Abstract
Objective:
To investigate pragmatic language abilities in young children with an increased risk for adverse neurobehavioral and neurocognitive outcomes due to an extra X or Y chromosome (sex chromosome trisomy; SCT) and to investigate to what degree early structural and pragmatic language abilities are predictive of neurobehavioral problems one year later.
Method:
In total, 72 children with SCT and 71 controls aged 3–7 years were included. Language assessments included parent-reported pragmatic language skills and direct assessment of structural language abilities. Parent-reported behavioral outcomes were measured one year after the initial language assessment.
Results:
Children with SCT demonstrated weaker pragmatic language skills compared to controls. These differences were not driven by karyotype, time of diagnosis, or ascertainment bias and irrespective of the presence of structural language impairment. Odds of having pragmatic difficulties was 23 times higher in the SCT group, with 25% of the children not meeting age-expectations. In addition, language, in particular pragmatic language, was an important predictor for later affective, oppositional defiant, pervasive developmental, attention deficit, and social-emotional problems in young children with SCT.
Conclusions:
This study is one of the first studies that directly illustrates the relationship between language and behavioral outcomes in children with SCT. Our results stress the importance to closely monitor pragmatic language in addition to structural language in clinical care of children with SCT, as pragmatic language abilities could serve as an early marker for children at risk for developing behavioral problems.
Keywords: Structural language, pragmatic language, sex chromosome trisomy, early development, neurobehavioral outcomes
Introduction
Approximately 1 in 650 to 1 in 1000 children is born with an extra X or Y chromosome, or sex chromosome trisomy (SCT; Bojesen et al., 2003; Groth et al., 2013; Morris et al., 2008). This leads to a 47,XXY or 47,XYY chromosomal pattern in males or a 47,XXX pattern in females. SCT is a relatively common genetic variation, associated with an increased risk for neurocognitive difficulties (for a review see Urbanus et al., 2020), neurodevelopmental disorders (for a review see Van Rijn, 2019) and for social-emotional and behavioral problems (Urbanus et al., 2020). As children with SCT can be diagnosed prenatally, this gives a unique opportunity to prospectively follow a group of children from an early age who biologically have a heightened risk to develop neurodevelopmental difficulties, and to investigate mechanisms of developmental vulnerability. It is likely that neurodevelopmental difficulties are anchored in early brain maturation; on both the X and the Y chromosome, genes are located that play an important role in neural development and cognitive functioning (Lenroot et al., 2014; Raznahan et al., 2016). Global intellectual functioning is variable in children with SCT, ranging from impaired to above average with mean functioning in the average to low-average range (for a review see Urbanus et al., 2020). Some studies found relative strengths on non-verbal reasoning and spatial intellectual functioning in contrast to performance on verbal intellectual tests (e.g. Ross et al., 2008; Cordeiro et al., 2012; Netley, 1986; Rovet et al., 1995; Rovet et al., 1996). In addition, neurocognitive difficulties have been reported in children with SCT regardless of level of intellectual functioning.
Neurocognitive functions could serve as early markers for behavioral problems in later life. Knowledge about early neurocognitive functions that underlie behavioral outcomes is important, as these functions could serve as important targets for early treatment and intervention. Among these neurocognitive difficulties are disturbances in language development, with studies reporting language difficulties in 70–80% of included SCT individuals (Boada et al., 2009). Recent studies including very young children with SCT indicate that these language difficulties can already be identified before children are one-year old (Urbanus et al., 2022; Zampini et al., 2021). Language problems are considered one of the most prominent neurocognitive vulnerabilities associated with SCT. Recent studies have shown difficulties in areas of early non-verbal communication (Zampini et al., 2018), early vocabulary (Zampini et al., 2018a, 2018b), and semantic skills (Ross et al., 2008; Ross et al., 2009; St John et al., 2019). However, the primary focus within these studies has been on structural aspects of language, which encompasses all aspects of language related to form (i.e. phonology, morphology, syntax) and content (i.e. semantics), whereas the use of language in a social context or pragmatic language is also important for social interaction and communicating with others.
Pragmatic language consists of a variety of skills; these include understanding and use of communicative intentions, presupposition, and discourse management. Pragmatic language encompasses paralinguistic and nonverbal aspects of language (Parsons et al., 2017). For example, in conversation it is important to take the other’s needs into account and to adapt to these needs if necessary (Asada et al., 2010). Within the SCT population, pragmatic language has been largely understudied. One study of boys with XXY aged 1–18 years reported deficits in pragmatic language that were more pronounced in older children (St John et al., 2019). Another study reported lower pragmatic language skills, including inappropriate initiation of conversation, difficulties with understanding and using scripted language, and difficulties with nonverbal communication, in children and adolescents (aged 4–22 years) with an extra X or Y chromosome compared to typically developing peers (Lee et al., 2012). Two studies with children and adolescents aged 5–16 years also reported increased rates of pragmatic language difficulties in all three karyotypes, including inappropriate initiation of conversation, difficulties with using conversational contexts, and difficulties with nonverbal communication. The authors reported more pronounced difficulties in subgroups of children with a postnatal diagnosis or children with behavioral or neurodevelopmental problems (Bishop et al., 2011; Bishop et al., 2018). In addition, there is some evidence that “higher order language levels” are affected in children with SCT, such as understanding of ambiguous sentences, figurative speech, and understanding meaning in context (Ross et al., 2008; Ross et al., 2009; Melogno et al., 2019).
Both structural and pragmatic aspects of language are part of the larger concept of communication. Adequate communication depends not only on structural language abilities, but also on one’s ability to use language in a social context. Studies have shown a relationship between structural and pragmatic language and behavioral outcomes in a diverse range of populations. Children with developmental language delays show more behavioral problems (Gallagher, 1999) and early language difficulties are commonly reported in children with (neuro)developmental disorders such as autism spectrum disorders (ASD; Miranda et al., 2020; Volden et al., 2009), attention-deficit hyperactivity disorder (ADHD; Staikova et al., 2013), oppositional defiant disorder (Gremillion & Martel, 2014) and conduct disorder (Gilmour et al., 2004).
Studies have pointed at an increased risk for psychopathology in individuals with SCT, including risks for ASD and ADHD (see for example Ross et al., 2012; Samango-Sprouse et al., 2018; Tartaglia et al., 2010; Urbanus et al., 2020; Van Rijn, 2019; van Rijn et al., 2014). Although it has been suggested that (structural) language difficulties could be linked to social difficulties in later life (Visootsak & Graham, 2009), studies that investigate the relationship between language and behavioral outcomes are lacking. In addition, to fully understand the relationship between language and risk for behavioral and social-emotional problems, it is important to take into account not only structural language, but pragmatic language as well. Lastly, by studying this relationship in young children, building blocks of later behavioral outcomes can be identified, which is important to identify targets for early interventions.
The present study focuses on pragmatic language abilities in young children with SCT (aged 3–7 years) and investigates the role of structural and pragmatic language in predicting behavioral outcomes one year later. The aims of this paper are two-fold. First, to determine if the presence of an extra X or Y chromosome not only affects structural language development, but also affects pragmatic skills in young children. In other words: Do children with SCT have communication deficits beyond structural language? Several questions will be answered to pinpoint which children are vulnerable for adverse pragmatic language outcomes: (1) Do children with SCT have similar pragmatic abilities compared to controls? Factors that could be relevant for interpretation of the results (e.g. specific SCT karyotype, time of diagnosis, ascertainment bias) were explored. (2) Is the proportion of children with age-appropriate pragmatic skills similar in both groups? (3) Within the SCT group, do only children with structural language problems experience problems with pragmatic language or are pragmatic language difficulties a more common deficit within this group? (4) Is the developmental pathway of pragmatic language skills comparable in children with and without SCT? The second aim of this paper is to determine if language abilities predict neurobehavioral outcomes in later development; more specifically, if pragmatic language abilities can predict these outcomes above and beyond the predictive value of structural language abilities.
As children with SCT have a biological risk to develop language difficulties and have an increased risk for unfavorable behavioral outcomes, it is important to investigate possible underlying mechanisms of these behavioral outcomes, for example early language and communication abilities. Focusing on pragmatic language, thus considering communication in a broader perspective than structural language alone, may yield important insights in this, and could help identify early markers for children with vulnerable behavioral development.
Materials and methods
Participants
The present study is part of a larger ongoing project (TRIXY Early Childhood Study) at Leiden University, which included children with SCT and nonclinical controls aged 1–7 years. The TRIXY Early Childhood Study is a longitudinal study that aims to identify neurodevelopmental risk in young children with an extra X or Y chromosome. For the present study, both children with SCT and children in the control group aged 3–7 years during the initial visit were included.
Clinical genetic departments, pediatricians, and national advocacy or support groups in the Netherlands, Colorado USA, and Belgium participated in the recruitment of children with SCT. Assessment took place in the Netherlands (Trisomy of the X and Y—TRIXY—Expert Center) and the USA (Children’s Hospital Colorado eXtraordinarY Kids Clinic in Developmental Pediatrics at University of Colorado). The control group was recruited in the western part of the Netherlands. With the help of government institutions, the civil registry was accessed, and information brochures were distributed among families with children of eligible age. In addition, public sites such as daycare centers and public schools were asked to distribute information brochures as well. If parents were interested in the study, they were able to contact the researchers to discuss enrollment.
In both participant groups, the child as well as the (primary) parent/caregiver had to speak Dutch or English. Children were excluded when there was a history of traumatic brain injury, severely impaired hearing or sight, neurological illness, or color-blindness. Specific for the SCT group, the trisomy had to be present in at least 80% of the cells (confirmed by standard karyotyping). Within the control group genetic screening was not performed due to ethical reasons. However, based on the prevalence of SCT, the risk of a SCT karyotype in the control group was considered minimal and acceptable.
In the present study, 72 children with SCT (Mage = 4.80, SD = 1.29) and 71 controls (Mage = 4.51, SD = .99) were included. There were no significant age or age-distribution differences between the children with SCT and controls (p = .138), nor were there differences in average age between children with XXX, XXY, or XYY (p = .605). Global intellectual functioning (GIF) was assessed with the Wechsler Preschool and Primary Scale of Intelligence third edition (WPPSI-III; Wechsler, 2002), or the Wechsler Nonverbal Scale of Ability (Wechsler and Naglieri, 2006). There was a significant difference in average GIF between the SCT and control group (p < .001, Cohen’s d = .81), but no significant difference between children with XXX, XXY, or XYY (p = .304). Highest level of parental education was used as an indication of socio-economic status (SES), if a child had two caregivers, SES was calculated as an average for both caregivers. There was a significant difference in SES between the SCT and control group (p = .021, Cohen’s d = .39); parents of children with SCT had a higher level of education. There were no significant differences in SES between children with XXX, XXY, or XYY (p = .525). Children recruited in the USA where White (85.3%), Black or African American (2.9%), Asian (2.9%), or ethnoracial background was unknown (8.8%). Ethnoracial background for the sample recruited in Western Europe was not available. Descriptive statistics for age, GIF, and SES can be found in Table 1.
Table 1.
Descriptives: children with sex chromosome trisomy versus controls.
| SCT | XXX | XXY | XYY | Control | XX | XY | p d | SCT comparisonse | |
|---|---|---|---|---|---|---|---|---|---|
| Total N | 72 | 27 | 29 | 16 | 71 | 40 | 31 | ||
| Age—Mean (SD) | 4.80 (1.29) | 4.89 (1.19) | 4.61 (1.35) | 4.98 (1.40) | 4.51 (.99) | 4.53 (1.08) | 4.49 (.90) | .138 | n.s. |
| Global intellectual functioningaRange | 95.19 (19.37) 55–138 | 92.85 (16.88) 60–122 | 99.59 (20.11) 55–138 | 91.07 (21.89) 59–125 | 108.31 (13.80) 72–140 | 106.50 (12.81) 76–137 | 110.65 (14.86) 72–40 | <.001 | n.s. |
| Socio-economic statusb | 5.90 (.96) | 5.94 (.93) | 5.98 (.95) | 5.66 (1.03) | 5.42 (1.45) | 5.21 (1.37) | 5.68 (1.52) | .021 | n.s. |
| Time of Diagnosis (prenatal/postnatal) | 40/32 | 15/12 | 16/13 | 9/7 | n.s. | ||||
| Ascertainment bias (A/B/C)c | 31/21/20 | 7/10/10 | 15/8/6 | 9/3/4 | n.s. |
Note: Scores represent Means (SD).
Abbreviations: n.s. = not significant; SCT = Sex Chromosome Trisomy.
Measured with the WPPSI-III or the Wechsler Nonverbal Scale of Ability; Data for 5 children with SCT was incomplete (1 XXX, 2 XXY, 2 XYY).
Classified according to the criteria of Hollingshead: 0) No formal education; 1) Less than 7th grade; 2) Junior high school; 3) Partial high school; 4) High school graduate; 5) Partial college or specialized training; 6) Standard college/university graduation; 7) Graduate/professional training.
A = Active prospective follow-up, B = Information seeking parents, C = Clinically referred.
p-value SCT versus Control comparison.
SCT comparisons: XXX versus XXY versus XYY.
Within the SCT group, both time of diagnosis and ascertainment bias were assessed. Regarding time of diagnosis, 40 children received the diagnosis prenatally (i.e. because of prenatal screening or advanced maternal age). Of the children that received a postnatal diagnosis (N = 32), reasons for genetic screening included developmental delay (N = 14), physical and/or growth problems (N = 9), or medical concerns (N = 9). Regarding ascertainment bias, children were divided into three subgroups: A) “Active prospective follow-up” (43.1%), including families that were actively followed after a prenatal diagnosis; B) “Information seeking parents” (29.2%), including families who enrolled into the study to learn more about their child’s condition, but without having specific concerns about their child’s development, and C) “Clinically referred cases” (27.8%), including families who enrolled into the study after receiving professional help because of specific concerns about the development of their child. The distribution of prenatal and postnatal diagnoses was similar across the three karyotypes (p = .998). There were no differences in the distribution of ascertainment bias across the three SCT karyotypes (p = .232). Descriptives of time of diagnosis and ascertainment bias can be found in Table 1.
Behavioral outcomes one year after initial assessment were studied. Data was available for 48 children with SCT (23 XXY, 16 XXX, 9 XYY) and 58 children in the control group. The high number of dropouts was mostly due to the worldwide COVID-19 pandemic, where assessments had to be canceled or postponed (NSCT = 16; Ncontrol = 5), other reasons for dropout were developmental concerns (NSCT = 2; Ncontrol = 1), family circumstances (NSCT = 1), or the child being too old for the specific assessment battery (NSCT = 2; Ncontrol = 1). For the remaining participants, reason for dropout was unknown (NSCT = 3; Ncontrol = 6). On average, the behavioral assessments took place 52 weeks after the initial assessment (range 50–61 weeks). Ages during the follow-up assessment ranged from 4.08–8.03 years (Mage = 5.61, SD = 1.07). Baseline scores for neurocognitive and behavioral outcomes were compared between SCT children who were included in the follow-up assessment and children with missing follow-up data. Multivariate analyses of variance indicated no significant multivariate difference for cognitive outcomes (i.e. GIF, structural language, pragmatic language), Wilk’s Lambda = .98, F(3,61) = .42, p = .738, partial η2 = .02, or behavioral outcomes that were available for the entire age range, Wilk’s Lambda = .93, F(5,64) = 1.00, p = .428, partial η2 = .07. Participant demographics, neurocognitive outcomes and behavioral outcomes of the initial assessment are reported in Table 2 for the entire SCT group and the SCT group with follow-up data.
Table 2.
Baseline descriptives for children with sex chromosome trisomy: total baseline group versus group included in predictive analyses (one-year follow-up).
| Baseline |
Follow-up |
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Instrument | N | M (SD) | N | M (SD) | |||
| Demographics | Age (years) | 72 | 4.80 (1.29) | 48 | 4.67 (1.19) | |||
| Socio-economic status | Hollingshead criteriaa | 72 | 5.90 (.96) | 48 | 5.95 (1.01) | |||
| Research siteb | Dutch speaking | 33 | 21 | |||||
| English speaking | 39 | 27 | ||||||
| Baseline |
Follow-up |
|||||||
| Measure | Instrument | N | Raw score M (SD) | Standardized score M (D) | N | Raw score M (SD) | Standardized score M (D) | |
|
| ||||||||
| Cognitive outcomes | Global intellectual functioning | WPPSI-III(Wechsler, 2002) or Wechsler Nonverbal Scale of Ability (Wechsler & Naglieri, 2006) (standardized total IQ score) | 67 | N/A | 95.19 (19.37) | 44 | N/A | 96.75 (18.27) |
| Structural language | Combined score of expressive semantic skills (CELF-P) (Wiig et al., 2004; Wiig et al., 2012), receptive semantic skills (PPVT) (Dunn & Dunn, 1997; Dunn & Dunn, 2005), and syntax (CELF-P) (Wiig et al., 2004; Wiig et al., 2012) (z-score) | 69 | N/A | −.32 (.85) | 46 | N/A | −.23 (.85) | |
| Pragmatic language | CELF-Ppragmatics profile (Wiig et al., 2004; Wiig et al., 2012) (total raw score) | 72 | 76.17 (12.58) | N/A | 48 | 76.44 (12.95) | N/A | |
| Behavioral outcomes | Anxiety | CBCLDSM scores (Achenbach & Ruffle, 2000) (corrected raw scorec / T score) | 70 | 22.29 (20.56) | 57.36 (9.71) | 48 | 18.99 (18.11) | 56.19 (8.94) |
| Affective | 70 | 17.13 (12.57) | 58.67 (8.07) | 48 | 16.43 (12.27) | 58.15 (7.70) | ||
| Oppositional defiant | 70 | 37.71 (25.75) | 57.07 (8.32) | 48 | 37.74 (25.46) | 57.04 (8.12) | ||
| Attention deficit | 70 | 39.01 (22.88) | 54.47 (6.11) | 48 | 38.22 (23.64) | 54.15 (6.08) | ||
| Pervasive developmental | 55 | 25.80 (16.19) | 63.29 (9.85) | 40 | 26.63 (17.36) | 63.73 (10.35) | ||
| Social-emotional | ASQ-SE-2 (Squires et al., 2015) (corrected raw scorec) | 71 | 13.22 (11.55) | N/A | 48 | 12.61 (11.21) | N/A | |
Abbreviations: N/A = not applicable.
Hollingshead criteria: 0) No formal education; 1) Less than 7th grade; 2) Junior high school; 3) Partial high school; 4) High school graduate; 5) Partial college or specialized training; 6) Standard college/university graduation; 7) Graduate/professional training.
The testing set-up and research protocols were identical for all sites to permit standardization of the testing set-up. The same instruments were used on all sites; tests and questionnaires were administered in either Dutch or English.
Raw scores were corrected for the maximum possible score and multiplied by 100 to correct for differences in the number of items between age specific forms.
Procedure
This study was approved by the Ethical committee of Leiden University Medical Center, the Netherlands, and the Colorado Multiple Institutional Review Board (COMIRB) in Colorado, USA. Written informed consent according to the declaration of Helsinki was obtained after providing a description of the study to the parent(s) of the child.
The primary caregiving parent (92% biological mother) was asked to complete several questionnaires, including questionnaires regarding social-emotional, behavioral, and language outcomes. The child was assessed either in a quiet room at the university or at home. Assessments took place at various sites (Colorado USA, the Netherlands, Belgium). The testing set-up and research protocols were identical for all sites to permit standardization of the testing set-up. Researchers from Leiden University were responsible for project and data management (i.e. training and supervision of researchers, processing and scoring of data).
Due to the inclusion of participants from multiple sites, the tasks and questionnaires were administered in either Dutch or English. Tasks and questionnaires in both languages are formally validated and have sufficient psychometric properties. When applicable language-specific norms based on population samples were used.
Instruments
Structural language
Receptive language skills were assessed with the Peabody Picture Vocabulary Test (PPVT; Dunn & Dunn, 1997; Dunn & Dunn, 2005). Expressive language skills with the Expressive Vocabulary subtest of the Clinical Evaluation of Language Fundamentals Preschool edition (CELF-P EW) and syntax with the Sentence Structure subtest of the CELF-P (CELF-P SS; Wiig et al., 2004; Wiig et al., 2012).
The PPVT assesses the child’s ability to comprehend spoken words. For each item, four black and white pictures were shown to the child, and the child was instructed to identify the word that was orally presented by the researcher. The CELF-P EV test assesses the child’s ability to label people, objects, and actions by looking at colored images. The CELF-P SS test assesses the child’s ability to interpret sentences of increasing length and structural complexity by identifying a picture out of four options that illustrates the orally presented sentence.
Pragmatic language
The primary caregiving parent of the child completed the pragmatics profile of the CELF-P (Wiig et al., 2004; Wiig et al., 2012). The CELF-P pragmatics profile is a checklist including 26 statements that the parent rates on a 4-point scale (never, sometimes, often, always). The pragmatics profile assesses three subdomains: (1) The child’s non-verbal communication abilities (7 statements; e.g. the child appropriately responds to a familiar person’s angry, happy, or sad tone of voice), (2) the child’s ability to request, give, and respond to information (12 statements; e.g. the child appropriately asks questions if he or she is confused), and (3) the child’s conversational routines and skills (7 statements; e.g. the child appropriately introduces new conversation topics). Answers for the statements on the three subdomains were added to total sub-scores and answers on all statements were summed to a total (raw) score. Higher scores indicate better pragmatic abilities.
Behavioral outcomes
The primary caregiving parent of the child completed two questionnaires to assess behavioral outcomes: The Ages-and-Stages Social-Emotional Questionnaire (ASQ-SE-2; Squires et al., 2015) and the Child Behavior Checklist (CBCL; Achenbach and Ruffle, 2000). For both questionnaires, the primary caregiving parent completed the age-appropriate version.
The ASQ-SE-2 assesses social- and emotional development on seven behavioral constructs. The used form depends on the age of the child, with number of questions ranging from 19 to 33. Items were answered on a 3-point scale (rarely or never, sometimes, most of the time) and for each item parents indicated if the specific behavior was a concern. Answers on the items and the number of concerns indicated add up to a total raw score, with higher scores indicating an increased risk for social-emotional deficits or delays.
The CBCL is a standardized measure of behavioral problems. Answers on the items yield several outcomes, including the DSM-oriented scales. Depending on the used form (i.e. 1.5–5 or 6–18 years), the DSM-oriented scales consist of five or six profiles. In this study, the following profiles were assessed (with number of items on the 1.5–5- and 6–18-year version respectively): 1) Affective problems (as an indication for mood disorders, 10/13 items), 2) Anxiety problems (10/6 items), 3) Pervasive developmental problems (as indication of disorders on the autism spectrum, included in 1.5–5 year old version only), 4) Attention deficit/hyperactivity problems (6/7 items), and 5) Oppositional defiant problems (6/5 items). Items were answered on a 3-point scale (not true, somewhat or sometimes true, very true or often true), with higher scores indicating more behavioral problems.
As the number of total items differs between the ASQ-SE-2 versions and between the CBCL 1.5–5 and 6–18 versions, raw scores were corrected for the maximum possible score and multiplied by 100. Raw scores were preferred due to greater variability in scores and as raw scores are more appropriate for parametric statistical analyses. By correcting these scores, we were able to include children of all ages in the analyses (with the exception of the DSM pervasive developmental problems scale), with higher scores denoting more problems. Due to the small sample of children with scores on the CBCL somatic problems and conduct problems (N < 20), these scales were discarded.
Statistical analyses
Data were analyzed with the Statistical Package for the Social Sciences (SPSS) Version 25. Level of significance was set at p ≤ .05. Effect sizes were calculated with partial η2 and interpreted according to the guidelines by Cohen (1988).
Types of scores
Several scores were used. First, summed scores on the three pragmatic subdomains were used for the pragmatic language outcomes. Second, a criterion score was used to assess if the total pragmatic score is appropriate for the child’s chronological age (e.g. children between the ages of 3.5 and 4.5 years are expected to have a raw total score of at least 67). Children where then classified as having “met” or “not met” age expectations. This age-criterion is provided for the American version of the CELF-P pragmatics profile and to evaluate if the same age-criterion scores could be used in the European sample, the total CELF-P pragmatic scores were compared between the research sites (USA vs NL/BE). As the USA group was younger, age was included in this analysis. No significant differences were found, F(1,69) = .02, p = .882, partial η2 < .01, therefore the age-criterion scores were used in the European sample as well. Third, to compare children with and without language difficulties in the SCT group, raw scores for expressive semantic and receptive semantic skills were converted to normed scores according to the instrument manual. Next normed scores for these subtests were individually converted into z-scores with a psychometric conversion table for neuropsychological tests (Lezak et al., 2004). Children were considered as having a “language impairment” if they had a z-score of −1.25 on the receptive (PPVT) and/or expressive (CELF-P EV) structural language task(s); a deviation of 1.25 SD or more below the mean on either receptive or expressive language is often specified as a specific language impairment in the literature (Tomblin et al., 1996). Lastly, a “structural language score” was calculated by averaging the child’s converted z-scores on the PPVT, CELF-P EV, and CELF-P SS. At least two of the three scores had to be available in order for the “structural language score” to be calculated.
Covariates
As we used raw scores, average age of the groups and the age distribution per group was assessed with t-tests and Kolmogorov-Smirnov Z tests respectively. If there was a significant age difference and/or significant difference in the age distribution, age was included in the analysis as covariate.
As there were differences in GIF and SES between the SCT and control group, correlations were calculated between the total pragmatic language score, GIF and SES for the SCT and control group separately. There were significant correlations between the total pragmatic language score and GIF in both groups (SCT: r = .24, p = .050; Control: r = .32, p = .006), but no significant correlations between the total pragmatic language score and SES in either group (SCT: r = .20, p = .095; Control: r = .04, p = .756). For that reason, only GIF was included as covariate in analyses comparing the SCT and control group.
Analyses
Group differences SCT versus controls.
Multivariate analysis of covariance (MANCOVA) was used to compare pragmatic language (i.e. nonverbal communication; requesting, giving, and responding to information; conversational routines) outcomes between the SCT and control group. As specific SCT karyotype, time of diagnosis, and ascertainment bias could be relevant for the interpretation of the SCT versus control group results, the impact of these factors was explored with MANCOVA.
First, regarding karyotype specific outcomes, as there were no significant differences between boys and girls in the control group on pragmatic language outcomes (p ranged from .064 to .220), sex dependent effects were also not expected in the SCT group, therefore the three SCT karyotypes (XXX, XXY, XYY) were compared directly. There were no significant differences between the three SCT karyotypes on average age (p = .605) or distribution of ascertainment bias (χ2 = 5.59, p =.242), therefore only GIF was included as a covariate in this analysis. There was no significant multivariate effect for SCT karyotype after controlling for GIF, Wilk’s Lambda = .88, F(6,122) = 1.30, p = .263, partial η2 = .06, indicating that pragmatic language outcomes are comparable across karyotypes. Second, regarding time of diagnosis, children with a prenatal diagnosis were significantly younger than children with a postnatal diagnosis (p = .024), therefore age was included in the analysis as a covariate in addition to GIF. There was no significant multivariate effect for time of diagnosis after controlling for age and GIF, Wilk’s Lambda = .98, F(3,61) = .51, p = .675, partial η2 = .03, indicating that pragmatic language outcomes are comparable between children with a prenatal or postnatal diagnosis. Lastly, regarding ascertainment bias, there were no differences between the three ascertainment groups (i.e. prospective follow-up, information seeking parents, or clinically referred cases) in average age (p = .660), therefore only GIF was included as a covariate in this analysis. There was no significant multivariate effect for ascertainment bias after controlling for GIF, Wilk’s Lambda = .84, F(6,122) = 1.85, p = .096, partial η2 = .08, indicating that pragmatic language outcomes are comparable between the three ascertainment bias groups. For each of these factors, the estimated marginal means per pragmatic subdomain can be found in Table 3.
Table 3.
Pragmatic language abilities as measured with the clinical evaluation of language fundamentals preschool pragmatics profile: effects of karyotype, time of diagnosis and ascertainment bias.
| SCT karyotype |
Time of Diagnosis |
Ascertainment Biasa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| XXX | XXY | XYY | p | Prenatal | Postnatal | p | A | B | C | p | |
| N | 26 | 27 | 14 | 39 | 28 | 26 | 27 | 14 | |||
| Nonverbal communication | 23.54 (.71) | 24.42 (.70) | 21.56 (.97) | .066 | 23.59 (.60) | 23.32 (.72) | .784 | 23.30 (.71) | 23.38 (.85) | 23.85 (.87) | .875 |
| Requesting, giving, and responding to information | 19.52 (.73) | 20.75 (.72) | 18.39 (.96) | .157 | 19.56 (.57) | 20.08 (.68) | .577 | 20.26 (.70) | 19.26 (.84) | 18.84 (.85) | .168 |
| Conversational routines | 34.16 (1.07) | 34.74 (1.06) | 30.92 (1.46) | .102 | 33.44 (.80) | 34.10 (.95) | .604 | 34.38 (1.07) | 32.95 (1.27) | 33.38 (1.29) | .682 |
Abbreviations: SCT = Sex Chromosome Trisomy.
Note: Scores represent estimated marginal means (SE) and are co-varied for global level of intellectual functioning (SCT comparisons and ascertainment bias) or global level of intellectual functioning and age (time of diagnosis); higher scores denote better pragmatic skills (raw scores).
A = Active prospective follow-up, B = Information seeking parents, C = Clinically referred.
As no effects were found for karyotype, time of diagnosis, or ascertainment bias, these factors were not included in the subsequent SCT versus control group analyses. In addition, there were no differences in average age (p = .138) or age distributions (p = .137) between the SCT and control group, therefore age was not included as a covariate in this analysis. Due to the significant correlations between GIF and pragmatic language, GIF was included as a covariate in analyses comparing the SCT and control group.
Associations with structural language.
To assess if difficulties with pragmatic language were associated with structural language impairments, three groups were compared: SCT with structural language impairment, SCT without structural language impairment, and controls. See “types of scores” for our definition of language impairment. As there were two children (1 SCT and 1 control) without a score on either the expressive or receptive structural language task, data from these children was discarded from this analysis. There was no difference in the distribution of SCT karyotypes between the SCT with language impairment and without language impairment, χ2 = .97, p = .617. There was a significant difference in average age between the three groups (p = .039), therefore, age was included as a covariate.
Clinical classification.
With frequencies and a Chi-square test, the classification of children who did and did not meet the age-criterion was compared between the SCT and control group. With odds ratio, the risk of having a “clinical score” (i.e. not meeting the age-criterion) was assessed.
Developmental stability.
To test if possible SCT versus control differences on pragmatic language are stable across ages, a PROCESS moderation analysis (Hayes, 2017) was used. Research group (SCT versus controls) was included as predictor, age as moderator, and pragmatic total score as dependent variable. First, the research group x age interaction was assessed. In case of a nonsignificant interaction effect, a linear hierarchical regression analysis followed to assess the effect of research group (step 1) and to assess the effect of age on top of research group (step 2; method = Enter). If including age improved the initial model, the results from the second model were interpreted.
Predictive value of structural and pragmatic language abilities on behavioral outcomes.
Linear hierarchical regression analyses (enter method) were used to assess the predictive value of structural and pragmatic language abilities on behavioral outcomes (i.e. ASQ social-emotional problems and CBCL-DSM scales; affective, anxiety, pervasive developmental, attention deficit, and oppositional defiant problems) one year later. For each behavioral outcome separately, structural language outcome was added to the model in the first step, and pragmatic language outcome in the second step (enter method). When including pragmatic language in the second model resulted in an improvement with respect to the first model (significant F change < .05), the model including both structural and pragmatic language was interpreted and reported. Multicollinearity was assessed with the variance inflation factor (VIF). VIF values below 10 were deemed acceptable (Meyers et al., 2006). Part correlations were used as an indication of the percentage of variance accounted for uniquely by each predictor.
Results
Pragmatic language: SCT versus controls
There was a significant multivariate effect for research group after controlling for GIF, Wilk’s Lambda = .89, F(3,133) = 5.53, p = .001, partial η2 = .11, indicating a moderate to large effect. Univariate effects showed significantly lower scores in the SCT group on all three subdomains, with effect sizes indicating small to medium effects for nonverbal communication and conversational routines and a moderate to large effect for requesting, giving, and responding to information. Univariate outcomes per subdomain can be found in Table 4.
Table 4.
Pragmatic language abilities as measured with the Clinical Evaluation of Language Fundamentals Preschool Pragmatics Profile: Children with sex chromosome trisomy versus controls and associations with language impairment.
| SCT versus controla |
Associations with language impairmentb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCT | Control | p | Partial η2 | SCT with language impairment (SCT+) | SCT without language impairment (SCT−) | Control | p | Partial η2 | Pairwise comparisons | |
| N | 67 | 71 | 19 | 52 | 70 | |||||
| Nonverbal communication | 23.68 (.41) | 25.11 (.40) | .016 | .04 | 23.19 (.76) | 23.43 (.45) | 25.30 (.39) | .003 | .08 | SCT+ = SCT− < C |
| Requesting, giving, and responding to information | 20.11 (.44) | 22.62 (.43) | < .001 | .10 | 18.28 (.80) | 20.03 (.48) | 23.13 (.41) | <.001 | .23 | SCT+ = SCT− < C |
| Conversational routines | 34.41 (.68) | 36.59 (.66) | .028 | .04 | 30.73 (1.22) | 34.31 (.72) | 37.66 (.63) | <.001 | .18 | SCT+ = SCT− < C |
Abbreviations: SCT = Sex Chromosome Trisomy.
Note: Scores represent estimated marginal means (SE); higher scores denote better pragmatic skills (raw scores).
Scores co-varied for global level of intellectual functioning (GIF). GIF data for 5 children with SCT was incomplete, therefore these children were not included in this analysis.
Scores are co-varied for age. Data for two children (1 SCT and 1 control) was missing for either the expressive structural language task, the receptive structural language task, or both, therefore these children were not included in this analysis.
Within the SCT group, 25% of the children did not meet their age-criterion (18 out of 72 children), whereas in the control group 1.4% of the children did not meet their age-criterion (1 out of 71 children). A Chi-square test indicated that that the distribution between SCT children and the control group was significantly different, χ2 = 17.27, p < .001. Odds ratio indicated that the risk of a “clinical score” (i.e. not meeting the age-criterion) was 23 times higher in the SCT group compared to the control group.
Pragmatic language: associations with language impairment
There was a statistically significant multivariate effect of group (SCT with structural language impairment, SCT without structural language impairment, control) after controlling for age, Wilk’s Lambda = .74, F(6,270) = 7.45, p < .001, partial η2 = .14, indicating a large effect. Univariate effects showed significant differences between the three groups for all three subdomains, with effect sizes indicating a moderate to large effect for nonverbal communication and large effects for requesting, giving, and responding to information and conversational routines. Significant univariate effects were further explored with pairwise comparisons based on estimated means. For the subdomains nonverbal communication and requesting, giving, and responding to information, children with SCT regardless of structural language abilities had lower outcomes than controls, with no differences between the SCT group with and without language impairment. For the subdomain conversational routines, children with SCT regardless of structural language abilities had lower outcomes than the control group, but in addition, children with SCT with structural language impairment also had lower scores than children with SCT without language impairment (p = .013). Estimated marginal means and pairwise comparisons can be found in Table 4.
Pragmatic language: developmental stability
The PROCESS analysis did not yield a significant research group (SCT vs controls) x age interaction, p = .989. The inclusion of research group as predictor in the linear regression analysis resulted in a significant model, F(1,141) = 24.02, p < .001. The addition of age significantly improved the model, F(2,140) = 17.02, p < .001 (R2adjusted = .18, significance F change = .004). These results indicate that in both groups pragmatic language scores increase with age, and that children in the control group had higher pragmatic scores than children in the SCT group across age-bins. A visualization of results can be found in Figure 1.
Figure 1.
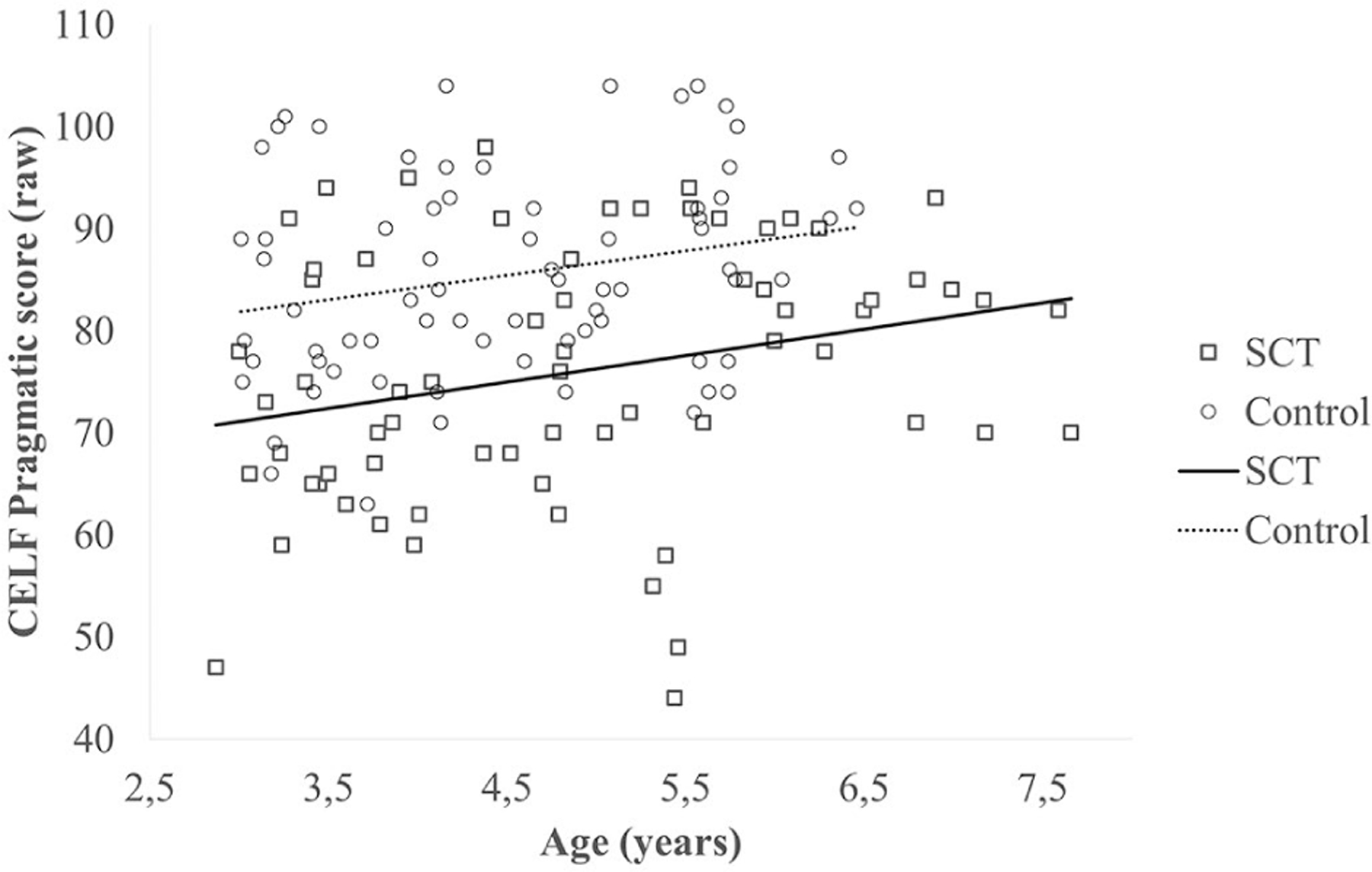
Pragmatic language abilities in the two groups (sex chromosome trisomy and control) at different ages (cross-sectional; NSCT = 72, Ncontrol = 71).
Predictive value of structural and pragmatic language on behavioral outcomes one year later: SCT group
For all outcomes, results for each predictor included in the final model are presented in Table 5. A visualization of results can be found in Figure 2.
Table 5.
Predictive value of structural language and pragmatic language on behavioral problems one year later in children with sex chromosome trisomy and controls.
| Total model |
Structural |
Pragmatic |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | R2 | p | β | Part corr. | VAF | β | Part corr. | VAF | ||
| SCT | Anxiety | .23 | .05 | .308 | −.08 | −.08 | −.21 | −.20 | ||
| Affective | .45 | .20 | .009 | −.21 | −.21 | −.36* | −.35 | 12.5% | ||
| Oppositional defiant | .41 | .17 | .021 | .02 | .01 | −.41** | −.41 | 16.5% | ||
| Attention deficit | .63 | .39 | <.001 | −.29* | −.29 | 8.1% | −.51*** | −.50 | 24.7% | |
| Pervasive developmental | .81 | .66 | <.001 | −.53*** | −.52 | 26.7% | −.53*** | −.53 | 27.6% | |
| Social-emotional | .73 | .53 | <.001 | −.26* | −.25 | 6.5% | −.64*** | −.63 | 39.1% | |
| Controls | Anxiety | .26 | .07 | .379 | −.17 | −.16 | −.14 | −.13 | ||
| Affective | .19 | .04 | .157 | −.08 | −.07 | −.15 | −.14 | |||
| Oppositional defianta | .30 | .09 | .021 | −.30* | −.30 | 9.1% | – | – | ||
| Attention deficita | .39 | .16 | .002 | −.39** | −.39 | 15.5% | − | – | ||
| Pervasive developmental | .23 | .05 | .404 | −.12 | −.11 | −.17 | −.16 | |||
| Social-emotional | .34 | .12 | .031 | −.01 | −.01 | −.34* | −.32 | 10.4% | ||
p < .05
p <.01
p < .001.
Abbreviations: SCT = sex chromosome trisomy; Part corr. = part correlation; VAF = unique variance accounted for by this variable.
As adding pragmatic language did not improve the model in the control group, the model that includes only structural language was reported and interpreted.
Note: NSCT group = 48 for anxiety, affective, oppositional defiant, attention deficit, and social-emotional; N= 29 for pervasive developmental. N control group = 58 for anxiety, affective, oppositional defiant, attention deficit, and social-emotional; N= 36 for pervasive developmental.
Figure 2.
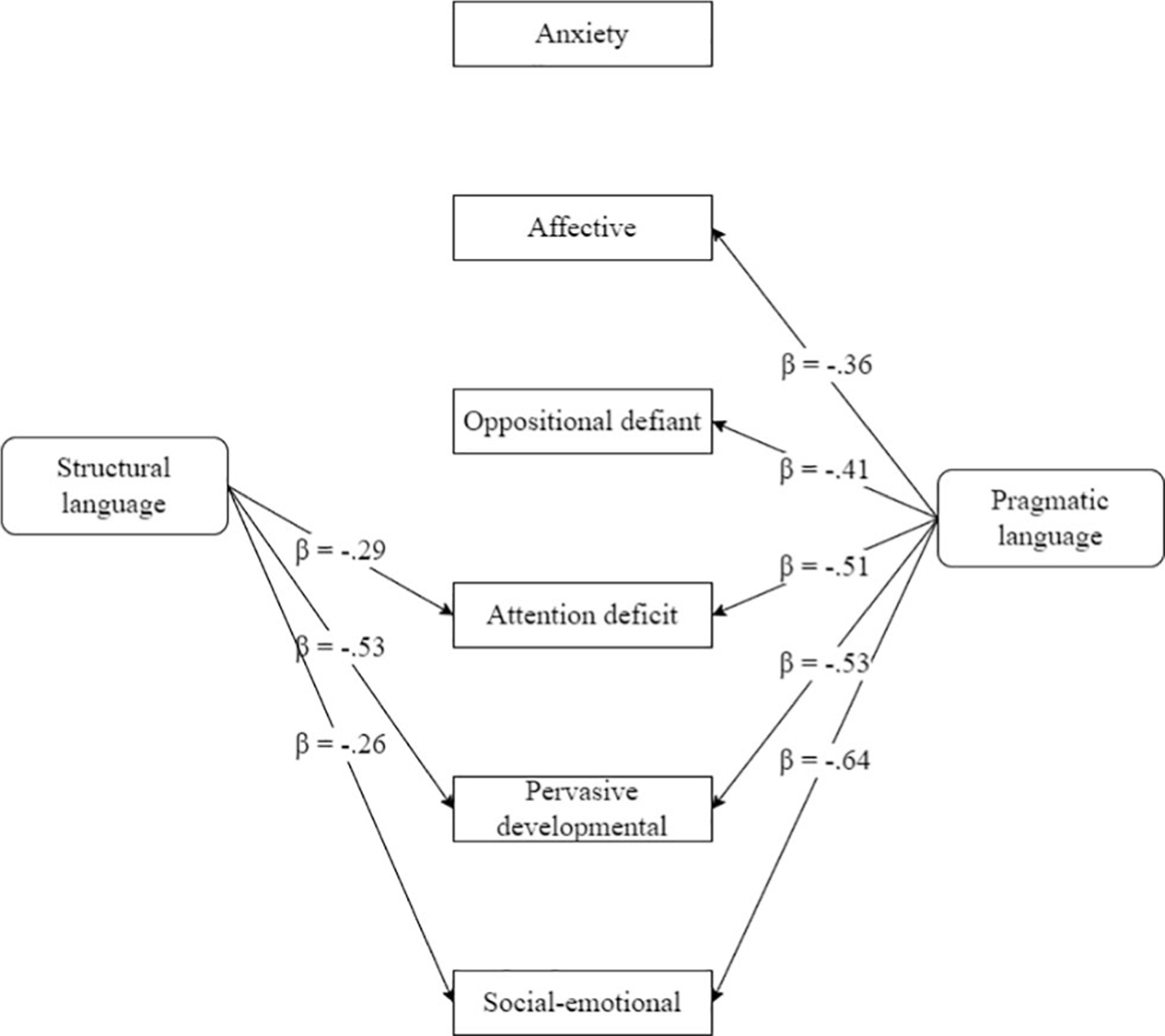
Predictive value of structural language and pragmatic language on behavioral outcomes one year later in children with sex chromosome trisomy (Nrange = 29–48).
Unique predictive value of pragmatic language
For two of the behavioral outcomes, only pragmatic language was a significant predictor in the model. Taken together, results indicated that more affective problems and more oppositional defiant problems one year later were predicted by more pragmatic language difficulties.
First, structural and pragmatic language together explained 19.9% of the variance in longitudinal affective problems, F(2,42) = 5.23, p = .009, with pragmatic language uniquely accounting for 12.5% of the variance (p = .014). Structural language was not a significant predictor once pragmatic language was taken into account (p = .144), nor was it a significant predictor on its own (p = .070)
Second, structural and pragmatic language together explained 16.8% of the variance in longitudinal oppositional defiant problems, F(2,42) = 4.24, p = .021, with pragmatic language uniquely accounting for 16.5% of the variance (p = .006). Structural language was not a significant predictor once pragmatic language was taken into account (p = .919), nor was it a significant predictor on its own (p = .704)
Combined predictive value of pragmatic language and structural language
For three of the behavioral outcomes, both structural language and pragmatic language were significant predictors in the model. Taken together, results indicated that more attention deficit problems, more pervasive developmental problems, and more social-emotional problems one year later were predicted by more pragmatic language difficulties and more structural language difficulties.
First, structural and pragmatic language together explained 39.1% of the variance in longitudinal attention deficit problems, F(2,42) = 13.49, p < .001. Pragmatic language (p <.001) uniquely accounted for 24.7% of the variance and structural language (p = .022) uniquely accounted for 8.1% of the variance in attention deficit problems.
Second, structural and pragmatic language together explained 66.0% of the variance in longitudinal pervasive developmental problems, F(2,25) = 24.25, p < .001. Pragmatic language (p < .001) uniquely accounted for 27.6% of the variance and structural language (p < .001), uniquely accounted for 26.7% of the variance in pervasive developmental problems.
Third, structural and pragmatic language together explained 52.8% of the variance in social-emotional problems, F(2,42) = 23.50, p < .001. Pragmatic language (p < .001), uniquely accounted for 39.1% of the variance and structural language (p = .021), accounted for 6.5% of the variance in social-emotional problems
No predictive value of pragmatic language and structural language
For anxiety problems regression results did not yield a significant model, F(2,42) = 1.21, p = .308. Structural and pragmatic language were not predictive of longitudinal anxiety problems.
Predictive value of structural and pragmatic language on behavioral outcomes one year later: control group
Structural and/or pragmatic language were predictive for behavioral outcomes one year later in the control group as well. Structural language on its own was predictive for both oppositional defiant problems and attention deficit problems one year later, uniquely accounting for 9.1% and 15.5% of the variance respectively. Pragmatic language on its own was predictive for social-emotional problems one year later, uniquely accounting for 10.4% of the variance. No predictive value of structural or pragmatic language was found for anxiety, affective, and pervasive developmental problems one year later. For all outcomes, results for each predictor included in the model are presented in Table 5.
Discussion
The aim of this study was two-fold. First, to determine if children with SCT also have compromised pragmatic language abilities; in other words, do children with SCT have communication deficits beyond structural language difficulties. Second, to determine if pragmatic language, above and beyond structural language, is predictive of neurobehavioral outcomes in later development.
With regard to the first aim, we addressed several questions. First, regarding average pragmatic language abilities, children in the SCT group had lower average scores on all included domains. These differences were not driven by SCT specific characteristics (i.e. karyotype, time of diagnosis, ascertainment bias). In addition, 25% of the children with SCT did not meet age expectations. Odds ratio indicates that the risk of having inadequate pragmatic language abilities is 23 times higher in the SCT group, compared to the control group. As the present study is one of the first studies to investigate pragmatic language abilities in children with SCT, it is important that findings of this study are replicated in other cohorts as our findings indicate that pragmatic language is a vulnerable domain for children with SCT. Pragmatic language abilities include nonverbal communication abilities, ability to request, give and respond to information, and the ability to engage in conversational routines. When nonverbal communication abilities are affected, this is possibly not only associated to someone’s ability to use nonverbal communication to send a message, but also to someone’s ability to understand nonverbal communication. When the ability to request, give, or respond to information or the ability to engage in conversational routines is affected, this could go together with someone’s ability to use language for different purposes or with one’s ability to follow the unspoken rules of conversation. These results show that in addition to structural language difficulties, pragmatic language can also be affected in this population. We suggest that these pragmatic language difficulties should be considered as part of a broader communication deficit. This is in line with findings that illustrate other difficulties in individuals with SCT that are part of or related to social communication; such as difficulties with understanding someone else’s perspective (i.e. Theory of Mind; van Rijn et al., 2014; Bouw et al., 2022), the ability to adapt adequately to the situation, and further language and communication development (Matthews et al., 2018). Individuals with SCT are often described as shy, timid, and withdrawn (for a review see Leggett et al., 2010). In addition, social difficulties, for example difficulties with reading social signals such as facial emotional recognition (van Rijn et al., 2014; van Rijn et al., 2018; Bouw et al., 2021) and tone of voice (Van Rijn et al., 2007) have been reported. Since pragmatic language abilities are interconnected with social skills and emotional understanding (Parsons et al., 2017), it is likely that these social difficulties in individuals with SCT are the result of a global communication deficit.
Second, we addressed the question whether pragmatic language problems were associated with language difficulties. Results indicated that not only children with language impairments experience difficulties with the social use of language, but rather that pragmatic difficulties are a more common characteristic within the SCT group. For nonverbal communication and requesting, giving, or responding to information, children in the SCT group on average had lower abilities than children in the control group, regardless of the presence of a language impairment. Children with SCT showed more challenges with engaging in conversational routines than controls, regardless of the presence of a language impairment, but these skills appeared to be even more compromised in children with SCT and language impairment. Taken together, these results show that pragmatic language abilities are a vulnerable domain in the SCT group, and that some pragmatic language abilities can be more pronounced when they co-occur with structural language abilities.
Third, looking at age-effects within this cross-sectional sample, results show that pragmatic language abilities continue to develop in both children with SCT and controls. However, across all ages, children in the SCT group had lower outcomes than controls. This suggests that, although pragmatic language abilities improve in children with SCT and that children with SCT do not necessarily deviate more from the norm when they get older, pragmatic language difficulties can be considered persistent in the SCT group.
Regarding our second aim—the predictive value of structural and pragmatic language on later behavior outcomes—our findings illustrate the relevance of language skills for a variety of neurobehavioral outcomes in both children with SCT as well as controls. In the SCT group, pragmatic language was predictive of a broader variety of behavioral outcomes than structural language, and for some behavioral outcomes the ability to use language as a social tool was the sole predictor. Thus, pragmatic abilities are important skills to consider in children with SCT, uniquely contributing to behavioral problems when also taking structural language into account. Although structural and pragmatic language were also predictive of behavioral outcomes in the control group, the pattern of the results differed from the results in the SCT group; the groups differed primarily in the predictive value of pragmatic language and to a lesser extend in the predictive value of structural language. The differences in the relation between pragmatic language and behavioral outcomes, in particular, were remarkable. For example, in the SCT group pragmatic language accounted for 39.1% of the variance in social-emotional outcomes, with a standardized beta coefficient of −.64. In the control group however, pragmatic language accounted for 10.4% in the variance of social-emotional outcomes, with a standardized beta coefficient of −.34. These results illustrate the importance of pragmatic language in identifying children with an increased risk for later adverse behavioral outcomes. In a study with 4-year-old children from a community sample, children who met the criteria for pragmatic language impairment and thus had lower pragmatic scores showed more behavioral problems than their peers without pragmatic language impairment (Ketelaars et al., 2010). This finding is in line with the current paper and the current paper adds to this, by studying a group of children who biologically are at increased risk for language difficulties and unfavorable behavioral outcomes. As SCT has a clear genetic cause, the SCT population can serve as a “risk-model,” providing a unique opportunity to study developmental pathways and underlying mechanisms of neurobehavioral problems and psychopathology. Taken together, the results of this study illustrate that the development of children with SCT follows a different path compared to controls and that difficulties with pragmatic language can serve as an early indicator for children with a more pronounced “at-risk” development, not only for children with SCT, but possibly in the general population as well. The striking finding that early social-communicative abilities explain a large part of the variance in neurobehavioral outcomes highlights the importance of early monitoring and the need for early support and intervention opportunities.
The results of this study have important clinical implications; they illustrate that early social-communicative abilities can be an important marker to identify children with SCT who are at risk for unfavorable outcomes at an early age, and for outcomes that are possibly also related to the risk for more severe psychopathology in later life. Thus, it is important to not only include structural language abilities, but also pragmatic language abilities in routine monitoring, and to look at the broader communication abilities of children with SCT. In addition, this shows that pragmatic language might be an important target for interventions as it is possible that supporting the development of pragmatic language could also have positive effects on behavioral outcomes. Lastly, it should be noted that although some children appear to be severely affected, other children are less affected or do not have notable differences from peers. In order to understand which children are vulnerable, it is important to gain more knowledge on the development of pragmatic language in young children with SCT.
The presence of an extra X or Y chromosome impacts the development of the brain (Raznahan et al., 2016); possibly including structures that are important for social communication. Although causality is not implied, the fact that difficulties with pragmatic language occur at an early age could be an important signal for deviant brain maturation. As SCT can be diagnosed prenatally, the impact of early mechanisms of developmental risk can already be studied from birth, providing the unique opportunity to study the earliest forms of communicative development in a homogenous group with a clear genetic cause. In contrast, studying groups of children with behavioral diagnoses, such as specific language impairment, limits this opportunity, as these children often form a heterogeneous group and children will not be identified until problems in daily functioning have presented themselves. In addition, as the results of this study illustrate the impact of the X and Y chromosome on pragmatic language outcomes, genes on these chromosomes could serve as possible candidate genes to explain variability in outcomes in the general population. In sum, studying communication skills in young children with SCT could give valuable insight in underlying mechanisms and developmental pathways to neurodevelopmental impact and psychopathology, and therefore increase our understanding of development and developmental risk, not only in the SCT population, but in the general population as well.
Within the present study, we were able to include a relatively large group of children at a young age. Due to the longitudinal design, we were able to make predictions in behavioral outcomes over time, although some data was missing, primarily due to the worldwide COVID-19 pandemic. There were some limitations in this study. First, only children aged 3 years or older were included, whereas social interaction and communication can already be assessed in younger children. It is important to learn more about the social communication abilities in children who are followed from birth, to pinpoint if difficulties in social communication can already be detected from birth or if they occur as a result of the development of the brain. Second, with this international sample, we were able to include a large cohort of children. Although our findings did not indicate differences in children with SCT from the USA and from the Dutch speaking parts of Western Europe, future studies could further explore cultural differences. In addition, other factors that could possibly play a mediating role in pragmatic language outcomes could be explored further. In our study, there were differences in SES and GIF between the SCT and control group. This difference was accounted for by including GIF as covariate in the analyses. However, it should be noted that by including GIF as a covariate, shared covariance between GIF and pragmatic language is filtered out. This possibly could have led to an underestimation of pragmatic language difficulties. SES however, although different between the SCT and control group, did not appear to play a role in pragmatic abilities, as illustrated by the non-significant correlation. Also, we cannot rule out that some children may have received some form of care as usual intervention, targeting language and/or communication skills within the timeframe of the study, which could possibly impact the studied associations with later behavioral outcomes. Future studies should further look into the contribution of both environmental factors (e.g. the “language-richness” of the environment) and interpersonal factors (e.g. services received, including hormonal treatments in the XXY group). Third, the composite structural language score in this study was based on children’s expressive semantic skills, receptive semantic skills, and syntactic abilities. There is more to language and communication than the included parameters in this study and future studies are encouraged to add to the growing body of literature examining the development of language and communication skills and how these skills are related to behavioral outcomes in children with SCT. A fourth limitation of this study is the use of a parent questionnaire to assess pragmatic language outcomes. Pragmatic language can also be assessed with performance-driven measures, participant transcript, or semi-naturalistic measures. Future studies are encouraged to incorporate a combination of these measures to gain a better understanding of the reach of pragmatic abilities in children with SCT. In addition, as the current study used parent questionnaires for pragmatic language outcomes and behavioral outcomes, but child-performance task as a measure for structural language, there is a possibility of shared-method variance which is a limitation of the design of the current study. However, although both pragmatic language outcomes and behavioral outcomes relied on parent reports (i.e. data was obtained from the same source), there was temporal dissociation between the measurements (i.e. there was a one-year time lag between the measurement of the predictive pragmatic language measurement and the measurements of the behavioral outcomes (Tehseen et al., 2017; Podsakoff et al., 2003). In addition, the correlations between pragmatic language, structural language, and the behavioral outcomes were not substantially large (i.e. no correlations > .90; Tehseen et al., 2017); see supplementary materials Table A2. Therefore, we are confident that the reported results reflect meaningful associations in the light of the different methods we used. Nonetheless, caution is warranted. We therefore suggest that future studies only use one method for all predictive variables (i.e. use parent questionnaires or child performance task for both structural and pragmatic outcomes). Finally, while studies designed to analyze predictors of later outcomes such as this study are unique, it is important that future studies investigate the developmental trajectory of pragmatic language, behavioral outcomes, and the predictive value of language outcomes for behavioral outcomes across a longer time span. Within this study, we identified functions on the language and communication domain as building blocks for later behavioral outcomes. It is important to further explore other neurocognitive domains, for example social cognitive functioning or executive functioning, to further unravel which mechanisms underly adverse behavioral outcomes. It should be noted that the development of children is dynamic; child characteristics interact with behavioral outcomes. For example, a child with language difficulties may socially isolate, resulting in less language learning experiences, which eventually could lead to worse language outcomes. It is important to take this dynamic interaction into account. Taken together, future studies should look into the social communicative abilities of children under the age of three, investigate possible mediating factors, and project outcomes over a longer time period.
To conclude, our data suggest that children with SCT are at risk for communication deficits that extend beyond structural language abilities, including difficulties to use language in a social context. The relevance of early assessment of a broad spectrum of communication skills in addition to structural language skills is illustrated by the fact that pragmatic deficits are not limited to children with structural language deficits but can be identified in those without structural language deficits as well. Most importantly, the social use of language seems to have stronger predictive value than structural language abilities for a broad range of neurobehavioral outcomes one year later. Thus, it is important to monitor not only structural language development, but also pragmatic language development in young children with SCT, since pragmatic language development, can serve as a marker for children who are at risk of developing behavioral and social-emotional problems.
Supplementary Material
Acknowledgments
The authors thank the families that participated in our study, and the research assistants and students for their help with data collection and processing.
Funding
This study was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (grant/award number to SvR: 016.165.397).
Footnotes
Supplemental data for this article is available online at https://doi.org/10.1080/13854046.2022.2067078.
Disclosure statement
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
The Ethical committee of Leiden University Medical Center, the Netherlands, and the Colorado Multiple Institutional Review Board (COMIRB) in Colorado, USA, provided ethical approval for this study.
Consent to participate
Written informed consent according to the declaration of Helsinki was obtained after providing a description of the study to the parent(s) of the child.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Achenbach T, & Ruffle T (2000). The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review, 21(8), 265–271. 10.1542/pir.21-8-265 [DOI] [PubMed] [Google Scholar]
- Asada K, Tomiwa K, Okada M, & Itakura S (2010). Fluent language with impaired pragmatics in children with Williams syndrome. Journal of Neurolinguistics, 23(6), 540–552. 10.1016/j.jneuroling.2010.04.001 [DOI] [Google Scholar]
- Bishop DVM, Brookman-Byrne A, Gratton N, Gray E, Holt G, Morgan L, Morris S, Paine E, Thornton H, & Thompson PA (2018). Language phenotypes in children with sex chromosome trisomies. Wellcome Open Research, 3, 143. 10.12688/wellcomeopenres.14904.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, Fryer A, Middlemiss P, Smithson S, Metcalfe K, Shears D, Leggett V, Nation K, & Scerif G (2011). Autism, language and communication in children with sex chromosome trisomies. Archives of Disease in Childhood, 96(10), 954–959. 10.1136/adc.2009.179747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, & Tartaglia N (2009). The cognitive phenotype in Klinefelter syndrome: A review of the literature including genetic and hormonal factors. Developmental Disabilities Research Reviews, 15(4), 284–294. 10.1002/ddrr.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen A, Juul S, & Gravholt CH (2003). Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. The Journal of Clinical Endocrinology and Metabolism, 88(2), 622–626. 10.1210/jc.2002-021491 [DOI] [PubMed] [Google Scholar]
- Bouw N, Swaab H, Tartaglia N, Cordeiro L, & van Rijn S (2021). The Impact of Sex Chromosome Trisomies (XXX, XXY, XYY) on Social Attention and Affect Recognition: A Cross-Sectional Eye Tracking Study. [Manuscript submitted for publication]. Clinical Neurodevelopmental Sciences, Leiden University. [Google Scholar]
- Bouw N, Swaab H, Tartaglia N, & van Rijn S (2022). The impact of sex chromosome trisomies (XXX, XXY, XYY) on early social cognition: Social orienting, joint attention and theory of mind. Archives of Clinical Neuropsychology, 37(1), 63–77. 10.1093/arclin/acab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates. [Google Scholar]
- Cordeiro L, Tartaglia N, Roeltgen D, & Ross J (2012). Social deficits in male children and adolescents with sex chromosome aneuploidy: A comparison of XXY, XYY, and XXYY syndromes. Research in Developmental Disabilities, 33(4), 1254–1263. 10.1016/j.ridd.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, & Dunn LM (1997). Peabody Picture Vocabulary Test—Third Edition Manual. American Guidance Service. [Google Scholar]
- Dunn LM, & Dunn LM (2005). Peabody Picture Vocabulary Test-III-NL. Harcourt Test Publishers. [Google Scholar]
- Gallagher TM (1999). Interrelationships among children’s language, behavior, and emotional problems. Top Lang Disord, 19(2), 1–15. [Google Scholar]
- Gilmour J, Hill B, Place M, & Skuse DH (2004). Social communication deficits in conduct disorder: A clinical and community survey. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45(5), 967–978. 10.1111/j.1469-7610.2004.t01-1-00289.x [DOI] [PubMed] [Google Scholar]
- Gremillion ML, & Martel MM (2014). Merely misunderstood? Receptive, expressive, and pragmatic language in young children with disruptive behavior disorders. Journal of Clinical Child and Adolescent Psychology : The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 43(5), 765–776. 10.1080/15374416.2013.822306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth KA, Skakkebaek A, Høst C, Gravholt CH, & Bojesen A (2013). Clinical review: Klinefelter syndrome-a clinical update. The Journal of Clinical Endocrinology and Metabolism, 98(1), 20–30. 10.1210/jc.2012-2382 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Second edition ed: Guilford Press. [Google Scholar]
- Ketelaars MP, Cuperus J, Jansonius K, & Verhoeven L (2010). Pragmatic language impairment and associated behavioural problems. International Journal of Language & Communication Disorders, 45(2), 204–214. 10.3109/13682820902863090 [DOI] [PubMed] [Google Scholar]
- Lee NR, Wallace GL, Adeyemi EI, Lopez KC, Blumenthal JD, Clasen LS, & Giedd JN (2012). Dosage effects of X and Y chromosomes on language and social functioning in children with supernumerary sex chromosome aneuploidies: Implications for idiopathic language impairment and autism spectrum disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(10), 1072–1081. 10.1111/j.1469-7610.2012.02573.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett V, Jacobs P, Nation K, Scerif G, & Bishop DV (2010). Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: A systematic review. Developmental Medicine and Child Neurology, 52(2), 119–129. 10.1111/j.1469-8749.2009.03545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Blumenthal JD, Wallace GL, Clasen LS, Lee NR, & Giedd JN (2014). A case-control study of brain structure and behavioral characteristics in 47,XXX syndrome. Genes, Brain, and Behavior, 13(8), 841–849. 10.1111/gbb.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson D, Loring D, & Hannay H (2004). Neuropsychological assessment. 4th ed. Oxford University Press. [Google Scholar]
- Matthews D, Biney H, & Abbot-Smith K (2018). Individual differences in children’s pragmatic Ability: A review of associations with formal language, social cognition, and executive functions. Language Learning and Development, 14(3), 186–223. 10.1080/15475441.2018.1455584 [DOI] [Google Scholar]
- Melogno S, Pinto MA, Badolato F, Sist E, Esposito A, Orsolini M, & Tarani L (2019). High-level language competencies and theory of mind in a group of children with Klinefelter syndrome. American Journal of Medical Genetics. Part A, 179(2), 183–189. 10.1002/ajmg.a.12 [DOI] [PubMed] [Google Scholar]
- Meyers LS, Gamst G, & Guarino AJ (2006). Applied multivariate research: Design and interpretation. Sage Publications, Inc. [Google Scholar]
- Miranda A, Berenguer C, Rosello B, & Baixauli I (2020). Relationships between the social communication questionnaire and pragmatic language, socialization skills, and behavioral problems in children with autism spectrum disorders. Applied Neuropsychology. Child, 9(2), 141–152. 10.1080/21622965.2018.1550403 [DOI] [PubMed] [Google Scholar]
- Morris JK, Alberman E, Scott C, & Jacobs P (2008). Is the prevalence of Klinefelter syndrome increasing? European Journal of Human Genetics: EJHG, 16(2), 163–170. 10.1038/sj.ejhg.5201956 [DOI] [PubMed] [Google Scholar]
- Netley CT (1986). Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy. In Ratcliffe SGP N (Ed.), Prospective studies on children with sex chromosome aneuploidy (pp. 296–306). Alan R. Liss, Inc.. [PubMed] [Google Scholar]
- Parsons L, Cordier R, Munro N, Joosten A, & Speyer R (2017). A systematic review of pragmatic language interventions for children with autism spectrum disorder. PloS One, 12(4), e0172242. 10.1371/journal.pone.0172242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsakoff PM, Mackenzie SB, Lee J-Y, & Podsakoff NP (2003). Common method biases in behavioral research: a critical review of the literature and recommended remedies. The Journal of Applied Psychology, 88(5), 879–903. 10.1037/0021-9010.88.5.879 [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee NR, Greenstein D, Wallace GL, Blumenthal JD, Clasen LS, & Giedd JN (2016). Globally divergent but locally convergent X- and Y-chromosome influences on cortical development. Cerebral Cortex (New York, N.Y. : 1991), 26(1), 70–79. 10.1093/cercor/bhu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, McCauley E, & Tartaglia N (2012). Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics, 129(4), 769–778. 10.1542/peds.2011-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MPD, Kushner H, Ramos P, Elder FF, & Zinn AR (2008). Cognitive and motor development during childhood in boys with Klinefelter syndrome. American Journal of Medical Genetics. Part A, 146A(6), 708–719. 10.1002/ajmg.a.32232 [DOI] [PubMed] [Google Scholar]
- Ross JL, Zeger MP, Kushner H, Zinn AR, & Roeltgen DP (2009). An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Developmental Disabilities Research Reviews, 15(4), 309–317. 10.1002/ddrr.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet J, Netley C, Bailey J, Keenan M, & Stewart D (1995). Intelligence and achievement in children with extra X aneuploidy: A longitudinal perspective. American Journal of Medical Genetics, 60(5), 356–363. ). 10.1002/ajmg.1320600503 [DOI] [PubMed] [Google Scholar]
- Rovet J, Netley C, Keenan M, Bailey J, & Stewart D (1996). The psychoeducational profile of boys with Klinefelter syndrome . Journal of Learning Disabilities, 29(2), 180–196. 10.1177/002221949602900208 [DOI] [PubMed] [Google Scholar]
- Samango-Sprouse C, Stapleton E, Chea S, Lawson P, Sadeghin T, Cappello C, de Sonneville L, & van Rijn S (2018). International investigation of neurocognitive and behavioral phenotype in 47,XXY (Klinefelter syndrome): Predicting individual differences. American Journal of Medical Genetics. Part A, 176(4), 877–885. 10.1002/ajmg.a.38621 [DOI] [PubMed] [Google Scholar]
- Squires J, Bricker D, & Twombly E (2015). Ages & stages questionnaires®: Social-emotional. second edition (ASQ®:SE-2): A parent-completed child monitoring system for social-emotional behaviors. Paul H. Brookes Publishing Co., Inc. [Google Scholar]
- St John M, Ponchard C, van Reyk O, Mei C, Pigdon L, Amor DJ, & Morgan AT (2019). Speech and language in children with Klinefelter syndrome. Journal of Communication Disorders, 78, 84–96. 10.1016/j.jcomdis.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Staikova E, Gomes H, Tartter V, McCabe A, & Halperin JM (2013). Pragmatic deficits and social impairment in children with ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 54(12), 1275–1283. 10.1111/jcpp.12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia NR, Cordeiro L, Howell S, Wilson R, & Janusz J (2010). The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome). Pediatric Endocrinology Reviews, 8, 151–159. [PMC free article] [PubMed] [Google Scholar]
- Tehseen S, Ramayah T, & Sajilan S (2017). Testing and controlling for common method variance: a review of available methods. Journal of Management Sciences, 4(2), 142–168. 10.20547/jms.2014.1704202 [DOI] [Google Scholar]
- Tomblin JB, Records NL, & Zhang X (1996). A system for the diagnosis of specific language impairment in kindergarten children. Journal of Speech and Hearing Research, 39(6), 1284–1294. 10.1044/jshr.3906.1284 [DOI] [PubMed] [Google Scholar]
- Urbanus E, Swaab H, Tartaglia N, Boada R, & Van Rijn S (2022). A cross-sectional study of early language abilities in children with sex chromosome trisomy (XXY, XXX, XYY) aged 1–6 years. Child Neuropsychology, 28(2), 171–196. 10.1080/09297049.2021.1960959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus E, Swaab H, Tartaglia N, Cordeiro L, & Van Rijn S (2020). The behavioral profile of children aged 1–5 years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY)). American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 184(2), 444–455. 10.1002/ajmg.c.31788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus E, Van Rijn S, & Swaab H (2020). A review of neurocognitive functioning of children with sex chromosome trisomies: Identifying targets for early intervention. Clinical Genetics, 97(1), 156–167. 10.1111/cge.13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn S (2019). A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47, XYY)). Current Opinion in Psychiatry, 32(2), 79–84. 10.1097/YCO.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn S, Aleman A, Swaab H, Krijn T, Vingerhoets G, & Kahn RS (2007). What it is said versus how it is said: Comprehension of affective prosody in men with Klinefelter (47,XXY) syndrome. Journal of the International Neuropsychological Society : JINS, 13(6), 1065–1070. 10.1017/S1355617707071044 [DOI] [PubMed] [Google Scholar]
- van Rijn S, de Sonneville L, & Swaab H (2018). The nature of social cognitive deficits in children and adults with Klinefelter syndrome (47,XXY). Genes, Brain, and Behavior, 17(6), e12465. 10.1111/gbb.12465 [DOI] [PubMed] [Google Scholar]
- van Rijn S, Stockmann L, Borghgraef M, Bruining H, van Ravenswaaij-Arts C, Govaerts L, Hansson K, & Swaab H (2014). The social behavioral phenotype in boys and girls with an extra X chromosome (Klinefelter syndrome and Trisomy X): A comparison with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(2), 310–320. 10.1007/s10803-013-1860-5 [DOI] [PubMed] [Google Scholar]
- van Rijn S, Stockmann L, van Buggenhout G, van Ravenswaaij-Arts C, & Swaab H (2014). Social cognition and underlying cognitive mechanisms in children with an extra X chromosome: a comparison with autism spectrum disorder. Genes, Brain, and Behavior, 13(5), 459–467. 10.1111/gbb.12134 [DOI] [PubMed] [Google Scholar]
- Visootsak J, & Graham JM Jr. (2009). Social function in multiple X and Y chromosome disorders: XXY, XYY, XXYY, XXXY. Developmental Disabilities Research Reviews, 15(4), 328–332. 10.1002/ddrr.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volden J, Coolican J, Garon N, White J, & Bryson S (2009). Brief report: Pragmatic language in autism spectrum disorder: Relationships to measures of ability and disability. Journal of Autism and Developmental Disorders, 39(2), 388–393. 10.1007/s10803-008-0618-y [DOI] [PubMed] [Google Scholar]
- Wechsler D (2002). WPPSI-III: Technical and interpretative manual. The Psychological Corporation. [Google Scholar]
- Wechsler D, & Naglieri JA (2006). Wechsler nonverbal scale of ability. Harcourt Assessment. [Google Scholar]
- Wiig EH, Secord Wa., & Semel E (2012). Clinical evaluation of language fundamentals preschool (CELF-2NL)–nederlandse versie. Pearson Assessment and Information. [Google Scholar]
- Wiig EH, Secord WA, & Semel E (2004). Clinical evaluation of language fundamentals–preschool (2nd ed.). Harcourt Assessment. [Google Scholar]
- Zampini L, Burla T, Silibello G, Capelli E, Dall’Ara F, Rigamonti C, Ajmone PF, Monti F, Zanchi P, Lalatta F, Costantino MA, & Vizziello PG (2021). Preverbal skills in 8-month-old children with sex chromosome trisomies. First Language, 41(2), 200–217. 10.1177/0142723720962944 [DOI] [Google Scholar]
- Zampini L, Burla T, Silibello G, Dall’Ara F, Rigamonti C, Lalatta F, & Vizziello P (2018). Early communicative skills of children with Klinefelter syndrome. Clinical Linguistics & Phonetics, 32(7), 577–586. 10.1080/02699206.2017.1384853 [DOI] [PubMed] [Google Scholar]
- Zampini L, Draghi L, Silibello G, Dall’Ara F, Rigamonti C, Suttora C, Zanchi P, Salerni N, Lalatta F, & Vizziello P (2018). Vocal and gestural productions of 24-month-old children with sex chromosome trisomies. International Journal of Language & Communication Disorders, 53(1), 171–181. 10.1111/1460-6984.12334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


