Abstract
Telehealth abortion has become critical to addressing surges in demand in states where abortion remains legal but evidence on its effectiveness and safety is limited. California Home Abortion by Telehealth (CHAT) is a prospective study that follows pregnant people who obtained medication abortion via telehealth from three virtual clinics operating in 20 states and Washington, DC between April 2021 and January 2022. Individuals were screened using a standardized no-test protocol, primarily relying on their medical history to assess medical eligibility. We assessed effectiveness, defined as complete abortion after 200 mg mifepristone and 1,600 μg misoprostol (or lower) without additional intervention; safety was measured by the absence of serious adverse events. We estimated rates using multivariable logistic regression and multiple imputation to account for missing data. Among 6,034 abortions, 97.7% (95% confidence interval (CI) = 97.2–98.1%) were complete without subsequent known intervention or ongoing pregnancy after the initial treatment. Overall, 99.8% (99.6–99.9%) of abortions were not followed by serious adverse events. In total, 0.25% of patients experienced a serious abortion-related adverse event, 0.16% were treated for an ectopic pregnancy and 1.3% abortions were followed by emergency department visits. There were no differences in effectiveness or safety between synchronous and asynchronous models of care. Telehealth medication abortion is effective, safe and comparable to published rates of in-person medication abortion care.
Subject terms: Epidemiology, Outcomes research, Health services, Adverse effects
In a prospective study across US states where abortion remains legal, telehealth medication abortion, provided primarily without tests, was effective, safe and comparable to previous findings from large US studies investigating in-person medication abortion care.
Main
In 2021, the US Food and Drug Administration (FDA) removed the in-person dispensing requirement on mifepristone, the first drug used in a medication abortion. This ruling allowed clinicians to begin offering a ‘no-test’ telehealth model of medication abortion care. Clinicians could now offer entirely remote consultations, using the patient’s self-reported medical history instead of ultrasonography or other tests to screen for medical eligibility.
Moving abortion out of the clinic reduced travel, cost and stigma-related barriers and increased convenience for patients1,2. While telehealth abortion is usually conducted through synchronous communication, with a real-time scheduled videoconference appointment with the patient, some virtual clinics rely on entirely asynchronous communication, using secure text messaging without a scheduled interaction. Follow-up for both models is usually asynchronous, through secure text messaging.
This expansion of services became critical after the June 2022 Supreme Court Dobbs v. Jackson Women’s Health Organization decision allowed states to ban abortion. In states such as Illinois, Kansas and Colorado, where abortion remained legal but neighboring states banned abortion, clinics experienced large increases in patient volume3. Telehealth became vital to meeting increased demand by reducing appointment waiting times and serving patients from states with abortion bans4. Some individuals from US states with an abortion ban use methods such as mail forwarding and mailing medications to a friend or Post Office box close to the border in states where abortion is permitted, minimizing the travel required5. Additionally, some clinicians have begun to use the legal protections of their state’s “shield laws” to provide medication abortion via telehealth to patients in banned states6.
However, access to mifepristone for medication abortion has been under threat, with a federal court ruling to reverse FDA regulatory approvals of mifepristone, including the 2021 decision that allowed telehealth for abortion to continue even after the pandemic. This ruling was issued despite multiple FDA reviews and abundant evidence demonstrating the effectiveness and safety of mifepristone7. According to the mifepristone label, 97.4% of 16,794 patients in US clinical trials of in-person medication abortion had a complete abortion and less than 0.5% had a serious adverse event8.
While decades of evidence support the effectiveness and safety of mifepristone provided in person, the evidence supporting no-test direct-to-patient telehealth abortion is more limited. Before 2021, US research on the effectiveness and safety of telehealth abortion was limited to clinic-to-clinic9–11 or direct-to-patient models that required pre-abortion ultrasonography or other tests12. To date, only five US studies have examined the outcomes of no-test direct-to-patient telehealth abortion models; four of these had small (fewer than 350) samples of patients receiving such care; thus, they were underpowered to examine outcomes as rare as serious adverse events13–16. The fifth study was a retrospective examination of no-test medication abortion provided either in-person or by telehealth and mail. Among 3,779 medication abortions, 95% were complete without procedural intervention and 0.5% experienced a serious adverse event. Effectiveness and safety were similar whether medications were dispensed in-person or by mail17,18. However, this study did not report the effectiveness and safety outcomes of asynchronous telehealth abortion.
In this study, we used data from the California Home Abortion by Telehealth (CHAT) study to follow a large sample of patients across the US from three virtual clinics to estimate the effectiveness and safety of medication abortion care provided via telehealth. Clinicians provided telehealth abortion care via either synchronous (video) or asynchronous (secure text messaging) methods. They screened patients using a published, standardized no-test protocol, primarily relying on patient medical history to assess medical eligibility19. Patients who had any risk factors for or symptoms of ectopic pregnancy or were potentially beyond the gestational limit of the virtual clinic were referred for pre-abortion ultrasonography. Eligible patients received 200 mg mifepristone and 800 or 1,600 μg buccal or vaginal misoprostol via mail order pharmacy. Outcome data were collected by scheduled follow-up interactions conducted remotely 3–7 days after intake and again 2–4 weeks after medication administration (Fig. 1). Our primary aim was to assess the effectiveness and safety of telehealth medication abortion care. Our secondary aim was to compare effectiveness and safety outcomes between synchronous and asynchronous models of telehealth.
Fig. 1. CHAT study data sources.
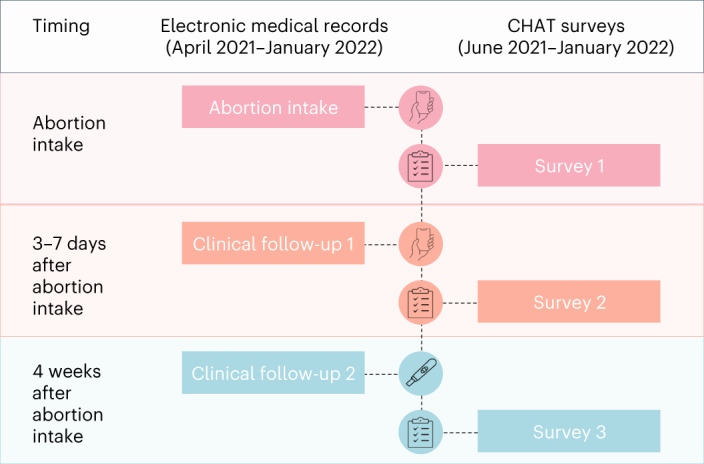
Timing and content of the electronic medical records and survey data analyzed in the CHAT study.
Results
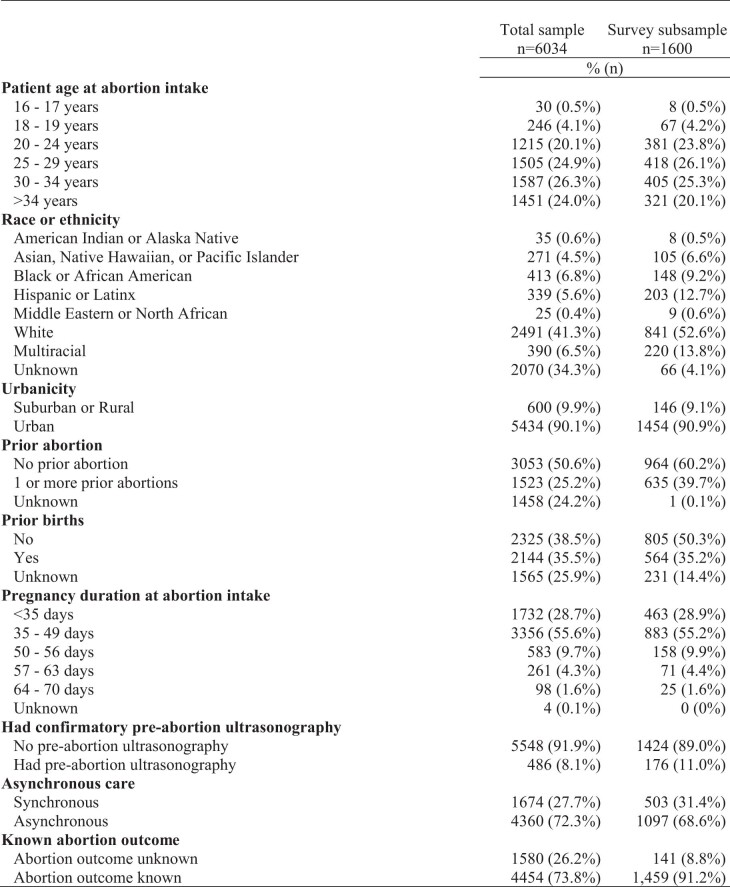
We received electronic medical records for 6,974 encounters. Among those, 6,154 patients met the eligibility criteria and had abortion medications dispensed to them in 20 states and Washington, DC. We excluded cases where the patient took neither mifepristone nor misoprostol (n = 120) leaving 6,034 patients in the analytical sample (Fig. 2). Among these, 1,600 patients provided supplementary self-reported data on their outcomes via surveys (Extended Data Table 1).
Fig. 2. Patient flow chart.
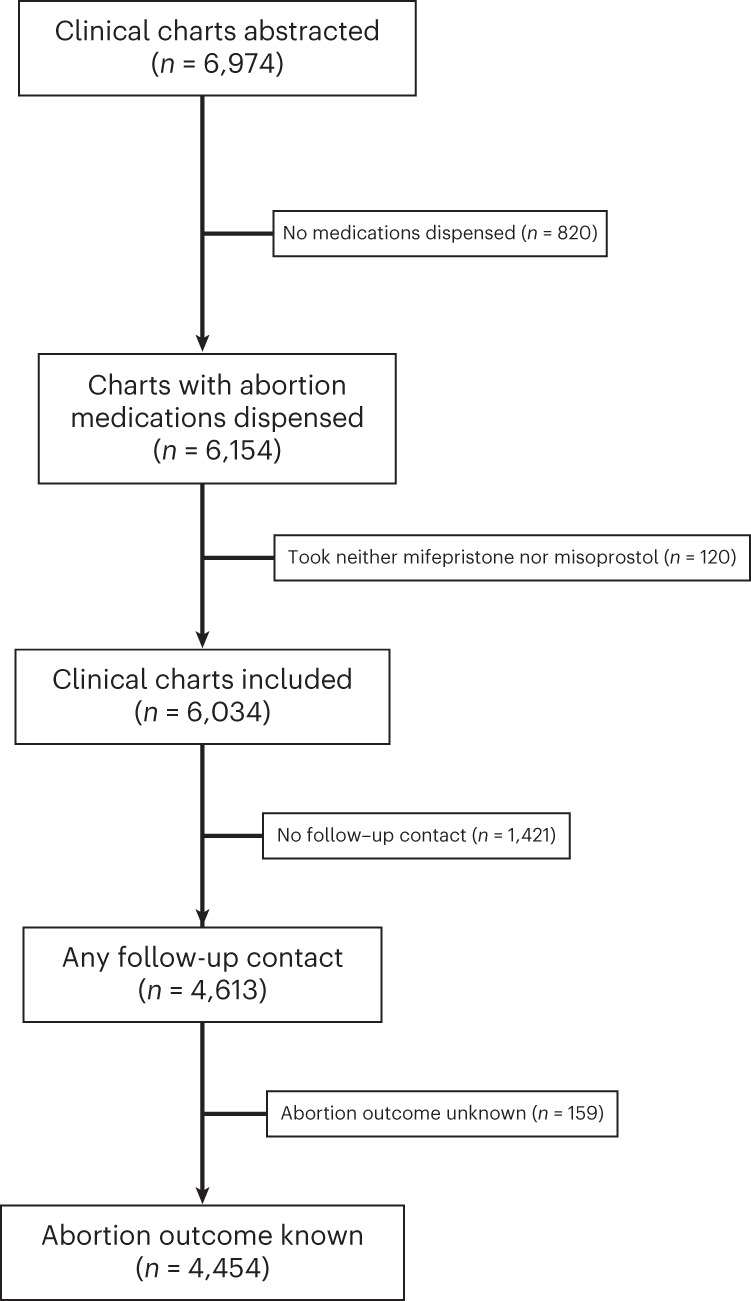
Patient flow chart depicting the exclusion criteria.
Extended Data Table 1.
Characteristics of the medical records sample and survey subsample
All patients were pregnant and seeking abortion. Half (50.3%) were 30 years or older and 4.6% were aged under 20 years (Table 1). Race, ethnicity or ethnic grouping was unknown for one-third (34.3%) of patients because one of the clinics did not record these data in their medical records for the first half of the study period. Among the subsample with known race, ethnicity or ethnic grouping, nearly two-thirds (62.7%) were white. Most (84.3%) patients had pregnancy durations under 7 weeks (≤49 days). Medical records did not document patient sex or gender.
Table 1.
Characteristics of the individuals who obtained synchronous and asynchronous telehealth medication abortion care
| Characteristics | Overall | Synchronous | Asynchronous | |
|---|---|---|---|---|
|
n = 6,034 (100.0%) |
n = 1,674 (27.7%) |
n = 4,360 (72.3%) |
||
| n (column %) | n (row %) | P | ||
| Patient age at abortion intake | ||||
| 16–17 years | 30 (0.5%) | 0 (0%) | 30 (100.0%) | <0.001 |
| 18–19 years | 246 (4.1%) | 51 (20.7%) | 195 (79.3%) | |
| 20–24 years | 1,215 (20.1%) | 324 (26.7%) | 891 (73.3%) | |
| 25–29 years | 1,505 (24.9%) | 422 (28.0%) | 1,083 (72.0%) | |
| 30–34 years | 1,587 (26.3%) | 476 (30.0%) | 1,111 (70.0%) | |
| >34 years | 1,451 (24.0%) | 401 (27.6%) | 1,050 (72.4%) | |
| Race, ethnicity or ethnic grouping | ||||
| American Indian or Alaska Native | 35 (0.6%) | 9 (25.7%) | 26 (74.3%) | <0.001 |
| Asian, Native Hawaiian or Pacific Islander | 271 (4.5%) | 48 (17.7%) | 223 (82.3%) | |
| Black or African American | 413 (6.8%) | 170 (41.2%) | 243 (58.8%) | |
| Hispanic or Latinx | 339 (5.6%) | 135 (39.8%) | 204 (60.2%) | |
| Middle Eastern or North African | 25 (0.4%) | 5 (20.0%) | 20 (80.0%) | |
| White | 2,491 (41.3%) | 746 (29.9%) | 1,745 (70.1%) | |
| Multiracial | 390 (6.5%) | 100 (25.6%) | 290 (74.4%) | |
| Unknown | 2,070 (34.3%) | 461 (22.3%) | 1,609 (77.7%) | |
| Urbanicity | ||||
| Suburban or rural | 600 (9.9%) | 189 (31.5%) | 411 (68.5%) | 0.030 |
| Urban | 5,434 (90.1%) | 1,485 (27.3%) | 3,949 (72.7%) | |
| Previous abortion | ||||
| No previous abortion | 3,053 (50.6%) | 945 (31.0%) | 2,108 (69.0%) | <0.001 |
| One or more previous abortions | 1,523 (25.2%) | 574 (37.7%) | 949 (62.3%) | |
| Unknown | 1,458 (24.2%) | 155 (10.6%) | 1,303 (89.4%) | |
| Previous births | ||||
| No previous birth | 2,325 (38.5%) | 721 (31.0%) | 1,604 (69.0%) | <0.001 |
| One or more previous births | 2,144 (35.5%) | 879 (41.0%) | 1,265 (59.0%) | |
| Unknown | 1,565 (25.9%) | 74 (4.7%) | 1,491 (95.3%) | |
| Pregnancy duration at abortion intake | ||||
| <35 days | 1,732 (28.7%) | 566 (32.7%) | 1,166 (67.3%) | <0.001a |
| 35–49 days | 3,356 (55.6%) | 960 (28.6%) | 2,396 (71.4%) | |
| 50–56 days | 583 (9.7%) | 147 (25.2%) | 436 (74.8%) | |
| 57–63 days | 261 (4.3%) | 1 (0.4%) | 260 (99.6%) | |
| 64–70 days | 98 (1.6%) | 0 (0%) | 98 (100.0%) | |
| Unknown | 4 (0.1%) | 0 (0%) | 4 (100.0%) | |
| Had confirmatory pre-abortion ultrasonography | ||||
| No pre-abortion ultrasonography | 5,548 (91.9%) | 1,527 (27.5%) | 4,021 (72.5%) | 0.198 |
| Had pre-abortion ultrasonography | 486 (8.1%) | 147 (30.2%) | 339 (69.8%) | |
| Known abortion outcome | ||||
| Abortion outcome unknown | 1,580 (26.2%) | 256 (16.2%) | 1,324 (30.4%) | <0.001 |
| Abortion outcome known | 4,454 (73.8%) | 1,418 (31.8%) | 3,036 (69.6%) | |
aP value was derived from a two-sided Fisher’s exact test.
P values were derived from two-sided chi-squared tests, unless otherwise noted.
Overall, 72.3% of patients received asynchronous care. Among patients of the clinic that offered asynchronous care but allowed patients to request a phone or video call, 0.3% requested a call with the provider. Patients who were younger (100.0% for 16–18 years, 79.3% for 18–19 years), Asian, Native Hawaiian or Pacific Islander (82.3%), Middle Eastern or North African (80.0%), living in an urban area (72.7%) and who had pregnancy durations over 56 days (74.8% for 50–56 days, 99.6% for 57–63 days and 100.0% for 64–70 days) were more likely to have received asynchronous care.
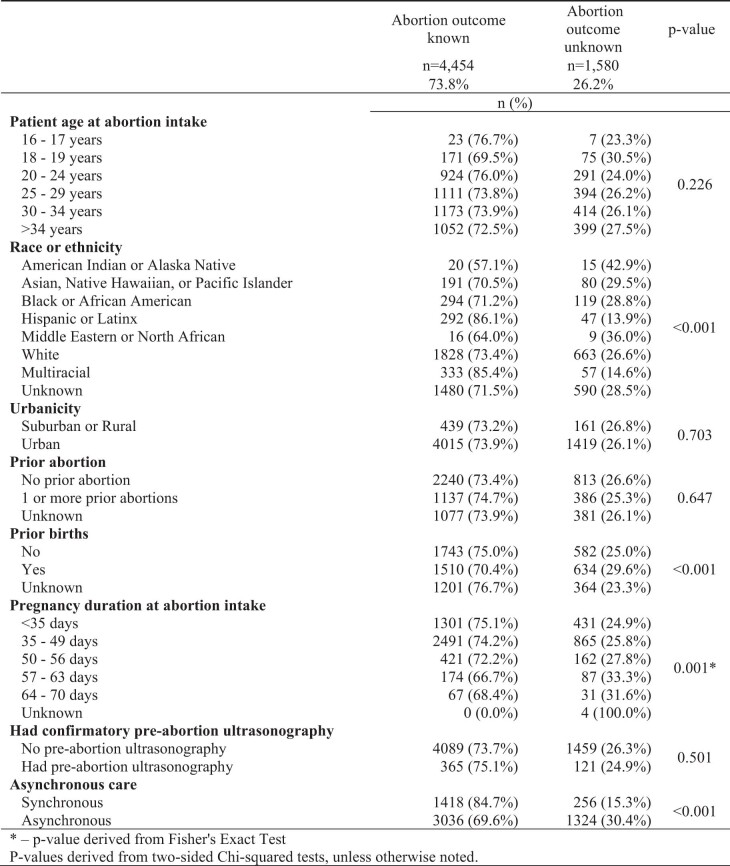
Of the sample, 76% (4,613 of 6,034) of cases had any follow-up contact with the virtual clinic or by surveys (Fig. 2). Abortion outcomes were known (ascertained using a test or the patient’s history) for 74% (4,454 of 6,034) of the analytical sample. There were few sociodemographic characteristics associated with unknown outcomes. Outcomes were less likely to be known for American Indian or Alaska Native patients (57.1%), Middle Eastern or North African patients (64.0%), patients with a previous birth (70.4%), patients with a pregnancy duration of 57–63 days (66.7%) and 64–70 days (68.4%), and patients receiving asynchronous care (69.6%) (Extended Data Table 2). Among patients with unknown outcomes, two requested abortion pill reversal after they took mifepristone but before misoprostol. Both were advised that evidence-based reversal treatment does not exist and referred to urgent in-person care. No further information on their outcomes was available.
Extended Data Table 2.
Characteristics of the sample with and without known abortion outcomes (n = 6,034)
Effectiveness
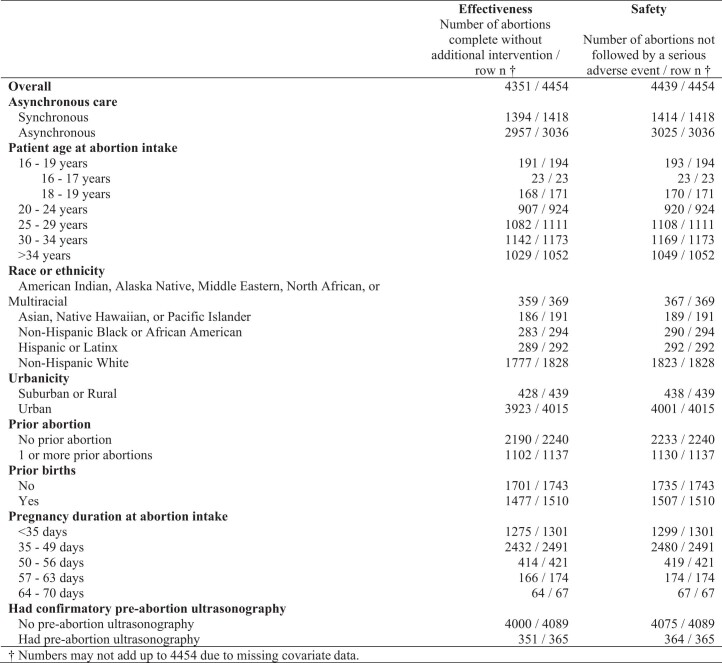
Overall, results from both the complete case analysis and the imputed models found that 97.7% (95% confidence interval (CI) = 97.2–98.1%) of abortions were complete without a subsequent known intervention or ongoing pregnancy after initial treatment (Table 2 and Extended Data Table 3). The effectiveness of synchronous and asynchronous telehealth was similar; in the complete case analysis effectiveness was 98.3% (95% CI = 97.5–99.0%) in the synchronous group and 97.4% (95% CI = 96.9–98.0%) in the asynchronous group. In the final imputed analysis, effectiveness was 98.3% (95% CI = 97.7–99.0%) in the synchronous group and 97.4% (95% CI = 96.9–98.0%) in the asynchronous group. Effectiveness also did not differ according to patient age, pregnancy duration, race, ethnicity or ethnic grouping, urbanicity, previous birth, previous abortion or whether the patient had screening ultrasonography.
Table 2.
Rate of effectiveness and safety according to the characteristics of the sample, derived from complete case and multiple imputation analyses
| Characteristics | Effectiveness | Safety | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted complete case rate | Adjusted imputed rate | Unadjusted complete case rate | Adjusted imputed rate | |||||
| n = 4,454 | P | n = 6,034 | P | n = 4,454 | P | n = 6,034 | P | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |||||
| Overall | 97.7 (97.2–98.1) | 97.7 (97.2–98.1) | 99.7 (99.5–99.8) | 99.8 (99.6–99.9) | ||||
| Asynchronous care | ||||||||
| Synchronous (ref) | 98.3 (97.6–99.0) | Ref | 98.3 (97.7–99.0) | Ref | 99.7 (99.4–100.0) | Ref | 99.8 (99.5–100.0) | Ref |
| Asynchronous | 97.4 (96.8–98.0) | 0.062 | 97.4 (96.9–98.0) | 0.071 | 99.6 (99.4–99.9) | 0.668 | 99.7 (99.6–99.9) | 0.926 |
| Patient age at abortion intake | ||||||||
| 16–19 years (ref)a | 98.5 (96.7–100.0) | Ref | 98.4 (96.7–100.0) | Ref | 99.5 (98.5–100.0) | Ref | 99.6 (98.9–100.0) | Ref |
| 16–17 years | 100.0 (100.0–100.0) | 100.0 (100.0–100.0) | ||||||
| 18–19 years | 98.2 (96.3–100.0) | 99.4 (98.3–100.0) | ||||||
| 20–24 years | 98.2 (97.3–99.0) | 0.780 | 98.1 (97.2–99.0) | 0.789 | 99.6 (99.1–100.0) | 0.876 | 99.7 (99.3–100.0) | 0.932 |
| 25–29 years | 97.4 (96.5–98.3) | 0.382 | 97.5 (96.6–98.3) | 0.408 | 99.7 (99.4–100.0) | 0.575 | 99.8 (99.6–100.0) | 0.604 |
| 30–34 years | 97.4 (96.4–98.3) | 0.370 | 97.4 (96.5–98.3) | 0.398 | 99.7 (99.3–100.0) | 0.711 | 99.7 (99.5–100.0) | 0.745 |
| >34 years | 97.8 (96.9–98.7) | 0.569 | 97.7 (96.7–98.6) | 0.534 | 99.7 (99.4–100.0) | 0.608 | 99.8 (99.6–100.0) | 0.627 |
| Race, ethnicity or ethnic grouping | ||||||||
| American Indian, Alaska Native, Middle Eastern, North African or Multiracialb | 97.3 (95.6–98.9) | 0.932 | 97.8 (96.6–99.0) | 0.733 | 99.0 (97.5–100.0) | 0.413 | 99.7 (99.3–100.0) | 0.474 |
| Asian, Native Hawaiian or Pacific Islander | 97.4 (95.1–99.6) | 0.89 | 97.9 (96.2–99.5) | 0.716 | 99.9 (99.7–100.0) | 0.108 | 99.5 (98.8–100.0) | 0.185 |
| Black or African American | 96.3 (94.1–98.4) | 0.37 | 97.1 (95.5–98.7) | 0.590 | 98.6 (97.3–100.0) | 0.017 | 99.3 (98.7–100.0) | 0.037 |
| Hispanic or Latinx | 99.0 (97.8–100.0) | 0.089 | 98.7 (97.5–100.0) | 0.206 | 100.0 (100.0–100.0) | N/A | 100.0 (100.0–100.0) | N/A |
| White (ref) | 97.2 (96.5–98.0) | Ref | 97.6 (97.0–98.2) | Ref | 99.7 (99.5–100.0) | Ref | 99.8 (99.7–100.0) | Ref |
| Urbanicity | ||||||||
| Suburban or rural (ref) | 97.5 (96.0–99.0) | Ref | 97.4 (95.9–98.9) | Ref | 99.8 (99.3–100.0) | Ref | 99.8 (99.5–100.0) | Ref |
| Urban | 97.7 (97.2–98.2) | 0.777 | 97.7 (97.2–98.2) | 0.728 | 99.7 (99.5–99.8) | 0.68 | 99.7 (99.6–99.9) | 0.674 |
| Previous abortion | ||||||||
| No previous abortion (ref) | 97.8 (97.2–98.4) | Ref | 97.9 (97.3–98.4) | Ref | 99.7 (99.5–99.9) | Ref | 99.8 (99.7–99.9) | Ref |
| One or more previous abortions | 96.9 (95.9–97.9) | 0.140 | 97.3 (96.4–98.1) | 0.247 | 99.4 (98.9–99.8) | 0.204 | 99.6 (99.4–99.9) | 0.188 |
| Previous births | ||||||||
| No previous birth (ref) | 97.6 (96.9–98.3) | Ref | 97.6 (96.9–98.3) | Ref | 99.5 (99.2–99.9) | Ref | 99.7 (99.5–99.9) | Ref |
| One or more previous births | 97.8 (97.1–98.6) | 0.671 | 97.8 (97.1–98.5) | 0.696 | 99.8 (99.6–100.0) | 0.215 | 99.8 (99.7–100.0) | 0.292 |
| Pregnancy duration at abortion intake | ||||||||
| <35 days (ref) | 98.0 (97.2–98.8) | Ref | 97.9 (97.1–98.7) | Ref | 99.8 (99.6–100.0) | Ref | 99.9 (99.7–100.0) | Ref |
| 35–49 days | 97.6 (97.0–98.2) | 0.465 | 97.6 (97.0–98.2) | 0.619 | 99.6 (99.3–99.8) | 0.169 | 99.7 (99.5–99.9) | 0.174 |
| 50–56 days | 98.3 (97.1–99.6) | 0.663 | 98.2 (97.0–99.5) | 0.610 | 99.5 (98.9–100.0) | 0.259 | 99.7 (99.2–100.0) | 0.276 |
| 57–63 days | 95.4 (92.3–98.5) | 0.037 | 96.6 (94.3–99.0) | 0.277 | 100.0 (100.0–100.0) | . | 100.0 (100.0–100.0) | . |
| 64–70 days | 95.5 (90.6–100.0) | 0.182 | 96.7 (93.1–100.0) | 0.493 | 100.0 (100.0–100.0) | . | 100.0 (100.0–100.0) | . |
| Had confirmatory pre-abortion ultrasonography | ||||||||
| No pre-abortion ultrasonography (ref) | 97.8 (97.4–98.3) | Ref | 97.8 (97.4–98.3) | Ref | 99.7 (99.5–99.8) | Ref | 99.7 (99.6–99.9) | Ref |
| Had pre-abortion ultrasonography | 96.2 (94.2–98.1) | 0.046 | 96.2 (94.1–98.2) | 0.063 | 99.7 (99.2–100.0) | 0.829 | 99.8 (99.4–100.0) | 0.844 |
aThe two youngest age categories (16–17 and 18–19 years) were collapsed in the multivariable models to facilitate model convergence. bAmerican Indian, Alaska Native, Middle Eastern, North African, and Multiracial groups were collapsed in the multivariable models to facilitate model convergence.
Estimates were derived from marginal estimates from logistic regressions. Estimates were not adjusted for multiple comparisons. Imputation models included patient age, urbanicity, whether the patient obtained screening ultrasonography, whether the patient obtained synchronous or asynchronous telehealth care, whether the patient participated in CHAT surveys or a virtual clinic, and whether the patient used an abortion fund to pay for any portion of their abortion.
Extended Data Table 3.
Rate of abortions not followed by a serious adverse event according to the characteristics of the sample from complete cases and multiple imputation analysis
Among the 2.3% (95% CI = 1.9–2.8%) of patients whose abortion was not initially complete, 0.56% were treated with more than 200 mg mifepristone, more than 1,600 μg misoprostol or other uterotonic medication to complete the abortion, 1.4% were treated with an aspiration or other abortion procedure, 0.16% were treated for an ectopic pregnancy and 0.94% had a confirmed or suspected continuing pregnancy (Table 3).
Table 3.
Medication abortion additional interventions and serious adverse events
| Effectiveness and safety outcomes | n = 4,454 | Complete case n = 4,454 | Imputeda n = 6,034 |
|---|---|---|---|
| No. | Estimate (95% CI) | Estimate (95% CI) | |
| Effectiveness | |||
| Complete abortion without intervention | 4,351 | 97.7 (97.2–98.1) | 97.7 (97.2–98.1) |
| Intervention to complete abortionb | 103 | 2.3 (1.9–2.8) | 2.3 (1.9–2.8) |
| Procedure, aspiration or surgery | 63 | 1.4 (1.1–1.8) | 1.4 (1.1–1.8) |
| Prescribed >1,600 μg misoprostol, mifepristone or other medications | 22 | 0.49 (0.29–0.70) | 0.56 (0.32–0.81) |
| Treatment for an ectopic pregnancyc | 6 | 0.13 (0.03–0.24) | 0.16 (0.01–0.31) |
| Suspected or confirmed continuing pregnancy | 41 | 0.92 (0.64–1.20) | 0.94 (0.65–1.23) |
| Safety | |||
| No major abortion-related adverse eventsc | 4,439 | 99.7 (99.5–99.8) | 99.8 (99.6–99.9) |
| Major abortion-related adverse eventsb,c | 15 | 0.34 (0.17–0.51) | 0.25 (0.12–0.37) |
| Blood transfusionc | 6 | 0.13 (0.03–0.24) | 0.10 (0.02–0.18) |
| Other major surgery, including treatment of an ectopic pregnancyc | 1 | 0.02 (0–0.07) | 0.02 (0–0.05) |
| Hospital admissionc | 10 | 0.22 (0.09–0.36) | 0.17 (0.06–0.27) |
| Other outcomes | |||
| Emergency department visits | 81 | 1.8 (1.4–2.2) | 1.3 (1.1–1.6) |
aImputation models included patient age, urbanicity, whether the patient obtained screening ultrasonography, whether the patient obtained synchronous or asynchronous telehealth care, whether the patient participated in the CHAT study surveys or a virtual clinic, and whether the patient used an abortion fund to pay for any portion of their abortion.
bSubcategories are not mutually exclusive.
cOutcomes are unadjusted because of small cells.
Models for estimates with n > 15 were adjusted for synchronous versus asynchronous care, patient age, race, ethnicity or ethnic grouping, urbanicity, previous abortion, pregnancy duration at intake and pre-abortion screening ultrasonography. Estimates were calculated from logistic regression models with missing outcomes and covariates imputed using multiple imputation with chained equations.
Overall, six (0.16%) patients had ectopic pregnancies; three (0.12%) were suspected ectopic pregnancies treated with methotrexate; one (0.07%) was an ectopic pregnancy treated with an unknown treatment; one (0.12%) was a cesarean scar ectopic pregnancy treated with an unknown treatment; and one (0.09%) was a ruptured ectopic pregnancy treated with a salpingectomy.
Safety
Overall, the rate of abortions that were not followed by a serious adverse event was 99.7% (95% CI = 99.5–99.8%) in the complete case analysis and 99.8% (95% CI = 99.6–99.9%) in the final imputed model (Table 2 and Extended Data Table 3). Safety was similar between patients who received synchronous and asynchronous care; in the complete case analysis, the safety rate was 99.7% (95% CI = 99.4–100.0%) in the synchronous group and 99.6% (95% CI = 99.4–99.9%) in the asynchronous group. In the final imputed model, safety was 99.8% (95% CI = 99.5–100.0%) among synchronous patients and 99.7% (95% CI = 99.6–99.9%) among asynchronous patients. In the final imputed models, safety was lower among Black or African American patients (99.3%, 95% CI = 98.7–100.0%) than among white patients (99.8%, 95% CI = 97.0–100.0%). No other factors were significantly associated with reduced safety.
Among the 0.25% of patients who experienced a serious adverse event, 0.10% received blood transfusions and 0.02% had abdominal surgery to treat a ruptured ectopic pregnancy; 0.17% of patients had hospital admissions requiring overnight stays. Among the ten (0.17%) hospital admissions, four (0.12%) received inpatient aspiration procedures, two (0.10%) were treated for infection and received an aspiration, one (0.09%) involved a blood transfusion and aspiration, one (0.09%) underwent surgery to treat a ruptured ectopic pregnancy, one (0.08%) was treated with intravenous antibiotics and one (0.09%) had a uterine infection treated with unknown treatment.
Other outcomes
Overall, 1.3% (95% CI = 1.1–1.6%) of abortions were followed by a known emergency department visit, 38.3% of which resulted in no treatment. Emergency department visits were similar between synchronous patients (1.2%, 95% CI = 0.7–1.7%) and asynchronous patients (1.4%, 95% CI = 1.0–1.7%). We identified no cases where, at the subsequent follow-up, it was determined that the abortion occurred beyond 70 days’ gestation.
Sensitivity analyses
The first sensitivity analysis, where we conservatively categorized the 25 patients who were referred to in-person care and were subsequently lost to follow-up as requiring additional intervention to complete the abortion, resulted in effectiveness rates that were not significantly different from the primary analysis; overall 97.1% (95% CI = 96.5–97.6%), with 98.1% (95% CI = 97.3–98.8%) among synchronous patients and 96.7% (95% CI = 96.0–97.3%) among asynchronous patients.
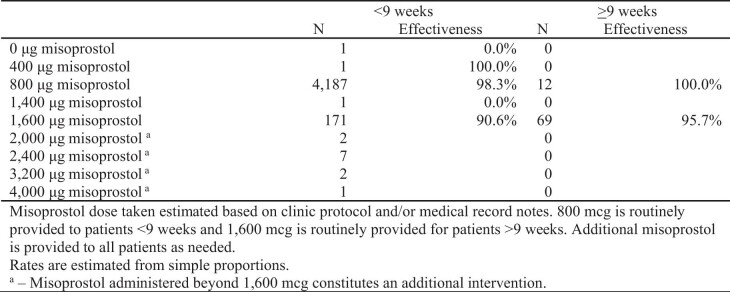
In the second sensitivity analysis modeling effectiveness, we considered patients as having complete abortions regardless of the amount of misoprostol they received, which is consistent with the Medical Abortion Reporting of Efficacy (MARE) guidelines20. (Total misoprostol dosages according to pregnancy duration are reported in Extended Data Table 4.) This also resulted in effectiveness rates that were not significantly different from the primary analysis: 97.9% (95% CI = 97.4–98.3%) overall, 98.4% (95% CI = 97.8–99.0%) among patients who received synchronous care and 97.7% (95% CI = 97.1–98.2%) among patients who received asynchronous care.
Extended Data Table 4.
Total misoprostol dosage administered after 200 mg mifepristone according to pregnancy duration (n = 4,454)
The third sensitivity analysis, where we examined effectiveness and safety only among the subsample of patients with supplementary self-reported data on their outcomes via surveys in addition to standard clinical follow-up (n = 1,600), resulted in effectiveness rates that were not significantly different from the primary analysis: 96.7% (95% CI = 95.7–97.6%), with 97.1% (95% CI = 95.6–98.6%) among those who received synchronous care and 96.4% (95% CI = 95.2–97.6%) among those who received asynchronous care. This sensitivity analysis resulted in a similar safety rate of 99.3% (95% CI = 98.9–99.7%), and rates of 99.4% (95% CI = 98.7–100.0%) among those who received synchronous care versus 99.3% (95% CI = 98.8–99.8%) of those who received asynchronous care.
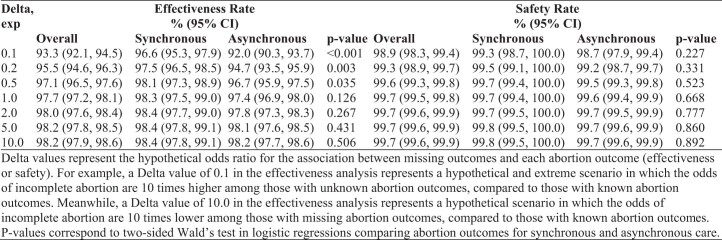
In the fourth sensitivity analysis, we conducted delta-adjusted pattern-mixture modeling to examine the potential impact of loss to follow-up on the observed results (Extended Data Table 5). Across a range of delta values, we found that the results were largely consistent with the main analysis. Under an extreme scenario in which those with unknown outcomes had ten times the odds of an incomplete abortion or serious adverse event, effectiveness for the entire sample would be 93.3% (95% CI = 92.1–94.5%) and safety would be 98.9% (95% CI = 98.3–99.4%). Under this scenario, effectiveness would be higher in the synchronous group than the asynchronous group, but there would be no differences in safety. Under the opposite and also extreme scenario in which those with unknown outcomes had ten times lower odds of an incomplete abortion, effectiveness would be 98.2% (95% CI = 97.9–98.6%) and safety would be 99.7% (95% CI = 99.6–99.9%), with no significant differences in effectiveness and safety between synchronous and asynchronous groups.
Extended Data Table 5.
Pattern-mixture model imputation with delta correction to explore the potential impact of informative missingness in abortion outcomes
Discussion
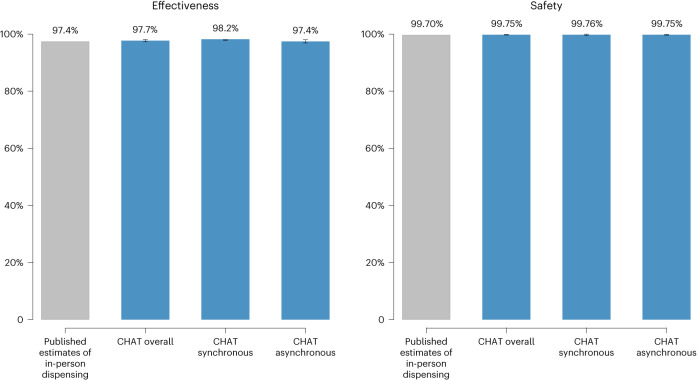
In this large prospective cohort study, telehealth medication abortion provided primarily without tests was effective and safe. The overall 98% effectiveness rate of our primary analysis, and the effectiveness rates from the sensitivity analyses, were similar to previous large US studies of in-person medication abortion care, which found rates of 95–98%21–24. The serious adverse event rate of 0.25% and ectopic pregnancy rate of 0.14% were also similar to previous studies of in-person medication abortion care, which found adverse event rates of 0.2–0.5%, and ectopic pregnancy rates of 0.2%8,23–25. Both effectiveness and safety rates were similar to the rates for medication abortions with in-person screening tests as published on the FDA label (Fig. 3)8.
Fig. 3. Abortion effectiveness and safety estimates: CHAT study and published estimates of in-person care.
The gray bars represent published estimates from the FDA label for in-person dispensing of mifepristone; the blue bars represent the rates found in the CHAT study. Estimates for the CHAT study were calculated using marginal estimates from logistic regression analyses conducted on n = 6,034 patients. The published estimates of in-person dispensing represent the published rates drawn from the FDA label for mifepristone in 2016. The 95% CIs are represented by the black error bars.
The effectiveness and safety rates found in this study are consistent with, although slightly lower than, those found in studies of no-test telehealth abortion in other countries. A national study in the UK, which included 18,435 telehealth medication abortions, found that 99% were complete without intervention and serious adverse events occurred in 0.02% (refs. 26–28). This higher documented effectiveness rate may be explained by the lack of routine follow-up after medication abortion care in the UK; additional interventions that patients may receive may not be systematically reported to the original abortion provider.
The rates in our study are also similar to the effectiveness and safety rates documented from self-managed medication abortion models (defined as using abortion pills to end a pregnancy outside of the formal healthcare system), in the USA29 and internationally, including in contexts where abortion is legally restricted30,31.
The effectiveness rates for both synchronous and asynchronous services were very high and similar to in-person care. These findings have important implications for service delivery and health equity. Synchronous models with videoconferencing require strong Internet connectivity. Asynchronous models can be accessed using more types of devices; they may be more private, require shorter waiting times and can be more easily integrated into work or home schedules because no appointment is needed32–34. Offering patients a choice between synchronous and asynchronous care is consistent with patient-centered care and may increase access for people historically excluded from healthcare, particularly those living in rural areas or those who live far from an abortion-providing facility1,35,36.
We used a more conservative definition of effectiveness than recommended by the MARE guidelines20 but used in previous studies17,37. Our definition included an additional 22 patients who received a second medication abortion (mifepristone plus misoprostol) or more than one additional dose of misoprostol. In the context of telehealth and in the wake of the Dobbs decision, patients living in states that have banned abortions may experience more barriers to procedural treatment for incomplete abortion and thus be more likely to obtain additional medications to complete the abortion. Therefore, our definition of effectiveness may better account for patient experience.
While safety was over 99% among all ethnic groups, Black patients had significantly higher rates of serious adverse events than white patients. This finding is consistent with research showing higher rates of adverse obstetric outcomes among Black patients. Growing consensus finds that these disparities in obstetric health are rooted in implicit biases and structural racism38,39.
This analysis provides an initial picture of the real-world effectiveness and safety of a rapidly expanding model of abortion care among a large US cohort. However, this analysis has several limitations. One is the lack of clinic-level variation in synchronous and asynchronous models, which may limit generalizability. However, each virtual clinic had multiple providers offering care, thereby increasing variation within each clinic and thus the generalizability of our findings. For example, different providers may use different thresholds or criteria for when to refer patients to in-person care for an ultrasound or exam, which may impact effectiveness rates. This natural variation strengthens the premise that these results could be applied to other providers offering synchronous or asynchronous care. While there was no direct comparison group, we were able to compare our results to widely accepted rates in the published literature using standardized guidelines for measuring medication abortion outcomes.
Additionally, we identified no cases of unexpected pregnancy durations beyond 70 days. This is surprising given that a previous study of no-test medication abortion found a rate of 0.38%17. This lack of evidence may be due to underreporting. Although most patients can accurately assess their pregnancy duration40,41, patients who later learned that they provided a date of last menstrual period that underestimated their pregnancies may have felt that they could be held responsible and thus not reported it to the virtual clinic, particularly if it resulted in an abortion beyond 70 days.
Finally, another limitation is the follow-up rate; at 74% it was similar or higher than other studies on abortion17,31,42,43; attrition may have introduced selection bias given that some groups had lower follow-ups than others. In particular, we observed lower follow-up rates in the asynchronous group than the synchronous group. Telehealth is a less medicalized healthcare model, and asynchronous care even less so; those who opt for it may prefer a more autonomous experience. This differential follow-up may overestimate effectiveness and safety rates for asynchronous patients if those with concerning symptoms seek additional care without informing the virtual clinic. On the other hand, it might underestimate effectiveness rates if patients who have a negative pregnancy test or clear signs of complete abortion do not feel that they must report their outcome back to the virtual clinic. We attempted to limit this potential bias with multiple imputation. We also explored this limitation through a sensitivity analysis simulating higher and lower odds of incomplete abortions and serious adverse events among those lost to follow-up relative to those with known outcomes. This analysis demonstrated that differences in effectiveness between synchronous and asynchronous groups could reach significance under extreme scenarios, but differences in safety remained nonsignificant in all scenarios tested.
These findings provide evidence that telehealth for abortion is effective and safe, with rates similar to in-person care. Additionally, synchronous and asynchronous care are comparably effective and safe. Although telehealth models cannot serve the needs and preferences of everyone, such as those who do not have electronic devices or those who are beyond the first trimester of pregnancy, offering people telehealth options has the potential to expand access to abortion care. These results are reassuring as more clinicians begin to provide telehealth abortion care to patients in US states with a ban, under the legal protections of their state’s shield laws. At the same time, 11 states continue to permit abortion but have prohibitions on no-test telehealth abortion (https://www.rhites.org/state-based-resources). This study demonstrates that policies that restrict telehealth abortion owing to concerns or claims about effectiveness or safety need to be revisited and revised to ensure equitable access to this essential healthcare service.
Methods
Ethics
The CHAT study was approved by the University of California, San Francisco institutional review board (no. 20-32951) and registered with ClinicalTrials.gov (registration: NCT04432792). We used Strengthening the Reporting of Observational studies in Epidemiology guidelines to design and report the results of this study. All survey respondents provided consent to participate in the research.
Data source and study cohort
The CHAT study followed the patients of three US virtual abortion clinics: Choix (which opened in October 2020); Hey Jane (which opened in January 2021); and Abortion on Demand (which opened in April 2021). These virtual clinics were selected because they were among the first to open in the USA after the FDA temporarily suspended the in-person dispensing requirement during the COVID-19 emergency, and because they operated in states with large populations.
Medication protocols included 200 mg mifepristone orally and 800 µg misoprostol buccally or vaginally for pregnancy durations less than 63 days or 1,600 µg for pregnancy durations of 63 or more days. Care was provided based on a published protocol19 by nurse practitioners, nurse midwives, physician assistants and physicians who specialize in abortion care. Clinics offered synchronous (video) or asynchronous (secure text messaging) telehealth abortion with mail order pharmacy delivery. One clinic offered only synchronous medication abortion care, one offered only asynchronous care and one offered asynchronous care with an option to have a phone or video call with the provider if preferred. Patients learned about the services through Web searches, social media or referrals.
During the study period, one clinic offered abortion care up to 56 days (8 weeks) of pregnancy, whereas the two other clinics offered it up to 70 days (10 weeks). As per the published protocol, patients were evaluated for medical eligibility based on the reported medical history. Pregnancy duration at intake was primarily based on self-reported date of last menstrual period or by ultrasonography, if available. Some patients had already had ultrasonography before contacting the virtual clinic. Additionally, patients were referred for pre-abortion ultrasonography if they had any risk factors for, or symptoms of, ectopic pregnancy19 or were potentially beyond the gestational limit of the virtual clinic. Some of these patients returned to the virtual clinic after their eligibility was confirmed by ultrasonography and obtained a telehealth abortion; thus, they were included in the study. Others opted for in-person care and thus were excluded.
Each clinic had two scheduled follow-up interactions. The first confirmed medication administration and assessed symptoms of complete abortion 3–7 days after intake. The second was a low-sensitivity pregnancy test at 2 weeks or a high-sensitivity test at 4 weeks after medication administration. Follow-up interactions were conducted by text messaging, secure messaging or telephone. At each scheduled follow-up, clinicians made up to four attempts to contact patients. Clinicians referred patients to in-person care if any adverse event or incomplete abortion was suspected and outcomes of care were documented whenever possible.
For this analysis, we evaluated data collected from two sources, both imported into REDCap44. We obtained anonymized medical record data of consecutive patients receiving care from the participating virtual clinics between April 2021 and January 2022.
Additionally, each virtual clinic invited all patients seen between June 2021 and January 2022 to enroll in three surveys about their abortion experience, including any additional treatments received. After providing electronic informed consent, participants completed a baseline survey on the date of the intake, which included sociodemographic characteristics and medical history. Participants completed a second survey 3–7 days after the intake, to assess medication administration, additional medical care and any adverse events, and a final survey 4 weeks after the intake to assess additional medical care and adverse events (Fig. 1). The survey sample was powered to assess the acceptability of telehealth (published separately2); thus, we aimed to collect complete sets of surveys from 1,600 participants. Survey participants received a US$50 electronic debit card on completion of all three surveys.
Outcomes
The primary outcomes were effectiveness and safety based on standard definitions in previous studies17,24,37,45. We generally followed the MARE guidelines for reporting outcomes20. We defined effectiveness as the proportion of medication abortions that were complete after initial treatment with 200 mg mifepristone and 1,600 µg or less of misoprostol without known subsequent intervention. Abortions were not considered complete if (1) the patient had an aspiration, dilation and evacuation, other procedure or surgical intervention to complete the abortion; (2) the patient received more than 200 mg mifepristone, more than 1,600 µg misoprostol, or a uterotonic medication to complete the abortion; (3) the patient received treatment for suspected or confirmed ectopic pregnancy; or (4) the patient had a continuing pregnancy confirmed by ultrasonography or suspected at last contact. While MARE guidelines define effectiveness as successful expulsion of pregnancy without the need for procedural intervention, we chose a more conservative definition, recognizing that patients may view the need to have what constitutes a second medication abortion treatment as a failure of the medication abortion protocol.
We defined safety using standardized definitions from the Procedural Abortion Incident Reporting and Surveillance Framework45 and Standardizing Abortion Research Outcomes protocol46 as the proportion of abortions that were not followed by a known abortion-related serious adverse event. Serious adverse events included: blood transfusion; abdominal surgery (including salpingectomy, laparotomy and laparoscopy to treat an ectopic pregnancy); hospital admission requiring overnight stay; or death.
Effectiveness and safety outcomes were determined from all information collected in the medical records and surveys. Abortion completion was determined based on the virtual clinic’s designation, either using a test (urine pregnancy test, ultrasonography or serum human chorionic gonadotrophin) or using the patient’s medical history (using a checklist reflecting symptoms of complete abortion) without further contact related to the abortion for at least 6 weeks after the intake visit. Patients without outcomes noted in the medical records were determined to have complete abortions if they completed a survey at least 28 days after screening and did not report an intervention or ongoing pregnancy.
Secondary outcomes included the number of cases where, at the subsequent follow-up, it was determined that at intake the patient had been beyond 70 days’ gestation. We also evaluated rates of suspected or confirmed ectopic pregnancy and emergency department visits.
Covariates
We examined the categorical covariates reflecting participant age at abortion intake in years (16–17 years, 18–19 years, 20–24 years, 25–29 years, 30–34 years and 35 years or older), and pregnancy duration in days at abortion intake (less than 35 days, 35–49 days, 50–56 days, 57–63 days, 64–70 days or unknown). We also included a measure of race, ethnicity or ethnic grouping indicated by participants on an intake form or in the surveys (American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, Black or African American, Middle Eastern or North African, White, Multiracial or Unknown). We included binary covariates for urbanicity (suburban or rural versus urban), whether the patient had a previous abortion, whether the patient had a previous birth and whether the patient had confirmatory pre-abortion ultrasonography.
Exposure
The key exposure was a binary measure reflecting whether the patient received care synchronously (video) or asynchronously (secure text messaging).
Statistical analysis
The study was powered to detect differences in the rarest primary outcome, that is, serious adverse events. We aimed to have outcome data from 4,202 patients. The study was designed to detect a difference of 0.4% or more in the rate of serious adverse events compared to 0.5%, the rate for in-person medication abortions as published in the FDA label8, with 90% power and a two-sided alpha of 0.05. With a final sample size of 4,454, the study had more than 90% power to detect a difference of 2% or more in the effectiveness rate compared to the 3% rate for in-person medication abortions as published on the FDA label8.
We described the characteristics of the overall sample and the subsample of patients who completed the surveys. We examined the extent of loss to follow-up and whether loss to follow-up differed between those who obtained synchronous and asynchronous care. We then conducted multiple imputation by chained equations to account for missing covariate and outcome data with 100 replications for primary regression analyses, assuming that missing data were related to observed patient and abortion characteristics. Multiple imputation by chained equations iteratively impute missing data using predictive models based on other variables in the dataset, and accounts for statistical uncertainty in the imputations47. Imputation models included patient age, urbanicity, whether the patient obtained screening ultrasonography, whether the patient obtained synchronous or asynchronous telehealth care, whether the patient participated in CHAT surveys, virtual clinics, and whether the patient used an abortion fund to pay for any portion of their abortion.
We developed logistic regression models for all effectiveness and safety outcomes. We used multivariable models for outcomes n > 15, adjusting for a binary measure of whether the patient received screening via synchronous or asynchronous methods. These models were also adjusted for baseline patient and abortion characteristics, including patient age, race, ethnicity or ethnic grouping, and pregnancy duration. We included binary measures reflecting whether the patient had a previous abortion or birth, and whether the patient had pre-abortion ultrasonography21. For rare outcomes (n < 15), we used unadjusted logistic regression models.
We calculated marginal estimates, the corresponding 95% CIs and P values from the logistic regression results to estimate the predicted probability of each effectiveness and safety outcome. Primary estimates came from logistic regression analyses performed on imputed data. P values correspond to a Wald test in the logistic regressions, comparing each group to the reference group. We then compared results with published estimates of effectiveness and safety. All statistical tests were two-tailed with significance set at 0.05. All analyses were conducted using Stata v.17.0 (StataCorp LLC).
We conducted several sensitivity analyses to assess the robustness of our findings. First, we replicated the effectiveness analysis, assuming that patients who were referred to in-person care after taking the medications and were then lost to follow-up required further intervention to complete the abortion. Second, we replicated the effectiveness analysis by categorizing all patients who received any additional misoprostol as completed abortions. This is consistent with the MARE guidelines and previous studies26,48, which classified patients who received more than 1,600 μg of misoprostol (more than two doses) as successful abortions. Third, we examined both effectiveness and safety outcomes only among the subsample of patients who completed the surveys to evaluate whether the main findings held true among this sample with supplementary self-reported data on their outcomes. Finally, to test how robust our results were to the follow-up rates, we used delta-adjusted pattern-mixture model imputation49 to simulate the outcomes under different assumptions regarding patients with missing outcome data, hypothesizing results if they had lower or higher odds of incomplete abortion or serious adverse events than those with outcome data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-024-02834-w.
Supplementary information
Acknowledgements
We thank M. Cervantes, L. Shin, K. Song, A. Becker and L. Peters for their contributions to data collection and management and other input on the CHAT study. We also thank C. Adam, M. Adam, K. Baron, S. Bussmann, L. Coplon, L. Dubey, L. DuBois, K. Freedman, G. Izarra, J. Phifer and A. Wagner for their support with data acquisition. We thank E. Wells and F. Coeytaux for their early input on study design, and E. Raymond for thoughtful guidance on classifying adverse events. We thank W. J. Boscardin for his input on pattern-mixture modeling. The CHAT study was supported by the BaSe Family Fund, the Erik E. and Edith H. Bergstrom Foundation, the Isabel Allende Foundation, Jess Jacobs, the Kahle/Austin Foundation, the Lisa and Douglas Goldman Fund, Preston-Werner Ventures (all to U.D.U.) and a Resource Allocation Program Award from the University of California, San Francisco National Center of Excellence in Women’s Health (to M.A.B.). L.R.K. was funded in part by a training grant from the National Institute of Child Health and Human Development of the National Institutes of Health under award no. F31HD111277 for the duration of the study. The funders had no role in study design, data collection and analysis, the writing of the manuscript or the decision to submit the manuscript for publication.
Extended data
Author contributions
U.D.U. obtained the funding for the study. U.D.U., L.R.K., E.S.V. and K.M. conceptualized and designed the study. L.R.K. conducted the data analysis. U.D.U. supervised the data analysis. J.K. provided management and administration for the study. U.D.U., L.R.K. and M.A.B. drafted the manuscript. All authors interpreted the data, reviewed the manuscript drafts, provided substantive input on its content and approved the final version of the manuscript. U.D.U. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Peer review
Peer review information
Nature Medicine thanks Alimu Dayimu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil and Jennifer Sargent, in collaboration with the Nature Medicine team.
Data availability
The datasets analyzed during this study are not publicly available because the patients who underwent an abortion did not consent to sharing their data beyond the primary researchers and because the legal status of abortion care is continually changing. The de-identified, individual-level data used to reach the study conclusions are available to qualified investigators from the corresponding author. Requesters must include a description of their research project, the qualifications of the research team, whether the analysis has institutional review board approval and how the results will be disseminated. Requesters must also sign a data use agreement to (1) use the data only for research purposes, (2) not attempt to re-identify the data or contact the study participants, (3) secure the data using appropriate computer technology and (4) destroy the data after the analyses are completed. Responses can be expected within 1 month of a request.
Code availability
Data analyses were carried out using Stata v.17.0 (StataCorp LLC) as specified in the Methods. The code is available on GitHub (https://github.com/Upadhyay-Lab).
Competing interests
K.M. reports receiving personal fees from Danco Laboratories, a distributor of mifepristone, for staffing a US Food and Drug Administration-mandated expert hotline. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41591-024-02834-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-024-02834-w.
References
- 1.Koenig LR, Becker A, Ko J, Upadhyay UD. The role of telehealth in promoting equitable abortion access in the United States: a spatial analysis. JMIR Public Health Surveill. 2023;9:e45671. doi: 10.2196/45671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koenig LR, et al. Patient acceptability of telehealth medication abortion care in the United States, 2021‒2022: a cohort study. Am. J. Public Health. 2024;0:e1–e10. doi: 10.2105/AJPH.2023.307437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Society of Family Planning. #WeCount Report April 2022 to June 2023; 10.46621/218569qkgmbl (2023).
- 4.Thomson-DeVeaux, A. Virtual abortions surged after Roe was overturned—but the Texas ruling could change that. ABC News (11 April 2023).
- 5.Rowland, C. To get banned abortion pills, patients turn to legally risky tactics. Washington Post (6 July 2022).
- 6.Belluck, P. & Bazelon, E. New York passes bill to shield abortion providers sending pills into states with bans. The New York Times (20 June 2023).
- 7.Walker, A. S., Corum, J., Khurana, M. & Wu, A. Are Abortion Pills Safe? Here’s the Evidence. The New York Times (7 April 2023).
- 8.US Food and Drug Administration. Mifeprex Label. U.S. FDA Label for Mifepristone (2016); www.accessdata.fda.gov/drugsatfda_docs/label/2016/020687s020lbl.pdf
- 9.Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet. Gynecol. 2017;130:778–782. doi: 10.1097/AOG.0000000000002212. [DOI] [PubMed] [Google Scholar]
- 10.Grossman D, Grindlay K, Buchacker T, Lane K, Blanchard K. Effectiveness and acceptability of medical abortion provided through telemedicine. Obstet. Gynecol. 2011;118:296–303. doi: 10.1097/AOG.0b013e318224d110. [DOI] [PubMed] [Google Scholar]
- 11.Kohn JE, et al. Medication abortion provided through telemedicine in four U.S. states. Obstet. Gynecol. 2019;134:343–350. doi: 10.1097/AOG.0000000000003357. [DOI] [PubMed] [Google Scholar]
- 12.Raymond E, et al. TelAbortion: evaluation of a direct to patient telemedicine abortion service in the United States. Contraception. 2019;100:173–177. doi: 10.1016/j.contraception.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Chong E, et al. Expansion of a direct-to-patient telemedicine abortion service in the United States and experience during the COVID-19 pandemic. Contraception. 2021;104:43–48. doi: 10.1016/j.contraception.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anger HA, et al. Clinical and service delivery implications of omitting ultrasound before medication abortion provided via direct-to-patient telemedicine and mail in the U.S. Contraception. 2021;104:659–665. doi: 10.1016/j.contraception.2021.07.108. [DOI] [PubMed] [Google Scholar]
- 15.Upadhyay UD, Koenig LR, Meckstroth KR. Safety and efficacy of telehealth medication abortions in the US during the COVID-19 pandemic. JAMA Netw. Open. 2021;4:e2122320. doi: 10.1001/jamanetworkopen.2021.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerestes C, et al. Provision of medication abortion in Hawai’i during COVID-19: practical experience with multiple care delivery models. Contraception. 2021;104:49–53. doi: 10.1016/j.contraception.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upadhyay UD, et al. Outcomes and safety of history-based screening for medication abortion: a retrospective multicenter cohort study. JAMA Intern. Med. 2022;182:482–491. doi: 10.1001/jamainternmed.2022.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koenig LR, et al. Mailing abortion pills does not delay care: a cohort study comparing mailed to in-person dispensing of abortion medications in the United States. Contraception. 2023;121:109962. doi: 10.1016/j.contraception.2023.109962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond EG, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361–366. doi: 10.1016/j.contraception.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creinin MD, Chen MJ. Medical abortion reporting of efficacy: the MARE guidelines. Contraception. 2016;94:97–103. doi: 10.1016/j.contraception.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Gatter M, Cleland K, Nucatola DL. Efficacy and safety of medical abortion using mifepristone and buccal misoprostol through 63 days. Contraception. 2015;91:269–273. doi: 10.1016/j.contraception.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas D, Chong E, Raymond EG. Outpatient medical abortion is safe and effective through 70 days gestation. Contraception. 2015;92:197–199. doi: 10.1016/j.contraception.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Raymond EG, Shannon C, Weaver MA, Winikoff B. First-trimester medical abortion with mifepristone 200 mg and misoprostol: a systematic review. Contraception. 2013;87:26–37. doi: 10.1016/j.contraception.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Upadhyay UD, et al. Incidence of emergency department visits and complications after abortion. Obstet. Gynecol. 2015;125:175–183. doi: 10.1097/AOG.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 25.Cleland K, Creinin MD, Nucatola D, Nshom M, Trussell J. Significant adverse events and outcomes after medical abortion. Obstet. Gynecol. 2013;121:166–171. doi: 10.1097/AOG.0b013e3182755763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiken A, Lohr PA, Lord J, Ghosh N, Starling J. Effectiveness, safety and acceptability of no-test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464–1474. doi: 10.1111/1471-0528.16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds-Wright JJ, Johnstone A, McCabe K, Evans E, Cameron S. Telemedicine medical abortion at home under 12 weeks’ gestation: a prospective observational cohort study during the COVID-19 pandemic. BMJ Sex. Reprod. Health. 2021;47:246–251. doi: 10.1136/bmjsrh-2020-200976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiebe ER, Campbell M, Ramasamy H, Kelly M. Comparing telemedicine to in-clinic medication abortions induced with mifepristone and misoprostol. Contracept. X. 2020;2:100023. doi: 10.1016/j.conx.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiken ARA, Romanova EP, Morber JR, Gomperts R. Safety and effectiveness of self-managed medication abortion provided using online telemedicine in the United States: a population based study. Lancet Reg. Health Am. 2022;10:100200. doi: 10.1016/j.lana.2022.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moseson H, et al. Effectiveness of self-managed medication abortion with accompaniment support in Argentina and Nigeria (SAFE): a prospective, observational cohort study and non-inferiority analysis with historical controls. Lancet Glob. Health. 2022;10:e105–e113. doi: 10.1016/S2214-109X(21)00461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endler M, Beets L, Gemzell Danielsson K, Gomperts R. Safety and acceptability of medical abortion through telemedicine after 9 weeks of gestation: a population-based cohort study. BJOG. 2019;126:609–618. doi: 10.1111/1471-0528.15553. [DOI] [PubMed] [Google Scholar]
- 32.Godfrey EM, Thayer EK, Fiastro AE, Aiken ARA, Gomperts R. Family medicine provision of online medication abortion in three US states during COVID-19. Contraception. 2021;104:54–60. doi: 10.1016/j.contraception.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiastro AE, et al. Remote delivery in reproductive health care: operation of direct-to-patient telehealth medication abortion services in diverse settings. Ann. Fam. Med. 2022;20:336–342. doi: 10.1370/afm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens J, Greenberg GM. Asynchronous telehealth. Prim. Care. 2022;49:531–541. doi: 10.1016/j.pop.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Budhwani S, et al. Challenges and strategies for promoting health equity in virtual care: findings and policy directions from a scoping review of reviews. J. Am. Med. Inform. Assoc. 2022;29:990–999. doi: 10.1093/jamia/ocac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiastro AE, Zheng Z, Ruben MR, Gipson J, Godfrey EM. Telehealth vs in-clinic medication abortion services. JAMA Netw. Open. 2023;6:e2331900. doi: 10.1001/jamanetworkopen.2023.31900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raymond EG, et al. Clinical outcomes of medication abortion using misoprostol-only: a retrospective chart review at an abortion provider organization in the United States. Contraception. 2023;126:110109. doi: 10.1016/j.contraception.2023.110109. [DOI] [PubMed] [Google Scholar]
- 38.Montalmant KE, Ettinger AK. The racial disparities in maternal mortality and impact of structural racism and implicit racial bias on pregnant black women: a review of the literature. J. Racial Ethn. Health Disparities. 2023 doi: 10.1007/s40615-023-01816-x. [DOI] [PubMed] [Google Scholar]
- 39.Chambers BD, et al. Black women’s perspectives on structural racism across the reproductive lifespan: a conceptual framework for measurement development. Matern. Child Health J. 2021;25:402–413. doi: 10.1007/s10995-020-03074-3. [DOI] [PubMed] [Google Scholar]
- 40.Ralph LJ, et al. Accuracy of self-assessment of gestational duration among people seeking abortion. Am. J. Obstet. Gynecol. 2022;226:710.e1–710.e21. doi: 10.1016/j.ajog.2021.11.1373. [DOI] [PubMed] [Google Scholar]
- 41.Bracken H, et al. Alternatives to routine ultrasound for eligibility assessment prior to early termination of pregnancy with mifepristone-misoprostol: alternatives to ultrasound prior to medical abortion. BJOG. 2011;118:17–23. doi: 10.1111/j.1471-0528.2010.02753.x. [DOI] [PubMed] [Google Scholar]
- 42.Horning EL, Chen BA, Meyn LA, Creinin MD. Comparison of medical abortion follow-up with serum human chorionic gonadotropin testing and in-office assessment. Contraception. 2012;85:402–407. doi: 10.1016/j.contraception.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Upadhyay UD, Johns NE, Meckstroth KR, Kerns JL. Distance traveled for an abortion and source of care after abortion. Obstet. Gynecol. 2017;130:616–624. doi: 10.1097/AOG.0000000000002188. [DOI] [PubMed] [Google Scholar]
- 44.Harris PA, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor D, et al. Standardizing the classification of abortion incidents: the Procedural Abortion Incident Reporting and Surveillance (PAIRS) Framework. Contraception. 2017;96:1–13. doi: 10.1016/j.contraception.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Whitehouse KC, et al. Standardizing abortion research outcomes (STAR): results from an international consensus development study. Contraception. 2021;104:484–491. doi: 10.1016/j.contraception.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 48.Grossman D, et al. Mail-order pharmacy dispensing of mifepristone for medication abortion after in-person clinical assessment. Contraception. 2022;107:36–41. doi: 10.1016/j.contraception.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 49.White IR, Carpenter J, Horton NJ. A mean score method for sensitivity analysis to departures from the missing at random assumption in randomised trials. Stat. Sin. 2018;28:1985–2003. doi: 10.5705/ss.202016.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during this study are not publicly available because the patients who underwent an abortion did not consent to sharing their data beyond the primary researchers and because the legal status of abortion care is continually changing. The de-identified, individual-level data used to reach the study conclusions are available to qualified investigators from the corresponding author. Requesters must include a description of their research project, the qualifications of the research team, whether the analysis has institutional review board approval and how the results will be disseminated. Requesters must also sign a data use agreement to (1) use the data only for research purposes, (2) not attempt to re-identify the data or contact the study participants, (3) secure the data using appropriate computer technology and (4) destroy the data after the analyses are completed. Responses can be expected within 1 month of a request.
Data analyses were carried out using Stata v.17.0 (StataCorp LLC) as specified in the Methods. The code is available on GitHub (https://github.com/Upadhyay-Lab).