Abstract
Endothelial progenitor cells (EPCs) have been recently found to exist circulating in peripheral blood of adults, and home to sites of neovascularization in peripheral tissues. They can also be differentiated from peripheral blood mononuclear cells (PBMNCs). In tumor tissues, EPCs are found in highly vascularized lesions. Few reports exist in the literature concerning the characteristics of EPCs, especially related to their surface antigen expressions, except for endothelial markers. Here, we aimed to investigate the surface expression of differentiation markers, and the functional activities of early-outgrowth of EPCs (EO-EPCs), especially focusing on their antigen-presenting ability. EO-EPCs were generated from PBMNCs, by culture in the presence of angiogenic factors. These EO-EPCs had the morphological and functional features of endothelial cells and, additionally, they shared antigen-presenting ability. They induced the proliferation of allogeneic lymphocytes in a mixed-lymphocyte reaction, and could generate cytotoxic lymphocytes, with the ability to lyze tumor cells in an antigen-specific manner. The antigen-presenting ability of EO-EPCs, however, was weaker than that of monocyte-derived dendritic cells, but stronger than peripheral blood monocytes. Since EO-EPCs play an important role in the development of tumor angiogenesis, targeting EPCs would be an effective anti-angiogenic strategy. Alternatively, due to their antigen-presenting ability, EO-EPCs can be used as the effectors of anti-tumor immunotherapy. Since they share endothelial antigens, the activation of a cellular immunity against angiogenic vessels can be expected. In conclusion, EO-EPCs should be an interesting alternative for the development of new therapeutic strategies to combat cancer, either as the effectors or as the targets of cancer immunotherapy.
Keywords: Early-outgrowth of endothelial progenitor cells, Endothelial progenitor cells, Angiogenesis, Antigen presenting cells
Introduction
While vasculogenesis is defined as the creation of primordial vessels from mesodermal-derived endothelial progenitor cells (EPCs), namely angioblasts, angiogenesis is the formation of new vessels by sprouting of preexisting mature endothelial cells (ECs) [12, 24]. Angiogenesis plays a beneficial role in the healing response to wound or tissue ischemia and infarction [12]. However, they also contribute for tumor progression and metastasis. Recent studies have characterized EPCs, and demonstrated that they can home to sites of angiogenesis and differentiate into ECs in situ, in a manner consistent with the process of vasculogenesis [3, 4]. With the discovery of circulating EPCs in peripheral blood, it is currently believed that, even in adult tissues, a combination of angiogenesis and vasculogenesis contributes to neovascularization.
EPCs administered systemically to animals with operatively induced hindlimb ischemia were found to incorporate into sites of neovascularization in ischemic parts [3, 19]. EPCs share many properties with mature ECs, such as the expression of CD31, CD34, Flk-1 and Tie-2 [3], and the reactivity with lectin from Ulex europaeus as well as the uptake of acetylated low-density lipoprotein [3].
Few reports exist in the literature concerning the surface antigen expression of EPCs, except for endothelial markers. In the present study, we aimed to better characterize this population of endothelial precursor cells, and for this purpose, we generated EO-EPCs from human peripheral blood mono-nuclear cells (PBMNCs), and evaluated the surface expression of differentiation markers, and their functional activities, especially focusing on their antigen-presenting ability.
Materials and methods
EO-EPC isolation and identification
PBMNCs were isolated from blood of normal human volunteers, using the density gradient centrifugation on Ficoll (Ficoll-Paque, Pharmatica), according to the manufacturer’s protocol. 6×107 PBMNCs were plated on 100 mm-diameter dishes pre-coated with fibronectin and were maintained in M199 medium supplemented with 15% FCS and 2.0 ng/ml of acidic fibroblast growth factor (aFGF) and 5 μg/ml of heparin (complete medium for ECs) and incubated in a 5% CO2 incubator at 37°C. After 4 days in culture, non-adherent cells were removed and thereafter, media were changed every other day. After 7 days of culture, adherent cells were removed by trypsinization, and incubated with 1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein (DiLDL, 2 μg/ml) at 37°C for 1 h. Then, cells were fixed with 4% paraformaldehyde at 4°C for 15 min, and stained with fluorescein isothiocyanate (FITC)-labeled lectin from Ulex europaeus (Sigma). After the staining, samples were observed in a fluorescent microscope (Olympus, Japan). DiLDL and lectin double positive cells were considered to be EO-EPCs.
Isolation of monocytes, monocytes-derived dendritic cells (Mo-DCs), and human umbilical vein endothelial cells
Monocytes were obtained from venous blood drawn from normal, healthy volunteers. Briefly, monocytes were obtained by using a magnetic cell separation system (MACS; Miltenyi Biotec, Bergish Glandbach, Germany). For this purpose, PBMNCs were incubated with microbead-coupled anti-CD14 monoclonal antibody, and the magnetically labeled cells were obtained by positive selection. Mo-DCs were generated from monocytes using granulocyte-macrophage colony-stimulating factor (GM-CSF, 1,000 IU/ml; Leukomax; Novartis, Basel, Switzerland) and interleukin-4 (IL-4, 500 IU/ml; Cell Genix, Freiburg, Germany) for 7 days [5].
Human umbilical vein endothelial cells (HUVECs) were isolated from the umbilical cord blood vessels, as previously described [6]. Umbilical cords were obtained from normal pregnants at delivery, after informed consent was obtained. Briefly, the umbilical vein was cannulated at both the edges, and 0.2% collagenase-I in Ca2+-Mg2+-free phosphate-buffered saline [PBS(−)] was added to the lumen of the vessel. The umbilical cord was incubated at 37°C for 20 min, and then the PBS(−) containing collagen was recovered and centrifuged to obtain the isolated cells. Cells were washed twice with PBS(−), suspended in complete medium consisting of M199 medium supplemented with 15% fetal calf serum (FCS), 2.0 ng/ml of aFGF and 5 μg/ml of heparin, and seeded in culture dished previously coated with 0.1% gelatin.
Cells were routinely cultured in complete medium in an atmosphere of 5% CO2 at 37°C, passaged by trypsinization, and used up to six passages for the experiments.
Characterization of EPCs
To detect the surface expression of EC, antigen presenting cell, and monocyte marker proteins, EO-EPCs were harvested by trypsinization, washed twice with PBS(−), and incubated for 30 min in the presence of the antibodies, as follows. CD83 was used as the DCs marker, CD14, as the monocytes marker, HLA-DR, CD40, CD54, CD80, and CD86, as the antigen presenting marker and VEGFR2, vWF, and CD105, as endothelial cell markers (all from BD PharMingen, San Jose, CA, except vWF from Dako, Copenhagen, Denmark and VEGFR2 from SIGMA, Saint Louis, USA). Single and two-color flow-cytometric analyses (FACS) were performed using the flow cytometer (Facs Calibur, Becton-Dickinson, San Jose, CA, USA).
Vascular-like tube formation on Matrigel
The ability of EO-EPCs to form vascular-like structures on Matrigel (Matrigel Basement Membrane Matrix, Becton Dickinson, Bedford, MA, USA), was assessed. A six-well flat-bottomed plate was coated with Matrigel (100 μl/well), and EO-EPCs were seeded at 1×105 cells/well in complete medium. After incubation at 37°C for 24 h, the wells were observed using a phase contrast microscope. Images were captured with a digital camera.
Phagocytotic activity of EO-EPCs
The phagocytotic function of EO-EPCs was assessed using fluorescent micro-beads. EO-EPCs were incubated with DiLDL for 1 h, then harvested by trypsinization and incubated in a tube with 0.16% (w/v) 0.5 μm fluoresbrite carboxylate latex beads (Polyscience, Warrington, PA) for 2 h. After washing thrice with PBS, EO-EPCs were observed under fluorescent microscope.
Mixed leukocyte reaction
The ability of EO-EPCs to stimulate allogeneic lymphocytes was assessed by the mixed-lymphocyte reaction (MLR), quantified by the MTS assay (Cell Tilter 96TM Non-Radioactive Cell Proliferation Assay, Promega Corporation, Madison, WI, USA) method, according to manufacturer’s recommendations. Cultured EO-EPCs were washed with PBS and fixed with 0.025% glutaraldehyde-PBS for 20 min at room temperature. Fixed EO-EPCs were washed thrice with PBS, and. then co-cultured at different ratios (1:1, 1:10, and 1:100) with allogeneic lymphocytes as responders in a 96-well microculture plate for 7 days. The culture medium was the RPMI-1640 (Gibco/BRL), supplemented with 5% heat-inactivated FCS and IL-2 (5 U/ml, Genzyme, Cambridge, MA, USA). The ability of EO-EPCs to activate allogeneic lymphocytes to proliferate was compared to that of monocytes, Mo-DCs, lymphocytes, and HUVECs. All experiments were performed in triplicate wells. After 7 days of co-culture, MTS solution was added 20 μl/well, and after 4 h, the conversion of MTS to formazan was measured in a microplate reader at 490 nm. As a control, freshly isolated lymphocytes were cultured in the absence of EO-EPCs. Proliferative activity was calculated as the ratio of each experimental condition to the control one.
CEA derived synthetic peptide
The CEA-HLA-A24 peptide, TYACFVSNL was provided by TAKARA (Japan).
Induction of peptide-specific cytotoxic T lympocytes
EO-EPCs from HLA-A24+ healthy donors were obtained. HLA-A specificities were performed by lymphocytotoxicity test. EO-EPCs were pulsed with 40 μg/ml peptide and 3 μg/ml of β2-microglobulin for 4 h at 20°C. Then peptide pulsed EO-EPCs were irradiated (55 Gy). The autologous non-adherent PBMNCs and peptide pulsed EO-EPCs were mixed at a ratio of 20:1 and cultured in RPMI-1640 with 10 ng/ml IL-7. The responder cells were re-stimulated on day 3 and 5 with peptide and β2-microglobulin. Non-specific CTL without peptide induction and fleshly isolated lymphocytes were used as control. After 7 days of culture, responder cells were tested for cytotoxicity assay. MKN-45 (CEA+, HLA-A24+), MKN-1 (CEA−, HLA-A24+), and KATO-III (CEA+, HLA-A2+).
Cytotoxicity assay
Target cells, MKN1, MKN45, and KATO3 were labeled by incubation with calcein (10 μg/ml, Calcein-AM solution, Dojindo, Kumamoto, Japan) for 30 min. After labeling, target cells were cultured together with effecter cells in a 96-well plate at effecter/target ratio of 10:1 in RPMI-1640 with 0.2% bovine serum albumin. Triplicate wells were prepared for each group. The plate was cultured at 5% CO2, 37°C for 4 h, and then the fluorescence intensity of the target cells measured in a fluorometer (TerascanVP, Minervateck, Tokyo, Japan). Negative control was determined by wells of target cells in lysis buffer (RPMI-1640 with 0.2% bovine serum albumin and 4% Tween 80) and target cells without effecter cells were defined as positive control.
Ratio of lysis = 1 − (experimental fluorescence − negative control fluorescence)/(positive control fluorescence − negative control fluorescence).
Statistical analysis of the data
For the statistical analysis of the data, the Student’s t test was used, and P<0.05 was considered statistically significant.
Results
EO-EPCs characterization
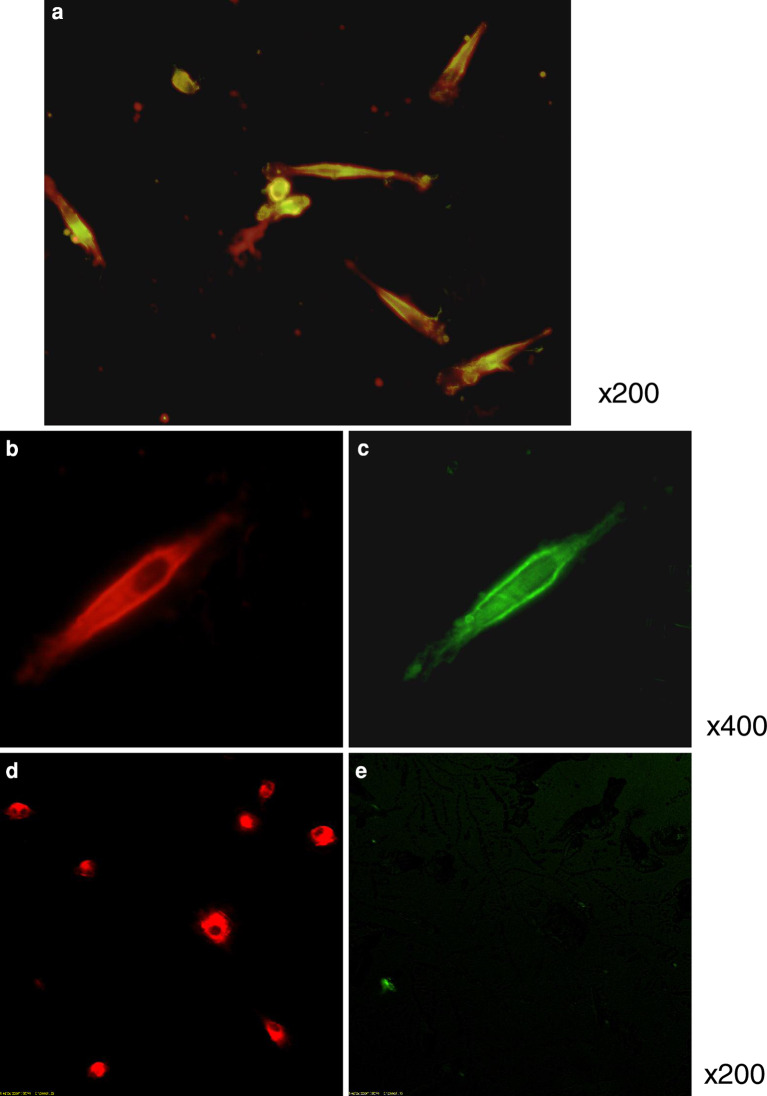
PBMNCs cultured for 7 days in complete medium, formed spindle-shaped cells and clusters resembling blood island cells (Fig. 1). The majority of the spindle-shaped cells incorporated DiLDL and bound lectin, similar to ECs (Fig. 2a–c). These are the characteristics of EPCs described in some articles [19, 30]. In contrast, Mo-DCs are able to incorporate DiLDL (Fig. 2d), but not to bind lectin (Fig. 2e).
Fig. 1.
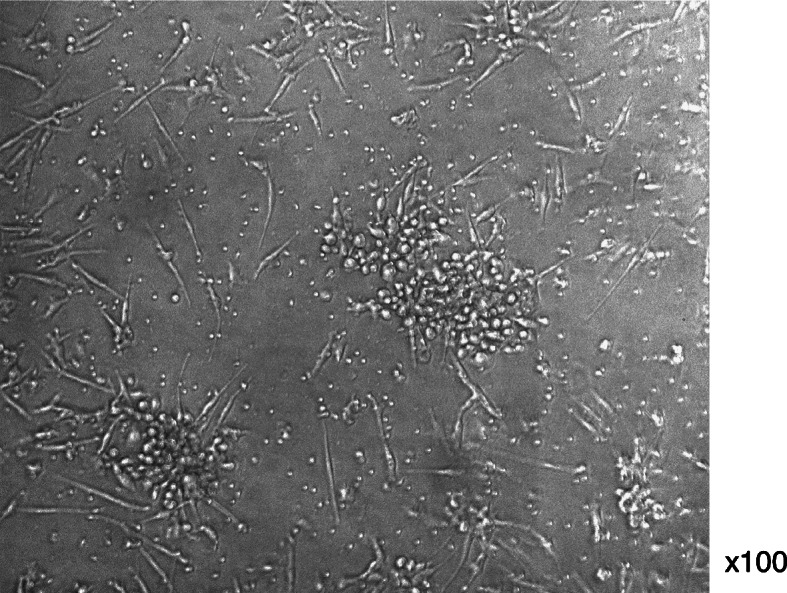
In vitro differentiation of EO-EPCs from PBMNCs in culture. PBMNCs isolated and cultured for 7 days formed spindle-shaped cells and clusters resembling blood island cell
Fig. 2.
EO-EPCs obtained by 7-day culture of PBMNCs in the presence of angiogenic factors. EO-EPCs were spindle-shaped, morphologically similar to ECs (a). EO-EPCs were tested for their ability to uptake DiLDL (red fluorescence, b) and bind lectin (green fluorescence, c). a shows the double-stained EO-EPCs. On the other hand, Mo-DCs are able to incorporate DiLDL, but not to bind lectin (d, e)
Cell surface markers of EO-EPCs
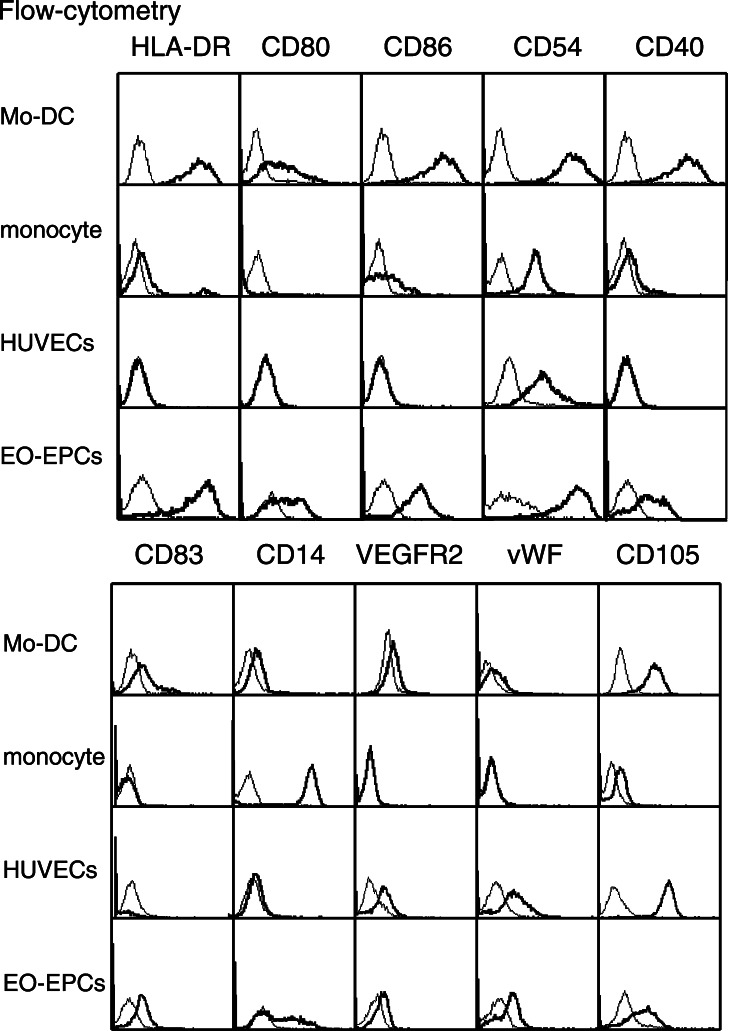
Flow-cytometric analysis revealed the cell surface expression of the antigen presenting marker proteins, namely HLA-DR, CD40, CD54, CD80, and CD86, and the expression pattern was comparable with that of DCs (Fig. 3). EO-EPCs not only expressed the DCs marker, CD83, but also a weak expression of CD14, the marker for monocytes was observed. In addition, the endothelial cell markers were also present.
Fig. 3.
Expression of antigen-presenting molecules on EO-EPCs. Flow-cytometric analysis revealed the expression of HLA-DR, CD40, CD54, CD80, and CD86 on EO-EPCs, which are the molecules expressed on Mo-DCs. The DC marker, CD83, was also present on EO-DCs. CD14 is highly expressed on peripheral blood monocytes, and weakly on Mo-DCs, but EO-EPCs could be divided into the CD14(+) and (−) subpopulations. Although weaker than HUVECs, EO-EPCs expressed endothelial cell markers (VEGRF2, vWF, and CD105)
Phagocytotic activity of EO-EPCs
Fluorescence microscopy revealed that most of the EO-EPCs that had incorporated DiLDL, also had the ability to phagocytose micro-beads. This result indicated that most EO-EPCs had the phagocytotic activity (Fig. 4).
Fig. 4.
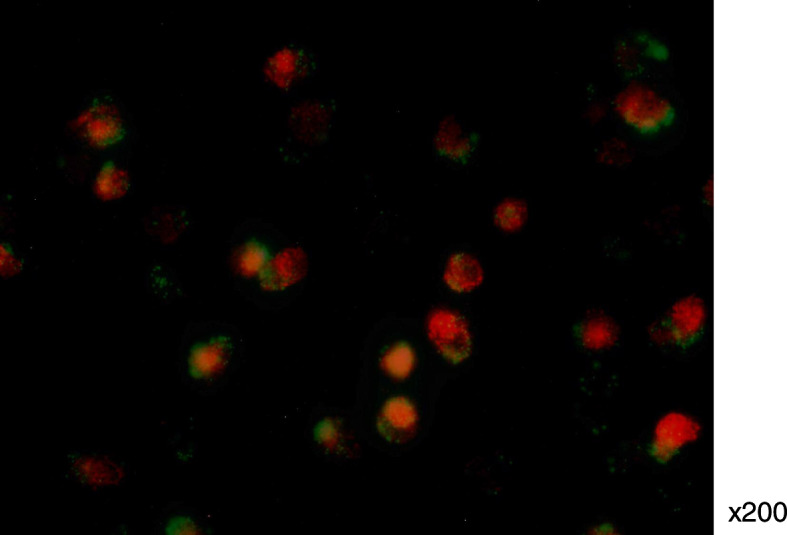
Phagocytotic ability of EO-EPCs evaluated by the fluorescent microscope. DiLDL-positive cells (red fluorescence) had the ability to phagocytose latex microbeads (green fluorescence)
Vascular-like tube formation on Matrigel
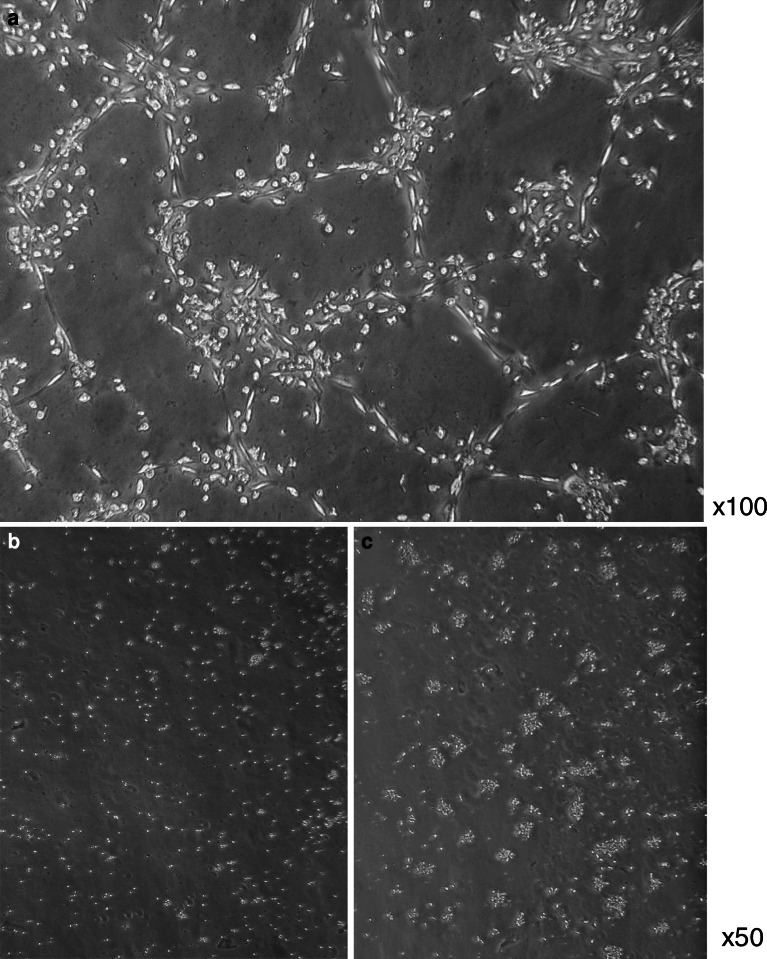
EO-EPCs, similar to the ECs, had the ability to form vascular-like tube formation on Matrigel, in the presence of complete medium of ECs. Phase contrast micrographs were taken after removal of non-adherent cells (Fig. 5a). On the other hand, monocytes (Fig. 5b), Mo-DCs (Fig. 5c) and the other peripheral blood cells did not form vascular-like tube structures on Matrigel.
Fig. 5.
In vitro capillary-like tube formation on Matrigel. EO-EPCs were seeded on Matrigel, and after 24-h incubation at 37°C, their ability to form capillary-like structures was evaluated. Representative picture (100× magnification) was obtained in a light microscope. EO-EPCs were spindle-shaped and formed clear vascular-like structures, confirming their endothelial nature (a). Monocytes (b) and Mo-DCs (c) did not form vascular-like tube structures on Matrigel
Mixed-leukocyte reaction
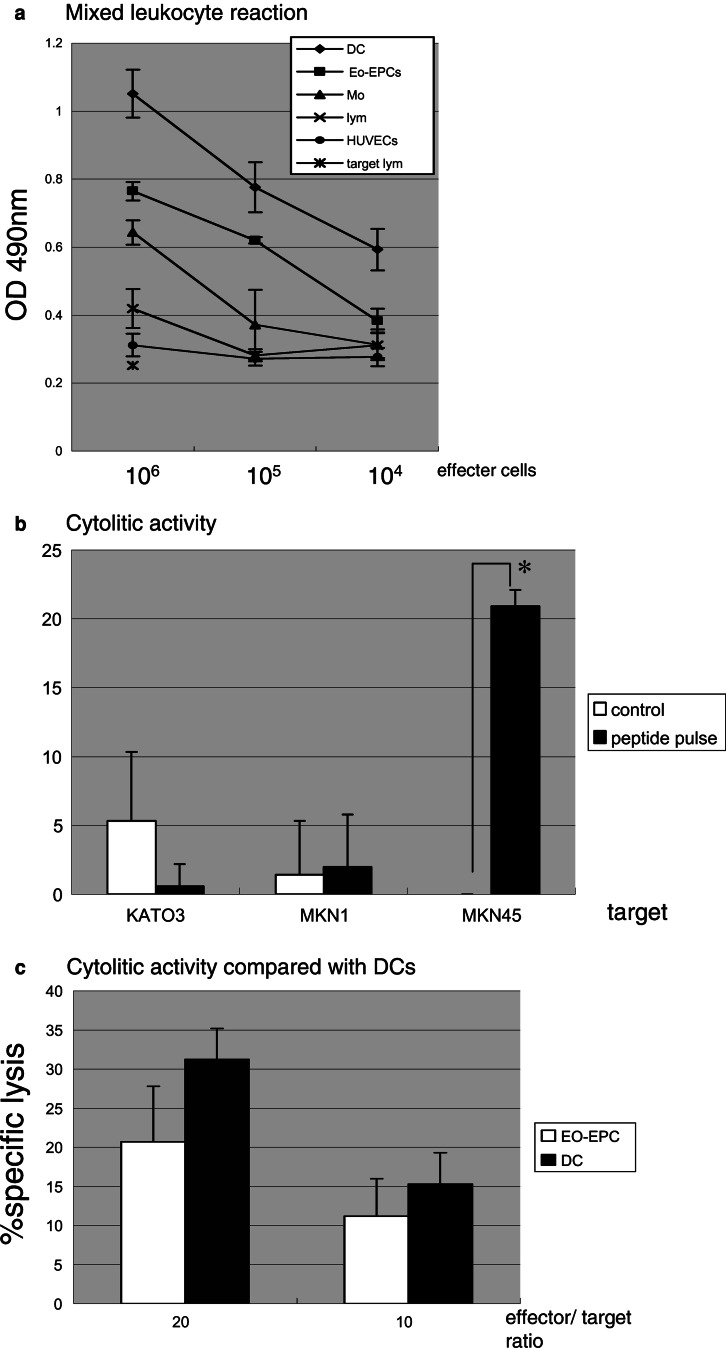
Allogeneic lymphocytes showed proliferative responses when cultured in the presence of EPCs, and the proliferative response rate correlated with the EO-EPCs/lymphocytes ratio. This ability to induce lymphocytes reaction was stronger than that of lymphocytes and monocytes, but weaker than that of DCs (Fig. 6a).
Fig. 6.
a The effect of EO-EPCs to stimulate the proliferation of allogeneic lymphocytes. EO-EPCs effectively stimulated the proliferation of allogeneic lymphocytes, but compared to DCs, the effect was weaker. b EO-EPCs were tested for their ability to stimulate HLA-restricted, CEA-specific autologous CTLs, and their ability to lyze tumor cells in vitro. CTL generated by co-culture with CEA peptide-pulsed EO-EPCs specifically and effectively lyzed the HLA-A24+, CEA+ gastric cancer cells, but not the HLA-A24(−) and/or CEA(−) cells. *P<0.05 (c) Comparison between CTLs generated by EO-EPCs and those generated by DCs, related to the lytic activity against gastric cancer cells. Both EO-EPCs and DCs were pulsed with HLA-A24-restricted CEA peptide, and then co-cultured with autologous lymphocytes. Cytotoxity of EO-EPCs-induced CTLs was weaker than that induced by DCs
Cytotoxicity assay
CTL lines elicited with or without CEA/HLA-24 binding peptide were tested for their capacity to kill the targets. CTL line pulsed by EO-EPCs with peptide had cytolytic activity against CEA+, HLA-A24 allogeneic cell line. However, they did not show cytolytic activity against CEA+, HLA-A2 cell and CEA−, HLA-A24 cell. On the other hand, CTL without peptide did not lyse any cell lines (Fig. 6b).
The cytolytic activity of CTL cultured with EO-EPC was weaker than DCs (Fig. 6c).
Discussion
Angiogenesis, the formation of new microvessels, is fundamental for tumor growth of solid tumors, by supplying nutrients to cancer cells [11, 12]. Until recently, in-growth from preexisting vessels surrounding the tumor was considered to be the principal source of this vascular supply, but recent evidence suggests that EPCs migrating from the bone marrow to the tumor site and differentiating into endothelium in the tumor bed, provide an important component of the neovasculature [2, 31].
EPCs have been shown to exist circulating in peripheral blood, but can also be obtained by culture of CD34-positive peripheral blood cells in the presence of angiogenic factors, such as bovine brain extract [3].
Here, we investigated on the characteristics of PBMNCs derived EO-EPCs, cultured in complete medium of ECs for 7 days. Analysis of the markers revealed that EO-EPCs express various levels of endothelial cell markers, such as VEGER2, von Willebrand factor, CD105. EO-EPCs also incorporated DiLDL and bound UEA-I, which are endothelial features, and additionally, they formed vascular-like structures on Matrigel. The peripheral blood monocytes, Mo-DCs, and the other peripheral blood cells were unable to form vascular-like structures when seeded on Matrigel. From these evidences, we confirmed their endothelial nature.
In addition to the endothelial cell markers, EO-EPCs expressed high levels of HLA-DR, CD80 and CD86, molecules essential for antigen presentation. Thus, we evaluated the potential antigen-presenting ability of EO-EPCs. Firstly, we investigated on the phagocytic activity of EO-EPCs, which is essential for antigen presentation. Previous works have suggested that the phagocytic ability is restricted to relatively immature DCs, monocytes, and macrophages [1, 18], but here we could demonstrate that EO-EPCs also bear phagocytic ability. Next, to evaluate the antigen-presenting function of EO-EPCs, their ability to stimulate proliferation of allogeneic lymphocytes, and additionally, to induce antigen-specific CTLs were investigated. Mo-DCs strongly stimulated the proliferation of allogeneic lymphocytes. Moreover, Mo-DCs from HLA-A24 individuals pulsed with the HLA-A24-restricted CEA peptide, efficiently induced CEA-specific CTL, which was able to specifically lyze CEA(+) HLA-A24 gastric cancer cells. Neither CEA(−) cells nor HLA-A24(−) cells could be lyzed by this CTL. The antigen-presenting ability of EO-EPCs was weaker than that of DCs, but stronger than that of monocytes and macrophages. Although previous reports [3, 8, [9, 17] have demonstrated the expression of antigen-presenting molecules on monocytes-derived endothelial-like cells, their antigen presenting ability remained to be confirmed.
Antigen-presenting cells (APC) capture antigens, process them into peptides and then present antigens to the T lymphocytes [7, 20], to stimulate antigen-specific CTLs. Among the known APCs, DCs are considered the most potent [27], and have been extensively investigated for their ability to induce anti-tumor immunity. Several clinical trials of DCs immunotherapy have been performed for lymphoma, melanoma, prostate, and renal cell cancer, with good clinical and immunological responses [15, 16, 21, 25]. Although not as potent as DCs, here we could clearly demonstrate that EO-EPCs also bear antigen-presenting abilities, stimulating antigen-specific and HLA-restricted immune responses.
Cell surface expression of HLA-DR and CD86 alone or together with CD80, are typical features of APCs, including DCs [14], and EO-EPCs, as shown here. Recently, however, microvascular ECs were also found to express CD86 and HLA-DR [22, 29], and their activation resulted in the additional expression of CD80 [13]. Moreover, IFN-gamma-treated allogeneic HUVECs and microvascular ECs were found to be capable of inducing the proliferative response of T cells [26]. These findings together indicated that ECs bear antigen-presenting properties. Inconsistent with those reports, however, we could not detect either HLA-DR, CD80 or CD86 on HUVECs, which, consistently, were unable to stimulate the proliferative activity of allogeneic lymphocytes. This discrepancy may be dependent on the differences of the activation status, since we used unstimulated HUVECs, as well as the different origins of the endothelial cell tested. EO-EPCs, independent of the activation status, expressed high levels of these molecules.
Rehman et al. [23] reported that the majority of cells that were characterized by DiLDL uptake and lectin binding were derived from the monocytes lineage. Urbich et al. [28] demonstrated that the origin of EPCs isolated from peripheral blood was not restricted to CD14-positive cells. Peripheral mononuclear cells and CD14-positive and -negative cells differentiated into EPCs and were incorporated into vascular structure of ischemic hind limb of nude mice. In the present study, EPCs included both CD14(+) and (−) populations. In a recent report [10], CD14-positive Mo-DCs were shown to differentiate into endothelial-like cells when cultured in the presence of angiogenic factors. Since Mo-DCs are CD14-weak positive cells, there is the possibility that EO-EPCs differentiate through at least two different pathways, i.e, the CD14(−) subpopulation through the DCs pathway, and the CD14(+) subpopulation directly from monocytes. Mo-DCs and EO-EPCs are quite similar cells, sharing many surface receptors, such as CD80, CD83, CD86, HLA-DR, and CD40. Additionally, DCs and monocytes, similar to EO-EPCs and HUVECs, are able to incorporate DiLDL, but they neither bind lectin nor form vascular-like structures on Matrigel.
The present results strongly suggest that EO-EPCs bear, in addition to endothelial functions, antigen-presenting abilities. Due to their endothelial functions, EO-EPCs can home and incorporate into sites of neovascularization, and in tumor tissues, they can encourage tumor growth and progression. On the other hand, by their antigen-presenting ability, EO-EPCs can activate the immune system of the tumor-bearing host, especially against tumor angiogenesis.
To the best of our knowledge, this is the first report on the ability of PBMNCs-derived EO-EPCs to activate the proliferation of allogeneic T-cells and induce peptide-specific, HLA-restricted CTL activity against cancer cells.
The selective targeting of EO-EPCs would be a promising strategy to combat cancer, since it will interrupt the oxygen and nutrients supply to tumor cells, but EO-EPCs would also serve as the APC for the induction of specific anti-angiogenic immunity by presenting self-antigens. Identification of EO-EPCs-specific antigens would help to develop new therapeutic strategies based on EO-EPCs either as the effectors or the targets of immunotherapy.
Acknowledgements
This study was supported partly by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, partly by a grant from the Ministry of Health, Labour and Welfare of Japan, and partly by a grant from the Sankyo Foundation of Life Science.
Abbreviations
- EPCs
Endothelial progenitor cells
- EO-EPCs
Early-outgrowth of endothelial progenitor cells
- ECs
Endothelial cells
- PBMNCs
Peripheral blood mononuclear cells
- aFGF
Acidic fibroblast growth factor
- DiLDL
1, 1′-Dioctadecyl-3, 3, 3′, 3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein
- APC
Antigen-presenting cells
- CTL
Cytotoxic T lymphocytes
- Mo-DCs
Monocyte-derived dendritic cells
- HUVECs
Human umbilical vein endothelial cells
- FCS
Fetal calf serum
- MLR
Mixed lymphocytes reaction
- FITC
Fluorescein isothiocyanate
References
- 1.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asakage M, Kitayama J, Tsuno NH, Komuro Y, Kaisaki S, Hori N, Nagawa H, Takahashi K. Primary malignant melanoma of the esophagus treated by esophagectomy and adjuvant dendritic-cell therapy. J Gastroenterol. 2005;40:545–546. doi: 10.1007/s00535-004-1582-8. [DOI] [PubMed] [Google Scholar]
- 6.Asakage M, Tsuno NH, Kitayama J, Kawai K, Okaji Y, Yazawa K, Kaisaki S, Takahashi K, Nagawa H. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (pravastatin) inhibits endothelial cell proliferation dependent on G1 cell cycle arrest. Anticancer Drugs. 2004;15:625–632. doi: 10.1097/01.cad.0000131680.83518.91. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Eggermann J, Kliche S, Jarmy G, Hoffmann K, Mayr-Beyrle U, Debatin KM, Waltenberger J, Beltinger C. Endothelial progenitor cell culture and differentiation in vitro: a methodological comparison using human umbilical cord blood. Cardiovasc Res. 2003;58:478–486. doi: 10.1016/S0008-6363(03)00252-9. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, Adamkiewicz J, Elsasser HP, Muller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez Pujol B, Lucibello FC, Zuzarte M, Lutjens P, Muller R, Havemann K. Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial-like cells. Eur J Cell Biol. 2001;80:99–110. doi: 10.1078/0171-9335-00136. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 13.Hancock WW, Sayegh MH, Zheng XG, Peach R, Linsley PS, Turka LA. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart PH, Whitty GA, Burgess DR, Croatto M, Hamilton JA. Augmentation of glucocorticoid action on human monocytes by interleukin-4. Lymphokine Res. 1990;9:147–153. [PubMed] [Google Scholar]
- 15.Holtl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurnher M. CD83+ blood dendritic cells as a vaccine for immunotherapy of metastatic renal-cell cancer. Lancet. 1998;352:1358. doi: 10.1016/S0140-6736(05)60748-9. [DOI] [PubMed] [Google Scholar]
- 16.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 17.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Naito M, Steinman RM. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin D, Constant S, Pasqualini T, Flavell R, Bottomly K. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J Immunol. 1993;151:6742–6750. [PubMed] [Google Scholar]
- 21.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 22.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- 23.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.CIR.0000058702.69484.A0. [DOI] [PubMed] [Google Scholar]
- 24.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 25.Salgaller ML, Lodge PA, McLean JG, Tjoa BA, Loftus DJ, Ragde H, Kenny GM, Rogers M, Boynton AL, Murphy GP. Report of immune monitoring of prostate cancer patients undergoing T-cell therapy using dendritic cells pulsed with HLA-A2-specific peptides from prostate-specific membrane antigen (PSMA) Prostate. 1998;35:144–151. doi: 10.1002/(SICI)1097-0045(19980501)35:2<144::AID-PROS8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Seino K, Azuma M, Bashuda H, Fukao K, Yagita H, Okumura K. CD86 (B70/B7-2) on endothelial cells co-stimulates allogeneic CD4+ T cells. Int Immunol. 1995;7:1331–1337. doi: 10.1093/intimm/7.8.1331. [DOI] [PubMed] [Google Scholar]
- 27.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 28.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 29.Vallee I, Guillaumin JM, Thibault G, Gruel Y, Lebranchu Y, Bardos P, Watier H. Human T lymphocyte proliferative response to resting porcine endothelial cells results from an HLA-restricted, IL-10-sensitive, indirect presentation pathway but also depends on endothelial-specific costimulatory factors. J Immunol. 1998;161:1652–1658. [PubMed] [Google Scholar]
- 30.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 31.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]