Abstract
Preclinical and clinical studies indicate that psychostimulants, in addition to having abuse potential, may elicit brain dysfunctions and/or neurotoxic effects. Central toxicity induced by psychostimulants may pose serious health risks since the recreational use of these substances is on the rise among young people and adults. The present review provides an overview of recent research, conducted between 2018 and 2023, focusing on brain dysfunctions and neurotoxic effects elicited in experimental models and humans by amphetamine, cocaine, methamphetamine, 3,4-methylenedioxymethamphetamine, methylphenidate, caffeine, and nicotine. Detailed elucidation of factors and mechanisms that underlie psychostimulant-induced brain dysfunction and neurotoxicity is crucial for understanding the acute and enduring noxious brain effects that may occur in individuals who use psychostimulants for recreational and/or therapeutic purposes.
Keywords: 3,4-methylenedioxymethamphetamine; amphetamine; caffeine; cell cultures; cocaine; methamphetamine; methylphenidate; neurotoxicity; nicotine
Introduction
Psychostimulants are a heterogeneous group of substances historically classified as “direct or indirect sympathomimetics” or “non-sympathomimetics”, according to their ability to mimic the action of the catecholamines dopamine, epinephrine, and norepinephrine (Koob et al., 2020; Additional Table 1). Sympathomimetic psychostimulants mimic the peripheral actions of catecholamines in the autonomic system by directly or indirectly activating their receptors, whereas non-sympathomimetic psychostimulants act on several neurotransmitter systems using different mechanisms of action (Koob et al., 2020).
Additional Table 1.
Classification of psychostimulants
| Direct sympathomimetics | Indirect sympathomimetics | Non-sympathomimetics |
|---|---|---|
| Apomorphine | Amphetamine | Caffeine |
| Epinephrine | Cocaine | Modafinil |
| Isoproterenol | Methamphetamine | Nicotine |
| Norepinephrine | 3,4-methylendioxymethamphetamine | Pentylenetetrazol |
| Phenylephrine | Methylphenidate | Scopolamine |
| Phenylpropanolamine | Pemoline | Strychnine |
| Phenmetrazine | ||
| Pipradrol | ||
| Tyramine |
Data are adapted from Koob et al. (2020). The psychostimulants in bold are those analyzed in the present review.
Although indirect sympathomimetic and non-sympathomimetic psychostimulants have different mechanisms of action, as described below, both ultimately increase the synaptic levels of dopamine and other monoamines (e.g., norepinephrine and serotonin) (Rothman and Baumann, 2003; Howell and Kimmel, 2007; Docherty and Alsufyani, 2021). This effect underlies their major central actions, which include elevation of mood, suppression of appetite, reduction of fatigue, and improvement of executive functions (Fast et al., 2021; Mckenzie et al., 2022). Elevated libido and heightened sexual pleasure and activity have also been documented in psychostimulant users, albeit often in conjunction with high-risk behaviors (Nazlı and Sevindik, 2020; Berry et al., 2022; Suyama et al., 2023).
Certain psychostimulants can improve brain function for specific pathological conditions and are currently used as therapeutic medications. For example, methylphenidate and dextroamphetamine, the dextrorotatory enantiomer of amphetamine (AMPH), can be prescribed to treat attention deficit hyperactivity disorder (ADHD) or narcolepsy due to their ability to increase attention, vigilance, and wakefulness (Cortese et al., 2018; Bassetti et al., 2021). Moreover, the Australian Therapeutic Goods Administration has recently announced that, starting July 2023, licensed psychiatrists will be able to prescribe 3,4-methylenedioxymethamphetamine (MDMA) to treat post-traumatic stress disorder, due to its empathogenic effects (Australian Government, Department of Health and Aged Care, Therapeutic Goods Administration). Non-sympathomimetic psychostimulants such as caffeine and nicotine can be used as performance enhancers by certain professionals (e.g., aviation, military corps, and truck drivers) for their anti-fatigue effects (Gore et al., 2010; McLellan et al., 2019; Kagabo et al., 2020). Indeed, caffeine has been reported to elevate mood and working memory, even though some authors have postulated that these effects could be due to its reversal of caffeine withdrawal (Lin et al., 2023). Moreover, both preclinical models and human studies have demonstrated that nicotine enhances cognitive performance by improving learning, memory, and attention (Valentine and Sofuoglu, 2018).
At the same time, the ability of psychostimulants to increase the synaptic levels of dopamine also underlies their ability to generate addictive behaviors, psychotic-like symptoms, and deficits in executive functions (Volkow and Swanson, 2003). AMPH, cocaine, methamphetamine (METH), and nicotine possess high abuse liability and may induce overt addictive behaviors in users. While the existence of addictive-like behaviors has also been suggested for users of caffeine and MDMA, drug consumption behaviors in these individuals rarely meet substance abuse criteria according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Psychotic-like symptoms may be observed in users of AMPH, METH, or cocaine, all of which increase extracellular levels of dopamine by acting at the dopamine transporter (DAT). For example, cocaine can elicit symptoms such as paranoia, hallucinations, violence, and aggression, even following a single administration (Gicas et al., 2022); similar effects have been observed in AMPH and METH users (Morton and Stock, 2000; Gicas et al., 2022). In contrast, users of caffeine, MDMA, or methylphenidate are less prone to exhibit psychotic-like behaviors (Patel et al., 2011; Virani et al., 2018). Nevertheless, MDMA consumption can be associated with anxiety and depressed mood, as well as deficits in working memory, attention, and verbal processing (Costa and Gołembiowska, 2022; Montgomery and Roberts, 2022). Moreover, caffeine consumption may facilitate relapse in psychiatric patients (Rizkallah et al., 2011).
The manifestation of behavioral abnormalities in psychostimulant users suggests that brain dysfunctions, and possibly neurotoxic phenomena, may occur in these individuals, especially in brain regions that regulate cognitive and emotional domains. Although the induction of neurotoxicity by psychostimulants has not conclusively been demonstrated in humans, imaging studies have reported the existence of altered brain morphology and connectivity in psychostimulant users (Boxler et al., 2018; Vuletic et al., 2018; Elbejjani et al., 2019; Bittencourt et al., 2021; Avram et al., 2022). Importantly, psychostimulant-induced alterations in brain function depend on several factors, such as the amount of drug consumed, the routes of drug intake, and the age and sex of the consumer (Moratalla et al., 2017). Indeed, it is noteworthy that prescription psychostimulants (e.g., AMPH, methylphenidate) may elicit detrimental, rather than beneficial, effects on the brain when they are taken outside of a therapeutic plan (e.g., at higher amounts and/or for longer times than prescribed). Moreover, people who use psychostimulants for recreational purposes often consume multiple psychostimulants (polydrug use), which further complicates ascribing a specific substance with the induction of definite dysfunctional/neurotoxic effects in the brain (Rudin et al., 2021).
To elucidate risk factors (e.g. age, dose, sex) and mechanisms related to psychostimulant-induced neurotoxicity, the present review provides a narrative overview of recently published findings in experimental models and humans regarding brain dysfunctions and neurotoxic effects following administration/consumption of the most used psychostimulants worldwide: the sympathomimetics AMPH, methylphenidate, cocaine, METH and MDMA, and the non-sympathomimetics caffeine and nicotine. The implications of these findings are briefly discussed in terms of risks associated with psychostimulant use. Due to its concise format, the present review focuses on brain dysfunctions and neurotoxicity induced by psychostimulants; it does not provide extensive coverage of the beneficial effects of psychostimulants on brain function.
Search Strategy
We began with an extensive search strategy to conduct a comprehensive review of the relevant literature, focusing on original research articles, meta-analyses, and review articles published in the last 5 years (2018–2023). Articles were searched by using the specific search terms “psychostimulants” AND “brain dysfunction” AND “neurotoxicity” in PubMed, to focus on articles on the brain dysfunction and neurotoxicity induced by AMPH, cocaine, METH, MDMA, methylphenidate, caffeine, or nicotine in experimental models and humans. The initial search yielded 296 articles. After reading the titles and abstracts of these articles, we included in the search strategy a series of additional keywords (e.g., the name of specific psychostimulants) focusing on the drugs' biochemical effects and mechanisms of toxicity at the central level, rather than on the behavioral effects of specific psychostimulants. Pure behavioral studies were not included in the present review. When a limited number of relevant articles were found, we adjusted the search criteria, for example by extending the search period to the most recent articles available, in order to obtain more relevant literature. Finally, we conducted filtering and article selection in accordance with the journal's criteria (e.g., selection of no more than three articles from the same author). Overall, the recent findings reported in this narrative review are derived from 120 articles published between 2018 and 2023. In consideration of the journal's limitations on the number of references, we could not include all possible relevant literature.
Indirect Sympathomimetic Psychostimulants
Indirect sympathomimetic psychostimulants have chemical structures that resemble that of the catecholamines. Cocaine, AMPH, and the AMPH-related drugs methylphenidate, METH, and MDMA are all indirect sympathomimetics. These drugs bind to DAT and to the norepinephrine and serotonin (SERT) transporters to increase the synaptic levels of monoamines (John and Jones, 2007). The precise mechanism by which indirect sympathomimetic psychostimulants exert their central effects is not yet fully defined. It is widely believed that these drugs produce their effects by enhancing dopamine neurotransmission in the brain, and in particular by inducing DAT-mediated reverse transport and/or by blocking dopamine reuptake through DAT (Koob et al., 2020). These drugs are also called “psychomotor stimulants” because their primary biological activity, as indirect dopamine agonists, is to produce behavioral activation, which is usually expressed as arousal, alertness, and increased motor activity (Koob et al., 2020).
AMPH and AMPH-related drugs also bind to the vesicular monoamine transporter-2 (VMAT-2) (Freyberg et al., 2016; Cholanians et al., 2019; Jayanthi et al., 2021), thus inhibiting vesicular uptake of dopamine and promoting dopamine release from synaptic vesicles, thereby increasing the amount of cytosolic dopamine available for reverse transport by DAT (Freyberg et al., 2016). The enhanced synaptic availability of monoamines results in the activation of receptors for dopamine, norepinephrine, and serotonin in the mesocorticolimbic and nigrostriatal systems (Drouin et al., 2002; Salamone and Correa, 2012; Müller and Homberg, 2015).
Amphetamine
AMPH is a psychostimulant utilized as a racemic mixture or as dextroamphetamine for the treatment of ADHD, narcolepsy, and binge eating disorder (Cortese et al., 2018; Bassetti et al., 2021). Besides its therapeutic application, AMPH is popular as a recreational drug due to its stimulant and euphoriant effects. According to recent self-reported survey responses, it was estimated that approximately 36 million individuals had used AMPH-related drugs (either AMPH or METH) in 2021, with a male-to-female ratio of 55:45 (UNODC, 2023). AMPH is highly consumed in Europe, where it ranks as the second most used psychostimulant drug after cocaine (UNODC, 2023). While AMPH-based prescriptions are safe when used for therapeutic purposes (Weyandt et al., 2016), the recreational use of AMPH raises concerns since users could be exposed to high and potentially neurotoxic amounts of the drug. In this respect, several pathological mechanisms have been implicated in the neurotoxic effects of AMPH including, but not limited to, hyperthermia (Miller and O'Callaghan, 1995), apoptosis (Stumm et al., 1999), bioenergetic failure (Wan et al., 1999) and increased production of reactive oxygen (ROS) and nitrogen (RNS) species (Bashkatova et al., 2004; Wan et al., 2006; Figure 1). Recent evidence in rats indicates that the different mechanisms underlying AMPH-induced neurotoxicity may occur sequentially, with energy failure preceding excitotoxicity and excitotoxicity preceding the production of free radicals (Tung et al., 2017; Additional Table 2). Moreover, a study using PC12 cells suggests that the protein phosphatase 2A (PP2A)/AKT/glycogen synthase kinase-3beta (GSK3β) pathway may be an important player in AMPH-induced apoptotic cell death (He et al., 2018; Additional Table 2). Preclinical investigations in mice, rats, and nonhuman primates have demonstrated that systemic administration of AMPH, either as a racemic mixture or dextroamphetamine, may induce marked neurotoxicity in striatal dopaminergic terminals, the severity of which varies depending on the dosing and frequency of AMPH administration. Indeed, AMPH may reduce the synthesis and levels of dopamine, as well as the density of tyrosine hydroxylase (TH) and of DAT in the caudate-putamen nucleus (CPu) (Ricaurte et al., 2005; Levi et al., 2012; Tung et al., 2017), although a progressive recovery of striatal dopaminergic functionality has been also reported with time (Melega et al., 1997). Conversely, and differently from what observed after the administration of other AMPH-related drugs, AMPH does not induce neurotoxic damage in dopaminergic neurons located in the substantia nigra pars compacta (SNc) (Gramage et al., 2010). Results from a recent preclinical investigation suggest that AMPH may induce the appearance of motor deficits before the emergence of signs of dopaminergic neurotoxicity. Indeed, Apóstol del Rosal et al. (2021) demonstrated that repeated administration of AMPH in rats induced microgliosis and astrogliosis in the dorsal CPu paired with an impairment in locomotor activity and motor coordination, but without affecting the immunoreactivity of TH-positive dopaminergic neurons and terminals in the nigrostriatal system (Apóstol del Rosal et al., 2021; Additional Table 2). In addition, AMPH may induce neuronal degeneration in non-dopaminergic populations, most notably in the hippocampus, parietal cortex, and piriform cortex, as demonstrated by studies in adult rats (Bowyer et al., 2008).
Figure 1.
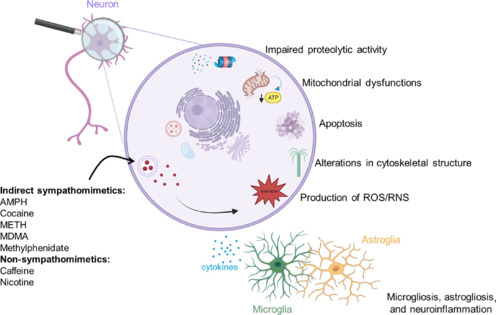
Scheme of the proposed mechanisms contributing to psychostimulant-induced brain dysfunctions and neurotoxicity.
Created with BioRender.com. AMPH: Amphetamine; MDMA: 3,4-methylenedioxymethamphetamine; METH: methamphetamine; RNS: reactive nitrogen species; ROS: reactive oxygen species.
Additional Table 2.
Recent preclinical and clinical studies evaluating amphetamine (AMPH)- and cocaine-induced brain dysfunctions and neurotoxicity
| Drug | Species (n, sex)/age | Treatment/duration | Main findings | References |
|---|---|---|---|---|
| PC12 cells | AMPH (1,2,3,4,5,6,7,8, or 10 mM) for 24 h | The study revealed that the inhibition of the AKT/GSK3β pathway is involved in AMPH-mediated dopaminergic neurotoxicity. | He et al., 2018 | |
| Wistar male rats (n=42); 8 weeks old | Pretreatment: cysteine (500 mg/kg, i.p.), MK-801 (1 mg/kg, i.p.), desferrioxamine (200 mg/kg, i.p.), or nicotinamide (500 mg/kg, i.p.); Treatment: dextroamphetamine (10 mg/kg, i.p.) plus desipramine (10 mg/kg, i.p.) | The study unveiled a sequential occurrence of energy failure, excitotoxicity, and free radical formation in AMPH-induced dopaminergic neurotoxicity | Tung et al., 2017 | |
| AMPH | Wistar male rats (n=42); age N.A. | Dextroamphetamine (2 mg/kg, p.o.), or clobenzorex (30 mg/kg, p.o.) for 31 days | The study revealed significant and comparable motor impairments and striatal gliosis in rats following either dextroamphetamine or clobenzorex, without evidence of dopaminergic nigrostriatal neurodegeneration. | Apóstol del Rosal et al., 2021 |
| Humans (n=28, 14 women); 24-32 years old | Dextroamphetamine (40 mg, p.o.), lysergic acid diethylamide (0.1 mg, p.o.), or MDMA (125 mg, p.o.) | The study revealed the ability of all substances tested to elicit auditory-sensorimotor-thalamic hyperconnectivity compared with placebo treatment. | Avram et al., 2022 | |
|
| ||||
| C57BL/6 mice (n=74); 4 months old | Saline or cocaine (30 mg/kg, i.p.) for 14 days | The study found sex-dependent neuronal alterations (e.g., higher D2R density) in the mPFC and VTA of cocaine-treated mice. | Clare et al., 2021 | |
| Sprague-Dawley male rats (n=76); PND28-42 | Cocaine hydrochloride (15 mg/kg, i.p.) or saline | The study found alterations in the protein levels and morphology of the synapses in the mPFC of cocaine- treated rats. | Zhuetal.,2017 | |
| Cocaine | Humans (n=18 men crack-cocaine users; n=17 men non cocaine users); 21-35 years old | N.A. | The study found cortical and hippocampal grey matter alterations in crack-cocaine users compared with non-users. | Bittencourt et al., 2021 |
| Humans (n=55 cocaine users, 27 women; n=56 non cocaine users, 26 women); 24-28 years old | N.A. | The study found a sex-specific association between stress-relief craving and insular morphometry. | Abdel Malek et al., 2022 | |
The articles reported in the table were arranged by model/species (cells, mice, rats, monkeys, humans) and in chronological order. AMPH: Amphetamine; D2R: dopamine D2 receptors; GSK3β: glycogen synthase kinase-3 beta; i.p.: intraperitoneal injection; MDMA: 3,4-methylenedioxymethamphetamine; mPFC: medial prefrontal cortex; MRI: magnetic resonance imaging; p.o.: per os; VTA: ventral tegmental area.
Neuroimaging studies suggest that neurotoxic effects of AMPH may also occur in humans, which is consistent with evidence that individuals with a history of AMPH consumption may display deficits in memory, decision-making, and set-shifting (Ersche et al., 2005; Schouw et al., 2013). A recent meta-analysis evaluated the occurrence of dopaminergic dysfunction in stimulant users, by comparing 31 positron emission tomography (PET) and single-photon emission computed tomography (SPECT) studies, revealing a significant reduction in the striatal availability of dopamine D2 (D2R) and D3 (D3R) receptors, as well as of DAT, in recreational AMPH users (Ashok et al., 2017). Conversely, striatal dopamine synthesis capacity, VMAT availability, and expression of dopamine D1 (D1R) receptors remained unaffected in this population (Ashok et al., 2017). Moreover, magnetic resonance imaging (MRI) investigations demonstrated that chronic AMPH users showed lower striatal activity in anticipation of reward (Schouw et al., 2013), as well as reduced cortico-striatal-thalamic and prefrontal-limbic-thalamic connectivity when acutely exposed to AMPH (Schrantee et al., 2016; Avram et al., 2022). Intriguingly, Avram and collaborators also reported that acute AMPH administration to healthy volunteers may promote auditory-sensorimotor-thalamic hyperconnectivity (Avram et al., 2022; Additional Table 2), a finding that mirrors what has been previously observed in psychiatric subjects (Woodward and Heckers, 2016), and highlights the inability of the thalamus to properly filter incoming sensory signals.
Cocaine
Approximately 22 million people are estimated to have used cocaine or cocaine-containing products at least once in 2021, according to the World Drug Report (2023). These figures make cocaine the first or second most used drug of abuse worldwide, according to the country considered. The widespread use of cocaine is related to its potent psychostimulant action, resulting in euphoria, increased energy, and mental alertness (Frank et al., 1992; Hutten et al., 2018). Cocaine is generally available in two forms: as a free base (or “crack”), consumed by smoking, and as a salt, consumed by snorting or injection.
The central effects of cocaine stem from its blockade of the reuptake of monoamine neurotransmitters, causing an increase in their synaptic concentrations (Koob et al., 2020). The DAT is a major target of cocaine; increased extracellular levels of dopamine in the mesocorticolimbic system are responsible for cocaine-induced euphoria and sense of well-being, but also for its addiction potential and other central untoward effects, such as irritability and induction of psychotic-like states (Koob et al., 2020). It is noteworthy that dopamine can undergo autooxidation, and several studies have suggested that extracellular dopamine may readily autoxidize to produce ROS, which are responsible for oxidative stress (Schieber and Chandel, 2014), one of the causative factors of neurotoxicity (Rudin et al., 2021; Figure 1). Another contributing factor to the neurotoxic effects of cocaine may be neuroinflammation. Research conducted using both in vitro and in vivo models has demonstrated that cocaine activates microglia, leading to the release of pro-inflammatory mediators, particularly in brain regions such as the prefrontal cortex (PFC), ventral tegmental area (VTA), and nucleus accumbens (NAc) (Liao et al., 2016; Vallender et al., 2017; Mai et al., 2019; Thangaraj et al., 2020). Interestingly, evidence also indicates that cocaine-induced production of pro-inflammatory mediators may stem from pericytes, a cellular constituent implicated in the preservation and integrity of the blood-brain barrier, as the result of impaired autophagy (Sil et al., 2019). In humans, a recent study has substantiated preclinical data by demonstrating that cocaine use disorder alters gene networks implicated in neuroinflammation in the NAc and CPu (Mews et al., 2023); however, other studies have failed to demonstrate microgliosis in cocaine users (Narendran et al., 2014).
Collectively, in vitro and in vivo studies have suggested that cocaine may induce dysfunctions and structural damage in cortical and mesencephalic regions (Camarini et al., 2017; Hirsiger et al., 2019; Bittencourt et al., 2021; Clare et al., 2021; Nicolucci et al., 2021; Tondo et al., 2021; Angarita et al., 2022). While some debate persists, these pathological alterations may be linked to cocaine's capacity to induce mitochondrial dysfunction and increase the vulnerability of neuronal and glial cells, thereby promoting cell death via apoptosis (De Oliveira and Jardim, 2016).
Recent studies in experimental animals indicate that the ability of cocaine to induce synaptic dysfunctions depends on the age and sex of the animals. Zhu et al. (2017) reported that rats treated with cocaine during adolescence showed behavioral abnormalities at adulthood, associated with neurochemical alterations in specific brain regions (Additional Table 2). For example, compared to rats treated with vehicle during adolescence, adult rats exposed to cocaine as adolescents exhibited cognitive deficits along with increased locomotor and anxiety-like behaviors, paired with a decrease in the levels of synapse-related proteins (synapsin I and PSD-95) and in the density of synapses and dendritic spines in the medial prefrontal cortex (mPFC) (Zhu et al., 2017; Additional Table 2). The finding that cocaine administration during adolescence induced altered morphology in the rat brain at adulthood suggests that cocaine may have long-term neurotoxic effects that persist after drug exposure. On the other hand, as emphasized by recent reviews on the topic, it is noteworthy that alterations in cognitive function, which may potentially be related to neurotoxic events, can be either mitigated or exacerbated depending on the timing of cocaine use during adolescence (Kantak, 2020; Caffino et al., 2022). These data in experimental animals are relevant to humans, since it is during adolescence that the use/abuse of illicit drugs, including cocaine, typically begins, as demonstrated by several longitudinal studies (Gerra et al., 2020; Moska et al., 2021). With regard to the possible influence of sex on the neurotoxic effects of cocaine, Clare et al. (2021) investigated changes in total neuronal density measured by NeuN (a marker of mature neurons) and the concentration of neurons expressing D2R in the mPFC, dorsal CPu, VTA, and NAc in both male and female mice treated with cocaine (Clare et al., 2021; Additional Table 2). They reported that cocaine-treated female mice had higher numbers of D2R-expressing neurons in the mPFC and NAc compared to vehicle-treated female mice, and also in the dorsal CPu and VTA compared to vehicle- and cocaine-treated male mice. Moreover, cocaine-treated female mice displayed higher D2R mRNA levels in the dorsal CPu, compared to their respective vehicle-treated female controls, as well as higher D2R and proenkephalin mRNA levels in the mPFC, compared to vehicle- and cocaine-treated male mice (Clare et al., 2021; Additional Table 2). Nevertheless, cocaine-treated female mice showed a decrease in the number of NeuN-positive neurons in the dorsal CPu and VTA, compared to saline-treated female mice, and also in the mPFC, compared to saline- and cocaine-treated male mice (Clare et al., 2021; Additional Table 2). On the other hand, cocaine-treated male mice had a higher number of D2R-expressing neurons in the dorsal CPu, NAc, and VTA, higher prodynorphin mRNA levels in the VTA, higher proenkephalin mRNA levels in the NAc and less NeuN-positive neurons in the mPFC, NAc, and VTA, compared to male controls (Clare et al., 2021; Additional Table 2). Taken together, these observations are of particular interest, since they demonstrate the existence of sex-related differences in cocaine-induced neurotoxicity and identify D2R expressing neurons as a target of this effect.
In humans, imaging studies have demonstrated the existence of morphological and functional differences in specific brain regions between cocaine users and non-users. These differences, though not always significant, have been identified in several cortical regions (e.g., anterior cingulate cortex, cortical insula, lateral frontal cortex) (Hirsiger et al., 2019; Bittencourt et al., 2021), CPu (Ashok et al., 2017) and NAc (Schuch-Goi et al., 2017). Abnormalities in the brains of cocaine users have also been found in white matter microstructure, suggesting the presence of demyelination and axonal damage (King et al., 2022; Gaudreault et al., 2023). Moreover, evidence indicates that cocaine-induced synaptic dysfunctions and neurotoxicity in cocaine users may not necessarily be affected by the duration of drug use and sex of the user. An MRI study by Bittencourt and coworkers (2021) reported that, compared to healthy controls, male crack users displayed decreased cortical thickness in the left inferior temporal cortex, which was negatively correlated with the duration of crack use (Bittencourt et al., 2021; Additional Table 2). Moreover, another MRI study by Abdel Malek and et al. (2022) found no significant sex-specific differences in the morphometry of the cortical insula between male and female cocaine users (Additional Table 2).
Methamphetamine
METH is an extremely addictive psychostimulant with significant abuse liability, characterized by rapid brain penetration and quick onset of its desired effects. According to the World Drug Report (2023), METH is the second most used illicit psychostimulant worldwide after cocaine. Traditionally, METH use has been concentrated in North America, but more recently, there is increasing presence of METH in East and South-East Asia, South-Eastern Europe, as well as Australia and New Zealand (UNODC, 2023). In the United States, METH ranked as the fourth most implicated drug in overdose deaths in 2017 (Hedegaard et al., 2019).
Multiple pathological alterations follow METH administration, which likely contribute to its neurotoxic potential. Among these are hyperthermia (Masai et al., 2021), dysfunction of the proteasomal system (Meng et al., 2020), increased autophagy (Subu et al., 2020), apoptosis (Huang et al., 2019), persistent neuroinflammation (Xu et al., 2023), oxidative and nitrosative stress (Xie et al., 2018; Qiao et al., 2019), and programmed necrosis (Zhao et al., 2021; Figure 1). Moreover, recent studies indicate the disruption of the gut microbiota as a potentially novel mechanism implicated in the neurotoxic effects of METH. Indeed, METH can decrease gut microbial diversity, promote intestinal colon inflammation, and damage its barrier functions, thereby compromising the release of anti-inflammatory metabolites (especially sphingolipids and serotonin) from the intestinal microbiota into the systemic circulation (Zhang et al., 2022; Additional Table 3).
Additional Table 3.
Recent preclinical and clinical studies evaluating methamphetamine (METH)-induced brain dysfunctions and neurotoxicity
| Drug | Species (n, sex)/age | Treatment/duration | Main findings | References |
|---|---|---|---|---|
| BALB/c male mice (n=27); 9 weeks old | Pre-treatment: anti-high mobility group box-1 (HMGB1) antibody, control immunoglobulin, or phosphate-buffered saline; treatment: saline or METH (2×4 mg/kg, s.c.) | The study revealed a potential role of HMGB1 in METH-induced neuroinflammation and dopaminergic neurotoxicity in the striatum. | Masai et al., 2021 | |
| C57BL/6J male mice (sample size N.A.); 4-8 weeks old | Saline or METH (2 or 10 mg/kg, i.p.) for 4 days on alternate days | The study showed the divergent effects elicited by low and high METH doses on memory and synaptic plasticity in the cortex and hippocampus of mice. | Ding et al., 2022 | |
| C57BL/6J male mice (sample size N.A.); 8 weeks old | Pre-treatment: saline or icariside (10 or 30 mg/kg, i.p.); treatment: saline or METH (from 1 to 5 mg/kg, i.p.) for 14 days | The study demonstrated that icariside treatment alleviated METH-induced neurotoxicity by activating the Keap1-Nrf2 pathway. | Huang et al., 2022 | |
| METH | C57BL/6 male mice (sample size N.A.); 8-10 weeks old | Saline or METH (2×15 mg/kg, i.p.) for 4 days | The study revealed the ability of METH to disrupt the gut microbiota and generate neurotoxic effects via the serum metabolism pathway. | Zhang et al., 2022 |
| Wistar male rats (n=60); age N.A. | Saline, crocin (90 mg/kg, i.p.), METH (4 × 40 mg/kg, i.p.), or METH plus crocin (30, 60, or 90 mg/kg, i.p.) | The study showed crocin's capacity to mitigate METH- induced neurotoxicity within the hippocampus through its anti-inflammatory, anti-apoptotic, and neuroprotective properties. | Shafahi et al., 2018 | |
| Sprague-Dawley male rats (sample size N.A.); 20-25 weeks old | Saline or METH (9 mg/kg, s.c.) | The study revealed the negative effects caused by aversive and stressful conditions on METH-induced neurotoxicity. | Lafuente et al., 2018 | |
| Sprague-Dawley male rats (sample size N.A.); 3 months old | Saline or METH (4 × 5 or 10 mg/kg, s.c.) | The study revealed that METH treatment in rats resulted in dose-dependent changes in the binding levels of 4-[18F]-ADAM. | Huang et al., 2019 | |
| Wistar adult male rats (n=48); age | Saline, METH (4 × 10 mg/kg, i.p.) for 1 day, or | The study revealed that the neurochemical changes in | Schweppe et al., | |
| N.A. | METH self-administration (0.1 mg/kg/infusion, i.v.) for 16 days | the dopaminergic and serotonergic systems in the CPu and hippocampus of METH-treated rats were not indicative of the presence of cognitive impairment. | 2020 | |
| Sprague-Dawley male rats (n=24); age N.A. | METH self-administration (0.1 mg/kg/infusion, i.v.) for 42 days | The study found that rats with compulsive METH self-administration had varying levels of striatal D1R, D2R, and dopamine metabolites compared to rats that did not self-administer METH. | Jayanthi et al., 2022 | |
| Cynomolgus female monkeys (n=6); 6-7 years old | Saline, METH (2 mg/kg, i.m.), or escalating doses of METH (from 0.1 to 0.75 mg/kg, i.m.) for 8 weeks | The study revealed alterations in the hippocampal expression of genes associated with cell proliferation, synaptic plasticity, neuron differentiation, and neurogenesis in METH-treated monkeys. | Choi et al., 2018 | |
| Humans (n=17 healthy volunteers, 8 women; n=23 METH users, 8 women); 18-55 years old | N.A. | The study indicated the presence of a negative association between cortical grey thickness and cumulative METH use. | Okita et al., 2016 | |
| Humans (n=14 healthy volunteers, 7 women; n=11 METH users, 6 women; n=14 METH users with psychosis, 5 women); 26-29 years old | N.A. | The study demonstrated impairments in glucose metabolism and cerebral blood flow in individuals dependent on METH who were experiencing psychotic symptoms. | Vuletic et al., 2018 | |
| Humans (n=65 healthy volunteers, 54 women; n=61 METH-dependent individuals, 53 women); 19-45 years old | N.A. | The study suggested decreased neuronal integrity, viability, number, and mitochondrial dysfunction in the mPFC of METH-dependent individuals. | Wu et al., 2018 |
The articles reported in the table were arranged by model/species (cells, mice, rats, monkeys, humans) and in chronological order. 4-[18F]-ADAM: N,N-dimethyl-2-(2-amino-4-(18)F- fluorophenylthio)benzylamine; CPu: caudate-putamen nucleus; D1R: dopamine D1 receptors; D2R: dopamine D2 receptors; HMGB1: high mobility group box 1 protein; i.m.: intramuscular injection; i.p.: intraperitoneal injection; i.v.: intravenous injection; keap-1: kelch-like ECH-associated protein 1; METH: methamphetamine; mPFC: medial prefrontal cortex; Nrf2: nuclear factor erythroid-2-related factor; p.o.: per os; s.c.: subcutaneous injection.
Rodents and nonhuman primates receiving either acute administration of high doses or repeated “binge” administration of METH display neurotoxicity in the nigrostriatal and, to a lesser extent, mesolimbic dopaminergic systems (Moratalla et al., 2017). In line with previous reports, recent investigations in rodents that have evaluated the impact of METH-induced dopaminergic dysfunctions on cognitive performance and drug-taking behavior have confirmed a significant reduction in TH, DAT, and VMAT-2 proteins, along with a decrease in the concentration of dopamine and its metabolites in the CPu of METH-treated mice (Masai et al., 2021; Huang et al., 2022) and rats (Schweppe et al., 2020; Jayanthi et al., 2022). Interestingly, these studies demonstrated that while METH-induced variations in monoaminergic markers are not correlated with the occurrence of cognitive deficits in mice (Schweppe et al., 2020; Additional Table 3), these neurochemical changes might be useful to predict animals' sensitivity to the addictive effects of METH (Jayanthi et al., 2022; Additional Table 3). Moreover, these studies highlight the key role of neuroinflammation and oxidative stress in METH-mediated neurotoxicity. Indeed, either blockade of high mobility group box-1 (HMGB1), a nuclear transcriptional activator that drives inflammatory responses (Masai et al., 2021; Additional Table 3), or activation of pathways that regulate the cytoprotective responses to ROS/RNS (Huang et al., 2022; Additional Table 3), attenuated the detrimental effects of METH on the dopaminergic system. These findings hold substantial significance, especially considering in vitro evidence that established a direct correlation between HMGB1 protein levels and D2R expression (Mao et al., 2015). The latter finding, in particular, may contribute to enhancing our understanding of the mechanisms underlying the robust protection against METH-induced dopaminergic neurotoxicity that has been observed in D2R knock-out mice (Granado et al., 2011).
Acute or binge administration of high doses of METH can also induce the degeneration of dopaminergic neurons in the SNc of mice, rats, and nonhuman primates (Harvey et al., 2000; Kousik et al., 2014; Shin et al., 2014), as well as severe deficits in motor activity and coordination in rats and mice (Jiang et al., 2014; Dang et al., 2017; Shin et al., 2019). Although a partial recovery has been reported over time (Granado et al., 2018), evidence in adult male rats indicates that METH-induced dopaminergic neurotoxicity persists for months after treatment discontinuation (Schweppe et al., 2020).
Besides affecting dopaminergic neurons, METH administration may impact brain regions that contain serotonergic (Silva et al., 2014), γ-aminobutyric acid (GABA)-ergic (Fujáková-Lipski et al., 2017), and glutamatergic neurons (Zhang et al., 2014). Recent findings in mice have shown that repeated METH administration negatively affects synapses located in the PFC and hippocampus, leading to lower synaptic density, loss of mature spines, and compromised post-synaptic structure (Ding et al., 2022; Additional Table 3). Other studies reported that METH increases the expression of neuroinflammatory, such as tumor necrosis factor-α and pro-apoptotic (e.g. caspase-3) markers in the hippocampus of rats (Shafahi et al., 2018; Additional Table 3). In addition, blood-brain barrier leakage and lowered signaling for 4-[18F]ADAM/PET, an in vivo marker of SERT availability, have been reported in the hypothalamus, thalamus, hippocampus, and PFC of METH-treated rats (Lafuente et al., 2018; Huang et al., 2019; Additional Table 3). In female cynomolgus monkeys, both acute and chronic METH treatments were found to reduce the hippocampal gray matter volume and to alter the expression of genes implicated in vesicle localization, cytoskeleton organization, synaptic transmission, and regulation of neuronal differentiation and neurogenesis (Choi et al., 2018; Additional Table 3).
In line with preclinical findings, studies in humans indicate that METH use may be associated with neurotoxic damage at the level of dopaminergic and serotonergic systems, along with neuroanatomical, neurochemical, and functional alterations in cortical and subcortical structures (Moszczynska and Callan, 2017). For example, people with a history of METH use have a 2-to-3-fold increased risk to develop Parkinson's disease (PD) compared to the general population (Lappin et al., 2018), which supports the possible occurrence of neurotoxicity at the level of mesencephalic nigrostriatal dopaminergic neurons.
Neuroimaging studies in humans have demonstrated the presence of several structural and functional abnormalities in the brain of METH users, with either a reduction or an increase in gray matter volume (London et al., 2015). In more recent studies, the thickness of cortical gray matter was negatively associated with cumulative drug intake and drug craving in METH users (Okita et al., 2016; Additional Table 3). In addition, loss of integrity in the white matter has been reported in METH users, as measured by changes in the mean diffusivity and fractional anisotropy (FA) (Beard et al., 2019; Ghavidel et al., 2020; Ottino-González et al., 2022; Zhou et al., 2023).
Changes in biological markers of neuronal function have also been detected in METH users, such as a decreased glucose metabolism in the left insula, left precentral gyrus, and anterior cingulate cortex (Vuletic et al., 2018; Additional Table 3). Furthermore, a recent proton magnetic resonance spectroscopy study identified pathological features regarding levels of n-acetyl-aspartate, myo-inositol, and glutamate in the mPFC of METH users, likely reflecting a decrease in neuronal integrity and the occurrence of mitochondrial dysfunction (Wu et al., 2018; Additional Table 3).
Even though neuroimaging studies strongly suggest the existence of neurotoxic phenomena in recreational METH users, conclusive evidence is still lacking (Kish et al., 2017). Moreover, earlier studies have observed that METH-associated abnormalities in gray matter volume, DAT density, and levels of vesicular dopamine may recover after protracted abstinence from METH (Volkow et al., 2015). Nonetheless, the recovery of monoaminergic markers does not exclude per se the possibility that METH may induce neuronal degeneration, since the increased synthesis of monoaminergic markers may occur in the neuronal population that remains following a neurotoxic insult; this adaptive mechanism may reflect an attempt to curb the loss of neuronal signaling, as previously observed in the context of neurodegenerative diseases, such as PD (Brotchie and Fitzer-Attas, 2009).
3,4-Methylenedioxymethamphetamine
MDMA, also known as “ecstasy” or “Molly”, is a ring-substituted AMPH-related drug extremely popular due to its stimulant and euphoriant effects. According to the World Drug Report (2023), in 2021 around 20 million people aged 15–64 had used MDMA in the past year, with a male-to-female ratio of 62:38.
Several pathological mechanisms have been implicated in the neurotoxic effects of MDMA. Among them are hyperthermia (Capela et al., 2006), formation of ROS/RNS (Górska et al., 2014), alterations in cytoskeletal structure (García-Cabrerizo and García-Fuster, 2015), apoptosis (Bakhshayesh et al., 2017), mitochondrial complex inhibition associated with bioenergetic metabolic dysfunction (Taghizadeh et al., 2016) and astrogliosis (Miner et al., 2017; Figure 1).
In rats and nonhuman primates, MDMA elicits neurotoxicity mainly in serotonergic systems. Indeed, MDMA has been found to reduce striatal, limbic, and cortical levels of serotonin, to decrease the activity of tryptophan hydroxylase, the rate-limiting enzyme in serotonin biosynthesis, and to lower SERT density and availability (Biezonski and Meyer, 2010; Beaudoin-Gobert et al., 2015; Lizarraga et al., 2015; Shih et al., 2016). These noxious effects may persist long after administration of MDMA. In this respect, a recent PET/MRI follow-up investigation conducted in Formosan rock monkeys demonstrated that the neurotoxic effects of MDMA on the serotonergic system may persist even 66 months after drug discontinuation (Yeh et al., 2022; Additional Table 4). Interestingly, Yeh and coworkers also reported a region-specific self-recovery of SERT availability in the occipital and cingulate cortex, but not in the CPu, hippocampus, thalamus, hypothalamus, midbrain, amygdala, frontal and orbitofrontal cortex (Yeh et al., 2022).
Additional Table 4.
Recent preclinical and clinical studies evaluating 3,4-methylenedioxymethamphetamine (MDMA)- and methylphenidate-induced brain dysfunctions and neurotoxicity
| Drug | Species (n, sex)/age | Treatment/duration | Main findings | References |
|---|---|---|---|---|
| C57BL/6J male mice (n=34), age N.A. | Saline or MDMA (10 mg/kg, two administrations per day, twice a week, i.p.) for 22,47, or 64 days. | The study found that MDMA elicited dopaminergic neurotoxicity in the CPu, SNc, and hippocampus, whose severity depended on the treatment regimen employed. | Costa et al., 2017 | |
| Swiss-Webster male mice (sample size N.A.); 6-7 weeks old | Saline or MDMA (4 × 20 mg/kg, i.p.) | The study found that adolescent mice are more susceptible to neurotoxicity induced by acute MDMA compared to adult mice, due to greater metabolism of dopamine. | Chitre et al., 2020 | |
| C57BL/6J male and female mice (n=45); 12 weeks old | Saline or MDMA (4 × 20 mg/kg, i.p.) | The study showed that MDMA causes sex-dependent dopaminergic neurotoxicity. Male mice had increased SOD 2 expression and reduced ubiquitin-proteasome system activity. | Costa et al., 2021 | |
| MDMA | Sprague-Dawley male rats (n=112); 5-6 weeks old | Pre-treatment: saline (s.c.) or caffeine (10 mg/kg, i.p.); treatment: saline (s.c.) or MDMA (5 mg/kg, s.c.) for 10 days | The study observed that rats exposed to MDMA during adolescence exhibited persistent and widespread dopaminergic neurotoxicity, leading to subsequent memory impairments at adulthood. | Cadoni et al., 2017 |
| Wistar male rats (n=33); 8-10 weeks old | Saline or MDMA (20 mg/kg, i.p.) | The study found that nitrosative stress from inducible nitric oxide synthase activity may play a pivotal role in mediating MDMA-induced neurotoxicity | Schiavone et al., 2019 | |
| Male Cynomolgus monkeys (Macaca fascicularisi (n=14); 3-6 years old | Saline or MDMA (5 mg/kg, twice a day, s.c.) for 4 consecutive days. | The study unveiled that MDMA-induced neurotoxicity in monkeys extends beyond the serotonergic system to impact the dopaminergic system. Moreover, MDMA was found to exacerbate the dopaminergic neurotoxicity induced by MPTP. | Millot et al., 2020 | |
| Formosan rock (Macaca cyclopis) monkeys (n=9, 3 females), 4-6 years old | Saline (s.c.), MDMA (5 mg/kg, twice a day, s.c.), or dextromethorphan (5 mg/kg, twice a day, s.c.) plus MDMA (5 mg/kg, twice a day, s.c.) for 4 days. | The study found that MDMA elicited long-lasting and region-specific serotonergic damage. In the hippocampus and amygdala, MDMA-induced neurotoxicity was partially contrasted by dextromethorphan. | Yeh et al., 2022 | |
| Humans (n=16 healthy volunteers, 8 women); 20-27 years old | Placebo or MDMA (125 mg, p.o., corresponding to 1.8 ± 0.2 mg/kg body weight) | The study identified significant changes in the plasma levels of molecules associated with heightened stress, increased serotonergic activity, and enhanced inflammatory responses in individuals receiving MDMA. | Boxler et al., 2018 | |
| Humans (n=39 healthy volunteers, 23 women; n=39 MDMA users, 22 women); 18-45 years old. | N.A. | The study revealed that the use of MDMA is not associated with white matter lesions. However, MDMA use was found to be associated with alterations in white matter diffusion, as evidenced by the heightened FA observed in multiple white tracts. | Zimmermann et al., 2022 | |
|
| ||||
| Wistar male rats (n=150); 8 weeks old | Saline, methylphenidate (10 mg/kg, i.p.), topiramate (10, 30, 50, 70 or 100 mg/kg, i.p.), methylphenidate plus topiramate for 14 or 21 days. | The study observed that methylphenidate triggered oxidative stress, inflammation, and neuronal damage, through the activation of NMDA, AMPA/kainate, GABA A, and Alpha 2 receptors. These effects were mitigated by topiramate administration. | Motaghinejad et al., 2017a,b | |
| Methylphenidate | Sprague-Dawley male rats (sample size N.A.), PND 20 and 35 | Saline, methylphenidate (2 mg/kg, twice a day, i.p.) for 14 days | The study discovered that chronic, but not acute, methylphenidate treatment alters in catecholaminergic targets in the parietal cortex and ventral CPu of young rats. | Quansah and Zetterström, 2019 |
| Wistar male rats (n=70); age N.A. | Saline, methylphenidate (10 mg/kg, i.p.), minocycline (10, 20, 30, or 40 mg/kg, i.p.), methylphenidate plus minocycline for 21 days. | The study found that minocycline treatment counteracted methylphenidate-induced toxic effects in the hippocampus, likely via the activation of the pCREB/BDNF or Akt/GSK3β pathways. | Motaghinejad and Motevalian, 2022 | |
The articles reported in the table were arranged by model/species (cells, mice, rats, monkeys, humans) and in chronological order. AMPA: α-ammo-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF: brain-derived neurotrophic factor; CPu: caudate-putamen nucleus; FA: fractional anisotropy; GABA: γ-aminobutyric acid; GSK3β: glycogen synthase kinase-3 beta; i.p.: intraperitoneal injection; MDMA: 3,4-methylenedioxymethamphetamine; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NMDA: N-methyl-D-aspartate; p.o.: per os; s.c.: subcutaneous injection; SERT: serotonin transporter; SNc: substantia nigra pars compacta; SOD: superoxide dismutases.
Evidence suggests that the neurotoxic effects of MDMA extend beyond the serotonergic system. Two recent studies have demonstrated that MDMA may induce neurodegeneration in the dopaminergic system of both rats (Cadoni et al., 2017) and macaques (Millot et al., 2020). Indeed, rats exposed to multiple MDMA injections during adolescence showed marked signs of dopaminergic damage at adulthood, as indicated by a reduced number of TH-positive neurons in the SNc and VTA, and decreased immunoreactivity for DAT and TH in the CPu (Cadoni et al., 2017; Additional Table 4). Moreover, Millot and coworkers (2020) reported that repeated MDMA treatment may elicit a significant reduction in DAT availability in the CPu of male macaques, along with lower SERT availability in the CPu and thalamus (Additional Table 4). The same study also found that prior exposure to MDMA aggravated the parkinsonian-like symptoms induced by the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in macaques (Millot et al., 2020; Additional Table 4).
In contrast to rats and nonhuman primates, the neurotoxic effects of MDMA in mice are more evident in the dopaminergic nigrostriatal and mesolimbic systems (Moratalla et al., 2017), since damage to serotonergic pathways in mice has only been detected following high doses of MDMA (Górska et al., 2018). For example, MDMA has been found to reduce striatal levels of dopamine, DAT, and TH, and to produce a persistent loss of dopaminergic cell bodies in the SNc of mice (Granado et al., 2008; Costa et al., 2017; Miner et al., 2017). Of note and consistent with prior data, abnormal activation of D1R and D2R seems to play a pivotal role in mediating MDMA-induced neurotoxic effects on the dopaminergic system of mice (Granado et al., 2011, 2014). A partial sprouting of TH- and DAT-positive fibers has been observed in the CPu of MDMA-treated mice starting from the third day following drug administration, which may suggest that dopaminergic damage induced by MDMA in this brain area may recover over time (Granado et al., 2008). Interestingly, age and sex are two factors known to affect MDMA neurotoxicity in experimental animals. In this respect, two recent investigations provide new evidence, which may account for such differential susceptibility to the noxious effects of MDMA (Chitre et al., 2020; Costa et al., 2021). Adolescent mice have a higher metabolic turnover of dopamine following MDMA compared to adult mice (Chitre et al., 2020; Additional Table 4), and MDMA-treated male mice display an increased expression of the superoxide dismutase (SOD) type 2 enzyme in striatal dopaminergic terminals as well as a reduced striatal proteolytic activity when compared to female mice (Costa et al., 2021; Additional Table 4).
Other studies indicate that the neurotoxic effects of MDMA may extend beyond the dopaminergic system. Indeed, a decrease in the number of GABAergic interneurons has been detected in the hippocampus of rats subjected to repeated MDMA exposure (Anneken et al., 2013). In line with this finding, chronic intermittent MDMA treatment has been reported to lower the density of glutamic acid decarboxylase 67-positive fibers, an index of neurotoxicity affecting the GABAergic system, in the hippocampus, CPu, and mPFC of mice (Costa et al., 2017; Additional Table 4).
Recent studies have also shown that MDMA treatment affects the NO system. Indeed, acute administration of MDMA in rats was found to increase the expression of the inducible nitric oxide synthase, but not that of the endothelial or neuronal nitric oxide synthase, in the PFC (Schiavone et al., 2019; Additional Table 4). In the same cortical area, the authors also reported a significant elevation in immunoreactivity for 3-nitrotyrosine specifically in DAT-positive neurons, but not in glial cells (Schiavone et al., 2019). These observations are relevant, as increased 3-NT levels have been also documented in major neurodegenerative diseases, such as PD, Alzheimer's, and Huntington's diseases, and are thought to reflect the presence of massive nitrosative stress (Bandookwala and Sengupta, 2020). Moreover, in vitro and in vivo investigations have demonstrated that pharmacological inhibition of NO synthesis significantly decreases serotonergic and dopaminergic neurotoxicity induced by MDMA (García-Pardo et al., 2022). Nevertheless, clinical validation of these findings is still needed.
In humans, chronic MDMA use has been associated with the appearance of enduring neurochemical and functional alterations in serotonergic pathways. Reduced levels of serotonin and its major metabolite, 5-hydroxyindoleacetic acid, have been detected in the CPu (Kish et al., 2000) and cerebrospinal fluid (McCann et al., 1999) of MDMA users. PET investigations in MDMA users have demonstrated a significant reduction in the availability of SERT in multiple cortical brain regions and in the hippocampus (Kish et al., 2010; Roberts et al., 2016), as well as an increase in 5-HT2A receptor density in the occipital cortex (Reneman et al., 2000). Interestingly, a recent meta-analysis of 10 PET/SPECT studies in MDMA users reported that the reduction in SERT density was not directly connected with lifetime episodes of MDMA use (Müller et al., 2019). This suggests that other factors, such as the amount of drug taken on each occasion, should be taken into account during the evaluation of potential drivers of MDMA-induced neurotoxicity. This consideration is especially relevant with respect to MDMA's evolving role as a therapeutic agent for the treatment of post-traumatic stress disorder. Furthermore, consistent with observations in nonhuman primates (Yeh et al., 2022), Müller et al. (2019) also reported a partial region-specific recovery in markers of serotonergic function after drug abstinence. Once more, this suggests that the noxious central effects of MDMA may be reversible to some extent (Müller et al., 2019). Nevertheless, additional studies are required to ascertain whether the restoration of SERT density indicates a full functional recovery of the serotonergic system.
A few studies have also investigated the presence of white matter alterations in MDMA users (De Win et al., 2008; Liu et al., 2011), with conflicting results (Roberts et al., 2018). A recent study found an elevation of FA in the corpus callosum and corticospinal tract of chronic MDMA users, which negatively correlated with MDMA use frequency (Zimmermann et al., 2022; Additional Table 4). While these results exclude the presence of a severe white matter lesion, they nevertheless suggest that MDMA use may cause abnormalities in axonal organization (Zimmermann et al., 2022).
Outside of changes in the central nervous system, a single oral dose of MDMA to human subjects increased plasma levels of cortisol and of inflammatory mediators (e.g. hydroxyeicosatetraenoic acid), and reduced levels of calcitriol (vitamin D), a hormone involved in the production of trophic factors (Boxler et al., 2018; Additional Table 4). In summary, data collected from human users suggest that MDMA induces changes in brain function primarily impacting the serotonergic system. Nonetheless, it remains unclear whether such changes reflect the presence of neurodegeneration in serotonergic pathways or neuroadaptive responses (Biezonski and Meyer, 2011). Moreover, it is important to state that most of the findings described here have been obtained from polydrug users, while studies involving pure/primary MDMA users are quite limited in number (Wunderli et al., 2017). Therefore, our knowledge of the precise effects, associated risks, and potential benefits of MDMA use remains to be further elucidated.
Methylphenidate
When taken orally according to a defined therapeutic plan, methylphenidate has negligible abuse potential and may elicit psychostimulant-like and sympathomimetic-like side effects of moderate intensity (e.g., repetitive movements, loss of appetite, insomnia, increase in blood pressure and heart rate) that can be controlled by dose adjustment. Nevertheless, concerns have been raised about possible long-term consequences that may arise from methylphenidate exposure during childhood and adolescence, which are two crucial phases of brain development (Loureiro‐Vieira et al., 2017). In this regard, it has been estimated that globally, the 8–17% of college students aged 18–24 have consumed methylphenidate at least once without a valid medical indication, with rates of misuse reaching up to 35% depending on the country considered (Sharif et al., 2021; Zahavi et al., 2023). The primary incentives driving the misuse of methylphenidate include cognitive and academic enhancement, heightened alertness, weight loss, and personal curiosity (Finger et al., 2013). Since the chemical structure and mechanism of action of methylphenidate are similar to AMPH, prolonged (chronic) use of methylphenidate raises concerns about its neurotoxicity. Early studies in cell cultures and experimental animals provide conflicting results, since the neurotoxic effects of methylphenidate have been demonstrated by some (Gopal et al., 2007; Schmitz et al., 2016), but not others (Yuan et al., 1997; Ludolph et al., 2006). In addition, there is evidence to indicate that methylphenidate, administered after METH treatment in rats, prevents the persistent dopamine deficits and reverses the acute decreases in vesicular dopamine uptake and content and VMAT-2 ligand binding observed following METH alone (Sandoval et al., 2003). However, more recent investigations in rats have demonstrated that chronic methylphenidate may elicit neurotoxic effects (Motaghinejad et al., 2017a, b; Motaghinejad and Motevalian, 2022) and dysfunctions in the ventral CPu (Quansah and Zetterström, 2019; Additional Table 4). For example, chronic administration of methylphenidate reduced the number of granular cells in the dentate gyrus and pyramidal cells in the cornu ammonis region 1 (CA1) of the hippocampus (Motaghinejad et al., 2017b; Additional Table 4). These cellular reductions were accompanied by decreased expression of brain-derived neurotrophic factor, cAMP response element-binding protein (total and phosphorylated), protein kinase B (total and phosphorylated) and B-cell lymphoma-2 (Bcl-2) proteins, as well as increased expression of pro-apoptotic marker Bcl-2-like protein 4 (Bax) and glycogen synthase kinase 3 proteins in the hippocampus (Motaghinejad et al., 2017a, b; Additional Table 4). Furthermore, the same group found that methylphenidate altered markers of oxidative stress, such as malondialdehyde, glutathione, glutathione disulfide, and SOD, as well as markers of inflammation, such as interleukin-1β and tumor necrosis factor-α, in the hippocampus and/or mitochondria (Motaghinejad and Motevalian, 2022; Additional Table 4). Overall, these observations suggest the existence of pro-apoptotic, pro-oxidant, and pro-inflammatory effects of chronic methylphenidate (Motaghinejad et al., 2017a, b; Motaghinejad and Motevalian, 2022), and thereby raise concerns about its potential neurotoxic effects in humans who use methylphenidate chronically. However, it is important to note that these studies, which demonstrated neurotoxicity by chronic methylphenidate in rats, used a dose (10 mg/kg) that far exceeds therapeutic doses of methylphenidate, which are roughly 10 mg/day in children and 20-30 mg/day in adults, as indicated by the U.S. Food and drug administration guidelines. These doses correspond to 0.3–0.4 mg/kg, if an average weight of 32 kg or 70 kg is assumed for children and adults, respectively. Indeed, the available evidence from studies of structural and functional neuroimaging in unmedicated and medicated patients with ADHD indicates that methylphenidate is safe overall and improves brain function (Loureiro‐Vieira et al., 2017; Krinzinger et al., 2019). Nevertheless, the same studies also indicate that brain toxicity (e.g., cerebral arteritis, hallucinations) may be induced by methylphenidate, although this effect seems to disappear after drug discontinuation (Loureiro‐Vieira et al., 2017; Krinzinger et al., 2019). Overall, there is a substantial lack of data on the long-term neurological evaluation of patients treated therapeutically with methylphenidate (Krinzinger et al., 2019). Similarly, only a few studies have explored the detrimental effects of low doses of methylphenidate in experimental animals. Hence, further studies on the neurotoxic effects of methylphenidate in humans are warranted. Special focus should be placed on individuals who use methylphenidate for recreational purposes or as a performance enhancer, since they may be exposed to amounts of methylphenidate that far exceed therapeutic doses, which could result in overt neurotoxic effects.
Non-Sympathomimetic Psychostimulants
Non-sympathomimetic psychostimulants possess dissimilar chemical structures and include caffeine and nicotine. Caffeine is a methylxanthine alkaloid, which at doses used during recreational consumption, induces its central effects by antagonizing adenosine A1 and A2A receptors (Ferré et al., 2018). Caffeine also indirectly modulates other neurotransmitter systems, including dopaminergic pathways. This action contributes to the full manifestation of caffeine's central effects as well as to its interactions with other psychoactive substances (Simola et al., 2021). Nicotine is a dinitrogen alkaloid that activates presynaptic nicotinic acetylcholine receptors, thus stimulating acetylcholine release (Benowitz, 2009). Nicotine also activates the dopaminergic system by increasing dopamine release in mesolimbic regions, CPu, and PFC (Benowitz, 2009). In addition to eliciting psychostimulant effects, caffeine, and nicotine may both induce neurochemical alterations possibly leading to brain dysfunctions and/or neurotoxicity.
Caffeine
Caffeine is a natural xanthinic alkaloid contained in several plant species (e.g., Coffea arabica, Thea sinensis) and consumed worldwide due to its mild euphoriant and psychostimulant effects. According to the latest European Food Safety Authority (EFSA) dietary survey, the daily intake of caffeine in Europe (expressed as mg/kg × body weight) ranges from 0.1 to 1.4 in adolescents (10–17 years old), from 0.5 to 4.3 in adults (18–65 years old), and from 0.3 to 4.8 in the elderly (60–74 years old) (Verster and Koenig, 2018). For adults, major sources of caffeine are coffee and tea, followed by carbonated soft drinks, while adolescents and children mostly consume carbonated soft drinks and tea, followed by coffee [EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), 2015].
Caffeine consumption is generally considered a harmless habit, which, in some cases, may even be beneficial for an individual's performance or health. For example, caffeine has been shown to be protective in experimental models of neurotoxicity (Ren and Chen, 2020). In addition, epidemiological studies suggest that caffeine-mediated neuroprotection could occur in humans, since there is an inverse correlation between caffeine consumption and the incidence of neurodegenerative diseases (Janitschke et al., 2021). Nevertheless, harmful effects associated with caffeine consumption have been also described. For example, caffeine may amplify the behavioral effects of dopaminergic psychostimulants of abuse, such as AMPH-related drugs and cocaine, in both experimental animals and humans (Morelli and Simola, 2011). Furthermore, preclinical studies have demonstrated that caffeine may elicit neurotoxic effects and exacerbate the neurotoxicity elicited by other substances (Frau et al., 2016).
Earlier investigations that employed in vitro or in vivo models demonstrated that caffeine-induced neurotoxicity may be sustained by diverse mechanisms, such as induction of apoptosis (Kang et al., 2002), alteration in the cellular levels of Ca2+ (Gepdi̇Remen et al., 1998), inhibition of neuronal repair and induction of neuroinflammation (Yang and Jou, 2016), inhibition of SOD and catalase enzymes and induction of lipid peroxidation (Bavari et al., 2016; Figure 1). On the other hand, activation of necrotic and excitotoxic pathways is not involved in caffeine-mediated neurotoxicity (Kang et al., 2002), whereas the involvement of oxidative stress has been suggested by some studies (Bavari et al., 2016), but not others (Kang et al., 2002). More recent in vitro investigations have shed light on the mechanisms by which caffeine induces neurotoxicity and how it could amplify the neurotoxic effects of other substances. A study in PC12 cells provides support to the involvement of oxidative stress pathways in caffeine-mediated neurotoxicity, by demonstrating that concentrations of caffeine that inhibited cell viability also induced apoptosis and increased the generation of ROS (Chian et al., 2022; Additional Table 5). Moreover, caffeine treatment reduced protein expression and mRNA levels of Nrf2 protein, a key regulator of oxidative stress response, as well as of the mRNA encoding Nrf2 and its target genes NADPH quinone oxidoreductase 1, glutamate cysteine ligase catalytic subunit and modifier subunit (Chian et al., 2022; Additional Table 5). Further support to the role of the Nrf2 pathway in the toxic effects of caffeine comes from the finding that caffeine treatment had a reduced effect on the viability of PC12 cells bearing a knockdown of Nrf2 expression (Chian et al., 2022; Additional Table 5). A study performed in SH-SY5Y cells demonstrated that concentrations of caffeine within the range of those detected in caffeine-contaminated environments (e.g., wastewater, seawater) under-expressed several genes encoding key elements of other neurotransmitter pathways, such as the serotonin 5HT3A receptor, the D2R, the enzyme GABA-transaminase, the protein synaptotagmin 10, which regulates neurotransmitter release, and the subunit a3 of the ATPase Na+/K+ pump (Vulin et al., 2022; Additional Table 5). Although this study found no overt cytotoxicity in SH-SY5Y cells exposed to the concentrations of caffeine tested (Vulin et al., 2022), the observed changes in gene expression deserve further consideration, since they identify new molecular targets that could potentially sustain caffeine's neurotoxic effects through prolonged exposure to low amounts or acute exposure to high amounts of caffeine.
Additional Table 5.
Recent preclinical and clinical studies evaluating caffeine- and nicotine-induced brain dysfunctions and neurotoxicity
| Drug | Species (n, sex)/age | Treatment/duration | Main findings | References |
|---|---|---|---|---|
| SH-SY5Y cells | 200 mM of ethanol (24 h) followed by 20 mM of caffeine (24 h). | The study found that caffeine potentiates cell death induced by ethanol through mTOR/p70S6K/4E-BP1 inhibition. | Sangaunchom et al., 2020 | |
| PC12 cells | Caffeine (0.5-4 mM) at different time points (24-72 h). | The study found that caffeine induced cell death by inhibiting the Nrf2 pathway. | Chian et al., 2022 | |
| SH-SY5Y cells | Caffeine (10 ng/L, 10 μg/L or 10 mg/L) or carbonyl cyanide 3-chlorophenylhydrazone (5 μΜ) (24 h) | The study found that caffeine altered the expression of genes encoding for serotonin receptor 3A, D2R, monoamine oxidase B, and GABA-transaminase. | Vulin et al., 2022 | |
| Caffeine | ICR mouse pups (sample size N.A.); PND 3 | Caffeine (a bolus of 80 mg/kg) alone or in combination with midazolam (a bolus of 6 mg/kg), ketamine (a bolus of 40 mg/kg), or fentanyl (a bolus of 40 μg/kg). | The study found that caffeine increased the neurotoxic effects of sedative/anaesthetic drugs. | Cabrera et al., 2017 |
| C57BL/6J male mice (sample size N.A.); 8-10 weeks old | Caffeine (2×5 mg/kg, i.p.), MDMA (4 × 10 mg/kg, i.p.), or caffeine plus MDMA for 2 days. After 5 days, mice received again the same treatment. In between treatments, mice received caffeine (2×5 mg/kg, i.p.) for 5 days | The study found that caffeine potentiated MDMA- induced neurotoxic effects. | Górska et al., 2018 | |
| C57BL/6J Fcen male mice (sample size N.A.); 25-35 days old | Vehicle, caffeine (3 × 5 mg/kg, i.p.), cocaine (3 × 10 mg/kg, i.p.), caffeine plus cocaine on alternate days for 13 days. | The study found that caffeine plus cocaine treatment induced alterations in thalamocortical physiology. | Rivero-Echeto et al., 2021 | |
| Sprague-Dawley male and female rat pups (sample size N.A.); PND 3 | Saline, caffeine citrate (100 mg/kg, s.c.), morphine sulphate (2 mg/kg, s.c.), or caffeine plus morphine for 4 days. | The study found that caffeine plus morphine treatment induced an age- and sex-dependent increase in apoptosis and mitochondrial dysfunction in the brain of neonatal rats. | Kasala et al., 2020 | |
| Pregnant rhesus macaques (gestational day 100-120) | Isoflurane (a bolus of 60 mg/kg, infusion 25 mg/kg/h for 5 h), isoflurane plus caffeine (a bolus of 80 mg/kg for 8 h), or no anaesthesia. Fetal brains were collected 3 h after anaesthesia. | The study found that caffeine augmented the neurotoxic of isoflurane | Noguchi et al., 2018 | |
|
| ||||
| C57BL/6 male mice perinatally exposed to nicotine (sample size N.A.); PND 21 | Nicotine (100 μg/mL, p.o.), saccharin (2%, p.o.), or water from 3 weeks before mating until weaning of pups. At PND 21, male offspring received methylphenidate (0.75 mg/kg, i.p.) or norbinaltorphimine (20 mg/kg, i.p.). | The study showed that perinatal exposure to nicotine reduced dopamine release in the frontal cortex and caused significant deficits in object-based attention and spatial working memory in the offspring. Such alterations were contrasted by norbinaltorphimine. | Zhang et al., 2021 | |
| C57BL/6 male and female mice perinatally exposed to nicotine (sample size N.A.); PND 20 | Nicotine (200 μg/mL plus saccharin 1% in drinking water), saccharin (1% in drinking water), or water from 2 weeks before mating until weaning of pups. At PND 20, offspring were evaluated. | The study found that maternal nicotine exposure dysregulates immune-related gene expression by skewing the polarity of M2 microglia in the hippocampus. | Zhou et al., 2021 | |
| Nicotine | Sprague-Dawley male and female rats prenatally exposed to nicotine (sample size N.A.); PSD 32 | Prenatal treatment: saline or nicotine (3 mg/kg/day) via osmotic mini-pumps. | The study found that prenatal nicotine treatment sex- dependently altered the development of the corticostriatal dopamine system. | Dwyer et al., 2019 |
| Humans (n=41 non-smokers, 7 women; n=41 smokers, 6 women); 22-70 years old. | N.A. | The study found that cigarette smoking is associated with thinner cortices in regions implicated in the development of substance abuse or in Alzheimer’s disease. | Durazzo et al., 2018 | |
| Humans (n=362 no smokers, 189 women; n=219 former smokers, 120 women; n=177 current smokers, 57 women); 18-30 years old | N.A. | The study identified a correlation between smoking and reduced grey matter volume in middle-aged individuals. | Elbejjani et al., 2019 | |
| Humans (n=1747, divided in nicotine exposed and non-exposed); 12-27 years old | N.A. | The study found that prenatal exposure to maternal cigarette smoking was associated with lower diffusivity in the corpus callosum. | Björnholm et al., 2020 | |
| Humans (n=1344 non-smokers, n=905 smokers); ageN.A. | N.A. | The study found altered grey matter volumes in chronic smokers. | Yang et al., 2020 | |
| Humans (n=41 children, 24 women, born to non-smoking mothers who were exposed to environmental tobacco smoke); 7 years old or older | N.A. | The study found that environmental tobacco smoke exerts detrimental effects on both structure and function of the thalamus. | Margolis et al., 2021 | |
| Humans (n=35 non-smokers, n=19 smokers, n=31 cannabis and tobacco smokers), 18-37 years old | N.A. | The study revealed that both tobacco and cannabis smoking affect grey matter volume. | Daniju et al., 2022 | |
The articles reported in the table were arranged by model/species (cells, mice, rats, monkeys, humans) and in chronological order. CPu: caudate-putamen nucleus; D2R: dopamine D2 receptor; GABA: γ-aminobutyric acid; i.p.: intraperitoneal injection; mTOR: mammalian target of rapamycin; Nrf2: nuclear factor erythroid-2-related factor; ROS: reactive oxygen species; s.c.: subcutaneous injection; SERT: serotonin transporter.
Additional studies have identified the induction of apoptotic cell death as a key mechanism by which caffeine potentiates the neurotoxic effects of other substances. A study in SH-SY5Y cells exposed to ethanol found that subsequent treatment with caffeine exacerbated the reduction in cell viability and increased early apoptosis (Sangaunchom and Dharmasaroja, 2020). Specifically, caffeine potentiated ethanol-induced inhibition of signaling mediated by mechanistic target of rapamycin, p70S6 kinase, and eukaryotic translation initiation factor 4E-binding protein 1, and eventually reduced mitochondrial membrane potential, as compared to either control conditions or ethanol alone (Sangaunchom and Dharmasaroja, 2020; Additional Table 5). Moreover, an investigation in premature newborn mice found that the co-administration of caffeine together with the sedative/anesthetic drugs midazolam, fentanyl, or ketamine potentiated the number of activated caspase 3 apoptotic cells in colliculi, hippocampus, neocortex, CPu, and thalamus, as compared to treatment with either drug alone (Cabrera et al., 2017; Additional Table 5). Caffeine has also been shown to exacerbate apoptosis induced by morphine in the premature rat brain (Kasala et al., 2020). In this model, combined administration of caffeine and morphine, compared to either drug alone, caused a more marked and sex-dependent increase in the expression of Bax and a more marked reduction in the expression of Bcl-2 (Kasala et al., 2020; Additional Table 5). Furthermore, caffeine has been reported to worsen the neurotoxicity induced by in utero exposure to isoflurane anesthesia in the fetal rhesus macaque brain (gestational days 100–120), by exacerbating neuronal apoptosis, especially in the basal ganglia and cerebellum (Noguchi et al., 2018; Additional Table 5). Interestingly, the brains of rhesus macaques prenatally exposed to isoflurane and caffeine displayed a decreased number of oligodendrocytes throughout their white matter (Noguchi et al., 2018; Additional Table 5).
It appears that mechanisms other than induction of apoptotic cell death may participate in the amplification of drug-induced neurotoxicity by caffeine. For example, caffeine potentiates MDMA-induced damage in dopaminergic and serotonergic terminals in the mouse brain following binge co-administration of caffeine and MDMA (Górska et al., 2018). This effect was associated with an increase in extracellular dopamine and serotonin levels in the CPu and an exacerbation of the oxidative damage of nuclear DNA induced by MDMA in the mPFC, compared to either drug alone (Górska et al., 2018; Additional Table 5). Another study found that co-administration of caffeine and cocaine, compared to either drug alone, exacerbated the increase in intracellular Ca2+ and dysregulated the hyperpolarization-activated cyclic nucleotide-gated and T-type VGC channels in thalamic ventrobasal neurons of mice (Rivero-Echeto et al., 2021; Additional Table 5). Although this study did not assess the neurotoxic effects of the caffeine-cocaine combination, the observed increase in intracellular Ca2+ suggests the existence of other mechanisms by which caffeine could potentiate the neurotoxic effects of other drugs, since dysregulation of Ca2+ homeostasis is a key player in neurotoxicity phenomena.
Despite evidence that caffeine may induce neurotoxicity and can exacerbate the neurotoxic effects of other substances, the neuroprotective effects of caffeine have also been extensively demonstrated (Simola et al., 2021). Various factors may influence the induction of neuroprotection/neurotoxicity by caffeine, including levels of caffeine exposure, the presence of interindividual and interspecies differences in caffeine pharmacokinetics and metabolism, as well as other drugs with which caffeine is consumed. Caffeine acts as an antagonist of adenosine A1 and A2A receptors at plasma concentrations attained following recreational consumption (Fredholm et al., 1999). In this regard, it is noteworthy that several of the studies that demonstrated caffeine neurotoxicity used concentrations/doses that exceeded those associated with the recreational consumption of caffeine. As such, these high concentrations/doses of caffeine may act on targets other than adenosine receptors (Fredholm et al., 1999), and, in turn, may activate molecular pathways that cause neurotoxicity. Nevertheless, the potential of caffeine to induce, or exacerbate, neurotoxicity should be regarded as a source of concern at least under specific circumstances, such as the use of high doses of caffeine for the treatment of infants for apnea of prematurity (Noguchi et al., 2018) and its recreational consumption in combination with other psychoactive substances that bear neurotoxic potential (Frau et al., 2016). Furthermore, the increasing presence of caffeine and its metabolites as environmental contaminants raises concern regarding potential neurotoxicity following the consumption of caffeine-contaminated food and water (Vieira et al., 2022).
Nicotine
Nicotine is a natural alkaloid contained in plant species from the genus Nicotiana and is consumed worldwide due to its euphoriant properties. It is found in several products, such as cigarettes, e-cigarettes, and nicotine cessation gums and patches. In 2019, more than 1 billion people worldwide were reported to regularly smoke cigarettes, and almost 8 million deaths were attributable to cigarette use (GBD 2019 Tobacco Collaborators). Nicotine elicits its euphoriant and psychostimulant properties by activating nicotinic acetylcholine receptors in the brain, which causes the release of a variety of neurotransmitters including norepinephrine (Verplaetse et al., 2015), acetylcholine (Wonnacott, 1997), serotonin (Fletcher et al., 2008), GABA and glutamate (Li et al., 2014), and dopamine (Di Chiara, 2000). The release of these neurotransmitters, in particular of dopamine, serves as the basis of the psychoactive, rewarding, and addictive properties of nicotine (Di Chiara, 2000). Several pathological mechanisms have been implicated in the brain dysfunctions that can be observed following nicotine use, including, but not limited to, apoptosis (Anbarasi et al., 2006), bioenergetic failure (Malińska et al., 2019), and neuroinflammation (Piao et al., 2009; Figure 1).
Multiple factors influence the long-term effects of nicotine, leading to either brain dysfunctions or neuroprotection depending on the age at which it is consumed (Ren et al., 2022), the amount consumed, and the duration of its use (Peng et al., 2018). For example, nicotine use during adulthood is associated with increased risk for psychiatric and neurological conditions, including major depression (Laviolette, 2021), alcohol use disorder (King and Meyer, 2022), lower processing speed, poorer general cognitive ability, poorer decision-making, increased impulsivity (Conti and Baldacchino, 2021, 2022), and Alzheimer's disease (Durazzo et al., 2018). It may be hypothesized that the increased risk of brain disease in nicotine users may depend, at least in part, on the induction of abnormalities in cerebral structure and function, as suggested by neuroimaging studies (see below). Conversely, preclinical and clinical evidence indicates that nicotine administration/consumption may have a protective effect on the underlying neurodegeneration and/or phenotypical manifestation of PD (Nicholatos et al., 2018; Mappin-Kasirer et al., 2020; Carvajal-Oliveros et al., 2021; Wang et al., 2022).
Nicotine is often consumed by pregnant women, and gestational nicotine exposure has been shown to negatively affect the developing fetal brain, leading to neuronal and glial alterations in several cerebral regions that may affect the offspring later in their life (Dwyer et al., 2019; Zhou et al., 2021). For example, a recent study demonstrated the existence of abnormalities in cortical regions of adolescent rats that were exposed to nicotine in utero from gestational days 4 to 18 (Dwyer et al., 2019; Additional Table 5). Specifically, male and female adolescent rats prenatally exposed to nicotine displayed an increase in dopamine content, a decrease in the turnover ratio of homovanillic acid/dopamine, and a decrease in norepinephrine transporter expression in the PFC, compared to adolescent rats not prenatally exposed to nicotine (Dwyer et al., 2019; Additional Table 5). There was also an increase in striatal and pallidal D3R binding, as measured by [125I]-7-OH-PIPAT, and D2R-G-protein functional coupling in the ventral CPu and pallidum in adolescent rats prenatally exposed to nicotine (Dwyer et al., 2019; Additional Table 5). In the same study, adolescent female rats prenatally exposed to nicotine exhibited a decrease in striatal DAT binding, as measured by [125I] RTI-55 (Dwyer et al., 2019; Additional Table 5). A different study using mice that were prenatally exposed to nicotine reported an increased number of microglial cells and Nissl-positive neurons in the CA1 of the hippocampus, as well as alterations in the expression of genes related to neuroinflammation, synaptic plasticity, and neurotransmitter function, compared to mice prenatally treated with saline (Zhou et al., 2021; Additional Table 5). Nicotine exposure in utero can also affect an offspring's response to subsequent challenges with a different psychostimulant. For example, in male adolescent mice born to mothers treated with nicotine during pregnancy methylphenidate treatment led to more pronounced release of dopamine and noradrenaline, but not serotonin, in the PFC (measured by in vivo microdialysis), compared to mice prenatally exposed to saline (Zhang et al., 2021; Additional Table 5).
Neuroimaging investigations in humans have obtained evidence supporting the possibility that in utero exposure to nicotine may elicit enduring detrimental effects on brain function in offspring. A recent MRI study demonstrated the presence of smaller volumes of the left and right thalami and inferior frontal gyrus in children aged 7–9 years old who were prenatally exposed to environmental tobacco smoke, compared to age-matched children not exposed to tobacco smoke during gestation (Margolis et al., 2021; Additional Table 5). Another MRI study found lower callosal volume, higher FA, and lower mean diffusivity in adolescent and young adults prenatally exposed to maternal cigarette smoking, compared to age-matched non-exposed individuals (Björnholm et al., 2020; Additional Table 5).
Studies of neuroimaging performed in cigarette smokers have also demonstrated that chronic nicotine consumption during adulthood is associated with brain abnormalities in gray or white matter (Elbejjani et al., 2019; Yang et al., 2020; Fan et al., 2023). Specifically, Elbejjani et al. (2019) reported a reduction in gray matter volume in adult cigarette smoker participants, compared to adult non-smokers (Additional Table 5). In line with this, a recent meta-analysis of 17 voxel-based morphometry studies found that chronic smokers had a robust decrease in gray matter volume in the right superior frontal gyrus, right middle frontal gyrus, left insular and left superior frontal gyrus (Yang et al., 2020; Additional Table 5). Furthermore, reductions in the thickness of the medial and lateral orbitofrontal cortex, entorhinal cortex, fusiform and middle temporal gyrus, and insula have been observed in cigarette smokers, compared to people who never smoked (Durazzo et al., 2018; Additional Table 5). Conversely, Yang et al. (2020) found an increased gray matter volume in the right lingual cortex and left occipital cortex of cigarette smokers (Additional Table 5). In line with this, a more recent MRI study found that cigarette smokers had higher gray matter volume in the left but not right putamen, compared to non-smokers, whereas no differences in gray matter volume were observed in the putamen, compared to cannabis and tobacco smokers (Daniju et al., 2022; Additional Table 5).
Conclusions
Of late, much progress has been achieved in clarifying the central noxious effects of psychostimulants. Indeed, while consistent evidence supports the therapeutic applications of some psychostimulants (e.g., amphetamine, methylphenidate) under closely monitored conditions, it is now clear that their misuse may potentially result in brain impairments and functional neurotoxicity.
We have reviewed the recent findings derived from experimental models and human studies that examined brain dysfunctions and neurotoxicity that may be triggered by the most commonly used sympathomimetic and non-sympathomimetic psychostimulants. The preclinical investigations reported in this review provide new insights into factors that may contribute to psychostimulant-induced neurotoxicity. These data also substantiate previous findings, indicating that consumption of psychostimulants, especially at high amounts and for extended periods of time, could result in the emergence of neurotoxic phenomena and brain dysfunctions which involve monoaminergic systems. Of note, oxidative stress, mitochondrial dysfunction, neuroinflammation, and apoptosis dysregulation emerge as prominent and common mechanisms involved in the neurotoxic effects of psychostimulants. At the same time, each psychostimulant may have its own specific mechanism(s) that mediates its detrimental effects on the brain, as well as unique noxious interactions with other substances that may lead to/contribute to brain damage.
It is important to note that the extent of psychostimulant-induced neurotoxic effects varies and depends on the specific psychostimulant considered, the experimental procedure, as well as the stage of life at which it is consumed or administered. Furthermore, recent findings indicate that detrimental alterations outside the central nervous system, for example in the gut microbiota, are of particular interest since they may provide new tools for monitoring the noxious effects of psychostimulants in users. Conversely, data derived from neuroimaging investigations in users provide compelling evidence that psychostimulants may elicit central structural and functional changes that impact the activity of several cortical and subcortical brain areas; interestingly, some of these changes appear to undergo recovery following extended periods of drug abstinence. Nevertheless, the question remains as to whether the psychostimulant-induced alterations observed in neuroimaging studies reflect the occurrence of neuronal cell death or should be attributed to adaptive responses triggered by aberrant monoaminergic signaling. Moreover, potential confounding variables such as polydrug use, inclusion of current or abstinent users, drug intake patterns, and amount of drug taken per occasion, introduce complexity into the interpretation of clinical studies. In this regard, further research involving animal models designed to more accurately replicate the patterns of drug consumption observed in humans will be crucial for gaining novel insights into the neurotoxic effects and brain dysfunction that psychostimulants may induce in the human brain.
Additional files:
Additional Table 1: Classification of psychostimulants.
Additional Table 2: Recent preclinical and clinical studies evaluating amphetamine (AMPH)- and cocaine-induced brain dysfunctions and neurotoxicity.
Additional Table 3: Recent preclinical and clinical studies evaluating methamphetamine (METH)-induced brain dysfunctions and neurotoxicity.
Additional Table 4: Recent preclinical and clinical studies evaluating 3,4-methylenedioxymethamphetamine (MDMA)- and methylphenidate-induced brain dysfunctions and neurotoxicity.
Additional Table 5: Recent preclinical and clinical studies evaluating caffeine-and nicotine-induced brain dysfunctions and neurotoxicity.
Funding Statement
Funding: This work was supported by PON AIM (PON RICERCA E INNOVAZIONE 2014-2020, - AZIONE I.2. D.D. N.407 DEL 27 FEBBRAIO 2018 - “ATTRACTION AND INTERNATIONAL MOBILITY”) (to GC); Zardi-Gori Foundation (research grant 2021) (to MS); intramural funds from the University of Cagliari (to NS); Fondazione CON IL SUD and The U.S.-Italy Fulbright Commission (to AEP).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: All data relevant to the work are included in the article or uploaded as Additional files.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Abdel Malek GS, Goudriaan AE, Kaag AM. The relationship between craving and insular morphometry in regular cocaine users: Does sex matter? Addict Biol. 2022;27:e13157. doi: 10.1111/adb.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbarasi K, Kathirvel G, Vani G, Jayaraman G, Shyamala Devi CS. Cigarette smoking induces heat shock protein 70 kDa expression and apoptosis in rat brain: modulation by bacoside A. Neuroscience. 2006;138:1127–1135. doi: 10.1016/j.neuroscience.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Angarita GA, Worhunsky PD, Naganawa M, Toyonaga T, Nabulsi NB, Li CR, Esterlis I, Skosnik PD, Radhakrishnan R, Pittman B, Gueorguieva R, Potenza MN, Finnema SJ, Huang Y, Carson RE, Malison RT. Lower prefrontal cortical synaptic vesicle binding in cocaine use disorder: an exploratory 11 C‐UCB‐J positron emission tomography study in humans. Addict Biol. 2022;27:e13123. doi: 10.1111/adb.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Cunningham JI, Collins SA, Yamamoto BK, Gudelsky GA. MDMA increases glutamate release and reduces parvalbumin-positive GABAergic cells in the dorsal hippocampus of the rat: role of cyclooxygenase. J Neuroimmune Pharmacol. 2013;8:58–65. doi: 10.1007/s11481-012-9420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apóstol del Rosal GD, Limón ID, Martínez I, Patricio-Martínez A. The chronic oral administration of clobenzorex or amphetamine decreases motor behavior and induces glial activation in the striatum without dopaminergic degeneration. Neurotox Res. 2021;39:1405–1417. doi: 10.1007/s12640-021-00395-1. [DOI] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:511. doi: 10.1001/jamapsychiatry.2017.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram M, Müller F, Rogg H, Korda A, Andreou C, Holze F, Vizeli P, Ley L, Liechti ME, Borgwardt S. Characterizing thalamocortical (dys)connectivity following D-amphetamine, LSD, and MDMA administration. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:885–894. doi: 10.1016/j.bpsc.2022.04.003. [DOI] [PubMed] [Google Scholar]
- Bakhshayesh M, Golab F, Kermanian F, Mehdizadeh M, Katebi AR, Soleimani M, Mohammadzadeh F, Shabani R, Movahed E, Katebi M. The mediating role of A2A adenosine receptors in the mitochondrial pathway of apoptotic hippocampal cell death, following the administration of MDMA in rat. Basic Clin Neurosci J. 2017;8:317–324. doi: 10.18869/nirp.bcn.8.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandookwala M, Sengupta P. 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. Int J Neurosci. 2020;130:1047–1062. doi: 10.1080/00207454.2020.1713776. [DOI] [PubMed] [Google Scholar]
- Bashkatova V, Kraus MM, Vanin A, Hornick A, Prast H. Comparative effects of NO-synthase inhibitor and NMDA antagonist on generation of nitric oxide and release of amino acids and acetylcholine in the rat brain elicited by amphetamine neurotoxicity. Ann N Y Acad Sci. 20041025:221–230. doi: 10.1196/annals.1316.027. [DOI] [PubMed] [Google Scholar]
- Bassetti CLA, Kallweit U, Vignatelli L, Plazzi G, Lecendreux M, Baldin E, Dolenc‐Groselj L, Jennum P, Khatami R, Manconi M, Mayer G, Partinen M, Pollmächer T, Reading P, Santamaria J, Sonka K, Dauvilliers Y, Lammers GJ. European guideline and expert statements on the management of narcolepsy in adults and children. Eur J Neurol. 2021;28:2815–2830. doi: 10.1111/ene.14888. [DOI] [PubMed] [Google Scholar]
- Bavari M, Tabandeh MR, Najafzadeh Varzi H, Bahramzadeh S. Neuroprotective, antiapoptotic and antioxidant effects of l -carnitine against caffeine-induced neurotoxicity in SH-SY5Y neuroblastoma cell line. Drug Chem Toxicol. 2016;39:157–166. doi: 10.3109/01480545.2015.1063062. [DOI] [PubMed] [Google Scholar]
- Beard CL, Schmitz JM, Soder HE, Suchting R, Yoon JH, Hasan KM, Narayana PA, Moeller FG, Lane SD. Regional differences in white matter integrity in stimulant use disorders: A meta-analysis of diffusion tensor imaging studies. Drug Alcohol Depend. 2019;201:29–37. doi: 10.1016/j.drugalcdep.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin-Gobert M, Epinat J, Météreau E, Duperrier S, Neumane S, Ballanger B, Lavenne F, Liger F, Tourvielle C, Bonnefoi F, Costes N, Bars DL, Broussolle E, Thobois S, Tremblay L, Sgambato-Faure V. Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain. 2015;138:2632–2647. doi: 10.1093/brain/awv183. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MS, Bruner NR, Herrmann ES, Johnson PS, Johnson MW. Methamphetamine administration dose effects on sexual desire, sexual decision making, and delay discounting. Exp Clin Psychopharmacol. 2022;30:180–193. doi: 10.1037/pha0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biezonski DK, Meyer JS. Effects of 3,4-methylenedioxymethamphetamine (MDMA) on serotonin transporter and vesicular monoamine transporter 2 protein and gene expression in rats: implications for MDMA neurotoxicity. J Neurochem. 2010;112:951–962. doi: 10.1111/j.1471-4159.2009.06515.x. [DOI] [PubMed] [Google Scholar]
- Bittencourt AML, Bampi VF, Sommer RC, Schaker V, Juruena MFP, Soder RB, Franco AR, Sanvicente-Vieira B, Grassi-Oliveira R, Ferreira PEMS. Cortical thickness and subcortical volume abnormalities in male crack-cocaine users. Psychiatry Res Neuroimaging. 2021;310:111232. doi: 10.1016/j.pscychresns.2020.111232. [DOI] [PubMed] [Google Scholar]
- Björnholm L, Nikkinen J, Kiviniemi V, Niemelä S, Drakesmith M, Evans JC, Pike GB, Richer L, Pausova Z, Veijola J, Paus T. Prenatal exposure to maternal cigarette smoking and structural properties of the human corpus callosum. NeuroImage. 2020;209:116477. doi: 10.1016/j.neuroimage.2019.116477. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Thomas M, Schmued LC, Ali SF. Brain region-specific neurodegenerative profiles showing the relative importance of amphetamine dose, hyperthermia, seizures, and the blood-brain barrier. Ann N Y Acad Sci. 20081139:127–139. doi: 10.1196/annals.1432.005. [DOI] [PubMed] [Google Scholar]
- Boxler MI, Streun GL, Liechti ME, Schmid Y, Kraemer T, Steuer AE. Human metabolome changes after a single dose of 3,4-methylenedioxymethamphetamine (MDMA) with special focus on steroid metabolism and inflammation processes. J Proteome Res. 2018;17:2900–2907. doi: 10.1021/acs.jproteome.8b00438. [DOI] [PubMed] [Google Scholar]
- Brotchie J, Fitzer-Attas C. Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology. 2009;72:S32–S38. doi: 10.1212/WNL.0b013e318198e0e9. [DOI] [PubMed] [Google Scholar]
- Cabrera OH, O'Connor SD, Swiney BS, Salinas-Contreras P, Manzella FM, Taylor GT, Noguchi KK. Caffeine combined with sedative/anesthetic drugs triggers widespread neuroapoptosis in a mouse model of prematurity. J Matern Fetal Neonatal Med. 2017;30:2734–2741. doi: 10.1080/14767058.2016.1261400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Simola N, Frau L, Porceddu PF, Corongiu S, Dessì C, Sil A, Plumitallo A, Wardas J, Di Chiara G. Widespread reduction of dopamine cell bodies and terminals in adult rats exposed to a low dose regimen of MDMA during adolescence. Neuropharmacology. 2017;123:385–394. doi: 10.1016/j.neuropharm.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Caffino L, Mottarlini F, Zita G, Gawliński D, Gawlińska K, Wydra K, Przegaliński E, Fumagalli F. The effects of cocaine exposure in adolescence: Behavioural effects and neuroplastic mechanisms in experimental models. Br J Pharmacol. 2022;179:4233–4253. doi: 10.1111/bph.15523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Scavone C, Marcourakis T. Long-lasting changes following repeated cocaine use: behavioral sensitization and neurotoxicity. In: Preedy VR, editor. The neuroscience of cocaine. New York: Academic Press; 2017. pp. 353–361. [Google Scholar]
- Capela JP, Meisel A, Abreu AR, Branco PS, Ferreira LM, Lobo AM, Remião F, Bastos ML, Carvalho F. Neurotoxicity of ecstasy metabolites in rat cortical neurons, and influence of hyperthermia. J Pharmacol Exp Ther. 2006;316:53–61. doi: 10.1124/jpet.105.092577. [DOI] [PubMed] [Google Scholar]
- Carvajal-Oliveros A, Domínguez-Baleón C, Zárate RV, Campusano JM, Narváez-Padilla V, Reynaud E. Nicotine suppresses Parkinson's disease like phenotypes induced by Synphilin-1 overexpression in Drosophila melanogaster by increasing tyrosine hydroxylase and dopamine levels. Sci Rep. 2021;11:9579. doi: 10.1038/s41598-021-88910-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian S, Jiang Z, Jiang L, Wang K, Fan Y, Liao T, Chen W, Yao W. Caffeine‐induced neurotoxicity mediated by Nrf2 pathway in PC12 cells and zebrafish larvae. J Appl Toxicol. 2022;42:629–637. doi: 10.1002/jat.4244. [DOI] [PubMed] [Google Scholar]
- Chitre NM, Bagwell MS, Murnane KS. The acute toxic and neurotoxic effects of 3,4-methylenedioxymethamphetamine are more pronounced in adolescent than adult mice. Behav Brain Res. 2020;380:112413. doi: 10.1016/j.bbr.2019.112413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MR, Chun JW, Kwak SM, Bang SH, Jin YB, Lee Y, Kim HN, Chang KT, Chai YG, Lee SR, Kim DJ. Effects of acute and chronic methamphetamine administration on cynomolgus monkey hippocampus structure and cellular transcriptome. Toxicol Appl Pharmacol. 2018;355:68–79. doi: 10.1016/j.taap.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Cholanians AB, Phan AV, Lau SS, Monks TJ. Concurrent inhibition of vesicular monoamine transporter 2 does not protect against 3,4-methylenedioxymethamphetamine (Ecstasy) induced neurotoxicity. Toxicol Sci. 2019;170:157–166. doi: 10.1093/toxsci/kfz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare K, Pan C, Kim G, Park K, Zhao J, Volkow ND, Lin Z, Du C. Cocaine reduces the neuronal population while upregulating dopamine d2-receptor-expressing neurons in brain reward regions: sex-effects. Front Pharmacol. 2021;12:624127. doi: 10.3389/fphar.2021.624127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AA, Baldacchino AM. Neuroanatomical correlates of impulsive choices and risky decision making in young chronic tobacco smokers: a voxel-based morphometry study. Front Psychiatry. 2021;12:708925. doi: 10.3389/fpsyt.2021.708925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AA, Baldacchino AM. Chronic tobacco smoking, impaired reward-based decision-making, and role of insular cortex: a comparison between early-onset smokers and late-onset smokers. Front Psychiatry. 2022;13:939707. doi: 10.3389/fpsyt.2022.939707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, Atkinson LZ, Tessari L, Banaschewski T, Coghill D, Hollis C, Simonoff E, Zuddas A, Barbui C, Purgato M, Steinhausen HC, Shokraneh F, Xia J, Cipriani A. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5:727–738. doi: 10.1016/S2215-0366(18)30269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G, Morelli M, Simola N. Progression and persistence of neurotoxicity induced by MDMA in dopaminergic regions of the mouse brain and association with noradrenergic, GABAergic, and serotonergic damage. Neurotox Res. 2017;32:563–574. doi: 10.1007/s12640-017-9761-6. [DOI] [PubMed] [Google Scholar]
- Costa G, Caputi FF, Serra M, Simola N, Rullo L, Stamatakos S, Sanna F, Germain M, Martinoli M-G, Candeletti S, Morelli M, Romualdi P. Activation of antioxidant and proteolytic pathways in the nigrostriatal dopaminergic system after 3,4-methylenedioxymethamphetamine administration: sex-related differences. Front Pharmacol. 2021;12:713486. doi: 10.3389/fphar.2021.713486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G, Gołembiowska K. Neurotoxicity of MDMA: main effects and mechanisms. Exp Neurol. 2022;347:113894. doi: 10.1016/j.expneurol.2021.113894. [DOI] [PubMed] [Google Scholar]
- Dang DK, Shin EJ, Mai AT, Jang CG, Nah SY, Jeong JH, Ledent C, Yamamoto T, Nabeshima T, Onaivi ES, Kim HC. Genetic or pharmacological depletion of cannabinoid CB1 receptor protects against dopaminergic neurotoxicity induced by methamphetamine in mice. Free Radic Biol Med. 2017;108:204–224. doi: 10.1016/j.freeradbiomed.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Daniju Y, Faulkner P, Brandt K, Allen P. Prefrontal cortex and putamen grey matter alterations in cannabis and tobacco users. J Psychopharmacol (Oxf) 2022;36:1315–1323. doi: 10.1177/02698811221117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira MR, Jardim FR. Cocaine and mitochondria-related signaling in the brain: a mechanistic view and future directions. Neurochem Int. 2016;92:58–66. doi: 10.1016/j.neuint.2015.12.006. [DOI] [PubMed] [Google Scholar]
- De Win MML, Jager G, Booij J, Reneman L, Schilt T, Lavini C, Olabarriaga SD, Ramsey NF, Den Heeten GJ, Van Den Brink W. Neurotoxic effects of ecstasy on the thalamus. Br J Psychiatry. 2008;193:289–296. doi: 10.1192/bjp.bp.106.035089. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Ding J, Huang J, Tang X, Shen L, Hu S, He J, Liu T, Yu Z, Liu Y, Wang Q, Wang J, Zhao N, Qi X, Huang J. Low and high dose methamphetamine differentially regulate synaptic structural plasticity in cortex and hippocampus. Front Cell Neurosci. 2022;16:1003617. doi: 10.3389/fncel.2022.1003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JR, Alsufyani HA. Pharmacology of drugs used as stimulants. J Clin Pharmacol 61 Suppl. 2021;2:S53–S69. doi: 10.1002/jcph.1918. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. α1b-Adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK. Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer's Disease. Drug Alcohol Depend. 2018;192:277–284. doi: 10.1016/j.drugalcdep.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, Cardenas A, Franke RM, Chen Y, Bai Y, Belluzzi JD, Lotfipour S, Leslie FM. Prenatal nicotine sex-dependently alters adolescent dopamine system development. Transl Psychiatry. 2019;9:304. doi: 10.1038/s41398-019-0640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific opinion on the safety of caffeine. EFSA J. 2015;13:4102. [Google Scholar]
- Elbejjani M, Auer R, Jacobs DR, Haight T, Davatzikos C, Goff DC, Bryan RN, Launer LJ. Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA brain MRI sub-study. Transl Psychiatry. 2019;9:78. doi: 10.1038/s41398-019-0401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJG, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Zha R, Liu Y, Wei Z, Wang Y, Song H, Lv W, Ren J, Hong W, Gou H, Zhang P, Chen Y, Zhou Y, Pan Y, Zhang X. Altered white matter functional network in nicotine addiction. Psychiatry Res. 2023;321:115073. doi: 10.1016/j.psychres.2023.115073. [DOI] [PubMed] [Google Scholar]
- Fast K, Björk A, Strandberg M, Johannesson E, Wentz E, Dahlgren J. Half of the children with overweight or obesity and attention‐deficit/hyperactivity disorder reach normal weight with stimulants. Acta Paediatr. 2021;110:2825–2832. doi: 10.1111/apa.15881. [DOI] [PubMed] [Google Scholar]
- Ferré S, Díaz-Ríos M, Salamone JD, Prediger RD. New developments on the adenosine mechanisms of the central effects of caffeine and their implications for neuropsychiatric disorders. J Caffeine Adenosine Res. 2018;8:121–130. doi: 10.1089/caff.2018.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger G, Da Silva ER, Falavigna A. Use of methylphenidate among medical students: a systematic review. Rev Assoc Médica Bras Engl Ed. 2013;59:285–289. doi: 10.1016/j.ramb.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Lê A, Higgins GA. Serotonin receptors as potential targets for modulation of nicotine use and dependence. Prog Brain Res. 2008;172:361–383. doi: 10.1016/S0079-6123(08)00918-7. [DOI] [PubMed] [Google Scholar]
- Frank RA, Manderscheid PZ, Panicker S, Williams HP, Kokoris D. Cocaine euphoria, dysphoria, and tolerance assessed using drug-induced changes in brain-stimulation reward. Pharmacol Biochem Behav. 1992;42:771–779. doi: 10.1016/0091-3057(92)90028-e. [DOI] [PubMed] [Google Scholar]
- Frau L, Costa G, Porceddu PF, Khairnar A, Castelli MP, Ennas MG, Madeddu C, Wardas J, Morelli M. Influence of caffeine on 3,4-methylenedioxymethamphetamine-induced dopaminergic neuron degeneration and neuroinflammation is age-dependent. J Neurochem. 2016;136:148–162. doi: 10.1111/jnc.13377. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, Pizzo AB, Zhang Y, Farino ZJ, Chen A, Martin CA, Kopajtic TA, Fei H, Hu G, Lin YY, Mosharov EV, McCabe BD, Freyberg R, Wimalasena K, Hsin LW, et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun. 2016;7:10652. doi: 10.1038/ncomms10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujáková-Lipski M, Kaping D, Šírová J, Horáček J, Páleníček T, Zach P, Klaschka J, Kačer P, Syslová K, Vrajová M, Bubenikova-Valešová V, Beste C, Šlamberová R. Trans-generational neurochemical modulation of methamphetamine in the adult brain of the Wistar rat. Arch Toxicol. 2017;91:3373–3384. doi: 10.1007/s00204-017-1969-y. [DOI] [PubMed] [Google Scholar]
- García-Cabrerizo R, García-Fuster MJ. Chronic MDMA induces neurochemical changes in the hippocampus of adolescent and young adult rats: down-regulation of apoptotic markers. Neurotoxicology. 2015;49:104–113. doi: 10.1016/j.neuro.2015.06.001. [DOI] [PubMed] [Google Scholar]
- García-Pardo MP, Calpe-López C, Martínez-Caballero MÁ, Aguilar MA. The biology of nitric oxide signaling and MDMA. In: Patel VB, Preedy VR, editors. Handbook of Substance Misuse and Addictions. Cham: Springer International Publishing; 2022. pp. 2337–2364. [Google Scholar]
- Gaudreault PO, King SG, Malaker P, Alia-Klein N, Goldstein RZ. Whole-brain white matter abnormalities in human cocaine and heroin use disorders: association with craving, recency, and cumulative use. Mol Psychiatry. 2023;28:780–791. doi: 10.1038/s41380-022-01833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepdi̇Remen A, Sönmez S, İKbal M, Düzenli̇ S, Tuna S. Response to nimodipine in caffeine-induced neurotoxicity in cerebellar granular cell culture of rat pups. Pharmacol Res. 1998;38:239–242. doi: 10.1006/phrs.1998.0358. [DOI] [PubMed] [Google Scholar]
- Gerra G, Benedetti E, Resce G, Potente R, Cutilli A, Molinaro S. Socioeconomic status, parental education, school connectedness and individual socio-cultural resources in vulnerability for drug use among students. Int J Environ Res Public Health. 2020;17:1306. doi: 10.3390/ijerph17041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavidel N, Khodagholi F, Ahmadiani A, Khosrowabadi R, Asadi S, Shams J. Inflammation but not programmed cell death is activated in methamphetamine-dependent patients: Relevance to the brain function. Int J Psychophysiol. 2020;157:42–50. doi: 10.1016/j.ijpsycho.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Gicas KM, Parmar PK, Fabiano GF, Mashhadi F. Substance-induced psychosis and cognitive functioning: A systematic review. Psychiatry Res. 2022;308:114361. doi: 10.1016/j.psychres.2021.114361. [DOI] [PubMed] [Google Scholar]
- Gopal KV, Miller BR, Gross GW. Acute and sub-chronic functional neurotoxicity of methylphenidate on neural networks in vitro. J Neural Transm. 2007;114:1365–1375. doi: 10.1007/s00702-007-0759-8. [DOI] [PubMed] [Google Scholar]
- Gore RK, Webb TS, Hermes EDA. Fatigue and stimulant use in military fighter aircrew during combat operations. Aviat Space Environ Med. 2010;81:719–727. doi: 10.3357/asem.2755.2010. [DOI] [PubMed] [Google Scholar]
- Górska AM, Noworyta-Sokołowska K, Gołembiowska K. The effect of caffeine on MDMA-induced hydroxyl radical production in the mouse striatum. Pharmacol Rep. 2014;66:718–721. doi: 10.1016/j.pharep.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Górska AM, Kamińska K, Wawrzczak-Bargieła A, Costa G, Morelli M, Przewłocki R, Kreiner G, Gołembiowska K. Neurochemical and neurotoxic effects of MDMA (Ecstasy) and caffeine after chronic combined administration in mice. Neurotox Res. 2018;33:532–548. doi: 10.1007/s12640-017-9831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramage E, Rossi L, Granado N, Moratalla R, Herradón G. Genetic inactivation of Pleiotrophin triggers amphetamine-induced cell loss in the substantia nigra and enhances amphetamine neurotoxicity in the striatum. Neuroscience. 2010;170:308–316. doi: 10.1016/j.neuroscience.2010.06.078. [DOI] [PubMed] [Google Scholar]
- Granado N, O'Shea E, Bove J, Vila M, Colado MI, Moratalla R. Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J Neurochem. 2008;107:1102–1112. doi: 10.1111/j.1471-4159.2008.05705.x. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, O´Shea E, Martin ED, Colado MI, Moratalla R. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis. 2011;42:391–403. doi: 10.1016/j.nbd.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Moratalla R. D1 but not D4 dopamine receptors are critical for MDMA-induced neurotoxicity in mice. Neurotox Res. 2014;25:100–109. doi: 10.1007/s12640-013-9438-8. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Tizabi Y, Moratalla R. Striatal reinnervation process after acute methamphetamine-induced dopaminergic degeneration in mice. Neurotox Res. 2018;34:627–639. doi: 10.1007/s12640-018-9925-z. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Laćan G, Melega WP. Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp Brain Res. 2000;133:349–358. doi: 10.1007/s002210000386. [DOI] [PubMed] [Google Scholar]
- He W, Yan X, Pan S. Amphetamine neurotoxicity in PC12 cells through the PP2A/AKT/GSK3β pathway. Neurotox Res. 2018;34:233–240. doi: 10.1007/s12640-018-9880-8. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Bastian BA, Trinidad JP, Spencer MR, Warner M. Regional differences in the drugs most frequently involved in drug overdose deaths: United States, 2017. Natl Vital Stat Rep. 2019;68:1–16. [PubMed] [Google Scholar]
- Hirsiger S, Hänggi J, Germann J, Vonmoos M, Preller KH, Engeli EJE, Kirschner M, Reinhard C, Hulka LM, Baumgartner MR, Chakravarty MM, Seifritz E, Herdener M, Quednow BB. Longitudinal changes in cocaine intake and cognition are linked to cortical thickness adaptations in cocaine users. NeuroImage Clin. 2019;21:101652. doi: 10.1016/j.nicl.2019.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Psychostimulants. In: Sibley DR, Kuhar M, Hanin I, Skolnick P, editors. Handbook of contemporary neuropharmacology. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2007. p. hcn038. [Google Scholar]
- Huang J, Ding J, Wang Z, Li Y, He Y, Wang X, Fan H, Xie Q, Qiu P. Icariside II attenuates methamphetamine-induced neurotoxicity and behavioral impairments via activating the Keap1-Nrf2 pathway. Oxid Med Cell Longev. 2022;2022:8400876. doi: 10.1155/2022/8400876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WS, Chen GJ, Tsai TH, Cheng CY, Shiue CY, Ma KH, Yeh SHH. In vivo long-lasting alterations of central serotonin transporter activity and associated dopamine synthesis after acute repeated administration of methamphetamine. EJNMMI Res. 2019;9:92. doi: 10.1186/s13550-019-0557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten NR, Kuypers KP, van Wel JH, Theunissen EL, Toennes SW, Verkes RJ, Ramaekers JG. A single dose of cocaine enhances prospective memory performance. J Psychopharmacol Oxf Engl. 2018;32:883–892. doi: 10.1177/0269881118783299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitschke D, Lauer AA, Bachmann CM, Grimm HS, Hartmann T, Grimm MOW. Methylxanthines and neurodegenerative diseases: an update. Nutrients. 2021;13:803. doi: 10.3390/nu13030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Daiwile AP, Cadet JL. Neurotoxicity of methamphetamine: main effects and mechanisms. Exp Neurol. 2021;344:113795. doi: 10.1016/j.expneurol.2021.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Sullivan P, McCoy MT, Krasnova IN, Goldstein DS, Cadet JL. Biochemical neuroadaptations in the rat striatal dopaminergic system after prolonged exposure to methamphetamine self-administration. Int J Mol Sci. 2022;23:10092. doi: 10.3390/ijms231710092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Li J, Zhang Z, Wang H, Wang Z. Epigenetic upregulation of alpha-synuclein in the rats exposed to methamphetamine. Eur J Pharmacol. 2014;745:243–248. doi: 10.1016/j.ejphar.2014.10.043. [DOI] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biezonski DK, Meyer JS. The nature of 3, 4-methylenedioxymethamphetamine (mdma)-induced serotonergic dysfunction: evidence for and against the neurodegeneration hypothesis. Curr Neuropharmacol. 2011;9:84–90. doi: 10.2174/157015911795017146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagabo R, Thiese MS, Eden E, Thatcher AC, Gonzalez M, Okuyemi K. Truck drivers' cigarette smoking and preferred smoking cessation methods. Subst Abuse Res Treat. 2020;14:117822182094926. doi: 10.1177/1178221820949262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Ae Lee Y, Won SJ, Rhee K-H, Gwag BJ. Caffeine-induced neuronal death in neonatal rat brain and cortical cell cultures. Neuroreport. 2002;13:1945–1950. doi: 10.1097/00001756-200210280-00023. [DOI] [PubMed] [Google Scholar]
- Kantak KM. Adolescent-onset vs. adult-onset cocaine use: impact on cognitive functioning in animal models and opportunities for translation. Pharmacol Biochem Behav. 2020;196:172994. doi: 10.1016/j.pbb.2020.172994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasala S, Briyal S, Prazad P, Ranjan AK, Stefanov G, Donovan R, Gulati A. Exposure to morphine and caffeine induces apoptosis and mitochondrial dysfunction in a neonatal rat brain. Front Pediatr. 2020;8:593. doi: 10.3389/fped.2020.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CP, Meyer PJ. The incentive amplifying effects of nicotine: roles in alcohol seeking and consumption. Adv Pharmacol. 2022;93:171–218. doi: 10.1016/bs.apha.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SG, Gaudreault PO, Malaker P, Kim J, Alia-Klein N, Xu J, Goldstein RZ. Prefrontal-habenular microstructural impairments in human cocaine and heroin addiction. Neuron. 2022;110:3820–3832. doi: 10.1016/j.neuron.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS. Striatal serotonin is depleted in brain of a human MDMA (Ecstasy) user. Neurology. 2000;55:294–296. doi: 10.1212/wnl.55.2.294. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Lerch J, Furukawa Y, Tong J, McCluskey T, Wilkins D, Houle S, Meyer J, Mundo E, Wilson AA, Rusjan PM, Saint-Cyr JA, Guttman M, Collins DL, Shapiro C, Warsh JJ, Boileau I. Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[11C]DASB and structural brain imaging study. Brain. 2010;133:1779–1797. doi: 10.1093/brain/awq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Boileau I, Callaghan RC, Tong J. Brain dopamine neurone ‘damage’: methamphetamine users vs. Parkinson's disease - a critical assessment of the evidence. Eur J Neurosci. 2017;45:58–66. doi: 10.1111/ejn.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Arends MA, McCracken ML, Le moal M. Psychostimulants. New York: Academic Press; 2020. Psychostimulants; pp. 1–245. [Google Scholar]
- Kousik SM, Carvey PM, Napier TC. Methamphetamine self-administration results in persistent dopaminergic pathology: implications for Parkinson's disease risk and reward-seeking. Eur J Neurosci. 2014;40:2707–2714. doi: 10.1111/ejn.12628. [DOI] [PubMed] [Google Scholar]
- Krinzinger H, Hall CL, Groom MJ, Ansari MT, Banaschewski T, Buitelaar JK, Carucci S, Coghill D, Danckaerts M, Dittmann RW, Falissard B, Garas P, Inglis SK, Kovshoff H, Kochhar P, McCarthy S, Nagy P, Neubert A, Roberts S, Sayal K, et al. Neurological and psychiatric adverse effects of long-term methylphenidate treatment in ADHD: a map of the current evidence. Neurosci Biobehav Rev. 2019;107:945–968. doi: 10.1016/j.neubiorev.2019.09.023. [DOI] [PubMed] [Google Scholar]
- Lafuente JV, Sharma A, Muresanu DF, Ozkizilcik A, Tian ZR, Patnaik R, Sharma HS. Repeated forced swim exacerbates methamphetamine-induced neurotoxicity: neuroprotective effects of nanowired delivery of 5-HT3-receptor antagonist ondansetron. Mol Neurobiol. 2018;55:322–334. doi: 10.1007/s12035-017-0744-7. [DOI] [PubMed] [Google Scholar]
- Lappin JM, Darke S, Farrell M. Methamphetamine use and future risk for Parkinson's disease: evidence and clinical implications. Drug Alcohol Depend. 2018;187:134–140. doi: 10.1016/j.drugalcdep.2018.02.032. [DOI] [PubMed] [Google Scholar]
- Laviolette SR. Exploring the impact of adolescent exposure to cannabinoids and nicotine on psychiatric risk: insights from translational animal models. Psychol Med. 2021;51:940–947. doi: 10.1017/S0033291719003325. [DOI] [PubMed] [Google Scholar]
- Levi MS, Divine B, Hanig JP, Doerge DR, Vanlandingham MM, George NI, Twaddle NC, Bowyer JF. A comparison of methylphenidate-, amphetamine-, and methamphetamine-induced hyperthermia and neurotoxicity in male Sprague–Dawley rats during the waking (lights off) cycle. Neurotoxicol Teratol. 2012;34:253–262. doi: 10.1016/j.ntt.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Li X, Semenova S, D'Souza MS, Stoker AK, Markou A. Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology. 2014;76:554–565. doi: 10.1016/j.neuropharm.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation. 2016;13:33. doi: 10.1186/s12974-016-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Weibel J, Landolt HP, Santini F, Slawik H, Borgwardt S, Cajochen C, Reichert CF. Brain activity during a working memory task after daily caffeine intake and caffeine withdrawal: a randomized double-blind placebo-controlled trial. Sci Rep. 2023;13:1002. doi: 10.1038/s41598-022-26808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Chou MC, Chung HW, Cho NY, Chiang SW, Wang CY, Kao HW, Huang GS, Chen CY. Potential long-term effects of MDMA on the basal ganglia–thalamocortical circuit: a proton MR spectroscopy and diffusion-tensor imaging study. Radiology. 2011;260:531–540. doi: 10.1148/radiol.11101918. [DOI] [PubMed] [Google Scholar]
- Lizarraga LE, Cholanians AB, Phan AV, Herndon JM, Lau SS, Monks TJ. Vesicular monoamine transporter 2 and the acute and long-term response to 3,4-(±)-methylenedioxymethamphetamine. Toxicol Sci. 2015;143:209–219. doi: 10.1093/toxsci/kfu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 20151628:174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro‐Vieira S, Costa VM, Lourdes Bastos M, Carvalho F, Capela JP. Methylphenidate effects in the young brain: friend or foe? Int J Dev Neurosci. 2017;60:34–47. doi: 10.1016/j.ijdevneu.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Schaz U, Storch A, Liebau S, Fegert JM, Boeckers TM. Methylphenidate exerts no neurotoxic, but neuroprotective effects in vitro. J Neural Transm. 2006;113:1927–1934. doi: 10.1007/s00702-006-0487-5. [DOI] [PubMed] [Google Scholar]
- Mai HN, Nguyen LTT, Shin EJ, Kim DJ, Jeong JH, Chung YH, Lei XG, Sharma N, Jang CG, Nabeshima T, Kim HC. Astrocytic mobilization of glutathione peroxidase-1 contributes to the protective potential against cocaine kindling behaviors in mice via activation of JAK2/STAT3 signaling. Free Radic Biol Med. 2019;131:408–431. doi: 10.1016/j.freeradbiomed.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Malińska D, Więckowski MR, Michalska B, Drabik K, Prill M, Patalas-Krawczyk P, Walczak J, Szymański J, Mathis C, Van Der Toorn M, Luettich K, Hoeng J, Peitsch MC, Duszyński J, Szczepanowska J. Mitochondria as a possible target for nicotine action. J Bioenerg Biomembr. 2019;51:259–276. doi: 10.1007/s10863-019-09800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao M, Yu T, Hu J, Hu L. Dopamine D2 receptor blocker thioridazine induces cell death in human uterine cervical carcinoma cell line SiHa. J Obstet Gynaecol Res. 2015;41:1240–1245. doi: 10.1111/jog.12691. [DOI] [PubMed] [Google Scholar]
- Mappin-Kasirer B, Pan H, Lewington S, Kizza J, Gray R, Clarke R, Peto R. Tobacco smoking and the risk of Parkinson disease: A 65-year follow-up of 30,000 male British doctors. Neurology. 2020;94:e2132–2138. doi: 10.1212/WNL.0000000000009437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Pagliaccio D, Ramphal B, Banker S, Thomas L, Robinson M, Honda M, Sussman T, Posner J, Kannan K, Herbstman J, Rauh V, Marsh R. Prenatal environmental tobacco smoke exposure alters children's cognitive control circuitry: a preliminary study. Environ Int. 2021;155:106516. doi: 10.1016/j.envint.2021.106516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai K, Kuroda K, Isooka N, Kikuoka R, Murakami S, Kamimai S, Wang D, Liu K, Miyazaki I, Nishibori M, Asanuma M. Neuroprotective effects of anti-high mobility group box-1 monoclonal antibody against methamphetamine-induced dopaminergic neurotoxicity. Neurotox Res. 2021;39:1511–1523. doi: 10.1007/s12640-021-00402-5. [DOI] [PubMed] [Google Scholar]
- McCann UD, Mertl M, Eligulashvili V, Ricaurte GA. Cognitive performance in (±) 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users: a controlled study. Psychopharmacology (Berl) 1999;143:417–425. doi: 10.1007/s002130050967. [DOI] [PubMed] [Google Scholar]
- Mckenzie A, Meshkat S, Lui LMW, Ho R, Di Vincenzo JD, Ceban F, Cao B, McIntyre RS. The effects of psychostimulants on cognitive functions in individuals with attention-deficit hyperactivity disorder: a systematic review. J Psychiatr Res. 2022;149:252–259. doi: 10.1016/j.jpsychires.2022.03.018. [DOI] [PubMed] [Google Scholar]
- McLellan TM, Riviere LA, Williams KW, McGurk D, Lieberman HR. Caffeine and energy drink use by combat arms soldiers in Afghanistan as a countermeasure for sleep loss and high operational demands. Nutr Neurosci. 2019;22:768–777. doi: 10.1080/1028415X.2018.1443996. [DOI] [PubMed] [Google Scholar]
- Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME. Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res. 1997;766:113–120. doi: 10.1016/s0006-8993(97)00548-9. [DOI] [PubMed] [Google Scholar]
- Meng Y, Qiao H, Ding J, He Y, Fan H, Li C, Qiu P. Effect of Parkin on methamphetamine‐induced α‐synuclein degradation dysfunction in vitro and in vivo. Brain Behav. 2020;10:e01574. doi: 10.1002/brb3.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mews P, Cunningham AM, Scarpa J, Ramakrishnan A, Hicks EM, Bolnick S, Garamszegi S, Shen L, Mash DC, Nestler EJ. Convergent abnormalities in striatal gene networks in human cocaine use disorder and mouse cocaine administration models. Sci Adv. 2023;9:eadd8946. doi: 10.1126/sciadv.add8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol. 1995;11:177–192. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- Millot M, Saga Y, Duperrier S, Météreau E, Beaudoin-Gobert M, Sgambato V. Prior MDMA administration aggravates MPTP-induced Parkinsonism in macaque monkeys. Neurobiol Dis. 2020;134:104643. doi: 10.1016/j.nbd.2019.104643. [DOI] [PubMed] [Google Scholar]
- Miner NB, O'Callaghan JP, Phillips TJ, Janowsky A. The combined effects of 3,4-methylenedioxymethamphetamine (MDMA) and selected substituted methcathinones on measures of neurotoxicity. Neurotoxicol Teratol. 2017;61:74–81. doi: 10.1016/j.ntt.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery C, Roberts CA. Neurological and cognitive alterations induced by MDMA in humans. Exp Neurol. 2022;347:113888. doi: 10.1016/j.expneurol.2021.113888. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Khairnar A, Simola N, Granado N, García-Montes JR, Porceddu PF, Tizabi Y, Costa G, Morelli M. Amphetamine-related drugs neurotoxicity in humans and in experimental animals: main mechanisms. Prog Neurobiol. 2017;155:149–170. doi: 10.1016/j.pneurobio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Morelli M, Simola N. Methylxanthines and drug dependence: a focus on interactions with substances of abuse. Handb Exp Pharmacol. 2011:483–507. doi: 10.1007/978-3-642-13443-2_20. [DOI] [PubMed] [Google Scholar]
- Morton WA, Stock GG. Methylphenidate abuse and psychiatric side effects. Prim Care Companion J Clin Psychiatry. 2000;2:159–164. doi: 10.4088/pcc.v02n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moska C, Goudriaan AE, Blanken P, van de Mheen D, Spijkerman R, Schellekens A, de Jonge J, Bary F, Vollebergh W, Hendriks V. Youth in transition: study protocol of a prospective cohort study into the long-term course of addiction, mental health problems and social functioning in youth entering addiction treatment. BMC Psychiatry. 2021;21:605. doi: 10.1186/s12888-021-03520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszczynska A, Callan SP. Molecular, behavioral, and physiological consequences of methamphetamine neurotoxicity: implications for treatment. J Pharmacol Exp Ther. 2017;362:474–488. doi: 10.1124/jpet.116.238501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaghinejad M, Motevalian M, Fatima S. Mediatory role of NMDA, AMPA/kainate, GABA A and Alpha 2 receptors in topiramate neuroprotective effects against methylphenidate induced neurotoxicity in rat. Life Sci. 2017a;179:37–53. doi: 10.1016/j.lfs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Motaghinejad M, Motevalian M, Shabab B. Possible involvements of glutamate and adrenergic receptors on acute toxicity of methylphenidate in isolated hippocampus and cerebral cortex of adult rats. Fundam Clin Pharmacol. 2017b;31:208–225. doi: 10.1111/fcp.12250. [DOI] [PubMed] [Google Scholar]
- Motaghinejad M, Motevalian M. Neuroprotective properties of minocycline against methylphenidate-induced neurodegeneration: possible role of CREB/BDNF and Akt/GSK3 signaling pathways in rat hippocampus. Neurotox Res. 2022;40:689–713. doi: 10.1007/s12640-021-00454-7. [DOI] [PubMed] [Google Scholar]
- Müller CP, Homberg JR. The role of serotonin in drug use and addiction. Behav Brain Res. 2015;277:146–192. doi: 10.1016/j.bbr.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Müller F, Brändle R, Liechti ME, Borgwardt S. Neuroimaging of chronic MDMA (“ecstasy”) effects: a meta-analysis. Neurosci Biobehav Rev. 2019;96:10–20. doi: 10.1016/j.neubiorev.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Narendran R, Lopresti BJ, Mason NS, Deuitch L, Paris J, Himes ML, Kodavali CV, Nimgaonkar VL. Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J Neurosci. 2014;34:9945–9950. doi: 10.1523/JNEUROSCI.0928-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazlı ŞB, Sevindik M. Use of methylphenidate in co-existing major depression and loss of libido and erectile dysfunction: a case report. Anatol J Psychiatry. 2020 doi: 10.5455/apd.112405. doi: 10.5455/apd.112405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholatos JW, Francisco AB, Bender CA, Yeh T, Lugay FJ, Salazar JE, Glorioso C, Libert S. Nicotine promotes neuron survival and partially protects from Parkinson's disease by suppressing SIRT6. Acta Neuropathol Commun. 2018;6:120. doi: 10.1186/s40478-018-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolucci C, Pais ML, Santos AC, Ribeiro FM, Encarnação PMCC, Silva ALM, Castro IF, Correia PMM, Veloso JFCA, Reis J, Lopes MZ, Botelho MF, Pereira FC, Priolli DG. Single low dose of cocaine–structural brain injury without metabolic and behavioral changes. Front Neurosci. 2021;14:589897. doi: 10.3389/fnins.2020.589897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi KK, Johnson SA, Manzella FM, Masuoka KL, Williams SL, Martin LD, Dissen GA, Ikonomidou C, Schenning KJ, Olney JW, Brambrink AM. Caffeine augments anesthesia neurotoxicity in the fetal macaque brain. Sci Rep. 2018;8:5302. doi: 10.1038/s41598-018-23560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ghahremani DG, Payer DE, Robertson CL, Mandelkern MA, London ED. Relationship of alexithymia ratings to dopamine d2-type receptors in anterior cingulate and insula of healthy control subjects but not methamphetamine-dependent individuals. Int J Neuropsychopharmacol. 2016;19:pyv129. doi: 10.1093/ijnp/pyv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottino-González J, Uhlmann A, Hahn S, Cao Z, Cupertino RB, Schwab N, Allgaier N, Alia-Klein N, Ekhtiari H, Fouche JP, Goldstein RZ, Li CR, Lochner C, London ED, Luijten M, Masjoodi S, Momenan R, Oghabian MA, Roos A, Stein DJ, et al. White matter microstructure differences in individuals with dependence on cocaine, methamphetamine, and nicotine: Findings from the ENIGMA-Addiction working group. Drug Alcohol Depend. 2022;230:109185. doi: 10.1016/j.drugalcdep.2021.109185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Moreland T, Haq F, Siddiqui F, Mikul M, Qadir H, Raza S. Persistent psychosis after a single ingestion of “Ecstasy” (MDMA) Prim Care Companion CNS Disord. 2011;13 doi: 10.4088/PCC.11l01200. PCC.11l01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P, Li M, Liu H, Tian YR, Chu SL, Van Halm-Lutterodt N, Jing B, Jiang T. Brain structure alterations in respect to tobacco consumption and nicotine dependence: a comparative voxel-based morphometry study. Front Neuroanat. 2018;12:43. doi: 10.3389/fnana.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao WH, Campagnolo D, Dayao C, Lukas RJ, Wu J, Shi FD. Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin. 2009;30:715–722. doi: 10.1038/aps.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta E, Hervias I, Goñi-Allo B, Zhang SF, Jordán J, Starkov AA, Aguirre N. Methylenedioxymethamphetamine inhibits mitochondrial complex I activity in mice: a possible mechanism underlying neurotoxicity: MDMA inhibits complex I activity in mice. Br J Pharmacol. 2010;160:233–245. doi: 10.1111/j.1476-5381.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao HH, Zhu LN, Wang Y, Hui JL, Xie WB, Liu C, Chen L, Qiu PM. Implications of alpha-synuclein nitration at tyrosine 39 in methamphetamine-induced neurotoxicity in vitro and in vivo. Neural Regen Res. 2019;14:319. doi: 10.4103/1673-5374.244795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quansah E, Zetterström TSC. Chronic methylphenidate preferentially alters catecholamine protein targets in the parietal cortex and ventral striatum. Neurochem Int. 2019;124:193–199. doi: 10.1016/j.neuint.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Ren M, Lotfipour S, Leslie F. Unique effects of nicotine across the lifespan. Pharmacol Biochem Behav. 2022;214:173343. doi: 10.1016/j.pbb.2022.173343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Chen JF. Caffeine and Parkinson's disease: multiple benefits and emerging mechanisms. Front Neurosci. 2020;14:602697. doi: 10.3389/fnins.2020.602697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Booij J, Schmand B, Van Den Brink W, Gunning B. Memory disturbances in ”Ecstasy” users are correlated with an altered brain serotonin neurotransmission. Psychopharmacology (Berl) 2000;148:322–324. doi: 10.1007/s002130050057. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Mechan AO, Yuan J, Hatzidimitriou G, Xie T, Mayne AH, McCann UD. Amphetamine treatment similar to that used in the treatment of adult attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates. J Pharmacol Exp Ther. 2005;315:91–98. doi: 10.1124/jpet.105.087916. [DOI] [PubMed] [Google Scholar]
- Rivero-Echeto MC, Perissinotti PP, González-Inchauspe C, Kargieman L, Bisagno V, Urbano FJ. Simultaneous administration of cocaine and caffeine dysregulates HCN and T-type channels. Psychopharmacology (Berl) 2021;238:787–810. doi: 10.1007/s00213-020-05731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkallah É, Bélanger M, Stavro K, Dussault M, Pampoulova T, Chiasson JP, Potvin S. Could the use of energy drinks induce manic or depressive relapse among abstinent substance use disorder patients with comorbid bipolar spectrum disorder? Bipolar Disord. 2011;13:578–580. doi: 10.1111/j.1399-5618.2011.00951.x. [DOI] [PubMed] [Google Scholar]
- Roberts CA, Quednow BB, Montgomery C, Parrott AC. MDMA and brain activity during neurocognitive performance: an overview of neuroimaging studies with abstinent ‘Ecstasy’ users. Neurosci Biobehav Rev. 2018;84:470–482. doi: 10.1016/j.neubiorev.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Roberts CarlA, Jones A, Montgomery C. Meta-analysis of molecular imaging of serotonin transporters in ecstasy/polydrug users. Neurosci Biobehav Rev. 2016;63:158–167. doi: 10.1016/j.neubiorev.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rudin D, Liechti ME, Luethi D. Molecular and clinical aspects of potential neurotoxicity induced by new psychoactive stimulants and psychedelics. Exp Neurol. 2021;343:113778. doi: 10.1016/j.expneurol.2021.113778. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J Pharmacol Exp Ther. 2003;304:1181–1187. doi: 10.1124/jpet.102.045005. [DOI] [PubMed] [Google Scholar]
- Sangaunchom P, Dharmasaroja P. Caffeine potentiates ethanol-induced neurotoxicity through mTOR/p70S6K/4E-BP1 inhibition in SH-SY5Y cells. Int J Toxicol. 2020;39:131–140. doi: 10.1177/1091581819900150. [DOI] [PubMed] [Google Scholar]
- Schiavone S, Neri M, Maffione A, Frisoni P, Morgese M, Trabace L, Turillazzi E. Increased iNOS and nitrosative stress in dopaminergic neurons of MDMA-exposed rats. Int J Mol Sci. 2019;20:1242. doi: 10.3390/ijms20051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. ROS function in Redox signaling and oxidative stress. Curr Biol. 2014;24:R453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Pierozan P, Rodrigues AF, Biasibetti H, Coelho DM, Mussulini BH, Pereira MSL, Parisi MM, Barbé-Tuana F, De Oliveira DL, Vargas CR, Wyse ATS. Chronic treatment with a clinically relevant dose of methylphenidate increases glutamate levels in cerebrospinal fluid and impairs glutamatergic homeostasis in prefrontal cortex of juvenile rats. Mol Neurobiol. 2016;53:2384–2396. doi: 10.1007/s12035-015-9219-x. [DOI] [PubMed] [Google Scholar]
- Schouw MLJ, De Ruiter MB, Kaag AM, van den Brink W, Lindauer RJL, Reneman L. Dopaminergic dysfunction in abstinent dexamphetamine users: results from a pharmacological fMRI study using a reward anticipation task and a methylphenidate challenge. Drug Alcohol Depend. 2013;130:52–60. doi: 10.1016/j.drugalcdep.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Schrantee A, Ferguson B, Stoffers D, Booij J, Rombouts S, Reneman L. Effects of dexamphetamine-induced dopamine release on resting-state network connectivity in recreational amphetamine users and healthy controls. Brain Imaging Behav. 2016;10:548–558. doi: 10.1007/s11682-015-9419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch-Goi SB, Goi PD, Bermudez M, Fara LS, Kessler FP, Pechansky F, Gama CS, Massuda R, Von Diemen L. Accumbens volumes are reduced among crack-cocaine users. Neurosci Lett. 2017;645:86–89. doi: 10.1016/j.neulet.2017.02.073. [DOI] [PubMed] [Google Scholar]
- Schweppe CA, Burzynski C, Jayanthi S, Ladenheim B, Cadet JL, Gardner EL, Xi Z-X, van Praag H, Newman AH, Keck TM. Neurochemical and behavioral comparisons of contingent and non-contingent methamphetamine exposure following binge or yoked long-access self-administration paradigms. Psychopharmacology (Berl) 2020;237:1989–2005. doi: 10.1007/s00213-020-05513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafahi M, Vaezi G, Shajiee H, Sharafi S, Khaksari M. Crocin inhibits apoptosis and astrogliosis of hippocampus neurons against methamphetamine neurotoxicity via antioxidant and anti-inflammatory mechanisms. Neurochem Res. 2018;43:2252–2259. doi: 10.1007/s11064-018-2644-2. [DOI] [PubMed] [Google Scholar]
- Sharif S, Guirguis A, Fergus S, Schifano F. The use and impact of cognitive enhancers among university students: a systematic review. Brain Sci. 2021;11:355. doi: 10.3390/brainsci11030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JH, Ma KH, Chen CFF, Cheng CY, Pao LH, Weng SJ, Huang YS, Shiue CY, Yeh MK, Li IH. Evaluation of brain SERT occupancy by resveratrol against MDMA-induced neurobiological and behavioral changes in rats: A 4-[18 F]-ADAM/small-animal PET study. Eur Neuropsychopharmacol. 2016;26:92–104. doi: 10.1016/j.euroneuro.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Shin SW, Nguyen TTL, Park DH, Wie MB, Jang CG, Nah SY, Yang BW, Ko SK, Nabeshima T, Kim HC. Ginsenoside re rescues methamphetamine-induced oxidative damage, mitochondrial dysfunction, microglial activation, and dopaminergic degeneration by inhibiting the protein kinase cδ gene. Mol Neurobiol. 2014;49:1400–1421. doi: 10.1007/s12035-013-8617-1. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Jeong JH, Sharma G, Sharma N, Kim DJ, Pham DT, Trinh QD, Dang DK, Nah SY, Bing G, Kim HC. Protein kinase Cδ mediates methamphetamine-induced dopaminergic neurotoxicity in mice via activation of microsomal epoxide hydrolase. Food Chem Toxicol. 2019;133:110761. doi: 10.1016/j.fct.2019.110761. [DOI] [PubMed] [Google Scholar]
- Sil S, Niu F, Tom E, Liao K, Periyasamy P, Buch S. Cocaine mediated neuroinflammation: role of dysregulated autophagy in pericytes. Mol Neurobiol. 2019;56:3576–3590. doi: 10.1007/s12035-018-1325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CD, Neves AF, Dias AI, Freitas HJ, Mendes SM, Pita I, Viana SD, de Oliveira PA, Cunha RA, Fontes Ribeiro CA, Prediger RD, Pereira FC. A single neurotoxic dose of methamphetamine induces a long-lasting depressive-like behaviour in mice. Neurotox Res. 2014;25:295–304. doi: 10.1007/s12640-013-9423-2. [DOI] [PubMed] [Google Scholar]
- Simola N, Pinna A, Frau L, Costa G, Marongiu J, Parekh P, Serra M, Morelli M. Protective agents in Parkinson's disease: caffeine and adenosine A2A receptor antagonists. In: Kostrzewa RM, editor. Handbook of neurotoxicity. Cham: Springer International Publishing; 2021. pp. 1–24. [Google Scholar]
- Stumm G, Schlegel J, Schäfer T, Würz C, Mennel HD, Krieg JC., Vedder H. Amphetamines induce apoptosis and regulation of bcl‐x splice variants in neocortical neurons. FASEB J. 1999;13:1065–1072. doi: 10.1096/fasebj.13.9.1065. [DOI] [PubMed] [Google Scholar]
- Subu R, Jayanthi S, Cadet JL. Compulsive methamphetamine taking induces autophagic and apoptotic markers in the rat dorsal striatum. Arch Toxicol. 2020;94:3515–3526. doi: 10.1007/s00204-020-02844-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama J, Dubinskaya A, Eilber K, Anger J. (267) Ecstasy' - accurate or deceptive name? A systematic review of MDMA effects on the male sexual response cycle. J Sex Med. 2023;20 doi: 10.1093/sxmrev/qead046. qdad060.251. [DOI] [PubMed] [Google Scholar]
- Taghizadeh G, Pourahmad J, Mehdizadeh H, Foroumadi A, Torkaman-Boutorabi A, Hassani S, Naserzadeh P, Shariatmadari R, Gholami M, Rouini MR, Sharifzadeh M. Protective effects of physical exercise on MDMA-induced cognitive and mitochondrial impairment. Free Radic Biol Med. 2016;99:11–19. doi: 10.1016/j.freeradbiomed.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Thangaraj A, Periyasamy P, Guo ML, Chivero ET, Callen S, Buch S. Mitigation of cocaine-mediated mitochondrial damage, defective mitophagy and microglial activation by superoxide dismutase mimetics. Autophagy. 2020;16:289–312. doi: 10.1080/15548627.2019.1607686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondo LP, Viola TW, Fries GR, Kluwe-Schiavon B, Rothmann LM, Cupertino R, Ferreira P, Franco AR, Lane SD, Stertz L, Zhao Z, Hu R, Meyer T, Schmitz JM, Walss-Bass C, Grassi-Oliveira R. White matter deficits in cocaine use disorder: convergent evidence from in vivo diffusion tensor imaging and ex vivo proteomic analysis. Transl Psychiatry. 2021;11:252. doi: 10.1038/s41398-021-01367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung CS, Chang ST, Huang CL, Huang NK. The neurotoxic mechanisms of amphetamine: Step by step for striatal dopamine depletion. Neurosci Lett. 2017;639:185–191. doi: 10.1016/j.neulet.2017.01.002. [DOI] [PubMed] [Google Scholar]
- UNODC World drug report, 2023. https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2023.html. 2023 Available at: Accessed November 3, 2023. [Google Scholar]
- Valentine G, Sofuoglu M. Cognitive effects of nicotine: recent progress. Curr Neuropharmacol. 2018;16:403–414. doi: 10.2174/1570159X15666171103152136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallender EJ, Goswami DB, Shinday NM, Westmoreland SV, Yao W-D, Rowlett JK. Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug Alcohol Depend. 2017;175:9–23. doi: 10.1016/j.drugalcdep.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Smith PH, Cosgrove KP, Mineur YS, Picciotto MR, Mazure CM, McKee SA. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine Tob Res. 2015;17:486–495. doi: 10.1093/ntr/ntu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Koenig J. Caffeine intake and its sources: a review of national representative studies. Crit Rev Food Sci Nutr. 2018;58:1250–1259. doi: 10.1080/10408398.2016.1247252. [DOI] [PubMed] [Google Scholar]
- Vieira LR, Soares AMVM, Freitas R. Caffeine as a contaminant of concern: a review on concentrations and impacts in marine coastal systems. Chemosphere. 2022;286:131675. doi: 10.1016/j.chemosphere.2021.131675. [DOI] [PubMed] [Google Scholar]
- Virani S, Daya GN, Brainch N, Kotapati VP, Zaveri D, Ahmed S. Persistent psychosis due to single dose of ecstasy. Cureus. 2018;10:e3058. doi: 10.7759/cureus.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Smith L, Fowler JS, Telang F, Logan J, Tomasi D. Recovery of dopamine transporters with methamphetamine detoxification is not linked to changes in dopamine release. Neuroimage. 2015;121:20–28. doi: 10.1016/j.neuroimage.2015.07.035. [DOI] [PubMed] [Google Scholar]
- Vuletic D, Dupont P, Robertson F, Warwick J, Zeevaart JR, Stein DJ. Methamphetamine dependence with and without psychotic symptoms: a multi-modal brain imaging study. Neuroimage Clin. 2018;20:1157–1162. doi: 10.1016/j.nicl.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulin I, Tenji D, Teodorovic I, Kaisarevic S. Assessment of caffeine neurotoxicity using novel biomarkers of neural function in SH-SY5Y cells – Is there a need for environmental concern? Chem Biol Interact. 2022;365:110082. doi: 10.1016/j.cbi.2022.110082. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Lin HC, Kang BH, Tseng CJ, Tung CS. d-amphetamine-induced depletion of energy and dopamine in the rat striatum is attenuated by nicotinamide pretreatment. Brain Res Bull. 1999;50:167–171. doi: 10.1016/s0361-9230(99)00185-9. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Tung CS, Shiah IS, Lin HC. Effects of α-phenyl-N-tert-butyl nitrone and N-acetylcysteine on hydroxyl radical formation and dopamine depletion in the rat striatum produced by d-amphetamine. Eur Neuropsychopharmacol. 2006;16:147–153. doi: 10.1016/j.euroneuro.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhou C, Guo T, Huang P, Xu X, Zhang M. Association between cigarette smoking and Parkinson's disease: a neuroimaging study. Ther Adv Neurol Disord. 2022;15:175628642210925. doi: 10.1177/17562864221092566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyandt LL, Oster DR, Marraccini ME, Gudmundsdottir BG, Munro BA, Rathkey ES, McCallum A. Prescription stimulant medication misuse: Where are we and where do we go from here? Exp Clin Psychopharmacol. 2016;24:400–414. doi: 10.1037/pha0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79:1016–1025. doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Qi C, Long J, Liao Y, Wang X, Xie A, Liu J, Hao W, Tang Y, Yang B, Liu T, Tang J. Metabolites alterations in the medial prefrontal cortex of methamphetamine users in abstinence: a 1H MRS study. Front Psychiatry. 2018;9:478. doi: 10.3389/fpsyt.2018.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderli MD, Vonmoos M, Fürst M, Schädelin K, Kraemer T, Baumgartner MR, Seifritz E, Quednow BB. Discrete memory impairments in largely pure chronic users of MDMA. Eur Neuropsychopharmacol. 2017;27:987–999. doi: 10.1016/j.euroneuro.2017.08.425. [DOI] [PubMed] [Google Scholar]
- Xie XL, Zhou WT, Zhang KK, Chen LJ, Wang Q. METH-induced neurotoxicity is alleviated by lactulose pretreatment through suppressing oxidative stress and neuroinflammation in rat striatum. Front Neurosci. 2018;12:802. doi: 10.3389/fnins.2018.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang T, Lou X, Chen J, Wang X, Hu M, An D, Gao R, Wang J, Chen X. Role of the Peli1–RIPK1 signaling axis in methamphetamine-induced neuroinflammation. ACS Chem Neurosci. 2023;14:864–874. doi: 10.1021/acschemneuro.2c00623. [DOI] [PubMed] [Google Scholar]
- Yang CC, Jou IM. Caffeine treatment aggravates secondary degeneration after spinal cord injury. Brain Res. 20161634:75–82. doi: 10.1016/j.brainres.2015.12.053. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang Y, Cheng J, Zheng R. Meta-analysis of brain gray matter changes in chronic smokers. Eur J Radiol. 2020;132:109300. doi: 10.1016/j.ejrad.2020.109300. [DOI] [PubMed] [Google Scholar]
- Yeh SHH, Kuo YY, Huang WS, Chiu CH, Yu TH, Ii LGF, Tsai CJ, Cheng CY, Ma KH. Preliminary results on the long-term effects of dextromethorphan on MDMA-mediated serotonergic deficiency and volumetric changes in primates based on 4-[18F]-ADAM PET/MRI. Front Neurosci. 2022;16:837194. doi: 10.3389/fnins.2022.837194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, McCann U, Ricaurte G. Methylphenidate and brain dopamine neurotoxicity. Brain Res. 1997;767:172–175. doi: 10.1016/s0006-8993(97)00771-3. [DOI] [PubMed] [Google Scholar]
- Zahavi E, Lev-Shalem L, Yehoshua I, Adler L. Methylphenidate use and misuse among medical residents in Israel: a cross-sectional study. Hum Resour Health. 2023;21:5. doi: 10.1186/s12960-023-00792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang KK, Chen LJ, Li JH, Liu JL, Wang LB, Xu LL, Yang JZ, Li XW, Xie XL, Wang Q. Methamphetamine disturbs gut homeostasis and reshapes serum metabolome, inducing neurotoxicity and abnormal behaviors in mice. Front Microbiol. 2022;13:755189. doi: 10.3389/fmicb.2022.755189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McCarthy DM, Eskow Jaunarajs KL, Biederman J, Spencer TJ, Bhide PG. Frontal cortical monoamine release, attention, and working memory in a perinatal nicotine exposure mouse model following kappa opioid receptor antagonism. Cereb Cortex. 2021;31:483–496. doi: 10.1093/cercor/bhaa238. [DOI] [PubMed] [Google Scholar]
- Zhang S, Jin Y, Liu X, Yang L, Ge Z juan, Wang H, Li J, Zheng J. Methamphetamine modulates glutamatergic synaptic transmission in rat primary cultured hippocampal neurons. Brain Res. 20141582:1–11. doi: 10.1016/j.brainres.2014.07.040. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lu J, Chen X, Gao Z, Zhang C, Chen C, Qiao D, Wang H. Methamphetamine exposure induces neuronal programmed necrosis by activating the receptor‐interacting protein kinase 3 ‐related signalling pathway. FASEB J. 2021;35:e21561. doi: 10.1096/fj.202100188R. [DOI] [PubMed] [Google Scholar]
- Zhou L, Tao X, Pang G, Mu M, Sun Q, Liu F, Hu Y, Tao H, Li B, Xu K. Maternal nicotine exposure alters hippocampal microglia polarization and promotes anti-inflammatory signaling in juvenile offspring in mice. Front Pharmacol. 2021;12:661304. doi: 10.3389/fphar.2021.661304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hu Y, Wang Q, Yang Z, Li J, Ma Y, Wu Q, Chen S, Yang D, Hao Y, Wang Y, Li M, Peng P, Liu T, Yang WFZ. Association between white matter microstructure and cognitive function in patients with methamphetamine use disorder. Hum Brain Mapp. 2023;44:304–314. doi: 10.1002/hbm.26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Ge X, Gao P, Li M, Guan Y, Guan X. Adolescent cocaine exposure induces prolonged synaptic modifications in medial prefrontal cortex of adult rats. Brain Struct Funct. 2017;223:1829–1838. doi: 10.1007/s00429-017-1590-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Friedli N, Bavato F, Stämpfli P, Coray R, Baumgartner MR, Grandgirard D, Leib SL, Opitz A, Seifritz E, Stock AK, Beste C, Cole DM, Quednow BB. White matter alterations in chronic MDMA use: evidence from diffusion tensor imaging and neurofilament light chain blood levels. Neuroimage Clin. 2022;36:103191. doi: 10.1016/j.nicl.2022.103191. [DOI] [PMC free article] [PubMed] [Google Scholar]


