Abstract
Ras proteins are binary switches that, by cycling through inactive GDP- and active GTP-bound conformations, regulate multiple cellular signaling pathways, including those that control growth and differentiation. For some time, it has been known that receptor-mediated increases in the concentration of intracellular free calcium ([Ca2+]i) can modulate Ras activation. Increases in [Ca2+]i often occur as repetitive Ca2+ spikes or oscillations. Induced by electrical or receptor stimuli, these repetitive Ca2+ oscillations increase in frequency with the amplitude of receptor stimuli, a phenomenon critical for the induction of selective cellular functions. Here, we show that Ca2+ oscillations are optimized for Ca2+-mediated activation of Ras and signaling through the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) cascade. We present additional evidence that Ca2+ oscillations reduce the effective Ca2+ threshold for the activation of Ras and that the oscillatory frequency is optimized for activation of Ras and the ERK/MAPK pathway. Our results describe a hitherto unrecognized link between complex Ca2+ signals and the modulation of the Ras/ERK/MAPK signaling cascade.
Keywords: RASAL, CAPRI, GRF, GDP
For a wide variety of cell surface receptors, activation leads to an increase in the concentration of intracellular free calcium ([Ca2+]i) (1–3). Once induced, the elevation in [Ca2+]i is responsible for controlling a diverse array of cellular processes, including secretion, contraction, learning, and proliferation (3). Understanding how receptor-mediated increases in [Ca2+]i are capable of modulating so many physiological processes is one of the major challenges in the study of Ca2+ signaling. It appears that such control is achieved through a complex relationship between the amplitude and spatiotemporal patterning of the Ca2+ signal and its resultant ability to couple to an extensive molecular repertoire of Ca2+-sensing proteins (3).
Receptor-mediated increases in [Ca2+]i are often observed as repetitive Ca2+ spikes or oscillations that increase their frequency with the amplitude of the receptor stimuli (refs. 4 and 5; reviewed in ref. 3). These frequency-encoded signals appear to be critical for the induction of selective cellular functions (3). For example, the frequency of receptor-mediated Ca2+ oscillations determines the efficiency of gene expression driven by the transcription factors NF-AT, OAP, and NF-κB (6–8) and mitochondrial ATP production (9). To decode the information contained within Ca2+ oscillations, cells have evolved a number of frequency-modulated decoders. Such proteins include calmodulin (10), protein kinase C (11–15), calpain (16), calmodulin-dependent protein kinase II (17, 18), and the Ras GTPase-activating protein RASAL (19).
Ras proteins are binary molecular switches that regulate multiple signaling pathways, including those controlling growth and differentiation, through an ability to cycle between inactive GDP- and active GTP-bound conformations (20–23). The magnitude and duration of Ras signaling is controlled by two classes of proteins: Guanine nucleotide exchange factors modulate Ras activation by enhancing the exchange of GDP for GTP, and GTPase-activating proteins regulate inactivation by increasing the intrinsic Ras GTPase activity (20–23). Although it has been known for some time that increases in [Ca2+]i can modulate Ras activation (for example, Ca2+ influx through voltage-operated ion channels or release from internal stores can activate Ras in neuronal cells) (24), only recently have molecular entities been described that allow for this coupling (reviewed in ref. 25).
Two families of Ras guanine nucleotide exchange factors (GEFs), RasGRFs (26–29) and RasGRPs (30–36), the latter also being known as CalDAG-GEFs, are modulated by increases in [Ca2+]i. For RasGRFs, this modulation occurs indirectly through association with Ca2+/calmodulin, whereas for RasGRPs, a more direct control is achieved through association of Ca2+ with atypical EF hands (25). In addition to stimulating Ras activation, increases in [Ca2+]i also mediate Ras inactivation through the Ca2+-triggered RasGTPase-activating proteins (RasGAPs) RASAL and CAPRI (19, 37). These proteins are cytosolic, inactive RasGAPs that, upon a receptor-mediated elevation in [Ca2+]i, undergo a rapid, C2 domain-dependent association with the plasma membrane, an association that leads to an increase in their RasGAP activity (19, 37). Unlike CAPRI, which undergoes a transient association with the plasma membrane and does not sense receptor-mediated Ca2+ oscillations, the plasma membrane association of RASAL occurs in an oscillatory manner (19). This oscillatory association occurs in synchrony with underlying receptor-mediated Ca2+ oscillations and is frequency-modulated such that, upon increasing the amplitude of receptor stimuli, the frequency of RASAL membrane association is enhanced (19). CAPRI and RASAL therefore constitute molecular entities that can sense the amplitude and frequency, respectively, of complex Ca2+ signals, decoding these distinct temporal signals through a modulation of plasma-membrane-associated Ras.
The characterization of such distinct Ca2+ sensors, tuned to detect different temporal Ca2+ signals, has raised the issue of whether the temporal dynamics of receptor-mediated Ca2+ oscillations are optimized for efficient Ca2+-mediated activation of Ras and downstream Ras-dependent signaling (25, 38). Here, we have addressed this issue, presenting data showing that the temporal dynamics of Ca2+ signals are indeed optimized for activation of Ras and the downstream extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) cascade.
Methods
Cell Culture. HeLa cells were cultured in DMEM (Invitrogen) containing 10% (vol/vol) fetal-calf serum, 100 units·ml–1 penicillin, and 100 μg·ml–1 streptomycin. Cell cultures were maintained at 37°C in a 95% air/5% CO2 humidified incubator.
Single-Cell Ca2+ Measurements. HeLa cells were grown on 22-mm coverslips and washed with 2 ml of extracellular medium [buffer A: 121 mM NaCl/5.4 mM KCl/1.6 mM MgCl2/6 mM NaHCO3/9 mM glucose/25 mM Hepes (pH 7.4)] supplemented with 1.8 mM CaCl2. These cells were incubated in 1 ml of extracellular medium containing 2 μM Fura-2/AM for 30 min at room temperature before being washed twice with 2 ml of extracellular medium. Ca2+ imaging was performed by using the Merlin ratiometric Ca2+-imaging system, an UltraPix FKL300 digital camera (PerkinElmer), and an Olympus IX50 inverted microscope fitted with a ×40 oil-immersion-objective lens (39). The data were processed by using merlin software (PerkinElmer). All imaging was performed at 37°C with constant perfusion. [Ca2+] calibrations were calculated with merlin software by using 340:380 ratio maxima and minima obtained after treatment of the cells with 2 μM ionomycin followed by 20 mM EGTA.
Ca2+ Clamp. This procedure was a modified version of that described by Dolmetsch et al. (6). Serum-starved HeLa cells, cultured on coverslips for Ca2+ imaging or in 60- or 100-mm Petri dishes for biochemical analysis, were incubated with 1 μM thapsigargin in extracellular solution (buffer A) supplemented with 1 mM EGTA (Ca2+-free solution) at 37°C. After a 15-min incubation, when the elevation in [Ca2+]i had returned to basal levels, the cells were rapidly switched between Ca2+-containing (buffer A supplemented with 1.3 mM CaCl2) and Ca2+-free solutions to elicit Ca2+ spikes. For experiments in which we performed single-cell Ca2+ measurements, buffer exchange was achieved through a rapid-perfusion system. In biochemical assays employing cells cultured on Petri dishes, exchange was achieved by rapidly moving the dishes between 37°C water baths containing 5 liters of the relevant buffer. In both methods, buffer exchange was achieved in <2 s. The amplitude of individual Ca2+ spikes was varied by manipulating the period of exposure to Ca2+-containing media, and the frequency was determined by varying the interval between exposures.
Ras Pull-Down Assays. Glutathione S-transferase fusion of the Ras-GTP-binding domain from Raf-1 (GST-RBD) was purified from BL21(DE3) Escherichia coli cells harboring the plasmid pGEX KG containing the Raf Ras-binding domain (amino acids 1–149). After induction of a bacterial culture (OD600 between 0.4 and 0.6) for 3 h at 37°C with 1 mM isopropyl-1-thio-β-d-galactopyranoside, cells were lysed by sonication in PBS containing 1 μM EDTA, 1% (vol/vol) Triton X-100, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. The lysate was clarified by centrifugation, and the resultant supernatant was stored in aliquots at –80°C. On the required day, aliquots were thawed before incubation with glutathione-agarose (Amersham Pharmacia Biotech) for 1 h at room temperature. The agarose beads were washed twice in PBS, 1 mM EDTA, and 1% (vol/vol) Triton X-100 before being suspended as a 1:1 slurry, which was used immediately in pull-down assays.
Here, dishes (100 mm) of HeLa cells (8 × 105) were transiently transfected by lipofection (GeneJuice, Novagen) with 2 μg of H-Ras cDNA. The cells were serum-starved for 2 h at 37°C in serum-free DMEM before the experimental procedures. Upon completion of the manipulation, cells were lysed in 1 ml of ice-cold extraction buffer [50 mM Hepes (pH 7.5)/100 mM NaCl/1 mM EGTA/0.5 μg/ml benzamidine/5 μg/ml aprotinin/5 μg/ml leupeptin/5 μg/ml pepstatin A/5 μg/ml trypsin inhibitor/1 mM DTT containing 1% (vol/vol) Triton X-100 and 10 mM MgCl2]. Nuclear-free supernatants were incubated with GST-RBD on glutathione-agarose beads at 4°C for 30 min. The beads were then collected by centrifugation and washed three times with ice-cold PBS, 0.1% Triton X-100, and 10 mM MgCl2. Ras proteins were separated by SDS/PAGE and visualized by immunoblotting on nitrocellulose filters with pan-Ras antibodies (Oncogene Science) and enhanced chemiluminescence (Amersham Pharmacia Biotech). Blots were analyzed by volume integration in imagequant (Molecular Dynamics) as described in refs. 37 and 40.
Analysis of Signaling Through the ERK/MAPK Cascade. HeLa cells grown on 60-mm dishes were starved in serum-free DMEM at 37°C for 2 h before experimental manipulation. Cells were then lysed in 500 μl of ice-cold lysis buffer [50 mM β-glycerolphosphate/1.5 mM EDTA/1 mM benzamidine/1 mM DTT/0.5 mM Na3VO4/0.1 mM PMSF/1 μg/ml each pepstatin, antipain, and leupeptin (pH 7.4)] and centrifuged at 10,000 × g for 10 min at 4°C. Equal quantities of total protein from each sample were resolved by 12% SDS/PAGE and transferred to Immobilin-P transfer membrane (Millipore) by wet-blotting. Activated ERK1 and ERK2 or total ERK 1 and 2 were detected with phospho-p44/p42 MAP kinase (Thr-202/Tyr-204) or p44/p42 MAP kinase antibody (Cell Signaling Technology, Beverly, MA), respectively, according to the manufacturer's recommendations. Detection was performed by using an ECL Western blotting system (Amersham Pharmacia Biotech), and developed films were analyzed by volume-integration in imagequant as described in refs. 37 and 40.
Cell Treatment with Inhibitors to Mitogen-Activated Protein Kinase Kinase (MEK), Calmodulin-Dependent Protein Kinase Kinase (CaMKK), and Calmodulin-Dependent Protein Kinase II (CaMKII). HeLa cells, cultured on 60-mm dishes, were starved in serum-free DMEM at 37°C for 2 h. The cells were pretreated with 10 μM U0126 (MEK 1 and 2 inhibitor, Calbiochem), 5 μM KN-93 (CaMKII inhibitor, Calbiochem), or 5 μM KN-92 (inactive analogue of KN-93, Calbiochem) for 30 min or with 2.6 μM STO-609 (CaMKK inhibitor, Calbiochem) for 60 min before experimental manipulation. Cells were incubated with 1 μM thapsigargin in buffer A supplemented with 1 mM EGTA for 15 min at 37°C in the presence of the inhibitors. Subsequent generation of Ca2+ oscillations was achieved through a rapid-perfusion system, alternating between 3 ml of buffer A (supplemented with 1.3 mM CaCl2 and inhibitors) and 3 ml Ca2+-free buffer A (supplemented with 1 mM EGTA and inhibitors). Activation of the ERK/MAPK cascade was determined as described above.
Results and Discussion
To establish the relationship among Ca2+ oscillations, coupling to Ras activation, and downstream signaling through the ERK/MAPK cascade, experiments were conducted to first define the receptor-mediated Ca2+ signals observed within the HeLa cells used in this study. Single-cell analysis revealed that, within a population of cells, agonist stimulation induced oscillatory increases in [Ca2+]i that were heterogeneous in nature, occurring with a range of frequencies and amplitudes (Fig. 1A). More detailed analysis revealed that, when stimulated with histamine, 65.1 ± 5.1% of Ca2+ oscillations were observed to display amplitudes between 200 and 500 nM (n = 52 oscillations in total) and interspike intervals that, depending on the concentration of the applied agonist, ranged from 30 s to >600 s (data not shown). These data are similar to those previously studied for HeLa cells stimulated with histamine and a variety of other agonists (41). In addition to defining the parameters of receptor-mediated Ca2+ oscillations observed in the HeLa cells used in this study, this analysis reveals one of the major problems in biochemically defining the coupling of Ca2+ oscillations to signaling pathways. The heterogeneous nature of receptor-mediated Ca2+ oscillations means that the information contained within the temporal dynamics of individual Ca2+ oscillations are averaged and hence lost within a cellular population. It is therefore impossible to biochemically examine the relationship between the temporal dynamics of Ca2+ oscillations and signaling through the Ras/ERK/MAPK cascade in a cellular population after receptor activation.
Fig. 1.
The use of the Ca2+ clamp to generate synchronous Ca2+ oscillations within a cellular population. (A) Single-cell imaging of four HeLa cells randomly chosen from a cellular population during stimulation with 10 μM histamine. Although each individual cell responds to the addition of histamine by increasing intracellular free Ca2+, each does so with distinct temporal kinetics inducing Ca2+ oscillations that vary in their frequency and amplitude. (B) The use of the Ca2+ clamp for generating synchronous Ca2+ oscillations of varying frequency. Here, HeLa cells were treated with thapsigargin in Ca2+-free extracellular media before eliciting Ca2+ oscillations by exposure for 15 s to Ca2+-containing media at a frequencies of 120 s (Bi) and 200 s (Bii). Traces are the average of 15 cells randomly imaged within the population. (C) By altering the duration of exposure to Ca2+-containing media from 5 through 10, 15, and 20 s, synchronous oscillations of varying amplitude can be generated in a population of cells. For each condition, traces are the average of 15 cells randomly imaged within the population. (D) The temporal dynamics of individual Ca2+ oscillations generated by agonist stimulation and through the Ca2+ clamp are similar. Here, single-cell Ca2+ imaging was performed on HeLa cells stimulated with either ATP or histamine, comparing the kinetics of the response with that elicited by a 15-s exposure to Ca2+-containing media in the Ca2+ clamp after treatment with thapsigargin. Each trace is the average of 15 cells randomly imaged within the population.
To overcome this heterogeneity and the related problem that cell surface receptors often couple to multiple signaling pathways (making it difficult to ascribe downstream effects to Ca2+ alone), we used a Ca2+ clamp for generating homogenous and synchronous receptor-independent Ca2+ spikes (6). Here, a population of cells cultured on 22-mm coverslips and mounted at 37°C on the stage of a Ca2+ imaging system were treated with media lacking extracellular Ca2+ with either the Ca2+ ionophore ionomycin or the irreversible inhibitor of the endoplasmic reticulum Ca2+-ATPase, thapsigargin (42). Each of these reagents generates a plasma membrane that is freely permeable to Ca2+. In the case of thapsigargin, this permeability is achieved through depletion of internal Ca2+ stores and the activation of plasma membrane store operated Ca2+ channels (42). Under either condition, the addition of Ca2+-containing media induced an elevation in [Ca2+]i through Ca2+ influx, whereas removal of extracellular Ca2+ allowed plasma-membrane Ca2+ pumps to extrude the cytosolic Ca2+, thereby lowering [Ca2+]i back to baseline values. Thus, by rapidly changing the concentration of extracellular Ca2+, it was possible to generate a Ca2+ oscillation that, unlike receptor-mediated responses, had a uniform frequency across cells and amplitudes that were relatively constant in each cell over time (SD, 16.3%) and among cells in the population (SD, 18.7%; n = 25). Furthermore, by varying the interval between which the cells were exposed to extracellular Ca2+ (Fig. 1B) and the duration of each exposure (Fig. 1C), it was possible to generate a range of Ca2+ oscillations of defined frequencies and amplitudes. Importantly, the temporal dynamics of individual Ca2+ oscillations elicited by either ionomycin or thapsigargin treatment were similar to those observed with agonist stimulation (Fig. 1D).
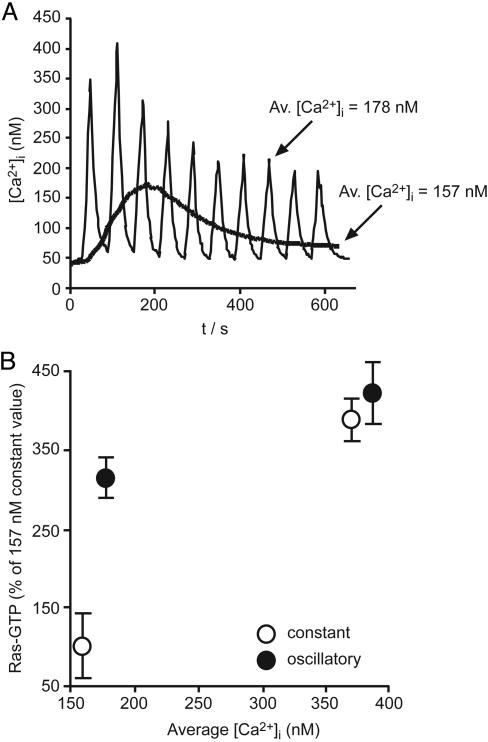
In initial experiments, Ca2+ oscillations were generated of a constant interspike interval of 60 s, but the amplitude was adjusted to produce defined average increases in [Ca2+]i that lay within the window of amplitudes observed upon receptor stimulation (Fig. 2A). To determine whether the temporal features of the Ca2+ signal provided any advantage on the activation of Ras independently of the amount of Ca2+ that entered the cell, data generated by using Ca2+ oscillations were compared with control cells in which the same average increase in [Ca2+]i was elevated constantly throughout the experimental period (Fig. 2 A). In cells in which the average [Ca2+]i was ≈165 nM, analysis of Ras activation revealed a 3.2-fold enhancement in cells stimulated with Ca2+ oscillations compared with cells with a constant elevation of [Ca2+]i, even though the same amount of Ca2+ had entered the cells (Fig. 2B). In contrast, in cells stimulated to an average of ≈376 nM, Ca2+ oscillations activated Ras to an extent similar to a constant elevation of [Ca2+]i (Fig. 2B). In other words, activation of Ras by Ca2+ oscillations increases as average [Ca2+]i decreases, enhancing the ability of small Ca2+ signals to activate and signal through this GTPase. A similar ability of Ca2+ oscillations to reduce the effective threshold for the activation of signaling has also been reported for the activation of the transcription factors NF-AT, Oct/OAP, and NF-κB (6).
Fig. 2.
Complex Ca2+ signals regulate Ras activation. (A) Generation of Ca2+ oscillations within a population of HeLa cells. Before recording, serum-starved HeLa cells were incubated for 15 min with thapsigargin in media lacking extracellular Ca2+ to deplete internal Ca2+ stores. Ca2+ oscillations of a fixed interspike interval (60 s) were induced for a period of 10 min by exposure for 10 s to Ca2+-containing media. Under these conditions, the average increase in [Ca2+]i was 178 nM. To generate the nonoscillatory increase in [Ca2+]i, the cells were exposed to extracellular media-containing 0.1 mM Ca2+ for the entire duration of the experiment. The average constant [Ca2+]i under these conditions was 157 nM. Each trace is the average of 15 cells randomly imaged within the population. (B) [Ca2+]i oscillations enhance the Ca2+ sensitivity of endogenous H-Ras activation at low-level stimulation. The comparative ability of [Ca2+]i oscillations (•) or constant [Ca2+]i elevation (○) to couple to Ras activation was determined under two conditions, oscillating Ca2+ (165 and 364 nM average) and constant Ca2+ (185 and 383 nM, respectively). Activation of Ras was determined as described in Methods after 10 min of Ca2+ manipulation.
To examine the dependency of Ca2+ oscillatory frequency, a series of oscillations of a fixed amplitude (365 ± 44 nM) were generated with interspike intervals that spanned the physiological range observed with agonist stimulation, varying between 60 and 600 s. It was not possible to generate oscillations with a frequency >60 s by using this methodology (data not shown). Analysis of Ras activation revealed a strong dependency on Ca2+ oscillatory frequency, significant activation being observed only as interspike intervals approached 120 s (Fig. 3A). Upon further increasing the oscillatory frequency, a nonlinear increase in Ras activation was observed, with maximal Ca2+-mediated Ras activation occurring at an interspike interval of 60 s. The complex temporal patterning of Ca2+ oscillations are therefore optimized for efficient coupling to the Ca2+-mediated activation of Ras.
Fig. 3.
The frequency of Ca2+ oscillations is optimized for activation of Ras and the MAPK pathway. (A) Activation of Ras after generation of [Ca2+]i spikes with varying interspike intervals. HeLa cells were transiently transfected with 2 μg of H-Ras cDNA for 24 h. Serum-starved cells were treated with thapsigargin for 15 min, after which Ca2+ spikes of a fixed amplitude (365 ± 44 nM) were generated for 10 min over a range of interspike intervals (60–600 s). Activation of Ras was determined as described in Methods. Similar data were obtained in an examination of endogenous Ras activation (data not shown). (B) Activation of MAPK after the generation of [Ca2+]i spikes with varying interspike intervals. Ca2+ spikes of fixed amplitude were generated for 10 min over a range of interspike intervals (60–600 s). Activation of MAPK was determined as described in Methods. (C) Ca2+-mediated activation of the ERK/MAPK pathway depends on MEK. Serum-starved HeLa cells were treated with U0126, STO-609, KN-93, and KN-92 as described in Methods. The cells were treated with thapsigargin for 15 min, after which Ca2+ spikes of fixed amplitude and interspike interval (120 s) were induced over a period of 10 min. Activation of ERK/MAPK was determined as described in Methods.
To extend these observations, we also examined the role of Ca2+ oscillations in modulating downstream Ras signaling. Here, a slightly distinct dependency on the frequency of Ca2+ oscillations was observed, with significant activation of the ERK/MAPK cascade occurring as interspike intervals approached 300 s (Fig. 3B). Inhibitory studies revealed that, under these conditions, where cell surface receptors are not being stimulated, the Ca2+-mediated activation of the ERK/MAPK cascade was mediated by MEK [the MEK1/2 inhibitor U0126 (43) completely blocked ERK activation] but appeared to be independent of calmodulin-dependent protein kinase kinase and calmodulin-dependent protein kinase II because STO-609 (44) and KN-93 (45), respectively, were without significant effect (Fig. 3C). Thus, Ca2+ oscillations of defined frequencies can couple through a MEK-dependent pathway to downstream signaling through the ERK/MAPK cascade.
Although agonists such as histamine and ATP are able to activate the ERK/MAPK cascade (e.g., refs. 46 and 47 and data not shown), one of the major inputs into Ras signaling is through growth-factor stimulation (20). Indeed, compared with the activation of the ERK/MAPK cascade observed upon the addition of a maximal dose of epidermal growth factor (EGF), a train of high-frequency Ca2+ oscillations (having an interspike interval of 60 s) elicited only 44.9 ± 15.0% (n = 3) of this activation (Fig. 4A). Thus, Ca2+ signals appear to have a modulatory rather than a decisive role in regulating Ras signaling through the ERK/MAPK cascade. To test this hypothesis, we stimulated serum-starved cells with 300 pg/ml of EGF, a suboptimal dose that does not induce a significant elevation in intracellular free Ca2+ (data not shown), and compared the level of activation of the ERK/MAPK cascade in the presence or absence of Ca2+ oscillations. Compared with a control in which cells were not exposed to any elevation in intracellular free Ca2+, Ca2+ oscillations in a frequency-dependent manner, potentiated the ability of the suboptimal EGF to induce activation of the ERK/MAPK cascade (Fig. 4B). These data establish a level of cross-talk between the frequency of [Ca2+]i oscillations and the growth-factor-mediated activation of the Ras/ERK/MAPK cascade, consistent with distinct Ca2+-dependent and -independent pathways, leading to ERK/MAPK signaling in which increases in [Ca2+]i can modulate Ca2+-independent ERK/MAPK signaling in a frequency-dependent manner.
Fig. 4.
Ca2+ signals have a modulatory rather than a decisive role in regulating Ras signaling. (A) Activation of ERK/MAPK after generation of rapid [Ca2+]i oscillations compared with ERK/MAPK activation after stimulation with 100 ng/ml EGF. Serum-starved HeLa cells were treated with thapsigargin. After 15 min, Ca2+ oscillations of fixed amplitude and interspike interval (60 s) were generated over a period of 10 min. Alternatively, cells were treated with 100 ng/ml EGF for 1 min. Activation of ERK/MAPK was determined as described in Methods. (B) Ca2+ oscillations can potentiate ERK/MAPK activation induced by suboptimal EGF concentration. Serum-starved HeLa cells were treated with thapsigargin for 15 min, stimulated with 300 pg/ml EGF, and either left untreated (TG) or exposed to fixed-amplitude Ca2+ oscillations over a range of interspike intervals (60–600 s). Activation of ERK/MAPK was determined as described in Methods and is plotted as fold activation over the control treatment, where cells were left untreated.
In summary, we have used a Ca2+ clamp to generate synchronous, receptor-independent Ca2+ oscillations within a cellular population (6). Although this technique does have its limitations, for example, the spatial patterning of the Ca2+ elevation is different from that achieved with receptor stimulation (6), it is currently the only available method for biochemically analyzing the coupling of Ca2+ oscillations to Ras activation and signaling through the ERK/MAPK cascade. Biosensors that report Ras activation in single living cells are available (reviewed in ref. 48); however, we have so far been unable to examine the coupling of Ca2+ oscillations to Ras activation by using either the Raichu-Ras (49, 50) or GFP-RBD (51) probes (S.K. and P.J.C., unpublished data). Given that, compared with growth factor stimulation, Ca2+ signals give a relatively small activation of Ras signaling, this inability may result from a lack of sensitivity of these probes.
By using the Ca2+ clamp, we have established that Ca2+ oscillations effectively reduce the threshold for Ca2+-mediated activation of Ras and that the frequencies of Ca2+ oscillations are optimized for coupling to Ras activation and downstream signaling. These data therefore add Ras signaling to a growing list of pathways, including the efficiency of gene expression driven by the transcription factors NF-AT, OAP, and NF-κB (6–8) and mitochondrial ATP production (9), which are regulated by the frequency of [Ca2+]i oscillations. That the temporal dynamics of Ca2+ oscillations are optimized for efficient Ca2+-mediated Ras activation and signaling through the ERK/MAPK cascade adds to our understanding of the biological role of this second messenger in the regulation of Ras signaling. With the identification of molecular entities that couple an elevation of [Ca2+]i to the activation of Ras at the plasma membrane, Golgi complex, and endoplasmic reticulum (52, 53) and the large body of evidence pointing to an important role for Ca2+ signaling through the Ras/ERK/MAPK cascade in synaptic plasticity (reviewed in ref. 54), the data presented in this study are consistent with the concept that cells utilize the complex nature of cytosolic Ca2+ signals to achieve a dynamic spatial and temporal modulation of Ras activation and downstream Ras-mediated signaling.
Acknowledgments
We thank Mark Jepson and Alan Leard for assistance. This work was supported in part by Component and Career Establishment grants from the Medical Research Council and The Wellcome Trust and a Medical Research Council Infrastructure Award (G4500006) to establish the School of Medical Sciences Cell Imaging Facility. P.J.C. acknowledges the financial support of the Lister Institute of Preventive Medicine during the early stages of this work.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: [Ca2+]i, concentration of intracellular free calcium; EGF, epithelial growth factor; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase.
References
- 1.Berridge, M. J., Lipp, P. & Bootman, M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11–21. [DOI] [PubMed] [Google Scholar]
- 2.Bootman, M. D., Lipp, P. & Berridge, M. J. (2001) J. Cell Sci. 114, 2213–2222. [DOI] [PubMed] [Google Scholar]
- 3.Berridge, M. J., Bootman, M. D. & Roderick, H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529. [DOI] [PubMed] [Google Scholar]
- 4.Berridge, M. J. & Prince, W. T. (1972) J. Exp. Biol. 56, 139–153. [DOI] [PubMed] [Google Scholar]
- 5.Woods, N. M., Cuthbertson, K. S. R. & Cobbold, P. H. (1986) Nature 319, 600–602. [DOI] [PubMed] [Google Scholar]
- 6.Dolmetsch, R. E., Xu, K. & Lewis, R. S. (1998) Nature 392, 933–936. [DOI] [PubMed] [Google Scholar]
- 7.Li, W. H., Llopis, J., Whitney, M., Zlokarnik, G. & Tsien, R. Y. (1998) Nature 392, 936–941. [DOI] [PubMed] [Google Scholar]
- 8.Tomida, T., Hirose, K., Takizawa, A., Shibasaki, F. & Iino, M. (2003) EMBO J. 22, 3825–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajnoczky, G., Robb-Gaspers, L. D., Seitz, M. B. & Thomas, A. P. (1995) Cell 82, 415–424. [DOI] [PubMed] [Google Scholar]
- 10.Craske, H., Takeo, T., Gerasimenko, O., Vaillant, C., Torok, K., Petersen, O. H. & Tepikin, A. V. (1999) Proc. Natl. Acad. Sci. USA 96, 4426–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oancea, E. & Meyer, T. (1998) Cell 95, 307–318. [DOI] [PubMed] [Google Scholar]
- 12.Pinton, P., Tsuboi, T., Ainscow, E. K., Pozzan, T., Rizzuto, R. & Rutter, G. A. (2002) J. Biol. Chem. 277, 37702–37710. [DOI] [PubMed] [Google Scholar]
- 13.Violin, J. D., Zhang, J., Tsien, R. Y. & Newton, A. C. (2003) J. Cell Biol. 161, 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogami, H., Zhang, H., Suzuki, Y., Urano, T., Saito, N., Kojima, I. & Petersen, O. H. (2003) J. Biol. Chem. 278, 9896–9904. [DOI] [PubMed] [Google Scholar]
- 15.Babwah, A. V., Dale, L. B. & Ferguson, S. S. G. (2003) J. Biol. Chem. 278, 5419–5426. [DOI] [PubMed] [Google Scholar]
- 16.Tompa, P., Toth-Boconadi, R. & Friedrich, P. (2001) Cell Calcium 29, 161–170. [DOI] [PubMed] [Google Scholar]
- 17.De Koninck, P. & Schulman, H. (1998) Science 279, 227–230. [DOI] [PubMed] [Google Scholar]
- 18.Dupont, G., Houart, G. & De Koninck, P. (2003) Cell Calcium 34, 485–497. [DOI] [PubMed] [Google Scholar]
- 19.Walker, S. A., Kupzig, S., Bouyoucef, D., Davies, L. C., Tsuboi, T., Cozier, G. E., Lockyer, P. J., Bivona, T., Buckler, A., Rutter, G. A., et al. (2004) EMBO J. 23, 1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downward, J. (2003) Nat. Rev. Cancer 3, 11–22. [DOI] [PubMed] [Google Scholar]
- 21.Hingorani, S. R. & Tuveson, D. A. (2003) Curr. Opin. Genet. Dev. 13, 6–13. [DOI] [PubMed] [Google Scholar]
- 22.Bivona, T. & Philips, M. R. (2003) Curr. Opin. Cell Biol. 15, 136–142. [DOI] [PubMed] [Google Scholar]
- 23.Hancock, J. F. (2003) Nat. Rev. Mol. Cell Biol. 4, 373–384. [DOI] [PubMed] [Google Scholar]
- 24.Rosen, L. B., Ginty, D. D., Weber, M. J. & Greenberg, M. E. (1994) Neuron 12, 1207–1221. [DOI] [PubMed] [Google Scholar]
- 25.Cullen, P. J. & Lockyer, P. J. (2002) Nat. Rev. Mol. Cell Biol. 3, 339–348. [DOI] [PubMed] [Google Scholar]
- 26.Shou, C., Farnsworth, C. L., Nell, B. G. & Feig, L. A. (1992) Nature 358, 351–354. [DOI] [PubMed] [Google Scholar]
- 27.Martegani, E., Vanoni, M., Zippel, R., Coccetti, P., Brambilla, R., Ferrari, C., Sturani, E. & Alberghina, L. (1992) EMBO J. 11, 2151–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farnsworth, C. L., Freshney, N. W., Rosen, L. B., Ghosh, A., Greenberg, M. E. & Feig, L. A. (1995) Nature 376, 524–527. [DOI] [PubMed] [Google Scholar]
- 29.Fam, N. P., Fan, W. T., Wang, Z. X., Zhang, L. J., Chen, H. & Moran, M. F. (1997) Mol. Cell. Biol. 17, 1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebinu, J. O., Bottorff, D. A., Chan, E. Y. W., Stang, S. L., Dunn, R. J. & Stone, J. C. (1998) Science 280, 1082–1086. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki, H., Sprihtgett, G. M., Toki, S., Canales, J. J., Harlan, P., Blumenstiel, J. P., Chen, E. J., Bany, I. A., Mochizuki, N., Ashbacher, A., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tognon, C. E., Kirk, H. E., Passmore, L. A., Whitehead, I. P., Der, C. J. & Kay, R. J. (1998) Mol. Cell. Biol. 18, 6995–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clyde-Smith, J., Silins, G., Gartside, M., Grimmond, S., Etheridge, M., Apolloni, A., Hayward, N. & Hancock, J. F. (2000) J. Biol. Chem. 275, 32260–32267. [DOI] [PubMed] [Google Scholar]
- 34.Ohba, Y., Mochizuki, N., Yamashita, S., Chan, A. M., Schrader, J. W., Hattori, S., Nagashima, K. & Matsuda, M. (2000) J. Biol. Chem. 275, 20020–20026. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita, S., Mochizuki, N., Ohba, Y., Tobiume, M., Okada, Y., Sawa, H., Nagashima, K. & Matsuda, M. (2000) J. Biol. Chem. 275, 25488–25493. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzo, P. S., Kung, J. W., Bottorff, D. A., Garfield, S. H., Stone, J. C. & Blumberg, P. M. (2001) Cancer Res. 61, 943–949. [PubMed] [Google Scholar]
- 37.Lockyer, P. J., Kupzig, S. & Cullen, P. J. (2001) Curr. Biol. 11, 51–56. [DOI] [PubMed] [Google Scholar]
- 38.Walker, S. A., Lockyer, P. J. & Cullen, P. J. (2003) Biochem. Soc. Trans. 31, 966–969. [DOI] [PubMed] [Google Scholar]
- 39.Walker, S. A., Kupzig, S., Lockyer, P. J., Bilu, S., Zharhary, D. & Cullen, P. J. (2002) J. Biol. Chem. 277, 48779–48785. [DOI] [PubMed] [Google Scholar]
- 40.Cozier, G. E., Bouyoucef, D. & Cullen, P. J. (2003) J. Biol. Chem. 275, 39489–39496. [DOI] [PubMed] [Google Scholar]
- 41.Bootman, M. D., Young, K. W., Young, J. M., Moreton, R. B. & Berridge, M. J. (1996) Biochem. J. 314, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thastrup, O., Cullen, P. J., Drøbak, B. K., Hanley, M. R. & Dawson, A. P. (1990) Proc. Natl. Acad. Sci. USA 87, 2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Favata, M. F., Horiuchi, K. Y., Manos, E. J., Daulerio, A. J., Stradley, D. A., Feeser, W. S., Van Dyk, D. E., Pitts, W. J., Earl, R. A., Hobbs, F., et al. (1998) J. Biol. Chem. 273, 18623–18632. [DOI] [PubMed] [Google Scholar]
- 44.Tokumitsu, H., Inuzuka, H., Ishikawa, Y., Ikeda, M., Saji, I. & Kobayashi, R. (2002) J. Biol. Chem. 277, 15813–15818. [DOI] [PubMed] [Google Scholar]
- 45.Tokumitsu, H., Chijiwa, T., Hagiwara, M., Mizutani, A., Terasawa, M. & Hidaka, H. (1990) J. Biol. Chem. 265, 4315–4320. [PubMed] [Google Scholar]
- 46.Cammarota, M., Bevilaqua, L. R. M., Rostas, J. A. P. & Dunkley, P. R. (2003) J. Neurochem. 84, 453–458. [DOI] [PubMed] [Google Scholar]
- 47.Muscella, A., Elia, M. G., Greco, S. & Marsigliante, S. (2003) J. Cell. Physiol. 195, 234–240. [DOI] [PubMed] [Google Scholar]
- 48.Walker, S. A. & Lockyer, P. J. (2004) J. Cell Sci. 117, 2879–2886. [DOI] [PubMed] [Google Scholar]
- 49.Mochizuki, N., Yamashita, S., Kurokawa, K., Ohba, Y., Nagai, T., Miyawaki, A. & Matsuda, M. (2001) Nature 411, 1065–1068. [DOI] [PubMed] [Google Scholar]
- 50.Ohba, Y., Kurokawa, K. & Matsuda, M. (2003) EMBO J. 22, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu, V. K., Bivona, T., Hach, A., Sajous, J. B., Silletti, J., Wiener, H., Johnson, R. L., Cox, A. D. & Philips, M. R. (2002) Nat. Cell Biol. 4, 343–350. [DOI] [PubMed] [Google Scholar]
- 52.Bivona, T. G., de Castro, I. P., Ahearn, I. M., Grana, T. M., Chiu, V. K., Lockyer, P. J., Cullen, P. J., Pellicer, A., Cox, A. D. & Philips, M. R. (2003) Nature 424, 694–698. [DOI] [PubMed] [Google Scholar]
- 53.Arozarena, I., Matallanas, D., Berciano, M. T., Sanz-Moreno, V., Calvo, F., Munoz, M. T., Egea, G., Lafarga, M. & Crespo, P. (2004) Mol. Cell. Biol. 24, 1516–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas, G. M. & Huganir, R. L. (2004) Nat. Rev. Neurosci. 5, 173–183. [DOI] [PubMed] [Google Scholar]