Abstract
We recently reported that inhibition of Cyclooxygenase-2 (Cox-2) reduced human B-CLL proliferation and survival. Herein, we investigated the mechanisms whereby small molecule Cox-2 selective inhibitors, SC-58125 (a Celebrex analog) and CAY10404 blunt survival of human B-cell lymphomas and chronic lymphocytic leukemia B-cells. SC-58125 and OSU03012 (a Celebrex analog that lacks Cox-2 inhibitory activity) both decreased intracellular glutathione (GSH) content in malignant human B-cells, as well as in Cox-2 deficient mouse B-cells. This new finding supports Cox-2 independent effects of SC-58125. Interestingly, SC-58125 also significantly increased B-cell reactive oxygen species (ROS) production, suggesting that ROS are a pathway that reduces malignant cell survival. Addition of GSH ethyl ester protected B lymphomas from the increased mitochondrial membrane permeability and reduced survival induced by SC-58125. Moreover, the SC-58125-mediated GSH depletion resulted in elevated steady-state levels of the glutamate cysteine ligase catalytic subunit mRNA and protein. These new findings of increased ROS and diminished GSH levels following SC-58125 exposure support novel mechanisms whereby a Cox-2 selective inhibitor reduces malignant B-cell survival. These observations also support the concept that certain Cox-2 selective inhibitors may have therapeutic value in combination with other drugs to kill malignant B lineage cells.
Keywords: Cox-2, Oxidative stress, Glutathione, Lymphoma, B-cells
Introduction
Cyclooxygenase-2 (Cox-2) plays a significant role in the development and progression of cancers [1, 2]. Non-steroidal anti-inflammatory drugs (NSAIDs) and Cox-2 selective inhibitors significantly reduce the incidence and progression of tumors in animal models and in some cancer patients [3, 4]. Cox-2 has multiple procancerous effects including stimulation of angiogenesis by promoting prostaglandin E2 (PGE2), thromboxane A2, and PGI2 production and by increasing VEGF expression [5, 6]. Increased Cox-2 may also contribute to elevation of matrix metalloproteinases, inhibition of IL-12 synthesis, and increase in Bcl-2 expression [7, 8]. While the inhibition of Cox-2 activity is one method by which Cox inhibitory drugs dampen malignant cell proliferation, emerging evidence using Cox-2 deficient and Cox-2 knockdown cells suggests that Cox-2-independent effects are also involved [9–16]. However, little is known about the mechanisms by which small molecule Cox-2 selective inhibitors alter cellular processes independent of blocking Cox-2.
We and others recently reported that constitutive and inducible Cox-2 expression by malignant B lineage cells promotes their survival [17–21]. The mechanism by which the Cox-2 selective inhibitor, SC-58125, a close structural analog of Celebrex, reduces human B lymphoma/leukemia cell survival may relate to intracellular glutathione (GSH) content. Elevated GSH levels were reported in malignant B-cells and GSH can regulate prostaglandin biosynthesis [22–24]. Thus, we investigated the ability of SC-58125 to modulate intracellular GSH levels in human B-cell malignancies.
Glutathione is the major intracellular non-protein thiol anti-oxidant defense against free radicals and is an essential cofactor for many cellular functions [25]. An intact intracellular anti-oxidant system is essential for B-cells to overcome cellular damage caused by reactive oxygen species (ROS). ROS are implicated as modulators of B lymphocyte proliferation, differentiation, and apoptosis [26]. Since intracellular GSH content is critical for determining cellular sensitivity to oxidative stress and chemotherapeutic agents, we investigated the ability of the Cox-2 selective inhibitor, SC-58125, to modulate intracellular GSH levels in malignant human B-cells. In this report, we provide evidence that Cox-2 selective inhibition by SC-58125 reduces B lymphoma/leukemic cell survival by depleting intracellular GSH content and increasing oxidative stress (i.e., ROS generation).
Materials and methods
Cell culture and reagents
Ramos (ATCC CRL-1596) is a widely used human Burkitt lymphoma cell line that is Epstein Barr virus negative and whose phenotype is representative of germinal center B-cells [27]. Normal human B lymphocytes and chronic lymphocytic leukemia B-cells (B-CLL) were isolated from peripheral blood obtained from consented donors at the University of Rochester. Ethical permission for blood collection was obtained from the Research Subjects Review Board at the University of Rochester. The isolation of highly purified B lymphocytes and B-CLL cells has been previously described [21, 28]. Normal human B lymphocytes, fresh B-CLL cells, and B lymphoma cells were cultured in RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% FBS, 5 × 105 M 2-ME, 10 mM HEPES, 2 mM l-glutamine, and 50 μg/ml gentamicin. Normal B lymphocytes and B-CLL cells were cultured with or without recombinant CD40L [29]. GSH ethyl ester was purchased from Sigma, St. Louis, MO, and 100 mM stock concentrations were made in PBS and diluted to working concentrations in culture medium.
Ptgs2-knockout mouse studies
Eight- to twelve-week old Cox-2 deficient (B6.129P2-Ptgs2tm1Smi) mice and their wild-type barrier colony controls were purchased from Taconic Farms, Frederick, MD. The Animal Care and Use Committee of the University of Rochester approved all mouse protocols. Mice were anesthetized with sodium pentobarbital (60 mg/kg). Mouse B lymphocytes were isolated from spleen using the B-cell isolation kit (Miltenyi Biotec, Bisley, UK) according to the manufacturer’s protocol. The cells were washed and counted, and viability was determined using the trypan blue exclusion method. B lymphocytes isolated in this manner are >97% surface B220 positive. Purified mouse B lymphocytes were cultured in RPMI 1640 (Life Technologies) supplemented with 5% FBS, 5 × 105 M 2-ME, 10 mM HEPES, 2 mM l-glutamine, and 50 μg/ml gentamicin. Ten μg/ml of lipopolysaccharide (LPS) (Sigma) was added to cultures to stimulate mouse B-cells.
Small molecule Cyclooxygenase inhibitors
SC-58125, a highly selective Cox-2 inhibitor, was purchased from Cayman Chemical Company, Ann Arbor, MI. Experiments were also performed using CAY10404, also a highly selective Cox-2 inhibitor (Cayman Chemical Co., data not shown). SC-58125 and CAY10404 were dissolved in DMSO (10 mM stock stored at −20°C) and diluted to working concentrations in culture medium. Pharmacological doses of these drugs that have been previously shown to block PG production in vitro range from 5 to 20 μM [30] OSU03012 (Cayman Chemical Co.) is an analog of Celecoxib™ that exhibits anti-cancer activity in a Cox-2-independent manner [31]. OSU03012 was dissolved in ethanol (4 mM stock stored at −20°C) and diluted to working concentrations in culture medium.
Measurement of intracellular glutathione (GSH) levels
Total intracellular GSH levels were determined in 4 × 106 normal or malignant B-cells using the 5,5′-dithiobis-(2-nitro-benzoic acid)-GSH reductase recycling method [32]. B Lymphocytes/leukemic cells were washed twice with 1× PBS and lysed in 300 μl extraction buffer (0.1 M KH2PO4, 5 mM EDTA, 0.1% Triton X-100, 0.6% sulfosalicylic acid, pH 7.5) followed by 30 s sonication. A 10 μl aliquot of lysate was used for protein quantitation by BCA (Pierce, Rockford, IL) according to the manufacturer’s protocol. The GSH assay reagents were added to the samples in the following order: 120 μl of a 1:1 mixture of 3 U/ml GSH reductase/0.6 mg/ml 5, 5′-dithiobis (2-nitrobenzoic acid) (Sigma) for 30 s. The reaction was started by addition of 60 μl of 0.6 mg/ml βNADPH (Sigma), and the absorbance at 415 nm was monitored for 5 min. The amount of total GSH was determined from a standard curve that was linear from 0.4 to 26 nmol/ml. Monochlorobimane (MCB) (Molecular Probes, Eugene, OR) was used to determine cellular GSH levels as previously described [33]. Ten mM stock concentrations of MCB (stored at −20°C) were diluted to working concentrations in staining media containing 1× PBS/5% FBS and 2.5 mM probenecid (Sigma). Cells were incubated with 50 μM MCB in the dark for 30 min at room temperature. Cells were washed with ice cold 1× PBS/50% FBS and immediately analyzed by flow cytometry. Data were collected from analysis of 30,000 live cells. Buthionine sulfoximine (BSO) treated Ramos cells were used to set gate parameters (>98% GSH negative). Bimane-GSH expression was visualized in lymphoma cells on a Nikon Eclipse TE 2000 microscope using a 20× (numerical aperture 0.45) and 40× (numerical aperture 0.60) objective.
ESI MS/MS analysis
Small molecule drugs SC-58125 and OSU03012 were infused with GSH to the LTQ ion-trap mass spectrometer (Thermo Corporation, San Jose, CA). Each sample was loaded in solvent (0.1% acetic acid in solution of 50% acetonitrile and 50% water). Survey full scan MS spectra (from m/z 150 to 2,000) were used.
Measurement of intracellular ROS
Reactive oxygen species generation was evaluated by the ROS indicator dye 5-(and-6)-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) (Molecular Probes) [34]. Superoxide anion production was measured using dihydroethidium (Molecular Probes) [35]. Purified human B-cells, B lymphoma or B-CLL cells were loaded with 10 μM DCFDA or 20 μM dihydroethidium at the end of culture for 20 min at 37°C. DCF peroxidation or ethidium incorporation was determined as detected by flow cytometry.
Imaging cytometry
After carboxy-H2DCFDA staining, nuclei was counterstained with 5 μM Draq-5 (Axxora, LLC, San Diego, CA) and dead cell excluded by staining with 50 μg/ml 7-AAD (Invitrogen, Carlsbad, CA). Images were acquired on the ImageStream imaging flow cytometer (Amnis Corp., Seattle, WA), using the manufacturer’s acquisition software. Spectral compensation was performed as previously described and data analysis was performed using the ImageStream Data Exploration and Analysis Software (IDEAS, Amnis Corp.) [36, 37]. Briefly, debris and cell aggregates were excluded and individual cells identified by gating on nuclear intensity and aspect ratio (ratio of length to width, a measure of circularity). Individual cell populations were identified by gating on cells expressing surface makers, and confirmed by visual inspection of the pattern of fluorescence of the images.
Reverse-transcription PCR (RT-PCR)
Total RNA was extracted using Qiagen RNAeasy mini kit (Valencia, CA) following the supplier’s protocol. RNA was measured on a Bio-Rad SmartSpec 3000. One microgram of RNA was reverse-transcribed using MMLV RT primed by an oligo (dT) primer. PCR reactions for human glutamate cysteine ligase (GCL) catalytic subunit and GAPDH were performed using Platinum Taq DNA polymerase (Invitrogen). Human GCL catalytic subunit primer sequences were 5′ GTGGTACTGCTCACCAGAGTGAT CCT 3′ and 5′ TGATCCAAGTAACTCTGGACATTCACA 3′. Human GAPDH sequences were 5′ ACCACAGTCCATGCCATCAC 3′ and 5′ TCCACCACCCTGTTG CTGTA 3′. DNA products were electrophoresed on a 1.2% agarose gel.
Real-time RT-PCR
Ten ng of cDNA was analyzed by real-time RT-PCR using the following GCL catalytic subunit primer sequences:
- Sense:
5′TCCCAGATTAGGCTGTCCTG 3′ and
- Anti-sense:
5′GGACTTGGAAGCTCCTCCTT 3′ and
- Probe:
6FAM GAGGTCAAACCCAACCCAGT TAMRA.
The primers (400 nM) and probe (250 nM) were added to a final 1× universal Taqman PCR master mix (Applied Biosystems, Foster City, CA). The following PCR conditions were used: 95°C for 10 min (Amplitaq activation) followed by 50 cycles of 95°C for 30 s and 60°C for 30 s. Cycle threshold values were determined using a standard curve and analyzed on Bio-Rad Icycler Software. Cycle threshold values were normalized to GAPDH, and fold induction was calculated in drug-treated cells compared to untreated.
SDS PAGE and Western blotting
Total cellular protein was harvested after culture for 24 h. Cells were lysed in protein isolation buffer (1% IGEPAL, 150 mM sodium chloride, 50 mM TRIS, 10% protease inhibitor cocktail) and quantified by BCA protein assay kit (Pierce). Ten micrograms of protein was fractionated by SDS-PAGE and electrophoretically transferred onto nitrocellulose membranes. After blocking in 10% non-fat powdered milk in 1× PBS/0.1% Tween 20, membranes were incubated with polyclonal rabbit anti-GCL catalytic subunit antibody (Santa cruz, Santa Cruz, CA), polyclonal rabbit anti-GCL modulatory subunit antibody (Santa cruz), or a mouse anti-Actin (Oncogene Research Products, San Diego, CA) antibody for 2 h at 20°C. After washing in PBS/Tween buffer, membranes were incubated with secondary goat anti-rabbit-HRP conjugated antibody (Jackson Immunoresearch Laboratories, West Grove, PA) for 1 h at 20°C. Bands were visualized with enhanced chemiluminescence (Amersham, Piscataway, NJ) and autoradiography film according to the manufacturer’s protocol.
Mitochondrial dehydrogenase activity (MTT assay)
B-cells were incubated in triplicate at 5 × 105 cells/ml in the presence or absence of SC-58125 (or CAY10404) and compared to untreated (vehicle) cells as control. The MTT assay measures mitochondrial dehydrogenase activity and was used to assess viability. For the final 4 h of culture, a solution of 5 mg/ml of MTT diluted in PBS (10 μl/well) was added to the cells. After a total incubation of 48 h at 37°C, the plate was centrifuged, the media removed, and the insoluble precipitate dissolved in DMSO. The plate was read at 560 nm on a Benchmark ELISA plate reader (Bio-Rad, Hercules, CA).
Mitochondrial membrane permeability
B-cells were incubated in triplicate with vehicle (DMSO) or SC-58125 in the presence and absence of GSH ethyl ester (Sigma) for 12 h. Forty nM 3, 3′-dihexyloxacarbocyanine iodide (DiOC6) (Molecular Probes) was diluted in culture media and added to the cells for 15 min. Cells were harvested, washed in 1× PBS and immediately analyzed on a Beckton Dickson FACS calibur flow cytometer (BD Biosciences, San Jose, CA). Cells with intact mitochondrial membrane potential incorporate DiOC6 into the mitochondria [38].
Statistical analysis
A Student’s t-test was used to evaluate statistical significance of the data comparing two groups. Correlation coefficients were calculated to determine the dose–response relationships of SC-58125. Statistical significance was determined by paired two-way analysis of variance (ANOVA). All data are represented as mean ± SEM and statistical significance was assigned for P < 0.05.
Results
Decreased intracellular GSH content in normal and malignant B-cells in response to SC-58125
Higher levels of GSH biosynthesis enzymes and GSH levels were reported in human B-cell malignancies compared to normal B lymphocytes, supporting a role for enhanced survival with increased GSH [22, 23, 39]. We and others reported that Cox-2 inhibition can reduce B lymphoma and B-CLL survival [19–21]. We first investigated whether modulation of GSH levels was the mechanism by which the Cox-2 selective inhibitor SC-58125 reduced malignant B lineage cell survival. This was accomplished by measuring the total intracellular GSH content in the presence and absence of SC-58125 at 3, 6, 12, and 24 h. At 6 h, the highest dose of SC-58125 decreased GSH content by ∼50% compared to vehicle control. Figure 1a also shows that SC-58125 dose-dependently diminished intracellular GSH levels in human Ramos B lymphoma cells after 12 and 24 h. At the 12 h time point there was ∼15% (10 μM), ∼72% (20 μM), and ∼90% (40 μM) depletion of intracellular GSH content. Ten μM SC-58125 and 20 μM SC-58125 for 24 h depleted GSH content by 35 and 70%, respectively. This 70% decrease in total intracellular GSH (GSH + GSSG) levels by SC-58125 was not associated with increased GSSG. Five μM OSU03012, a celebrex analog that lacks Cox-2 inhibitory activity [31], also significantly reduced GSH levels ∼45%. Ramos cells treated with BSO, an agent that depletes GSH, showed ∼95% GSH depletion and was used as a positive control (data not shown).
Fig. 1.

The Cox-2 selective inhibitor, SC-58125, depletes intracellular GSH in normal and malignant B lineage cells. a Ramos B lymphomas treated with vehicle (DMSO), 10, 20, or 40 μM SC-58125 for 3, 6, 12, and 24 h were analyzed for total intracellular GSH content. A dose-dependent decrease in GSH levels was detected by 10, 20, and 40 μM SC-58125 treated cells after 6, 12, and 24 h. Five μM OSU03012 (which lacks Cox-2 inhibitory activity) decreased GSH by ∼45%. b Untreated or CD40L-stimulated normal human B lymphocytes and B-CLL cells were cultured in the presence and absence of SC-58125 for 24 h. Significantly increased GSH levels were measured in CD40L stimulated cells compared to untreated. SC-58125 induced larger dose-dependent decreases in GSH levels in B-CLL cells, compared to normal human B lymphocytes. c Total GSH content was analyzed in wildtype and Cox-2 deficient mouse B-cells cultured with nothing or LPS in the presence and absence of SC-58125 (20 μM). LPS stimulated wildtype and Cox-2 deficient B-cells treated with SC-58125 showed decreased GSH levels compared to non-drug treated cells. No differences between wildtype and Cox-2 deficient B-cells were detected in untreated or LPS stimulated B-cells. Data are shown as mean ± SEM (n = 3, *P < 0.05). d Ramos B lymphomas were treated with SC-58125 for 6, 12, 18, and 24 h and the percentage of bimane-GSH positive cells was determined by flow cytometry using the thiol reactive dye, monochlorobimane (MCB). A dose-dependent decrease in the percentage of Ramos B lymphomas was seen as early as 6 h, and persisted until 24 h. BSO (100 μM) was used to set gate parameters and show complete GSH depletion. Data shown are representative of three experiments with similar results
We next determined whether or not SC-58125 decreased intracellular GSH in rigorously purified human peripheral blood B lymphocytes and in B-CLL cells. Figure 1b shows that CD40 ligand (CD40L) stimulation significantly increased GSH levels over untreated normal and leukemic B-cells (P < 0.05). Five million normal human B lymphocytes or leukemic cells stimulated with CD40L in the presence of SC-58125 showed lower GSH levels over non-drug treated cells (Fig. 1b). The larger SC-58125-mediated decreases in GSH measured in B-CLL cells compared to normal human B lymphocytes was irrespective of baseline intracellular GSH levels. A statistically significant dose-dependent effect of SC-58125 on GSH depletion in normal B-cells and B-CLL cells was detected (P < 0.05). CD40L-stimulated B-CLL cells demonstrated 60% decreased GSH levels with 10 μM SC-58125 and 85% reductions with 40 μM SC-58125 over untreated cells. Normal B-cells showed up to a 40% decrease with SC-58125.
The finding of decreased GSH produced by OSU03012 suggests that Cox-2 inhibition is not required for GSH depletion. In order to evaluate whether or not the SC-58125-mediated GSH depletion was Cox-2 independent, GSH content was measured in wild-type and Cox-2 deficient mouse B-cells. No difference in GSH content was detected between mouse wild-type and Cox-2 deficient B-cells cultured for 24 h with nothing or LPS (Fig. 1c). Interestingly, a 33% decrease in GSH content was detected in both wild-type and Cox-2 deficient LPS-stimulated B-cells treated with SC-58125. These new findings reveal that the SC-58125-mediated GSH depletion was Cox-2 independent.
The fraction of cells that was sensitive to SC-58125-mediated GSH depletion was determined using the thiol reactive dye, MCB. Histogram plots demonstrate reductions in the percentage of bimane-GSH positive cells after SC-58125 treatment, with <1% detection in BSO (100 μM) treated cells. A total of 30,000 live cells were analyzed to determine the percent of bimane-GSH positive cells. As shown in Fig. 1d, there was a time and dose-dependent effect of SC-58125 on the percent of GSH depleted Ramos B lymphomas compared to vehicle control. Consistent with the findings of lower GSH content (Fig. 1a), significantly fewer (28%) bimane-GSH positive cells were detected as early as 6 h after treatment with SC-58125. SC-58125 exposure for 12 h resulted in a dose-dependent 5–7% decrease (5–80 μM) in the percent of bimane-GSH positive Ramos B lymphomas. A less than fourfold reduction in the percentage of bimane-GSH positive lymphoma cells was detected after 18 and 24 h of treatment with SC-58125 (80 μM). The decrease in the percentage of cells expressing bimane-GSH and the lower fluorescence intensity with SC-58125 were also visualized in Ramos B lymphomas by fluorescence microscopy (data not shown).
Increased ROS production following GSH depletion by SC-58125
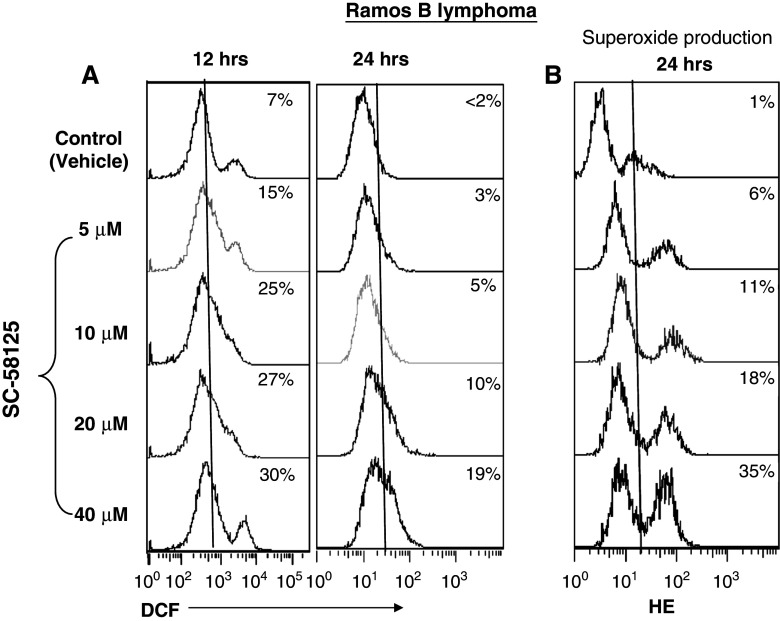
We next evaluated whether or not increased ROS production was responsible for the GSH depletion by SC-58125 (and CAY10404, data not shown). As shown in Fig. 2a, there was a dose-dependent increase in ROS production by Ramos B lymphomas as measured by DCF fluorescence and detected by flow cytometry. H2O2 was used as a positive control for ROS production (data not shown). An increase in ROS was detected in 15% of cells 12 h after incubation with 5 μM SC-58125. Thirty percent of cells demonstrated increased ROS production after 12 h of treatment with 40 μM SC-58125 compared to vehicle treated control (7%). Significantly less than twofold increased mean fluorescence intensity of DCF positive cells was seen after 12 h of drug treatment (P < 0.05) (Fig. 2a). Elevated ROS levels remained detectable 24 h after treatment with SC-58125 compared to untreated cells. Dihydroethidium was next used to evaluate whether superoxide anion generation was a contributor to the increased ROS production. Figure 2B shows a dose-dependent increase in the percentage of cells staining positive for superoxide anion production after treatment with SC-58125. Doses as low as 5 μM SC-58125 were shown to induce increases in superoxide production. Higher doses of SC-58125 produced even larger increases in ethidium incorporation after 24 h as detected by flow cytometry.
Fig. 2.
Increased ROS production by SC-58125 treated Ramos B lymphoma cells. a Ramos B lymphoma cells treated with SC-58125 showed an increase in the percentage of DCF positive cells as detected by flow cytometry. Histogram plots show the percentage of DCF positive cells increases with the dose of SC-58125 treated Ramos B lymphomas compared to vehicle control. b An increase in the percentage of cells positive for superoxide anion production was detected by dihydroethidium (HE) staining 24 h after exposure to SC-58125 at doses as low as 5 μM
The SC-58125 mediated induction of ROS as determined by DCF fluorescence was also visualized by imaging flow cytometry (see Materials and methods) in Ramos B lymphomas (Fig. 3). DCF intensity was determined only in live cells that were positive for Draq-5 and negative for 7-AAD. Draq-5 is rapidly taken up by live cells. Apoptotic or necrotic cells, due to loss of membrane permeability, will be positive for 7-AAD and negative for Draq-5. Cells that were double positive for Draq-5 and 7-AAD were considered early apoptotic (data not shown). Figure 3 shows that live SC-58125 treated cells had enhanced production of ROS compared to live OSU03012 treated B lymphoma cells. However, a subset of early apoptotic cells treated with OSU03012 (R2 gate, Fig. 3) had substantially greater ROS production suggesting a delay in the Cox-2 independent induction of ROS.
Fig. 3.
Cox-2 independent ROS induction in Ramos B lymphoma cells. DCF fluorescence intensity was analyzed by imaging flow cytometry in Ramos B lymphoma cells either a untreated or treated with b SC-58125 (20 μM) or c OSU03012 (5 μM). 7-AAD (cell impermeable) and Draq5 (cell permeable) dyes were used to analyze for cell viability. SC-58125 and OSU03012 showed an increased percentage of ROS positive cells compared to untreated. SC-58125 induced ROS in a larger proportion of live cells compared to OSU03012. A subset of early apoptotic OSU03012 treated cells (R2 gate-Draq5 and 7-AAD double positive) showed the largest increase in DCF fluorescence
The findings of increased ROS production by SC-58125 in B lymphomas prompted us to investigate whether or not this also occurred in normal human B lymphocytes. Consistent with our findings in Figs. 2 and 3, SC-58125 increased ROS production in both unactivated and CD40L-stimulated normal human B lymphocytes as detected by DCF fluorescence (Fig. 4a). There was a dramatic 85% increase in ROS production by unactivated B-cells (20 μM SC-58125) compared to vehicle control. The 85% of DCF-positive B-cells with elevated ROS had fourfold increased mean fluorescence intensity with 20 and 40 μM SC-58125 compared to untreated B lymphocytes (Fig. 4b). CD40L-stimulated B-cells also showed a dose-dependent increase in DCF-positive cells after 24 h. Five μM SC-58125 induced ROS in 41% of cells compared to 16% in vehicle treated cells. Treatment with 20 μM SC-58125 showed 66% of cells with increased ROS production. These data reveal that SC-58125 may decrease survival by causing oxidative stress in normal and malignant human B-cells.
Fig. 4.
Increased ROS production by SC-58125 in normal human B lymphocytes. a Normal human B lymphocytes cultured with nothing or CD40L and treated in the presence of SC-58125 for 24 h showed significantly (85%) increased ROS production as detected by DCF compared to vehicle control treated cells. b Significantly increased mean fluorescence intensity of normal human B lymphocytes (cultured with nothing or CD40L) treated with SC-58125 were detected compared to control cells (n = 3, *P < 0.05)
Steady-state glutamate cysteine ligase catalytic subunit (GCLC) mRNA levels induced by SC-58125
To further investigate the mechanism by which SC-58125 depleted GSH in Ramos B lymphomas, we next examined whether or not there were differences in the expression of the rate limiting enzyme in GSH biosynthesis, namely GCL [40]. The effect of SC-58125 on GCL mRNA expression was first investigated in normal and malignant human B-cells. GCL is comprised of two subunits: the catalytic subunit (GCLC, 73 kDa) that is inducible by oxidative stress, and the modulatory subunit (GCLM, 23 kDa) which increases the affinity of the catalytic subunit for glutamate [41]. The data in Fig. 5a show that CD40L plus anti-IgM antibody-activated B lymphocytes treated for 48 and 72 h with 20 or 40 μM SC-58125 showed increased steady-state GCLC mRNA levels as detected by semi-quantitative RT-PCR. No changes in GCLM mRNA were detected (data not shown). In contrast to normal human B lymphocytes that express low basal levels of GCLC mRNA, Ramos B lymphomas and B-CLL cells constitutively expressed high levels of GCLC mRNA (Fig. 5a).
Fig. 5.
Increased steady-state GCLC mRNA levels in SC-58125 treated normal and malignant human B-cells. a GCLC mRNA levels were measured in SC-58125 treated normal human B-cells, Ramos B lymphomas and B-CLL cells. PCR products were analyzed on a 1.2% agarose gel. Increased GCLC mRNA expression was detected by normal B-cells treated with SC-58125 for 48 or 72 h. b An ∼4–12-fold induction of GCLC mRNA levels in SC-58125 treated Ramos B lymphomas over untreated controls was detected by real-time RT-PCR. Cycle threshold values were normalized to GAPDH. c Unstimulated or CD40L stimulated B-CLL cells treated with SC-58125 for 24 h showed a less than fivefold induction over non-drug treated samples (n = 3, *P < 0.05)
We next utilized a sensitive real-time RT-PCR approach to quantify changes in GCLC mRNA levels in malignant B lymphoma/leukemic cells. Cycle threshold values were generated for each sample using a standard curve for GCLC and GAPDH. Following normalization to GAPDH levels, 8-fold and 12-fold increased steady-state GCLC mRNA levels were detected in Ramos B lymphomas treated with as low as 5 μM SC-58125 for 24 h compared to vehicle control (Fig. 5b). Purified primary human B-CLL cells showed less than fourfold inductions in SC-58125 treated cells compared to untreated after 24 h (Fig. 5c). CD40L stimulation modestly elevated GCLC expression over unstimulated (approximately twofold); however, GCLC mRNA levels were further significantly induced (less than fourfold) by CD40L-stimulated B-CLL cells treated with 20 or 40 μM SC-58125 over non-drug treated CD40L-stimulated B-CLL cells (P < 0.05).
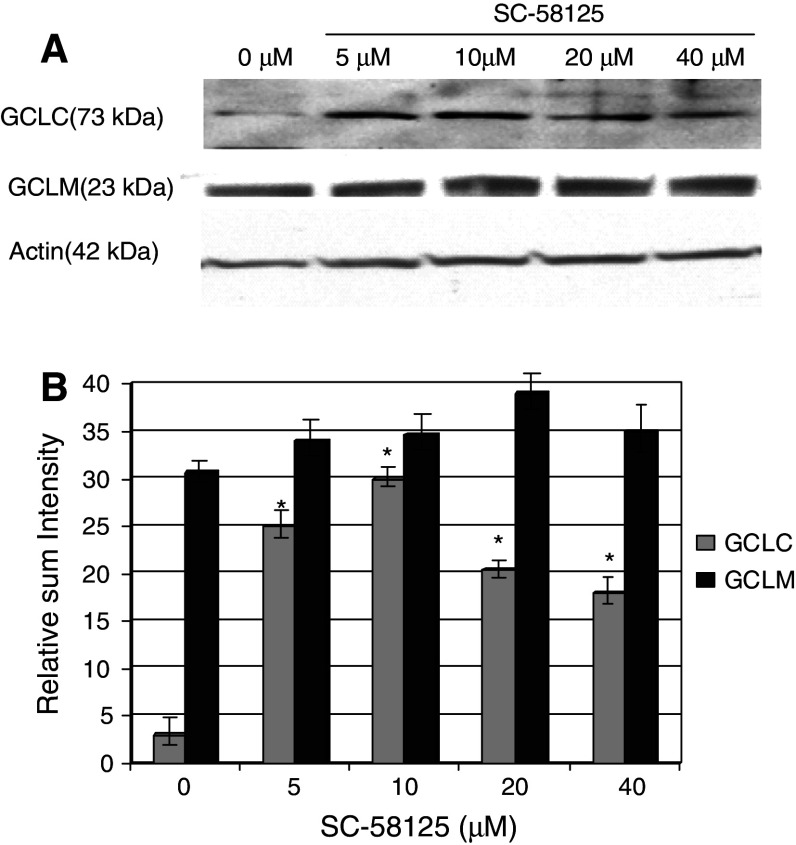
The results of increased GCLC mRNA levels by SC-58125 prompted us to determine whether or not there was enhanced GCLC protein expression. Ramos B lymphomas treated with SC-58125 showed increased expression of GCLC protein after 24 h as detected by Western blot analysis (Fig. 6a). No major differences following drug treatment were seen in GCLM protein expression. Densitometry analysis revealed that Ramos B lymphomas treated with 5 μM SC-58125 demonstrate a fivefold induction in the relative sum intensity of GCLC expression over untreated cells (Fig. 6b). The increased GCLC expression by SC-58125 resulted in modest increases in GCL activity (data not shown). These new data show that depletion of GSH by SC-58125 enhances oxidative stress in normal and malignant human B-cells and promotes GSH synthesis via the up-regulation of GCLC expression.
Fig. 6.
Increased GCLC protein expression in Ramos B lymphoma cells by SC-58125. a Ramos B lymphomas treated with SC-58125 increased GCLC protein levels in a dose-dependent manner after 24 h as detected by Western blot. No major changes in GCLM protein expression were seen following treatment with SC-58125. b Densitometry analysis of Western blot in panel A shows significant fourfold increased GCLC expression in SC-58125 treated cells compared to untreated (n = 3, *P < 0.05)
Reduced survival of B lymphomas rescued by GSH ethyl ester
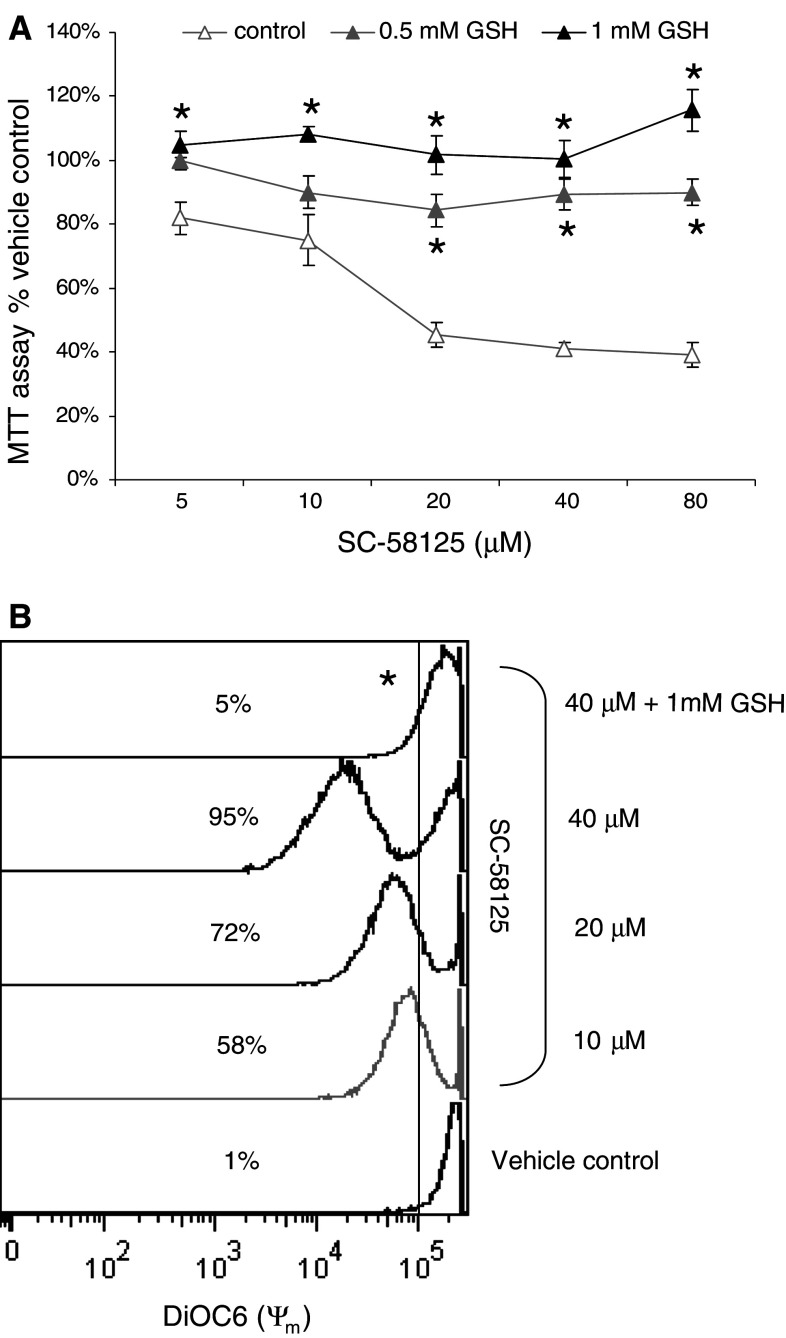
To gain insight into the ability of GSH to protect against the reduced viability of Cox-2 selective inhibition, GSH was added to SC-58125-treated B lymphomas. Mitochondrial dehydrogenase activity was measured after 48 h by MTT assay. As shown in Fig. 7a, 1 mM GSH completely restored the dose-dependent reduction in mitochondrial dehydrogenase activity. A statistically significant rescue in survival by GSH was detected at 5 μM SC-58125. Further, the anti-oxidant and GSH pre-cursor n-acetyl-l cysteine produced protective effects against SC-58125-mediated reduction in mitochondrial dehydrogenase activity (data not shown). These findings suggest that GSH depletion by SC-58125 induces mitochondrial changes in B lymphomas that adversely affect their survival. Encouraged by the finding that GSH restored mitochondrial dehydrogenase activity, we investigated the ability of GSH to prevent SC-58125-mediated mitochondrial membrane permeability in Ramos B lymphomas using DIOC6 incorporation. Live cells fully incorporate DIOC6 whereas apoptotic cells do not because of increased mitochondrial membrane permeability. Figure 7b shows that GSH prevented ∼90% of B lymphoma cells from the SC-58125-induced changes in mitochondrial membrane potential. These findings indicate that modulation of GSH levels by SC-58125 induces changes in mitochondrial dehydrogenase activity and mitochondrial membrane permeability that reduce B lymphoma cell viability.
Fig. 7.
GSH restores SC-58125 induced mitochondrial alterations in Ramos B lymphoma cells. a SC-58125 dose-dependently reduced mitochondrial dehydrogenase activity in Ramos B lymphomas after 48 h as detected by MTT assay. Addition of GSH ethyl ester (1 mM) significantly restored the dose-dependent SC-58125 mediated reduction in mitochondrial activity. About 0.5 mM GSH ethyl ester showed partial rescue of the reduced mitochondrial activity. b SC-58125 increased mitochondrial membrane permeability in ∼90% of Ramos B lymphomas after 12 h as determined by DIOC6 incorporation and detected by flow cytometry. Co-incubation of 1 mM GSH ethyl ester and SC-58125 (4 μM) completely restored mitochondrial membrane potential (n = 3, *P < 0.05)
Discussion
This report provides the first evidence that the Cox-2 selective inhibitor SC-58125 decreases intracellular GSH content in normal and malignant human B-cells. We also show significantly increased production of ROS in response to SC-58125 that may also contribute to reduced survival. Neoplastic cells may be more vulnerable to agents that augment oxidative stress because they function with elevated basal levels of ROS [42]. Celecoxib (also known as Celebrex), a clinically used Cox-2 selective inhibitor with close structural similarity to SC-58125, was shown to induce apoptosis via a mitochondrial-dependent pathway [43–45]. Our new data show that addition of GSH restores B lymphoma/leukemic cell mitochondrial alterations caused by SC-58125, namely increased mitochondrial membrane permeability and reduced mitochondrial dehydrogenase. This finding suggests that GSH depletion may be an upstream mediator of mitochondrial dysfunction and apoptosis in these cancer cells. Interference with DNA binding transcription factors (e.g., Sp1 and hypoxia inducible factor-1) was also reported as a Cox-2-independent anti-cancer effect of Cox-2 selective inhibitors [10, 46]. Our results suggest that depletion of GSH is involved in mediating such Cox-2 independent effects, as GSH is a critical cofactor for many intracellular processes, including regulation of transcription factors [25]. GSH depletion by SC-58125 in Cox-2-deficient mouse B-cells and Cox-2 negative naive human B-cells indicates that GSH is a viable Cox-2-independent target. Furthermore, the celecoxib analog that lacks Cox-2 inhibitory activity, OSU03012, also reduced intracellular GSH. Taken together, these data support that the dramatic decrease in GSH by these small molecules represents an important mechanism that triggers multiple downstream consequences, ultimately leading to cell death.
The finding that SC-58125 depletes GSH in malignant human B-cells has important implications for elucidating the mechanisms by which Cox-2 selective inhibitors are beneficial chemotherapeutic agents. Our mass spectrometry analysis did not reveal direct interactions between GSH and SC-58125 nor OSU03012 (data not shown). However, several studies demonstrate that chemotherapeutic agents reduce malignant B-cell survival in part by increased formation of ROS [35, 47]. A decrease in GSH alone was shown to act as a potent early activator of apoptotic signaling in a B lymphoma cell line. However, increased ROS production following mitochondrial GSH depletion was a crucial event, which irreversibly commits some B lymphomas to apoptosis [22]. Our data show that SC-58125 induced a dose-dependent increase in ROS production by normal and malignant human B-cells. These findings demonstrate that increased ROS production is a new mechanism by which the Cox-2 selective inhibitor SC-58125 influences normal and malignant human B lineage cell survival. Increased ROS production was detected 12 h after drug treatment. The time course of events post-SC-58125 treatment suggests that ROS production may contribute to and be a result of GSH depletion (seen as early as 6 h, Fig. 1a, d). Given that naive human B lymphocytes do not express Cox-2 [28], these new findings of SC-58125 mediated GSH depletion/ROS production by unstimulated human B lymphocytes indicates that the effects of SC-58125 on GSH depletion and ROS generation were Cox-2-independent. The Cox-2-independent nature of these effects was further supported by the fact that SC-58125 mediated GSH depletion in Cox-2 deficient mouse B-cells.
Interestingly, SC-58125 enhanced the expression of GCLC, the rate-limiting step in GSH biosynthesis. SC-58125 increased both steady-state GCLC mRNA and protein levels. These data reveal activation of a modulatory pathway of GSH biosynthesis in response to decreased GSH by the Cox-2 selective inhibitor, SC-58125. Further investigation of cellular GSH metabolism in B-cells is necessary to determine which processes are involved in SC-58125-mediated depletion of GSH. Moreover, we suggest that high levels of dietary anti-oxidant supplementation may reduce the efficacy of potential chemotherapeutic benefits of Cox-2 selective inhibitors and warrant further investigation of the effects of Cox-2 selective inhibition on GSH depletion in malignant B lineage cells in humans.
Strict regulation of reduction-oxidation reactions is critical for B-cells to avoid oxidative stress and cellular damage that may ultimately be tumorigenic [42, 48]. B-cell malignancies can arise in an environment of chronic B-cell activation, and we speculate that increased Cox-2 levels in lymphomas were a result of inducible Cox-2 in activated normal B-cells. Elevated and constitutive Cox-2 expression in cancer cells can promote tumor progression by promoting cell growth and angiogenesis, as well as by decreasing immune surveillance [49, 50]. We determined a range of GSH levels in malignant human B-cells (lymphomas/leukemias) that overlap and exceed GSH levels in normal human B lymphocytes. These findings are consistent with previous reports; however, it is largely unknown at what stage of tumorigenesis elevated GSH levels are detectable. Furthermore, B-CLL is a heterogeneous disease with a variable clinical course and therefore may exhibit a spectrum of GSH levels. Our finding that exposure to Cox-2 selective drugs modulates cellular GSH levels also contributes to understanding the potential chemopreventive properties of these drugs. Epidemiological evidence has shown a role for anti-inflammatory drugs in reducing the risk of developing non-Hodgkins lymphoma [51–53]. To our knowledge, there are no reports that elucidate the cellular and molecular mechanisms by which the use of Cox-2 selective inhibitors can reduce the risk of lymphoma. Further investigation is needed to determine if a decrease in GSH levels is a mechanism by which non-steroidal anti-inflammatory drug use and Cox-2 deficient mice demonstrate resistance to developing cancers [54]. Therefore, pre-malignant B-cells treated with SC-58125 or similar compounds may prevent development of B lymphoma by a combination of both Cox-2-dependent and Cox-2-independent mechanisms. Future studies will be necessary to identify the precise mechanism(s) of SC-58125-mediated GSH depletion and whether or not this is a mechanism by which Cox inhibitors reduce the risk of developing B-cell malignancies in humans.
Acknowledgments
This work was supported by the following NIH grants DE011390, ES01247, HL078603, T32-ES07026, AI071064 and R25CA102618 and a grant from the Leukemia and Lymphoma Society at the James P. Wilmot Foundation.
Abbreviations
- Cox-2
Cyclooxygenase-2
- GSH
Glutathione
- ROS
Reactive oxygen species
- GCL
Glutamate cysteine ligase
References
- 1.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26:1393–1399. [PubMed] [Google Scholar]
- 2.Chen Q, Shinohara N, Abe T, Watanabe T, Nonomura K, Koyanagi T. Significance of COX-2 expression in human renal cell carcinoma cell lines. Int J Cancer. 2004;108:825–832. doi: 10.1002/ijc.11646. [DOI] [PubMed] [Google Scholar]
- 3.Dubois RN. Review article: cyclooxygenase—a target for colon cancer prevention. Aliment Pharmacol Ther. 2000;14(Suppl 1):64–67. doi: 10.1046/j.1365-2036.2000.014s1064.x. [DOI] [PubMed] [Google Scholar]
- 4.Samoha S, Arber N. Cyclooxygenase-2 inhibition prevents colorectal cancer: from the bench to the bed side. Oncology. 2005;69(Suppl 1):33–37. doi: 10.1159/000086630. [DOI] [PubMed] [Google Scholar]
- 5.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhi YH, Liu RS, Song MM, Tian Y, Long J, Tu W, Guo RX. Cyclooxygenase-2 promotes angiogenesis by increasing vascular endothelial growth factor and predicts prognosis in gallbladder carcinoma. World J Gastroenterol. 2005;11:3724–3728. doi: 10.3748/wjg.v11.i24.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito H, Duxbury M, Benoit E, Clancy TE, Zinner MJ, Ashley SW, Whang EE. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res. 2004;64:7439–7446. doi: 10.1158/0008-5472.CAN-04-1177. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Kang KH, Kim SH, Lee JH, Cho CM, Kweon YO, Kim SK, Choi YH, Bae HI, Kim MS. Expression of Cyclooxygenase-2 and Bcl-2 in human gastric adenomas. Korean J Intern Med. 2005;20:198–204. doi: 10.3904/kjim.2005.20.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charames GS, Bapat B. Cyclooxygenase-2 knockdown by RNA interference in colon cancer. Int J Oncol. 2006;28:543–549. [PubMed] [Google Scholar]
- 10.Han S, Roman J. COX-2 inhibitors suppress lung cancer cell growth by inducing p21 via COX-2 independent signals. Lung Cancer. 2006;51(3):283–296. doi: 10.1016/j.lungcan.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Palayoor ST, Arayankalayil MJ, Shoaibi A, Coleman CN. Radiation sensitivity of human carcinoma cells transfected with small interfering RNA Targeted against cyclooxygenase-2. Clin Cancer Res. 2005;11:6980–6986. doi: 10.1158/1078-0432.CCR-05-0326. [DOI] [PubMed] [Google Scholar]
- 12.Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AJ, Hsu AL, Lin HP, Song X, Chen CS. The cyclo-oxygenase-2 inhibitor celecoxib perturbs intracellular calcium by inhibiting endoplasmic reticulum Ca2+-ATPases: a plausible link with its anti-tumour effect and cardiovascular risks. Biochem J. 2002;366:831–837. doi: 10.1042/BJ20020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier TJ, Janssen A, Schmidt R, Geisslinger G, Grosch S. Targeting the beta-catenin/APC pathway: a novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. Faseb J. 2005;19:1353–1355. doi: 10.1096/fj.04-3274fje. [DOI] [PubMed] [Google Scholar]
- 15.Weber A, Casini A, Heine A, Kuhn D, Supuran CT, Scozzafava A, Klebe G. Unexpected nanomolar inhibition of carbonic anhydrase by COX-2-selective celecoxib: new pharmacological opportunities due to related binding site recognition. J Med Chem. 2004;47:550–557. doi: 10.1021/jm030912m. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Song X, Lin HP, Young DC, Yan S, Marquez VE, Chen CS. Using cyclooxygenase-2 inhibitors as molecular platforms to develop a new class of apoptosis-inducing agents. J Natl Cancer Inst. 2002;94:1745–1757. doi: 10.1093/jnci/94.23.1745. [DOI] [PubMed] [Google Scholar]
- 17.Phipps RP, Ryan E, Bernstein SH. Inhibition of cyclooxygenase-2: a new targeted therapy for B-cell lymphoma? Leuk Res. 2004;28:109–111. doi: 10.1016/S0145-2126(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 18.Hazar B, Ergin M, Seyrek E, Erdogan S, Tuncer I, Hakverdi S. Cyclooxygenase-2 (Cox-2) expression in lymphomas. Leuk Lymphoma. 2004;45:1395–1399. doi: 10.1080/10428190310001654032. [DOI] [PubMed] [Google Scholar]
- 19.Wun T, McKnight H, Tuscano JM. Increased cyclooxygenase-2 (COX-2): a potential role in the pathogenesis of lymphoma. Leuk Res. 2004;28:179–190. doi: 10.1016/S0145-2126(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 20.Secchiero P, Barbarotto E, Gonelli A, Tiribelli M, Zerbinati C, Celeghini C, Agostinelli C, Pileri SA, Zauli G. Potential pathogenetic implications of cyclooxygenase-2 overexpression in B chronic lymphoid leukemia cells. Am J Pathol. 2005;167:1599–1607. doi: 10.1016/S0002-9440(10)61244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan EP, Pollock SJ, Kaur K, Felgar RE, Bernstein SH, Chiorrazi N, Phipps RP. Constitutive and activation-inducible cyclooxygenase-2 expression enhances survival of chronic lymphocytic leukemia B cells. Clin Immunol. 2006;120(1):76–90. doi: 10.1016/j.clim.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 23.Ferraris AM, Rolfo M, Mangerini R, Gaetani GF. Increased glutathione in chronic lymphocytic leukemia lymphocytes. Am J Hematol. 1994;47:237–238. doi: 10.1002/ajh.2830470318. [DOI] [PubMed] [Google Scholar]
- 24.Margalit A, Hauser SD, Zweifel BS, Anderson MA, Isakson PC. Regulation of prostaglandin biosynthesis in vivo by glutathione. Am J Physiol. 1998;274:R294–R302. doi: 10.1152/ajpregu.1998.274.2.R294. [DOI] [PubMed] [Google Scholar]
- 25.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–1503. doi: 10.1016/S0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 26.Fedyk ER, Phipps RP. Reactive oxygen species and not lipoxygenase products are required for mouse B-lymphocyte activation and differentiation. Int J Immunopharmacol. 1994;16:533–546. doi: 10.1016/0192-0561(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 27.Padilla J, Leung E, Phipps RP. Human B lymphocytes and B lymphomas express PPAR-gamma and are killed by PPAR-gamma agonists. Clin Immunol. 2002;103:22–33. doi: 10.1006/clim.2001.5181. [DOI] [PubMed] [Google Scholar]
- 28.Ryan EP, Pollack SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 29.Kehry MR, Castle BE. Regulation of CD40 ligand expression and use of recombinant CD40 ligand for studying B cell growth and differentiation. Semin Immunol. 1994;6:287–294. doi: 10.1006/smim.1994.1037. [DOI] [PubMed] [Google Scholar]
- 30.Capone ML, Tacconelli S, Sciulli MG, Patrignani P. Clinical pharmacology of selective COX-2 inhibitors. Int J Immunopathol Pharmacol. 2003;16:49–58. [PubMed] [Google Scholar]
- 31.Johnson AJ, Smith LL, Zhu J, Heerema NA, Jefferson S, Mone A, Grever M, Chen CS, Byrd JC. A novel celecoxib derivative, OSU03012, induces cytotoxicity in primary CLL cells and transformed B-cell lymphoma cell line via a caspase- and Bcl-2-independent mechanism. Blood. 2005;105:2504–2509. doi: 10.1182/blood-2004-05-1957. [DOI] [PubMed] [Google Scholar]
- 32.Eady JJ, Orta T, Dennis MF, Stratford MR, Peacock JH. Glutathione determination by the Tietze enzymatic recycling assay and its relationship to cellular radiation response. Br J Cancer. 1995;72:1089–1095. doi: 10.1038/bjc.1995.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staal FJ, Roederer M, Herzenberg LA, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, Arbiser JL. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr Virus (EBV)-positive versus EBV-negative Burkitt’s lymphoma. Proc Natl Acad Sci USA. 2005;102:175–179. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- 36.George TC, Fanning SL, Fitzgeral-Bocarsly P, Medeiros RB, Highfill S, Shimizu Y, Hall BE, Frost K, Basiji D, Ortyn WE, Morrissey PJ, Lynch DH. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J Immunol Methods. 2006;311:117–129. doi: 10.1016/j.jim.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Ortyn WE, Hall BE, George TC, Frost K, Basiji DA, Perry DJ, Zimmerman CA, Coder D, Morrissey PJ. Sensitivity measurement and compensation in spectral imaging. Cytometry A. 2006;69:852–862. doi: 10.1002/cyto.a.20306. [DOI] [PubMed] [Google Scholar]
- 38.Ray DM, Akbiyik F, Phipps RP. The peroxisome proliferator-activated receptor {gamma} (PPAR{gamma}) ligands 15-deoxy-{delta}12,14-prostaglandin J2 and ciglitazone induce human B lymphocyte and B cell lymphoma apoptosis by PPAR{gamma}-independent mechanisms. J Immunol. 2006;177:5068–5076. doi: 10.4049/jimmunol.177.8.5068. [DOI] [PubMed] [Google Scholar]
- 39.Inoue H, Takemura H, Kawai Y, Yoshida A, Ueda T, Miyashita T. Dexamethasone-resistant human Pre-B leukemia 697 cell line evolving elevation of intracellular glutathione level: an additional resistance mechanism. Jpn J Cancer Res. 2002;93:582–590. doi: 10.1111/j.1349-7006.2002.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seelig GF, Simondsen RP, Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem. 1984;259:9345–9347. [PubMed] [Google Scholar]
- 41.Rahman I, Bel A, Mulier B, Lawson MF, Harrison DJ, Macnee W, Smith CA. Transcriptional regulation of gamma-glutamylcysteine synthetase-heavy subunit by oxidants in human alveolar epithelial cells. Biochem Biophys Res Commun. 1996;229:832–837. doi: 10.1006/bbrc.1996.1888. [DOI] [PubMed] [Google Scholar]
- 42.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Ding H, Han C, Zhu J, Chen CS, D’Ambrosio SM. Celecoxib derivatives induce apoptosis via the disruption of mitochondrial membrane potential and activation of caspase 9. Int J Cancer. 2005;113:803–810. doi: 10.1002/ijc.20639. [DOI] [PubMed] [Google Scholar]
- 44.Jendrossek V, Handrick R, Belka C. Celecoxib activates a novel mitochondrial apoptosis signaling pathway. Faseb J. 2003;17:1547–1549. doi: 10.1096/fj.02-0947fje. [DOI] [PubMed] [Google Scholar]
- 45.Johnson AJ, Smith LL, Zhu J, Heerema NA, Jefferson S, Mone A, Grever M, Chen CS, Byrd JC. A novel celecoxib derivative, OSU03012, induces cytotoxicity in primary CLL cells and transformed B-cell lymphoma via a caspase and Bcl-2 independent mechanism. Blood. 2005;15(6):2504–2509. doi: 10.1182/blood-2004-05-1957. [DOI] [PubMed] [Google Scholar]
- 46.Zhong H, Willard M, Simons J. NS398 reduces hypoxia-inducible factor (HIF)-1alpha and HIF-1 activity: multiple-level effects involving cyclooxygenase-2 dependent and independent mechanisms. Int J Cancer. 2004;112:585–595. doi: 10.1002/ijc.20438. [DOI] [PubMed] [Google Scholar]
- 47.Carew JS, Zhou Y, Albitar M, Carew JD, Keating MJ, Huang P. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003;17:1437–1447. doi: 10.1038/sj.leu.2403043. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson J, Soderberg O, Nilsson K, Rosen A. Differentiation-associated redox-regulation in human B cell lines from stem cell/pro-B to plasma cell. Immunol Lett. 2004;94:83–89. doi: 10.1016/j.imlet.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/S0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 50.Chu AJ, Chou TH, Chen BD. Prevention of colorectal cancer using COX-2 inhibitors: basic science and clinical applications. Front Biosci. 2004;9:2697–2713. doi: 10.2741/1429. [DOI] [PubMed] [Google Scholar]
- 51.Chang ET, Zheng T, Weir EG, Borowitz M, Mann RB, Spiegelman D, Mueller NE. Aspirin and the risk of Hodgkin’s lymphoma in a population-based case-control study. J Natl Cancer Inst. 2004;96:305–315. doi: 10.1093/jnci/djh038. [DOI] [PubMed] [Google Scholar]
- 52.Beiderbeck AB, Holly EA, Sturkenboom MC, Coebergh JW, Stricker BH, Leufkens HG. Prescription medications associated with a decreased risk of non-Hodgkin’s Lymphoma. Am J Epidemiol. 2003;157:510–516. doi: 10.1093/aje/kwg004. [DOI] [PubMed] [Google Scholar]
- 53.Ellen T, Chang KEmS. Medication use and risk of non-Hodgkin’s Lymphoma. Am J Epidemiol. 2005;162:965–974. doi: 10.1093/aje/kwi311. [DOI] [PubMed] [Google Scholar]
- 54.Rao CV, Reddy BS. NSAIDs and chemoprevention. Curr Cancer Drug Targets. 2004;4:29–42. doi: 10.2174/1568009043481632. [DOI] [PubMed] [Google Scholar]