Abstract
In photoreceptor cells the Ca2+-binding protein recoverin controls phosphorylation of the visual receptor rhodopsin by inhibiting rhodopsin kinase (GRK-1). It can also serve as a paraneoplastic antigen in the development of retinal degeneration in some patients with cancer. The aberrant expression of recoverin in cancer cells and the presence of autoantibodies against recoverin are essential for the occurrence of cancer-associated retinopathy, which finally results in the apoptosis of photoreceptor cells. Noteworthy in cancer patients, the aberrant recoverin expression and the appearance of autoantibodies against recoverin are more frequent than paraneoplastic syndromes. We suggest the term “cancer-retina antigens” for this kind of proteins like recoverin that are solely expressed in retina and tumor tissues and evoke antibodies and/or T cells in patients with cancer. The rare development of a paraneoplastic syndrome is possibly caused by this immune response and probably depends on further events allowing to overcome the blood–retina barrier and the immune privileged status of the retina. It is still unknown whether aberrantly expressed recoverin could have a specific function in cancer cells, though it is suggested that it can be functionally associated with G-protein-coupled receptor kinases. This paper reviews the present knowledge on paraneoplastic syndromes associated with the aberrant expression of recoverin. A possible application of recoverin as a potential target for immunotherapy of cancer is discussed.
Keywords: Paraneoplastic syndromes, Paraneoplastic antigens, Antibody, T cell, Tumor antigen
Paraneoplastic neurological syndromes associated with loss of vision
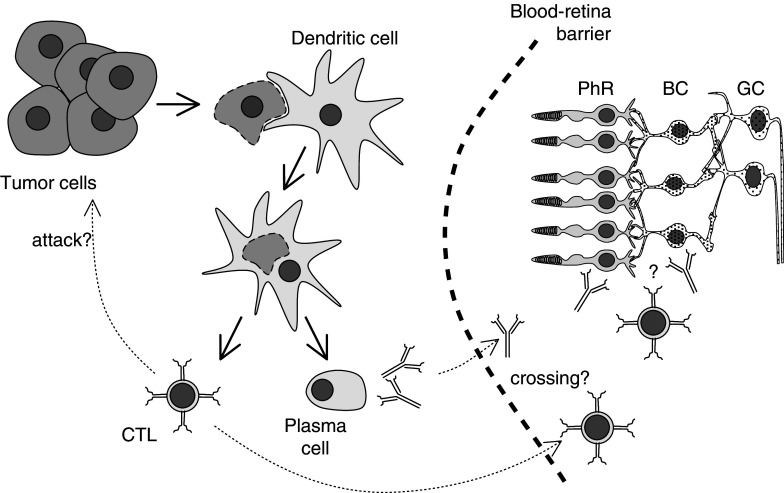
Paraneoplastic neurological syndromes (PNS) are rare neurological disorders that are related to cancer, even though the primary tumor and its metastases have not invaded the nervous system. PNS are thought to be autoimmune diseases that develop when malignant tumors express proteins, paraneoplastic antigens (PNA), which are normally present only in neurons. PNA expression outside the nervous system triggers the host’s immune response toward the corresponding PNA or its epitopes present in neurons, resulting in the development of PNS [31]. A pivotal point in pathogenesis of the disorders was the recognition by J. Posner and coworkers that patients with PNS harbor high-titer antibodies both in their serum and spinal fluid that recognize apparently identical antigens in normal brain and tumor tissues as shown by Western blot analysis [59]. Retinopathies as paraneoplastic syndromes are also thought to be induced by antibodies or cytotoxic T cells of the patient to antigens expressed both in the retina and the tumor (Fig. 1).
Fig. 1.
Hypothesis of the induction of retinopathies associated with cancers. Dendritic cells take up necrotic or apoptotic tumor cells and present paraneoplastic antigens to the immune system. This induces the production of specific antibodies and/or CD8 positive, cytotoxic T cells (CTLs). These CTLs might play a role in tumor defense. Antibodies and/or CTLs might eventually cross the blood–retina barrier and subsequently damage either photoreceptor cells (PhR) or bipolar cells (BC). GC ganglion cells
Cancer-associated retinopathy (CAR) is a very rare disease with only 55 cases published so far. An interesting feature of CAR is that it can be observed several weeks or even months before the underlying tumor is detected [16]. Indications are blurred vision, complaints of flashing lights, loss of peripheral and color vision, and night blindness [36]. The concept that cancer can promote visual dysfunction was proposed for the first time by Sawyer et al. in 1976 [63]. Seven years later, Keltner et al. postulated that autoimmunity plays a crucial role in retinopathy mediated by cancer [39]. The autoimmunity theory was supported by experimental data showing that serum antibodies from lung cancer patients with CAR can recognize certain cell types in retina sections [30, 42, 43]. The first indication that a retinal protein with an apparent molecular weight of 23 kDa could be “a CAR antigen” was obtained by Thirkill and coworkers [69]. Recoverin as a Ca2+-binding protein in photoreceptor cells was parallelly discovered by several groups [21, 22, 44]. Subsequently, recoverin was identified as being the CAR antigen [57, 71].
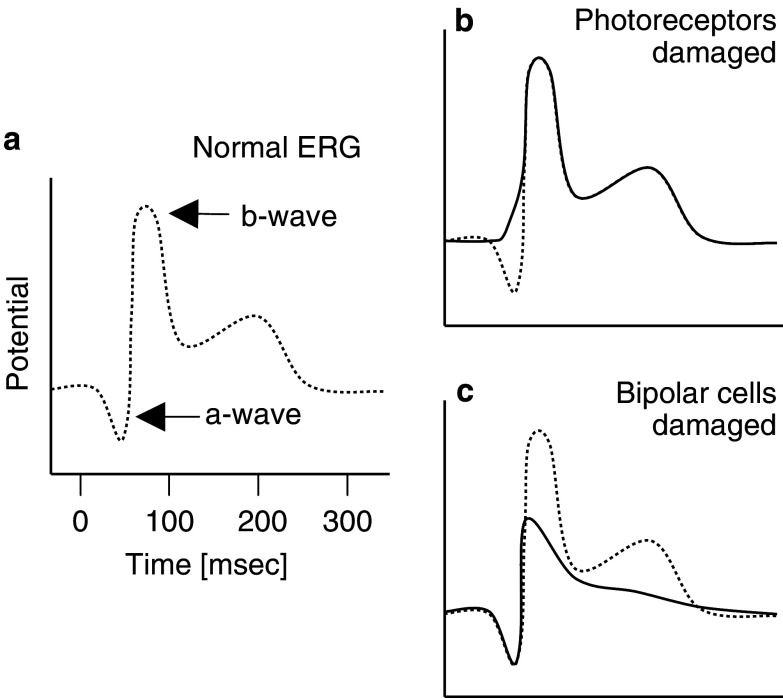
Melanoma-associated retinopathy (MAR) is distinguished from CAR by clinical differences. Today, the ophthalmological components of both CAR and MAR are well studied. In CAR patients, full field flush electroretinograms (ERG) show a reduction of the a-wave indicating the degeneration of photoreceptor cells (Fig. 2). Flush ERG of MAR patients show often a reduction of the b-wave reflecting a dysfunction of bipolar cells, which create Na+-currents through the Muller glia [12, 54, 73]. CAR patients’ sera strongly stain photoreceptor cells and weakly stain bipolar cells, while MAR sera have been reported to exhibit strong immunoreactivity in bipolar cells [12, 54]. The histological examination of the retina from MAR patients also indicated degeneration of the bipolar cells [27, 70]. This explains why MAR patients suffer from sudden visual loss and night blindness, whereas their color vision is often normal. However, in some MAR patients, the a-wave in the ERG can be reduced, degenerative regions are detected in retina and pigment epithelium, and sera recognize photoreceptor cells [13, 40]. These data suggest that in addition to bipolar cells, photoreceptor cells can also be affected in MAR patients.
Fig. 2.
Normal and pathological electroretinogram (ERG). (a) The ERG is a field potential in the whole eye normally composed of an a-wave and a b-wave. Photoreceptor degenerations manifest in a reduced or changed a-wave (b), while malfunction in the bipolar cells are seen as altered b-waves (c)
While the mechanism of paraneoplastic retina degeneration in CAR is rather well investigated, the mechanism of retinopathy in MAR is still unknown. The molecular mechanism of CAR is the apoptotic death of the photoreceptor cells attributed to autoantibodies reacting to the photoreceptor protein recoverin [4, 6]. Lei and colleagues have shown that serum autoantibodies of MAR patients injected in monkeys induce the degeneration of depolarized bipolar cells [45]. Antigenic mimicry has been implicated in the autoimmune response in MAR; cancer cells may have epitopes, which are similar to those in bipolar cells [60]. Still, at present there are no data about specific target(s) for autoantibodies in bipolar cells. In contrast, Potter et al. showed that photoreceptors can also be damaged in MAR patients [60]. The authors detected autoantibodies against the photoreceptor protein transducin, which is part of the visual signal transduction, in the serum of one MAR patient.
Recoverin as a paraneoplastic antigen in CAR
Recoverin is a Ca2+-binding protein present only in vertebrate photoreceptors, in certain other retinal neurons, and in pineal glands [53]. It represents a family of neuron-specific Ca2+-binding proteins, the so called neuronal calcium sensors [15], containing Ca2+-binding sites of an EF-hand type. Four potential Ca2+-binding sites of such a type are present in the recoverin molecule of which only two (the second and the third) are capable of binding calcium ions [25]. In vitro recoverin can form a complex with rhodopsin kinase (GRK-1) [17, 28] thus inhibiting phosphorylation of the visual receptor rhodopsin in a Ca2+-dependent manner [41, 64, 65]. It has been confirmed recently that recoverin serves as a Ca2+-sensor of rhodopsin phosphorylation under physiological conditions [50].
To date, many authors have described the presence of autoantibodies against recoverin in the sera of CAR patients with different types of malignancies located outside the nervous system as summarized in Table 1. The frequency of such cases is very low. Only about 25 cases have been described so far. The majority of the autoantibody-positive CAR cases have been detected in patients with small cell lung cancer, the most malignant lung tumor of neuroendocrine origin.
Table 1.
Published cases of patients with CAR and anti-recoverin autoantibodies
| Tumor type | Number of patients | Reference |
|---|---|---|
| Small cell lung cancer | 2 | [36] |
| 5 | [7] | |
| 1 | [58] | |
| 1 | [14] | |
| 1 | [37] | |
| 1 | [51] | |
| Non-small cell lung cancer | 1 | [62] |
| Endometrial carcinoma | 1 | [5] |
| 1 | [7] | |
| Ovarian carcinoma | 1 | [33] |
| Cervical cancer | 1 | [32] |
| Uterine sarcoma | 1 | [24] |
| 1 | [67] | |
| Breast cancer | 1 | [67] |
| 2 | [35] | |
| 1 | [7] | |
| Colon carcinoma | 1 | [7] |
| Skin squamous cell carcinoma | 1 | [7] |
| Invasive thymoma | 1 | [38] |
A rat model system has been established to elucidate the contribution of recoverin autoantibodies to the development of retinopathy [3, 26]. The injection of recoverin led to the induction of uveitis and retina degeneration. In a guinea pig model, animals were sensitized with small cell lung cancer cell lines that induced production of anti-recoverin autoantibodies in the animals and caused retinopathy in them [68]. Based on the models described, it was concluded that autoantibodies against recoverin can be a principle cause of retina degeneration in patients with CAR.
How can autoantibodies reach the retinal cells and how do they induce cell death? The first hurdle is the blood–retina barrier (see also Fig. 1). It is still unclear why this barrier does not prevent autoantibodies from penetrating into the retina. But function or malfunction of the blood–ocular barrier may account for the low frequency of CAR (and possibly also MAR). Even when this first obstacle is overcome, autoantibodies have to enter the cell, as recoverin is located intracellularly. This issue was addressed by experiments incubating immortalized rat retinal cells with sera either from CAR patients or from animals immunized with recoverin [4]. It could be shown that the autoantibodies were internalized by the cells and produced their destruction in a dose-dependent and time-dependent manner. The authors suggested that internalization may occur through nonspecific endocytosis, as specific and unspecific IgGs were taken up similarly. The observed cell death was characterized by apoptotic features, which is in line with experiments of the same group showing induction of apoptosis in photoreceptor and bipolar cells in vivo by antibodies against recoverin [6].
The next question is as to how these internalized autoantibodies can account for apoptosis. Adamus and colleagues speculate in the same paper [6] that blocking of intracellular recoverin by the antibodies may lead to an increase in cytoplasmic free calcium and subsequently to the activation of a nuclear endonuclease, which finally results in DNA fragmentation. This assumption is supported by the observation that conformational changes of recoverin induced by calcium enhance binding of antibodies to the sequence within residues 64–70 of the second calcium-binding domain which is found to be the major antigenic region of recoverin molecule [1, 2].
Recoverin autoantibodies can be detected in CAR patients and can induce photoreceptor apoptosis. But is the autoantibody response against recoverin indeed triggered by recoverin expressed in the tumor cells? In fact, it has been shown that tumor samples and one cell line from CAR patients are recoverin positive [52, 58, 68].
Recoverin as a paraneoplastic antigen without PNS
From the above one might assume that autoantibodies against recoverin should be detected only in CAR patients. But this is not the case! Many PNA have the capacity to induce low titer autoantibodies without any signs of paraneoplastic syndromes as, for example, those described for the Hu-antigen [18, 29]. In the process of raising antibodies against recoverin, we found that immunized rabbits developed an immune response to recoverin at variable titers. Investigations of the eye bottoms and light microscopy of retina slices detected retinal degeneration in rabbits with high titers of the antibody, while the eye characteristics of rabbits with low titers did not differ from those of control animals [9]. These findings stimulated us to screen serum samples of patients with lung cancers irrespective of the presence of CAR. Indeed, we have revealed 15 patients with SCLC of the 99 individuals investigated (15%) and 9 of 44 patients with non-small cell lung cancers (20%) with relatively low titers of the autoantibodies in their sera, but without the manifestation of CAR at the time of the serum sampling [8, 11]. Thus, we have concluded that autoantibodies against recoverin can be detected in sera of patients with lung cancer without manifestation of paraneoplastic syndrome.
What is the reason for the development of retinopathy in some patients with autoantibodies against PNA but not in others? One can suggest the following explanations: (1) To overcome the blood–ocular barrier, the titer of the autoantibodies in the blood stream of patients should exceed a certain level. Otherwise the autoantibodies cannot penetrate into the cells, get the corresponding intracellular antigen and initiate apoptosis of the cells. The same might be true for the hemato–encephalic barrier in case of other PNS. (2) The PNS development might depend on the actual epitopes recognized by the autoantibodies. In the case of recoverin, autoantibodies from CAR patients mostly bind to residues 64–70 representing the EF-hand 2 domain and initiate apoptosis by blocking Ca2+-binding [2, 58]. It would be interesting to identify the recoverin epitopes against which the autoantibodies in patients without CAR are directed. (3) The manifestation of CAR might well depend on a second event allowing the transmissibility of the blood–ocular barrier.
Recoverin is a cancer-retina antigen
Investigation of recoverin expression in cancer tissues and cell lines irrespective of the CAR-syndrome was started by Maeda et al. [46]. The authors analyzed 33 cell lines of various types of cancer and detected recoverin expression in about half of them (Table 2). Our own studies revealed recoverin expression in 68% of small cell lung cancers and in 85% of non-small cell lung cancers [11], which is more frequent than many other tumor-associated antigens [23]. Furthermore, we detected recoverin expression in tumors and/or cell lines of melanoma, head and neck cancer as well as in colon cancer cell lines (Bazhin et al. in preparation; Table 2).
Table 2.
Recoverin expression in tumors and cell lines (positive/total)
| mRNA | Protein | ||
|---|---|---|---|
| Small cell lung carcinoma | Cell lines |
0/7a 1/4e |
0/16a 1/4e |
| Non-small cell lung carcinoma | Cell lines | 0/2a | 0/9a |
| Tissues | 25/29b | ||
| Lung adenocarcinoma | Cell lines |
0/1a 2/5e |
0/3a 0/5e |
| Tissues | 9/11b | ||
| Lung cancer (without typing) | Cell lines | 10/14c | |
| Tissues | 18/27c | ||
| Melanoma | Cell lines | 20/32d | 11/21d |
| Tissues | 37/47d | 11/30d | |
| Endometrium adenocarcinoma | Cell lines | 3/3e | 2/3e |
| Breast adenocarcinoma | Cell lines |
9/12c 3/3e |
3/3e |
| Tissues | 8/14c | ||
| Pancreas adenocarcinoma | Cell lines | 0/3e | 0/3e |
| Cervix adenocarcinoma | Cell lines | 2/2e | 2/2e |
| Squamous cell carcinoma of the cervix | Cell lines | 1/2e | 1/2e |
| Squamous cell carcinoma of the oral cavity | Cell lines | 3/3e | 3/3e |
| Squamous cell carcinoma of the esophagus | Cell lines | 1/1e | 1/1e |
| Squamous cell carcinoma of the stomach | Cell lines | 3/3e | 2/3e |
| Colon (without typing) | Cell lines | 1/5d | |
| Squamous cell carcinoma of head and neck | Tissues | 0/5d | |
| Leukemia | Cell lines |
0/3d 0/1e |
0/1e |
| B-cell lymphoma | Cell lines | 1/1e | 1/1e |
Thus, in addition to the retina, recoverin can also be aberrantly expressed in various cancer cells at high frequencies. Its aberrant expression can induce autoantibody and T cell responses [47]. Importantly, recoverin is not the only photoreceptor protein with these characteristics. Recently, we detected rhodopsin and arrestin in some melanoma cells and found autoantibodies in the serum of melanoma patients against these proteins, though these patients did not suffer from any paraneoplastic retinal degenerations [34].
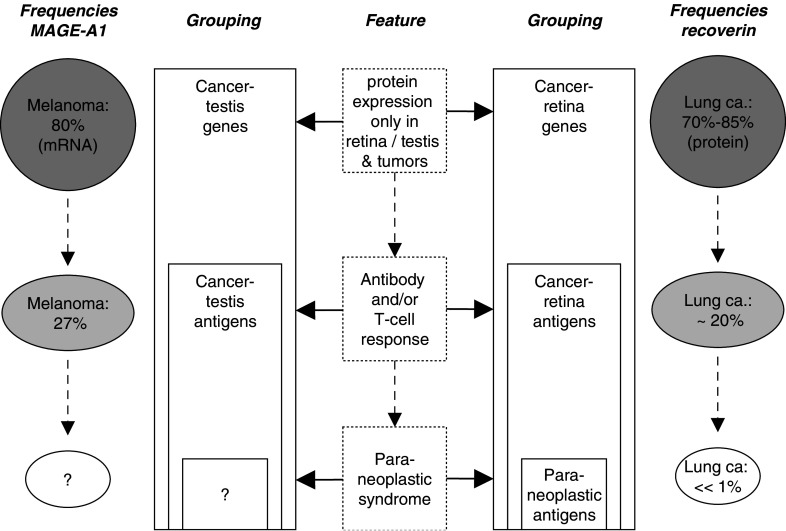
Therefore, by analogy with cancer-testis antigens [56], we suggest the expression cancer-retina antigens for this type of proteins for the following reasons (Fig. 3): (1) Normal expression is restricted to immunoprivileged tissues (retina/testis), (2) proteins are aberrantly expressed in tumor cells, (3) and show subsequently high antigenicity (autoantibodies and/or T cells); (4) the term “cancer-retina antigens” does not reduce this kind of proteins to the rare events of CAR or MAR as does “paraneoplastic antigens”. Interestingly, there is evidence that the aberrant expression of both, cancer-testis antigens and cancer-retina antigens (at least recoverin), might be due to promoter demethylation [10, 20].
Fig. 3.
Comparison of cancer-testis antigens and cancer-retina antigens and the features characterizing these groups. Frequencies of expressions of and responses from MAGE-A1 and recoverin are taken from [11] and [72], respectively
Does recoverin have a function in tumor cells?
It is known that in photoreceptor cells, recoverin serves as a Ca2+-dependent regulator of rhodopsin kinase (GRK 1) [66]. Thus, one may speculate that in tumor cells, recoverin might also be responsible for regulation of certain GRKs. Miyagawa et al. [55] have shown that recoverin can functionally be associated with GRKs through calveolin-1 in cancer cell lines. If so, recoverin may play a role in tumor progression, metastasis and drug resistance Also, Maeda et al. transfected the lung adenocarcinoma cell line A549 with recoverin cDNA and showed a reduction in cell proliferation [48]. However, it cannot be excluded that the aberrant expression of recoverin is merely a consequence of disturbance in regulation of gene expression in cancer cells and thus has no function at all in these. In any case further investigations are needed to answer these questions.
Could recoverin be suitable for cancer diagnosis and therapy?
The presence of autoantibodies against recoverin in the serum of lung cancer patients is highly specific (98%), but only moderately sensitive (20%) [11]. This makes recoverin autoantibodies unattractive for consideration as a single markers. Although considering that the autoantibodies appear well before the cancer can be diagnosed by the usual diagnostic techniques, these could be used in combination with other markers of cancer. The recoverin protein is expressed at rather high frequencies in lung cancer, ranging from 68 to 85% depending on the tumor subtype [11]. In non-small cell lung cancer, the grade of differentiation is inversely correlated to the frequency of recoverin positive tumor cells. This qualifies recoverin as a possible prognostic marker, which should be further analyzed.
It was suggested that SCLC patients with CAR have a better prognosis than those without CAR [19]. Maeda et al. hypothesized that this benefit might be due to the presence of recoverin-specific cytotoxic T lymphocytes (CTL) [47], which could attack the tumor cells (see also Fig. 1). In fact, they described recoverin-specific CTL in two HLA-A24+ CAR-positive patients and in three of ten CAR-negative patients, but unfortunately also in two of six healthy individuals. The CTL precursor frequency of CAR-positive patients and that of CAR-negative patients was higher than that of healthy donors. Moreover, the authors identified three recoverin peptides which can induce peptide-specific CTL. Rong et al. [61] used tetramers with HLA-A24 dependent peptides of recoverin and showed recoverin-specific CTL precursors in colon, stomach, breast cancer patients and in some healthy individuals. The tetramer analysis had a good correlation with cytotoxic response.
In an experimental in BALB/c mouse model Maeda et al. could induce specific CTLs in response to the peptide R64 (AYQHVFRSF) and observed a tumor-preventive effect by the vaccination [49]. Though, this peptide also induced the generation of antibodies and retinal degenerations.
Thus, recoverin can be targeted both by antibodies and T cells. The circumstances which do or do not lead subsequently to a PNS still have to be clarified before recoverin can be considered as a target in therapy.
Conclusions
The expression and antigenicity of recoverin distinguish this molecule as a cancer-retina antigen. Immune responses against recoverin can have beneficial effects for the tumor patient, but might also induce paraneoplastic syndromes. The induction of the latter seems to depend on the presence of autoantibodies directed against a certain functional domain of the molecule. The known function of recoverin in photoreceptor cells did not give any hints as to its function in tumor cells, though an involvement in proliferation might be speculated. From the therapeutic point of view, it seems to be pivotal to unravel differences in antibody responses against different epitopes of the protein. The proper selection of specificities might be responsible for whether or not CAR/MAR is induced during tumor defense via recoverin.
Acknowledgements
This work was supported in parts by grants from the Cancer Research Institute/Elaine R. Shepard Memorial Investigator Award to S.B.E., the Ludwig Institute for Cancer Research and the Russian Foundation for Basic Research NN 04-04-48438 to P.P.Ph.
Abbreviations
- CAR
Cancer-associated retinopathy
- CTL
Cytotoxic T cell(s)
- MAR
Melanoma-associated retinopathy
- PNA
Paraneoplastic antigen(s)
- PNS
Paraneoplastic neurological syndrome(s)
- SCLC
Small cell lung cancer
Footnotes
This article is a symposium paper from the conference “Progress in Vaccination against Cancer 2005 (PIVAC 5)”, held in Athens, Greece, on 20–21 September 2005.
References
- 1.Adamus G, Amundson D. Epitope recognition of recoverin in cancer associated retinopathy: evidence for calcium-dependent conformational epitopes. J Neurosci Res. 1996;45:863–872. doi: 10.1002/(SICI)1097-4547(19960915)45:6<863::AID-JNR23>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Adamus G, Guy J, Schmied JL, Arendt A, Hargrave PA. Role of anti-recoverin autoantibodies in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:2626–2633. [PubMed] [Google Scholar]
- 3.Adamus G, Ortega H, Witkowska D, Polans A. Recoverin: a potent uveitogen for the induction of photoreceptor degeneration in Lewis rats. Exp Eye Res. 1994;59:447–455. doi: 10.1006/exer.1994.1130. [DOI] [PubMed] [Google Scholar]
- 4.Adamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1997;38:283–291. [PubMed] [Google Scholar]
- 5.Adamus G, Amundson D, MacKay C, Gouras P. Long-term persistence of antirecoverin antibodies in endometrial cancer-associated retinopathy. Arch Ophthalmol. 1998;116:251–253. [PubMed] [Google Scholar]
- 6.Adamus G, Machnicki M, Elerding H, Sugden B, Blocker YS, Fox DA. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun. 1998;11:523–533. doi: 10.1006/jaut.1998.0221. [DOI] [PubMed] [Google Scholar]
- 7.Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4:5. doi: 10.1186/1471-2415-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazhin AV, Shifrina ON, Savchenko MS, Tikhomirova NK, Goncharskaia MA, Gorbunova VA, Senin II, Chuchalin AG, Philippov PP. Low titre autoantibodies against recoverin in sera of patients with small cell lung cancer but without a loss of vision. Lung Cancer. 2001;34:99–104. doi: 10.1016/S0169-5002(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 9.Bazhin AV, Slepova OS, Tikhomirova NK. Retinal degeneration under the effect of antibodies to recoverin. Bull Exp Biol Med. 2001;131:350–352. doi: 10.1023/A:1017952102887. [DOI] [PubMed] [Google Scholar]
- 10.Bazhin AV, Savchenko MS, Belousov EV, Jaques G, Philippov PP. Stimulation of the aberrant expression of a paraneoplastic antigen, recoverin, in small cell lung cancer cell lines. Lung Cancer. 2004;45:299–305. doi: 10.1016/j.lungcan.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Bazhin AV, Savchenko MS, Shifrina ON, Demoura SA, Chikina SY, Jaques G, Kogan EA, Chuchalin AG, Philippov PP. Recoverin as a paraneoplastic antigen in lung cancer: the occurrence of anti-recoverin autoantibodies in sera and recoverin in tumors. Lung Cancer. 2004;44:193–198. doi: 10.1016/j.lungcan.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Boeck K, Hofmann S, Klopfer M, Ian U, Schmidt T, Engst R, Thirkill CE, Ring J. Melanoma-associated paraneoplastic retinopathy: case report and review of the literature. Br J Dermatol. 1997;137:457–460. doi: 10.1046/j.1365-2133.1997.18701958.x. [DOI] [PubMed] [Google Scholar]
- 13.Borkowski LM, Grover S, Fishman GA, Jampol LM. Retinal findings in melanoma-associated retinopathy. Am J Ophthalmol. 2001;132:273–275. doi: 10.1016/S0002-9394(01)00915-1. [DOI] [PubMed] [Google Scholar]
- 14.Boucher MC, Allaire GS. Cancer-associated retinopathy: a clinicopathological case report. Can J Ophthalmol. 1997;32:46–49. [PubMed] [Google Scholar]
- 15.Braunewell KH, Gundelfinger ED. Intracellular neuronal calcium sensor proteins: a family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res. 1999;295:1–12. doi: 10.1007/s004410051207. [DOI] [PubMed] [Google Scholar]
- 16.Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48:12–38. doi: 10.1016/S0039-6257(02)00416-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- 18.Dalmau J, Furneaux HM, Gralla RJ, Kris MG, Posner JB. Detection of the anti-Hu antibody in the serum of patients with small cell lung cancer—a quantitative western blot analysis. Ann Neurol. 1990;27:544–552. doi: 10.1002/ana.410270515. [DOI] [PubMed] [Google Scholar]
- 19.Darnell RB, DeAngelis LM. Regression of small-cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet. 1993;341:21–22. doi: 10.1016/0140-6736(93)92485-C. [DOI] [PubMed] [Google Scholar]
- 20.De Smet C, De Backer O, Faraoni I, Lurquin C, Brasseur F, Boon T. The activation of human gene MAGE-1 in tumor cells is correlated with genome-wide demethylation. Proc Natl Acad Sci USA. 1996;93:7149–7153. doi: 10.1073/pnas.93.14.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dizhoor AM, Nekrasova ER, Philippov PP. A novel photoreceptor cell-specific protein with molecular weight 26 kDa capable of binding to immobilized delipidated rhodopsin. Biokhimiya. 1991;56:225–229. [Google Scholar]
- 22.Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- 23.Eichmüller S, Usener D, Jochim A, Schadendorf D. mRNA expression of tumor-associated antigens in melanoma tissues and cell lines. Exp Dermatol. 2002;11:292–301. doi: 10.1034/j.1600-0625.2002.110402.x. [DOI] [PubMed] [Google Scholar]
- 24.Eltabbakh GH, Hoogerland DL, Kay MC. Paraneoplastic retinopathy associated with uterine sarcoma. Gynecol Oncol. 1995;58:120–123. doi: 10.1006/gyno.1995.1194. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty KM, Zozulya S, Stryer L, McKay DB. Three-dimensional structure of recoverin, a calcium sensor in vision. Cell. 1993;75:709–716. doi: 10.1016/0092-8674(93)90491-8. [DOI] [PubMed] [Google Scholar]
- 26.Gery I, Chanaud NP, III, Anglade E. Recoverin is highly uveitogenic in Lewis rats. Invest Ophthalmol Vis Sci. 1994;35:3342–3345. [PubMed] [Google Scholar]
- 27.Gittinger JW, Jr, Smith TW. Cutaneous melanoma-associated paraneoplastic retinopathy: histopathologic observations. Am J Ophthalmol. 1999;127:612–614. doi: 10.1016/S0002-9394(98)00431-0. [DOI] [PubMed] [Google Scholar]
- 28.Gorodovikova EN, Philippov PP. The presence of a calcium-sensitive p26-containing complex in bovine retina rod cells. FEBS Lett. 1993;335:277–279. doi: 10.1016/0014-5793(93)80746-H. [DOI] [PubMed] [Google Scholar]
- 29.Graus F, Elkon KB, Cordon-Cardo C, Posner JB. Sensory neuronopathy and small cell lung cancer. Antineuronal antibody that also reacts with the tumor. Am J Med. 1986;80:45–52. doi: 10.1016/0002-9343(86)90047-1. [DOI] [PubMed] [Google Scholar]
- 30.Grunwald GB, Kornguth SE, Towfighi J, Sassani J, Simmonds MA, Housman CM, Papadopoulos N. Autoimmune basis for visual paraneoplastic syndrome in patients with small cell lung carcinoma. Retinal immune deposits and ablation of retinal ganglion cells. Cancer. 1987;60:780–786. doi: 10.1002/1097-0142(19870815)60:4<780::AID-CNCR2820600413>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Gure AO, Stockert E, Scanlan MJ, Keresztes RS, Jager D, Altorki NK, Old LJ, Chen YT. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA. 2000;97:4198–4203. doi: 10.1073/pnas.97.8.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol. 1999;117:471–477. doi: 10.1001/archopht.117.4.471. [DOI] [PubMed] [Google Scholar]
- 33.Harmon JP, Purvin VA, Guy J, Aptsiauri N, Sutton GP. Cancer-associated retinopathy in a patient with advanced epithelial ovarian carcinoma. Gynecol Oncol. 1999;73:430–432. doi: 10.1006/gyno.1999.5342. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann TB, Bazhin AV, Schadendorf D, Eichmüller SB. SEREX identification of new tumor antigens linked to melanoma-associated retinopathy. Int J Cancer. 2005;114:88–93. doi: 10.1002/ijc.20762. [DOI] [PubMed] [Google Scholar]
- 35.Huober J, Holz FG, Schmid H, Nolle B, Bellmann C, Krastel H, Wallwiener D, Bastert G. Paraneoplastic retinopathy in 2 patients with breast carcinoma. Zentralbl Gynakol. 1997;119:278–281. [PubMed] [Google Scholar]
- 36.Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28:162–167. doi: 10.1002/ana.410280208. [DOI] [PubMed] [Google Scholar]
- 37.Kashiwabara K, Nakamura H, Kishi K, Yagyu H, Sarashina G, Kobayashi K, Matsuoka T. Cancer-associated retinopathy during treatment for small-cell lung carcinoma. Intern Med. 1999;38:597–601. doi: 10.2169/internalmedicine.38.597. [DOI] [PubMed] [Google Scholar]
- 38.Katsuta H, Okada M, Nakauchi T, Takahashi Y, Yamao S, Uchida S. Cancer-associated retinopathy associated with invasive thymoma. Am J Ophthalmol. 2002;134:383–389. doi: 10.1016/S0002-9394(02)01598-2. [DOI] [PubMed] [Google Scholar]
- 39.Keltner JL, Roth AM, Chang RS. Photoreceptor degeneration. Possible autoimmune disorder. Arch Ophthalmol. 1983;101:564–569. doi: 10.1001/archopht.1983.01040010564006. [DOI] [PubMed] [Google Scholar]
- 40.Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neuroophthalmol. 2001;21:173–187. doi: 10.1097/00041327-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Klenchin VA, Calvert PD, Bownds MD. Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J Biol Chem. 1995;270:16147–16152. doi: 10.1074/jbc.270.41.24127. [DOI] [PubMed] [Google Scholar]
- 42.Kornguth SE, Klein R, Appen R, Choate J. Occurrence of anti-retinal ganglion cell antibodies in patients with small cell carcinoma of the lung. Cancer. 1982;50:1289–1293. doi: 10.1002/1097-0142(19821001)50:7<1289::AID-CNCR2820500711>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Kornguth SE, Kalinke T, Grunwald GB, Schutta H, Dahl D. Anti-neurofilament antibodies in the sera of patients with small cell carcinoma of the lung and with visual paraneoplastic syndrome. Cancer Res. 1986;46:2588–2595. [PubMed] [Google Scholar]
- 44.Lambrecht HG, Koch KW. A 26 kd calcium binding protein from bovine rod outer segments as modulator of photoreceptor guanylate cyclase. EMBO J. 1991;10:793–798. doi: 10.1002/j.1460-2075.1991.tb08011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei B, Bush RA, Milam AH, Sieving PA. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41:262–266. [PubMed] [Google Scholar]
- 46.Maeda A, Ohguro H, Maeda T, Wada I, Sato N, Kuroki Y, Nakagawa T. Aberrant expression of photoreceptor-specific calcium-binding protein (recoverin) in cancer cell lines. Cancer Res. 2000;60:1914–1920. [PubMed] [Google Scholar]
- 47.Maeda A, Ohguro H, Nabeta Y, Hirohashi Y, Sahara H, Maeda T, Wada Y, Sato T, Yun C, Nishimura Y, Torigoe T, Kuroki Y, Sato N. Identification of human antitumor cytotoxic T lymphocytes epitopes of recoverin, a cancer-associated retinopathy antigen, possibly related with a better prognosis in a paraneoplastic syndrome. Eur J Immunol. 2001;31:563–572. doi: 10.1002/1521-4141(200102)31:2<563::AID-IMMU563>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Maeda T, Maeda A, Maruyama I, Ogawa KI, Kuroki Y, Sahara H, Sato N, Ohguro H. Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 2001;42:705–712. [PubMed] [Google Scholar]
- 49.Maeda A, Maeda T, Ohguro H, Palczewski K, Sato N. Vaccination with recoverin, a cancer-associated retinopathy antigen, induces autoimmune retinal dysfunction and tumor cell regression in mice. Eur J Immunol. 2002;32:2300–2307. doi: 10.1002/1521-4141(200208)32:8<2300::AID-IMMU2300>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 50.Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol. 2004;123:729–741. doi: 10.1085/jgp.200308994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masaoka N, Emoto Y, Sasaoka A, Fukushima A, Ueno H, Ohguro H. Fluorescein angiographic findings in a case of cancer-associated retinopathy. Retina. 1999;19:462–464. doi: 10.1097/00006982-199919050-00022. [DOI] [PubMed] [Google Scholar]
- 52.Matsubara S, Yamaji Y, Sato M, Fujita J, Takahara J. Expression of a photoreceptor protein, recoverin, as a cancer-associated retinopathy autoantigen in human lung cancer cell lines. Br J Cancer. 1996;74:1419–1422. doi: 10.1038/bjc.1996.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milam AH, Dacey DM, Dizhoor AM. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis Neurosci. 1993;10:1–12. doi: 10.1017/S0952523800003175. [DOI] [PubMed] [Google Scholar]
- 54.Milam AH, Saari JC, Jacobson SG, Lubinski WP, Feun LG, Alexander KR. Autoantibodies against retinal bipolar cells in cutaneous melanoma-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:91–100. [PubMed] [Google Scholar]
- 55.Miyagawa Y, Ohguro H, Odagiri H, Maruyama I, Maeda T, Maeda A, Sasaki M, Nakazawa M. Aberrantly expressed recoverin is functionally associated with G-protein-coupled receptor kinases in cancer cell lines. Biochem Biophys Res Commun. 2003;300:669–673. doi: 10.1016/S0006-291X(02)02888-7. [DOI] [PubMed] [Google Scholar]
- 56.Old LJ. Cancer/testis (CT) antigens—a new link between gametogenesis and cancer. Cancer Immun. 2001;1:1. [PubMed] [Google Scholar]
- 57.Polans AS, Buczylko J, Crabb J, Palczewski K. A photoreceptor calcium binding protein is recognized by autoantibodies obtained from patients with cancer-associated retinopathy. J Cell Biol. 1991;112:981–989. doi: 10.1083/jcb.112.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polans AS, Witkowska D, Haley TL, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci USA. 1995;92:9176–9180. doi: 10.1073/pnas.92.20.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Posner JB, Furneaux HM. Paraneoplastic syndromes. Res Publ Assoc Res Nerv Ment Dis. 1990;68:187–219. [PubMed] [Google Scholar]
- 60.Potter MJ, Adamus G, Szabo SM, Lee R, Mohaseb K, Behn D. Autoantibodies to transducin in a patient with melanoma-associated retinopathy. Am J Ophthalmol. 2002;134:128–130. doi: 10.1016/S0002-9394(02)01431-9. [DOI] [PubMed] [Google Scholar]
- 61.Rong S, Ikeda H, Sato Y, Hirohashi Y, Takamura Y, Sahara H, Sato T, Maeda A, Ohguro H, Sato N. Frequent detection of anti-recoverin cytotoxic T-lymphocyte precursors in peripheral blood of cancer patients by using an HLA-A24-recoverin tetramer. Cancer Immunol Immunother. 2002;51:282–290. doi: 10.1007/s00262-002-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salgia R, Hedges TR, Rizk M, Reimer RH, Skarin AT. Cancer-associated retinopathy in a patient with non-small-cell lung carcinoma. Lung Cancer. 1998;22:149–152. doi: 10.1016/S0169-5002(98)00062-2. [DOI] [PubMed] [Google Scholar]
- 63.Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81:606–613. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 64.Senin II, Zargarov AA, Alekseev AM, Gorodovikova EN, Lipkin VM, Philippov PP. N-myristoylation of recoverin enhances its efficiency as an inhibitor of rhodopsin kinase. FEBS Lett. 1995;376:87–90. doi: 10.1016/0014-5793(95)01187-2. [DOI] [PubMed] [Google Scholar]
- 65.Senin II, Dean KR, Zargarov AA, Akhtar M, Philippov PP. Recoverin inhibits the phosphorylation of dark-adapted rhodopsin more than it does that of bleached rhodopsin: a possible mechanism through which rhodopsin kinase is prevented from participation in a side reaction. Biochem J. 1997;321(Pt 2):551–555. doi: 10.1042/bj3210551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Senin II, Koch KW, Akhtar M, Philippov PP. Ca2+-dependent control of rhodopsin phosphorylation: recoverin and rhodopsin kinase. Adv Exp Med Biol. 2002;514:69–99. doi: 10.1007/978-1-4615-0121-3_5. [DOI] [PubMed] [Google Scholar]
- 67.Sobottka B, Schlote T, Besch D, Djelebova T, Wilhelm H, Zrenner E. Carcinoma-associated retinopathy: a review with clinical examples. Klin Monatsbl Augenheilkd. 2000;216:17–24. doi: 10.1055/s-2000-10511. [DOI] [PubMed] [Google Scholar]
- 68.Thirkill CE. Experimental, cancer-induced retinopathy. Ocul Immunol Inflamm. 1997;5:55–65. doi: 10.3109/09273949709085051. [DOI] [PubMed] [Google Scholar]
- 69.Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987;105:372–375. doi: 10.1001/archopht.1987.01060030092033. [DOI] [PubMed] [Google Scholar]
- 70.Thirkill CE, Roth AM, Takemoto DJ, Tyler NK, Keltner JL. Antibody indications of secondary and superimposed retinal hypersensitivity in retinitis pigmentosa. Am J Ophthalmol. 1991;112:132–137. doi: 10.1016/s0002-9394(14)76691-7. [DOI] [PubMed] [Google Scholar]
- 71.Thirkill CE, Tait RC, Tyler NK, Roth AM, Keltner JL. The cancer-associated retinopathy antigen is a recoverin-like protein. Invest Ophthalmol Vis Sci. 1992;33:2768–2772. [PubMed] [Google Scholar]
- 72.Usener D, Gerhardt A, Schadendorf D, Eichmüller S. Sero-reactivity against MAGE-A and LAGE-1 proteins in melanoma patients. Br J Dermatol. 2003;149:282–288. doi: 10.1046/j.1365-2133.2003.05410.x. [DOI] [PubMed] [Google Scholar]
- 73.Weinstein JM, Kelman SE, Bresnick GH, Kornguth SE. Paraneoplastic retinopathy associated with antiretinal bipolar cell antibodies in cutaneous malignant melanoma. Ophthalmology. 1994;101:1236–1243. doi: 10.1016/s0161-6420(94)31183-3. [DOI] [PubMed] [Google Scholar]