Abstract
Adjuvant treatment is still only working in a small percentage of breast cancer patients. Therefore, new strategies need to be developed. Immunotherapies are a very promising approach because they could successfully attack tumor cells in the stage of dormancy. To assess the feasibility of using an allogeneic approach for vaccination of breast cancer patients, we selected a CD80-transfected breast cancer cell line based on its immunogenic properties. Using CD80+ KS breast cancer cells and human leukocyte antigen (HLA)-A*02–matched peripheral blood mononuclear cells (PBMCs) of breast cancer patients in allogeneic mixed lymphocyte–tumor cell cultures (MLTCs), it was possible to isolate HLA-A*02–restricted cytotoxic T cells (CTLs). Furthermore, a genetically modified KS variant expressing influenza A matrix protein serving as a surrogate tumor-associated antigen (TAA) was able to stimulate flu peptide-specific T cells alongside the induction of alloresponses in MLTCs. KS breast cancer cells were demonstrated to express already known TAAs such as CEA, MUC-1, MAGE-1, MAGE-2, and MAGE-3. To further improve antigenicity, HER-2/neu was added to this panel as a marker antigen known to elicit HLA-A*02–restricted CTLs in patients with breast cancer. Thus, the antigen-processing and antigen-presentation capacity of KS cells was further demonstrated by the stimulation of HER-2/neu–specific CD8+ T cells in PBMCs of breast cancer patients in vitro. These results gave a good rationale for a phase I/II trial, where the CD80+ HER-2/neu–overexpressing KS variant is actually used as a cellular vaccine in patients with metastatic breast cancer. As a proof of principle, we present data from two patients where a significant increase of interferon-γ (IFN-γ) release was detected when postvaccination PBMCs were stimulated by allogeneic vaccine cells as well as by HLA-A*02–restricted HER-2/neu epitopes. In whole cell vaccine trials, monitoring is particularly challenging because of strong alloresponses and limited knowledge of TAAs. In this study, a panel of HER-2/neu epitopes, together with the quantitative real time (qRT)-PCR method to analyze vaccine-induced cytokines secreted by T cells, proved to be highly sensitive and feasible to perform an “immunological staging” following vaccination.
Keywords: Breast cancer, Cellular vaccines, HER-2/neu, Immunotherapy, T-cell diagnostic
Introduction
The rationale behind the use of allogeneic tumor cell lines as therapeutic vaccines is that multiple antigens shared by both the immunizing cell line and the patient’s tumor are presented by shared human leukocyte antigen (HLA) molecules. However, most tumor cells are of poor immunogenicity per se and do not effectively induce antigen-specific immune responses. This is frequently due to low densities of HLA/antigen complexes and a lack of costimulatory molecules at their cell surfaces [7, 8, 23, 45, 46, 55]. Earlier studies in mouse models revealed that tumor cells modified to express CD80 costimulatory molecules were capable of inducing immune responses in naïve animals that could protect against challenge with unmodified tumor cells [2, 7, 22, 35, 55]. Introduction of CD80 into several human tumor cell lines also improved their immunogenicity and in some cases led to the activation of specific cytotoxic T cells (CTLs) in vitro [24, 28, 31, 39, 56]. For clinical vaccination studies, such allogeneic approaches have advantages in that in vitro genetically modified and well-characterized tumor variants can be selected for their immunogenicity and easily expanded under GMP conditions. This would circumvent the elaborative and inefficient generation of individual tumor vaccines and guarantee that a rather larger group of patients expressing frequent HLA alleles will obtain a standardized treatment. However, it has been speculated that strong allogeneic responses by patient’s T cells directed against mismatched HLA molecules of the cellular vaccine may be dominant, thus, weaker tumor antigen–associated responses either will not develop at all or will not be detectable. In contrast, several in vitro immunization approaches, as well as animal models using partial HLA-matched tumor cells as stimulators, suggest that allogeneic responses support the generation of antigen-specific T cells [6, 9, 31, 33, 39, 42, 49, 52, 54].
To assess the feasibility of using an allogeneic approach for vaccination of breast cancer patients we selected a breast cancer cell line based on its immunogenic properties. The cell line KS was established from a malignant effusion and was found to express an array of tumor-associated antigens (TAAs) such as MUC-1, CEA, SSX-2, and members of the MAGE family which are known to be expressed ubiquitously by breast carcinoma tissues. As a result of interferon γ (IFN-γ) stimulation, the KS cells express high levels of HLA molecules [32], which probably present multiple peptide ligands for specific T-cell activation. This KS cell line was genetically modified to express CD80 providing costimulatory signals to T lymphocytes [18, 19], and further transfected to overexpress HER-2/neu in order to provide a well-characterized TAA as a marker antigen for immunodiagnostics. HER-2/neu is an oncogene with extensive homology to the epidermal growth factor (EGF) receptor and is known to be overexpressed in approximately 25% of all ovarian and breast cancers. Preexisting cellular and humoral immunity has been observed in cancer patients confirming the immunogenicity of HER-2/neu and encouraging vaccination trials with HER-2/neu–derived proteins and peptides (reviewed in [3]). In this context, several HLA-A*02–restricted epitopes of HER-2/neu are characterized, providing exellent diagnostic tools for the analysis of vaccine-induced T cells using allogeneic but HLA-matched immunocompetent cell variants (reviewed in [43]).
We demonstrated that KS breast cancer cells have antigen-processing and presentation capacity as well as the potential to stimulate TAA-specific effector T cells in vitro. These results gave a good rationale for a phase I/II trial, where a CD80+ HER-2/neu-overexpressing KS variant is used as a cellular vaccine in patients with metastatic breast cancer. Here, we would like to share the first clinical results as a proof of principle demonstrating the induction of HLA-A*02–restricted HER-2/neu-specific T cells following vaccination with an HLA-A*02–matched CD80-transfected tumor cell vaccine, as shown, for example, in two selected patients.
Material and methods
Cell lines
The cell line K562 and T2 cells were purchased from the American Type Cell Collection (ATCC, Rockville, MD, USA). T2, a TAP-deficient and antigen-processing defective T/B-hybrid cell line, does express HLA-B/C at rather low levels and HLA-A*02 at higher levels [47]. The Epstein-Barr virus–transformed B lymphoid cell line LAZ509 was a gift of U. Moebius (German Cancer Research Center, Heidelberg, Germany). LAZ509, T2, and K562 were maintained as suspension cultures in RPMI 1640 medium containing 10% FCS, 4 mM L-glutamine, and 1% penicillin/streptomycin (all from Invitrogen, Paisley, UK). The breast carcinoma cell lines KS and AR were established in our laboratory from a malignant effusion and from a metastasis at the primary site of a tumor, respectively [18, 32]. SkBr3.A2, a HLA-A*02–expressing variant of the breast cancer cell line, was provided by S. Kaul (Department of Obstetrics and Gynecology, University of Heidelberg). SW480, a colon carcinoma cell line, was purchased from the tumor cell collection of the German Cancer Research Center. All tumor cell lines were kept in DMEM supplemented as described above and maintained adherently as monolayers.
Previous work has demonstrated the influence of cytokines on the expression of HLA antigens and adhesion receptors of tumor cell lines—resulting in augmented T-cell stimulation [18, 32]. Cytokine pretreated tumor cells were obtained by incubating the cells overnight with 250 U/ml IFN-γ (Roche, Mannheim, Germany).
Transfections and KS variants
HLA-typing of KS cells was performed in the Institute of Immunology, University of Heidelberg, Germany: HLA-A*0201, x; HLA-B*35x, 40x; DRB1*15011, 1302; DQB1*0602, 06041.
The CD80-transfected subline of KS (KS24) has been described previously and was maintained as per their nontransfected parental counterparts, with the exception of G418 supplemented at 0.5 mg/ml (Invitogen) [18]. The KS24 cell line was further transfected using a plasmid coding for HER-2/neu, generating the KS24.22 cell variant. Briefly, the cDNA of HER-2/neu was amplified by PCR in two fragments coding for amino acids 1–813 (primer 1: 5′-CCAAGCTTATGGAGCTGGCGGCCTTGTGCCGCTGG-3′, containing HindIII restriction site; primer 2: 5′-GGCCGCGGTTTTCCCGGACATGGTCTAAGAGG-3′, containing SacII-restriction site) and amino acids 813–1256 (primer 3: 5′-GGCCGCGGACGCCTGGGCTCCCAGGACCTG-3′; primer 4: 5′-CCGCGGCCGCTCACACTGGCACGTCCAGACCCAGG-3′, includes NotI restriction site after stop codon). In a three-fragment ligation step the HER-2/neu-coding sequences were subcloned into the pcDNA3.1-Zeo expression vector (all reagents by Promega, Madison, USA). KS24 cells (1.5×107) were stably transfected using 15 μg pcDNA3-1-Zeo/HER-2 by electroporation using a Gene Pulser (Biorad, München, Germany) at 100 Ω, 500 μF, and 380 V in PBS. HER-2/neu–expressing cells were expanded in the presence of G418 and Zeocin (all Invitrogen) which were supplemented at 0.5 and 0.25 mg/ml, respectively.
The matrix protein (MP, here M1 of influenza A virus)–transfected subline of KS24 (KS24-M1) has been described previously [39]. Briefly, MP cDNA was cloned into the plasmid pUHD-10.1 [10]. KS24 cells were cotransfected with 25 μg pUHD-10.1-M1 and 2.5 μg pX242 (providing hygromycin resistance) using Lipofectin (Invitrogen) according to the manufacturer’s protocol. Transfectants were selected using 100 μg/ml hygromycin (Merck, Darmstadt, Germany). MP expression was controlled by Western blot.
Flow cytometry
Adherent tumor cells were harvested using PBS w/o Mg2+/Ca2+, 1% EDTA (all from Invitrogen), and incubated with the anti-HER-2/neu monoclonal antibody (mAb) c-neu Ab5 according to the manufacturer’s instructions (Oncogene, San Diego, CA, USA). For indirect immunofluorescence, PE-labeled goat-F(ab′)2antimouse immunoglobulin (Ig) (DAKO, Hamburg, Germany) was used as second stage reagent. Background fluorescence was determined by secondary Ab staining alone. Events numbering 1×105 were acquired for each analysis on a FACS Calibur cytometer (Becton Dickinson, Heidelberg, Germany); dead cells were excluded by appropriate gating. The data are presented as histograms showing mean fluorescence intensities.
Blood samples, patients and clinical immunization protocol
Healthy blood donors were selected on the basis of HLA-A*02 antigen expression determined by FACS staining using BB7.2 mAb (hybridoma supernatant, ATCC).
Here, peripheral blood mononuclear cells (PBMCs) of two patients enrolled in a still-ongoing clinical vaccination trial were chosen as an example for HER-2/neu–specific T-cell diagnosis prevaccination and postvaccination. Therefore, the study design is described briefly: women with metastatic breast cancer were enrolled in a phase I/II vaccination trial using a CD80-modified HLA-A*02+ allogeneic breast cancer cell line (KS24.22) [20]. Eligibility criteria included the following: (1) histologically proven, measurable metastatic breast cancer, (2) patient already received either anthracyclin- or taxane-based chemotherapy, (3) HLA-A*0201 positivity, (4) in vitro activation of patient’s T cells by mitogenic antibodies and KS24.22 vaccine cells, and (5) written informed consent. Patients were excluded for the following reasons: (1) immunosuppressive or autoimmune diseases, (2) active acute or systemic infections, (3) chemotherapy or radiotherapy within 4 weeks, or (4) treatment with antibodies, cytokines, or other immune therapies within 6 weeks. Patients received 107 lethally irradiated KS24.22 cells from a GMP working cell bank per injection. The first four vaccinations, which were given every 2 weeks intradermally, were followed by four monthly vaccinations. PBMCs obtained by venipuncture prior to treatment and periodically during the vaccination protocol were cryopreserved for subsequent analyses. Written informed consent was obtained from all patients according to the policies of the local ethical committee. The protocol was also approved by the Paul-Ehrlich-Institut (Langen, Germany) and the Committee for Somatic Gentherapy of the Deutsche Ärztekammer (Köln, Germany).
HLA-A*02–restricted synthetic peptides
All peptides were kindly provided by S. Stevanoviç (Department of Immunology, Institute for Cell Biology, University of Tübingen, Germany). Peptides were synthesized by solid-phase Fmoc chemistry using peptide synthesizer 432A (Applied Biosystems, Foster City, CA, USA). Identity and purity (>90%) were analyzed by reversed-phase HPLC and matrix-assisted laser desorption/ionization/time of flight (MALDI-TOF) mass spectrometry. Peptides were dissolved in DMSO at 10 mg/ml, further diluted in H2Obidest to a final concentration of 1 mg/ml, and stored at −20°C. The following HLA-A*0201–binding peptides were used: HER-2/neu369-377 (“E75”, KIFGSLAFL); HER-2/neu654-662 (“GP2”, IISAVVGIL), HER-2/neu789-797 (CLTSTVQLV) [15, 16]; HER-2/neu1023-1032 (YLVPQQGFFL) [48]; HER-2/neu752-761 (VMAGVGSPYV) [37]; MUC-1950-958 (STAPPVHNV); MUC-112-20 (LLLLTVLTV) [5]; influenza matrix protein flu58-66 (GILGFVFTL); flu57-68 (KGILGFVFTLTV) [4]; and RNA-dependent helicase p68146-154 (YLLPAIVHI) [50].
The antigen-pulse of T2 cells or tumor cell variants was performed in serum-free X-Vivo medium (BioWhittaker, Verviers, Belgium) at a peptide concentration of 5–10 μg/ml for 2 h.
T-cell cultures
PBMCs were isolated from heparinized blood by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density centrifugation, washed twice with PBS, and cryopreserved in fetal calf serum (FCS), 10% DMSO (all from Invitogen). Vials contained 5–10×106 PBMCs/ml and were frozen gradually to −80°C prior to storage in liquid nitrogen.
For mixed lymphocyte–tumor cell cultures (MLTCs) T cells were enriched by plastic adherence followed by rosetting with sheep erythrocytes. T cells were >90% CD3+, >95% CD2+, and <1% CD14+. T cells (106) were cocultured in 24 wells with γ-irradiated (200 Gy), IFN-γ–pretreated KS cells or variants derived thereof in weekly intervals at a T lymphocyte to tumor cell ratio of 10:1. T-cell lines were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated human AB serum (HS; ccPro, Neustadt, Germany), 1% penicillin/streptomycin, and 4 mM L-glutamine (all from Life Technologies) which contained 30–50 U/ml IL-2 after the first restimulation. T-cell lines were analyzed by ELISpot assay or cytotoxic T-cell assay. T-cell cloning was performed in V-bottomed 96-well plates by limiting dilution using 5×103 inactivated tumor cells as stimulator cells together with a feeder cell layer consisting of inactivated 5×104 allogeneic PBMCs and 3×104 cells of an immortalized B-cell line. Medium was supplemented with 50–100 U/ml interleukin-2 (IL-2) and 1 μg/ml phytohemagglutinin (PHA)-L in the beginning.
Frozen PBMCs of KS24.22-vaccinated patients collected during our clinical study were thawed and seeded into round-bottomed 96-well plates in RPMI 1640 medium as described above. At a concentration of 1×105 cells per well, PBMCs were cultured overnight for reconstitution. PBMCs containing CD8+ effector T cells as well as subsets of antigen-presenting cells (APCs) were stimulated directly with peptide antigens added in final concentrations of 5 μg/ml without the addition of further cytokines. Presensitized PBMCs were harvested after 1 week of peptide stimulation and restimulated for an additional 2 h using peptide-pulsed T2 cells as APCs, before RNA isolation and further functional analysis by quantitative RT-PCR [44].
Proliferation assay
For assessment of proliferation, T cells were cultured at 1×105 cells/well in 96-well flat-bottomed microtiter plates (Nunc, Roskilde, Denmark) together with 1×104 mitomycin C inactivated stimulator cells as indicated. Cell cultures were incubated for 5 days and pulsed for an additional 18 h with 37 kBq of [3H]thymidine (74.0 GBq/mM; New England Nuclear, Boston, USA) per well. Thymidine incorporation was determined by liquid scintillization counting in triplicates and measured as mean counts per minute (cpm). Stimulator cell background was subtracted from all values. To facilitate a comparative analysis of different proliferative activities, results were expressed as stimulative index (SI), where SI = T cells stimulated (cpm) divided by unstimulated T cells (cpm).
Cytotoxic T-cell assay
The cytolytic activity of T-cell lines and clones was tested in a nonradioactive cytotoxicity assay detecting the release of lactate dehydrogenase (LDH) (CytoTox 96; Promega). Briefly, 5–10×103 target cells were plated in V-bottomed 96-well plates in triplicates. T2 cells were pulsed before with 10 μg/ml peptide. Varying numbers of CTLs were added to a final volume of 150 μl and incubated for 4 h at 37°C. Supernatants were harvested and LDH was quantified by fluorescence measurement. Spontaneous and maximal LDH release of target cells alone as well as calculations of specific lysis were determined according to the manufacturer’s instructions.
For antibody inhibition experiments, target cells were preincubated for 30 min (4°C) with anti-HLA-A*02 mAb (BB7.2, hybridoma supernatant) or isotype antibody (murin IgG1 4F7, kindly provided by C. Sachsenmmaier, Clinic of Tumor Biology, Freiburg, Germany).
IFN-γ enzyme-linked immunospot (ELISpot) assay
Enzyme-linked immunospot assay was carried out as described before [44]. In brief, multiscreen 96-well nitrocellulose plates (Millipore, Bedford, MA, USA) were coated overnight at 4°C with antihuman IFN-γ mAb (1-D1 K, 2 μg/ml; Mabtech, Stockholm, Sweden) diluted in 0.1 M carbonate-bicarbonate buffer (pH 9.6). Plates were blocked with RPMI 1640 containing 10% HS for 2 h at 37°C. Different numbers of T cells stimulated with KS variants or peptide-pulsed autologous PBMCs were added to each well and coincubated with 3.5×104 peptide-pulsed T2 cells for 18 h in RPMI 1640, 10% HS. Background controls were performed using p68-loaded T2 cells. As positive controls, T cells were stimulated with anti-CD3/anti-CD28-coated magnetic beads (Miltenyi, Bergisch-Gladbach, Germany) (data not shown). After incubation, plates were washed extensively with PBS, 0.05% Tween 20 (Sigma, Taufkirchen, Germany), and were further incubated with 100 μl/well anti-IFN-γ Ab (7-B6-1-biotin, 0.2 μg/ml; Mabtech). After incubation for 3 h at room temperature, plates were washed and developed for additional 2 h with streptavidin-alkaline phosphatase (1 μg/ml; Mabtech). Spots were visualized by adding the substrate (5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium) (Sigma) and counted using an automated ELISpot reader (Zeiss Vision, Göttingen, Germany). Numbers of spot-forming T cells in response to the control peptide p68 are subtracted from indicated frequencies of T cells reactive to the antigen of interest.
Quantification of antigen-induced IFN-γ–specific mRNA by real time (RT) PCR
PBMCs that experienced a peptide presensitization were restimulated with 1×104 peptide-loaded T2 cells per well as described previously [44]. Briefly, after 2 h of restimulation 1–3×105 cells were harvested, lysed in 800 μl Trizol (Invitrogen), and stored at −80°C. Total RNA was isolated using Trizol according to the manufacturer’s instructions for small cell numbers (Invitrogen). After extraction, RNA was resuspended in 10 μl RNAse-free water (Promega). Five microliters of RNA was reverse transcribed into cDNA using the Omniscript Kit (Qiagen, Hilden, Germany) and 10 U RNAsin (Promega) according to manufacturer’s instructions and stored at −20°C until use. The quantification of IFN-γ gene expression was performed by using the ABI Prism 7700 sequence detection system (PerkinElmer, Foster City, CA, USA) with probes and primers as described elsewere [29, 30, 34, 44]. The granzyme B probes and primers were as follows: 5′-TGCAACCAATCCTGCTTCTG-3′ (forward), 5′-CCGATGATCTCCCCTGCAT-3′ (reverse), FAM-TGGCCTTCCTCCTGCTGCCCAG-TAMRA (probe). Amplification of a CD8 sequence for normalization was done in separate tubes with primers and probes as described before [29]. Probes were labeled at the 5′ end with the reporter dye molecule FAM (6-carboxy-fluorescein; emission λmax=518 nm), and at the 3′ end with the quencher dye molecule TAMRA (6-carboxytetramethyl-rhodamine; emission λmax=582 nm).
The qRT-PCR was performed with the TaqMan Universal Master Mix (Applied Biosystems) using 5 μl of 1:10 diluted cDNA, fluorescence-labeled probe at a final concentration of 150 nM, and primers at final concentrations of 400 nM in a reaction volume of 20 μl. Cycling conditions were as follows: one cycle (50°C, 2 min; 95°C, 10 min) followed by 40 cycles (95°C, 15 sec; 60°C, 1 min). Cycle threshold (CT)-values of IFN-γ and granzyme B were normalized to CT values of CD8. IFN-γ–specific and granzyme B–specific mRNA expressions were presented as an x-fold increase (stimulation index) in comparison to the p68 stimulations. A reaction was considered as positive if it was twofold above the background control (p68 stimulation) plus two times the standard deviation [44].
Results
The CD80+ KS breast cancer cell variant activates HLA-A*02–restricted CTLs in allogeneous MLTCs
In contrast to the nontransfected parental breast cancer line KS, its CD80 expressing variant KS24 was previously described to induce strong T-cell proliferation in allogeneic MLTC of healthy donors. These studies emphasize the requirement for CD80-mediated costimulation in the induction phase of an antitumoral immune response [18, 19]. Using T cells of breast cancer patients we could confirm these results. Table 1 summarizes examples of 15 patients demonstrating that CD80+ KS transfectants induced a marked T-cell proliferation in 11/15 cases, in contrast to the untransfected KS parental line which did not (Table 1).
Table 1.
CD80 expression by breast cancer cell line KS enhances its stimulatory capacity
| Breast cancer patient | Stimulator cellsa | |||
|---|---|---|---|---|
| KS | KS-mock | KS-CD80 | LAZ509 | |
| BCP1 | 2.3b | 3.3 | 78.4 | 770.0 |
| BCP2 | 0 | 0.1 | 78.4 | 263.8 |
| BCP3 | 0.1 | 0 | 0.3 | 1,148.0 |
| BCP4 | 0.5 | 0.8 | 326.8 | 1,521.6 |
| BCP5 | 2.9 | 1.5 | 73.0 | 2,376.0 |
| BCP6 | 1.6 | 0.7 | 25.8 | 58.7 |
| BCP7 | 0.2 | 0.1 | 34.7 | 231.6 |
| BCP8 | 2.0 | 1.7 | 84.0 | 31.0 |
| BCP9 | 0 | 1.2 | 25.0 | 317.0 |
| BCP10 | 0.1 | 0.1 | 22.0 | 57.0 |
| BCP11 | 0.3 | 2.0 | 3.0 | 108.0 |
| BCP12 | 1.0 | 0.5 | 5.0 | 59.0 |
| BCP13 | 0.3 | 1.0 | 17.0 | 61.0 |
| BCP14 | 3.1 | 2.8 | 1.5 | 24.1 |
| BCP15 | 7.7 | 6.4 | 11.1 | 99.7 |
aT cells of breast cancer patients were stimulated with the allogeneic KS cell line, its CD80-transfected variant, or a mock-transfectant (all IFN-γ-pretreated). The allogeneic B-cell line LAZ509 was included as a positive control
bProliferation of T cells was assessed by [3H]thymidine incorporation after 5 days of coculture. Proliferation rates were given as stimulative index (SI). For details see “Material and methods”
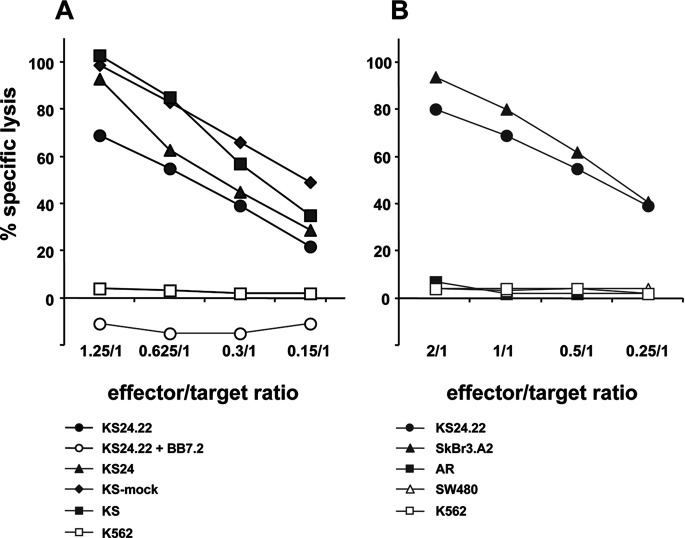
To further estimate likely HLA-A*02–restricted CTL induction in a more clinical situation, T lymphocytes from two HLA-A*02+ breast cancer patients (BCP1, BCP2) were further stimulated in long-term allogeneic MLTCs with the KS24 breast cancer cell variant. T cell lines generated from both patients showed strong lysis of KS24 target cells and no natural killer (NK) cell activity (Fig. 1). Cultures stimulated with unmodified KS cells could not be propagated, instead the cells gradually died (not shown). As shown in Fig. 1, HLA-A*02–specific mAbs inhibit cytolysis of KS24 by >50%. The T-cell line of patient BCP2 was further subcloned generating the CTL clone P2 which was clearly HLA-A*02 restricted as demonstrated by BB7.2-mAb–blocking (Fig. 2a). Noteworthy is the fact that the CD80− KS parental cells were lysed as effectively as its CD80-expressing variants, indicating that CD80 plays no dominant role in the effector phase of T-cell activation (Fig. 2a). The HLA-A*02+ colon carcinoma cell line SW480 was not recognized (Fig. 2b).
Fig. 1.
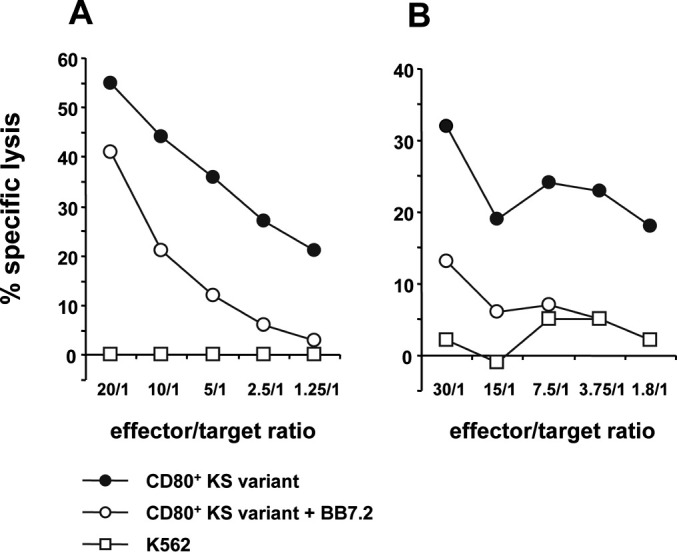
Induction of HLA-A*02–restricted CTLs by CD80+ KS variant in allogeneic MLTCs. CD8+ T lymphocytes of two different HLA-A*02+ breast cancer patients, patients BCP1 (a) and BCP2 (b), were stimulated in an allogeneic MLTCs culture using IFN-γ–pretreated CD80+ KS transfectants as stimulator cells. The generated T-cell lines were tested after four restimulation cycles in a LDH release assay. Cytolysis was determined on the NK-sensitive cell line K562 (open squares) and CD80+ KS transfectants, either BB7.2 mAb pretreated (open circles) or not (filled circles).
Fig. 2.
HLA-A*02 restriction and lytic profile of KS24.22-stimulated CTL clone P2. The CTL clone P2 was generated by KS24.22 stimulation as described in “Materials and methods” and tested in a LDH release assay. a Cytolysis was determined on the KS24.22 transfectants (HLA-A*02+), either BB7.2 mAb pretreated (open circles) or not (filled circles), on KS24 transfectants (filled triangles), KS-mock transfectants (filled diamonds), on the parental cell line KS (filled squares), and on K562 (open squares). b The cytolytic profile of the CTL clone P2 was further analyzed using the breast cancer lines SkBr3.A2 (HLA-A*02+) (filled triangles) and AR (HLA-A*02−) (filled squares), as well as the colon carcinoma cell line SW480 (HLA-A*02+) (open triangles). KS24.22 killing was included as positive control (open squares).
The CD80+ KS breast cancer variant activates peptide-specific T cells following endogeneous antigen processing
Besides the lack of costimulatory molecules, tumor cells are often described to have less immunostimulating potential because of defects in antigen-processing and weak HLA expression. The MP of influenza A virus was used as a surrogate antigen to confirm the antigen-processing capacity of KS24 breast cancer cells. KS24 cells were either exogenously loaded with the HLA-A*02–restricted peptide MP57-68 or transfected using an M1-including expression vector, and subsequently used as APCs in an HLA-A*02–matched MLTC. After restimulation, MP57-68-specific T cells were quantified in an IFN-γ ELISpot assay. Both, exogenously antigen-loading as well as endogenously antigen-processing of KS24 cells resulted in MP57-68-specific T-cell activation. As a positive control, peptide-pulsed autologous PBMCs were used as APCs, which stimulated antigen-specific T cells at a comparable degree (Table 2). As expected, M1-transfected tumor cells had less stimulative potency, probably due to lower HLA/antigen surface density. In this setting the frequencies of KS24-reactive T cells run up to >500/10,000.
Table 2.
Induction of MP57-68-specific T cells by allogeneic KS24 breast cancer cells following exogenous antigen loading or endogenous antigen processing. NT Not tested
| Number of effector cells tested per well | Frequencies of MP57-68-specific T cells as determined by IFN-γ secretion in ELISpot assaya | ||||
|---|---|---|---|---|---|
| Stimulib | |||||
| PBMC/p68 | PBMC/MP57-68 | KS24-mock/p68 | KS24/MP57-68 | KS24-M1 | |
| 10,000 | 0 | 101 | 0 | 76 | 27 |
| 3,000 | 0 | 31 | 0 | 26 | 11 |
| 1,000 | 0 | 12 | 0 | 12 | 5 |
| 300 | 1 | 5 | 0 | NT | 2 |
aT cells of a HLA-A*0201+ healthy donor were stimulated weekly using different stimulator cells. Frequencies of MP57-68-specific T cells were determined by IFN-γ ELISpot out of different MLTCs on day 14 using MP57-68-pulsed T2 cells as targets. T cells responding to the control peptide p68 are subtracted from T cells reactive to MP57-68
bT cells were stimulated respectively, with unpulsed and MP57-68-pulsed autologous PBMCs, unpulsed and MP57-68-pulsed IFN-γ–pretreated KS24 cells (mock transfectants), and IFN-γ–pretreated KS24 cells transfected with M1
HLA-A*02–restricted HER-2/neu–specific T cells are generated in allogeneic MLTCs by using the KS24.22 breast cancer variant
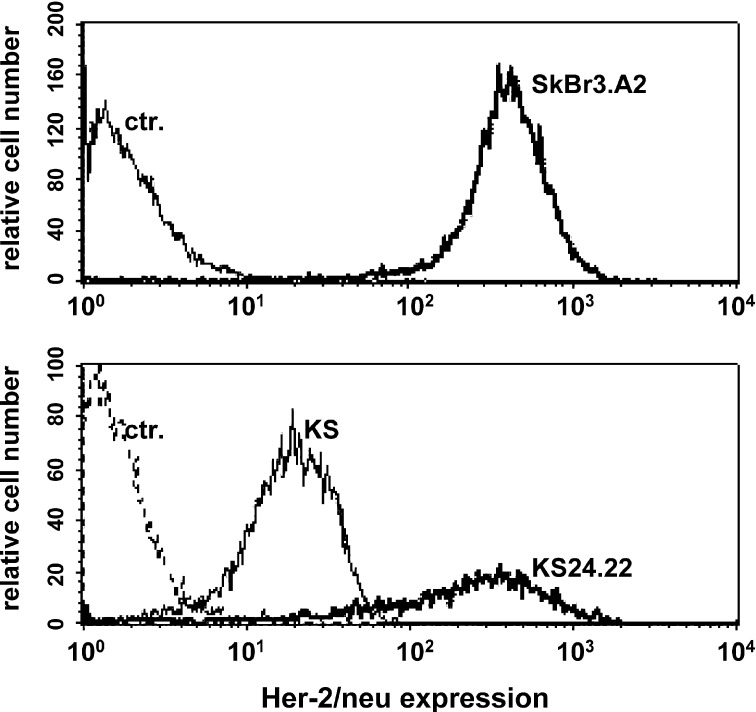
KS cells were demonstrated to express already known TAAs such as CEA, MUC-1, and MAGE-1, MAGE-2, MAGE-3 [32]. HER-2/neu was added to this panel known to elicit HLA-A*02–restricted CTLs in patients with breast cancers. KS24 cells were transfected using a HER-2/neu–including expression vector, generating the KS24.22 cell variant. Its HER-2/neu overexpression was confirmed by FACS staining in comparison to the well-characterized HER-2/neu+ breast cancer line SkBr3.A2 (Fig. 3). KS24.22 cells were used to stimulate PBMCs of breast cancer patients in a HLA-A*02–matched MLTC. The generated T-cell lines were tested for HLA-A*02–restricted HER-2/neu specificity using peptide-pulsed T2 cells as targets in an IFN-γ ELISpot assay. In addition, patient’s T cells were analyzed directly ex vivo. Table 3 summerizes the data of a representative patient. In contrast to MP58-66-specific T cells, HER-2/neu–specific T cells could not be detected without antigen presensitization in vitro. After three rounds of stimulation in MLTCs, T cells with specificity against two of three tested HLA-A*02–restricted HER-2/neu epitopes could be quantified at evident low frequencies (40–72/500,000). As expected, higher frequencies of MP58-66-specific T cells could be detected ex vivo as well as after stimulation by MP58-66-loaded KS24.22 (Table 3). They could be expanded under these conditions, yielding a substantial number of MP58-66-specific T cells on day 28.
Fig. 3.
HER-2/neu overexpression of KS24.22 transfectant. HER-2/neu–specific immunofluorescence of HER-2/neu–transfected KS24.22 cells, together with nontransfected parental cell KS. The SkBr3.A2 breast cancer cell line, which expresses constitutively high levels of HER-2/neu, was included as a positive control.
Table 3.
Induction of HLA-A*02–restricted, HER-2/neu–specific T cells by allogeneic KS24.22 breast cancer cells in vitro
| Stimulus in MLTC | Target antigen in ELISpotb | Frequencies of IFN-γ–secreting T cells in 100,000 or 500,000 effector cellsa | ||
|---|---|---|---|---|
| Day 28 | Day 14 | Day 0 (ex vivo) | ||
| KS24.22/MP58-66 | MP58-66 | 1,800/100,000c | 700/100,000 | 56/100,000 |
| KS24.22 | HER-2/neu369-377 | 0/500,000 | 0/500,000 | 0/500,000 |
| KS24.22 | HER-2/neu654-662 | 40/500,000 | 0/500,000 | 0/500,000 |
| KS24.22 | HER-2/neu1023-1032 | 72/500,000 | 0/500,000 | 0/500,000 |
aT cells of a HLA-A*0201+ breast cancer patient were stimulated weekly using either unpulsed or MP58-66-pulsed IFN-γ–pretreated KS24.22 cells. Frequencies of MP58-66-specific or HER-2/neu–specific T cells were determined by IFN-γ ELISpot ex vivo (without stimulation), on day 14, and day 28 (T cells in different MLTC)
bAntigen-specificity was determined using T2 cells loaded with HLA-A*0201–restricted epitopes of either MP or HER-2/neu
cNumber of antigen-specific T cells per 100,000 and 500,000 plated effector cells, respectively. T cells responding to the control peptide p68 are subtracted from T cells reactive to the antigens of interest
HLA-A*02–restricted HER-2/neu–specific CTLs could be detected in breast cancer patients following vaccination with the KS24.22 variant
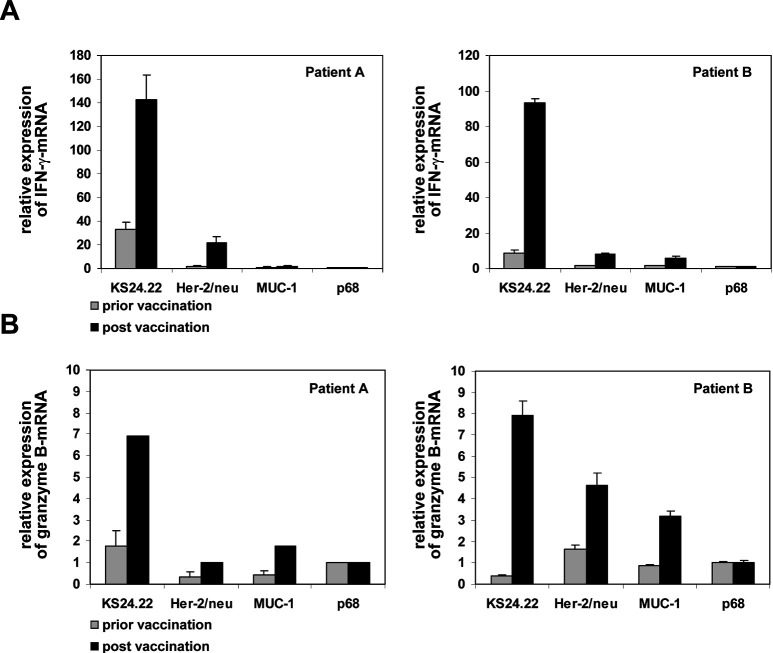
Having confirmed the antigen-processing and antigen-presenting capacity of KS24.22 cells as well as their T-cell stimulating potential, we employed this cell line as a therapeutic vaccine in a clinical phase I/II trial. So far, 10 of 15 planned patients with metastatic breast cancer (stage IV) and no other therapeutical options were included in this study. In the context of our correlative scientific programm, we intended to analyze the induction of HLA-A*02–restricted TAA-specific T cells in the presence of an alloresponse toward the KS24.22 vaccine. As a prove of principle, we would like to submit the data of two patients showing HER-2/neu–specific T-cell reactions after one KS24.22 vaccination (patient A) or seven (patient B). Using quantitative real-time (qRT)-PCR we measured antigen-dependent IFN-γ release of CD8+ T cells using either KS24.22 or a cocktail of HLA-A*02–restricted HER-2/neu epitopes as stimulus (Fig. 4a). As expected, we observed an increase of KS24.22-related T-cell responses postvaccination (fourfold and tenfold, respectively). More interestingly, HER-2/neu–specific T-cell responses increased as well postvaccination (12-fold and fivefold). The HLA-A*02–restricted HER-2/neu–specific T-cell responses figured in up to 15% and 8% of the KS24.22-related T-cell responses, which include allospecific reactions (Fig. 4a). To analyze a functional marker more related to lytic capacity, we included granzyme B in our investigation (Fig. 4b). At least for patient B, a clear HER-2/neu–induced granzyme B expression could be detected.
Fig. 4.
Induction of HLA-A*02–restricted, HER-2/neu–specific T cells by KS24.22 cell line following vaccination. PBMCs (3×105) of breast cancer patients prior to vaccination (shaded column) or post KS24.22 vaccination (solid column) were cocultured with a mixture of four HLA-A*02–restricted HER-2/neu peptides, two HLA-A*02–restricted MUC-1 peptides, or p68 peptide as negative control (each 5 μg/ml). PBMCs were tested for IFN-γ–specific (a) or granzyme B–specific (b) mRNA by qRT-PCR after peptide presensitization for 7 days followed by an additional 2-h restimulation with 3×104 peptide-loaded T2 cells (for details, see “Material and methods”). IFN-γ–specific and granzyme B–specific mRNA expressions were normalized to CD8 expressions and shown as an x-fold increase (stimulation index) in comparison to the p68 stimulations. A reaction was considered positive if it was twofold above the background control (set as 1 for p68 stimulation) plus two times the standard deviation. Patient A: HLA-A*0201, 23x; HLA-B*40x, 44x; HLA-DRB1*0404, 07011; HLA-DQB1*0302, 02x. Patient B: HLA-A*0201, 24x; HLA-B*07x, x; HLA-DRB1*04011, 15011; HLA-DQB1*0302, 0602.
Discussion
The prognosis for breast cancer patients has been improved by the use of postoperative systemic therapy; however, despite radiation, chemotherapy, and the use of antiestrogens, up to 50% of patients with nonmetastatic breast cancer at diagnosis will relapse, and as many as 30% will die from metastatic disease [53]. Thus, treatment failure and adverse events associated with current adjuvant approaches have led to the search for complementary systemic strategies with improved efficacy and decreased toxicity. More recently, attempts have been made to activate the patient’s own immune system by the stimulation of TAA-specific CTLs and memory T cells in order to eliminate already existing micrometastases and prevent the formation of new metastases.
The generation of cell-mediated antitumor responses requires the activation of antigen-specific CD4+ and CD8+ T cells. For breast cancer, a small panel of antigens is characterized including overexpressed TAAs such as MUC-1, CEA, and HER-2/neu, as well as cancer-testis antigens such as NY-ESO-1 and members of the MAGE family [26]. Several groups have compared peptide-based cancer vaccines with cellular immunization protocols: In a murine melanoma model it was shown that combinations of immunodominant melanoma-associated antigens were less potent in inducing systemic antitumor immunity than IL-2–secreting or GM-CSF–secreting tumor vaccines [51]. In a study by Zaks and Rosenberg, ovarian carcinoma patients were vaccinated with HER-2/neu peptides. Postvaccination CTLs could recognize peptide-pulsed targets but failed to lyse HER-2/neu–overexpressing tumors [58]. So, it was speculated that vaccinating with tumor cells could induce T cells of higher avidity able to recognize endogenously processed peptides and subsequently lyse tumor cells. In addition, the rationale of using whole tumor cell vaccines instead of a few defined peptides is that they provide a more comprehensive antigen repertoire allowing a “multi-epitop” vaccination. However, optimal activation of TAA-specific T cells requires both the interaction between T-cell receptors plus HLA/peptide complexes and so-called costimulatory signals. These are often provided by members of the B7 family found on professional APCs but not on tumors [7, 55]. Current strategies designed to improve the immunogenicity of tumor cells by inserting genes that encode for costimulatory molecules or cytokines can be performed more easily with allogeneic tumor cell lines [38].
We improved the antigenicity as well as the immunogenicity of a breast cancer cell line by transfecting genes encoding the TAA HER-2/neu and the costimulatory molecule CD80. We have shown previously that in contrast to the nontransfected parental breast cancer line KS, its CD80-expressing variants induced strong T-cell proliferation in allogeneic MLTC. Moreover, CD80-transfected KS cells promoted the expansion of MHC class I–restricted CD8+ T-cell lines with cytolytic activity against unmodified tumor cells [18, 19, 38, 39]. Furthermore, nonfunctional mass spectrometry–based methods supported their antigen-processing capacity by identifying an endogenously processed MAGE-1 epitope (KVLEYVIKV) in the pool of HLA-A*02 ligands of the KS cell line. Moreover, MAGE-1–specific CTL lines generated in HLA-A*02–transgeneic mice were able to lyse KS target cells indicating their stimulative capacity in the effector phase of T cells [41].
The results presented in this paper were chosen to demonstrate the possible activation of TAA-specific HLA-restricted T cells by CD80+ KS cells in an allogeneic setting—in vitro as well as in a clinical situation following vaccination. By first analyzing the stimulator phase of T-cell activation, we showed in this investigation that HLA-A*02–matched T cells of breast cancer patients could be primed by KS24.22 and maintained as T-cell lines which recognized the wild-type KS as well as the genetically modified variants derived thereof. Part of this lysis could be blocked by the mAb BB7.2, demonstrating the induction of HLA-A*02–restricted T cells as already demonstrated for T cells of healthy donors [18]. From these allogeneic MLTCs a HLA-A*02–restricted T-cell clone derived that was able to lyse HLA-A*02+ breast cancer cell lines but no HLA-A*02+ colon cancer cell line. However, previous work demonstrated that SW480 cells showed HLA-A*02 expression densities comparable to the breast cancer cell lines and could be killed by HLA-restricted T-cell lines [21, 36]. These results indicate that CTLs, able to recognize nonallogeneic ligands on the KS cells, develop after long-term stimulation despite the induction of broad allogeneic responses. To look for antigen specificity, we initially used a defined viral antigen—the influenza A MP and HLA-A*02–restricted peptides derived thereof—serving as a surrogate TAA. This had the advantage of a higher memory T-cell frequency which could be expected and could probably be quantified by the ELISpot technique. Flu peptide-loaded autologous PBMCs, as well as antigen-pulsed allogeneic CD80+ KS variants, were able to stimulate flu-specific T cells at comparable frequencies. More importantly, endogenous expression of the viral M1 by the tumor cell variant following stable transfection resulted in the induction of MP57-68-specific T cells as well, alongside the induction of alloresponses. Since the M1 antigen represents a transgeneic foreign protein that is highly visible to the immune system, it was speculated that alloreactive CTLs did not overcome the response. Therefore, using the TAA HER-2/neu we extended our observations by demonstrating that even naturally expressed ligands of tumors can induce specific T cells in vitro. We chose HER-2/neu because by analyzing preexistent cellular immune responses to frequently expressed breast cancer–associated antigens we recently demonstrated that approximately 19% of the patients showed CD8+ T-cell reactions specific for HER-2/neu epitopes, wheras none of the investigated healthy donors did, probably due to lower precursor frequencies [44]. Patients’ PBMCs were analyzed following 1 week of in vitro peptide presensitization using autologous APCs, revealing a frequency of approximately 40–100/50,000 HER-2/neu–specific T cells by ELISpot [44]. Here, using HER-2/neu–overexpressing CD80+ KS variants as allogeneic but HLA-A*02–matched APCs, we detected HER-2/neu–specific T cells alongside KS24.22-reactive T cells; however, these appeared at lower frequencies and not before 3 weeks of in vitro presensitization.
Other groups have previously analyzed CD80-modified tumor cells for their capacity to induce specific CTL responses in HLA-matched MLTCs as well [9, 14, 25, 31, 39, 49, 51]. However, they often used culture conditions that did not support the induction of an alloresponse, such as applying purified CD8+ T cells or analyzing limiting dilution cultures. By doing so, the induction of melanocyte-specific or HPV 16-specific T cells, using allogeneic CD80-modified melanoma or cervical cancer cell lines, respectively, was possible [14, 25, 31]. Taken together, these conditions did not reflect the clinical situation of administering allogeneic tumor cells as vaccine in vivo. Recent results of Schendel et al. [49] have clearly supported our data demonstrating the expansion of TAA-associated CTLs by a CD80-modified renal cell carcinoma (RCC) line. In contrast to our study and not knowing RCC-associated TAAs, they used T-cell receptor analysis (CDR3 amino acid sequences of Vα chains) instead of HLA-restricted peptides to confirm CTL specificity [49].
Our characterization of KS24.22 cells clearly shows its functional integrity in antigen processing and HLA-restricted presentation, as well as its potential to stimulate antigen-specific effector T cells in HLA-matched situations. Induction of complex allospecific responses did not impair the development of antigen-specific CTLs in vitro and might act as a nonspecific adjuvant in vivo [33, 39, 42]. These results formed the basis for a clinical trial where KS24.22 is used as a cellular vaccine in patients with metastatic breast cancer. The primary objectives of this phase I/II study were toxicity and feasibility. To analyze vaccine-induced immune responses, T cells will be evaluated using a combination of molecular and cellular immunodiagnostic tests [20]. KS24.22-responding T cells as well as T cells specific for HLA-A*02–restricted TAA epitopes probably expressed by the vaccine cells were detected by quantifying antigen-induced IFN-γ mRNA. As a proof of principle, we first of all present data from two patients with stable disease undergoing KS24.22 vaccination, developing HLA-A*02–restricted, HER-2/neu–specific CD8+ T cells alongside KS24.22-related alloresponses. Furthermore, we could also demonstrate the induction of granzyme B as a functional parameter for their lytic capacity. To our knowledge, our study is the first presentation of TAA-specific T-cell induction using a CD80-modified allogeneic breast cancer cell in vitro as well as in a clinical setting.
Older studies using unmodified allogeneic breast cancer cell lines together with adjuvants such as BCG, IL-2, or low-dose GM-CSF did not report on analyses of specific T-cell responses (reviewed in [11, 17, 57]). Applying MCF-7, a well-characterized breast cancer line expressing several TAAs, one group could demonstrate an increase of proliferative responses of the patients’ PBMCs to the autologous tumor and tumor markers such as CA15-2, CEA, and CA125. Surprisingly, no significant reactions toward the allogenous vaccine cells could be shown [27]. Dols et al. [12, 13] vaccinated 30 HLA-A*02+ breast cancer patients with a CD80-modified MB-231 breast cancer cell line together with BCG or GM-CSF. Their immunodiagnostic program was focused on the analysis of prevaccination and postvaccination sera, where they could detect vaccine-induced antibodies toward HER-2/neu (8/24 cases), MUC-1 (4/24), and p53 (1/24) [13]. Quantifying vaccine-induced cytokine induction using the ELISA technique, they found an IFN-γ increase in postvaccination PBMCs in a minority of patients [12]. Besides our own study, HLA-restricted TAA-specific responses were analyzed in two studies with melanoma patients. Malignant melanoma is the tumor were the greatest experience in administering allogeneic tumor vaccines has been gained. Using genetically modified melanoma cell lines secreting either IL-2 or IL-4, a minority of patients exhibited postvaccination T-cell responses toward tyrosinase368-376 and gp100289-288 [1, 40].
In whole cell vaccine trials, monitoring is particularly challenging because of strong alloresponses and limited knowledge of TAAs. We demonstrated the potential of allogeneic KS24.22 breast carcinoma variants to induce TAA-specific CD8+ T cells in parallel with the development of alloreactivity in vitro and in vivo following vaccination. Here, the qRT-PCR method proved to be highly sensitive and can be used to perform an “immunological staging” under vaccination. However, to date we can not comment on the correlation of the clinical outcome and immune response of our yet open clinical trial.
Acknowledgements
Parts of this research were supported by grants from the Angewandte Klinische Forschung (AKF)-programm of the Medizinische Fakultät, University of Tübingen, the Forschungskommission (FoKo) der Medizinische Fakultät, University of Heidelberg, and by the Bundesministerium für Bildung und Forschung (BMBF), Germany.
References
- 1.Arienti F, Belli F, Napolitano F, Sule-Suso J, Mazzocchi A, Gallino GF, Cattelan A, Santantonio C, Rivoltini L, Melani C, Colombo MP, Cascinelli N, Maio M, Parmiani G, Sanantonio C. Vaccination of melanoma patients with interleukin 4 gene-transduced allogeneic melanoma cells. Hum Gene Ther. 2000;10:2907–2916. doi: 10.1089/10430349950016320. [DOI] [PubMed] [Google Scholar]
- 2.Baskar S, Nabavi N, Glimcher LH, Ostrand-Rosenberg S. Tumor cells expressing major histocompatibility complex class II and B7 activation molecules stimulate potent tumor-specific immunity. J Immunother. 1993;14:209–215. doi: 10.1097/00002371-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Baxevanis CN, Sotiropoulou PA, Sotiriadou NN, Papamichail M. Immunobiology of Her-2/neu oncoprotein and its potential application in cancer immunotherapy. Cancer Immunol Immunother. 2004;53:166–175. doi: 10.1007/s00262-003-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bednarek MA, Engl SA, Gammon MC, Lindquist JA, Porter G, Williamson AR, Zweerink HJ. Soluble HLA-A2.1 restricted peptides that are recognized by influenza virus specific cytotoxic T lymphocytes. J Immunol Methods. 1991;139:41–47. doi: 10.1016/0022-1759(91)90349-K. [DOI] [PubMed] [Google Scholar]
- 5.Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, Muhm A, Rammensee HG, Kanz L, Brugger W. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- 6.Cayeux S, Beck C, Aicher A, Dorken B, Blankenstein T. Tumor cells cotransfected with interleukin-7 and B7.1 genes induce CD25 and CD28 on tumor-infiltrating T lymphocytes and are strong vaccines. Eur J Immunol. 1995;25:2325–2331. doi: 10.1002/eji.1830250831. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 8.Courmier JN, Panelli MC, Hackett JA, Bettinotti MP, Mixon A, Wunderlich J, Parker LL, Restifo NP, Ferrone S, Marincola FM. Natural variation of the expression of HLA and endogenous antigen modulates CTL recognition in an in vitro melanoma model. Int J Cancer. 1999;80:781–790. doi: 10.1002/(SICI)1097-0215(19990301)80:5<781::AID-IJC24>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley NJ, Slingluff CL, Jr, Vervaert CE, Darrow TL, Seigler HF. Inhibition of the growth of human melanoma xenografts in nude mice by human tumor-specific cytotoxic T-cells. J Surg Oncol. 1990;43:67–72. doi: 10.1002/jso.2930430202. [DOI] [PubMed] [Google Scholar]
- 10.Deuschle U, Pepperkok R, Wang FB, Giordano TJ, McAllister WT, Ansorge W, Bujard H. Regulated expression of foreign genes in mammalian cells under the control of coliphage T3 RNA polymerase and lac repressor. Proc Natl Acad Sci U S A. 1989;86(14):5400–5404. doi: 10.1073/pnas.86.14.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dols A, Meijer SL, Smith JW, II, Fox BA, Urba WJ. Allogeneic breast cancer cell vaccines. Clin Breast Cancer. 2003;3(suppl 4):173–180. doi: 10.3816/cbc.2003.s.008. [DOI] [PubMed] [Google Scholar]
- 12.Dols A, Smith JW, II, Meijer SL, Fox BA, Hu HM, Walker E, Rosenheim S, Moudgil T, Doran T, Wood W, Seligman M, Alvord WG, Schoof D, Urba WJ. Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Hum Gene Ther. 2003;14:1117–1123. doi: 10.1089/104303403322124828. [DOI] [PubMed] [Google Scholar]
- 13.Dols A, Meijer SL, Hu HM, Goodell V, Disiss ML, von Mensdorff-Pouilly S, Verheijen R, Alvord WG, Smith JW, II, Urba WJ, Fox BA. Identification of tumor-specific antibodies in patients with breast cancer vaccinated with gene-modified allogeneic tumor cells. J Immunother. 2003;26:163–170. doi: 10.1097/00002371-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Fenton RG, Turcovski-Corrales SM, Taub DD. Induction of melanoma antigen-specific cytotoxic T lymphocytes in vitro by stimulation with B7-expressing human melanoma cell lines. J Immunother. 1998;21:95–108. [PubMed] [Google Scholar]
- 15.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of Her-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisk B, Hudson JM, Kavanagh J, Wharton JT, Murray JL, Ioannides CG, Kudelka AP. Existent proliferative response of peripheral blood mononuclear cells from healthy donors and ovarian cancer patients to Her-2 peptides. Anticancer Res. 1997;17:45–53. [PubMed] [Google Scholar]
- 17.Giuliano AE, Sparks FC, Patterson K, Spears I, Morton DL. Adjuvant chemo-immunotherapy in stage II carcinoma of the breast. J Surg Oncol. 1986;31:255–259. doi: 10.1002/jso.2930310407. [DOI] [PubMed] [Google Scholar]
- 18.Gückel B, Lindauer M, Rudy W, Habicht A, Siebels M, Kaul S, Bastert G, Meuer SC, Moebius U. CD80-transfected human breast and ovarian tumor cell lines: improved immunogenicity and induction of cytolytic CD8+ T lymphocytes. Cytokines Mol Ther. 1995;1:211–221. [PubMed] [Google Scholar]
- 19.Gückel B, Meyer GC, Rudy W, Batrla R, Meuer SC, Bastert G, Wallwiener D, Moebius U. Interleukin-12 requires initial CD80-mediated T-cell activation to support immune responses toward human breast and ovarian carcinoma. Cancer Gene Ther. 1999;6:228–237. doi: 10.1038/sj.cgt.7700050. [DOI] [PubMed] [Google Scholar]
- 20.Gückel B, Meuer S, Bastert G, Wallwiener D. Tumor-associated antigens as tools in immunodiagnostics and immunotherapy of breast cancer. Geburtsh Frauenheilk. 2003;64:130–139. doi: 10.1055/s-2003-37461. [DOI] [Google Scholar]
- 21.Habicht A, Lindauer M, Galmbacher P, Rudy W, Gebert J, Schackert HK, Meuer SC, Moebius U. Development of immunogenic colorectal cancer cell lines for vaccination: expression of CD80 (B7.1) is not sufficient to restore impaired primary T cell activation in vitro. Eur J Cancer. 1995;31:2396–2402. doi: 10.1016/0959-8049(95)00435-1. [DOI] [PubMed] [Google Scholar]
- 22.Hellström KE, Hellström I, Chen L. Can co-stimulated tumor immunity be therapeutically efficacious? Immunol Rev. 1995;145:123–145. doi: 10.1111/j.1600-065x.1995.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 23.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–186. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 24.Hirano N, Takahashi T, Takahashi T, Azuma M, Okumura K, Yazaki Y, Yagita H, Hirai H. Protective and therapeutic immunity against leukemia induced by irradiated B7-1 (CD80)-transduced leukemic cells. Hum Gene Ther. 1997;8:1375–1384. doi: 10.1089/hum.1997.8.11-1375. [DOI] [PubMed] [Google Scholar]
- 25.Imro MA, Dellabona P, Manici S, Heltai S, Consogno G, Bellone M, Rugarli C, Protti MP. Human melanoma cells transfected with the B7-2 co-stimulatory molecule induce tumor-specific CD8+ cytotoxic T lymphocytes in vitro. Hum Gene Ther. 1998;9:1335–1344. doi: 10.1089/hum.1998.9.9-1335. [DOI] [PubMed] [Google Scholar]
- 26.Jäger E, Jager D, Knuth A. Strategies for the development of vaccines to treat breast cancer. Recent Results Cancer Res. 1998;152:94–102. doi: 10.1007/978-3-642-45769-2_9. [DOI] [PubMed] [Google Scholar]
- 27.Jiang XP, Yang DC, Elliott RL, Head JF. Vaccination with a mixed vaccine of autogenous and allogeneic breast cancer cells and tumor associated antigens CA15-3, CEA and CA125–results in immune and clinical responses in breast cancer patients. Cancer Biother Radiopharm. 2000;15:495–505. doi: 10.1089/cbr.2000.15.495. [DOI] [PubMed] [Google Scholar]
- 28.Jung D, Jaeger E, Cayeux S, Blankenstein T, Hilmes C, Karbach J, Moebius U, Knuth A, Huber C, Seliger B. Strong immunogenic potential of a B7 retroviral expression vector: generation of HLA-B7-restricted CTL response against selectable marker genes. Hum Gene Ther. 1998;9:53–62. doi: 10.1089/hum.1998.9.1-53. [DOI] [PubMed] [Google Scholar]
- 29.Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;163:6867–6875. [PubMed] [Google Scholar]
- 30.Kammula US, Marincola FM, Rosenberg SA. Real-time quantitative polymerase chain reaction assessment of immune reactivity in melanoma patients after tumor peptide vaccination. J Natl Cancer Inst. 2000;92:1336–1344. doi: 10.1093/jnci/92.16.1336. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann AM, Gissmann L, Schreckenberger C, Qiao L. Cervical carcinoma cells transfected with the CD80 gene elicit a primary cytotoxic T lymphocyte response specific for HPV 16 E7 antigens. Cancer Gene Ther. 1997;4:377–382. [PubMed] [Google Scholar]
- 32.Kayser S, Watermann I, Rentzsch C, Weinschenk T, Wallwiener D, Gückel B. Tumor-associated antigen profiling in breast and ovarian cancer: mRNA, protein or T cell recognition? J Cancer Res Clin Oncol. 2003;129:397–409. doi: 10.1007/s00432-003-0445-7. [DOI] [PubMed] [Google Scholar]
- 33.Knight BC, Souberbielle BE, Rizzardi GP, Ball SE, Dalgleish AG. Allogeneic murine melanoma cell vaccine: a model for the development of human allogeneic cancer vaccine. Melanoma Res. 1996;6:299–306. doi: 10.1097/00008390-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kruse N, Pette M, Toyka K, Rieckmann P. Quantification of cytokine mRNA expression by RT PCR in samples of previously frozen blood. J Immunol Methods. 1997;210:195–203. doi: 10.1016/S0022-1759(97)00188-9. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, McGowan P, Hellstrom I, Hellstrom KE, Chen L. Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J Immunol. 1994;153:421–428. [PubMed] [Google Scholar]
- 36.Lindauer M, Rudy W, Gückel B, Doeberitz MV, Meuer SC, Moebius U. Gene transfer of costimulatory molecules into a human colorectal cancer cell line: requirement of CD54, CD80 and class II MHC expression for enhanced immunogenicity. Immunology. 1998;93:390–397. doi: 10.1046/j.1365-2567.1998.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lustgarten J, Theobald M, Labadie C, LaFace D, Peterson P, Disis ML, Cheever MA, Sherman LA. Identification of Her-2/neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD8. Hum Immunol. 1997;52:109–118. doi: 10.1016/S0198-8859(96)00292-3. [DOI] [PubMed] [Google Scholar]
- 38.Meuer SC, Gückel B, Lindauer M, Rudy W, Moebius U. Construction of immunogenic tumor cell surfaces by somatic gene transfer. Recent Results Cancer Res. 1998;144:78–85. doi: 10.1007/978-3-642-46836-0_10. [DOI] [PubMed] [Google Scholar]
- 39.Meyer GC, Batrla R, Rudy W, Meuer SC, Wallwiener D, Gückel B, Moebius U. Potential of CD80-transfected human breast carcinoma cells to induce peptide-specific T lymphocytes in an allogeneic human histocompatibility leukocyte antigens (HLA)-A2.1+-matched situation. Cancer Gene Ther. 1999;6:282–288. doi: 10.1038/sj.cgt.7700054. [DOI] [PubMed] [Google Scholar]
- 40.Osanto S, Schiphorst PP, Weijl NI, Dijkstra N, Van Wees A, Brouwenstein N, Vaessen N, Van Krieken JH, Hermans J, Cleton FJ, Schrier PI. Vaccination of melanoma patients with an allogeneic, genetically modified interleukin 2-producing melanoma cell line. Hum Gene Ther. 2000;11:739–750. doi: 10.1089/10430340050015635. [DOI] [PubMed] [Google Scholar]
- 41.Pascolo S, Schirle M, Gückel B, Dumrese T, Stumm S, Kayser S, Moris A, Wallwiener D, Rammensee H-G, Stevanović S. A MAGE-A1 HLA-A*0201 epitope identified by mass spectrometry. Cancer Res. 2001;61:4072–4077. [PubMed] [Google Scholar]
- 42.Plautz GE, Yang ZY, Wu BY, Gao X, Huang L, Nabel GJ. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci U S A. 1993;90:4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rammensee HG, Bachmann J, Emmerich NN, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 44.Rentzsch C, Kayser S, Stumm S, Watermann I, Walter S, Stevanoviæ S, Wallwiener D, Gückel B. Evaluation of pre-existent immunity in patients with primary breast cancer: molecular and cellular assays to quantify antigen-specific T lymphocytes in peripheral blood mononuclear cells. Clin Cancer Res. 2003;9:4376–4386. [PubMed] [Google Scholar]
- 45.Riker AI, Kammula US, Panelli MC, Wang E, Ohnmacht GA, Steinberg SM, Rosenberg SA, Marincola FM. Threshold levels of gene expression of the melanoma antigen gp100 correlate with tumor cell recognition by cytotoxic T lymphocytes. Int J Cancer. 2000;86:818–826. doi: 10.1002/(SICI)1097-0215(20000615)86:6<818::AID-IJC10>3.3.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Cabello F, Garrido F. HLA and cancer: from research to clinical impact. Immunol Today. 1998;19:539–542. doi: 10.1016/S0167-5699(98)01349-8. [DOI] [PubMed] [Google Scholar]
- 47.Salcedo M, Momburg F, Hämmerling GJ, Ljunggren HG. Resistance to natural killer cell lysis conferred TAP1/2 genes in human antigen-processing mutant cells. J Immunol. 1994;152:1702–1708. [PubMed] [Google Scholar]
- 48.Scardino A, Alves P, Gross DA, Tourdot S, Graff-Dubois S, Angevin E, Firat H, Chouaib S, Lemonnier F, Nadler LM, Cardoso AA, Kosmatopoulos K. Identification of HER-2/neu immunogenic epitopes presented by renal cell carcinoma and other human epithelial tumors. Eur J Immunol. 2001;31:3261–3270. doi: 10.1002/1521-4141(200111)31:11<3261::AID-IMMU3261>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Schendel DJ, Frankenberger B, Jantzer P, Cayeux S, Nobetaner E, Willimsky G, Maget B, Pohla H, Blankenstein T. Expression of B7.1 (CD80) in a renal cell carcinoma line allows expansion of tumor-associated cytotoxic T lymphocytes in the presence of an alloresponse. Gene Ther. 2000;7:2007–2014. doi: 10.1038/sj.gt.3301349. [DOI] [PubMed] [Google Scholar]
- 50.Schirle M, Keilholz W, Weber B, Gouttefangeas C, Dumrese T, Becker HD, Stevanovic S, Rammensee HG. Identification of tumor-associated MHC class I ligands by a novel T cell-independent approach. Eur J Immunol. 2000;30:2216–2225. doi: 10.1002/1521-4141(2000)30:8<2216::AID-IMMU2216>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt W, Buschle M, Zauner W, Kirlappos H, Mechtler K, Trska B, Birnstiel ML. Cell-free tumor antigen peptide-based cancer vaccines. Proc Natl Acad Sci U S A. 1997;94:3262–3267. doi: 10.1073/pnas.94.7.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens EJ, Jacknin L, Robbins PF, Kawakami Y, el Gamil M, Rosenberg SA, Yannelli JR. Generation of tumor-specific CTLs from melanoma patients by using peripheral blood stimulated with allogeneic melanoma tumor cell lines: fine specificity and MART-1 melanoma antigen recognition. J Immunol. 1995;154:762–771. [PubMed] [Google Scholar]
- 53.Sundquist M, Thorstenson S, Brudin L, Wingren S, Nordenskjold B. Incidence and prognosis in early onset breast cancer. Breast. 2002;11:30–35. doi: 10.1054/brst.2001.0358. [DOI] [PubMed] [Google Scholar]
- 54.Toes RE, Blom RJ, van der Voort E, Offringa R, Melief CJ, Kast WM. Protective antitumor immunity induced by immunization with completely allogeneic tumor cells. Cancer Res. 1996;56:3782–3787. [PubMed] [Google Scholar]
- 55.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 56.Wang YC, Zhu L, McHugh R, Graham SD, Jr, Hillyer CD, Dillehay D, Sell KW, Selvaraj P. Induction of autologous tumor-specific cytotoxic T-lymphocyte activity against a human renal carcinoma cell line by B7-1 (CD80) costimulation. J Immunother Emphasis Tumor Immunol. 1996;19:1–8. doi: 10.1097/00002371-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Wiseman C, Jessup JM, Smith TL, Hersh E, Bowen J, Blumenshein G. Inflammatory breast cancer treated with surgery, chemotherapy and allogeneic tumor cell/BCG immunotherapy. Cancer. 1982;49:1266–1271. doi: 10.1002/1097-0142(19820315)49:6<1266::aid-cncr2820490631>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 58.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer. 1998;58:4902–4908. [PubMed] [Google Scholar]