Abstract
Root extracts from the arsenic (As) hyperaccumulating Chinese brake fern (Pteris vittata) were shown to be able to reduce arsenate to arsenite. An arsenate reductase (AR) in the fern showed a reaction mechanism similar to the previously reported Acr2p, an AR from yeast (Saccharomyces cerevisiae), using glutathione as the electron donor. Substrate specificity as well as sensitivity toward inhibitors for the fern AR (phosphate as a competitive inhibitor, arsenite as a noncompetitive inhibitor) was also similar to Acr2p. Kinetic analysis showed that the fern AR had a Michaelis constant value of 2.33 mm for arsenate, 15-fold lower than the purified Acr2p. The AR-specific activity of the fern roots treated with 2 mm arsenate for 9 d was at least 7 times higher than those of roots and shoots of plant species that are known not to tolerate arsenate. A T-DNA knockout mutant of Arabidopsis (Arabidopsis thaliana) with disruption in the putative Acr2 gene had no AR activity. We could not detect AR activity in shoots of the fern. These results indicate that (1) arsenite, the previously reported main storage form of As in the fern fronds, may come mainly from the reduction of arsenate in roots; and (2) AR plays an important role in the detoxification of As in the As hyperaccumulating fern.
Arsenic (As) is a highly toxic metalloid that poses hazards to microbes, plants, animals, and humans (Kaise et al., 1985). Arsenic is introduced into soil environments through both anthropogenic and geogenic sources, as found in groundwater (Smith et al., 1998). Long-term exposure to As can affect the nervous system in humans or lead to cancer. The cancer risks to the population due to As in U.S. water supplies may be comparable to those from environmental tobacco smoke and radon in homes (Smith et al., 1992). In Bangladesh, over 40 million people drink well water containing toxic levels of As (Smedley and Kinniburgh, 2002) and, in West Bengal, India, out of a total of 18 districts, nine have been identified where the groundwater contains As above 0.05 mg L−1 (well above the permissible limit of As in drinking water [0.01 mg L−1] recommended by the World Health Organization [WHO]; Roychowdhury et al., 2002). Decontamination of As pollution has become a major environmental concern around the globe, particularly in Southeast Asia (Meharg, 2004).
Even though As and most other heavy metals are toxic to plants, a range of plants has been described as so-called metallophytes or hyperaccumulators, growing preferably or exclusively in soils contaminated with high levels of heavy metals or metalloids. Hyperaccumulators (Salt et al., 1995; Brooks, 1998) have attracted particular interest. They are not only able to grow in soils with high heavy-metal concentrations, but also accumulate those metals in their shoots to levels that may be higher than the levels lethal for other living organisms. These plants have fueled the idea of remediating heavy metal-contaminated soils via removal of the contaminants after accumulation into their above-ground parts: the process of phytoremediation or, more exactly, phytoextraction (Wenzel et al., 1999; Tu et al., 2002).
The relatively recent discovery of an As hyperaccumulator, the Chinese brake fern (Pteris vittata; Ma et al., 2001), has offered the hope that phytoextraction might be developed into an efficient, environmentally friendly, and cost-effective technology for As decontamination (Salt et al., 1998; Tu et al., 2002). This fern can accumulate up to 22.6 g As kg−1 shoot (frond) dry weight, i.e. 2% As in the above-ground biomass. The As in the above-ground biomass is much higher than that for most common plant species (<10 mg kg−1; Matschullat, 2000). The bioconcentration factor (ratio of shoot As concentration to soil As concentration) is greater than 10 (Ma et al., 2001; Zhao et al., 2002). Knowledge of the mechanisms of As uptake and detoxification in Chinese brake fern may contribute to optimization of phytoremediation processes (Clements et al., 2002).
As occurs mostly in its oxidized form, arsenate, in aerobic environments and has been reported to be taken up by plants via the phosphate transport systems (Asher and Reay, 1979; Lee, 1982; Ullrich-Eberius et al., 1989; Meharg and Macnair, 1991, 1992; Meharg and Hartley-Whitaker, 2002; Wang et al., 2002). Mechanisms behind As detoxification in plants have yet to be investigated. Many plants use enhanced synthesis of phytochelatins to detoxify toxic metals (Grill et al., 1985; Kneer et al., 1992; Schmoger et al., 2000). However, the proportion of As complexed with phytochelatins relative to the total As was small (about 1%) in As-treated Chinese brake fern (Zhao et al., 2003; Raab et al., 2004), and the actual mechanism is not yet fully known. Nevertheless, in the fronds of Chinese brake fern grown in the presence of arsenate, arsenite is the main storage form of As (>85%; Lombi et al., 2002; Wang et al., 2002; Zhang et al., 2002) and is mainly stored in the vacuoles (Lombi et al., 2002). This information suggests that the reduction of arsenate to arsenite may be a key step in the detoxification pathways. The reduction of arsenate to arsenite occurs in a number of plant species (Pickering et al., 2000; Meharg and Hartley-Whitaker, 2002; Salt et al., 2002; Quaghebeur and Rengel, 2003), but so far this reduction mechanism is not well understood in plants.
In contrast, the mechanisms of As detoxification in bacteria and yeast (Saccharomyces cerevisiae) are well understood, and arsenate reduction to arsenite is thought to be the key step for As detoxification (Ghosh et al., 1999; Shi et al., 1999; Mukhopadhyay et al., 2000, Mukhopadhyay and Rosen, 2002a; Rosen, 2002). In these microorganisms, arsenate is reduced enzymatically in the first step to arsenite and then, in the second step, arsenite is transported out of the cell or into vacuoles via arsenite transporters (Rosen, 1999; Doucleff and Terry, 2002). Both arsenate reductase (AR) and arsenite transporters have been characterized from these microorganisms (Rosen, 1999; Mukhopadhyay et al., 2002b).
Significant transformation of As species in Chinese brake fern indicates that AR should also be present in this plant, as proposed by Pickering et al. (2000) and Meharg and Hartley-Whitaker (2002). However, so far little is known about this enzyme in plant. In this article, we describe the detection and partial characterization of an AR from roots of Chinese brake fern and its relevance for the As hyperaccumulation mechanism of this fern.
RESULTS
Detection of AR Activity in Chinese Brake Fern
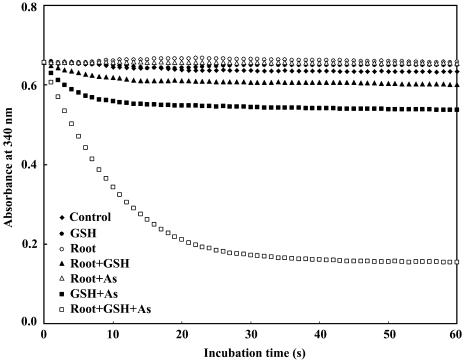
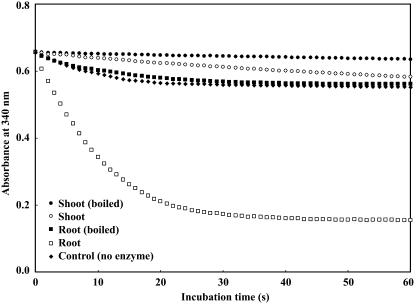
Preliminary analysis showed that Chinese brake fern did not contain detectable amounts of glutathione reductase (GR) in either roots or shoots. GR was therefore supplied to the assay mixture in order to measure AR activity successfully via the coupled assay described by Mukhopadhyay et al. (2000) and Shi et al. (1999). Figure 1 clearly shows fast oxidation of NADPH in roots of Chinese brake fern when root extract, glutathione (GSH), and arsenate were all added to the reaction mixture. Figure 2 shows that fast NADPH oxidation could only be detected in root extract and that oxidation completely disappeared when the protein in the extract was denatured by boiling for 10 min at 100°C. No measurable amount of AR activity was present in shoots (Fig. 2).
Figure 1.
Requirements for arsenate reduction by AR in Chinese brake fern. The root extracts were from Chinese brake fern treated with 2 mm arsenate for 9 d. Preparation of plants and root extracts was described in “Materials and Methods.” All measurements were performed at 30°C, pH 6.5 (as described in “Materials and Methods”), and the protein concentrations are the same (0.12 mg mL−1). (1) Control, buffer + 1.5 mm NADPH + 1 unit GR; (2) GSH, control + 1 mm GSH; (3) root, control + 100 μL root extract; (4) root + GSH, control + 100 μL root extract + 1 mm GSH; (5) root + As, control + 100 μL root extract + 10 mm As(V); (6) GSH + As, control + 1 mm GSH + 10 mm As(V); (7) root + GSH + As, control + 100 μL root extract + 1 mm GSH + 10 mm As(V).
Figure 2.
AR activity in and boiled extracts of Chinese brake fern. The root extracts were from Chinese brake fern treated with 2 mm arsenate for 9 d. Preparation of plants and root extracts was described in “Materials and Methods.” All measurements were performed at 30°C, pH 6.5 (as described in “Materials and Methods”), and the protein concentrations are the same (0.12 mg mL−1). (1) Control, buffer + 1.5 mm NADPH + 1 unit GR + 1 mm GSH + 10 mm arsenate; (2) shoot (boiled), control + 100 μL boiled shoot extract; (3) shoot, control + 100 μL shoot extract; (4) root (boiled), control + 100 μL boiled root extract; (5) root, control + 100 μL root extract.
AR Levels in Response to Arsenate Levels in the Culture Medium
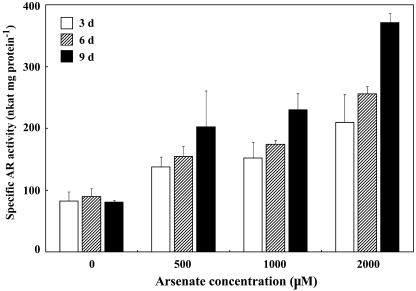
A moderate basic level of AR activity (about 80 nkat [nmol products s−1] mg protein−1) was present in roots at all times, but the activity of the enzyme increased almost 5-fold compared to the controls after 9 d in the presence of 2 mm arsenate (Fig. 3). Enzyme activity increased significantly as the arsenate concentration (r = 0.983) and the exposure time (r = 0.968) increased. These results clearly showed that the AR activities of Chinese brake fern, even though present at moderate levels constitutively, were arsenate inducible and dosage dependent. For this reason, all further experiments were conducted with extracts from roots of Chinese brake fern treated with 2 mm arsenate for 9 d.
Figure 3.
Specific AR activities in roots of Chinese brake fern. Chinese brake fern plants were cultured as described in “Materials and Methods,” and were then treated with arsenate for periods indicated in the graph. The measurements were performed at 30°C, pH 6.5, using the following reaction mixture: buffer + 1.5 mm NADPH + 1 unit GR + 1 mm GSH + 10 mm arsenate + 100 μL root extract.
AR Levels in Other Plant Species
In the short-term exposure experiment, the As concentrations chosen for different plant species did not produce any significant As toxicity symptoms. Except in the Arabidopsis (Arabidopsis thaliana) mutant with disruption in a putative AR gene, AR activities could be detected in roots and also sometimes in shoots of all plants tested, but they were at least 7 times lower than that in Chinese brake fern roots (Table I).
Table I.
Comparison of AR activities (±sd, n = 3) in extracts of various plant species
ND, Not detectable.
| Plant Species
|
Treatmentsa
|
Specific AR Activity
|
|
|---|---|---|---|
| Shoots | Roots | ||
| μm | nkat/mg protein | ||
| Chinese brake fern | 0 | ND | 80.43 ± 2.81 |
| 500 | ND | 202.11 ± 58.27 | |
| 1,000 | ND | 230.32 ± 26.75 | |
| 2,000 | ND | 371.10 ± 15.34 | |
| C. fortunei | 2,000 | 16.77 ± 1.64 | 19.07 ± 1.94 |
| M. caffrorum | 2,000 | ND | 33.01 ± 6.17 |
| B. chrenbergiana | 2,000 | ND | 32.39 ± 0.79 |
| O. sativa | 55 | 63.97 ± 1.35 | 40.82 ± 5.80 |
| Arabidopsis (mutant) | 300 | ND | ND |
| Arabidopsis (wild type) | 300 | 33.36 ± 0.67 | 59.14 ± 5.14 |
Treatment concentrations of As in the growth medium for nonhyperaccumulators were selected as the near maximum concentrations that led to acute toxicity.
Partial Characterization of the Enzyme
Kinetic Parameters of Arsenate Reduction
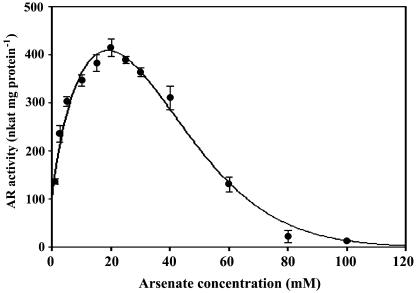
The rate of arsenate reduction as a function of arsenate concentration was determined (Fig. 4). The data were transformed to the Michaelis-Menten equation by using SigmaPlot 8.0, and the curve before it reached inflection was best fitted with a hyperbolic relationship. The Km for sodium arsenate was 2.3 mm, and Vmax was 445 nkat mg protein−1 (r = 0.9952; P < 0.0001). The curve peaked at 20 mm and the AR activity gradually decreased with increasing arsenate concentrations.
Figure 4.
AR kinetics for arsenate. Reaction mixture: buffer + 1.5 mm NADPH + 1 unit GR + 1 mm GSH + 100 μL root extract; arsenate was added as indicated. Each point is the average of three separate assays with independent root extract of Chinese brake fern roots treated with 2 mm arsenate for 9 d. All measurements were performed at 30°C, pH 6.5 (as described in “Materials and Methods”), and the protein concentrations are the same (0.21 mg mL−1) The data were transformed to the Michaelis-Menten equation by using SigmaPlot 8.0; the curve before it reached inflection was best fitted with a hyperbolic relationship. The Km for sodium arsenate was 2.3 mm and Vmax was 445 nkat mg protein−1. r = 0.9952; P < 0.0001.
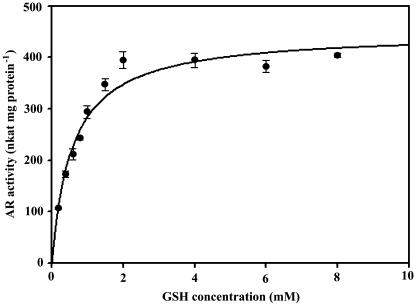
The rate of arsenate reduction as a function of GSH concentration was also determined (Fig. 5). The data were again transformed to the Michaelis-Menten equation by SigmaPlot 8.0, and the curve was best fitted with a typical Michaelis-Menten hyperbolic relationship. The Km for GSH was 0.57 mm and Vmax was 447 nkat mg protein−1 (r = 0.9770; P < 0.0001).
Figure 5.
AR kinetics for GSH. Reaction mixture: buffer + 1.5 mm NADPH + 1 unit GR + 10 mm arsenate + 100 μL root extract; GSH was added as indicated. Each point is the average of three separate assays with independent root extract of Chinese brake fern roots treated with 2 mm arsenate for 9 d. All measurements were performed at 30°C, pH 6.5 (as described in “Materials and Methods”), and the protein concentrations are the same (0.21 mg mL−1). The data were transformed to the Michaelis-Menten equation by using SigmaPlot 8.0; the curve was best fitted with a hyperbolic relationship. The Km for GSH was 0.58 mm and Vmax was 448 nkat mg protein−1. r = 0.9779; P < 0.0001.
Optimum pH and Temperature for the Reaction
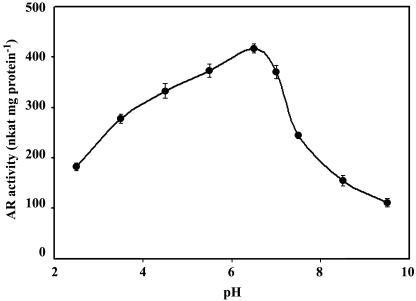
The AR from Chinese brake fern showed a pH optimum of about 6.5 (Fig. 6) and corresponded closely to the optimum pH of the yeast enzyme. The enzyme exhibited a broad pH optimum, and enzyme activity changed only slightly from pH 5.5 to 7.0. The drop in activity at alkaline pH was more pronounced than at acidic pH; it showed 60% of maximum activity at pH 7.5 and 90% at pH 5.5.
Figure 6.
pH optimum for the reaction. Reaction mixture: buffer + 1.5 mm NADPH + 1 unit GR + 1 mm GSH + 100 μL root extract + 10 mm arsenate. Each point is the average of three separate assays with independent extract of Chinese brake fern roots treated with 2 mm arsenate for 9 d. All measurements were performed at 30°C (as described in “Materials and Methods”) and the protein concentrations are the same (0.21 mg mL−1).
The activity of the enzyme was determined at a range of temperatures from 20°C to 50°C using sodium arsenate as substrate. The enzyme showed a broad temperature optimum, and enzyme activity changed only slightly from 20°C to 50°C (data not shown).
Substrate Specificity and Inhibitory Factors
The AR from Chinese brake fern appeared to be highly specific for arsenate. When arsenate was replaced by various concentrations of sodium phosphate or sodium nitrate, no NADPH oxidation was observed (data not shown).
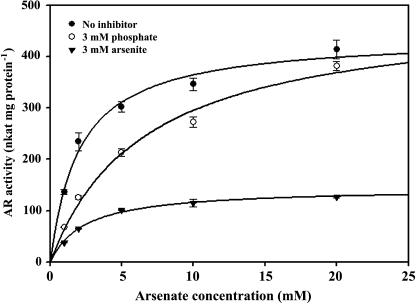
Both phosphate and arsenite inhibited the AR activity (Fig. 7). AR could be competitively inhibited by phosphate. When the enzyme was incubated with 3 mm phosphate for 5 min, the Vmax was unchanged, but the Km for arsenate was increased from 2.1 to 6.6 mm. The enzyme could be noncompetitively inhibited by the reaction product arsenite: When it was incubated with 3 mm arsenite for 5 min, the Km for arsenate was unchanged, but the Vmax was decreased from 490 to 144 nkat mg protein−1.
Figure 7.
Inhibition of AR. Reaction mixture: buffer + 1.5 mm NADPH + 1 unit GR + 1 mm GSH + 100 μL root extract + 10 mm As. No inhibitor, use root extract, Km = 2.1 mm, Vmax = 439 nkat mg protein−1; 3 mm P(V), root extract was preincubated for 5 min with sodium phosphate, Km = 6.6 mm, Vmax = 490 nkat mg protein−1; 3 mm AS(III), root extract was preincubated for 5 min with sodium arsenite, Km = 2.4 mm Vmax = 144 nkat mg protein−1. Each point is the average of three separate assays with independent extract of Chinese brake fern roots treated with 2 mm arsenate for 9 d. All measurements were performed at 30°C, pH 6.5 (as described in “Materials and Methods”), and the protein concentrations are the same (0.21 mg mL−1).
DISCUSSION
It is generally accepted that Chinese brake fern takes up As mainly in the form of arsenate, but in the fronds As has been shown to be primarily present in the reduced form, arsenite (Meharg and Hartley-Whitaker, 2002; Wang et al., 2002; Zhang et al., 2002). Although arsenate can be reduced nonenzymatically in the presence of GSH (Delnomdedieu et al., 1994), this process is too slow to be significant biologically. Data from this study showed that arsenate reduction by GSH alone was usually very slow and incomplete, whereas the enzymatic reduction under optimum conditions was much faster (Figs. 1 and 2). The NADPH oxidation rates were 0.92 nmol s−1 without root extract and 8.4 nmol s−1 with root extract containing 0.02 mg protein.
The observed transformation of As species in Chinese brake fern and in other plant species (Meharg and Hartley-Whitaker, 2002; Salt et al., 2002) is a good indication that AR is not only present in microorganisms, but also in higher plants. Although the exact reduction steps in Chinese brake fern are not yet resolved, results from this study clearly suggest that the reaction mechanism is analogous to the arsenate reduction in yeast and bacteria that involves four crucial steps (Shi et al., 1999; Mukhopadhyay et al., 2000):
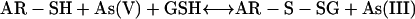 |
(1) |
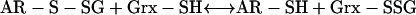 |
(2) |
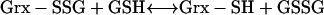 |
(3) |
 |
(4) |
In (1), arsenate is reduced to arsenite with one electron transferred from a protein Cys thiolate and the second from GSH; in (2), Grx acts as the electron donor for reduction of the AR-S-SG mixed disulfide; in (3), Grx is regenerated by GSH, forming oxidized glutathione. Grx is an abundant protein in most cells (Fernandes and Holmgren, 2004) and is unlikely to be a limiting factor of the reaction; in (4), oxidized glutathione is reduced by GR via oxidation of NADPH, a reaction that can be quantified easily by photometrically monitoring the decrease of A340 caused by the consumption of NADPH, and it has been indicated that the NADPH oxidized in moles is equal to arsenate reduced (or arsenite formed) in moles (Shi et al., 1999; Mukhopadhyay et al., 2000).
Data from this study clearly demonstrated that AR from Chinese brake fern utilizes a reduction mechanism very similar to AR from yeast (Figs. 1 and 2). Substrate specificity as well as sensitivity to inhibitors is also in close agreement with AR from yeast (Fig. 7). Furthermore, the pH optimum and temperature optimum correspond closely to those of the enzyme from yeast (Mukhopadhyay et al., 2000).
Even though very similar to the AR from yeast, the AR from Chinese brake fern has some significantly different characteristics. Reaction rate of AR from Chinese brake fern dropped at high concentrations of arsenate (>20 mm). This drop was not observed by Mukhopadhyay et al. (2000) for the yeast enzyme. It is still unclear what is responsible for this difference. No obvious precipitations of any kind were observed in the reaction mixture at high arsenate concentrations. It was unlikely to be due to arsenate inhibition of the used GR, since neither arsenate nor arsenite inhibited GR activity, even at high concentrations (20 mm; data not shown). We speculate that high arsenate concentration might inhibit Grx activity, but further investigation is needed to confirm this.
The largest difference is that AR from Chinese brake fern has a 15-fold lower Km for arsenate than that of yeast AR (Mukhopadhyay et al., 2000), i.e. a much higher substrate affinity (Fig. 4). The observed Km of 2.3 mm for Chinese brake fern seems to be high considering the cytosolic arsenate concentrations in nonhyperaccumulating plants, but root arsenate concentrations in Chinese brake fern could be over 10 mm (Wang et al., 2002). AR activity from Chinese brake fern was also at least 7 times higher (Table I) than AR from other plant species that did not tolerate As toxicity. This feature of Chinese brake fern AR is very likely one of the reasons why this fern is able to withstand high concentrations of As in soils and is the first key to understanding its mechanism of As hyperaccumulation.
The AR of Chinese brake fern, even though present at moderate levels constitutively, is highly arsenate inducible and dosage dependent (Fig. 3). The fact that there was no measurable AR activity in the extracts of fronds suggests that arsenate reduction may occur mainly in roots (Fig. 2). Arsenite is then transported to shoots, where it may be stored in the vacuoles (Lombi et al., 2002). Pickering et al. (2000) showed a similar behavior of As in Indian mustard, where arsenate was also reduced to arsenite in roots and transported to shoots in the reduced form. Zhao et al. (2002) hypothesized that in Chinese brake fern arsenate is likely to be reduced to arsenite in roots, because there was almost no competition between As and phosphate during the transport from roots to fronds. Previously, Tu et al. (2003) conducted experiments with excised roots and fronds of Chinese brake fern that were incubated in solution containing arsenate and observed that the ratios of arsenate conversion to arsenite were 3- to 4-fold higher in shoots compared to roots. This observation led these authors to draw the conclusion that the reduction of arsenate in Chinese brake fern would take place mainly in shoots. In this article, however, we give unambiguous proof that the enzymatic reduction of arsenate in Chinese brake fern took place mainly in roots. The observed high levels of arsenite in the fronds and pinnae in the study of Tu et al. (2003) might have been caused by nonenzymatic reduction of arsenate by GSH, which is present in all plant cells at concentrations between 1 and 10 mm (Alscher, 1989). The lower ratios of arsenite found in roots by those authors after arsenate treatment may have been caused by a fast transport after reduction into the xylem where remaining positive root pressure could have pumped the ions through the cuts back into the incubation medium.
With the information reported so far (Wang et al., 2002; Zhao et al., 2003; Raab et al., 2004) and obtained from this study, it is expected that arsenate is taken up by roots via phosphate transporters and is then reduced to arsenite. Although arsenite is more toxic than arsenate, plants may have specific transporters to pump arsenite to the vacuole, as Lombi et al. (2002) reported that As is stored primarily in the vacuole of Chinese brake fern. The reason for the reduction could then be looked at as a means of preparing for arsenite translocation (out of the cell or into the vacuole) without interfering with the phosphate status of the cells.
In conclusion, with the data presented in this article, the As detoxification and accumulation mechanism in Chinese brake fern seems to be established with some certainty: Arsenate is taken up by Chinese brake fern and reduced to arsenite by a root-specific AR and transported to the fronds in the reduced form. Enzymatic reduction does not take place in measurable amounts in the fronds. Based on these and reported results (Wang et al., 2002; Zhao et al., 2003; Raab et al., 2004), we believe that arsenate reduction in roots is an important step in arsenic hyperaccumulation by Chinese brake fern.
MATERIALS AND METHODS
Plant Material and Culture
Chinese Brake Fern
Spores of Chinese brake fern (Pteris vittata) were collected from hyperaccumulating individual plants growing on As-contaminated soil (about 100 mg kg−1) in Chenzhou, Hunan province, China (N25°35.360′, E113°00.346′). For germination, the spores were sprinkled on a seed tray containing an As-free mixture of sandy soil and vermiculite (2:1 v/v) as growth medium. The tray was covered with plastic cling film to maintain moisture. The seedlings were carefully removed from trays and thoroughly washed with tap water at the four- to five-frond stage. Uniform seedlings were then transferred to hydroponic pots (PVC, 7.5-cm diameter, 15-cm height) containing 500 mL of nutrient solution with vigorous aeration. Modified Hoagland-Arnon nutrient solution (Hoagland and Arnon, 1938) was used as a growth medium [full-strength composition 5 mm Ca(NO3)2, 5 mm KNO3, 2 mm MgSO4, 1 mm KH2PO4, 50 μm Fe(II)-EDTA, 46 μm H3BO4, 1 μm ZnSO4, 0.5 μm CuSO4, 10 μm MnSO4, 0.1 μm (NH4)6Mo7O24] at 0.3× strength in the first month and 0.5× strength in the second month. The nutrient solution was changed every 3 d and the pH of the solution adjusted to 6.3 using HCl or NaOH. The plants were cultivated in a growth room at a 14-h light period (260–350 μE m−2 s−1). The temperature was regulated to 28°C day and 20°C night and the relative humidity was maintained at 60%.
After 2 months, the most uniform individuals were selected for the experiments. Treatments with arsenate (supplied as Na3AsO4) were administered at 0, 500, 1,000, and 2,000 μm. During the arsenate treatments, nutrient solution was replaced every 3 d. When the plants were harvested, they were thoroughly washed first with tap water, then with deionized water. Adhering water was then removed with filter paper and each plant was separated into roots and fronds, shock frozen with liquid nitrogen, and crushed to powder with mortar and pestle. The frozen powder was stored in liquid nitrogen until further use.
Arabidopsis (Arabidopsis thaliana) T2 seeds of the putative ACR2 knockout mutant of Arabidopsis (SALK_143282.55.75.x, carrying a T-DNA insertion on chromosome 5 in the At5g03455 locus with start position 863,366 bp) were purchased from the Arabidopsis Biological Resource Center. T4 Arabidopsis plants (screened by using kanamycin and confirmed by PCR in T3 plants) were used. The seeds of the mutant and its wild type were germinated in sterilized Eppendorf tubes (0.5 mL) containing 0.8% sterilized agar, one seed per tube. After germination, the bottom of the tubes was cut off and the tubes were fitted into holes in styrofoam floats (four tubes per float); each float was then transferred to hydroponic pots (PVC, 7.5-cm diameter, 15-cm height) containing 500 mL of the nutrient solution (0.5× strength; Gibeaut et al., 1997). The solution was aerated continuously and renewed every 4 d. All other cultivation conditions were identical to those described for Chinese brake fern. After a growth period of 3 weeks in the hydroponic pots, the most uniform plants were selected; they were treated with 300 μm arsenate and harvested after 9 d. Harvest and storage conditions were identical to those described for Chinese brake fern.
Other Plant Species
Seeds of rice (Oryza sativa) were germinated and cultured as previously described (Liu et al., 2004). Plants were treated with 55 μm arsenate for 9 d. Ferns (Cyrtomium fortunei, Bommeria chrenbergiana, and Mohria caffrorum) were purchased from a local supplier. They were cultivated in soil culture, watered with water containing 2,000 μm arsenate, and harvested after 9 d in the presence of arsenate. Other cultivation conditions, as well as harvest and storage procedures, were identical to those described for Chinese brake fern.
Extraction of Plant Material
Powdered plant material (5 g) was homogenized with quartz sand and 15 mL of extraction buffer (50 mm MOPS, 50 mm MES [both from Sigma, St. Louis], adjusted to pH 6.5 with NaOH). After homogenization of the plant material to a fine paste, debris was removed by passing the paste through four layers of cheesecloth. The filtrate was then centrifuged for 30 min (4°C, 10,000g), and the supernatant was filtered through Whatman Number 1 filter paper. All steps were performed on ice.
The resulting extracts were passed through Sephadex PD-10 desalting columns (Amersham Biosciences, Uppsala) to remove interfering low-Mr compounds.
Determination of Protein Concentrations
Protein contents were determined according to Bradford (1976), using Coomassie Brilliant Blue G-250 (Sigma) as dye and albumin (Bovine V; Sigma) as a standard.
Determination of AR Activity
AR activity was assayed using the coupled enzymatic reaction described by Shi et al. (1999) with some modifications. In this assay, AR activity is measured by observing NADPH oxidation. The assay was performed in 50 mm MOPS, 50 mm MES, pH 6.5, containing 1.5 mm NADPH, 1 unit yeast (Saccharomyces cerevisiae) GR (Sigma), 1 mm GSH, and 10 mm sodium arsenate, in a total volume of 1.5 mL. Chinese brake fern extracts used in these experiments were all from plants treated with 2 mm arsenate for 9 d, with the exception of the experiment to investigate AR levels in response to arsenate levels in the culture medium. Generally, 100 μL of extract were preincubated for 5 min in buffer containing GR and GSH at 30°C for temperature adjustment (preliminary analysis showed that the protein concentration was within linear range of the standard assay conditions). To start the reaction, NADPH and arsenate were added as small volumes and mixed thoroughly prior to measurement; all the measurements were performed at 30°C. GR-specific NADPH oxidation was monitored by recording the decrease in A340 (U-3010 spectrophotometer; Hitachi, Tokyo, Japan) and the amount of NADPH oxidized was calculated using a molar extinction coefficient of 6,200 m−1 cm−1 for NADPH at 340 nm.
Reagents
All reagents were obtained from local suppliers at analytical grade or better, if not otherwise stated in the text.
Statistical Analysis
Data analysis, curve fitting, and statistical calculations were performed using Sigma Plot 8.0 (Jandel Scientific, Erkrath, Germany).
Acknowledgments
We thank Professor F. Andrew Smith for his critical reading of the manuscript.
This work was supported by the Natural Science Foundation of China (40225002) and by the Chinese Academy of Sciences through its Hundred Talent Program.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.057422.
References
- Alscher RG (1989) Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant 77: 457–464 [Google Scholar]
- Asher CJ, Reay PF (1979) Arsenic uptake by barley seedlings. Aust J Plant Physiol 6: 459–466 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brooks RR (1998) Plants That Hyperaccumulate Heavy Metals. CABI Publishing, Wallingford, UK, pp 379
- Clements S, Palmgren MG, Krämer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7: 309–315 [DOI] [PubMed] [Google Scholar]
- Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ (1994) Reduction and binding of arsenate and dimethylarsenate by glutathione: a magnetic resonance study. Chem-Biol Interact 90: 139–155 [DOI] [PubMed] [Google Scholar]
- Doucleff M, Terry N (2002) Pumping out the arsenic. Nat Biotechnol 20: 1094–1096 [DOI] [PubMed] [Google Scholar]
- Fernandes AP, Holmgren A (2004) Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxidants Redox Signaling 6: 63–74 [DOI] [PubMed] [Google Scholar]
- Ghosh M, Shen J, Rosen BP (1999) Pathways of As (III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 96: 5001–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Hulett J, Cramer GR, Seemann JR (1997) Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115: 317–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH (1985) Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science 230: 674–676 [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. Calif Agric Expt Stn Circ 347: 1–39 [Google Scholar]
- Kaise T, Watanabe S, Itoh K (1985) The acute toxicity of arsenobetaine. Chemosphere 14: 1327–1332 [Google Scholar]
- Kneer R, Kutchan TM, Hochberger A, Zenk MH (1992) Saccharomyces cerevisiae and Neurospora crassa contain heavy metal sequestering phytochelatin. Arch Microbiol 157: 305–310 [DOI] [PubMed] [Google Scholar]
- Lee RB (1982) Selectivity and kinetics of ion uptake by barley plants following nutrient deficiency. Ann Bot (Lond) 50: 429–449 [Google Scholar]
- Liu WJ, Zhu YG, Smith FA, Smith SE (2004) Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture. New Phytol 162: 481–488 [Google Scholar]
- Lombi E, Zhao FJ, Fuhrmann M, Ma LQ, McGrath SP (2002) Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol 156: 195–203 [DOI] [PubMed] [Google Scholar]
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic: a hardy, versatile, fast-growing plant helps to remove arsenic from contaminated soils. Nature 409: 579. [DOI] [PubMed] [Google Scholar]
- Matschullat J (2000) Arsenic in the geosphere: a review. Sci Tot Environ 249: 297–312 [DOI] [PubMed] [Google Scholar]
- Meharg AA (2004) Arsenic in rice: understanding a new disaster for South-East Asia. Trends Plant Sci 9: 415–417 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154: 29–43 [Google Scholar]
- Meharg AA, Macnair MR (1991) The mechanisms of arsenate tolerance in Deschampsia cespitosa (L.) Beauv. and Agrostis capillaries L. New Phytol 119: 291–297 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Macnair MR (1992) Suppression of the high-affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43: 519–524 [Google Scholar]
- Mukhopadhyay R, Rosen BP (2002. a) Arsenate reductase in prokaryotes and eukaryotes. Environ Health Perspect 110: 745–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Rosen BP, Phung LT, Silver S (2002. b) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol Rev 26: 311–325 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Shi J, Rosen BP (2000) Purification and characterization of Acr2p, the Saccharomyces cerevisiae arsenate reductase. J Biol Chem 275: 21149–21157 [DOI] [PubMed] [Google Scholar]
- Pickering IJ, Prince RC, Salt DE, George GN (2000) Reduction and coordination of arsenic in Indian mustard. Plant Physiol 122: 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaghebeur M, Rengel Z (2003) The distribution of arsenate and arsenite in shoots and roots of Holcus lanatus is influenced by arsenic tolerance and arsenate and phosphate supply. Plant Physiol 132: 1600–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab A, Feldmann J, Meharg AA (2004) The nature of arsenic phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol 134: 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BP (1999) Families of arsenic transporters. Trends Microbiol 7: 207–212 [DOI] [PubMed] [Google Scholar]
- Rosen BP (2002) Biochemistry of arsenic detoxification. FEBS Lett 529: 86–92 [DOI] [PubMed] [Google Scholar]
- Roychowdhury T, Uchino T, Tokunaga H, Ando M (2002) Survey of arsenic in food composites from an arsenic-affected area of West Bengal, India. Food Chem Toxicol 40: 1611–1621 [DOI] [PubMed] [Google Scholar]
- Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 13: 468–474 [DOI] [PubMed] [Google Scholar]
- Salt DE, Prince RC, Pickering IJ (2002) Chemical speciation of accumulated metals in plants: evidence from x-ray absorption spectroscopy. Microchem J 71: 255–259 [Google Scholar]
- Salt DE, Smith RD, Raskin E (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49: 643–668 [DOI] [PubMed] [Google Scholar]
- Schmoger MEV, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Vlamis-Gardikas A, Åslund F, Holmgren A, Rosen BP (1999) Reactivity of glutaredoxins 1, 2, and 3 from Escherichia coli shows that glutaredoxin 2 is the primary hydrogen donor to arsC-catalyzed arsenate reduction. J Biol Chem 274: 36039–36042 [DOI] [PubMed] [Google Scholar]
- Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17: 517–568 [Google Scholar]
- Smith AH, Hopenhayn RC, Bates MN, Goeden HM, Hertz PI, Duggan HM, Wood R, Kosnett MJ, Smith MT (1992) Cancer risks from arsenic in drinking water. Environ Health Perspect 97: 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Naidu R, Alston AM (1998) Arsenic in the soil environment: a review. Adv Agron 64: 149–195 [Google Scholar]
- Tu C, Ma LQ, Bondada B (2002) Arsenic accumulation in the hyperaccumulator Chinese brake and its utilization potential for phytoremediation. J Environ Qual 31: 1671–1675 [DOI] [PubMed] [Google Scholar]
- Tu S, Ma LQ, MacDonald GE, Bondada B (2003) Effects of arsenic species and phosphorus on arsenic absorption, arsenate reduction and thiol formation in excised parts of Pteris vittata L. Environ Exp Bot 51: 121–131 [Google Scholar]
- Ullrich-Eberius CI, Sanz A, Novacky AJ (1989) Evaluation of arsenate- and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba-G1. J Exp Bot 40: 119–128 [Google Scholar]
- Wang JR, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130: 1552–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel WW, Adriano DC, Salt D, Smith R (1999) Phytoremediation: a plant-microbe-based remediation system. In DC Adriano, JM Bollag, WT Frankenberger Jr, RC Sims, eds, Bioremediation of Contaminated Soils. Agronomy Monograph No. 37. Soil Science Society of America, Madison, WI, pp 456–508
- Zhang WH, Cai Y, Tu C, Ma LQ (2002) Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci Tot Environ 300: 167–177 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Dunham SJ, McGrath SP (2002) Arsenic hyperaccumulation by different fern species. New Phytol 56: 27–31 [Google Scholar]
- Zhao FJ, Wang JR, Barker JHA, Schat H, Bleeker PM, McGrath SP (2003) The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol 159: 403–410 [DOI] [PubMed] [Google Scholar]