Abstract
Background
A combination of weekly pegylated interferon (peginterferon) alpha and daily ribavirin still represents standard treatment of chronic hepatitis C infection in the majority of patients. However, it is not established which of the two licensed peginterferon products, peginterferon alpha‐2a or peginterferon alpha‐2b, is the most effective and has a better safety profile.
Objectives
To systematically evaluate the benefits and harms of peginterferon alpha‐2a versus peginterferon alpha‐2b in head‐to‐head randomised clinical trials in patients with chronic hepatitis C.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and LILACS until October 2013. We also searched conference abstracts, journals, and grey literature.
Selection criteria
We included randomised clinical trials comparing peginterferon alpha‐2a versus peginterferon alpha‐2b given with or without co‐intervention(s) (for example, ribavirin) for chronic hepatitis C. Quasi‐randomised studies and observational studies as identified by the searches were also considered for assessment of harms. Our primary outcomes were all‐cause mortality, liver‐related morbidity, serious adverse events, adverse events leading to treatment discontinuation, other adverse events, and quality of life. The secondary outcome was sustained virological response in the blood serum.
Data collection and analysis
Two authors independently used a standardised data collection form. We meta‐analysed data with both the fixed‐effect and the random‐effects models. For each outcome we calculated the relative risk (RR) with 95% confidence interval (CI) based on intention‐to‐treat analysis. We used domains of the trials to assess the risk of systematic errors (bias) and trial sequential analyses to assess the risks of random errors (play of chance). Intervention effects on the outcomes were assessed according to GRADE.
Main results
We included 17 randomised clinical trials which compared peginterferon alpha‐2a plus ribavirin versus peginterferon alpha‐2b plus ribavirin in 5847 patients. All trials had a high risk of bias. Very few trials reported data on very few patients for the patient‐relevant outcomes all‐cause mortality, liver‐related morbidity, serious adverse events, and quality of life. Accordingly, we were unable to conduct meta‐analyses on all‐cause mortality, liver‐related morbidity, and quality of life. Twelve trials reported on adverse events leading to discontinuation of treatment without clear evidence of a difference between the two peginterferons (197/2171 (9.1%) versus 311/3169 (9.9%); RR 0.84, 95% CI 0.57 to 1.22; I2 = 44%; low quality evidence). A trial sequential analysis showed that we could exclude a relative risk reduction of 20% or more on this outcome. Peginterferon alpha‐2a significantly increased the number of patients who achieved a sustained virological response in the blood serum compared with peginterferon alpha‐2b (1069/2099 (51%) versus 1327/3075 (43%); RR 1.12, 95% CI 1.06 to 1.18; I2= 0%, 12 trials; moderate quality evidence). Trial sequential analyses supported this result. Subgroup analyses based on risk of bias, viral genotype, and treatment history yielded similar results. Trial sequential analyses supported the results in patients with genotypes 1 and 4, but not in patients with genotypes 2 and 3.
Authors' conclusions
There is lack of evidence on patient‐important outcomes and paucity of evidence on adverse events. Moderate quality evidence suggests that peginterferon alpha‐2a is associated with a higher sustained virological response in serum than with peginterferon alpha‐2b. This finding may be affected by the high risk of bias of the included studies . The clinical consequences of peginterferon alpha‐2a versus peginterferon alpha‐2b are unknown, and we cannot translate an effect on sustained virological response into comparable clinical effects because sustained virological response is still an unvalidated surrogate outcome for patient‐important outcomes. The lack of evidence on patient‐important outcomes and the paucity of evidence on adverse events means that we are unable to draw any conclusions about the effects of one peginterferon over the other.
Keywords: Humans; Antiviral Agents; Antiviral Agents/therapeutic use; Drug Administration Schedule; Drug Therapy, Combination; Drug Therapy, Combination/methods; Hepatitis C, Chronic; Hepatitis C, Chronic/drug therapy; Interferon alpha‐2; Interferon‐alpha; Interferon‐alpha/therapeutic use; Polyethylene Glycols; Polyethylene Glycols/therapeutic use; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/therapeutic use; Ribavirin; Ribavirin/therapeutic use
Plain language summary
Peginterferon alpha‐2a versus peginterferon alpha‐2b for chronic hepatitis C
Importance of the review or background on the condition
Hepatitis C is a disease of the liver caused by the hepatitis C virus. Globally, an estimated 170 million people are chronically infected with hepatitis C virus. Chronic hepatitis C can cause liver damage in the form of inflammation and scarring of the liver (cirrhosis). Liver damage can lead to liver failure and other complications, including liver cancer. The aim of the treatment for chronic hepatitis C is to prevent complications of hepatitis C infection. This might be achieved by clearing the virus from the blood of the patient. However, we still need to understand if clearance of virus from blood has any association with patient‐relevant and clinically‐relevant outcomes. A combination of weekly injections of peginterferon alpha and daily oral ribavirin still represents the standard of care for the majority of patients with chronic hepatitis C. Currently, there are two licensed products of peginterferon, peginterferon alpha‐2a and peginterferon alpha‐2b, on the market.
The main findings of the review
The review identified 17 randomised clinical trials. The trials reported on patient‐relevant outcomes only occasionally. All trials had high risk of bias ie, a trial might systematically overestimate benefits or underestimate harms of the treatments). Both treatments were associated with a high risk of experiencing adverse events, which may lead to discontinuation of the treatment. Twelve trials reported on clearing the virus from blood six months after the end of treatment. A summary of the current evidence in this review suggests that peginterferon alpha‐2a has higher chances of clearing the virus from the patient's blood than peginterferon alpha‐2b (in 50% compared with 43%).
Conclusions
We were unable to identify any evidence on the benefits of one peginterferon over the other on patient‐important outcomes.
Any limitations of the review
There is lack of data regarding patient‐important outcomes on this topic.
Summary of findings
Summary of findings for the main comparison. Peginterferon alpha‐2a versus peginterferon alpha‐2b for chronic hepatitis C.
| Peginterferon alpha‐2a versus peginterferon alpha‐2b for chronic hepatitis C | ||||||
| Patient or population: patients with chronic hepatitis C. Settings: mainly out‐patients in tertiary and teaching hospitals. Intervention: peginterferon alpha‐2a versus peginterferon alpha‐2b. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Peginterferon alpha‐2b | Peginterferon alpha‐2a | |||||
| All‐cause mortality Deaths during and after the treatment Follow‐up: 48 to 72 weeks | Study population | RR 1.97 (0.64 to 6.08) | 3070 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 3 per 1000 | 6 per 1000 (2 to 18) | |||||
| Moderate | ||||||
| 3 per 1000 | 6 per 1000 (2 to 18) | |||||
| Liver‐related morbidity Number of events Follow‐up: 8 weeks | Study population | RR 3 (0.7 to 12.93) | 36 (1 study) | ⊕⊝⊝⊝ very low2 | ||
| 111 per 1000 | 333 per 1000 (78 to 1000) | |||||
| Moderate | ||||||
| 111 per 1000 | 333 per 1000 (78 to 1000) | |||||
| Serious adverse events Number of events Follow‐up: 48 to 72 weeks | Study population | RR 1.12 (0.95 to 1.3) | 3900 (4 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 114 per 1000 | 127 per 1000 (108 to 148) | |||||
| Moderate | ||||||
| 70 per 1000 | 78 per 1000 (66 to 91) | |||||
| Adverse events leading to treatment discontinuation Number of events Follow‐up: 48‐72 weeks | Study population | RR 0.84 (0.57 to 1.22) | 5340 (12 studies) | ⊕⊕⊝⊝ low1,4,5,6 | ||
| 99 per 1000 | 83 per 1000 (56 to 120) | |||||
| Moderate | ||||||
| 80 per 1000 | 67 per 1000 (46 to 98) | |||||
| All other (non‐serious) adverse events Follow‐up: 48 to 72 weeks | See comment | See comment | Not estimable | 4981 (9 studies) | ⊕⊝⊝⊝ very low4,5,6 | |
| Quality of life SF 36 and CLDQ Follow‐up: 48 to 71 weeks | See comment | See comment | 434 (1 study) | ⊕⊝⊝⊝ very low7,8 | ||
| Sustained virological response Absence of viraemia 24 weeks after the treatment Follow‐up: 48 to 72 weeks | Study population | RR 1.12 (1.06 to 1.18) | 5013 (12 studies) | ⊕⊕⊕⊝9,10 moderate | ||
| 421 per 1000 | 480 per 1000 (451 to 510) | |||||
| Moderate | ||||||
| 510 per 1000 | 581 per 1000 (546 to 617) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The trial is at low risk of bias due to the allocation sequence generation and allocation concealment. 2Data from only one trial, wide confidence interval. Incomplete outcome data. Very low due to imprecision. 3Post hoc required information size calculation based on a 10% risk of adverse events in the peginterferon alpha‐2b group, a minimally important difference of 10%, a 5% type I error, and a 80% power, suggests that a minimum of 27,000 patients need to be randomised for a conclusive meta‐analysis on adverse events. The current number of patients is only approximately 5000. 4Wide confidence interval. Low due to imprecision. 5Trials yield widely differing estimates of effect. Low due to imprecision. 6 Reporting of all other adverse events was poor and inconsistent across all included trials. The proportions of observed adverse events differ substantially across the trials, and the direction of effect is heterogeneous. Because the event proportion is relatively low across all trials, all of the included trials may be subject to considerable random errors, thus explaining the apparent heterogeneity in direction of estimates. 7 Data from only one trial. Low due to imprecision 8 Investigators fail to report the details necessary for calculating the effect estimate of the quality of life assessment. Very low due to imprecision. 9Sustained virological response does not seem to be a valid surrogate marker for assessing HCV treatment efficacy of interferon retreatment. Moderate quality of evidence due to indirectness due to surrogate and risk of bias. 10All trials are with high risk of bias. Sensitivity analyses did not show any important change in the intervention effects when we focused on trials with lower risk of bias.
Background
Description of the condition
Globally, an estimated 170 million people are chronically infected with hepatitis C virus, and three to four million people are newly infected each year (WHO 1999). In the majority of patients, acute hepatitis C infection is asymptomatic. Hepatitis C infection is generally recognised in the chronic phase (Hodgson 2003). Around 85% of patients who become infected with hepatitis C fail to clear the virus and become chronic carriers of hepatitis C virus. Among these individuals, 5% to 20% are reported to develop cirrhosis over a period of approximately 20 to 25 years (Seeff 2002; Seef 2009). Patients with advanced fibrosis or cirrhosis develop liver complications such as hepatocellular carcinoma with an annual proportion of 2% to 4% (Benvegnu 2001; Fattovich 2002). Furthermore, chronic hepatitis C is the single most common indication for liver transplantation (OPTN 2008).
Hepatitis C virus is an enveloped RNA virus that constitutes the genus Hepacivirus within the Flaviviridae family (van Regenmorte 2000; Penin 2004). Hepatitis C virus is divided into six genotypes, which differ from each other by up to 30% in the nucleotide sequence, and has a large and growing number of subtypes (Rosenberg 2001). Hepatitis C virus genotypes differ with geographic region (Davis 1999). Although a genotype does not predict the outcome of the infection, it does predict the likelihood of virologic treatment response and, in many cases, determines the duration of treatment (Manns 2001; Fried 2002; Hadziyannis 2004).
Description of the intervention
The aim of treatment of chronic hepatitis C is to prevent complications of the hepatitis C infection. This is principally sought by eradication of the infection in the serum (Ghany 2009). Accordingly, treatment is aimed to achieve a virological response, defined as the absence of hepatitis C virus RNA in the blood serum, measured by a sensitive test six months after the end of treatment (that is, a sustained virological response). Monotherapy with interferon produces a sustained virological response in less than 20% of patients (Myers 2002). The introduction of combination therapy with interferon plus ribavirin was considered a major advance due to a greater effect on the sustained virological response in the blood (Brok 2005). The next improvement in chronic hepatitis C treatment was the development of direct‐acting antiviral (DAA) agents, boceprevir (BOC) and teleprevir (TVR). The two DAAs have demonstrated significant inhibition of hepatitis C virus (HCV) genotype 1 replication and markedly improved the sustained virological response rate (Ghany 2011). Unfortunately due to the high price of such a triple regimen it is still not affordable to patients in many countries. Furthermore, we do not have sufficient data about the efficacy of BOC and TVR in the treatment of HCV genotype 2 and 3 infections, which leaves pegylated interferon (peginterferon) plus ribavirin as the major treatment option for the majority of patients. Combination therapy with interferon and ribavirin produces a sustained virological response in approximately 40% of previously untreated patients (Brok 2005). The majority of randomised clinical trials primarily assess sustained virological response, which is a surrogate marker, instead of outcomes which might be of more interest for patients and clinicians such as all‐cause mortality, liver‐related morbidity, and progression to hepatocellular carcinoma (HCC) (Gluud 2007). Recently, some groups have published results of trials with longer duration of follow‐up of patients with advanced hepatitis C who achieved a sustained virological response (Fernandez‐ Rodriguez 2010; Morgan 2010; Di Biscceglie 2011). The authors reported marked reductions in liver‐related morbidity, no significant difference in liver‐related mortality, and a significantly higher mortality in those treated with long‐term peginterferon (Di Biscceglie 2011). Furthermore, we still lack data about adverse events, quality of life during the treatment, all‐cause morbidity and mortality due to suicide, anaemia, or infections. Therefore, sustained virological response should still be considered as a non‐validated surrogate outcome, that is, an outcome which should not be used to guide clinical decision making (Gluud 2007; Koretz 2013; Gurusamy 2014). A combination of weekly subcutaneous injections of long‐acting peginterferon alpha and oral ribavirin has achieved the highest overall sustained virological response rates of 56% (Ghany 2009). This still represents the standard of care for the majority of patients according to The American Association for the Study of Liver Diseases and European Association for the Study of Liver Disease guidelines (Ghany 2009; Ghany 2011; EASL 2012). However, approximately 75% of those treated with either peginterferon alpha or interferon alpha experience one or more adverse events (for example, influenza‐like symptoms, depression, neutropenia, thrombocytopenia, etc.) (Ghany 2009). Pegylation involves the addition of polyethylene glycol molecules to the interferon molecule, thus decreasing renal clearance, altering metabolism, and increasing the half life of the peginterferon molecule in the circulation. This necessitates fewer doses (Reddy 2001). Currently, there are two licensed products of peginterferon, peginterferon alpha‐2a (Pegasys®, Hoffmann‐La Roche), which consists of a 40 kDa branched pegylated chain linked to the interferon molecule (Bailon 2001), and peginterferon alpha‐2b (Peg‐Intron®, Schering‐Plough Corporation) consisting of a 12 kDa linear pegylated chain linked to the interferon molecule (Glue 2000; Foster 2004). In particular, pegylation reduces the rapid kidney clearance of a given protein by increasing its hydrodynamic volume, prevents immunogenicity by acting at different levels, reduces protein aggregation owing to a repulsion between pegylated surfaces, and increases the thermal stability of proteins (Pasut 2011). The pegylate and its conjugates are mainly excreted by kidney clearance and the excretion rate is significantly reduced for molecular weights over 40 kDa (Pasut 2011). The different mechanisms of the pegylated interferon induced different pharmacokinetic and pharmacodynamic properties include that peginterferon alpha‐2a has a higher molecular weight (40 kDa versus 12 kDa), a longer half‐life, a lower body distribution volume, and different routes of elimination because peginterferon alpha‐2a is mainly eliminated by the liver while peginterferon alpha‐2b is mainly eliminated by the kidney (Glue 2000; Bailon 2001; Foster 2004).
Why it is important to do this review
Lately, there has been considerable controversy over which treatment option of peginterferon is the most effective one (McHutchison 2009; Lee 2010; Kamal 2011; Miyase 2012). A large randomised clinical trial has recently concluded that the two peginterferons are comparable in both benefits and harms (McHutchison 2009) but the majority of other trials, although with smaller numbers of patients, conclude that there are significant differences between the two peginterferons (Kamal 2011; Mach 2011; Miyase 2012). However, findings from a single randomised trial, even a very large one, are rarely definitive and caution should be taken to ensure reproducibility of the findings (Lau 1995; Lacchetti 2002; Trikalinos 2004; Ioannidis 2005; Ioannidis 2005a; Thorlund 2009). Systematic reviews and meta‐analyses including all available trials are considered the highest level of evidence as they provide valuable information on the quality of the available evidence and provide the greatest statistical strength. Hence, the risks of systematic errors as well as random errors are smaller in systematic reviews than in single trials. We have, therefore, conducted a Cochrane Hepato‐Biliary Group systematic review to identify, assess, and analyse all randomised trials to add to the existing body of evidence and strengthen inferences about which peginterferon would work best with fewer possible harms to the patient. A previous version of this review that was published in Hepatology suggested that peginterferon alpha‐2a leads to a significantly higher proportion of patients with sustained virological response than with peginterferon alpha‐2b (Awad 2010), while the safety profile remained comparable. The present review provides an update with improved methodology and includes three more randomised clinical trials. There are several meta‐analyses that have been published recently which compare the efficacy and safety of the two pegylated interferons (Alavian 2010; Barros 2010c; Cheinquer 2010; Zhao 2010; Coppola 2011; Singal 2011; Druyits 2012; Romero‐Gomez 2012; Flori 2013; Yang 2013). Four of them have been published as abstracts only (Barros 2010c; Cheinquer 2010; Coppola 2011; Romero‐Gomez 2012) and despite the different numbers of included studies and different outcomes observed, their common conclusion is that pegylated interferon alpha‐2a has advances in terms of efficacy over the pegylated interferon alpha‐2b. Six meta‐analyses that were published as full papers uniformly report superior efficacy of pegylated interferon alpha‐2a over pegylated interferon alpha‐2b while the safety profile remains comparable for both treatments (Alavian 2010; Zhao 2010; Singal 2011; Druyits 2012; Flori 2013; Yang 2013). One meta‐analysis (Druyits 2012) is not comparable to ours because it has a different search strategy and inclusion criteria. The author did not assess the risk of bias and included studies which were not head‐to‐head comparisons. Four meta‐analyses (Alavian 2010; Zhao 2010; Singal 2011; Yang 2013) have similar search strategies and outcomes as in Awad 2010, but they included fewer trials and smaller numbers of patients. They included seven trials with 3518 patients (Alavian 2010); seven trials with 3212 patients (Zhao 2010); nine trials with 3546 patients (Singal 2011); and seven trials with 3668 patients (Yang 2013). They excluded conference abstracts or limited participants only to patients naive to previous antiviral intervention. However, results of those meta‐analyses are in concordance with our conclusions on the higher efficacy of pegylated interferon alpha‐2a and similar safety profile among the pegylated interferons (Awad 2010).
Objectives
To systematically evaluate the benefits and harms of peginterferon alpha‐2a versus peginterferon alpha‐2b for patients with chronic hepatitis C.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials irrespective of language, publication status, or year of publication for assessment of benefits and harms. We also included for assessment of harms quasi‐randomised studies and observational studies that were identified during our searches for randomised trials.
Types of participants
Patients with chronic hepatitis C were included. Patients could have been treatment naive (not previously treated with antivirals), relapsers (patients with a transient response to previous antiviral treatment), or non‐responders (patients without a response to previous antiviral treatment). We also included patients with comorbidities such as liver cirrhosis and human immunodeficiency virus (HIV) co‐infection. Patients who had undergone liver transplantation or were positive for chronic hepatitis B infection were excluded.
Types of interventions
Peginterferon alpha‐2a compared with peginterferon alpha‐2b given with or without co‐intervention(s) (for example, ribavirin, telaprevir) regardless of the dose or the duration of the interventions. Co‐interventions were permitted if received equally by all intervention groups and applied equally.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Liver‐related morbidity: number of patients who developed ascites, variceal bleeding, progression of bilirubinaemia, hepatic encephalopathy, or hepatocellular carcinoma.
Adverse events: serious adverse events, adverse events leading to treatment discontinuation, and all other (non‐serious) adverse events. The number and type of adverse events are defined as patients with any untoward medical occurrence not necessarily having a causal relationship with the treatment. We defined serious adverse events according to the International Conference on Harmonisation (ICH) Guidelines (ICH‐GCP 1997) as "any event that leads to death, is life‐threatening, requires in‐patient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability, and any important medical event, which may jeopardise the patient or requires intervention to prevent it". All other adverse events were considered non‐serious.
Quality of life as defined in the individual trials.
Secondary outcomes
Sustained virological response: number of patients with undetectable hepatitis C virus RNA in their serum by a sensitive test six months after the end of treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2013), Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded (Royle 2003), and LILACS using the search strategies and time spans given in Appendix 1. The last search was conducted in October 2013.
Searching other resources
We identified further trials by searching national and topic‐specific databases, bibliographies, conference abstracts, journals, and the grey literature. Furthermore, we reviewed the reference lists of the other meta‐analyses and the included studies and contacted the principal authors of the identified trials if needed.
Data collection and analysis
We performed the review and meta‐analyses following the recommendations of The Cochrane Collaboration (Higgins 2011) and the Cochrane Hepato‐Biliary Group (Gluud 2013). The analyses were performed using Review Manager 5.1 (RevMan 2012) and Trial Sequential Analysis (TSA) version 0.9 (CTU 2011; Thorlund 2011).
Selection of studies
Two authors (GH and TA) independently screened titles and abstracts for potential eligibility and the full‐texts for final eligibility. Disagreements were resolved by discussion and arbitrated with a third author (CG).
Data extraction and management
Two authors (GH and TA) independently extracted data using a standardised data collection form to record trial design and methodological characteristics, patient characteristics, interventions, outcomes, and missing outcome data. Authors of included trials were contacted for additional information that was not described in the published reports. Disagreements were resolved by discussion and arbitration with a third author (CG). Any further information required from the original authors was requested by written correspondence and any relevant information obtained in this manner was included in the review.
Assessment of risk of bias in included studies
Trials with adequate generation of the allocation sequence, adequate allocation concealment, adequate blinding, adequate outcome data reporting, no selective outcome reporting, and without vested interests were considered as trials with low risk of bias (high methodological quality) (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012; Savovic 2012a). Trials with one or more unclear or inadequate quality component were considered as trials with high risk of bias (low methodological quality) (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savovic 2012; Savovic 2012a). The methodological quality of the trials, hence risk of bias, was assessed based on the following domains.
Sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent person not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (for example, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to induce bias on the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes were likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to introduce bias in the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: all outcomes were predefined and reported, or all clinically relevant and reasonably expected outcomes were reported.
Uncertain risk of bias: it is unclear whether all predefined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
Other sources of bias
Low risk of bias: the trial appears to be free of other components (for example, academic bias) that could put it at risk of bias.
Uncertain risk of bias: the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias: there are other factors in the trial that could put it at risk of bias (for example, authors have conducted trials on the same topic, etc).
All the above bias risk domains were assessed independently by two authors (GH and TA). Disagreements were resolved by discussion and arbitrated by a third author (CG). To minimise bias in our findings and recommendations, we used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) summary of findings (SoF) table for outcomes to rate the available evidence (Guyatt 2008).
Measures of treatment effect
Dichotomous data were expressed as risk ratios (RR) with 95% confidence intervals (CI). Furthermore, the number needed to treat (NNT) was derived from the risk differences (RD) in meta‐analyses where the 95% confidence interval did not include zero. Rare events (morbidity and mortality) were estimated using the odds ratios as a measure of association.
Dealing with missing data
We planned to perform all analyses according to the intention‐to‐treat method, including all participants irrespective of compliance or follow‐up. However, we performed analyses according to the intention‐to‐treat method only for dichotomous outcomes. For continuous outcomes we performed available case analysis and included data only on those whose results were known. Regarding the primary outcome measures we planned to include patients with incomplete or missing data in the sensitivity analyses by imputing them according to the two scenarios below (Hollis 1999; Gluud 2013).
'Best‐worst' case scenario analyses: participants with missing outcome data are considered successes in the experimental group and failures in the control group. The denominator will include all the participants in the trial.
'Worst‐best' case scenario analyses: participants with missing outcome data are considered failures in the experimental group and successes in the control group. The denominator will include all the participants in the trial.
For trials with missing data we assessed the adequacy of the methods used to deal with missing data. When patients were lost to follow‐up and missing data methods were not applied, data were analysed according to the intention‐to‐treat principle. The intention‐to‐treat analysis was performed assuming poor outcome in both groups where dropouts were considered as failures and the total number of patients was used as denominator.
Assessment of heterogeneity
Heterogeneity was explored by the Chi2 test, and the quantity of heterogeneity was measured by the I2 statistic (Higgins 2002;Higgins 2011). Sources of heterogeneity were assessed with subgroup analysis and meta‐regression whenever possible. Subgroup analyses were only carried out when data from at least two trials were available for each subgroup. Meta‐regression was only carried out for meta‐analyses including more than 10 trials. Sensitivity analyses were identified during the review process.
Assessment of reporting biases
Different types of reporting biases (for example, publication bias, time lag bias, outcome reporting bias, etc.) were handled following the recommendations of The Cochrane Collaboration (Higgins 2011). For continuous outcomes with intervention effects measured as mean difference, the test proposed by Egger 1997 was planned to be used to test for funnel plot asymmetry. For dichotomous outcomes with intervention effects measured as odds ratios, the arcsine test proposed by Rücker 2008 was planned to be used to test for funnel plot asymmetry. Due to sufficient trials (Higgins 2011) included in the meta‐analyses, we could perform the test for funnel plot asymmetry for two outcomes, namely adverse events leading to treatment discontinuation and sustained virological response.
Data synthesis
For all analyses, we used both random‐effects (DerSimonian 1986) and fixed‐effect model (DeMets 1987) analyses. Due to the underlying assumptive differences, results from the random‐effects model and the fixed‐effect model may differ to an extent that cannot be ignored. In case such discrepancies were observed, results were interpreted according to the implications of the subgroup and heterogeneity analyses, and according to the confidence intervals of the two models.
Assessment of risks of random errors (play of chance)
Random errors may play an important role in the evaluation of meta‐analyses due to sparse data and multiplicity from repetitive testing. To assess the reliability of inferences from our meta‐analysis on sustained virological response, we calculated the required information size which is the required sample size for the meta‐analysis to detect a 10% relative risk reduction in sustained virological response. We assumed an average event proportion of 50% in the control group, assuming that 30% of the variation in the meta‐analysis would be explained by variation across trials, and used statistical error levels of alpha = 5% and beta = 10% (90% power) or beta = 20% (80% power). Meta‐analyses conducted before surpassing the required sample size are considered analogous to interim analyses in a single randomised trial, and thus they necessitate adjustment of the threshold for statistical significance to maintain the predetermined maximum risk of obtaining a false positive result (set to alpha = 5% in our analysis). We, therefore, substituted the conventional 5% threshold for statistical significance with those of the Lan‐DeMets trial sequential monitoring boundaries (Bangalore 2008; Brok 2008; Rambaldi 2008; Wetterslev 2008; Brok 2009; Thorlund 2009). We used trial sequential analysis (CTU 2011; Thorlund 2011). On the basis of the required information size and risk for type I (5%) and type II (10% or 20%) errors, trial sequential monitoring boundaries were constructed (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size. If a trial sequential monitoring boundary is crossed by the cumulative Z‐score before the required information size is reached in a cumulative meta‐analysis, firm evidence may have been established and further trials may be superfluous. On the other hand, if the boundaries are not surpassed, it is most probably necessary to continue doing further trials in order to detect or reject a certain intervention effect. We used as the default a type I error of 5%, type II error of 10% or 20%, and adjusted the information size for heterogeneity within diversity unless otherwise stated (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010).
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were considered and performed when possible.
Risk of bias: trials that were assessed to be at low risk of bias compared to trials at high risk of bias.
Risk of detection bias: trials with blinded outcome assessment compared to trials without blinded outcome assessment.
Participants: trials with treatment‐naive patients compared to trials with relapsers or non‐responders.
Genotype: trials with patients infected with different hepatitis C virus genotypes were compared.
Co‐infections and comorbidities: patients with HIV, haemolytic disease, etc. compared to patients without any of these.
Sensitivity analysis
Suitable sensitivity analyses were identified during the review process. We did not plan specific sensitivity analyses but screened our results to examine if suitable sensitivity analyses could examine the robustness of our results. We conducted a sensitivity analysis excluding trials that included patients with HIV.
Results
Description of studies
Results of the search
We identified a total of 6638 references through electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register and Cochrane Central Register of Controlled Trials inThe Cochrane Library (n = 1663), MEDLINE (n = 1087), EMBASE (n = 2070), Science Citation Index Expanded (n = 1794), LILACS (n = 24), and in the reference lists of other meta‐analyses (n = 1) until October 2013. After removing 1906 duplicates, limiting the search to humans, the number of references in the final list was 4732. Reading the titles and abstracts of the remaining references we excluded clearly irrelevant references and, accordingly, 39 references were retrieved for further assessment. Twelve publications were excluded due to irrelevant outcome measures (for example, cost effectiveness analysis) or being a review article or a retrospective, non‐randomised study. Twenty‐seven publications describing 17 trials were eligible for inclusion in our meta‐analysis.
Included studies
Seventeen trials, published in 27 publications, fulfilled our inclusion criteria and included a total number of 5847 patients (Bruno 2004; Sinha 2004; Berak 2005; Silva 2006; Sporea 2006; Yenice 2006; Di Bisceglie 2007; Kolakowska 2008; Scotto 2008; Laguno 2009; McHutchison 2009;Ascione 2010; Rumi 2010; Kamal 2011; Mach 2011; Marcellin 2011; Miyase 2012). All trials compared peginterferon alpha‐2a (180 µg/week) versus peginterferon alpha‐2b (1.0 to 1.5 µg/kg/week). All trials administered ribavirin as a co‐intervention to both peginterferon groups. The dose of ribavirin was according to the weight of the patient, ranging from 800 mg to 1400 mg. One trial included telaprevir as a co‐intervention to both peginterferon groups (Marcellin 2011). The hepatitis C genotype of the included patients varied among the trials. Eleven trials included patients with no previous chronic hepatitis C treatment (naive patients) (Sinha 2004; Kolakowska 2008; Laguno 2009; McHutchison 2009; Ascione 2010; Rumi 2010; Kamal 2011; Mach 2011; Marcellin 2011; Miyase 2012) and two trials included non‐responders (Scotto 2008;Kamal 2011). Three trials included patients with a clear or unclear history of previous hepatitis C treatment (Berak 2005; Scotto 2008; Kamal 2011). One trial included patients with HIV co‐infection (Laguno 2009). Three trials were published in an abstract form only (Sinha 2004; Berak 2005; Kolakowska 2008).
Excluded studies
Fourteen publications were excluded for the reasons shown in the table 'Characteristics of excluded studies' (Excluded studies).
Risk of bias in included studies
All trials had one or more domains with high risk of bias. Accordingly, all information in our review originated from trials that were assessed as trials with high risk of bias (Figure 1; Figure 2). Eleven trials had sequence generation and nine had allocation concealment with low risk of bias. Blinding of the outcome assessors, however, was not clear in most of the trials. We considered this bias less important for the outcome sustained virological response. Two trials without risk of blinding bias reported results regarding sustained virological response (Kamal 2011; Miyase 2012). Incomplete outcome data were adequately addressed in 10 trials. It was difficult to assess selective outcome reporting due to the unavailability of the trial protocols. Most trials did not report on the primary outcomes of our review. Five trials had funding with possible conflict of interest (Bruno 2004; Silva 2006; Di Bisceglie 2007; McHutchison 2009; Marcellin 2011).
1.
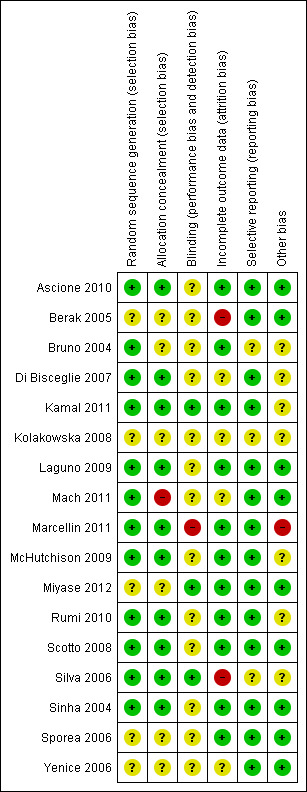
Methodological quality summary: review authors' judgements about each methodological quality item for each included trial.
2.
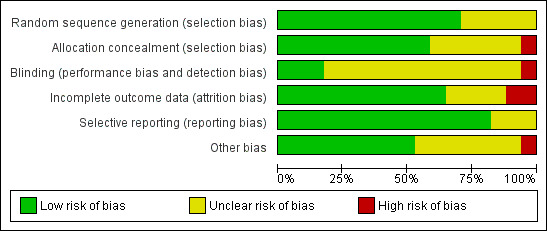
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
Effects of interventions
See: Table 1
All‐cause mortality
Only one trial reported all‐cause mortality (McHutchison 2009). The authors reported 12 patients who died during the treatment or follow‐up period. Six patients died in the peginterferon alpha‐2a group and six patients died in the peginterferon alpha‐2b group (RR 1.97, 95% CI 0.64 to 6.08) (Analysis 1.1). Only two deaths were considered by the authors to be possibly related to the intervention drug: one person, who was treated with peginterferon alpha‐2b, committed suicide six months after the end of treatment; and another, who was treated with peginterferon alpha‐2a, died due to myocardial infarction. In order to detect or reject a RR reduction of 20%, the calculated required information size was n = 132,938 patients. Accordingly, we had less than 1% of the required information size, and we could not make any conclusions about the potential similarities or differences regarding the effects of the two peginterferons on all‐cause mortality.
1.1. Analysis.
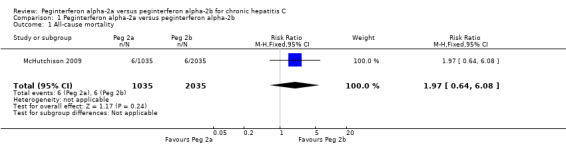
Comparison 1 Peginterferon alpha‐2a versus peginterferon alpha‐2b, Outcome 1 All‐cause mortality.
Liver‐related morbidity
One trial with 36 patients reported on liver‐related morbidity (hyperbilirubinaemia) (Silva 2006). Six patients in the peginterferon alpha‐2a group and two patients in the peginterferon alpha‐2b group had hyperbilirubinaemia (RR 3.00, 95% CI 0.70 to12.93) (Analysis 1.2). In order to detect or reject a RR reduction of 20%, the required information size should be at least n = 1480 patients. This was far above the number we had in the included trials, and we could not make any firm conclusions about potential similarities or differences regarding the effects of the two peginterferons on liver‐related morbidity.
1.2. Analysis.
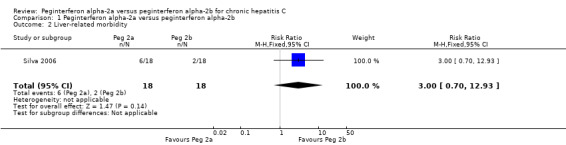
Comparison 1 Peginterferon alpha‐2a versus peginterferon alpha‐2b, Outcome 2 Liver‐related morbidity.
Adverse events
Serious adverse events
The authors of four trials reported serious adverse events according to the International Conference on Harmonisation (ICH) Guidelines (ICH‐GCP 1997) (Laguno 2009; McHutchison 2009; Rumi 2010; Kamal 2011). The proportion of patients with serious adverse events, in four trials, was quite low. In the trial which included HIV co‐infected patients, adverse events occurred in 55% of the patients (Laguno 2009). The meta‐analysis yielded a RR of 1.12 (95% CI 0.95 to 1.30) using the fixed ‐effect model (Analysis 1.3). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.78 and the heterogeneity was I2 = 0%. Because our meta‐analysis did not reach the required information size (n = 6115), we used trial sequential monitoring boundaries, calculated with TSA, to adjust the thresholds for statistical significance accordingly. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) did not cross the adjusted threshold for statistical significance, thus yielding a non‐significant difference between the two peginterferons regarding serious adverse events (Figure 3).
1.3. Analysis.
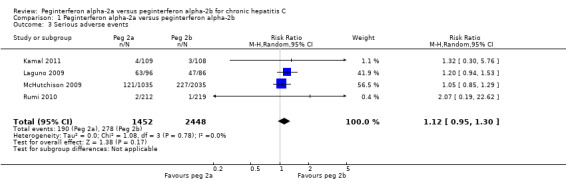
Comparison 1 Peginterferon alpha‐2a versus peginterferon alpha‐2b, Outcome 3 Serious adverse events.
3.
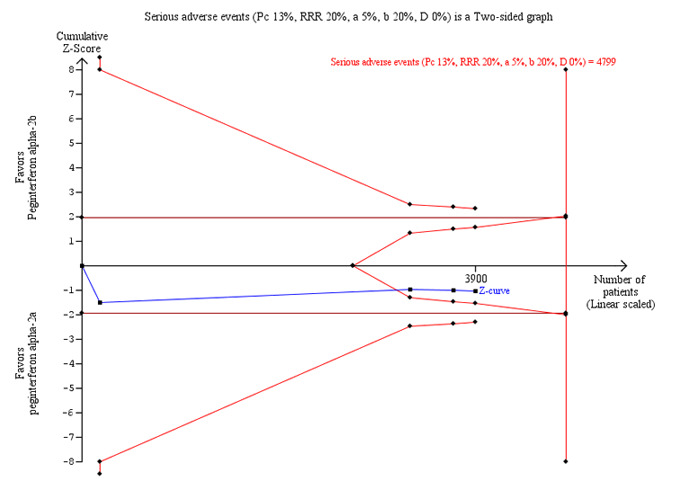
Trial sequential analysis (TSA): peginterferon alpha‐2a versus peginterferon alpha‐2b on the outcome serious adverse events. The diversity‐adjusted required information size of n = 4799 patients was calculated based upon a proportion of 13.0% of patients with serious adverse events in the peginterferon alpha‐2b group, a relative risk reduction of 20% in peginterferon alpha‐2a group, an alpha (type I error) of 5%, a beta (type II error) of 20%, and a diversity (D) of 0%. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The cumulative Z‐score reaches the area of futility delineated by the two trial sequential monitoring boundaries.
Adverse events leading to treatment discontinuation
The meta‐analysis of adverse events leading to treatment discontinuation, using data from 12 trials, yielded a RR of 0.84 (95% CI 0.57 to 1.22) using the random‐effects model (Analysis 1.4) (Bruno 2004; Sinha 2004; Berak 2005; Silva 2006; Yenice 2006; Di Bisceglie 2007; Scotto 2008; Laguno 2009; McHutchison 2009; Ascione 2010; Rumi 2010; Kamal 2011; Miyase 2012). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.05 and the heterogeneity was I2 = 44%. Due to funnel plot asymmetry we could not exclude possible bias (Figure 4).
1.4. Analysis.
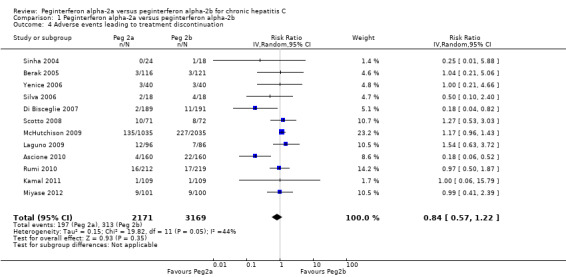
Comparison 1 Peginterferon alpha‐2a versus peginterferon alpha‐2b, Outcome 4 Adverse events leading to treatment discontinuation.
4.
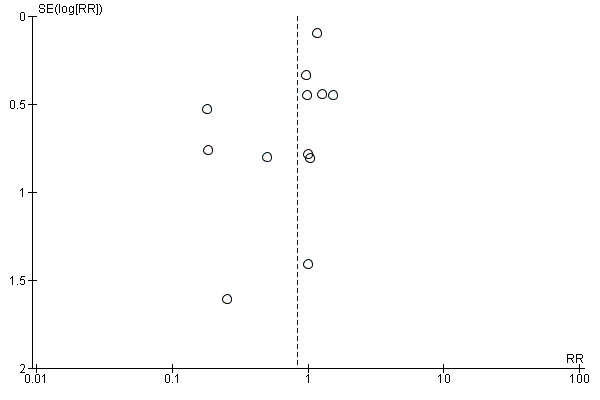
Funnel plot of comparison: 1 Peginterferon alpha‐2a versus peginterferon alpha‐2b, outcome: 1.4. Adverse events leading to treatment discontinuation.
Because our meta‐analysis did not reach the required information size (n = 12,382), we used trial sequential monitoring boundaries calculated with TSA to adjust the thresholds for statistical significance accordingly. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) did not cross the adjusted threshold for statistical significance. The Z‐score crossed the trial sequential monitoring boundary for futility. Accordingly, we could reject a 20% difference in causation of adverse events leading to discontinuation of the treatment between the two peginterferons (Figure 5).
5.
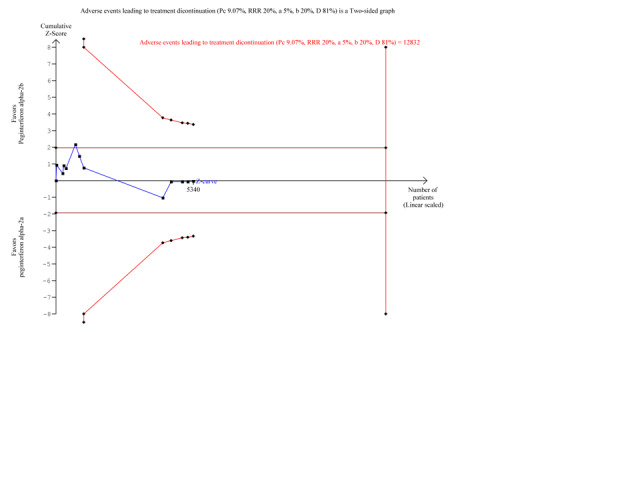
Trial sequential analysis (TSA): peginterferon alpha‐2a versus peginterferon alpha‐2b on the outcome adverse events leading to treatment discontinuation. The diversity‐adjusted required information size of n = 12,832 patients was calculated based upon a proportion of 9.0% of patients with treatment discontinuation in the peginterferon alpha‐2b group, a relative risk reduction of 20% in peginterferon alpha‐2a group, an alpha (type I error) of 5%, a beta (type II error) of 20%, and a diversity (D) of 81%. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The cumulative Z‐score does not cross any of the monitoring boundaries and does not reach the area of futility delineated by the two trial sequential monitoring boundaries which are not even drawn by the program due to the fact that the distance between the acquired and the required information size is too large.
Other (non‐serious) adverse events
In nine included trials, the authors reported on numerous adverse events not leading to treatment discontinuation (Silva 2006; Di Bisceglie 2007; Scotto 2008; Laguno 2009; McHutchison 2009; Ascione 2010; Rumi 2010; Kamal 2011; Miyase 2012) but which surely could have influenced adherence to the treatment protocol (Laguno 2009). Adverse events included haematological changes (for example, neutropenia, thrombocytopenia, and anaemia), psychological (for example, depression), and other systemic adverse events (for example, fatigue, headache, insomnia, fever, nausea, and dyspnoea). However, the reporting of adverse events not leading to treatment discontinuation was poor and inconsistent across all included trials and prevented any statistical analysis.
Assessing quality of life
Although quality of life is a very important outcome for the patients, it was rarely reported in the randomised clinical trials. Only one trial assessed quality of life in both treatment groups (peginterferon alpha‐2a versus peginterferon alpha‐2b) during and after the treatment (Kamal 2011). Using the Short Form 36 and Chronic Liver Disease Questionnaires (CLDQ), Kamal et al concluded that quality of life after the treatment was significantly better in the peginterferon alpha‐2a group than in the peginterferon alpha‐2b group regarding physical functioning, vitality, emotional role, bodily pain, and almost all domains of the CLDQ, overall score 5.9 versus 5.5 (P = 0.01) (peginterferon alpha‐2a versus peginterferon alpha‐2b).
Sustained virological response
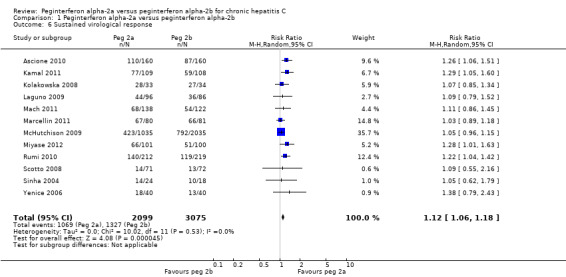
The meta‐analysis using intention‐to‐treat analysis for sustained virological response included 12 trials assessing 5013 patients (Sinha 2004; Yenice 2006; Kolakowska 2008; Scotto 2008; Laguno 2009; McHutchison 2009; Ascione 2010; Rumi 2010; Kamal 2011; Mach 2011; Marcellin 2011; Miyase 2012) and yielded an estimated effect in favour of peginterferon alpha‐2a in the random‐effects model (RR 1.12, 95% CI 1.06 to 1.18) (Analysis 1.6). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.53 and the heterogeneity was I2 = 0%. The number needed to treat (NNT) to obtain an extra patient with a sustained virological response was estimated to be 25 patients (95% CI 14 to 100 patients).
1.6. Analysis.
Comparison 1 Peginterferon alpha‐2a versus peginterferon alpha‐2b, Outcome 6 Sustained virological response.
A funnel plot of the included trials showed no significant asymmetry (Figure 6). For the outcome sustained virological response we estimated that the meta‐analysis needed to include a total of 5471 patients in order to detect or reject a RR reduction of 10%. We used trial sequential monitoring boundaries to assess statistical significance. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) crossed the trial sequential monitoring boundary, thus yielding a robust statistically significant difference between the two peginterferons regarding sparse data and repetitive testing (Figure 7).
6.
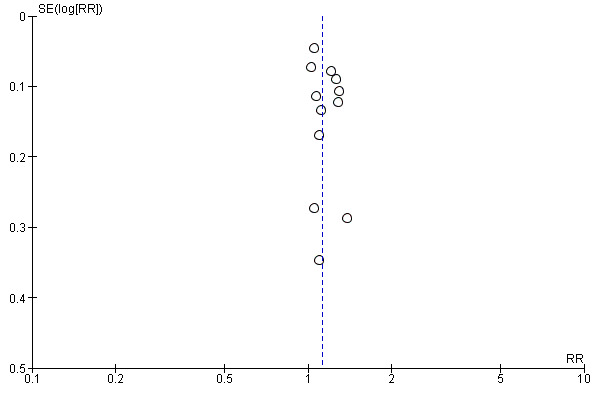
Funnel plot of comparison: Peginterferon alpha‐2a versus peginterferon alpha‐2b, outcome: 1.8 Sustained virological response.
7.
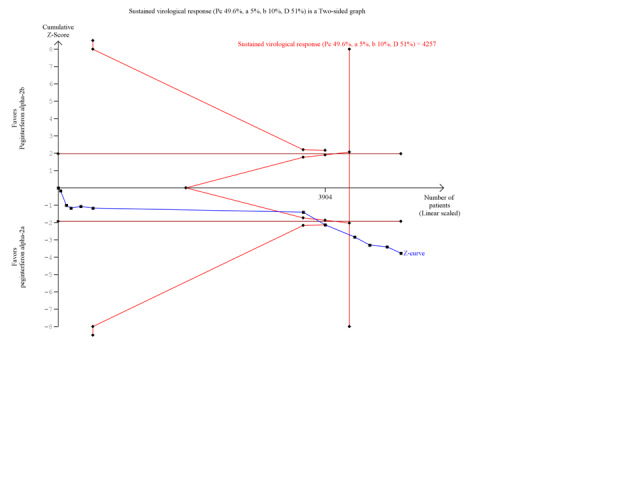
Trial sequential analysis (TSA): peginterferon alpha‐2a versus peginterferon alpha‐2b on the outcome sustained virological response. The diversity‐adjusted required information size of n = 4257 patients was calculated based upon a proportion of 49.6 % of patients with sustained virological response in the peginterferon alpha‐2b group, a relative risk reduction of a 10% in peginterferon alpha‐2a group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity (D) of 51%. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the adjusted threshold for statistical significance according to the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The cumulative Z‐score reaches the area of futility (delineated by the two trial sequential monitoring boundaries), but then it crosses both the conventional significance boundary (two tailed P = 0.05) and the trial sequential monitoring boundary.
Subgroup and sensitivity analyses
Data from 10 trials of patients infected with hepatitis C genotype 1 and genotype 4 (Yenice 2006; Scotto 2008; Laguno 2009; McHutchison 2009; Ascione 2010; Rumi 2010; Kamal 2011; Mach 2011; Marcellin 2011; Miyase 2012) yielded a RR in favour of peginterferon alpha‐2a on the outcome sustained virological response (RR 1.15, 95% CI 1.06 to 1.26) using the random‐effects model (Analysis 2.1). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.0004 and the heterogeneity was I2 = 23% (Analysis 2.1). For sustained virological response in the genotype 1 and 4 infected patients, we estimated that for the required information size a meta‐analysis needed to include a total of n = 6375 patients to detect or reject a RR reduction of 10%. Because our present meta‐analysis did not reach the required information size, we used trial sequential monitoring boundaries to assess the risk of random error. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) crossed the monitoring boundary, thus yielding a robust statistically significant difference between the two peginterferons (Figure 8). Data from five trials of patients infected with hepatitis C genotype 2 and genotype 3 (Kolakowska 2008; Scotto 2008; Laguno 2009; Ascione 2010; Rumi 2010) yielded a RR in favour of peginterferon alpha‐2a on the outcome sustained virological response (RR 1.11, 95% CI 1.02 to 1.22) using the random‐effects model (Analysis 2.1). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.89 and the heterogeneity was I2 = 0% (Analysis 2.1). For sustained virological response in the genotype 2 and 3 infected patients, we estimated that a meta‐analysis needed to include a total of n = 1113 patients in order to detect or reject a RR reduction of 10%. Because our present meta‐analysis did not reach the required information size, we used trial sequential monitoring boundaries to assess the risk of random error. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) did not cross any of the monitoring boundaries, thus showing that we may lack evidence in this subgroup (Figure 9). Data from 11 trials of patients naive to previous antiviral intervention (Sinha 2004; Yenice 2006; Kolakowska 2008; Laguno 2009; McHutchison 2009; Ascione 2010; Rumi 2010; Kamal 2011; Mach 2011; Marcellin 2011; Miyase 2012) yielded a RR in favour of peginterferon alpha‐2a on the outcome sustained virological response (RR 1.12, 95% CI 1.06 to 1.18) using the random‐effects model (Analysis 2.2; Figure 10). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.0001 and the heterogeneity was I2 = 0% (Analysis 2.2). For sustained virological response in naive patients, we estimated that a required information size of 4083 patients was needed in order to detect or reject a RR of 10%. Because our present meta‐analysis did not reach the required information size, we used trial sequential monitoring boundaries to assess the risk of random error. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) crossed the monitoring boundary, thus yielding a robust statistically significant difference between the two peginterferons. We used a funnel plot to explore the bias of the included trials and there was no significant asymmetry in the trials with naive patients (Figure 10).
2.1. Analysis.
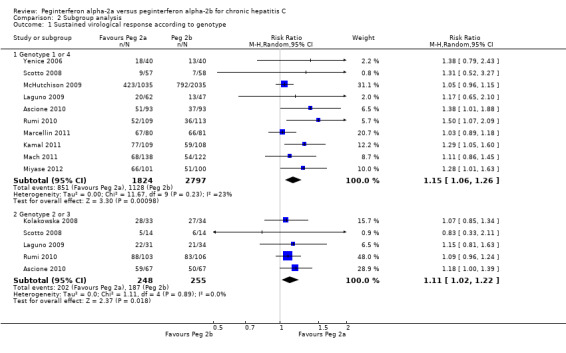
Comparison 2 Subgroup analysis, Outcome 1 Sustained virological response according to genotype.
8.
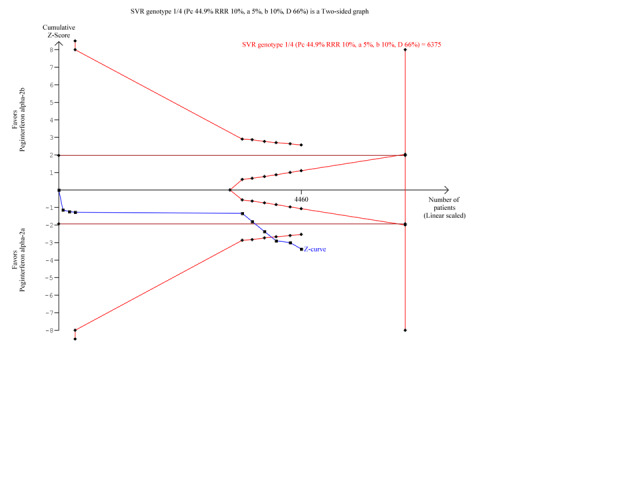
Trial sequential analysis (TSA): peginterferon alpha‐2a versus peginterferon alpha‐2b on the two subgroup analysis sustained virological response in participants infected with hepatitis C genotype 1 and 4. The diversity‐adjusted required information size of n = 6375 patients was calculated based upon a proportion of 44.9% of patients with sustained virological response in the peginterferon alpha‐2b group, a relative risk reduction of a 10% in peginterferon alpha‐2a group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity (D) of 45%. The solid blue curve presents the cumulative meta‐analysis test Z‐score and the inward sloping red curves present the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The cumulative Z‐score almost reaches the area of futility (delineated by the two trial sequential monitoring boundaries), but then it crosses both the conventional significance boundary (two tailed P = 0.05 ) and the trial sequential monitoring boundary.
9.
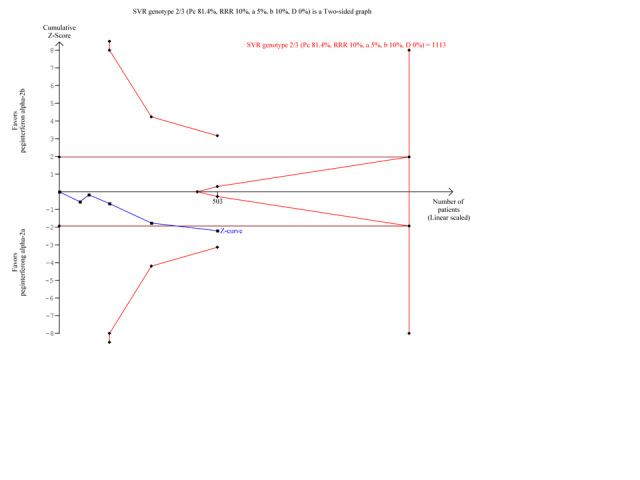
Trial sequential analysis (TSA): peginterferon alpha‐2a versus peginterferon alpha‐2b on the two subgroup analysis sustained virological response in patients infected with hepatitis C genotype 2 and 3.The diversity‐adjusted required information size of n = 1113 patients was calculated based upon a proportion of 81.4% of patients with sustained virological response in the peginterferon alpha‐2b group, a relative risk reduction of a 10% in peginterferon alpha‐2a group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity (D) of 0%. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The cumulative Z‐score does not reach the area of futility (delineated by the two trial sequential monitoring boundaries), but it crosses the conventional significance boundary (two tailed P = 0.05). However, the cumulative Z‐score does not cross the trial sequential monitoring boundary.
2.2. Analysis.
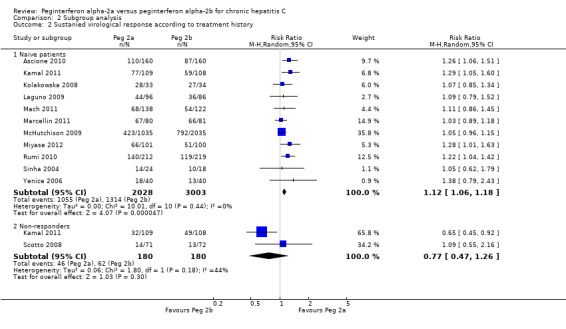
Comparison 2 Subgroup analysis, Outcome 2 Sustanied virological response according to treatment history.
Data from eight trials with low risk of randomisation bias (Sinha 2004; Scotto 2008; Laguno 2009; McHutchison 2009; Ascione 2010;Rumi 2010; Kamal 2011; Marcellin 2011) yielded a RR in favour of peginterferon alpha‐2a on sustained virological response (RR 1.12, 95% CI 1.05 to 1.20) using the random‐effects model (Analysis 2.3). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.34 and the heterogeneity was I2 = 13% (Analysis 2.3). Sustained virological response in the four trials with high risk of randomisation bias (Yenice 2006; Kolakowska 2008; Mach 2011; Miyase 2012) remained the same (RR 1.16, 95% CI 1.02 to 1.33) using the random‐effects model (Analysis 2.3). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.62 and the heterogeneity was I2 = 0% (Analysis 2.3). For the sustained virological response in the trials with low risk of randomisation bias, we estimated that a meta‐analysis needed to include a total of 5624 patients in order to detect or reject a RR reduction of 10%. Because our present meta‐analysis did not reach the required information size, we used trial sequential monitoring boundaries to assess the risk of random error. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) reached the area of futility, thus yielding a robust statistically significant difference between the two peginterferons regarding sparse data and repetitive testing (Figure 10).
2.3. Analysis.
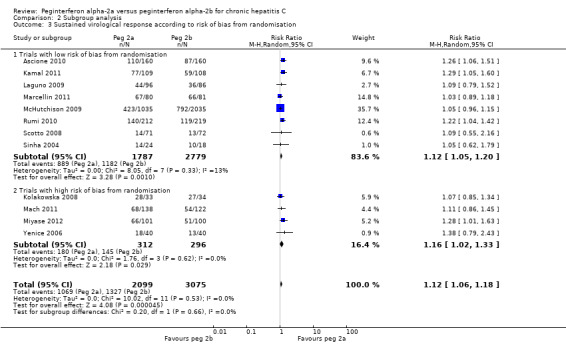
Comparison 2 Subgroup analysis, Outcome 3 Sustained virological response according to risk of bias from randomisation.
10.
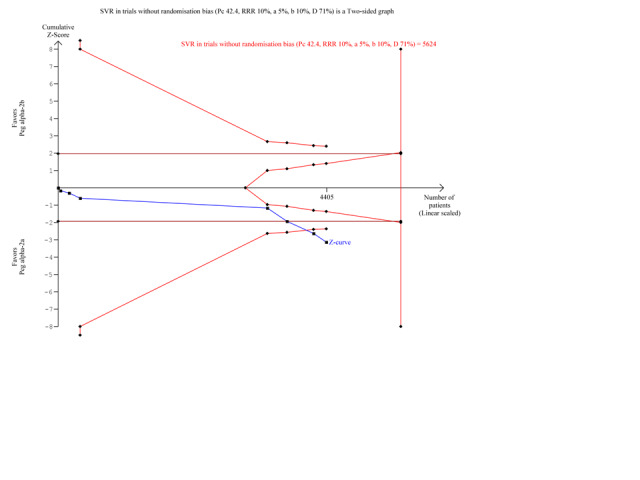
Trial sequential analysis (TSA): peginterferon alpha‐2a versus peginterferon alpha‐2b on the subgroup analysis on the outcome sustained virological response in trials with low risk of randomisation bias. The diversity‐adjusted required information size of n = 5,624 patients was calculated based upon a proportion of 42.4 % of patients with sustained virological response in the peginterferon alpha‐2b group, a relative risk reduction of a 10% in peginterferon alpha‐2a group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity (D) of 71%. The solid blue curve presents the cumulative meta‐analysis Z‐score which reaches the area of futility (delineated by the two trial sequential monitoring boundaries), but then crosses both the conventional significance boundary (two tailed P = 0.05 not shown on the figure) and the trial sequential monitoring boundary.
A subgroup analysis in the two trials with low risk of bias according to blinding (Kamal 2011; Miyase 2012) yielded a RR in favour of peginterferon alpha‐2a on sustained virological response (RR 1.29, 95% CI 1.10 to 1.51) using the random‐effects model (Analysis 2.4). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.95 and the heterogeneity was I2 = 0% (Analysis 2.4). For sustained virological response in trials with low risk of bias according to blinding, we estimated that the needed required information size was 2079 patients in order to detect or reject a RR of 10%. Because our present meta‐analysis did not reach the required information size of 2079, we used trial sequential monitoring boundaries to assess the risk of random error. Using the random‐effects model, the resulting cumulative test statistic (Z‐score) did not break the monitoring boundaries, thus yielding no statistically significant difference between the two peginterferons (Figure 11).
2.4. Analysis.
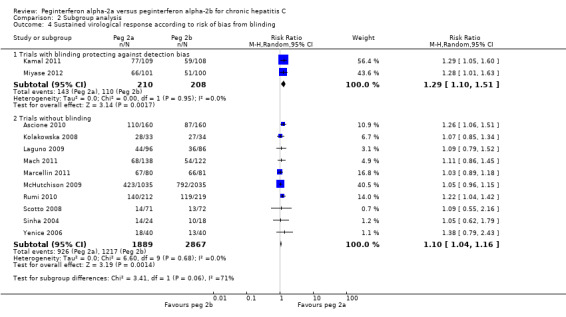
Comparison 2 Subgroup analysis, Outcome 4 Sustained virological response according to risk of bias from blinding.
11.
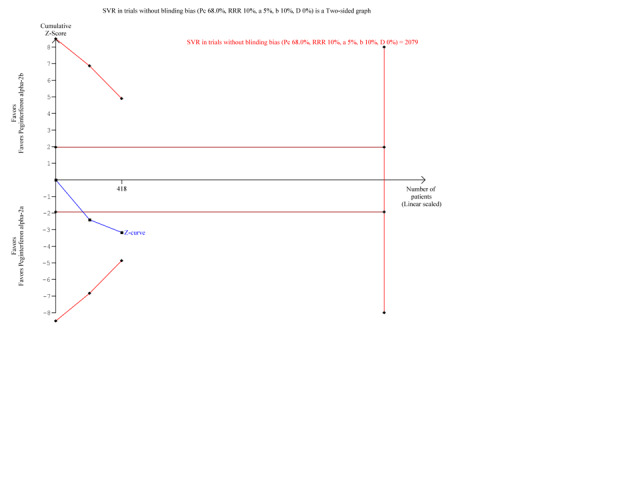
Trial sequential analysis (TSA): peginterferon alpha‐2a versus peginterferon alpha‐2b on the subgroup analysis on the outcome sustained virological response in trials with low risk of blinding bias. The diversity‐adjusted required information size of n = 2079 patients was calculated based upon a proportion of 68% of patients with sustained virological response in the peginterferon alpha‐2b group, a relative risk reduction of a 10% in peginterferon alpha‐2a group, an alpha (type I error) of 5%, a beta (type II error) of 10%, and a diversity (D) of 0%. The solid blue curve presents the cumulative meta‐analysis Z‐score and the inward sloping red curves present the two‐sided Lan‐DeMets trial sequential monitoring boundaries. The trial sequential monitoring boundaries were not broken by the cumulative Z‐curve.
Ten trials which had a high risk of bias due to lack of blinding (Sinha 2004; Yenice 2006; Kolakowska 2008; Scotto 2008; Laguno 2009; McHutchison 2009; Ascione 2010; Rumi 2010; Mach 2011; Marcellin 2011) also yielded a RR in favour of peginterferon alpha‐2a on sustained virological response (RR 1.10, 95% CI 1.06 to 1.14). Using RR as the measure of effect, the Cochran homogeneity test statistic yielded a P value of 0.69 and the heterogeneity was I2 = 0% (Analysis 2.4). Excluding the trial that included patients with HIV co‐infection did not noticeably change the meta‐analysed estimate (Analysis 2.5).
2.5. Analysis.
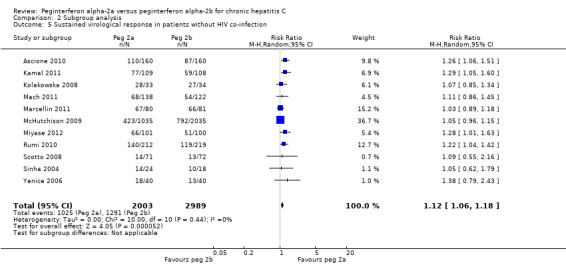
Comparison 2 Subgroup analysis, Outcome 5 Sustained virological response in patients without HIV co‐infection.
Summary of findings
We constructed 'Summary of findings' table using The Grading of Recommendations Assessment, Development, and Evaluation guidelines (GRADE) (Guyatt 2008). The information provided on the 'Summary of findings' table shows that we are very uncertain regarding the effects of the interventions on all‐cause mortality, liver‐related morbidity, all other adverse events, and quality of life which we have judged to be of low or very low quality evidence (Table 1). We have only low level confidence in the current evidence on harms measured as serious adverse events and adverse events leading to discontinuation of treatment for a number of different reasons. Furthermore, the table reveals that we had moderate confidence in the current evidence on treatment benefits measured as sustained virological response because all trial are with high risk of bias. The reason was that 5 out of 17 trials did not report on this outcome, raising suspicion of outcome reporting bias. Because the meta‐analysis for sustained virological response included 12 trials, we drew a funnel plot to explore bias and did not find significant asymmetry. The meta‐analysis included a seemingly reasonable mix of small and large trials yielding fairly consistent results, thus giving little concern regarding the presence of bias in the trials reporting the outcome (Table 1).
Discussion
Summary of main results
In this systematic review we have summarised the available evidence from randomised clinical trials comparing peginterferon alpha‐2a versus peginterferon alpha‐2b, both given in combination with orally administered ribavirin administered in doses in accordance with the weight of the patient. Our results suggest that only one trial reported on mortality (McHutchison 2009); only one trial reported on liver‐related morbidity (Silva 2006); and no trial reported on liver‐related mortality. Therefore, our present knowledge regarding patient‐important outcomes is very sparse. Our results also suggest that the two peginterferons are comparable in regard to adverse events leading to treatment discontinuation and serious and non‐serious adverse events. However, evidence on adverse events is also sparse and the meta‐analysis on adverse events is likely to be underpowered to detect any significant differences. Likewise, evidence on quality of life was sparse. The combination of peginterferon alpha‐2a and weight‐based ribavirin may achieve a significantly higher sustained virological response than the combination of peginterferon alpha‐2b and weight‐based ribavirin.
Overall completeness and applicability of evidence
The GRADE (Guyatt 2008) summary of findings table reveals that, in general, we can have moderate confidence in the current evidence on treatment benefits measured as sustained virological response and we can only have low confidence in the current evidence on harms measured as all‐cause mortality, liver‐related morbidity, serious adverse events, and adverse events leading to treatment discontinuation (Table 1). Information to assess the risk of bias was incomplete in a few trials with small numbers of participants. However, our sensitivity analyses did not show any important change in the intervention effects when we focused on trials with lower risk of bias. In our study, the trials that adequately reported on the trial methodology are large trials and they dominate the estimates of intervention effects from the meta‐analyses. Therefore, it is less likely that the pooled estimates are biased. In the meta‐analysis for sustained virological response, there were no serious inconsistencies across trials and the meta‐analysis crossed the Lan‐DeMets monitoring boundary (trial sequential monitoring boundary) leaving any random error less likely. The comparison of the largest trial (McHutchison 2009) with the second and third largest trials (Ascione 2010; Rumi 2010) yielded discrepancies however. The largest trial, which was funded by the manufacturer of peginterferon alpha‐2b, observed the smallest benefit of peginterferon alpha‐2a whereas the second and third largest trials both reported peginterferon alpha‐2a to be significantly superior to peginterferon alpha‐2b, and these trials were not funded by either of the two manufacturers. It is well known that industry bias may affect the outcomes and interpretation of trials (Als‐Nielsen 2003; Lexchin 2003; Lundh 2012).
Quality of the evidence
Because the meta‐analysis for sustained virological response included 12 trials, we drew a funnel plot to explore bias (Figure 6). There was no significant asymmetry. The meta‐analysis included a seemingly reasonable mix of small and large trials yielding fairly consistent results, which gives little concern about the presence of publication bias and other biases. When we included only trials without risk of bias due to other factors than lack of blinding, the results remained the same regarding sustained virological response. This effect did not pass the test of trial sequential analysis (Figure 11). However, we have some concerns in regard to indirectness. In the identified trials, virological response was the predominant measure of benefit. Many of the trials measured sustained virological response, which is currently the commonly used non‐validated surrogate outcome (Gluud 2007). Recent large cohort studies show a correlation between the presence of viraemia and mortality (Adeel 2009; Hirofumi 2009). However, it is important to remember that sustained virological response (and early virological response and end of treatment virological response) are still only non‐validated surrogate outcomes for antiviral intervention effects (Gluud 2007; Koretz 2013; Gurusamy 2014). We do not know the effects of the interventions on patient‐relevant outcomes (Gluud 2007). Because randomised clinical trials need to inform clinical practice, patient‐relevant outcomes such as risk of liver failure, hepatocellular carcinoma, and mortality would be of greater interest to patients and clinicians. Nevertheless, to be able to report on these outcomes, a much larger sample size and a follow‐up of several years would be required. Currently, no randomised clinical trials comparing the two peginterferons are of such a size or duration.
There were serious discrepancies across trials in the meta‐analysis on adverse events. The proportions of observed adverse events differed greatly across the trials, and the direction of effect was also heterogeneous. It is noteworthy that the IDEAL trial (McHutchison 2009) included three intervention groups, one for peginterferon alpha‐2a and two for peginterferon alpha‐2b. The two peginterferon alpha‐2b groups consisted of the usual 1.5 µg/kg/week dose and a low 1.0 µg/kg/week dose. The usual dose group yielded a similar proportion of adverse events as the peginterferon alpha‐2a group, whereas the low dose peginterferon alpha‐2b group yielded a lower proportion of the group with adverse events. Including or excluding the low dose peginterferon alpha‐2b group from the meta‐analysis had no visible impact on the estimated adverse events however. Furthermore, the meta‐analysis on adverse events leading to treatment discontinuation had low precision. The frequency of all other adverse events varied greatly among the included trials. In the majority of the included trials each patient had at least four to six adverse events during the treatment (Di Bisceglie 2007; Laguno 2009; Ascione 2010; Rumi 2010; Miyase 2012), but there are some trials where patients experienced no or only one adverse event (Scotto 2008; McHutchison 2009; Kamal 2011). Such a discrepancy in reporting adverse events imposes the necessity of uniform reporting of the adverse events in future trials. A post hoc calculation of the required information size to detect a minimally important difference of 10% relative risk reduction, based on the assumption of an average population risk of 10% and employing a 5% maximum type I error and 80% power, suggested that a minimum of 27,000 patients should be randomised to obtain a conclusive meta‐analysis on adverse events. The current number of patients in the meta‐analysis on adverse events is approximately 5000 (that is, less than 20% of what is required).
There have been some concerns regarding the non‐standardisation of the ribavirin dose across trials. The weight‐based dose of ribavirin ranged from 800 mg to 1400 mg. However, the weight cut‐off varied among trials as well as within the same trial. In the largest included trial (McHutchison 2009), patients weighing from 40 kg to 65 kg received a lower dose of ribavirin (800 mg) in the peginterferon alpha‐2b group compared with a higher dose of ribavirin (1000 mg) in the peginterferon alpha‐2a group. Patients in the peginterferon alpha‐2b group achieved a lower sustained virological response compared with patients in the peginterferon alpha‐2a group (39% versus 41%). Patients weighing more than 105 kg received a higher dose of ribavirin in the peginterferon alpha‐2b group (1400 mg) compared with a lower dose of ribavirin (1200 mg) in the peginterferon alpha‐2a group. Patients in the peginterferon alpha‐2b group achieved a slightly higher sustained virological response compared with patients in the peginterferon alpha‐2a group (43% versus 39%) (McHutchison 2009). It is also interesting that in the same trial, patients who developed anaemia and thus required ribavirin dose reduction achieved a higher sustained virological response than the patients who did not require a ribavirin dose reduction (McHutchison 2009). Accordingly, we do not think that the varying doses of ribavirin have any major confounding influence on our observations regarding the effects of the type of peginterferon. More research needs to be done to explore the optimal ribavirin dose. A ribavirin dose reduction due to adverse events was reported in only seven trials (Yenice 2006; Scotto 2008; McHutchison 2009; Ascione 2010; Rumi 2010; Kamal 2011; Miyase 2012). Six of these trials applied one and the same dose reduction to all trial groups (Yenice 2006; Scotto 2008; Ascione 2010; Rumi 2010; Kamal 2011; Miyase 2012). Only one trial applied a different ribavirin dose reduction to the intervention groups (McHutchison 2009). Our estimate did not change noticeably when we excluded the latter trial from our meta‐analysis for the outcome sustained virological response.
Selective outcome reporting was difficult to assess in this review. Most of the included trials were not adequately registered or did not have their protocols publicly available prior to the trial completion. The risk of bias from selective reporting was considered low if the trial protocol was available and all of the pre‐specified outcomes that were of interest in the review were reported. It turned out that protocols were not available for all trials but two (McHutchison 2009; Kamal 2011). The outcomes reported in the protocols (sustained virological response and adverse events) matched the outcomes that were reported (McHutchison 2009; Kamal 2011). We also considered that low risk of reporting bias was present if the trial reported both the sustained virological response and adverse events. However, since the other primary outcomes that were of interest in this review related to morbidity and mortality and were rarely reported in any of the included trials, we could have chosen to assess all the trials as having high risk of reporting bias due to the lack of reporting of important, patient‐relevant outcomes. Hopefully, the initiation of the World Health Organization (WHO) International Clinical Trials Registry Platform coupled with timely and correct registrations of trials will facilitate such assessments for future trials (WHO 2009). Another limitation in this review was insufficient reporting of the included trials. Investigators of future trials are, therefore, well advised to adhere to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Chan 2013) and the Consolidated Standards for Reporting of Trials (CONSORT) (Moher 2012) in order to improve the quality of trial reports.
Potential biases in the review process
The strengths of this Cochrane Hepato‐Biliary Group systematic review are that it builds on a peer‐reviewed published protocol (Awad 2010), uses extensive searches until October 2013, considers the risks of systematic errors (bias, that is overestimation of benefits and underestimation of harms), and considers risks of random errors (play of chance) by adjusting the threshold for statistical significance according to the information and strength of evidence in the cumulative meta‐analysis.
A possible limitation is the unavailability of full reports of all the included trials. Two out of the 12 trials that provided data on sustained virological response for the meta‐analysis are only available as abstracts. However, we were able to successfully retrieve the necessary data for one of the two abstracts via e‐mail correspondence with the authors, and thus the risk of bias assessment of the included trial was carried out satisfactorily. Our sensitivity analyses did not show any important changes in our results whether including or excluding the trials. In our review, the trials that were published as a full paper are large and dominated the meta‐analysed estimates of effects. Moreover, empirical evidence suggests that trials that fail to refute the null hypothesis have lower odds of getting published, especially those not funded by industry (Als‐Nielsen 2003; Lee 2008; Hopewell 2009). Thus, many of the included abstracts may have a low probability of getting published as full articles. In fact, including these abstracts in our systematic review may likely be a strength rather than a limitation. By including abstracts we consider the complete available body of evidence. By excluding abstracts we would only obtain a subset that is defined through present‐day biased publication mechanisms. This would considerably increase the likelihood of publication bias. These potential limitations and concerns may lower our confidence in the estimates of intervention effect. However, in our meta‐analysis of sustained virological response there is no apparent heterogeneity (I2 = 0%) and the direction of the treatment effect is the same across all included trials. Further research is unlikely to change our confidence in the estimate of the effect. It is a common misconception that large randomised trials are generally more reliable than meta‐analyses. The reason this misconception has prevailed is due to a number of highly cited papers that compared large trials with low risk of bias to meta‐analyses of small trials with high risk of bias. This is of course an unfair comparison. In empirical studies where large trials with low risk of bias are compared to a meta‐analysis of small trials with low risk of bias, the results from the two are typically non‐discrepant. In the case of the IDEAL trial (McHutchison 2009), the results still show an effect, albeit small, in favour of peginterferon alpha‐2a. There are many examples of large trials that, merely by the play of chance, underestimate (or overestimate) the true treatment effect.
Agreements and disagreements with other studies or reviews
There are several meta‐analyses that have been published recently which compare the efficacy and safety of the two pegylated interferons (Alavian 2010; Barros 2010c; Cheinquer 2010; Zhao 2010; Coppola 2011; Singal 2011; Druyits 2012; Romero‐Gomez 2012; Flori 2013; Yang 2013). Four of them have been published as abstracts only (Barros 2010c; Cheinquer 2010; Coppola 2011; Romero‐Gomez 2012) and, despite the different numbers of included studies, the common conclusion is that pegylated interferon alpha‐2a has advances over pegylated interferon alpha‐2b. Six meta‐analyses that were published as full papers (Alavian 2010; Zhao 2010; Singal 2011; Druyits 2012; Flori 2013; Yang 2013) also uniformly report the superior efficacy of pegylated interferon alpha‐2a over pegylated interferon alpha‐2b while the safety profile remains similar for both treatments. Two meta‐analyses are not comparable to ours because of the different search strategy and inclusion criteria in one (Druyits 2012) and because non‐randomised trials were included for assessment of benefits in the other (Flori 2013). The authors did not assess the risk of bias of the included trials (Druyits 2012). The other four meta‐analyses have similar search strategies and outcomes as ours but included both fewer trials and smaller number of patients (Alavian 2010; Zhao 2010; Singal 2011; Yang 2013). They excluded conference abstracts or limited the participants to only naive patients. The conclusions of those three meta‐analyses are in concordance with our conclusions on the higher efficacy of pegylated interferon alpha‐2a on sustained virological response and comparable safety profile with pegylated interferon alpha‐2b.
Current evidence suggests that peginterferon alpha‐2a is significantly better than peginterferon alpha‐2b regarding sustained virological response, which is the clearance of the virus from the blood. However, there is insufficient evidence to detect any differences regarding effects on clinical outcomes (liver complications and mortality) as well as harms (adverse events). Future trials need to study the correlation between sustained virological response and risk of cirrhosis, hepatocellular carcinoma, and mortality.
Authors' conclusions
Implications for practice.
There is lack of evidence on patient‐important outcomes such as mortality, liver complications, cirrhosis, hepatocellular carcinoma, and quality of life. Both drugs look comparable regarding harms (adverse events), but the reporting of adverse events in trials was insufficient. Moderate quality evidence suggests that peginterferon alpha‐2a is superior to peginterferon alpha‐2b in achieving a sustained virological response. However, sustained virological response is still an unvalidated surrogate outcome for patient‐important outcomes. The lack of evidence on patient‐important outcomes and the paucity of evidence on adverse events means we are unable to draw definitive conclusions about the effects of one peginterferon over the other.
Implications for research.
Future randomised clinical trials need to study the association between achieving a sustained virological response and patient‐relevant outcomes such as liver complications, quality of life, and mortality. Furthermore, future trials ought to be designed according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Chan 2013) and be reported following the CONSORT statement (Moher 2012).
Acknowledgements
Peer reviewers: Daniele Prati, Italy; Carlo Camma, Italy; Lior Katz, Israel. Contact Editor: Kurinchi Gurusamy, UK.
Thanks to the authors who provided us with additional data regarding their trials: Laguno M, Kamal MS, Mach TH, Rumi M. Special thanks to Dimitrinka Nikolova from the Cochrane Hepato‐Biliary Group for her advice, encouragement, and continuous support during the preparation of this review. Thanks to Luit Penninga for his assistance with statistics and Sarah Klingenberg from the Cochrane Hepato‐Biliary Group for conducting the database searches. Thanks to Marija Simin for drafting the previous version of the protocol for this systematic review.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | October 2013 | ('pegylated interferon' or peginterferon or peg‐interferon or peg‐IFN or (polyethylene and glycol) or pegasus or pegasys or pegintron or 'viraferon peg') AND ('hepatitis C' OR 'hep C' OR HCV) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Wiley) | Issue 9 of 12, 2012 | #1 MeSH descriptor Polyethylene Glycolsexplode all trees #2 pegylated interferon or peginterferon or peg‐interferon or peg‐ifn or (polyethylene and glycol) or pegasus or pegasys or pegintron or viraferon peg #3 (#1 OR #2) #4 MeSH descriptor Hepatitis C, Chronicexplode all trees #5 hepatitis c #6 (#4 OR #5) #7 (#3 AND #6) |
| MEDLINE (OvidSP) | 1946 to October 2013 | 1. exp Polyethylene Glycols/ 2. (pegylated interferon or peginterferon or peg‐interferon or peg‐IFN or (polyethylene and glycol) or pegasus or pegasys or pegintron or viraferon peg).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 3. 1 or 2 4. exp Hepatitis C, Chronic/ 5. hepatitis c.mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. 7 and 8 |
| EMBASE (OvidSP) | 1974 to October 2013 | 1. exp macrogol derivative/ 2. exp peginterferon/ 3. (pegylated interferon or peginterferon or peg‐interferon or peg‐IFN or (polyethylene and glycol) or pegasus or pegasys or pegintron or viraferon peg).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 4. 1 or 2 or 3 5. exp hepatitis C/ 6. hepatitis c.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 7. 5 or 6 8. 4 and 7 9. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 10. 8 and 9 11. limit 10 to human |
| Science Citation Index Expanded | 1900 to October 2013 | # 5 #4 AND #3 # 4 TS=(random* or placebo* or blind* or meta‐analysis) # 3 #2 AND #1 # 2 TS=(hepatitis c) # 1 TS=(pegylated interferon or peginterferon or peg‐interferon or peg‐ifn or (polyethylene and glycol) or pegasus or pegasys or pegintron or viraferon peg) |
| LILACS | 1982 to October 2013 | peginterferon [Words] |
Data and analyses
Comparison 1. Peginterferon alpha‐2a versus peginterferon alpha‐2b.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | 3070 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.64, 6.08] |
| 2 Liver‐related morbidity | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.70, 12.93] |
| 3 Serious adverse events | 4 | 3900 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.30] |
| 4 Adverse events leading to treatment discontinuation | 12 | 5340 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.57, 1.22] |
| 5 All other (non serious) adverse events | 9 | 4981 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sustained virological response | 12 | 5174 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.06, 1.18] |
1.5. Analysis.
Comparison 1 Peginterferon alpha‐2a versus peginterferon alpha‐2b, Outcome 5 All other (non serious) adverse events.
Comparison 2. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained virological response according to genotype | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Genotype 1 or 4 | 10 | 4621 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.26] |
| 1.2 Genotype 2 or 3 | 5 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.02, 1.22] |
| 2 Sustanied virological response according to treatment history | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Naive patients | 11 | 5031 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.06, 1.18] |
| 2.2 Non‐responders | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.47, 1.26] |
| 3 Sustained virological response according to risk of bias from randomisation | 12 | 5174 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.06, 1.18] |
| 3.1 Trials with low risk of bias from randomisation | 8 | 4566 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.05, 1.20] |
| 3.2 Trials with high risk of bias from randomisation | 4 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.33] |
| 4 Sustained virological response according to risk of bias from blinding | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Trials with blinding protecting against detection bias | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.10, 1.51] |
| 4.2 Trials without blinding | 10 | 4756 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.04, 1.16] |
| 5 Sustained virological response in patients without HIV co‐infection | 11 | 4992 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.06, 1.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ascione 2010.
| Methods | Study design: randomised clinical trial. Inclusion criteria: Detectable serum HCV RNA level, have an ALT level > 1.5 times the upper limit of normal for > 6 months, have a liver biopsy performed within 12 months of starting treatment graded according to Scheuer’s criteria (unless not indicated or refused), have a negative pregnancy test result, use contraceptive methods during therapy and for 6 months after the end of treatment, and have abstained from alcohol use for at least 6 months. Exclusion criteria: patients were excluded if they had a haemoglobin level < 120 g/L; had a neutrophil count < 1.5 x 109/L or a platelet count < 70 x 109/L; had an abnormal serum creatinine level; were hepatitis B surface antigen positive or human immunodeficiency virus positive; had any other cause of liver disease; had a history of liver decompensation; had clinically relevant depression or any other psychiatric disease; had cancer; had severe cardiac, pulmonary, or renal disease; or had uncontrolled diabetes or severe hypertension with vascular complications, including retinopathy. ITT analysis: yes, the trial used the worst‐case scenario for intention‐to‐treat analysis 'Patients who withdrew from the study for any reason were considered to be nonresponders in the efficacy assessment'. Sample size calculation: yes (160 participants for each group). |
|
| Participants | Study location: Italy. Total number (sample size): 320. Mean (SD) age: 50.2 (10.9). Sex (male sex (n (%)): n = 175 (54.7%). Comorbidity: liver cirrhosis. Genotype: 1 to 4. Previous HCV treatment: naive patients. Viral load (median HCV RNA (IU/mL x 103)): 600 (0.20 to 10,800). Histology at biopsy: only for cirrhotic patients (230 patients). Fibrosis grade was 2.13 (± 1.03). |
|
| Interventions | Group A: n = 160. Drug: peginterferon alpha‐2a: 180 μg/week. Drug: ribavirin 1000 mg/d (< 75 kg) or 1200 mg/d (75 kg). Group B: n = 160. Drug: peginterferon alpha‐2b (PEG‐Intron): 1.5 μg/kg/week. Drug: ribavirin 1000 mg/d (< 75 kg) or 1200 mg/d (75 kg). Patients affected by genotypes 1/4 received 48 weeks of treatment, while those affected by genotypes 2/3 were treated for 24 weeks. |
|
| Outcomes | Sustained virological response. Adverse events. |
|
| Notes | Published data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation was computer generated. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients who accepted the treatment were assigned to one of the 2 treatment arms on the basis of a computer‐generated randomisation list that was not available to the treating physician. The physician received the communication on the allocation of each patient from an independent researcher who did not know the patient or his or her characteristics". |
| Blinding (performance bias and detection bias) svr | Unclear risk | Quote: "The phase of the analysis of data, the person who did it received the results under code: treatment A and treatment B without any information on the type of drug used". Comment: open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The trial reports the reasons for all patients (in according to the treatment groups) who interrupt the therapy or needed dose modification. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Comment: The study seems to be free of other sources of bias. |
Berak 2005.
| Methods | Study design: randomised trial. Inclusion criteria: patients with chronic hepatitis C. Exclusion criteria: not mentioned. ITT: yes, however it is not clear which intention‐to‐treat analysis scenario was used. Sample size calculation: not mentioned. |
|
| Participants | Study location: Poland. Total number (sample size): 237; at follow‐up 208. Age: not mentioned. Sex (male sex (n (%)): not mentioned. Co‐morbidity: not mentioned. Genotype: all other except for 2 and 3. Previous HCV treatment: not mentioned. Viral load (mean HCV RNA (log10 IU/ml)): not mentioned. Histology at biopsy: analysed according to Knodell´s and Scheuer´s score. |
|
| Interventions | Group A: n = 116. Drug: peginterferon alpha‐2a (Pegasys, 40 KD). Drug: ribavirin. Group B: n = 121. Drug: peginterferon alpha‐2b (PegIntron, 12KD). Drug: ribavirin. |
|
| Outcomes | Adverse events and early virological response. | |
| Notes | Only the abstract was available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised.....". Comment: The method of sequence generation is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method of allocation concealment is not mentioned. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: Blinding of the outcome assessor is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were six patients lost to follow‐up, however, intention‐to‐treat analysis was adopted. It is not clear which intention‐to‐treat analysis scenario was used.Sample size is 237, finally analysed 208 and authors explained only 6 dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Comment: Authors have indicated they have no relationships to disclose. |
Bruno 2004.
| Methods | Study design: randomised trial. Inclusion criteria: interferon‐naive, HCV RNA levels greater or equal to 2000 copies per mL of serum by PCR assay, serum alanine aminotransferase (ALT) above the upper limit of normal within six months before entry into the trial, and a liver biopsy result consistent with the diagnosis of CHC performed within six months of trial entry. Exclusion criteria: absolute neutrophil count of < 1500 cells/mm3, a platelet count of < 90 000/mm3, a haemoglobin concentration of < 12 g/dL (women) or < 13 g/dL (men), or a serum creatinine level of > 1.5 times the upper limit of normal. Coinfection with the human immunodeficiency virus, decompensated liver disease, poorly controlled psychiatric disease, alcohol or drug dependence within one year before entry into the trial, and substantial coexisting medical conditions were additional exclusion criteria. Duration: 12 weeks. ITT analysis: yes. Sample size calculation: not mentioned. |
|
| Participants | Study location: Italy. Total number (sample size): (n = 22). Age: peginterferon alpha‐2a: 47 ± 8 years, peginterferon alpha‐2b: 40 ± 10 years. Sex (male sex (n (%))): peginterferon alpha‐2a: 70%, peginterferon alpha‐2b: 75%. Comorbidity: not mentioned. Genotype: 13 patients with HCV genotype 1; 9 patients with genotype 2/3; peginterferon alpha‐2a: genotype 1 was 70% (7/10), peginterferon alpha‐2b: genotype 1 was 50% (6/12). Previous HCV treatment: naive patients. Viral load (mean HCV RNA (log10 IU/ml)): peginterferon alpha‐2a: 5.75 ± 0.38, peginterferon alpha‐2b: 5.65 ± 0.50. Histology at biopsy: cirrhosis; peginterferon alpha‐2a: 20%, peginterferon alpha‐2b: 16%. |
|
| Interventions | Group A: n = 10. Drug: peginterferon alpha‐2a: 180 μg/week. Drug: ribavirin 1000 mg/d (≲ 75 kg) or 1200 mg/d (≥ 75 kg). Group B: n = 12. Drug: peginterferon alpha‐2b (PEG‐Intron): 1.0 μg/kg/week. Drug: ribavirin 1000 mg/d (≲ 75 kg) or 1200 mg/d (≥ 75 kg). |
|
| Outcomes | Pharmacokinetics and viral kinetics. | |
| Notes | Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was done by a computer". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the investigators allocated the intervention based on patients agreement to take part in the study". |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: It is not clear if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was not patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: The protocol is not available and the study does not report on SVR . |
| Other bias | Unclear risk | Comment: The trial was not stopped early for benefit, but there might be a conflict of interest bias. Hoffman‐LaRoche provided the drug peginterferon alpha‐2a. |
Di Bisceglie 2007.
| Methods | Study design: randomised, active control, parallel assignment, pharmacodynamics trial. Inclusion criteria: adult patients at least 18 years of age, chronic hepatitis C infection, genotype 1, use of two forms of contraception during the trial in both men and women. Exclusion criteria: previous systemic therapy with anti‐viral, anti‐neoplastic, or immunomodulatory agents; medical condition associated with chronic liver disease (eg, haemochromatosis, autoimmune hepatitis, metabolic liver disease, alcoholic liver disease, toxin exposure); decompensated liver disease; women who are pregnant or breastfeeding. ITT: yes. However it is not clear which intention‐to‐treat analysis scenario was used. Sample size calculation: yes (sample size of 172 for each group). |
|
| Participants | Study location: Italy. Total number: pre‐randomisation: 385, post‐randomisation: 380. Age: peginterferon alpha‐2a: 48.4 ± 0.56 years (age > 40 years 157 (83.1%)), peginterferon alpha‐2b: 40 ± 10 years (age > 40 years 70 (89.0%)). Sex (male sex (n (%))): peginterferon alpha‐2a: 121 (64%), peginterferon alpha‐2b: 136 (71%). Comorbidity: cirrhotic: peginterferon alpha‐2a: 28(14.8%), peginterferon alpha‐2b: 29 (15.2%). Genotype: 1. Previous HCV treatment: naive patients. Viral load (log10 IU/ml, mean ± SE): peginterferon alpha‐2a: 6.5 ± 0.03; peginterferon alpha‐2b: 6.5 ± 0.03. Histology at biopsy: not mentioned. |
|
| Interventions | Experimental: n = 189. Drug: peginterferon alpha‐2a 180 µg weekly. Drug: ribavirin 1000 mg/d to 1200 mg/day. Active comparator: n = 191. Drug: peginterferon alpha 2b1.5 µg/kg weekly. Drug: ribarivin1000 mg/d to 1200 mg/day. |
|
| Outcomes | Adverse events, EVR, and RVR. | |
| Notes | Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study randomisation was done by a central randomisation centre (a professional third party). The computer‐generated schedule of randomisation was only known to the randomisation centre." "The randomisation schedule was generated in block size of two in 1:1 ratio balanced for the whole study regardless of centre. A sequential number in the chronological order of randomisation for the entire study was recorded. A separate four‐digit patient number, which identified the centre and was sequential within each centre, was also assigned at randomisation. This patient number was used to identify the patient throughout the trial". |
| Allocation concealment (selection bias) | Low risk | Quote: "The study randomisation was done by a central randomisation centre (a professional third party). The computer generated schedule of randomisation was only known to the randomisation centre. The site had no control over the treatment assignment. The site, after verifying the eligibility of the patient, would call the randomisation centre to provide the patient information and the centre would fax the treatment assignment to the site." |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: It is not clear if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Five patients did not receive any medication and were not included in the ITT analysis (n = 380). There was 45 patients lost to follow‐up but the trial adhered to the intention‐to‐treat analysis. However it is not clear which intention‐to‐treat analysis scenario was used. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. There were no differences between the protocol and the trial report, and all the outcomes were defined prior to the beginning of the trial. The study was located on clinicaltrials.gov Identifier: NCT 00087607. |
| Other bias | Unclear risk | Comment: There might be conflict of interest bias. The trial was supported by Roche Laboratories. |
Kamal 2011.
| Methods | Prospective, randomised, open label, parallel‐group clinical trial. Inclusion criteria: chronic hepatitis C patients IFN naive with proven chronic HCV‐4; elevated serum ALTat least two times above the upper limit of normal (ULN: 40 U/l) during the preceding 6 months, detectable anti‐HCV, detectable HCV RNA by PCR. Exclusion criteria: previously an IFN‐a‐based regimen, evidence of other liver diseases, including hepatitis A, hepatitis B, autoimmune hepatitis, alcoholic liver disease, drug‐induced hepatitis and decompensated liver disease, coinfection with schistosomiasis or HIV, leucocyte count < 3000/mm3, neutropenia (< 1500 cells/mm3), haemoglobin level < 12 g/dl for women and <13 g/dl for men, thrombocytopenia (< 90 000 cells/mm3), creatinine concentration 1.5 times above ULN, organ transplantation, malignant conditions, severe cardiac or pulmonary disease, unstable thyroid dysfunction, severe depression or psychiatric disorder, active substance abuse, current pregnancy or breast feeding, body mass index (BMI) > 30 kg/m2 or known sensitivity to the drugs tested or therapy with immunomodulatory agents within the last 6 months. Sample size calculation: yes. ITT analysis: yes. |
|
| Participants | Study location: Egypt. Total number (sample size): 226 screened, 9 have been excluded 217 eligible for randomisation. Age: the mean age of the entire cohort was 41.4 years for females and 41.0 for males. Sex (male sex (n (%)): n = 107 (49.31%). Comorbidity: no. Genotype: 4. Previous HCV treatment: naive patients. Viral load (mean HCV RNA (log10 IU/ml)): 765.61 kIU (Group A) and 762.065 kIU (Group B). Histology at biopsy: Grading scores 5.0 for both groups. Fibrosis score 1.0 for both groups. |
|
| Interventions | Group A 109 patients: Drug: peginterferon alpha‐2a injections: 180 μg once per week plus ribavirin.Dose ranged between 1000 and 1200 mg/day according to body weight. ≥75 kg, 1200 mg/day and < 75 kg 1000 mg/day respectively. Group B 108 patients: Drug: peginterferon alpha‐2b:1.5 mg/kg one per week plus ribavirin.Dose ranged between 1000 and 1200 mg/day according to body weight (≥75 kg, 1200 mg/day and <75 kg 1000 mg/day respectively. |
|
| Outcomes | Primary outcome measures: SVR, defined as undetectable serum hepatitis C virus RNA (< 5 IU/ml). [Time frame: 24 weeks after discontinuation of treatment] [Designated as safety issue: yes] Secondary outcome measures:
|
|
| Notes | Contacted author due to serious adverse events and all cause mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomised in blocks of four..." |
| Allocation concealment (selection bias) | Low risk | Quote: "...the randomisation process, which was concealed from both the investigators and patients". |
| Blinding (performance bias and detection bias) svr | Low risk | Quote: An independent coordinator (A. A.) blinded to the patients’ demographics or clinical characteristics used computer‐generated lists for the randomisation process, which was concealed from both the investigators and patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote. "The primary efficacy analysis was an intention to‐treat analysis (ITT) that included all participants allocated to a given regimen whether they completed it or not". |
| Selective reporting (reporting bias) | Low risk | All clinically relevant and reasonably expected outcomes were reported. Protocol has been registered www.clinicaltrials.gov (NCT00502099). |
| Other bias | Unclear risk | Authors provided no conflict of interest statement (positive or negative), no data about study funding. |
Kolakowska 2008.
| Methods | Randomised open label clinical trial. Inclusion criteria: chronic hepatitis C patients. Exclusion criteria: not mentioned. Sample size calculation: not mentioned. ITT analysis: not clear. |
|
| Participants | Study location: Poland. Total number: 67. Group A: peginterferon alfa‐2a: 33. Group B: peginterferon alfa‐2b: 34. Genotype: genotype 3. Previous HCV treatment: naive. |
|
| Interventions | Group A: n = 33. Drug: PEG 2a plus weight‐based ribavirin. Group B: n = 34. Drug: PEG 2b plus weight‐based ribavirin. |
|
| Outcomes | Sustanied virological response. | |
| Notes | Only the abstract was available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised study.....". Comment: The method of sequence generation is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method of allocation concealment is not mentioned. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: It is not clear if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: There seems to be no patients lost to follow‐up, but it is not clear if the trial adhered to the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | Comment: The study was not stopped for early stopping, but it is not clear if the study is free of other sources of bias. |
Laguno 2009.
| Methods | Study design: randomised, multi‐centre clinical trial. Inclusion criteria: untreated chronic hepatitis C, HIV (CD4+ cell count above 250 cells/mm3 and viral load lower than 50.000 copies/mL). Exclusion criteria: known contraindication for PEG INF or RBV; decompensated liver disease; pregnancy. ITT: yes, however it is not clear which intention‐to‐treat analysis scenario was used. Sample size calculation: yes (total sample size of 182). |
|
| Participants | Study location: Spain. Total number: 182. Age: peginterferon alpha‐2a: 40.6 years, peginterferon alpha‐2b: 40.7. Sex (male sex (n (%))): peginterferon alpha‐2a: 64 (66.7%), peginterferon alpha‐2b: 68 (79.1%). Comorbidity: HIV. Genotype: 1 to 4. Previous HCV treatment: naive patients. Histological findings: 68% had fibrosis index ≥ 2. Viral load: 600,000 IU in 59% of patients and ≤ 400,000 in 24% of patients. |
|
| Interventions | Group A: n = 96. Drug: peginterferon alpha‐2a 180 μg. Drug: ribavirin 1000 mg/d to 1200 mg/day. Group B: n = 86. Drug: peginterferon alpha‐2b 80 to 150 μg. Drug: ribavirin 1000 mg/d to1200 mg/day. |
|
| Outcomes | Sustained virological response. Adverse events. |
|
| Notes | Contacted author due to serious adverse events and all‐cause mortality. Answer: no patients died. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of the two study treatments in equal proportions by means of a computer‐generated table of random numbers". |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was performed centrally. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: Blinding to the outcome assessor is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 34 patients were lost to follow‐up, but intention‐to‐treat analysis was adopted. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Comment: The study seems to be free of other sources of bias. |
Mach 2011.
| Methods | Study design: Randomised, prospective, open‐label study, single centre in Poland. Inclusion criteria: antiHCV and HCV‑RNA in serum and elevated alanine aminotransferase (ALT) levels at least 6 months before the inclusion, chronic hepatitis confirmed by histological examination, body mass index (BMI) below 30 kg/m2. Exclusion criteria: decompensated liver cirrhosis, autoimmune liver disease, alcohol abuse, liver cancer, hepatitis B virus or HIV coinfection, any severe chronic disease, diabetes, dyslipidaemia, metabolic syndrome, haemochromatosis, and immunosuppressive therapy. ITT: No. Sample size calculation: No. |
|
| Participants | Study location: Poland. Total number: 260. Age: peginterferon alpha‐2a: 45.2 ± 10.5 years, peginterferon alpha‐2b: 44.2 ± 13.6. Sex (male sex (n (%))): peginterferon alpha‐2a: 80 (50.8%), peginterferon alpha‐2b: 73 (59.9%). Comorbidity: not mentioned. Genotype: 1a. Previous HCV treatment: not mentioned. Histological findings Batts‐Ludwig score: F0‐2 64 (78.1%), F 3‐4 18 (21.9%). Viral load x106 IU/ml: peginterferon alpha‐2a: 4.01 ± 2.17, peginterferon alpha‐2b: 3.45 ± 0.92. |
|
| Interventions | Group A: peginterferon alfa‑2a 180 μg subcutaneously once a week and oral ribavirin 1.0–1.2 g daily. Group B: peginterferon alfa‑2b 1.5 mg/kg of body weight once a week and oral ribavirin 1–1.2 g daily. |
|
| Outcomes | Early virological response; end of treatment response; sustained virological response. | |
| Notes | Correspondace with the contact author: naive patients were treated and they used sealed envelopes in randomisation process. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomly assigned to 1 of the 2 treatment groups". Comment: see notes. |
| Allocation concealment (selection bias) | High risk | Comment: Open label study. Authors did not mention allocation process. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: Blinding to the outcome assessor is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No patients were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Quote: "The study was supported by the Polish National Health Fund and conducted according to the relevant recommendations." |
Marcellin 2011.
| Methods | Study design: randomised clinical trial. Inclusion criteria: treatment‐naive patients aged 18 to 65 years with chronic HCV genotype 1 infection. Additional inclusion criteria were serum HCV RNA level 10,000 IU/mL, absolute neutrophil count 1500 mm3, and platelet count 100,000 mm3. Liver fibrosis status had to have been documented within 18 months (no or minimal fibrosis, portal fibrosis, bridging fibrosis, or cirrhosis) with a liver biopsy or transient elastography. Exclusion criteria: co‐infection with human immunodeficiency virus or hepatitis B, any other cause of liver disease, poorly controlled diabetes mellitus (glycated haemoglobin value >8.5%), morbid obesity (weight >125 kg), severe depression or a severe psychiatric disorder, or active substance abuse. Sample size calculation: yes. ITT: yes. |
|
| Participants | Study location: Europe ‐ Austria, Belgium, France, Germany, Italy, Spain, The Netherlands. Total number of patients: 161. Age: Group A 47.5, Group B 47.5 Group C 40.0, Group D 49.0. Sex male n (%): Group A 20 (50.0%), Group B 20 (47.6%), Group C 21 (52.5%), Group D 19 (48.7%). Comorbidity: N/A. Previous HCV treatment: naive. Genotype: 1. Histological findings n (%): Cirrhosis: Group A 1 (2.5), Group B 1 (2.4), Group C 0, Group D 2 (5.1). Bridging fibrosis: Group A 8 (20.0%), Group B 10 (23.8%), Group C 7 (17.5%), Group D 12 (30.8%). Portal fibrosis: Group A 16 (40.0%), Group B 16 (38.1%), Group C 11 (27.5%), Group D 13 (33.3%). No or minimal fibrosis: Group A 15 (37.5%), Group B 15 (35.7%), Group C 22 (55.0%), Group D 11 (28.2%). Virological load: < 800.000: Group A 10 (25.0%), Group B 8 (19.0%), Group C 7 (17.5%), Group D 5 (12.8%). > 800.000: Group A 30 (75.0%), Group B 34 (81.0%), Group C 33 (82.5%), Group D 34 (87.2%). |
|
| Interventions | Group A telaprevir 750 mg q8h plus peginterferon alfa‐2a/ribavirin (q8h alfa‐2a). Group B telaprevir 750 mg q8h plus peginterferon alfa‐2b/ribavirin (q8h alfa‐2b). Group C telaprevir 1125 mg q12h plus peginterferon alfa‐2a/ribavirin (q12h alfa‐2a). Group D telaprevir 1125 mg q12h plus peginterferon alfa‐2b/ribavirin (q12h alfa‐2b). Peginterferon alfa‐2a was administered at 180 μg/wk with ribavirin at 1000 to 1200 mg/day; peginterferon alfa‐2b was administered at 1.5 μg/kg/wk1 with ribavirin at 800 to 1200 mg/day. |
|
| Outcomes | Sustained virological response. Adverse events. Pharmacokinetics. |
|
| Notes | In the published article safety data has been presented only for the whole cohort (161 patients). It is not possible extract data regarding different treatment groups. Corresponding author has been contacted for further information regarding adverse events but no answer obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: "The randomisation lists were generated by means of permuted blocks before the start of the trial, under the supervision of the sponsor". |
| Allocation concealment (selection bias) | Low risk | Comment: Central allocation before the star of the trial under supervision of the sponsor. |
| Blinding (performance bias and detection bias) svr | High risk | Comment: "Because this was an open label trial, blinding procedures were not applicable". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 79.5% of patients completed the treatment, but intention‐to‐treat analysis, worst‐case scenario, was adopted and all patients have been included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. Study protocol registered in ClinicalTrials.gov (NCT00528528). |
| Other bias | High risk | Quote: "This clinical trial was funded by Janssen Pharmaceuticals, and by Vertex Pharmaceuticals Inc. The study sponsor was involved in the trial design and conduct, data collection, and data analysis. Several authors are employees of the sponsor". |
McHutchison 2009.
| Methods | Study design: randomised clinical trial. Inclusion criteria: HCV genotype 1 infection and a detectable plasma HCV RNA level, and had not been previously treated for hepatitis C infection. The patients had an absolute neutrophil count of 1500/mm3, a platelet count of 80,000 or more/mm3, and haemoglobin level of 12 g (for women) or 13 g (for men) or more per dL. Exclusion criteria: co‐infection with human immunodeficiency virus or hepatitis B, any other cause of liver disease, poorly controlled diabetes mellitus (glycated haemoglobin value >8.5%), morbid obesity (weight > 125 kg), severe depression or a severe psychiatric disorder, or active substance abuse. Sample size calculation: yes. ITT: yes. However it is not clear which intention‐to‐treat analysis scenario was used. |
|
| Participants | Study Location: USA. Total number of patients: 3070. Age: Group A 47.6 ± 8.2, Group B 47.5 ± 7.8, Group C 47.5 ± 8.1. Sex male n (%): Group A 613 (59.2%) Group B 613 (60.2%), Group C 607 (59.7%). Comorbidity: steatosis. Previous HCV treatment: naive. Genotype: 1. Histological findings: METAVIR Group A 102 (10.6%), Group B 111 (10.9%), Group C 107 (10.5%). Virological load: > 600.000: Group A 852 (82.3%), Group B 836 (82.0%), Group C 830 (81.7%). |
|
| Interventions | Group A: n = 1035. Drug: peginterferon alpha‐2a: 180 μg/week. Drug: ribavirin 1000 mg/d to 1200 mg/d. Group B: n = 1016. Drug: peginterferon alpha‐2b (PEG‐Intron): 1.5 μg/kg/week. Drug: ribavirin 1000 mg/d to 1200 mg/d. Group C: n = 1019. Drug: peginterferon alpha‐2b (PEG‐Intron): 1.0 μg/kg/week. Drug: ribavirin 1000 mg/d to 1200 mg/d. |
|
| Outcomes | Failure of sustained virological response. Adverse events. Failure of end of treatment response. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The randomisation was done centrally so we assumed it was conducted with low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Comment: Centralised telephone‐based IVRS was used. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Quote: "The study was double‐blinded with regard to the dose of peginterferon alfa‐2b". Comment: It is not mentioned if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were 653 patients lost to follow‐up, but the trial adopted intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported.The study protocol was published in ClinicalTrial.gov (NCT00081770) |
| Other bias | Unclear risk | Quote: "The principal investigators had unrestricted access to the data, wrote the manuscript, and vouch for the accuracy and integrity of the data and analyses". Comment: The study was not stopped early for benefit, but there might be conflict of interest bias, as the trial is sponsored by Schering‐Plough. |
Miyase 2012.
| Methods | Study design: prospective, randomised, open label, single centre. Inclusion criteria: antiHCV and HCV‑RNA in serum and elevated alanine aminotransferase (ALT) levels at least 6 months before the inclusion, chronic hepatitis confirmed by histological examination, body mass index (BMI) below 30 kg/m2. Exclusion criteria: decompensated liver cirrhosis, autoimmune liver disease, alcohol abuse, liver cancer, hepatitis B virus or HIV coinfection, any severe chronic disease, diabetes, dyslipidaemia, metabolic syndrome, haemochromatosis, and immunosuppressive therapy. ITT: yes. Sample size calculation: yes. |
|
| Participants | Study location: Japan. Total number: 206 randomised, but 201 were eligible to receive at least one dose of treatment. Age: peginterferon alpha‐2a: 59.2 ± 9.1 years, peginterferon alpha‐2b: 58.9.2 ± 10.8. Sex (male sex (n (%))): peginterferon alpha‐2a: 39 (38.6%), peginterferon alpha‐2b: 40 (40.0%). Comorbidity: not mentioned. Genotype: 1. Previous HCV treatment: naive. Histological findings METAVIR score: peginterferon alpha‐2a fibrosis 81 (80.2%), peginterferon alpha‐2b 83 (83.0%). Viral load log IU/ml: peginterferon alpha‐2a: 6.3 ± 0.6, peginterferon alpha‐2b: 6.2 ± 0.7. |
|
| Interventions | Group A: peginterferon alpha‐2a at a dosage of 180 µg once weekly. Group B: peginterferon alpha‐2b once weekly at a dosage of 60 to 150 µg/kg of body. Weight (35 to 45 kg, 60 µg; 46 to 60 kg, 80 µg; 61to 75 kg 100 µg; 76 to 90 kg, 120 µg; 91 to 120 kg, 150 µg). The ribavirin dosage was determined by body weight in both regimens (600 mg/day in patients ≤ 60 kg; 800 mg/day in patients 60–80 kg; 1000 mg/day in patients > 80 kg. |
|
| Outcomes | Sustained virological response and adverse events. | |
| Notes | Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients who accepted the treatment were randomly assigned to 1 of 2 treatment arms" Comment: authors did not explain randomisation method they used. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Authors did not explain stated allocation process. |
| Blinding (performance bias and detection bias) svr | Low risk | Quote: "The patients who accepted the treatment were randomly assigned to 1 of 2 treatment arms, to which the treating physician was blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 43 patients were excluded, but intention‐to‐treat analysis, worst‐case scenario, was adopted and all patients have been included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Quote: "The authors declare that they have no conflict of interest." |
Rumi 2010.
| Methods | Study design: randomised clinical trial. Inclusion criteria: treatment‐naive patients infected with chronic hepatitis C, serum HCV‐RNA, higher than normal alanine aminotransferase (ALT) activity, and a diagnostic liver biopsy done in the previous 24 months. Exclusion criteria: patients with persistently normal ALT; haemoglobin 12 g/dL for women and 13 g/dL for men; white blood cell count 2.5 x 103/mm3; neutrophil count 1.5 x 103/mm3; platelet count 75 x 103/mm3; serum creatinine level 1.5 times the upper limit of normal; any other liver disease; human immunodeficiency virus coinfection; autoimmune diseases; and general contraindications to the IFN and RBV. Sample size calculation: yes (n = 210 for each group). ITT: yes, the trial used the worst‐case scenario for intention‐to‐treat analysis 'By intention‐to‐treat analysis, patients for whom HCVRNA levels had not been measured by the end of the follow‐up period as well as those who discontinued treatment for any reason were categorized as nonresponders'. |
|
| Participants | Study location: Italy. Total number: eligible number was 431 after of initial assessment of 473 patients. Previous HCV treatment: treatment naive. Median age: peginterferon alpha 2a: 54 years versus peginterferon alpha 2b: 56 years. Sex: peginterferon alpha 2a: 60% males versus peginterferon alpha 2b:55% males. Body mass index: peginterferon alpha 2a: 25.5 kg/m2 versus peginterferon alpha 2b: 24.8 kg/m2. Comorbidity: cirrhosis: peginterferon alpha 2a: 20% versus peginterferon alpha 2b:18%. Genotype distribution 1 to 4: peginterferon alpha 2a: 51% versus peginterferon alpha 2b: 52%. Viral load > 800,000 IU: peginterferon alpha 2a: 102 (48.1%) versus peginterferon alpha 2b: 103 (47.0%). Histological findings: Ishak score S5,6 peginterferon alpha 2a: 43 (20.3%) versus peginterferon alpha 2b: 39 (17.8%). |
|
| Interventions | Group A: n = 212. Drug: peginterferon alpha‐2a: 180 μg/week. Drug: ribavirin 800 mg/d to 1200 mg/d. Group B: n = 219. Drug: peginterferon alpha‐2b (PEG‐Intron): 1.5 μg/kg/week. Drug: ribavirin 800 mg/d to 1400 mg/d. Ribavirin was weight‐dosed in patients receiving peginterferon alpha‐2a but patients with genotype 2 and 3 on peginterferon alpha‐2a received a fixed dose of 800 mg/day ribavirin. |
|
| Outcomes | Sustained virological response. Adverse events. |
|
| Notes | Contacted author due to serious adverse events and all‐cause mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Patients were randomised using a computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Comment: The allocation was telephone‐based (central). |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: Blinding of the outcome assessor is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 119 patients were lost to follow‐up but the trial adhered to the intention‐to‐treat analysis. The trial used the worst case scenario for intention‐to‐treat analysis 'By intention‐to‐treat analysis, patients for whom HCV RNA levels had not been measured by the end of the follow‐up period as well as those who discontinued treatment for any reason were categorized as nonresponders'. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | Quote: one author disclosed grants and research support from different pharma companies. |
Scotto 2008.
| Methods | Study design: randomised, single centre. Inclusion criteria: HCV patients with positive HCV‐RNA detectable in plasma by means of real time PCR in plasma (sensitivity < 100IU/mL), ALT levels greater than two‐fold than upper limit of normal for at least three times during the six months before screening, liver biopsy performed within one year before trial enrolment with histological diagnosis of chronic hepatitis according to Knodell’s histological activity classification, no ongoing antiviral and or immunosuppressive treatment during the six months before the trial enrolment. Exclusion criteria: presence of other causes of chronic liver disease (HBV infection, Wilson’s disease, alpha‐1‐antitrypsin deficiency, haemochromatosis, and autoimmune hepatitis), HIV positive patients, active drug abusers, patients with pre‐existing and or social contraindications, patients with prior cirrhotic decompensation, pregnancy, and breastfeeding women. ITT analysis: yes. However, it is not clear which intention‐to‐treat analysis scenario was used. Sample size calculation: not mentioned. |
|
| Participants | Study location: Italy. Total number (sample size): 143. Age: peginterferon alpha‐2a: 45.86 years, peginterferon alpha‐2b: 47.82 years. Sex (male sex): peginterferon alpha‐2a : 42, peginterferon alpha‐2b: 40. Comorbidity: not declared. Genotype
Previous HCV treatment: non‐responders to standard interferon. Viral load IU/ml: Group A mean 2.4 ± 5 x 106, Group B mean 2.1 ± 3 x 106. Histological assessment Knodell histological activity: Group A mean score 10 ± 4, Group B mean score 9 ± 1. |
|
| Interventions | Group A: n = 71. Drug: peginterferon alpha 2a. Dosage: 180 μg weekly plus RBV 15 mg/kg daily. Duration: 48 weeks. Group B: n = 72. Drug: peginterferon alpha 2b. Dosage: 1.5 μg/kg weekly plus RBV 15mg/kg daily. Duration: 48 weeks. |
|
| Outcomes | Sustained virological response. Adverese events. |
|
| Notes | Published and unpublished data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were enrolled by a pre‐established numerical randomisation method determined by the Clinic’s medical staff" "Computer generated random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "While allocation concealment was sequentially numbered, and we used sealed opaque envelopes". |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: Blinding to the outcome assessor is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 18 patients were lost to follow‐up. The trial adopted intention‐to‐treat analysis. However it is not clear which intention‐to‐treat analysis scenario was used. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Quote: "We disclose any potential conflict of interest. The authors do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript." Comment: The trial seems to be free of other sources of bias. |
Silva 2006.
| Methods | Study design: Double‐blinded, randomised, multi centre, parallel‐group trial. Inclusion criteria: patients between the ages of 18 and 65 years who were infected with HCV genotype 1a or 1b, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels less‐than‐or‐equals, slant 10 times the upper limit of normal (ULN), normal haemoglobin. Exclusion criteria: liver disease due to causes other than chronic HCV infection, HIV positivity, haemoglobinopathy, haemophilia, severe pre‐existing psychiatric disease, poorly controlled diabetes mellitus, significant Ischaemic heart disease, chronic obstructive lung disease, or active autoimmune disease. ITT: no. Sample size calculation: yes (18 for each group). |
|
| Participants | Study location: Argentina, Mexico, and Germany. Total number: 36. Age: peginterferon alpha‐2a plus ribavirin: 45.6 (± 11.8), peginterferon alpha‐2b plus ribavirin: 48.3 (± 9.7). Sex (male sex (n (%))): peginterferon alpha‐2a plus ribavirin: 9, peginterferon alpha‐2b plus ribavirin: 10. Comorbidity: not mentioned. Genotype: 1. Previous HCV treatment: naive patients. Viral load (mean HCV RNA (log 10 IU/ml)): peginterferon alpha‐2a plus ribavirin 1.8 ( ± 0.1), peginterferon alpha‐2b plus ribavirin 1.8 (± 0.2). Histology at biopsy: not mentioned. |
|
| Interventions | Group A: n = 18. Drug: peginterferon alpha‐2a 180 μg/week. Group B: n = 18. Drug: peginterferon alpha‐2b 1.5 μg/kg/week. After the fourth week of treatment, oral ribavirin therapy was added to the regimen at a dose of 13 mg/kg, in a divided twice a day dose. |
|
| Outcomes | Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The investigator requested randomisation by faxing the central randomisation service. Only the site pharmacist responsible for medication preparation received confirmation of both the assigned treatment and the subject number. The investigator received the subject number only". |
| Allocation concealment (selection bias) | Low risk | Quote: "The investigator requested randomisation by faxing the central randomisation service. Only the site pharmacist responsible for medication preparation received confirmation of both the assigned treatment and the subject number. The investigator received the subject number only". |
| Blinding (performance bias and detection bias) svr | Low risk | Quote: "Study drug was prepared by a site pharmacist and administered by a qualified, independent third party who was blinded to protocol assignments". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Six patients dropped out, and the trial did not adhere to the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Unclear risk | Comment: There might be conflict of interest bias. Some of the authors are employees of Schering‐Plough and own company stock. |
Sinha 2004.
| Methods | Study design: randomised trial. Inclusion criteria: chronic hepatitis C patients. Exclusion criteria: not mentioned. Sample size: not mentioned. Intention‐to‐treat analysis: yes, however it is not clear which intention‐to‐treat analysis scenario was used. |
|
| Participants | Study location: USA. Total number (sample size): 42. Age: Group A: peginterferon alfa‐2a: 24; Group B: peginterferon alfa‐2b: 18. Genotype: all: mixed, 13/42 (30%) had genotype other than genotype 1. Previous HCV treatment: naive. |
|
| Interventions | Group A: n =24. Drug: PEG 2a plus weight‐based ribavirin (1000 mg to 1200 mg). Group B: n =18. Drug: PEG 2b plus weight‐based ribavirin (1000 mg to 1200 mg). |
|
| Outcomes | Sustanied virological response. Adverse events. |
|
| Notes | Abstract publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The sequence generation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation concealment was performed by the mean of sealed envelopes. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: Blinding of the outcome assessor is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 patient was lost to follow‐up but the trial adhered to the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported |
| Other bias | Low risk | Comment: The trial was not stopped early for benefit, and the study seems to be free of other sources of bias. |
Sporea 2006.
| Methods | Study design: randomised open label trial. Inclusion criteria: presence of chronic C viral hepatitis (proven by liver biopsy performed maximum 6 months before the treatment) and the quantification of the viral load (by PCR) before treatment and after 12 weeks of treatment. Exclusion criteria: not mentioned. ITT: not mentioned. Sample size calculation: not mentioned. |
|
| Participants | Study location: Romania. Total number: 116 Age: peginterferon alfa‐2a: 49.3 years; peginterferon alfa‐2b: 50.9 years. Sex (male sex (n (%))): peginterferon alfa‐2a: 21 (36%); peginterferon alfa‐2b: 14 (24%). Co‐morbidity: not mentioned. Genotype: all genotypes. Previous HCV treatment. Peginterferon alfa‐2a: 48 patients were naïve (N1), 7 were relapsers after previous treatment (RL1) and 3 non‐responders to previous treatment (NR1). Peginterferon alfa‐2b: 33 patients were naïve (N2), 18 relapsers (RL2) and 7 non‐responders (NR2) Viral load (mean HCV RNA (log10 IU/mL)): peginterferon alfa‐2a: 1.20 ± 0.43 (MIU/mL). peginterferon alfa‐2b: 1.38 ± 1.85 (MIU/mL). Histology at biopsy (total Knodell score): peginterferon alfa‐2a: 10 ± 2.4 peginterferon alfa‐2b: 10.7 ± 2.8. |
|
| Interventions | Group A: n = 58. Drug: peginterferon alfa‐2a: 180mg/week. Drug: ribavirin 800‐1200 mg/day. Group B: n = 58. Drug: peginterferon alfa‐2b :1.5 mg/kg/week. Drug: ribavirin 800 to 1200 mg/day. |
|
| Outcomes | Failure of early virological response. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised in chronological order to be treated with either one of the two products". Comment: quasi‐randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No statement about allocation concealment. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: Blinding of the outcome assessor is not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: The trial did not mention any dropouts or missing data. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Quote: "This study was not financed by any of the pharmaceutical companies producing the Peg‐IFNs." Comment: The study seems to be free of other sources of bias. |
Yenice 2006.
| Methods | Study design: randomised clinical trial. Inclusion criteria: positive antiHCV; normal and/or elevated serum transaminase levels; positive HCV RNA by quantitative polymerase chain reaction (PCR); and at least stage 1 fibrosis according to Knodell Scoring System on liver biopsy. Biochemical criteria: haemoglobin 12 g/dl for women and 13 g/dl for men; leukocyte 3 x 103/mm3; neutrophils 1.5 x 103/mm3; and platelets 100 x 103/mm3; and bilirubin, albumin, and creatinine levels had to be within the normal range. Exclusion criteria: patients with abdominal ascites; history of bleeding from oesophageal varicosities; HCC or other malignant disorders; positive test results for hepatitis B virus (HBV), hepatitis D virus (HDV) or human immunodeficiency virus (HIV) antibodies or antigens; use of antidepressants or tranquillising agents for more than three months; a history of depression, psychosis or suicide attempt; and significant cardiac or pulmonary problems were excluded. ITT: no, only per protocol analysis. Sample size calculation: not mentioned. |
|
| Participants | Study location: Turkey. Total number: 80. Age ‐ mean: peginterferon alpha 2a: M/F 48.2/50.9, peginterferon alpha 2b: M/F 50.8/50.85. Sex (male sex (n (%))): peginterferon alpha 2a: 13 (35%), peginterferon alpha 2b: 10 (27%). Comorbidity: none of the patients had positive test results for hepatitis B virus (HBV), hepatitis D virus (HDV), or human immunodeficiency virus (HIV) antibodies or antigens. Genotype: 1. Previous HCV treatment: naive. Viral load (mean HCV RNA (log10 IU/ml)): not mentioned. Histology at biopsy: all patients had least stage 1 fibrosis according to Knodell scoring system on liver biopsy. |
|
| Interventions | Group A: allocated 40 patients but finally analysed n = 37. Drug: peginterferon alpha‐2a: 180 μg/week. Drug: ribavirin (40 to 64 kg: 800 mg; 65 to 85 kg: 1000 mg; > 85 kg: 1200 mg). Group B: allocated 40 patients but finally analysed n = 37. Drug: peginterferon alpha‐2b (PEG‐Intron): 1.5 μg/kg/week. Drug: ribavirin (40 to 64 kg: 800 mg; 65 to 85 kg: 1000 mg; > 85 kg: 1200 mg). |
|
| Outcomes | Sustained virological response. Adverse events. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who met the selection criteria were randomly assigned into two treatment groups". Comment: the method used for sequence generation is not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method used for allocation concealment was not mentioned. |
| Blinding (performance bias and detection bias) svr | Unclear risk | Comment: It is not mentioned if the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The assessments were limited to patients who received the full dose of pegylated interferon for 48 weeks". Comment: Six patients were excluded due to adverse events , so they were not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All clinically relevant and reasonably expected outcomes were reported. |
| Other bias | Low risk | Comment: The trial seems to be free of other sources of bias. |
SVR: sustained virological response. ITT: intention‐to‐treat analysis. HCV: hepatitis C virus. HCC: hepatocellular carcinoma. PCR: polymerase chain reaction. RBV: ribavirin. EVR: early virological response. ETR: end of treatment response.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrade 2006 | Retrospective study of a randomised trial to assess the vascular ophthalmalgic side effects associated with antiviral therapy for chronic hepatitis C. |
| Barros 2010a | Cost effectiveness analysis, not a randomised clinical trial. |
| Barros 2010b | Cost effectiveness analysis, not a randomised clinical trial. |
| Bruchfeld 2006 | Non‐randomised clinical study. |
| Cozzolongo 2006 | Retrospective study. |
| Craxi 2008 | Non‐randomised clinical study. |
| El Raziky 2013 | Retrospective study. |
| Escudero 2008 | Non‐randomised clinical study. |
| Espinosa 2007 | Retrospective study to assess anaemia associated with antiviral therapy for chronic hepatitis C. |
| Hofmann 2006 | Retrospective study. |
| Lee 2010 | Retrospective, non‐randomised study. |
| Rumi 2012 | Review article. |
| Villa 2012 | Non‐randomised clinical study. |
| Witthoeft 2008 | Retrospective study. |
Differences between protocol and review
We redefined the 'Risk of bias' subgroup analysis as analysis between trials that are assessed to be at a low or high risk of bias instead of trials that are assessed to be at a low, unclear, or high risk of bias. This is in accordance with the recommendation from the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011) and previous research on the topic (Wood 2008; Savovic 2012; Savovic 2012a).
We changed the outcome failure of sustained virological response into sustained virological response for ease of interpretation.
We changed the sequence of outcomes in accordance with recommendations in the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011). We decided to change the outcomes in order to give priority to the outcomes which are of greater interest for patients and clinicians such as liver‐related morbidity, liver‐related mortality, all‐cause mortality, and quality of life instead of unvalidated surrogate outcomes such as sustained virological response. We reported the primary and secondary outcomes. As randomised clinical trials need to inform clinical practice, clinical outcomes such as risk of liver failure, hepatocellular carcinoma, and mortality would be of greater interest to patients and clinicians, but unfortunately these data are lacking in the current literature.
A Summary of Findings table was constructed, rating the evidence on the review outcomes.
The original protocol outcomes were as follows.
Primary outcome
1. Failure of sustained virological response: number of patients with detectable hepatitis C virus RNA in serum by sensitive test six months later. 2. Liver‐related morbidity plus all‐cause mortality: number of patients who developed cirrhosis, ascites, variceal bleeding, hepatic encephalopathy, hepatocellular carcinoma, or died. 3. Adverse events: number of patients with any untoward medical occurrence not necessarily having a causal relationship with the treatment. We will report on adverse events that lead to treatment discontinuation and those that have not lead to treatment discontinuation separately. We have defined serious adverse events according to the International Conference on Harmonisation (ICH) Guidelines (ICH‐GCP 1997) as any event that leads to death, is life‐threatening, requires in‐patient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability, and any important medical event, which may have jeopardised the patient or requires intervention to prevent it. All other adverse events will be considered non‐serious.
Secondary outcomes
1. Failure of end of treatment virological response: number of patients with detectable hepatitis C virus RNA at the end of treatment. 2. Failure of early virological response: number of patients with detectable hepatitis C virus RNA or without 2 log IU/ml reduction in relation to the baseline amount assessed 12 weeks after the introduction of the treatment. 3. Failure of biochemical response:number of patients without improvement in the liver enzymes levels (eg, AST and ALT). 4. Failure of histological response: number of patients without improvement of histology (inflammation score or fibrosis score as defined by the individual trials). 5. Quality of life as defined in the individual trials.
The updated review protocol outcomes are as follows.
Primary outcomes
All‐cause mortality.
Liver‐related morbidity: number of patients who developed ascites, variceal bleeding, hepatic encephalopathy, hepatocellular carcinoma, progression of bilirubinaemia, or died.
Adverse events: serious adverse events, adverse events leading to treatment discontinuation, and all other (non‐serious) adverse events.
Quality of life as defined in the individual trials.
Secondary outcomes
Sustained virological response (SVR): number of patients with undetectable hepatitis C virus RNA in their serum by a sensitive test six months after the end of treatment.
In our protocol we included baseline imbalance and early stopping as potential bias components. According to updated guidelines from The Cochrane Collaboration, this should no longer be used (Gluud 2013).
Contributions of authors
Goran Hauser performed the literature search, data extraction, assessed the risk of bias of the included trials, performed all statistical analyses that could be performed in RevMan, TSA, and GRADE, contributed to the interpretation of results, and revised the review. Tahany Awad drafted the protocol (Awad 2009; Awad 2010), performed the literature search, performed the data extraction, assessed the risk of bias of the included trials, contributed to the interpretation of results, and drafted the review. Kristian Thorlund revised the protocol, contributed to the interpretation of results, and revised the review. Davor Stimac revised the protocol, contributed to the interpretation of results, and revised the review. Mahasen Mabrouk revised the protocol, acted as arbitrator when there were disagreements in data extraction and the bias risk assessment, contributed to the interpretation of results, and revised the review. Christian Gluud revised the protocol, acted as arbitrator when there were disagreements in data extraction and the bias risk assessment, contributed to the interpretation of results, and revised the review.
Sources of support
Internal sources
Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Denmark.
External sources
-
ERASMUS project, Croatia.
Erasmus staff mobility was organised by the University of Rijeka and realised with the financial support of the European Commission under the auspices of the LLP Erasmus program.
Declarations of interest
Tahany Awad was an invited speaker for Roche and is now employed by AbbVie.
New
References
References to studies included in this review
Ascione 2010 {published data only}
- Ascione A, Luca M, Tartaglione MT, Lampasi F, Lanza AG, Picciotto FP, et al. Peginterferon alfa‐2a plus ribavirin Is more effective than peginterferon alfa‐2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology 2010;138(1):116‐22. [DOI] [PubMed] [Google Scholar]
- Ascione A, Luca M, Tartaglione MT, Lampasi F, Lanza AG, Picciotto FP, et al. Peginterferon alpha‐2a plus ribavirin versus peginterferon alpha‐2b plus ribavirin in naive patients with chronic hepatitis C virus infection: results of a prospective randomised trial. Abstracts of the 43rd Annual Meeting of the European Association for the Study of the Liver. Hepatology 2008;48(S2):370. [Google Scholar]
Berak 2005 {published data only}
- Berak H, Horban A, Wasilewski M, Stanczak JJ, Kolakowska‐Rzadzka A. Randomized, open label trial comparing efficacy and safety of pegylated interferon alfa 2A vs alfa 2B treatment of patients with chronic hepatitis C infected with non 2/3 genotypes‐12 week virological response analysis. Hepatology 2005;42(4 S1):684A. [Google Scholar]
Bruno 2004 {published data only}
- Bruno R, Sacchi P, Ciappina V, Zochetti C, Patruno S, Maiocchi L, et al. Viral dynamics and pharmacokinetics of peginterferon alpha‐2a and peginterferon alpha‐2b in naive patients with chronic hepatitis C: a randomized, controlled study. Antiviral Therapy 2004;9(4):491‐7. [PubMed] [Google Scholar]
- Bruno R, Sacchi P, Maiocchi L, Zocchetti C, Ciappina V, Patruno S, et al. Area‐under‐the‐curve for peginterferon alpha‐2a and peginterferon alpha‐2b is not related to body weight in treatment‐naive patients with chronic hepatitis C. Antiviral Therapy 2005;10(2):201‐5. [PubMed] [Google Scholar]
- Bruno R, Sacchi P, Scagnolari C, Torriani F, Maiocchi L, Patruno S, et al. Pharmacodynamics of peginterferon alpha‐2a and peginterferon alpha‐2b in interferon‐naïve patients with chronic hepatitis C: a randomized, controlled study. Alimentary Pharmacology & Therapeutics 2007;26(3):369‐76. [DOI] [PubMed] [Google Scholar]
Di Bisceglie 2007 {published data only}
- Bisceglie AM, Ghalib RH, Hamzeh FM, Rustgi VK. Early virologic response after peginterferon alpha‐2a plus ribavirin or peginterferon alpha‐2b plus ribavirin treatment in patients with chronic hepatitis C. Viral Hepatitis 2007;14(10):721‐9. [DOI] [PubMed] [Google Scholar]
- Bisceglie AM, Rustgi VK, Thuluvath P, Davis M, Ghalib R, Lyons MF, et al. Pharmacokinetics and pharmacodynamics of pegylated interferon alfa‐2A or alfa‐2B with ribavirin in treatment naive patients with genotype 1 chronic hepatitis C. Hepatology 2004;40(S4):734A. [Google Scholar]
Kamal 2011 {published data only}
- Kamal MS, Ahmed A, Mahmoud S, Nabegh L, Gohary I, Obadan I, et al. Enhanced efficacy of pegylated interferon alpha‐2a over pegylated interferon and ribavirin in chronic hepatitis C genotype 4A randomised trial and quality of life analysis. Liver International 2011;31:401‐11. [DOI] [PubMed] [Google Scholar]
Kolakowska 2008 {published data only}
- Kolakowska A, Berok H, Wasilewski M, Horbon A. Relevance between fibrosis and response to treatment with peginterferon alfa2a vs alfa2b with ribavirin in chronic hepatitis C genotype 3 patients. Randomized open label study. Hepatology 2008;48(4):1278. [Google Scholar]
Laguno 2009 {published data only}
- Laguno M, Cifuentes C, Murillas J, Veloso S, Larrousse M, Payeras A, et al. Randomized trial comparing pegylated interferon alpha‐2b versus pegylated interferon alpha‐2a, both plus ribavirin, to treat chronic hepatitis C in human immunodeficiency virus patients. Hepatology 2009;49(1):22‐31. [DOI] [PubMed] [Google Scholar]
Mach 2011 {published data only}
- Mach TH, Cieśla A, Warunek W, Janas‐Skulina U, Cibor D, Owczarek D. Efficacy of pegylated interferon alfa‑2aor alfa‑2b in combination with ribavirin in the treatment of chronic hepatitis caused by hepatitis C virus genotype 1b. Polskie Archiwum Medycyny Wewnetrznej 2011;121:434‐40. [PubMed] [Google Scholar]
Marcellin 2011 {published data only}
- Marcellin P, Forns X, Goeser T, Ferenci P, Nevens F, Carosi G, et al. Telaprevir is effective given every 8 or 12 hours with ribavirin and peginterferon alfa‐2a or ‐2b to patients with chronic hepatitis C. Gastroenterology 2011;140:459‐68. [DOI: 10.1053/j.gastro.2010.10.046] [DOI] [PubMed] [Google Scholar]
McHutchison 2009 {published data only}
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa‐2b or alfa‐2a with ribavirin for treatment of hepatitis C infection. New England Journal of Medicine 2009;361(6):580‐93. [DOI] [PubMed] [Google Scholar]
Miyase 2012 {published data only (unpublished sought but not used)}
- Miyase S, Haraoka K, Ouchida Y, Morishita Y, Fujiyama S. Randomized trial of peginterferon a‐2a plus ribavirin versus peginterferon a‐2b plus ribavirin for chronic hepatitis C in Japanese patients. Journal of Gastroenterology 2012;47(9):1014‐21. [DOI] [PubMed] [Google Scholar]
- Miyase S, Morishita Y, Haraoka K, Ouchida Y, Fujiyama S. PEGIFNalpha‐2A plus ribavirin shows higher SVR rate compare with PEGIFNalpha‐2B plus ribavirin in elderly patients with chronic hepatitis C specially in IL‐28B major allele. Journal of Hepatology 2012;56(Suppl 2):S451. [Google Scholar]
Rumi 2010 {published data only}
- Rumi M, Aghemo A, Prati GM, D'Ambrosio R, Donato MF, Russo R, et al. Randomized study comparing peginterferon‐alfa2a plus ribavirin and peginterferon‐alfa2b plus ribavirin in naïve patients with chronic hepatitis C: final results of the Milan Safety Tolerability (MIST) study. Hepatology 2008;48(4):A212. [Google Scholar]
- Rumi M, Aghemo A, Prati GM, D'Ambrosio R, Donato MF, Russo R, et al. Randomized study of peginterferon‐∝2a plus ribavirin vs peginterferon‐∝2b plus ribavirin in chronic hepatitis C. Gastroenterology 2010;138(1):108‐15. [DOI] [PubMed] [Google Scholar]
Scotto 2008 {published data only}
- Scotto G, Fazio V, Fornabaio C, Tartaglia A, Tullio R, Saracino A, et al. Early and sustained virological response in non‐responders with chronic hepatitis C; a randomized open‐label study of pegylated interferon‐α‐2a versus pegylated interferon‐α‐2b. Drugs 2008;68(6):791‐801. [DOI] [PubMed] [Google Scholar]
- Scotto G, Fazio V, Fornabaio C, Tartaglia A, Tullio R, Saracino A, et al. Peg‐interferon alpha‐2a versus peg‐interferon alpha‐2b in non responders with HCV active chronic hepatitis: A pilot study. Interferon and Cytokine Research 2008;28:623–30. [DOI] [PubMed] [Google Scholar]
Silva 2006 {published data only}
- Silva M, Poo J, Wagner F, Jackson M, Cutler D, Grace M, et al. A randomised trial to compare the pharmacokinetic, pharmacodynamic, and antiviral effects of peginterferon alfa‐2b and peginterferon alfa‐2a in patients with chronic hepatitis C (COMPARE). Hepatology 2006;45(2):204‐13. [DOI] [PubMed] [Google Scholar]
- Silva M, Poo‐Ramirez JL, Wagner F, Jackson M, Laughlin M. Compare trial: Updated data. Hepatology 2008;49(2):288‐9. [DOI] [PubMed] [Google Scholar]
- Silva M, Poo‐Ramirez JL, Wagner F, Jackson M, Laughlin M. Comparison of peginterferon‐alpha2a and peginterferon‐alpha2B pharmacokinetics and pharmacodynamic in compare, a randomized, prospective, blinded trial. Hepatology 2004;40(4 S1):192A. [Google Scholar]
Sinha 2004 {published data only}
- Sinha S, Gulur P, Patel V, Hage‐Nassar G, Tenner S. A randomized prospective clinical trial comparing pegylated interferon alpha 2a/ribavirin versus pegylated interferon alpha 2b/ribavirin in the treatment of chronic hepatitis C. American Journal of Gastroenterology 2004;99(10):237. [Google Scholar]
Sporea 2006 {published data only}
- Sporea I, Danila M, Sirli R, Popescu A, Laza A, Baditoiu L. Comparative study concerning the efficacy of Peg‐IFN alpha‐2a versus Peg‐IFN alpha‐2b on the early virological response (EVR) in patients with chronic viral C hepatitis. Journal of Gastrointestinal and Liver Diseases 2006; Vol. 15, issue 2:125‐30. [PubMed]
Yenice 2006 {published data only}
- Yenice N, Mehtap O, Gumrah M, Arican N. The efficacy of pegylated interferon alpha 2a or 2b plus ribavirin in chronic hepatitis C patients. Turkish Journal of Gastroenterology 2006;17(2):94‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Andrade 2006 {published data only}
- Andrade RJ, González FJ, Vázquez L, Cilvetti A, Camargo R, García‐Cortés M, et al. Vascular ophthalmological side effects associated with antiviral therapy for chronic hepatitis C are related to vascular endothelial growth factor levels. Antiviral Therapy 2006;11(4):491‐8. [PubMed] [Google Scholar]
Barros 2010a {published data only}
- Barros FMR, Cheinquer H, Borges LG, Santos E. Cost‐effectiveness analysis of treatment with peginterferon‐alfa‐2a versus peginterferon‐alfa‐2b for patients with genotypes 2/3 chronic hepatitis C under the public payer perspective in Brazil. Value in Health 2010;13(3):A71‐A72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barros 2010b {published data only}
- Barros FMR, Cheinquer H, Borges LG, Santos E. Cost‐effectiveness analysis of treatment with peginterferon‐alfa‐2a versus peginterferon‐alfa‐2b for patients with genotype 1 chronic hepatitis C under the public payer perspective in Brazil. Value in Health 2010;13(3):A72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruchfeld 2006 {published data only}
- Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R. Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. Journal of Viral Hepatology 2006;13(5):316‐21. [DOI] [PubMed] [Google Scholar]
Cozzolongo 2006 {published data only}
- Cozzolongo R, Sartorie M, Lanzilotta E, Tonello N, Manghisi OG. Comparison between the two peginterferon's a in the treatment of chronic hepatitis C. Journal of Hepatology 2006;44(S2):209. [Google Scholar]
Craxi 2008 {published data only}
- Craxi A, Piccinino F, Alberti A. Predictors of SVR in naïve HCV G1 patients in real life practice: the probe. Journal of Hepatology 2008;2(48):S291. [Google Scholar]
El Raziky 2013 {published data only}
- Raziky M, Fathalah WF, El‐Akel WA, Salama A, Esmat G, Mabrouk M, et al. The effect of peginterferon alpha‐2a vs. peginterferon alpha‐2b in treatment of naive chronic HCV genotype‐4 patients: A single centre Egyptian study. Hepatitis Monthly 2013;13:e100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Escudero 2008 {published data only}
- Escudero A, Rodríguez F, Serra MA, Olmo J, Montes F, Rodrigo JM. Pegylated interferon‐2a plus ribavirin compared with pegylated interferon‐2b plus ribavirin for initial treatment of chronic hepatitis C virus: Prospective, non‐randomized study. Journal of Gastroenterology and Hepatology 2008;23(6):861‐6. [DOI] [PubMed] [Google Scholar]
Espinosa 2007 {published data only}
- Espinosa M, Arenas MD, Aumente MD, Barril G, Buades JM, Aviles B, et al. Anemia associated with pegylated interferon‐alpha 2a and alpha 2b therapy in haemodialysis patients. Clinical Nephrology 2007;67:366‐73. [DOI] [PubMed] [Google Scholar]
Hofmann 2006 {published data only}
- Hofmann WP, Bock H, Weber C, Tacke W, Pfaff R, Kihn R, et al. Effectiveness of antiviral therapy in patients with chronic hepatitis C treated by private practice gastroenterologists. Zeitschrift für Gastroenterologie 2006;44(1):25‐31. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee S, Kim IH, Kim SH, Kim SW, Lee SO, Lee ST. Efficacy and tolerability of pegylated interferon‐alpha 2a plus ribavirin versus pegylated interferon‐ alpha 2b plus ribavirin in treatment‐naive chronic hepatitis C patients. Intervirology 2010;53:146‐53. [DOI] [PubMed] [Google Scholar]
Rumi 2012 {published data only}
- Rumi M, Aghemo A, Prati GM. Comparative trials of peginterferon alpha 2a and peginterferon alpha 2b for chronic hepatitis C. Journal of Viral Hepatitis 2012;19:37‐41. [DOI] [PubMed] [Google Scholar]
Villa 2012 {published data only}
- Villa E, Camma C, Leo A, Karampatou A, Enea M, Gitto S, et al. Peginterferon‐A 2B plus ribavirin is more effective than peginterferon‐A 2A plus ribavirin in menopausal women with chronic hepatitis C. Journal of Viral Hepatitis 2012;19:640–9. [DOI] [PubMed] [Google Scholar]
Witthoeft 2008 {published data only}
- Witthoeft T, Hueppe D, John C, Goelz J, Meyer U, Heyne R, et al. Efficacy and safety of peginterferon alfa‐2a or ‐2b plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Germany: The practice study. Abstracts of the 43rd Annual Meeting of the European Association for the Study of the Liver. Hepatology 2008;48(S2):315. [Google Scholar]
Additional references
Adeel 2009
- Adeel AB, Xiao QW, Charity GM. Effect of hepatitis C virus and its treatment on survival. Hepatology 2009;50(2):387‐92. [DOI] [PubMed] [Google Scholar]
Alavian 2010
- Alavian SM, Behnava B, Tabatabaei SV. The comparative efficacy and safety of peginterferon alpha‐2a vs. 2b for the treatment of chronic HCV infection: a meta‐analysis. Hepatitis Monthly 2010;10:121‐31. [PMC free article] [PubMed] [Google Scholar]
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: A reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. [DOI] [PubMed] [Google Scholar]
Bailon 2001
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung W‐J, Porter JE, et al. Rational design of a potent, long‐lasting form of interferon: a 40 kDa branched polyethylene glycol‐conjugated interferon alpha‐2a for the treatment of hepatitis. Bioconjugate Chemistry 2001;12(2):195‐202. [DOI] [PubMed] [Google Scholar]
Bangalore 2008
- Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli F. Perioperative β blockers in patients having non‐cardiac surgery: a meta‐analysis. Lancet 2008;372(9654):1962‐76. [DOI] [PubMed] [Google Scholar]
Barros 2010c
- Barros FMR, Cheinquer H, Borges LG, Santos E. Is there any difference between the effects of therapy with peginterferon‐alpha‐2a versus standard‐dose peginterferon‐alpha‐2b? A meta‐analysis comparing both treatments plus ribavirin in genotype 1/4 chronic hepatitis C virus (HCV) infection patients. Value in Health 2010;13:A368. [Google Scholar]
Benvegnu 2001
- Benvegnu L, Alberti A. Patterns of hepatocellular carcinoma development in hepatitis B virus and hepatitis C virus‐related cirrhosis. Antiviral Research 2001;52:199‐207. [DOI] [PubMed] [Google Scholar]
Brok 2005
- Brok J, Gluud LL, Gluud C. Ribavirin plus interferon versus interferon for chronic hepatitis C. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005445] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Gluud C, Wetterslev J. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment for random error risk in conclusive Cochrane neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI: 10.1093/ije/dyn188] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheinquer 2010
- Cheinquer H, Barros FMR, Borges LG, Santos E, Buschinelli CT. Systematic review and meta‐analysis of published randomised controlled trials comparing the efficacy of peginterferon‐alpha‐2a versus peginterferon alpha‐2b both plus ribavirin in chronic hepatitis C patients. Value in Health 2010;13:A69. [Google Scholar]
Coppola 2011
- Coppola N, Pisaturo M, Sagnelli C, Tonziello G, Sagnelli E, Angelillo IF. Efficacy and tolerability of peginterferon α‐2a and α‐2b in patients with chronic hepatitis C by genotype 1: A meta‐analysis. Digestive and Liver Diseases 2011;43(Suppl):S94. [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ 2011 (accessed 27 May 2013).
Davis 1999
- Davis GL. Hepatitis C virus genotypes and quasi species. American Journal of Medicine 1999;107(6B):21S‐6S. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Di Biscceglie 2011
- Bisceglie AM, Stoddard AM, Dienstag JL, Shiffman ML, Seeff LB, Bonkovsky HL, et al. Excess mortality in patients with advanced chronic hepatitis C treated with long‐term peginterferon. Hepatology 2011;53:1100‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Druyits 2012
- Druyts E, Mills EJ, Nachega J, O’Regan C, Cooper CL. Differences in clinical outcomes among hepatitis C genotype 1‐infected patients treated with peginterferon alpha‐2a or peginterferon alpha‐2b plus ribavirin: a meta‐analysis. Clinical and Experimental Gastroenterology 2012;5:11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
EASL 2012
- European Association for the Study of the Liver. European Association for the Study of the Liver, EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. Journal of Hepatology 2011;55(2):245‐64. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fattovich 2002
- Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E, European Concerted Action on Viral Hepatitis (EUROHEP). Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. American Journal of Gastroenterology 2002;97(11):288‐95. [DOI] [PubMed] [Google Scholar]
Fernandez‐ Rodriguez 2010
- Fernández‐Rodríguez CM, Alonso S, Martinez SM, Forns X, Sanchez‐Tapias JM, Rincón D. Peginterferon plus ribavirin and sustained virological response in HCV‐related cirrhosis: outcomes and factors predicting response. American Journal of Gastroenterology 2010;105:2164‐72. [DOI] [PubMed] [Google Scholar]
Flori 2013
- Flori N, Funakoshi N, Duny Y, Valats JC, Bismuth M, Christophorou D, et al. Pegylated interferon‐a2a and ribavirin versus pegylated interferon‐a2b and ribavirin in chronic hepatitis C. A meta‐analysis. Drugs 2013;73:263‐77. [DOI] [PubMed] [Google Scholar]
Foster 2004
- Foster GR. Pegylated interferons: chemical and clinical differences. Alimentary Pharmacology & Therapeutics 2004;20(8):825‐30. [DOI] [PubMed] [Google Scholar]
Fried 2002
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa‐2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Human Services 2002;347:975–82. [DOI] [PubMed] [Google Scholar]
Ghany 2009
- Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49(4):1335‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghany 2011
- Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:1433–44. [DOI: 10.1002/hep.24641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glue 2000
- Glue P, Fang JWS, Rouzier‐Panis R, Raffanel C, Sabo R, Gupta SK, et al. Pegylated interferon‐alpha 2b: Pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Clinical Pharmacology and Therapeutics 2000;68(5):556‐67. [DOI] [PubMed] [Google Scholar]
Gluud 2007
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. [DOI] [PubMed] [Google Scholar]
Gluud 2013
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 12. Art. No.: LIVER.
Gurusamy 2014
- Gurusamy KS, Wilson E, Koretz RL, Allen VB, Davidson BR, Burroughs AK, et al. Is sustained virological response a marker of treatment efficacy in patients with chronic hepatitis C viral infection with no response or relapse to previous antiviral intervention?. PLoS ONE 2014;8(12):e83313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadziyannis 2004
- Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon alfa‐2a (40 kilodaltons) and ribavirin combination therapy in chronic hepatitis C: randomized study of the effect of treatment duration and ribavirin dose. Annals of Internal Medicine 2004;140:346–55. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirofumi 2009
- Hirofumi U, Sherri OS, Katsuhiro H, Kotaro K, Fumisato S, Shuji K, et al. Increased rate of death related to presence of viremia among hepatitis C virus antibody‐positive subjects in a community‐based cohort study. Hepatology 2009;50(2):393‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodgson 2003
- Hodgson HJF. Viral hepatitis ‐ clinical aspects. In: Warrell DA, Cox TM, Firth JD, Benz EJ Jr editor(s). Oxford Textbook of Medicine. 4th Edition. Oxford/ New York: Oxford University Press, 2003. [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ (Clinical Research Ed.) 1999;319:670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Ioannidis 2005
- Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005;294(2):218‐28. [DOI] [PubMed] [Google Scholar]
Ioannidis 2005a
- Ioannidis JP. Why most published research findings are false. PLoS Medicine 2005;2(8):e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between small and large randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Koretz 2013
- Koretz RL, Pleguezuelo M, Arvaniti V, Baena PB, Ciria R, Gurusamy KS, et al. Interferon for interferon nonresponding and relapsing patients with chronic hepatitis C. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003617.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacchetti 2002
- Lacchetti C, Guyatt G. Therapy and validity: surprising results of randomized controlled trials. In: Guyatt G, Rennie D editor(s). Users' Guides to the Medical Literature: A Manual for Evidence‐Based Clinical Practice. Chicago: AMA Press, 2002:247‐65. [Google Scholar]
Lau 1995
- Lau J, Schmid C, Chalmers T. Cumulative meta‐analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):45‐57. [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee K, Bacchetti P, Sim I. Publication of clinical trials supporting successful new drug applications: A literature analysis. PLoS Medicine 2008;5(9):e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ (Clinical Research Ed.) 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Manns 2001
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358(9286):958‐65. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2012
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2012;10:28‐55. [DOI] [PubMed] [Google Scholar]
Morgan 2010
- Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52:833‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2002
- Myers RP, Regimbeau C, Thevenot T, Leroy V, Mathurin P, Opolon P, et al. Interferon for interferon naive patients with chronic hepatitis C. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000370] [DOI] [PMC free article] [PubMed] [Google Scholar]
OPTN 2008
- United Network for Organ Sharing. Chapter VI ; Liver and Intestine Transplantation in the United States, 1998‐2007. optn.transplant.hrsa.gov/ar2008/chapter_iv_AR_cd.htm?cp=5 (accessed 27 May 2013).
Pasut 2011
- Pasut G, Veronese FM. State of the art in PEGylation: The great versatility achieved after forty years of research. Journal of Controlled Release 2011;161:461‐72. [DOI] [PubMed] [Google Scholar]
Penin 2004
- Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky J. Structural biology of hepatitis C virus. Hepatology 2004;39(1):5‐19. [DOI: 10.1002/hep.20032] [DOI] [PubMed] [Google Scholar]
Rambaldi 2008
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Glucocorticosteroids for alcoholic hepatitis – a Cochrane Hepato‐Biliary Group Systematic Review with meta‐analyses and trial sequential analyses of randomised clinical trials. Alimentary Pharmacology & Therapeutics 2008;27(12):1167‐78. [DOI] [PubMed] [Google Scholar]
Reddy 2001
- Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, et al. Efficacy and safety of pegylated (40‐kd) interferon alpha‐2a compared with interferon alpha‐2a in noncirrhotic patients with chronic hepatitis C. Hepatology 2001;33(2):433–8. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Romero‐Gomez 2012
- Romero‐Gomez M, Planas R, Sola R, Garcıa‐Samaniego J, Diago M, Crespo J, et al. Peginterferon alpha‐2A achieves higher early virological responses (RVR and CEVR) than peginterferon alpha‐2B in chronic Hepatitis C: Meta‐analysis of randomized clinical trials (RCT). Journal of Hepatology 2012;56:S456. [Google Scholar]
Rosenberg 2001
- Rosenberg S. Recent advances in the molecular biology of hepatitis C virus. Journal of Molecular Biology 2001;313:451‐64. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157:429‐38. [DOI] [PubMed] [Google Scholar]
Savovic 2012a
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Seef 2009
- Seeff LB. The history of the "natural history" of hepatitis C (1968‐2009). Liver International 2009;29(S1):89‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seeff 2002
- Seeff LB, Hoofnagle JH. National institutes of health consensus development conference: Management of hepatitis C: 2002. Hepatology 2002;36(S1):S1‐S2. [DOI: 10.1002/hep.1840360702] [DOI] [PubMed] [Google Scholar]
Singal 2011
- Singal AK, Jampana SC, Anand BS. Peginterferon alfa‐2a is superior to peginterferon alfa‐2b in the treatment of naïve patients with hepatitis C virus infection: meta‐analysis of randomized controlled trials. Digestive Diseases and Sciences 2011;56:2221‐26. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JPA, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI: 10.1093/ije/dyn179] [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 23 April 2013):1‐115.
Trikalinos 2004
- Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. Effect sizes in cumulative meta‐analyses of mental health randomized trials evolved over time. Journal of Clinical Epidemiology 2004;57(11):1124‐30. [DOI] [PubMed] [Google Scholar]
van Regenmorte 2000
- Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, et al. Virus Taxonomy: The Classification and Nomenclature of Viruses. The Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press, 2000:599‐621. [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses.. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Global surveillance and control of hepatitis C. Journal of Viral Hepatitis 1999;6(1):35‐47. [PubMed] [Google Scholar]
WHO 2009
- WHO. International Clinical Trials Registry Platform (ICTRP). www.who.int/ictrp/en/ (accessed 27 May 2013).
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2013
- Yang Z, Zhuang L, Yang L, Chen X. Efficacy and tolerability of peginterferon A‐2a and peginterferon A‐2b, both plus ribavirin, for chronic hepatitis C: A meta‐analysis of randomized controlled trials. Gastroenterology Research and Practice 2013;2013:739029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2010
- Zhao S, Liu E, Chen P, Cheng D, Lu S, Yu Q, et al. A comparison of peginterferon α‐2a and α‐2b for treatment‐naive patients with chronic hepatitis C virus: A meta‐analysis of randomised trials. Clinical Therapeutics 2010;32:1565‐77. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Awad 2009
- Awad T, Thorlund K, Hauser G, Mabrouk M, Stimac D, Gluud C. Pegylated interferon alpha 2a versus pegylated interferon alpha 2b for chronic hepatitis C. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005642.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Awad 2010
- Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha‐2a is associated with higher sustained virological response than peginterferon alfa‐2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51(4):1176‐84. [DOI] [PubMed] [Google Scholar]


