Abstract
Background and Objectives
Prospective measures of plasma and cerebral MRI biomarkers of Alzheimer disease (AD) and vascular neuropathology provide an opportunity to investigate possible mechanisms linking liver disease and dementia. We aimed to quantify the association of midlife nonalcoholic fatty liver disease (NAFLD) with change in plasma and brain MRI biomarkers of AD and vascular neuropathology.
Methods
We included participants from the Atherosclerosis Risk in Communities Study with brain MRI measurements of white matter hyperintensity (WMH) volume and temporal-parietal lobe cortical thickness meta region of interest (ROI) at up to 2 different visits, in 2011–13 and 2016–19, and plasma biomarkers of β-amyloid (Aβ)42:40, phosphorylated tau at threonine 181, and neurofilament light (NfL) were measured up to 3 times in 1993–95, 2011–13, and 2016–19. NAFLD was categorized using the fatty liver index in 1990–92. Multivariate linear regression was performed for associations between midlife NAFLD and change in plasma and brain MRI biomarkers of AD and vascular neuropathology. The primary models adjusted for demographics, Apolipoprotein E, alcohol use, and kidney function.
Results
Among 1,706 participants (mean age 56 years, 62% female, 28% Black), midlife NAFLD vs no NAFLD was associated with greater late-life WMH volume (difference per SD 0.19, 95% CI 0.06–0.31) and faster late-life WMH increase over 6 years (difference in annual change, SD 0.28, 95% CI 0.05–0.51), suggesting accumulating vascular pathology. Midlife NAFLD vs no NAFLD was also associated with AD biomarkers in midlife (lower Aβ42:40 [SD −0.21, 95% CI −0.39 to −0.04] measured in 1993–95) and late life (lower Aβ42:40 [SD −0.13, 95% CI −0.23 to −0.03] and lower temporal-parietal lobe cortical thickness meta ROI [SD −0.16, 95% CI −0.28 to −0.05] measured in 2011–13). Although midlife NfL was lower in individuals with vs without midlife NAFLD, those with NAFLD exhibited a faster rate of NfL increase that accelerated over time.
Discussion
Midlife NAFLD shows associations with AD and accumulating vascular pathology, revealing potential pathways linking liver function to dementia. Plasma biomarkers of neuropathology and neuronal injury may serve as easily measurable and dynamic indicators for monitoring the impacts of impaired liver function on brain health.
Introduction
Nonalcoholic fatty liver disease (NAFLD) affects 20%–35% of the US population.1 Animal studies have demonstrated that hepatic and metabolic dysfunction associated with NAFLD can trigger inflammation, cerebrovascular dysfunction, and neuronal apoptosis2,3 decrease peripheral clearance of total β-amyloid (Aβ) and increase brain Aβ deposition.3,4 The human evidence, however, is limited. It is not clear whether NAFLD and liver fibrosis is associated with dementia-related brain Aβ deposition and vascular changes.5-11
This study focused on understanding the mechanisms underlying the relationship between NAFLD and dementia using repeated measures of plasma and cerebral MRI biomarkers of Alzheimer disease (AD) and vascular neuropathology (2 primary pathways to dementia12), obtained from the community-based Atherosclerosis Risk in Communities (ARIC) Study. Our primary aim sought to quantify the relationship between midlife NAFLD and brain imaging and plasma biomarkers of neuropathology. Secondarily, we examined the mediating role of change in plasma biomarkers in the association between midlife NAFLD and late-life MRI markers.
Methods
Study Population
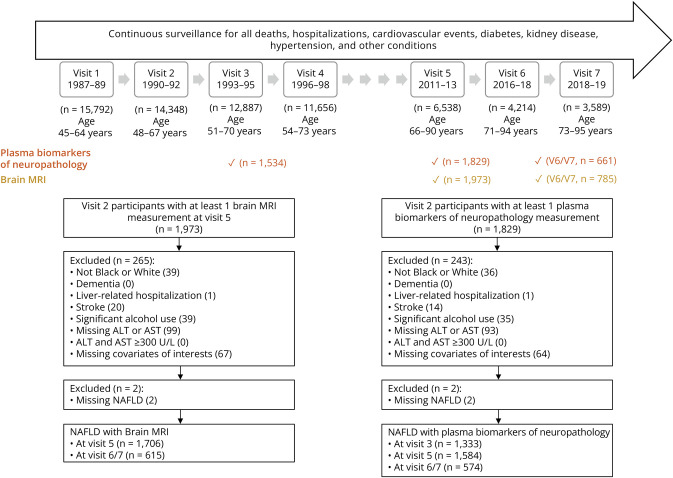
ARIC is a prospective community-based cohort that enrolled 15,792 participants aged 45–64 years from 4 US communities (Forsyth County, NC; Minneapolis, MN; Washington County, MS; and Jackson, MS) in 1987–89.13 In 2011–13 (visit 5), a cohort subset (N = 1,973) was selected for 3T MRI brain scans if they (1) participated in the 2004–2006 brain MRI examination; (2) had evidence of low cognitive test scores or cognitive decline; or (3) were randomly selected from cognitively unimpaired participants.14 Sampling weights were assigned to each participant to take into account the MRI selection criteria and the probability of refusal to participate for eligible participants.15 Plasma biomarkers of neuropathology were assayed for this subcohort on stored specimens obtained in 1993–95 (visit 3) and 2011–13 (visit 5). Between 2016 and 2019 (visit 6 and 7), 785 participants received another brain MRI scan, and plasma biomarkers of neuropathology were measured.
Midlife NAFLD was assessed in 1990–92 (visit 2), when the average age of participants was 55.6 (SD 5.2) years. Visit 2 served as the baseline visit. Baseline study exclusions are displayed in Figure 1. The median follow-up durations between the baseline visit and visit 3, visit 3 and visit 5, and visit 5 and visit 6/7 are 3.0, 17.7, and 6.3 years, respectively.
Figure 1. Study Timeline and Study Eligibility Criteria.
Liver-related hospitalization was identified by hospital discharge diagnoses (International Classification of Diseases, Ninth Revision, Clinical Modification codes 571.0–571.9); dementia and stroke were ascertained through follow-up visits, annual follow-up contacts, and community-wide hospital surveillance; reported significant alcohol consumption was defined as current alcohol consumption of >21 standard drinks per week for men and >14 standard drinks per week for women. ALT = alanine aminotransferase; AST = aspartate aminotransferase; NAFLD = nonalcoholic fatty liver disease.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the Institutional Review Boards of all ARIC study sites, and all participants provided written informed consent.
NAFLD Classification
NAFLD was quantified using the validated fatty liver index (FLI),16 following the equation shown below. FLI< 30, 30-<60, and ≥60 was used to classify no NAFLD, indeterminate NAFLD, and NAFLD, respectively.
where y = 0.953 × ln (triglycerides, mg/dL) + 0.139 × body mass index (BMI), kg/m2 + 0.718 × ln (gamma-glutamyl transferase, U/L) + 0.053 × waist circumference, cm − 15.745
Plasma Biomarkers of Neuropathology
AD pathology (Aβ40, Aβ42, phosphorylated tau at threonine 181 [p-tau181]) and neurodegeneration (neurofilament light [NfL]) were measured using commercially available ultrasensitive single-molecule array (Simoa) assays from Quanterix using stored plasma (visits 3, 5, and 6/7).17 Aβ40, Aβ42, and NfL were measured using the Neurology 4-PLEX E. Glial fibrillary acidic protein was excluded from the study because of our specific focus on AD-related neuropathology and neurodegeneration. Aβ42:40 ratio was calculated by dividing Aβ42 over Aβ40. Biomarkers of p-tau181 and NfL were right skewed and base 2 log transformed. Lower plasma Aβ42:40 levels and higher p-tau181 levels reflect greater Aβ pathology in early stages of AD development, and higher and faster increasing levels of NfL reflect greater neurodegeneration.18
MRI-Based Biomarkers of Neuropathology
Brain morphology was measured with 3T MRI in 2011–13 (visit 5), with quantification at a central image processing center at the Mayo Clinic.19 Magnetization-prepared rapid acquisition gradient echo (MPRAGE) and sagittal T2 fluid-attenuated inversion recovery (FLAIR) were obtained. FLAIR images assessed white matter hyperintensities (WMHs) in 2-dimension using a semiautomated algorithm, and MPRAGE images assessed brain volume and thickness in 3-dimension.20 The Freesurfer atlas was used to quantify regional brain volume and thickness.21 Participants with a 2016–19 MRI follow-up adhered to visit 5 methodology, except that WMH was quantified using 3-dimension technology. Brain MRI data were reanalyzed in 2020 to produce harmonized measures to reconcile inconsistency across visits.
WMH volume was summed over the frontal, parietal, temporal, and occipital lobes and log transformed because of a right skewed distribution. The brain cortical thickness was quantified for the temporal-parietal lobe meta region of interest (ROI), which is particularly vulnerable to age-related neurodegeneration. This ROI encompasses entorhinal, fusiform, inferior temporal, middle temporal, hippocampus, amygdala, and precuneus and performed the best in the ROI selection studies conducted in the Mayo Clinic Study of Aging and Mayo Alzheimer Disease Research Center to discriminate between amyloid PET-negative cognitively unimpaired and amyloid PET-positive cognitively impaired individuals.22 The lower values of temporal-parietal lobe cortical thickness meta ROI suggest greater AD-related atrophy.
Covariates
Information about date of birth, sex, race, and years of education was collected at visit 1 by interview (1987–1989). Years of education was categorized as less than high school, high school or vocational school, and at least some college. APOE ε4 was genotyped using the TaqMan assay (Applied Biosystems, Foster City, CA) at visit 1.23 Participants' behavioral and clinical characteristics were assessed using standardized protocols at visit 2.13 Habitual alcohol use was self-reported and categorized as current, former, or never. Standardized anthropometric measurements of weight, height, and waist circumference were obtained at examination visits. BMI was calculated using weight in kilograms divided by the square of height in meters. Sitting arm blood pressures were measured after a 5-minute rest using a standardized sphygmomanometer.24 Three measures were taken for each individual, and the average of the last 2 readings was calculated. Serum aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase were measured on frozen specimens collected from visit 2 using a kinetic rate reaction method on the Roche Cobas 6000 chemistry analyzer (Roche Diagnostics, Indianapolis, IN) at the University of Minnesota in 2011–13.25 Total and high-density lipoprotein cholesterols and triglycerides were measured using automated enzymatic methods.26 Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI creatinine equation.27 Coronary heart disease, heart failure, stroke, cancer, and chronic obstructive pulmonary disease were ascertained through follow-up visits, annual follow-up contacts, and community-wide hospital surveillance.28 Glucose was measured by a hexokinase/glucose-6-phosphate dehydrogenase method on a Coulter DACOS device (Beckman Coulter, Fullerton, CA).29 Diabetes was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, self-reported diagnosis of diabetes by a physician, or using antidiabetic medications. Use of medications for hypertension, dyslipidemia, and diabetes in the previous 2 weeks was self-reported by the participants and validated by medication containers brought to the ARIC clinic.
Statistical Evaluation
Baseline characteristics were summarized across midlife NAFLD categories. We first quantified the cross-temporal associations of (1) midlife NAFLD (visit 2) with brain MRI markers (visit 5) and (2) midlife NAFLD (visit 2) with plasma biomarkers at midlife (visit 3) and late life (visit 5) using linear regression models. Annual change rate in MRI measurements from visit 5 to visit 6/7 and of plasma biomarkers from visit 3 to visit 5 and visit 5 to visit 6/7 were calculated by subtracting the measures taken at the earlier visit from the latter visit then dividing by the years between 2 measures. We examined midlife NAFLD in relation to annual change rate using linear regression.
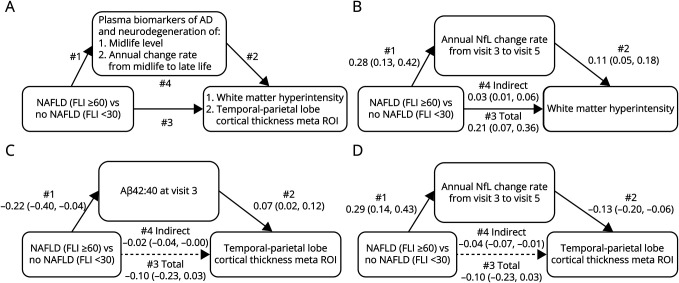
Formal mediation analyses were performed using structural equation modeling (Stata program “medeff”) to estimate the indirect effects of NAFLD on MRI biomarkers, which were attributed to variations in midlife and changes in plasma biomarkers.30 This analysis was restricted to individuals who had available plasma biomarkers at visit 3 and visit 5, as well as MRI biomarkers at visit 5. For a simplified mediation analysis, we examined NAFLD as a binary exposure (NAFLD vs no NAFLD). The final sample consisted of 426 individuals with midlife NAFLD and 502 individuals without. The mediation analysis framework is shown in Figure 2A.31
Figure 2. Mediation Analyses of Midlife Plasma Biomarkers of Neuropathology and Changes on the Midlife NAFLD Late-Life Brain MRI Associations.
Solid lines indicate significant pathway, whereas dashed lines represent nonsignificant pathways. (A) The framework for the mediation analysis. With mediating influences, path 1 and 2 are present, and path 3 attenuates when additionally adjusting for the potential mediator. Path 4 indicates indirect association through mediator. A significant path 4 is indicative of a mediation effect. (B) Mediating role of annual change rate of NfL in NAFLD-WMH association. (C) Mediating role of midlife Aβ42:40 in NAFLD temporal-parietal lobe cortical thickness meta ROI association. (D) Mediating role of annual change rate of NfL in NAFLD temporal-parietal lobe cortical thickness meta ROI association. Aβ = β-amyloid; NAFLD = nonalcoholic fatty liver disease; NfL = neurofilament light; ROI = region of interest; WMH = white matter hyperintensity.
To enable a direct comparison of the strength of the associations, the MRI and plasma biomarkers were scaled according to the SDs at the earliest visit available. Primary models were adjusted for age, sex, race center, education, APOE-ε4 genotype, baseline alcohol use, and eGFR. Model 2 additionally included baseline blood pressure, high density lipoprotein cholesterol, total cholesterol, hypertension, diabetes, and coronary heart disease. Model 2 was secondary because the shared underlying pathophysiologic mechanisms and bidirectional interactions may hinder the independence of those baseline factors and NAFLD.32 BMI was not included because it is a component of the NAFLD assessment model and highly correlated with NAFLD. Total intracranial volume was included as a covariate for WMH to adjust for differences in participant head size. Sampling weights were incorporated into all models to obtain inferences that generalize to the ARIC cohort at visit 5.19 To mitigate the concern of informative attrition from visit 5 to visit 6/7 (60%) due to death and nondeath dropout, we conducted a sensitivity analysis using a combined weight that multiplied the sampling weight by a stabilized inverse probability attrition weight. This weight was used for the analyses with annual change rate in MRI and plasma biomarkers from visit 5 to visit 6/7 as the outcomes (details in the eMethods, links.lww.com/WNL/D469).33
All analyses were performed with Stata version 17.0 (StataCorp LLC, College Station, TX). A p value <0.05 was considered nominally statistically significant.
Data Availability
Researchers can obtain ARIC data from the NIH public data repository (BioLINCC, biolincc.nhlbi.gov/studies/aric/) by signing a data use agreement.
Results
The brain MRI subcohort included 1,706 participants (mean age 55.6 [SD 5.2] years, 61.6% female, 28.3% Black) at visit 2. Among them, 36.7%, 29.0%, and 34.3% were classified as no NAFLD, indeterminate NAFLD, and NAFLD, respectively. Participants with NAFLD, compared with those without, were more likely to be male and Black, less educated, more likely to be APOE-ε4 allele carriers, less likely to be current drinkers, and had higher BMI, systolic and diastolic blood pressure, and triglyceride levels. In addition, participants with NAFLD had a higher prevalence of hypertension, diabetes, coronary heart disease, and had lower cognitive function (Table 1).
Table 1.
Characteristics of Participants in the ARIC Study Visit 2 (1990–92) by Categories of NAFLD (N = 1,706)
| Characteristics | Total (n = 1,706) | No NAFLD (n = 626) | Indeterminate NAFLD (n = 494) | NAFLD (n = 586) |
| Age, y, mean (SD) | 55.6 (5.2) | 55.3 (5.1) | 56.6 (5.3) | 55.2 (5.0) |
| Female, n (%) | 1,050 (61.5) | 477 (76.2) | 255 (51.6) | 318 (54.3) |
| Race (center), n (%) | ||||
| White (Forsyth County, NC) | 401 (23.5) | 197 (31.5) | 115 (23.3) | 89 (15.2) |
| White (Minneapolis, MN) | 384 (22.5) | 155 (24.8) | 91 (18.4) | 138 (23.5) |
| White (Washington, MD) | 439 (25.7) | 136 (21.7) | 129 (26.1) | 174 (29.7) |
| Black (Forsyth County, NC) | 32 (1.9) | 12 (1.9) | 13 (2.6) | 7 (1.2) |
| Black (Jackson, MS) | 450 (26.4) | 126 (20.1) | 146 (29.6) | 178 (30.4) |
| Education, n (%) | ||||
| <High school | 237 (13.9) | 63 (10.1) | 73 (14.8) | 101 (17.2) |
| High school or vocational school | 706 (41.4) | 269 (43.0) | 190 (38.5) | 247 (42.2) |
| At least some college | 763 (44.7) | 294 (47.0) | 231 (46.8) | 238 (40.6) |
| APOE ε4 status, n (%) | 506 (29.7) | 184 (29.4) | 132 (26.7) | 190 (32.4) |
| Alcohol use, n (%) | ||||
| Current | 990 (58.0) | 399 (63.7) | 281 (56.9) | 310 (52.9) |
| Former | 288 (16.9) | 79 (12.6) | 85 (17.2) | 124 (21.2) |
| Never | 428 (25.1) | 148 (23.6) | 128 (25.9) | 152 (25.9) |
| eGFR, mL/min/1.73 m2, mean (SD) | 98.0 (14.3) | 98.2 (13.1) | 97.2 (14.4) | 98.5 (15.2) |
| BMI, kg/m2, mean (SD) | 27.5 (5.1) | 23.5 (2.4) | 27.0 (2.3) | 32.3 (4.9) |
| SBP, mm Hg, mean (SD) | 117.2 (15.9) | 111.6 (15.2) | 118.6 (15.5) | 122.1 (15.1) |
| DBP, mm Hg, mean (SD) | 71.4 (9.7) | 68.8 (9.7) | 71.5 (9.5) | 74.2 (9.0) |
| Triglycerides, mg/dL, mean (SD) | 125.4 (77.2) | 88.7 (35.3) | 119.7 (53.6) | 169.4 (101.0) |
| Total cholesterol, mg/dL, mean (SD) | 208.1 (37.1) | 201.1 (34.2) | 211.3 (37.5) | 212.8 (38.6) |
| HDL, mg/dL, mean (SD) | 52.3 (17.0) | 61.0 (17.2) | 50.3 (15.3) | 44.9 (14.0) |
| Diabetes, n (%) | 146 (8.6) | 18 (2.9) | 30 (6.1) | 98 (16.7) |
| Hypertension, n (%) | 434 (25.5) | 91 (14.5) | 127 (25.9) | 216 (37.0) |
| Coronary heart disease, n (%) | 21 (1.2) | 2 (0.3) | 7 (1.4) | 12 (2.0) |
| Cognitive factor score, mean (SD) | 0.8 (0.8) | 1.0 (0.8) | 0.7 (0.9) | 0.7 (0.8) |
Abbreviations: ARIC = Atherosclerosis Risk in Communities; BMI = body mass index; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein cholesterol; NAFLD = nonalcoholic fatty liver disease; SBP = systolic blood pressure.
Midlife NAFLD, Late-Life Brain MRI Measurements, and Change
Compared with participants without NAFLD, those with NAFLD had a 0.19 SD higher WMH volume (0.19, 95% CI 0.06–0.31) and a SD 0.16 lower temporal-parietal lobe cortical thickness meta ROI (−0.16, 95% CI −0.28 to −0.05) (Table 2). After adjusting for baseline cardiometabolic factors, the association of NAFLD with WMH volume was attenuated (SD 0.13, 95% CI −0.01 to 0.28), but mostly unchanged for temporal-parietal lobe cortical thickness meta ROI (SD −0.15, 95% CI −0.29 to −0.01) (eTable 1, links.lww.com/WNL/D470). Midlife NAFLD and indeterminate NAFLD were associated with a faster rate of increase in WMH volume over 5 years in late life (difference in annual change rate of WMH volume for NAFLD vs no NAFLD, SD 0.28, 95% CI 0.05–0.51 and for indeterminate NAFLD vs no NAFLD, SD 0.26, 95% CI 0.01–0.51 in Table 2). These associations were robust to adjustment for attrition (eTable 2). The difference was slightly attenuated to borderline insignificant after adjusting for baseline cardiometabolic factors (eTables 1 and 2). There were no differences in the annual change rate in the temporal-parietal lobe cortical thickness meta ROI for participants with vs without NAFLD.
Table 2.
Difference (per SD, 95% CI) in Levels and Changes of MRI and Plasma Biomarkers of Neuropathology by Categories of NAFLD in the ARIC Study
| Per SD in levelsa | No NAFLD | Indeterminate NAFLD | NAFLD | |
| Brain MRI markers at visit 5 | log(WMH) | 0 (Ref.) | 0.14 (0.01 to 0.27) | 0.19 (0.06 to 0.31)b |
| Temporal-parietal lobe cortical thickness meta ROI | 0 (Ref.) | −0.04 (−0.16 to 0.08) | −0.16 (−0.28 to −0.05)b | |
| Plasma biomarkers of neuropathology at visit 3 | Aβ42:40 | 0 (Ref.) | −0.13 (−0.30 to 0.03) | −0.21 (−0.39 to −0.04)b |
| log2(p-tau181) | 0 (Ref.) | 0.08 (−0.08 to 0.24) | 0.15 (−0.02 to 0.31) | |
| log2(NfL) | 0 (Ref.) | −0.09 (−0.22 to 0.04) | −0.36 (−0.49 to −0.23)b | |
| Plasma biomarkers of neuropathology at visit 5 | Aβ42:40 | 0 (Ref.) | −0.10 (−0.19 to −0.01)b | −0.13 (−0.23 to −0.03)b |
| log2(p-tau181) | 0 (Ref.) | 0.03 (−0.08 to 0.14) | 0.10 (−0.01 to 0.21) | |
| log2(NfL) | 0 (Ref.) | −0.03 (−0.15 to 0.10) | −0.12 (−0.24 to 0.01) | |
| Per SD in annual change ratesc | No NAFLD | Indeterminate NAFLD | NAFLD | |
| Annual change rate in brain MRI markers from visit 5 to visit 6/7 | log(WMH) | 0 (Ref.) | 0.26 (0.01 to 0.51)b | 0.28 (0.05 to 0.51)b |
| Temporal-parietal lobe cortical thickness meta ROI | 0 (Ref.) | 0.02 (−0.22 to 0.26) | −0.01 (−0.23 to 0.22) | |
| Annual change rate in plasma biomarkers of neuropathology from visit 3 to visit 5 | Aβ42:40 | 0 (Ref.) | 0.07 (−0.09 to 0.23) | 0.10 (−0.08 to 0.28) |
| log2(p-tau181) | 0 (Ref.) | −0.05 (−0.21 to 0.12) | −0.03 (−0.19 to 0.13) | |
| log2(NfL) | 0 (Ref.) | 0.06 (−0.09 to 0.21) | 0.26 (0.11 to 0.40)b | |
| Annual change rate in plasma biomarkers of neuropathology from visit 5 to visit 6/7 | Aβ42:40 | 0 (Ref.) | 0.18 (−0.13 to 0.49) | −0.01 (−0.30 to 0.28) |
| log2(p-tau181) | 0 (Ref.) | 0.01 (−0.39 to 0.41) | −0.00 (−0.37 to 0.37) | |
| log2(NfL) | 0 (Ref.) | −0.01 (−0.66 to 0.64) | 0.74 (0.19 to 1.30)b |
Abbreviations: Aβ = β-amyloid; NAFLD = nonalcoholic fatty liver disease; NfL = neurofilament light; p-tau181 = phosphorylated tau at threonine 181; ROI = region of interest; WMH = white matter hyperintensity.
Models were adjusted for age, sex, race center, education, APOE ε4 genotype, alcohol use, estimated glomerular filtration rate, and total intracranial volume for WMH.
1 SD in levels: log(WMH), 0.8942; temporal-parietal lobe cortical thickness meta ROI, 0.1358; Aβ42:40, 0.0185; log2(p-tau181), 0.8075; log2(NfL), 0.6671.
Statistical significance at p < 0.05.
1 SD in annual change rates: log(WMH), 0.0724; temporal-parietal lobe cortical thickness meta ROI, 0.0123; Aβ42:40, 0.0010; log2(p-tau181), 0.0453; log2(NfL), 0.0364.
Midlife NAFLD, Midlife and Late-Life Plasma Biomarkers, and Change
We estimated the associations of midlife NAFLD (visit 2) with plasma biomarkers in midlife (visit 3) and late life (visit 5). Compared with participants without midlife NAFLD, those with NAFLD had SD 0.21 lower Aβ42:40 levels in midlife (−0.21, 95% CI −0.39 to −0.04) and SD 0.13 lower Aβ42:40 levels in late life (−0.13, 95% CI −0.23 to −0.03); NfL was SD 0.36 lower in midlife (−0.36, 95% CI −0.49 to −0.23) (Table 2). These estimates were robust to adjustment for baseline cardiometabolic factors (eTable 1, links.lww.com/WNL/D470). Individuals with midlife NAFLD vs without had a faster increase in NfL, but not a faster decrease in Aβ42:40, from midlife to late-life. The difference in annual change rate of NfL was even greater in late life (NAFLD vs no NAFLD, visit 3 to 5: SD 0.26, 95% CI 0.11–0.40, visit 5 to 6/7: SD 0.74, 95% CI 0.19–1.30 in Table 2 and materially unchanged after accounting for attrition in eTable 2). The difference in annual change rate of NfL was no longer significant after adjustment for baseline cardiometabolic factors (eTables 1 and 2). We did not observe associations between midlife NAFLD and p-tau181.
Mediating Role of Plasma Biomarkers in Associations Between Midlife NAFLD and Late-Life Brain MRI Markers
Increased annual change rate of NfL because of midlife NAFLD was associated with SD 0.03 higher WMH volume (path 4: 0.03, 95% CI 0.01–0.06), which explained 15% of the total association between midlife NAFLD and WMH volume (Figure 2B, eTable 3, links.lww.com/WNL/D470). We also observed a significant indirect effect of midlife Aβ42:40 (path 4: −0.02, 95% CI −0.04 to −0.00) or the annual change rate of NfL (path 4: −0.04, 95% CI −0.07 to −0.01) on the association between midlife NAFLD and temporal-parietal lobe cortical thickness meta ROI (Figure 2, C and D).
Discussion
This study estimated associations between midlife NAFLD and imaging and plasma biomarkers of neuropathology in the community-based ARIC cohort. Participants with midlife NAFLD had greater WMH volume and a faster increase in WMH volume in late life compared with those without midlife NAFLD, suggesting associations with accumulating vascular pathology. Participants with NAFLD also had lower temporal-parietal lobe cortical thickness meta ROI in late-life, as well as lower levels of midlife and late-life Aβ42:40, suggesting associations with AD pathology. Although midlife NfL was lower in individuals with midlife NAFLD than those without, there was a faster rate of increase in NfL from midlife to late life among participants with midlife NAFLD. The associations of midlife NAFLD with imaging and plasma biomarkers of AD neuropathology were consistent after adjustment for baseline cardiometabolic risk factors. NfL also partially mediated the association between midlife NAFLD and late-life WMH volume.
This study suggests that midlife NAFLD is associated with both AD and vascular pathology. Previous studies have reported associations between NAFLD and higher WMH volume.6,7,10 NAFLD is closely associated with vascular complications, in part because liver inflammation induces persistent systemic inflammation,32 which manifests in the brain as vascular endothelial cell proliferation, intimal thickening, media fibrosis, lumen narrowing,34 and microvascular hemodynamic change,35 progressively causing brain lesions and white matter alterations.2,8 Many shared cardiometabolic risk factors between NAFLD and dementia, such as hypertension, insulin resistance, and dyslipidemia, also contribute to cerebrovascular dysfunction.2,36 In addition, the urea cycle occurs exclusively in the liver and is the primary metabolic pathway for eliminating nitrogenous (ammonia) waste. Loss of liver function results in systemic ammonia accumulation, crossing the blood-brain barrier and increasing the brain's susceptibility to inflammatory responses.32 Adding to this feature, gut microbiota disturbance is often associated with progressive liver injury. Intestinal metabolites and proinflammatory bacterial products, including ammonia, could return to the liver through the portal vein, initiating a cascade of hepatic inflammation, lipogenesis, oxidative stress, insulin resistance, and fibrogenesis. These disturbances, together with systemic inflammation, are central elements of the gut-liver-brain axis, ultimately inducing neuroinflammation and interfering with astrocytic-neuronal communication.37
Few studies have examined the relationship between NAFLD and AD pathology. A cross-sectional study reported an association between the severity of liver fibrosis and brain Aβ and tau PET, independent of cardiometabolic factors.11 By contrast, Peng et al.7 did not find an association between all-cause liver disease and changes in the volume of brain regions specific to AD or with plasma biomarkers of AD and neurodegeneration. Similar to the first study, our results suggest a robust association between midlife NAFLD and markers of Aβ pathology, including smaller temporal-parietal lobe cortical thickness meta ROI and lower levels of Aβ42:40. The discrepancy with the second study is likely due to their use of a liver disease definition that was not restricted to NAFLD.
Some pathophysiologic mechanisms for our findings have been proposed. Aβ deposition in the brain is the result of Aβ overproduction and clearance deficiency that involves both the brain and periphery.38 The liver is one of the main insulin-responsive tissues controlling peripheral metabolism.39 Patients with NAFLD often exhibit altered hepatic and peripheral insulin resistance and metabolic dysregulation, which parallel the brain insulin and insulin-like growth factor (IGF-I) resistance seen in dementia.34,40 Aberrant brain insulin/IGF signaling interferes with normal Aβ and tau expression and protein processing and inhibits intracellular degradation of Aβ.34 In addition, the key protein mediating the relationship between liver and Aβ clearance is low-density lipoprotein-related protein-1 (LRP1), which is abundantly expressed in hepatocytes and sinusoidal cells.38,41,42 Liver dysfunction and hepatic insulin resistance appeared in NAFLD could reduce hepatic expression of LRP1 and impede translocation of LRP1 to the hepatocyte membrane,43 resulting in defective peripheral clearance of Aβ, which in turn may contribute to Aβ deposition in the brain.
Our study suggests that individuals with midlife NAFLD develop Aβ pathology (lower midlife Aβ42:40) earlier than those without NAFLD but that the rate of accumulation is not accelerated. By contrast, we observed an accelerated increase in WMH volume in late life in individuals with midlife NAFLD. It is plausible to consider that in this middle-aged cohort at baseline, even subclinical manifestations of NAFLD and slightly compromised liver function contribute to the Aβ pathology. On the other hand, NAFLD may also contribute to the development and accumulation of cardiometabolic risk factors, which increase the risk of brain structural changes that drive further dementia development, primarily through the vascular pathway. This may also explain the null associations between NAFLD and AD pathology in studies of older adults.
Our mediation analysis further supports this hypothesis by showing that faster increase in NfL from midlife to late life in individuals with midlife NAFLD accounts for approximately 15% of brain small vascular disease measures. Plasma NfL levels serve as a marker of ongoing neurodegeneration broadly, regardless of the underlying cause, and a faster increase in its levels suggests a more rapid vascular disease progression.44 We also observed indirect-only mediators of midlife Aβ42:40 and NfL change that mediate the association between midlife NAFLD and temporal-parietal lobe cortical thickness meta ROI. Taken together, our findings suggest that timely intervention in the liver in midlife can help preserve brain structural integrity, and the use of NfL as a general neurodegeneration biomarker may help monitor liver impact on brain health.
In this study, midlife NAFLD was associated with lower Aβ42:40, but not with p-tau181. Although both lower Aβ42:40 and higher p-tau181 are associated with brain Aβ deposition,18 the plasma Aβ42:40 ratio stands out for its ability to change in the early stages of the disease, even before detectable alterations in p-tau181 levels and in the absence of cognitive impairment.45,46 This is consistent with our findings that only a lower Aβ42:40, but not higher p-tau181, was observed in individuals with midlife NAFLD. In addition, individuals with NAFLD often have higher BMI (a proxy of larger blood volume),47 which is negatively associated with plasma biomarkers of neuropathology.48 This association may level out the difference of p-tau181 in those with vs without NAFLD. By contrast, the ratio of Aβ42 over Aβ40 uses Aβ40 as a reference peptide to cancel out the impact of BMI on the absolute levels of individual biomarker. Previous studies have indeed shown that renal function and BMI affect plasma Aβ40, Aβ42, NfL, and to a lesser extent, p-tau181 alone, but not the Aβ42:40 ratio.47,49,50
Strengths of this study include the use of repeat imaging and plasma biomarkers of neuropathology spanning midlife to late life, collected from a well-characterized, community-based prospective cohort. The sequence of measurements of midlife NAFLD, midlife and late-life plasma biomarkers of neuropathology, and late-life brain imaging allows a prospective assessment.
Our research has some limitations. Our study used a clinical predictive model of FLI, which used easily measurable clinical variables to identify NAFLD. Observed associations may have been weakened because of possible misclassification. In addition, FLI has a moderate correlation with BMI and waist circumference (variance inflation factor >2), precluding inclusion of these variables in our models. We therefore cannot determine whether any liver contributions are independent of body adiposity.9 Furthermore, the severity of NAFLD, ranging from simple steatosis to various stages of fibrosis, was not assessed in this study but may be more pertinent to the development of dementia-related pathology.10 The issue of large body mass associated with NAFLD potentially diluting the assessment of absolute levels of plasma biomarkers is relevant to this study47 and reflected in our observations of lower NfL in individuals with midlife NAFLD. However, this concern could be mitigated with the use of Aβ42:40 ratio which dissects away the confounding effect of the non–AD-associated factors or the use of rising NfL as an indicator of disease progression with self-referencing.
In conclusion, our findings suggest associations between midlife NAFLD and the presence of AD and accumulating vascular pathology. Although midlife NAFLD is associated with a pathologic Aβ state early in midlife, it may also contribute to the development and accumulation of cardiometabolic risk factors that drive further dementia development through a vascular pathway. This study also highlights the value of plasma biomarkers of neuropathology as easily measurable and dynamic indicators for monitoring impacts of impaired liver function on brain health.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions. The authors acknowledge the contributions of Dr. Gerardo Heiss, Kenan Distinguished Professor of Epidemiology at the University of North Carolina at Chapel Hill Gillings School of Global Public Health. Coauthor Gerardo Heiss, PhD, was central to the successful development of this manuscript; he passed away on June 11, 2022.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- eGFR
estimated glomerular filtration
- FLAIR
fluid-attenuated inversion recovery
- FLI
fatty liver index
- IGF
insulin-like growth factor
- LRP1
lipoprotein-related protein-1
- MPRAGE
magnetization-prepared rapid acquisition gradient echo
- NAFLD
nonalcoholic fatty liver disease
- NfL
neurofilament light
- p-tau181
phosphorylated tau at threonine 181
- ROI
region of interest
- WMH
white matter hyperintensity
Appendix. Authors
| Name | Location | Contribution |
| Yifei Lu, PhD | Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| James R. Pike, MBA | Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD | Drafting/revision of the manuscript for content, including medical writing for content |
| Ron Hoogeveen, PhD | Department of Medicine, Baylor College of Medicine, Houston, TX | Drafting/revision of the manuscript for content, including medical writing for content |
| Keenan Walker, PhD | Laboratory of Behavioral Neuroscience, National Institute on Aging, Bethesda, MD | Drafting/revision of the manuscript for content, including medical writing for content |
| Laura Raffield, PhD | Department of Genetics, School of Medicine, University of North Carolina at Chapel Hill | Drafting/revision of the manuscript for content, including medical writing for content |
| Elizabeth Selvin, PhD, MPH | Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD | Drafting/revision of the manuscript for content, including medical writing for content |
| Christy Avery, PhD | Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill | Drafting/revision of the manuscript for content, including medical writing for content |
| Stephanie Engel, PhD | Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill | Drafting/revision of the manuscript for content, including medical writing for content |
| Michelle M. Mielke, PhD | Department of Epidemiology and Prevention, Wake Forest University School of Medicine, Winston-Salem, NC | Drafting/revision of the manuscript for content, including medical writing for content |
| Tanya Garcia, PhD | Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill | Drafting/revision of the manuscript for content, including medical writing for content |
| Gerardo Heiss, PhD | Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill | Study concept or design |
| Priya Palta, PhD, MHS | Department of Neurology, School of Medicine, University of North Carolina at Chapel Hill | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
Study Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. The plasma biomarker measurements were in part supported by R00AG052830. K. Walker receives funding from the NIA Intramural Research Program. This study was funded, in part, by the NIA Intramural Research Program. L. Raffield is funded by R01AG075884. Drs. Lu and Palta were in part supported by R01AG066134.
Disclosure
Y. Lu, J.R. Pike, K.A. Walker, E. Selvin, C.L. Avery, S.M. Engel, T.P. Garcia, P. Palta report no disclosures relevant to the manuscript. R.C. Hoogeveen has received research grants from Denka Seiken (to his institution) and is a consultant for Denka Seiken unrelated to the current manuscript. L.M. Raffield is a consultant for the NHLBI Trans-Omics for Precision Medicine Program Administrative Coordinating Center (through Westat). M.M. Mielke has served on scientific advisory boards and/or has consulted for Biogen, LabCorp, Lilly, Merck, PeerView Institute, Siemens Healthineers, and Sunbird Bio unrelated to the current manuscript. G. Heiss is deceased, to the best of our knowledge, G. Heiss had no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Than NN, Newsome PN. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239(1):192-202. doi: 10.1016/j.atherosclerosis.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20(37):13306-13324. doi: 10.3748/wjg.v20.i37.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassendine MF, Taylor-Robinson SD, Fertleman M, Khan M, Neely D. Is Alzheimer's disease a liver disease of the brain? J Alzheimers Dis. 2020;75:1-14. doi: 10.3233/JAD-190848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutcliffe JG, Hedlund PB, Thomas EA, Bloom FE, Hilbush BS. Peripheral reduction of β-amyloid is sufficient to reduce brain β-amyloid: implications for Alzheimer's disease. J Neurosci Res. 2011;89(6):808-814. doi: 10.1002/jnr.22603 [DOI] [PubMed] [Google Scholar]
- 5.Weinstein G, Zelber-Sagi S, Preis SR, et al. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham Study. JAMA Neurol. 2018;75(1):97-104. doi: 10.1001/jamaneurol.2017.3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh NS, Kamel H, Zhang C, et al. Association of liver fibrosis with cognitive test performance and brain imaging parameters in the UK Biobank study. Alzheimers Dement. 2023;19(4):1518-1528. doi: 10.1002/alz.12795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Z, Duggan MR, Dark HE, et al. Association of liver disease with brain volume loss, cognitive decline, and plasma neurodegenerative disease biomarkers. Neurobiol Aging. 2022;120:34-42. doi: 10.1016/j.neurobiolaging.2022.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petta S, Tuttolomondo A, Gagliardo C, et al. The presence of white matter lesions is associated with the fibrosis severity of nonalcoholic fatty liver disease. Medicine (Baltimore). 2016;95(16):e3446. doi: 10.1097/MD.0000000000003446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanWagner LB, Terry JG, Chow LS, et al. Nonalcoholic fatty liver disease and measures of early brain health in middle-aged adults: the CARDIA study. Obesity (Silver Spring). 2017;25(3):642-651. doi: 10.1002/oby.21767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang H, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and cerebral small vessel disease in Korean cognitively normal individuals. Sci Rep. 2019;9(1):1814. doi: 10.1038/s41598-018-38357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein G, O'Donnell A, Davis-Plourde K, et al. Non-alcoholic fatty liver disease, liver fibrosis, and regional amyloid-β and tau pathology in middle-aged adults: the Framingham Study. J Alzheimers Dis. 2022;86(3):1371-1383. doi: 10.3233/JAD-215409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.2022 Alzheimer's Disease Facts and Figures. Alzheimer's Association; 2022. Accessed March 22, 2022. alz.org/media/documents/alzheimers-facts-and-figures.pdf. [DOI] [PubMed] [Google Scholar]
- 13.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687-702. [PubMed] [Google Scholar]
- 14.Atherosclerosis Risk in Communities Neurocognitive Study. Manual 17. Accessed November 24, 2023. aric.cscc.unc.edu/aric9/sites/default/files/public/visitdocuments/v5/17%20Neurocognitive%20Exam%20%28Stage%202%20and%203%29.pdf. [Google Scholar]
- 15.Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76(22):1879-1885. doi: 10.1212/WNL.0b013e31821d753f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson DH, Rissin DM, Kan CW, et al. The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. 2016;21(4):533-547. doi: 10.1177/2211068215589580 [DOI] [PubMed] [Google Scholar]
- 18.Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27(6):954-963. doi: 10.1038/s41591-021-01382-x [DOI] [PubMed] [Google Scholar]
- 19.Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46(2):433-440. doi: 10.1161/STROKEAHA.114.007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raz L, Jayachandran M, Tosakulwong N, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80(10):911-918. doi: 10.1212/WNL.0b013e3182840c9f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin. 2016;11:802-812. doi: 10.1016/j.nicl.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1-11. doi: 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherosclerosis Risk in Communities Study. Protocol Manual 11: Sitting Blood Pressure. Accessed March 22, 2022. www2.cscc.unc.edu/aric/sites/default/files/public/manuals/Sitting_Blood_Pressure_and_Postural_Changes_in_Blood_Pressure_and_Heart_Rate.1_11.pdf. [Google Scholar]
- 25.Parrinello CM, Grams ME, Couper D, et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61(7):938-947. doi: 10.1373/clinchem.2015.238873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29(6):1075-1080. doi: 10.1093/clinchem/29.6.1075 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Tang O, Brady TM, et al. Simplified blood pressure measurement approaches and implications for hypertension screening: the Atherosclerosis Risk in Communities study. J Hypertension. 2021;39(3):447-452. doi: 10.1097/HJH.0000000000002682 [DOI] [PubMed] [Google Scholar]
- 29.Schmidt MI, Watson RL, Duncan BB, et al. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Atherosclerosis Risk in Communities Study Investigators. Metabolism. 1996;45(6):699-706. doi: 10.1016/s0026-0495(96)90134-1 [DOI] [PubMed] [Google Scholar]
- 30.Hicks R, Tingley D. Causal mediation analysis. Stata J. 2011;11(4):605-619. doi: 10.1177/1536867x1201100407 [DOI] [Google Scholar]
- 31.Zhao X, Lynch JG, Chen Q. Reconsidering Baron and Kenny: myths and truths about mediation analysis. J Consum Res. 2010;37(2):197-206. doi: 10.1086/651257 [DOI] [Google Scholar]
- 32.Kjærgaard K, Mikkelsen ACD, Wernberg CW, et al. Cognitive dysfunction in non-alcoholic fatty liver disease-current knowledge, mechanisms and perspectives. J Clin Med. 2021;10(4):673. doi: 10.3390/jcm10040673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119-128. doi: 10.1097/EDE.0b013e318230e861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol. 2014;88(4):548-559. doi: 10.1016/j.bcp.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal-González D, López-Sánchez GN, Concha-Rebollar LA, et al. Cerebral hemodynamics in the non-alcoholic fatty liver. Ann Hepatol. 2020;19(6):668-673. doi: 10.1016/j.aohep.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 36.Weinstein G, Davis-Plourde K, Himali JJ, Zelber-Sagi S, Beiser AS, Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: the Framingham Study. Liver Int. 2019;39(9):1713-1721. doi: 10.1111/liv.14161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheon SY, Song J. Novel insights into non-alcoholic fatty liver disease and dementia: insulin resistance, hyperammonemia, gut dysbiosis, vascular impairment, and inflammation. Cell Biosci. 2022;12(1):99. doi: 10.1186/s13578-022-00836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deane R, Sagare A, Zlokovic BV. The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer's disease. Curr Pharm Des. 2008;14(16):1601-1605. doi: 10.2174/138161208784705487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke JR, Ribeiro FC, Frozza RL, De Felice FG, Lourenco MV. Metabolic dysfunction in Alzheimer's disease: from basic neurobiology to clinical approaches. J Alzheimers Dis. 2018;64(suppl 1):S405-S426. doi: 10.3233/JAD-179911 [DOI] [PubMed] [Google Scholar]
- 40.Karbalaei R, Allahyari M, Rezaei-Tavirani M, Asadzadeh-Aghdaei H, Zali MR. Protein-protein interaction analysis of Alzheimer's disease and NAFLD based on systems biology methods unhide common ancestor pathways. Gastroenterol Hepatol Bed Bench. 2018;11(1):27-33. [PMC free article] [PubMed] [Google Scholar]
- 41.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid β-peptide elimination from the brain. J Neurochem. 2010;115(5):1077-1089. doi: 10.1111/j.1471-4159.2010.07002.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sagare AP, Winkler EA, Bell RD, Deane R, Zlokovic BV. From the liver to the blood-brain barrier: an interconnected system regulating brain amyloid-β levels. J Neurosci Res. 2011;89(7):967-968. doi: 10.1002/jnr.22670 [DOI] [PubMed] [Google Scholar]
- 43.Wang YR, Wang QH, Zhang T, et al. Associations between hepatic functions and plasma amyloid-beta levels-implications for the capacity of liver in peripheral amyloid-beta clearance. Mol Neurobiol. 2017;54(3):2338-2344. doi: 10.1007/s12035-016-9826-1 [DOI] [PubMed] [Google Scholar]
- 44.Zetterberg H, Bendlin BB. Biomarkers for Alzheimer's disease-preparing for a new era of disease-modifying therapies. Mol Psychiatry. 2021;26(1):296-308. doi: 10.1038/s41380-020-0721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong JR, Ashton NJ, Karikari TK, et al. Plasma P-tau181 to Aβ42 ratio is associated with brain amyloid burden and hippocampal atrophy in an Asian cohort of Alzheimer's disease patients with concomitant cerebrovascular disease. Alzheimers Dement. 2021;17(10):1649-1662. doi: 10.1002/alz.12332 [DOI] [PubMed] [Google Scholar]
- 46.Bilgel M, An Y, Walker KA, et al. Longitudinal changes in Alzheimer's-related plasma biomarkers and brain amyloid. Alzheimers Dement. 2023;19(10):4335-4345. doi: 10.1002/alz.13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pichet Binette A, Janelidze S, Cullen N, et al. Confounding factors of Alzheimer's disease plasma biomarkers and their impact on clinical performance. Alzheimers Dement. 2023;19(4):1403-1414. doi: 10.1002/alz.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18(12):2669-2686. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehmann S, Schraen-Maschke S, Vidal JS, et al. Plasma Aβ42/Aβ40 ratio is independent of renal function. Alzheimers Dement. 2023;19(6):2737-2739. doi: 10.1002/alz.12949 [DOI] [PubMed] [Google Scholar]
- 50.Syrjanen JA, Campbell MR, Algeciras-Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2022;18(6):1128-1140. doi: 10.1002/alz.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researchers can obtain ARIC data from the NIH public data repository (BioLINCC, biolincc.nhlbi.gov/studies/aric/) by signing a data use agreement.