Abstract
Objective:
To study the association between the Sarnat Exam (SE) performed before and after therapeutic hypothermia (TH) and outcomes at 2-years in infants with moderate or severe hypoxic-ischemic encephalopathy (HIE).
Design:
Secondary analysis of the High-dose Erythropoietin for Asphyxia and EncephaLopathy trial. Adjusted odds ratios (aORs) for death or neurodevelopmental impairment (NDI) based on SE severity category and change in category were constructed, adjusting for sedation at time of exam. Absolute SE score and its change were compared for association with risk for death or NDI using locally estimated scatterplot smoothing curves.
Setting:
Randomized, double-blinded, placebo-controlled multicenter trial including 17 centers across the United States.
Patients:
479/500 enrolled neonates who had both a qualifying SE (qSE) before TH and a SE after rewarming (rSE).
Interventions:
Standardized SE was used across sites before and after TH. All providers underwent standardized SE training.
Main outcome measures:
Primary outcome was defined as the composite outcome of death or any NDI at 22-36 months.
Results:
Both qSE and rSE were associated with the primary outcome. Notably, an aOR for primary outcome of 6.2 (95%CI 3.1-12.6) and 50.3 (95%CI 13.3-190) was seen in those with moderate and severe encephalopathy on rSE, respectively Persistent or worsened severity on rSE was associated with higher odds for primary outcome compared to those who improved, even when qSE was severe.
Conclusion:
Both rSE and change between qSE and rSE were strongly associated with the odds of death/NDI at 22-36 months in infants with moderate or severe HIE.
BACKGROUND
In high-resource settings, hypoxic-ischemic encephalopathy (HIE) affects approximately 1-4/1,000 life births. Therapeutic hypothermia (TH) improves outcome after moderate or severe HIE, (1-4) though up to half of affected neonates still experience adverse outcomes such as death or neurodevelopmental impairment (NDI).(2, 5) The Sarnat Exam (SE) was initially developed in 1976 to describe the dynamic clinical nature of neonatal encephalopathy over time.(6, 7) It has been adapted several times and is now commonly referred to as the “modified SE”.(3, 4, 6, 8-12) The SE is widely used to assess the initial stage of encephalopathy and to determine eligibility for TH. The Thompson Encephalopathy Score (TS), developed to simplify the SE, is an alternative clinical assessment.(13) The two scoring systems vary slightly and are reported differently, the TS as a numerical score, and the SE generally as a categorical result.
The initial SE and TS when performed shortly after birth are poorly correlated with outcome.(14, 15) However, evolution of the TS over the first week better predicts outcomes.(16) In studies of SE performed on day of life 3, 4, or after rewarming, varying relations to short-term outcome are described; associations with long-term outcomes are promising but remain unclear due to confounding factors such as sedative medications and variable study populations.(17-19) Numerical assessments of the SE have shown superior performance compared to the categorical interpretation with respect to predicting magnetic resonance imaging (MRI) injury, but it is not known whether numerical SE or change in SE over time is associated with long-term outcome.(20, 21)
We hypothesized that the change in Sarnat category, the individual components of the modified SE, as well as its numerical equivalent, before and after TH would be associated with the risk of death or NDI at 22-36 months of age in a large contemporary cohort of neonates with moderate or severe HIE.
METHODS
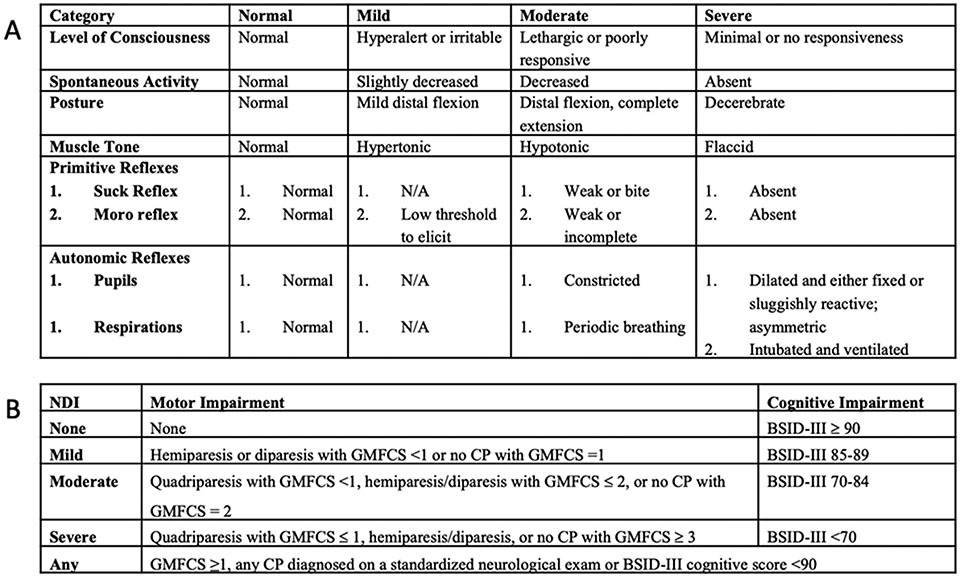
This is a secondary analysis of the multicenter, double-blinded, randomized, placebo-controlled High-Dose Erythropoietin for Asphyxia and EncephaLopathy (HEAL) trial which included 500 infants born ≥36 weeks gestational age and treated for 72 hours with TH for moderate or severe HIE based on qualifying SE (qSE) on day 0, between 1 and 6 hours after birth.(5) All participating sites agreed to use the same modified SE for the trial (Fig. 1A) and every enrolling provider received standardized training. Only trained providers performed or verified the SE. After enrollment, infants were randomized to either five doses of erythropoietin (1,000 IU/kg/dose) or placebo. Infants were reassessed with the same standardized SE after rewarming (rSE) on day of life 5. The use of sedating medications within 4 hours of the assessment was recorded. Infants with both a qSE and rSE were included in this analysis. SE findings were categorized as normal (no abnormalities), mild (<3 moderate or severe subcategories), moderate (≥3 moderate or severe subcategories, but <3 in severe category) or severe (≥ 3 severe subcategories) encephalopathy. A score of 0 was assigned to normal findings, 1 to mild findings, 2 to moderate findings, and 3 to severe findings. To generate a total Sarnat score, the assigned scores of each Sarnat category (Fig. 1A) were summed (possible range 0-18). For categories with two sub-scores, the worst of the two scores was counted. Primary outcome was defined as the composite outcome of death or any NDI at 22-36 months (Fig. 1B). Details of the trial protocol were published previously and approval by the Institutional Review Board (UCSF 16-19260) was obtained.(5, 22)
Figure 1:
Sarnat Exam and Outcome definitions. A - Modified Sarnat Exam used in this study. B -Definitions of neurodevelopmental outcome used in this study. NDI=Neurodevelopmental Impairment; GMFCS=Gross Motor Function Classification System; BSID-III=Bayley Scales of Infant Toddler Development3rd edition; CP=Cerebral Palsy.
Statistical Analysis
Demographics, illness severity, and outcome variables were compared between infants whose SE improved versus those whose score remained stable or worsened. Adjusted odds ratios (aORs) for death/NDI based on qSE and rSE severity categories, change in category, and the individual components (as dummy variables) at both time points were constructed using logistic regression, adjusting for extracorporeal membrane oxygenation and sedation at time of exam. The few missing Sarnat components were imputed using multiple imputation with chained equations (MICE, n=5 imputations), using information from the available Sarnat components given the high level of correlation between items. Absolute rSE score and change in score as continuous variables were visually compared against risk of death and level of NDI using locally estimated scatterplot smoothing curves. For these visualizations only, the median of imputed scores was used to determine total and change in score. For all other analyses, estimates from models using all five imputations were pooled using the “with” function from the mice package in R. Analyses were conducted in RStudio using the R statistical package (Version 4.1.2, Foundation for Statistical Computing, Vienna, Austria).(23)
RESULTS
Patients
Of the 500 neonates enrolled in HEAL, 479 had both a qSE and rSE and were included. On the qSE, 377 (79%) neonates had moderate and 102 (21%) had severe encephalopathy. The rSE was conducted at a median (IQR) age of 5 (5, 5) days after birth, 381 (80%) were assessed on day 5, and 467 (98%) on day 4-6 after birth. The rSE was normal in 99 (21%) neonates, whereas 230 (48%) had mild, 97 (20%) moderate, and 53 (11%) severe encephalopathy. Based on their rSE, neonates were grouped into two categories - improved exam or same/worsened exam (Table 1). The primary outcome was available for 460/479 (96%); neonates with missing outcome data were excluded from the outcome analyses.
Table 1:
Patient characteristics comparing those neonates who improved in their Sarnat Exam from birth to rewarming versus those who worsened or did not change.
| All Infants, n (%) |
Improved, n (%) |
No Change/ Worsened, n (%) |
||
|---|---|---|---|---|
| Total Included, n (% of total) | 479 | 360 (75.2) | 119 (24.8) | |
| Maternal Age, mean (SD) | 29.7 (6.4) | 29.8 (6.5) | 29.4 (6.0) | |
| Maternal Education | ||||
| · High school or less | 181 (37.8) | 132 (36.7) | 49 (41.2) | |
| · Some college | 102 (21.3) | 79 (21.9) | 23 (19.3) | |
| · College graduate or higher | 173 (36.1) | 139 (38.6) | 34 (28.6) | |
| · Not Reported | 23 (4.8) | 10 (2.8) | 23 (19.3) | |
| Maternal Parity | ||||
| · 1 | 276 (57.6) | 213 (59.2) | 63 (52.9) | |
| · 2 | 104 (21.7) | 76 (21.1) | 28 (23.5) | |
| · ≥ 3 | 99 (20.7) | 71 (19.7) | 28 (23.5) | |
| Maternal SSRI use | 28 (5.8) | 26 (7.2) | 2 (1.7) | |
| Pregnancy Complications | ||||
| · Pregnancy Induced Hypertension | 56 (11.7) | 42 (11.7) | 14 (11.8) | |
| · Preeclampsia or Eclampsia | 45 (9.4) | 34 (9.4) | 11 (9.2) | |
| · Gestational Diabetes/Insulin Dependent Diabetes Mellitus | 57 (11.9) | 40 (11.1) | 17 (14.3) | |
| · Thyroid Disease | 40 (8.4) | 31 (8.6) | 9 (7.6) | |
| · Maternal Chorioamnionitis or Fever | 75 (15.7) | 57 (15.8) | 18 (15.1) | |
| Labor & Delivery Complications and Sentinel Events | ||||
| · Placenta Abruption | 67 (14.0) | 44 (12.2) | 23 (19.3) | |
| · Cord Prolapse | 22 (4.6) | 22 (6.1) | 0 (0.0) | |
| · Uterine Rupture | 23 (4.8) | 12 (3.3) | 11 (9.2) | |
| · Shoulder Dystocia | 31 (6.5) | 25 (6.9) | 6 (5.0) | |
| · Chorioamnionitis | 63 (13.2) | 50 (13.9) | 13 (10.9) | |
| · Any Sentinel Event | 136 (28.4) | 99 (27.5) | 37 (31.1) | |
| Delivery Mode | ||||
| · Spontaneous Vaginal Delivery | 116 (24.2) | 92 (25.6) | 24 (20.2) | |
| · Instrumented Vaginal Delivery | 48 (10.0) | 38 (10.6) | 10 (8.4) | |
| · Elective Cesarean Delivery | 12 (2.5) | 9 (2.5) | 3 (2.5) | |
| · Emergent/Urgent Cesarean Delivery | 303 (63.3) | 221 (61.4) | 82 (68.9) | |
| Male Sex | 265 (55.3) | 203 (56.4) | 62 (52.1) | |
| Apgar (Median, IQR) | ||||
| · 5 min | 3 (2-5) | 4 (2-5) | 3 (1-5) | |
| · 10 min | 5 (4-7) | 5 (4-7) | 4 (3-6) | |
| Worst Blood Gas Parameters | ||||
| · pH, Mean (SD) | 6.93 (0.17) | 6.96 (0.16) | 6.85 (0.20) | |
| · Base Deficit, Mean (SD) | −18.3 (6.1) | −17.6 (5.8) | −20.5 (6.7) | |
| Resuscitation Measures: | ||||
| · Intubation | 330 (68.9) | 235 (65.3) | 95 (79.8) | |
| · Cardiac Compressions | 148 (30.9) | 95 (26.4) | 53 (44.5) | |
| · Epinephrine | 84 (17.5) | 47 (31.1) | 37 (31.1) | |
| Placenta Pathology | ||||
| · Chorioamnionitis | 122 (25.5) | 102 (28.3) | 20 (16.8) | |
| · Any Abnormality | 264 (55.1) | 213 (59.2) | 51 (42.9) | |
| · Any Acute Abnormality | 197 (41.1) | 158 (43.9) | 39 (32.8) | |
| · Any Chronic Abnormality | 200 (41.8) | 161 (44.7) | 39 (32.8) | |
| Sarnat Stage at Randomization (qSE) | ||||
| · Moderate | 377 (78.7) | 291 (80.8) | 86 (72.3) | |
| · Severe | 102 (21.3) | 69 (19.2) | 33 (27.7) | |
| · Total Sarnat Score, Median (IQR) | 12 (10-14) | 12 (10-13) | 13 (11-15) | |
| · Sedative Medications around Time of Exam | 53 (11.1) | 37 (10.3) | 16 (13.4) | |
| Sarnat Stage after Rewarming (rSE) | ||||
| · Normal | 99 (20.7) | 99 (27.5) | 0 (0.0) | |
| · Mild | 230 (48.0) | 230 (63.9) | 0 (0.0) | |
| · Moderate | 97 (20.3) | 31 (8.6) | 66 (55.5) | |
| · Severe | 53 (11.1) | 0 (0.0) | 53 (44.5) | |
| · Total Sarnat Score, Median (IQR) | 3 (1-9) | 2 (0-5) | 13 (10-16) | |
| · Sedative Medications around Time of Exam | 146 (30.5) | 69 (19.2) | 77 (64.7) | |
| End-Organ Injury | ||||
| · Liver Injury (AST > 100 IU/L) | 184 (38.4) | 115 (31.9) | 69 (58.0) | |
| · Disseminated Intravascular Coagulopathy (INR > 2.0) | 141 (29.4) | 84 (23.3) | 57 (47.9) | |
| · Anuria/Oliguria/Acute Kidney Injury (Creatinine > 1.5mg/dL) | 50 (10.4) | 24 (6.7) | 26 (21.8) | |
| · Thrombocytopenia | 13 (2.7) | 6 (1.7) | 7 (5.9) | |
| · Respiratory Support | ||||
| o Intubation | 330 (68.9) | 235 (65.3) | 95 (79.8) | |
| o iNO | 88 (18.4) | 45 (12.5) | 43 (36.1) | |
| · Extracorporeal Membrane Oxygenation | 19 (4.0) | 9 (2.5) | 10 (8.4) | |
| · Hypotension Treatment by Day 5 | ||||
| o Inotropic Support | 172 (35.9) | 98 (27.2) | 74 (62.2) | |
| o Hydrocortisone | 89 (18.6) | 41 (11.4) | 48 (40.3) | |
| · Seizures | 174 (36.3) | 87 (24.2) | 87 (73.1) | |
| · Erythropoietin Treatment | 244 (50.9) | 188 (52.2) | 56 (47.1) | |
| Discharge | ||||
| All Oral Feedings at Discharge | 370 (77.2) | 319 (88.6) | 51 (42.9) | |
| Outcomes | ||||
| · Death | 45 (9.4) | 9 (2.5) | 36 (30.3) | |
| · Day of Death , Median (IQR) | 11 (7-29) | 12 (6-30) | 10 (7-26) | |
| · Any Neurodevelopmental Impairment | 174 (36.3) | 121 (33.6) | 53 (44.5) | |
| · Not Known | 19 (4.0) | 15 (4.2) | 4 (3.4) |
Initial qualifying SE and outcome
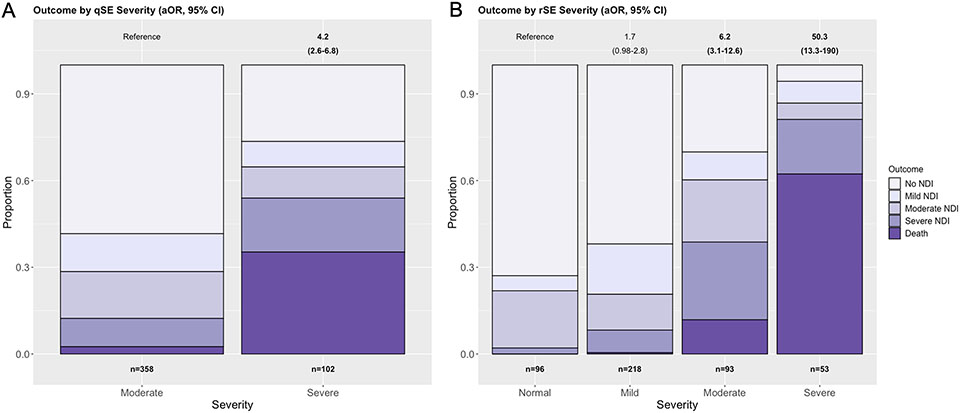
Fifty-seven percent of infants with moderate encephalopathy on qSE experienced a normal outcome compared to 24% with severe qSE (p<0.001, Figure 2A). At 22-36 months, infants with severe qSE had an aOR of 4.2 (95%CI 2.6-6.8) for primary outcome compared to those with moderate qSE. Death also occurred more commonly in neonates with severe qSE (35%) compared to those with moderate qSE (3%, p<0.001). Among survivors, NDI was less severe in the infants with moderate compared to severe qSE (no NDI 60% vs 39%, mild NDI 12% vs 9%, moderate NDI 13% vs 17%, severe NDI 11% vs 30%; p<0.001).
Figure 2:
A – Qualifying Sarnat Exam (qSE) and association with primary outcome. Only neonates with moderate or severe encephalopathy were included. In the moderate group, n=209 (58%) experienced disability-free survival, n=47 (13%) mild NDI, n=58 (16%) moderate NDI, n=35 (10%) severe NDI, and n=9 (3%) died. In the severe group, n=27 (27%) experienced disability-free survival, n=9 (9%) mild NDI, n=11 (11%) moderate NDI, n=19 (19%) severe NDI, and n=36 (35%) died. B - Sarnat Exam after rewarming (rSE) and association with primary outcome. In the normal group, n=70 (73%) experienced disability-free survival, n=5 (5%) mild NDI, n=19 (20%) moderate NDI, n=2 (2%) severe NDI, and n=0 (0%) died. In the mild group, n=135 (62%) experienced disability-free survival, n=38 (17%) mild NDI, n=27 (12%) moderate NDI, n=17 (8%) severe NDI , and n=1 (0%) died. In the moderate group, n=28 (30%) experienced disability-free survival, n=9 (10%) mild NDI, n=20 (22%) moderate NDI, n=25 (27%) severe NDI, and n=11 (12%) died. In the severe group, n=3 (6%) experienced disability-free survival, n=4 (8%) mild NDI, n=3 (6%) moderate NDI, n=10 (19%) severe NDI, and n=33 (62%) died. aORs with 95% CI for the HEAL primary outcome (death/NDI) are shown above the plot for each group compared to infants with a moderate (A, qSE) or normal (rSE, B) Sarnat exam. Bolded values display when the 95% CIs do not cross 1. Models are adjusted for sedative medications prior to assessment and ECMO. NDI = Neurodevelopmental Impairment; ECMO = Extracorporeal Membrane Oxygenation.
SE after rewarming and outcome
rSE was strongly associated with primary outcome (Figure 2B). A normal outcome was seen in 73% of infants with a normal rSE, and in 62% with mild, 22% with moderate, and 6% with severe encephalopathy on rSE, respectively. Severe encephalopathy was most associated with death, and surviving infants experienced worse NDI with increasing degree of encephalopathy on rSE: Mild encephalopathy on rSE showed a trend towards an abnormal outcome (aOR 1.7, 95%CI 0.98-2.8), whereas a significant association with primary outcome was seen for moderate (aOR 6.2, 95%CI 3.1-12.6) and severe encephalopathy (aOR 50.3, 95%CI 13.3-190).
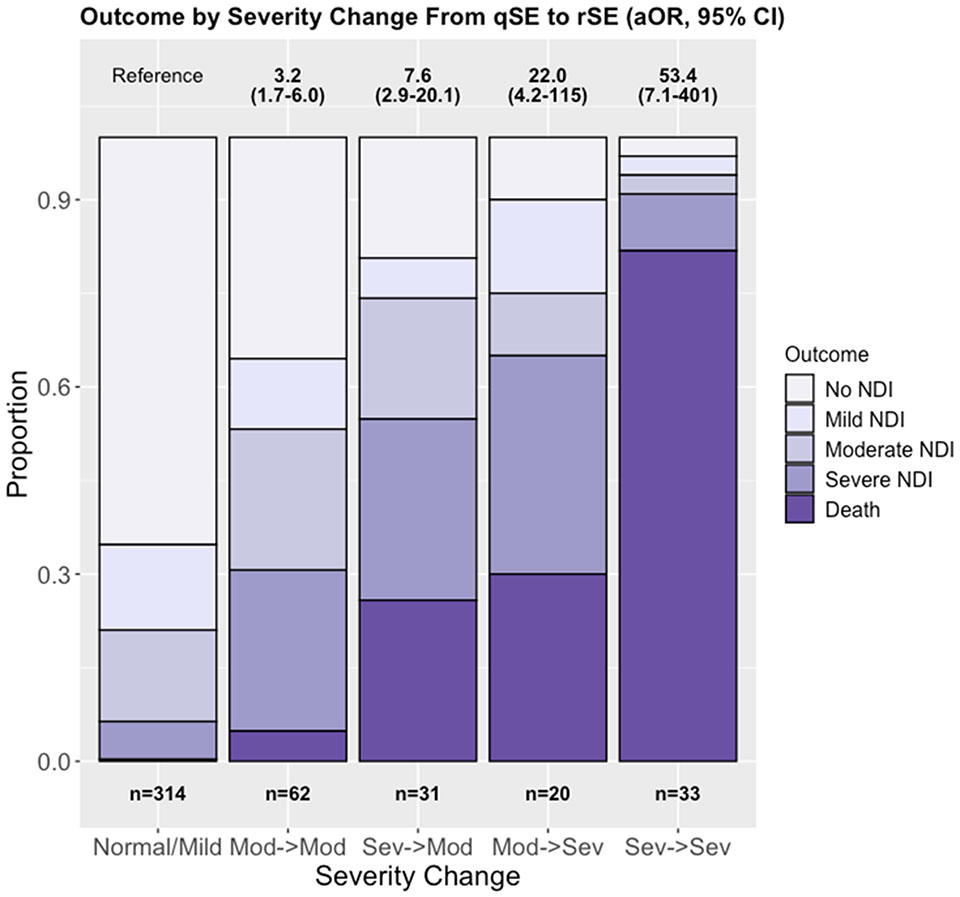
Change in SE and outcome at 22-36 months
Improvement was seen between qSE and rSE in 360 (75.2%) infants and 119 (24.8%) remained the same or worsened (Table 1). Change in SE was associated with primary outcome (Fig. 3). Infants whose SE improved to normal or mild, even when initially severe, were most likely to experience no or mild NDI, whereas an unchanged or worsened exam was associated with moderate-severe NDI or death. Among infants who started severe and remained severe, the aOR for primary outcome was 53.4 (95%CI 7.1-410) and most infants in this group died. If an initial severe exam improved to moderate, the aOR for primary outcome decreased to 7.6 (95%CI 2.9-20.1). Infants who started moderate and became severely encephalopathic had an aOR of 22.0 (95%CI 4.2-115) for death/NDI, whereas infants who had an initial moderate encephalopathy and stayed moderate after rewarming had an aOR of 3.2 (95%CI 1.7-6.0) for the primary outcome and most infants in this group survived.
Figure 3:
Primary Outcome by change in encephalopathy from qualifying Sarnat exam (qSE) to exam after rewarming (rSE). In the Normal/Mild group, n=205 (65%) experienced disability-free survival, n=43 (14%) mild NDI, n=46 (15%) moderate NDI, n=19 (6%) severe NDI, and n=1 (0%) died. In the Moderate->Moderate group, n=22 (36%) experienced disability-free survival, n=7 (11%) mild NDI, n=14 (23%) moderate NDI, n=16 (26%) severe NDI, and n=3 (5%) died. In the Severe->Moderate group, n=6 (19%) experienced disability-free survival, n=2 (6%) mild NDI, n=6 (19%) moderate NDI, n=9 (29%) severe NDI, and n=8 (26%) died. In the Moderate -> Severe group, n=2 (10%) experienced disability-free survival, n=3 (15%) mild NDI, n=2 (10%) moderate NDI, n=7 (35%) severe NDI, and n=6 (30%) died. In the Severe->Severe group, n=1 (3%) each experienced no NDI, mild NDI, and moderate NDI, with n=3 (9%) experiencing severe NDI and n=27 (82%) died. aORs with 95% CI for the HEAL primary outcome (death/NDI) are shown above the plot for each group compared to infants who improved from moderate or severe on qSE to mild or normal on rSE. Models are adjusted for medications prior to assessment and ECMO. NDI = Neurodevelopmental Impairment; HIE = Hypoxic-ischemic encephalopathy; ECMO = Extracorporeal Membrane Oxygenation.
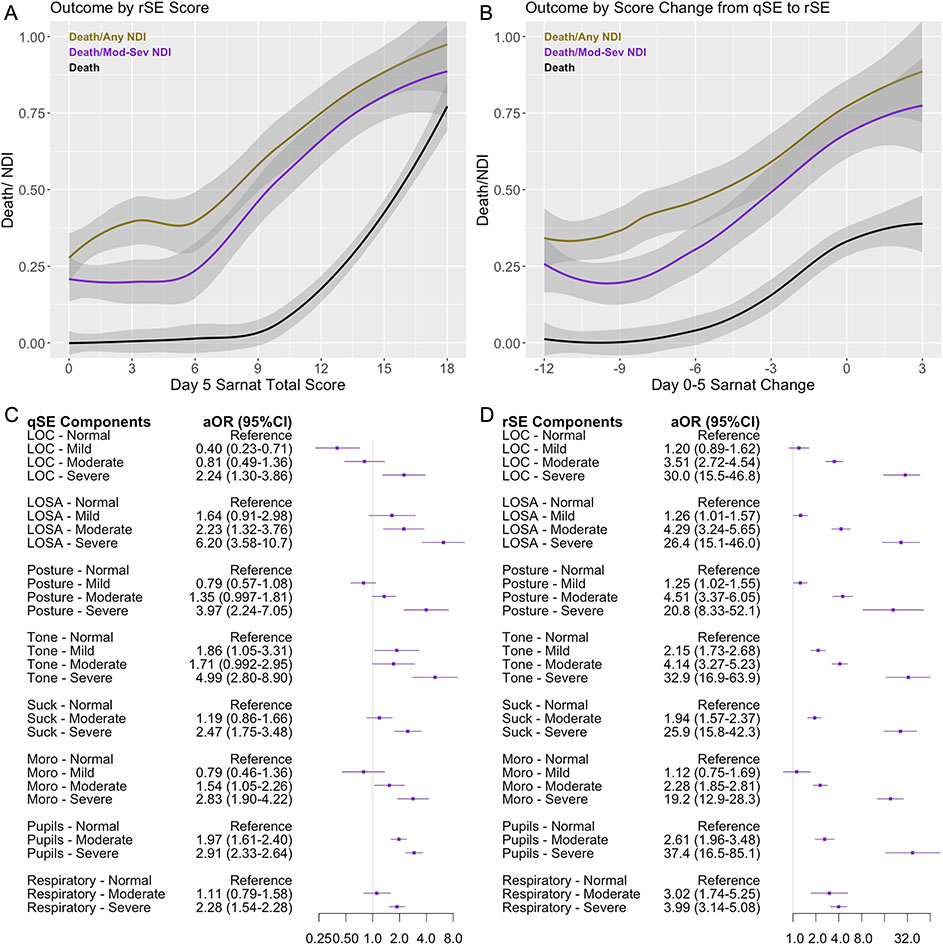
Total score and change in total score of the SE
The total numeric score on rSE was associated with primary outcome (Fig. 4A), with an aOR 1.22 (95% CI: 1.16-1.29) per each unit increase in total score (p<0.001). Each unit decrease in total Sarnat score from qSE to rSE was associated with an aOR of 0.85 (95%CI: 0.81-0.90; p<0.001, Fig. 4B) for primary outcome.
Figure 4:
Unadjusted locally estimated scatterplot smoothing (LOESS) curved depicting proportion of infants experiencing death or NDI by A: Absolute value of total rSE score, and B: Change in Sarnat score from qSE to rSE. C: Forest plot of Sarnat components and subcategories on qSE. D: Forest plot of the Sarnat components and subcategories on rSE. Each component was included on a continuous 0-3 scale - aOR is per 1 unit increase in score adjusted for ECMO and medications prior to assessment. qSE = Qualifying Sarnat Exam; rSE = Sarnat Exam after rewarming; NDI = Neurodevelopmental Impairment; aOR = adjusted Odds Ratio, ECMO = Extracorporeal Membrane Oxygenation.
Association of the qSE and rSE subcategories with outcome
Of the six exam categories, a mildly abnormal level of consciousness on qSE was associated with a decreased aOR of 0.40 (95%CI 0.23-0.71) for primary outcome, whereas most other categories were associated with either a trend or an increased odds for primary outcome (Fig. 4C). Severe findings in any category were associated with an increased odds for primary outcome. Moderate findings in the categories of spontaneous activity, Moro reflex, and pupillary exam were associated with an increased odds for abnormal outcome, while respirations, suck reflex, level of consciousness and posture were not. Both increased and decreased tone on qSE were similarly associated with higher odds of primary outcome even after adjusting for sedative medications. In contrast, on rSE, all categories, except mildly abnormal Moro reflex and mildly abnormal level of consciousness, were associated with higher odds of primary outcome with increasing severity (Fig. 4D).
DISCUSSION
We present a large cohort of neonates treated with TH for moderate or severe HIE who were assessed with a standardized SE prior to cooling, and again after rewarming on day 5 after birth. We show that the stage of encephalopathy on SE after rewarming and the change from qSE to rSE were strongly associated with death or NDI.
The modified SE is routinely used to determine eligibility for TH, and this early-stage SE has shown an association with long-term outcome. The TS and the standardized Amiel-Tison Neurological Assessment at Term, when applied after rewarming, result in a stronger association with short and long-term outcomes.(16, 24, 25) This has not consistently been demonstrated for the SE, and studies comparing the early SE with SE performed at 72-96 hours were done prior to the TH era and have not routinely accounted for sedative medications or the trajectory of the SE over time.(17, 18) In our study, while the qSE was associated with long-term outcome, this association appeared even more robust for the rSE performed on day 5, after rewarming. Additionally, the temporary evolution between qSE and rSE was highly predictive of long-term outcome. This emphasizes that while the qSE prior to TH provides important information, it may be confounded by dynamic factors from the initial insult, and that the rSE and the trajectory of the SE may be more reflective of permanent sequelae. Incorporating rSE in routine clinical practice can provide significant information regarding longer-term outcomes and might be particularly useful in settings where technologies such as MRI are unavailable.
To further optimize the reliability the SE, interest has been directed towards comparing numerical and categorical assessments.(20) When assessed within 6 hours of birth, the numerical score of the SE more accurately predicted an abnormal 2-year outcome than the categorical result in infants with mild-moderate HIE; however, in neonates with moderate or severe HIE no difference between numerical and categorical score on SE with respect to associations with MRI injury or long-term outcome were found.(20, 21, 24, 26, 27) By using a different approach, assessing the change in numerical score from qSE to rSE, we identified an association with death/NDI: for each unit increase in score, the odds for death or NDI increased whereas for each unit decrease, the odds lowered.
The contribution of the six individual SE components and their associations with long-term findings have not previously been described. We found that muscle tone was associated with poor outcome at any timepoint, a key finding considering that “tone” has been the most adjusted category throughout SE modifications. The original Sarnat Staging did not include hypertonia, whereas the Neonatal Research Network (NRN) included hypertonia as a marker of moderate encephalopathy across several trials.(6, 28) The NRN modification was also adopted for studies of mild encephalopathy, which highlighted tone abnormality as the most commonly identified component of the SE associated with disability.(29) However, investigators of other large trials have opted to not include increased tone as moderate abnormality,(3, 8, 9) or classified an increased tone as a symptom of mild encephalopathy instead.(10-12) Our findings show that hypertonia and hypotonia on qSE are similarly associated with death/NDI, but that on rSE hypotonia is associated with a greater odds of death/NDI than hypertonia (Fig. 4C&D). When interpreting these results, the timing and evolution of changes are therefore crucial. For instance, muscle tone after a hypoxic-ischemic insult evolves from decreased to increased tone, and in the most severe cases progresses into cerebral palsy.(30, 31) In contrast, irritability as sequela from an acute insult is more commonly observed in conjunction with an increased tone. Therefore, hypertonicity may represent a clinical expression of two very different origins - an older insult that is progressing in its clinical course or a very acute symptom associated with neuro-irritability following an acute insult. Correlation with timing of injury on MRI might assist with distinguishing between these two scenarios.
Dr. Harvey Sarnat himself recently proposed reevaluating the SE, advocating for studies weighing the predictive value of each component of the SE with the goal of modifying the scoring system and/or identifying specific risks based on severity and timing of component abnormality.(32) Using the rSE may enhance the prognostic ability, particularly when combined with other metrics such as blood glucose, cooling mattress temperature, MRI, MRS, and EEG.(33-36) Furthermore, it is plausible that the evolution of the SE and its individual components may assist in the characterization of an individualized injury profile and facilitate tailored therapies and resource allocation.(37, 38) Prospective studies examining combinations of these easily accessible yet powerful metrics to predict outcome are required.
A significant strength of this study is the standardization across sites of the SE at two time points, the large cohort-size, and the high follow-up rate. In addition, the ability to adjust for medications with sedative effects in this study minimized their confounding influence on the SE. Limitations of this study are that the specific sedative medication, dose, and precise timing within the 4 hours prior to the SE were not collected, and we were therefore unable to adjust or account for how the different medications may affect the SE. Furthermore, data regarding type and duration of selective serotonin uptake inhibitor exposure during pregnancy was unavailable, which limited the ability to adjust for the possible influence on SE, particularly tone. In addition, rSE and MRI occurred around the same time, and we were unable to determine how MRI findings influenced death. Due to a combination of fewer infants represented in the most severe Sarnat categories and a high percentage of those with poor outcome in those categories, high point-estimates and wide confidence intervals in the aOR reflect a degree of statistical uncertainty, however, the lower bounds of those aORs still suggest a notable increase in odds of poor outcome based on Sarnat trajectory, supporting the value of the rSE. Lastly, the etiology and sequalae of HIE are heterogeneous, therefore confounding variables associated with multi-organ involvement may have influenced the rSE and outcome at 22-36 months. Although perinatal factors and co-morbidities were assessed (Table 1), the long-term impact on outcomes cannot be precisely accounted for in this study.
CONCLUSION
In this study we found that compared to the qSE, the rSE is more predictive of death or NDI, and that the change from qSE to rSE is strongly associated with outcome at 22-36 months.
Key Messages:
What is already known on this topic
The Sarnat Exam, a tool to measure the degree of encephalopathy, is dynamic throughout the first week after birth.
What this study adds
Both the initial Sarnat Exam prior to initiation of therapeutic hypothermia and Sarnat Exam performed after rewarming were associated with outcome at 22-36 months. In particular, the trajectory of the Sarnat exam between the two time points shows a strong association with outcome at 22-36 months. Therefore, routine Sarnat exam performed after rewarming adds a valuable tool to clinical practice.
How this study might affect research, practice, or policy
Incorporating standardized Sarnat Exams after rewarming and comparison to the initial exam can assist clinicians and parents in assessing risk for death and neurodevelopmental impairment, facilitating resource allocation after hospital discharge.
ACKNOWLEDGEMENT
We like to thank the study participants and the entire HEAL consortium and for their dedication to this work. HEAL consortium: Kaashif A Ahmad, Pediatrix Medical Group of San Antonio, San Antonio, TX; Marianna Baserga, University of Utah, Salt Lake City, UT; Ellen Bendel-Stenzel, Mayo Clinic, Rochester, MN; Kristen L. Benninger, Nationwide Children’s Hospital, Columbus, OH; Lina Chalak, University of Texas Southwestern Medical Center, Dallas, TX; Taeun Chang, Children’s National Hospital, Washington, D.C.; John Flibotte, Children’s Hospital of Philadelphia, Philadelphia, PA; Andrea L. Lampland, Children’s Minnesota, Minneapolis/St. Paul, MN; Nathalie L. Maitre, Children’s Healthcare of Atlanta and Emory University, Atlanta, GA; Amit M. Mathur, Saint Louis University School of Medicine, St. Louis, MO; Stephanie Merhar, Cincinnati Children’s Hospital, Cincinnati, OH; Brenda B. Poindexter, Children’s Healthcare of Atlanta and Emory University, Atlanta, GA; Rakesh Rao, Washington University, St. Louis, MO; David Riley, Cook Children’s Medical Center, Fort Worth, TX; Christopher D. Smyser, Washington University, St. Louis, MO; Gregory M. Sokol, Indiana School of Medicine, Indianapolis, IN; Joern-Hendrik Weitkamp, Vanderbildt University Medical Center, Nashville, TN; Toby Yanowitz, University of Pittsburgh School of Medicine & Children’s Hospital of Pittsburgh of UPMC and Magee, Pittsburgh, PA
Funding:
The study was funded by the National Institute of Neurological Disease and Stroke (NINDS), 1U01NS092764, U01NS092553
Role of Funder/Sponsor:
The funder/sponsor did not participate in the work.
Footnotes
Conflict of Interest Disclosures:
YW, SJ, BC, PH receive support to their institution from the NIH/NINDS for the presented study. FG, HG, KVM, BC, PH receive grants paid to the institution from the NIH and NIH/NINDS, KVM receives grant support from PCORI, and NN receives support from Biogen and UCB Pharma paid to the institution. SJ receives royalties for editing Avery’s Disease of the Newborn. UM, HG, and KVM receive payments for expert testimony. FG receives grant support for travel/meeting attendance from the NIH. HG and SJ disclose participation on data safety monitoring board or advisory boards for NIH IACQUIRE (HG) and ALBINO (SJ). FG discloses membership of the Societies for Pediatric Research executive council, HG discloses a leadership/fiduciary role within the Pediatric Academic Societies, and NN discloses an unpaid position as board member of Wonderland Child and Family Services. HG discloses stocks/stock options with ELEMENO Health.
SK, TW, DM, SB have nothing to disclose.
Trial Registration: ClinicalTrials.gov: NCT02811263
REFERENCES
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–38. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013(1):CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. [DOI] [PubMed] [Google Scholar]
- 5.Wu YW, Comstock BA, Gonzalez FF, Mayock DE, Goodman AM, Maitre NL, et al. Trial of Erythropoietin for Hypoxic-Ischemic Encephalopathy in Newborns. N Engl J Med. 2022;387(2):148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705. [DOI] [PubMed] [Google Scholar]
- 7.Mrelashvili A, Russ JB, Ferriero DM, Wusthoff CJ. The Sarnat score for neonatal encephalopathy: looking back and moving forward. Pediatr Res. 2020;88(6):824–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692–700. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–70. [DOI] [PubMed] [Google Scholar]
- 10.Wu YW, Mathur AM, Chang T, McKinstry RC, Mulkey SB, Mayock DE, et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics. 2016;137(6). [DOI] [PubMed] [Google Scholar]
- 11.Ivy AS, Clark CL, Bahm SM, Meurs KP, Wusthoff CJ. Improving the Identification of Neonatal Encephalopathy: Utility of a Web-Based Video Tool. Am J Perinatol. 2017;34(5):520–2. [DOI] [PubMed] [Google Scholar]
- 12.Bonifacio SL, Hutson S. The Term Newborn: Evaluation for Hypoxic-Ischemic Encephalopathy. Clin Perinatol. 2021;48(3):681–95. [DOI] [PubMed] [Google Scholar]
- 13.Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, Molteno CD, Malan AF. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757–61. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor CM, Ryan CA, Boylan GB, Murray DM. The ability of early serial developmental assessment to predict outcome at 5years following neonatal hypoxic-ischaemic encephalopathy. Early Hum Dev. 2017;110:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Ambalavanan N, Carlo WA, Shankaran S, Bann CM, Emrich SL, Higgins RD, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118(5):2084–93. [DOI] [PubMed] [Google Scholar]
- 16.Mendler MR, Mendler I, Hassan MA, Mayer B, Bode H, Hummler HD. Predictive Value of Thompson-Score for Long-Term Neurological and Cognitive Outcome in Term Newborns with Perinatal Asphyxia and Hypoxic-Ischemic Encephalopathy Undergoing Controlled Hypothermia Treatment. Neonatology. 2018;114(4):341–7. [DOI] [PubMed] [Google Scholar]
- 17.Grass B, Scheidegger S, Latal B, Hagmann C, Held U, Brotschi B, et al. Short-term neurological improvement in neonates with hypoxic-ischemic encephalopathy predicts neurodevelopmental outcome at 18-24 months. J Perinat Med. 2020;48(3):296–303. [DOI] [PubMed] [Google Scholar]
- 18.Shankaran S, Laptook AR, Tyson JE, Ehrenkranz RA, Bann CM, Das A, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2012;160(4):567–72 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mietzsch U, Radhakrishnan R, Boyle FA, Juul S, Wood TR. Active cooling temperature required to achieve therapeutic hypothermia correlates with short-term outcome in neonatal hypoxic-ischaemic encephalopathy. J Physiol. 2020;598(2):415–24. [DOI] [PubMed] [Google Scholar]
- 20.Chalak LF, Adams-Huet B, Sant'Anna G. A Total Sarnat Score in Mild Hypoxic-ischemic Encephalopathy Can Detect Infants at Higher Risk of Disability. J Pediatr. 2019;214:217–21 e1. [DOI] [PubMed] [Google Scholar]
- 21.Walsh BH, Munster C, El-Shibiny H, Yang E, Inder TE, El-Dib M. Comparison of numerical and standard sarnat grading using the NICHD and SIBEN methods. J Perinatol. 2022;42(3):328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juul SE, Comstock BA, Heagerty PJ, Mayock DE, Goodman AM, Hauge S, et al. High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): A Randomized Controlled Trial - Background, Aims, and Study Protocol. Neonatology. 2018;113(4):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Team RC. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2014. [Available from: http://www.R-project.org/. [Google Scholar]
- 24.Murray DM, Bala P, O'Connor CM, Ryan CA, Connolly S, Boylan GB. The predictive value of early neurological examination in neonatal hypoxic-ischaemic encephalopathy and neurodevelopmental outcome at 24 months. Dev Med Child Neurol. 2010;52(2):e55–9. [DOI] [PubMed] [Google Scholar]
- 25.Aoki H, Shibasaki J, Tsuda K, Yamamoto K, Takeuchi A, Sugiyama Y, et al. Predictive value of the Thompson score for short-term adverse outcomes in neonatal encephalopathy. Pediatr Res. 2023;93(4):1057–63. [DOI] [PubMed] [Google Scholar]
- 26.Troha Gergeli A, Škofljanec A, Neubauer D, Paro Panjan D, Kodrič J, Osredkar D. Prognostic Value of Various Diagnostic Methods for Long-Term Outcome of Newborns After Hypoxic-Ischemic Encephalopathy Treated With Hypothermia. Front Pediatr. 2022;10:856615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales MM, Montaldo P, Ivain P, Pant S, Kumar V, Krishnan V, et al. Association of Total Sarnat Score with brain injury and neurodevelopmental outcomes after neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2021;106(6):669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312(24):2629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalak LF, Nguyen KA, Prempunpong C, Heyne R, Thayyil S, Shankaran S, et al. Prospective research in infants with mild encephalopathy identified in the first six hours of life: neurodevelopmental outcomes at 18-22 months. Pediatr Res. 2018;84(6):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drobyshevsky A, Derrick M, Luo K, Zhang LQ, Wu YN, Takada SH, et al. Near-term fetal hypoxia-ischemia in rabbits: MRI can predict muscle tone abnormalities and deep brain injury. Stroke. 2012;43(10):2757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpe JJ, Volpe JJ. Volpe's neurology of the newborn. Philadelphia, PA: Elsevier,; 2018. [Google Scholar]
- 32.Sarnat HB, Flores-Sarnat L, Fajardo C, Leijser LM, Wusthoff C, Mohammad K. Sarnat Grading Scale for Neonatal Encephalopathy after 45 Years: An Update Proposal. Pediatr Neurol. 2020;113:75–9. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Yang Q, Wei H, Dong W, Fan Y, Hua Z. Prognostic Value of Clinical Tests in Neonates With Hypoxic-Ischemic Encephalopathy Treated With Therapeutic Hypothermia: A Systematic Review and Meta-Analysis. Front Neurol. 2020;11:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mietzsch U, Flibotte JJ, Law JB, Puia-Dumitrescu M, Juul SE, Wood TR. Temperature dysregulation during therapeutic hypothermia predicts long-term outcome in neonates with HIE. J Cereb Blood Flow Metab. 2023:271678X231162174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124(3):e459–67. [DOI] [PubMed] [Google Scholar]
- 36.Kamino D, Widjaja E, Brant R, Ly LG, Mamak E, Chau V, et al. Severity and duration of dysglycemia and brain injury among patients with neonatal encephalopathy. EClinicalMedicine. 2023;58:101914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkan H, Kahraman A, Mutlu A. Early Spontaneous Movements of Infants With Hypoxic- Ischemic Encephalopathy. Pediatr Phys Ther. 2021;33(1):18–22. [DOI] [PubMed] [Google Scholar]
- 38.Pouppirt NR, Martin V, Pagnotto-Hammitt L, Spittle AJ, Flibotte J, DeMauro SB. The General Movements Assessment in Neonates With Hypoxic Ischemic Encephalopathy. J Child Neurol. 2021;36(8):601–9. [DOI] [PubMed] [Google Scholar]