Abstract
Purpose
Direct oral anticoagulants (DOACs) are not recommended in adult Fontan patients (Level of Evidence C). We hypothesized that DOACs are comparable to warfarin and do not increase thrombotic and embolic complications (TEs) or clinically significant bleeds.
Methods
We reviewed the medical records of adult Fontan patients on DOACs or warfarin at three major medical centers. We identified 130 patients: 48 on DOACs and 107 on warfarin. In total, they were treated for 810 months on DOACs and 5637 months on warfarin.
Results
The incidence of TEs in patients on DOACs compared to those on warfarin was not increased in a statistically significant way (hazard ratio [HR] 1.7 and p value 0.431). Similarly, the incidence of nonmajor and major bleeds in patients on DOACs compared to those on warfarin was also not increased in a statistically significant way (HR for nonmajor bleeds in DOAC patients was 2.8 with a p value of 0.167 and the HR for major bleeds was 2.0 with a p value 0.267). In multivariate analysis, congestive heart failure (CHF) was a risk factor for TEs across both groups (odds ratio [OR] = 4.8, 95% confidence interval [CI] = 1.3–17.6) and bleed history was a risk factor for clinically significant bleeds (OR = 6.8, 95% CI = 2.7–17.2).
Conclusion
In this small, retrospective multicenter study, the use of DOACs did not increase the risk of TEs or clinically significant bleeds compared to warfarin in a statistically significant way.
Keywords: ACHD, Anticoagulation, Fontan, DOAC, Thrombus
Introduction
Long-term thromboprophylaxis is recommended for adult Fontan patients with atrial arrhythmias [1–3]. Prescribing practice is largely based on prescriber preference due to conflicting efficacy evidence [1, 4–8]. DOACs, which include the direct thrombin inhibitor (dabigatran) and factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban), are accepted as first-line anticoagulants in many noncongenital patients [9, 10].
Despite favorable clinical trials [11–14] and established indications in select patients [15], experience with DOACs in adult Fontan patients is limited [3, 16–21]. There is a class III, Level of Evidence C, recommendation against the use of DOACs in adult Fontan patients due in part to a lack of safety and efficacy data, along with concerns regarding abnormal Fontan coagulation and liver function [2, 3, 22, 23]. While not well studied in adult Fontan patients specifically, the pharmacokinetic and pharmacodynamic properties of DOACs have been well established in clinical trials and after gaining guideline approval [9–14]. We published a single center study of DOACs in adult Fontan patients showing that DOACs may be reasonable in the short term [24]. Here, we include more patients and compare DOAC outcomes against warfarin.
Methods
Baylor College of Medicine/Texas Children’s Hospital, Emory University, and Vanderbilt University Medical Center identified adult patients with Fontan palliation treated with DOAC or warfarin therapy between 1995 and 2018. Heart transplant patients were excluded. Patients were considered lost to follow-up if there had unexpectedly been no communication for more than 6 months. Since some patients were initiated on therapy more than once, New York Heart Association Functional Classification (NYHA class), CHA2DS2-VASc, and HAS-BLED scores were calculated at each therapy initiation [9].
Definitions
Thrombotic and embolic complications (TEs) were defined as new stroke, transient ischemic attack, deep vein thrombosis, pulmonary embolism, Fontan circuit thrombus, or intracardiac thrombus identified on imaging that was obtained at the discretion of the provider. Arrhythmia warranting anticoagulation included atrial fibrillation and/or atrial flutter/intraatrial reentrant tachycardia as determined by the provider. Procedure prophylaxis included prophylaxis following pulmonary artery stent placement, coil embolization of collateral vessels, patients receiving cardioversion (electrical or chemical), or following arrhythmia procedure (catheter- or surgical-based modified Maze or Cox Maze III procedure). CHF represents history of heart failure documented in the chart whereas NYHA classification of heart failure symptoms was calculated based on what was documented at each independent therapy initiation.
Bleeding events were defined as major, nonmajor, or minor, using previously reported definitions from major DOAC trials [25, 26]. Major bleeding included acute clinical bleeds requiring cessation of therapy coupled with a hemoglobin drop ≥ 2 g/dL in 24 h, transfusion of blood products, or bleeding into a critical site (intracranial, intraspinal, intraocular, pericardial, intraarticular, intramuscular with compartment syndrome, or retroperitoneal). Nonmajor bleeding required cessation of therapy but did not meet major bleeding criteria. “Clinically significant” bleeding was defined as major plus nonmajor bleeding. Minor bleeding included easy bruising, epistaxis, menorrhagia, and any other events that met neither major nor nonmajor criteria.
Warfarin patients’ time in therapeutic range (TTR) was calculated using the Rosendaal linear interpolation method. Therapeutic range was defined as international normalized ratio (INR) values between 2 and 3. Patients were excluded if they had INR gaps longer than 2 months (total therapy ≤ 2 years) or 4 months (total therapy ≥ 2 years). INR was subtherapeutic if < 2 within 48 h of TE, and supratherapeutic if > 3 within 48 h of clinically significant bleed.
Statistical Analysis
Continuous variables were presented as mean with standard deviation (SD) and median with interquartile range. Student’s t test or one-way ANOVA was applied to compare the difference between two or among three groups, respectively, for data with normal distribution. For nonnormal distributed data, the Wilcoxon rank sum test or the Kruskal–Wallis test was used for the comparison in two or three groups, respectively. Categorical variables were expressed as numbers with percentages and compared using the Fisher exact test. To account for multiple initiation events for the same patient, the univariate and multivariable generalized estimating equations were applied to estimate odds ratios of risk factors associated with outcomes. The risk associated with each variable was expressed as an odds ratio (OR) with 95% confidence interval (CI). Propensity score matching was done using study institution, age at therapy initiation, sex, BSA, BMI, pre-surgical anatomy, Fontan type, indication for anticoagulation, CHA2DS2-VASc score, HAS-BLED score, and NYHA classification. SAS version 9.4 (SAS Institute Inc., Cary, NC) was applied for data analysis.
Results
We identified 130 unique adult Fontan patients, either started on therapy as adults or continued on therapy upon turning 18. Because of cross-over among treatment groups, this included 48 patients on a DOAC (apixaban [n = 21], dabigatran [n = 14], and rivaroxaban [n = 13]), and 107 patients on warfarin.
Patient Demographics
Patient demographics were recorded upon a patient’s first initiation of a specific therapy. There was no statistically significant difference between patients’ BSA, BMI, and sex (Tables 1 and 2); however, DOAC patients were older at initiation (median age: 29.5 years) than patients on warfarin (23 years). Most patients were started on anticoagulation because of a prior history of TEs or arrhythmias (72.9% for DOACs, 76.6% for warfarin). A higher proportion of DOAC patients were started on therapy because of a recent procedure (16.7%) compared to patients on warfarin (0.9%). Almost 1 in 6 patients on DOACs and 1 in 3 patients on warfarin were co-treated with antiplatelet medication or an NSAID. Of note, one patient on warfarin was concurrently treated with enoxaparin.
Table 1.
Baseline characteristics
| DOAC (n = 48) | Warfarin (n = 107) | p value | |
|---|---|---|---|
|
| |||
| Time on therapy (months) | |||
| Cumulative | 810 | 5637 | |
| Median | 11.0 | 46.0 | |
| Range | 0.5–68.1 | 0.7–209.9 | |
| Age at therapy start (years) | 29.5 (26–37) | 23 (19–31) | < 0.01 |
| Body surface area | 1.75 (1.64–1.93) | 1.76 (1.62–1.93) | 0.90 |
| Body mass index | 23.68 (21.22–28.93) | 23.39 (20.48–27.53) | 0.55 |
| Female | 22 (45.8) | 52 (48.6) | 0.75 |
| Predominant systemic ventricle anatomy | |||
| Left | 34 (70.8) | 78 (72.9) | 0.46 |
| Right | 7 (14.6) | 20 (18.7) | |
| Unspecified | 7 (14.6) | 9 (8.4) | |
| Fontan type | |||
| AP | 10 (20.8) | 23 (21.5) | 0.28 |
| LT | 22 (45.8) | 55 (51.4) | |
| ECC | 13 (27.1) | 28 (26.2) | |
| Other | 3 (6.3) | 1 (0.9) | |
| Indication for anticoagulation | |||
| Arrhythmia | 24 (50.0) | 43 (40.2) | < 0.01 |
| Thrombosis | 11 (22.9) | 39 (36.4) | |
| Procedure* | 8 (16.7) | 1 (0.9) | |
| Other† | 5 (10.4) | 10 (9.3) | |
| Empiric‡ | 0 | 14 (13.1) | |
| Other agents used | |||
| None | 41 (85.4) | 72 (67.3) | 0.06 |
| Aspirin, Clopidogrel, NSAID | 7 (14.6) | 34 (31.8) | |
| Enoxaparin | 0 | 1 (0.9) | |
If not expressly stated, values are median (interquartile range) or n (%).
Procedure = coiling, stents, antiarrhythmia, cardioversion;
Other = patent fenestration, right-to-left shunt, cyanosis, decreased ventricular function, prosthetic valve, hypercoagulability;
Empiric = Fontan physiology prophylaxis, not documented. Of the 48 patients on a DOAC, 10 were exclusively treated with a DOAC, 25 were at some point on warfarin, and 26 were at some point on aspirin. Of the 107 patients on warfarin, 64 were treated exclusively with warfarin, 25 were at some point on a DOAC, and 31 were at some point on aspirin. Of the 180 patients on aspirin, 136 were exclusively treated with aspirin, 26 were at some point on a DOAC, and 31 were at some point on warfarin. Thirteen patients were, at different points, treated with all three therapies
Table 2.
Baseline characteristics
| DOAC (n = 48) | Warfarin (n = 107) | p value | |
|---|---|---|---|
|
| |||
| CHA2DS2-VASc score | |||
| 0 | 11 (22.9) | 16 (15.0) | 0.25 |
| 1 | 13 (27.1) | 39 (36.4) | |
| 2 | 12 (25.0) | 23 (21.4) | |
| 3 | 8 (16.7) | 18 (16.8) | |
| 4 | 2 ( 4.2) | 6 (5.6) | |
| 5 | 2 ( 4.2) | 0 | |
| N/A | 0 | 5 (4.7) | |
| NYHA classification | |||
| No CHF | 18 (37.5) | 61 (57.0) | 0.15 |
| 1 | 7 (14.6) | 11 (10.3) | |
| 2 | 17 (35.4) | 28 (26.2) | |
| 3 | 6 (12.5) | 7 (6.5) | |
| HASBLED score | |||
| 0 | 22 (45.8) | 38 (35.5) | 0.61 |
| 1 | 15 (31.3) | 33 (30.8) | |
| 2 | 10 (20.8) | 23 (21.5) | |
| 3 | 1 ( 2.1) | 5 (4.7) | |
| 4 | 0 | 3 (2.8) | |
| N/A | 0 | 5 (4.7) | |
| Bleed Hx | 2 (4.2) | 5 (4.7)† | 1.00 |
| TE Hx | 11 (22.9) | 27 (25.2)* | 0.84 |
| Diabetes mellitus | 2 (4.2) | 4 (3.7)* | 1.00 |
| Renal dysfunction | 2 (4.2) | 4 (3.7) | 1.00 |
| Liver disease | 14 (29.2) | 30 (28.0) | 1.00 |
| Labile INR | 4 (8.3)* | 7 (6.5) | 0.74 |
Values are n (%). Bleed Hx, bleed history or predisposition; CHF, congestive heart failure; TE Hx, history of thrombotic/embolic complications; INR, international normalized ratio.
One patient converted to positive history during therapy re-initiation.
The number of warfarin patients with a bleed history/predisposition is underestimated because 7 patients initially did not have a bleed history/predisposition, and then had a bleeding event while on warfarin, and were ultimately restarted on warfarin because of high TE risk. Including these 7 patients who re-started warfarin after a bleeding event increases the proportion of warfarin patients with a bleed history/predisposition from 4.7 to 11.2%, although the p value is not statistically significant when compared to DOAC patients (p = 0.23)
There was no statistical difference between DOAC or warfarin patients’ CHA2DS2-VASc score. A larger proportion of DOAC patients had CHF, but this did not approach statistical significance (p value = 0.15). 45.8% of patients on DOACs had zero HAS-BLED points, compared to 35.5% of patients on warfarin. The presence and/or patency of Fontan fenestration was not clearly documented in a large proportion of cases and was not included in the demographic data.
The number of warfarin patients with a bleed history/predisposition is underestimated because 7 patients did not initially have a bleed history/predisposition, and then had a bleeding event while on warfarin, and were ultimately restarted on warfarin because of high TE risk. Including these 7 patients increases the proportion of warfarin patients with a bleed history/predisposition from 4.7 to 11.2%, although this did not lead to a statistically significant difference when compared to DOAC patients (p value = 0.23).
Multiple patients were started and stopped on the same, or different, therapies. Of the 48 patients on a DOAC, 10 were exclusively treated with a DOAC, 25 were at some point on warfarin, and 26 were at some point on aspirin. Of the 107 patients on warfarin, 64 were treated exclusively with warfarin, 25 were at some point on a DOAC, and 31 were at some point on aspirin.
Thrombotic and Embolic Complications
For patients initiated on therapy more than once, each therapy start event was considered separately when evaluating TE and bleeding event incidence per year. This resulted in 62 DOAC initiation events for the 48 DOAC patients and 140 warfarin initiation events for the 107 warfarin patients.
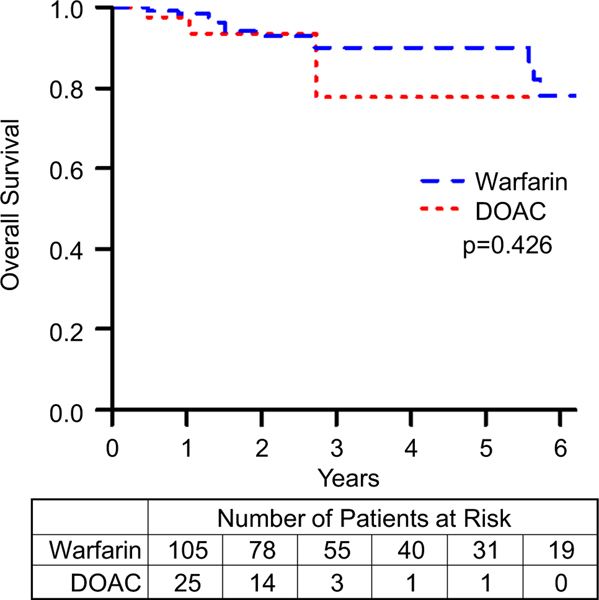
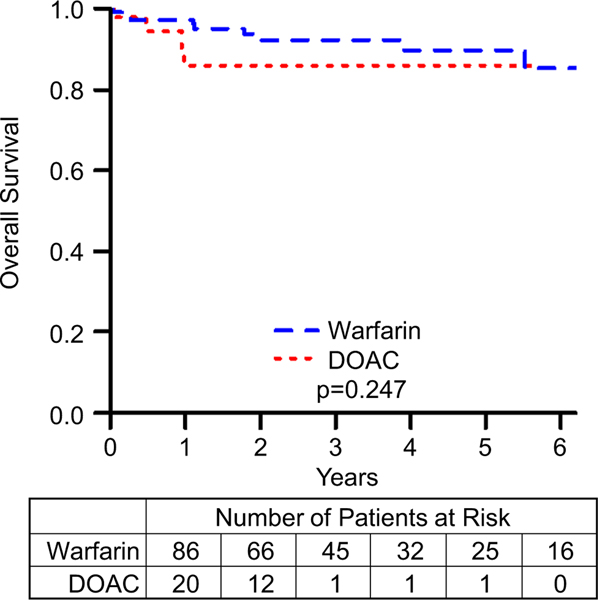
TEs are summarized in Table 3 and the event-free survival data for TEs is summarized in Fig. 1. The difference between the incidence of TEs in patients on DOACs compared to those on warfarin was not statistically significant (HR = 1.7, 95% CI = 0.4–6.3, p value = 0.431). Although the event-free survival data for TEs appears to diverge, favoring Warfarin, this difference did not approach statistical significance. After performing a propensity score matching analysis, we found that there was still no significant difference between the DOAC and warfarin groups (HR = 0.8, 95% CI = 0.2–3.2). Matching resulted in 48 observations in each group, with good balance between them (Supplemental Fig. 1).
Table 3.
Thrombotic and embolic complications (TEs)
| DOAC (n = 48) | Warfarin (n = 107) | p value | |
|---|---|---|---|
|
| |||
| TEs | 3 (6.3) | 12 (11.2) | 0.33 |
Values are n (%)
Fig. 1.
Freedom from thrombotic and embolic complications comparing patients on warfarin to those on DOACs. p represents log-rank p value. Kaplan–Meier curves comparing patients on warfarin to those on DOACs
On average, warfarin patients had TTR of 57% (SD = 24%) based on 39 separate therapy initiations (representing 28% of warfarin initiations); over two-thirds of the warfarin patients were excluded from this measurement for incomplete INR data. Four of the 12 TEs had a recorded INR immediately preceding the event, and of those, 2 were subtherapeutic. Only 1 patient with a TTR estimation (76%) had a TE; as such, an odds ratio relating TTR to risk of TE could not be reported.
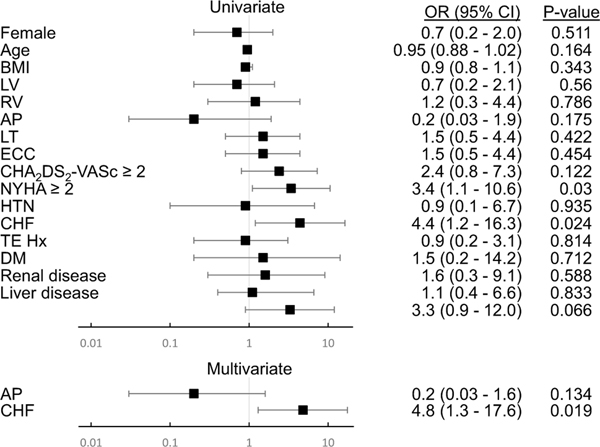
To identify risk factors for TEs, we completed univariate analysis of the data, summarized in Fig. 2, which revealed CHF (OR = 4.4, 95% CI = 1.2–16.3) and symptomatic heart failure (NYHA ≥ 2) (OR = 3.4, 95% CI = 1.1–10.6) as risk factors. On further analysis of the data, any history of CHF increased risk in the multivariate analysis (OR = 4.8, 95% CI = 1.3–17.6), but the NYHA classification at time of therapy initiation did not.
Fig. 2.
Risk factors for thrombotic and embolic complications. OR, odds ration; BMI, body mass index; LV, predominantly systemic left ventricle; RV, predominantly systemic right ventricle; AP, atriopulmonary Fontan subtype; LT, lateral tunnel Fontan subtype; ECC, extracardiac conduit Fontan subtype; CHA2DS2-VASc ≥ 2, CHA2DS2-VASc score for atrial fibrillation stroke risk 2 or greater; NYHA ≥ 2, New York Heart Association heart failure symptom classification 2 or greater; HTN, hypertension; CHF, congestive heart failure; TE Hx, history of thrombotic or embolic complication; DM, diabetes mellitus; Renal disease, history of chronic kidney disease; Liver disease, history of liver disease; Labile INR, labile international normalization ratio with time in therapeutic range < 60%. Forest plot
Bleeding Events
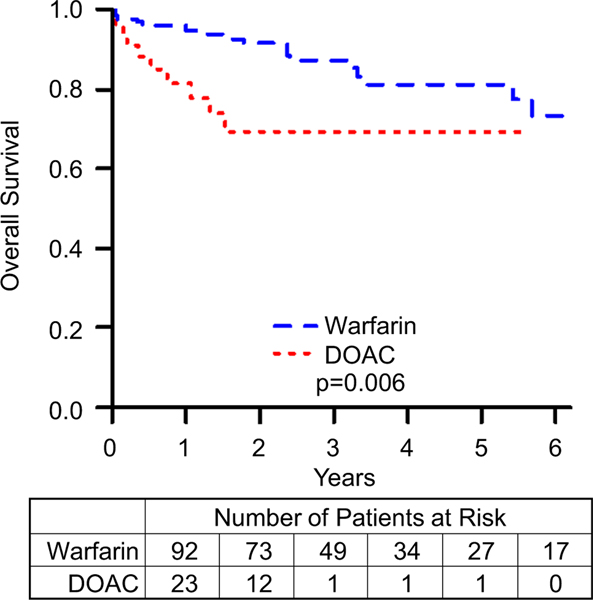
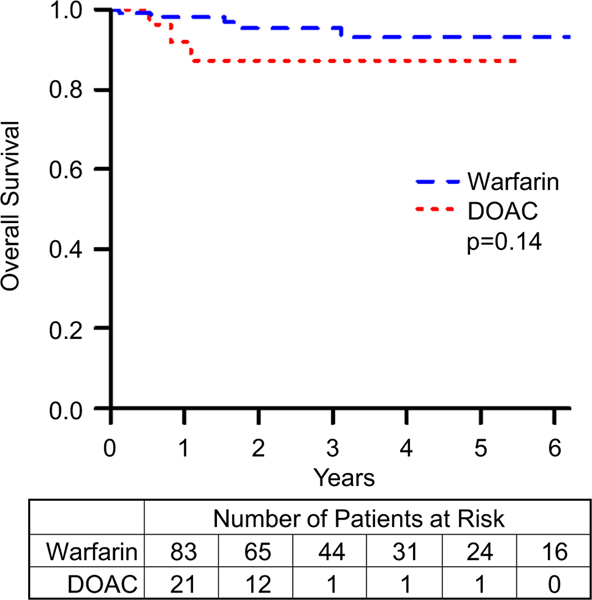
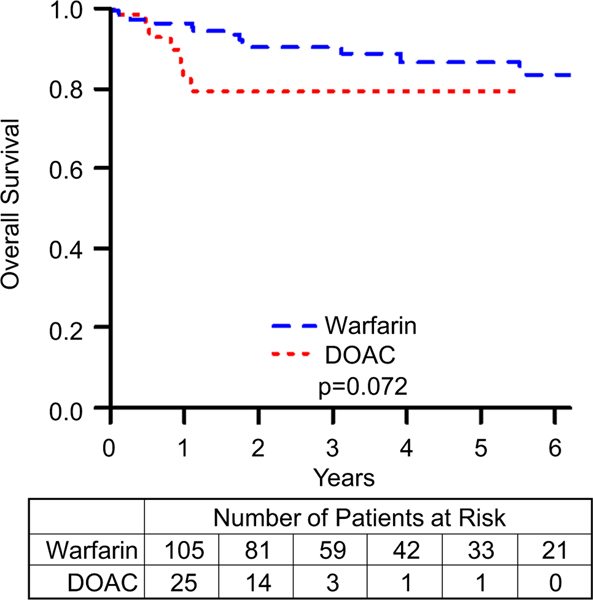
Bleeding events are summarized in Table 4. Freedom from minor, nonmajor, and major bleed data are summarized in Figs. 3, 4, and 5, respectively. Comparing patients on DOACs to those on warfarin, the incidence of nonmajor and major bleeds did not approach statistical significance (nonmajor bleeds: HR = 2.9, 95% CI = 0.7–12.7, p value = 0.159; major bleeds: HR = 2.0, 95% CI = 0.6–6.8, p value = 0.256). However, patients on a DOAC were more likely to have a minor bleeding event (HR = 3.1, 95% CI = 1.3–7.1, p value = 0.008). To further characterize bleeding risk by increasing the number of events, we combined nonmajor and major bleeding events, summarized in Fig. 6, and even here the risk of clinically significant bleeds for patients on DOACs compared to those on warfarin did not reach statistical significance (HR = 2.3, 95% CI = 0.9–5.9, p value = 0.08). Although the event-free survival data for nonmajor, major, and clinically significant bleeds appear to diverge, favoring Warfarin, these differences did not approach statistical significance. After performing a propensity score matching analysis, we found that there was still no significant difference between the DOAC and warfarin groups (RR = 1.4, 95% CI = 0.8–2.5). Matching resulted in 48 observations in each group, with good balance between them (Supplemental Fig. 1).
Table 4.
Bleeding events
| DOAC (n = 48) | Warfarin (n = 107) | p value | |
|---|---|---|---|
|
| |||
| Minor bleed | 11 (22.9) | 19 (17.8) | 0.89 |
| Nonmajor bleed | 3 (6.3) | 6 (5.6) | |
| Major bleed | 4 (8.3) | 9 (8.4) | |
Values are n (%)
Fig. 3.
Freedom from minor bleeds comparing patients on warfarin to those on DOACs. p represents log-rank p value. Kaplan–Meier curves comparing patients on warfarin to those on DOACs
Fig. 4.
Freedom from nonmajor bleeds comparing patients on warfarin to those on DOACs. p represents log-rank p value. Kaplan–Meier curves comparing patients on warfarin to those on DOACs
Fig. 5.
Freedom from major bleeds comparing patients on warfarin to those on DOACs. p represents log-rank p value
Fig. 6.
Freedom from clinically significant bleed comparing patients on warfarin to those on DOACs. p represents log-rank p value. Kaplan–Meier curves comparing patients on warfarin to those on DOACs
Five of the 15 warfarin patients with a clinically significant bleed had TTR data. Having a TTR less than 60% increased the odds of clinically significant bleeds in our study, but this did not reach statistical significance in our patient population (OR = 15.6, 95% CI = 0.80–304). Eight of the 15 clinically significant bleeds had a recorded INR immediately preceding the TE, and of those, only 1 was supratherapeutic.
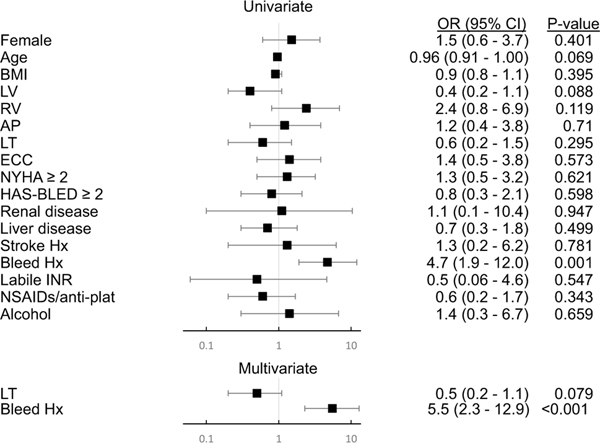
Using univariate and multivariate analysis (Fig. 7), we found a higher odds ratio of clinically significant bleeds among patients with a history of, or predisposition to, bleeding (univariate analysis OR = 4.7, 95% CI = 1.9–12.0; multivariate analysis OR = 5.5, 95% CI = 2.3–12.9).
Fig. 7.
Risk factors for clinically significant bleeds. OR, odds ratio; BMI, body mass index; LV, predominantly systemic left ventricle; RV, predominantly systemic right ventricle; AP, atriopulmonary Fontan subtype; LT, lateral tunnel Fontan subtype; ECC, extracardiac conduit Fontan subtype; NYHA, New York Heart Association heart failure symptom classification; HAS-BLED ≥ 2, HAS-BLED Score for Major Bleeding greater than 2; Renal disease, history of chronic kidney disease; Liver disease, history of liver disease; Stroke Hx, history of cerebrovascular accident; Bleed Hx, history of major bleed; Labile INR, labile international normalization ratio with time in therapeutic range < 60%. NSAIDs/antiplat: concurrent use of nonsteroidal antiinflammatory drugs or antiplatelet medication; Alcohol: drinking the equivalent of 8 or more alcoholic beverages per week. Forest plot
Discussion
Currently, only warfarin enjoys guideline recommendation for long-term thromboprophylaxis for adult Fontan patients with atrial arrhythmias. However, DOACs are much less cumbersome to use, both for the patient and the prescribing provider, in that they do not require close monitoring. The ease of use of DOACs is most evident in short-term prophylaxis (e.g., following an ablation procedure or direct current cardioversion). Based on our experience, prescribing practice is largely based on prescriber preference, with significant variation between institutions.
This is among the first multicenter studies comparing DOAC therapy in adult Fontan patients to warfarin therapy. Two recent studies by Yang et al. look at DOAC therapy in Fontan and ACHD patients (which include the same Fontan cohort) [27, 28]. The publications rely on historical warfarin use as a point of reference for DOACs (n = 74) and so there are fewer people in the warfarin group (n = 37). In both studies, the incidence of major bleeding and thromboembolic phenomenon are equivalent between those taking DOACs and those on warfarin (less than 3 per 100 patient-years for both outcomes). Similar yearly incidences were identified by Pujol et al. [29] when looking at DOACs in a wider variety of ACHD patients. Their paper included only 12 Fontan patients. A recent meta-analysis, which includes our prior publication on 21 Fontan patients [24], summarized the annual rate of thromboembolic and major bleeding events as approximately 3% annually for both [30]. Here we expand on the above by attempting a more rigorous comparison between DOACs and warfarin compared to our prior study: we now have data on a larger cohort, and for a longer period. Our exploratory data suggest that (1) patients on DOACs are not more likely to suffer TEs in the short term and (2) DOAC patients on short-term prophylaxis are not at higher risk of clinically significant bleeding than warfarin patients. Our patient population had an overall TE prevalence between 5.6 and 11.2%.
Thrombotic and Embolic Complications and Bleeding Events in DOAC Patients
We found that DOACs did not increase the risk of TEs when compared to warfarin. Most differences between DOAC and warfarin patient demographics were within statistical error (Table 1). There were, however, key ways in which the two populations differed. Patients on DOAC therapy were older than those on warfarin. More DOAC patients were started on therapy for procedure prophylaxis, an inherently shorter term indication. In contrast, more warfarin patients were on therapy secondary to a history of thrombosis. More warfarin patients had a bleed history, although the p value is not statistically significant (p value = 0.23, even when including warfarin patients who had a bleed on therapy). More DOAC patients had CHF or a higher NYHA classification, although the p value is not statistically significant. Looking at the trends within the Kaplan–Meier curves, we believe it is reasonable to conclude that, at least in short-term prophylaxis (6 months or less), DOACs do not present a statistically significant increased risk of clinically significant bleeds. While patients on DOACs were more likely to suffer minor bleeds, the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) study suggests nuisance bleeding is not associated with a higher risk of major bleeding, stroke, or systemic embolism within 6 months [31].
It is possible that we did not note a difference between risk of TEs and clinically significant bleeds when comparing DOACs to warfarin because the warfarin cohort did not have a sufficiently high TTR (57%). Our TTR only represents about one-third of the total warfarin cohort due to incomplete INR documentation, so it is difficult to say whether low TTR is representative of our patient population. The paucity of data highlights one of the difficulties of medication and testing compliance in this population of patients.
Thrombotic and Embolic Complications and Bleeding Events Overall
In our study, there was a TE prevalence between 5.6 and 11.2% depending on which therapy was used, with no statistically significant difference between the treatment groups. While we noted that C HA2DS2-VASc score was not predictive of TEs, univariate and multivariate analysis identified NYHA ≥ 2 and a diagnosis of heart failure as additional risk factors for TEs.
We found that DOAC and warfarin patients had similar rates of nonmajor and major bleeding events. While we noted that HAS-BLED score was not predictive of bleeding events, univariate and multivariate analysis identified bleed history/predisposition as a risk factor. We believe that, in those patients that meet indications for anticoagulation, have a bleed history/predisposition, and are likely to receive care in a setting without ready access to DOAC reversal agents, the use of warfarin may be more prudent.
Limitations
Although we present one of the larger studies of its kind, there are still fewer than 50 DOAC patients with median time on therapy of 11 months, making it difficult to assess lifelong risk. Furthermore, this is a retrospective study, with wide confidence intervals. In the warfarin cohort, only limited INR data was available; however, calculated TTR was incrementally lower than the presumed target and may have reduced relative effectiveness of warfarin therapy in our population. Given the above limitations, this study would at most be considered exploratory.
Conclusion
We show that there is no statistically significant difference in TEs or clinically significant bleeds, at least in the short term, when comparing patients on DOACs to those on warfarin. Both patient groups demonstrated similar rates of TEs. We identified CHF as a risk factor for TEs. We also identified bleed history/predisposition as a risk factor for clinically significant bleeding events. The current studies pave the way for a much-needed randomized control trial to evaluate the noninferiority of DOACs in comparison to warfarin for the prevention of TEs in adult Fontan patients. We look forward to the data that will become available in the next few years from the NOTE Registry (an international registry on the safety and efficacy of DOACs in ACHD) and the ongoing study by the team behind the PROTECT-AR study (a prospective, observational, multicenter study on the safety and efficacy of apixaban in the prevention of thromboembolism in ACHD patients and atrial arrhythmias).
Supplementary Material
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10557-021-07298-5.
This paper is dedicated to Dr. Justin Georgekutty, a friend and colleague. Without his vision and contributions, this paper would never have been started.
Code Availability Not applicable.
Declarations
Ethics Approval Not applicable (retrospective chart review only).
Declaration of Helsinki This study complies with the Declaration of Helsinki. Each institution’s ethics committee approved the research protocol. Due to the retrospective nature of the study, informed consent was not required to be obtained from the subjects.
Consent to Participate Not applicable (retrospective chart review only).
Consent for Publication Not applicable (retrospective chart review only).
Conflict of Interest The authors declare no competing interests.
Availability of Data and Material
Available upon request.
References
- 1.Jacobs ML, Pourmoghadam KK. Thromboembolism and the role of anticoagulation in the Fontan patient. Pediatr Cardiol. 2007;28:457–64. [DOI] [PubMed] [Google Scholar]
- 2.Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease. Heart Rhythm. 2014;11:e102–65. [DOI] [PubMed] [Google Scholar]
- 3.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):e81–192. [DOI] [PubMed] [Google Scholar]
- 4.Firdouse M, Agarwal A, Chan AK, Mondal T. Thrombosis and thromboembolic complications in Fontan patients: a literature review. Clin Appl Thromb Hemost. 2014;20:484–92. [DOI] [PubMed] [Google Scholar]
- 5.Marrone C, Galasso G, Piccolo R, et al. Antiplatelet versus anticoagulation therapy after extracardiac conduit Fontan: a systematic review and meta-analysis. Pediatr Cardiol. 2011;32:32–9. [DOI] [PubMed] [Google Scholar]
- 6.McCrindle BW, Manlhiot C, Cochrane A, et al. Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61:346–53. [DOI] [PubMed] [Google Scholar]
- 7.Potter BJ, Leong-Sit P, Fernandes SM, et al. Effect of aspirin and warfarin therapy on thromboembolic events in patients with univentricular hearts and Fontan palliation. Int J Cardiol. 2013;168:3940–3. [DOI] [PubMed] [Google Scholar]
- 8.Seipelt RG, Franke A, Vazquez-Jimenez JF, et al. Thromboembolic complications after Fontan procedures: comparison of different therapeutic approaches. Ann Thorac Surg. 2002;74:556–62. [DOI] [PubMed] [Google Scholar]
- 9.January CT, Wann LS, Alpert JS, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 10.Kirchhof P, Benussi S, Kotecha D, ESC Scientific Document Group, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 11.Connolly SJ, Ezekowitz MD, Yusuf S, RE-LY Steering Committee and Investigators, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 12.Giugliano RP, Ruff CT, Braunwald E, ENGAGE AF-TIMI 48 Investigators, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 13.Granger CB, Alexander JH, McMurray JJ, ARISTOTLE Committees and Investigators, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Mahaffey KW, Garg J, Pan G, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 15.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Bouma BJ, Mulder BJM. Non vitamin K antagonist Oral anticoagulants for ThromboEmbolic prevention in adult congenital heart disease (NOTE) investigators. Is initiating DOACs for atrial arrhythmias safe in adults with congenital heart disease? Cardiovasc Drugs Ther. 2017;31:413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarubbi B, Scognamiglio G, Merola A, et al. New oral anticoagulants in complex adult congenital heart disease patients: a single centre experience. J Atr Fibrilation (Venice Arrhythmias 2015). [Google Scholar]
- 18.Cheng K, Harrogate S, Orchard E. The use of novel oral anticoagulants in adult congenital heart disease: a single center experience. Am J Cardiol. 2016;117:312–3. [DOI] [PubMed] [Google Scholar]
- 19.Egbe AC, Connolly HM, McLeod CJ, et al. Thrombotic and embolic complications associated with atrial arrhythmia after Fontan operation: role of prophylactic therapy. J Am Coll Cardiol. 2016;68:1312–9. [DOI] [PubMed] [Google Scholar]
- 20.Pinto C, Samuel BP, Ratnasamy C, Vettukattil JJ. Thrombosis in Fontan patient on apixaban. Int J Cardiol. 2015;182:66–7. [DOI] [PubMed] [Google Scholar]
- 21.Pujol C, Niesert AC, Engelhardt A, et al. Usefulness of direct oral anticoagulants in adult congenital heart disease. Am J Cardiol. 2016;117:450–5. [DOI] [PubMed] [Google Scholar]
- 22.Khairy P, Van Hare GF. Reply to the Editor-Concern regarding adult congenital heart disease arrhythmia guidelines. Heart Rhythm. 2015;12:e33–4. [DOI] [PubMed] [Google Scholar]
- 23.Tomkiewicz-Pajak L, Hoffman P, Trojnarska O, Lipczynska M, Podolec P, Undas A. Abnormalities in blood coagulation, fibrinolysis, and platelet activation in adult patients after the Fontan procedure. J Thorac Cardiovasc Surg. 2014;147:1284–90. [DOI] [PubMed] [Google Scholar]
- 24.Georgekutty J, Kazerouninia A, Wang Y, et al. Novel oral anticoagulant use in adult Fontan patients: a single center experience. Congenit Heart Dis. 2018;13(4):541–7. [DOI] [PubMed] [Google Scholar]
- 25.Lopes RD, Alexander JH, Al-Khatib SM, et al. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–9. [DOI] [PubMed] [Google Scholar]
- 26.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Veldtman GR, Bouma BJ, et al. Non-vitamin K antagonist oral anticoagulants in adults with a Fontan circulation: are they safe. Open Heart. 2019;6(1):e000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Bouma BJ, Dimopoulos K, et al. Non-vitamin K antagonist oral anticoagulants (DOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. Int J Cardiol. 2020;15(299):123–30. [DOI] [PubMed] [Google Scholar]
- 29.Pujol C, Müssigmann M, Schiele S, et al. Direct oral anticoagulants in adults with congenital heart disease - a single centre study. Int J Cardiol. 2020;1(300):127–31. [DOI] [PubMed] [Google Scholar]
- 30.Stalikas N, Doundoulakis I, Karagiannidis E, et al. Non-vitamin K oral anticoagulants in adults with congenital heart disease: a systematic review. J Clin Med. 2020;9(6):1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brien EC, Holmes DN, Thomas LE, et al. Prognostic significance of nuisance bleeding in anticoagulated patients with atrial fibrillation. Circulation. 2018;138(9):889–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request.