Abstract
Cutaneous T-cell lymphomas (CTCL) belong to non-Hodgkin lymphomas, which are primarily manifested in the skin and mostly exhibit a T-helper memory phenotype. Mycosis fungoides (MF) and the leukemic variant Sézary syndrome (SzS) are the most common forms of CTCL. The aim of this study was to identify CTCL surface proteins with a tumor specific expression profile. A plasma membrane enriched fraction of the CTCL cell line HuT78 was used for immunization of two rabbits. Subsequently, a CTCL cDNA phage library was screened by a new variant of the SEREX method (serological identification of antigens by recombinant expression cloning) using the polyspecific rabbit antisera instead of patients’ sera. Isolated reactive transfectants were sequenced and 42 different genes identified including four known plasma membrane proteins: Ligatin, HLA-A, integrin α4 and MT5-MMP. The level of transcripts of the matrix metalloproteinase MT5-MMP was diminished in MF tumor specimens. MT5-MMP normally occurs in several different protein variants. Western blot analysis revealed that activated MT5-MMPs were reduced in tumor specimens, whereas the amounts of most of the inactivated variants were unchanged. The amount of mRNA coding for the adhesion protein integrin α4 was not altered in tumor specimens in comparison to controls when analyzed by quantitative real-time PCR analysis. Ku86, known to be predominantly located in the nucleus and cytosol, was frequently detected during the SEREX screening. Western blot analysis revealed higher protein amounts of Ku86 in HuT78 than in control cells. In addition, we could show, that Ku86 can also be detected in lipid rafts of CTCL cells as it has been described for other tumor types. Thus, Ku86 might be involved in homo- and heterotypic adhesion steps of CTCL tumor cells and might protect these cells against apoptosis triggered by irradiation as it was suggested for multiple myeloma cells. The design of this study enabled screening for all proteins on the plasma membrane, irrespectively of whether these are directly anchored within the membrane or associated with other membrane proteins. Further analysis will unravel whether the list of identified proteins harbors candidates, which might be accessible for antibodies from outside the cell.
Keywords: SEREX, MT5-MMP, Integrin α4, Ku86, Lipid rafts
Introduction
Cutaneous T-cell lymphomas (CTCL) are non-Hodgkin lymphomas primarily located in the skin [61]. The most common forms are mycosis fungoides (MF) and the more aggressive Sézary syndrome (SzS) [14]. Tumor cells are malignant, clonally expanded mature CD4+ T helper cells with a memory phenotype [8]. SzS is characterized by the appearance of tumor cells in the peripheral blood (Sézary cells), which can accumulate to a high number of cells. The pathogenesis of both types is poorly understood. Chronic antigen stimulation is hypothesized to be causative for the development of CTCL.
Previous studies have identified cell surface proteins overexpressed on CTCL-cells. A member of the killer immunoglobulin-like receptor (KIR) p140-KIR3DL2 which is normally expressed on a minor subset of natural killer lymphocytes was found as a novel allelic form on tumor cells of Sézary syndrome [58]. Therefore, it was suggested as a marker to distinguish between tumor cells of Sézary syndrome and of Mycosis fungoides. A second potential differentiation marker is SC5, which was found to be elevated in Sézary cells [38]. Additionally, SC5 is discussed to be involved in inhibition of CD3-induced cell proliferation.
Oligonucleotide array analysis using T-cell mRNAs isolated from the peripheral blood of SzS patients and subsequent real-time PCR analysis revealed increased amounts of tyrosine kinase receptor EphA4 [54]. A possible role in the pathogenesis of CTCL is suspected due to the overexpression of some targets downstream of its signal transduction pathways like Fyn, Grb2 and Abl or STAT3, a transcriptional activator constitutively phosphorylated in tumor cells of SzS patients [12].
The objective of this study was to identify membrane-bound proteins in CTCL. Therefore, we enriched plasma membrane of the CTCL cell line HuT78 by differential centrifugation. The plasma membrane fraction was used for immunizing rabbits to get reactive antisera, which were then applied in the SEREX method for the first time. Thereby, a cDNA phage library of the CTCL cell line SeAx was screened for reactive clones. The clones were identified using the HUSAR program “blast2n”. Finally, MT5-MMP, integrin α4 and Ku86 of all identified genes were chosen for further expression analyses (Real-time PCR and Western blotting).
Materials and methods
Cell lines
The CD4+ human cell lines HuT78 (derived from a SzS patient; TIB-161) and HH (aggressive cutaneous T-cell lymphoma; CRL-2105) were purchased from ATCC. The CD4+ cell lines SeAx (SzS patient) and MyLa (MF patient) were kindly provided by K. Kaltoft. The CD8+ Cou-L3 cell line has been established from a patient with MF evolved into a pleomorphic large T-cell lymphoma and has been kindly provided by A. Bensussan. Cells were routinely cultured in RPMI medium (Biochrom AG, Berlin) supplemented with 1% penicillin/streptomycin, 2% L-glutamine and 10% fetal calf serum (Pan biotecs) at 37°C and 5% CO2. SeAx and Cou-L3 cells were additionally cultured with 1,000 U/ml and 150 U/ml IL-2, respectively.
Patients tissues
Tumor tissues were collected during routine diagnostic procedures with a formal agreement signed by the patients and with the official permission of the local ethical review board. Skin specimens obtained from CTCL patients and patients with benign skin diseases served as a source for generating cDNA and/or protein: 7 MF (stage IIb–IVb), 2 SzS patients, 2 dermatitis, 3 eczema, and 6 control skin tissue samples. 5 SzS (stage III-IV) and 12 control PBMC samples were fractionated from whole blood samples by Ficoll density centrifugation. PBMC fractions were then partially used to isolate CD4+ T-cells with anti-CD4 Microbeads (Miltenyi Biotec). The control samples were received from the blood bank in Mannheim (Germany).
Antibodies
Primary antibodies against ICAM-1, CD3ɛ, calnexin, Tom40 and Ku86 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); antibodies against Lck were from BD Pharmingen (San Diego, USA); antibodies against Nuclear pore were from Oncogene research products (Schwalbach/Ts, Germany); and antibodies against MT5-MMP were from Neuromics antibodies (Northfield, MN, USA). Horseradish peroxidase-conjugated secondary antibodies against rabbit and mouse IgG were purchased from Santa Cruz Biotechnology, Inc. The protease inhibitor cocktail (Complete, Mini, EDTA-free) was purchased from Roche Applied Science (Mannheim, Germany). For immunocytochemistry, normal goat serum, Vectastain ABC kit, alkaline phosphatase substrate Kit I were purchased from Vector Laboratories Inc. (Burlingame, CA).
Plasma membrane enrichment
Plasma membrane enriched fractions from CTCL cell lines HuT78 and MyLa were prepared using the method described by Ellis et al. with some modifications [11]. Briefly, 1–1.5 × 109 cells were grinded in a mixer mill and homogenized in buffer A (250 mM sucrose, 12 mM Tris–HCl, pH 7.4, complete, Mini EDTA-free). The homogenate was first centrifuged for 10 min at 270 × g. The resulting supernatant was centrifuged at 920 × g for 10 min. MgCl2 was added to 10 mM to the following supernatant and the suspension was stirred for 15 min on ice and centrifuged for 15 min at 2,300 × g. The supernatant was decanted into a centrifuge tube and centrifuged at 100,000 × g for 45 min. The pellet was resuspended with a Dounce homogenizer in 0.5 ml buffer B (250 mM sucrose, 5 mM Na2-EDTA, 2 mM Tris–HCl, pH 7.4, protease inhibitor cocktail, Complete, Mini, EDTA-free), layered on 4.5 ml 30% sucrose and centrifuged at 68,000 × g for 2 h. The band at the interphase was supplemented with finally 8% sucrose in buffer A. The pellet obtained by centrifugation at 100,000 × g for 1 h was finally resuspended in a minimal volume PBS.
Western blot analysis
Whole cell extracts, plasma membrane fractions or lipid rafts fractions were boiled in 5 × sample buffer and separated by SDS-PAGE (16% polyacrylamide). For MT5-MMP analysis cells and tissues were mechanically disrupted by a mixer mill, treated with lysis buffer, centrifuged and the supernatant was used for cytosolic enriched proteins. The pellet was then treated with Triton X-100 and thereafter used as membrane fraction.
Protein gels were fixed in 40% methanol, 10% acetic acid and stained with colloidal Coomassie G250. Alternatively, proteins were transferred to nitrocellulose membrane. The transfer was controlled by incubation in 0.2% Ponceau S. For blocking of unspecific binding sites blots were incubated with 5% (w/v) skim milk in PBST buffer solution (1 xPBS, 0.1%(v/v) Tween) for 1 h at RT or overnight at 4°C.
The primary antibodies were used in 0.5% milk PBST buffer solution at a dilution of 1:500 for ICAM-1 (H-108), CD3ɛ (FL-207), Calnexin (H-70), Tom40 (H-300) and Ku86 (H-300); 1:100 for Lck (MOL171); 1:300 for Nuclear pore (Ab-2); 1:1,000 for MT5-MMP and 1:5,000 for the generated rabbit pre- and post-immune sera. The incubations were done for 1 h at room temperature or overnight at 4°C. After washing (3 × 5 min with PBST), anti-rabbit (NA934) or anti-mouse (NA931) IgG secondary antibodies coupled with horseradish peroxidase were added at a dilution of 1:10,000 and incubated for 1 h at room temperature. The filters were washed in PBST, developed with an ECL kit (Amersham Biosciences), and exposed to Hyperfilm (ECL) (Amersham Biosciences Europe). For re-use, blots were stripped with stripping buffer (100 mM β-mercaptoethanol, 62.5 mM Tris-HCl pH 6.8, 2% SDS) at 57°C for 30 min.
Immunization
Two rabbit polyclonal antisera were raised against the plasma membrane enriched fraction (DKFZ animal facilities or Eurogentec, Belgium). Briefly, protein was isolated from 2.8 × 1010 HuT78 cells and plasma membrane enriched fractions were established. Rabbits (chinchilla bastard) were immunized with 375 μg or 500 μg protein, respectively, dissolved in 250 μl PBS mixed with 250 μl complete Freund’s adjuvant. After one month, rabbits were boosted at two-week intervals for 3 times and 2 times, respectively, using between 375 and 500 μg protein dissolved in 250 μl PBS and 250 μl incomplete Freund’s adjuvant for each immunization. The antisera before and after immunization (pre-and post-immune serum) were collected and tested by ELISA. After final bleed we obtained 62 and 50 ml final serum, respectively.
Immunocytochemistry
The 1 × 105 cells were spun per slide at 1,500 rpm for 3 min and fixed in 3.7% formaldehyde at 4°C for 15 min. The slides were washed 4 times with PBS, permeabilized with PBST for 2 min and washed again with PBS for 4 min. After blocking with 5% normal goat serum in PBS the slides were incubated with the primary antiserum (1:50 diluted pre- or post-immune serum) for 1 h at RT or overnight at 4°C. The slides were then washed (PBST) and blocked with 0.5% skim milk in PBST and finally incubated with the secondary antibody (biotin coupled anti-rabbit IgG) for 30 min at RT. After washing, incubation with the ABC complex, and another wash binding of the primary antibody was visualized by development with alkaline phosphatase substrate resulting in a red staining (all Vector laboratories Inc.). Cells were counterstained with hemalaun (Applichem, Darmstadt, Germany).
RNA isolation and cDNA synthesis
RNA was extracted from frozen skin biopsies and from PBMC samples using the RNeasy kit (Qiagen, Hilden, Germany) and peqGOLD TriFast (Peq Biotechnology GmbH), respectively. RNA quality was controlled by a MOPS Gel. Approximately 1 μg DNase treated total RNA was then used for cDNA synthesis (iScript cDNA Synthesis Kit, Bio-Rad, Richmond, CA). A PCR with a specific primer pair for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was done to ensure the quality of the synthesized cDNA (denaturation for 30 s at 95°C; annealing and extension for 30 s at 57°C for 35 cycles).
Construction of a SeAx cDNA library
RNA was extracted from the CTCL cell line SeAx (formerly provided by R. Dummer) using a RNA isolation kit (RNeasy midi kit) and subsequently—5 μg mRNA (oligotex mRNA kit, both Qiagen, Hilden, Germany) were used for the construction of the λ-ZAP expression library (ZAP-cDNA synthesis Kit, ZAP-cDNA Gigapack III gold cloning Kit from Stratagene, La Jolla, CA). The library consisted of 1.7 × 106 primary recombinants with insert sizes larger than 0.4 kb.
SEREX (serological identification of antigens by recombinant expression cloning)
The identification of clones recognized by the post-immune serum was performed by SEREX as described by Hartmann et al. [18] with the only difference that instead of human serum the sera from immunized rabbits were used. The binding of antibodies of the rabbit serum was visualized by using a goat anti-rabbit antibody coupled with alkaline phosphatase and subsequent AP-substrate incubation (Dianova, Hamburg, Germany). The nucleotide sequence of their cDNA inserts was analyzed by sequencing. Database analysis was performed with the HUSAR package from the Biocomputing Service Group at the German Cancer Research Center, Heidelberg, using EMBL and GenBank databases.
Prediction of subcellular localization of proteins
PSort first predicts the presence of signal sequences by McGeoch’s method [34] modified by Nakai and Kanehisa, 1991. “TmHMM” is a prediction algorithm for transmembrane domains using a hidden Markov model and was developed by Sonnhammer et al. [46].
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR analysis was done using ABsolute™ QPCR SYBR® Green Mix (ABgene House, Surrey, GB) and iTaq DNA polymerase (Bio-Rad) with specific primers (Oligonucleotide Synthesis facility, DKFZ, see Table 1) on a MyiQ cycler (Bio-Rad). For each gene, amplification curves were produced and threshold values (Ct value) were obtained. Standard curves for extrapolation were performed using specific PCR products with determined copy numbers. Standardization of samples was achieved by measurement of the endogenous reference gene HMBS (hydroxylmethylbilane synthetase). By using the ΔC T method diagrams of all genes of interest were established. Primer specificity was tested by melting curves and gel electrophoresis. The cycle parameters for these transcripts and for the housekeeping genes HMBS used for normalization were as follows: denaturing for 15 s at 95°C; annealing and extension for 60 s at specific temperature (see Table 1) for 40 cycles. Differences between groups were calculated with the Mann–Whitney U test using the software Analyze-it.
Table 1.
Primer real-time RT-PCR analysis
| Name | Forward primer | Annealing temperature (°C) | Product size(bp) |
|---|---|---|---|
| Reverse primer | |||
| MT5-MMP | att agc tca cac ctg tcc act c | 66 | 106 |
| gca agt aac aac ctc tct gtg c | |||
| Integrin α4 | ggg aaa atg gaa agt gga aaa g | 59 | 142 |
| caa tta ctc ttg gat ttg gc | |||
| HMBS | ggc aat gcg gct gca a | 64 | 75 |
| ggg tac cca cgc gaa tca c |
The table shows sequences of forward and reverse primers in 5′3′ notation, as well as the annealing temperature and the size of the PCR product. MT5-MMP, membrane-type 5–matrix metalloproteinase, HMBS hydroxymethylbilane synthase
Lipid rafts isolation
Lipid rafts of HuT78 were obtained by a modified method described by Ilangumaran et al. [23]. Briefly, the cells were frozen in liquid nitrogen, grinded and homogenized with a Dounce homogenizer in TKM buffer (50 mM Tris–HCl pH 7.4, 25 mM KCl, 5 mM MgCl, 1 mM EGTA). Triton X-100 was added to the homogenate to 2 ml TKM buffer with a final concentration of 1% and incubated for 20 min at 4°C. The whole lysate was adjusted to 2 ml TKM buffer with a final concentration of 40% sucrose, overlaid with 6 ml of 30% sucrose and on top with 3 ml of 5% sucrose in TKM buffer. After centrifugation (250,000 × g for at least 16 h at 4°C), 1 ml fractions were collected from the top excluding the pellet, numbered 1–9 from top to bottom, and stored at −20°C. Aliquots of the lipid rafts enriched fractions (fraction 3, 4 and 5) were pooled for re-banding. Fractions were adjusted to 50% sucrose in a final volume of 2 ml TKM buffer. Six ml of 40% and 3 ml of 5% sucrose in TKM buffer were overlaid and the centrifugation was done again at 250,000 × g for at least 16 h at 4°C. Finally 1 ml fractions were collected from the top.
Results
Plasma membrane enrichment
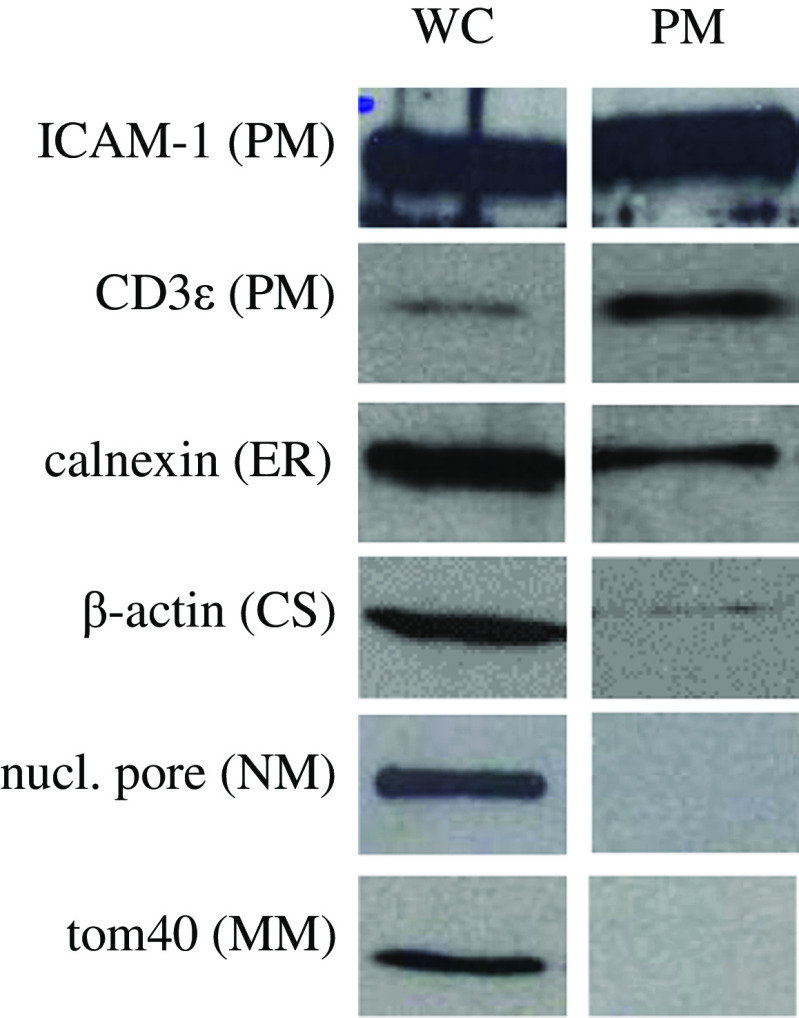
Plasma membrane fractions were used for immunization of rabbits and for Western blot analysis. Enrichment of the plasma membrane from the CTCL cell line HuT78 was established using a series of density centrifugations. Efficiency was controlled by antibodies against organelle-specific markers (Fig. 1): ICAM-1 and CD3ɛ for the plasma membrane, calnexin for the endoplasmic reticulum, β-actin for the cytoskeleton, Nuclear pore for the nuclear membrane and Tom40 for the mitochondrial membrane. In contrast to the whole cell lysate (WC) we did not detect any mitochondrial membrane marker (Tom40) or nuclear membrane marker (Nuclear pore) in the plasma membrane enriched fraction (PM, Fig. 1). Calnexin (ER) and β-actin (cytoskeleton), however, are still found in fraction PM though clearly reduced. Notably, the ER membrane has a very similar composition like the plasma membrane. β-actin is an important component of the cytoskeleton and linked to the inner side of the plasma membrane explaining the faint band of β-actin in lane PM. Appropriate amounts of PM fractions were used for immunizing rabbits.
Fig. 1.
Western blot analysis of plasma membrane enrichment of HuT78 cells. Marker protein distribution was used to control the purity of the plasma membrane fraction. Compared to lane WC (whole cell suspension) lane PM did not show mitochondrial or nuclear membrane components (Tom40, Nuclear pore). Small amounts of β-actin (CS) and some calnexin (ER) were still detected in fraction PM. Lane CS was loaded with 20 μg and lane PM with 5 μg protein. PM, plasma membrane; ER, endoplasmic reticulum; CS, cytoskeleton; NM, nuclear membrane; MM, mitochondrial membrane
Analysis of post-immune antisera for reactivity against PM fraction
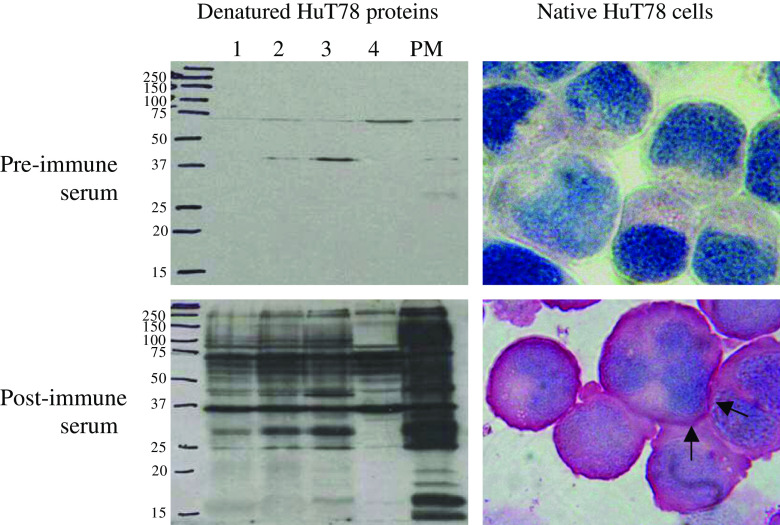
The reactivity of the antisera was first analyzed by ELISA (data not shown). The sero-reactivity against the whole PM fraction reached a plateau already after the first boost reaction. Additionally, the antisera were tested in Western blot and immunocytochemical analysis (Fig. 2). Excluded fractions of different enrichment steps and the PM fraction were loaded on a SDS-PAGE gel, blotted and first incubated with the 1:5,000 diluted pre-immune serum. The blot was then stripped and incubated with the post-immune serum (1:5,000). Apparently, the post-immune serum recognized a multitude of HuT78 proteins, especially of the PM fraction (Fig. 2, bottom left). In contrast, the incubation with the pre-immune serum labeled only a few faint bands. Similarly, cytospins of HuT78 cells were only stained by the post-immune serum (1:50; Fig. 2, bottom right) with a clear preference of the plasma membrane, and not by the pre-immune serum (1:50; Fig. 2 top right). Repetition of the immunocytochemistry using both rabbit antisera (1:200) and cytospins of the CTCL cell lines MyLa, SeAx, HH, and HuT78 revealed the same staining pattern (not shown).
Fig. 2.
Detection of HuT78 proteins by rabbit sera before and after immunization. The post-immune serum recognized HuT78 proteins, especially fraction PM, in Western blot analysis (bottom left) and stained the cell surface (see arrows) of HuT78 cells (bottom right). In contrast, the pre-immune serum labeled only 2 weak bands on the Western blot (top left) and was negative in immunocytochemistry (top right). The lanes 1, 2, 3, and 4 are excluded fractions during plasma membrane enrichment. The sera were diluted 1:5000 in Western blot and 1:50 in immunocytochemistry
Immunoscreening of a CTCL library with the rabbit post-immune serum by SEREX
A total of 1.7 × 106 recombinant clones of a cDNA phage library derived from CTCL cell line SeAx were screened using the rabbit post-immune serum. 90 phage clones were found to be reactive with IgG antibodies in the post-immune serum. Sequencing revealed 42 different genes, which are summarized in Table 2.
Table 2.
Identified proteins, their described predominant subcellular localization and accession number
| Subcellular localization | Identified protein | Accession no. |
|---|---|---|
| Plasma membrane | Integrin alpha 4 | L12002 |
| Ligatin | Bc058905 | |
| MT5-MMP | Bc047614 | |
| HLA-A | X13111 | |
| Golgi | Rab10 | Af106681 |
| Endoplasmic reticulum | Stearoyl-CoA desaturase 4 | Af389338 |
| Cytochrome b5 | Ab009282 | |
| Calnexina | M94859 | |
| Ribosomes | Translation initiation factor 4a | Bc009585 |
| Ribosomal protein S6 | Ab062123 | |
| Elongation factor 1a | X03558 | |
| Cytosol | RRM2 | X59618 |
| RAB2L | U68142 | |
| Nedd5 | Af038404 | |
| Lysyl-tRNA Synthetase | D32053 | |
| Mitochondria | ClpX-like protein | Aj006267 |
| MEG4 | Af151782 | |
| Nucleus | Splicing factor, arginine/serine-rich 11 | Bc017359 |
| Nuclear P1 | X62153 | |
| hZimp10 | Ay235683 | |
| FoxM1 | L16783 | |
| AIP | Bc062333 | |
| Homer homolog 1 | Bc015502 | |
| Braf25 | Bc002552 | |
| Various subcellular compartments | Ku86a | M30938 |
| Hsp70, transcript variant 1a | Bc019816 | |
| Cyclophilin A | Bc005982 | |
| CCT-zeta | L27706 | |
| Unknown | Bac clone RP11-555K2 | Ac018692 |
| Bac clone XXbac-55C20 | Ai591044 | |
| GAS5 | Bc038733 | |
| KIAA1191 protein | Cb155134 | |
| UBX domain containing 2 protein | D87684 | |
| Zinc finger protein 292 | Ab011102 | |
| Zinc finger protein 238 (RP58) | Bc036677 | |
| Chromosome 14, ORF 149 | Bi828749 | |
| FK506 binding protein 5 | Bc042605 | |
| Protein X 0004 | Bc008416 | |
| RNA-binding motif protein 25 | Ak125513 | |
| Hypothetical protein FLJ11730 | Bc056406 | |
| Clone CTB-193M12 | Ac026401 | |
| Cep290 or se2-2 | Af273044 |
aThese genes have been described to be also expressed at the plasma membrane
Homologous genes, their function and putative subcellular localization
Numerous proteins homologous to the identified clones have already been described for their predominant subcellular localization (Table 2). These compartments and organelles included plasma membrane (4 proteins), Golgi apparatus (1), endoplasmic reticulum (3), ribosomes (3), cytosol (4), mitochondria (2), nucleus (7), and proteins of various subcellular localizations (4). Fourteen clones resemble genes, which are scarcely characterized, and no information on the subcellular localization was available. We used computer algorithms for the deduced proteins of these genes, but did not observe any prediction for cell surface localization for any of the 14 tested proteins.
Beside the classification by their localization the identified proteins can be categorized by their functions: Ribonucleotide reductase M2 [59] is involved in DNA synthesis and the splicing factor arginine/serine-rich 11 [4] in RNA processing. A large group of genes encodes for proteins involved in protein synthesis and folding: Ribosomal protein S6 [20], translation initiation factor 4a [29], elongation factor 1α [52], lysyl-tRNA synthetase [15], calnexin [55], caseinolytic protease X homolog [6], hsp70 [62], cyclophilin A [17], and chaperonin containing TCP-1, subunit zeta [64]. Two factors, stearoyl-CoA desaturase 5 [1] and cytochrome b5 [39], have functions in different lipid synthesis steps.
The largest group of identified genes comprises proteins with regulatory roles in very different areas of the cell. First, Ku86 has main roles in the nucleus e.g. in non-homologous end-joining machinery [35, 63]. Nuclear P1, a homologue of the yeast protein Mcm3, regulates DNA replication [50]. Aurora A kinase interacting protein inhibits aurora A kinase, which has a role in regulation of structure and function of centrosomes [28]. Homer homologue 1 binds to the metabotropic glutamate receptor involved in release of Ca2+ ions of ER into the cytosol [3]. Braf25 (BRCA2-associated factor 35, transcript variant 25) forms a complex with BRCA2 (Breast cancer gene 2) and controls the cell cycle [57]. RAB2L is similar to RalGDS (Ral guanine nucleotide dissociation stimulator), an activator of Ral, but lacks the GDS activity domain [24]. The septin Nedd5 is involved in cytokinetics [30]. YME1-like 1 (yeast mitochondrial escape 1-like 1) has a function in protein metabolism in mitochondria [5]. hZimp10 (human zinc finger-containing, Miz1, PIAS-like protein on chromosome 10) shares a Miz1 domain with PIAS proteins and has been found to co-activate androgen receptor [45]. Finally, FoxM1 (Forkhead box M1) is a transcription factor, which is activated by the Hedgehog signaling pathway [31].
Ligatin, the first identified plasma membrane protein, is a base plate for the attachment of peripheral glycoproteins to the external cell surface [25]. HLA-A (human leukocyte antigen, alpha chain) is expressed on almost all cells and involved in cellular immune response by presenting antigens on the cell surface [2]. Integrin α4 has its role in adhesion to extracellular matrix and to other cells [49]. MT5-MMP (membrane type 5 matrix metalloproteinase; formerly named MMP24) is part of a family mainly responsible in degrading of extracellular matrix components [41].
Real-time PCR analysis of integrin α4 and MT5-MMP
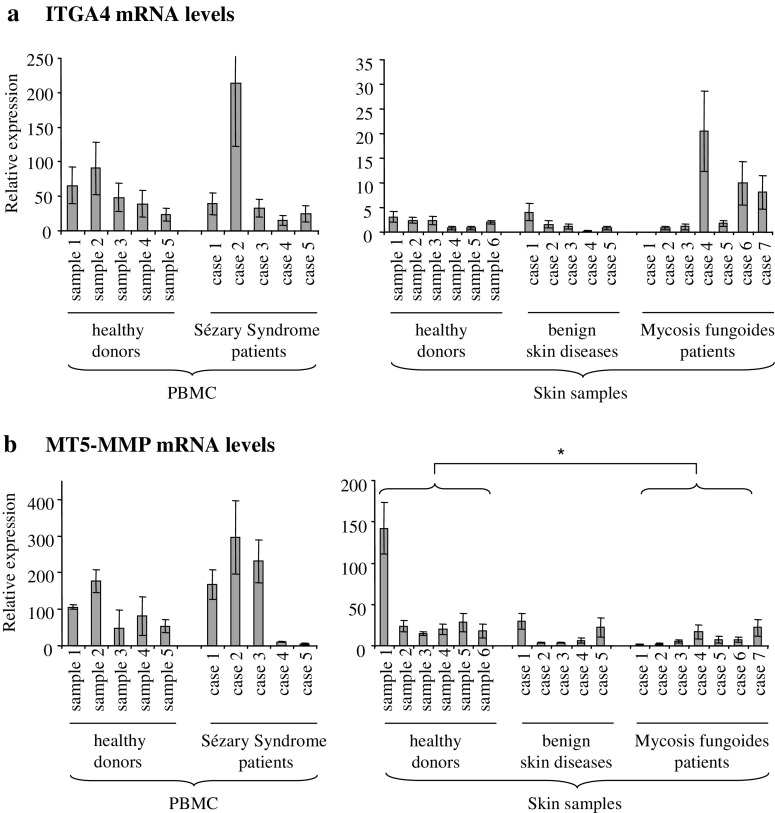
Transcriptional levels of both plasma membrane located genes were determined by real time PCR in CTCL compared to non-cancer tissues using PBMC of five SzS patients and five healthy volunteers, as well as skin samples of seven MF patients, five patients with benign inflammatory skin diseases (eczema and dermatitis) and six healthy persons.
In general, integrin α4 mRNA levels were much higher in PBMC samples than in skin specimens, irrespectively whether they were derived from tumor patients or healthy controls (Fig. 3a). The comparison between patients and healthy controls unraveled 1/5 SzS and 3/7 MF specimen with clearly elevated levels of integrin α4 mRNA in comparison to samples from benign lesions and/or healthy individuals. Using the Mann–Whitney U test we did not find any statistically significant differences between healthy controls and the corresponding CTCL group or benign skin diseases.
Fig. 3.
Quantification of integrin α4 and MT5-MMP mRNA in CTCL and controls. Samples included PBMC (left graphs) and skin specimens (right graphs) from healthy donors, patients with benign skin diseases, SzS and MF patients. a Integrin α4 transcript levels were higher in PBMCs than in skin samples (note different scale). Specimens of 1/5 SzS and 3/7 MF patients showed clearly elevated integrin α4 mRNA levels, but no significant differences were found between the groups (Mann–Whitney U test). b No difference in expression levels of MT5-MMP mRNA was detected between PBMCs of healthy donors and SzS patients, while specimens of MF patients had slightly decreased amounts in comparison to healthy controls (*p = 0.014)
MT5-MMP mRNA levels were also higher in the PBMC probes than in the skin probes, though not to the same degree like integrin α4 (Fig. 3b). PBMC samples of healthy donors did not differ significantly from those of SzS patients (Mann–Whitney U test). A significant down-regulation could be observed in skin specimens of MF patients in comparison to those of normal persons (Fig. 3b, right histogram; Mann–Whitney U test, 2-tailed p = 0.014).
Expression of MT5-MMP protein variants in CTCL
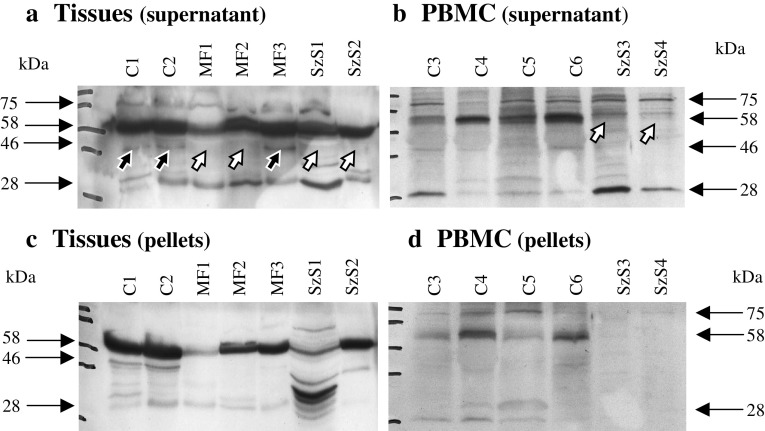
Western blot analysis with an MT5-MMP specific antibody, which recognizes the catalytic, the hinge and the hemopexin-like domain unraveled differences in the relative amounts of MT5-MMP variants in specimens of the different groups. To date, five variants of MT5-MMP are known [41]: (1) An inactive proform (64 kDa), (2) an active, membrane-located form (58 kDa), (3) active, soluble forms missing the transmembrane and the cytosolic domain (40–46 kDa), (4) the soluble catalytic form (28 kDa), and (5) the glycosylated form with unknown activity status (75 kDa).
We found several protein forms of MT5-MMP in the various PBMC and tissue samples analyzed with varying intensities (Fig. 4). The active 58 kDa form was present in almost all samples, except PBMCs of Sézary syndrome patients (four tested negative, one positive; examples see Fig. 4b, open arrows). The soluble, active 46 kDa form was missing in several skin tumor specimens of MF and SzS patients (Fig. 4a, open arrows), but was present in skin controls and one of the MF specimen (Fig. 4a, closed arrows).
Fig. 4.
MT5-MMP and its variants in CTCL. Cells and tissues were mechanically disrupted and supernatant and pellet were analyzed separately. a Specimen of control skin (C1 and C2), three MF and two SzS specimen displayed the active 58 kDa form of MT5-MMP. The 46 kDa form is missing in several tumor specimens (open arrows) but present in controls and one MF specimen (closed arrows). b PBMC samples of four controls (C3 to C6) and two SzS patients differ in the presence or absence of the active 58 kDa form. Panels c and d represent the corresponding pellet fractions
Lipid raft localization of Ku86
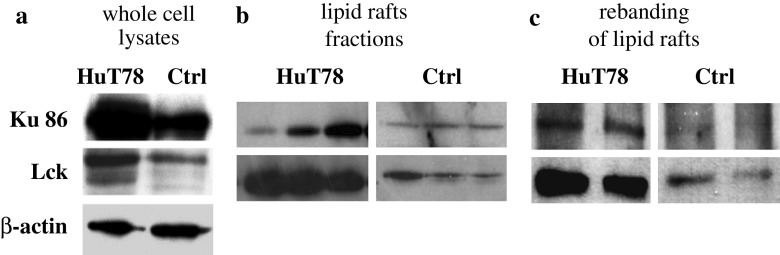
While Ku86 has clear-cut functions in the nucleus and cytosol [35], there are reports for expression of Ku86 in the plasma membrane of various tumors [33]. This and the very frequent isolation of Ku86 during the screening tempted us to analyze possible Ku86 expression in the plasma membrane and especially in the lipid rafts of CTCL cells. First, the total amount of Ku86 was analyzed by Western blotting of whole cell lysates, showing a strong overexpression in the CTCL cell line HuT78 in comparison to PBMC of a healthy donor (Fig. 5a). To test a possible localization of Ku86 in lipid rafts of HuT78 cells and control PBMC a Tween-20 treatment followed by sucrose density centrifugation were performed. After collecting 1 ml-fractions from the top of the gradient, fractions 3, 4, and 5 of both probes resembled the lipid rafts as demonstrated by a α-Lck antibody staining (Fig. 5b). Lck serves as a lipid raft marker in T-cells [26]. In both, HuT78 and control PBMC, Ku86 could be found in lipid raft fractions (Fig. 5b), whereas most of Ku86 proteins of HuT78 are still localized in the soluble fractions (data not shown). To confirm the lipid raft localization of Ku86 a second discontinuous gradient centrifugation was performed with the pooled lipid raft fractions (3, 4, and 5) at higher sucrose concentrations. Again, Ku86 could be detected in the lipid rafts fractions of HuT78 cells and very weakly in control PBMC (Fig. 5c).
Fig. 5.
Ku86 expression is elevated in the CTCL cell line HuT78 and partially located in lipid rafts. a Western blot analysis of whole cell lysates of HuT78 and control PBMCs (ctrl) reveals higher amounts of Ku86 and Lck in the cell line. b Western blotting of the lipid raft fractions (fractions 3–5) depicts a minor portion of Ku86 in the rafts of both HuT78 and control PBMC. c Localization of Ku86 in the lipid rafts has been confirmed by a second sucrose density gradient using the lipid raft fractions (3–5) of the first gradient. Both, Ku86 and Lck, appeared again in the lipid rafts fractions
Discussion
Numerous studies try to identify tumor-specific proteins to be used in immunotherapeutic strategies. For CTCL only very few specific antigens have been identified [9, 10]. Two plasma-membrane-located antigens, KIR3DL2 and SC5 were described for CTCL, which are not solely expressed on lymphoma cells, but also on natural killer cells or a subset of normal T cells, respectively [38, 58].
Attempts to directly isolate tumor-specific plasma membrane antigens are hampered by a variety of hurdles: The separation of membrane proteins is difficult [42] and the usage of technologies based on prokaryotic expression systems does not provide posttranslational modifications of the recombinant protein. While it is well known, that membrane proteins are often heavily glycosylated, there are examples of under-glycosylation: E.g. MUC-1 is less glycosylated in its tumor form than in normal cells [37]. This tempted us to use a variant of the SEREX method (serological identification of antigens by recombinant expression cloning, [44]), where polyclonal, polyspecific rabbit antisera were applied for screening. These rabbit antisera were generated against the plasma membrane enriched fraction of a CTCL tumor cell line. This allowed us to make usage of the high sensitivity of SEREX and the simplicity of identifying the isolated genes by sequencing the clone insert.
To get a native plasma membrane fraction enrichment was achieved by differential centrifugation [11]. Biotinylation of membrane proteins could not be applied due to resulting antibodies against biotin. Western blot analysis with pre- and post-immune sera showed that the post-immune serum is reactive against a large number of HuT78 proteins, especially from the plasma membrane. Using the described combination of methods we could isolate four known plasma membrane proteins and three proteins already described to be additionally located at the plasma membrane.
Integrin α4 was the first isolated membrane protein. Integrins are composed of α and β subunits and are mainly responsible for cell-cell and cell-extracellular matrix adhesion [21, 53]. Integrin α4 forms dimers with either integrin β1 or β7 and is expressed on leukocytes. We could not detect any major differences in integrin α4 mRNA levels between tumor samples and controls. Some individual MF specimens showed elevated integrin α4 mRNA in comparison to normal skin, but this might be explained with the increased number of malignant and reactive T cells in these samples. In general PBMCs of both SzS patients and controls had much higher integrin α4 levels than skin samples. In a mouse model with transduced lymphoma cells Gosslar et al. showed, that the expression of α4-integrins inhibits metastasis formation, but not tumor cell spread [16].
MT5-MMP, the second plasma membrane protein analyzed in detail, is the fifth member (of the membrane type matrix metalloproteinases (MT-MMPs). Like other MT-MMPs it is responsible for degrading extracellular matrix components and for activating soluble MMPs [41]. It was first found in brain tissues especially during embryogenesis and promotes axon growth [19]. Besides, MT5-MMP was detected in adult brain, kidney, pancreas and lung [32]. An overexpression was described for brain tumors (astrocytoma, glioblastoma) and diabetic kidney tissue [32, 43].
Our real-time RT-PCR analysis showed a decrease of MT5-MMP expression in skin specimens of MF patients compared to those of healthy persons. As MT5-MMP is a post-translationally modified protein and can appear in various active/inactive and membranous/soluble forms, it is important to analyze the expression of the different variants in detail. Western blotting unraveled a rather heterogeneous composition of MT5-MMP variants, but the main active 58 kDa form was missing in 4/5 SzS PBMC and the 46 kDa soluble active form was diminished in skin specimen of MF. The functional consequences of a reduction in active MT5-MMPs in malignant T cells remain to be clarified.
Ku86 was isolated 44 times from the cDNA phage library by our polyspecific rabbit antisera. This points towards a high expression level in the cell line used for library construction and a strong induction of rabbit IgGs against this protein. Ku86 together with Ku70 forms the heterodimeric Ku autoantigen. It was first discovered by analyzing autoantibodies from sera of patients with scleroderma polymyositis overlap syndrome and related diseases [36]. Ku86 has numerous functions, explaining its abundance. Still, we wondered why we isolated Ku86 and not other abundant proteins. One explanation might be an ectopic subcellular expression: Is Ku86 also expressed at the plasma membrane of CTCL-cells?
Our expression analysis on plasma membrane and especially lipid rafts unraveled, that Ku86 can be detected in lipid rafts of both, normal PBMCs and CTCL tumor cells. Moreover, Ku86 seems to be overexpressed in tumor cells, which might also explain the elevated amount of Ku86 protein in the rafts. Looking at the literature reveals that Ku86 can be found on the plasma membrane of a variety of tumor cells [33]. One study discovered Ku86/Ku70 translocation to the cell surface of multiple myeloma cells upon activation with CD40L and association of Ku86/Ku70 with CD40 [48]. In addition, CD40 activated tumor cells were protected against apoptosis induced by irradiation or doxorubicin, but antibodies against Ku86 prevented this protection. These results led the authors to assume that Ku86 could be an interesting target for the therapy of multiple myeloma.
The actual amount of Ku86 on the cell surface does probably also depend on the amount of lipid rafts. We used Lck, an Src-kinase family member, as T cell-specific marker for lipid rafts [26]. Interestingly, we found an increased level of Lck in the CTCL cell line HuT78 in comparison to normal PBMCs, which points towards a possible elevation of lipid rafts in this tumor cell line, though it has to be taken into account, that PBMCs enclose also non-T cells. As described for multiple myeloma cells (see above), Ku86 might be associated with CD40 in lipid rafts of CTCL cells, since CD40 and its ligand CD40L have also been detected in CTCL cells [27, 47].
Ku86 might not be the only protein encoded by the isolated clones (Table 2), which is—contrary to general assumptions—additionally expressed on the cell surface. In case of two proteins the ectopic expression in the plasma membrane is already known: Heat shock protein 70, a chaperone expressed in almost all cell types has been detected on the cell surface of HIV-infected cells [7], of tumor cells [13, 51] and of fibroblasts of patients with auto-immune diseases [22]. The second protein, calnexin, was found on the cell surface of immature thymocytes in complex with CD3. It seems to target the membrane by masking its ER retention signal [60]. Further studies also detected calnexin in the plasma membrane of mastocytoma cells, murine splenocytes, fibroblasts and HeLa cells [40].
In summary, we isolated 42 different reactive transfectants of a CTCL cDNA phage library by using rabbit antisera against a plasma membrane fraction of a CTCL cell line in a SEREX approach. This technique has proven to be very sensitive and feasible. The high number of proteins, which are published as being non-plasma membrane located, has to be taken with care: Besides four well known plasma membrane proteins (ligatin, HLA-A, integrin α4, and MT5-MMP), another three proteins (Ku86, Hsp70, and calnexin) have been described to be exceptionally expressed on the cell surface, e.g. by binding to other plasma membrane proteins. The same could apply for other proteins recognized by our rabbit antisera; thus it is difficult to judge specificity of this technique. The identified plasma membrane protein MT5-MMP could be shown to be downregulated in some tumors in its activated, but rather constant in its inactivated variants. The frequently identified Ku86 was overexpressed in HuT78 and for the first time detected in lipid rafts of this cell line and of normal PBMCs. As plasma membrane proteins are known to be glycosylated using a yeast expression systems for serological screening (RAYS) instead of E. coli would probably increase the rate of yield [56].
Acknowledgments
We are very grateful to Dr. Eva Frei (German Cancer Research Center, E080) for her help and support in the enrichment of membrane fractions, to Elke Dickes and Anita Heinzelmann for their excellent technical assistance, to Dr. K. Kaltoft (Aarhus University Hospital, University of Aarhus, Denmark) for providing us the cell lines SeAx and MyLa, and to Dr. A. Bensussan (Medical Faculty, Creteil, France) for the cell line Cou-L3.
Abbreviations
- CTCL
Cutaneous T cell lymphoma
- SEREX
Serological identification of antigens by recombinant expressed cloning
- MT5-MMP
Membrane-type 5 matrix metalloproteinase
References
- 1.Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, Edelson RL. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 3.Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary N, McMahon C, Blobel G. Primary structure of a human arginine-rich nuclear protein that colocalizes with spliceosome components. Proc Natl Acad Sci USA. 1991;88:8189–8193. doi: 10.1073/pnas.88.18.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppola M, Pizzigoni A, Banfi S, Bassi MT, Casari G, Incerti B. Identification and characterization of YME1L1, a novel paraplegin-related gene. Genomics. 2000;66:48–54. doi: 10.1006/geno.2000.6136. [DOI] [PubMed] [Google Scholar]
- 6.Corydon TJ, Wilsbech M, Jespersgaard C, Andresen BS, Borglum AD, Pedersen S, Bolund L, Gregersen N, Bross P. Human and mouse mitochondrial orthologs of bacterial ClpX. Mamm Genome. 2000;11:899–905. doi: 10.1007/s003350010173. [DOI] [PubMed] [Google Scholar]
- 7.Di Cesare S, Poccia F, Mastino A, Colizzi V. Surface expressed heat-shock proteins by stressed or human immunodeficiency virus (HIV)-infected lymphoid cells represent the target for antibody-dependent cellular cytotoxicity. Immunology. 1992;76:341–343. [PMC free article] [PubMed] [Google Scholar]
- 8.Edelson RL. Cutaneous T cell lymphoma: mycosis fungoides, Sezary syndrome, and other variants. J Am Acad Dermatol. 1980;2:89–106. doi: 10.1016/S0190-9622(80)80034-X. [DOI] [PubMed] [Google Scholar]
- 9.Eichmüller S, Usener D, Dummer R, Stein A, Thiel D, Schadendorf D. Serological detection of cutaneous T-cell lymphoma-associated antigens. Proc Natl Acad Sci USA. 2001;98:629–634. doi: 10.1073/pnas.021386498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichmüller S, Usener D, Thiel D, Schadendorf D. Tumor-specific antigens in cutaneous T-cell lymphoma: expression and sero-reactivity. Int J Cancer. 2003;104:482–487. doi: 10.1002/ijc.10967. [DOI] [PubMed] [Google Scholar]
- 11.Ellis JA, Jackman MR, Luzio JP. The post-synthetic sorting of endogenous membrane proteins examined by the simultaneous purification of apical and basolateral plasma membrane fractions from Caco-2 cells. Biochem J. 1992;283:553–560. doi: 10.1042/bj2830553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C, Nissen MH, Ropke C, Wasik MA, Odum N. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia. 2001;15:787–793. doi: 10.1038/sj.leu.2402093. [DOI] [PubMed] [Google Scholar]
- 13.Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- 14.Foss F. Mycosis fungoides and the Sezary syndrome. Curr Opin Oncol. 2004;16:421–428. doi: 10.1097/00001622-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Freist W, Gauss DH. Lysyl-tRNA synthetase. Biol Chem Hoppe Seyler. 1995;376:451–472. doi: 10.1515/bchm3.1995.376.8.451. [DOI] [PubMed] [Google Scholar]
- 16.Gosslar U, Jonas P, Luz A, Lifka A, Naor D, Hamann A, Holzmann B. Predominant role of alpha 4-integrins for distinct steps of lymphoma metastasis. Proc Natl Acad Sci USA. 1996;93:4821–4826. doi: 10.1073/pnas.93.10.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann TB, Thiel D, Dummer R, Schadendorf D, Eichmuller S. SEREX identification of new tumour-associated antigens in cutaneous T-cell lymphoma. Br J Dermatol. 2004;150:252–258. doi: 10.1111/j.1365-2133.2004.05651.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashita-Kinoh H, Kinoh H, Okada A, Komori K, Itoh Y, Chiba T, Kajita M, Yana I, Seiki M. Membrane-type 5 matrix metalloproteinase is expressed in differentiated neurons and regulates axonal growth. Cell Growth Differ. 2001;12:573–580. [PubMed] [Google Scholar]
- 20.Heinze H, Arnold HH, Fischer D, Kruppa J. The primary structure of the human ribosomal protein S6 derived from a cloned cDNA. J Biol Chem. 1988;263:4139–4144. [PubMed] [Google Scholar]
- 21.Hemler ME, Elices MJ, Parker C, Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990;114:45–65. doi: 10.1111/j.1600-065X.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 22.Heufelder AE, Wenzel BE, Bahn RS. Cell surface localization of a 72 kilodalton heat shock protein in retroocular fibroblasts from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1992;74:732–736. doi: 10.1210/jc.74.4.732. [DOI] [PubMed] [Google Scholar]
- 23.Ilangumaran S, Briol A, Hoessli DC. Distinct interactions among GPI-anchored, transmembrane and membrane associated intracellular proteins, and sphingolipids in lymphocyte and endothelial cell plasma membranes. Biochim Biophys Acta. 1997;1328:227–236. doi: 10.1016/S0005-2736(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 24.Isomura M, Okui K, Fujiwara T, Shin S, Nakamura Y. Isolation and mapping of RAB2L, a human cDNA that encodes a protein homologous to RalGDS. Cytogenet Cell Genet. 1996;74:263–265. doi: 10.1159/000134431. [DOI] [PubMed] [Google Scholar]
- 25.Jakoi ER, Kempe K, Gaston SM. Ligatin binds phosphohexose residues on acidic hydrolases. J Supramol Struct Cell Biochem. 1981;16:139–153. doi: 10.1002/jsscb.1981.380160205. [DOI] [PubMed] [Google Scholar]
- 26.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamarashev J, Burg G, Kempf W, Hess Schmid M, Dummer R. Comparative analysis of histological and immunohistological features in mycosis fungoides and Sezary syndrome. J Cutan Pathol. 1998;25:407–412. doi: 10.1111/j.1600-0560.1998.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 28.Kiat LS, Hui KM, Gopalan G. Aurora-A kinase interacting protein (AIP), a novel negative regulator of human Aurora-A kinase. J Biol Chem. 2002;277:45558–45565. doi: 10.1074/jbc.M206820200. [DOI] [PubMed] [Google Scholar]
- 29.Kim NS, Kato T, Abe N, Kato S. Nucleotide sequence of human cDNA encoding eukaryotic initiation factor 4AI. Nucleic Acids Res. 1993;21:2012. doi: 10.1093/nar/21.8.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 31.Korver W, Roose J, Heinen K, Weghuis DO, de Bruijn D, van Kessel AG, Clevers H. The human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and promoter characterization. Genomics. 1997;46:435–442. doi: 10.1006/geno.1997.5065. [DOI] [PubMed] [Google Scholar]
- 32.Llano E, Pendas AM, Freije JP, Nakano A, Knauper V, Murphy G, Lopez-Otin C. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase a overexpressed in brain tumors. Cancer Res. 1999;59:2570–2576. [PubMed] [Google Scholar]
- 33.Lynch EM, Moreland RB, Ginis I, Perrine SP, Faller DV. Hypoxia-activated ligand HAL-1/13 is lupus autoantigen Ku80 and mediates lymphoid cell adhesion in vitro. Am J Physiol Cell Physiol. 2001;280:C897–911. doi: 10.1152/ajpcell.2001.280.4.C897. [DOI] [PubMed] [Google Scholar]
- 34.McGeoch DJ. On the predictive recognition of signal peptide sequences. Virus Res. 1985;3:271–286. doi: 10.1016/0168-1702(85)90051-6. [DOI] [PubMed] [Google Scholar]
- 35.Meek K, Gupta S, Ramsden DA, Lees-Miller SP. The DNA-dependent protein kinase: the director at the end. Immunol Rev. 2004;200:132–141. doi: 10.1111/j.0105-2896.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 36.Mimori T, Ohosone Y, Hama N, Suwa A, Akizuki M, Homma M, Griffith AJ, Hardin JA. Isolation and characterization of cDNA encoding the 80-kDa subunit protein of the human autoantigen Ku (p70/p80) recognized by autoantibodies from patients with scleroderma-polymyositis overlap syndrome. Proc Natl Acad Sci USA. 1990;87:1777–1781. doi: 10.1073/pnas.87.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller S, Goletz S, Packer N, Gooley A, Lawson AM, Hanisch FG. Localization of O-glycosylation sites on glycopeptide fragments from lactation-associated MUC1. All putative sites within the tandem repeat are glycosylation targets in vivo. J Biol Chem. 1997;272:24780–24793. doi: 10.1074/jbc.272.40.24780. [DOI] [PubMed] [Google Scholar]
- 38.Nikolova M, Tawab A, Marie-Cardine A, Bagot M, Boumsell L, Bensussan A. Increased expression of a novel early activation surface membrane receptor in cutaneous T cell lymphoma cells. J Invest Dermatol. 2001;116:731–738. doi: 10.1046/j.1523-1747.2001.01305.x. [DOI] [PubMed] [Google Scholar]
- 39.Nobrega FG, Araujo PS, Pasetto M, Raw I. Some properties of cytochrome b5 from liver microsomes of man, monkey, pig and chicken. Biochem J. 1969;115:849–856. doi: 10.1042/bj1150849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okazaki Y, Ohno H, Takase K, Ochiai T, Saito T. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J Biol Chem. 2000;275:35751–35758. doi: 10.1074/jbc.M007476200. [DOI] [PubMed] [Google Scholar]
- 41.Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J Biol Chem. 1999;274:8925–8932. doi: 10.1074/jbc.274.13.8925. [DOI] [PubMed] [Google Scholar]
- 42.Rabilloud T. Membrane proteins ride shotgun. Nat Biotechnol. 2003;21:508–510. doi: 10.1038/nbt0503-508. [DOI] [PubMed] [Google Scholar]
- 43.Romanic AM, Burns-Kurtis CL, Ao Z, Arleth AJ, Ohlstein EH. Upregulated expression of human membrane type-5 matrix metalloproteinase in kidneys from diabetic patients. Am J Physiol Renal Physiol. 2001;281:F309–317. doi: 10.1152/ajprenal.2001.281.2.F309. [DOI] [PubMed] [Google Scholar]
- 44.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma M, Li X, Wang Y, Zarnegar M, Huang CY, Palvimo JJ, Lim B, Sun Z. hZimp10 is an androgen receptor co-activator and forms a complex with SUMO-1 at replication foci. Embo J. 2003;22:6101–6114. doi: 10.1093/emboj/cdg585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol. 1998;6:175–182. [PubMed] [Google Scholar]
- 47.Storz M, Zepter K, Kamarashev J, Dummer R, Burg G, Haffner AC. Coexpression of CD40 and CD40 ligand in cutaneous T-cell lymphoma (mycosis fungoides) Cancer Res. 2001;61:452–454. [PubMed] [Google Scholar]
- 48.Tai YT, Podar K, Kraeft SK, Wang F, Young G, Lin B, Gupta D, Chen LB, Anderson KC. Translocation of Ku86/Ku70 to the multiple myeloma cell membrane: functional implications. Exp Hematol. 2002;30:212–220. doi: 10.1016/S0301-472X(01)00786-X. [DOI] [PubMed] [Google Scholar]
- 49.Takada Y, Elices MJ, Crouse C, Hemler ME. The primary structure of the alpha 4 subunit of VLA-4: homology to other integrins and a possible cell-cell adhesion function. Embo J. 1989;8:1361–1368. doi: 10.1002/j.1460-2075.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thommes P, Fett R, Schray B, Burkhart R, Barnes M, Kennedy C, Brown NC, Knippers R. Properties of the nuclear P1 protein, a mammalian homologue of the yeast Mcm3 replication protein. Nucleic Acids Res. 1992;20:1069–1074. doi: 10.1093/nar/20.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uetsuki T, Naito A, Nagata S, Kaziro Y. Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha. J Biol Chem. 1989;264:5791–5798. [PubMed] [Google Scholar]
- 53.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 54.van Doorn R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R, Tensen CP. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- 55.Wada I, Rindress D, Cameron PH, Ou WJ, Doherty JJ, 2nd, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJ. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- 56.Wadle A, Mischo A, Imig J, Wullner B, Hensel D, Watzig K, Neumann F, Kubuschok B, Schmidt W, Old LJ, Pfreundschuh M, Renner C. Serological identification of breast cancer-related antigens from a Saccharomyces cerevisiae surface display library. Int J Cancer. 2005;117:104–113. doi: 10.1002/ijc.21147. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, McCarty IM, Balazs L, Li Y, Steiner MS. Immunohistological detection of BRAF25 in human prostate tumor and cancer specimens. Biochem Biophys Res Commun. 2002;295:136–141. doi: 10.1016/S0006-291X(02)00625-3. [DOI] [PubMed] [Google Scholar]
- 58.Wechsler J, Bagot M, Nikolova M, Parolini S, Martin-Garcia N, Boumsell L, Moretta A, Bensussan A. Killer cell immunoglobulin-like receptor expression delineates in situ Sezary syndrome lymphocytes. J Pathol. 2003;199:77–83. doi: 10.1002/path.1251. [DOI] [PubMed] [Google Scholar]
- 59.Whitfield JF, Sikorska M, Youdale T, Brewer L, Richards R, Walker PR. Ribonucleotide reductase–new twists in an old tale. Adv Enzyme Regul. 1989;28:113–123. doi: 10.1016/0065-2571(89)90067-8. [DOI] [PubMed] [Google Scholar]
- 60.Wiest DL, Burgess WH, McKean D, Kearse KP, Singer A. The molecular chaperone calnexin is expressed on the surface of immature thymocytes in association with clonotype-independent CD3 complexes. Embo J. 1995;14:3425–3433. doi: 10.1002/j.1460-2075.1995.tb07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 62.Wu B, Hunt C, Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yaneva M, Wen J, Ayala A, Cook R. cDNA-derived amino acid sequence of the 86-kDa subunit of the Ku antigen. J Biol Chem. 1989;264:13407–13411. [PubMed] [Google Scholar]
- 64.Yokota S, Yamamoto Y, Shimizu K, Momoi H, Kamikawa T, Yamaoka Y, Yanagi H, Yura T, Kubota H. Increased expression of cytosolic chaperonin CCT in human hepatocellular and colonic carcinoma. Cell Stress Chaperones. 2001;6:345–350. doi: 10.1379/1466-1268(2001)006<0345:IEOCCC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]