Abstract
Adoptive transfer of virus-specific memory lymphocytes can be used to identify factors and mechanisms involved in the clearance of persistent virus infections. To analyze the role of B cells in clearing persistent infection with lymphocytic choriomeningitis virus (LCMV), we used B-cell-deficient μMT/μMT (B−/−) mice. B−/− mice controlled an acute LCMV infection with the same kinetics and efficiency as B-cell-competent (B+/+) mice via virus-specific, major histocompatibility complex (MHC) class I-restricted CD8+ cytotoxic T lymphocytes (CTL). CTL from B−/− and B+/+ mice were equivalent in affinity to known LCMV CTL epitopes and had similar CTL precursor frequencies (pCTL). Adoptive transfer of memory cells from B+/+ mice led to virus clearance from persistently infected B+/+ recipients even after in vitro depletion of B cells, indicating that B cells or immunoglobulins are not required in the transfer population. In contrast, transfer of memory splenocytes from B−/− mice failed to clear virus. Control of virus was restored neither by transferring higher numbers of pCTL nor by supplementing B−/− memory splenocytes with LCMV-immune B cells or immune sera. Instead, B−/− mice were found to have a profound CD4 helper defect. Furthermore, compared to cultured splenocytes from B+/+ mice, those from B−/− mice secreted less gamma interferon (IFN-γ) and interleukin 2, with differences most pronounced for CD8 T cells. While emphasizing the importance of CD4 T-cell help and IFN-γ in the control of persistent infections, the CD4 T-helper and CD8 T-cell defects in B−/− mice suggest that B cells contribute to the induction of competent T effector cells.
Cytotoxic T lymphocytes (CTL) have in general been associated with the resolution of both acute and chronic viral infections. As first shown by studies of lymphocytic choriomeningitis virus (LCMV) in mice, its natural host, a critical component of immune responses to virus infection is the induction of virus-specific major histocompatibility complex (MHC) class I-restricted CTL (reviewed in reference 14). Evidence that these cells can curtail acute viral infections and clear virus and viral genetic material from sera, peripheral blood leukocytes, and infected tissues came from adoptive transfer of LCMV memory CTL into mice persistently infected with LCMV (1, 25, 33, 47, 53).
Studies with humans have correlated the presence of CTL with the control of acute infection and clearance of virus and the absence of CTL activity with persistent viral infections. Hence, humans with genetic deficiencies in the humoral compartment of the immune system but with an intact T-cell compartment overcome most viral infections and display immunological memory when challenged or reinfected with the same virus. For example, agammaglobulinemic children recover from acute measles infection as well as do fully immunocompetent individuals and resist reinfection (23). In contrast, individuals with genetic or acquired defects in the T-cell compartment generally cannot control viral infections. Similarly, activity of CTL specific for hepatitis B virus (HBV) is associated with control of acute HBV infection; in the absence of CTL, HBV persists (39). Additionally, anti-HIV CTL dramatically decrease the load of human immunodeficiency virus (HIV) in infected patients, whereas loss of CTL function is accompanied by regress from a relatively healthy clinical stage to AIDS or rapid development of disease after HIV infection (9, 32). Finally, diminished or missing CTL responses to human cytomegalovirus (HCMV) facilitate HCMV disease in individuals undergoing bone marrow transplantation (40). Adoptive transfer of HCMV MHC-restricted CTL into such patients prevented CMV viremia or CMV disease (55). Thus, understanding the requirements for initiation and maintenance of CTL activity is essential.
Earlier, we and others documented the requirement for CD4 T-cell help (5, 16, 29, 48) and gamma interferon (IFN-γ) (48) in maintaining sufficient CTL activity in vivo and resolution of a chronic LCMV infection. Here, we evaluate the role of B lymphocytes in this process. Under the appropriate signals, B lymphocytes can differentiate into plasma cells to function as antibody-secreting cells. Trapping of antibody-antigen complexes as well as processing of antigen and peptide presentation within the MHC complex allows B cells to also function as antigen-presenting cells (APC) to T cells (22). Furthermore, B cells release numerous growth factors and cytokines that regulate immune responses (44).
To ascertain the role of B lymphocytes in the clearance of both acute and persistent LCMV infections, we used μMT/μMT B-cell-deficient (B−/−) mice which lack functional B cells and antibody. Earlier studies showed that CD8 T cells from these mice were capable of controlling an acute LCMV infection and that there was no defect in generating CTL precursors (3). Our results confirm and expand these findings. We demonstrate that while adoptive transfer of memory cells from B+/+ mice easily clears infectious virus and viral material in an MHC-matched persistently infected recipient, transfer of similar cells from B−/− mice does not. However, failure to terminate the persistent infection does not result from absence of B cells in the transfer population. Apparently, B−/− mice have a fundamental defect in CD4 helper function as well as a quantitative deficiency in IFN-γ and interleukin 2 (IL-2) preferentially produced by CD8 T cells after LCMV infection. These results emphasize the essential role for CD4 T-lymphocyte help and IFN-γ in achieving CTL activity necessary for clearing a persistent LCMV infection and point to an expanded role for B cells in the development and maintenance of CD4 and CD8 T-cell functions.
MATERIALS AND METHODS
Mice.
B-cell-deficient μMT/μMT H-2b (B−/−) mice, originally created by targeted disruption of the membrane exon of the immunoglobulin μ-chain (28) and back-crossed for eight generations to the C57BL/6J background, were purchased from the Jackson Laboratory, Bar Harbor, Maine. B-cell-competent C57BL/6J H-2b (B+/+) were obtained from the Rodent Breeding Colony at the Scripps Research Institute, La Jolla, Calif. All mice were bred and maintained under specific-pathogen-free conditions.
Virus, virus quantitation, and routes of infection.
The virus used was LCMV Armstrong (ARM) clone 53b, whose origin, sequence, biological properties, growth on BHK cells, and plaquing on Vero cells have been detailed elsewhere (11, 18, 34, 43). Plaque assays on Vero cells were also used to quantitate virus-neutralizing antibody in LCMV immune serum. Serial dilutions of mouse sera were incubated for 30 min with 150 PFU of LCMV ARM at 37°C. Control serum was guinea pig anti-LCMV serum containing neutralizing activity at dilutions of >1:500. For acute infections, 8- to 12-week-old mice were inoculated intraperitoneally (i.p.) with 105 PFU of LCMV ARM in a volume of 0.2 ml. Persistently infected mice were generated by intracranial inoculation of newborns with 5 × 103 PFU of LCMV ARM or were the congenitally infected offspring from persistently infected mice (14, 33).
Cytotoxicity assays.
LCMV-specific CTL activity in spleens harvested 7 days after inoculation with 105 PFU of LCMV ARM i.p. was assessed in a standard 4- to 5-h 51Cr release assay on LCMV-infected and uninfected, MHC-matched (MC57 [H-2b]) and mismatched (BALB/c17 [H-2d]) target cells (34, 56). Effector-to-target cell (E:T) ratios were 50:1, 25:1, and 12.5:1. To determine the affinity of CD8 CTL for major LCMV epitopes, uninfected MC57 cells were coated with graded dilutions of the LCMV NP peptide FQPQNGQFI (amino acids [aa] 396 to 404) as well as GP1 KAVYNFATC (aa 33 to 41) or GP2 SGVENPGGYCL (aa 276 to 286) peptides immediately before adding effector cells at an E:T ratio of 50:1.
For determination of LCMV-specific CTL precursor (pCTL) frequency 60 days after infection, spleen cells from immunized mice were serial diluted and cultured in 96-well flat-bottom plates (24 wells per dilution; highest dilution, 64,000 cells per well) with LCMV-infected and irradiated (2,000 rads) macrophages as well as irradiated spleen cells. After 7 days, cells from each well were split and tested on LCMV-infected and uninfected MC57 targets in a 4- to 5-h 51Cr release assay. pCTL frequencies were assessed by plotting the fraction of negative cultures on a semilogarithmic scale against the number of splenocytes per culture. pCTL frequencies were defined by the slope of the linear regression among at least three separate data points (30, 54).
Adoptive transfers.
Spleens of immunized mice were harvested 50 to 70 days after infection and prepared as single-cell suspensions by pressing through a fine steel mesh, addition of 0.83% NH4Cl to lyse erythrocytes, and passage through a 70-μm-pore-size nylon filter to remove residual tissues and cell aggregates. In some cases, the cell preparations were depleted or enriched as described below. Transfer populations (cell counts are listed in the figure legends) were resuspended in phosphate-buffered saline (PBS) at various concentrations not exceeding 0.3 ml of transfer volume and were injected i.p. into persistently infected recipients irradiated 1 day before adoptive transfer (300 rads). To ensure histocompatibility between B+/+ and B−/− mice, naive splenocytes from B+/+ or B−/− mice were labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, Oreg.) and adoptively transferred into naive, irradiated B+/+ or B−/− mice. At 3 and 7 days after transfer, peripheral blood lymphocytes (PBL), spleens, and sublingual, axillary, mesenteric, and inguinal lymph nodes were harvested and analyzed by flow cytometry for the presence of CFSE-positive cells. In addition, proliferation of transferred cells was assessed by the loss of fluorescence intensity.
In vitro depletion and enrichment.
Transfer populations were depleted or enriched immediately before adoptive transfers by using antibody-coated magnetic beads according to protocols provided by the manufacturers (Dynal, Lake Success, N.Y., and StemCell Technology, Vancouver, British Columbia, Canada). For B-cell depletion, anti-B220 antibody coupled to magnetic beads (DYNABEADS Mouse pan B [B220]; Dynal) was incubated for 20 min at 4°C with splenocytes at a bead-to-target-cell ratio of 8:1 and a concentration of 107 beads per ml. Samples were gently rotated during incubation to avoid sedimentation of magnetic beads. For separation, samples were placed into a magnet stand, and the B-cell-depleted supernatant was removed. The cells were then washed, resuspended in PBS, and analyzed by flow cytometry. Fewer than 0.2% of these cells were positive for B220. For B-cell enrichment, macrophages and NK cells were partially removed by 2-h adherence; subsequently, incubation with magnetic beads coupled to Thy 1.2 antibody (DYNABEADS Mouse pan T [Thy 1.2]; Dynal) and magnetic separation were used to remove T cells. B-cell-enriched fractions contained <1.5% CD4 T cells and <0.4% CD8 T cells as determined by flow cytometric analysis. For CD4 enrichment, an antibody cocktail containing antibodies against CD11b, CD45R, CD8, myeloid differentiation antigen Gr-1, and TER 119 (StemCell Technology) was used. After 15 min of incubation of 5 × 107 splenocytes/ml with 100 μl of antibody cocktail per ml of cell suspension in the presence of 1% normal rat serum and 2% fetal calf serum (FCS) in Hanks balanced salt solution (HBSS), cells were washed, resuspended in PBS, and incubated at recommended concentrations with an anti-biotin tetramer and, subsequently, with magnetic colloid. The cells were then passed through a column placed in a magnet stand to remove non-CD4 T cells. The purity of enriched CD4 cells was >95% with <0.05% CD8 T cells as determined by fluorescence-activated cell sorter (FACS) analysis.
B-cell helper assays.
Human gamma globulin (HGG) was purified from Cohn fraction II of human plasma (Hyland laboratories, Glendale, Calif.) (35). 2,4,6-Trinitrobenzenesulfonic acid (TNP; J. T. Baker, Philipsburgh, N.J.) was conjugated to sheep erythrocytes (SRBC) according to Rittenberg and Pratt (41) and to HGG by the method of Eisen (19). Keyhole limpet hemocyanin (KLH) was obtained from Calbiochem, La Jolla, Calif. Mice were injected subcutaneously with 100 mg of HGG in complete Freund’s adjuvant (CFA; Sigma Chemical Co., St. Louis, Mo.). For primed T cells, single-cell suspensions from the draining lymph nodes were used 8 to 12 days after injection and enriched as described elsewhere (42). For primed B cells, mice were first injected i.p. with TNP-KLH. Splenic B cells were used 8 to 10 days after priming. Single-cell suspensions of enriched B cells were obtained following treatment of spleen cells (twice) with anti-Thy and rabbit complement. B-cell helper assays were performed as described elsewhere (35). Briefly, TNP-KLH-primed B cells were cultured with HGG-primed T cells in the presence of 10 ng of TNP-HGG. After 7 days, the cells were harvested and used in a hemolytic plaque assay (21) with TNP-coated SRBC targets.
ELISAs for LCMV-specific antibodies and cytokines.
LCMV-specific total immunoglobulin G (IgG) in LCMV-immunized mice was determined by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (10) with a peroxidase-conjugated secondary antibody (goat anti mouse IgG; no. 55550; Cappel, Durham, N.C.) (dilution, 1:50,000). Endpoint titers of virus-binding serum antibodies were determined as the last serum dilution giving an absorbance of more than 3 standard deviations above the mean of 10 negative control wells. For quantitation of cytokine production after antigen-specific stimulation in vitro, spleens were harvested 8, 30, and 60 days after acute infection with LCMV, and 6 × 106 splenocytes were cultured in the presence of LCMV-infected, irradiated macrophages. Supernatant was collected 2 days later and stored at −20°C for analysis in a sandwich ELISA (all antibodies from PharMingen, La Jolla, Calif.). Then, 96-well plates were precoated overnight at 4°C with 2 μg of capture antibodies against IFN-γ, IL-2, and IL-6 (18181D, 18161D, and 18071D) per ml. After washing and blocking with 10% FCS in PBS, serial dilutions of supernatants and recombinant cytokine standards (IFN-γ, 19301T; IL-2, 19211T; and IL-6, 19251V) were incubated for 2 to 4 h at room temperature. Washing and a 1-h incubation with 1 μg of matched biotinylated detection antibodies (IFN-γ, 18112D; IL-2, 18172D; and IL-6, 18082D) per ml were followed by a 30-min incubation with a streptavidin-peroxidase conjugate (1:1,000) (Boehringer Mannheim, Indianapolis, Ind.). For the color reaction, H2O2-activated ABTS (2,2′-azino-di-[3-ethylbenzthiazolin-6-sulfonic acid]; Sigma) solution in 0.1 M citric acid (pH 4.35) was added. The plates were read at 405 nm with an EL-800 microplate reader (Biotek, Winooski, Vt.) with DeltaSoft 3 (BioMetallics, Princeton, N.J.) software.
FACS analyses.
Staining of cell surface antigens was performed in 96-well V-bottom microtiter plates at 4°C. Antibodies (all antibodies were from PharMingen, La Jolla, Calif., unless otherwise noted) were purified rat anti-mouse monoclonal antibodies against CD4 and CD8 (01061D and 01041D) and a fluorescein isothiocyanate-conjugated F(ab′)2 fragment (goat anti-rat 112-096-072; Jackson Immunoresearch Laboratories, West Grove, Pa.) or directly conjugated Cy-Chrome-CD4 (01068A) and APC-CD8 (01049A). For CD44 staining, a phycoerythrin (PE)-conjugated monoclonal antibody was used (01225A). For staining of intracellular cytokines, 5 × 105 to 7 × 105 splenocytes were incubated overnight in the presence of 2 μg of anti-CD28 (01671D) per ml and 5 μg of Brefeldin A (BFA; B7651; Sigma) per ml in 96-well tissue culture plates precoated with 10 μg of anti-CD3ɛ (01081D) per ml. BFA blocks protein transport into post-Golgi compartments, allowing cytokines to accumulate within cells. For antigen-specific stimulation, splenocytes were cultured for 2 weeks on LCMV-infected, irradiated macrophages. For the final 15 h, the cells were put on fresh macrophages in the presence of 5 μg of BFA per ml. Before staining, Fc receptors were blocked with 10 μg of an anti-CD16/anti-CD32 antibody cocktail (01241D) per ml. After surface staining with directly conjugated antibodies in PBS staining buffer (1% FCS and 0.1% sodium azide [wt/vol] in PBS), the cells were fixed and permeabilized by a 10-min incubation at room temperature in HBSS containing 4% paraformaldehyde (P6148; Sigma), 0.1% saponin (S7900; Sigma), and 1% HEPES. The cells were then washed twice with a PBS-saponin buffer (0.1% saponin in PBS-staining buffer), and intracellular cytokine staining was performed by a 30-min incubation of 5 × 105 to 7 × 105 cells with 0.12 μg of PE-conjugated anti-cytokine antibody (PE–IFN-γ, 18115A; PE–IL-2, 18005A; and PE–IL-6, 18075A) in 50 μl of PBS-saponin buffer at 4°C. Negative controls were stained with cytokine-specific PE-conjugated antibodies preincubated for 30 min at 4°C with an excess of recombinant cytokine. The cells were subsequently washed twice with PBS-saponin buffer, resuspended in PBS-staining buffer to reverse permeabilization, and analyzed on a FACSort or FACScalibur flow cytometer (Beckton Dickinson Co., San Jose, Calif.) with Cell Quest software (Beckton Dickinson). Semiquantitative analysis of cytokine production was performed by assessing mean fluorescence intensity over background (geometric mean of distribution of cytokine-positive cells divided by geometric mean of distribution of cytokine-negative cells).
RESULTS
After acute infection, B−/− and B+/+ mice display similar kinetics of virus clearance in vivo and equivalent CTL activity and CTL affinities to the major LCMV epitopes in vitro.
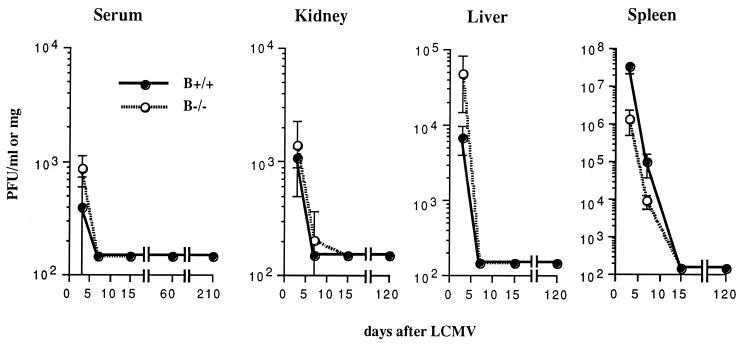
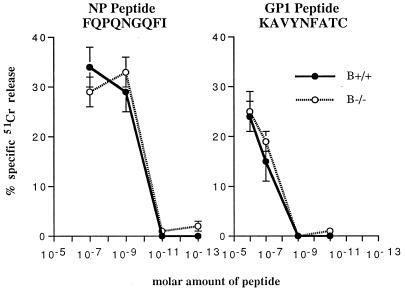
To analyze virus clearance after acute infection, amounts of infectious virus in sera and various tissues of B+/+ and B−/− mice at 3, 7, 15, 60, 120, and 210 days were measured after i.p. inoculation with 105 PFU of LCMV ARM. As shown in Fig. 1, both groups cleared virus from serum, liver, kidney, and spleen with similar kinetics. Viral clearance in B−/− mice remained effective over time, since no recurrence of virus was detected during a 7-month observation period. To evaluate the possible contribution of B cells in the induction of the primary immune response, the generation of virus-specific, MHC class I-restricted CTL activity of B−/− and B+/+ mice was assessed 7 days after LCMV immunization and was found to be equivalent in activity (data not shown). H-2b mice recognize three DbMHC class I-restricted peptides from LCMV, i.e., NP (aa 396 to 404), GP1 (aa 33 to 41), and GP2 (aa 276 to 286). B+/+ and B−/− mice recognized the same H-2b-restricted LCMV CTL epitopes and had similar affinities, as determined by specific lysis of uninfected, MHC-matched targets coated with serial dilutions of NP, GP1, and GP2 (Fig. 2 [data for GP2 not shown]). In the presence of equal numbers of B+/+ or B−/− effectors, half-maximal lysis of target cells was observed at molar concentrations of 10−10 for NP and 5 × 10−7 for GP1.
FIG. 1.
B−/− mice control acute infection with LCMV. Mice (6 to 8 weeks old) were inoculated i.p. with 105 PFU of LCMV ARM, and virus titers were determined 3, 7, 15, 60, 120, and 210 days later in a standard plaque assay on Vero cells. The detection level was 200 PFU. Data are averages ± 1 SE for three B+/+ and three B−/− mice.
FIG. 2.
B−/− and B+/+ CTL display similar CTL affinities to dominant LCMV epitopes. CTL assays were performed 7 days after i.p. infection of mice with 105 PFU of LCMV ARM as described in Materials and Methods. Briefly, splenic lymphocyte effectors were used at an E:T ratio of 50:1; targets were MC57 H-2b mouse fibroblasts coated immediately before addition of effectors with LCMV NP peptide FQPQNGQFI (aa 396 to 404) or GP1 peptide KAVYNFATC (aa 33 to 41) at dilutions ranging from 10−6 to 10−13. Results were obtained in a 5- to 6-h 51Cr release assay; percent killing of uncoated targets was subtracted from specific killing. Values are means ± 1 SE for three to four mice per each group.
Adoptively transferred LCMV memory splenocytes from B−/− mice cannot clear a persistent infection despite generating pCTL at frequencies similar to those of B+/+ mice.
Prior to adoptive transfer experiments with LCMV-primed memory cells, histocompatibility between B+/+ and B−/− mice was confirmed by use of CFSE-labeled cell transfers as described in Materials and Methods (31). Similar amounts of CFSE-positive B−/− splenocytes were found in all organs harvested from both B−/− and B+/+ recipients 3 and 7 days after transfer, excluding the possibility of a host versus graft disease. In vivo proliferation of transferred cells (graft versus host disease) is accompanied by loss of CFSE fluorescence intensity and was not observed in B−/− or B+/+ recipients (data not shown). We then determined the ability of memory cells from B−/− mice to clear a persistent infection in B+/+ mice. To ensure the transfer of equal amounts of LCMV-specific pCTL, precursor frequencies were assessed by limiting dilution analysis (30, 54). During the maintenance phase, i.e., beyond day 45 after infection, precursor frequencies of B+/+ and B−/− mice were stable and comparable, i.e., 1/200 ± 36 pCTL in B+/+ mice and 1/163 ± 14 in B−/− mice (means ± 1 standard errors [SE], three mice per group [Fig. 3A]). However, the absolute number of pCTL was lower in B−/− spleens due to the reduced number of lymphoid cells in the absence of B cells. Adoptive transfer of 3 × 107 memory splenocytes from B+/+ mice into persistently infected B+/+ recipients resulted in virus clearance within 2 to 3 weeks, as expected (1, 33). In contrast, adoptive transfer of as many as 108 B−/− memory cells failed to clear virus in B+/+ recipients (Fig. 3B). In addition, transfer of B−/− as well as B+/+ memory cells into persistently infected B−/− recipients also failed to clear virus (Fig. 3C). Since the transferred B+/+ cells were in principle capable of clearing the virus, failure of clearance indicates a requirement for B cells and/or antibody in the persistently infected recipient.
FIG. 3.
B−/− memory splenocytes fail to clear virus from persistently infected hosts. (A) B+/+ and B−/− mice show similar pCTL frequencies 60 days after acute infection with LCMV; three individual mice of each group, as well as pooled splenocytes for adoptive transfers, which yielded similar results (data not shown), were analyzed. (B) Adoptive transfer of B−/− memory splenocytes fails to clear virus from sera of persistently infected B+/+ recipients. Transfers of as many as 108 B−/− splenocyte populations were conducted to ensure that a sufficient number of CTL precursors was contained in the transfer population. Control mice received 3 × 107 B+/+ memory splenocytes. Experimental groups consisted of two to four mice. (C) Transfer of B−/− or B+/+ memory splenocytes into persistently infected B−/− recipients failed to clear virus from recipients. Experimental groups consisted of three to nine mice. Data are means ± 1 SE.
B cells must be present during generation of virus-specific T cells capable of clearing a persistent LCMV infection but are not required in the transfer population for virus clearance.
The failure to clear virus after adoptive transfer of B−/− memory cells could be due to several factors. To distinguish whether (i) B cells are required as APC (2, 20, 22, 38, 49, 58), or (ii) LCMV-specific antibody plays a protective role (4, 37, 57) or, alternatively, whether (iii) B cells are mandatory to generate effector T cells capable of clearing a persistent infection, we supplemented B−/− memory cells with LCMV-primed B cells isolated from B+/+ mice. Adoptive transfer of such B-cell-supplemented B−/− populations into persistently infected recipients did not lead to virus clearance (Fig. 4A). Similarly, transfer of B−/− memory cells in conjunction with immune sera derived from B+/+ mice 50 to 70 days after LCMV immunization and containing high titers of LCMV-specific antibody (ELISA anti-LCMV IgG titer, 1:43,740) but little to no neutralizing antibody had no effect on virus titers in the recipient (Fig. 4A). In contrast, transfer of splenocyte populations educated in a normal microenvironment (B+/+ mice) but depleted of B cells prior to adoptive transfer (containing <0.2% cells staining positive for B-cell marker B220) led to rapid and long-lasting virus clearance in persistently infected recipients (Fig. 4B). These results indicate that B cells are not required in the transfer population and are not needed to maintain CD4 T-cell help to support sufficient CTL activity. However, since B cells or B-cell products such as specific antibody cannot establish virus clearance after cotransfer with B−/− memory cells, this suggests that generation of memory cells known to participate in terminating a persistent infection, i.e., CD4 and CD8 T cells, may be affected in a B-cell-deprived microenvironment.
FIG. 4.
Virus persists after transfer of B−/− memory splenocytes combined with LCMV-primed B cells or LCMV-immune serum but not B-cell-depleted B+/+ memory splenocytes. (A) B−/− memory splenocytes reconstituted with either LCMV-primed B cells or LCMV-immune serum did not clear virus. LCMV-immune sera were obtained from B+/+ splenocyte donors 60 days after acute infection, and 0.2 ml was given i.p. on days 0, 5, and 11 after adoptive transfers. LCMV-specific sera contained an ELISA anti-LCMV IgG titer of 1:43,740 but little to no neutralizing antibody (data not shown). Experimental groups consisted of four mice. Controls included persistently infected recipients receiving LCMV-immune serum only, as well as B+/+ populations. (B) Adoptive transfer of 3 × 107 B-cell-depleted B+/+ memory splenocytes led to long-term virus clearance from serum in persistently infected B+/+ recipients. Three to four mice were analyzed at each time point. Data are means ± 1 SE.
T cells of B−/− mice provide less help to B cells in vitro.
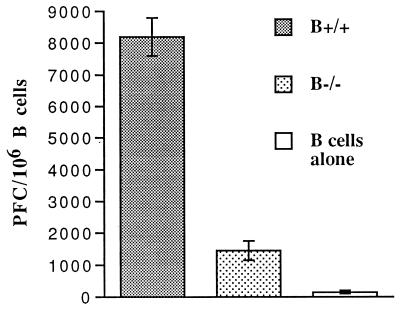
Whereas CD4 T cells are not required for the control of an acute LCMV ARM infection, their presence is critical to maintenance of CTL activity and successful resolution of a chronic LCMV infection (5, 16, 29, 37, 48). To evaluate the general ability of CD4 T cells in B−/− mice to provide help, B cells primed with TNP-KLH and T cells primed with HGG were cocultured for 7 days with TNP-HGG, after which the B-cell antibody response to TNP-coated SRBC was examined. B cells cocultured with primed, normal CD4 T cells from B+/+ mice mounted a vigorous response to TNP (Fig. 5). In comparison, the plaque-forming colony (PFC) response of B cells cocultured with primed B−/− T cells was markedly reduced, demonstrating that B−/− CD4 T cells are deficient in providing help to hapten-specific B cells.
FIG. 5.
In vitro CD4 helper activity from B−/− and B+/+ mice. CD4 T cells from B−/− mice exhibit decreased in vitro helper activity. Ten days after immunization with 100 μg of HGG in CFA, CD4 T cells were cultured with TNP-KLH-primed B cells and 10 ng of TNP-HGG. Anti-TNP PFC with TNP-SRBC targets were measured on day 7 of culture.
Reconstitution of B−/− memory cells with LCMV-primed CD4 T cells from B+/+ mice temporarily decreases virus titers.
Hypothesizing that the CD4 T-helper defect in B−/− mice may affect the activity of CD4 T cells in adoptive transfer populations, we determined whether a supplementation of B−/− memory splenocytes with LCMV-primed CD4 T cells from B+/+ mice could overcome failure of virus clearance. CD4 T cells were enriched by depletion of non-CD4 T cells with a cocktail of antibodies coated to magnetic beads. The resulting CD4 T-cell fractions were more than 95% pure with less than 0.05% CD8 T cells. Subsequent transfer of B−/− memory cells combined with LCMV-primed CD4 T cells from B+/+ mice decreased virus titers by about 1.5 log units (Fig. 6). Reconstitution with LCMV nonimmune B+/+ CD4 T cells reduced the viral load by less than 0.5 log units, a reduction similar to that in a control animal that received LCMV-primed CD4 memory cells alone. By day 20 after adoptive transfer, however, virus titers in recipients were again elevated, and transfer boosts with purified LCMV-primed B+/+ CD4 T cells did not further reduce virus titers (data not shown).
FIG. 6.
Reconstitution of B−/− memory splenocytes with LCMV-primed CD4 cells temporarily decreased virus titers by 1.5 log units. Reconstitution with naive B+/+ CD4 cells showed a less-pronounced effect. Four to five mice were analyzed per group; one control recipient received only LCMV-primed CD4 cells.
CD8 T cells from B−/− mice show reduced IFN-γ and IL-2 production after acute infection with LCMV.
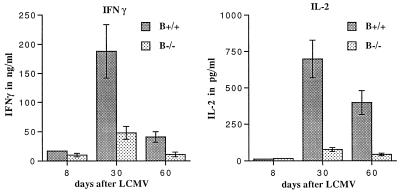
The inability to clear virus completely after transfer of B+/+ CD4 T-cell-supplemented B−/− memory cells suggested the possibility of additional T-cell defects. Analysis of the activation profile of CD4 and CD8 T cells as determined by CD44hi expression revealed similar patterns between B+/+ and B−/− mice (data not shown). As reported earlier, cytokines, in particular IFN-γ and tumor necrosis factor alpha (TNF-α), appear to play a critical role in virus clearance after adoptive transfer (24, 48). Analysis of cytokine profiles after acute LCMV infection of B+/+ and B−/− mice by ELISA revealed differences in IFN-γ, IL-2, and IL-6 production. After antigen-specific stimulation, B+/+ splenocytes produced significantly more IFN-γ than B−/− splenocytes, with a fourfold difference observed 30 and 60 days after infection. IL-2 was reduced by ninefold at the same time points in B−/− mice (Fig. 7). IL-6 production was consistently more pronounced in B−/− mice only 8 days after infection. At later time points, increased IL-6 production by B−/− splenocytes was not always observed. Other cytokines, such as TNF-α, IL-4, IL-10, and IL-12, were not detectable under these assay conditions. Control experiments with macrophages harvested from B−/− mice as APC in the stimulation culture yielded similar results. To phenotype the cytokine-producing cells, FACS analyses were conducted on cells stained for CD4, CD8, IFN-γ and IL-2 after polyclonal stimulation with anti-CD3 and anti-CD28 (Table 1). At 30 and 60 days after LCMV infection, B−/− mice had significantly fewer IL-2-producing CD8 T cells but similar amounts of IL-2-producing CD4 T cells compared to B+/+ mice. While the majority of IFN-γ-producing cells in both B+/+ and B−/− mice were CD8 T cells, B−/− mice had consistently fewer CD8 as well as CD4 T cells producing IFN-γ 8 and 30 days after infection. By day 60, similar percentages of IFN-γ-producing CD8 and CD4 T cells were found in B+/+ and B−/− mice. After culture on LCMV-infected macrophages (antigen-specific stimulation), the fractions of IFN-γ+ CD8 T cells per total CD8 T cells were comparable between B+/+ and B−/− mice at all time points. However, 30 and 60 days after infection, CD8 T cells from B−/− mice produced less IFN-γ, as determined by reduced mean fluorescence intensity. Antigen-specific stimulation did not generate enough IL-2 to be detectable by FACS analysis under these assay conditions.
FIG. 7.
Differential cytokine production by B+/+ and B−/− splenocytes after antigen-specific stimulation in vitro. Supernatant of 6 × 106 splenocytes cultured for 2 days with LCMV-infected and irradiated macrophages was analyzed by a sandwich ELISA. Splenocytes from uninfected B+/+ and B−/− mice did not produce detectable levels of IFN-γ or IL-2. B+/+ splenocytes produced significantly more IFN-γ at all time points after infection with LCMV (P < 0.05). At 30 and 60 days after acute LCMV infection, B+/+ splenocytes also produced more IL-2 (P < 0.01).
TABLE 1.
Cytokine production by CD4 and CD8 T cells after polyclonal and antigen-specific stimulationa
| Mouse genotype | Days after LCMV | Value after polyclonal activation
|
Value after antigen-specific activation for CD8 T cells
|
||||
|---|---|---|---|---|---|---|---|
| CD4 T cells
|
CD8 T cells
|
||||||
| % IL-2+ | % IFN-γ+ | % IL-2+ | % IFN-γ+ | % IFN-γ+ | MFI over backgroundb | ||
| B+/+ | 8 | 3.3 ± 0.1 | 9.6 ± 0.6 | 3.1 ± 0.8 | 45 ± 1 | 62 ± 11 | 20.3 ± 8.3 |
| B−/− | 8 | 3.2 ± 0.4 | 6.5 ± 0.8 | 3.3 ± 0.1 | 32 ± 6 | 73 ± 5 | 21.3 ± 6.5 |
| B+/+ | 30 | 3.0 ± 0.5 | 6.4 ± 1.9 | 7.0 ± 1.2 | 48 ± 1 | 76 ± 8 | 47.3 ± 1.9 |
| B−/− | 30 | 1.6 ± 0.2 | 2.8 ± 0.3 | 1.4 ± 0.4 | 41 ± 1 | 74 ± 6 | 30.3 ± 2.8 |
| B+/+ | 60 | 2.8 ± 0.3 | 2.6 ± 0.3 | 6.8 ± 1.0 | 32 ± 3 | 82 ± 3 | 57.1 ± 10.9 |
| B−/− | 60 | 3.1 ± 0.4 | 2.0 ± 1.0 | 2.6 ± 1.0 | 36 ± 7 | 78 ± 7 | 27.1 ± 6.9 |
Splenocytes were polyclonally or antigen specifically activated as described in Materials and Methods. At 30 and 60 days after LCMV infection, more B+/+ CD8 T cells but not CD4 T cells produced IL-2 (P < 0.04). More B+/+ CD4 and CD8 T cells produced IFN-γ 8 and 30 days after infection. At all time points, equal numbers of CD8 T cells in B+/+ and B−/− mice produced IFN-γ after antigen-specific stimulation. However, 30 and 60 days after infection, CD8 T cells from B−/− mice produced less IFN-γ, as determined by mean fluorescence intensity. Three mice per group were tested in two to three independent experiments.
MFI over background, geometric mean of fluorescence intensity of cytokine-positive cells divided by the geometric mean of fluorescence intensity of cytokine-negative cells.
DISCUSSION
We present here three important observations. First, B cells and antibodies are not required in the transfer population for clearing a persistent LCMV infection. Second, μMT/μMT B-cell-deficient mice, which are designated here as B−/− mice, display profoundly decreased CD4 T-helper function. Third, B−/− mice also exhibit reduced IL-2 and IFN-γ production by CD8 T cells after infection with LCMV.
We demonstrated that B−/− mice controlled acute LCMV infection with kinetics similar to those of C57BL/6J controls (B+/+ mice). As shown earlier in the B−/− model (3, 12), we confirmed that both B+/+ and B−/− mice generate virus-specific MHC class I-restricted CTL with similar activities, recognize the dominant LCMV NP and GP epitopes, and develop CD8+ T-cell memory as determined by similar pCTL frequencies. We also showed that B−/− and B+/+ CTL have similar affinities to the NP and GP epitopes. These results indicate that humoral immunity is not crucial for immune protection or pathology from acute LCMV infection and corroborate findings based on studies with mice depleted of B cells since birth (15, 26).
Adoptive transfer of memory cells obtained from the spleen after LCMV infection has been shown to lead to virus clearance in persistently infected recipients (1, 25, 33, 47, 50, 53). However, transfer of memory splenocytes from B−/− mice failed to terminate persistent LCMV infection, even after transfer of pCTL exceeding the required amounts of pCTL from B+/+ mice by more than threefold. This failure to reduce virus titers was not due to histoincompatibilities between B+/+ and B−/− mice and could not be remedied by supplementation of splenocytes from B−/− mice with LCMV-immune B cells or titers of LCMV-immune sera equivalent to those found in mice that successfully terminated the infection. Transfer of LCMV-immune sera alone had, as expected (52), no significant effect on lowering virus titers in persistently infected recipients. Interestingly, since transfer of B+/+ cells into B−/− mice also failed to clear virus (Fig. 3C), B cells in the recipient may be required for antigen presentation to CD4 T cells, which in turn are required in conjunction with CD8 T cells for virus clearance (37, 48). In agreement with this concept are other studies suggesting that bone marrow-derived host cells participate in the resolution of infection (25, 46).
We also showed that in vitro B-cell depletion of memory splenocytes from B+/+ mice did not affect the kinetics of successful virus clearance after adoptive transfers (Fig. 4B). This is in agreement with early T-cell enrichment and B-cell depletion studies suggesting no active role for B cells in virus clearance (51). Furthermore, studies with LCMV ARM and B cell-depleted transfer populations (25) support the idea that B cells in the transfer population play a minor, if any, role in clearing a persistent infection.
Our studies using LCMV ARM clearly document that neither B cells nor anti-LCMV antibodies play an important role in terminating an acute viral infection or clearing a persistent viral infection with adoptive immunotherapy. While different LCMV isolates generate comparable antibody levels by ELISA or complement fixation assay after immunization, titers of neutralizing antibodies differ quantitatively among various LCMV strains (13, 14, 37), and a role for neutralizing antibodies has been invoked after infection with LCMV WE (37). After immunization of B−/− mice with LCMV Traub (45) or WE (37), initial clearance was followed by dose-dependent (37) virus recrudescence 90 to 200 days later. The basis for the return of virus may also be related to immunosuppression induced by these viruses (7, 8, 23, 46). By contrast, in our study after infection with LCMV ARM, no return of virus occurred over a 210-day observation period. Our results that B-cell-depleted memory cells are capable of virus clearance from persistently infected recipients, while in agreement with earlier studies (25, 51), are in contrast to the recent report that B cells are required for clearing a persistent infection with LCMV ARM (37). Whereas we routinely observe virus clearance after 2 to 3 weeks in positive controls, Planz et al. (37) reported clearance of virus after adoptive transfer with marked delays (8 to 9 weeks). The reason for this discrepancy is not clear but may be related to differences in the virus strain or dilution used or in the route of administration.
The requirement for both CD8 and CD4 T cells to resolve a persistent LCMV infection by adoptive immunotherapy has been demonstrated by failure to abolish the persistent infection after transfer of memory splenocytes from CD4-deficient mice (48) or CD4 T-cell-depleted B+/+ mice (37). Although CD4 T cells of B−/− mice generate a normal antigen-specific proliferative response following in vitro stimulation (36), we have demonstrated here that they provide only minimal in vitro T-cell help to B cells (Fig. 5). This marked decrease in T-cell help in B−/− mice suggests that their subsequent response to recall antigen may also be compromised. Restoring of CD4 T-cell help in transfer populations by cotransfer of B−/− memory cells and LCMV-primed B+/+ CD4 T cells only temporarily decreased virus titers in persistently infected recipients (by approximately 95%), suggesting the possibility of additional T-cell defects.
The absence of immunopathology after adoptive transfers clearing virus from infected cells in the central nervous system (27, 33, 47) suggested the participation of factors other than CTL-mediated lysis of virus-infected cells. The importance of IFN-γ in controlling persistent LCMV infection was documented by failure of virus clearance, using mice with disrupted IFN-γ (48) or IFN-γ receptor genes (37). Our results extend these observations by finding that after antigen-specific stimulation in vitro, LCMV-immune B−/− splenocytes that fail to clear virus after adoptive transfer produce less IFN-γ. Control experiments conducted with B−/− and B+/+ offspring of F1 crosses between the original B+/+ and B−/− mice yielded similar results.
Phenotypic analysis of cytokine-producing cells by flow cytometry revealed CD8 T cells as the main source for IFN-γ. However, CD4 T cells produce more IFN-γ than on a per-cell basis (6). Depending on the time point after infection, B−/− mice had fewer IFN-γ-producing CD8 and CD4 T cells and/or CD8 T cells producing less IFN-γ. Furthermore, fewer CD8 T cells of B−/− mice produced IL-2, whereas comparable numbers of CD4 T cells produced IL-2 in B+/+ and B−/− mice. Interestingly, the absence of endogenous IL-2 has been associated with decreased IFN-γ production following LCMV infection (17). It is possible that the inability of B−/− memory splenocytes to terminate a persistent virus infection may also have resulted from decreased IL-2 as well as IFN-γ production by CD8 T cells in conjunction with the observed CD4 helper defect.
In conclusion, our results demonstrate that B cells participate in the generation and possibly maintenance of fully competent CD4 and CD8 T cells. B cells may have direct effects on CD8 T cells or may act indirectly via CD4 T cells. These findings have implications for interpreting some of the more than 30 studies using the B−/− (μMT/μMT) mouse model published in the past 2 years alone and, more importantly, for our understanding of intercellular communication between cells of the immune system as well as therapeutic applications such as adoptive immunotherapy and vaccination.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI-09484 (M.B.A.O.), NIH grant AI-11576 (W.O.W.), and grants R-29 DK 51091, AI41439, and JDF-CDA 02/03 (M.G.v.H.). It was also supported by NIH training grant AG-00080 and by grants from the Studienstiftung des Deutschen Volkes, Ludwig-Maximilians-Universität (D.H.), and Deutsche Forschungsgemeinschaft BE 1702/1-1 (D.P.B.).
REFERENCES
- 1.Ahmed R, Jamieson B D, Porter D D. Immune therapy of a persistent and disseminated viral infection. Virology. 1987;61:3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amigorena S, Bonnerot C. Role of B-cell and Fc receptors in the selection of T-cell epitopes. Curr Opin Immunol. 1998;10:88–92. doi: 10.1016/s0952-7915(98)80037-x. [DOI] [PubMed] [Google Scholar]
- 3.Asano M S, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldridge, J. R., and M. J. Buchmeier. 1992. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J. Virol. 4252–4257. [DOI] [PMC free article] [PubMed]
- 5.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak T W, Zinkernagel R M. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, D. P., D. Homann, and M. B. A. Oldstone. 1998. Unpublished observations.
- 7.Borrow P, Cao W, de la Torre J C, Oldstone M B A. Correlating in vivo pathogenicity of different LCMV isolates with binding to cell receptor and amino acid sequences of the virion attachment protein GP-1. 1998. Unpublished data. [Google Scholar]
- 8.Borrow P, Evans C F, Oldstone M B A. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 10.Borrow P, Tishon A, Lee S, Xu J C, Grewal I S, Oldstone M B A, Flavell R A. CD40L-deficient mice show deficits in antiviral immunity and have impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow P, Tishon A, Oldstone M B A. Infection of lymphocytes by a virus that aborts cytotoxic T lymphocyte activity and establishes persistent infection. J Exp Med. 1991;174:203–212. doi: 10.1084/jem.174.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brundler M-A, Aichele P, Bachmann M, Kitamura D, Rajewsky K, Zinkernagel R M. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur J Immunol. 1996;26:2257–2262. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 13.Buchmeier M J, Oldstone M B A. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis infection. J Immunol. 1978;1210:1297–1304. [PubMed] [Google Scholar]
- 14.Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 15.Cerny A, Huegin A W, Sutter S, Bazin H, Hengartner H H, Zinkernagel R M. Immunity to lymphocytic choriomeningitis virus in B cell-depleted mice: evidence for B cell and antibody-independent protection by memory T cells. Eur J Immunol. 1986;16:913–917. doi: 10.1002/eji.1830160807. [DOI] [PubMed] [Google Scholar]
- 16.Christensen J P, Marker O, Thomsen A R. The role of CD4+ T cells in cell-mediated immunity to LCMV: studies in MHC class I and class II deficient mice. Scand J Immunol. 1994;40:373–382. doi: 10.1111/j.1365-3083.1994.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 17.Cousens L P, Orange J S, Biron C A. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–5699. [PubMed] [Google Scholar]
- 18.Dutko F J, Oldstone M B A. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983;64:1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- 19.Eisen H N. Preparation of purified anti-2-4-dinitrophenyl antibodies. In: Eisen H N, editor. Methods in medical research. Vol. 10. Chicago, Ill: Year Book Medical Publishers; 1964. p. 94. [PubMed] [Google Scholar]
- 20.Garside P, Ingulli E, Merica R R, Johnson J G, Noelle R J, Jenkins M K. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 21.Golub E S, Mishell R I, Weigle W O, Dutton R W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968;100:133–137. [PubMed] [Google Scholar]
- 22.Gray D, Sipemann K, Vanessen D, Poudrier J, Wykes M, Jainandunsing S, Bergthorsdottir S, Dullforce P. B-T lymphocyte interactions in the generation and survival of memory cells. Immunol Rev. 1996;150:45–61. doi: 10.1111/j.1600-065x.1996.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 23.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1267–1312. [Google Scholar]
- 24.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 25.Jamieson B D, Butler L D, Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. Virology. 1987;61:3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson E D, Monjan A A, Morse H C., III Lack of B-cell participation in acute lymphocyte choriomeningitis disease of the central nervous system. Cell Immunol. 1978;36:143–150. doi: 10.1016/0008-8749(78)90257-5. [DOI] [PubMed] [Google Scholar]
- 27.Joly E, Mucke L, Oldstone M B. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 29.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald H R, Cerottini J C, Ryser J-E, Maryanski J L, Taswell C, Widmer M, Brunner K T. Quantitation and cloning of CTL and their precursors. Immunol Rev. 1980;51:93–123. doi: 10.1111/j.1600-065x.1980.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 31.Oehen S, Brduscha-Riem K, Oxenius A, Odermatt B. A simple method for evaluating the rejection of grafted spleen cells by flow cytometry and tracing adoptively transferred cells by light microscopy. J Immunol Methods. 1997;207:33–42. doi: 10.1016/s0022-1759(97)00089-6. [DOI] [PubMed] [Google Scholar]
- 32.Oldstone M B A. HIV versus cytotoxic T lymphocytes—the war being lost. N Engl J Med. 1997;337:1306–1309. doi: 10.1056/NEJM199710303371811. [DOI] [PubMed] [Google Scholar]
- 33.Oldstone M B A, Blount P, Southern P J, Lampert P W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321:239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- 34.Oldstone M B A, Whitton J L, Lewicki H, Tishon A. Fine dissection of a nine amino acid glycoprotein epitope, a major determinant recognized by lymphocytic choriomeningitis virus-specific class I-restricted H-2b cytotoxic T lymphocytes. J Exp Med. 1988;168:559–570. doi: 10.1084/jem.168.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parks D E, Walker S M, Weigle W O. Bacterial lipopolysaccharide (endotoxin) interferes with the induction of tolerance and primes thymus-derived lymphocytes. J Immunol. 1981;126:938–942. [PubMed] [Google Scholar]
- 36.Phillips J A, Romball C G, Hobbs M V, Ernst D N, Shultz L, Weigle W O. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Planz O, Ehl S, Furrer E, Horvath E, Brundler M A, Hengartner H, Zinkernagel R M. A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferon in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 39.Rehermann B, Lau D, Hoofnagle J H, Chisari F V. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Invest. 1996;97:1655–1665. doi: 10.1172/JCI118592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 41.Rittenberg M B, Pratt K L. Anti-trinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particular immunogen. Proc Soc Exp Biol Med. 1969;132:575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- 42.Romball C G, Weigle W O. In vivo induction of tolerance in murine CD4+ cell subsets. J Exp Med. 1993;178:1637–1644. doi: 10.1084/jem.178.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvato M S, Shimomaye E, Southern P, Oldstone M B A. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, clone 13 (CTL−) Virology. 1988;164:517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- 44.Sprent J. Immunological memory. Curr Opin Immunol. 1997;9:371–379. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen A R, Johansen J, Marker O, Christensen J P. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 46.Thomsen A R, Volkert M. Studies on the role of mononuclear phagocytes in resistance to acute lymphocytic choriomeningitis virus infection. Scand J Immunol. 1983;18:271–277. doi: 10.1111/j.1365-3083.1983.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 47.Tishon A, Eddleston M, de la Torre J C, Oldstone M B. Cytotoxic T lymphocytes cleanse viral gene products from individually infected neurons and lymphocytes in mice persistently infected with lymphocytic choriomeningitis virus. Virology. 1993;197:463–467. doi: 10.1006/viro.1993.1613. [DOI] [PubMed] [Google Scholar]
- 48.Tishon A, Lewicki H, Rall G, von Herrath M, Oldstone M B A. An essential role for type 1 interferon-γ in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 49.Townsend S E, Goodnow C C. Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J Exp Med. 1998;187:1611–1621. doi: 10.1084/jem.187.10.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkert M. Studies in immunological tolerance to LCM virus. 2. Treatment of virus carrier mice by adoptive immunization. Acta Pathol Microbiol Scand. 1963;57:465–487. [PubMed] [Google Scholar]
- 51.Volkert M, Bro-Jorgensen K, Marker O, Rubin B, Trier L. The activity of T and B lymphocytes in immunity and tolerance to the lymphocytic choriomeningits virus in mice. Immunology. 1975;29:455–464. [PMC free article] [PubMed] [Google Scholar]
- 52.Volkert M, Larsen J H. Studies in immunological tolerance to LCM virus. 6. Immunity conferred on tolerant mice by immune serum and by grafts of homologous lymphoid cells. Acta Pathol Microbiol Scand. 1965;63:172–180. doi: 10.1111/apm.1965.63.2.172. [DOI] [PubMed] [Google Scholar]
- 53.Volkert M, Marker O, Bro Jorgensen K. Two populations of T lymphocytes immune to the lymphocytic choriomeningitis virus. J Exp Med. 1974;139:1329–1343. doi: 10.1084/jem.139.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Herrath M G, Yokoyama M, Dockter J, Oldstone M B A, Whitton J L. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 56.Whitton J L, Southern P J, Oldstone M B A. Analysis of cytotoxic T lymphocyte responses to glycoproyein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988;162:321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- 57.Wright K E, Buchmeier M J. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J Virol. 1991;65:3001–3006. doi: 10.1128/jvi.65.6.3001-3006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zinkernagel R M. Immunology and immunity studied with viruses. Ciba Found Symp. 1997;204:105–125. doi: 10.1002/9780470515280.ch8. [DOI] [PubMed] [Google Scholar]