Abstract
The Vpr protein of human immunodeficiency virus type 1 (HIV-1) performs a number of functions that are associated with the nucleus. Vpr enhances the nuclear import of postentry viral nucleoprotein complexes, arrests proliferating cells in the G2 phase of the cell cycle, and acts as a modest transcriptional activator. For this paper, we have investigated the nuclear import of Vpr. Although Vpr does not encode a sequence that is recognizable as a nuclear localization signal (NLS), Vpr functions as a transferable NLS both in somatic cells and in Xenopus laevis oocytes. In certain contexts, Vpr also mediates substantial accumulation at the nuclear envelope and, in particular, at nuclear pore complexes (NPCs). Consistent with this, Vpr is shown to interact specifically with nucleoporin phenylalanine-glycine (FG)-repeat regions. These findings not only demonstrate that Vpr harbors a bona fide NLS but also raise the possibility that one (or more) of Vpr’s functions may take place at the NPC.
The accessory gene vpr is present in all viruses that belong to the five phylogenetic lineages of primate lentiviruses. The human immunodeficiency virus type 1 (HIV-1) Vpr protein is well conserved in terms of primary sequence and is approximately 96 amino acids in length. To date, a number of functions have been ascribed to HIV-1 Vpr (18). First, Vpr has been shown to act early in viral infection as a facilitator of postentry nucleoprotein complex (often referred to as the preintegration complex [PIC]) nuclear import (30, 40). This activity of Vpr is thought to be responsible for Vpr’s ability to enhance HIV-1 replication in nondividing cells, such as terminally differentiated macrophages (5, 12, 26, 40, 77). Consistent with this function, Vpr is efficiently packaged into virus particles (10, 49, 60) and is present in PICs (32, 40). Second, the expression of Vpr in proliferating cultures results in their arrest during the G2 phase of the cell cycle (39, 42, 64, 66). The induction of programmed cell death by Vpr (4, 70) and the ability of Vpr to activate differentiation of certain cell lines (46) may both represent alternative consequences of Vpr-mediated perturbation of the cell cycle. Third, Vpr can act as a modest transcriptional activator of the HIV-1 long terminal repeat (LTR) (2, 11, 76) as well as the promoter for the α- inhibitor of NF-κB (IκBα) (4); it has been suggested that these effects may also be related to interference with the cell cycle (18). Importantly, the finding that the cell cycle arrest and nuclear import phenotypes are mediated by different proteins— Vpr and Vpx, respectively—in viruses of the lineage that contains HIV-2 and sooty mangabey-derived simian immunodeficiency virus (SIVSM) strongly implies that these effects represent independent functions of HIV-1 Vpr (23, 61, 68).
The contribution of Vpr to HIV-1 replication in infected hosts is difficult to evaluate. However, the fact that the vpr gene is conserved in primary isolates of HIV-1 is strongly suggestive of a crucial role. This notion is further supported by the finding that the vpr alleles present in a mother-child pair of HIV-1-infected long-term nonprogressors had accumulated numerous, presumably deleterious, mutations towards their 3′ termini (75). Experimental infections of rhesus macaques with SIVMAC, a virus of the HIV-2-SIVSM lineage, have been used to examine the relative importance of the vpr and vpx genes in vivo (33). It was found that the loss of vpx alone led to a marked reduction in virus titer and a substantial delay in disease progression, whereas a loss of vpr alone only resulted in minimal differences compared to the wild-type counterpart. Interestingly, however, a clear selection for reversion back to intact vpr genes was still observed for challenges with vpr-deficient (Δvpr) viruses (45). Taken together, these findings suggest that both the cell cycle arrest and nuclear import functions of HIV-1 Vpr are required for maximum replication in vivo.
Site-directed mutagenesis and secondary structure predictions have been used to identify important functional and structural regions within the ∼15-kDa HIV-1 Vpr protein. In cells that do not express other HIV-1 proteins, Vpr is localized predominantly to the nucleus (15, 49, 51). Vpr does not, however, contain a sequence element that is recognizable as a nuclear localization signal (NLS) (see below), and nuclear accumulation has been shown to be dependent both on the proposed helical domain I (residues 19 to 37) and on additional residues positioned throughout the amino-terminal 70 amino acids (15, 50). The packaging of Vpr into cytoplasmically assembled viral particles is mediated by an interaction between Vpr and the p6 region of p55gag (43, 48, 60) and requires helical domains I and II (residues 56 to 70) (15, 50). For Vpr to be incorporated into virions, a certain amount of Vpr must be localized to the cytoplasm of virus-producing cells; although it seems probable that the same Vpr-Gag interaction(s) that determines encapsidation may also mediate cytoplasmic accumulation, this issue has not yet been addressed. The region of Vpr that lies between residue 73 and the extreme carboxy terminus is rich in basic amino acids and is essential for G2 arrest, but it is dispensable for both nuclear accumulation and virion incorporation (15, 50, 52). Interestingly, the relationship between nuclear localization and Vpr-mediated cell cycle arrest is unclear, since nuclear as well as cytoplasmic mutants of Vpr can induce cell cycle arrest (50). Taken together, these analyses have shown that the regions of Vpr that are predicted to form α-helices are essential for biological activity and suggest that it may not be possible to organize Vpr into readily definable discrete functional domains. The importance of secondary structure is exemplified by a mutation that substitutes the alanine at position 30 for proline (VprA30P) and disrupts the predicted helicity of domain I. This mutant protein has been shown to be deficient for nuclear localization, virion packaging, and cell cycle arrest (15).
The ability of HIV-1 Vpr to enter the nucleus, either in the context of PICs or independent of other viral proteins, is presumed to be important for at least some of its activities. Accordingly, it is important to determine how Vpr nuclear import occurs if the various functions of this viral accessory protein are to be fully understood. The transport of molecules and multicomponent complexes into the nucleus proceeds through gated channels of ∼125 MDa known as nuclear pore complexes (NPCs) (17, 35, 57). At least 50 different proteins are present in multiple copies in each NPC; these are termed nucleoporins and are often characterized by the presence of multiple tetrapeptide repeats that contain the dipeptide phenylalanine-glycine (FG-repeats). Molecules and particles that exceed the ∼40-kDa diffusion limit of NPCs are actively imported via specific pathways following the recognition of cis-acting targeting signals termed NLSs (34, 35, 57). These sequences have been defined for a wide variety of proteins and are frequently characterized by one or two stretches of basic amino acids (basic-type NLSs) (16, 44). Importantly, however, a number of NLSs have also been described which do not obviously conform to this consensus (44, 57). Regarding proteins that have molecular masses of less than 40 kDa, some are able to diffuse into the nucleus passively, whereas others are dependent on signal-mediated import (35).
A number of distinct pathways of protein nuclear import have been described so far, the most extensively characterized of which is known as the classical pathway and is utilized by basic-type NLSs (34, 35, 57). Here, the NLS-bearing substrate first binds to the α-subunit of a heterodimeric importin-α/β complex in the cytoplasm. This ternary complex subsequently docks at the NPC via importin-β and is transported through the pore. Following translocation, the complex is disassembled, and the importin subunits are recycled to the cytoplasm. The Ran GTPase is also essential for import and is thought to function in at least two ways: not only is GTP hydrolysis required for translocation through the pore, but Ran in its GTP-bound form (the predominant form in the nucleus) also terminates import by binding to importin-β in the nucleus and mediating the dissociation of the importin subunits from each other. Although fewer details are known concerning the other import pathways, a similar general mechanism is thought to be operating, in that proteins which share sequence similarity with importin-β appear to be the mediators of translocation through the NPC. One important difference between these emerging pathways and the classical pathway is that no counterparts of importin-α are required, and the import substrates bind directly to the importin-β-like factors (3, 27, 41, 62, 67).
There are a number of important unanswered questions concerning the nuclear import of Vpr. In particular, because Vpr lacks a canonical NLS and has a mass of ∼15 kDa, it is formally possible that it could, when not in a complex with other viral proteins, enter the nucleus by diffusion and then accumulate as a result of retention. Alternatively, it is possible that Vpr harbors a novel type of NLS and that this sequence mediates import by using a pathway (or pathways) that may or may not have been described. Here we describe experiments which demonstrate that HIV-1 Vpr encodes an NLS that can confer nuclear import on substrates that are too large to enter the nucleus by passive diffusion. Vpr is also shown to be capable of mediating the accumulation of certain chimeric proteins at NPCs and of interacting specifically with nucleoporin FG-repeat regions. These data not only support the idea that Vpr enhances the nuclear import of PICs but also raise the possibility that Vpr may function at the NPC itself.
MATERIALS AND METHODS
Molecular clones.
The provirus expression vector for the primary macrophage-tropic HIV-1YU-2 isolate (47) is termed pYU-2 and has been described previously (25). All HIV-1 sequences used in this study were obtained from this vector. A vpr-deficient derivative, pYU-2/Δvpr, in which the ATG initiation codon of vpr was changed to GTG and four nonsense mutations were introduced within the amino-terminal 11 codons, was derived from pYU-2 by PCR-mediated site-directed mutagenesis; importantly, no amino acid changes were introduced into the overlapping vif gene by these mutations. The alanine-to-proline substitution mutation at position 30 of vpr was also introduced into pYU-2 by PCR-mediated mutagenesis; this vector is termed pYU-2/VprA30P.
The pVpr-Myc and pVprA30P-Myc expression vectors have carboxy-terminal c-Myc epitope tags and were constructed by the simultaneous insertion of PCR-amplified BamHI-XhoI full-length vpr fragments and SalI-SmaI c-Myc fragments into BamHI-EcoRV-digested pcDNA3 (Clontech). A pcDNA1 (Clontech)-based expression vector that encodes maltose binding protein (MBP) with a polylinker at its carboxy terminus has been described previously (25). All full- length (wild-type and A30P mutant) and truncated vpr alleles were amplified by PCR and inserted at the 3′ terminus of MBP as XhoI-XbaI fragments. The MBP- NLS and NPLC-M9 control vectors, which express, respectively, the bipartite basic NLS of the heterogeneous nuclear ribonucleoprotein particle (hnRNP) K protein fused to the carboxy terminus of MBP and the nucleoplasmin core domain fused to the NLS of hnRNP A1, have been described previously (25, 54). To construct the β-galactosidase-based vectors, the entire β-galactosidase gene was inserted between the HindIII and BstEII sites of pBC12/CMV/I1-2 (13), such that NcoI and Asp718 sites were introduced at its 5′ terminus and an XbaI site was introduced at its 3′ terminus. Full-length wild-type and A30P vpr alleles were then inserted as XbaI fragments to create the pβ-gal–Vpr and pβ-gal–VprA30P expression vectors.
The pGBT9 (GAL4 DNA binding domain; bait vector) and pGAD-GH (GAL4 transcription activation domain; prey vector) parental plasmids for yeast two-hybrid analyses were purchased from Clontech. Wild-type and mutant vpr alleles were inserted as blunt-ended fragments into the SmaI site of pGAD-GH to create the prey vectors pGAD-GH-Vpr and pGAD-GH-VprA30P. All bait vectors were obtained by inserting PCR-amplified EcoRI-XhoI fragments between the EcoRI and SalI sites of pGBT9. These fragments encode the FG-repeat regions of the following nucleoporins (the species from which each cDNA was isolated is indicated by y for yeast, h for human, and r for rat), with the amino acid coordinates of the region given in parentheses: yNup1p (438 to 737), yNup2p (182 to 537), yNsp1p (296 to 606), yNup159p/RAT7 (497 to 701), yNup145p (24 to 216), yNup116p (459 to 672), yNup100p (278 to 539), yNup49p (7 to 239), yRip1p (151 to 275), rPom121 (796 to 1199), hCAN/Nup214 (full length), and hRIP/RAB1 (388 to 562) (28, 72).
The Escherichia coli vectors for expression of glutathione S-transferase (GST)–nucleoporin fusion proteins was generated by insertion of the EcoRI-XhoI fragments of yNsp1p, yNup100p, and rPom121 described above between the matching sites of pGEX-5X-3 (Pharmacia Biotech, Inc.).
Indirect immunofluorescence.
Thirty-five-millimeter-diameter subconfluent HeLa cell monolayers were transiently transfected with 5 μg of the indicated expression vectors with calcium phosphate. At 24 h, the cells were fixed with paraformaldehyde and permeabilized, and the staining patterns were determined by hybridization with various primary antibodies. For single-label analyses, the Vpr-Myc, MBP-Vpr, and β-gal–Vpr proteins were detected, respectively, with the Myc-specific monoclonal antibody 9E10 (19), a rabbit polyclonal anti-MBP antiserum (U.S. Biochemical), and a rabbit polyclonal anti-β-galactosidase antiserum (Organon Teknika). Following secondary hybridization with appropriate Texas red (TXRD)-conjugated antibodies raised in goats, samples were viewed by epifluorescence with a Nikon microphot-SA microscope at a magnification of ×400 (25, 53). Expression of fusion proteins with the expected molecular masses was confirmed by Western blot analysis of whole-cell lysates.
For double labeling of HeLa cells expressing β-galactosidase fusion proteins, fixed samples were initially hybridized with the polyclonal β-galactosidase antiserum together with either the importin-β-specific monoclonal antibody 3E9 (9) or the nucleoporin-specific monoclonal antibody QE5 (which recognizes the p250, Nup153, and p62 nucleoporins) (59). Bound antibodies were detected with a TXRD-conjugated goat anti-rabbit antibody and a fluorescein isothiocyanate-conjugated goat anti-mouse antibody. Ten consecutive horizontal sections of double-stained cells were scanned with a Leica TCS 4D confocal microscope and stored on a computer for subsequent superimpositioning of the staining patterns.
Microinjection of Xenopus laevis oocytes.
MBP, the MBP fusion proteins, and NPLC-M9 were synthesized in vitro and radiolabeled with [35S]methionine with a T7-reticulocyte lysate coupled transcription-translation system (Promega Corp.). The reaction products were examined for integrity by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Each MBP was then mixed with NPLC-M9 and injected directly into the cytoplasms of 10 to 20 stage VI oocytes. At 12 h, the oocytes were manually dissected into nuclear and cytoplasmic fractions, and the soluble proteins were analyzed by SDS-polyacrylamide gel electrophoresis, fluorography, and autoradiography (22, 25).
Yeast two-hybrid interactions.
Combinations of the pGBT9- and pGAD-GH-derived expression vectors were cotransformed into Saccharomyces cerevisiae HF7C, plated onto synthetic dropout minimal medium without l-leucine or l- tryptophan (SD/Leu−/Trp−), and incubated at 30°C. Single colonies of double transformants were then streaked onto Whatman no. 5 filters that had been placed on SD/Leu−/Trp− plates and incubated for 1 day at 30°C. The colonies were lysed by three cycles of being submerged in liquid nitrogen and then thawed at room temperature. Finally, expression of β-galactosidase was determined by incubation of the filters in indicator buffer (16.1 g of Na2HPO4 · 7H2O, 5.5 g of NaH2PO4 · H2O, 0.75 g of KCl, 0.246 g of MgSO4 · 7H2O, 2.7 ml of β-mercaptoethanol, 350 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [pH 7.0] per liter) for 1 to 5 h until a blue color developed. Equivalent expression of the wild-type and VprA30P mutant prey fusions in all cotransformed yeast strains was verified by the parallel growth of those strains in suspension followed by Western blot analysis of whole-cell lysates with a GAL4 transcription activation domain-specific monoclonal antibody (Clontech).
Purification of GST proteins and in vitro pull-down assays.
The pGEX-5X-3, pGEX-Nsp1p, pGEX-Nup100p, and pGEX-Pom121 expression vectors were transformed into E. coli BL21.pLys and grown at 37°C in ampicillin-containing medium to an optical density at 600 nm of ∼0.6 prior to induction for 4 h with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were harvested, resuspended in buffer A (10 mM HEPES-NaOH [pH 7.4], 250 mM NaCl, 1 mM EDTA, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per ml, 1 μg of pepstatin per ml, and 1 μg of leupeptin per ml) and disrupted by sonication on ice. Lysates were adjusted to 0.5% Nonidet P-40, rocked for 30 min at 4°C, and cleared by centrifugation at 15,000 × g for 15 min at 4°C. Glutathione-Sepharose 4B (Pharmacia Biotech, Inc.) was added to each lysate, the mixtures were rocked for 30 min at 4°C, and the beads were washed five times with buffer A. Bound proteins were examined for integrity and quantitated by SDS- polyacrylamide gel electrophoresis followed by staining with Coomassie blue.
MBP and MBP-Vpr (full length) were synthesized, radiolabeled with [35S]methionine, and examined for integrity as for oocyte microinjections. The binding reaction mixtures comprised 10 μl of translation mixture and 5 μg of GST, GST-Nsp1p, GST-Nup100p, or GST-Pom121 bound to 10 μl of beads in a final volume of 100 μl of buffer B [20 mM HEPES-NaOH (pH 7.3), 110 mM KC2H3O2, 5 mM NaC2H3O2, 2 mM Mg(C2H3O2)2, 0.5 mM EGTA, 1% Nonidet P-40]. After a 60-min incubation at 4°C, the beads were washed six times with 0.5 ml of buffer B and boiled for 5 min in Laemmli dissociation buffer containing 4% SDS. Eluted proteins were analyzed by SDS-polyacrylamide gel electrophoresis, fluorography, and autoradiography.
Cells and HIV-1 replication.
Peripheral blood mononuclear cells (PBMCs) and monocyte-derived macrophages (MDMs) were simultaneously derived from the blood of healthy volunteer donors following venipuncture as described previously (25). PBMCs were purified with Ficoll-Paque, stimulated with 5 μg of phytohemagglutinin (PHA) for 72 h, washed, challenged with virus, and maintained in RPMI 1640 medium containing 20% fetal bovine serum and 20 U of recombinant interleukin-2 per ml at a density of ∼106 cells/ml. MDMs were purified by gelatin-coated plastic adherence and maintained in 24-well culture dishes at a density of ∼4 × 105 cells per well in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum, 1% l-glutamine, and 1% nonessential amino acids, penicillin, and streptomycin for 14 days prior to viral challenge. Primary human microglia (MG) were isolated from fresh human brain tissue from donors undergoing temporal lobectomy surgery as described previously (71). Briefly, following tissue disruption, digestion with trypsin and DNase, filtration, and centrifugation in a continuous Percoll gradient, MG were purified by plastic adherence and maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum, 5% giant cell tumor supernatant (IGEN, Inc.), 1 mM sodium pyruvate, and gentamicin. After 7 to 10 days, MG were replated in 48-well culture dishes at a density of ∼105 cells per well and used for viral challenge. The immortalized T-cell line CEM-CCR5, which is susceptible to infection by macrophage-tropic HIV-1 isolates, has been described previously (25).
Stocks of wild-type and mutant viruses were generated by transient calcium phosphate-mediated transfection of 100-mm-diameter cultures of 293T cells with provirus expression vectors. At 24 h, the supernatants were harvested and stored in aliquots at −80°C. A total of 5 × 106 PBMCs, 4 × 105 MDMs, 2 × 105 MG, or 0.5 × 106 CEM-CCR5 cells were challenged with stocks corresponding to 10 ng of soluble p24gag as determined by enzyme-linked immunosorbent assay. The cultures were sampled and the media were replenished every 1 or 2 days; virus replication was measured as the accumulation of soluble p24gag in the culture supernatants.
RESULTS
Signal-mediated nuclear import of the HIV-1 Vpr protein.
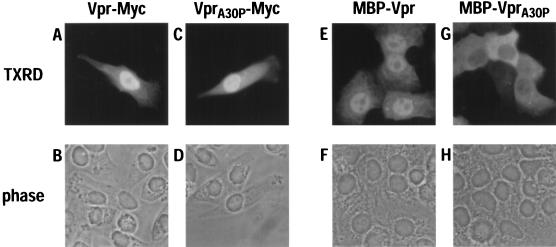
It has previously been demonstrated that HIV-1 Vpr expressed as a unit-length protein localizes primarily to the nucleus in transiently transfected cells (15, 51). The Vpr proteins used for these analyses were derived either from the laboratory-adapted T-cell line-tropic virus HIV-1IIIB or from the primary dualtropic (T-cell line and macrophage) isolate HIV-189.6. Because our long-term objective is to understand the viral and cellular factors which govern HIV-1 infection of tissue macrophages and other nondividing cell populations (infections that are essential for the establishment of pathogenic infections in humans), we have been focusing on the primary macrophage-tropic isolate HIV-1YU-2 for our experiments (47). To determine whether HIV-1YU-2 Vpr localizes to the nucleus in the absence of other viral proteins, HeLa cells were transfected with a vector that expressed a Myc epitope-tagged version of this 97-amino-acid protein and examined by indirect immunofluorescence with a Myc-specific monoclonal antibody (Fig. 1). Consistent with the findings of others, Vpr accumulated predominantly in the nucleoplasm (Fig. 1A and B). It has previously been shown that disruption of helical domain I yields proteins that are no longer capable of localizing to the nucleus. We therefore introduced the alanine-to-proline missense mutation at position 30 into HIV-1YU-2 Vpr and evaluated its consequences for subcellular localization (Fig. 1C and D). In contrast to earlier findings (15), this VprA30P mutant protein appeared to localize to the nucleus as efficiently as the wild-type protein.
FIG. 1.
Subcellular localization of HIV-1 Vpr in HeLa cells. Monolayer cultures were transfected with vectors that expressed wild-type Vpr (A, B, E, and F) or the VprA30P mutant (C, D, G, and H) as either Myc-tagged proteins (A to D) or fusions to MBP (E to H). Expressed proteins were detected by indirect immunofluorescence with either the Myc-specific monoclonal antibody (A and C) or an MBP-specific antiserum (E and G), TXRD-conjugated secondary antibodies, and epifluorescence. The corresponding phase-contrast analyses are also shown (B, D, F, and H).
As discussed above, certain proteins with masses of less than ∼40 kDa can enter the nucleus by passive diffusion rather than by a signal-mediated process. These two possibilities can be distinguished from each other by evaluating import function in the context of chimeric proteins that exceed the diffusion limit of NPCs. The ∼15-kDa wild-type Vpr protein as well as the A30P mutant were therefore expressed as fusions to the ∼45-kDa MBP and analyzed for nuclear localization in transfected HeLa cells (Fig. 1). As with unit-length wild-type Vpr, the majority of MBP-Vpr was localized to the nucleus (Fig. 1E and F). In contrast, the mutated MBP-VprA30P fusion protein was largely confined to the cytoplasm (Fig. 1G and H). Importantly, Western blot analysis of whole-cell lysates confirmed that fusion proteins with the predicted molecular masses were expressed in both samples (data not shown). We have concluded, therefore, that HIV-1 Vpr does indeed possess a specific nuclear targeting signal and that the A30P mutation inactivates it. It is unclear why a previous study was unable to show that Vpr can mediate the nuclear import of a coupled heterologous substrate (15); possible explanations include the nature of the fusion between Vpr and the heterologous sequence and/or the source of Vpr itself.
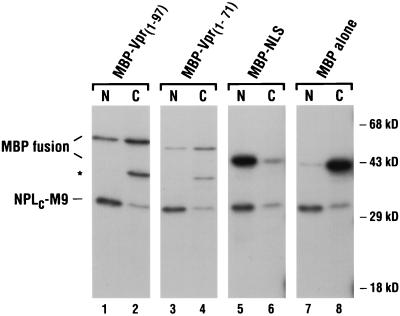
To validate the results obtained in transfected HeLa cells, MBP-Vpr fusion proteins were also tested for nuclear import function in microinjected X. laevis oocytes. MBP alone, MBP-Vpr1–97 (wild type), MBP fused to a 26-amino-acid carboxy-terminal truncation of Vpr (MBP-Vpr1–71), and MBP fused to the bipartite basic-type NLS of the hnRNP K protein (MBP-NLS) were each radiolabeled in vitro and injected into the cytoplasms of oocytes. As an internal positive control, all samples also included a labeled chimeric protein that comprised the pentameric nucleoplasmin core domain fused to the defined NLS of the hnRNP A1 protein (NPLC-M9). At ∼12 h, nuclear and cytoplasmic fractions were isolated, and the patterns of localization were visualized following gel electrophoresis (Fig. 2). As shown previously, NPLC-M9 (all lanes) and MBP-NLS (lanes 5 and 6) accumulated in the nucleus, whereas MBP as a nonfusion protein did not (lanes 7 and 8). Importantly, both the full-length MBP-Vpr and truncated MBP-Vpr1–71 fusions were targeted to the nucleus with moderate efficiency (30 to 40% accumulation in the nucleus [lanes 1 to 4]). These findings confirmed our earlier conclusion that Vpr harbors sequences sufficient for nuclear localization and revealed that the basic carboxy-terminal region of Vpr is dispensable for import; the latter observation is in agreement with earlier conclusions of others (15, 50). Of note, we could not determine the import capability of MBP-VprA30P in oocytes, because this protein was unstable and, as a result, was undetectable by 12 h postinjection (data not shown).
FIG. 2.
Nuclear import of MBP fusion proteins in microinjected X. laevis oocytes. The indicated MBP fusion proteins and NPLC-M9 were radiolabeled in vitro and coinjected into the cytoplasms of 10 to 20 stage VI oocytes. At 12 h, the oocytes were separated into nuclear (N) and cytoplasmic (C) fractions, and the soluble proteins were analyzed on an SDS-polyacrylamide gel. The bands corresponding to the MBP fusions and NPLC-M9 are indicated; the faster-migrating, and presumably truncated, protein present in the MBP-Vpr1–97 (lane 2) and MBP-Vpr1–71 (lane 4) samples is indicated by an asterisk.
Targeting of HIV-1 Vpr to the nuclear envelope.
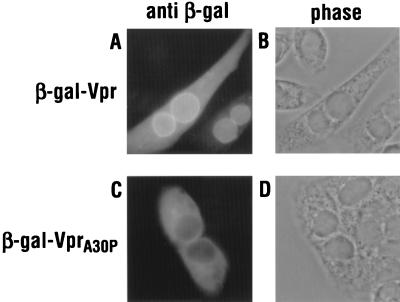
In addition to assessing Vpr nuclear localization in the context of MBP chimeras, fusions to β-galactosidase were also examined in HeLa cells (Fig. 3). Interestingly, in addition to accumulating in the nucleoplasm, a substantial fraction of wild-type Vpr, when expressed as a carboxy-terminal fusion, frequently localized to a narrow rim around the nucleus that coincided with the nuclear envelope (Fig. 3A and B). Of note, this pattern was not unique to this particular fusion protein, because the positioning of Vpr at the amino terminus of β-galactosidase also resulted in significant accumulation at the nuclear envelope (data not shown). As with the fusion to MBP, the β-gal–VprA30P chimera was localized entirely to the cytoplasm (Fig. 3C and D).
FIG. 3.
Subcellular localization of β-gal–Vpr fusion proteins in HeLa cells. Monolayers were transfected with wild-type (A) or A30P mutant (C) expression vectors and analyzed by indirect immunofluorescence with a β-galactosidase-specific antiserum. The phase-contrast analyses are also shown (B and D).
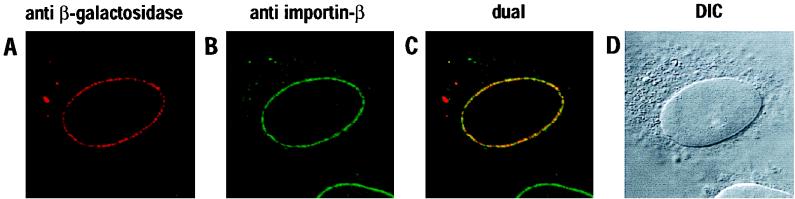
To examine more definitively the subcellular localization of wild-type β-gal–Vpr, transfected HeLa cells were subjected to double-label indirect immunofluorescence and laser-scanning confocal microscopy (Fig. 4). The primary antibodies used in this experiment were specific for β-galactosidase or the nuclear import factor importin-β (monoclonal antibody 3E9); the latter was chosen because importin-β has previously been shown to localize to the nuclear envelope and to interact with nucleoporins (36, 55, 56, 65). The patterns of localization for β-gal–Vpr and importin-β were virtually the same, in that both were detected as a ring of intense punctate staining that precisely coincided with the nuclear envelope (Fig. 4A, B, and D). Not surprisingly, a computer-generated superimposition of these images demonstrated that substantial amounts of β-gal–Vpr and importin-β were, indeed, colocalized (visualized in yellow in Fig. 4C). Previous analyses have shown that this punctate rim staining is characteristic of localization to the NPCs (14); this was confirmed for β-gal–Vpr by performing analogous double-labeling experiments with a nucleoporin-specific antibody (monoclonal antibody QE5) in place of 3E9 (data not shown). In light of these results, we also examined the localization of our Myc-tagged Vpr protein by confocal microscopy. Consistent with the epifluorescence experiments (Fig. 1), we could not discern any marked accumulation of this protein at the nuclear envelope (data not shown).
FIG. 4.
Colocalization of wild-type β-gal–Vpr and importin-β at NPCs. Transfected HeLa cells were subjected to double-label immunofluorescence with primary antibodies specific for β-galactosidase (A) or β-importin (B) and analyzed by laser-scanning confocal microscopy. The superimposed images are shown (dual [C]) together with the corresponding differential interference contrast image (DIC [D]).
Interaction of HIV-1 Vpr with nucleoporins.
Having shown that Vpr can target a heterologous protein to the NPC in a sequence-specific manner and can colocalize with importin-β, we wished to investigate whether Vpr, like importin-β, is capable of interacting with nucleoporins. To do this, we initially used the yeast two-hybrid system as a surrogate in vivo interaction assay. Wild-type and A30P mutant Vpr proteins were expressed as carboxy-terminal fusions to the activation domain of the yeast transcription factor GAL4 (preys), whereas the FG-repeat regions of assorted yeast and vertebrate nucleoporins were expressed as carboxy-terminal fusions to the GAL4 DNA binding domain (baits). Interactions between Vpr and a given FG-repeat region were scored in cotransformed yeast cells by the induction of β-galactosidase expression from the resident GAL4-responsive reporter cassette. As can be seen from Table 1, an interaction between wild-type Vpr and the FG- repeat region of Pom121 was readily and reproducibly detected. In contrast, none of the other nucleoporin fragments that were tested was found to be capable of interacting with Vpr. Importantly, the specificity of Vpr’s interaction with an FG-repeat region was established by the inability of VprA30P to interact with Pom121.
TABLE 1.
Yeast two-hybrid interaction of HIV-1 Vpr with a nucleoporin FG-repeat regiona
| Bait nucleoporin | Result with Vpr as prey |
|---|---|
| Empty vector (pGBT9) | − |
| yNup1p | − |
| yNup2p | − |
| yNsp1p | − |
| yNup159p/RAT7 | − |
| yNup145p | − |
| yNup116p | − |
| yNup100p | − |
| yNup49p | − |
| yRip1p | − |
| rPom121 | + |
| hCAN/Nup214 | − |
| hRIP/RAB1 | − |
Yeast two-hybrid interactions were scored by using cotransformants of S. cerevisiae HF7C. +, β-galactosidase expression detected in three independent colonies, and an interaction between the bait and prey fusion can be inferred; −, β-galactosidase expression not detected for that particular bait-prey combination. (Note that no β-galactosidase expression was detected with empty vector pGAD-GH or VprA30P as the prey for any of the bait nucleoporins shown.) Importantly, equivalent expression of the wild-type and VprA30P mutant prey fusions was verified by parallel Western blot analyses of all strains by using a GAL4 activation domain-specific antibody. The FG-repeat regions of a number of other nucleoporins were also tested as bait fusions in pGBT9; these induced expression of β-galactosidase in the absence of a prey fusion and therefore are not shown. No additional interactions were detected in the opposite orientation: namely, with Vpr or VprA30P as the bait fusion in pGBT9 and the FG-repeat regions as prey fusions in pGAD-GH (data not shown).
As an independent confirmation of Vpr’s ability to interact with Pom121, we also performed a series of in vitro GST pull- down assays (Fig. 5). MBP-Vpr and MBP were each radiolabeled and mixed with glutathione-Sepharose beads that had been prebound to equal quantities of either GST or GST fused to the FG-repeat region of Nsp1p, Nup100p, or Pom121. Following a brief incubation, the beads were washed, and the bound proteins (lanes 1 to 8), as well as a fraction of the labeled input proteins (lanes 9 and 10), were examined by gel electrophoresis. An interaction between Vpr and Pom121 was readily detected (lane 8), as was a comparatively weak interaction between Vpr and Nsp1p (lane 4). Importantly, the inability of Nup100p (lane 6) or GST alone (lane 2) to interact with Vpr, as well as the lack of interaction between MBP alone and any of the GST-containing proteins (lanes 1, 3, 5, and 7), served to illustrate the specificity of the Pom121 (and Nsp1p) interaction. Of note, our yeast two-hybrid analysis failed to provide evidence for the interaction between Vpr and Nsp1p that was detected in this experiment; we suspect that the comparative inefficiency of this interaction may place it below the threshold that is necessary for detection under the two-hybrid conditions employed here. Although not conclusive, we consider it likely that Vpr’s interaction with nucleoporin FG-repeat regions in two-hybrid and pull-down experiments is the consequence of direct binding.
FIG. 5.
Interaction of Vpr with nucleoporin FG-repeat regions in vitro. Radiolabeled MBP-Vpr or MBP alone was incubated with GST, GST-Nsp1p, GST-Nup100p, or GST-Pom121 that had been prebound to glutathione-Sepharose beads. After washing, bound proteins were eluted and analyzed on an SDS-polyacrylamide gel (lanes 1 to 8) together with the input proteins (lanes 9 and 10).
Vpr enhances the replication of a primary macrophage-tropic HIV-1 isolate in cultured cells.
As with earlier nuclear localization studies of HIV-1 Vpr, the effects of Vpr on virus replication have been limited to the analysis of the laboratory-adapted HIV-1IIIB strain and the dualtropic isolate HIV-189.6. (For experiments that have evaluated VprIIIB function during growth in macrophages, viral tropism was first altered by exchanging the env gene for one derived from a macrophage-tropic isolate [5, 11, 12, 26, 40, 58, 77].) Because we have used the Vpr protein of HIV-1YU-2 for our analyses, it was important to characterize the contribution of Vpr to this isolate’s replication phenotype. A variety of dividing and nondividing cultured cell systems were therefore challenged with normalized stocks of wild-type and vpr-deficient (Δvpr) HIV-1YU-2. For nondividing cell populations, macrophages derived from two sources were used: first, MDMs maintained for 14 days prior to challenge in the absence of additional cytokines; second, MG (also known as brain macrophages) purified from fresh human brain and cultured for 7 to 10 days prior to challenge. For cultures in which substantial proliferation was ongoing, matched PHA-stimulated PBMCs derived from the MDM donors and the immortalized T-cell line CEM-CCR5 were used; ectopic introduction of the CCR5 chemokine receptor into the latter confers susceptibility to infection by macrophage-tropic HIV-1 isolates (25). Virus replication was measured as the accumulation of viral p24gag in the culture supernatants. (A representative selection of growth curves is shown in Fig. 6.)
FIG. 6.
Replication of wild-type (squares) and vpr-deficient (triangles) HIV-1YU-2 in spreading infections of nondividing and dividing cells. PBMCs (A [PBMC-1] and B [PBMC-2]), MDMs (D [MDM-1], E [MDM-2], and F [MDM-3]), primary MG (G [MG-4] and H [MG-5]), and CEM-CCR5 cells (C) were challenged with normalized virus stocks produced by transfection of 293T cells. Virus production, and hence replication, was measured as the expression of soluble p24gag in the culture supernatants.
The most striking feature of these data is that Vpr’s effects on HIV-1 replication are quite similar for the various types of culture and for the different primary cell donors. In all cases, the Δvpr virus replicated less well than the wild-type counterpart—even though the absolute values for the magnitudes of replication varied substantially. The difference between the replication profiles was between two- and fourfold for the majority of the challenges. The greatest difference was noted with the MDMs of donor 1 (Fig. 6D), whereas the smallest difference was observed in the CEM-CCR5 cell line (Fig. 6C). In no case, however, did we obtain enhanced replication for the Δvpr virus (including numerous additional experiments that are not shown here). Thus, and in general agreement with observations made with other viral isolates, Vpr exerts a moderate stimulatory effect on the replication of HIV-1 in cultured cells. Although not an absolute rule, this effect tended to be more pronounced in nondividing cells than in dividing cells. As would be expected, the replication of a virus that harbored the A30P mutation was essentially indistinguishable from that of the Δvpr virus (data not shown).
DISCUSSION
The ∼15-kDa Vpr protein of HIV-1 functions during early as well as late stages of the viral life cycle. Because the localization of Vpr to the nucleus appears to contribute to function, it is important to determine how Vpr nuclear import occurs if all aspects of its biological activities are to be fully understood. In terms of the general principles of protein import, previous analyses had, first, indicated that Vpr lacks a recognizable basic- type NLS and, second, been unable to demonstrate signal-mediated nuclear import for Vpr by conventional criteria. In this paper, we show that Vpr fusion proteins that exceed the diffusion limit of NPCs are targeted to the nucleus of both somatic human cells (Fig. 1 and 3) and Xenopus oocytes (Fig. 2). The specificity of localization was established by the loss of import function for fusion proteins bearing the A30P mutation. In contrast, this mutation had no discernible effect on nuclear accumulation in the context of a unit-length Vpr protein (Fig. 1), thereby implying that Vpr is capable of passively diffusing into the nucleus. Taken together, these fusion protein results formally establish that HIV-1 Vpr does indeed harbor a bona fide NLS. By repeating experiments of this genre with mutant Vpr proteins, it should now be relatively straightforward to define which features (sequence and/or structure) constitute Vpr’s NLS.
Experiments performed with β-galactosidase fusion proteins revealed that Vpr can, in certain contexts, target heterologous substrates to the nuclear envelope and, specifically, to NPCs (Fig. 3 and 4). A molecular explanation for NPC localization was revealed by the novel finding that Vpr can interact specifically with the FG-repeat region of the vertebrate nucleoporin Pom121 (Table 1 and Fig. 5) and the yeast nucleoporin Nsp1p (Fig. 5). Previous studies have shown that Pom121 is an integral membrane protein and that aspects of its extensive carboxy-terminal FG-repeat region are positioned towards both the lumen and the cytoplasmic side of the NPC (38, 69). Although confocal microscopy did not allow us to determine the precise localization of β-gal–Vpr at the NPC, staining of cells that had been subjected to limited permeabilization with digitonin (a detergent which did not allow immunodetection of nucleoplasmic proteins) indicated that at least some accumulation occurred towards the cytoplasmic face (data not shown). Of note, importin-β and other members of the importin-β-like family of transport receptors have been shown to interact with a variety of nucleoporins (3, 24, 55, 56, 65, 67, 79); it will therefore be interesting to determine how many additional nucleoporins can interact with Vpr.
A number of important questions remain concerning both the nuclear import of HIV-1 Vpr and its ability to interact with NPCs. First, the nature of the mechanistic relationship between the import of Vpr in the absence of other viral proteins and its import as a component of the PIC is unknown. Given that these two forms of import substrate are very different, it seems plausible that more than one pathway for Vpr-mediated import may exist. Alternatively, it is possible that there is a single pathway for Vpr import but that it may be utilized in more than one way. Second, it needs to be determined whether the interactions between Vpr and nucleoporins are transient ones that take place as Vpr (either alone or in the context of the PIC) is translocated through the NPC or whether one of Vpr’s functions is actually performed at the NPC itself.
In terms of possible pathways of Vpr nuclear import, a number of features and prior observations are suggestive of novel aspects. First and foremost, the region of Vpr that harbors the NLS (the amino-terminal 70 amino acids) does not appear to resemble any previously characterized NLS. This suggests, perhaps, that yet-to-be identified cellular factors may be involved in the import process. Second, the accumulation of Vpr at the NPC is not an attribute ordinarily associated with NLSs; even though the import factor importin-β also localizes to NPCs (36) (Fig. 4), we consider it improbable that Vpr itself functions as a mediator of nuclear translocation. Third, it has been shown that the nuclear import of wild-type HIV-1 PICs is unaffected by a dominant-negative form of importin-α, whereas the import of vpr-deficient PICs is severely inhibited (30). This implies that PICs, at least when Vpr is present, can be imported into the nucleus by a mechanism that is distinct from that utilized by classical basic-type NLSs and either does not require importin-α or, alternatively, uses importin-α, but in an unconventional manner (63). By extension, these possibilities may also be applicable to the import of Vpr in the absence of other viral proteins. The analysis of the nuclear import of both Vpr and purified PICs (8, 20, 21) by using the permeabilized cell system (1) should help to answer some of these questions. In particular, it will be interesting to use various recombinant import factors and depleted cytosolic extracts to determine whether Vpr can enter the nucleus by multiple mechanisms.
As mentioned above, we do not know whether the marked accumulation of β-gal–Vpr fusion proteins at NPCs is indicative of Vpr functioning at the NPC or is merely reflective of a rate-limiting step during nuclear import that is accentuated by fusion to β-galactosidase. One intriguing possibility for a function at the NPC is that Vpr may help to bind PICs to the cytoplasmic side of the pore prior to transport into the nucleus. Clearly, such an activity cannot be an absolute requirement for PIC import, because vpr-deficient viruses are still able to replicate quite well in nondividing cells (5, 12, 26, 40) (Fig. 6). This implies, therefore, that the PIC must contain additional NLSs; although it has been proposed that both the matrix (MA, p17gag) and integrase (IN) proteins contain NLSs, the precise identity of the NLSs that function during viral infection remains a controversial issue (7, 25, 26, 29, 31, 40, 74). Thus, Vpr may act early in PIC import by stimulating NPC docking, whereas other NLSs may function later as PICs are translocated across the nuclear envelope. A stepwise mechanism for HIV-1 PIC import would be somewhat reminiscent of the events that culminate in the nuclear uptake of viral DNA during adenovirus infection. Here, postentry viral core structures are first transported to NPCs, where interaction with the pore triggers a major disassembly of the capsid. This, in turn, results in viral DNA and, at the minimum, DNA-associated protein VII and terminal protein entering the nucleus (37).
Although our replication experiments, as well as those of others, indicate that Vpr is more important for HIV-1 infection of nondividing cells, Vpr also tended to enhance replication in cultures of dividing cells (Fig. 6). It seems likely, therefore, that Vpr’s ability to stimulate the nuclear import of PICs may be beneficial to replication in all cell types but that the effect is more consequential in cells that are postmitotic. Consistent with the idea that import can occur via NPCs in proliferating cells, components of PICs have been shown to accumulate in the nuclei of such cells within 60 min of viral challenge, a time period that would have been insufficient for most cells to have undergone mitosis (6).
As noted earlier, it remains unclear what the subcellular localization requirements are for Vpr-mediated cell cycle arrest. Specifically, although Vpr mutants that fail to accumulate in the nucleus are generally unable to induce G2 arrest, one mutant protein (a substitution of the leucine at position 68 for serine) localizes predominantly to the cytoplasm but still retains the arrest phenotype (50). In light of the demonstration that mutant Vpr proteins can diffuse into and, by extrapolation, out of the nucleus (Fig. 2), it will be important to reevaluate the localization phenotypes of such mutants in the context of fusion proteins. In addition, it is possible that localization to NPCs is important for cell cycle arrest; the nucleoporin interaction assays discussed in this paper can now be used to address this point. It will also be of interest, therefore, to assess whether the Vpr or Vpx proteins of HIV-2 or SIVSM are capable of interacting with nucleoporins.
Nucleoporins are not the first cellular proteins to have been molecularly identified as interacting partners for HIV-1 Vpr. Recent studies have shown that two proteins associated with DNA repair, uracil DNA glycosylase (UNG) (68) and a human homolog of the S. cerevisiae Rad23 protein (HHR32A) (78), can interact with HIV-1 Vpr. It has been proposed that these proteins could be recruited during reverse transcription to limit the error rate of DNA synthesis or, alternatively, could participate in the induction of cell cycle arrest. Earlier reports also demonstrated that Vpr can interact with the cellular transcription factors Sp1 (76) and TFIIB (2). It seems likely that these interactions could contribute to the transcriptional effects of Vpr. Given that Vpr appears to have a number of diverse functions, one might anticipate that additional cellular proteins that interact with Vpr will be identified. Future analyses of the interplay between these proteins and Vpr should continue to provide important insights into the biological roles of this conserved lentivirus protein.
Subsequent to the completion of this work, and consistent with our findings, an independent series of experiments demonstrated that Vpr (HIV-1LAI isolate) colocalizes with NPCs in human and yeast cells and can interact with full-length forms of the yeast nucleoporins as well as with yeast importin-α (73).
ACKNOWLEDGMENTS
We thank Steve Adam, Hal Bogerd, Brian Burke, Bryan Cullen, Gideon Dreyfuss, Larry Gerace, Matt Michael, Mike Rosbash, and Françoise Stutz for sharing reagents; Livio Pellizzoni for assistance with confocal microscopy; Vicki Pollard for helpful discussions; and Laurie Zimmerman for excellent secretarial support.
This work was supported by the Howard Hughes Medical Institute and U.S. Public Health Service grant AI41933 (to M.H.M.) from NIAID. U.F. received support from an AIDS fellowship from the Deutsches Krebsforschungszentrum and a grant from the Deutsche Forschungsgemeinschaft (SFB 286).
REFERENCES
- 1.Adam S A, Marr R S, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agostini I, Navarro J-M, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- 3.Aitchison J D, Blobel G, Rout M P. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]
- 4.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 5.Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 6.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubei A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi N C, Adam J H, Adam S A. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. Identification of HIV-1 vpr product and function. J Acquired Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 12.Connor R I, Chen B K, Choe S, Landau N R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 13.Cullen B R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986;46:973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- 14.Davis L I, Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986;45:699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- 15.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau N R. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingwall C, Laskey R A. Nuclear targeting sequences—a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 17.Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 18.Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol. 1996;6:1096–1103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 19.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farnet C M, Bushman F D. HIV-1 cDNA integration: requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell. 1997;88:483–492. doi: 10.1016/s0092-8674(00)81888-7. [DOI] [PubMed] [Google Scholar]
- 21.Farnet C M, Haseltine W A. Integration of human immunodeficiency virus type 1 DNA in vitro. Proc Natl Acad Sci USA. 1990;87:4164–4168. doi: 10.1073/pnas.87.11.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer U, Huber J, Boelens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher T M, III, Brichacek B, Sharova N, Newman M A, Stivahtis G, Sharp P M, Emerman M, Hahn B H, Stevenson M. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-1/SIVSM. EMBO J. 1996;15:6155–6165. [PMC free article] [PubMed] [Google Scholar]
- 24.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti K G, Fransen J, Grosveld G. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component, Nup88. EMBO J. 1997;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fouchier R A M, Meyer B E, Simon J H M, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freed E O, Englund G, Martin M A. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;69:3949–3954. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridell R A, Truant R, Thorne L, Benson R E, Cullen B R. Nuclear import of hnRNP A1 is mediated by a novel cellular cofactor related to karyopherin-beta. J Cell Sci. 1997;110:1325–1331. doi: 10.1242/jcs.110.11.1325. [DOI] [PubMed] [Google Scholar]
- 28.Fritz C C, Green M R. HIV Rev uses a conserved cellular protein export pathway for the nucleocytoplasmic transport of viral RNAs. Curr Biol. 1996;6:848–854. doi: 10.1016/s0960-9822(02)00608-5. [DOI] [PubMed] [Google Scholar]
- 29.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 32.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs J S, Lackner A A, Lang S M, Simon M A, Sehgal P K, Daniel M D, Desrosiers R C. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Görlich D. Nuclear protein import. Curr Opin Cell Biol. 1997;9:412–419. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 35.Görlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 36.Görlich D, Vogel F, Mills A D, Hartmann E, Laskey R A. Distinct functions for the two importin subunits in nuclear protein import. Nature. 1995;377:246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- 37.Greber U F, Suomalainen M, Stidwill R P, Boucke K, Ebersold M W, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallberg E, Wozniak R W, Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J Cell Biol. 1993;122:513–521. doi: 10.1083/jcb.122.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izaurralde E, Kutay U, von Kobbe C, Mattaj I W, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo E, Göttlinger H G. A conserved LXXLF sequence is the major determinant in p6gag required for the incorporation of human immunodeficiency virus type 1 Vpr. J Virol. 1996;70:159–164. doi: 10.1128/jvi.70.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaCasse E C, Lefebvre Y A. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 1995;23:1647–1656. doi: 10.1093/nar/23.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang S M, Weeger M, Stahl-Hennig C, Coulibaly C, Hunsmann G, Müller J, Müller-Hermelink H, Fuchs D, Wachter H, Daniel M M, Desrosiers R C, Fleckenstein B. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy D N, Fernandes L S, Williams W V, Weiner D B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993;72:541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Hui H, Burgess C J, Price R W, Sharp P M, Hahn B H, Shaw G M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu Y-L, Bennett R P, Wills J W, Gorelick R, Ratner L. A leucine repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J Virol. 1995;69:6873–6879. doi: 10.1128/jvi.69.11.6873-6879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y-L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Weiner D B. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahalingam S, Collman R G, Patel M, Monken C E, Srinivasan A. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virology. 1995;212:331–339. doi: 10.1006/viro.1995.1490. [DOI] [PubMed] [Google Scholar]
- 52.Mahalingam S, Patel M, Collman R G, Srinivasan A. The carboxy-terminal domain is essential for stability and not for virion incorporation of HIV-1 Vpr into virus particles. Virology. 1995;214:647–652. doi: 10.1006/viro.1995.0079. [DOI] [PubMed] [Google Scholar]
- 53.Meyer B E, Malim M H. The HIV-1 Rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 54.Michael W M, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 55.Moroianu J, Hijikata M, Blobel G, Radu A. Mammalian karyopherin α1β and α2β heterodimers: α1 or α2 subunit binds nuclear localization signal and β subunit interacts with peptide repeat-containing nucleoporins. Proc Natl Acad Sci USA. 1995;92:6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neville M, Stutz F, Lee L, Davis L I, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 57.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 58.Ogawa K, Shibata R, Kiyomasu T, Higuchi I, Kishida Y, Ishimoto A, Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989;63:4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panté N, Bastos R, McMorrow I, Burke B, Aebi U. Interactions and three-dimensional localization of a group of nuclear pore complex proteins. J Cell Biol. 1994;126:603–617. doi: 10.1083/jcb.126.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paxton W, Connor R I, Landau N R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Planelles V, Jowett J B M, Li Q-X, Xie Y, Hahn B, Chen I S Y. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollard V W, Michael W M, Nakielny S, Siomi M C, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 63.Popov S, Dubrovsky L, Lee M-A, Pennathur S, Haffar O, Al-Abed Y, Tonge P, Ulrich P, Rexach M, Blobel G, Cerami A, Bukrinsky M. Critical role of reverse transcriptase in the inhibitory mechanism of CNI-H0294 on HIV-1 nuclear translocation. Proc Natl Acad Sci USA. 1996;93:11859–11864. doi: 10.1073/pnas.93.21.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 66.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rout M P, Blobel G, Aitchison J D. A distinct nuclear import pathway used by ribosomal proteins. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 68.Selig L, Benichou S, Rogel M E, Wu L I, Vodicka M A, Sire J, Benarous R, Emerman M. Uracil DNA glycosylase specifically interacts with Vpr of both human immunodeficiency virus type 1 and simian immunodeficiency virus of sooty mangabeys, but binding does not correlate with cell cycle arrest. J Virol. 1997;71:4842–4846. doi: 10.1128/jvi.71.6.4842-4846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Söderqvist H, Hallberg E. The large C-terminal region of the integral pore membrane protein, POM121, is facing the nuclear pore complex. Eur J Cell Biol. 1994;64:186–191. [PubMed] [Google Scholar]
- 70.Stewart S A, Poon B, Jowett J B M, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strizki J M, Albright A V, Sheng H, O’Connor M, Perrin L, González-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stutz F, Izaurralde E, Mattaj I W, Rosbash M. A role for nucleoporin FG repeat domains in export of human immunodeficiency virus type 1 Rev protein and RNA from the nucleus. Mol Cell Biol. 1996;16:7144–7150. doi: 10.1128/mcb.16.12.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang B, Ge Y C, Palasanthiran P, Xiang S-H, Ziegler J, Dwyer D E, Randle C, Dowton D, Cunningham A, Saksena N K. Gene defects clustered at the C-terminus of the vpr gene of HIV-1 in long-term nonprogressing mother and child pair: in vivo evolution of vpr quasispecies in blood and plasma. Virology. 1996;223:224–232. doi: 10.1006/viro.1996.0471. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Mukherjee S, Jia F, Narayan O, Zhao L-J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 77.Westervelt P, Henkel T, Trowbridge D B, Orenstein J, Heuser J, Gendelman H E, Ratner L. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J Virol. 1992;66:3925–3931. doi: 10.1128/jvi.66.6.3925-3931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Withers-Ward E S, Jowett J B M, Stewart S A, Xie Y-M, Garfinkel A, Shibagaki Y, Chow S A, Shah N, Hanaoka F, Sawitz D G, Armstrong R W, Souza L M, Chen I S Y. Human immunodeficiency virus type 1 Vpr interacts with HHR23A, a cellular protein implicated in nucleotide excision DNA repair. J Virol. 1997;71:9732–9742. doi: 10.1128/jvi.71.12.9732-9742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yaseen N R, Blobel G. Cloning and characterization of human karyopherin β3. Proc Natl Acad Sci USA. 1997;94:4451–4456. doi: 10.1073/pnas.94.9.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]