Abstract
Polyomavirus-like-particles (PLPs) are empty, non-replicative, non-infectious particles that represent a potent antigen-delivery system against malignant disease. Protective anti-tumour immunity can be induced under therapy conditions by subcutaneous (s.c.) treatment with particulate antigenic structures like chimerical polyomavirus-pentamers (PPs). These PPs displaying an immunodominant H-2Kb-restricted ovalbumin (OVA)257-264 epitope evoked nearly complete tumour remission in MO5 (B16-OVA) melanoma-bearing C57BL/6 mice by two s.c. applications in a weekly interval. The immunotherapeutic intervention started at day 4 after melanoma implant. Furthermore, 40% of melanoma-bearing mice vaccinated with heterologous PPs carrying a H-2Kb-restricted cytotoxic T lymphocyte (CTL) epitope derived from of tyrosinase-related protein 2 (TRP2) survived similar treatment conditions. However, a late immunotherapeutic onset at day 10 post melanoma inoculation revealed no significant differences between the therapeutic values (40–60% survival) of VP1-OVA252-270 and VP1-TRP2180-192 PPs, respectively. These experiments underlined the capacity of PPs to break T cell tolerance against a differentially expressed self-antigen. As a correlate for preventive and therapeutic immunity against MO5 melanoma the number of OVA257-264- or TRP2180-188-specific CD8 T cells were significantly increased within the splenocyte population of treated mice as measured by H-2Kb-OVA257-264-PE tetramer staining or appropriate ELISPOT assays, respectively. These results reveal that heterologous PLPs and even chimerical PPs represent highly efficient antigen carriers for inducing CTL responses underlining their potential as immunotherapeutics against cancer.
Keywords: CD8 T cell response, Pentamers, Virus-like-particles, T cell epitope, Melanoma therapy, TRP2 tumour antigen
Introduction
A strong antigen-specific cytotoxic T lymphocyte (CTL) response is considered to be critical for the treatment of cancer [31]. The induction CTL responses require the presentation of antigen-derived peptides associated with major histocompatibility complex (MHC) class I molecules on the surface of antigen presenting cells (APCs) to specific CD8 T cells. It is now well established that soluble exogenous antigens can also access the cytosol of APCs through an alternative pathway exclusive for macrophages and dendritic cells (DCs) [3, 17], although cell-associated antigens are more efficiently presented than soluble antigens [21]. For the generation of more efficacious CTL responses elicited by immunotherapeutic cancer vaccines, the appropriate antigen availability for MHC class I presentation has to be improved further. Several strategies have been developed for enhancing antigen delivery into the cytosol of APCs [17, 37]. The most important issue for novel CTL-stimulating vaccines is represented by safety concerns for replicating vectors capable of introducing antigens into MHC class I processing and presentation pathways. Therefore, the development of non-replicating antigen delivery systems is an important prerequisite in the design of new efficient CTL-priming cancer vaccines. In this regard, virus-like-particles (VLPs) receive growing attention due to their capability to elicit cell-mediated immunity (CMI) with emphasis on CD8 T cell responses in the absence of immunostimulatory substances [5–7, 13, 16, 29]. Therefore, we are currently developing a non-replicating antigen delivery system based on heterologous polyomavirus-like-particles (PLPs) formed (1) by the self-assembly of the viral VP1 coat protein displaying foreign T cell epitopes at its C-terminal end [6] or (2) by the encapsulation of full-length proteins into the VP1-capsoid shell [1]. We have already analysed the capacity of these non-replicative PLPs to prime in vivo CD8 T cell responses [6]. For this purpose, a protein fragment harbouring the immunodominant CD8 CTL epitope, residues 257-264 of ovalbumin (OVA), was genetically engineered to the C-terminal end of the VP1 protein resulting in the VP1-OVA252-270 entity [6]. Heterologously Escherichia coli-expressed VP1-OVA252-270 polyomavirus-like-pentamers (PPs) can be easily assembled in vitro under high salt conditions [6]. These recombinant PLPs were analysed for their capacity to elicit in vivo CD8 T cell responses and to protect mice against lethal thymoma (EG7, EL4-OVA) or melanoma (MO5, B16-OVA) challenge [6]. Two s.c. application of heterologous PLPs (VP1-OVA252-270) given in weekly intervals induced protective immunity against these respective OVA-expressing tumour cell lines. In contrast, one PLP-specific vaccine administration however failed to protect animals from lethal tumour challenge [6].
Data from Van Elsas et al. [32] demonstrated that therapeutic auto reactive CD8 T cell responses directed against the differentially expressed tyrosinase-related protein 2 (TRP2) can be effectively generated in melanoma-bearing mice and stressed the value of studying tumour immunity not only in preventive settings but also under therapeutic conditions. Importantly, both human and murine TRP2 proteins were recently identified as tumour antigens recognized by CD8 T cells [4, 34]. Specifically, the human HLA-A2-restricted TRP2180-188 peptide is identical to a murine peptide presented by H2-Kb molecules [25], thus representing an ideal human tumour antigen to be tested in animal models. Moreover, the use of TRP2-derived epitopes for preventive and therapeutic vaccination provides a mouse model that closely mimics human melanoma without introduction of xenogeneic or otherwise foreign antigen.
The present study demonstrates that particulate structures of chimerical PPs displaying a single OVA257-264-specific non-self epitope or a TRP2180-188-specific self-epitope for CD8 T cell induction have beneficial immunotherapeutic effects under early and late onset of treatment measures in a murine melanoma model.
Materials and methods
Cells
The C57BL/6-derived OVA-transfected B16 clone, MO5 [9], was generously provided by K.L. Rock and grown in RPMI 1640/10% FCS in the presence of 2 mg G418 and 40 μg hygromycin per ml. The H-2b-restricted MC57G fibrosarcoma cell line (ATCC number: CRL-2295) was cultured as recommended by the local distributor.
Construction of heterologous VP1 derivates
For the construction of the expression vector pET9a/VP1-TRP2180-192 the TRP2180-192 fragment (carrying the H2-Kb -restricted TRP2180-188-epitope SVYDFFVWL) was generated by hybridisation of two corresponding oligonucleotides each containing a 5×-SapI restriction site. This TRP2180-192 coding DNA-fragment was cloned into the SapI-sites of pET9a/VP1 (GenBank accession number for a murine polyomavirus VP1-sequence: M97647). The resulting construct, termed pET9a/VP1-TRP2180-192, codes for a fusion protein carrying the TRP2180-192 sequence at the C-terminus of the VP1 subunit. The construction of the pET9a/VP1-OVA252-270 was peviously described by Brinkman et al. [6].
Expression, purification, assembly and characterisation of heterologous PPs and PLPs
Recombinant E. coli BL21 microorganisms were grown in LB medium containing 70 μg/ml kanamycin at 37°C to a OD600 at 0.7 before the VP1-expression was induced by 1 mM isopropyl-β-D-thiogalactoside (IPTG). After subsequent incubation for 4 h, the microbes were harvested by centrifugation at 15,000g for 15 min at 4°C; the pellet was resuspended in QS-A buffer (20 mM Ethanolamine, 2 mM EDTA, 6 mM DTT, 5% glycerol, 50 mM NaCl pH 9.0) and lysed at 800 bar by using a gaulin LAB 1000 homogeniser. The suspension was centrifuged at 75,000g for 45 min at 4°C and VP1-TRP2180-192 was purified from the supernatant by an anion exchange column (Poros, Applied Biosystems, Germany) previously equilibrated with QS-A buffer. After elution of VP1- TRP2180-192 from the column by a linear gradient from 50 mM NaCl to 1 M NaCl, the target-protein VP1-TRP2180-192 was applied to a Q-Sepharose column (Amersham Biosciences, Germany) previously equilibrated with QS-A buffer. The VP1-TRP2180-192 was again eluted by a linear gradient of 50 mM NaCl to 1 M NaCl. In a final step the protein was applied to size exclusion chromatography (Superdex 200, Amersham Bioscience) equilibrated with KB1 buffer (50 mM Natriumphosphate, 2 mM EDTA, 6 mM DTT, 5% glycerol, pH 6.8) and the VP1-TRP2180-192 peak fraction was eluted. Homogeneity of the protein was verified by SDS-PAGE, photon correlation spectroscopy (PCS) and mass spectrometry. Protein concentration was determined by UV-Vis spectroscopy. The homogeneity of the protein was analysed by SDS-PAGE and mass spectrometry, and the concentration was determined by UV-Vis-spectroscopy. For each PLP-specific immunisation dose the endotoxin content ranged from 20 EU to 45 EU per mouse (2–4.5 ng lipopolysaccharide [LPS]) as determined by the limulus amebocyte lysate (LAL) assay. This endotoxin content did not significantly appear to influence antigen-specific CMI as VP1-PLPs per se failed to protect mice from lethal tumour challenge. Moreover, the impact of residual enodotoxin on vaccine efficacy of VP1-OVA252-270 PLPs was assessed as described in Results. The parental VP1 or VP1-OVA252-270 PLPs were assembled by adding KB2 buffer (10 mM Tris/HCl, 150 mM NaCl, 5% glycerol, 3 M (NH4)2SO4, pH 8.0) to a final ammonium sulphate concentration of 250 mM to generate PLPs. After incubation for 30 min at 25°C, the formed particles were oxidised by the addition of KB3 buffer (400 mM GSSG in KB2) to a final GSSG concentration of 7.2 mM. Finally the protein was dialysed overnight at 25°C against PBS (Biochrom KG, Germany) containing 0.7 mM CaCl2 to stabilise the respective capsoid preparation. The concentration was measured by UV-Vis spectroscopy and SEC and PCS determined the size of the capsoids. Similar procedures performed with VP1-TRP2180-192 PPs revealed that—in contrast to VP1-OVA252-270 pentamers—VP1-TRP2180-192 could not be assembled to PLPs. The TRP2180-192-fragment appears to impair the assembly of PLPs as PCS analysis failed to confirm expected size of capsoids (data not shown).
ELISA protocol
To give new insights into a beneficial vaccination schedule circumventing possible VP1-specific antibody interference, six C57BL/6 mice were s.c. immunised with a single dose of 100 μg VP1-OVA252-270. Another group of six C57BL/6 animals received two doses of 50 μg VP1-OVA252-270 in a weekly interval. The sera of these groups of mice was collected from the plexus orbitalis 1 or 2 weeks after the first immunisation for antibody detection by means of VP1-OVA252-270-specific ELISA. Thereto the wells of an ELISA-plate were each coated with 100 ng of VP1 PLPs or OVA252-270 peptide diluted in a volume of 50 μl PBS and incubated at 8°C overnight. In the next step, each of the wells were blocked with 300 μl 0.2% gelatine (in PBST) and incubated for 2 h at 37°C in a wet chamber. After that 50 μl of the appropriate blood serum were added to each well in various dilutions (1:2,500 to 1:100,000) and incubated for 1 h at 37°C. The following incubation with 50 μl of the rabbit anti-mouse immunoglobulin G (IgG) horseradish peroxidase-conjugated antibodies (a 1:1,000 dilution in blocking buffer) was performed for 45 min at 37°C. For the reaction with the substrate 50 μl of the substrate solution (ABTS) was added to each well and incubated at RT for 20–30 min before the reaction was stopped with 50 μl of 0.5 M oxalic acid. Between each step the plates were washed in an ELISA-washer with 300 μl PBST (PBS+0.05% Tween-20) per well. The samples were then analysed in an ELISA-reader at 490 nm wavelength.
MHC class I tetrameric complexes and determination of antigen-specific T cells
Three C57BL/6 mice were s.c. twice immunised with 50 μg VP1-OVA252-270 PLPs in a weekly interval. Similarly, mice that had been s.c. inoculated with a lethal dose of 105 MO5 melanoma cells were s.c. treated with two doses of 50 μg VP1-OVA252-270 PLPs given s.c. at day 4 and 11 or day 10 and 17 after melanoma implantation. Three days after the second VP1-OVA252-270 administration splenocyte populations from two different groups of treated and only immunised mice were isolated and pooled. Some samples including those from non-immunised mice were subsequently analysed by FACS and corresponding samples (107 splenocytes) were restimulated in vitro with 25 Uml−1 murine recombinant interleukin-2 and 4 μg ml−1 VP1-OVA252-270 capsoids in a 24-well plate for 3 days. Antigen-specific analysis was performed by staining the spleen cells with phycoerythrin (PE) labelled H2Kb-tetramers loaded with the OVA-octapeptide SIINFEKL (Proimmune). Cells were counterstained with anti-CD8α/FITC monoclonal antibodies (Ly-2; PharMingen) and analysed by using a FACSCalibur (Becton Dickinson). In addition, samples were stained with VIA-PROBE (Becton Dickinson) for dead cell exclusion. Within the CD8 T cell population the percentage of tetramer-positive cells was determined after gating on forward scatter and exclusion of VIA-PROBE positive cells using CELLQUEST software (Becton Dickinson).
ELISPOT analysis
The frequency of interferon-γ (IFNγ)-secreting T lymphocytes specific for the H2-Kb-epitope TRP2180-188 was determined by ELISPOT technique as described previously [6]. Briefly, 96-well nitro-cellulose plates (Millipore, Bedford, MA, USA) were coated with 5 μg/ml of the anti-mouse IFNγ-monoclonal antibody (mab) R4 (PharMingen) in 100 ml of carbonate buffer, pH 9.6. After overnight incubation at 4°C, PBS-washing and BSA-blocking, 5×104 splenocytes from naive VP1, VP1-TRP180-192 PPs immunised, VP1-TRP180-192 PPs early or late treated, or naive mice restimulated with or without 1 μg of the H-2Kb-restricted TRP2180-188 peptide for 3 days were added in 100 μl RPMI 1640/10% FCS per well. MC57G cells were coated with 1 μg TRP2180-188 peptide vice versa (peptides were purchased from Biosyntan, Berlin, Germany, and dissolved in DMSO/PBS) at 37°C for 1 h. Coated or uncoated 5×104 MC57G cells were added to splenocytes in 100 μl of RPMI 1640/10%FCS and after 20 h incubation at 37°C, 5% CO2 in the presence of 30 U/ml IL-2, plates were washed ten times with 0.05% Tween 20 in PBS (washing buffer). To detect IFNγ-spots, 0.25 μg/ml biotinylated anti-mouse IFNγ mab XMB1.2 (PharMingen) was added and incubated at 37°C for 2 h. Plates were washed ten times and incubated for 1 h at 37°C in the presence of 100 μl of a 1/20,000 dilution of alkaline phosphatase-coupled streptavidin (PharMingen). After five washes, spots of IFNγ-secreting cells were visualised by adding 50 μl of the ready-to-use substrate BCIP/NBT (Sigma, St. Louis, MO, USA). The reaction was stopped after 15 min at 37°C by several washes with H2O. After drying, spots were automatically scored with the Bioreader 2000 and subsequently analysed (Biosys, Frankfurt/Main, Germany).
Therapeutic anti-tumour treatment of mice
C57BL/6 mice were s.c. inoculated with 105 MO5 cells (i.e. tenfold the minimal tumorigenic dose of the B16-OVA melanoma) into the right flank. The first therapeutic immunisation was performed 4 days later by s.c. injection of 50 μg capsoids of VP1-OVA252-270 or wild-type VLP (early immunotherapy). In addition, vaccine entities consisting of 50 μg heterologous PPs, e.g., VP1-OVA252-270,VP1 or VP1-TRP2180-192 were also used in these therapeutic settings. A week later the second therapeutic dose of each appropriate vaccine, capsoids or PPs (50 μg) was administered. During a second series of therapy experiments groups of mice were twice vaccinated s.c. with PLPs of VP1-OVA252-270 in the presence of the adjuvant QuilA (100 μg per dose; Superfos, Copenhagen, Denmark). In a third series of therapeutic experiments, C57BL/6 mice anti-tumour treatment were performed at day 10 and 17 post MO5 inoculation at tumour sizes of 3 mm (the mean of the perpendicular diameters of the tumour; late immunotherapy). For addressing the immunostimulatory capacity of residual endotoxin in the capsoid preparations, five C57BL/6 mice per group were s.c. treated twice with parental VP1 PLPs in the presence of 50 μg OVA257-264 peptide, according to the early and late immunotherapy schedule. In addition, appropriate control groups (e.g. VP1-, VP1-OVA252-270-PLPs treated or naive) were used in these experiments. B16-derived tumours became usually palpable by day 8–14 after implant. Each therapeutic immunisation was s.c. given into the collateral flank [6]. The tumour take and size (formula tumour volume [mm3]=4r3 π/3) were monitored daily. Mice that became moribund were sacrificed. These criteria are in accordance with the German law for animal welfare.
Results
Construction of particulate structures carrying self and non-self epitopes for CD8 T cell induction
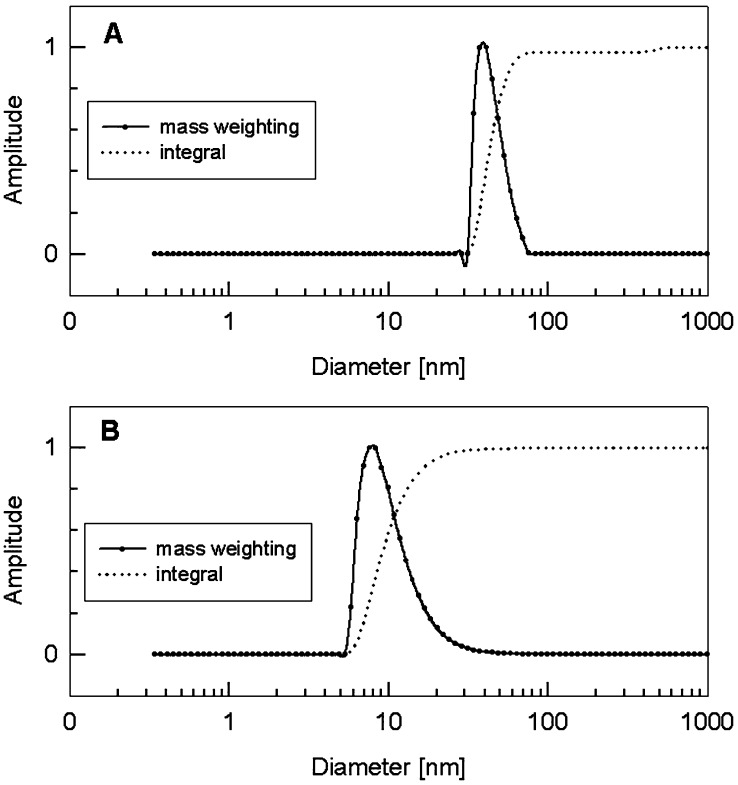
For the evaluation of the therapeutic value of particulate structures based on the polyomavirus VP1 major coat protein H-2Kb-restricted epitopes including neighbouring amino acid sequences were fused to the c-terminal end of VP1. Two different well-established CD8 T cell peptides OVA257-264 and TRP2180-188 were selected, originating from self or non-self antigens, respectively, in regard to the animal model. The VP1-OVA252-270 product, clearly shaped PLP at an average size of 45 nm, were described elsewhere [6]. Here, we also report on the construction of the VP1-TRP180-192 fusion protein and the subsequent purification steps resembling those of VP1-OVA252-270 capsoids. Unfortunately, VP1-TRP180-192 subunits failed to assemble to full-size capsoid-like structures as compared to VP1-OVA252-270 PLPs. However, PCS measurements clearly confirmed that VP1-TRP180-192 fusion proteins were spontaneously able to form PPs with about 9 nm in size (Fig. 1). We assume that the hydrophobic character of the TRP2 fusion partner could interfere with VP1-inherent ability for capsoid formation. This important VP1 property was not impaired in case of the OVA-specific fusion partner. Due to the encouraging findings of Öhlschlaeger et al. [24] reporting on CTL responses induced by human papillomavirus (HPV) type 16 L1-capsomers, we included VP1-TRP180-192 and VP1-OVA252-270 PPs into our further experimental design [24].
Fig. 1.
a, b Photon correlation spectroscopy of chimerical VP1 PPs and VP1 PLPs. a PCS profile of VP1-OVA252-270 PLPs and b PCS profile of VP1-TRP2180-192 PPs
Induction of humoral and cell-mediated immune responses against heterologous PLPs
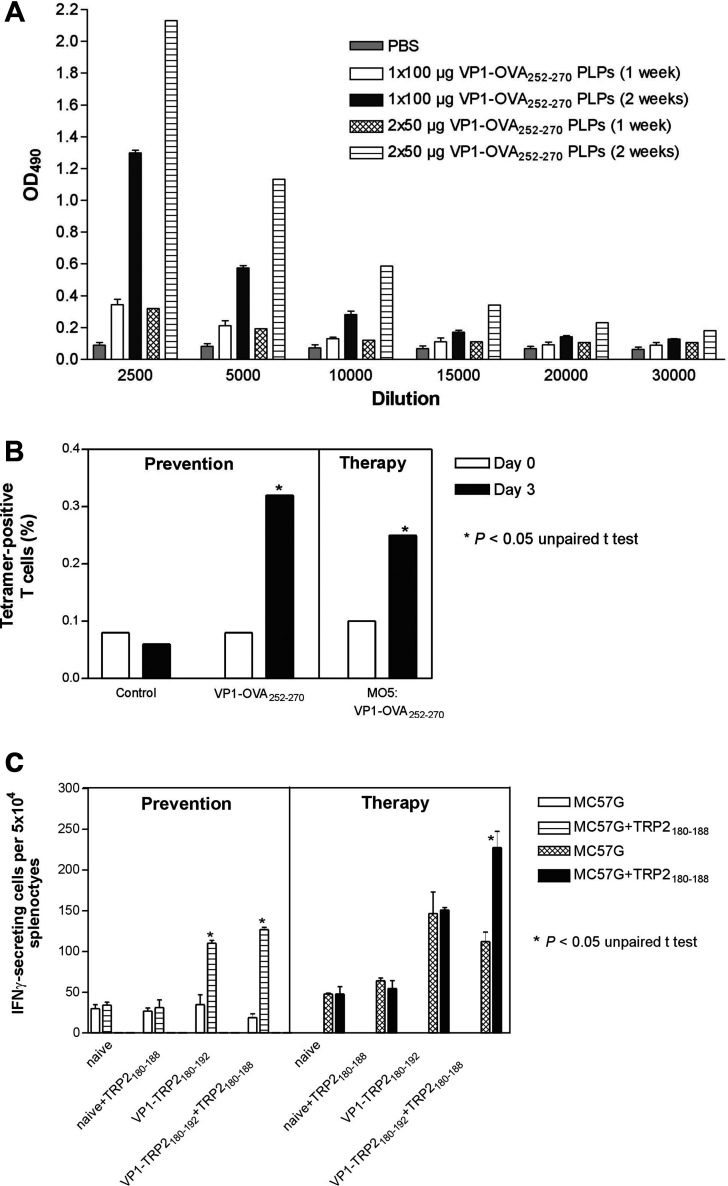
It was obvious that two therapeutic immunisations were required to achieve similar anti-tumour immunity when compared to the protection studies [6]. Here, we show that the weekly treatment schedule may be central for preventive and therapeutic efficacy of PLPs. C57BL/6 mice were s.c. vaccinated with a single dose of 100 μg VP1-OVA252-270 PLPs. A second group of mice were treated with two doses of 50 μg VP1-OVA252-270 PLPs given s.c. in weekly intervals. For both groups of immunised mice VP1 antibody endpoint titres were monitored 1 and 2 weeks post immunisation by means of ELISA (Fig. 2a). This experiment confirmed the beneficial effect of a weekly treatment interval as VP1 antibody titres were only slightly augmented after 1-week p.i. in comparison to the serum titres of non-immunised mice. Furthermore, no significant differences for VP1 antibody titres could be detected in sera of mice, which had received low (50 μg) and high (100 μg) PLPs single doses (Fig. 2a). In contrast, VP1 antibody titres were significantly increased at week 2 p.i., independent from single or two-dose schedule (Fig. 2a). In addition, OVA252-270-specific antibody responses could not be detected in VP1-OVA252-270 PLPs immunised mice by means of ELISA (data not shown).
Fig. 2.
a–c Humoral and cell-mediated immune responses induced by VP1-OVA252-270 PLPs or VP1-TRP2180-192PPs. a VP1-OVA252-270-specific antibody endpoint titres after single or double immunisation in weekly intervals. Three C57BL/6 have been s.c. immunised with 100 μg capsoids for the single dose schedule and with 50 μg VP1-OVA252-270 specific capsoids for each dose during the two vaccinations schedule. Sera were pooled and appropriately diluted for ELISA performance. b H2-Kb-OVA257-264-tetramer/anti-CD8 labelling of splenocytes after two s.c. immunisations of mice each with 50 μg VP1-OVA252-270 VLPs. The OVA257-264 -specific CD8 T cells within splenocyte population isolated 3 days after the second application could be significantly measured after in vitro restimulation with 4 μgml−1 VP1-OVA252-270 for 3 days (*P<0.05, unpaired t test). Antigen specific analysis was performed by staining the splenocytes with PE-labelled H-2Kb -tetramers loaded with the OVA257-264 peptide. Cells were counterstained with anti-CD8α/FITC monoclonal antibodies and analysed by using a FACSCalibur. Each experiment was repeated twice with similar results. c TRP2180-188-specific CD8 T cell responses were measured by IFNγ-ELISPOT as described in Materials and Methods. Five days before the restimulation of splenocytes in vitro (each sample with 1 μg TRP2180-188 peptides) three C57BL/6 mice were s.c. immunised twice with 50 μg VP1- or VP1-TRP2180-192 PPs in a weekly interval. In the therapeutic setting immunisation started at day 4 after s.c. implantation of 105 MO5 melanoma cells. The following H2-Kb -restricted CD8 T cell frequencies specific for the TRP2180-188 epitope were significantly different from controls (*P<0.05, unpaired t test, mean values of n=3± standard deviation SD). Each experiment was repeated twice with similar results
Naive and MO5-bearing C57BL/6 mice were s.c. immunised with two doses of 50 μg VP1-OVA252-270 or VP1-TRP2180-192 PLPs given in a weekly interval to address the induction of epitope-specific CD8 T cell responses. The latter group had been s.c. inoculated with 105 MO5 cells 4 days before treatment was started. Subsequently, 3 days after the second treatment 107 splenocytes of naive and the VP1-OVA252-270-treated group were pooled and restimulated in vitro with 4 μg VP1-OVA252-270 PLPs for additional 3 days. As demonstrated by H2-Kb-OVA tetramer and anti-CD8 co-staining, a significant proportion of OVA257-264-specific CD8 T cells evoked by VP1-OVA252-270 immunisation of mice could be detected during the preventive and early therapeutic settings (Fig. 2b). It should be noted that late immunotherapeutic intervention with VP1-OVA252-270 PLPs did correlate with comparable OVA257-264-specific CD8 T cell frequencies in MO5 melanoma-bearing mice (data not shown). In case of VP1-TRP2180-192-treated mice 107 splenocytes were pooled and restimulated in vitro with 1 μg TRP2180-188 peptide for 3 days. By means of TRP2180-188 peptide- and IFN-γ-specific ELISPOT analysis CD8 T cells were detected in both immunisation schedules, during preventive as well as early immunotherapeutic treatment (Fig. 2c). Similar TRP2180-188-specific CD8 T cell frequencies were assessed during late immunotherapy upon peptide restimulation (data not shown). In addition, it should be stressed that TRP2180-188 specific CD8 T cells could be monitored during prevention even without peptide restimulation for 3 days (Fig. 2c). These findings clearly demonstrate that heterologous PLPs elicit cell-mediated immunity against self and non-self CD8 T cell epitopes in the absence of an appropriate adjuvant and therefore fulfil a necessary requirement for an immunotherapeutic vaccine against cancer.
Possible contribution of residual endotoxin in capsoid preparations to therapeutic value
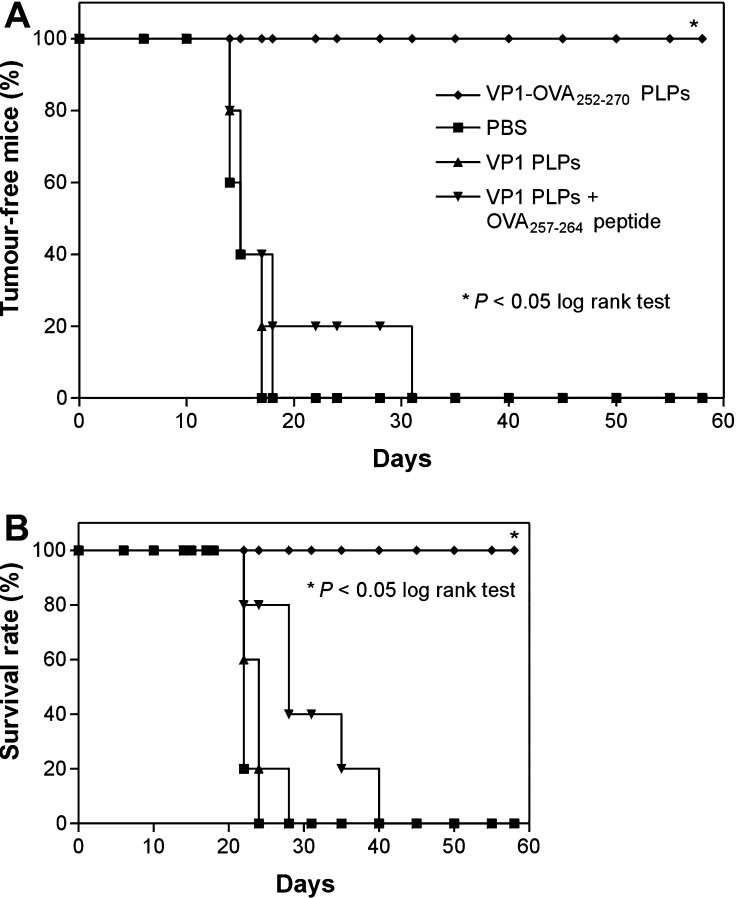
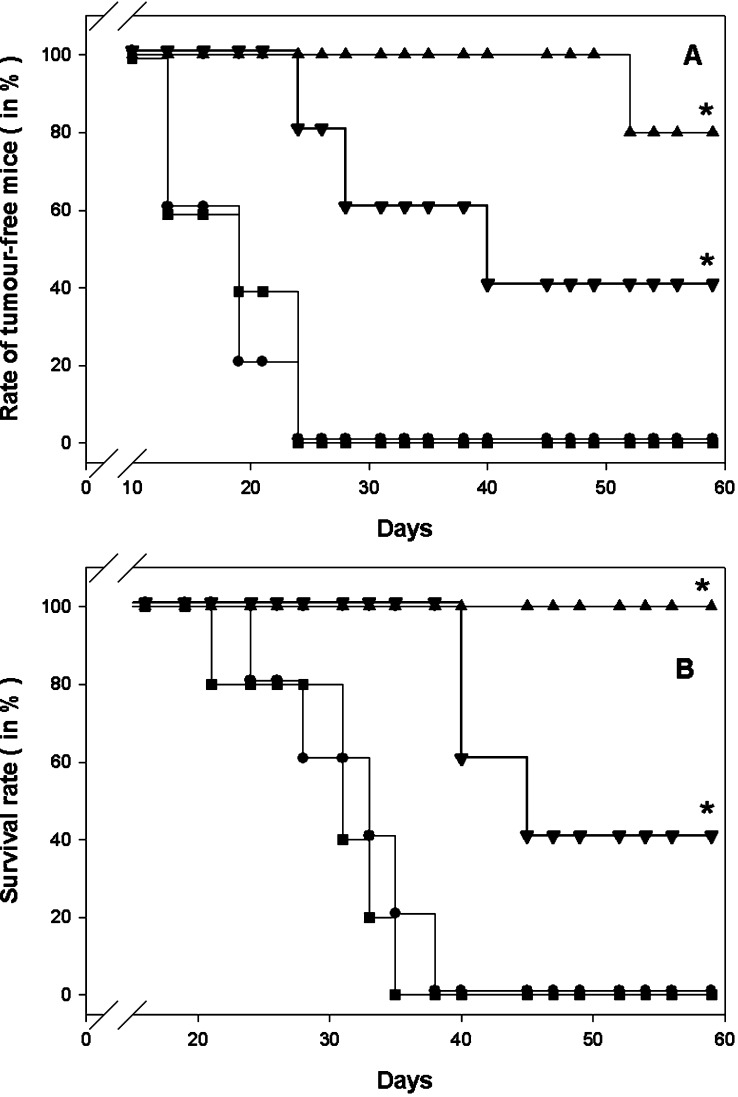
To address the contribution of residual endotoxin to the therapeutic efficacy of VP1-OVA252-270 PLPs, parental VP1 capsoids and OVA257-264 peptide were used in combination to treat tumour-bearing C57BL/6 mice that had been s.c. implanted with 105 MO5 melanoma cells (i.e. tenfold of the minimal tumorigenic dose) into the right flank. The rate of tumour-free mice and finally the survival rate of treated and untreated mice were monitored. The early and late immunotherapeutic interventions were performed at day 4 and 11 or day 10 and 17 post tumour cell implantation, respectively. Melanoma-bearing groups of C57BL/6 mice were s.c. treated twice with (1) PBS as negative control, (2) 50 μg VP1 PLPs as carrier control, (3) 50 μg VP1 PLPs and 50 μg OVA257-264 peptide and (4) VP1-OVA252-270 PLPs into the opposite flank (Fig. 3). The group of mice that had been treated with VP1-OVA252-270 PLPs significantly showed 100% survival within the observation period of 60 days post tumour cell inoculation (P< 0.05, log rank test, n=5) (Fig. 4). In contrast, all control mice and tumour-bearing animals, which had received the combination of peptide and VP1 PLPs, died of melanoma (Fig. 3). Similar data were obtained for the late immunotherapeutic schedule (data not shown). In summary, these results clearly showed that residual endotoxin in the VP1 capsoid preparation had no beneficial effect on the therapeutic value suggesting a definite correlation of particulate VP1-OVA252-270 structures with anti-tumour efficacy.
Fig. 3.
a, b Early immunotherapy in MO5 melanoma-bearing mice with VP1-OVA252-270 capsoids or VP1 PLPs in presence of OVA257-264 peptide. a Rate of tumour-free mice (in%) treated in each case twice with 50 μg of parental VP1 PLPs (filled triangle), VP1 PLPs plus 50 μg OVA257-264 peptide (filled inverted triangle) or VP1-OVA252-270 PLPs (filled diamond). The C57BL/6 mice were s.c. charged with 105 MO5 melanoma cells given into the right flank. The therapeutic immunisations or PBS administrations (filled square) were performed at day 4 and day 11 after s.c. melanoma implantation into the left flank. The animals were monitored for survival and tumour development for 60 days. b The survival rate of these treated mice was monitored daily (*P<0.05, log rank test, n=5). Mice that became moribund were sacrificed. Each experiment was repeated with similar results
Fig. 4.
a, b Early immunotherapy in MO5 melanoma-bearing mice with VP1-OVA252-270 capsoids. a Rate of tumour-free mice (in%) treated in each case twice with 50 μg of parental VP1 (filled square), VP1-OVA252-270 PLPs (filled inverted triangle) or OVA257-264 peptide (filled triangle). The latter was s.c. administered in the presence of 100 μg QuilA, a saponin adjuvant. The C57BL/6 mice were s.c. inoculated with 105 MO5 melanoma cells given into the right flank. The therapeutic immunisations or PBS administrations (filled circle) were performed at day 4 and day 11 after melanoma inoculation by s.c. injections into the left flank. The animals were monitored for survival and tumour development for 60 days. b The survival rate of these treated mice was monitored daily (*P<0.05, log rank test, n=5). Mice that became moribund were sacrificed. Each experiment was repeated with similar results
Early immunotherapy with chimerical PPs or capsoids against melanoma
C57BL/6 mice were s.c. inoculated with 105 MO5 melanoma cells into the right flank to evaluate the therapeutic potency of VP1-OVA252-270 PLPs. The rate of tumour-free mice and finally the survival rate of treated and untreated mice were monitored. The early immunotherapeutic interventions were performed at day 4 and 11 post tumour cell inoculation. Melanoma-bearing groups of C57BL/6 mice were s.c. immunised with (1) PBS as negative control, (2) 50 μg VP1 PLPs as carrier control, (3) 50 μg OVA257-264 in the presence of 100 μg QuilA adjuvant and (4) VP1-OVA252-270 PLPs into the opposite flank (Fig. 4). It turned out that the group of mice that had received VP1-OVA252-270 significantly showed 80% survival within the observation period of 60 days post tumour cell inoculation (P<0.05, log rank test, n=5) (Fig. 4). The peptide immunisation approach resulted in a survival rate of 40%, whereas all control mice died to melanoma within 36 days (Fig. 4). This experiment stressed the therapeutic value of chimerical PLPs carrying a foreign model epitope even in the absence of an appropriate adjuvant.
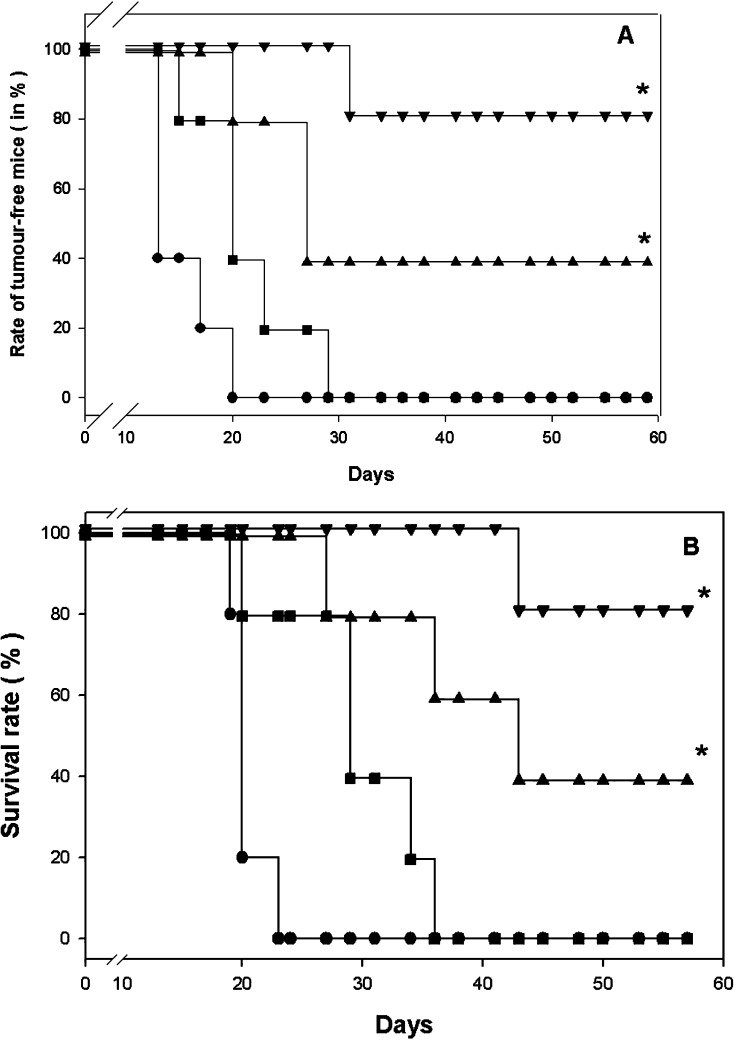
The question is still open whether less particulate structures like the VP1-OVA252-270 PPs could also evoke beneficial therapeutic effects against melanoma. In a similar therapy setting treatment with VP1-OVA252-270 PPs led to 100% survival within the given time suggesting that both entities, VP1-OVA252-270 PPs and PLPs, independent from the size of their ordered structures had a comparable capability to elicit beneficial immune responses in tumour-bearing mice (Fig. 5). To find out if pentameric structures like VP1-TRP180-192 displaying a differentially expressed self epitope for CD8 T cells were able to break T cell tolerance, melanoma-bearing C57BL/6 mice were similarly treated as described for VP1-OVA252-270 PPs. The VP1-TRP180-192 treatment elicited immune responses resulting in 40% survival of the melanoma-bearing mice and in a 40% rate of tumour-free mice, which clearly mirrored the less immunodominant capacity of the TRP180-188 epitope compared to the OVA257-264 peptide. Nevertheless, these results were encouraging as they showed for the first time that in a significant proportion of melanoma-bearing mice T cell tolerance had been broken leading to this therapeutic value.
Fig. 5.
a, b Early immunotherapy in MO5 melanoma-bearing mice with small particulate structures. a Rate of tumour-free mice (in%) treated in each case twice with 50 μg of parental VP1 PPs (filled square), VP1-OVA252-270 PPs (filled triangle) or VP1-TRP2180-192 PPs (filled inverted triangle). The C57BL/6 mice were s.c. charged with 105 MO5 melanoma cells given into the right flank. The therapeutic immunisations or PBS administrations (filled circle) were performed at day 4 and day 11 after melanoma inoculation by s.c. injections into the left flank. The animals were monitored for survival and tumour development for 60 days. b The survival rate of these treated mice was monitored daily (*P<0.05, log rank test, n=5). Mice that became moribund were sacrificed. Each experiment was repeated with similar results
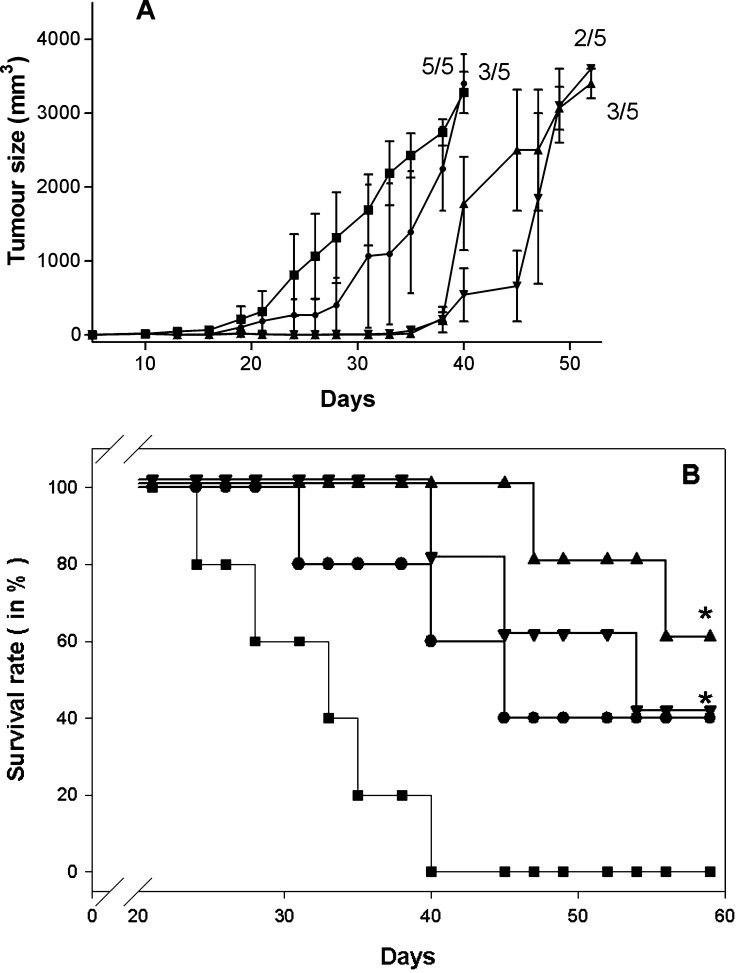
Late immunotherapy with particulate structures against melanoma
We used 105 MO5 melanoma cells to further stress the potential of PLP-based cancer vaccines. Tumour growth and survival of C57BL/6 mice under late immunotherapeutic conditions were monitored. PLP-specific treatments were performed at day 10 and 17 post tumour cell inoculation. At the beginning of therapy, tumours had already reached sizes of 3 mm representing the mean of the two perpendicular diameters of the tumour. Five animals per group were s.c. treated with (1) 50 μg VP1-OVA252-270 pentamers, (2) 50 μg VP1-OVA 252–270 PLPs, (3) 50 μg VP1-TRP180-192 PPs or PBS as negative control (Fig. 6). Surprisingly, these late interventions did not reveal the dramatic difference in the therapeutic value of VP1-TRP180-192 and VP1-OVA252-270 PPs observed during the early treatment setting (Fig. 6). It should be noted that OVA257-264 and TRP2180-188 peptide-specific and late treatment onset completely failed to protect animals against tumour growth (data not shown). Furthermore, VP1-OVA252-270-specific PPs or PLPs administration did not show the expected therapeutic value as observed during the early immunotherapeutic intervention. However, VP1-TRP180-192 PPs induced beneficial immunostimulatory effects with survival rates at 60% during this late therapeutic challenge.
Fig. 6.
a, b Late immunotherapy in MO5 melanoma-bearing mice with particulate structures. a Monitoring of MO5-tumour sizes of mice treated in each case twice with 50 μg of parental VP1-OVA252-270 PPs (filled inverted triangle), VP1-OVA252-270 PLPs (filled circle) or VP1-TRP2180-192 PPs (filled triangle). The C57BL/6 mice were s.c. charged with 105 MO5 melanoma cells given into the right flank. The therapeutic immunisations or PBS administrations (filled square) were performed at day 10 and day 17 after melanoma inoculation by s.c. injections into the left flank. The treatment was started at tumour sizes of 3 mm (the mean of the two perpendicular diameters of the tumour). The tumour size (formula tumour volume [mm3]=4r3π/3) of tumour-bearing mice/total number of mice was monitored daily for 60 days. Numbers above tumour sizes represent number of tumour-bearing mice/total number of treated mice per group. b The survival rate of these treated and melanoma-bearing mice was monitored daily for 60 days (*P<0.05, log rank test, n=5). Mice that became moribund were sacrificed. Each experiment was repeated with similar results
Taken together, PLPs and even PPs represent very efficient peptide delivery systems, which are both able to induce therapeutically efficacious CD8 T cell responses against self and non-self target antigens of melanoma.
Discussion
This report describes particulate structures like heterologous PPs at 9 nm average size and PLPs (45 nm in size) displaying a H-2Kb -restricted CTL-epitope of OVA capable of inducing OVA257-264-specific CD8 T cells against the melanoma transfectant MO5 expressing full-length OVA. Our early immunotherapeutic onset, 4 days post tumour cell inoculation, revealed that two s.c. applications of PPs or PLPs carrying the OVA257-264 epitope were already sufficient to cure mice from lethal MO5 melanoma. Using a similar treatment protocol chimerical PPs displaying the self H-2Kb -restricted epitope TRP2180-188 showed less therapeutic value in tumour-bearing mice as compared to that of VP1-OVA252-270 PPs. However, late immunotherapeutic interventions starting at day 10 post MO5 melanoma inoculation resulted in comparable curative efficacy of both PP entities, VP1-OVA252-270 and VP1-TRP2180-192. To the best of our knowledge, this is the first report that describes the induction of protective anti-tumour immunity in a therapeutic setting evoked by pentamers carrying a self-antigen/a tumour-specific protein epitope.
In a recent article, we reported on the potential of VP1-OVA252-270 PLPs to protect mice against MO5 melanoma challenge [6]. Upon single s.c. application these chimerical PLPs induced OVA257-264-specific CD8 T cells in the absence of an appropriate adjuvant. However, protective immunity towards lethal melanoma challenge could only be evoked by two s.c administrations following a weekly immunisation schedule [6]. Here, we generated evidence that this weekly schedule for PLP application may be central to vaccine efficacy due to low anti-VP1 antibody titres at day 7, the date for the second immunisation. The detrimental effects of antibodies directed against chimerical capsoids on the induction of protective anti-tumour CTL responses have been reported [11]. Nevertheless, this short treatment interval for capsoid-based immunotherapeutic has never been in the research focus of other groups. Prime-boost vaccination schedules were employed with heterologous HPV-specific capsoids originating from different animal sources to enhance the therapeutic anti-tumour value of VLPs [12].
For the application of PLPs in human tumour therapy, the question is still open whether cross-reactive anti-VP1 antibodies raised against the human polyomaviruses JCV and BKV could interfere with a successful treatment. It is worth mentioning that these human polyomaviruses JCV and BKV are endemic all over the world with infection rates of almost 100% by the age of ten in case of BKV and with JCV reaching almost 92% in adulthood [14]. To address this important issue we have already collected preliminary data of eight human sera in regard to VP1-crossreactivity, five sera were positive and three sera were negative for anti-JCV antibodies (A. Abbing, unpublished results). More importantly, all eight human sera did not show any cross reactivity with PLPs, suggesting that PLP-based treatment in humans is feasible without the detrimental vaccination interference due to pre-existing anti-JCV antibodies. In a clinical setting the antibody status of the patients can easily be monitored before the vaccination procedure.
First, the impact of residual endotoxin in capsoid preparations (2–4.5 ng LPS per ml) on the survival rate and rate of tumour-free mice should be determined. This issue was even more important as VLPs from human papillomavirus VLPs appeared to be more immunogenic in the presence of LPS [15]. Using early and late therapy onset twice treatments with VP1 PLPs and OVA257-264 peptide in combination absolutely failed to cure these tumour-bearing animals; they all died to melanoma. In contrast, the vaccine formulation OVA257-264 peptide plus QuilA adjuvant appeared to be more immunogenic than VP1 PLPs in terms of tumour take and survival rate (Fig. 4). These findings revealed that minor LPS concentration in PLP preparations led to negligible if any immunotherapeutic effects in this melanoma model. At the same time these data supported the notion that antigens have to be covalently attached to PLPs for inducing anti-tumoral immune responses.
Due to the prominent immunostimulatory capacity of VLPs, only capsoids have been addressed for the development of vaccines. However, two reports recently stressed the importance of particulate structures (e.g. HPV-capsomers with minor size) in regard to autoantibody or CTL induction and tumour remission [10, 24]. Considering their promising findings, we could show that two immunisations with VP1-OVA252-270- or VP1-TRP2180-192-specific PPs led to complete or 60% protection against a lethal MO5 melanoma challenge, respectively [6; M. Brinkman, unpublished data]. Furthermore, we succeeded in completely curing mice from MO5 melanoma by means of VP1-OVA252-270 PPs application with an early treatment onset after tumour implant. Although OVA257-264-specific CD8 T cell numbers were at a similar level during early and late immunotherapy, a later therapy onset did not lead to the expected tumour regression in MO5 melanoma-bearing mice. Therefore, we guess that these late immunotherapeutic interventions against an OVA-expressing melanoma cell line resembled the situation of natural immune escape mutants. Due to the lack of selection pressure the clonal expansion of OVA-deficient melanoma cells occurred with a higher frequency in regard to the proceeding presence of the MO5 transfectant in vivo. Therefore, immune responses induced by PPs or capsoids and directed against the OVA target antigen may obviously impair late immunotherapy, whereas the less immunodominant VP1-TRP2180-192 PPs showed similar therapeutic values under early and late therapy onset. These promising findings clearly indicate the therapeutic potential of particulate structures even with minor size (e.g. VP1-TRP2180-192 PPs). In contrast, DCs pulsed with TRP2180-188 peptides did not show any therapeutic value in similar melanoma models [2].
Addressing the findings during early treatment onset the minor therapeutic value of VP1-TRP2180-192 in comparison to VP1-OVA252-270 PPs could be explained by the following notions. Due to the low immunostimulatory capacity of the TRP2180-188 epitope additional TRP2-specific CD4 T cell should be provided which in turn may support TRP2180-188-specific CD8 T cell effector mechanisms by generating an appropriate cytokine milieu. As TRP2180-188-specific CD8 T cell numbers elicited by VP1-TRP180-192 PPs treatment during early and late therapeutic settings were similar to OVA257-264-specific CD8 T cell frequencies evoked by appropriate particles, the additional induction of TRP2-specific CD4 T cells may be beneficial for obtaining complete melanoma regression [32].
As both CD4 and CD8 T cells were required for optimal anti-melanoma immunity demonstrated by antibody depletion of T cell subsets, as well as with various gene knockout mice [33], we may predict that TRP2-specific induction of CD4 T cells besides CD8 T cell stimulation by PLP-based antigen delivery system could lead to complete remission of melanoma even under the conditions of late immunotherapy. It may be obvious that these CD8 T cells could exert cytolytic activity on tumour cells. To characterise the effector mechanism of anti-tumoral OVA257-264-specific CD8 T cells, we performed several lactate dehydrogenase-release assays on OVA257-264 peptide-pulsed target cells but always failed to get positive results for the detection of CTL. These findings were in agreement with data about the failure of determining in vitro CTL activity for splenocytes of mice acutely infected with murine polyomavirus [8]. In contrast, assessing the polyomavirus-specific CTL activity in vivo revealed a highly specific anti-polyoma cytotoxicity during acute infection [8]. To obtain further informations about the functional properties of antigen-specific CD8 T cells induced by heterologous PLPs or PPs, similar experiments towards the detection of CTL activity in vivo are under way.
In general, PLP derivatives belong to the group of protein-based vaccines, which usually require potent adjuvants to prime CTL responses. Here, we reported on the therapeutic potential in the absence of any further immunostimulatory compound. However, the question is still under our investigation if CTL-stimulating adjuvants like Montanide ISA51 or ISA720 [27, 36], which are currently used in clinical trials, could even enhance the inherent therapeutic value of our PLPs- or PPs-based vaccines.
Since self peptides or protein require the disruption of tolerating or down regulatory T cell circuits to prime CTL responses, only immunisation with appropriately loaded DCs appear to fulfil these immunotherapeutic criteria at the present time [37]. This notion mirrors current research activities in the field of anti-melanoma therapy. While immunisation of mice with DCs pulsed with TRP180-188 only generated partial protection against B16 melanoma [2, 28, 33], preventive approaches with enhanced intracellular TRP180-188 peptide delivery into DCs led to complete protective immunity towards melanoma challenge [30, 33, 35]. These latter approaches also resulted in significant inhibition of lung metastases in an immunotherapeutic 3-day tumour model [33, 35]. Unfortunately, the treatment of B16 melanoma-bearing mice with TRP2180-188-pulsed DCs failed to cure mice from melanoma even with a therapeutic onset at day 1 post tumour implant [2].
The central role of DCs for the presentation of antigens delivered by VLPs has been convincingly demonstrated by several research groups [20, 22, 23, 26]. By means of adoptive transfer of CD11c+ DCs or CD11b+ CD11c− macrophages from mice immunised with parvovirus-VLPs displaying the OVA257-264 epitope only DCs were capable of inducing OVA257-264-specific CTL responses in acceptor mice [23]. Nevertheless, VLPs of human JC- and BK-polyomavirus absolutely failed to induce phenotypic activation of murine bone marrow-derived DCs in vitro [19]. To clarify these contradictory evidences with human polyomavirus-VLPs murine bone marrow-derived DCs were loaded with murine VP1 PLPs and their phenotypic maturation was subsequently analysed by flow cytometry. Interestingly, the CD40, CD80, CD86 and MHC class II surface expression of DCs were significantly enhanced in a dose-dependent way by means of VP1 PLPs stimulation [T. Bickert, J. Hess and K. Erb, manuscript in preparation]. Furthermore, these VP1 PLPs-pulsed DCs were capable of secreting IL-12 underlining their important contribution for the induction of Th1-mediated immune responses towards melanoma [T. Bickert, J. Hess and K. Erb, manuscript in preparation].
The fact that chimerical VLPs which represent important antigen sources for MHC class I-specific processing and presentation pathways are efficiently taken up by DCs in vivo [22], could have important implications for the development of anti-cancer vaccines based on such VLP carrying complete tumour antigens, fragments of these polypeptides or CTL epitopes of the respective proteins [5, 6]. In addition, our data provides evidence that even less particulate structures like PPs are capable of breaking T cell tolerance obviously by these efficient DC priming events in vivo [18].
Along this line, we are currently developing a PLP-based antigen delivery system capable of directing complete proteins to DCs [1]. Together with the knowledge that PPs and PLPs are able to break T cell tolerance and that both antigen-specific CD4 and CD8 T cells are required for tumour remission, we are convinced that appropriate polyoma capsoid-based vaccines delivering complete tumour antigens or appropriate fragments will offer new therapeutic opportunities in the treatment of cancer. By using universal tumour antigens expressed in many types of tumour cells originating from a multitude of cancer patients, this novel vaccine generation may be applied on a non-individual basis. Unlike immunisations with short peptides, vaccination with complete proteins has the potential to prime CMI to multiple antigenic epitopes and should not be limited in its use to individuals expressing only one particular class of MHC molecules. In general, the major advantage of these non-replicative protein-based vaccine entities administered in the absence of an appropriate adjuvant is that PPs and PLPs should be well-tolerated and safe even in immunocompromised individuals.
Acknowledgements
This work was financially supported by the BMBF projects ‘Biochance‘ 0313044 and ‘Supramolecular Drug-Delivery-Systems’ 03C0308 A-C. Prof. K. L. Rock generously provided the MO5 melanoma cell line.
Abbreviations
- APCs
Antigen presenting cells
- CMI
Cell-mediated immunity
- CTL
Cytotoxic T lymphocytes
- DCs
Dendritic cells
- LPS
Lipopolysaccharide
- MHC
Major histocompatibility complex
- OVA
Ovalbumin
- PCS
Photon correlation spectroscopy
- PE
Phycoerythrin
- PLPs
Murine polyomavirus-like-particles
- PPs
Murine polyomavirus-like-pentamers
- TRP2
Tyrosinase-related protein 2
- VP1
Viral protein 1 of murine polyomavirus
References
- Abbing A, Blaschke UK, Grein S, Kretschmar M, Stark CMB, Thies MJW, Walter J, Weigand M, Woith DC, Hess J, Reiser COA. Efficient intracellular delivery of a protein and a low molecular weight substance via recombinant polyomavirus-like particles. J Biol Chem. 2004;279:27410–27421. doi: 10.1074/jbc.M313612200. [DOI] [PubMed] [Google Scholar]
- Bellone M, Cantarella D, Castiglioni P, Crosti MC, Ronchetti A, Moro M, Garancini MP, Casorati G, Dellabona P. Relevance of the tumor antigen in the validation of three vaccination strategies for melanoma. J Immunol. 2000;165:2651–2656. doi: 10.4049/jimmunol.165.5.2651. [DOI] [PubMed] [Google Scholar]
- Beyer T, Herrmann M, Reiser C, Bertling W, Hess J. Bacterial carriers and virus-like-particles as antigen delivery devices: role of dendritic cells in antigen presentation. Curr Drug Targets Infect Disord. 2001;1:287–302. doi: 10.2174/1568005014605973. [DOI] [PubMed] [Google Scholar]
- Bloom MB, Perry-Lalley D, Robbins PF, Li Y, El-Gamil M, Rosenberg SA, Yang JC. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgerault F, Moron G, Leclerc C. Virus-like particles: a new family of delivery systems. Exp Rev Vaccines. 2002;1:101–109. doi: 10.1586/14760584.1.1.101. [DOI] [PubMed] [Google Scholar]
- Brinkman M, Walter J, Jennes I, Neugebauer M, Bertling W, Grein S, Thies MJW, Weigand M, Beyer T, Herrmann M, Reiser COA, Hess J. Recombinant murine polyoma virus-like-particles induce protective anti-tumour immunity. Lett Drug Des Disc. 2004;1:137–147. [Google Scholar]
- Bungener L, Idema J, ter Veer W, Huckriede A, Daemen T, Wilschut J. Virosomes in vaccine development: induction of cytotoxic T lymphocyte activity with virosome-encapsulated protein antigens. J Liposome Res. 2002;12:155–163. doi: 10.1081/LPR-120004789. [DOI] [PubMed] [Google Scholar]
- Byers AM, Kemball CC, Moser JM, Lukacher AE. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol. 2003;171:17–21. doi: 10.4049/jimmunol.171.1.17. [DOI] [PubMed] [Google Scholar]
- Celluzzi CM, Falo LD., Jr Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J Invest Dermatol. 1997;108:716–720. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002;169(11):6120–6126. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- Da Silva DM, Pastrana DV, Schiller JT, Kast WM. Effect of preexisting neutralizing antibodies on the anti-tumor immune response induced by chimerical human papillomavirus virus-like particle vaccines. Virology. 2001;290:350–360. doi: 10.1006/viro.2001.1179. [DOI] [PubMed] [Google Scholar]
- Da Silva DM, Schiller JT, Kast WM. Heterologous boosting increases immunogenicity of chimerical papillomavirus virus-like-particle vaccines. Vaccine. 2003;21:3219–3227. doi: 10.1016/S0264-410X(03)00237-8. [DOI] [PubMed] [Google Scholar]
- Deml L, Wild J, Wagner R. Virus-like particles: a novel tool for the induction and monitoring for both T-helper and cytotoxic T-lymphocyte activity. Methods Mol Med. 2004;94:133–157. doi: 10.1385/1-59259-679-7:133. [DOI] [PubMed] [Google Scholar]
- Doerries K. Virus-host interactions and diagnosis of human polyomavirus-associated disease. Interviroloy. 1996;39:165–175. doi: 10.1159/000150492. [DOI] [PubMed] [Google Scholar]
- Freyschmidt EJ, Alsonso A, Hartmann G, Gissmann L. Activation of dendritic cells and induction of T cell responses by HPV 16 L1/E7 chimeric virus-like particles are enhanced by CpG ODN or sorbitol. Antivir Ther. 2004;9(4):479–489. [PubMed] [Google Scholar]
- Greenstone HL, Nieland JD, de Visser KE, de Brujin MLH, Kirnbauer R, Roden RBS, Lowy DR, Kast M, Schiller JT. Chimerical papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Schaible U, Raupach B, Kaufmann SHE. Exploiting the immune system: new vaccines against intracellular bacteria. Adv Immunol. 2000;75:1–88. doi: 10.1016/s0065-2776(00)75001-2. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, Delos Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/S1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz P, Day PM, Pang Y-YS, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- Lenz P, Thompson CD, Day PM, Bacot SM, Lowy DR, Schiller JT. Interactioon of papillomavirus virus-like particles with human myeloid antigen-presenting cells. Clin Immunol. 2003;106(3):231–237. doi: 10.1016/S1521-6616(02)00039-6. [DOI] [PubMed] [Google Scholar]
- Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- Moron G, Rueda P, Casal I, Leclerc C. CD8alpha- CD11b+ dendritic cells present exogenous virus-like particles to CD8+ T cells and subsequently express CD8alpha and CD205 molecules. J Exp Med. 2002;195(10):1233–1245. doi: 10.1084/jem.20011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron VG, Rueda P, Sedlik C, Leclerc C. In vivo, dendritic cells can cross-present virus-like-particles using an endosome-to-cytosol pathway. J Immunol. 2003;171(5):2242–2240. doi: 10.4049/jimmunol.171.5.2242. [DOI] [PubMed] [Google Scholar]
- Öhlschlaeger P, Osen W, Dell K, Faath S, Garcea RL, Jochmus I, Muller M, Pawlita M, Schafer K, Sehr P, Staib C, Sutter G, Gissmann L. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J Virol. 2003;77:4635–4645. doi: 10.1128/JVI.77.8.4635-4645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–4901. [PubMed] [Google Scholar]
- Ruedl C, Storni T, Lechner F, Baechi T, Bachmann MF. Cross-presentation of virus-like particles by skin-derived CD8− dendritic cells: a dispensable role for TAP. Eur J Immunol. 2002;32:818–825. doi: 10.1002/1521-4141(200203)32:3<818::AID-IMMU818>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Scalzo AA, Elliott SL, Cox J, Gardner J, Moss DJ, Suhrbier A. Induction of protective cytotoxic T cells to murine cytomegalovirus by using a nonapeptide and a human-compatible adjuvant (Montanide ISA 720) J Virol. 1995;69(2):1306–1309. doi: 10.1128/jvi.69.2.1306-1309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs MWJ, Eggert AAO, de Boer AJ, Vissers JLM, van Hall T, Offringa R, Figdor CG, Adema GJ. Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res. 2000;60:6995–7001. [PubMed] [Google Scholar]
- Sedlik C, Saron M, Sarraseca J, Casal I, Leclerc C. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc Natl Acad Sci USA. 1997;94:7503–7508. doi: 10.1073/pnas.94.14.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibagaki N, Udey MC. Dendritic cells transduced with TAT protein transduction domain-containing tyrosinase-related protein 2 vaccinate against murine melanoma. Eur J Immunol. 2003;33:850–860. doi: 10.1002/eji.200323709. [DOI] [PubMed] [Google Scholar]
- Stuhler G, Walden P (eds) (2002) T cells in tumor immunity in Cancer Immune Therapy. Wiley, Weinheim
- Van Elsas A, Sutmuller RPM, Hurwitz AA, Ziskin J, Villasenor J, Medema J-P, Overwijk WW, Restifo NP, Melief CJM, Offringa R, Allison JP. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on ctotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Wang HY. Enhancement of antitumor immunity by prolonging antigen presentation on dendritic cells. Nat Biotechnol. 2002;20:149–154. doi: 10.1038/nbt0202-149. [DOI] [PubMed] [Google Scholar]
- Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Fu T, Wang G, Zeng G, Perry-Lalley DM, Yang JC, Restifo NP, Hwu P, Wang R-F. Induction of CD4 T cell-dependent antitumor immunity by TAT-mediated tumor antigen delivery into dendritic cells. J Clin Invest. 2002;109:1463–1470. doi: 10.1172/JCI200215399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Teates D, Neese P, Grosh WW, Petroni G, Engelhard VH, Slingluff CL., Jr Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int J Cancer. 2001;92:703–711. doi: 10.1002/1097-0215(20010601)92:5<703::AID-IJC1250>3.3.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bosch ML, Salgaller ML. Current methods for loading dendritic cells with tumor antigen for the induction of antitumor immunity. J Immunother. 2002;25:289–303. doi: 10.1097/00002371-200207000-00001. [DOI] [PubMed] [Google Scholar]