Abstract
Tumor associated macrophages (TAMs) are well known to play a very important role in tumor angiogenesis and metastasis. The suppression of TAMs in the tumor-microenvironment (TME) provides a novel strategy to inhibit tumor growth and dissemination by remodeling the tumor’s stroma. Here, we tested our hypothesis that suppression of TAMs can be achieved in syngeneic BALB/c mice with oral minigene vaccines against murine MHC class I antigen epitopes of Legumain, an asparaginyl endopeptidase and a member of the C13 family of cystine proteases which is overexpressed on TAMs in the tumor stroma. Vaccine vectors were constructed and transformed into attenuated Salmonella typhimurium (Dam − , AroA −) for oral delivery. Groups of mice received either the expression vectors encoding the Legumain H-2D or 2K epitopes or the control empty vector by gavage. The efficacy of the minigene vaccines was determined by their ability to protect mice from lethal tumor cell challenges, the induction of a specific CTL response as well as IFN-γ release, and inhibition of tumor angiogenesis. We demonstrated that the Legumain minigene vaccine provided effective protection against tumor cell challenge by inducing a specific CD8+ T-cell response against Legumain+ TAMs in our breast tumor model. The protection, induced by this T-cell response, mediated by the Legumain Kd minigene, is also responsible for lysing D2F2 breast carcinoma cells in syngeneic BALB/c mice and for suppressing tumor angiogenesis. Importantly, in a prophylactic setting, the minigene vaccine proved to be of similar anti-tumor efficacy as a vaccine encoding the entire Legumain gene. Together, our findings establish proof of concept that a Legumain minigene vaccine provides a more flexible alternative to the whole gene vaccine, which may facilitate the future design and clinical applications of such a vaccine for cancer prevention.
Keywords: Legumain, Minigene vaccine, Tumor associated macrophages, Anti-angiogenesis, CTLs
Introduction
We were first to previously report on a novel anti-cancer strategy, establishing that the attenuation of tumor-associated macrophages (TAMs) in the tumor stroma could effectively remodel the tumor microenvironment (TME) involved in tumor progression and angiogenesis to markedly suppress tumor growth and metastasis induced by an oral DNA vaccine encoding the entire murine Legumain gene [1]. This vaccine produced these anti-tumor effects by inducing a cytotoxic CD8+ T-cell-mediated immune response, which specifically deleted and killed Legumain-positive macrophages, i.e., TAMs infiltrating the tumor stroma during tumor growth and progression. Abrogation of such TAMs markedly reduced the release from these cells of tumor growth factors, pro-angiogenesis factors and MMP-9 all of which play key roles during the formation of the tumor vasculature and initiation of tumor angiogenesis.
The selection of Legumain as a target for this DNA vaccine is based on the fact that the gene encoding this asparaginyl endopeptidase was found to be highly up-regulated in many murine and human tumor tissues, but absent or only present at very low levels in all normal tissues from which such tumors arose [2–4]. Furthermore, we recently found that Legumain is heavily overexpressed by TAMs in murine tumor stroma by using gene expression profiling and immunohistochemical staining [1]. Therefore, Legumain provides a most suitable target for a DNA minigene vaccine to induce a specific CTL response attacking TAMs.
The minigene vaccine strategy induces a specific immune response by CD8+ T-cells which recognizes antigens as 8–10 amino acid long peptides that are presented to T-cell receptors on the cell surface as complexes with major histocompatibility complex (MHC) class I antigens. These peptides, usually referred to as CTL epitopes, are generated in the cytosol of cells after proteolytic processing of antigen by the proteasome. The application of minigene vaccines provides an attractive approach because of their ease of synthesis and manipulation. Importantly, in contrast to vaccines encoding entire genes, minigene vaccines induce immune responses directed against specific antigen epitopes while avoiding the interference of irrelevant antigen epitopes, which can potentially cause serious side effects. In this regard, the minigene strategy has been reported to induce an effective T-cell mediated anti-tumor immune response in our previous studies of other tumor-associated antigens. [5, 6] Furthermore, the molecularly defined CTL epitopes of minigene vaccines facilitate further in depth mechanistic studies at the molecular level. Here, we used a similar strategy, which specifically targeted TAMs in the tumor stroma and demonstrated that a Legumain-based minigene vaccine provides a flexible alternative to a whole gene vaccine. This approach might facilitate the improved future design and clinical application of such minigene vaccine for breast cancer therapy and prevention.
Materials and methods
Animals, bacterial strains, and cell lines
Female BALB/c mice 6–8 week of age were purchased from The Scripps Institute Rodent Breeding Facility. All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The murine D2F2 breast cancer cell line was kindly provided by Dr. W-Z. Wei (Karmanos Cancer Institute, Detroit, MI) while 4T1 breast carcinoma cells were a gift from Dr. Suzanne Ostrand-Rosenberg (University of Maryland, Baltimore, MD) The murine Macrophage cell line (RAW 309 Cr.1) was purchased from ATCC. The doubly attenuated S. typhimurium AroA − and dam − strain RE88 was originally provided by Remedyne Corporation (Santa Barbara, CA) and is now established in our laboratory. These attenuated bacteria were transduced with the DNA vaccine plasmids encoding the Legumain minigenes and served as vaccine carriers as previously described [7–9].
Construction of expression vectors
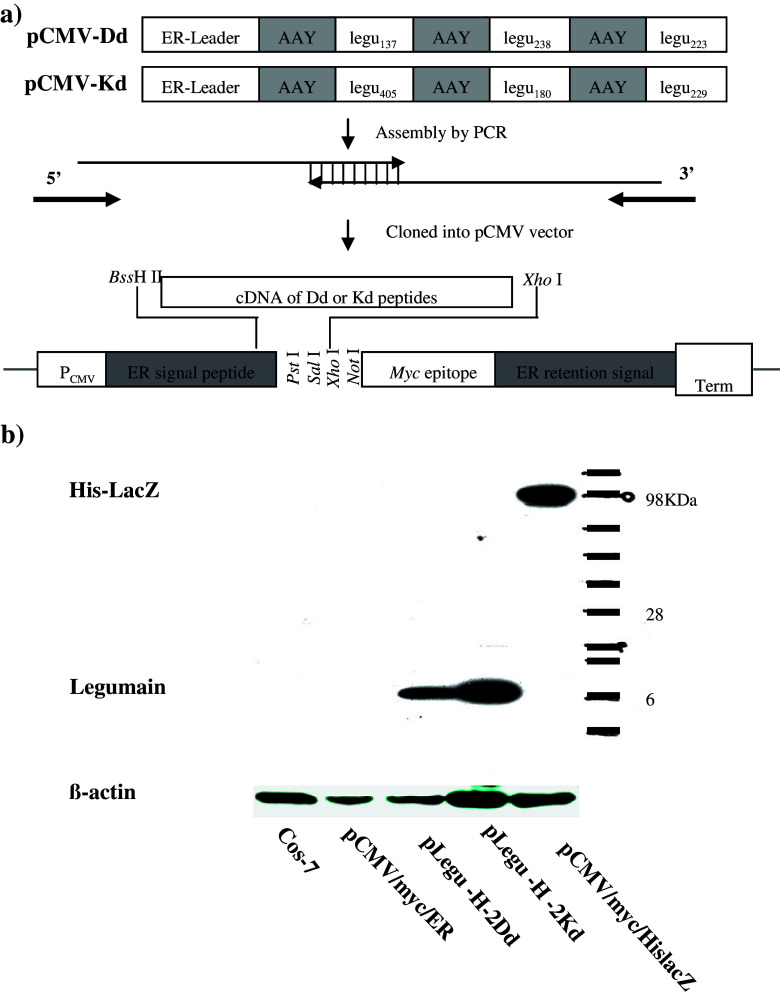
Vector construction is illustrated schematically by Fig. 1a. The expression vectors were established based on the pCMV/Myc/ER vector (Invitrogen, Carlsbad, CA). The peptides were cloned, and the sequence of each peptide is indicated in Table 1. All peptides were engineered to be in-frame with the myc epitope. All constructs were confirmed by DNA sequencing at The Scripps Research Institute’s Core Facility (La Jolla, CA). Peptide expression was demonstrated by Western blotting analysis of transfected COS-7 cells with monoclonal anti-myc antibody (Invitrogen). Once peptide expression was verified, a stop codon was introduced immediately in front of the myc epitope sequences. The resulting vectors, pLegu-H-2Dd and pLegu-H-2Kd were each verified by nucleotide sequencing and used to transform doubly attenuated S. typhimurium (dam − , AroA −) for subsequent immunization.
Fig. 1.
Construction and expression of DNA minigene vaccines against Legumain. (a) Schematic map. Minigenes encoding the murine H-2 Dd-/Kd -restricted Legumain nonapeptides and AAY spacers were assembled by PCR with overlapping oligonucleotides as templates. The sequence of each nonapeptide is shown in Table 1. The PCR fragments generated were cloned in a modified pcDNA expression vector by using Xho I and BassH II restriction sites. (b) Proteins encoded by minigenes were expressed in mammalian cells. To this end, COS-7 cells were transfected with either pLegu-H-2Dd-myc or pLegu-H-2Kd-myc for 24 h, harvested, lysed and analyzed by Western blotting with anti-myc monoclonal antibody
Table 1.
Prediction of legumain MHC class I antigen binding peptides
| H-2D/K | Epitope | Position | Score | Antigen Index for the peptides they usea |
|---|---|---|---|---|
| H-2Db/H-2Dd | Db1 or Dd1: SGPRDHVFI | 137 | 9/240 | 1.7 |
| Db2 or Dd2: LPPVTHLDL | 238 | 7/20 | 1.7 | |
| Db3 or Dd3: YDEERGTYL | 223 | –/6 | 1.7 | |
| H-2Kb/H-2Kd | Kb1 or Kd1: RYLYVLANL | 405 | 1/4,800 | 0.4 |
| Kb2 or Kd2: MYQKMVFYI | 180 | 1/2,400 | 0.8 | |
| Kb3 or Kd3: TYLGDWYSV | 229 | –/1,200 | 1.7 |
aAntigegnic Index was predicted by using DNAStar software
Oral immunization and tumor cell challenge
Groups of BALB/c mice (n = 8) were immunized three times at 1-week intervals by gavage with 100 μl PBS containing approximately 5 × 108 CFU of doubly mutated S. typhimurium harboring either empty vector, pLegu-H-2Dd or pLegu-H-2Kd plasmids. Mice were challenged i.v. or s.c. with the D2F2 carcinoma cell line 2 week after the last immunization.
Cytotoxicity assay
Cytotoxicity was measured by a standard 51Cr-release assay as previously described. [10] The percentage of specific target cell lysis was calculated by the formula
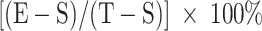 , where E is the average experimental release, S the average spontaneous release, and T the average total release. The splenocytes that isolated from immunized BALB/c mice were used as effector cells. Cells from the RAW murine macrophage cells line, which were previously cultured with IL-4, IL-10 and IL-13 cytokines to induce the expression of Legumain, were used as target cells.
, where E is the average experimental release, S the average spontaneous release, and T the average total release. The splenocytes that isolated from immunized BALB/c mice were used as effector cells. Cells from the RAW murine macrophage cells line, which were previously cultured with IL-4, IL-10 and IL-13 cytokines to induce the expression of Legumain, were used as target cells.
ELISPOT assay
Splenocytes were collected 2 weeks after D2F2 tumor cell challenge from all experimental groups of BALB/c mice, and cultured for 24 h with either irradiated (1000 Gy) 4T1 cells or 4T1 cells obtained from freshly harvested 4T1 breast tumor tissue. The assay was done according to instructions provided by the manufacturer (BD Bioscience, San Jose. CA).
Evaluation of anti-angiogenic activity
Suppression of angiogenesis was determined by the Matrigel assay as previously described [11]. Vessel growth in the Matrigel was determined by measuring the concentration of Hemoglobin using DRABKIN’S reagent. Matrigel plugs were removed 6 days after Matrigel implantation, fixed in Bouin’s solution for 24 h, and embedded in paraffin. All tissues were sectioned, mounted onto slides and stained with Masson’s trichrome. All images were captured by the SPOT Cooled Color digital camera system (Diagnostic Instruments Inc.).
Statistical analysis
The statistical significance of differential findings between experimental groups and controls was determined by Student’s t-test. Findings were regarded as significant, if two-tailed P values were <0.05.
Results
Minigenes encoded by expression vectors are expressed in mammalian cells
We previously demonstrated that a DNA vaccine encoding the entire murine Legumain gene effectively induced CD8+ T-cell-mediated anti-angiogenesis that protected mice from tumor cell challenges [1]. Here, using a minigene vaccine approach in syngeneic BALB/c mice, we attempt to further establish proof of concept for effective anti-TAM minigene vaccines with defined CTL epitopes, which down-regulate TAMs in the TME and suppress both, tumor growth and angiogenesis. To this end, six legumain peptides were evaluated as H-2 Dd-1, 2, 3, or H-2 Kd-1, 2, 3-restricted minigenes based on the binding predictions for these MHC class I antigen molecules by the HLA Peptide Binding Predictions program provided by the BioInformatics & Molecular Analysis Section (BIMAS) of NIH at their website: http://bimas.dcrt.nih.gov/molbio/hla_bind/. The amino acid sequences of these peptides and their binding activities are listed in Table 1 as predicted by DNAStar software (DNAStar. Inc. Madison, WI).
Expression vectors were constructed based on the backbone of pCMV/myc/ER (Fig. 1a). Gene expression of the resulting plasmids was verified by Western blotting analysis of COS-7 cells transfected with pLegu-H-2Dd (Dd-1, Dd-2 and Dd-3)-myc, or pLegu-H-2Kd (Kd-1, Kd-2 and Kd-3)-myc, respectively. As expected, COS-7 cells transfected with all plasmids displayed one major band of approximately 10 kDa (Fig. 1b). The vaccine vectors pLegu-H-2Dd and pLegu-H-2Kd were generated by introducing a stop codon immediately downstream from the peptide coding sequences, so that the translated protein would not contain the myc epitope. The correct constructs were confirmed by DNA sequencing. The empty pcDNA vector was also included for control purposes.
The pLegu-H-2Kd Minigene vaccine protects mice against D2F2 breast tumor cell challenge and prevents pulmonary metastasis
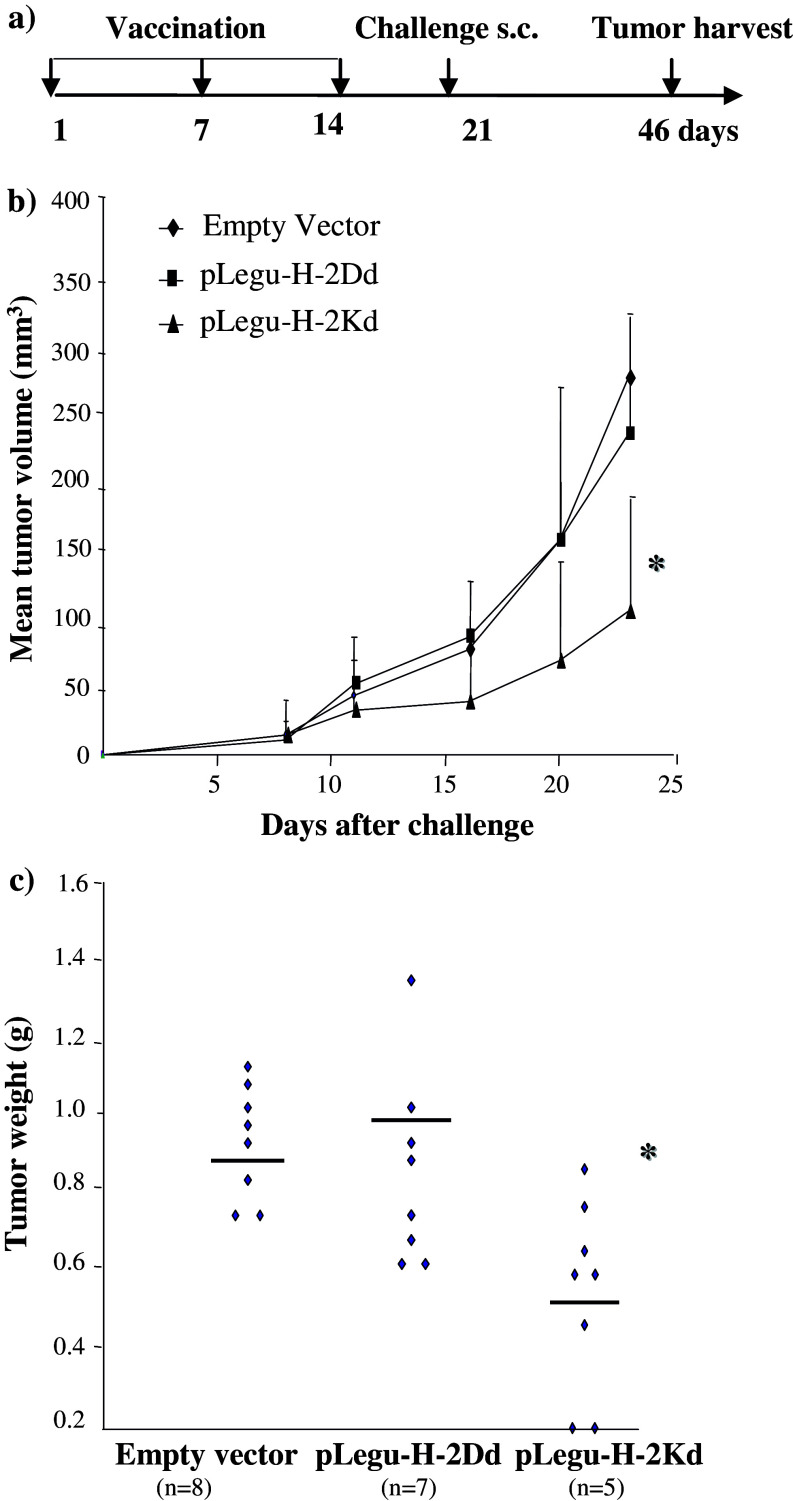
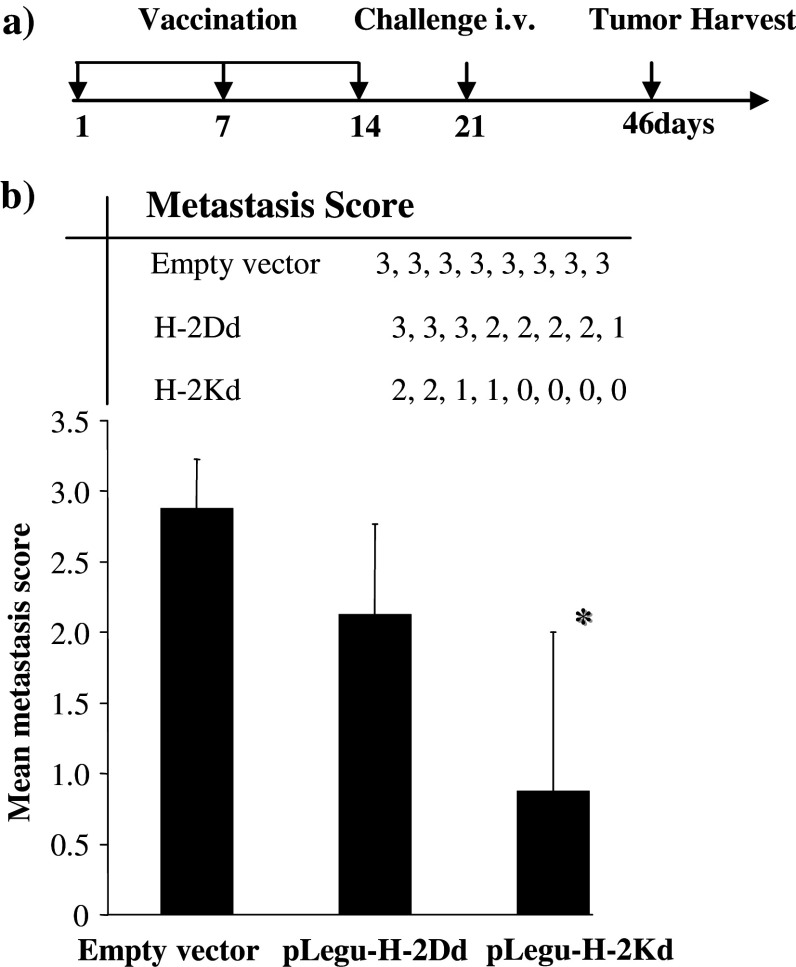
Initially, we tested the minigene vaccines in a prophylactic breast carcinoma model, where mice were first vaccinated with this vaccine and subsequently challenged s.c. with murine D2F2 breast carcinoma cells (Fig. 2). We then observed a marked inhibition of tumor growth in syngeneic BALB/c mice vaccinated with pLegu-H-2Kd, but not with pLegu-H-2Dd. In contrast, all mice vaccinated with only the empty vector control revealed rapid s.c. tumor growth. Furthermore, we demonstrated that the Legumain minigene vaccines could indeed suppress and/or inhibit pulmonary D2F2 metastasis by ablating TAMs, coupled with a marked inhibition of experimental metastases in BALB/c mice that were challenged by i.v. injection of D2F2 breast carcinoma cells 2 weeks after the third vaccination with the pLegu-H-2Kd, but not with the pLegu-H-2Dd minigene vaccine. In contrast, mice vaccinated with only the empty vector control revealed uniform, rapid metastatic pulmonary tumor growth (Fig. 3).
Fig. 2.
The pLegu-H-2Kd minigene vaccine protects mice from challenge with D2F2 breast carcinoma cells. Groups of BALB/c mice (n = 8) were immunized three times at 1-week intervals by gavage with 108 doubly attenuated Salmonella typhimurium RE-88 harboring the vectors indicated. Mice were challenged 1 week after the last immunization by s.c. injection of 2 × 105 D2F2 breast carcinoma cells. (a) Schematic of experimental protocol. (b) Mean tumor volume of mice 5–25 days after tumor cell challenge. (c) Tumor weight of mice 25 days after tumor cell challenge. *, P < 0.05 compared to empty vector control group. Experiments were repeated three times with similar results
Fig. 3.
The pLegu-H-Kd minigene vaccine reduces D2F2 breast carcinoma metastasis in syngeneic BALB/c mice Groups of mice (n = 8) were immunized three times at 1-week intervals by gavage with attenuated Salmonella typhimurium harboring the vectors indicated. Mice were challenged 2 week after the last immunization by i.v. injection of 1 × 105 D2F2 breast carcinoma cells. (a) Schematic of experimental protocol. (b) Average lung metastasis score of mice from each experimental group 25 days after tumor cell challenge. Tumor metastasis scores on lungs were established by estimating the % surface area covered by fused metastases as follows: 0, no metastases; 1, < 20%; 2, 20–50%; and 3, >50% represented by individual symbols for each treatment group. *, P < 0.05 compared to empty vector control group. Experiments were repeated three times with similar results
The minigene vaccine induces a CTL response, which is capable of killing Legumain+ cells
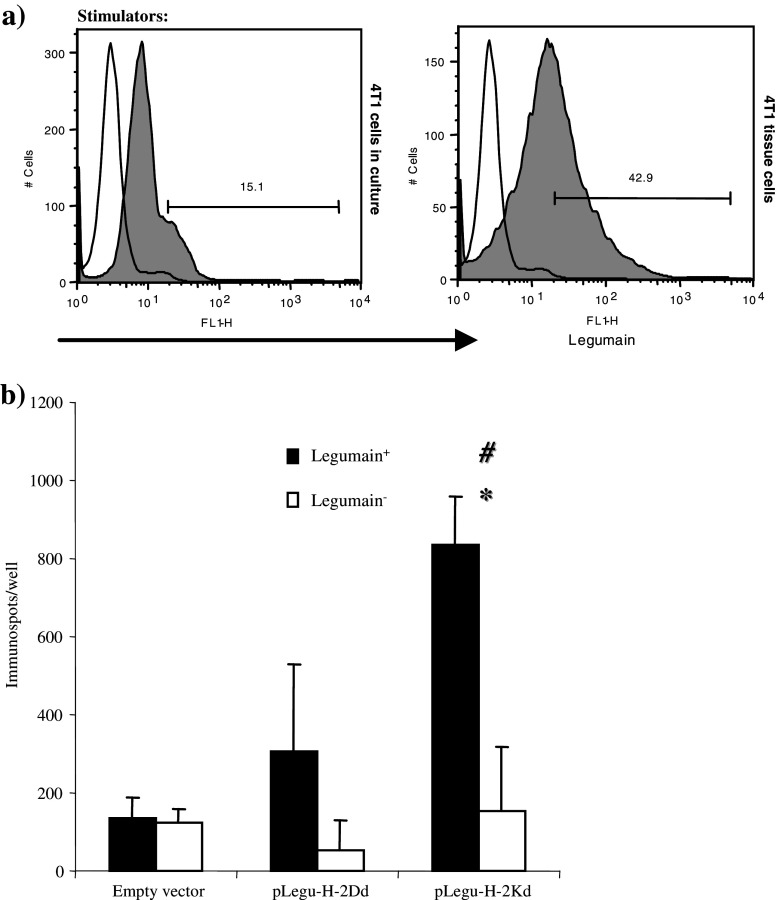
In order to demonstrate the specificity of the T-cells response achieved after minigene vaccinations, T-cell activation was demonstrated by the specific release of IFN-γ from activated T-cells (Fig. 4b). These cells were stimulated with cells harvested from fresh 4T1 tumor tissue that highly expressed Legumain as indicated by Flow cytometry analysis (Fig 4a). Importantly, cytotoxicity assays clearly demonstrated marked CTL activity only of immunized mice. The target cells used to prove this point were cells of the murine macrophage cell line RAW, which previously were cultured with IL-4, IL-10 and IL-13 cytokines to induce the expression of Legumain. This was done since these cytokines, derived from tumor cells, can switch the M1 macrophage to the M2 macrophage phenotype [12–14]. This finding was also verified by Western blot analysis (Fig. 5a), where wild type RAW cells were shown to be Legumain−, whereas their incubation with IL-4, IL-10 and IL-13 rendered them Legumain+. In contrast, splenocytes isolated from control mice treated only with empty vector showed similar background killing of RAW cells, either positive or negative for Legumain expression (Fig. 5b). However, splenocytes from pLegu-H-2Kd-vaccinated mice induced significantly stronger killing against Legumain+ target cells than against such cells harvested from mice treated with pLegu-H-2D vaccine or the empty vector. (Fig. 5b) These data demonstrate the specificity of the pLegu-H-2Kd minigene vaccine-induced CTL activity and its capability to specifically kill Legumain+ TAMs.
Fig. 4.
The pLegu-H-2Kd minigene vaccine induces IFN-γ release by Legumain specific T-cells (a) Legumain+ 4T1 cells freshly harvested from tumor tissues were used as stimulator cells. Flow Cytometry was performed to indicate the extent of Legumain expression on those cells. (b) Production of IFN-γ was verified at the single cell level by ELISPOT as lymphocytes from immunized mice were restimulated with either Legumain+ 4T1 tumor cells freshly harvested from tumor tissue or Legumain− 4T1 tissue culture cells. IFN-γ release is indicated by the number of immunospots formed per well. *, P < 0.05, compared with groups of mice whose lymphocytes were not stimulated by Legumain + tumor cells. # P < 0.05, compared with control groups. Experiments were repeated three times with similar results
Fig. 5.
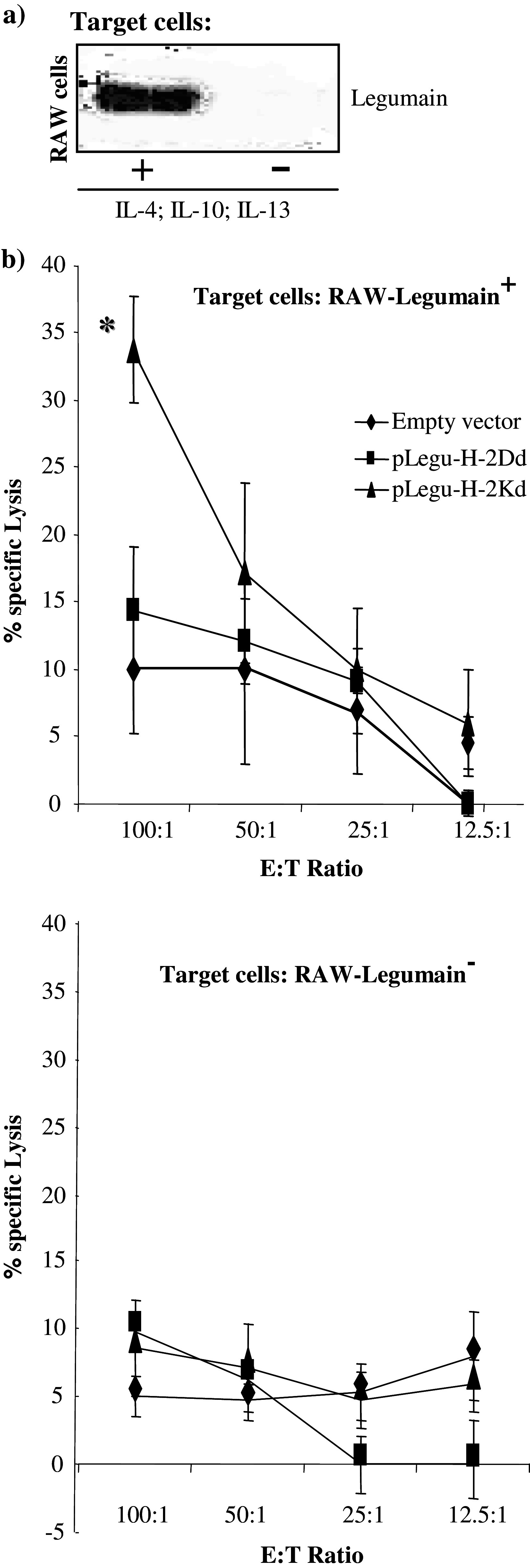
The pLegu-H-2Kd minigene vaccine induces specific CTL mediated killing of Legumain positive macrophage cells (a) The expression of Legumain by the murine macrophage cell line RAW is indicated after culture with IL-4, IL-10 and IL-13. Western blot analysis was performed to indicate Legumain expression on those cells. (b) Groups of immunized BALB/c mice (n = 4) were sacrificed 2 week after the last immunization and splenocytes isolated from them were stimulated with irradiated 4T1-Legumain+ cells for 5 days. Thereafter, cytotoxicity assays were performed with either wild type RAW–Legumain− cells (lower panel), or RAW–Legumain+ cells as target cells (upper panel). *, P < 0.05 compared to empty vector control group while using RAW Legumain+ cells as target cells. Experiments were repeated three times with similar results
The pLegu-H-2Kd minigene vaccine induces anti-angiogenic effects
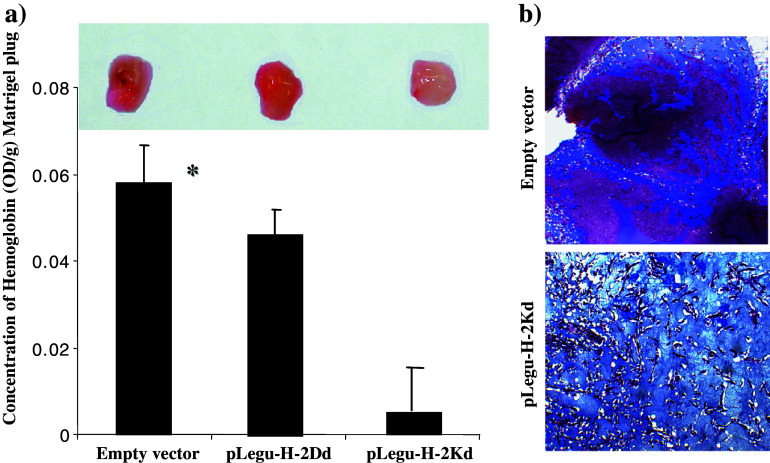
In an effort to investigate whether tumor anti-angiogenesis plays a key role in the pLegu-H-2Kd-vaccine-induced tumor protection, we substantiated this contention by performing Matrigel assays in which blood vessel formation was induced within the Matrigel by recombinant bFGF. The difference in vessel formation was quantified by measuring the relative concentrations of Hemoglobin (Hb) in Matrigel plug extracts obtained from either immunized or control mice. Thus, mice vaccinated with the pLegu-Kd vaccine displayed a clear reduction in the average relative concentration of Hb (Fig. 6a). Furthermore, when Matrigel sections from immunized and control mice were analyzed by Masson’s trichrome staining, those sections obtained from mice in the empty vector control group revealed ample and multiple blood vessels. In contrast, blood vessels were markedly reduced in Matrigel sections obtained from pLegu-H-2Kd-vaccinated mice (Fig. 6b). These data demonstrate that immunization with the pLegu-H-2Kd vaccine resulted in a reduction of tumor vasculature. Taken together, these findings indicate that the pLegu-H-2Kd minigene vaccine induced marked anti-angiogenic effects which aided in the protection of BALB/c mice from lethal challenges with D2F2 breast tumor cells in a prophylactic setting.
Fig. 6.
The pLegu-H-2Kd minigene vaccine suppresses angiogenesis in syngeneic BALB/c mice. (a) Results of Matrigel assay. Matrigel was implanted into mice vaccinated with either empty vector, pLegu-H-2Dd or pLegu-H-2Kd vaccines. The measurement of Hemoglobin (Hb) concentration in Matrigel plugs was performed for quantification of blood vessel growth. The average Hb concentration of Matrigel plugs from each group of mice is depicted by the bar graph (n = 4; mean + SD). *, P < 0.05 compared to empty vector control group. (b) Masson’s trichrome staining of Matrigel sections prepared 7 days after Matrigel plug implantation. Blue arrows indicate blood vessels in the Matrigel plug. Experiments were repeated two times with similar results
Discussion
In our prior study we established a novel strategy indicating that anti-tumor efficacy can be achieved by targeting tumor associated macrophages (TAMs) in the TME via the induction of a specific CD8+ T-cell response against Legumain, an asparaginyl endopeptides, which we identified for the first time as a highly over-expressed molecular target on TAMs. In these prior experiments, we also demonstrated that abrogation of TAMs in tumor tissues effectively decreased several pro-tumor growth and angiogenesis factors released by TAMs such as VEGF, TGF-β, TNF-α and MMP-9 [1]. In addition, we concluded that there are several advantages in targeting CD8+ T-cells against such tumor stroma components as TAMs. First, it is well known that tumor stromal cells, including TAMs, are genetically more stable than tumor cells. This makes TAMs in the tumor stroma more suitable target cells for immunotherapy than tumor cells per se [15–18]. Second, since TAMs play a critical role in the progression and dissemination of solid tumors, killing of TAMs in the tumor microenvironment is applicable for therapies of a variety of malignancies. Importantly, our minigene vaccine, which suppresses the expression of TAMs, can focus the immune system against certain universal epitopes in the tumor microenvironment and therefore represents a simple, safe and economical vaccine, which is effective against a variety of malignancies.
In our prior studies we generated a DNA vaccine encoding a full-length murine Legumain gene, which induced an effective anti-tumor response in several mouse tumor models, including breast, lung and colon carcinoma. However, on the negative side, the full-length Legumain gene utilized in these prior studies was 1.3 kb in length, which could easily lead to potential mutations during vaccine production because of the relatively large size of this gene. Such mutations could also occur in the host, once plasmids encoding the gene are delivered to secondary lymphoid tissues. Furthermore, the whole gene vaccine is bound to induce immune response not only against specific epitopes but also against non-relevant antigen epitopes, which could cause serious side effects. These potential safety and quality control issues might be of substantial concern before such a strategy would become clinically applicable. In this regard a DNA minigene vaccine strategy could overcome some of these strictures by offering a more specific and stable vaccine target.
In our current experiments, a mammalian expression vector with an ER signal (pCMV/Myc/ER) was utilized for construction of Legumain-based DNA minigene vaccines. The rationale for using this vector is that it has an-ER signal peptide as a targeting sequence, which directs the protein into the secretory compartment as well as a C-terminal peptide that retains the protein in the endoplasmic reticulum (ER). To this end, these vaccine peptides were processed in the ER and then bound to MHC class I antigen-binding sites to be finally presented to T-cell receptors on the cell surface which induced a Legumain-specific T-cell response. In fact, we took advantage of these constructs to create specific minigene vaccines, which can induce an effective T-cell response against breast tumors in syngeneic mouse tumor models. Based on these findings, we could potentially apply this same strategy against different tumors with different genetic background. This is of considerable practical importance, particularly since in the clinic patients present with different genetic backgrounds, and CTLs display a variety of MHC class I antigen–restrictions which depend on each individual’s genetic background. In this regard, it is of interest that all of our minigene vaccines of different genetic background induced tumor protection to a certain extent. Thus, the tumor protection induced by the Legumain Kd and Dd minigene was obtained against D2F2 breast carcinoma syngeneic in BALB/c mice, whereas both Legumain Db and Kb minigene were effective against D121 non-small cell lung carcinoma in syngenenicC57/BL6 mice (data not shown); however, only the Legumain Kd minigene vaccine was shown to induce a more effective immune response against D2F2 breast cancer. A possible explanation for this finding is that there could be a much greater number of Legumain peptides which may bind to H-2Dd/Kd with higher affinity than those binding to H-2Db/Kb molecules. This contention is strengthened since according to the BioInfomatics & Molecular Analysis Section (BIMAS) of NIH, the predictive scores of Legumain peptides binding to H-2Dd and H-2Kd are much higher than the corresponding scores for H-2Db and H-2Kb whereas the binding score of H-2Kd is the highest (Table 1).
Considering that the TCR repertoire is almost unlimited, there exist a much higher number of peptide-H-2Dd/Kd complexes that can be recognized by the CTLs in BALB/c mice. Moreover, the H-2D peptide had a much higher antigenicity index than two of three H-2K peptides (Table 1). Apparently, based on these data, antigenicity seems to be less important for our purpose than the MHC binding score. Similar results were found regarding the difference in tumor prevention effects in another of our studies involving different genetic backgrounds with a FLK-1 minigene strategy [5]. Taken together, such findings provide us with a better understanding of DNA based cancer vaccines as they suggest that the genetic background of the cancer patient maybe be one of the keys to the successful treatment with such a vaccine.
In summary, we report here that Legumain-based minigene vaccines induced an effective protection against tumors by ablating TAMs in the TME of our murine breast tumor model. The tumor protection induced by these Legumain-Kd minigene vaccines led to a T-cell-mediated attack on TAMs in the D2F2 breast carcinoma TME in syngeneic BALB/c mice. This protective immune response, which specifically killed Legumain+ TAMs, resulted in a marked suppression of tumor growth, metastasis and angiogenesis. Importantly, the minigene vaccine proved to be of relatively similar efficacy than a vaccine encoding the whole Legumain gene, at least in a prophylactic setting [1]. Taken together, these data validate the first anti-Legumain minigene vaccine to be effective against TAMs in mice which, based on our prior data, suggest that this strategy could potentially be applied clinically to individuals with different genetic backgrounds. Importantly, once validated in a therapeutic setting, this could provide a simple, more safe and flexible alternative to the whole-gene vaccine. This strategy could add a new dimension to antiangiogenic interventions in cancer immunotherapy and potentially facilitate future improved designs of DNA-based vaccines and their clinical application in breast cancer therapy and prevention.
Acknowledgements
We thank K. Cairns for editorial assistance. This work was supported by Grant 12RT-0002 from the California Tobacco-Related Disease Research Program (to R.A.R.), and E. Merck, Darmstadt-Lexigen Research Center, Billerica, MA Grant SFP1330 (to R.A.R.). Grants from The National Natural Science Foundation of China No.30672389 and No. 30572116 (to R.X.). This is The Scripps Research Institute’s manuscript number 17696-IMM.
Abbreviations
- TAMs
Tumor associated macrophages
- CTLs
Cytotoxic T lymphocytes
- MHC
Major histocompatibility complex
- TME
Tumor microenvironment
- Hb
Hemoglobin
Contributor Information
Rong Xiang, Phone: +1-858-7848109, FAX: +1-858-7842708, Email: rxiang@scripps.edu.
Yunping Luo, Email: ypluo@scripps.edu.
References
- 1.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van GE, van RN, Meijer S, van dS Jr, Beelen RH, van EM (2005) Macrophages direct tumor histology and clinical outcome in a colon cancer model. J Pathol 207:147 [DOI] [PubMed]
- 3.Liu C, Sun C, Huang H, Janda K, Edgington T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 2003;63:2957. [PubMed] [Google Scholar]
- 4.Murthy RV, Arbman G, Gao J, Roodman GD, Sun XF. Legumain expression in relation to clinicopathologic and biological variables in colorectal cancer. Clin Cancer Res. 2005;11:2293. doi: 10.1158/1078-0432.CCR-04-1642. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Luo Y, Mizutani M, Mizutani N, Xiang R, Reisfeld RA. T-cell-mediated suppression of angiogenesis results in tumor protective immunity. Blood. 2005;106:2026. doi: 10.1182/blood-2005-03-0969. [DOI] [PubMed] [Google Scholar]
- 6.Xiang R, Lode HN, Chao TH, Ruehlmann JM, Dolman CS, Rodriguez F, Whitton JL, Overwijk WW, Restifo NP, Reisfeld RA. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci USA. 2000;97:5492. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y, Zhou H, Mizutani M, Mizutani N, Reisfeld RA, Xiang R. Transcription factor Fos-related antigen 1 is an effective target for a breast cancer vaccine. Proc Natl Acad Sci USA. 2003;100:8850. doi: 10.1073/pnas.1033132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Y, Zhou H, Mizutani M, Mizutani N, Liu C, Xiang R, Reisfeld RA. A DNA vaccine targeting Fos-related antigen 1 enhanced by IL-18 induces long-lived T-cell memory against tumor recurrence. Cancer Res. 2005;65:3419. doi: 10.1158/0008-5472.CAN-05-1132. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Luo Y, Lo JF, Kaplan CD, Mizutani M, Mizutani N, Lee JD, Primus FJ, Becker JC, Xiang R, Reisfeld RA. DNA-based vaccines activate innate and adaptive antitumor immunity by engaging the NKG2D receptor. Proc Natl Acad Sci USA. 2005;102:10846. doi: 10.1073/pnas.0502208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Luo Y, Mizutani M, Mizutani N, Becker JC, Primus FJ, Xiang R, Reisfeld RA. A novel transgenic mouse model for immunological evaluation of carcinoembryonic antigen-based DNA minigene vaccines. J Clin Invest. 2004;113:1792. doi: 10.1172/JCI200421107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, Eliceiri BP, Reisfeld RA. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8:1369. doi: 10.1038/nm794. [DOI] [PubMed] [Google Scholar]
- 12.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 13.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, reuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Regev A, Lavi S. Small polydispersed circular DNA (spcDNA) in human cells: association with genomic instability. Oncogene. 1997;14:977. doi: 10.1038/sj.onc.1200917. [DOI] [PubMed] [Google Scholar]
- 17.Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 18.Vitale, Rezzani MR, Rodella L, Zauli G, Grigolato P, Cadei M, Hicklin DJ, Ferrone S (1998). HLA class I antigen and transpoter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinona lesions. Cancer Res 58:737 [PubMed]