Abstract
A major focus of academia, industry, and global governmental agencies is to develop and apply artificial intelligence and other advanced analytical tools to transform health care delivery. The American Heart Association supports the creation of tools and services that would further the science and practice of precision medicine by enabling more precise approaches to cardiovascular and stroke research, prevention, and care of individuals and populations. Nevertheless, several challenges exist, and few artificial intelligence tools have been shown to improve cardiovascular and stroke care sufficiently to be widely adopted. This scientific statement outlines the current state of the art on the use of artificial intelligence algorithms and data science in the diagnosis, classification, and treatment of cardiovascular disease. It also sets out to advance this mission, focusing on how digital tools and, in particular, artificial intelligence may provide clinical and mechanistic insights, address bias in clinical studies, and facilitate education and implementation science to improve cardiovascular and stroke outcomes. Last, a key objective of this scientific statement is to further the field by identifying best practices, gaps, and challenges for interested stakeholders.
Keywords: AHA Scientific Statements; artificial intelligence; electrocardiography; electronic health records; ethics; genetics; heart diseases; monitoring, physiologic
The objective of this scientific statement is to present the state of the art on the use of artificial intelligence (AI) or machine learning (ML) to enable precision medicine and implementation science in cardiovascular research and clinical care. For a primer on AI and ML, please see the Supplemental Material.
This task has been propelled by academia, industry, and global governmental agencies who are investing immense resources to transform health care delivery with AI, resulting in a rapid growth rate of scientific research articles on health care–related AI research in the past decade,1 which is likely to accelerate in coming years.
This work has led to several parallel initiatives, including the digitization and analysis of electronic health records (EHRs), to understand the heterogeneity of treatment effects,2 the comparative effectiveness of tests and interventions,3 and, more recently, to build prediction,4 classification,5 and optimization6 models to inform clinical decision-making (Figure).7,8
Figure.
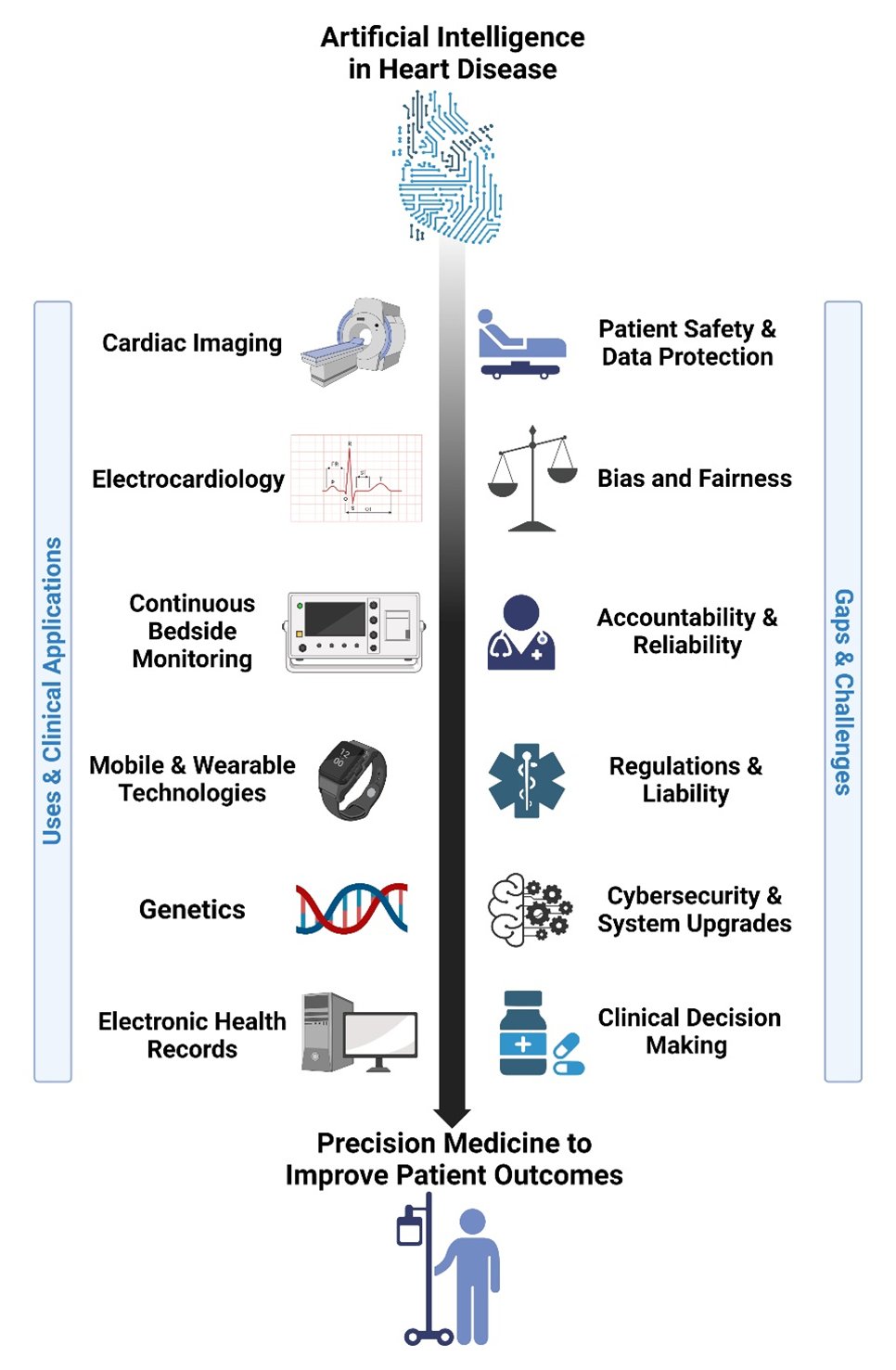
Artificial intelligence in heart disease.
Yet, despite enormous academic interest and industry financing, AI-based tools, algorithms, and systems of care have yet to improve patient outcomes at scale. Therefore, another objective of this scientific statement is to identify best practices, gaps, and challenges that may improve the applicability of AI tools in each domain. For each application, we will discuss the need to identify and mitigate bias and ensure education and access to AI/ML technologies by all stakeholders across diverse health care settings.
IMAGING
Overview
Imaging has become an essential diagnostic tool in clinical decision-making in cardiovascular diseases and stroke.9 However, expertise in image interpretation takes years to acquire, and experts are often overburdened with tasks such as image processing, segmentation, quantitation, and interpretation.10,11 Moreover, expertise in image interpretation is scarce, exacerbating inequities in access to high-quality patient care in underresourced areas, between lower and higher income populations, and between low- and rich-resource countries. AI/ML-based tools for imaging cardiovascular diseases and stroke address many of these concerns and are therefore of increasing interest.12
AI/ML Application on Different Modalities in Cardiac Diagnosis and Prognostication
Current-use cases of AI/ML algorithms in imaging are broad and include referring and scheduling image acquisition, image analysis including the reduction of image acquisition and processing times,13 reduction of radiation exposure and contrast dose use, assisting in diagnosis and reporting, with clinical decision support and with estimation of patient prognosis.3 These various AI applications broadly apply to multimodal cardiac imaging and include its use in echocardiography, cardiac CT, cardiac magnetic resonance imaging (CMR), and nuclear imaging.
With echocardiography, applications include automated segmentation and volumetric analysis of the cardiac chambers along with ejection fraction (EF) calculation, automated assessment of valvular structures, including valve geometry and associated flow gradient and measuring longitudinal strain and cardiac wall motion abnormalities.14 AI/ML applications in echocardiography have also been used for automated disease detection. Some examples include its use in automated diagnosis of myocardial infarction, differentiating hypertrophic cardiomyopathy from physiological hypertrophy, and in detecting heart failure and pulmonary artery hypertension automatically. These applications, when potentially combined with handheld echocardiography, can provide high-quality cardiac diagnosis in many places around the world that lack such capabilities, thereby democratizing the expertise gap that currently exists in cardiac diagnosis.
Cardiac CT (including CT angiography) is another modality with increasing use of AI. Uses include automated quantification of coronary artery plaques and blood flow and increasingly in cardiovascular risk assessment using coronary artery calcium scoring. Automated quantification of coronary plaque (both calcified and noncalcified) and of coronary lumen on cardiac CT compares favorably with manual measurements in multiple studies. In addition, cardiac CT is being used to compute fractional flow reserve and myocardial perfusion.15 With cardiovascular risk assessment using coronary artery calcium scoring gaining increasing importance, AI applications are now capable of automating the computing of coronary artery calcium scoring from low-dose chest CT or even from nuclear imaging studies, such as positron emission tomography CTs.
CMR applications of AI/ML include use in structural and volumetric analysis of cardiac chambers and in estimation of ventricular and myocardial blood flow and perfusion reserve.16 CMR is also being used for myocardial tissue characterization and prediction of risk of sudden cardiac death from ventricular late gadolinium CMR and to help plan treatment strategies, such as guiding ablation for ventricular tachycardia (VT) by analyzing patterns of late gadolinium CMR indicative of fibrosis that may indicate critical isthmuses for reentrant VT circuits.17,18 CMR is also being used to assess ischemic stroke risk from automated atrial chamber morphology and fibrosis burden measurements.10
Nuclear imaging applications of AI are also increasing with use in myocardial blood flow and flow reserve quantification and associated prognostication of cardiovascular mortality.
AI/ML in Cardiac Treatment Planning
Structural interventions are increasingly assisted by AI/ML by using fast automated coronary vessel centerline extractions or measuring stenosis for coronary interventions, or by assessing dynamic mitral annulus, left ventricular outflow tract, sinus of Valsalva, and sinotubular measurements for transcatheter aortic valve10 or mitral valve replacement or patent foramen ovale closure.
AI/ML in Stroke Diagnosis, Prognostication, and Treatment Planning
AI/ML has recently been used to facilitate the diagnosis of acute stroke,19 by automatically detecting intracranial hemorrhage on noncontrast CT of the head.20 AI/ML applied to baseline CT angiography images of the head are able to automatically detect large vessel occlusions, reducing the time to successful neurovascular intervention by ≥30 minutes.21 AI/ML applications on CT of the head can automatically detect early ischemic changes of the brain, without the need for diffusion-weighted MRI.22 AI/ML algorithms have improved quantitation of CT or MR brain perfusion imaging and enhanced their ability to predict recovery of cerebral function during the time taken to transport patients for reperfusion therapies.23 Other applications include neurointerventional planning for the management of acute ischemic stroke and cerebral aneurysms, and for patient recruitment in clinical trials for acute stroke.
Challenges in Applying AI/ML in Imaging
Key limitations specific to imaging include appropriate data sourcing, curating, and sharing (Table 1). Imaging data from clinical repositories are difficult to obtain and, when available, are often unstructured and unlabeled. Using appropriate learning techniques (eg, supervised learning when labeled data are available during training versus unsupervised learning when labeled data are difficult to come by or expensive to procure) is important. Additional techniques, such as transfer learning where pretrained models are applied to a new classification task, weak supervision when available data are imprecisely labeled, and a hybrid semisupervised learning approach when some data are appropriately labeled while the majority of data are not, may be considered in applying the appropriate AI/ML approach to the available data. A recently published 11-point framework/checklist provides guidance that includes defining the research question, choosing an appropriate ML/deep learning model for each type of problem, defining a priori sample size and study design, including the nature and type of training, validation, and test datasets, reporting on the reliability of data labeling and annotations especially in the reference datasets, and appropriate reporting of results using accepted statistical measures.11
Table 1.
Artificial Intelligence in Imaging
| Best practices | Description |
|---|---|
| Clinical problem addressed with imaging is defined in consultation with clinical experts, AI/ML experts, and experts in ethics and patient engagement | Universal clinical adoption of AI/ML-based imaging tools needs proper definition of the clinical problem and needs alongside consideration of ethical issues with such use of these tools as well as a patient’s perspective on the utility and effect of these tools. |
| Study design, methods, and resultant AI/ML techniques used in developing imaging solutions are defined a priori | Formulation of a priori hypothesis, study aims, and objectives and appropriate study designs and reporting measures are key when assessing algorithm quality and validity. |
| Imaging data are adequate, representative, well characterized, and reusable | Data annotation uses well-defined rules (eg, medical imaging data readiness [MIDaR] and Findability, Accessibility, Interoperability, and Reusability [FAIR] principles) that also takes into consideration interrater variability. |
| Gaps and challenges | Description |
| Define disease states for which AI/ML-based image classification is validated | Diagnostic accuracy reflects pathophysiology, patient demographics, or technical issues with respect to data representation (ie, bias and lack of generalizability). |
| Identify imaging systems that may detect stroke | AI/ML-based and computational imaging algorithms may predict stroke, using diverse imaging modalities such as cardiac MRI, strain, or nuclear imaging. |
| Lack of representative imaging data sets | Imaging data from clinical repositories may have class imbalances and other biases (eg, data coming from highly selected centers). |
| Lack of studies that test effect on clinical outcomes | Most AI/ML algorithms have been tested on retrospective data, with minimal prospective practical clinical workflow development and testing demonstrating utility. |
AI indicates artificial intelligence; ML, machine learning; and MRI, magnetic resonance imaging.
Tools such as the recently developed medical imaging data readiness scale can help to structure imaging data for developing ML/deep learning algorithms.24 Applying the Findability, Accessibility, Interoperability, and Reuse of digital assets (FAIR) framework to curate imaging data and storing it using formats like the Neuroimaging Informatics Technology Initiative data format for segmentations will help with reuse of this scarce resource among multiple research groups.25
For issues pertaining to data privacy, and ethical and legal challenges, techniques such as “federated learning” may accelerate algorithm development by enabling a collaborator to download a developed AI/ML tool for use on their local data.
ELECTROCARDIOGRAPHY
Overview
The application of AI/ML to the ECG has already dramatically affected electrocardiography.26–29 First, by automating interpretation, human capabilities can be massively scaled, enabling interpretation of an exponentially growing number of ECGs.26 Second, AI/ML algorithms can identify subtle and interrelated nonlinear patterns in the ECG often not recognizable to experts, enhancing disease phenotyping.30 Third, because cardiac electrical activity may be affected before mechanical or structural abnormalities are evident on imaging, such algorithms may enable the identification of occult disease and prediction of impending disease. By segregating subtypes of similar conditions, AI/ML of the ECG may reveal novel phenotypes.
AI/ML to Scale Current Expert Capabilities
Several studies have shown that AI/ML can scale current expert capabilities. The growing need for ECG interpretation, coupled with the limited skills and availability of human experts,31 motivates efforts for automated and accurate interpretation of ECGs. Rules-based interpretation of the ECG is widely used in existing devices, yet has known limitations32 that may adversely affect medical decision-making.33 In early studies, AI/ML algorithms may better mimic expert interpretation,34 yet their widespread adoption and clinical data are currently lacking.
AI/ML to Read ECGs Beyond Trained Experts
Application of AI/ML on the ECG appears effective in detecting occult structural heart disease up to 1 to 2 years earlier than traditional testing. In retrospective studies, independent groups report that AI of the ECG can identify left ventricular dysfunction in diverse populations27,35 irrespective of sex, race, or ethnicity,36 from diverse causes, including peripartum cardiomyopathy.27,37 A prospective, pragmatic trial of AI/ML applied to the ECG in >20 000 patients without previous heart failure in primary care clinics in Minnesota and Wisconsin improved first detection of ventricular dysfunction by 32% over usual care (area under the curve [AUC]=0.92; P<0.007).38 Similar results were reported in the United Kingdom by a stethoscope-based ECG with a similar algorithm compared prospectively with usual care.39 AI/ML of the ECG can identify other structural heart disease, including hypertrophic cardiomyopathy,40 amyloid heart disease,41 aortic stenosis,41 and pulmonary hypertension.42 Detecting hypertrophic cardiomyopathy by AI/ML of the ECG can also guide strategies to improve outcomes.43
In 36 280 patients in sinus rhythm (of whom 8.4% had known paroxysmal atrial fibrillation [AF]), Attia et al27 reported that a single-lead ECG had an AUC for identifying silent AF of 0.87 (95% CI, 0.86–0.88). Other studies support these findings.44,45 It remains to be determined if such AI/ML tools can be combined with other P-wave metrics that predict AF.46 The role of the AI/ML in predicting stroke from ECGs in sinus rhythm is less well defined.27
AI/ML on Electrocardiographic Phenotyping
Attia et al47 applied AI/ML on ECG to predict sex and biological age (an indicator of health) in 275 056 patients (52% male). The AI/ML algorithm provided 90.4% accuracy for identifying sex, with AUC 0.97. Age estimates fell within 6.9±5.6 years of chronological age and, intriguingly, patients in whom AI/ML-based prediction of age exceeded chronological age by >7 years had factors of “advanced biological age,” such as low ejection fraction (left ventricular EF), hypertension, and coronary disease.48
Challenges in the Clinical Application of AI/ML on ECG
Robust clinical validation in large diverse populations that minimizes bias is essential to address uncertainties,49 such as automation bias, vulnerability to adversarial attacks (ie, imperceptible data may cause AI/ML misclassification), and overfitting (ie, poor generalizability), which reduce clinical acceptance and adoption49 (Table 2). Hybrid approaches during model development that combine domain- and data-driven knowledge, clinician familiarity with AI/ML, and “stress testing” of electrocardiographic algorithms may also increase adoption.50,51 Last, the limited availability of digitized and well-labeled electrocardiographic data and open-source datasets may limit research and development of AI/ML algorithms.52 The AUC is frequently reported to describe AI/ML model performance, but the optimal statistical metrics or combination of metrics to assess the performance of this new class of tests is not yet defined.
Table 2.
Electrocardiography
| Best practices | Description |
|---|---|
| The acquisition environment of ECGs used to train AI/ML should match those used clinically | Electrocardiographic signals are affected by body position, lead placement, motion, and signal processing issues such as sampling rate and dynamic range. |
| Accounting of bias enables generalization of results to diverse populations | Different populations show different “normal” electrocardiographic features. These factors should be incorporated into AI/ML models to ensure generalizability. |
| AI/ML algorithms must be tested in independent, external cohorts | AI/ML algorithm generalizability is ensured by their testing on data structures other than the ones in which they were created, considering different populations, equipment, and clinical workflows. |
| Gaps and challenges | Description |
| Develop a robust framework to apply AI/ML algorithms for scenarios that appear superficially similar but differ in important respects | Some AI/ML algorithms work well across different clinical scenarios, yet others do not (eg, an algorithm applied on ECG to detect AF in outpatients may not apply to postoperative AF). On the other hand, ECG-based AI/ML algorithms can detect ventricular dysfunction irrespective of mechanism. |
| Clinical outcome data are limited | Development and testing of practical workflows that integrate AI/ML ECG-based algorithms may demonstrate real-world utility. |
| Develop a framework to address the consistency of ground truth labels. | Accurate ground-truth labels are needed for AI/ML algorithm training. Tools to rapidly generate labels, such as natural language processing, may be prone to errors. Semisupervised models are still in the research phase. |
AF indicates atrial fibrillation; AI, artificial intelligence; and ML, machine learning.
IN-HOSPITAL MONITORING
Overview
Bedside monitoring has been a standard of care for decades. Traditional systems apply expert static rules to generate an alarm once a vital sign exceeds a given threshold. However, assigning scores to individual vital signs heuristically and ignoring potential covariance between different physiological signals53 has contributed to the modest accuracy of these systems. Application of AI/ML on streaming physiological signals from bedside monitors31 provides tools to harvest subtle signatures across simultaneously acquired vital sign signals, which holds significant promise in improving outcomes.
False Alarm Reduction
Only 5% to 13% of alarms from bedside monitors are actionable, whereas the remaining 87% to 95% may actually distract clinicians and compromise patient safety.54 Applications of AI/ML on in-hospital monitors has been shown to increase the accuracy of alarms, improving patient outcomes and allocation of resources.55 Convolutional neural networks (CNNs) applied on intensive care unit (ICU) vital-sign data could differentiate true from false monitor alarms,32 thus reducing alarm fatigue.
Clinical Deterioration
AI/ML models applied to bedside monitors can detect worsening of heart failure56 and decompensation,57,58 in ICU and emergency department settings. These models can detect subtle physiological signatures before clinical deterioration, broadening the diagnostic and therapeutic window for early intervention.59 AI/ML systems have been shown to improve accuracy over traditional diagnostic systems, although with a broad range of accuracy.60 Prospective studies on the clinical validation of AI models for forecasting clinical deterioration are important, yet are relatively sparse.
Sepsis and Hypotension
Several studies have used AI/ML algorithms for the early detection of sepsis61–63 and hypotension,64,65 with high accuracy, 3 to 40 hours ahead of traditional approaches. In a meta-analysis of 36 studies including 6 randomized controlled trials, AI/ML-based prediction of sepsis coupled with early intervention may reduce mortality rate (relative risk, 0.56 [95% CI, 0.39–0.80]) more effectively than alternative strategies.66 The beneficial effect of AI/ML predictions was higher in the emergency department and general wards, where patients are less frequently monitored, than in the ICU. This has important implications for deploying such systems in clinical practice.
Cardiac Arrest
AI/ML tools may predict impending in-hospital cardiac arrest and enable early intervention. However, at the present time, most proof-of-concept studies have been retrospective. An Extreme Gradient Boosting–based model using heart rate and respiratory rate data predicted VT 1 hour before its onset with sensitivity and specificity >0.80,67 using ECG, noninvasive blood pressure, and percutaneous oxygen saturation (Pao2),68 Hidden Markov and Gaussian mixture models predicted imminent ventricular fibrillation (VF), from ≈5 minutes to 6 hours before onset with accuracies of 0.83 to 0.94.68–71 In the pediatric ICU, AI/ML predicted cardiac arrest up to 50 minutes before onset in 91% of patients, compared with only 6% by clinicians, albeit with modest positive predictive value (0.11).72 Thus, although AI/ML algorithms may predict imminent ventricular arrhythmias in reference datasets, prospective validation and testing are urgently required.
Atrial Fibrillation
Several AI/ML applications can detect AF in the acute care setting.73 In 6040 patients in the well-described MIMIC-III (Medical Information Mart for Intensive Care) database of patients undergoing cardiac surgery, AI/ML tools predicted postoperative AF, a major cause of delayed discharge and morbidity, with AUCs of 0.59 to 0.74 that were better than standard clinical scores. In this study, saliency analysis was used to provide personalized risk profiles for each patient,74 which may improve management and shed mechanistic insights. AI/ML has been shown to predict in-hospital stroke/transient ischemic attack and major bleeding in critically ill patients with preexisting AF from EHRs with an AUC of 0.931 for stroke/transient ischemic attack, and 0.93 for major bleeding.75
Drug-Related Proarrhythmia
A common cause for admission to acute care settings is to monitor risk of proarrhythmia from medications. Several studies now report that QTc duration is accurately estimated by AI/ML of the ECG, including electrocardiographic data from smartphone-based systems (some with US Food and Drug Administration [FDA] approval for QT measurement76), although clinical experience is limited.77 For drug discovery, an AI/ML model termed deepHerg predicted if a specific agent blocks the hERG potassium channel and provided a c-statistic of 0.967 for torsades de pointes, while it revealed that 29.6% of 1824 of the FDA-approved drugs may inhibit hERG.78 Other AI/ML systems can predict 70.3% of drugs that are known to cause torsades.79 A computational “atom to rhythm” pipeline that combines AI/ML with computer models of drug structure was able to infer channel binding and hERG block from drugs such as dofetilide and moxifloxacin.80 Several AI/ML models have been reported to predict proarrhythmia from drugs that block the delayed rectifier, L-type calcium, and late sodium channels.80,81 Nevertheless, the clinical actionability of such approaches remains undetermined.
Perioperative Risk Assessment
Application of AI/ML on large numbers of discrete variables or physiological inputs may be superior to clinical risk scores for assessing perioperative risk.82 In patients undergoing valve or bypass surgery, application of CNNs to the ECG to screen for ventricular dysfunction predicted long-term mortality of inpatients (with EF>35%).83 Intraoperatively, AI/ML applied to the electroencephalogram revealed spectral features that can assess the depth of anesthesia, guide anesthetic drug dosing, and potentially mitigate postoperative delirium.84,85 AI/ML of other intraoperative variables may also predict hypotension, arrhythmias, and hypoxemia minutes before occurrence,86 whereas reinforcement learning algorithms have been used to manage complex control rules to enable continuous anesthetic dosing in synthetic models.87 AI/ML systems able to reliably predict perioperative complications and mortality from various surgical procedures could dramatically improve patient selection, clinical trial design, and informed consent.
Challenges on the Use of AI/ML in In-Hospital Monitoring
A major challenge to current AI/ML-based monitoring systems is the lack of rigorous prospective evaluation. Moreover, few studies have been shown to affect clinical end points such as mortality,88–90 or make predictions that could directly inform clinical decision-making. Although some studies reported dramatic reductions in mortality,91,92 such effects could reflect altered behavior in individuals being monitored (the Hawthorne effect), as revealed from the algorithm use during the COVID-19 pandemic.93,94 AI/ML tools may also be limited in practice by the a lack of standardized platforms to report predictions to clinicians95 and noise in ambulatory data,55 with some studies reporting that valid data are present for as little as half of the monitoring time.96 Solutions may involve deriving more informative time-varying metrics for longer periods of time,97 and the adoption of best practices for designing trial protocols, as well (Table 3).
Table 3.
In-Hospital Monitoring
| Best practices | Description |
|---|---|
| AI/ML algorithms track cardiovascular status from in-hospital monitoring | Development of in-hospital electronic monitoring which is integrated with other technologies may predict events such as cardiac arrest, heart failure, AF, and stroke. |
| AI/ML algorithms may identify conditions such as sepsis, hemorrhage, delirium, and overall clinical deterioration | AI/ML-based algorithms may provide early warning for many types of clinical deterioration, each of which may need different integrated workflows. |
| AI/ML algorithms reduce alarm fatigue among staff | Alarm fatigue is a major issue in ICU settings. AI/ML algorithms may reduce excessive alarms that result from current rule-based systems. |
| AI/ML algorithms improve allocation of services and resources | Use of AI/ML of in-hospital data streams may improve allocation of resources. |
| AI/ML algorithms for in-hospital use assist in procedures | Procedures may be improved by AI/ML methods, such as robotic surgery. |
| Gaps and challenges | Description |
| Translation performance of predictive AI/ML algorithms across centers | AI/ML-based alerting algorithms exhibit robust performance when tested across institutions and places that reflect differences in clinical settings or study designs. |
| Identification of patients, conditions where monitoring may improve outcomes | It is unclear which patients benefit from automated alerting systems, and if that affects disparities in in-hospital outcomes. |
| Evaluation of the effect of alarms across conditions and patient groups | Limited evaluation has been performed on the effect of false positive triggers and their reduction on clinician workload and health system cost. |
| Acceleration and scaling annotation of in-hospital monitoring data | Because the annotation of in-hospital monitoring data is labor intensive, and complicated by noise and artifacts, the limited availability of large, well-labeled datasets hampers progress. Open-source data sets may be noise free and not representative. New techniques (eg, semisupervised ML) may be effective. |
| Real-time operation of alert triggering AI/ML algorithms, across hospital settings | Few hospitals have pipelines that integrate physiological monitoring with other systems, which may widen the gap between safety net and high cost among hospitals. |
AF indicates atrial fibrillation; AI, artificial intelligence; ICU, intensive care unit; and ML, machine learning.
IMPLANTABLE AND WEARABLE TECHNOLOGIES
Overview
The ability to interpret physiological data on a near continuous basis may provide unprecedented data on disease progression, new time points for intervention, and redefine the boundary between inpatient and outpatient care.98 This technology also has the potential to reduce disparities of care.99 An important unaddressed theme is to identify those patients and disease types most amenable for AI/ML-enabled monitoring, and to develop and validate practical pathways of care for each.
Device Types
Consumer wearables may or may not contain FDA-cleared components,49 and may differ in the types of signal captured, signal processing, data security and governance, level of clinical validation, and data integration into medical records. There are several forms of FDA-cleared implanted devices.49,98 The efficacy and utility of each device depends on its form factor, sensor type, anatomical placement, and analytics, including noise reduction and interpretation algorithms.
Motion detection is important because inactivity is associated with adverse cardiovascular outcomes and mortality and because activity provides a context for physiological signals. Motion sensors use piezoresistive, piezoelectric, or differential capacitive accelerometers to record linear acceleration in 3 planes and process it on the basis of anatomical locations to identify motion,100 corresponding to sleep, steps, or activity.101 The wristwatch is commonly used, but ankle recordings are superior for step counting. Global positioning system data can augment analysis for outdoor activities, and micro-electromechanical barometers can sense changes in elevation to detect activities such as stairs climbed or a fall.98 Other form factors include chest patches, chest straps, wearable garments with embedded sensors, smart phones, and head-mounted devices.98
Photoplethysmography (PPG) or ECG-based devices can both detect heart rate or rhythm. ECG-based devices are considered the gold standard for rhythm diagnosis. Chest strap devices can record the ECG but are less well-tolerated than watches that typically record a pseudo lead I between 1 finger on the crown and the watch base.102 Smart watches have been used in small and large studies with >400 000 participants103 to screen for AF with positive predictive values from 84% to 99%. PPGs require good skin contact and may be adversely affected by tattoos and darker skin tones.104 PPG-based devices can detect arrhythmias such as AF but may be sensitive to movement artifacts.105
Additional sensors in wearables include acoustic sensors to provide a phonocardiogram and skin-impedance sensors for use in garments.98 Sensors in implantable devices can detect impedance to electrical current to quantify pulmonary congestion (which reduces thoracic impedance) and direct pressure sensors (eg, pulmonary artery) for heart failure management.98
Detection of Near-Term Atrial Fibrillation
There is a substantial literature that AF can be detected by AI-enabled PPG-based devices including the Apple Heart study (Assessment of Wristwatch-Based Photoplethysmography to Identify Cardiac Arrhythmias),103 WATCH-AF trial (Smartwatches for Detection of Atrial Fibrillation),106 and others.105 In 91 232 annotated ambulatory patch ECGs from 53 549 patients, Hannun et al107 used AI/ML to ECG-based devices to detect 12 rhythm classes with an F1 score superior to cardiologists (0.837 versus 0.78). Adding smartphone accelerometry108 or gyroscope107 data (to measure chest micromovements of cardiac motion) may push the accuracy for AF detection >90%. Mobile devices can also detect VT/VF. AI/ML applied to 3 public ECG databases provided an accuracy of 96.3%.109 As described above, AI/ML applied to electrocardiographic and other vital sign data can predict imminent ventricular arrhythmias.68–71
Blood pressure can be estimated from PPG devices98,110 by using AI/ML. Key indices are the pulse transit time, which is the time that the pulse takes to travel between 2 arterial sites, and pulse arrival time, which refers to the time between the ECG R wave and the peak of the PPG signal (the pulse wave). The pulse transit time can be found either by using a single-source PPG and the electrocardiographic signal, or using PPG signals from 2 sensors at different locations. Pulse arrival time requires both the PPG and electrocardiographic signals.
Monitors can increasingly measure numerous indices of cardiovascular disease and health.110 To guide management of patients with heart failure (HF), the multicenter LINK-HF study (Multisensor Non-Invasive Telemonitoring System for Prediction of Heart Failure Exacerbation)111 applied AI/ML to a smartphone-accessed wearable multisensor chest patch and detected HF exacerbation and impending rehospitalization with sensitivities of up to 88% and specificities of up to 85%. Although some studies show improved clinical utility over conventional care, others show no improvement.112
Implantable devices provide monitoring data that can also improve cardiovascular care. This includes AF management using data from implanted arrhythmia monitors, pacemakers, or defibrillators,98 and management of HF using data from implanted pressure monitoring (but not impedance monitoring).113
In general, prospective studies are needed not only to further establish the accuracy and generalizability of such approaches, but also their translation to actionable care pathways that can demonstrate clinical utility.
Challenges in Applying AI/ML in Mobile and Wearable Technologies
The form factor of wearables affects signal quality or patient comfort, and this must be taken into account when comparing devices. AI/ML of mobile device data opens specific ethical issues, because data are owned by patients, yet data privacy, operability, and integrity114 must be maintained among all stakeholders115 (Table 4). Regulatory pathways must be developed for AI/ML-enabled wearable and implantable devices in the United States.116 However, a greater scientific knowledge base is also required. Prospectively collected data, clinical trials, and development of workflows are urgently needed. For example, a notable recent study showed that AF diagnosed by wearables could be confirmed by cardiologists in only 34% to 65% of cases,117 and >90% of alerts did not lead to clinically actionable diagnoses.118 In terms of acceptance, 35% of clinicians in a recent survey stated that they would refuse to integrate AI/ML-enabled wearables in their care and 11% considered them “a great danger.”119 Heterogeneity exists in how AF is labeled in various AI/ML-based systems. It remains to be determined if acceptance will improve as clinicians and patients become more familiar with such technology.
Table 4.
Implantable and Wearable Technologies
| Best practices | Description |
|---|---|
| Identification of disease states and patient types in whom wearable technologies can provide hospital grade information | Identification of the accuracy of each application may result in some applications converting in-patient to “at-home hospital” monitoring. |
| Identification of disease states and patients in whom implanted devices are preferable | Certain scenarios may be better served by implanted devices, such as patients with existing pacemakers and defibrillators at risk for serious adverse outcomes. |
| Definition of states of wellness that can be tracked by wearable devices | Tracking and maintaining some states of wellness may effectively prevent transition to disease. |
| Gaps and challenges | Description |
| Interoperability standards between devices and electronic health systems | Data ownership needs to be defined, while interoperability standards enable data sharing and auditing between stakeholders, thus reducing barriers for third-party firms to innovate. |
| Definition of new sensor reference standards for key cardiovascular metrics | Not all sensors are equally accurate across clinical scenarios. |
| Identification of robust, disease-based applications for each device | Clinical trials may reveal differential accuracy among devices across populations (eg, atrial fibrillation screening for an older patient versus a young athlete). |
| Cost-effectiveness, implementation, ethics, privacy, and safety | Effect assessment of wearable and implantable devices on resource utilization, costs, and clinical outcomes. |
| Evolution of regulatory boundaries | Establishment of regulatory approaches between different groups, even for the same disease. |
GENETICS
Overview
The development of high-throughput DNA-sequencing technologies over the past decade has provided the means to generate large-scale genomic data well suited for AI/ML. The ability to generate 3 billion nucleotides uniquely arranged in a single individual in just 24 hours, coupled with the generation of these data collectively from >1 million individuals involved in government-funded DNA-sequencing projects,120 has made available large volumes of human genomic data that is ≈4% non-European.121 These initiatives, integrated with longitudinal phenotypic information and lifestyle behaviors, provide the training datasets necessary to robustly predict future risk of disease in individuals of European ancestry and open a new era of surveillance and potential intervention for both rare and common diseases, redefining cardiovascular prevention.
AI/ML in Genome-Wide Association Studies
Genome-wide association studies (GWAS) seek to find statistical associations between genetic variants and health-related traits in populations.122 GWAS use relatively common (>1% minor allele frequency) single-nucleotide polymorphisms (SNPs) at up to >4 million loci in the genome to identify health-related associations.123 The NHGRI-EBI GWAS study catalog (a collaboration between the National Human Genome Research Institute [NHGRI] and the European Bioinformatics Institute [EBI] to create a publicly available resource of GWAS studies and their results) contains findings from nearly 6000 publications reporting ≈420 000 genotype-phenotype associations that met some nominal level of significance.124 GWAS data have been used in meta-analyses, pathway analyses, and in the construction of polygenic risk scores; these approaches have sometimes offered insights into disease biology, prompted drug development, and improved risk stratification.125,126
AI/ML using GWAS data to identify variants for risk classification of cardiovascular disease is in its developmental phase. As an illustrative proof of concept, Jo et al126 used CNNs to identify SNPs associated with Alzheimer disease in a 3-step process. First, they divided the whole genome into nonoverlapping, optimally sized fragments, then applied CNNs to each fragment to identify Alzheimer disease–associated fragments. Second, they used deep learning to generate a “phenotype influence score” for each SNP in the most highly associated fragments to identify Alzheimer disease–associated SNPs. Third, they used deep learning with the most highly associated SNPs from step 2 to develop a classification model. This approach identified significant SNPs that differed from those identified using a standard GWAS method,127 although both approaches implicated similar regions of the genome (coding Apoprotein E).
Extending Polygenic Risk Scores
GWAS data are most frequently used to characterize univariate associations between traits of interest and individual variants, which can be used to construct polygenic risk scores (PRS). However, PRS often explain only a small percentage of the variance in a phenotype, potentially because they do not account for interactions among SNPs or for nonlinearities in variant trait associations. Elgart et al127 sought to overcome these limitations by using data from a multiethnic genomic dataset of ≈29 000 individuals with an ensemble method of SNP selection followed by a gradient-boosting AI/ML technique (XGBoost) to identify 9 complex phenotypes. Compared with the standard, linear PRS, the AI/ML approach resulted in relative increases in explained variance in phenotypes ranging from 22% (height) to 100% (diastolic blood pressure). The multiancestry-trained AI/ML models performed as well as racial and ethnic group–trained models and better than standard linear PRS models. Leveraging AI/ML (such as from XGBoost) to integrate enhanced PRS with clinical information from EHR holds promise to advance the application and implementation of precision medicine in cardiovascular disease.
Ancestry Characterization
Stratification may be necessary to produce meaningful genotype-phenotype associations. Panels of autosomal ancestry-informative SNPs historically have been used for this purpose but sometimes with crude resolution. For example, some methods create a single East Asian racial group despite known genetic differences in subgroups. AI/ML approaches may enable the creation of ancestry-informative SNP panels with higher-resolution ancestry inferences. Gu et al128 applied ML methods (Softmax, Random Forest) to screen a candidate panel of 1185 ancestry-informative SNPs (collected from 13 previously published panels) to develop an optimized classification model that used 272 SNPs to distinguish Northern Han, Southern Han, Korean, and Japanese individuals. Their ancestry-informative SNP panel correctly classified individuals to the 4 East Asian groups with >90% accuracy.
Phenotype to Gene Identification
There is an emerging use of AI/ML in a “reverse direction,” applied to phenotypes to predict genetic conditions. DeepGestalt, an AI/ML-based facial image analysis algorithm, has been shown to be superior to experts in identifying monogenic genetic syndromes with facial anomalies, including several cardiovascular diseases and correctly prioritizing pathogenic genetic variants.129 This deep learning model can accurately distinguish distinct genetic subtypes of Noonan syndrome.130 Likewise, deep learning models have been suggested to outperform cardiologists in detecting long QT syndrome from electrocardiographic analysis, and potentially distinguish between the common genetic causes of long QT syndrome (LQT1-KCNQ1, LQT2-KCNH2, LQT3-SCN5A).131
Determining the Clinical Relevance of Genetic Variants
More than 6000 genetic variants are now implicated as Mendelian causes of human disease, yet the vast majority of observed genetic changes are classified as variants of uncertain significance. AI/ML has been applied to assist in more confidently classifying the benign or deleterious nature of variants of uncertain significance. The Combined Annotation Dependent Depletion approach uses AI/ML that integrates multiple data sources to predict variant pathogenicity (eg, evolutionary conservation and functional predictions from the variant). Deep learning that builds on Combined Annotation Dependent Depletion can enhance classification accuracy compared with non-AI/ML models.132 PrimateAI, a uniquely trained deep CNN based merely on DNA or protein sequence from data of >100 000 human sequence alignments, has shown promise in accurately classifying variants of uncertain significance.133 Extensions of such AI/ML-based models may improve the prioritization of variants and candidate genes identified through unbiased gene discovery methods such as whole exome sequencing, whole genomic sequencing, or GWAS in patients and cohorts with gene-elusive disease.134
Challenges in Applying AI/ML in Genetics
It is important to note that, although AI/ML models are making significant progress in enhancing variant interpretation, their use as a definitive classification tool still requires caution (Table 5). As with all deep learning models, those used in genomics require training on human-derived data which itself is prone to errors and inaccuracies. Although optimism remains high that AI/ML will accelerate the discoveries of complex interactions that will inform future prevention and treatment efforts, in the cardiovascular domain, we are currently at the bottom of a steep hill with many steps to make to reach the summit. Step by step, AI/ML will evolve to affect our understanding of human genomic data in relation to cardiovascular disease prevention and treatment.
Table 5.
Genetics
| Best practices | Description |
|---|---|
| AI/ML algorithms predict common cardiovascular disease (coronary artery disease, diabetes, hypertension, arrhythmia) using personal genomics | Effective preventive medicine and clinical surveillance may be used to decrease cardiovascular disease morbidity and mortality for large, at-risk populations. |
| AI/ML algorithm-based identification of monogenic causes of cardiovascular disease, for targeted drug development | Discovery of genes that cause cardiovascular disease identify potential targets for highly efficacious novel drug therapies (eg, statin drugs). |
| AI/ML algorithm-based classification improvement for predicting rare genetic variants as benign or pathogenic | Targeted genetic testing in clinical genetics is fraught with the frequent observation of genetic variants of uncertain relevance. |
| Gaps and challenges | Description |
| Implementation of universal standards to clinically translate genomic AI/ML algorithms | AI/ML-based models must be validated and robust in prediction for routine use in clinical genetics. |
AI indicates artificial intelligence; and ML, machine learning.
AI/ML IN INTERPRETING EHRs
Overview
In principle, appropriate analysis of the EHR could improve disease detection, stratify patients into treatable disease types (novel “phenotypes”), and identify novel clinical workflows. Randomized controlled trials evaluate 1 treatment at a time and at a single time point, typically at the time of enrollment, and provide an average treatment effect across a heterogeneous cohort of patients. On the other hand, AI/ML applied to EHR could simulate sequential decision-making at different time points, enrolling every patient who has been treated or not treated, with little exclusion criteria and with less patient dropout.6 Several EHR-based applications have been described, although most have not been generalized outside their development cohorts.
Predicting In-Hospital Mortality
In a review of 21 studies using elements from the EHR, AI/ML achieved an accuracy of ≈0.86 for predicting mortality in the ICU.135 The Super ICU Learner Algorithm (SICULA) used 17 static variables to achieve an AUC of 0.94 (95% CI, 0.90–0.98) for predicting mortality in a test population.136 AI/ML applied to clinical features in 217 289 ICU patients predicted 30-day mortality with an AUC of 0.89, improving on the Simplified Acute Physiology Score-3 with AUC 0.85.137
Predicting General Cardiovascular Outcomes
Several models have been trained on large numbers of variables from the EHR. Zhao et al138 reported better prediction of cardiovascular events at 10 years in 109 490 individuals from their HER-based AI/ML tool than from the American College of Cardiology/American Heart Association pooled cohort risk equation. In 7686 patients, analysis of 1000 variables, from the EHR, predicted major adverse cardiovascular and cerebrovascular events with an AUC of 0.81 (95% CI, 0.80–0.83).
Predicting Specific Cardiovascular Disease
AI/ML applied to EHR chart data has been reported to predict impending HF rehospitalization better than individual cardiologists.112 Ye et al139 developed an XGBoost-based AI/ML risk prediction model for incident hypertension in 823 627 patients, which provided an AUC of 0.870 for incident primary hypertension within 1 year in 680 810 patients studied prospectively. Guan et al140 used EHR to define features of ischemic stroke in 1598 patients from the Massachusetts General Hospital Ischemic Stroke Registry and found that the best model had 92.2% accuracy with AUC of 0.911 (95% CI, 87.5–93.9). Predictors were AF, age, cardiomyopathy, HF, patent foramen ovale, mitral annulus calcification, and recent myocardial infarction.
Disease Classification
Although existing disease phenotypes have often been based on readily available data using traditional grouping elements, AI/ML provides an opportunity to better characterize disease types by integrating several, often complex data categories. In addition to nuanced definitions of HF beyond conventional classification of HF with reduced EF and HF with preserved EF, AI/ML-based phenotypes are increasingly reported to integrate multimodal data to identify patients at risk for adverse outcomes from HF,138 at heightened risk for sudden cardiac arrest,16,141 or with AF who are more likely to respond to ablation.142,143
Challenges in Applying AI/ML in EHR
EHR data are only as good as their curation and consistency. Raw EHR data are extracted from different information systems and must be linked and prepared for analysis by individuals familiar with local practice patterns (Table 6). This may introduce variation in data collection compared with centralized clinical trials.144 EHR analysis introduces several potential biases. For example, the likelihood of an abnormal measure correlates strongly with frequency of measurement, which in turn reflects the severity of illness because clinicians order more tests in unstable patients. Hence, “routinely” collected data implicitly encode clinician judgment that may be highly variable across clinicians.145 Sampling biases may lead to spurious associations unless input is obtained from domain experts.146 For example, when analyzing EHR data from the hospital, modeling is affected by the criteria for admission, which vary from 1 facility to another, and even within the same hospital at different times.147 Treatment administration is subject to differences in inter- and intraclinician decision-making. EHRs often lack relevant social determinants for treatment and other confounding variables. In addition, differences between institutions and regions vary over time so that results may not generalize beyond the original data source.148 With the advent of generative AI/ML, there are opportunities to leverage these technologies to assist clinicians and researchers using EHR. Generative AI/ML develops new content by applying advanced algorithms to existing data from sources such as the internet. Generative pretrained transformer language models have demonstrated the ability to answer complex, context-specific medical knowledge questions accurately, and to structure and summarize clinical data, as well.149 However, the accuracy of such systems has not been widely tested, particularly for guiding health care decisions. It is thus imperative that data scientists discuss design choices and study assumptions with clinicians or other clinicians who are knowledgeable of local clinical protocols, and researchers adopt causal frameworks where possible to avoid introducing bias by indication. A causal diagram can be helpful to infer the generalizability of models by making explicit which relationships in the data are likely to differ between institutions and across time.150 Last, model evaluation should be tailored to the intended use of the system, for example, screening versus triage recommendation.151
Table 6.
Electronic Health Records
| Best practices | Description |
|---|---|
| Use of the largest and best curated electronic EHR to develop AI/ML algorithms | EHR-based optimization of AI/ML algorithms for each application that takes into consideration the number and types of elements to maximize their generalizability. |
| The data represent the whole population for each application | Consideration to differences between centers in the accuracy and frequency of data collection, varying modalities, and clinical actions helps to avoid exacerbating disparities. |
| Development of predictive models and clinical decision support systems using EHR | Clinical conditions should be clearly defined for best use of EHR data. |
| Iterate future EHR structures on the basis of learning from current experience | The structure of current EHR borrows heavily from historical paper records. Future EHR may benefit from different data curation, structures, and analytic systems. |
| Gaps and challenges | Description |
| Ensure the accuracy and generalizability of predictive AI tools on the basis of the EHR. | EHR-based AI/ML algorithms can predict cardiovascular disease better than the American College of Cardiology/American Heart Association pooled cohort risk equation, yet more salient analyses may improve their rigor and robustness. |
| EHR-based AI/ML algorithms may complement randomized clinical trials | It is increasingly difficult and expensive to conduct randomized clinical trials. Robust “real-world trial emulation” may fill the gap between such trials. |
| Integration of EHR data from diverse electronic systems | EHR systems differ around the world. AI/ML algorithms developed in large national databases, or claims data, are expected to be applicable to diverse health care systems. |
| Integration of EHR data from different languages | Multilingual EHR may promote diversity, equity, and inclusion, enabling AI/ML algorithms trained with data from underrepresented races and ethnicities to be applied to these groups in the United States. |
| Ensure that EHR are available to all | Making EHR-based AI/ML algorithms cost feasible, including in remote and underresourced areas, helps avoid exacerbating inequities and perpetuating bias of these algorithms. |
AI indicates artificial intelligence; EHRs, electronic health records; and ML, machine learning.
A FRAMEWORK FOR THE SUCCESSFUL IMPLEMENTATION OF AI/ML IN CARDIOVASCULAR MEDICINE
Implementation Science for AI/ML-Based Precision Medicine
Implementation science is defined as the study and use of methods aiming to promote the systematic uptake of research findings and other evidence-based practices into routine practice, thereby improving the quality and effectiveness of health services among all people.28 Implementation science for AI/ML is essential to ensure that personal and public data are integrated appropriately to address core unmet clinical needs to achieve precision cardiovascular medicine (Table 7).152
Table 7.
A Framework for Successful Implementation of AI/ML in Cardiovascular Medicine
| Best practices | Description |
|---|---|
| AI/ML algorithm triangulation in different data sets, by allowing data sharing | Several best practices have been reported by the American Heart Association Precision Medicine Platform to facilitate generalizability of results and data sharing. |
| Study benchmarking against current standards for gain and cost-effectiveness analysis. | Validation of AI/ML-based precision medicine algorithms (eg, using cluster randomized clinical trials to assess the utility of the developed decision support tools). |
| Involvement of a multidisciplinary team in AI/ML algorithm development | Use of interdisciplinary teams of clinicians and researchers who leverage AI/ML and informatics, may improve treatment for patients. |
| Explainability of AI/ML algorithms increases trust and adoption | Scepticism regarding the wide application of “big data” analysis and AI/ML algorithms can be eased by explainable algorithms for interested stakeholders. |
| Gaps and challenges | Description |
| Algorithms need to be transferable | Translating precision medicine platforms from the original development cohort to other external patient populations introduces uncertainty in clinical decisions. |
| Social determinants or measures of deprivation are not used for prediction, classification, or optimization | Inclusion of social determinants or measures of social deprivation have been shown to improve cardiovascular risk scores. |
| Regulations ensure that AI/ML algorithms are safe, effective, efficient | The diversity of devices, AI/ML algorithms, and databases introduces several risks. The US Food and Drug Administration provides guidance on data use and algorithm development. |
| Protection of at-risk communities from further discrimination by AI/ML algorithms | It is critical to devise strategies to eradicate rather than exacerbate existing health inequalities. |
AI indicates artificial intelligence; and ML, machine learning.
Clinical Utility and Integration in Patient Care
Robustly designed AI/ML systems can identify informative and hidden patterns in complex clinical data to personalize cardiovascular medicine from screening and diagnosis, to find novel classification and phenotypes, to predict adverse outcomes, to guide therapy, and to guide trial design.51
AI/ML should augment and support clinical decision-making, rather than replace clinical judgment needed for evidence-based practice.49 However, to realize this potential, AI/ML analytics must be presented to clinicians through intuitive and interpretable human–computer interfaces that enhance user trust and integrate with existing clinical workflows.95 Interpretability in AI/ML, however, is an imprecise and controversial science. Moreover, it is not clear that complete understanding of a complex algorithm is essential for its robust use, given that algorithms in some instances have already outperformed the expert annotator. For instance, it is not necessary to understand the complex mechanisms of action of a drug to use it according to its labeling on the basis of clinical evidence. As a result, the efficacy of AI/ML algorithms should be FDA “labeled” with precise descriptions of the subject population and intended clinical scenarios for use.49 As new patient groups are studied, their details should be added to labeling. A unique hazard to AI/ML-based systems is that algorithm performance may degrade over time as a consequence of such changes in patient demographics, clinical context, or other factors, and may have to be updated and reevaluated as part of clinical practice evidence.153 Reimbursement models for AI/ML in cardiovascular disease must be developed to ensure wide access and avoid the risk of inadvertently widening health care disparities.
At present, there remains a paucity of evidence that AI/ML can positively affect patient outcomes compared with current standards of care.154 The future adoption of AI in cardiovascular medicine will ultimately require such evidence that AI/ML applications measurably improve patient outcomes.155
Clinician Education and Decision-Making
With the avalanche of reported AI/ML applications in medical practice, there is pressure for clinicians to understand AI/ML to at least the same level they apply for any technology that influences decision-making.156 A useful model may be one where, first, clinicians must be able to identify when a technology is appropriate for a given clinical scenario, and what inputs are required; and, second, clinicians should be able to interpret results in the context of errors and biases that may limit applicability for specific patient groups. In a model for the future, clinicians’ progressively incremental data science training may take the form of progressively adding statistical courses during training, or as continuing education for current practitioners. It is critical that all stakeholders appreciate the context-specific nature of AI/ML and that performance of a given application may not always be transferable.157
Data Handling
Issues pertaining to detailed descriptions of data handling (preprocessing),158 such as which and how features are extracted and excluded,159 and how final model predictions are validated, are required to ensure transparency and clinical acceptability of AI/ML-derived decision support systems, and may be of a different level of interest among stakeholders. Furthermore, details such as recoding, newly derived variables, data reduction techniques or transformations may substantially affect the interpretation and accuracy of models, yet may differ from conventional statistical approaches and require advanced training. It is essential to assess the gain from using an AI/ML decision support system over conventional methods. There are emerging reporting guidelines that aim to enhance both the rigor and reproducibility in the design of AI/ML-derived decision support systems.160
Ethics
Those who contribute their data to AI/ML databases to improve the care of others should be treated with thoughtfulness and respect. Individuals likely have differing views on how their data should be used in the future. Many contributors are not comfortable with their data being sold to third parties for commercial purposes, without notice or consent.161,162 Individuals generally want to be informed about the commercial use of their data regardless of whether it is identified or deidentified (as is typical for AI/ML databases). Self-identified race and ethnicity can also be associated with data sharing preferences.163,164
Stakeholders must also assess which communities are contributing to AI models and which are benefiting from those advances to balance equity considerations.165
Equitable Distribution of Benefits and Burdens
AI/ML offers the means for implementing precision medicine and personalized care, yet the increasing extraction of personal data by public and private stakeholders may negatively affect health and well-being through many effects pertaining to environmental, social, political, and commercial determinants of health.166,167
The World Health Organization defines equity as the absence of avoidable, unfair, or remediable differences among groups of people defined socially, economically, demographically, or geographically.152 For health equity to be achieved, every citizen should have a reasonable opportunity to fully access all available health care. Therefore, to reach the aspirational goal of health care equity, population-representative datasets must be included in AI/ML algorithm development. On the other hand, the scaling inherent in AI/ML may further exacerbate existing inequities.168 Therefore, prioritizing in equity should be a noticeably articulated goal in health care AI/ML algorithm development.49
Bias
AI models can perform differently across subpopulations which may reflect societal and statistical bias. Societal bias is due to systemic forms of discrimination that drive disproportionate cardiovascular health outcomes and differential data quality across historically and contemporarily oppressed and excluded populations.169 These biases can manifest at the structural level, the institutional level, or interpersonally. Statistical bias comes from nonrepresentative samples in the training data, for instance, undersampling or excluding certain populations. Exclusion may be due to certain subpopulations not being represented in the data or have incomplete data due to inadequate health care access, and other socioeconomic factors that prevent robust integration into health care systems.170 Model bias relates to the specific mechanics of most AI/ML and statistical models whereby the tools work by minimizing overall prediction error without attention to performance among underrepresented racial and ethnic groups. As a consequence, AI models can exhibit overall strong performance (low error) while still performing poorly for people of underrepresented races and ethnicities who exhibit the worst health outcomes.171
Attention to Those Historically Excluded by Medical Advances
Digital technologies and AI/ML raise important issues about the way we perceive and represent sexuality, race, ethnicity, gender, class, geography, age, underlying health condition, and ability. Therefore, in the context of AI/ML-driven digital health, a new understanding of inclusion will involve forms of context-aware technical development, and innovative, local- and community-led approaches aiming the redesign, deployment, and validation of digital technologies.
Fairness
AI/ML models will not be completely fair until the various forms of discrimination that drive health inequities, and thus data bias, are removed. The current status quo is such that populations most harmed by algorithmic bias are not centered in the development of algorithms or the processes to make them more just.172
Therefore, to mitigate societal bias at the institutional level, individuals from people of underrepresented races and ethnicities must be incorporated into the AI/ML model building process with community-based participatory frameworks on more diverse research teams to make sure that the model-building process, from defining the question, to outcome selection, and feature engineering, are applicable to all populations or designed specifically for historically excluded ones. Such evaluations will need to be tailored to the specific disease context and model task and may include consideration of which subgroups have the highest incidence of disease, greatest risk of adverse events, or least access to treatment.173,174
The intersection of social identities should consider, when necessary, debiasing techniques to decrease variation in performance across subgroups.173,175 Although debiasing provides an opportunity to incorporate social determinants of health to better identify populations for AI/ML models,176 some clinicians have called for a reevaluation of this practice. In some cases, race correction may exacerbate inequities in disease outcomes and treatments among groups that already experience disparities. The American Heart Association is committed to assessing current algorithms with race correction.
Consideration to Community Input
For AI/ML technologies to earn the trust of the public, a continuous effort will be required by all stakeholders. Public engagement and dialogue are means that will ensure that use of AI/ML technologies in health care meets certain core societal expectations and values, and builds and maintains broad trust and acceptance, as well. Public dialogue will also ensure that societal views on AI/ML-based tools are incorporated across the digital health ecosystem.
Approaches that promote inclusivity include concepts such as: (1) open-source software, which improves transparency and participation in the design of an AI/ML technology; (2) citizen science, which refers to the direct involvement and contribution of nonprofessional scientists to scientific research; (3) increased diversity, of the data on which AI/ML algorithms are based, by promoting greater involvement of people who are familiar with the nature of potential bias, context, and regulations throughout the process of the algorithm development, including the labeling of the data, and the algorithm design, testing, and deployment, as well.177
Law
In general, the law can be applied to AI/ML in 2 ways: (1) regulatory attempts to mitigate AI/ML harms before they happen, and (2) through medical malpractice/case law system to attempt to rectify harms already allegedly caused by AI/ML. A new challenge that AI/ML presents in case law is the lack of transparency in how AI/ML mechanisms formulate clinical recommendations.168 AI/ML generated by a “black box” can make it difficult to establish both how the standard of care was defined and whether that care “caused” the injury in question. Although clinicians should use an AI/ML algorithm as labeled,49 it remains to be seen whether clinicians will be held liable for injuries associated with the use of AI/ML tools, and whether such tools will shift the standard of care.178
The FDA regulates AI/ML as a medical device, and they recently reaffirmed their commitment to improvement of AI/ML algorithms, mitigating against bias and improving robustness.169 Today, FDA’s list of cardiovascular medical devices incorporating AI/ML functionality includes 50 technologies that have received 510(k) clearance, and 5 that they were granted De Novo request.
AI/ML Governing Architectures
Because the health care sector is expected to be the fastest growing data-producing industry,29 the uptake of AI/ML in health care will rely heavily on the trust of patients, doctors, and other health professionals.121 However, trust can be eroded by several personal, technological, and institutional factors, including fear of data exploitation, lack of digital skills, paucity of accessibility, and poor reputation of clinicians.163
There is a need to build governing architectures that create trust in AI/ML and digital health. Such approaches may accelerate innovation in task-focused directions, protecting the collection and use of digital data to protect individual rights, promoting the public benefit of using such data, and building a culture of equity.142 Governing architectures for AI/ML digital health would have the goals of empowering patients, people of underrepresented races and ethnicities, and disenfranchised groups, as well, ensuring affordable digital health, ensuring digital rights, and regulating business in the digital-health ecosystem. Country or regional policymakers could promote digital-health strategies that prioritize such technologies through investment roadmaps.
Digital models of governance must be adapted in different societal contexts and account for implications on an individual’s health and well-being.164 As such, digital-health technologies that create value for the general public will require mission-oriented innovation,169 such that these technologies are not developed or inadvertently repurposed in ways that threaten human rights, or reinforce discrimination.179 At the institutional level, AI/ML technologies that rely on data that are both accurate and representative may help reduce inefficiency and errors and ensure more appropriate allocation of resources.177
Development of algorithm “auditing” processes that can recognize a group (or even an individual) for which a decision may not be reliable, can reduce the implications of such a decision, for example, due to bias.167 As a result, health care–related AI/ML algorithms have the capacity to influence confidence in a health care system, particularly if these tools result for some groups in worse outcomes or increased inequities.48
Liability
Assessing the liability of AI/ML algorithms is crucial to balance their risks and benefits. Thus, AI/ML governing architectures need to engage all stakeholders (developers, clinicians, and researchers) to continuously evaluate the safety and effectiveness of these algorithms. Companies should file an application with the FDA to allow marketing of an algorithm. After approval, there should be postmarket safety monitoring similar to phase IV drug development evaluation. In this ongoing phase, if the use of the algorithm results in potential adverse events/system failure, it would be the responsibility of the AI/ML algorithm developers to report and investigate such outcomes. Therefore, the critical issue of a physician’s professional liability in case of an incorrect decision and a potentially harmful outcome,168 as with any other medical product, narrows down to a responsibility to use such algorithm as “labeled,” which minimizes liability concerns.48
Adverse Event Reporting
From a quality and safety perspective, institutional metrics designed to evaluate patient safety and subsequently mechanisms targeted to reduce adverse events may have to be modified for AI/ML-based applications.
The digitization of EHR facilitates the automation of many aspects of patient safety, but efficacy is contingent on reliable data. Even if we can ensure that future algorithms are trained on more representative patient populations, there remain certain components of data collection that invariably involve a human element (eg, bias of the reporter).180 Patient safety is of paramount importance and the use of decision support systems in clinical settings must be monitored long term to avoid hidden stratification181 or other unintended consequences.182
System Upgrading
Because data quality, population characteristics, and clinical practice will all change over time, decision support systems need to be regularly updated49 to mitigate the effect of these changes on their reliability, validity and clinical utility.183 It may also be necessary to update outcome definitions to retrain models as scientific understanding of disease progresses (eg, better phenotyping of subtypes), or the demographics of the areas in which the AI/ML algorithms are used change.
The system-upgrading process ideally should be streamlined in some way to allow the decision support system to be upgraded in a timely manner, but this can be costly and can lead to unintended consequences if prespecified processes are not in place.49
Some AI/ML algorithms may be designed to continue to learn (train) continuously, refine their internal model, and improve performance (refinement/adaptation).48,166 In particular, the algorithm learns how to update from the addition of new cases (inputs) resulting in different outputs with the same inputs (compared with the outputs before the update). Such algorithms require frequent real-world performance monitoring, although the ongoing development of these systems increases the difficulty of applying a regulatory framework.166
Cybersecurity
Although questions remain with respect to privacy and patient control over their data,184 subtle approaches to reidentification of (potentially improperly) anonymized health data stand in stark contrast to the illegal, forcible acquisition of personal health data by means of a data breach (eg, illegal disclosure, attainment, or use of information without authorization). Theft of medical records allows access to financial services and health care for criminals.185 Although the risks to patient privacy should be minimized, an acceptable risk threshold needs to be decided by all stakeholders, below which data sharing can occur, for the benefit of a global medical knowledge system, by placing appropriate firewalls and other key cybersecurity measures that are regularly updated.186
CONCLUSIONS
The American Heart Association aims to advance cardiovascular health for all, including identifying and removing barriers to health access and quality.
At this dawn in the era of precision medicine, scientists and clinicians, computer and data scientists, patient advocacy groups, health care organizations, and policymakers must develop principles and guidance for the development and application of AI/ML-based digital health. Numerous applications already exist where AI/ML-based digital tools can improve disease screening, extract insights into what makes individual patients healthy, and develop precision treatments for complex diseases.
There is an urgent need to develop implementation science for AI/ML tools to create tractable cost-effective workflows for AI/ML-based precision medicine that address core unmet clinical (or translational) needs, the evidence of which can be robustly tested in trials. This process must organically incorporate the need to avoid bias and maximize generalizability of findings to avoid perpetuating existing health care inequalities.
Supplementary Material
ARTICLE INFORMATION
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on September 25, 2023, and the American Heart Association Executive Committee on October 25, 2023. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or Meredith.Edelman@wolterskluwer.com
The American Heart Association requests that this document be cited as follows: Armoundas AA, Narayan SM, Arnett DK, Spector-Bagdady K, Bennett DA, Celi LA, Friedman PA, Gollob MH, Hall JL, Kwitek AE, Lett E, Menon BK, Sheehan KA, Al-Zaiti SS; on behalf of the American Heart Association Institute for Precision Cardiovascular Medicine; Council on Cardiovascular and Stroke Nursing; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Cardiovascular Radiology and Intervention; Council on Hypertension; Council on the Kidney in Cardiovascular Disease; and Stroke Council. Use of artificial intelligence in improving outcomes in heart disease: a scientific statement from the American Heart Association. Circulation. 2024;149:e•••–e•••. doi: 10.1161/CIR.0000000000001201
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Disclosures
Writing Group Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Antonis A. Armoundas | Massachusetts General Hospital | AHA Institute of Precision Medicine (17UNPG33840017)†; RICBAC Foundation†; NIH (1 R01 HL135335–01, 1 R01 HL161008–01, 1 R21 HL137870–01, 1 R21EB026164–01,3R21EB026164–02S1)† | None | None | None | None | None | None |
| Sanjiv M. Narayan | Stanford University | Laurie C. McGrath Foundation†; NIH (R01 HL83359, R01 HL149134, R01 HL122384 and T32 HL166155 [“Computational Medicine in the Heart”])† | None | None | None | Dr Narayan is inventor or co-inventor on intellectual property owned by Stanford University and UC Regents.† | None | None |
| Salah S. Al-Zaiti | University of Pittsburgh | NHLBI (R01HL137761)†; NINR (NR013912)† | None | None | None | None | None | None |
| Donna K. Arnett | University of South Carolina | None | None | None | None | None | None | None |
| Derrick A. Bennett | University of Oxford (United Kingdom) | None | None | None | None | None | None | None |
| Leo Anthony Celi | Beth Israel Deaconess Medical Center | NIH (Our group receives research funding from the NIH to maintain and distribute de-identified EHR databases, NIBIB R01 grant EB017205)†; Philips (Our group research funding from Philips to develop and maintain the publicly available eICU-CRD)* | None | None | None | None | None | None |
| Paul A. Friedman | Mayo Clinic | Anumana (A number of AI ECG algorithms have been licensed by Mayo Clinic to Anu- mana, Eko Health, and AliveCor, and Mayo Clinic. Dr Friedman, and other inventors may benefit financially from their commercialization.)*; Eko Health (A number of AI ECG algorithms have been licensed by Mayo Clinic to Eko Health and Mayo Clinic. Dr Friedman, and other inventors may benefit financially from their commercialization.)*; AliveCor (A number of AI ECG algorithms have been licensed by Mayo Clinic to AliveCor, and Mayo Clinic. Dr Friedman, and other inventors may benefit financially from their commercialization.)* | None | None | None | None | None | None |
| Michael H. Gollob | University of Toronto; Toronto General Hospital (Canada) | None | None | None | None | None | None | None |
| Jennifer L. Hall | American Heart Association | None | None | None | None | None | None | None |
| Anne E. Kwitek | Medical College of Wisconsin | NIH (R01HL064541, U24HG010859, P01HL084207 P01HL149620, U24HG010423, and R24OD024617)† | None | None | None | None | None | Medical College of Wisconsin (pro-fessor)† |
| Elle Lett | University of Pennsylvania, Perelman School of Medicine | None | None | None | None | None | None | None |
| Bijoy K. Menon | University of Calgary, Hotchkiss Brain Institute, Calgary (Canada) | None | None | None | None | Circle CVI* | Circle CVI* | None |
| Katherine A. Sheehan | American Heart Association | None | None | None | None | None | None | None |
| Kayte Spector-Bagdady | University of Michigan Medical School | NHGRI (K01HG010496)†; NCATS (R01TR004244)† | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/ honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Andrew D. McCulloch | University of California San Diego | NIH (I am PI of the Cardiac Atlas Project that uses AI and ML for analysis of congenital heart diseases)†; Additional Ventures (I am PI of an Extension Award that uses AI and ML for analysis of single ventricle disease)† | None | None | None | Vektor* | None | None |
| Akhil Narang | Northwestern University Feinberg School of Medicine | None | None | None | None | None | None | None |
| Jagmeet Singh | Massachusetts General Hospital, Harvard Medical School | None | None | Abbott Laboratories†; Biosense Webster, Biotronik†; Boston Scientific†; CVRx†; Impulse Dynamics†; Medtronic†; Microport Inc†; Sanofi† | None | None | Abbott Laboratories†; Biotronik†; Boston Scientific†; Cardiologs†; CVRx†; EBR Systems Inc†; Implicity Inc†; Impulse Dynamics†; Medtronic†; Microport Inc†; New Century Health†; Sanofi†; | None |
| Patrick J. Trainor | New Mexico State University | NIH (NIH Grant SC-1GM139730 provides support to -omics analyses of blood samples from myocardial infarction and includes using AI/ ML towards diagnostics; NIH Grant R01HL158976 seeks to re-adjudicate myocardial infarction cases from the Multi-Ethnic Study of Atherosclerosis to enable better risk prediction and understanding of risk factor associations.† | None | None | None | BioinfoScientist LLC† | None | None |
| Marly van Assen | Emory University School of Medicine | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
REFERENCES
- 1.CBInsights. State of AI 2021 report. Accessed September 26, 2022. https://www.cbinsights.com/research/report/ai-trends-2021/
- 2.Hsu DJ, Feng M, Kothari R, Zhou H, Chen KP, Celi LA. The association between indwelling arterial catheters and mortality in hemodynamically stable patients with respiratory failure: a propensity score analysis. Chest. 2015;148:1470–1476. doi: 10.1378/chest.15-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JT, de la Hoz MAA, Kuo PC, Paguio JA, Yao JS, Dee EC, Yeung W, Jurado J, Moulick A, Milazzo C, et al. Developing and validating multimodal models for mortality prediction in covid-19 patients: a multicenter retrospective study. J Digit Imaging. 2022;35:1514–1529. doi: 10.1007/s10278-022-00674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehrmann S, Dernoncourt F, Li Y, Carlson ET, Wu JT, Welt J, Foote J Jr., Moseley ET, Grant DW, Tyler PD, et al. Comparing deep learning and concept extraction based methods for patient phenotyping from clinical narratives. PLoS One. 2018;13:e0192360. doi: 10.1371/journal.pone.0192360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716–1720. doi: 10.1038/s41591-018-0213-5 [DOI] [PubMed] [Google Scholar]
- 6.Cowie MR, Blomster JI, Curtis LH, Duclaux S, Ford I, Fritz F, Goldman S, Janmohamed S, Kreuzer J, Leenay M, et al. Electronic health records to facilitate clinical research. Clin Res Cardiol. 2017;106:1–9. doi: 10.1007/s00392-016-1025-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop CM. Pattern Recognition and Machine Learning. Springer; 2006. [Google Scholar]
- 9.Seetharam K, Brito D, Farjo PD, Sengupta PP. The role of artificial intelligence in cardiovascular imaging: state of the art review. Front Cardiovasc Med. 2020;7:618849. doi: 10.3389/fcvm.2020.618849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon BK. Neuroimaging in acute stroke. Continuum (Minneap Minn). 2020;26:287–309. doi: 10.1212/CON.0000000000000839 [DOI] [PubMed] [Google Scholar]
- 11.Weikert T, Francone M, Abbara S, Baessler B, Choi BW, Gutberlet M, Hecht EM, Loewe C, Mousseaux E, Natale L, et al. Machine learning in cardiovascular radiology: ESCR position statement on design requirements, quality assessment, current applications, opportunities, and challenges. Eur Radiol. 2021;31:3909–3922. doi: 10.1007/s00330-020-07417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatsugami F, Higaki T, Nakamura Y, Yu Z, Zhou J, Lu Y, Fujioka C, Kitagawa T, Kihara Y, Iida M, et al. Deep learning-based image restoration algorithm for coronary CT angiography. Eur Radiol. 2019;29:5322–5329. doi: 10.1007/s00330-019-06183-y [DOI] [PubMed] [Google Scholar]
- 13.Howard JP, Fisher L, Shun-Shin MJ, Keene D, Arnold AD, Ahmad Y, Cook CM, Moon JC, Manisty CH, Whinnett ZI, et al. Cardiac rhythm device identification using neural networks. JACC Clin Electrophysiol. 2019;5:576–586. doi: 10.1016/j.jacep.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Oever LB, Vonder M, van Assen M, van Ooijen PMA, de Bock GH, Xie XQ, Vliegenthart R. Application of artificial intelligence in cardiac CT: from basics to clinical practice. Eur J Radiol. 2020;128:108969. doi: 10.1016/j.ejrad.2020.108969 [DOI] [PubMed] [Google Scholar]
- 15.Rau A, Soschynski M, Taron J, Ruile P, Schlett CL, Bamberg F, Krauss T. [Artificial intelligence and radiomics: value in cardiac MRI]. Radiologie (Heidelb). 2022;62:947–953. doi: 10.1007/s00117-022-01060-0 [DOI] [PubMed] [Google Scholar]
- 16.Narayan SM, Wang PJ, Daubert JP. New concepts in sudden cardiac arrest to address an intractable epidemic: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:70–88. doi: 10.1016/j.jacc.2018.09.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakosa A, Arevalo HJ, Deng D, Boyle PM, Nikolov PP, Ashikaga H, Blauer JJ, Ghafoori E, Park CJ, Blake RC 3rd, et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng. 2018;2:732–740. doi: 10.1038/s41551-018-0282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheth SA, Giancardo L, Colasurdo M, Srinivasan VM, Niktabe A, Kan P. Machine learning and acute stroke imaging. J Neurointerv Surg. 2023;15:195–199. doi: 10.1136/neurintsurg-2021-018142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chilamkurthy S, Ghosh R, Tanamala S, Biviji M, Campeau NG, Venugopal VK, Mahajan V, Rao P, Warier P. Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet. 2018;392:2388–2396. doi: 10.1016/S0140-6736(18)31645-3 [DOI] [PubMed] [Google Scholar]
- 20.Yahav-Dovrat A, Saban M, Merhav G, Lankri I, Abergel E, Eran A, Tanne D, Nogueira RG, Sivan-Hoffmann R. Evaluation of artificial intelligence-powered identification of large-vessel occlusions in a comprehensive stroke center. AJNR Am J Neuroradiol. 2021;42:247–254. doi: 10.3174/ajnr.A6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu W, Kuang H, Teleg E, Ospel JM, Sohn SI, Almekhlafi M, Goyal M, Hill MD, Demchuk AM, Menon BK. Machine learning for detecting early infarction in acute stroke with non-contrast-enhanced CT. Radiology. 2020;294:638–644. doi: 10.1148/radiol.2020191193 [DOI] [PubMed] [Google Scholar]
- 22.Qiu W, Kuang H, Ospel JM, Hill MD, Demchuk AM, Goyal M, Menon BK. Automated prediction of ischemic brain tissue fate from multiphase computed tomographic angiography in patients with acute ischemic stroke using machine learning. J Stroke. 2021;23:234–243. doi: 10.5853/jos.2020.05064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey H, Glocker B. A standardised approach for preparing imaging data for machine learning tasks in radiology. In: Ranschaert ER, Morozov S, Algra PR, eds. Artificial Intelligence in Medical Imaging: Opportunities, Applications and Risks. Springer International Publishing; 2019:61–72. [Google Scholar]
- 24.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro AH, Ribeiro MH, Paixao GMM, Oliveira DM, Gomes PR, Canazart JA, Ferreira MPS, Andersson CR, Macfarlane PW, Meira W Jr, et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat Commun. 2020;11:1760. doi: 10.1038/s41467-020-15432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn JC, Attia ZI, Rattan P, Mullan AF, Buryska S, Allen AM, Kamath PS, Friedman PA, Shah VH, Noseworthy PA, et al. Development of the AI-cirrhosis-ECG score: an electrocardiogram-based deep learning model in cirrhosis. Am J Gastroenterol. 2022;117:424–432. doi: 10.14309/ajg.0000000000001617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0 [DOI] [PubMed] [Google Scholar]
- 28.Al-Zaiti S, Besomi L, Bouzid Z, Faramand Z, Frisch S, Martin-Gill C, Gregg R, Saba S, Callaway C, Sejdic E. Machine learning-based prediction of acute coronary syndrome using only the pre-hospital 12-lead electrocardiogram. Nat Commun. 2020;11:3966. doi: 10.1038/s41467-020-17804-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouzid Z, Faramand Z, Martin-Gill C, Sereika SM, Callaway CW, Saba S, Gregg R, Badilini F, Sejdic E, Al-Zaiti SS. Incorporation of serial 12-lead electrocardiogram with machine learning to augment the out-of-hospital diagnosis of non-ST elevation acute coronary syndrome. Ann Emerg Med. 2022;81:57–69. doi: 10.1016/j.annemergmed.2022.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Zaiti SS, Martin-Gill C, Zegre-Hemsey JK, Bouzid Z, Faramand Z, Alrawashdeh MO, Gregg RE, Helman S, Riek NT, Kraevsky-Phillips K, et al. Machine learning for ECG diagnosis and risk stratification of occlusion myocardial infarction. Nat Med. 2023;29:1804–1813. doi: 10.1038/s41591-023-02396-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bollepalli SC, Sevakula RK, Au-Yeung WM, Kassab MB, Merchant FM, Bazoukis G, Boyer R, Isselbacher EM, Armoundas AA. Real-time arrhythmia detection using hybrid convolutional neural networks. J Am Heart Assoc. 2021;10:e023222. doi: 10.1161/JAHA.121.023222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guglin ME, Thatai D. Common errors in computer electrocardiogram interpretation. Int J Cardiol. 2006;106:232–237. doi: 10.1016/j.ijcard.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 33.Smith SW, Walsh B, Grauer K, Wang K, Rapin J, Li J, Fennell W, Taboulet P. A deep neural network learning algorithm outperforms a conventional algorithm for emergency department electrocardiogram interpretation. J Electrocardiol. 2019;52:88–95. doi: 10.1016/j.jelectrocard.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 34.Adedinsewo DA, Johnson PW, Douglass EJ, Attia IZ, Phillips SD, Goswami RM, Yamani MH, Connolly HM, Rose CH, Sharpe EE, et al. Detecting cardiomyopathies in pregnancy and the postpartum period with an electrocardiogram-based deep learning model. Eur Heart J Digital Health. 2021;2:586–596. doi: 10.1093/ehjdh/ztab078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao X, Rushlow DR, Inselman JW, McCoy RG, Thacher TD, Behnken EM, Bernard ME, Rosas SL, Akfaly A, Misra A, et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med. 2021;27:815–819. doi: 10.1038/s41591-021-01335-4 [DOI] [PubMed] [Google Scholar]
- 36.Brito BOF, Attia ZI, Martins LNA, Perel P, Nunes MCP, Sabino EC, Cardoso CS, Ferreira AM, Gomes PR, Luiz Pinho Ribeiro A, et al. Left ventricular systolic dysfunction predicted by artificial intelligence using the electrocardiogram in Chagas disease patients: the SaMI-Trop cohort. PLoS Negl Trop Dis. 2021;15:e0009974. doi: 10.1371/journal.pntd.0009974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harmon DM, Carter RE, Cohen-Shelly M, Svatikova A, Adedinsewo DA, Noseworthy PA, Kapa S, Lopez-Jimenez F, Friedman PA, Attia ZI. Real-world performance, long-term efficacy, and absence of bias in the artificial intelligence enhanced electrocardiogram to detect left ventricular systolic dysfunction. Eur Heart J Digital Health. 2022;3:238–244. doi: 10.1093/ehjdh/ztac028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachtiger P, Petri CF, Scott FE, Ri Park S, Kelshiker MA, Sahemey HK, Dumea B, Alquero R, Padam PS, Hatrick IR, et al. Point-of-care screening for heart failure with reduced ejection fraction using artificial intelligence during ECG-enabled stethoscope examination in London, UK: a prospective, observational, multicentre study. Lancet Digit Health. 2022;4:e117–e125. doi: 10.1016/S2589-7500(21)00256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siontis KC, Liu K, Bos JM, Attia ZI, Cohen-Shelly M, Arruda-Olson AM, Zanjirani Farahani N, Friedman PA, Noseworthy PA, Ackerman MJ. Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents. Int J Cardiol. 2021;340:42–47. doi: 10.1016/j.ijcard.2021.08.026 [DOI] [PubMed] [Google Scholar]
- 40.Grogan M, Lopez-Jimenez F, Cohen-Shelly M, Dispenzieri A, Attia ZI, Abou Ezzedine OF, Lin G, Kapa S, Borgeson DD, Friedman PA, et al. Artificial intelligence–enhanced electrocardiogram for the early detection of cardiac amyloidosis. Mayo Clin Proc. 2021;96:2768–2778. doi: 10.1016/j.mayocp.2021.04.023 [DOI] [PubMed] [Google Scholar]
- 41.Cohen-Shelly M, Attia ZI, Friedman PA, Ito S, Essayagh BA, Ko WY, Murphree DH, Michelena HI, Enriquez-Sarano M, Carter RE, et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur Heart J. 2021;42:2885–2896. doi: 10.1093/eurheartj/ehab153 [DOI] [PubMed] [Google Scholar]
- 42.Tison Geoffrey H, Siontis Konstantinos C, Abreau S, Attia Z, Agarwal P, Balasubramanyam A, Li Y, Sehnert Amy J, Edelberg Jay M, Friedman Paul A, et al. Assessment of disease status and treatment response with artificial intelligence–enhanced electrocardiography in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2022;79:1032–1034. doi: 10.1016/j.jacc.2022.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christopoulos G, Graff-Radford J, Lopez CL, Yao X, Attia ZI, Rabinstein AA, Petersen RC, Knopman DS, Mielke MM, Kremers W, et al. Artificial intelligence-electrocardiography to predict incident atrial fibrillation: a population-based study. Circ Arrhythm Electrophysiol. 2020;13:e009355. doi: 10.1161/CIRCEP.120.009355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khurshid S, Friedman S, Reeder C, Di Achille P, Diamant N, Singh P, Harrington LX, Wang X, Al-Alusi MA, Sarma G, et al. ECG-based deep learning and clinical risk factors to predict atrial fibrillation. Circulation. 2022;145:122–133. doi: 10.1161/CIRCULATIONAHA.121.057480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasmussen MU, Kumarathurai P, Fabricius-Bjerre A, Larsen BS, Dominguez H, Davidsen U, Gerds TA, Kanters JK, Sajadieh A. P-wave indices as predictors of atrial fibrillation. Ann Noninvasive Electrocardiol. 2020;25:e12751. doi: 10.1111/anec.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attia ZI, Friedman PA, Noseworthy PA, Lopez-Jimenez F, Ladewig DJ, Satam G, Pellikka PA, Munger TM, Asirvatham SJ, Scott CG, et al. Age and sex estimation using artificial intelligence from standard 12-lead ECGs. Circ Arrhythm Electrophysiol. 2019;12:e007284. doi: 10.1161/CIRCEP.119.007284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad A, Corban MT, Toya T, Attia ZI, Noseworthy PA, Lopez-Jimenez F, Cohen MS, Sara JD, Ozcan I, Lerman LO, et al. Coronary microvascular dysfunction and the risk of atrial fibrillation from an artificial intelligence-enabled electrocardiogram. Circ Arrhythm Electrophysiol. 2021;14:e009947. doi: 10.1161/CIRCEP.121.009947 [DOI] [PubMed] [Google Scholar]
- 48.Bazoukis G, Hall J, Loscalzo J, Antman EM, Fuster V, Armoundas AA. The inclusion of augmented intelligence in medicine: a framework for successful implementation. Cell Rep Med. 2022;3:100485. doi: 10.1016/j.xcrm.2021.100485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Zaiti SS, Alghwiri AA, Hu X, Clermont G, Peace A, Macfarlane P, Bond R. A clinician’s guide to understanding and critically appraising machine learning studies: a checklist for Ruling Out Bias Using Standard Tools in Machine Learning (ROBUST-ML). Eur Heart J Digit Health. 2022;3:125–140. doi: 10.1093/ehjdh/ztac016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sevakula RK, Au-Yeung WM, Singh JP, Heist EK, Isselbacher EM, Armoundas AA. State-of-the-art machine learning techniques aiming to improve patient outcomes pertaining to the cardiovascular system. J Am Heart Assoc. 2020;9:e013924. doi: 10.1161/JAHA.119.013924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al Hinai G, Jammoul S, Vajihi Z, Afilalo J. Deep learning analysis of resting electrocardiograms for the detection of myocardial dysfunction, hypertrophy, and ischaemia: a systematic review. Eur Heart J Digit health. 2021;2:416–423. doi: 10.1093/ehjdh/ztab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruppel H, Funk M, Whittemore R. Measurement of physiological monitor alarm accuracy and clinical relevance in intensive care units. Am J Crit Care. 2018;27:11–21. doi: 10.4037/ajcc2018385 [DOI] [PubMed] [Google Scholar]
- 53.Au-Yeung WM, Sahani AK, Isselbacher EM, Armoundas AA. Reduction of false alarms in the intensive care unit using an optimized machine learning based approach. NPJ Digit Med. 2019;2:86. doi: 10.1038/s41746-019-0160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rush B, Celi LA, Stone DJ. Applying machine learning to continuously monitored physiological data. J Clin Monit Comput. 2019;33:887–893. doi: 10.1007/s10877-018-0219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clifton DA, Wong D, Clifton L, Wilson S, Way R, Pullinger R, Tarassenko L. A large-scale clinical validation of an integrated monitoring system in the emergency department. IEEE J Biomed Health Inf. 2013;17:835–842. doi: 10.1109/JBHI.2012.2234130 [DOI] [PubMed] [Google Scholar]
- 56.Chen L, Dubrawski A, Hravnak M, Clermont G, Pinsky M. Modelling risk of cardio-respiratory instability as a heterogeneous process. AMIA Annu Symp Proc. 2015;2015:1841–1850. [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L, Ogundele O, Clermont G, Hravnak M, Pinsky MR, Dubrawski AW. Dynamic and personalized risk forecast in step-down units. Implications for monitoring paradigms. Ann Am Thorac Soc. 2017;14:384–391. doi: 10.1513/AnnalsATS.201611-905OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinsky MR, Dubrawski A, Clermont G. Intelligent clinical decision support. Sensors (Basel). 2022;22:1408. doi: 10.3390/s22041408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muralitharan S, Nelson W, Di S, McGillion M, Devereaux PJ, Barr NG, Petch J. Machine learning-based early warning systems for clinical deterioration: systematic scoping review. J Med Internet Res. 2021;23:e25187. doi: 10.2196/25187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duncan HP, Fule B, Rice I, Sitch AJ, Lowe D. Wireless monitoring and real-time adaptive predictive indicator of deterioration. Sci Rep. 2020;10:11366. doi: 10.1038/s41598-020-67835-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu R, Greenstein JL, Granite SJ, Fackler JC, Bembea MM, Sarma SV, Winslow RL. Data-driven discovery of a novel sepsis pre-shock state predicts impending septic shock in the ICU. Sci Rep. 2019;9:6145. doi: 10.1038/s41598-019-42637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fagerström J, Bång M, Wilhelms D, Chew MS. LiSep LSTM: a machine learning algorithm for early detection of septic shock. Sci Rep. 2019;9:15132. doi: 10.1038/s41598-019-51219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J, Mark RG. An investigation of patterns in hemodynamic data indicative of impending hypotension in intensive care. Biomed Eng Online. 2010;9:62. doi: 10.1186/1475-925X-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee J, Mark R. A hypotensive episode predictor for intensive care based on heart rate and blood pressure time series. Comput Cardiol (2010). 2011:2010;81–84. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z, Chen L, Xu P, Wang Q, Zhang J, Chen K, Clements CM, Celi LA, Herasevich V, Hong Y. Effectiveness of automated alerting system compared to usual care for the management of sepsis. NPJ Digit Med. 2022;5:101. doi: 10.1038/s41746-022-00650-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syed M, Syed S, Sexton K, Syeda HB, Garza M, Zozus M, Syed F, Begum S, Syed AU, Sanford J, et al. Application of machine learning in intensive care unit (ICU) settings using MIMIC dataset: systematic review. Informatics (MDPI). 2021;8:16. doi: 10.3390/informatics8010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yijing L, Wenyu Y, Kang Y, Shengyu Z, Xianliang H, Xingliang J, Cheng W, Zehui S, Mengxing L. Prediction of cardiac arrest in critically ill patients based on bedside vital signs monitoring. Comput Methods Programs Biomed. 2022;214:106568. doi: 10.1016/j.cmpb.2021.106568 [DOI] [PubMed] [Google Scholar]
- 68.Matam BR, Duncan H, Lowe D. Machine learning based framework to predict cardiac arrests in a paediatric intensive care unit: prediction of cardiac arrests. J Clin Monit Comput. 2019;33:713–724. doi: 10.1007/s10877-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 69.Tonekaboni S, Mazwi M, Laussen P, Eytan D, Greer R, Goodfellow SD, Goodwin A, Brudno M, Goldenberg A. Prediction of cardiac arrest from physiological signals in the pediatric ICU. Proceedings of the 3rd Machine Learning for Healthcare Conference, PMLR. 2018;85:534–550. [Google Scholar]
- 70.Layeghian Javan S, Sepehri MM, Layeghian Javan M, Khatibi T. An intelligent warning model for early prediction of cardiac arrest in sepsis patients. Comput Methods Programs Biomed. 2019;178:47–58. doi: 10.1016/j.cmpb.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 71.Tsuji T, Nobukawa T, Mito A, Hirano H, Soh Z, Inokuchi R, Fujita E, Ogura Y, Kaneko S, Nakamura R, et al. Recurrent probabilistic neural network-based short-term prediction for acute hypotension and ventricular fibrillation. Sci Rep. 2020;10:11970. doi: 10.1038/s41598-020-68627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon JH, Mu L, Chen L, Dubrawski A, Hravnak M, Pinsky MR, Clermont G. Predicting tachycardia as a surrogate for instability in the intensive care unit. J Clin Monit Comput. 2019;33:973–985. doi: 10.1007/s10877-019-00277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karri R, Kawai A, Thong YJ, Ramson DM, Perry LA, Segal R, Smith JA, Penny-Dimri JC. Machine learning outperforms existing clinical scoring tools in the prediction of postoperative atrial fibrillation during intensive care unit admission after cardiac surgery. Heart Lung Circ. 2021;30:1929–1937. doi: 10.1016/j.hlc.2021.05.101 [DOI] [PubMed] [Google Scholar]
- 74.Falsetti L, Rucco M, Proietti M, Viticchi G, Zaccone V, Scarponi M, Giovenali L, Moroncini G, Nitti C, Salvi A. Risk prediction of clinical adverse outcomes with machine learning in a cohort of critically ill patients with atrial fibrillation. Sci Rep. 2021;11:18925. doi: 10.1038/s41598-021-97218-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee CK, Hofer I, Gabel E, Baldi P, Cannesson M. Development and validation of a deep neural network model for prediction of postoperative in-hospital mortality. Anesthesiology. 2018;129:649–662. doi: 10.1097/ALN.0000000000002186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.ALIVECOR. FDA clears personal ECG device for measurement of QTc interval, a critical marker for patient safety. July 8, 2021. https://www.Alivecor.Com/press/press_release/fda-clears-personal-ecg-device-for-measurement-of-qtc-interval/
- 77.Rabinstein AA, Yost MD, Faust L, Kashou AH, Latif OS, Graff-Radford J, Attia IZ, Yao X, Noseworthy PA, Friedman PA. Artificial intelligence-enabled ECG to identify silent atrial fibrillation in embolic stroke of unknown source. J Stroke Cerebrovasc Dis. 2021;30:105998. doi: 10.1016/j.jstrokecerebrovasdis.2021.105998 [DOI] [PubMed] [Google Scholar]
- 78.Sharifi M, Buzatu D, Harris S, Wilkes J. Development of models for predicting Torsade de Pointes cardiac arrhythmias using perceptron neural networks. BMC Bioinformatics. 2017;18:497. doi: 10.1186/s12859-017-1895-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang PC, DeMarco KR, Aghasafari P, Jeng MT, Dawson JRD, Bekker S, Noskov SY, Yarov-Yarovoy V, Vorobyov I, Clancy CE. A computational pipeline to predict cardiotoxicity: from the atom to the rhythm. Circ Res. 2020;126:947–964. doi: 10.1161/CIRCRESAHA.119.316404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Costabal FS, Matsuno K, Yao J, Perdikaris P, Kuhl E. Machine learning in drug development: characterizing the effect of 30 drugs on the QT interval using gaussian process regression, sensitivity analysis, and uncertainty quantification. Comput Methods Appl Mech Eng. 2019;348:313–333. doi: 10.1016/j.cma.2019.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maille B, Wilkin M, Million M, Resseguier N, Franceschi F, Koutbi-Franceschi L, Hourdain J, Martinez E, Zabern M, Gardella C, et al. Smartwatch electrocardiogram and artificial intelligence for assessing cardiac-rhythm safety of drug therapy in the COVID-19 pandemic. The QT-logs study. Int J Cardiol. 2021;331:333–339. doi: 10.1016/j.ijcard.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahayni AA, Attia ZI, Medina-Inojosa JR, Elsisy MFA, Noseworthy PA, Lopez-Jimenez F, Kapa S, Asirvatham SJ, Friedman PA, Crestenallo JA, et al. Electrocardiography-based artificial intelligence algorithm aids in prediction of long-term mortality after cardiac surgery. Mayo Clin Proc. 2021;96:3062–3070. doi: 10.1016/j.mayocp.2021.06.024 [DOI] [PubMed] [Google Scholar]
- 83.Abel JH, Badgeley MA, Meschede-Krasa B, Schamberg G, Garwood IC, Lecamwasam K, Chakravarty S, Zhou DW, Keating M, Purdon PL, et al. Machine learning of EEG spectra classifies unconsciousness during GABAergic anesthesia. PLoS One. 2021;16:e0246165. doi: 10.1371/journal.pone.0246165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lapp L, Young D, Kavanagh K, Bouamrane M-M, Schraag S. Using machine learning for predicting severe postoperative complications after cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32:S84–S85. doi: 10.1053/j.jvca.2018.08.156 [DOI] [Google Scholar]
- 85.Miyaguchi N, Takeuchi K, Kashima H, Morita M, Morimatsu H. Predicting anesthetic infusion events using machine learning. Sci Rep. 2021;11:23648. doi: 10.1038/s41598-021-03112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schamberg G, Badgeley M, Meschede-Krasa B, Kwon O, Brown EN. Continuous action deep reinforcement learning for propofol dosing during general anesthesia. Artif Intell Med. 2022;123:102227. doi: 10.1016/j.artmed.2021.102227 [DOI] [PubMed] [Google Scholar]
- 87.Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383:1951–1960. doi: 10.1056/NEJMsa2001090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henry KE, Adams R, Parent C, Soleimani H, Sridharan A, Johnson L, Hager DN, Cosgrove SE, Markowski A, Klein EY, et al. Factors driving provider adoption of the TREWS machine learning-based early warning system and its effects on sepsis treatment timing. Nat Med. 2022;28:1447–1454. doi: 10.1038/s41591-022-01895-z [DOI] [PubMed] [Google Scholar]
- 89.Warttig S, Alderson P, Evans DJ, Lewis SR, Kourbeti IS, Smith AF. Automated monitoring compared to standard care for the early detection of sepsis in critically ill patients. Cochrane Database Syst Rev. 2018;6:CD012404. doi: 10.1002/14651858.CD012404.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimabukuro DW, Barton CW, Feldman MD, Mataraso SJ, Das R. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4:e000234. doi: 10.1136/bmjresp-2017-000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo H, Li J, Liu H, He J. Learning dynamic treatment strategies for coronary heart diseases by artificial intelligence: real-world data-driven study. BMC Med Inform Decis Mak. 2022;22:39. doi: 10.1186/s12911-022-01774-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh K, Valley TS, Tang S, Li BY, Kamran F, Sjoding MW, Wiens J, Otles E, Donnelly JP, Wei MY, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized patients with COVID-19. Ann Am Thorac Soc. 2021;18:1129–1137. doi: 10.1513/AnnalsATS.202006-698OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong A, Otles E, Donnelly JP, Krumm A, McCullough J, DeTroyer-Cooley O, Pestrue J, Phillips M, Konye J, Penoza C, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Internal Med. 2021;181:1065–1070. doi: 10.1001/jamainternmed.2021.2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Helman S, Terry MA, Pellathy T, Williams A, Dubrawski A, Clermont G, Pinsky MR, Al-Zaiti S, Hravnak M. Engaging clinicians early during the development of a graphical user display of an intelligent alerting system at the bedside. Int J Med Inform. 2022;159:104643. doi: 10.1016/j.ijmedinf.2021.104643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krittanawong C, Rogers AJ, Johnson KW, Wang Z, Turakhia MP, Halperin JL, Narayan SM. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol. 2021;18:75–91. doi: 10.1038/s41569-020-00445-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frizzell JD, Liang L, Schulte PJ, Yancy CW, Heidenreich PA, Hernandez AF, Bhatt DL, Fonarow GC, Laskey WK. Prediction of 30-day all-cause readmissions in patients hospitalized for heart failure: comparison of machine learning and other statistical approaches. JAMA Cardiol. 2017;2:204–209. doi: 10.1001/jamacardio.2016.3956 [DOI] [PubMed] [Google Scholar]
- 97.Guidi G, Pollonini L, Dacso CC, Iadanza E. A multi-layer monitoring system for clinical management of congestive heart failure. BMC Med Inform Decis Mak. 2015;15:S5. doi: 10.1186/1472-6947-15-S3-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bonsignore L, Bloom N, Steinhauser K, Nichols R, Allen T, Twaddle M, Bull J. Evaluating the feasibility and acceptability of a telehealth program in a rural palliative care population: TapCloud for palliative care. J Pain Symptom Manage. 2018;56:7–14. doi: 10.1016/j.jpainsymman.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 99.Krittanawong C, Rogers AJ, Aydar M, Choi E, Johnson KW, Wang Z, Narayan SM. Integrating blockchain technology with artificial intelligence for cardiovascular medicine. Nat Rev Cardiol. 2020;17:1–3. doi: 10.1038/s41569-019-0294-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, Martin SS, Muse ED, Turakhia MP, Tarakji KG, et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat Rev Cardiol. 2021;18:581–599. doi: 10.1038/s41569-021-00522-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bent B, Goldstein BA, Kibbe WA, Dunn JP. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit Med. 2020;3:18. doi: 10.1038/s41746-020-0226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas AA. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1582–1592. doi: 10.1016/j.jacc.2020.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Q, Rajagopalan C, Clifford GD. Ventricular fibrillation and tachycardia classification using a machine learning approach. IEEE Trans Biomed Eng. 2014;61:1607–1613. doi: 10.1109/TBME.2013.2275000 [DOI] [PubMed] [Google Scholar]
- 104.Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ, Vittinghoff E, Lee ES, Fan SM, Gladstone RA, et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018;3:409–416. doi: 10.1001/jamacardio.2018.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, et al. ; Apple Heart Study Investigators. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shashikumar SP, Shah AJ, Li Q, Clifford GD, Nemati S. A deep learning approach to monitoring and detecting atrial fibrillation using wearable technology. 2017. IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Orlando, FL. 2017:141–144. doi: 10.1109/BHI.2017.7897225 [DOI] [Google Scholar]
- 107.Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, Ng AY. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–69. doi: 10.1038/s41591-018-0268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lahdenoja O, Hurnanen T, Iftikhar Z, Nieminen S, Knuutila T, Saraste A, Kiviniemi T, Vasankari T, Airaksinen J, Pankaala M, et al. Atrial fibrillation detection via accelerometer and gyroscope of a smartphone. IEEE J Biomed Health Inform. 2018;22:108–118. doi: 10.1109/JBHI.2017.2688473 [DOI] [PubMed] [Google Scholar]
- 109.Dorr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S, Raichle CJ, Rhinisperger M, Stockli R, Eckstein J. The WATCH AF trial: smartWATCHes for detection of atrial fibrillation. JACC. Clin Electrophysiol. 2019;5:199–208. doi: 10.1016/j.jacep.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 110.Stehlik J, Schmalfuss C, Bozkurt B, Nativi-Nicolau J, Wohlfahrt P, Wegerich S, Rose K, Ray R, Schofield R, Deswal A, et al. Continuous wearable monitoring analytics predict heart failure hospitalization: the LINK-HF multicenter study. Circ Heart Fail. 2020;13:e006513. doi: 10.1161/CIRCHEARTFAILURE.119.006513 [DOI] [PubMed] [Google Scholar]
- 111.Shah AJ, Isakadze N, Levantsevych O, Vest A, Clifford G, Nemati S. Detecting heart failure using wearables: a pilot study. Physiol Meas. 2020;41:044001. doi: 10.1088/1361-6579/ab7f93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaji DA, Zech JR, Kim JS, Cho SK, Dangayach NS, Costa AB, Oermann EK. An attention based deep learning model of clinical events in the intensive care unit. PLoS One. 2019;14:e0211057. doi: 10.1371/journal.pone.0211057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Varma N, Cygankiewicz I, Turakhia M, Heidbuchel H, Hu Y, Chen LY, Couderc JP, Cronin EM, Estep JD, Grieten L et al. 2021 ISHNE/HRS/EHRA/APHRS collaborative statement on mhealth in arrhythmia management: Digital medical tools for heart rhythm professionals: from the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society. Eur Heart J Digital Health. 2021;2:7–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Slotwiner DJ, Tarakji KG, Al-Khatib SM, Passman RS, Saxon LA, Peters NS, McCall D, Turakhia MP, Schaeffer J, Mendenhall GS, et al. Transparent sharing of digital health data: a call to action. Heart Rhythm. 2019;16:e95–e106. doi: 10.1016/j.hrthm.2019.04.042 [DOI] [PubMed] [Google Scholar]
- 115.Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel). 2010;10:7772–7788. doi: 10.3390/s100807772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frisch DR. Diagnosing atrial fibrillation by mobile technology: physician decision or device provision? Heart. 2020;106:629–630. doi: 10.1136/heartjnl-2019-316390 [DOI] [PubMed] [Google Scholar]
- 117.Wyatt KD, Poole LR, Mullan AF, Kopecky SL, Heaton HA. Clinical evaluation and diagnostic yield following evaluation of abnormal pulse detected using Apple Watch. J Am Med Inform Assoc. 2020;27:1359–1363. doi: 10.1093/jamia/ocaa137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tran V-T, Riveros C, Ravaud P. Patients’ views of wearable devices and AI in healthcare: findings from the ComPaRe e-cohort. NPJ Digit Med. 2019;2:53. doi: 10.1038/s41746-019-0132-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaiser J 200,000 whole genomes made available for biomedical studies. Science. 2021;374:1036. doi: 10.1126/science.acx9689 [DOI] [PubMed] [Google Scholar]
- 120.Uffelmann E, Posthuma D. Emerging methods and resources for biological interrogation of neuropsychiatric polygenic signal. Biol Psychiatry. 2021;89:41–53. doi: 10.1016/j.biopsych.2020.05.022 [DOI] [PubMed] [Google Scholar]
- 121.Morales J, Welter D, Bowler EH, Cerezo M, Harris LW, McMahon AC, Hall P, Junkins HA, Milano A, Hastings E, et al. A standardized framework for representation of ancestry data in genomics studies, with application to the NHGRI-EBI GWAS catalog. Genome Biol. 2018;19:21. doi: 10.1186/s13059-018-1396-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verlouw JAM, Clemens E, de Vries JH, Zolk O, Verkerk A, Am Zehnhoff-Dinnesen A, Medina-Gomez C, Lanvers-Kaminsky C, Rivadeneira F, Langer T, et al. A comparison of genotyping arrays. Eur J Human Genet. 2021;29:1611–1624. doi: 10.1038/s41431-021-00917-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, et al. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang K, Zhang H, Kugathasan S, Annese V, Bradfield JP, Russell RK, Sleiman PM, Imielinski M, Glessner J, Hou C, et al. Diverse genome-wide association studies associate the IL12/IL23 pathway with Crohn disease. Am J Hum Genet. 2009;84:399–405. doi: 10.1016/j.ajhg.2009.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jo T, Nho K, Bice P, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative. Deep learning-based identification of genetic variants: application to Alzheimer’s disease classification. Brief Bioinform. 2022;23:bbac022. doi: 10.1093/bib/bbac022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elgart M, Lyons G, Romero-Brufau S, Kurniansyah N, Brody JA, Guo X, Lin HJ, Raffield L, Gao Y, Chen H, et al. Non-linear machine learning models incorporating SNPs and PRS improve polygenic prediction in diverse human populations. Commun Biol. 2022;5:856. doi: 10.1038/s42003-022-03812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gu JQ, Zhao H, Guo XY, Sun HY, Xu JY, Wei YL. A high-performance SNP panel developed by machine-learning approaches for characterizing genetic differences of Southern and Northern Han Chinese, Korean, and Japanese individuals. Electrophoresis. 2022;43:1183–1192. doi: 10.1002/elps.202100184 [DOI] [PubMed] [Google Scholar]
- 129.Gurovich Y, Hanani Y, Bar O, Nadav G, Fleischer N, Gelbman D, Basel-Salmon L, Krawitz PM, Kamphausen SB, Zenker M, et al. Identifying facial phenotypes of genetic disorders using deep learning. Nat Med. 2019;25:60–64. doi: 10.1038/s41591-018-0279-0 [DOI] [PubMed] [Google Scholar]
- 130.Bos JM, Attia ZI, Albert DE, Noseworthy PA, Friedman PA, Ackerman MJ. Use of artificial intelligence and deep neural networks in evaluation of patients with electrocardiographically concealed long QT syndrome from the surface 12-lead electrocardiogram. JAMA Cardiol. 2021;6:532–538. doi: 10.1001/jamacardio.2020.7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sundaram L, Gao H, Padigepati SR, McRae JF, Li Y, Kosmicki JA, Fritzilas N, Hakenberg J, Dutta A, Shon J, et al. Predicting the clinical impact of human mutation with deep neural networks. Nat Genet. 2018;50:1161–1170. doi: 10.1038/s41588-018-0167-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.De La Vega FM, Chowdhury S, Moore B, Frise E, McCarthy J, Hernandez EJ, Wong T, James K, Guidugli L, Agrawal PB, et al. Artificial intelligence enables comprehensive genome interpretation and nomination of candidate diagnoses for rare genetic diseases. Genome Med. 2021;13:153. doi: 10.1186/s13073-021-00965-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davis SE, Lasko TA, Chen G, Matheny ME. Calibration drift among regression and machine learning models for hospital mortality. AMIA Annu Symp Proc. 2018;2017:625–634. [PMC free article] [PubMed] [Google Scholar]
- 135.Pirracchio R, Petersen ML, Carone M, Rigon MR, Chevret S, van der Laan MJ. Mortality prediction in intensive care units with the super ICU learner algorithm (SICULA): a population-based study. Lancet Respir Med. 2015;3:42–52. doi: 10.1016/S2213-2600(14)70239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holmgren G, Andersson P, Jakobsson A, Frigyesi A. Artificial neural networks improve and simplify intensive care mortality prognostication: a national cohort study of 217,289 first-time intensive care unit admissions. J Intensive Care 2019;7:44. doi: 10.1186/s40560-019-0393-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu TT, Zheng RF, Lin ZZ, Gong HR, Li H. A machine learning model to predict critical care outcomes in patient with chest pain visiting the emergency department. BMC Emerg Med. 2021;21:112. doi: 10.1186/s12873-021-00501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao J, Feng Q, Wu P, Lupu RA, Wilke RA, Wells QS, Denny JC, Wei WQ. Learning from longitudinal data in electronic health record and genetic data to improve cardiovascular event prediction. Sci Rep. 2019;9:717. doi: 10.1038/s41598-018-36745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ye C, Fu T, Hao S, Zhang Y, Wang O, Jin B, Xia M, Liu M, Zhou X, Wu Q, et al. Prediction of incident hypertension within the next year: prospective study using statewide electronic health records and machine learning. J Med Internet Res. 2018;20:e22. doi: 10.2196/jmir.9268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guan W, Ko D, Khurshid S, Trisini Lipsanopoulos AT, Ashburner JM, Harrington LX, Rost NS, Atlas SJ, Singer DE, McManus DD, et al. Automated electronic phenotyping of cardioembolic stroke. Stroke. 2021;52:181–189. doi: 10.1161/STROKEAHA.120.030663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kolk MZH, Deb B, Ruiperez-Campillo S, Bhatia NK, Clopton P, Wilde AAM, Narayan SM, Knops RE, Tjong FVY. Machine learning of electrophysiological signals for the prediction of ventricular arrhythmias: systematic review and examination of heterogeneity between studies. EBioMedicine. 2023;89:104462. doi: 10.1016/j.ebiom.2023.104462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roney CH, Sim I, Yu J, Beach M, Mehta A, Alonso Solis-Lemus J, Kotadia I, Whitaker J, Corrado C, Razeghi O, et al. Predicting atrial fibrillation recurrence by combining population data and virtual cohorts of patient-specific left atrial models. Circ Arrhythm Electrophysiol. 2022;15: e010253. doi: 10.1161/CIRCEP.121.010253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang S, Razeghi O, Kapoor R, Alhusseini MI, Fazal M, Rogers AJ, Rodrigo Bort M, Clopton P, Wang PJ, Rubin DL, et al. Machine learningenabled multimodal fusion of intra-atrial and body surface signals in prediction of atrial fibrillation ablation outcomes. Circ Arrhythm Electrophysiol. 2022;15:e010850. doi: 10.1161/CIRCEP.122.010850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bower JK, Patel S, Rudy JE, Felix AS. Addressing bias in electronic health record-based surveillance of cardiovascular disease risk: finding the signal through the noise. Curr Epidemiol Rep. 2017;4:346–352. doi: 10.1007/s40471-017-0130-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Subbaswamy A, Schulam P, Saria S. Preventing failures due to dataset shift: predictive models that transport [internet]. arXiv. Accessed December 9, 2023. https://arxiv.org/abs/1812.04597 [Google Scholar]
- 146.Andaur Navarro CL, Damen JAA, Takada T, Nijman SWJ, Dhiman P, Ma J, Collins GS, Bajpai R, Riley RD, Moons KGM, et al. Risk of bias in studies on prediction models developed using supervised machine learning techniques: systematic review. BMJ. 2021;375:n2281. doi: 10.1136/bmj.n2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Futoma J, Simons M, Panch T, Doshi-Velez F, Celi LA. The myth of generalisability in clinical research and machine learning in health care. Lancet Digit Health. 2020;2:e489–e492. doi: 10.1016/S2589-7500(20)30186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Etminan M, Collins GS, Mansournia MA. Using causal diagrams to improve the design and interpretation of medical research. Chest. 2020;158:S21–S28. doi: 10.1016/j.chest.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 149.Sallam M Chatgpt utility in healthcare education, research, and practice: systematic review on the promising perspectives and valid concerns. Healthcare (Basel). 2023;11:887. doi: 10.3390/healthcare11060887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lauritsen SM, Thiesson B, Jorgensen MJ, Riis AH, Espelund US, Weile JB, Lange J. The framing of machine learning risk prediction models illustrated by evaluation of sepsis in general wards. NPJ Digit Med. 2021;4:158. doi: 10.1038/s41746-021-00529-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee H, Shin SY, Seo M, Nam GB, Joo S. Prediction of ventricular tachycardia one hour before occurrence using artificial neural networks. Sci Rep. 2016;6:32390. doi: 10.1038/srep32390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.National Institutes of Health. Bridge to artificial intelligence (Bridge2AI). Accessed December 10, 2023. https://commonfund.nih.gov/bridge2ai [Google Scholar]
- 153.Castaneda C, Nalley K, Mannion C, Bhattacharyya P, Blake P, Pecora A, Goy A, Suh KS. Clinical decision support systems for improving diagnostic accuracy and achieving precision medicine. J Clin Bioinforma. 2015;5:4. doi: 10.1186/s13336-015-0019-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Asselbergs FW, Fraser AG. Artificial intelligence in cardiology: the debate continues. Eur Heart J Digit Health. 2021;2:721–726. doi: 10.1093/ehjdh/ztab090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McCoy LG, Nagaraj S, Morgado F, Harish V, Das S, Celi LA. What do medical students actually need to know about artificial intelligence? NPJ Digit Med. 2020;3:86. doi: 10.1038/s41746-020-0294-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van Smeden M, Heinze G, Van Calster B, Asselbergs FW, Vardas PE, Bruining N, de Jaegere P, Moore JH, Denaxas S, Boulesteix AL, et al. Critical appraisal of artificial intelligence-based prediction models for cardiovascular disease. Eur Heart J. 2022;43:2921–2930. doi: 10.1093/eurheartj/ehac238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spencer R, Thabtah F, Abdelhamid N, Thompson M. Exploring feature selection and classification methods for predicting heart disease. Digit Health 2020;6:2055207620914777. doi: 10.1177/2055207620914777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rios R, Miller RJH, Hu LH, Otaki Y, Singh A, Diniz M, Sharir T, Einstein AJ, Fish MB, Ruddy TD, et al. Determining a minimum set of variables for machine learning cardiovascular event prediction: results from REFINE SPECT registry. Cardiovasc Res. 2022;118:2152–2164. doi: 10.1093/cvr/cvab236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kotecha D, Asselbergs FW, Achenbach S, Anker SD, Atar D, Baigent C, Banerjee A, Beger B, Brobert G, Casadei B, et al. CODE-EHR best-practice framework for the use of structured electronic health-care records in clinical research. Lancet Digit Health. 2022;4:e757–e764. doi: 10.1016/S2589-7500(22)00151-0 [DOI] [PubMed] [Google Scholar]
- 160.Foryciarz A, Pfohl SR, Patel B, Shah N. Evaluating algorithmic fairness in the presence of clinical guidelines: the case of atherosclerotic cardiovascular disease risk estimation. BMJ Health Care Inform. 2022;29:e100460. doi: 10.1136/bmjhci-2021-100460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Trinidad MG, Platt J, Kardia SLR. The public’s comfort with sharing health data with third-party commercial companies. Humanit Soc Sci Commun. 2020;7:149. doi: 10.1057/s41599-020-00641-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wiens J, Price WN, Sjoding MW. Diagnosing bias in data-driven algorithms for healthcare. Nat Med. 2020;26:25–26. doi: 10.1038/s41591-019-0726-6 [DOI] [PubMed] [Google Scholar]
- 163.Jagsi R, Griffith KA, Sabolch A, Jones R, Spence R, De Vries R, Grande D, Bradbury AR. Perspectives of patients with cancer on the ethics of rapid-learning health systems. J Clin Oncol. 2017;35:2315–2323. doi: 10.1200/JCO.2016.72.0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spector-Bagdady K, Trinidad G, Kardia S, Krenz CD, Nong P, Raj M, Platt JE. Reported interest in notification regarding use of health information and biospecimens. JAMA. 2022;328:474–476. doi: 10.1001/jama.2022.9740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lenert MC, Matheny ME, Walsh CG. Prognostic models will be victims of their own success, unless… J Am Med Inform Assoc. 2019;26:1645–1650. doi: 10.1093/jamia/ocz145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, et al. ; and for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998 [DOI] [PubMed] [Google Scholar]
- 167.Schulam P, Saria S. Reliable decision support using counterfactual models. Adv Neural Inf Processing Syst. 2017;30:1698–1709. [Google Scholar]
- 168.Price WN 2nd, Gerke S, Cohen IG. Potential liability for physicians using artificial intelligence. JAMA. 2019;322:1765–1766. doi: 10.1001/jama.2019.15064 [DOI] [PubMed] [Google Scholar]
- 169.Streed CG Jr, Beach LB, Caceres BA, Dowshen NL, Moreau KL, Mukherjee M, Poteat T, Radix A, Reisner SL, Singh V; on behalf of the American Heart Association Council on Peripheral Vascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Hypertension; and Stroke Council. Assessing and addressing cardiovascular health in people who are transgender and gender diverse: a scientific statement from the American Heart Association. Circulation. 2021;144:e136–e148. doi: 10.1161/CIR.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Walton-Moss B, Samuel L, Nguyen TH, Commodore-Mensah Y, Hayat MJ, Szanton SL. Community-based cardiovascular health interventions in vulnerable populations: a systematic review. J Cardiovasc Nurs. 2014;29:293–307. doi: 10.1097/JCN.0b013e31828e2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. Potential biases in machine learning algorithms using electronic health record data. JAMA Internal Med. 2018;178:1544–1547. doi: 10.1001/jamainternmed.2018.3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kearns M, Neel S, Roth A, Wu ZS. Preventing fairness gerrymandering: auditing and learning for subgroup fairness. Proceedings of the 35th International Conference on Machine Learning. PMRL. 2018;80:2564–2572 [Google Scholar]
- 173.Fazelpour S, Lipton ZC. Algorithmic fairness from a non-ideal perspective. Proceedings of the AAAI/ACM Conference on AI, Ethics, and Society. 2020:57–63. doi: 10.1145/3375627.3375828 [DOI] [Google Scholar]
- 174.Bellamy RKE, Dey K, Hind M, Hoffman SC, Houde S, Kannan K, Lohia P, Martino J, Mehta S, Mojsilović A, et al. AI fairness 360: an extensible toolkit for detecting and mitigating algorithmic bias. IBM J Res Dev. 2019;63:4:1–4:15. doi: 10.1147/jrd.2019.2942287 [DOI] [Google Scholar]
- 175.American Heart Association Professional Heart Daily. Policies governing all research awards. American Heart Association Intellectual Property Policy For Research Funding. Effective Date: October 28, 2021. Accessed December 9, 2023. https://professional.heart.org/en/research-programs/awardee-policies/policies-governing-all-research-awards [Google Scholar]
- 176.Mao Q, Jay M, Hoffman JL, Calvert J, Barton C, Shimabukuro D, Shieh L, Chettipally U, Fletcher G, Kerem Y, et al. Multicentre validation of a sepsis prediction algorithm using only vital sign data in the emergency department, general ward and ICU. BMJ Open. 2018;8:e017833. doi: 10.1136/bmjopen-2017-017833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leach B, Parkinson S, Lichten C, Marjanovic S. Emerging developments in citizen science: reflecting on areas of innovation. RAND Corporation; 2020. https://www.rand.org/pubs/research_reports/RR4401.html
- 178.Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. doi: 10.1038/s41746-020-00324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Anderson J, Rainie L. Net threats. 2014. Accessed August 2, 2023. https://www.pewresearch.org/internet/2014/07/03/net-threats/
- 180.Oakden-Rayner L, Dunnmon J, Carneiro G, Ré C. Hidden stratification causes clinically meaningful failures in machine learning for medical imaging. Proc ACM Conf Health, Inference, Learn. 2020;2020:151–159. doi: 10.1145/3368555.3384468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ash JS, Sittig DF, Campbell EM, Guappone KP, Dykstra RH. Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007;2007:26–30. [PMC free article] [PubMed] [Google Scholar]
- 182.Kim J, Kim H, Bell E, Bath T, Paul P, Pham A, Jiang X, Zheng K, Ohno-Machado L. Patient perspectives about decisions to share medical data and biospecimens for research. JAMA Netw Open. 2019;2:e199550. doi: 10.1001/jamanetworkopen.2019.9550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tapuria A, Porat T, Kalra D, Dsouza G, Xiaohui S, Curcin V. Impact of patient access to their electronic health record: systematic review. Inform Health Soc Care. 2021;46:192–204. doi: 10.1080/17538157.2021.1879810 [DOI] [PubMed] [Google Scholar]
- 184.Seh AH, Zarour M, Alenezi M, Sarkar AK, Agrawal A, Kumar R, Khan RA. Healthcare data breaches: insights and implications. Healthcare (Basel). 2020;8:133. doi: 10.3390/healthcare8020133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Offner KL, Sitnikova E, Joiner K, MacIntyre CR. Towards understanding cybersecurity capability in Australian healthcare organisations: a systematic review of recent trends, threats and mitigation. Intelligence Natl Secur. 2020;35:556–585. doi: 10.1080/02684527.2020.1752459 [DOI] [Google Scholar]
- 186.Price WN 2nd, Gerke S, Cohen IG. How much can potential jurors tell us about liability for medical artificial intelligence? J Nucl Med. 2021;62:15–16. doi: 10.2967/jnumed.120.257196 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


