Abstract
The lipid-rich, sticky exudate covering the stigma of solanaceous species such as tobacco (Nicotiana tabacum) and petunia (Petunia hybrida) contains several proteins, of which only some have been characterized to date. Proteome analysis of the stigmatic exudate in both species revealed the presence of a cysteine-rich, slightly acidic 12-kD protein called stigma-specific protein 1 (STIG1). In both tobacco and petunia, Stig1 is highly expressed at the mRNA level in very young and developing flowers, whereas hardly any Stig1 transcript is detected in mature flowers. This expression pattern coincides with the differentiation of the secretory zone, forming the intercellular spaces into which the exudate is secreted. Using reverse genetics, we show that STIG1 is involved in the secretion and merging of exudate lipids in the intercellular spaces of the secretory zone and that plants lacking STIG1 show an accelerated deposition of exudate onto the stigmatic surface. This phenotype was observed both in a petunia knockout mutant and in tobacco transgenic plants. We therefore propose that STIG1 plays a role in the temporal regulation of the essential exudate secretion onto the stigma.
Many interactions between pollen and pistil have evolved to ensure successful sexual reproduction in plants (for review, see Sanchez et al., 2004). These interactions may differ depending on whether the species in which they occur have a wet or dry stigma. Solanaceous species, such as petunia (Petunia hybrida) and tobacco (Nicotiana tabacum), have wet stigmas, on which a sticky exudate is secreted. When mature pollen land on a mature stigma, they first come into contact with the exudate that completely covers the grains and the emerging pollen tubes, which grow further through the exudate in the intercellular spaces toward the transmitting tissue. The exudate consists of a complex mixture of mainly lipids, proteins, and saccharides (Konar and Linskens, 1966; Cresti et al., 1986). Wolters-Arts et al. (1998) showed that the lipids present in the exudate are sufficient and essential for pollen tube initiation and can functionally replace both the exudate and the stigmatoid tissue. The lipid composition influences water uptake by pollen grains and enables successful pollen-pistil interactions (Wolters-Arts et al., 2002). This implies that the proteins present in the exudate are apparently not an absolute requirement for compatible pollen tube growth through the stigma. However, pollen tube growth with whole exudate is more efficient than when pollen is grown with lipids only (Wolters-Arts et al., 1998). In addition, the in vivo growth rate of pollen tubes, such as that in tobacco, is always much greater than the rate in in vitro-growing pollen tubes. All together, this suggests that other components present in the pollen-pistil environment, such as the exudate, may be needed to improve pollen germination and pollen tube growth.
Kuboyama (1998) and Kuboyama et al. (2001) reported on several proteins present in the exudate of tobacco and hypothesized that they could be involved in pathogen resistance based on sequence homology to pathogenesis-related proteins. Our aim is to further investigate the role of exudate proteins in plant reproduction, using tobacco and petunia as model species with wet-type stigmas. We previously reported on the pollen-pistil allergen-like protein PPAL, a β-expansin homolog present in the exudate of tobacco (Pezzotti et al., 2002). This protein shows a high homology to β-expansins, but in contrast to β-expansins isolated from maize pollen (Li et al., 2003) does not enhance cell wall loosening. Instead, cell wall loosening was enhanced by a Cys-rich protein called lipid transfer protein (LTP), also present in the tobacco exudate (Nieuwland, 2004).
In this study, we describe the presence of another small Cys-rich protein in the exudate of tobacco and petunia, stigma-specific protein 1 (STIG1), which shows no homology to any other protein. Many Cys-rich proteins, such as thionins, LTPs, and defensins, have been implicated in plant defense against pathogen attack (Garcia-Olmedo et al., 1998; Thomma et al., 2002). Wet-type stigmas, such as are present in solanaceous species, can be seen as more accommodating to pathogens than dry-type stigmas (Dickinson, 1995), and several Cys-rich proteins have been found and characterized in the former. While a role in plant defense is not ruled out, some of the Cys-rich proteins found in the pistil have specific roles in pollen-pistil interactions. For example, the stigma/stylar Cys-rich adhesin, present in the stigma of lily, has been shown to increase adhesion of the pollen tube and tip (Park et al., 2000; Zhao et al., 2004), suggesting a role in pollen tube guidance. In Brassica species, some Cys-rich proteins present on the pollen coat have been implicated in pollen-pistil interactions, especially in self-incompatibility (Doughty et al., 2000). Even though Brassica species have dry-type stigmas, the proteins found on the pollen coat of these species are thought to fulfill a similar role as those present in the exudate of species with wet-type stigmas (Wolters-Arts et al., 1998). Interestingly, some of these pollen coat proteins were found to bind an S locus-related glycoprotein, SLR1 (Doughty et al., 1998), which is thought to play a role in pollen adhesion rather than in self-incompatibility (Luu et al., 1997).
The STIG1 protein sequence (Goldman et al., 1994) contains a putative secretory pathway signal peptide, without which the protein has a calculated molecular mass of 12 kD and a predicted pI at pH 6.2. The Cys-motif present in the STIG1 protein sequence has been identified in several other proteins; for instance, there are at least 6 genes in the Arabidopsis (Arabidopsis thaliana) genome that are annotated to encode proteins of the Stig1 family. However, apart from the conservation of 13 to 15 Cys residues, STIG1 shares little homology with known proteins from Arabidopsis and other species outside the Solanaceae.
Stig1 is specifically expressed in the stigmatic secretory zone of tobacco (Goldman et al., 1994) from the onset of flower differentiation. Tang et al. (2004) reported on the interaction of LeSTIG1 with pollen-specific receptor kinases in tomato (Lycopersicon esculentum). In addition, they show that exogenous LeSTIG1 promotes pollen tube growth in vitro. Here, we show that the STIG1 protein is present in the exudate of petunia and tobacco pistils. We highlight the role of this particular protein during the development of the secretory zone and deposition of the exudate onto the stigma. By using reverse genetics, we discovered that loss of the STIG1 protein enhances the speed of exudate secretion in both tobacco and petunia pistils. We conclude that this protein is necessary for normal exudate deposition in these species.
RESULTS
Proteome Analysis of the Exudate of Petunia and Tobacco
To identify proteins specifically present in the exudate of petunia and tobacco stigmas, total exudate was subjected to SDS-PAGE and stained with Coomassie Brilliant Blue. Approximately 10 protein bands ranging from 4 to 70 kD were clearly visible. Both tobacco and petunia exudate showed similar protein band patterns (data not shown). Sections of the gels containing protein bands ranging between 4 to 16 kD, 16 to 30 kD, and 30 to 50 kD were excised, digested with trypsin, and the resulting peptides were extracted and sequenced using a mass spectrometer equipped with an electrospray ion source. The most abundant peptides were derived from the β-expansin PPAL and the Cys-rich proteins LTP and STIG1. Both LTP and PPAL had previously been identified in the exudate of tobacco (Pezzotti et al., 2002; Nieuwland, 2004) but not in the exudate of petunia, whereas the presence of STIG1 in the exudate of either species has not been reported. Table I lists the STIG1 peptides identified by quadrupole time-of-flight analysis of petunia exudate. To further investigate the role of STIG1 in the exudate of petunia and tobacco stigmas, we decided to select for or produce plants not expressing Stig1 in either of these species.
Table I.
STIG1 peptides identified by quadrupole time-of-flight analysis of exudate from petunia
Several of the peptides were present in two forms. In each case, one of the forms contained a Cys residue reduced from the expected 160D to 44D. These Cys residues are underlined.
| Peptide Molecular Mass | Peptide m/z | Peptide Sequence |
|---|---|---|
| D | D | |
| 1,165.6 | 583.8++ | CCGGLCVDITK |
| 1,440.6 | 721.3++ | FNCGSCGIVCDLR |
| 1,556.6 | 779.4++ | FNCGSCGIVCDLR |
| 1,902.9 | 635.3+++ | ASCVDLSSNR |
| 1,786.6 | 894.3++ | LTCCFNASCVDLSSNR |
Developmental Expression Pattern of Stig1 in Petunia
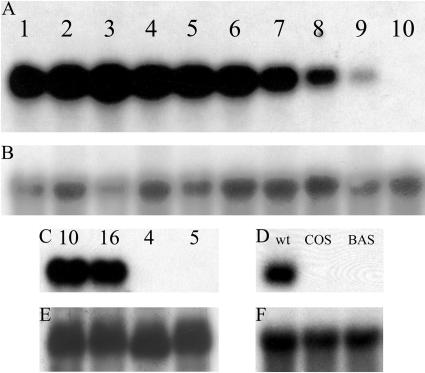
To test whether the developmental expression pattern of Stig1 in petunia was similar to that found in tobacco (Goldman et al., 1994), we analyzed the Stig1-mRNA levels in stigmas from developmental stages 1 to 10 (see “Materials and Methods”). This showed that Stig1 was expressed from the first developmental stage onwards. The expression peaked at early- to mid-stage of development and gradually declined toward the end of flower development. No Stig1 mRNA was detected in mature open flowers (stage 10; Fig. 1), as is also the case in tobacco.
Figure 1.
Expression of Stig1 in petunia and tobacco wild-type, knockout, and transgenic plants. A, RNA gel-blot analysis of Stig1 transcript levels in developing stigmas of wild-type petunia plants, stages 1 to 10 (see “Materials and Methods”). Ten micrograms of total RNA was loaded in each lane. B, E, and F, Control for equal loading using a random-primed 32P radioactive-labeled 25S cDNA probe. C, RNA gel-blot analysis of Stig1 transcript levels in stigmas of wild-type (10 and 16) and petunia knockout (4 and 5) plants. Mutants carry the stabilized transposon in the Stig1 gene. Ten micrograms of total RNA was loaded in each lane. D, RNA gel-blot analysis of Stig1 transcript level in stigmas of wild-type, COS, and BAS transgenic tobacco plants. Ten micrograms of total RNA was loaded in each lane.
Screening a Petunia Transposon Library for a Transposon Insertion in the Stig1 Gene
Screening petunia transposon libraries enabled us to detect knockout mutants in the Stig1 gene, as described by Koes et al. (1995) and Vandenbussche et al. (2003). This resulted in the identification of one W138 plant with a dTph1 transposon inserted in the Stig1 gene (supplemental data), of which the seeds of a selfing were sown. The presence of the transposon insertion and Stig1-mRNA levels of the resulting plants were verified. The insertion was present in the coding sequence of the gene and exhibited a normal Mendelian transmission rate. Pollen from plants homozygous for the transposon insertion was used to pollinate wild-type petunia var Mitchell flowers; petunia Mitchell plants do not produce transposase, which stabilizes the transposon insertions. The seeds resulting from a self of this cross were sown, and the resulting F2 plants were analyzed for Stig1-mRNA levels. Figure 1C shows 2 selected stable mutant plants (4 and 5) that do not contain Stig1 mRNA. F2 plants 10 and 16 (Fig. 1C) do contain Stig1 mRNA and were used as control plants in mRNA, protein, and phenotypic analysis.
Generation of STIG1 Transgenic Tobacco Plants
Transgenic plants carrying the Stig1 transgene in either the sense (cosuppression [COS] plants) or anti-sense (BIN19 anti-sense [BAS] plants) orientation were generated and selected on the basis of a PCR on genomic DNA using kanamycin primers. Plants were screened by northern-blot analysis to determine Stig1-mRNA levels. Plants that lacked Stig1 mRNA were allowed to self-pollinate and the resulting seeds were sown and grown. Expression levels of the resulting F1 plants were again analyzed using northern-blot analysis (data not shown). Three BAS and three COS plants were selected for phenotypic analysis. Representative Stig1 expression levels in 1 COS and 1 BAS tobacco plant compared to those in a wild-type tobacco plant are represented in Figure 1D.
Proteome Analysis of Petunia and Tobacco Plants Not Expressing Stig1
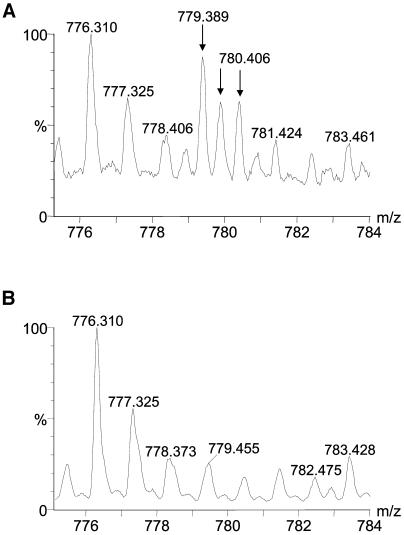
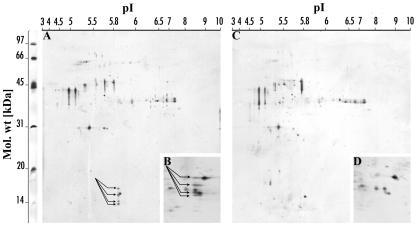
No Stig1 mRNA could be detected using northern-blot analysis in either the petunia knockout mutant or tobacco Stig1-COS or -BAS plants. Therefore, no detectible STIG1 protein should be present in the exudate of these plants. To verify this, we decided to subject the exudate of wild-type and petunia knockout mutants to one- and two-dimensional gel electrophoresis. The one-dimensional gels were stained with Coomassie Brilliant Blue, proteins were excised and digested, and peptides extracted and analyzed. The results obtained confirmed that no STIG1 was present in the exudate of the petunia knockout mutant (Fig. 2). Two-dimensional gels were silver stained and analyzed using the PDQuest Two-Dimensional Analysis Software, which showed that 4 spots with a molecular mass between 12 and 16 kD and a pI of approximately 6.1 were missing in the exudate of the knockout mutant (Fig. 3). This was consistent with the expected molecular mass and pI of STIG1. Three potential N-glycosylation sites were identified in the STIG1 protein sequence; therefore, the four spots found on the two-dimensional gel from wild-type exudate may indicate four different states of glycosylated STIG1.
Figure 2.
Mass spectra from the analysis of proteins in petunia exudate. A, Exudate from wild-type petunia plants. B, Exudate from petunia knockout plants The arrows indicate the isotopic cluster of the peptide 779.4++ from STIG1.
Figure 3.
Silver-stained two-dimensional gels showing the difference in exudate proteome of wild-type and petunia knockout stigmas. Ten to 50 μg of total protein was loaded for first-dimension electrophoresis. Gels containing 12.5% (w/v; A and C) and 15% (w/v; B and D) polyacrylamide were used for second-dimension electrophoresis. All gels were made using the same low molecular mass protein standard, with a range from approximately 97 to 14 kD, shown on the left. Above the gels the pI range from 3 to 10 is indicated. Four spots (arrows) of approximately 14 to 16 kD in size and with a pI of 6.1 were consistently missing in both 12.5% gels (main sections) and 15% gels (detailed inlay) run with exudate from petunia knockout stigmas, compared to similar gels run with exudate from wild-type petunia stigmas.
Characterization of the stig1 Phenotype
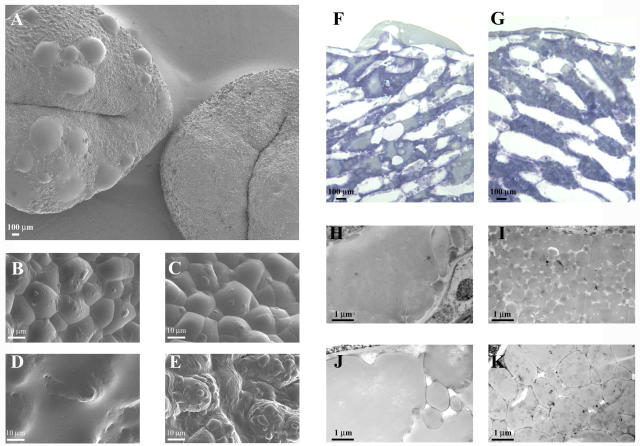
On the whole, petunia knockout mutants and tobacco transgenic (COS and BAS) plants grew normally, flowered, and set seed as successfully as their wild-type counterparts. However, mature stigmas of flowers of the petunia knockout mutant and tobacco transgenic plants seemed to produce more exudate compared to wild-type plants. We therefore decided to look at the exudate secretion in the petunia knockout mutant and transgenic tobacco plants in more detail, using different microscopical techniques. First, stigmas of different developmental stages of wild-type (petunia and tobacco), petunia knockout mutant, and transgenic tobacco plants were viewed using cryo-scanning electron microscopy (Fig. 4, A–E). This showed a remarkable difference in the amount of exudate present at different stages of development. At the earliest stages (stage 1; Fig. 4, B and C), there is no discernible difference between wild-type and petunia knockout mutant or wild-type and transgenic tobacco stigmas. However, as the stigmas mature, those that lack STIG1 consistently have more exudate than those that have STIG1 in the exudate (Fig. 4, A, D, and E). Second, wild-type (petunia and tobacco), petunia knockout mutant, and transgenic tobacco stigmas were prepared for light microscopy and stained with toluidine blue, a basic blue general dye, to identify general differences in the secretory zone of the stigma. This showed that the appearance of the exudate in mutant and transgenic stigmas differed from that of their respective wild types (Fig. 4, F and G). Third, we decided to use transmission electron microscopy to look at the secreted exudate in the intercellular spaces in more detail. In near-mature wild-type stigmas of petunia and tobacco (stages 9 and 6, respectively), the secreted exudate in the intercellular spaces consisted of small, individual lipid droplets surrounded by an electron-dense coat (Fig. 4, I and K). From mid-stage onwards, these small droplets tended to merge, forming larger droplets and eventually lipid pools of varying sizes, toward the middle of the intercellular space. In contrast, large lipid pools were observed early on in stigmas of both petunia knockout mutant and tobacco transgenic plants (Fig. 4, H and J). It therefore seemed that the lack of STIG1 resulted in accelerated merging of secreted lipid droplets in the intercellular spaces.
Figure 4.
Morphological comparison of stigmas from petunia (A–I) and tobacco (J and K) plants expressing and not expressing Stig1. Micrographs were made using different microscopy techniques: A to E, Cryo-scanning electron microscopy; F and G, Light microscopy; H to K, Transmission electron microscopy. A, Mature stigmas from knockout (on the left) and wild-type (on the right) plants. Note the large droplets of exudate present on the mutant stigmas, absent on the wild-type stigmas. B, Detail of a knockout mutant stigma at the earliest stage of flower development (stage 1) and (D) at mid-stage of development (stage 5). At stage 1, only a few small exudate droplets are present, whereas at stage 5 the stigma is almost completely covered. C, Detail of a wild-type stigma at the earliest stage of flower development (stage 1) and (E) at mid-stage of development (stage 5). As for the mutant, only small droplets can be seen at stage 1, whereas these have increased in size by stage 5, but do not completely cover the stigmatic surface. F, Transverse section from a nearly mature (stage 9) knockout stigma, stained with toluidine blue. The intercellular spaces are predominantly filled with a light-staining substance, probably lipid, whereas in the wild type the intercellular spaces are mostly filled with blue-staining globules (G). H, Transverse section from a knockout mutant and (I) wild-type stigma, at a nearly mature stage of development (stage 9). The mutant has large lipid pools in the secretory zone, whereas in the wild type the lipid globules have not yet merged to form lipid pools. J, Transverse section from a tobacco transgenic and (K) wild-type stigma, at mid-stage of development (stage 6). Again, the transgenic stigma has large lipid pools similar to those observed in petunia transposon mutants, whereas the wild-type stigma does not.
The Loss of STIG1 Does Not Affect in Vivo Pollen Tube Germination, Pollen Tube Growth, or Seed Set in Petunia and Tobacco
It has previously been shown that lipids play an important role in successful pollen hydration, germination, and growth of the pollen tube through the style (Wolters-Arts et al., 1998). Although this indicates that proteins may not be necessary for these processes, we decided to study the role of STIG1 in pollen germination and pollen tube growth, since the absence of STIG1 affected the appearance of the lipids in the exudate. Wild-type and petunia knockout stigmas were pollinated and pollen was allowed to grow for 3, 6, or 24 h. After 3 h, the amount of pollen tubes germinated and the mean distance of penetration through wild-type and mutant stigmas was compared. The amount of pollen tubes germinated did not differ between wild-type and mutant stigmas (data not shown). The average length of pollen tubes in wild-type stigmas was 0.78 mm and in mutant stigmas 0.63 mm, with a sd of 0.24 mm and 0.15 mm, respectively (not statistically significant; Student's t test, P < 0.1). Similarly, 6 and 24 h after pollination, no significant differences in mean lengths of pollen tubes growing through mutant and wild-type styles were observed (data not shown). In addition, nearly mature fruits from both wild-type and knockout mutant plants were harvested and their seeds were counted. Seeds per fruit did not differ significantly between wild-type and petunia knockout mutants (418 ± 49 and 380 ± 56, respectively; Student's t test, P < 0.1). Therefore, the number of pollen tubes successfully reaching the ovules in petunia knockout mutants is not affected by the absence of STIG1. These in vivo experiments show that STIG1 is not necessary for pollen germination, nor is the lack of STIG1 negatively affecting pollen tube growth through the style of petunia knockout mutants under the test conditions used.
DISCUSSION
Stig1 has previously been shown to be exclusively expressed in the stigmatic secretory zone and the Stig1 promoter has successfully been used to generate stigma-less tobacco plants (Goldman et al., 1994). However, the specific location and function of the STIG1 protein in planta has not been further investigated until now. The STIG1 protein sequence contains a putative signal peptide for secretion, and we have shown that it is present in the exudate. This suggests that STIG1 is secreted into the intercellular spaces of the stigmatic secretory zone.
Tobacco and petunia plants not expressing STIG1 exhibited increased amounts of exudate on the stigmatic surface, which seemed to result from accelerated exudate deposition onto the stigma. Our microscopical studies showed that in the secretory zones of stigmas lacking STIG1, lipid droplets present in the intercellular spaces merged to form large lipid pools at a much earlier developmental stage than found in wild-type plants, coinciding with larger amounts of exudate on the stigmatic surface. In wild-type plants, such large lipid pools are found in almost-mature stigmas, and this is also when most of the exudate is present on top of the stigma. It is therefore possible that the fusion of lipid droplets in the secretory zone and the deposition of large amounts of exudate onto the stigma are linked processes. From this observation, we suggest that STIG1 is involved in timing the fusion and subsequent release of lipid droplets, thus controlling the crucial moment at which the exudate is present on the stigma. In plant sexual reproduction, timing is all important. Pollen not only has to find its way to the stigma, but it also has to remain there to hydrate and germinate. The receptivity of a stigma can vary between species and also greatly depends on the appropriate stage of development (Heslop-Harrison, 2000). The exudate is indispensable for proper pollen germination and initial growth. Thus, timing the deposition of the exudate with stigma receptivity, thereby preventing unnecessary loss of precious exudate, would greatly enhance a plant's chances of successfully reproducing in a natural environment. In view of this hypothesis, STIG1 would play an indirect role in the establishment of successful pollen-pistil interactions in tobacco and petunia. STIG1 could be one of many components present in the extracellular matrix that may be coordinately regulated when optimal female fertility is needed. To determine precisely how STIG1 influences the coalescence of exudate droplets, more detailed localization of STIG1 in the exudate is needed. Such studies will be included in our further investigations of the function of STIG1. It also remains to be determined whether the bigger exudate droplets on the mutant stigmas merely represent an earlier release or (also) an actual increase in exudate production.
In both tobacco and petunia, Stig1 expression starts when the stigmatic secretory zone begins to differentiate and decreases when the exudate is fully formed and secreted on top of the stigma (Goldman et al., 1994; this study). Our hypothesis that Stig1 is involved in timing the deposition of the exudate is in agreement with the fact that Stig1 is expressed early in stigma development, when the secretory zone is differentiating and the exudate is being formed. The STIG1 protein was isolated from exudate from mature stigmas, even though at that stage of flower development expression of Stig1 had ceased, indicating that the protein is fairly stable. When comparing initial pollen tube germination and growth through the style in plants expressing and not expressing Stig1, no difference in pollen germination was observed. The small decrease in average length in 3-h-old pollen tubes growing through stigmas without STIG1 in the exudate was not significant. Similarly, during later stages of pollen tube growth through the style, we did not see any significant differences. This is not remarkable, since once compatible pollen have germinated and penetrated the stigmatic tissue, arrest of pollen tube growth rarely occurs. It is specifically during pollen germination and initial penetration of the pollen tube through the stigma that differences in the stigmatic and stylar environment matter. In addition, it has been shown that lipids alone are essential for successful pollen tube growth and fertilization (Wolters-Arts et al., 1998). Our data on seed set, which was unaffected in the petunia knockout mutants, support the hypothesis that STIG1 does not affect pollen tube growth in vivo. Tang et al. (2004) reported on the presence of STIG1 in tomato pistils. They showed with in vitro pollen tube growth assays that exogenous STIG1 binds to pollen tubes and promotes growth. It is important to note that the natural variation present in in vivo experiments is very large. Therefore, in contrast to similar in vitro experiments as reported by Tang et al., an in vivo difference in pollen tube length of approximately 0.5 mm would be attributed to natural variation. In addition, the growth medium used for in vitro pollen tube growth experiments is much impoverished compared to the highly enriched natural growth medium found in the pistil. Finally, we showed that STIG1 is glycosylated in vivo. The recombinant STIG1 used by Tang et al. (2004) was an unglycosylated glutathione S-transferase-fused protein produced in bacterial hosts, and both modifications may alter protein structure and/or activity significantly. STIG1 may have an additional role in facilitating and/or enhancing initial pollen tube germination and growth in a naturally competitive environment, but based on our expression data and microscopical studies, we conclude that the primary role of STIG1 is controlling exudate secretion and timing its deposition onto the stigmatic surface.
MATERIALS AND METHODS
Plant Growth Conditions and Defining of Flower Developmental Stages
Plants were grown in a greenhouse with supplementary lighting from October until May providing a 16-h photoperiod. Temperature was maintained at 25°C during the day and 20°C during the night. Ten stages of development were identified in petunia (Petunia hybrida) var Mitchell flowers (also referred to as petunia Mitchell), based on the length of the corolla. Stage 1, 5 mm; stage 2, 10 mm; stage 3, 15 mm; stage 4, 20 mm; stage 5, 25 mm; stage 6, 30 mm; stage 7, 35 mm; stage 8, 40 mm; stage 9, 40+ mm prior to anthesis; and stage 10, 40+ mm anthesis. Developmental stages of tobacco (Nicotiana tabacum) referred to in this article correspond to those previously described in Goldberg (1988).
Preparation of Protein Extracts for One- and Two-Dimensional Gel Electrophoresis
Exudate was isolated by submerging 10 mature stigmas of petunia in 100 μL of 50 mm NaAc, pH 4.5, for 30 min, centrifuging for 20 min, and removing the aqueous phase. A total of 1,000 stigmas were extracted and the resulting exudate was pooled and concentrated 10-fold using a CM10 column (Millipore, Bedford, MA). Tobacco exudate was isolated as for petunia but no concentration was necessary. Exudate from both species was frozen at −20°C until used in one- or two-dimensional gel electrophoresis.
One-Dimensional Gel Electrophoresis
Gel electrophoresis was performed on 12.5% (w/v) polyacrylamide gels (0.4 m Tris, pH 8.8, and 1% [w/v] SDS) with a SeeBlue Pre-Stained Protein Standard (Invitrogen, Carlsbad, CA) used according to the manufacturer's instructions, using an SDS running buffer (25 mm Tris, 192 mm Gly, and 0.1% [w/v] SDS). After electrophoresis, gels were stained with Coomassie Brilliant Blue. Areas with protein bands corresponding to a molecular mass of 4 to 16 kD, 16 to 30 kD, and 30 to 50 kD were excised from the gel and used for peptide analysis by mass spectrometry.
Two-Dimensional Gel Electrophoresis and Silver Staining
For the first dimension, the method described in Eklund and Edqvist (2003) was followed with the exception that proteins were not precipitated but whole exudate extract containing 10 to 50 μg of total protein was used. Second-dimension gel electrophoresis was performed on 12.5% and 15% (w/v) polyacrylamide gels (0.4 m Tris, pH 8.8, and 1% [w/v] SDS) with a low Mr protein standard (SDS-PAGE Molecular Weight Standards, Bio-Rad Laboratories, Hercules, CA) used according to the manufacturer's instructions, using an SDS running buffer (25 mm Tris, 192 mm Gly, and 0.1% [w/v] SDS).
Gels were stained with silver nitrate as described in Eklund and Edqvist (2003). Silver-stained gels were scanned using a scanner (GS-710 Calibrated Imaging Densitometer, Bio-Rad Laboratories) equipped with PDQuest Two-Dimensional Analysis Software (Bio-Rad Laboratories).
Peptide Analysis by Mass Spectrometry
In-gel digestion, peptide extraction, and peptide analysis by mass spectrometry were performed according to the method described by Eklund and Edqvist (2003).
Bioinformatics
Peptide sequences obtained by Mass Lynx were subjected to BLAST using BLASTp (Altschul et al., 1997) at the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/BLAST/) and MS BLAST (Shevchenko et al., 2001) at the European Molecular Biology Laboratory (http://dove.embl-heidelberg.de/blast2/msblast.html). Determination of N-glycosylation sites was done using the NetNGlyc server at http://www.cbs.dtu.dk/services/NetNGlyc/.
PCR Screening of the Transposon Insertion Libraries
PCR-based screening was performed following a three-dimensional method devised by Koes et al. (1995). In this particular experiment, we screened a three-dimensional transposon library containing genomic DNA of 4,096 plants. For one PCR reaction, the following components were mixed together: 5 μL of template DNA (10–20 ng/μL), 2.5 μL of 10× PCR buffer (with 15 mm MgCl2), 1 μL of gene-specific primer (1 pmol/μL; o-stig06, 5′-ATAATCATCCTCACTCTTTCTAGCACACC-3′ or o-stig07, 5′-AGACAGGGGAATAGTTATTTGACCAGC-3′), 0.5 μL of deoxynucleotide triphosphates (10 mm), 1 μL of inverted-repeat primer (5′-GAATTCGCTCCGCCCCTG-3′) complementary to the terminal-inverted repeats of dTph1 (1 pmol/μL), 0.08 μL of platinum Taq DNA polymerase (Invitrogen), and deionized water to 25 μL. The mixture was amplified in an Applied Biosystems (Foster City, CA) 9600 thermocycler with the following PCR profile: 14 cycles of 94°C for 15 s, 71°C for 30 s minus 1°C/cycle, and 72°C for 60 s, followed by 40 cycles of 94°C for 15 s, 56°C for 30 s, and 72°C for 60 s.
Five microliters of 5× loading dye (1 mm Tris, 10 mm EDTA, 0.06% bromphenol blue, and 0.06% xylene cyanol, 50% glycerol) were added to the PCR product and 5 μL of this mix were used for electrophoresis in a 1% agarose gel. This gel was blotted to a NytranSuPerCharge nylon transfer membrane (Schleicher & Schuell, Keene, NH) and hybridized with a random primed 32P radioactive-labeled Stig1 cDNA probe.
Plants giving a signal of the same size in all three dimensions were used for further analysis.
Analysis of the Transposon Insertion Site
Plants identified as containing a dTph1-DNA insertion in Stig1 (see above) were used for DNA isolation. PCR was performed using the inverted-repeat and gene-specific primers under the same conditions as described above. To determine the transposon insertion position, PCR fragments were cloned and sequenced according to Sanger et al. (1977).
Constructs for Silencing Stig1 in Tobacco
Both cosuppression (COS) and anti-sense (BAS) constructs were generated. The Stig1 full-length cDNA (accession no. X77823) was cloned in sense (COS plants) and anti-sense (BAS plants) orientation under the control of the 35S cauliflower mosaic virus promoter (Angenent et al., 1994). The constructs were transferred to Agrobacterium tumefaciens LBA4404 using the triparental mating approach. The recombinant A. tumefaciens strains were used to transform tobacco SR1 by using a standard leaf disc transformation and regeneration method (Tavazza et al., 1989). Transgenic plants were selected in vitro on the basis of kanamycin resistance and further grown in the greenhouse.
Total RNA Isolation and Gel-Blot Hybridization Analysis
Total RNA was isolated using the method of Frankis and Mascarenhas (1980) with some modifications (Goldberg et al., 1981; Van Eldik et al., 1996). Nonpollinated stigmas and styles of petunia and tobacco were used for the extraction of RNA.
Total RNA concentrations were determined from the A260. From each sample, 10 μg of total RNA was separated in 1.2% formaldehyde agarose gels. Following a prehybridization for 4 h, the RNA gel blots were probed with a random-primed 32P-labeled Stig1-cDNA probe (Goldman et al., 1994). Hybridization was performed at 60°C for 16 h in 6× SSC/0.1% SDS, 5× Denhardt's solution. The filters were washed in 1× SSC/0.1% SDS, 0.5× SSC/0.1% SDS, and 0.2× SSC/0.1% SDS, respectively, at 60°C and exposed to CEAX-Pro films at −80°C with intensifying screens. Finally, the filters were stripped and probed with a random-primed 32P-labeled 25S ribosomal RNA probe from tobacco.
Light Microscopy and Transmission Electron Microscopy
Stigmas were fixed in 2% glutaraldehyde in 0.1 m phosphate buffer at pH 7.2 for 2 h at room temperature, followed by postfixation in 1% (w/v) osmium tetroxide in water. Tissues were dehydrated in ethanol and propylene oxide and embedded in Spurr's resin. For light microscopy, sections of 1 μm were stained by toluidine blue (0.1% in 1% borax). For transmission electron microscopy, thin sections were poststained with uranyl acetate and lead citrate according to standard procedures and viewed with a JEOL JEM 100CX II.
Cryo-Scanning Electron Microscopy
Stigmas were glued onto a stub with colloidal carbon adhesive and frozen in liquid nitrogen. The samples were transferred in a transfer holder under vacuum at liquid-nitrogen temperature to the cold stage at −95°C into a cryo-preparation chamber CT 1500 HF (Oxford Instruments, High Wycomb, UK). The specimens were sputter coated with 5 nm platinum. The specimens were conveyed under high vacuum to the cold stage of a scanning electron microscope equipped with a cold-field emission electron gun (JSM 6300F; Jeol, Tokyo), analyzed, and recorded at −180°C using a 5-kV accelerating voltage.
Pollen Tube Growth in Vivo
Flowers of wild-type and petunia knockout plants were emasculated before anthesis and pollinated 24 h later with an excess of pollen from one anther. Three, 6, and 24 h after pollination, pistils were collected and fixed in ethanol/acetic acid (3:1) for 12 h at 4°C and pretreated in 2 n NaOH for 1 h at 60°C to soften the tissue, rinsed in water, and placed directly into a drop of aniline blue. The pistils were squashed, the number of pollen tubes that had penetrated the stigma was counted, and their mean length measured (10 pistils of each) using a Leitz fluorescence microscope with a UV excitation-blue emission filter.
To verify the number of pollen tubes successfully reaching the ovule, 10 nearly mature fruits of both wild-type and petunia knockout plants were harvested, the pericarp was removed, and the seeds were scraped from the placenta and counted.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number X77823.
Supplementary Material
Acknowledgments
We thank Ingrid Schenning for excellent technical support and Håkan Larsson for technical advice.
This work was supported by the European Union Training Network TIPNET (grant no. HPRN–CT–2002–00265 to T.V.). J.E. received financial support from the Carl Trygger Foundation and The Swedish Research Council.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.054809.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenent GC, Franken J, Busscher M, Weiss D, Van Tunen AJ (1994) Co-suppression of the petunia homeotic gene Fbp2 affects the identity of the generative meristem. Plant J 5: 33–44 [DOI] [PubMed] [Google Scholar]
- Cresti M, Keijzer CJ, Ciampolini F, Focardi S (1986) Stigma of Nicotiana: ultrastructural and biochemical studies. Am J Bot 73: 1713–1722 [Google Scholar]
- Dickinson H (1995) Dry stigmas, water and self-incompatibility in Brassica. Sex Plant Reprod 8: 1–10 [Google Scholar]
- Doughty J, Dixon S, Hiscock SJ, Willis AC, Parkin IAP, Dickinson HG (1998) PCP-A1, a defensin-like Brassica pollen coat protein that binds the S locus glycoprotein, is the product of gametophytic gene expression. Plant Cell 10: 1333–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty J, Wong HY, Dickinson HG (2000) Cysteine-rich pollen coat proteins (PCPs) and their interactions with stigmatic S (incompatibility) and S-related proteins in Brassica: putative roles in SI and pollination. Ann Bot (Lond) 85: 161–169 [Google Scholar]
- Eklund DM, Edqvist J (2003) Localization of nonspecific lipid transfer proteins correlate with programmed cell death responses during endosperm degradation in Euphorbia lagascae seedlings. Plant Physiol 132: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankis R, Mascarenhas JP (1980) Messenger-Rna in the ungerminated pollen grain: a direct demonstration of its presence. Ann Bot (Lond) 45: 595–599 [Google Scholar]
- Garcia-Olmedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P (1998) Plant defense peptides. Biopolymers 47: 479–491 [DOI] [PubMed] [Google Scholar]
- Goldberg RB (1988) Plants: novel developmental processes. Science 240: 1460–1467 [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Hoschek G, Tam SH, Ditta GS, Breidenbach RW (1981) Abundance, diversity, and regulation of messenger-RNA sequence sets in soybean embryogenesis. Dev Biol 83: 201–217 [DOI] [PubMed] [Google Scholar]
- Goldman MHS, Goldberg RB, Mariani C (1994) Female sterile tobacco plants are produced by stigma-specific cell ablation. EMBO J 13: 2976–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison Y (2000) Control gates and micro-ecology: the pollen-stigma interaction in perspective. Ann Bot 85: 5–13 [Google Scholar]
- Koes R, Souer E, Vanhouwelingen A, Mur L, Spelt C, Quattrocchio F, Wing J, Oppedijk B, Ahmed S, Maes T, et al (1995) Targeted gene inactivation in petunia by PCR-based selection of transposon insertion mutants. Proc Natl Acad Sci USA 92: 8149–8153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konar RN, Linskens HF (1966) The morphology and anatomy of the stigma of Petunia hybrida. Planta 71: 356–371 [DOI] [PubMed] [Google Scholar]
- Kuboyama T (1998) A novel thaumatin-like protein gene of tobacco is specifically expressed in the transmitting tissue of stigma and style. Sex Plant Reprod 11: 251–256 [Google Scholar]
- Kuboyama T, Yoshida KT, Takeda G (2001) Antiserum against a stigma-exudate protein of tobacco, SE32, which was identical with PPAL, a beta-expansin-like protein specific to stigma, cross-reacted with another stigma-exudate protein, SE35. Breed Sci 51: 131–135 [Google Scholar]
- Li LC, Bedinger PA, Volk C, Jones AD, Cosgrove DJ (2003) Purification and characterization of four beta-expansins (Zea m 1 isoforms) from maize pollen. Plant Physiol 132: 2073–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu DT, Heizmann P, Dumas C (1997) Pollen-stigma adhesion in kale is not dependent on the self-(in)compatibility genotype. Plant Physiol 115: 1221–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland JH (2004) Cell wall loosening proteins of the stigma exudate. PhD thesis. University of Nijmegen, The Netherlands
- Park S-Y, Jauh G-Y, Mollet J-C, Eckard KJ, Nothnagel EA, Walling LL, Lord EM (2000) A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzotti M, Feron R, Mariani C (2002) Pollination modulates expression of the PPAL gene, a pistil-specific β-expansin. Plant Mol Biol 49: 187–197 [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Bosch M, Bots M, Nieuwland J, Feron R, Mariani C (2004) Pistil factors controlling pollination. Plant Cell 16: S98–S106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulsen AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Sunyaev S, Loboda A, Shevehenko A, Bork P, Ens W, Standing KG (2001) Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time of flight mass spectrometry and BLAST homology searching. Anal Chem 73: 1917–1926 [DOI] [PubMed] [Google Scholar]
- Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S (2004) LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vivo. Plant J 39: 343–353 [DOI] [PubMed] [Google Scholar]
- Tavazza R, Tavazza M, Ordas RJ, Ancora G, Benvenuto E (1989) Genetic transformation of potato (Solanum tuberosum): an efficient method to obtain transgenic plants. Plant Sci 59: 175–181 [Google Scholar]
- Thomma BPHJ, Cammue BPA, Thevissen K (2002) Plant defensins. Planta 216: 193–202 [DOI] [PubMed] [Google Scholar]
- Van Eldik GJ, Wingens M, Ruiter RK, Van Herpen MMA, Schrauwen JAM, Wullems GJ (1996) Molecular analysis of a pistil-specific gene expressed in the stigma and stylar cortex of Solanum tuberosum. Plant Mol Biol 30: 171–176 [DOI] [PubMed] [Google Scholar]
- Vandenbussche M, Zethof J, Souer E, Koes R, Tornielli GB, Pezzotti M, Ferrario S, Angenent GC, Gerats T (2003) Toward the analysis of the petunia MADS box gene family by reverse and forward transposon insertion mutagenesis approaches: B, C, and D floral organ identity functions require SEPALLATA-like MADS box genes in petunia. Plant Cell 15: 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392: 818–821 [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, Van der Weerd L, Van Aelst AC, Van der Weerd J, Van As H, Mariani C (2002) Water-conducting properties of lipids during pollen hydration. Plant Cell Environ 25: 513–519 [Google Scholar]
- Zhao J, Mollet J-C, Lord EM (2004) Lily (Lilium longiflorum L.) pollen protoplast adhesion is increased in the presence of the peptide SCA. Sex Plant Reprod 16: 227–233 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.