over a duration of 1 h. The estimated temperature of the liver achieved by this heating procedure was 40°C, based on the data collected by the actual measurements of tissue temperature during HT treatment of liver tumors [16]. As an additional control group, legs and feet of four healthy volunteers were placed in a water bath and kept at 41°C for 1 h. Axillary or sublingual temperatures were measured at the end of each heating procedure in the volunteers of each group: 38.5±0.5°C in the liver-targeted HT, 38.9±0.3°C in the lung-targeted HT, and 37.8±0.3°C in the leg-heating group, without significant differences in the body temperature between the groups. Vital signs including blood pressure and pulse rate were monitored during and after the HT treatment. No adverse effect due to the above heating procedures was reported, except regional irritation of the skin adjacent to the electrodes and flushing of the face, which were well tolerated.
Preparation of peripheral blood mononuclear cells (PBMC)
Blood samples were drawn from each volunteer through a subcutaneous vein in the forearm just before and soon after a 1 h HT treatment, and 1, 2, 6, 24, 36, 48, and 72 h after the end of the heating procedure. Twenty microliters of heparinized peripheral blood was collected at each time point and kept at room temperature. Within 1 h after blood collection, each peripheral blood sample was diluted with an equal volume of phosphate-buffered balanced salt solution (PBBS), and mononuclear cells were separated by density gradient centrifugation using Ficoll-Paque PLUS (Amersham Bioscience, Piscateway, New Jersey). Mononuclear cells were washed twice with PBBS, counted in a hemocytometer, and resuspended in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) at 107 cells/ml.
Analyses of cell-surface markers and cell numbers
A total of 106 PBMC were incubated with the following combinations of fluorescent-labeled antibodies. For analyses of lymphocyte subsets, we used fluorescein isothiocyanate (FITC)-labeled anti-human CD4, FITC-labeled anti-human CD8, phycoerythrin-cyanin 5.1 (PC5)-labeled anti-human CD3, PC5-labeled anti-human CD16, FITC-labeled anti-human HLA-DR reactive to a monomorphic α-chain epitope, and PC5-labeled anti-human CD19. For staining of monocyte subsets and dendritic cells (DCs), we utilized phycoerythrin (PE)-labeled anti-human CD14, PC5-labeled anti-human CD16, FITC or PC5-labeled anti-HLA-DR, and FITC-labeled mixture of multiple lineage-specific antibodies (Lin 1; Becton-Dickinson Immunocytometry Systems, San Jose, CA, USA) that contains antibodies reactive to human CD3, CD14, CD16, CD19, CD20, and CD56 to distinguish peripheral blood DCs and basophils from other leucocytes [19, 30]. To examine the levels of T-cell activation, PE-labeled anti-human CD69 was used in combination with FITC-labeled anti-CD4 or anti-CD8, and PC5-labeled anti-CD3. Isotype-matched mouse myeloma proteins labeled with the above fluorescent dyes were used as negative control antibodies in all analyses. All antibodies except Lin 1 cocktail were purchased from Immunotech Coulter, Marseille, France. A number of 0.3–3.5×105 cells stained with the antibodies were analyzed with a Becton-Dickinson FACSCalibur (Becton-Dickinson) and CellQuest software. Dead cells and debris were excluded based on their forward and side scatter profiles, and percentages of each leukocyte subpopulation among viable mononuclear cells were determined by multi-color staining with appropriate antibodies. Absolute numbers of each cell subset were then calculated by multiplying the number of total mononuclear cells with the corresponding cell percentage determined. Geometric mean fluorescence intensity for CD14 expression was obtained from the appropriate histograms for the CD14+ CD16− subset of monocytes.
In vitro stimulation of isolated PBMC and measurements of cytokine production
A number of 8×105 PBMC were cultured in each well of 96-well microculture plates in RPMI-1640 medium supplemented with 10% FBS and 10 μg/ml (final) PHA (PHA-P, Sigma Chemical Co., St. Louis, MO). After 48 h of culture at 37°C, supernates were collected, and the amounts of IFN-γ and IL-4 were measured in duplicate by an enzyme-linked immunosorbent assay using OptEIA human kits (Pharmingen, San Diego, CA) according to the manufacturer’s instructions.
Measurements of plasma IL-1β and IL-6
Plasma were separated from a small aliquot of blood drawn at the same time when peripheral blood was collected for the isolation of PBMC. The concentrations of IL-1β and IL-6 in each plasma sample were measured by using the Opt EIA human kits as described above.
Statistical analyses
The paired t test for samples exhibiting Gaussian distribution and the Wilcoxon’s test for samples exhibiting non-Gaussian distribution were employed to compare the values at each time-point after the HT treatment with the pre-treatment values in the same volunteer group by using the online tools archived in the Multifunctional Web Calculator established and maintained by Prof. S. Aoki, Faculty of Social and Information Studies, Gunma University, Maebashi, Japan (http://www.aoki2.si.gunma-u.ac.jp/calculator/index.html), and a software for statistical analysis, JSTAT, which was developed by M. Sato and downloadable from the following website: http://www.vector.co.jp. P values less than 0.05 were considered to represent statistically significant differences.
Results
Changes in the number of blood mononuclear cells following the regional HT treatment
To investigate the effects of the regional HT aimed at the liver on immune cells, we first assessed possible changes in the numbers of peripheral mononuclear cells that consist of lymphocytes, monocytes, and DCs in healthy volunteers exposed to the regional HT treatment. Following a 1 h HT treatment aimed at the liver, the absolute numbers of mononuclear cells in the peripheral blood slightly decreased throughout the 48 h of observation, although significant differences were not found in comparison with the pre-treatment value (Table 1). This change in the number of mononuclear cells was mainly due to the decrease in lymphocytes. On the other hand, there was a drastic decrease in the number of monocytes soon after the treatment in four of the six subjects who received the HT aimed at the liver. The average number of monocytes per 1 ml of blood significantly decreased from 8.5×104 at pre-treatment to 4.5×104 at 1 h post-treatment. Due to the decreased number of monocytes, a reduction in the monocyte/lymphocyte ratio was also found at 1 h post-treatment.
Table 1.
Absolute numbers and the percentages of mononuclear cells in volunteers exposed to the regional hyperthermic treatment aimed at the liver (n=6)
| Cell numbers (×104/ml blood) | Percent among mononuclear cells | Ratio | ||||
|---|---|---|---|---|---|---|
| Total mononuclear cells | Lymphocytes | Monocytes | Lymphocytes | Monocytes | Monocyte/lymphocyte | |
| Pre-HT | 184±31 | 165.1±32.1 | 8.5±0.8 | 87.2±2.3 | 5.4±1.1 | 6.4±1.4 |
| 1 h after HT | 158±14 | 142.5±13.5 | 4.5±1.4* | 89.2±1.3 | 2.8±0.8 | 3.2±1.0 |
| 6 h | 148±15 | 131.5±13.9 | 7.6±1.7 | 88.6±0.5 | 4.8±0.6 | 5.4±0.7 |
| 24 h | 173±29 | 155.2±27.3 | 7.9±2.0 | 89.4±1.2 | 4.6±1.0 | 5.2±1.2 |
| 48 h | 147±9 | 130.4±7.7 | 7.2±2.1 | 89.0±1.6 | 4.7±1.2 | 5.4±1.3 |
*Significant difference (P<0.05) in comparison with the pre-treatment value
To evaluate whether the above changes were distinctive to the heating of the liver, the same parameters were analyzed in healthy volunteers exposed to a regional HT treatment aimed at the chest, in which the lungs were the primary target of heating, or in those received heating of the legs. No significant changes in the number of monocytes at 1 h post-treatment were observed in any of the subjects who underwent the lung-targeted HT or the heating of the legs (data not shown), indicating a specific role of the liver in the HT-induced decrease in the monocyte numbers.
Changes in the percentages of lymphocyte subpopulations
We further investigated whether the regional HT treatment affected the percentages of lymphocyte subpopulations. In both groups of the volunteers exposed to the HT treatment aimed at the liver and that aimed at the lungs, the number of CD4+ T cells decreased, while that of CD8+ T cells was unchanged (Table 2). The CD4+/CD8+ ratio at 1 h post-treatment was reduced in five of the six subjects who received the liver-targeted HT and in all who received the lung-targeted HT treatment; however, significant differences were observed only in those whose chest was heated. Only in the volunteers exposed to the regional HT aimed at the liver, the numbers of B cells were slightly decreased at all time-points following the treatment, although the changes in comparison with the pre-treatment value were not statistically significant, and no significant changes in NK cell numbers were observed. No significant changes were observed in the subjects whose legs were heated (data not shown).
Table 2.
Absolute numbers (×104/ml blood) of lymphocyte subsets in volunteers exposed to the regional hyperthermic treatment
| CD4+T | CD8+T | CD4+/CD8+ | B (CD19+) | NK (CD16+ HLA-DR−) | |
|---|---|---|---|---|---|
| Liver-targeted HT (n=6) | |||||
| Pre-HT | 71.8±20.0 | 28.3±3.8 | 2.4±0.4 | 20.6±6.8 | 16.5±4.0 |
| 1 h after HT | 55.6±6.8 | 28.8±4.9 | 2.1±0.4 | 14.9±2.2 | 17.2±3.0 |
| 6 h | 52.0±6.8 | 24.3±3.0 | 2.2±0.3 | 15.1±2.6 | 16.8±4.4 |
| 24 h | 65.9±17.3 | 28.5±4.2 | 2.3±0.4 | 15.6±2.5 | 17.8±3.7 |
| 48 h | 49.7±5.0 | 24.4±3.9 | 2.4±0.5 | 16.4±2.0 | 16.9±3.2 |
| Lung-targeted HT (n=5) | |||||
| Pre-HT | 63.5±9.8 | 27.8±5.3 | 2.6±0.6 | 15.4±3.2 | 16.1±1.2 |
| 1 h after HT | 54.6±8.8 | 29.9±7.4 | 2.1±0.4* | 15.9±3.7 | 15.5±2.3 |
| 6 h | 60.2±12.2 | 24.9±6.1 | 2.7±0.6 | 14.7±4.9 | 11.6±2.4 |
| 24 h | 58.0±11.4 | 26.2±4.6 | 2.4±0.5 | 18.6±5.6 | 16.4±1.7 |
| 48 h | 50.4±7.9 | 24.4±3.8 | 2.2±0.4 | 09.1±2.8 | 13.8±2.9 |
*Significant difference (P<0.05) in comparison with the pre-treatment value
Enhancement in the activation of peripheral T cells
To examine whether T-cell functions were influenced by the regional HT treatment, we next analyzed the expression of the early activation antigen CD69 on blood T cells. Figure 1a shows representative patterns of CD69 expression in T-cell subsets before and at different time-points after the HT treatment aimed at the liver. The frequency of CD69-expressing cells in both CD4+ and CD8+ populations of T cells was significantly elevated at 1 h after the treatment in all subjects: the mean percentages of CD69-expressing cells among CD4+ and CD8+ T cells increased by 1.7- and 1.5-fold, respectively, compared to the corresponding percentages at pre-treatment (Fig. 1b). They returned to basal levels at 6 h after the treatment, but again increased in both T-cell subsets at 24 h post-treatment, although only the changes in CD8+ T cells were significant. The CD69-expressing cells in both T-cell subsets showed a tendency to increase in the subjects whose lungs were heated, although there were no significant differences in comparison with the pre-treatment values. No changes in CD69 expression were found in the subjects who received the heating of the legs.
Fig. 1.
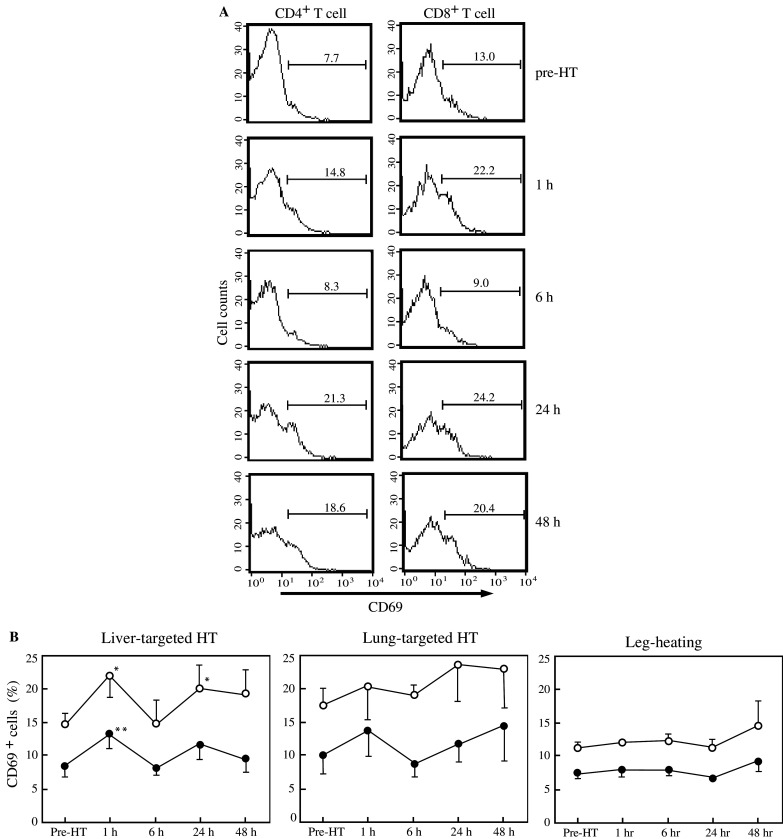
Effects of regional hyperthermic treatment aimed at the liver on peripheral T-cell activation. PBMC were prepared before and at the indicated time-points after the 1 h HT treatment aimed either at the liver or at the lungs, or after 1 h heating of the legs, and CD69 expression on CD4+ and CD8+ T cells was monitored by flow cytometry. a Histograms showing CD69 expression on each T-cell subset in a representative volunteer who received the liver-targeted HT treatment. Numbers indicate the percentage of CD69-positive cells. b Time course of CD69 induction on CD4+ (closed circles), and CD8+ (open circles) T cells after the HT treatment aimed at the liver (left panel) or at the lungs (middle panel), or heating of the legs (right panel). Each circle and bar show a mean ± SEM calculated from the data obtained from four to six volunteers. *Significantly different from the mean value observed before the HT treatment, P<0.05; **significantly different from the same pre-treatment value, P<0.01
Changes in IFN-γ production from PBMC
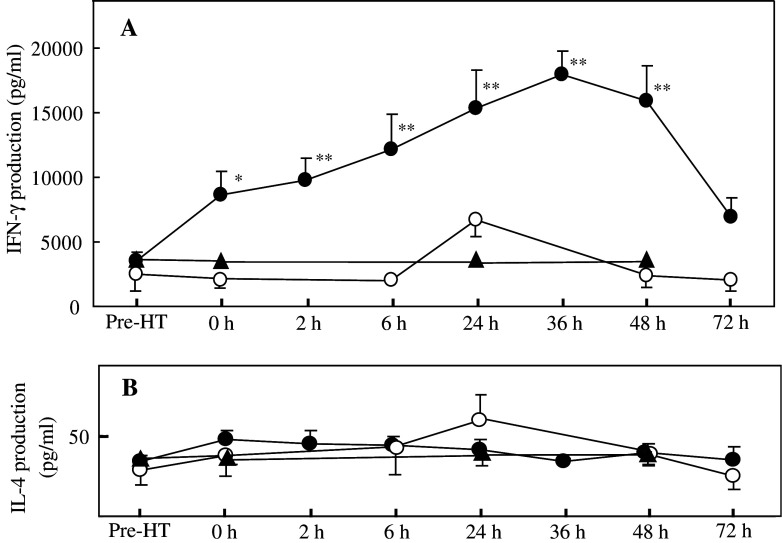
To further evaluate the possible effect of the regional HT treatment on T-cell activation, cytokine productions from PBMC were also analyzed. PBMC collected before and at different time-points after the treatment were stimulated with PHA, and the accumulation of interferon (IFN)-γ and interleukin (IL)-4 in the medium during 48 h of cell culturing was measured. In the volunteers who received the liver-targeted HT, the amount of IFN-γ produced gradually and significantly increased in the 2 days following the heating procedure, peaking at 36 h after the end of the HT treatment (Fig. 2a). In the subjects exposed to heating of the lungs or the legs, there were no significant changes in the PHA-induced production of IFN-γ. On the other hand, the amount of IL-4 produced after PHA stimulation showed no change at all during the 3 days of observation in all groups (Fig. 2b).
Fig. 2.
Changes in PHA-induced cytokine production from cultured PBMC after the 1 h regional HT aimed either at the liver (closed circles) or at the lungs (open circles), or after 1 h heating of the legs (closed triangles). PBMC prepared from each subject at each indicated time-point were stimulated with PHA, and the concentrations of IFN-γ (a) and IL-4 (b) produced into culture medium were measured after 2 days of incubation. Each symbol and bar show mean ± SEM calculated from the data obtained from 4 to 11 volunteers. * Significantly different from the mean value observed before the HT treatment, P<0.02; **significantly different from the same pre-treatment value, P<0.01
Elevation of plasma cytokine levels
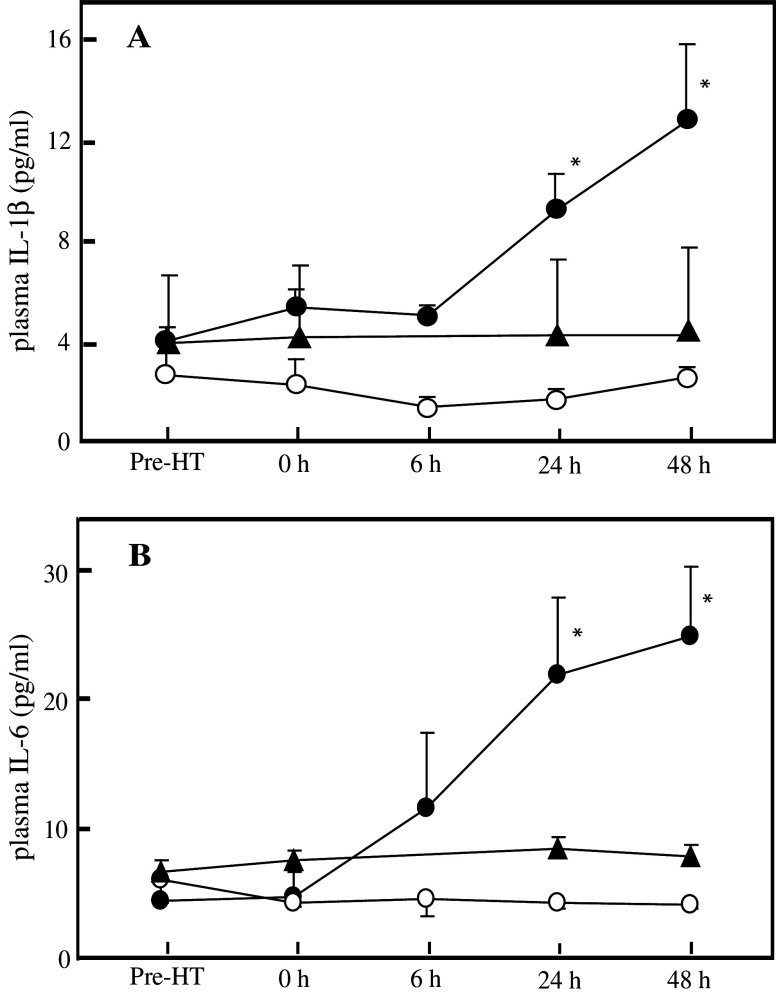
Plasma levels of proinflammatory cytokines, IL-1β and IL-6 were measured before and following the regional HT. In subjects who underwent HT aimed at the liver, both cytokines showed no significant change immediately after the treatment, but continued to increase gradually towards the end of the observation period, exhibiting significant differences at 24 and 48 h post-treatment (Fig. 3). Again, no changes in the plasma cytokine levels were found in the volunteers who underwent heating of the lungs or the legs.
Fig. 3.
Average plasma concentrations of IL-1β (a) and IL-6 (b) after the 1 h regional HT aimed either at the liver (closed circles) or the lungs (open circles), or after 1 h heating of the legs (closed triangles). Each symbol and bar represent mean ± SEM calculated from the data obtained from 4 to 11 volunteers. *Significantly different from the mean value observed before the HT treatment, P<0.01
Changes in the numbers of monocyte subsets
Recently, functional heterogeneity of human blood monocytes has been demonstrated based on the expression levels of the lipopolysaccharide (LPS) receptor CD14 and the Fcγ receptor III (CD16) [10]: CD14+ CD16− cells that correspond to classical inflammatory monocytes and CD14lo CD16+ cells that have been characterized as resident monocytes (Fig. 4a). As shown in Table 1, a marked reduction in the number of the whole monocyte population in the peripheral blood was observed following the HT treatment aimed at the liver. In order to examine which subset among the monocytes was affected by the treatment, we further analyzed both frequencies and absolute numbers of the above monocyte subsets as well as those of DCs which also exist within the blood monocyte population defined by conventional gating based on the forward and side scatter profiles (Fig. 4b). The DC population was distinguished in the present study by their expression of HLA-DR and the lack of the expression of CD3, CD14, CD16, CD19, CD20, and CD56. At 1 h after the liver-targeted HT, the frequency of CD14+ CD16− cells among the gated monocyte population was markedly reduced in five of the six volunteers, the mean values of which were 87.0±1.4% at pre-treatment and significantly lower 76.2±4.2% at 1 h after the HT treatment. A remarkable reduction in the absolute number of CD14+ CD16− monocytes was also observed at 1 h post-treatment. In contrast to the sharp decrease in the percentage of CD14+ CD16− monocytes, CD14lo CD16+ monocytes increased significantly in their percentage at 1 h after the liver-targeted HT treatment. However, due to the decrease in the total number of peripheral monocytes (Table 1), their absolute numbers were unchanged. With respect to DCs, their number tended to decrease soon after the HT treatment, and remained low throughout the 48 h of observation following the heating of the liver. There were no significant changes in the frequencies and absolute numbers of monocyte subsets in volunteers exposed to the lung-targeted HT. In addition, cell surface expression of CD14 molecules on the CD14+ CD16− monocytes increased starting from 6 h following the heating procedure, exhibiting significant differences (P<0.05) at 6 and 24 h post-treatment in the liver-targeted HT, and at 6 h post-treatment in the lung-targeted HT group (Fig. 4c). No significant changes in the monocyte-counts or the CD14 expression were observed in the volunteers exposed to the heating of the legs (data not shown).
Fig. 4.
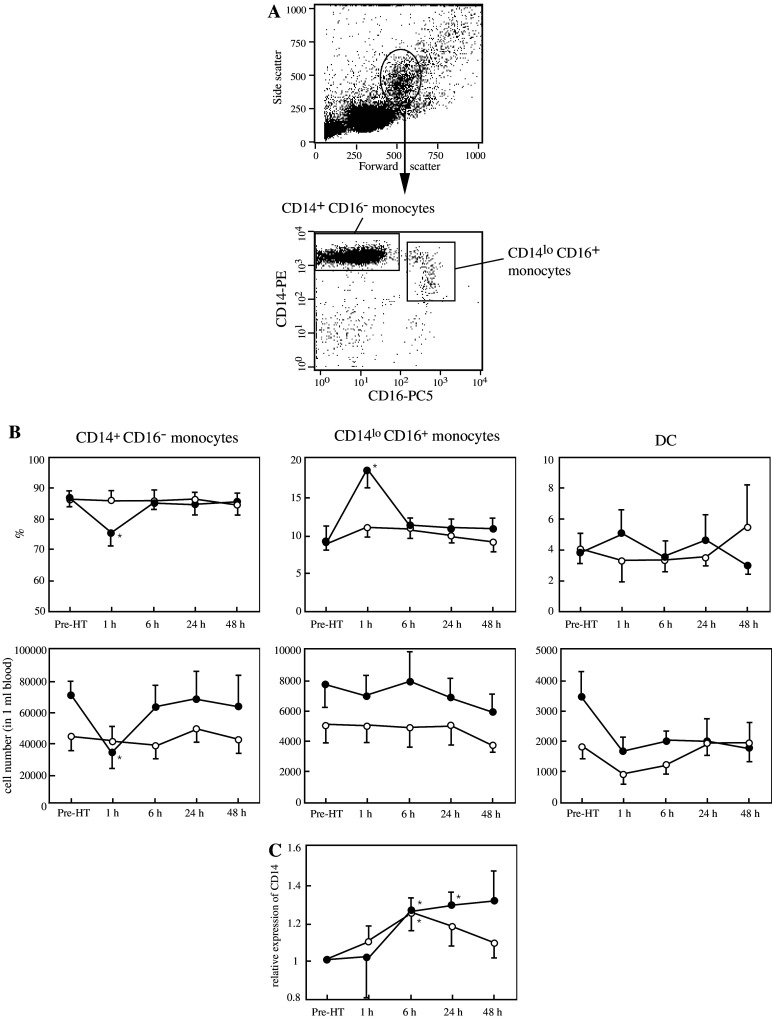
Effects of the 1 h regional HT on cell numbers of monocyte subsets and their CD14 expression. a Monocytes and DCs in PBMC prepared fell into an oval gate in the dot plot of forward and side scatters (the upper panel). The monocyte subsets were distinguished by multi-color staining (lower panels). b Percentages of the above-defined monocyte populations and DCs (upper panels) among the whole peripheral monocytes and their absolute numbers (lower panels) were analyzed in both groups of volunteers that were exposed to either the liver-targeted (closed circles) or lung-targeted (open circles) HT treatment. The cell numbers of each subset were calculated by the product of numbers of the entire mononuclear cells counted and the corresponding cell percentages among the mononuclear cells. c Cell surface CD14 expression on the CD14+ CD16− monocytes obtained from the volunteers exposed to either liver-targeted (closed circles) or lung-targeted (open circles) HT treatment was evaluated by measuring the geometric mean of the fluorescent intensities. The results are represented relative to the expression of CD14 molecules at pre-HT. Each circle and bar represent mean ± SEM determined from data obtained from five or six volunteers. *Significantly different from the mean value observed before the HT treatment, P<0.05
Discussion
The findings described herein provide evidence that the regional HT treatment aimed at the liver induces activation of peripheral T cells, production of proinflammatory cytokines, and changes in the number of blood monocyte subpopulations and their CD14 expression. To heat the liver selectively in the present study, we placed a pair of electrodes on the front and back of the subject’s right upper abdominal region; however, it is also conceivable that other lymphoid organs that lie adjacent to the liver might have been heated. With respect to the spleen, however, there happened to be a volunteer who had undergone splenectomy among our subjects. Importantly, we observed the same changes in the above immunological parameters in the splenectomized volunteer following the HT aimed at the liver, ruling out the possibility that the effects of the right upper abdominal heating depend on the presence of the spleen. When administering HT to the chest, in which the lungs were supposed to be the primary target organs, the subject’s body temperatures reached levels as high as those measured in the volunteers treated with the liver-targeted HT; however, the lung-targeted treatment resulted in no significant activation of T cells (Figs. 1 and 2) or changes in the numbers of blood monocyte subsets (Fig. 4). Thus, it is highly conceivable that the changes observed following the heating of the right upper abdominal region can be mainly attributed to the heating of the liver.
Immunological changes in humans who received systemic hyperthermic treatment have been described, most of which refer to its effect on the composition of lymphocyte subpopulations, the concentration of serum cytokines, and the NK cell activity (reviewed in [12]). On the other hand, there are very few reports describing the effect of regional HT on the host’s immune system. Regarding the changes in lymphocyte subpopulations, we observed a slight decrease in the peripheral CD4+/CD8+ ratio following the HT treatment aimed at the liver and lungs, which was statistically significant in the latter case. The decrease in CD4+/CD8+ ratio is mainly accounted for by the reduction in the number of CD4+ T cells, rather than an increase in CD8+ T-cell counts, in the both experimental groups. In line with this result, a decrease in CD4+/CD8+ ratio has been shown in patients with hepatocellular carcinoma immediately after the regional HT at the upper abdominal region [20], as well as in the persons who underwent whole-body hyperthermia (WBH) [1, 7]. By way of experiment with mice, WBH has been shown to enhance the homing of antigen-specific T cells to the inflammatory site; however, differences in sensitivity to this treatment of each T-cell subset have not been described [21]. As a possibility, chemotaxis of CD4+ T cells into tissues might be more quickly and markedly affected by heat than that of CD8+ T cells, thus resulting in the sharp decrease in CD4+/CD8+ ratio we observed.
In the present study, T-cell activation was assessed by the expression of the activation marker CD69 and by the generation of cytokines after the stimulation with PHA. CD69 molecule is a phosphorylated and disulfide-linked 27/33 kD homodimeric protein [26], and signals triggered by anti-CD69 antibodies result in the synthesis of different cytokines and their receptors, and the enhancement of T cell proliferation [5, 17]. The expression of CD69 on human T cells is induced in vitro by a wide variety of stimuli: an increase in CD69 expression is observed at as early as 1–4 h after stimulation with anti-CD3 antibody or polyclonal mitogens including phorbol ester 12-myristate 13-acetate (PMA), an activator of intracellular protein kinase C (PKC), whereas it takes 24–72 h after an in vitro stimulation through antigen–T-cell receptor interactions for the induction of CD69 expression [5, 11]. In the volunteers exposed to the HT treatment aimed at the liver, we observed the increase in the fraction of CD69-expressing cells in both CD4+ and CD8+ populations of T cells in two waves (Fig. 1). The first elevation, accompanied by the nearly twofold increase in PHA-induced IFN-γ production (Fig. 2a), was found at 1 h after the liver-targeted HT treatment. Based on the above knowledge, this “early phase” of T-cell activation, as demonstrated by the CD69 expression, is not attributable to an antigen-dependent reaction. Our observation is rather consistent with a report demonstrating that the expression of CD69 molecule on the surface of resting human T cells was induced at as early as 1 h after an incubation at 44°C for 30 min, which subsided 3 h later, due to a rapid translocation of the molecules already present in the cell cytoplasm [23]. In addition, fever-range (39.5°C) WBH has been shown to result in a rapid increase in PKC activity within T cells [32]. Thus, the liver-targeted HT treatment may have caused the very early induction of CD69 molecules to the cell surface via yet undescribed PKC signal transduction pathways, which in turn lead to the observed T-cell activation and the resultant increase in IFN-γ production. Of note, an extreme WBH of 41.8–42.2°C has led to a reversible and transient immune impairment in the patients with metastatic cancers during or immediately after the heating procedure [1, 4]. The regional heating aimed at the liver may have an advantage in that no intense immune impairment is induced during the treatment.
On the other hand, the “late phase” of CD69 induction was observed at 24 h post-HT treatment aimed at the liver. At the same time-point, a more than threefold increase in IFN-γ production from the PHA-stimulated PBMC was observed as compared to the pre-treatment value, suggesting that the peripheral T cells were fully activated at 24 h after the HT treatment of the liver. Furthermore, the plasma concentrations of IL-1 and IL-6, which are known to be secreted predominantly from vascular endothelial cells and professional phagocytes and act on the activation of lymphocytes [2, 6], were also elevated in our volunteers at this time-point, while there were no significant elevations in the early phase. In contrast, the plasma IL-6 increased during or just after the heating treatment in patients treated with an extreme WBH of 41.8°C, and returned to basal levels at 24-h post-treatment [4, 25]. The rapid increase in plasma IL-6 may be attributable to an augmentation in IL-6 release from the circulating monocytes or T cells. In fact, the TNF-α release from PBMC has been shown to increase immediately after the WBH [36]. On the other hand, the increase in plasma IL-6 was observed only in the subject who received the liver-targeted HT, but not in those receiving the heating of the lungs or legs, at the late phase. Liver cells can secrete a variety of cytokines including IL-1, IL-6 and TNF-α [2]. Among these, TNF-α is a potent inducer of IL-1 and IL-6 expression [2, 14]. Interestingly, the HT treatment has been shown to enhance TNF-α release from Kupffer cells in LPS-challenged mice [13]. It is possible, therefore, that the TNF-α locally produced by the HT-treated hepatic tissue may have, in turn, stimulated IL-1 and IL-6 release from the liver cells, which resulted in the observed late-phase increases in the plasma cytokines.
Unprecedented heterogeneity in their surface marker phenotypes and functions of blood monocytes has been demonstrated only recently: classical CD14+ CD16− monocytes that migrate into the sites of inflammation, where they differentiate into macrophages and DCs, and CD14lo CD16+ monocytes that extravasate into tissues, where they serve as specific resident myeloid cells such as Kupffer cells, alveolar macrophages, or Langerhans cells [10, 37]. Interestingly, our study revealed a remarkable decrease in only the CD14+ CD16− monocyte subset in the peripheral blood, but not in the CD14lo CD16+ monocytes and circulating DCs, at 1 h after the HT treatment. So far, there have been no reports demonstrating the decrease in the number of monocytes by HT treatment, whereas an increased number of total monocytes has been shown in the peripheral blood of healthy volunteers at 6 h after a fever-range WBH treatment [36]. Our observation raises two possibilities for the action of the heating of the liver: (1) apoptotic loss of the peripheral CD14+ CD16− monocytes may have been selectively induced, or (2) the entrance of CD14+ CD16− monocytes into tissues such as the liver may have been facilitated, resulting in their disappearance from the peripheral blood. Regarding the heat-induced apoptosis, a number of experiments both in vitro and in vivo have shown that HT treatments influence cell viability in various types of tumors [3, 34]. Exposing monocytes to 41°C for 1 h, however, had only a minor effect on their viability [22]. We also observed no effect of an in vitro treatment at 40°C for 1 h on the viability of blood monocytes (S. Kinoshita et al. unpublished observation). Taken together, it is not likely that the decrease in blood monocytes at 1 h post-HT treatment is due to a heat-induced apoptosis. With respect to the other hypothesis concerning the trafficking of CD14+ CD16− monocytes, chemokines produced locally in the liver should be taken into consideration. It has recently been reported in fulminant hepatic failure that the expression of intrahepatic chemokines, MCP-1, MIP-1α, MIP-1β, and RANTES, which are produced from Kupffer cells, sinusoidal endothelial cells, and hepatocytes, was closely correlated with the extent of infiltration by macrophages or monocytes and T cells into the liver [15]. Moreover, mRNAs of these intrahepatic chemokines have started to be expressed at as early as 1 h after an administration of toxins which elicit liver damage in mouse models of fulminant hepatic failure [15]. Of note, inflammatory chemokine receptors, CCR1, CCR2, and CCR5, which bind to MCP-1, MIP-1α, MIP-1β, and RANTES, are expressed on human CD14+ CD16− monocytes at much higher levels than on CD14lo CD16+ monocytes [10]. Collectively, the heat-exposed liver may produce the above-described inflammatory chemokines immediately after an HT treatment, resulting in the entrance into the liver of blood CD14+ CD16− monocytes. Further, upregulation of the CD14 expression levels on the CD14+ CD16− monocytes in the blood was found at as early as 6 h following the liver-targeted HT treatment, and this increase lasted at least until 24 h post-HT (Fig. 4). In contrast to the above result, a short-lasting increase in the CD14 expression on monocytes was observed in healthy volunteers exposed to fever-range WBH [36] and in our volunteers treated with the lung-targeted HT. The CD14 expression level on the monocytes was associated with an ability to release TNF-α following the stimulation with LPS [36]. Taken together, the possible early migration of CD14+ CD16− monocytes into the heated liver may have contributed to the observed long-lasting activation of peripheral monocytes after the HT treatment and the observed increase in plasma levels of IL-1β and IL-6.
As there is still no enough evidence demonstrating substantial direct anticancer effect of regional HT when used as a single treatment modality, we believe that it is currently applicable only in combination with other modalities, as a way to improve the efficacy of chemo-, radio- and, possibly other immunotherapies. The chemo- and radiotherapies often inevitably cause the suppression of host immune responses. Reduced immune responses have also been observed in patients with liver tumors after transcatheter arterial embolization [18]. In contrast, various regimens of HT are known to cause an augmentation in immune responses [4, 12, 24, 25]. This study has provided the evidence that the liver-targeted regional HT treatment induces the full activation of peripheral T cells and suggests the possible enhancement in chemotaxis of monocytes into the liver without severe stress reactions, indicating that this HT treatment may be a promising modality for liver tumors in combination with other anticancer treatments.
Acknowledgements
We thank M. Patrick Gorman for critically reviewing the manuscript. This work was supported by a grant from the Japan Health Research Foundation.
References
- 1.Ahlers O, Hildebrandt B, Dieing A, Deja M, Bohnke T, Wust P, Riess H, Gerlach H, Kerner T. Stress induced changes in lymphocyte subpopulations and associated cytokines during whole body hyperthermia of 41.8–42.2 degrees C. Eur J Appl Physiol. 2005;95:298. doi: 10.1007/s00421-005-0009-4. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1. doi: 10.1016/S0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 3.Allan DJ, Harmon BV. The morphologic categorization of cell death induced by mild hyperthermia and comparison with death induced by ionizing radiation and cytotoxic drugs. Scan Electron Microsc. 1986;3:1121–1133. [PubMed] [Google Scholar]
- 4.Atanackovic D, Nierhaus A, Neumeier M, Hossfeld DK, Hegewisch-Becker S. 41.8 degrees C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various malignant diseases. Cancer Immunol Immunother. 2002;51:603. doi: 10.1007/s00262-002-0327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cebrian M, Yague E, Rincon M, Lopez-Botet M, de Landazuri MO, Sanchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- 7.Downing JF, Martinez-Valdez H, Elizondo RS, Walker EB, Taylor MW. Hyperthermia in humans enhances interferon-gamma synthesis and alters the peripheral lymphocyte population. J Interferon Res. 1988;8:143. doi: 10.1089/jir.1988.8.143. [DOI] [PubMed] [Google Scholar]
- 8.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97:2727. doi: 10.1182/blood.V97.9.2727. [DOI] [PubMed] [Google Scholar]
- 9.Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17:1. doi: 10.1080/02656730150201552. [DOI] [PubMed] [Google Scholar]
- 10.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Hara T, Jung LK, Bjorndahl JM, Fu SM. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med. 1986;164:1988. doi: 10.1084/jem.164.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33. doi: 10.1016/S1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q, Detolla L, Singh IS, Gatdula L, Fitzgerald B, van Rooijen N, Cross AS, Hasday JD. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999;276:R1653. doi: 10.1152/ajpregu.1999.276.6.R1653. [DOI] [PubMed] [Google Scholar]
- 14.Le JM, Weinstein D, Gubler U, Vilcek J. Induction of membrane-associated interleukin 1 by tumor necrosis factor in human fibroblasts. J Immunol. 1987;138:2137. [PubMed] [Google Scholar]
- 15.Leifeld L, Dumoulin FL, Purr I, Janberg K, Trautwein C, Wolff M, Manns MP, Sauerbruch T, Spengler U. Early up-regulation of chemokine expression in fulminant hepatic failure. J Pathol. 2003;199:335. doi: 10.1002/path.1298. [DOI] [PubMed] [Google Scholar]
- 16.Nagata Y, Hiraoka M, Nishimura Y, Masunaga S, Mitumori M, Okuno Y, Fujishiro M, Kanamori S, Horii N, Akuta K, Sasai K, Abe M, Fukuda Y. Clinical results of radiofrequency hyperthermia for malignant liver tumors. Int J Radiat Oncol Biol Phys. 1997;38:359. doi: 10.1016/S0360-3016(96)00625-6. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S, Sung SS, Bjorndahl JM, Fu SM. Human T cell activation. IV. T cell activation and proliferation via the early activation antigen EA 1. J Exp Med. 1989;169:677. doi: 10.1084/jem.169.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiguchi S, Tamori A, Shiomi S, Enomoto M, Tatsumi N, Koh N, Habu D, Sakaguchi H, Takeda T, Seki S, Nakamura K, Kubo S, Kinoshita H. Cimetidine reduces impairment of cellular immunity after transcatheter arterial embolization in patients with hepatocellular carcinoma. Hepatogastroenterology. 2003;50:460. [PubMed] [Google Scholar]
- 19.Olweus J, BitMansour A, Warnke R, Thompson PA, Carballido J, Picker LJ, Lund-Johansen F. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci USA. 1997;94:12551. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostapenko VV, Tanaka H, Miyano M, Nishide T, Ueda H, Nishide I, Tanaka Y, Mune M, Yukawa S. Immune-related effects of local hyperthermia in patients with primary liver cancer. Hepatogastroenterology. 2005;52:1502. [PubMed] [Google Scholar]
- 21.Ostberg JR, Gellin C, Patel R, Repasky EA. Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependent cellular immune response. J Immunol. 2001;167:2666. doi: 10.4049/jimmunol.167.5.2666. [DOI] [PubMed] [Google Scholar]
- 22.Pollheimer J, Zellner M, Eliasen MM, Roth E, Oehler R. Increased susceptibility of glutamine-depleted monocytes to fever-range hyperthermia: the role of 70-kDa heat shock protein. Ann Surg. 2005;241:349. doi: 10.1097/01.sla.0000152028.19115.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risso A, Smilovich D, Capra MC, Baldissarro I, Yan G, Bargellesi A, Cosulich ME. CD69 in resting and activated T lymphocytes. Its association with a GTP binding protein and biochemical requirements for its expression. J Immunol. 1991;146:4105. [PubMed] [Google Scholar]
- 24.Roberts NJ., Jr Impact of temperature elevation on immunologic defenses. Rev Infect Dis. 1991;13:462. doi: 10.1093/clinids/13.3.462. [DOI] [PubMed] [Google Scholar]
- 25.Robins HI, Kutz M, Wiedemann GJ, Katschinski DM, Paul D, Grosen E, Tiggelaar CL, Spriggs D, Gillis W, d’Oleire F. Cytokine induction by 41.8 degrees C whole body hyperthermia. Cancer Lett. 1995;97:195. doi: 10.1016/0304-3835(95)03976-4. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Mateos P, Sanchez-Madrid F. Structure-function relationship and immunochemical mapping of external and intracellular antigenic sites on the lymphocyte activation inducer molecule, AIM/CD69. Eur J Immunol. 1991;21:2317. doi: 10.1002/eji.1830211005. [DOI] [PubMed] [Google Scholar]
- 27.Shen RN, Lu L, Young P, Shidnia H, Hornback NB, Broxmeyer HE. Influence of elevated temperature on natural killer cell activity, lymphokine-activated killer cell activity and lectin-dependent cytotoxicity of human umbilical cord blood and adult blood cells. Int J Radiat Oncol Biol Phys. 1994;29:821. doi: 10.1016/0360-3016(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 28.SugaharaT, Ostapenko VV, Yamamoto I (2001). In: Kosaka M, Sugahara T, Schmidt KL, Simon E (eds) Thermotherapy for neoplasis, inflammation, and pain. Springer, Tokyo, p 480
- 29.Tanaka H, Ostapenko VV, Miyano M, Nishide T, Sonobe M, Toda K, Nishide I, Mune M, Yukawa S. Successful treatment of hepatocellular carcinoma with percutaneous ethanol injection therapy and local hyperthermia. Hepatogastroenterology. 2002;49:1666. [PubMed] [Google Scholar]
- 30.Thomas R, Lipsky PE. Human peripheral blood dendritic cell subsets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol. 1994;153:4016. [PubMed] [Google Scholar]
- 31.van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 32.Wang XY, Ostberg JR, Repasky EA. Effect of fever-like whole-body hyperthermia on lymphocyte spectrin distribution, protein kinase C activity, and uropod formation. J Immunol. 1999;162:3378. [PubMed] [Google Scholar]
- 33.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487. doi: 10.1016/S1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 34.Yonezawa M, Otsuka T, Matsui N, Tsuji H, Kato KH, Moriyama A, Kato T. Hyperthermia induces apoptosis in malignant fibrous histiocytoma cells in vitro. Int J Cancer. 1996;66:347. doi: 10.1002/(SICI)1097-0215(19960503)66:3<347::AID-IJC14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Yumoto Y, Jinno K, Tokuyama K, Wada T, Kobashi H, Okamoto T, Toki H, Inatsuki S, Hara K, Moriwaki S, et al. Trans-catheter hepatic arterial injection of lipiodol soluble anti-cancer agent SMANCS and ADR suspension in lipiodol combined with arterial embolization and local hyperthermia for treatment of hepatocellular carcinoma. Int J Hyperthermia. 1991;7:7. doi: 10.3109/02656739109004972. [DOI] [PubMed] [Google Scholar]
- 36.Zellner M, Hergovics N, Roth E, Jilma B, Spittler A, Oehler R. Human monocyte stimulation by experimental whole body hyperthermia. Wien Klin Wochenschr. 2002;114:102. [PubMed] [Google Scholar]
- 37.Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17:424. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]