Abstract
One immunization with murine polyomavirus (MPyV) VP1 virus-like particles containing a fusion protein between MPyV VP2 and the extra cellular and transmembrane domain of Her2 (Her21–683PyVLPs) efficiently protects BALB/c mice from outgrowth of the Her2 expressing tumor D2F2/E2. To possibly enhance the anti-Her2 immune response and abrogate the induced anti-VLP antibody response, immunization with murine dendritic cells (DCs) loaded with Her21–683PyVLPs was performed. Mice were immunized once or more with 5 or 50 μg Her21–683PyVLPs alone or loaded on DCs, and challenged 14 days after the last immunization with a lethal dose of Her2-positive D2F2/E2 cells. Mice were protected from tumor outgrowth, when immunized only once with 5 or 50 μg Her21–683PyVLPs loaded on DCs, or 50 μg of Her21–683PyVLPs alone, whereas immunization once or more with 5 μg of Her21–683PyVLPs alone only protected half of the mice. Immunization with recombinant Her2 protein alone, or loaded on DCs, did not induce tumor immunity. Using both immunization strategies, Her2-specific T cell immunity was demonstrated, while Her2-specific antibodies were not detected. Loading VLPs on DCs reduced anti-VLP antibodies sixfold, but did not influence the efficiency of subsequent immunizations. Notably, DC maturation by Her21–683PyVLPs in vitro was not demonstrated although the IL-12 production was significantly increased. In conclusion, loading of VLPs on DCs can enhance specific VLP immunization considerably.
Keywords: Her2, Breast cancer, Virus-like particle, Polyomavirus and immunotherapy
Introduction
Virus-like particles (VLPs) from polyomaviruses or human papillomaviruses (HPV) are formed spontaneously when their major capsid proteins (VP1 or L1) are produced in baculovirus or in bacterial protein expression systems [7, 19]. These non-replicating VLPs attach to the cell surface and enter cells in the same way as the native virus. VLPs have been attractive candidates in gene therapy for their potential as carriers for foreign DNA, but are now also receiving growing attention as a delivery system for proteins in immune therapy, since they are capable of inducing cellular immunity without additional adjuvant [1, 3, 4, 8].
Murine polyomavirus (MPyV) VLPs are of particular interest for their use as vaccine or gene therapy vectors in humans, since humans lack pre-existing immunity towards MPyV. Although MPyV-VLPs have not been used in vivo in humans yet, VLPs from, e.g., different HPV types have been used in clinical trials for prevention against HPV type specific infection [9, 11]. Today immunization with VLPs is not only recognized as efficient, but also very safe, since VLPs are devoid of viral and oncogenic DNA [9, 11].
The proto-oncogene Her2/neu (Her2) is a member of the epidermal growth factor receptor family with tyrosine kinase activity and is amplified in many tumors leading to overexpression of the Her2 oncoprotein. Her2 overexpression, which occurs in, e.g., 25% of breast cancers, is associated with poor prognosis and is also implicated as a possible marker of resistance to tamoxifien [16, 21]. Her2 is a suitable target for immune therapy, due to its selective overexpression in tumor tissue, and since Her2-specific antibodies and T-cells have been reported to occur naturally in patients with Her2-positive tumors [6, 18]. Her2 derived vaccines based on, e.g., plasmid DNA, proteins or peptides have been developed in order to induce rejection of Her2 positive tumors. When tested in animal models and in clinical trials, these vaccines have clearly shown that it is possible to break tolerance to Her2 and generate effective T and B cell responses leading to tumor resistance [10].
Recently, we have shown that one immunization with MPyV-VLPs, containing a fusion protein between MPyV VP2 and the extra cellular and transmembrane domain of Her2 (Her21-683PyVLPs), protects against outgrowth of Her2 expressing tumors in two different in vivo systems [23]. More specifically, Her21–683PyVLP immunization protected BALB/c mice against a lethal challenge with D2F2/E2, a mammary carcinoma transfected with human Her2. In addition, immunization with Her21–683PyVLPs prevented the normally spontaneous outgrowth of mammary carcinomas in BALB-neuT mice, which are transgenic for rat Her2 [5]. This protective effect was presumably cell mediated, since a Her2-specific T-cell response was shown in an ELISPOT assay, whereas antibodies against Her2 were not detected [23]. Nevertheless, a high titer of anti-VP1 antibodies was obtained, indicating that the efficiency of a similar second immunization could be reduced, or abolished, something that has been described for HPV-VLPs [4].
Dendritic cells (DCs) are important for the induction of a tumor-specific immune response due to their unique capacity to activate a broad range of immune cells. In this study, the possibility of murine DCs loaded with Her21–683PyVLPs, to further enhance the immunization effect against the Her2 expressing tumor D2F2/E2 was examined. The possibility to obtain abrogate or reduce the antibody response to VLPs by DC immunization was also investigated. Immunizations with two different doses of Her21–683PyVLP (5 and 50 μg) alone, or loaded onto DCs, were performed to optimize the VLP dose and to examine the anti-VLP antibody response. The latter was followed by a VLP specific ELISA. In addition, an ELISPOT was used to monitor T-cell responses, while possible maturation of DCs by Her21–683PyVLPs in vitro was analyzed by flow cytometry using monoclonal antibodies against CD80, CD83, CD86, and MHC class II markers.
Materials and methods
Mice
Female BALB/c mice (6–10 weeks old), bred and maintained in pathogen free facilities at the Microbiology and Tumor Biology Center, Karolinska Institutet, were used for rejection tests.
Cell line
The D2F2/E2 cell line was obtained by transfection of the murine mammary cell line D2F2 with the Her2 expression vector pCMV/E2 and the selectable plasmid pRSV/neo, as described previously [17]. D2F2/E2 cells were maintained in vitro in DMEM supplemented with 10% heat-inactivated FCS, 10% NCTC 109 medium, 50 μM 2-ME, 0.5 mM sodium pyruvate, 2 mM L-glutamine, 0.1 mM MEM nonessential amino acids and 800 μg/ml of G418 (Sigma-Aldrich). The expression of Her2 in D2F2/E2 was confirmed by staining with a PE-conjugated anti human Her2 antibody (Becton Dickinson) one day before cells were used for tumor challenge.
Expression and purification of Her21–683PyVLPs and VP2PyVLPs
Recombinant baculoviruses expressing VP1 and VP2 or VP1 and VP2Her21–683, for the production of VP2PyVLPs and HER-21–683PyVLPs respectively, were obtained as described previously [23]. Both VP2PyVLPs and Her21–683PyVLPs were produced in Sf9 cells infected with recombinant baculoviruses and purified by an equilibrium density CsCl-gradient as described previously [22]. Purified VLPs were analyzed for their content of VP2 or VP2Her21–683 on SDS-PAGE and by a Her2 specific immunoblot.
DC purification
Murine spleen derived DCs were isolated with CD11c micro beads (Miltenyi Biotec) according to manufacturer’s protocol. Briefly, spleens were removed and cut into pieces and digested with 2 mg/ml of collagenase type D (Roche Diagnostics) for 45 min at 37°C. The cells were washed and resuspended in buffer (PBS with 0,5% BSA and 2 mM EDTA) and were labeled with anti-CD11c magnetic beads and enriched on MACS columns. The purity of the eluted cells was determined by flow cytometry using a FITC conjugated anti-CD11c antibody and CD11c positive cells (90% pure) were used in the in vivo experiments described below.
Murine bone marrow derived DCs (BMDCs) were generated according to Lutz et al. 1999 [13]. Femurs from BALB/c mice were flushed with PBS, and cells obtained this way were washed and seeded at 2 × 106 per 100 mm dish in 10 ml R10 medium (RPMI-1640 supplemented with 10% heat-inactivated FCS, 2mM L-glutamate, 2.5 mM 2-ME) containing 20 ng/ml rmGM-CSF (Peprotech). At days 3, 6 and 8 fresh R10 containing 20 ng/ml rmGM-CSF was added.
For comparison, human DCs were prepared from peripheral blood mononuclear cells (PBMC) and were maintained in X-vivo medium (Bio-Whittaker) supplemented with 50 ng/ml human granulocyte macrophage-colony stimulating factor (GM-CSF) and 40 ng/ml human interleukin 4 (IL-4) and assayed after 5–7 days of culture.
All cells were stored at 37°C, 10% CO2.
Analysis of binding and uptake of Her21–683PyVLPs
Purified Her21–683PyVLPs were labeled with Alexa Flour 488 succinimidyl ester (AF) as described previously [23].
Human or murine DCs (106) were incubated with 10 μg of Alexa Flour 488 labeled Her21–683PyVLPs in 0.5 ml of medium for 1 h at 37°C and analyzed by flow cytometry as described elsewhere [22].
For the analysis of uptake of Her21–683PyVLPs in vitro, human DCs (3 × 106) were incubated with 10 μg of VLPs at 4°C for 30 min and then half of the cells were transferred to 37°C for 1 h, whereas the other half was left at 4°C. Treatment at 37°C allows internalization of VLPs, whereas 4°C does not [22]. Thereafter each sample was divided into two, one was immediately washed in PBS to remove any non-attached VLPs, whereas the other was treated with trypsin for 5 min at 37°C in order to release VLPs bound to the cell surface [22]. Cell extracts from the different samples were analyzed for the amount of VP2HER21–683 protein using a western blot. Briefly, cell extracts were run on SDS-gel and transferred to a PVDF membrane. After blocking the membrane, it was incubated with an anti-ErbB2 antibody diluted 1:2250 (BD Biosciences Pharmingen) followed by incubation with a peroxidase conjugated anti mouse IgG antibody diluted 1:4000. Development was performed with the ECL™ Western blotting analysis system according to manufacturer’s protocol (Amersham Bioscience).
VLP-loading of DCs
Spleen derived CD11c + DCs (2 × 105) were incubated with VP2PyVLPs or Her21–683PyVLPs (5 or 50 μg) for 1 h on ice then washed twice and resuspended in PBS.
Immunization and tumor challenge
Five similar experiments were performed with 5–10 mice in each group. Two doses (5 or 50 μg) of VLPs were used. Mice were immunized with VP2PyVLPs or Her21–683PyVLPs alone or loaded on spleen derived DCs (2 × 105). Non-immunized mice were not included, since VP2PyVLPs have no protective effect against D2F2/E2 [23]. In addition, one experiment was performed with ten mice in each group, where some groups of mice were initially immunized once or two times with 5 μg of VP2PyVLPs alone, or loaded on DCs, and subsequently immunized with 5 μg of Her21–683PyVLPs and in all cases in 14 days intervals. In the final type of experiment the immunization efficiency of one, or three immunizations with 5 μg Her21–683PyVLPs alone, or loaded on DCs (in 14 day intervals), was tested. In addition, the efficiency of one immunization with 200 ng of recombinant full-length Her2 protein (kindly obtained from GENETEC) alone, or loaded on DCs was also tested. In all experiments mice were challenged subcutaneously (s.c) with 105 Her2 positive D2F2/E2 cells 14 days after the last immunization and tumor growth was followed twice a week for up to 60 days. Once the tumor reached a diameter of 10 mm, the mice were sacrificed. Mice shown as survivors at the end of the experiment did not develop tumors.
Measurement of VLP specific antibodies
Sera from immunized mice were obtained one day prior to tumor challenge. Titers of VP1 specific antibodies in mouse sera were measured by ELISA as described elsewhere [24]. Briefly, microtiter plates were coated with 0.5 μg VP2PyVLPs per well o.n. at 4°C and then the plates were blocked in milk-solution for 1 h at r.t. All sera were diluted 1/50 in milk-solution before serial dilution was performed. After 1 h incubation in r.t the plates were washed and incubated with a secondary AP-conjugated goat anti-mouse IgG (Sigma- Aldrich). All plates were washed and developed with NPP-tablets (Sigma-Aldrich) and absorbance was measured at 405 nm.
Measurement of Her2 specific antibodies
Anti Her2 IgG concentrations in sera were measured by flow cytometry. Serum samples were diluted 1:50 and added to D2F2/E2 cells and left to incubate 30 min on ice. Cells were washed and stained with FITC-conjugated goat anti mouse IgG antibody (DAKO A/S, Denmark).
ELISPOT assay
ELISPOT assay was performed according to Mabtech’s guidelines for Mouse IFN-γ ELISPOT (Mabtech, Nacka, Sweden). Briefly, splenocytes (4 × 106) from non-immunized mice and mice immunized with VP2PyVLPs (50 μg), Her21–683PyVLPs (50 μg) or DCs loaded with Her21–683PyVLPs (50 μg) were incubated in 24-well plates alone or together with D2F2/E2 cells (3 × 105), VP2PyVLPs (10 μg/ml) or Her21–683PyVLPs (10 μg/ml). The plates were incubated 24 h at 37°C and then cells (1.2 × 105) in triplicate from each well were transferred to an anti-mouse IFN-γ antibody coated ELISPOT plate and incubated 24 h at 37°C before spots were detected and counted in an ELISPOT reader (Zeiss, Germany).
Maturation assay
Non-attached immature murine BMDCs or human DCs from day 7–10 were seeded in 24-well plates at 106 cells/ml in R10 medium and cultured with Her21–683PyVLPs at different concentrations (5–50 μg/ml). LPS (1 μg/ml) was used as positive control. Phenotypic changes of DCs after interaction with Her21–683PyVLPs were investigated after 24 h of culture by flow cytometry.
PE-conjugated monoclonal antibodies (PharMingen) against the following molecules were used: CD80, CD86, CD11c and MHC class II I-Ab for mouse DCs and CD83 and CD86 for human DCs.
DCs were stained with antibodies for 30 min at 4°C, washed twice in PBS + 0.1% BSA and analyzed by flow cytometry using Cell Quest software (Becton Dickinson). Appropriate isotype controls were always included.
IL-12 production by BMDCs was assessed by an IL-12 sandwich ELISA according to manufacturer’s protocol (MabTech). Briefly, microtiter plates were coated with an anti IL-12 capture antibody at 4°C o.n. After blocking, 100 μl from the culture supernatants were added to the first row of wells, and serial dilutions were performed and plates were incubated 1 h at r.t. Plates were washed and incubated with a biotinylated anti-IL-12 antibody for 1 h at r.t. The binding reaction was visualized with AP-labeled streptavidin and NPP substrate.
Statistical analysis
Differences in survival after D2F2/E2 challenge were calculated by Fischer’s exact two-tailed test using Statistica software (StatSoft Inc.). Differences in IL-12 production and in ELISPOT data were evaluated by Student’s t test.
Results
Her21–683PyVLPs bind to and enter DCs in vitro
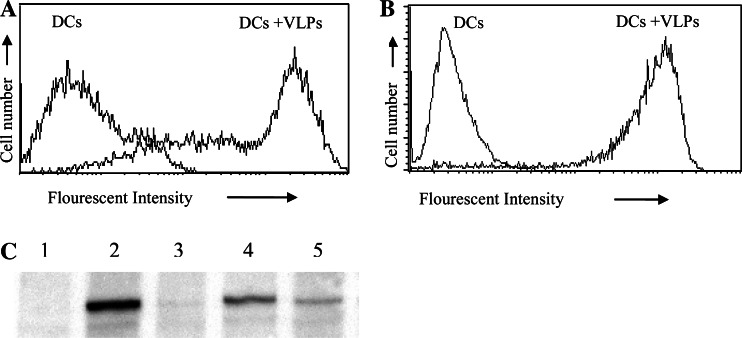
To assess the ability of the Her21–683PyVLPs to be taken up by DCs, VLPs were labeled with a fluorescent dye (Alexa Flour 488) and incubated with murine and human DCs. VLP binding was analyzed using flow cytometry and VLPs were shown to bind readily to both murine DCs (Fig. 1a) and human DCs (Fig. 1b).
Fig. 1.
Her21–683PyVLPs bind to DCs of murine (a) and human (b) origin demonstrated by flow cytometry. DCs alone or incubated with fluorescently conjugated Her21–683PyVLPs. c Her21–683PyVLPs enter DCs, confirmed with a Her2-specific immunoblot. DCs alone (Lane 1) or incubated with Her21–683PyVLPs, at 4°C (Lane 2), at 4°C followed by trypsin treatment (Lane 3), at 37°C (Lane 4) or at 37°C followed by trypsin treatment (Lane 5)
To certify that Her21–683PyVLPs not only bound to the surface of DCs, but also were internalized together with the Her2 fusion protein, we analyzed the presence of Her2 inside the cells. Trypsin treatment of cells after incubation with VLPs, releases VLPs bound to the cell surface, whereas internalized VLPs are protected from the effects of trypsin treatment. Hence, using trypsin treatment of DCs incubated with Her21–683PyVLPs for 1 h at 37°C, or at 4°C, we could demonstrate on a Her2 specific immunoblot the internalization of the Her2 fusion protein after incubation at 37°C, but not at 4°C (Fig. 1c). DCs alone did not exhibit the presence of Her2 (Lane 1). The Her2 fusion protein is demonstrated both with and without trypsin treatment of DCs incubated with Her21–683PyVLPs at 37°C (Lanes 4 and 5 respectively). However, after DC incubation at 4°C, Her2 was demonstrated only without and not after trypsin treatment (Lanes 2 and 3 respectively).
Her21–683PyVLPs loaded DCs immunize mice more efficiently than Her21–683PyVLPs alone against outgrowth of a Her2-expressing tumor
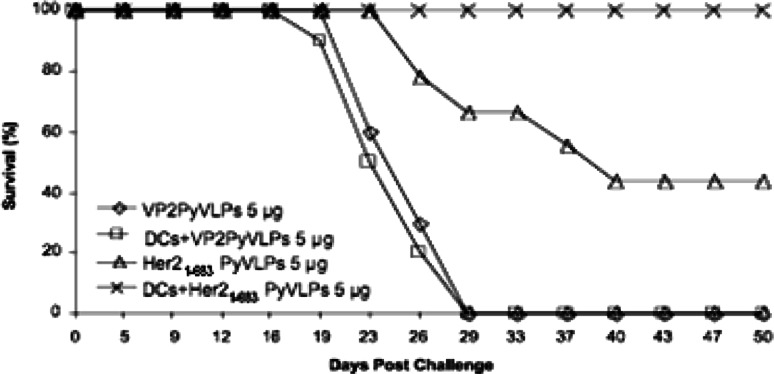
To test if Her21–683PyVLPs could induce protective immunity against a Her2-expressing tumor, D2F2/E2, five experiments were performed with two doses of VLPs (5 or 50 μg). In each test, groups of 5–10 BALB/c mice were immunized with one s.c. injection of Her21–683PyVLPs alone or with DCs (2 × 105) loaded with Her21–683PyVLPs. As controls, mice were immunized with VP2PyVLPs alone or DCs loaded with VP2PyVLPs. Fourteen days later, mice were challenged with a lethal dose of the Her2-positive D2F2/E2 cell line and followed for tumor outgrowth.
A typical experiment showing that incubation of Her21–683PyVLPs with DCs improves immunization efficiency is illustrated in Fig. 2. Clearly, Her21 –683PyVLPs loaded on DCs were more efficient immunogens than Her21–683PyVLPs alone. Only one out of ten mice incubated with 5 μg Her21–683PyVLPs loaded on DCs developed a tumor, in comparison with the group inoculated with 5 μg Her21–683PyVLPs alone, where half of the mice developed tumors. As expected, mice inoculated with either 5 μg of VP2PyVLPs alone, or VP2PyVLPs loaded on DCs all rapidly develop tumors.
Fig. 2.
Her21–683PyVLP loaded DCs efficiently protect BALB/c mice against a challenge with D2F2/E2 tumor cells. Results from one experiment with ten mice per group are shown. Mice shown as survivors at the end of the experiment did not develop tumors
A synopsis of all five experiments is presented in Table 1. As expected, mice in the control groups, immunized with VP2PyVLPs, or VP2PyVLP-loaded DCs, developed tumors (93–100% tumor takes). Immunization with both doses of Her21–683PyVLPs, either alone, or loaded on DCs, induced a significant reduction of tumor takes compared to controls. Furthermore, there was a significant difference in tumor take in mice immunized with 5 μg of Her21–683PyVLPs loaded on DCs, giving full protection, compared to immunization with Her21–683PyVLP alone, resulting in a 50% tumor take (P < 0.0001). Moreover, no tumors developed after immunization with 50 μg of Her21–683PyVLPs loaded on DCs, while 20% tumor takes was observed after immunization with 50 μg Her21–683PyVLPs alone. However, this difference was not statistically significant.
Table 1.
Her21–68 3PyVLPs loaded DCs protect BALB/c mice more efficiently than Her21–68 3PyVLPs alone against a challenge with D2F2/E2 tumor cells
| Immunogen | Amount of VLPs (μg) | Tumor takesa | Total takes | Takes (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Exp. 1b | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | ||||
| VP2PyVLPs | 50 | 5/5 | 5/5 | 100 | ||||
| 5 | 5/5 | 4/5 | 10/10 | 19/20 | 95 | |||
| DCs + VP2PyVLPs | 50 | 5/5 | 5/5 | 10/10 | 100 | |||
| 5 | 4/5 | 10/10 | 14/15 | 93 | ||||
| Her21–683PyVLPs | 50 | 0/5 | 2/5 | 2/10 | 20 | |||
| 5 | 3/5 | 2/5 | 1/5 | 6/9 | 12/24c | 50 | ||
| DCs + Her21–683PyVLPs | 50 | 0/5 | 0/5 | 0/10c | 0 | |||
| 5 | 0/5 | 0/5 | 0/10 | 0/10 | 0/25c | 0 | ||
aResults are reported as the number of tumor-positive animals per total number of animals challenged with D2F2/E2 cells
bExperiment number
cDCs loaded with 50 μg Her21–683PyVLPs gave a significant protection (P < 0.0001), compared to DCs loaded with 50 μg VP2PyVLPs (calculated from experiments 1 and 2). DCs loaded with 5 μg Her21–683PyVLPs gave a significant protection (P < 0.0001) compared to DCs loaded with 5 μg VP2PyVLPs (calculated from experiments 3 and 5), and compared to 5 μg Her21–683PyVLPs alone (calculated from experiments 2–5). Immunization with 5 μg Her21–683PyVLPs gave a significant protection (P < 0.01), compared to 5 μg VP2PyVLPs (calculated from experiments 3–5)
A separate group of mice were immunized with 5 μg of Her21–683PyVLPs loaded on murine BMDCs and the tumor take was 40% in this group (data not shown).
Immunization with Her21–683PyVLP alone or Her21–683PyVLP loaded DCs induce Her2-specific T cells
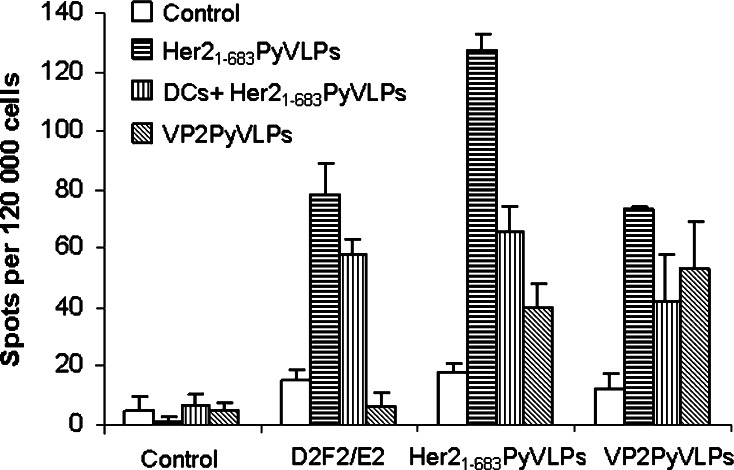
To evaluate and quantify Her2-specific T cells, an ELISPOT assay was performed. Spleens from mice, immunized once with VP2PyVLPs, Her21–683PyVLPs alone or DCs loaded with Her21–683PyVLPs, were taken 14 days after immunization. Splenocytes were incubated alone or with VP2PyVLPs, Her21–683PyVLPs or D2F2/E2 cells and the frequency of specific T cells was calculated. As expected, both T cells from mice immunized with Her21–683PyVLPs alone or loaded on DCs elicited a significant (P < 0.01) response to D2F2/E2 cells as well as against Her21–683PyVLPs. No response could be detected against D2F2/E2 cells from VP2PyVLPs immunized mice or non-immunized mice, indicating a specific Her2 response (Fig. 3).
Fig. 3.
Presence of Her2-specific T cells was demonstrated in an ELISPOT assay in mice immunized with Her21–683PyVLPs alone, and in mice immunized with dendritic cells (DCs) loaded with Her21–683PyVLPs, but not in naïve mice
Immunization with Her21–683PyVLP alone or Her21–683PyVLP loaded DCs do not induce Her2 specific antibodies
Previously, we have shown that immunization with Her21–683PyVLPs do not induce Her2-specific antibodies [23]. To investigate if that also could be the case after Her21–683PyVLP loaded DC immunization, sera from immunized mice were screened for anti-Her2 antibodies and as expected no antibodies could be detected (data not shown).
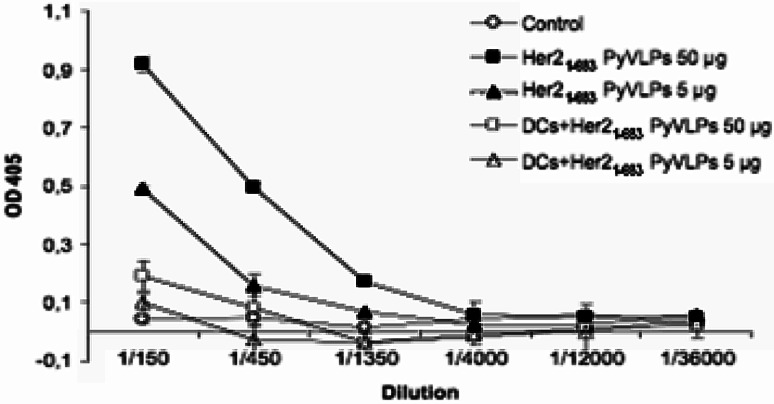
Immunization with Her21–683PyVLP loaded DCs induces lower titers of anti-VLP antibodies than immunization with Her21–683PyVLPs alone
To compare antibody titers towards VLPs after immunization with Her21–683PyVLPs alone, and DCs loaded with Her21–683PyVLPs, sera were collected 14 days after immunization and a VLP specific ELISA was performed. Immunization with Her21–683PyVLPs (both doses) loaded DCs induced approximately sixfold lower titers of anti-VLP antibodies, than immunization with Her21–683PyVLPs alone (Fig. 4). The antibody titers were also compared after one and three consecutive immunizations and using both immunization strategies the titers increased two fold after three immunizations comparison to what was obtained after one immunization (data not shown).
Fig. 4.
Immunization with Her21–683PyVLP loaded DCs induces lower titers of anti-VP1 antibodies than immunization with Her21–683PyVLPs alone. Antibody titers to VLP in BALB/c mice after one immunization as measured by ELISA. Sera were pooled from several mice in each group and error bars represent standard deviation from duplicates
Two pre-immunizations with either VP2PyVLPs alone, or loaded on DCs only marginally reduce the anti-tumor effect of a subsequent Her21–683PyVLPs immunization
To investigate to which extent anti-VLPs antibodies could influence a subsequent Her21–683PyVLP immunization, mice were pre-immunized once or two times with 5 μg VP2PyVLPs alone, or loaded on DCs, before immunization with 5 μg of Her21–683PyVLPs. A tumor challenge of 105 D2F2/E2 cells was given s.c. 2 weeks after the last immunization. After one pre-immunization with either VP2PyVLPs alone, or loaded on DCs, the tumor takes were similar to those induced in non-pre-immunized mice, and 4/10, 5/10 and 4/10 mice respectively, were protected against tumor outgrowth as shown in Table 2. Two pre-immunizations slightly reduced the anti-tumor effect, since 7/10 mice pre-immunized with VP2PyVLPs alone, or loaded on DCs, developed tumors compared to 4/10 non-pre-immunized mice, however this difference was not statistically significant.
Table 2.
Pre-immunizations with VP2PyVLPs alone or loaded on DCs do not significantly reduce the anti-tumor effect of a subsequent Her21–68 3PyVLPs immunization
| 1st immunization | 2nd immunization | 3rd immunization | Tumor takes a |
|---|---|---|---|
| DCs + VP2PyVLPs | DCs + VP2PyVLPs | Her21–683PyVLPs | 7/10 |
| – | DCs + VP2PyVLPs | Her21–683PyVLPs | 5/10 |
| VP2PyVLPs | VP2PyVLPs | Her21–683PyVLPs | 7/10 |
| – | VP2PyVLPs | Her21–683PyVLPs | 4/10 |
| – | – | Her21–683PyVLPs | 4/10 |
| – | – | – | 10/10 |
aResults are reported as the number of tumor-positive animals per total number of animals challenged with D2F2/E2 cells
A single immunization with Her21–683PyVLPs loaded on DCs, results in a more efficient anti-tumor effect than three immunizations with Her21–683PyVLPs alone
To examine if several immunizations could influence the anti-tumor effect compared to one immunization, mice were immunized once or three times with 5 μg Her21–683PyVLPs alone, or loaded on DCs. Fourteen days after the last immunization the mice were challenged with D2F2/E2 cells. As shown in Table 3, immunization once or three times using DCs completely protected mice against tumor outgrowth. Accordingly, immunization once or three times with 5 μg of Her21–683PyVLPs gave similar partial protection, resulting in tumor outgrowth in 50% of the animals.
Table 3.
A single immunization with Her21–68 3PyVLPs loaded on DCs gives stronger anti-tumor effect than three immunizations with Her21–68 3PyVLPs alone, while immunization with recombinant Her2 protein alone or loaded on DCs has no anti-tumor effect
| 1st immunization | 2nd immunization | 3rd immunization | Tumor takesa |
|---|---|---|---|
| DCs + Her21–683PyVLPs | DCs + Her21–683PyVLP | DCs + Her21–683PyVLPs | 0/10 |
| – | – | DCs + Her21–683PyVLPs | 0/10 |
| Her21–683PyVLPs | Her21–683PyVLPs | Her21–683PyVLPs | 5/10 |
| – | – | Her21–683PyVLPs | 5/10 |
| – | – | DCs + Her2 protein | 10/10 |
| – | – | Her2 protein | 9/10 |
| – | – | – | 10/10 |
aResults are reported as the number of tumor-positive animals per total number of animals challenged with D2F2/E2 cells
Immunization with recombinant Her2 protein alone or loaded on DCs does not have any anti-tumor effect
The influence of VLPs in enhancing the anti-tumor response was also investigated separately. A single immunization with 200 ng recombinant Her2 protein (which is more than the corresponding maximum amount of 80 ng of Her2 included in 5 μg of Her21–683PyVLPs) alone, or loaded on DCs, was compared to a single immunization with 5 μg of Her21–683PyVLPs alone, or loaded on DCs. As shown in Table 3, neither the recombinant Her2 protein itself or loaded on DCs protected the mice against a lethal dose of D2F2/E2 cells, as compared to full protection with Her21–683PyVLPs loaded on DCs and 50% protection with Her21–683PyVLP-loaded DCs. We therefore conclude that the specific construct of VLPs containing Her2 is of significant importance for the anti-tumor effect.
Her21–683PyVLPs induce a significant increase in IL-12 production, but do not induce maturation of human or murine DCs in vitro
To investigate if Her21–683PyVLPs induce maturation of DCs, murine BMDCs and human DCs were grown in vitro for 7–10 days, and were thereafter incubated with 5–50 μg Her21–683PyVLPs /106 DCs. After 24 h the expression of several maturation surface markers was analyzed by flow cytometry. CD80, CD86 or MHC II on murine BMDCs and CD83 and CD86 markers on human DCs were not upregulated, in contrast to the upregulation of these markers on LPS stimulated DCs (data not shown).
However, a significant (P < 0.01) increase in production of IL-12 was observed by ELISA, when comparing culture supernatants from murine DCs incubated with Her21–683PyVLPs and culture supernatants from DCs alone (0.90 ng/ml and 0.39 ng/ml, respectively). As expected, a substantial IL-12 production was observed after LPS stimulation (6.0 ng/ml).
Discussion
In this study, murine dendritic cells (DCs) loaded with Her21–683PyVLPs were compared to the use of Her21–683PyVLPs alone, for their ability to vaccinate BALB/c mice against outgrowth of the Her2 expressing tumor D2F2/E2. The possibility to obtain a lower antibody response to VLP by the DC immunization strategy and its influence on repeated immunizations was also investigated.
Both immunization strategies, i.e., Her21–683PyVLPs alone, as well as DCs loaded with Her21–683PyVLPs, protected the mice from tumor outgrowth, whereas controls, i.e., mice immunized with VP2PyVLPs alone, or loaded on DCs, developed tumors. In addition, immunization with a corresponding amount of recombinant Her2 protein alone, or loaded on DCs did not protect against tumor outgrowth, confirming the efficiency of VLP immunization. These results are thus in accordance with our previous findings, where immunization with 50 μg of Her21–683PyVLPs protected mice against outgrowth of the Her2 expressing tumor D2F2/E2 [23].
Notably, Her21–683PyVLP loaded DCs were much more efficient as immunogens compared to Her21–683PyVLPs alone. A significant difference in tumor protection was seen when mice were immunized with 5 μg of Her21–683PyVLPs loaded on DCs, which protected all mice against tumor outgrowth, compared to using 5 μg of Her21–683PyVLPs alone, which only gave a 50% protection. Furthermore, immunization with 50 μg of Her21–683PyVLPs on DCs protected all, whereas immunization with 50 μg of Her21–683PyVLPs alone protected 80% of the mice against tumor outgrowth. Moreover, one immunization with 5 μg Her21–683PyVLP-loaded DCs was still more efficient (100% protection) than three immunizations of 5 μg Her21–683PyVLPs alone (50% protection).
Immunization with Her21–683PyVLPs loaded on DCs was clearly successful. This strategy was even more efficient than immunization with Her21–683PyVLPs alone, and Her21–683PyVLPs must therefore be very effectively presented to the immune system when loaded on DCs. DC-immunization obviously requires a smaller quantity of Her21–683PyVLPs to be efficient. Incubation of 2 × 105 DCs with 5 μg of VLPs corresponds to approximately 106 VLPs/DC. Hence, both 5 and 50 μg of VLPs should be well in excess for binding to 2 × 105 DCs. It is therefore not surprising that the immunogenic effect of 5 and 50 μg of Her21–683PyVLPs loaded on to DCs was similar.
On the contrary, when immunizing with Her21–683PyVLPs alone, a high dose of Her21–683PyVLPs was necessary to get full protection against tumor outgrowth. This could be anticipated, since the low dose (5 μg) of Her21–683PyVLPs corresponds to a very small amount of Her2 molecules (approximately 80 ng). Furthermore, when introduced this way, only a minor fraction of the VLPs bind directly to antigen presenting cells.
Immunization with Her21–683PyVLPs alone, or loaded onto DCs, was not able to reject previously implanted tumors (data not shown). Still, this is not so surprising due to the aggressive nature of the D2F2/E2 tumor model.
The immunization effect of Her21–683PyVLPs has previously been shown to be T cell mediated and not antibody dependant [23]. In this study, a Her2-specific T cell response could be demonstrated after immunization with Her21–683PyVLPs alone or loaded on DCs in an ELISPOT assay, although the T cell response did not differ significantly when Her21–683PyVLPs were loaded on DCs. As expected, no anti-Her2 antibodies could be detected using either immunization strategies. In view of the fact that immunization with Her21–683PyVLPs protect against a Her2-expressing tumor in a presumably cell mediated manner, a possibility would be that Her21–683PyVLPs induce maturation of DCs. However, no upregulation of MHC II, CD80, DC83 or CD86 maturation markers on human or murine DCs could be demonstrated, when DCs were incubated with Her21–683PyVLPs in vitro. This is in accordance with recent data from Boura et al. 2005 [2], who were also unable to detect upregualtion of the same markers. Moreover, human polyomaviruses BK and JC also failed to induce maturation of human DCs in vitro, whereas human papillomavirus has been shown to do so [12]. Nevertheless, Her21–683PyVLPs do induce a slight but significant increase of IL-12 production (P > 0.01) a key cytokine in the induction of Th1 and CTL responses [14] and might still activate DCs in vivo, although this cannot be demonstrated in vitro. Boura et al. 2005 [2], suggest an alternative mechanism of cross-presentation by gap-junction-mediated immunological coupling as a possible explanation for induction of cellular immunity in vivo, the latter recently described by Neijssen et al. 2005 [15].
DC-immunization with Her21–683PyVLPs reduced the antibody response VLPs six fold, compared to the response obtained by immunizing with Her21–683PyVLPs alone. This could be due to that VLPs are not fully exposed when bound to DCs, and that the actual number of VLPs on the surface of DCs is reduced compared to when introducing VLPs alone. The reduced antibody response was expected to have some influence on the effect of repeated immunizations, since neutralizing antibodies have been shown to limit the effect of a boosting immunization [8]. However, there is also evidence that antibody-antigen complexes might even increase the efficiency of antigen presentation and T cell priming in vivo [20]. In this study, we observed that one pre-immunization with empty VLPs alone or loaded on DCs did not affect, while two pre-immunizations with the same antigens showed an insignificant tendency to decrease, the immunization efficiency of a later of Her21–683PyVLP immunization. Nevertheless, one immunization with Her21–683PyVLPs loaded on DCs, was still more efficient compared to three immunizations with Her21–683PyVLPs alone.
In summary, DC-immunization with Her21–683PyVLPs against tumor outgrowth is an attractive vaccine concept, since it increases the effect of an already efficient vaccine, i.e., immunization with Her21–683PyVLPs alone. Immunization with DCs loaded with a low dose of Her21–683PyVLP fully protected against a lethal challenge of the Her2-expressing tumor D2F2/E2, while the same dose alone did not induce full protection. We conclude that it its possible to further improve the efficiency of antigen specific VLP immunization by loading them on DCs.
Acknowledgments
We thank Fredrik Eriksson and Pavel Pisa for help with dendritic cells and Margareta Hagelin and Maj-Britt Alter for excellent technical assistance. The Swedish Cancer Foundation, the Swedish Medical Research Council (VR), the Gustav Vth Jubileum Society, the Stockholm Cancer Society, the Stockholm City Council, the EC 6th Frame Work program: COMPUVAC and the Karolinska Institute are acknowledged for financial support.
References
- 1.Boisgerault F, Moron G, Leclerc C. Virus-like particles: a new family of delivery systems. Expert Rev Vaccines. 2002;1:101–109. doi: 10.1586/14760584.1.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Boura E, Liebl D, Spisek R, Fric J, Marek M, Stokrova J, Holan V, Forstova J. Polyomavirus EGFP-pseudocapsids: analysis of model particles for introduction of proteins and peptides into mammalian cells. FEBS Lett. 2005;579:6549–6558. doi: 10.1016/j.febslet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 3.Bungener L, Idema J, ter Veer W, Huckriede A, Daemen T, Wilschut J. Virosomes in vaccine development: induction of cytotoxic T lymphocyte activity with virosome-encapsulated protein antigens. J Liposome Res. 2002;12:155–163. doi: 10.1081/LPR-120004789. [DOI] [PubMed] [Google Scholar]
- 4.Da Silva DM, Schiller JT, Kast WM. Heterologous boosting increases immunogenicity of chimeric papillomavirus virus-like particle vaccines. Vaccine. 2003;21:3219–3227. doi: 10.1016/S0264-410X(03)00237-8. [DOI] [PubMed] [Google Scholar]
- 5.Di Carlo E, Diodoro MG, Boggio K, Modesti A, Modesti M, Nanni P, Forni G, Musiani P. Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Lab Invest. 1999;79:1261–1269. [PubMed] [Google Scholar]
- 6.Disis ML, Smith JW, Murphy AE, Chen W, Cheever MA. In vitro generation of human cytolytic T-cells specific for peptides derived from the HER-2/neu protooncogene protein. Cancer Res. 1994;54:1071–1076. [PubMed] [Google Scholar]
- 7.Forstova J, Krauzewicz N, Sandig V, Elliott J, Palkova Z, Strauss M, Griffin BE. Polyoma virus pseudocapsids as efficient carriers of heterologous DNA into mammalian cells. Hum Gene Ther. 1995;6:297–306. doi: 10.1089/hum.1995.6.3-297. [DOI] [PubMed] [Google Scholar]
- 8.Greenstone HL, Nieland JD, de Visser KE, De Bruijn ML, Kirnbauer R, Roden RB, Lowy DR, Kast WM, Schiller JT. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 10.Kiessling R, Wei WZ, Herrmann F, Lindencrona JA, Choudhury A, Kono K, Seliger B. Cellular immunity to the Her-2/neu protooncogene. Adv Cancer Res. 2002;85:101–144. doi: 10.1016/S0065-230X(02)85004-7. [DOI] [PubMed] [Google Scholar]
- 11.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 12.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 13.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 14.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 15.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 16.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 17.Piechocki MP, Pilon SA, Kelly C, Wei WZ. Degradation signals in ErbB-2 dictate proteasomal processing and immunogenicity and resist protection by cis glycine-alanine repeat. Cell Immunol. 2001;212:138–149. doi: 10.1006/cimm.2001.1853. [DOI] [PubMed] [Google Scholar]
- 18.Pupa SM, Menard S, Andreola S, Colnaghi MI. Antibody response against the c-erbB-2 oncoprotein in breast carcinoma patients. Cancer Res. 1993;53:5864–5866. [PubMed] [Google Scholar]
- 19.Rose RC, Bonnez W, Reichman RC, Garcea RL. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993;67:1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuurhuis DH, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief CJ, Verbeek JS, Ossendorp F. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol. 2006;176:4573–4580. doi: 10.4049/jimmunol.176.8.4573. [DOI] [PubMed] [Google Scholar]
- 21.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 22.Tegerstedt K, Andreasson K, Vlastos A, Hedlund KO, Dalianis T, Ramqvist T. Murine pneumotropic virus VP1 virus-like particles (VLPs) bind to several cell types independent of sialic acid residues and do not serologically cross react with murine polyomavirus VP1 VLPs. J Gen Virol. 2003;84:3443–3452. doi: 10.1099/vir.0.19443-0. [DOI] [PubMed] [Google Scholar]
- 23.Tegerstedt K, Lindencrona JA, Curcio C, Andreasson K, Tullus C, Forni G, Dalianis T, Kiessling R, Ramqvist T. A single vaccination with polyomavirus VP1/VP2Her2 virus-like particles prevents outgrowth of HER-2/neu-expressing tumors. Cancer Res. 2005;65:5953–5957. doi: 10.1158/0008-5472.CAN-05-0335. [DOI] [PubMed] [Google Scholar]
- 24.Vlastos A, Andreasson K, Tegerstedt K, Hollanderova D, Heidari S, Forstova J, Ramqvist T, Dalianis T. VP1 pseudocapsids, but not a glutathione-S-transferase VP1 fusion protein, prevent polyomavirus infection in a T-cell immune deficient experimental mouse model. J Med Virol. 2003;70:293–300. doi: 10.1002/jmv.10394. [DOI] [PubMed] [Google Scholar]