Abstract
Although the proinflammatory cytokine interferon-γ (IFN-γ) has been generally thought to enhance antitumor immune responses and be involved in antitumor mechanisms of many other immunotherapy molecules, it has also been reported that IFN-γ could promote tumor immune evasion. In this report, by using an ideal mouse model that expresses IFN-γ locally in muscle, we demonstrate that sustained low-level expression of IFN-γ promotes the development of several types of tumor including H22 hepatoma, MA782/5S mammary adenocarcinoma and B16 melanoma. However, transitory expression of IFN-γ does not have such an effect. On the other hand, sustained high-level expression of IFN-γ mediates significant antitumor effect on H22 hepatoma. Low level of IFN-γ upregulates expression of PD-L1, PD-L2, CTLA-4 and Foxp3, which may partly account for the tumor immune evasion promoted by IFN-γ. Furthermore, blockade of PD-L inhibits IFN-γ’s tumor-promoting effect. Our findings provide a mechanistic link between chronic inflammation and cancer and would have potential implications for cancer prevention and also for the design of cytokine–based cancer immunotherapy.
Keywords: Interferon-γ, Immune evasion, Tumor development, PD-L, Tumor immunotherapy
Introduction
Cytokines regulate the survival, proliferation, differentiation and function of immune cells as well as cells of most organ systems [15] and are also involved in the mechanisms of many diseases including cancer. Cytokines have been widely employed as biological drugs for cancer targets [5]. On the other hand, many cytokines such as TNF-α and IL-1β produced in chronic inflammation may contribute to cancer growth and progression through mechanisms such as DNA damage, by functioning as growth and survival factors, by promoting angiogenesis, and so on [1, 6].
Interferon-γ (IFN-γ), a potent proinflammatory cytokine, is mainly secreted by T lymphocytes under certain conditions of activation and by natural killer cells. Although originally defined as an agent with direct antiviral activity, IFN-γ is capable of upregulating both MHC classes I and II, and has demonstrated direct inhibitory effects on tumor proliferation [13]. However, it has also been reported that IFN-γ could promote tumor development through downregulation of the endogenous tumor antigen [2, 17] and enhance the survival of tumor cells and their metastatic potential under certain conditions [14, 25]. Taken together, these results indicate that the action of IFN-γ in cancer is paradoxical.
In this report, we explored the possibility of the involvement of IFN-γ in tumor development by using experimental tumor models. By using naked plasmid to express IFN-γ locally in muscle, we demonstrate that sustained low-level expression of IFN-γ promotes tumor development. However, sustained high-level expression of IFN-γ mediates significant antitumor effect. These results indicate that IFN-γ may serve as a “two-edged sword” either in tumor development or in tumor immunotherapy.
Materials and methods
Tumor initiation and progression models
All studies involving mice were approved by the institute’s Animal Care and Use Committee. All tumor models were established in hind thigh muscle of BALB/c mice. To determine the minimal dose of tumor cells that can form tumor, mice were inoculated i.m. with different doses of tumor cells. For the tumor initiation models of H22 hepatoma and MA782/5S mammary adenocarcinoma, mice were inoculated with 8×103 and 1×105 tumor cells respectively. In some experiments BALB/c mice were inoculated with 2×105 B16 melanoma cells. For the tumor progression model, mice were inoculated with 5×104 H22 tumor cells. All mice received the indicated plasmid injections beginning on day 2 after tumor cell inoculation. When tumor is palpable, the two perpendicular diameters were monitored and the tumor diameter was calculated using the formula (a+b)/2, with a as the larger diameter and b as the smaller diameter. In some experiments, mice were sacrificed and tumors were dissected and weighed on the indicated days.
Plasmids
The mIFNG plasmid was constructed by insertion of the mouse IFN-γ cDNA into a simple eukaryotic expression vector, pCDx [22], which does not express any other unwanted protein except for the one encoded by the inserted cDNA. The mIFNG plasmid can express biologically active IFN-γ as determined by an antiviral assay as described [20]. The pGRA and pPD-1A plasmids were constructed by insertion of the cDNA encoding extracellular region of mouse IFN-γ receptor α-chain or PD-1 receptor into the expression vector pcDNA3.1 (Invitrogen), respectively. The eukaryotic-expressed extracellular domain of IFN-γ receptor has been proved to bind IFN-γ specifically and efficiently [9]. We also confirmed this by NO release assay [16] after pGRA was transfected into mouse muscle in vivo (data not shown). Expression product of pPD-1A is PD-L specific and binds efficiently to PD-L as determined in vitro by an antibody-blocking assay [10]. Plasmid DNA was prepared by selective compaction with spermine (Sigma) as described in [18] and endotoxin levels were less than 0.15 EU/μg of plasmid DNA. Spectrophotometric analysis of the plasmid DNA preparation revealed 260/280 nm ratios ≥1.80. Purity and conformation of the prepared DNA were confirmed by agarose gel electrophoresis.
Gene transfection and expression detection
Naked plasmid DNA at the indicated dose in 100 μl of saline was injected into the tumor inoculation site every other day. Mice of control groups received equal volume of saline or equal amount of empty vector plasmids. For short- or long-term expression, mice received injections twice or eight times respectively. To detect IFN-γ expression, RT-PCR and real-time PCR were performed with RNA extracted from muscle tissues of injection sites at the indicated time. Also the treated muscle tissues were excised, minced and cultured in vitro and the culture supernatants were assayed for IFN-γ expression by ELISA as described [20].
RT-PCR and real-time PCR
Total RNA was isolated and treated with RNase-free DNase I. To detect IFN-γ expression, an equal amount of RNA was reverse-transcribed and amplified by PCR for 26 cycles using gene-specific primers. ß-actin mRNA was used as an internal control and co-amplified. Amplified products were analyzed by electrophoresis on agarose gel and stained with ethidium bromide.
Relative quantitative real-time PCR was also performed using the TaqMan probe to detect expression level of IFN-γ, as described in [24]. To detect the expression level of PD-L1, PD-L2, Foxp3 and CTLA-4, RNA was isolated from peritumor tissues of tumor-bearing mice. The primer and probe sequences were as follows: PD-L1, sense 5′-GGAATTGTCTCAGAATGGTC-3′, antisense 5′-GTAGTTGCTTCTAGGAAGGAG-3′, and probe 5′-CACCAAACCAGCTCTATTCCCTCAGCCTAT-3′; PD-L2, sense 5′-AAGACTGACAATCTTCCCTC-3′, antisense 5′-CCTGAAAGTCATTAGGAGCC-3′, and probe 5′-TCAAGACTCTCTGAACAGCAAGACCCCAAT-3′; CTLA-4, sense 5′-GTCTTCTCTGAAGCCATACAG-3′, antisense 5′-GACCTCATCAGTGTTGTGTGA-3′, and probe 5′-TACAGGTGACCCAACCTTCAGTGGTGTT-3′. The primers and probe used for Foxp3 were as described in [11]. Each gene was normalized to the housekeeping gene (ß-actin) from the same sample before fold change was calculated to account for variations between different samples. The mRNA level in normal muscle tissue was used as the calibrator.
Cell proliferation assay and flow cytometry analysis
Proliferation of tumor cells was tested using the Boehringer Mannheim Cell Proliferation Kit II (XTT). H22 tumor cells were plated in 96-well plates at a concentration of 5×104 cells/ml and cultured in the presence of recombinant mIFN-γ or 36-h tissue culture supernatants from BHK cells transfected with either mIFNG or control pCDx plasmid at different dilution rates. Three days later the cells were analyzed for expression of PD-L1 and PD-L2 using PE-labeled monoclonal antibodies (eBioscience, San Diego, CA, USA) by flow cytometry, or after every 3 days 12–15% of cells were passaged and supplemented with the corresponding IFN-γ. The XTT labeling reagent was added to the rest of the cells and the OD value was determined at 490 nm 6–24 h later. The proliferation index was determined by the formula: OD490 of cells incubated with IFN-γ-containing supernatants or recombinant mIFN-γ/OD490 of cells incubated with control supernatants.
Data analysis
Tumor incidence was compared by applying Fisher’s exact test on treatment and control group. Other data were expressed as mean ± SEM and results were interpreted using one-way ANOVA (or followed by Student–Newman–Keuls test when needed). Differences were considered to be statistically significant when P< 0.05.
Results
Sustained low-level expression of IFN-γ promotes tumor development in several mouse tumor models
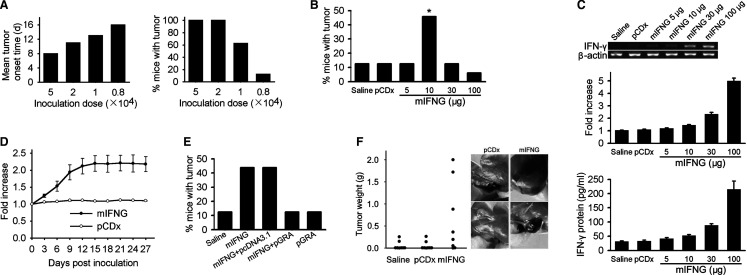
To evaluate the potential role of IFN-γ in tumor development, we first injected different doses of H22 hepatoma cells into the hind thigh muscle of mouse and tumor growth was monitored. The mean tumor onset time was prolonged when less number of tumor cells were inoculated. Only 12.5% of mice (2/16) inoculated with 8×103 H22 cells developed tumors by day 18 and others remained free of tumors up to 100 days after inoculation (Fig. 1a). The role of IFN-γ in tumor development was evaluated by i.m. injection of an IFN-γ-expressing plasmid, mIFNG, into mice inoculated with 8×103 H22 cells. The mice received different doses of mIFNG plasmid at inoculation site every other day eight times, beginning on day 2 after inoculation. The treatment with 10 μg of mIFNG plasmid significantly increased tumor incidence, with 45.8% of the recipients (11/24) developing palpable tumors by day 40 after inoculation. On the other hand, the treatment with control plasmid did not increase tumor incidence neither did the treatment with higher doses (30 μg or 100 μg) and lower dose (5 μg) of mIFNG plasmid (Fig. 1b).
Fig. 1.
Sustained low-level expression of IFN-γ promotes tumor development. a Mean tumor onset time (left) and tumor incidence (right) in BALB/c mice (n=16 per group) inoculated i.m. with different doses of H22 tumor cells. b BALB/c mice were inoculated with 8×103 H22 cells and received treatment with saline (n=16), 10 μg of pCDx plasmid (n=16) or different doses of mIFNG plasmid (n=24 per group) for eight times. *, P=0.040 by Fisher’s exact test, two-sided, compared with pCDx control. c BALB/c mice (n=5 per group) received mIFNG plasmid injections twice and 5 days after the second injection, RT-PCR (up, showing a representative sample in each group) and relative quantitative real-time PCR (middle) of IFN-γ mRNA expression or ELISA detection of IFN-γ protein expression (down, 1 ng/ml approximately 8 IU/ml). d IFN-γ mRNA expression in BALB/c mouse muscle injected with 10 μg of mIFNG or pCDx plasmid for eight times (n=3 for each point). e Sustained blockade of IFN-γ inhibits the tumor-promoting effect of mIFNG plasmid. After 8×103 H22 tumor cell inoculation, BALB/c mice (n=16 per group) received the indicated treatment for eight times (mIFNG, 10 μg; pcDNA3.1 or pGRA, 100 μg). f IFN-γ promotes B16 melanoma development in BALB/c mice. Mice (n=8 per group) received saline, 10 μg of pCDx or mIFNG plasmid for eight times after 2×105 B16 cells inoculation. Tumor weight by day 40 after inoculation (left) and two representative individuals in pCDx or mIFNG plasmid group were shown (right). Data are representative of two experiments
The reason that why we used plasmid DNA to express IFN-γ is that the expression level of IFN-γ could be controlled by the injection dose and frequency. IFN-γ mRNA expression level increased in step with the increment of mIFNG plasmid dose as determined by RT-PCR. We also confirmed this result by real-time PCR. IFN-γ protein level also increased following the same pattern (Fig.1c). When we injected mIFNG plasmid into mouse muscle every other day for eight times, IFN-γ mRNA expression level became relatively stable from day 12 (Fig. 1d). This sustained expression model may be similar to the cytokine expression profile in physiopathological state such as chronic inflammation in vivo, maintaining a lower-level but more stable concentration of IFN-γ in local site and avoiding the sharp peaks and drops that occur after bolus protein injection [12].
To further determine whether the tumor-promoting effect of mIFNG plasmid injection was mediated by expressed IFN-γ, we used a plasmid, pGRA, carrying the cDNA encoding extracellular region of mouse IFN-γ receptor α-chain, to make a sustained blockade of IFN-γ in the local sites. As compared to the injection of mIFNG plasmid alone, the tumor incidence in mice that received the injection of both pGRA and mIFNG plasmid was reduced to the saline control level by day 40 after 8×103 tumor cell inoculation. In contrast, coinjection of the empty vector pcDNA3.1 with mIFNG plasmid did not reduce the tumor incidence (Fig. 1e). In addition, the expression product of pGRA had no effect on H22 tumor growth in vitro in a proliferation assay (data not shown). Taken together, these results indicate that pGRA mediated the reduced tumor incidence through blockade of IFN-γ and further support that low level of IFN-γ expressed by mIFNG plasmid at local site promotes tumor development.
We then explored whether the tumor-promoting effect of IFN-γ could be applied to other tumor models. The effect of low dose of mIFNG plasmid was evaluated in BALB/c mice inoculated with i.m. injection of 1×105 MA782/5S mammary adenocarcinoma cells. The tumor incidence was 12.5% (2/16) in saline control group by day 45 after inoculation but increased to 43.8% (7/16, P=0.057) if mice received 10 μg of mIFNG plasmid at inoculation site for eight times. Interestingly, we found that sustained low-level expression of IFN-γ also promoted B16 melanoma (H-2b) development in BALB/c mice (H-2d) (Fig. 1f). In these two models, treatment with either empty vector or 5, 30 or 100 μg of mIFNG plasmid did not increase tumor incidence as compared to saline control treatment (data not shown).
Short-term expression of IFN-γ does not promote tumor development
To determine whether the tumor-promoting effect of IFN-γ depends on its sustained expression, mice received local injection of mIFNG plasmid only twice after inoculation with 8×103 H22 tumor cells. Neither lower doses (5 μg or 10 μg) nor higher doses (30 μg or 100 μg) of mIFNG plasmid could increase the tumor incidence as compared with saline or empty vector controls (Fig. 2a). Real-time PCR revealed that IFN-γ mRNA expression level began to decline by day 7 after the second injection (Fig. 2b), and the same profile was observed when protein expression was determined (data not shown). These results suggest that short-term and sharp expression of IFN-γ has no tumor-promoting effect regardless of the expression level of IFN-γ.
Fig. 2.
Effect of short-term expression of IFN-γ on tumor development. Mice were treated as in Fig. 1b but only for twice and the tumor incidence in each group (a, n=16 per group) as well as the time course of IFN-γ mRNA expression (b, n=4 for each point) are shown. Data shown in A are representative of two experiments
Sustained high-level expression of IFN-γ mediates significant antitumor effect
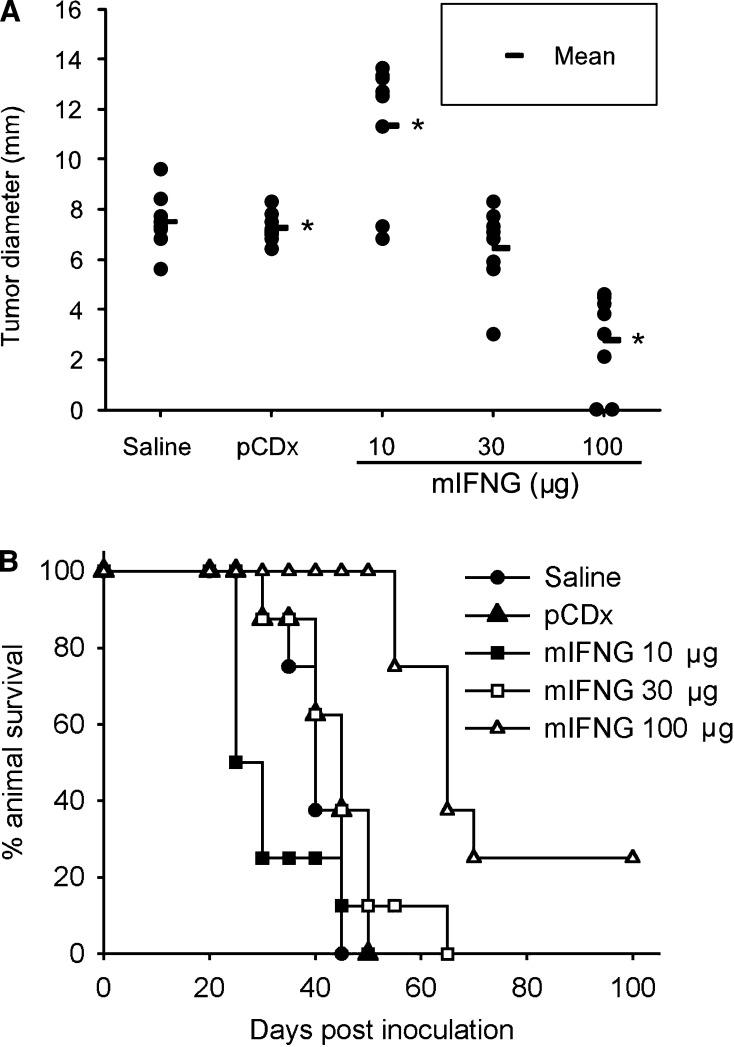
We also tested the effect of IFN-γ on mice inoculated with a higher dose of H22 tumor cells. On day 20 after inoculation, mice that had received 10 μg of mIFNG plasmid showed a significant increment in tumor diameter as compared to the controls (Fig. 3a). In contrast, however, injection of 100 μg of mIFNG plasmid mediated significant antitumor effect, which resulted in long-term survival in some cases (Fig. 3a, b), consistent with the previous results showing that intratumoral injection of 100 μg of naked IFN-γ-expressing plasmid significantly inhibited growth of CT-26 tumors [20]. Our results thus suggest that IFN-γ may function as a “two-edged sword” in tumor development and the expression pattern of IFN-γ could alter the balance of its tumor promoting and suppressor activity. Sustained low-level expression of IFN-γ mainly has tumor-promoting activity while high level of IFN-γ mainly has tumor-inhibiting activity.
Fig. 3.
IFN-γ functions as a “two-edged sword” in tumor growth. Mice (n=8 per group) were inoculated with 5×104 H22 tumor cells and received treatment with saline, 100 μg of pCDx plasmid or different doses of mIFNG plasmid for eight times. a Individual tumor diameter by day 20 after inoculation. *, P<0.01 by ANOVA, followed by Student-Newman-Keuls test, compared with each other. b Long-term survival follow up of these groups. Data shown are representative of two experiments
IFN-γ promotes tumor evasion of the immune system through upregulation of the expression of negative costimulatory molecules
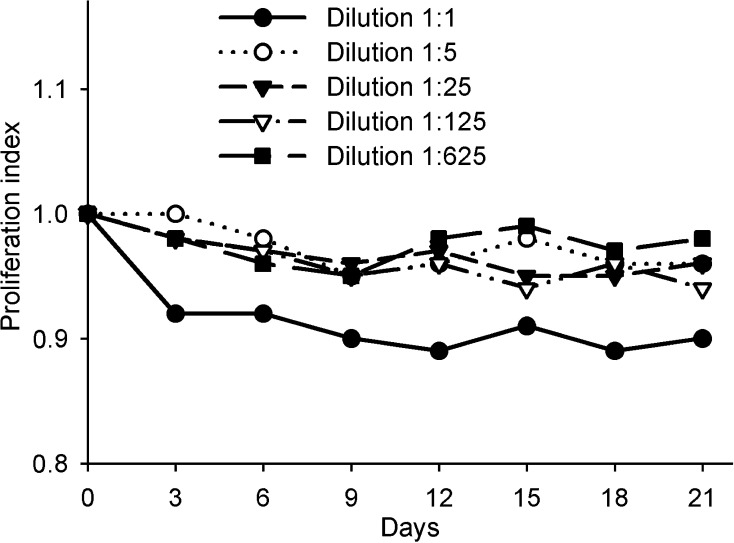
To determine whether the tumor-promoting effect of IFN-γ is mediated directly by its action on tumor cell growth, we transfected BHK cells with mIFNG plasmid and incubated H22 tumor cells with the supernatants of transfected cells (containing about 200 IU/ml of IFN-γ) at different dilution rates. We did not observe a significant promoting effect of IFN-γ on H22 cell proliferation during 21 days (Fig. 4), and the same results were obtained when recombinant mIFN-γ was used (data not shown). These results indicate that there are indirect mechanisms involved in the tumor-promoting effect of IFN-γ. We supposed that IFN-γ may promote tumor immune evasion.
Fig. 4.
Effect of IFN-γ on H22 tumor cell proliferation in vitro. H22 tumor cells were cultured in the presence of supernatants of BHK cells transfected with mIFNG plasmid at different dilution rates and cell proliferation was tested and calculated as compared with the control cells. Data are representative of three separate experiments
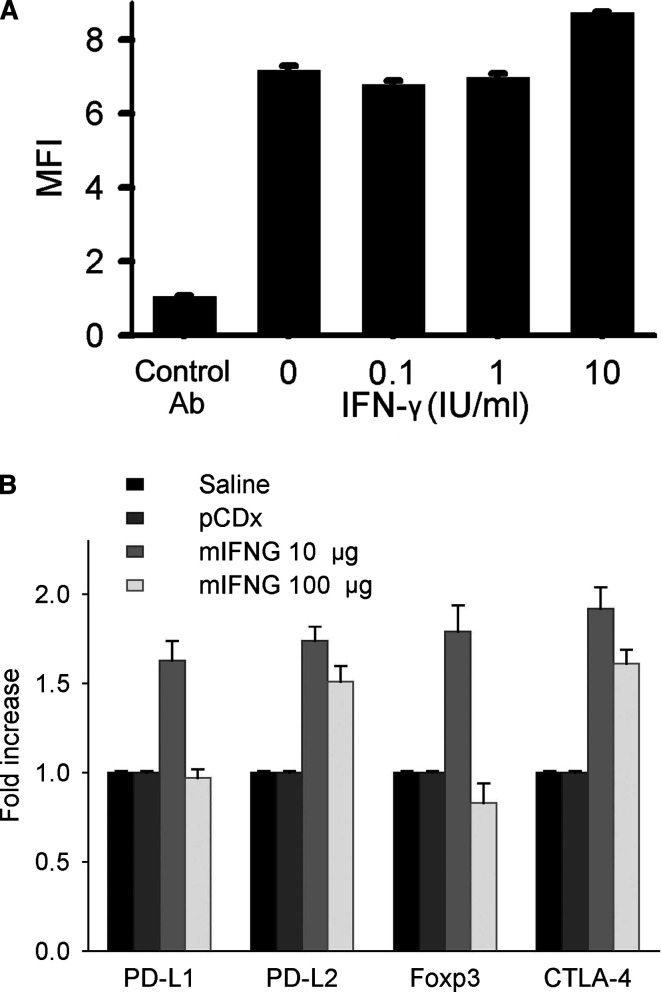
We then examined IFN-γ’s role in the regulation of the expression level of some genes involved in T cell immune tolerance. Previous studies showed that the negative signal provided by interactions of PD-1 and its ligands, costimulatory molecules PD-L1 and PD-L2, is involved in the mechanism of tumor immune evasion [4, 7, 8, 27]. We found that H22 tumor cells expressed PD-L1, which could be upregulated by treatment with IFN-γ (Fig. 5a), while PD-L2 was not expressed on H22 cells (data not shown). Both PD-L1 and PD-L2 mRNAs were expressed in peritumor tissues and upregulated (P<0.01) when low level of IFN-γ was expressed in local sites. However, such an effect was abolished (for PD-L1) or diminished (for PD-L2, P<0.05) when high level of IFN-γ was expressed (Fig. 5b). We also found that mRNA expression of Foxp3, a specific marker of regulatory T cells [11], was upregulated by low but not high level of IFN-γ in peritumor tissues. Consistent with this result, expression of CTLA-4, also an important marker of regulatory T cells, was upregulated by low level of IFN-γ and the upregulation was diminished at high level of IFN-γ (P<0.05, Fig. 5b), indicating that regulatory T cells may be involved in the mechanism of IFN-γ-induced tumor immune evasion. Taken together, these results suggest that low level of IFN-γ may induce T cell immune tolerance to tumor.
Fig. 5.
Interferon-γ promotes tumor evasion of the immune system. a H22 tumor cells were treated with recombinant mIFN-γ at indicated concentration and harvested 3 days later and stained for PD-L1 expression. Mean fluorescence intensity (MFI) of the total stained cells is shown. b Mice (n=5 per group) were treated as in Fig. 3a and PD-L1, PD-L2, Foxp3 and CTLA-4 mRNA expression in peritumor tissues by day 20 after tumor cell inoculation were examined by relative quantitative real-time PCR. Data in a are representative of three separate experiments. Data in b are representative of two experiments
To further determine whether the upregulated PD-L is involved in IFN-γ-induced tumor promotion, we used a plasmid, pPD-1A, which carries the cDNA encoding extracellular region of mouse PD-1 receptor, to make a sustained blockade of PD-L in local sites. Compared with the treatment with mIFNG plasmid alone, treatment with mIFNG plus pPD-1A plasmid significantly reduced the tumor incidence and inhibited the tumor growth (Fig. 6). These results thus support that PD-L/PD-1 pathway may be involved in the mechanism of IFN-γ-induced tumor immune evasion and suggest the possibility of using the blockade of this pathway to prevent and treat cancer. However, whether blockade of other molecules such as CTLA-4 also can inhibit IFN-γ’s tumor-promoting effect needs to be further studied.
Fig. 6.
Blockade of PD-L inhibits IFN-γ’s tumor-promoting effect. Mice (n=8 per group) were inoculated with 8×103 (left) or 5×104 (right) H22 tumor cells and treated with saline, 10 μg of mIFNG plasmid and 100 μg of pcDNA3.1 or pPD-1A plasmid for eight times. Tumor incidence by day 40 after inoculation (a) and mean tumor weight (MTW) by day 20 after inoculation (b) are shown. Data are representative of two experiments
Discussion
Generally, there are two stages of cancer development: initiation and progression. If cancer cells are not eliminated by the immune system at both stages, they may continue to grow and subsequently form tumor. Our study shows that when tumors are initiated (by the intramuscular injection of a small number of tumor cells), sustained low level of IFN-γ, as may occur in chronic inflammation, can promote tumor progression. As low level of IFN-γ can promote tumor evasion of immune system, we suppose that IFN-γ may also be involved in the tumor initiation process.
As IFN-γ is involved in many chronic inflammatory diseases including Helicobacter pylori-related gastritis [21], the inflammatory bowel diseases [3], chronic active hepatitis [26], and so on, which have strong association with malignant tumors [6], our finding that the sustained low-level expression of IFN-γ promotes tumor development provides a mechanistic link between chronic inflammation and cancer and also provides the potential applications for cancer prevention. Either strategy of anti-inflammatory or anti-IFN-γ therapy, or blockade of such negative immune regulators as PD-L may be useful for the prevention of cancer development in chronic inflammation associated with cancer risk.
Interferon-γ is also a cytokine involved in antitumor mechanisms of many other immunotherapy molecules such as IL-12 [19] and secondary lymphoid tissue chemokine (SLC) [23]. In our previous study, we demonstrated that immune evasion was enhanced during tumor immunotherapy by using SLC [10]. Thus, there is the possibility that IFN-γ produced in SLC therapy may account for the immune evasion. Similarly, it is important to evaluate the possibility whether IFN-γ’s tumor-promoting effect would occur during immunotherapy using other cytokines that could induce IFN-γ expression directly or indirectly.
A variety of strategies may be attributed to the IFN-γ’s tumor-promoting effect. These include downregulation of endogenous tumor antigen of some kinds of tumors, such as CT-26 colon carcinoma [2] and melanoma [17], and enhancing the survival of tumor cells partly through resistance to natural killer cell-mediated lysis[14]. In our study, we found that some negative costimulatory molecules were involved in the mechanisms of tumor immune evasion promoted by IFN-γ. It was not observed that IFN-γ could enhance the survival of H22 tumor cells in vitro. Whether the downregulation of the endogenous tumor antigen of the tumors by IFN-γ is related to the expression level of IFN-γ in vivo needs to be further explored.
Generally, use of high dose of cytokines often results in severe side effects and toxicity [5]. Our previous study also showed that high doses of SLC induced tumor immune evasion [10]. In this study, however, we showed that the cytokine IFN-γ, if expressed at a relatively stable low level, had no antitumor effect, but on the contrary could promote tumor immune evasion and enhance the tumor development. This suggests that using low dose of certain cytokines could also result in severe side effects. As cytokines have been widely employed as biological drugs for cancer therapy, use of the optimal dose is extremely important. The dose of cytokines used needs to be optimized according to the different cytokines used (and also may be different immune state).
Acknowledgments
We thank S. Hemmi, University of Zurich, for providing mouse IFNGR1-encoding cDNA and Hui-Fen Zhu for FACS analysis. This work was supported by the National Development Program (973) For Key Basic Research (2002CB513100) of China.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001;357:539. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Beatty GL, Paterson Y. IFN-gamma can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J Immunol. 2000;165:5502. doi: 10.4049/jimmunol.165.10.5502. [DOI] [PubMed] [Google Scholar]
- 3.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 4.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 5.Chada S, Ramesh R, Mhashilkar AM. Cytokine- and chemokine-based gene therapy for cancer. Curr Opin Mol Ther. 2003;5:463. [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 8.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 9.Fountoulakis M, Schlaeger EJ, Gentz R, Juranville JF, Manneberg M, Ozmen L, Garotta G. Purification and biochemical characterization of a soluble mouse interferon-gamma receptor produced in insect cells. Eur J Biochem. 1991;198:441. doi: 10.1111/j.1432-1033.1991.tb16034.x. [DOI] [PubMed] [Google Scholar]
- 10.He YF, Zhang GM, Wang XH, Zhang H, Yuan Y, Li D, Feng ZH. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol. 2004;173:4919. doi: 10.4049/jimmunol.173.8.4919. [DOI] [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 12.Horton HM, Anderson D, Hernandez P, Barnhart KM, Norman JA, Parker SE. A gene therapy for cancer using intramuscular injection of plasmid DNA encoding interferon alpha. Proc Natl Acad Sci USA. 1999;96:1553. doi: 10.1073/pnas.96.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 14.Kelly SA, Gschmeissner S, East N, Balkwill FR. Enhancement of metastatic potential by gamma-interferon. Cancer Res. 1991;51:4020. [PubMed] [Google Scholar]
- 15.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 16.Lawson BR, Prud’homme GJ, Chang Y, Gardner HA, Kuan J, Kono DH, Theofilopoulos AN. Treatment of murine lupus with cDNA encoding IFN-gammaR/Fc. J Clin Invest. 2000;106:207. doi: 10.1172/JCI10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Poole IC, Riker AI, Quevedo ME, Stennett LS, Wang E, Marincola FM, Kast WM, Robinson JK, Nickoloff BJ. Interferon-gamma reduces melanosomal antigen expression and recognition of melanoma cells by cytotoxic T cells. Am J Pathol. 2002;160:521. doi: 10.1016/s0002-9440(10)64871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourich DV, Munks MW, Murphy JC, Willson RC, Hill AB. Spermine compaction is an efficient and economical method of producing vaccination-grade DNA. J Immunol Methods. 2003;274:257. doi: 10.1016/s0022-1759(02)00516-1. [DOI] [PubMed] [Google Scholar]
- 19.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697. [PubMed] [Google Scholar]
- 20.Nomura T, Yasuda K, Yamada T, Okamoto S, Mahato RI, Watanabe Y, Takakura Y, Hashida M. Gene expression and antitumor effects following direct interferon (IFN)-gamma gene transfer with naked plasmid DNA and DC-chol liposome complexes in mice. Gene Ther. 1999;6:121. doi: 10.1038/sj.gt.3300792. [DOI] [PubMed] [Google Scholar]
- 21.Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70:3295. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okayama H, Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983;3:280. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX, Batra RK, Dubinett SM. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol. 2000;164:4558. doi: 10.4049/jimmunol.164.9.4558. [DOI] [PubMed] [Google Scholar]
- 24.Stordeur P, Poulin LF, Craciun L, Zhou L, Schandene L, de Lavareille A, Goriely S, Goldman M. Cytokine mRNA quantification by real-time PCR. J Immunol Methods. 2002;259:55. doi: 10.1016/S0022-1759(01)00489-6. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi K, Petersson M, Hoglund P, Kiessling R, Klein G, Karre K. Interferon gamma induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. Proc Natl Acad Sci USA. 1987;84:3405. doi: 10.1073/pnas.84.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyonaga T, Hino O, Sugai S, Wakasugi S, Abe K, Shichiri M, Yamamura K. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc Natl Acad Sci USA. 1994;91:614. doi: 10.1073/pnas.91.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462. [PubMed] [Google Scholar]