Abstract
Radical prostatectomy and radiation therapy provide excellent localized prostate cancer (PC) control. Although the majority of prostate carcinoma is nowadays diagnosed at early stages with favourable risk features, in patients up to 30–40% it recurs within 10 years. Furthermore, the lack of effective therapies, once prostate carcinoma becomes refractory to androgen deprivation, mandates the development of alternative therapeutic options. There is a growing interest in harnessing the potency and specificity of anti-tumour immunity through the generation of fully competent dendritic cells and tumour reactive effector lymphocytes. Several strategies to treat or prevent the development of metastatic PC have been explored in clinical trials and are summarized in this review, considering also the feasibility and safety of these approaches. In some cases clinical responses were achieved showing that vaccine-primed T cells induced anti-tumour activity in vivo. The present findings and perspectives of the immunologic interventions in PC patients will be discussed.
Keywords: Prostate cancer, Antigens, Cancer vaccine, Tumour immunity, Androgen deprivation therapy, Radiotherapy
Introduction
Prostate cancer (PC) is one of the most frequent diseases diagnosed in adult male population in the Western countries [1]. Its incidence rose continuously for more than 20 years. In fact, the application of modern diagnostic methods, such as the prostate-specific antigen (PSA) dosage and the trans-rectal ultrasound-guided biopsies above all, has resulted in a consistent increase in the number of early-detected PCs [2]. At the same time, the therapeutic approaches traditionally utilized in the management of PC, like surgery, radiotherapy (RT) and androgen deprivation therapy (ADT), have resulted in a more efficacious treatment, improving patients’ quality of life [3, 4].
Nonetheless, biochemical relapse-free survival at 10 years of patients affected by clinically localized PC is only 25–55%, based on the individual risk of disease recurrence. Therefore, a loco-regional treatment is still unable to guarantee a positive outcome in a considerable percentage of patients affected by the organ-confined disease. Rising levels of PSA following radical prostatectomy or RT are usually regarded as a treatment failure [2] that requires salvage therapy in order to avoid the development of metastases [4]. At the moment there are no diagnostic tools able to detect early relapsed PC; however, factors such as the Gleason pattern score, seminal vesicle invasion, absolute pre-treatment PSA level and PSA doubling time are well-known important determinants of clinical outcome after therapy.
Many non-conventional salvage therapies have been employed and nowadays hormonal therapy represents the ‘gold standard’ for relapsed patients [5], even though the optimal timing of androgen deprivation is still debated. Despite its proven efficacy, most of the treated patients develop resistance to neoadjuvant ADT between 1 and 5 years [6]. Thus, it seems that the hormonal therapy allows the selection of androgen-independent neoplastic clones. Moreover, osteoporosis, anaemia, weight gain and neurocognitive decline are associated with ADT [7].
Chemotherapy based on docetaxel and prednisone is considered the standard treatment for patients who have progressed on androgen deprivation, but this approach has not been shown to be curative. In fact, this regimen can achieve a median survival benefit of only a few months [8]. Novel therapeutic strategies for the management of hormone refractory PC are therefore urgently needed; among them, immunotherapy appears to be the most promising one.
Immunotherapy
Tumour-associated antigens of PC
Since the discovery of the first genetically defined tumour antigen MAGE-1 [9], many attempts have been made to identify other potential targets of clinical interest for an immunotherapeutic intervention. On the basis of their expression pattern, tumour-associated antigens (TAA) can be classified into differentiation antigens, germ line-related antigens and unique antigens [10]. The identification of the epitopes recognized by T cells paved the way for the development of antigen-specific active immunotherapy protocols in which protein or peptides injected into cancer patient may induce de novo or stimulate a pre-existing systemic T cell-mediated immunization to recognize and destroy tumour cells [11].
Although the majority of tumour antigens have been identified in patients affected by melanoma, PC appears to express a wide array of potential immunological targets too (see Table 1). Moreover, self-reactivities to prostate proteins are not unusual; clinical data describing autoimmune responses directed against the gland have been reported and, although the majority of prostate antigens derive from normal proteins for which immune tolerance may prevent immunogenicity, these reactions could sometime allow the complete destruction of the glandular epithelium [42].
Table 1.
A list of PC antigens containing epitopes recognized by T cells
| Gene | HLA restriction | Peptide epitope | Amino acid sequence | References |
|---|---|---|---|---|
| PSA | A1 | PSA 68–77 | VSHSFPHPLY | [12] |
| A2 | PSA 141–150 | FLTPKKLQCV | [13] | |
| A2 | PSA 154–163 | VISNDVCAQV | [13] | |
| A2 | PSA 146–154 | KLQCVDLHV | [14] | |
| A3 | PSA 16–24 | GAAPLILSR | [15] | |
| A24 | PSA 152–160 | CYASGWGSI | [16] | |
| A24 | PSA 248–257 | HYRKWIKDTI | [16] | |
| DRB1 | PSA 49–63 | ILLGRMSLFMPEDTG | [12] | |
| DRB1 | PSA 55–67 | SLFHPEDTGQVFQ | [12] | |
| DRB1 | PSA 64–78 | QVFQVSHSFPHPLYD | [12] | |
| DRB1 | PSA 95–109 | NDLMLLRLSEPAELT | [12] | |
| DRB1 | PSA 148–160 | KKLQCVQLHVISM | [12] | |
| DRB1 | PSA 171–190 | LQCVDLHVISNDVCAQVHPQ | [17] | |
| DRB1 | PSA 221–240 | GVLQGITSWGSEPCALPERP | [17] | |
| PSMA | A1 | PSMA 347–356 | HSTNGVTRIY | [12] |
| A1 | PSMA 557–566 | ETYELVEKFY | [12] | |
| A2 | PSMA 4–12 | LLHETDSAV | [18] | |
| A2 | PSMA 711–719 | ALFDIESKV | [18] | |
| A3 | PSMA 207–215 | KVFRGNKVK | [15] | |
| A3 | PSMA 431–440 | STEWAEENSR | [15] | |
| A24 | PSMA 178–186 | NYARTEDFF | [19] | |
| A24 | PSMA 227–235 | LYSDPADYF | [19] | |
| DRB1 | PSMA 334–348 | TGNFSTQKVKMHIHS | [20] | |
| DRB1 | PSMA 687–701 | YRHVIYAPSSHNKYA | [20] | |
| DRB1 | PSMA 730–744 | RQIYVAAFTVQAAAE | [20] | |
| DRB1 | PSMA 459–473 | NYTLRVDCTPLMYSL | [21] | |
| PAP | A2 | PAP 135–143 | ILLWQPIPV | [22] |
| A3 | PAP 155–163 | LYLPFRNCPR | [15] | |
| A3 | PAP 248–257 | GIHKQKEKSR | [15] | |
| DRB1 | PAP 199–213 | GQDLFGIWSKVYDPL | [23] | |
| DRB1 | PAP 228–242 | TEDTMTKLRELSELS | [23] | |
| PSCA | A2 | PSCA 14–22 | ALQPGTALL | [24] |
| A2 | PSCA 7–15 | ALLMAGLAL | [25] | |
| A2 | PSCA 21–30 | LLCYSCKAQV | [25] | |
| A24 | PSCA 76–84 | DYYVGKKNI | [26] | |
| STEAP | A2 | STEAP | LLLGTIHAL | [22] |
| A2 | STEAP 86–94 | FLYTLLREV | [27] | |
| TARP | A2 | TARP 4–13 | FPPSPLFFFL | [28] |
| A2 | TARP 27–35 | FVFLRNFSL | [29] | |
| A2 | TARP 29–37 | FLRNFSLML | [29] | |
| DRB1 | TARP 1–14 | MQMFPPSPLFFFLQ | [30] | |
| DRB1 | TARP 14–27 | QLLKQSSRRLEHTF | [30] | |
| PTH rp | A2 | PTH rp 59–68 | FLHHLIAEIH | [31] |
| A2 | PTH rp 165–173 | TSTTSLELD | [31] | |
| A2 | PTH rp 42–51 | QLLHDKGKSI | [32] | |
| A2 | PTH rp 59–67 | FLHHLIAEI | [32] | |
| Prostein | A2 | Prostein 31–39 | CLAAGITYV | [33] |
| Eph | A2 | Eph A2 58–66 | IMNDMPIYM | [34] |
| Eph A2 550–558 | VLAGVGFFI | [34] | ||
| KLK4 | DRB1 | KLK4 155–169 | LLANGRRMPTVLQCVN | [35] |
| DRB1 | KLK4 160–174 | RMPTVLQCVNVSVVS | [35] | |
| DPB1 | KLK4 125–139 | SVSESDTIRSISIAS | [35] | |
| SVV | A2 | SVV 96–104 | LTLGEFLKL | [36] |
| HER2 | A2 | HER2 435–443 | ILHNGAYSL | [37] |
| A2 | HER2 665–673 | VVLGVVFGI | [37] | |
| A2 | HER2 952–960 | YMIMVKCWM | [37] | |
| A2 | HER2 369–377 | KIFGSLALF | [38] | |
| hTERT | A2 | hTERT 540–548 | ILAKFLHWL | [39] |
| A2 | hTERT 865–873 | RLVDDFLLV | [40] | |
| A3 | hTERT 973–981 | KLFGVLRLK | [41] |
A great variety of targets of a potential anti-tumour response have been identified so far in PC cells [43] (see Table 1). Antigens like PSA [44], prostate-specific membrane antigen (PSMA) [45], prostatic acid phosphatase (PAP) [46], prostate stem cell antigen (PSCA) [47] or molecules of different origin like HER-2 and Ep-CAM have been shown to be overexpressed by the PC cells as compared to normal counterparts. These antigens, therefore, represent possible targets of immunotherapy depending on the frequency and stability of their expression by PC cells and their immunogenicity. Each of these TAAs includes one or more T cell epitopes recognized in the context of class I and/or class II HLA.
PSA is a kallikrein-like protease highly expressed by normal prostatic epithelial cells and represents one of the most characterized TAAs in PC; PSA can provide several T cell epitopes (see Table 1). PSMA is a metallopeptidase expressed particularly in undifferentiated, metastatic and hormone-resistant carcinomas, although its biological function in PC cells remains poorly understood. Its sequence contains at least two different MHC class I HLA-A*02 [18] and two MHC class I HLA-A*24 highly immunogenic peptides [19], both already utilized in clinical trials. Other potentially immunogenic and overexpressed molecules are represented by factors involved in the regulation of cell cycle and survival of PC cells like survivin [48], livin and telomerase. These proteins have been defined as universal antigens, thanks to their wide distribution among tumour cells of different histology [49]. The employment of such a class of TAA appears to be particularly promising. In fact, one of the major escape strategies exploited by tumour cells relies in the loss or down-regulation of expression of TAA and/or HLA molecules [50]. In such a case, given their biological function, this would limit the proliferation of tumour cells and activate apoptosis [51, 52]. Encouraging pre-clinical data [53] prompted the development of clinical studies to assess the therapeutic potential of vaccination strategies based on the above-mentioned TAA given alone, in combination with different adjuvants or presented by dendritic cells (DCs). It has been demonstrated that the activation of the immune system by immunotherapeutic approaches allows the development of specific CD8+ and CD4+ T cell clones. This response may associate with a stabilization or reduction of PSA levels even in metastatic hormone refractory PC patients.
Different vaccination strategies have been used in cancer patients according to the vaccine formulation, type of adjuvant, route and schedule of immunization, etc. As far as the PC patients are concerned, some of these strategies are summarized in the following sections.
Vaccination studies in PC patients
Peptide-based vaccines
Even though several T cell epitopes have been characterized and evaluated for their immunogenicity in vitro, only a limited number of prostatic peptides have been clinically tested to assess both a favourable toxicity profile and immunological responses. Most of them are HLA-A*02- and HLA-A*24-restricted epitopes, recognized by CD8+ T cells (see Table 1).
Noguchi et al. [54] established a pre-vaccination evaluation of peptide-specific CTL precursors in the peripheral blood of PC patients to choose the most immunogenic epitopes of a multipeptide trial of vaccination. Two phase I studies were carried out in 10 HLA*A02 [54] and 14 HLA*A24 [55] metastatic hormone-resistant PC patients showing increased cellular as well as humoral immune responses to the selected targets. The vaccination strategy was safe, well tolerated with no major toxic effects apart from grade I dermatological reactions at the injection site. Stabilization or reduction of PSA levels was also observed and one HLA*A02 patient showed disappearance of a bone metastasis after the fifth vaccination. The main limitation of this approach that makes it difficult for clinical applications relies in the need of a priori knowledge of patient HLA haplotype as well as of peptide expression by PC cells. However, peptide-based vaccination strategies have not been extensively studied in PC patients even though some clinical studies have already assessed their therapeutic potential.
Cytokine and GM-CSF gene-transduced cellular vaccines
Effective T cell activation requires antigen presentation by professional antigen-presenting cells (APC). As DCs are considered the most powerful APCs, studies aimed at manipulating their immunological properties have been undertaken. The design of a DC-based trial of vaccination may vary upon DC generation and maturation status, together with antigen loading and treatment schedule [56]. Although the development of DC-based clinical trials allowed the induction of immunological responses in a high percentage of subjects, cancer patient-derived DCs may be hampered in their stimulatory capabilities by tumour-released factors, thus limiting clinical efficacy [57]. The use of GM-CSF, a cytokine known to promote differentiation and activation of DCs, has been introduced to improve the immune response of cancer patients including PC-bearing subjects.
GM-CSF as systemic treatment
Systemically administered GM-CSF has been studied in many clinical settings and also in metastatic hormone refractory PC (HRPC) patients [58]. A first group (n = 23) of men received 250 μg/m2/day of GM-CSF s.c. on days 1–14 in a 28-day cycle. PSA declined in 10 out of 23 subjects during GM-CSF administration ranging from 6 to 64% (median PSA decline 37%), but increased during the following 14 days. Therefore, in a second group of patients a maintenance therapy based on 250 μg/m2/day GM-CSF given s.c. three times weekly until progressive disease (PD) was added to the previous treatment schedule. Although clinical differences between the two groups were found, fluctuations in PSA kinetics were less prominent and of shorter duration in group II. All but one patient experienced PSA decline (median PSA decline 32.4%). One patient experienced a decreased PSA from 77 to 0.1 ng/ml with an improvement in bone scan. Systemic GM-CSF was also administered in biochemically relapsed PC patients [59]. Among 16 treated patients receiving 250 μg s.c. three times weekly, only one experienced a PSA decline of >50%. In a recent work by Rini et al. [60] 250 μg/m2/day of GM-CSF was administered s.c. on days 1–14 of a 28-day cycle to 30 patients with serologic progression of PC. Three men experienced a PSA decline of >50% while additional 18 subjects achieved a variable biochemical response. Schwaab et al. [61] randomized 26 men with clinical or biochemical evidence of PD to receive 125 or 250 μg/m2 GM-CSF. Both clinical and immunological responses were monitored. Only two patients (one for each group) mounted a specific CD4+ and CD8+ T cell responses to PSA with a statistically significant positive correlation between pre-treatment PSA levels and T cell precursors. Nine patients experienced a PSA decline of ≥25% but only one was >50%.
In conclusion, although GM-CSF possesses critical regulatory functions on DC survival and differentiation, its activity on PC cells and on the development of a specific T cell response remains to be confirmed despite its potential effects on PSA serum levels.
GM-CSF gene transduced cellular vaccines
Irradiated GM-CSF-secreting cancer cell vaccines have been shown to induce strong anti-tumour immunity in animal models [62] and, to a lesser extent, in clinical settings through the local recruitment of APCs. Simons et al. [63] established primary PC cultures from radical prostatectomy specimens and transduced them with a cDNA encoding GM-CSF using a retroviral vector. Before clinical use, transduced PC cells were lethally irradiated. Eleven patients with metastatic PC were eligible for the phase I study but PC cultures were obtained only from 8 of 11 patients. Up to six i.d. injections were administered at 3-week intervals. The treatment was relatively safe with low-grade local or systemic toxicities. This strategy allowed the development of anti-tumour B and T lymphocyte-mediated responses directed against many PC antigens. The main limitation of this approach relies in the establishment of autologous PC lines to be used as vaccine. For this reason two phase II trials were carried out in 33 patients using two already established, GM-CSF gene-transduced allogeneic cell lines (LnCaP and PC3). Encouraging results were achieved in terms of reduction of PSA velocity; furthermore, repeated vaccinations with high doses seemed to extend time to disease progression (TTP). Phase III clinical trials with this vaccine (GVAX®, Cell Genesis) are currently ongoing.
Modulation of immune response by antibodies
CTLA4 is an inducible receptor engaged by the B7 family ligands (CD80 e CD86) that switch activated CD4 and CD8 T cells off, thus hindering the development of uncontrolled immune reactions [64]. It has been shown that antibody-based inhibition of this molecule can enhance the development of a specific anti-tumour T cell response even though serious adverse autoimmune events were commonly seen among the responder patients [65]. These data suggest that forcing multiple mechanisms of peripheral tolerance may allow the development of T cell responses against self-antigens.
In a preliminary single agent single dose phase I study, 3 mg/kg anti CTLA4 antibody (MDX-010®, Medarex Inc, Bloomsbury, NJ, USA) were generally well tolerated and 2 out of 14 HRPC patients experienced a PSA reduction of >50% [66]. Phase I and phase II studies are currently ongoing in PC patients to assess the combined activity of CTLA4 blocking together with GM-CSF, G-VAX, docetaxel-based chemotherapy regimens or ADT.
DC-based vaccines
Peptide or protein-loaded DCs
A preliminary phase I trial of vaccination was carried out by Murphy et al. [67] in an attempt to evaluate the potential immune response to two HLA-A*0201 PSMA epitopes (PSMA-P1 and PSMA-P2) given alone or pulsed onto DCs. Fifty-one patients affected by advanced hormone refractory PC were enrolled. These subjects were divided into five groups each receiving four or five injections of PSMA-P1 peptide (group I), PSMA-P2 peptide (group II), autologous DCs (group III only) or DCs pulsed either with PSMA-P1 (group IV) or with PSMA-P2 (group V).
Patients received four infusions of the vaccine over 6-week intervals and clinical and immunological responses were assessed (Table 2). According to NPCP standard criteria and a serum reduction of PSA >50%, seven partial responses (PRs) were observed. Moreover, 5 out of 7 PRs were shown by patients enrolled in group IV or V. The treatment was well tolerated, and the duration of the response varied between 100 and 200 days. These results prompted the development of a phase II trial in which 62 patients (33 of the phase I participants were subsequently enrolled) with local disease recurrence after first-line treatment failure or metastatic hormone refractory PC received six infusions of autologous DCs pulsed with HLA-A*0201 PSMA-P1 and PSMA-P2 peptides at 6-week intervals [68] (Table 2). In the responder group of non-metastatic patients, 11 experienced a PSA reduction of >50% while 8 had a SD based on PSA measurements.
Table 2.
Clinical and immunological results of phase I and II studies (DC-based vaccines)
| Vaccine | No. of patients treated | Clinical responses | Duration (months) | Immune responses | References |
|---|---|---|---|---|---|
| Dendritic cells with | |||||
| PSMA peptides | 51 | 7 PR (14%) | 6 | Not determined | [67] |
| PSMA peptides | 62 | 11 PR (18%) 8 SD (13%) | 9–18 | Not determined | [68] |
| PAP/GM-CSF (Provenge) | 31 | 3 PR (10%) | 12 | 100% | [74] |
| PAP/GM-CSF (Provenge) | 19 | 3 PR (16%) 1 SD (5%) | 4–48 | Not determined | [75] |
| PSA mRNA (viral vector) | 13 | 6 PR (46%) | 6 | 100% | [78] |
| Telomerase mRNA (viral vector) | 20 | 6 PR (30%) | 4 | 25% | [79] |
| PSA mRNA (viral vectors) only (no DCs) | 64 | 37 SD (58%) | 10 | 32% | [82] |
Similar strategies have been recently developed by Fuessel et al. [69] and Waeckerle-Men et al. [70]. A cocktail of HLA*A-0201 epitopes derived from different prostatic antigens was used to load autologous DCs. Although these studies were carried out on a small number of patients, detectable immune responses as well as temporary PSA declines or stabilizations were reported.
Since high-avidity T cells recognizing self-antigens can be negatively selected, an approach to overcome tolerance could be based on vaccination with homologous xenogenic antigens to enhance the immunogenicity of weak self TAA [71]. Mouse PAP (mPAP) shows 81% homology with human PAP and cross reactivity against the homologous TAA has been described [72]. Moreover, tissue expression of PAP seems to be limited to the prostatic tissue, while PSA and PSMA have a wider pattern of tissue distribution. Two monthly vaccination with autologous DCs (mean DC dose: 11.2 × 106 per vaccination) pulsed with mPAP was carried out in 21 patients with recurrent or metastatic PC [73]. The treatment was relatively well tolerated but six patients developed elevated anti-nuclear antibodies or elevated rheumatoid factors following vaccination in the absence of any clinical sign of autoimmune disease.
Following immunization six patients showed SD as assessed by PSA kinetics and radiological imaging. These clinical responses were associated to the generation of a cross-reactive specific T cell immunity against the hPAP. In the non-responder group only 5 out of 15 patients developed immunological responses to the relevant self-antigen hPAP, although a specific T cell response from all the patients was seen towards the mPAP xenogenic peptide.
A promising phase I study has been carried out by Small et al. [74]; the clinical efficacy of a previously described recombinant fusion protein PAP/GM-CSF (PA2024 or Provenge) was evaluated. Preliminary data in rats demonstrated that DCs loaded with PA2024 were able to induce a strong immune response towards PAP-expressing tissues.
Autologous DC precursors were isolated from leukapheresis through sequential buoyant density centrifugations and co-incubated with the recombinant peptide for 40 h; the pellet contained not only CD54+ cells but also variable amounts of CD3+, CD19+ and CD56+ cells. A total of 31 patients affected by androgen-independent PC were enrolled (Table 2). A first group of 12 men received different doses of Provenge ranging from 0.2 × 109 to 2 × 109 nucleated cells/m2, while additional 19 patients received the maximum dose of the vaccine on weeks 0, 4 and 8. An additional dose was given to the responding patients on week 24. Men belonging to the first group had been more heavily pre-treated than the second group of patients. Altogether three patients experienced a PSA reduction of >50% and three additional patients between 25 and 49%. TTP correlated both with the development of an immune response to PAP (median TTP of responders 34 weeks; median TTP of non-responders 13 weeks) and with the dose of Provenge (median TTP of patients receiving >10 × 107 cells 31.7 weeks; median TTP of patients receiving <10 × 107 cells 12.1 weeks). In a phase II trial 21 men affected by metastatic hormone refractory PC received two infusions of Provenge on week 0 and 2 followed by 3 s.c. injections of the recombinant protein PAP-GM-CSF [75] (Table 2). Of 19 evaluated patients, two exhibited a PSA reduction between 25 and 50%, while another patient experienced an increasing PSA serum level from 221 to 251 ng/ml and then it dropped to undetectable levels by week 24 and beyond. At the same time a regression of his retroperitoneal and pelvic lymphadenopathy was observed.
A double-blinded placebo controlled phase III trial with this vaccine has been recently described by Small [76]. Upon PD patients assigned to the control arm were eligible to an open label salvage protocol receiving Provenge. One hundred and twenty-seven metastatic HRPC patients were enrolled. TTP in the Provenge arm was 11.7 weeks compared to the 10.0 weeks of the control arm. Patients with a Gleason pattern score (GPS) of ≤7 receiving Provenge experienced better TTP. This group of patients also developed a stronger T cell response to PAP compared to the patients with GPS >8.
DC-transfected approach
A promising approach to vaccination treatment of PC is based on the transfection of total mRNA derived from the autologous tumour into DCs. This strategy has the theoretical advantage of targeting multiple HLA class I and class II patient specific TAAs without prior HLA typing. Moreover, even stromal antigens (including endothelial ones) could be targeted by this strategy since mRNA was obtained from surgical samples and not from tumour cell lines [77].
Heiser et al. [78] developed a DC-based immunotherapy protocol in which DCs were exposed to a single mRNA species encoding for PSA. Sixteen patients with metastatic PC were enrolled but only 13 subjects could be fully evaluated; the low dose group (n = 3) received 107 DCs, the intermediate dose group (n = 3) received 3 × 107 DCs while the high dose group (n = 6) received 5 × 107 DCs by i.v. infusion on weeks 2, 4 and 6 (Table 2). Vaccination was well tolerated and induced an increased T cell response to PSA regardless of the vaccination doses in all 13 subjects. Nonetheless, the patients of the intermediate–high dose group exhibited increased responses as compared to the low dose group. Furthermore, PSA velocity post-treatment declined in six of seven evaluated patients. The authors applied RT-PCR to detect haematogenous micrometastases in three patients. Complete but short-lasting clearance of circulating cancer cells was observed. Interestingly, the reappearance of PC cells in peripheral blood was associated with radiological progression of disease. The main features of these trials are summarized in Table 2. This approach also allows the modification of mRNA to increase its stability or improve antigen presentation. In fact the same group recently demonstrated that the chimeric transcript containing human telomerase mRNA fused to lysosome-associated membrane protein 1 (LAMP-1 hTERT) could be used to transfect DCs, thus allowing the development of a stronger CD4+ immunity in metastatic PC patients [79].
In conclusion, DC-based anti-PC vaccines appear to generate strong T cell response, which may be accompanied by clinical response though the frequency of the latter still remains unsatisfactory.
Recombinant vaccines
Recombinant vaccinia viruses (rVV) are among the most studied vectors for gene therapy. Some of the characteristics that make them suitable for gene delivery are their stability, the lack of nuclear integration and the large size of their genome (130–360 kb) that allows the introduction of multiple transgenes [80]. Moreover, the inflammatory response triggered by rVV highly immunogenic peptides may enhance the immunogenicity of the foreign protein.
Recombinant vaccinia virus-expressing TAAs have been tested in many clinical settings. Phase I trials were carried out using rVV-expressing PSA [81]. The authors demonstrated that the treatment was well tolerated, apart from low-grade injection site reactions. Specific T cell immune responses to PSA as well as serum PSA stabilizations were observed in selected patients. When GM-CSF was added s.c., toxicity was increased but an improved TTP was also seen.
Although the immunogenicity of vaccinia proteins may help the development of an immune response toward weak immunogens, this characteristic could also limit their use of rVV vectors. In fact, the host immune response has been shown to control and reduce rVV replication in subsequent vaccinations thus limiting clinical efficacy. For this reason a diversified prime and boost strategy using recombinant vaccinia and fowlpox viruses has been employed [81]. Recombinant fowlpox viruses (rFV) are replication defective vectors in mammalian cells that induce a weak neutralizing immune response. Furthermore they can express the transgene for longer period of time compared to rVV thus enhancing immunization.
A phase II trial was carried out by Kaufman et al. [82] to evaluate the feasibility and tolerability of different prime and boost vaccination schedules using rVV and rFv (Table 2). A tendency for a more favourable response was seen in the group receiving a priming dose of rVV followed by three boosting doses of rFV which, however, did not reach statistical differences. Although the authors did not see any objective biochemical responses, 45.3% of 64 evaluated patients remained free of PSA progression and 78% were free of clinical progression at 19.1 months of follow-up. In 46% of patients a specific T cell response against PSA was detected.
T cell activation requires a stimulation via the T cell receptor but also an additional signal provided by the engagement of co-stimulatory molecules on APCs is needed. Thus, a poxviral vector containing a triad of co-stimulatory molecules B7.1, ICAM-1 and LFA-3 has been developed (TRICOM). Ten patients affected by hormone refractory PC or with objective progression were enrolled [83]. They received one dose of PSA-rVV-TRICOM followed by a dose of PSA-rFV-TRICOM after 4 weeks. The vaccination was well tolerated and four patients experienced a SD (less than 25% increase in PSA) during the 8-week follow-up period [83]. Using the same strategy, Gulley [84] recently demonstrated decreasing serum PSA levels and one soft tissue response in a lymph node in hormone refractory PC patients.
In conclusion, recombinant vaccines have shown immunogenicity and evidence of tumour response in several trials but this result needs to be substantiated in phase III clinical studies.
Glycolipids/glycoproteins-based vaccines
Oncogenesis is responsible for complex structural and functional cellular changes. Among these, qualitative and quantitative alterations in the expression of cell surface carbohydrates have been described [85]. It has been demonstrated that the injection of purified cancer carbohydrates linked to T helper activating protein keyhole limpet haemocyanin (KLH) and mixed with immunological adjuvant QS21 induced an IgM and IgG antibody response against tumour cells [86]. This strategy of active immunization might be useful to detect and lyse micrometastases or circulating tumour cells through complement-mediated lysis or antibody-dependent, cell-mediated cytotoxicity.
Globo H is a hexasaccharide expressed on many normal tissues such as breast, pancreas, small bowel and prostate. Its enhanced expression on both primary and metastatic PC specimens makes it a candidate target antigen [87]. A phase I study has been carried out by Slovin et al. [88] using Globo H conjugated to KLH and QS21 in 20 patients who failed first-line prostatectomy or RT. Four doses of Globo H-KLH given s.c. at weekly intervals for 3 weeks were tested. No major toxicities were seen apart from local injection site reaction due to QS21; an IgM and IgG antibody response to glycolipid and glycoprotein antigens was detected at all dose level after 3 weeks. The same group recently reported the results of a similar trial of vaccination in which MUC-2-KLH was added to a Globo H-KLH vaccine [89]. IgM and IgG antibody directed against Globo H and MUC-2 were elicited. Another molecule able to induce a strong humoral anti-tumour response in PC patients is the monosaccharide alpha-N-acetylgalactosamine-O-serine/threonine (Tn) [87]. Tn antigenicity is found exclusively in glycopeptides containing a cluster of three or four consecutive residues of GalNAc-Ser/Thr (cTn) [90]. cTn was linked to KLH or to palmitic acid (PAM) and injected s.c. with the immunological adjuvant QS21 to 25 patients with biochemically relapsed PC [91]. IgM and IgG responses were seen even if PAM induced a lower IgG antibodies production.
Thus these types of vaccines, while inducing Ab response, have yet to prove their clinical efficacy.
Combined approaches to PC therapy
Ionizing radiation
Radiation therapy (RT) not only represents an effective alternative to surgery for the management of clinically localized PC but also for the treatment of those patients undergoing radical prostatectomy with high-risk pathological feature of relapse (positive margins or involvement of seminal vesicles). Important advances have been made in the development and use of different forms of RT, providing a variety of treatment options [92].
Although traditionally considered immune suppressive, ionizing radiation also possesses immune-modulating properties (see Table 3); encouraging pre-clinical and clinical data show that RT can modify the anti-tumour immune response and can therefore be coupled with different strategies of immunotherapy [93]. RT is characterized by the ability to exert direct toxic effects on tumour cells and, though controversies over the different processing, presentation and T cell response to apoptotic or necrotic cellular antigens exist, RT may induce both kinds of cell death [94]. The wide array of antigens released following RT, potentially allows the development of an immune response directed to multiple, patient-specific relevant targets, not only expressed by neoplastic cells but also by stromal cells.
Table 3.
RT and ADT induce immunomodulatory effects that favour the development of cancer immunity
| Treatment | Effect |
|---|---|
| Radiation therapy | Proinflammatory microenvironment |
| Radiation-induced antigenic peptides expression | |
| ↑ Release of antigenic material | |
| ↑ Recruitment of APCs and lymphocytes | |
| ↑ TAA expression | |
| ↑ MHC I antigen presentation | |
| Androgen deprivation therapy | Activates immune responses |
| ↑ Naive T cells | |
| ↑ Recruitment of APCs and lymphocytes | |
| ↓ Systemic tolerance |
Moreover, the so-called ‘danger’ model of immunity, proposed by Matzinger [95] in 1994, postulates that the outcome of an antigen-specific immune response depends upon the status of the surrounding microenvironment. The inflammatory response triggered within the irradiated tissue can provide the ‘danger signals’ necessary to gain optimal DCs maturation and TAA presentation through the release of pro-inflammatory cytokines as IL1, IL2, IL6, IL8, IL12, TNFα, TNFβ and the up-regulation of other molecules such as PgE2 and heat shock proteins. In addition, the bystander effect allows reciprocal influences on cytokine secretion from irradiated and non-irradiated cells [96].
Radiation also influences the expression of different molecules since it can up-regulate the expression of MHC class I, TAAs and death receptors on tumour cells, thus allowing a better recognition and killing of the transformed cells by incoming T cell clones.
Furthermore, RT induced inflammation, together with the up-regulation of adhesion, cell trafficking and chemotactic molecules contributes to the extravasation of both APCs and effector T lymphocytes [97], limiting the metastatic potential of tumour cells through the up-regulation of VE-cadherin.
Hormonal therapy
Prostate organogenesis as well as PC development are strictly dependent on androgens [98]. Hence, the employment of ADT in the management of patients with advanced PC, although a consensus on the more appropriate timing, dose and patients’ selection does not exist [99]. Some investigators prefer to delay the beginning of ADT to prevent the onset of androgen-resistant PC cells while others administer ADT earlier in the natural history of patients to obtain massive cell death in a low tumour burden setting. However, once PC becomes resistant to ADT, effective treatments are scanty.
Pre-clinical and clinical data suggest that ADT may be used to modulate the immune system [100] (see Table 3). Actually, the inhibitory role of androgens on immunity has been defined based on epidemiological and experimental data. Moreover, PC arises in adult, aged patients in a setting of chronic inflammation and, therefore, age-induced immune system dysfunctions may contribute to the down-modulation of immunological functions [101].
Immunosenescence comprises many alterations in the T cell compartment: an increased number of memory T cells and a reduction of naive T cells have been described due to thymic involution. Furthermore, these subsets appear to be hyporesponsive to different antigenic stimuli and an increased NK function compensates the decreased CTL activity [102]. An immune compartment predominantly made of memory T cells allows the recognition of previously encountered antigens, which is accompanied by an impairment in the identification of novel TAAs. Therefore, the response to vaccination relies on pre-existing memory T cells. After the onset of puberty, circulating sex steroid levels are responsible for thymus atrophy and thus ADT may be used to hinder age-induced narrowing of the naive pool. The role of ADT on T cell function has been studied by several groups. Sutherland et al. [103] recently showed that sex steroid ablation therapy based on LHRH analogues induced the restoration of thymopoiesis with an increase in the ratio of naive and memory T lymphocytes in both animals and men. Moreover, these cells appear to be phenotipically and functionally normal.
One of the major problems regarding vaccine strategies for the treatment of cancer concerns the overcoming of tolerance toward self-antigens [11]. An elegant work from Drake et al. [104] showed that androgen ablation in mouse model may contribute to the development of an immune response towards PC antigens through attenuation of systemic tolerance. Moreover, ADT-induced involution of prostatic tissue allows the release of antigens and alters the structure of the gland, which is accompanied by infiltration of T cells, macrophages and DCs [105]. Therefore, these mechanisms might act synergistically within a vaccination treatment strategy in the development of a clinically relevant immune response.
Although the employment of RT, ADT and chemotherapy remains confined to their cytotoxic effects, the combination of these standard treatments with vaccination strategies appears rationale. Studies in animal models and pilot clinical trials suggest a potential therapeutic efficacy of such strategies.
In an attempt to evaluate the effects of immunotherapy and standard treatment, Gulley et al. [84] randomized 30 patients affected by PC and eligible for external beam RT to receive RT alone or RT combined to a poxviral-PSA/B7.1-based vaccine. Similar treatment schedules have been applied to evaluate the combined role of poxviral vaccination with nilutamide or docetaxel + dexamethasone [106]. Although information about the clinical outcome of patients has been limited, these approaches do not appear to alter the ability of the immune system to mount a specific T cell response against multiple prostate-specific targets.
A phase I and phase I/II studies were planned to evaluate the potential additive anti-tumour effect of a combined approach based on a multipeptide vaccination associated to estramustine phosphate [107]. High dose estramustine (560 mg/die) was shown ex vivo to induce severe functional impairment on peripheral blood mononuclear cells while 280 mg/die had no such a detrimental effect. Epitopes were chosen after a pre-vaccination measurement of peptide-specific CTL precursors and mainly derived from PSA, PAP, SART1/2/3 and Lck: up to four peptides were injected s.c. six times at 2-week intervals. The response rates, defined as PSA reduction of ≥50%, were 46% in the HLA-A*0201 group and 73% in the HLA-A*24 group; these results are comparable with those obtained by chemotherapy-based approaches. Interestingly, PSA responses were also noted in patients who previously failed to estramustine administration.
A different approach has been taken by Freytag et al. [108]. They developed a suicide gene therapy approach based on a replication competent oncolytic adenovirus. The viral vector contains a fusion gene composed by cytosine deaminase (CD) and herpes simplex virus thymidine kinase (HSV-tk). CD converts 5-fluorocytosine (FC) in 5-fluorouracil (FU) that inhibits DNA synthesis by blocking thymidylate synthase and HSV-tk confers the ability to turn a non-toxic prodrug (vGCV) into the toxic anti-viral agent ganciclovir thus allowing the selective killing of the transduced cells. Pre-clinical data indicate that both CD and HSV-tk are potent tumour cell radiosensitizers that possess synergistic mechanism of action. A total of 15 patients were treated in a phase I study. Adenovirus was injected transrectally into the prostate under ultrasound guidance. Prodrugs were administered orally: 150 mg/Kg/die of 5-FC and 1,800 mg/die of vGCV were given for 1, 2 or 3 weeks. At the end patients received standard RT on prostate and seminal vesicles, while ADT was offered to high-risk patients. Grade I and II toxicities were commonly seen: lymphopenia, urinary symptoms, leucopenia, diarrhoea and anaemia due to prodrug administration or RT. Elevation in liver transaminases and monocytosis may be due to systemic shedding of the viral vector. Transgene expression was detected in prostatic tissue up to 3 weeks after the last adenovirus injection; these findings underline that the chemotherapeutic and radiosensitizing effect of the vaccination may persist during time.
Tumour microenvironment in PC and its role on the immune response
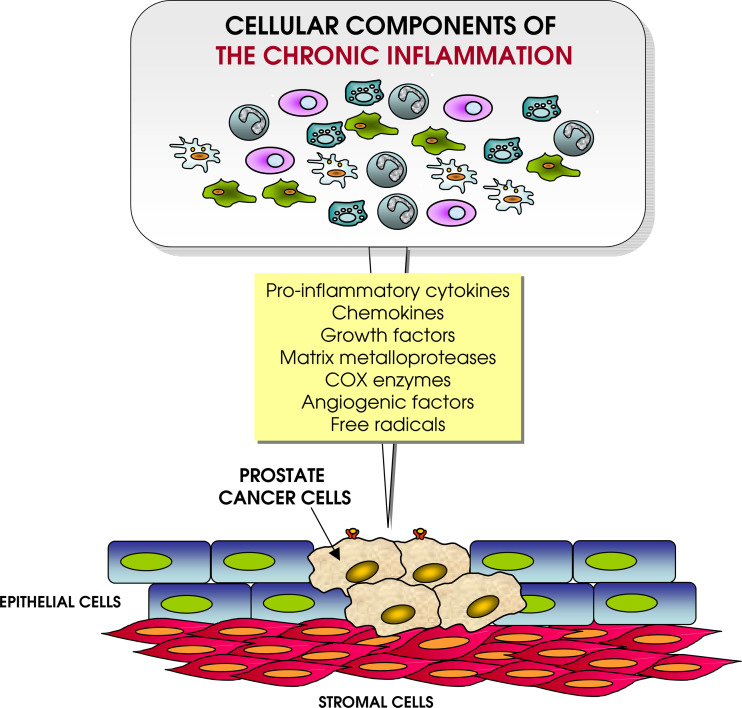
In 1863 Rudolph Virchow postulated a connection between inflammation and cancer. Asymptomatic prostatitis is almost ubiquitous in prostate specimens or biopsies. Although different potential microbial agents have been described to infect the prostate gland, the offending pathogen is often unknown. Moreover, an increased risk to develop PC is seen among men with a history of exposure to gonorrhoea, human papilloma virus or other sexually transmitted diseases [109]. These findings suggest that despite a specific aetiology, PC may stem from the inflammatory response mounted by the host (see Fig. 1).
Fig. 1.
Influence of chronic inflammation on tumour microenvironment. Different immune cells, including regulatory T cells, M2 macrophages, granulocytes and B cells, chronically stimulated by the presence of flogistic stimuli and by the transformed cells, release a large array of soluble factors promoting tumour growth (even through epigenetic mechanisms), impair function of anti-tumour immune effector cells and facilitate tissue invasion and eventually dissemination
Chronic inflammation is now emerging as a major player in the development of PC [110]. The causal link between chronic inflammation and the development of different types of cancer in humans is well established. Colorectal cancer, cervical, gastric, lung and also PC seem to develop as a consequence of proliferating epithelial cells that fails to acquire a mature cell phenotype. The term ‘proliferative inflammatory atrophy’ refers to areas of atrophic epithelium in the context of the prostatic parenchyma [111]. Recent evidences showing an increased expression of Ki67, bcl-2, GTSTP1 stress-induced detoxifying enzyme and low levels of the p27 suggest that this lesion may represent proliferative or regenerative areas resulting from the replacement of prostate epithelial cellular loss. Macrophages and polymorphonuclear leucocyte infiltration is a common finding. Tumour infiltrates predominantly made of CD4+ and CD8+ T effector lymphocytes are usually associated to a better prognosis [112] compared with the presence of innate immune cells that instead may yield a wide array of chemokines, angiogenic factors and matrix-degrading enzymes, creating an environment prone to tumour growth [113]. Moreover, the release of reactive oxygen and nitrogen species contributes to DNA damage [114]. Thus, the role of non-steroidal anti-inflammatory drugs (NSAIDs) in chemoprevention trials had been tested both in animals and humans. Evidences supporting the protective role of NSAID against PC have been reported [115], even though immunohistochemical studies on the expression of the enzymatic target of NSAID, i.e. cyclo-oxygenase 2 in PC are conflicting [116, 117].
Prostate gland embryogenesis and development, as well as carcinogenesis, relies on the complex signalling between stromal and epithelial elements [118]. Cancer cells microenvironment appears to be crucial to survival, progression and metastasis. In fact, it has been shown that normal fibroblasts could prevent transformation of initiated epithelial cells [119] and even reverse an already established neoplastic phenotype [120]. In PC a stromal reaction evolves together with carcinoma progression: an enrichment in myofibroblasts and fibroblasts, decreasing differentiated smooth muscle cells and matrix remodelling have been described [121, 122]. Transforming growth factor β (TGFβ) seems to be one of the key mediators of such changes [123]. The role of TGFβ in prostate homeostasis and PC development is quite complex. It generally inhibits proliferation and induces apoptosis in epithelial cells and controls smooth muscle cells differentiation [124]. Loss in the sensitivity to TGFβ inhibitory effects or overexpression of TGFβ is a common event in PC, thus promoting tumour growth [125]. In fact, the down-regulation of TGFβ receptor correlates with Gleason pattern score and clinical stage and it is considered a marker of poor outcome [126]. TGFβ may also alter the host–tumour interaction, thus supporting angiogenesis, remodelling of the extracellular matrix and inhibiting the immune response [127]. PSA also contributes to create an immune suppressive environment within the prostate. In fact, the primary function of PSA relies in the cleavage of semenogelin and fibronectin, causing liquefaction of semen [128], but this protease can also activate insulin growth factor 1 [129] which is a potent mitogen to stroma and epithelial cells and digests components of the basement membrane thus allowing tumour spread. On the contrary, PSA is a negative regulator of cell-cycle and when it cleaves plasminogen, anti-angiogenic factors are released [130]. In vitro experiments show that PSA inhibits T cell proliferation in a TGFβ- and IL2-independent fashion [131].
Most solid tumours contain tumour-infiltrating lymphocytes (TILs) but often these cells lack the ability to kill tumour cells. It is now well established that a lymphocytic infiltration predominantly made of effector memory T cells is associated with good prognosis in patients affected by different tumours while abundance of CD4+CD25+FOXP3+ regulatory T cells predicts unfavourable outcomes [112]. TILs are often unable to recognize and lyse autologous tumour cells; their anergic/tolerant state seems to be related to PC microenvironment. Bronte et al. [132] recently demonstrated that enhanced intratumoural metabolism of l-arginine in PC negatively affects T cell function through defects in signal transduction and effector function. Rescue of TIL responsiveness can be achieved by adding specific inhibitors of l-arginine metabolizing enzymes.
PSA detrimental effects seem to be directed also towards DCs. Such APCs should infiltrate cancer tissue to pick up TAAs, become activated and migrate to lymph nodes [133]. Tumour and stromal cells can alter the immune stimulatory capabilities of DCs through different pathways involving, for example VEGF, IL6, IL10 or TGFβ [57]. Thus, the inhibition of maturation, function and survival are commonly seen among cancer patients. PSA seems to inhibit the generation of DCs from CD34+ haematopoietic precursors and to hamper their stimulatory activity towards T cells in vitro [131, 134]. Furthermore, Troy et al. [135] have shown that the number of DCs infiltrating PC is low and their expression of co-stimulatory and activation markers is decreased. On the contrary macrophages and lymphocytes were a common and comparable finding in both healthy and neoplastic areas.
Another immune escape strategy adopted by cancer cells consists in the release of membrane vesicles, which proved to induce detrimental effects on the immune system in terms of induction of T cell apoptosis [136], impairment of monocyte differentiation into DCs and generation of myeloid suppressor cells [137] in different tumour histologies. With this regard it has been recently demonstrated that PC cells also release microvesicles, which could potentially exert similar negative effect on the anti-tumour immune response [138] (see Fig. 2).
Fig. 2.
Systemic immunological factors impairing the efficient recognition of tumour cells by T lymphocytes. Defects in DC maturation and accumulation of myeloid suppressor cells and expansion of regulatory T cells are promoted by tumour cells themselves. These cell subsets, together with a more complex network of soluble and cellular factors, provoke functional alterations in anti-tumour T lymphocytes leading to their inability of efficiently counteract tumour growth. Defects at tumour site, for instance involving altered processing and presentation of antigenic peptide by cancer cells can also contribute to the phenomenon
Hence, altogether the PC microenvironment may be considered immune suppressive. Moreover, the existence of a blood prostate barrier has been postulated based on the similarities between blood testis and blood epididymis barrier and the layer of basal cells that lie upon the tubular basement membrane of the prostate [139]. Thus, a combination of both physical and active mechanisms may lead to the constitution of local deficient immune surveillance.
Conclusions
Despite the improvement in the management of organ-confined PC, therapeutic options for castration-resistant and metastatic PC patients are limited and palliative. After the failure of surgery or RT, ADT is generally administered. Vaccination trials have been performed with different strategies and some of them provided encouraging biological or clinical responses observed even in phase III trials. Many authors underlined that vaccination strategies and standard treatments could be combined without increased toxicities.
Thus, the major problem regarding cancer vaccination relies on ‘how’ to induce a clinically relevant immune response [140]. The development of effective PC vaccines requires the identification of the right immunological targets, the induction of a potent immune response as well as long-term memory. Functional and phenotypical features of these responses are unfortunately still unclear. Moreover, vaccination strategies to treat cancer require the development of different complementary approaches to overcome immunological tolerance and tumour escape mechanisms.
Another problem concerning PC patients is ‘when’ to introduce an experimental vaccine in the treatment schedule. Given the acceptable toxicity profile, vaccination strategies may be employed after a first-line conventional therapy (surgery or RT) to prevent relapses thus allowing the development of an immune response in patients with minimal residual disease. For the same reason another favourable clinical setting may be that of biochemically relapsing patients. However, in these cases the evaluation of responses represents a critical issue. Indeed, many authors pointed out that PSA serum level (and slope) and its changes may not reliably reflect the outcomes of biological as well as standard treatments [141]. Moreover, these patients lack a measurable disease making the assessment of the clinical response rather questionable. Thus, more sophisticated monitoring techniques are needed.
As pointed out by Finn, small phase I and II studies are still required to evaluate different strategies of vaccination (antigen formulation, adjuvants, treatment schedules, etc.) and to dissect biological and clinical issues [142]. This applies also for PC patients. In fact, promising data obtained in vitro and in animal models often failed when tested in clinical trials. Thus, a better understanding of PC immune escape is mandatory because even a vaccination strategy that elicits a strong immune response may fail to improve patients’ clinical conditions [143].
Abbreviations
- ADT
Androgen deprivation therapy
- APC
Antigen-presenting cells
- DCs
Dendritic cells
- PAP
Prostatic acid phosphatase
- PC
Prostate cancer
- PSA
Prostate-specific antigen
- PSMA
Prostate-specific membrane antigen
- RT
Radiotherapy
- TAA
Tumour-associated antigens
- TGFβ
Transforming growth factor β
References
- 1.Parkim DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632–1642. [PubMed] [Google Scholar]
- 3.Samson DJ, Seidenfeld J, Schmitt B, Hasselblad V, Albertsen PC, Bennett CL, Wilt TJ, Aronson N. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95:361–376. doi: 10.1002/cncr.10647. [DOI] [PubMed] [Google Scholar]
- 4.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20:3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 5.Swanson GP, Riggs M, Earle J. Failure after primary radiation or surgery for prostate cancer: differences in response to androgen ablation. J Urol. 2004;172:525. doi: 10.1097/01.ju.0000132412.74468.57. [DOI] [PubMed] [Google Scholar]
- 6.Oefelein MG, Ricchiuti VS, Conrad PW, Goldman H, Bodner D, Resnick MI, Seftel A. Clinical preictors of androgen-indipendent prostate cancer and survival in the prostate-specific antigen era. Urology. 2002;60:120–124. doi: 10.1016/s0090-4295(02)01633-3. [DOI] [PubMed] [Google Scholar]
- 7.Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology. 2003;61(Suppl 1):32–38. doi: 10.1016/s0090-4295(02)02397-x. [DOI] [PubMed] [Google Scholar]
- 8.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 9.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 10.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004) update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst USA. 2002;94:805–818. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 12.Corman JM, Sercarz EE, Nanda NK. Recognition of prostate-specific antigenic peptide determinants by human CD4 and CD8 T cells. Clin Exp Immunol. 1998;114:166–172. doi: 10.1046/j.1365-2249.1998.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst USA. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 14.Xue BH, Zhang Y, Sosman JA, Peace DJ. Induction of human cytotoxic T lymphocytes specific for prostate-specific antigen. Prostate. 1997;30:73–78. doi: 10.1002/(sici)1097-0045(19970201)30:2<73::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Matsueda S, Takedatsu H, Yao A, Tanaka M, Noguchi M, Itoh K, Harada M. Identification of peptide vaccine candidates for prostate cancer patients with HLA-A3 supertype alleles. Clin Cancer Res. 2005;11:6933–6943. doi: 10.1158/1078-0432.CCR-05-0682. [DOI] [PubMed] [Google Scholar]
- 16.Harada M, Kobayashi K, Matsueda S, Nakagawa M, Noguchi M, Itoh K. Prostate-specific antigen-derived epitopes capable of inducing cellular and humoral responses in HLA-A24+ prostate cancer patients. Prostate. 2003;57:152–159. doi: 10.1002/pros.10280. [DOI] [PubMed] [Google Scholar]
- 17.Klyushnenkova EN, Link J, Oberle WT, Kodak J, Rich C, Vandenbark AA, Alexander RB. Identification of HLA-DRB1*1501-restricted T-cell epitopes from prostate-specific antigen. Clin Cancer Res. 2005;11:2853–2861. doi: 10.1158/1078-0432.CCR-04-1927. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Celis E. Recognition of prostate tumor cells by cytotoxic T lymphocytes specific for prostate-specific membrane antigen. Cancer Res. 2002;62:5807–5812. [PubMed] [Google Scholar]
- 19.Horiguchi Y, Nukaya I, Okazawa K, Kawashima I, Fikes J, Sette A, Tachibana M, Takesako K, Murai M. Screening of HLA-A24-restricted epitope peptides from prostate-specific membrane antigen that induce specific antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8:3885–3892. [PubMed] [Google Scholar]
- 20.Kobayashi H, Omiya R, Sodey B, Yanai M, Oikawa K, Sato K, Kimura S, Senju S, Nishimura Y, Tateno M, Celis E. Identification of naturally processed helper T-cell epitopes from prostate-specific membrane antigen using peptide-based in vitro stimulation. Clin Cancer Res. 2003;9:5386–5393. [PubMed] [Google Scholar]
- 21.Schroers R, Shen L, Rollins L, Xiao Z, Sonderstrup G, Slawin K, Huang XF, Chen SY. Identification of MHC class II-restricted T-cell epitopes in prostate-specific membrane antigen. Clin Cancer Res. 2003;9:3260–3271. [PubMed] [Google Scholar]
- 22.Machlenkin A, Paz A, Bar Haim E, Goldberger O, Finkel E, Tirosh B, Volovitz I, Vadai E, Lugassy G, Cytron S, Lemonnier F, Tzehoval E, Eisenbach L. Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res. 2005;65:6435–6442. doi: 10.1158/0008-5472.CAN-05-0133. [DOI] [PubMed] [Google Scholar]
- 23.McNeel DG, Nguyen LD, Disis ML. Identification of T helper epitopes from prostatic acid phosphatase. Cancer Res. 2001;61:5161–5167. [PubMed] [Google Scholar]
- 24.Dannull J, Diener PA, Prikler L, Furstenberger G, Cerny T, Schmid U, Ackermann DK, Groettrup M. Rostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res. 2000;60:5522–5528. [PubMed] [Google Scholar]
- 25.Matsueda S, Kobayashi K, Nonaka Y, Noguchi M, Itoh K, Harada M. Identification of new prostate stem cell antigen-derived peptides immunogenic in HLA-A2(+) patients with hormone-refractory prostate cancer. Cancer Immunol Immunother. 2004;53:479–489. doi: 10.1007/s00262-003-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsueda S, Yao A, Ishihara Y, Ogata R, Noguchi M, Itoh K, Harada M. A prostate stem cell antigen-derived peptide immunogenic in HLA-A24- prostate cancer patients. Prostate. 2004;60:205–213. doi: 10.1002/pros.20038. [DOI] [PubMed] [Google Scholar]
- 27.Alves PM, Faure O, Graff-Dubois S, Cornet S, Bolonakis I, Gross DA, Miconnet I, Chouaib S, Fizazi K, Soria JC, Lemonnier FA, Kosmatopoulos K (2006) STEAP, a prostate tumor antigen, is a target of human CD8(+) T cells. Cancer Immunol Immunother 19 April 2006 (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 28.Carlsson B, Totterman TH, Essand M. Generation of cytotoxic T lymphocytes specific for the prostate and breast tissue antigen TARP. Prostate. 2004;61:161–170. doi: 10.1002/pros.20091. [DOI] [PubMed] [Google Scholar]
- 29.Oh S, Terabe M, Pendleton CD, Bhattacharyya A, Bera TK, Epel M, Reiter Y, Phillips J, Linehan WM, Kasten-Sportes C, Pastan I, Berzofsky JA. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res. 2004;64:2610–2618. doi: 10.1158/0008-5472.can-03-2183. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H, Nagato T, Oikawa K, Sato K, Kimura S, Aoki N, Omiya R, Tateno M, Celis E. Recognition of prostate and breast tumor cells by helper T lymphocytes specific for a prostate and breast tumor-associated antigen, TARP. Clin Cancer Res. 2005;11:3869–3878. doi: 10.1158/1078-0432.CCR-04-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francini G, Scardino A, Kosmatopoulos K, Lemonnier FA, Campoccia G, Sabatino M, Pozzessere D, Petrioli R, Lozzi L, Neri P, Fanetti G, Cusi MG, Correale P. High-affinity HLA-A(*)02.01 peptides from parathyroid hormone-related protein generate in vitro and in vivo antitumor CTL response without autoimmune side effects. J Immunol. 2002;169:4840–4849. doi: 10.4049/jimmunol.169.9.4840. [DOI] [PubMed] [Google Scholar]
- 32.Yao A, Harada M, Matsueda S, Ishihara Y, Shomura H, Takao Y, Noguchi M, Matsuoka K, Hara I, Kamidono S, Itoh K. New epitope peptides derived from parathyroid hormone-related protein which have the capacity to induce prostate cancer-reactive cytotoxic T lymphocytes in HLA-A2+ prostate cancer patients. Prostate. 2005;62:233–242. doi: 10.1002/pros.20133. [DOI] [PubMed] [Google Scholar]
- 33.Kiessling A, Stevanovic S, Fussel S, Weigle B, Rieger MA, Temme A, Rieber EP, Schmitz M. Identification of an HLA-A*0201-restricted T-cell epitope derived from the prostate cancer-associated protein prostein. Br J Cancer. 2004;90:1034–1040. doi: 10.1038/sj.bjc.6601642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alves PM, Faure O, Graff-Dubois S, Gross DA, Cornet S, Chouaib S, Miconnet I, Lemonnier FA, Kosmatopoulos K. EphA2 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Cancer Res. 2003;63:8476–8480. [PubMed] [Google Scholar]
- 35.Hural JA, Friedman RS, McNabb A, Steen SS, Henderson RA, Kalos M. Identification of naturally processed CD4 T cell epitopes from the prostate-specific antigen kallikrein 4 using peptide-based in vitro stimulation. J Immunol. 2002;169:557–565. doi: 10.4049/jimmunol.169.1.557. [DOI] [PubMed] [Google Scholar]
- 36.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 37.Sotiropoulou PA, Perez SA, Voelter V, Echner H, Missitzis I, Tsavaris NB, Papamichail M, Baxevanis CN. Natural CD8+ T-cell responses against MHC class I epitopes of the HER-2/ neu oncoprotein in patients with epithelial tumors. Cancer Immunol Immunother. 2003;52:771–779. doi: 10.1007/s00262-003-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sotiropoulou PA, Perez SA, Iliopoulou EG, Missitzis I, Voelter V, Echner H, Baxevanis CN, Papamichail M. Cytotoxic T-cell precursor frequencies to HER-2 (369–377) in patients with HER-2/neu-positive epithelial tumours. Br J Cancer. 2003;89:1055–1061. doi: 10.1038/sj.bjc.6601244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 40.Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci USA. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vonderheide RH, Anderson KS, Hahn WC, Butler MO, Schultze JL, Nadler LM. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin Cancer Res. 2001;7:3343–3348. [PubMed] [Google Scholar]
- 42.Klyushnenkova EN, Ponniah S, Rodriguez A, Kodak J, Mann DL, Langerman A, Nishimura MI, Alexander RB. CD4 and CD8 T-lymphocyte recognition of prostate specific antigen in granulomatous prostatitis. J Immunother. 2004;27:136–146. doi: 10.1097/00002371-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Zellweger T, Ninck C, Bloch M, Mirlacher M, Koivisto PA, Helin HJ, Mihatsch MJ, Gasser TC, Bubendorf L. Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer. 2005;113:619–628. doi: 10.1002/ijc.20615. [DOI] [PubMed] [Google Scholar]
- 44.Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21:383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 45.Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 46.Peshwa MV, Shi JD, Ruegg C, Laus R, van Schooten WC. Induction of prostate tumor-specific CD8+ cytotoxic T-lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptide. Prostate. 1998;36:129–138. doi: 10.1002/(sici)1097-0045(19980701)36:2<129::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 47.Kiessling A, Schmitz M, Stevanovic S, Weigle B, Holig K, Fussel M, Fussel S, Meye A, Wirth MP, Rieber EP. Prostate stem cell antigen: identification of immunogenic peptides and assessment of reactive CD8+ T cells in prostate cancer patients. Int J Cancer. 2002;102:390–397. doi: 10.1002/ijc.10713. [DOI] [PubMed] [Google Scholar]
- 48.Kishi H, Igawa M, Kikuno N, Yoshino T, Urakami S, Shiina H. Expression of the survivin gene in prostate cancer: correlation with clinicopathological characteristics, proliferative activity and apoptosis. J Urol. 2004;171:1855–1860. doi: 10.1097/01.ju.0000120317.88372.03. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt SM, Schag K, Muller MR, Weck MM, Appel S, Kanz L, Grunebach F, Brossart P. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez P, Cabrera T, Mendez R, Esparza C, Cozar JM, Tallada M, Lopez-Nevot MA, Ruiz-Cabello F, Garrido F. A nucleotide insertion in exon 4) is responsible for the absence of expression of an HLA-A*0301 allele in a prostate carcinoma cell line. Immunogenetics. 2001;53:606–610. doi: 10.1007/s002510100371. [DOI] [PubMed] [Google Scholar]
- 51.Altieri DC. Survivin and apoptosis control. Adv Cancer Res. 2003;88:31–52. doi: 10.1016/s0065-230x(03)88303-3. [DOI] [PubMed] [Google Scholar]
- 52.Casati C, Dalerba P, Rivoltini L, Gallino G, Deho P, Rini F, Belli F, Mezzanzanica D, Costa A, Andreola S, Leo E, Parmiani G, Castelli C. The apoptosis inhibitor protein survivin induces tumor-specific CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res. 2003;63:4507–4515. [PubMed] [Google Scholar]
- 53.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, Thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 54.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 55.Noguchi M, Itoh K, Suekane S, Yao A, Suetsugu N, Katagiri K, Yamada A, Yamana H, Noda S. Phase I trial of patient-oriented vaccination in HLA-A2-positive patients with metastatic hormone-refractory prostate cancer. Cancer Sci. 2004;95:77–84. doi: 10.1111/j.1349-7006.2004.tb03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 57.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 58.Small EJ, Reese DM, Um B, Whisenant S, Dixon SC, Figg WD. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 59.Dreicer R, See WA, Klein EA. Phase II trial of GM-CSF in advanced prostate cancer. Invest New Drugs. 2001;19:261–265. doi: 10.1023/a:1010637105066. [DOI] [PubMed] [Google Scholar]
- 60.Rini BI, Weinberg V, Bok R, Small EJ. Prostate-specific antigen kinetics as a measure of the biologic effect of granulocyte-macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J Clin Oncol. 2003;21:99–105. doi: 10.1200/JCO.2003.04.163. [DOI] [PubMed] [Google Scholar]
- 61.Schwaab T, Tretter CP, Gibson JJ, Cole BF, Schned AR, Harris R, Fisher JL, Crosby N, Stempkowski LM, Heaney JA, Ernstoff MS. Tumor-related immunity in prostate cancer patients treated with human recombinant granulocyte monocyte-colony stimulating factor (GM-CSF) Prostate. 2006;66:667–674. doi: 10.1002/pros.20266. [DOI] [PubMed] [Google Scholar]
- 62.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, Weber CE, Baccala AA, Goemann MA, Clift SM, Ando DG, Levitsky HI, Cohen LK, Sanda MG, Mulligan RC, Partin AW, Carter HB, Piantadosi S, Marshall FF, Nelson WG. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 64.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 65.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis TA, Korman A, Keler T. MDX-010 (human anti CTLA-4): a phase I trial in hormone refractory prostate carcinoma (HRPC) Proc Am Soc Clin Oncol. 2002;21:74. [Google Scholar]
- 67.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 68.Tjoa BA, Simmons SJ, Bowes VA, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, Salgaller ML, Boynton AL, Murphy GP. Evaluation of phase I/II clinical trials in prostate cancer with dendritic cells and PSMA peptides. Prostate. 1998;36:39–44. doi: 10.1002/(sici)1097-0045(19980615)36:1<39::aid-pros6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 69.Fuessel S, Meye A, Schmitz M, Zastrow S, Linne C, Richter K, Lobel B, Hakenberg OW, Hoelig K, Rieber EP, Wirth MP. Vaccination of hormone-refractory prostate cancer patients with peptide cocktail-loaded dendritic cells: results of a phase I clinical trial. Prostate. 2006;66:811–821. doi: 10.1002/pros.20404. [DOI] [PubMed] [Google Scholar]
- 70.Waeckerle-Men Y, Uetz-von Allmen E, Fopp M, von Moos R, Bohme C, Schmid HP, Ackermann D, Cerny T, Ludewig B, Groettrup M, Gillessen S (2006) Dendritic cell-based multi-epitope immunotherapy of hormone-refractory prostate carcinoma. Cancer Immunol Immunother 13 April 2006 (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 71.Naftzger C, Takechi Y, Kohda H, Hara I, Vijayasaradhi S, Houghton AN. Immune response to a differentiation antigen induced by altered antigen: a study of tumor rejection and autoimmunity. Proc Natl Acad Sci USA. 1996;93:14809–14814. doi: 10.1073/pnas.93.25.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roiko K, Janne OA, Vihko P. Primary structure of rat secretory acid phosphatase and comparison to other acid phosphatases. Gene. 1990;89:223–229. doi: 10.1016/0378-1119(90)90009-g. [DOI] [PubMed] [Google Scholar]
- 73.Fong L, Brockstedt D, Benike C, Breen JK, Strang G, Ruegg CL, Engleman EG. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol. 2001;167:7150–7156. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 74.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 75.Burch PA, Croghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, Richardson RL, Valone FH, Vuk-Pavlovic S. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 76.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 77.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 78.Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E, Vieweg J. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 80.Guo ZS, Bartlett DL. Vaccinia as a vector for gene delivery. Expert Opin Biol Ther. 2004;4:901–917. doi: 10.1517/14712598.4.6.901. [DOI] [PubMed] [Google Scholar]
- 81.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 82.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, Whiteside TL, Schlom J, Wilding G, Weiner LM. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 83.Dipaola R, Plante M, Kaufman H, Petrylak D, Israeli R, Lattime E, Manson K, Schuetz T. A phase I trial of pox PSA vaccines (PROSTVAC(R)-VF) with B7–1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM trade mark) in patients with prostate cancer. J Transl Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 85.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 86.Livingston PO, Ragupathi G. Carbohydrate vaccines that induce antibodies against cancer. Previous experience and future plans. Cancer Immunol Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang S, Zhang HS, Reuter VE, Slovin SF, Scher HI, Livingston PO. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 88.Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Slovin SF, Ragupathi G, Fernandez C, Jefferson MP, Diani M, Wilton AS, Powell S, Spassova M, Reis C, Clausen H, Danishefsky S, Livingston P, Scher HI. A bivalent conjugate vaccine in the treatment of biochemically relapsed prostate cancer: a study of glycosylated MUC-2-KLH and Globo H-KLH conjugate vaccines given with the new semi-synthetic saponin immunological adjuvant GPI-0100 or QS-21. Vaccine. 2005;23:3114–3122. doi: 10.1016/j.vaccine.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 90.Nakada H, Inoue M, Numata Y, Tanaka N, Funakoshi I, Fukui S, Mellors A, Yamashina I. Epitopic structure of Tn glycophorin A for an anti-Tn antibody (MLS 128) Proc Natl Acad Sci USA. 1993;90:2495–2499. doi: 10.1073/pnas.90.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky S, Livingston PO, Scher HI. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 92.Speight JL, Roach M., III Radiotherapy in the management of clinically localized prostate cancer: evolving standards, consensus, controversies and new directions. J Clin Oncol. 2005;23:8176–8185. doi: 10.1200/JCO.2005.03.4629. [DOI] [PubMed] [Google Scholar]
- 93.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 96.Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20:7085–7095. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- 97.Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer. 1999;82:385–395. doi: 10.1002/(sici)1097-0215(19990730)82:3<385::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 98.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signalling in androgen-refractory prostate cancer. J Natl Cancer Inst USA. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 99.Ryan CJ, Small EJ. Early versus delayed androgen deprivation for prostate cancer: new fuel for an old debate. J Clin Oncol. 2005;23:8225–8231. doi: 10.1200/JCO.2005.03.5311. [DOI] [PubMed] [Google Scholar]
- 100.Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 101.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 102.Provinciali M, Smorlesi A. Immunoprevention and immunotherapy of cancer in ageing. Cancer Immunol Immunother. 2005;54:93–106. doi: 10.1007/s00262-004-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 104.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Noguchi M, Itoh K, Suekane S, Morinaga A, Sukehiro A, Suetsugu N, Katagiri K, Yamada A, Noda S. Immunological monitoring during combination of patient-oriented peptide vaccination and estramustine phosphate in patients with metastatic hormone refractory prostate cancer. Prostate. 2004;60:32–45. doi: 10.1002/pros.20011. [DOI] [PubMed] [Google Scholar]
- 108.Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, DePeralta-Venturina M, Xia X, Brown S, Lu M, Kim JH. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- 109.Correa P. Commentary: is prostate cancer an infectious disease? Int J Epidemiol. 2005;34:197–198. doi: 10.1093/ije/dyh403. [DOI] [PubMed] [Google Scholar]
- 110.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parmiani G. Tumor-infiltrating T cells—friend or foe of neoplastic cells? N Engl J Med. 2005;353:2640–2641. doi: 10.1056/NEJMp058236. [DOI] [PubMed] [Google Scholar]
- 113.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 114.Maeda H, Akaike T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (Mosc) 1998;63:854–865. [PubMed] [Google Scholar]
- 115.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90:93–99. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zha S, Gage WR, Sauvageot J, Saria EA, Putzi MJ, Ewing CM, Faith DA, Nelson WG, De Marzo AM, Isaacs WB. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- 117.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11:3250–3256. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 118.Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol. 2004;92:221–236. doi: 10.1016/j.jsbmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 119.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hayashi N, Cunha GR. Mesenchyme-induced changes in the neoplastic characteristics of the Dunning prostatic adenocarcinoma. Cancer Res. 1991;51:4924–4930. [PubMed] [Google Scholar]
- 121.Timme TL, Truong LD, Merz VW, Krebs T, Kadmon D, Flanders KC, Park SH, Thompson TC. Mesenchymal-epithelial interactions and transforming growth factor-beta expression during mouse prostate morphogenesis. Endocrinology. 1994;134:1039–1045. doi: 10.1210/endo.134.3.8119140. [DOI] [PubMed] [Google Scholar]
- 122.Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res. 1997;232:208–215. doi: 10.1006/excr.1997.3525. [DOI] [PubMed] [Google Scholar]
- 123.Lee C, Sintich SM, Mathews EP, Shah AH, Kundu SD, Perry KT, Cho JS, Ilio KY, Cronauer MV, Janulis L, Sensibar JA. Transforming growth factor-beta in benign and malignant prostate. Prostate. 1999;39:285–290. doi: 10.1002/(sici)1097-0045(19990601)39:4<285::aid-pros9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 124.Sutkowski DM, Fong C-J, Sensibar JA, Rademaker AW, Sherwood ER, Kozlowski JM, Lee C. Interaction of epidermal growth factor and transforming growth factor-β1 in human prostatic epithelial cells in culture. Prostate. 1992;21:133–143. doi: 10.1002/pros.2990210206. [DOI] [PubMed] [Google Scholar]
- 125.Steiner MS, Barrack ER. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol Endocrinol. 1992;6:15–25. doi: 10.1210/mend.6.1.1738367. [DOI] [PubMed] [Google Scholar]
- 126.Kim IY, Ahn HJ, Zelner DJ, Shaw JW, Lang S, Kato M, Oefelein MG, Miyazono K, Nemeth JA, Kozlowski JM, Lee C. Loss of expression of transforming growth factor beta type I and type II receptors correlates with tumor grade in human prostate cancer tissues. Clin Cancer Res. 1996;2:1255–1261. [PubMed] [Google Scholar]
- 127.Mulé JJ, Schwarz SL, Roberts AB, Sporn MB, Rosenberg SA. Transforming growth factor-beta inhibits the in vitro generation of lymphokine-activated killer cells and cytotoxic T cells. Cancer Immunol Immunother. 1988;26:95–100. doi: 10.1007/BF00205600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lilja H, Oldbring J, Rannevik G, Laurell CB. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987;80:281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pollak M, Beamer W, Zhang JC. Insulin-like growth factors and prostate cancer. Cancer Met Rev. 1998–1999;17:383–390. doi: 10.1023/a:1006154108619. [DOI] [PubMed] [Google Scholar]
- 130.Fortier AH, Nelson BJ, Grella DK, Holaday JW. Antiangiogenic activity of prostate-specific antigen. J Natl Cancer Inst USA. 1999;91:1635–1640. doi: 10.1093/jnci/91.19.1635. [DOI] [PubMed] [Google Scholar]
- 131.Kennedy-Smith AG, McKenzie JL, Owen MC, Davidson PJ, Vuckovic S, Hart DN. Prostate specific antigen inhibits immune responses in vitro: a potential role in prostate cancer. J Urol. 2002;168:741–747. [PubMed] [Google Scholar]
- 132.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 134.Aalamian M, Tourkova IL, Chatta GS, Lilja H, Huland E, Huland H, Shurin GV, Shurin MR. Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol. 2003;170:2026–2030. doi: 10.1097/01.ju.0000091264.46134.b7. [DOI] [PubMed] [Google Scholar]
- 135.Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol. 1998;160:214–219. [PubMed] [Google Scholar]
- 136.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 137.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with TGFβ-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 138.Carlsson L, Nilsson O, Larsson A, Stridsberg M, Sahlen G, Ronquist G. Characteristics of human prostasomes isolated from three different sources. Prostate. 2003;54:322–330. doi: 10.1002/pros.10189. [DOI] [PubMed] [Google Scholar]
- 139.Leibovitz A, Baumoehl Y, Segal R. Increased incidence of pathological and clinical prostate cancer with age: age related alterations of local immune surveillance. J Urol. 2004;172:435–437. doi: 10.1097/01.ju.0000131908.19114.d3. [DOI] [PubMed] [Google Scholar]
- 140.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 141.Vicini FA, Vargas C, Abner A, Kestin L, Horwitz E, Martinez A. Limitations in the use of serum prostate specific antigen levels to monitor patients after treatment for prostate cancer. J Urol. 2005;173:1456–1462. doi: 10.1097/01.ju.0000157323.55611.23. [DOI] [PubMed] [Google Scholar]
- 142.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 143.Miller AM, Pisa P (2005) Tumour escape mechanisms in prostate cancer. Cancer Immunol Immunother 16 December 2005 (Epub ahead of print) [DOI] [PMC free article] [PubMed]