Abstract
Deciphering non-canonical WNT signaling is proving to be both fascinating and challenging. Discovered almost 30 years ago, non-canonical WNT ligands signal independently of the transcriptional co-activator β-catenin to regulate a wide range of morphogenetic processes during development. The molecular and cellular mechanisms that underlie non-canonical WNT function however, remain nebulous. Recent results from various model systems have converged to define a core non-canonical WNT pathway consisting of the prototypic non-canonical WNT ligand, WNT5A, the receptor tyrosine kinase ROR, the seven transmembrane receptor Frizzled and the cytoplasmic scaffold protein Dishevelled. Importantly, mutations in each of these signaling components cause Robinow syndrome, a congenital disorder characterized by profound tissue morphogenetic abnormalities. Moreover, dysregulation of the pathway has also been linked to cancer metastasis. As new knowledge concerning the WNT5A-ROR pathway continues to grow, modeling these mutations will likely provide crucial insights into both the physiological regulation of the pathway and the etiology of WNT5A-ROR-driven diseases.
Keywords: WNT5A, ROR, Frizzled, FZD, Dishevelled, DVL, Non-canonical WNT signaling, Robinow syndrome, Omodysplasia, Tissue morphogenesis, Cancer metastasis
1. A brief history of canonical and non-canonical WNT pathways
WNTs make up a highly conserved family of glycoproteins that mediatecell-cell communication in diverse contexts within multicellular organisms. First discovered as drivers of mammary tumors in mice (Nusse and Varmus, 1982) and then as segment polarity genes in Drosophila (Baker, 1987; Nusslein-Volhard and Wieschaus, 1980), WNT ligands have garnered several decades of intense study from researchers worldwide. Today, it is recognized that WNT ligands play a pivotal role in most aspects of metazoan biology, from the earliest stages of embryonic development to the most severe states of various diseases and many other physiological and pathological activities in-between.
The WNTs identified in these early studies are known as canonical WNTs. Ectopic expression of canonical WNT ligands in Xenopus embryos caused alterations in tissue patterning (McMahon and Moon, 1989), most notably an axis duplication phenotype resulting from the dorsalizing effect of WNT misexpression (Figure 1A). In cultured cells, expression of canonical WNTs induced cell transformation (Nusse and Varmus, 1982; Ramakrishna and Brown, 1993; Rijsewijk et al., 1987; Wong et al., 1994). Together, these robust and tractable assays provided the first crucial tools for functional characterization of the pathway. Progress in the field was further propelled by epistasis experiments in Drosophila (Noordermeer et al., 1994; Peifer et al., 1994; Siegfried et al., 1992) which helped provide a molecular framework for the canonical WNT signaling cascade.
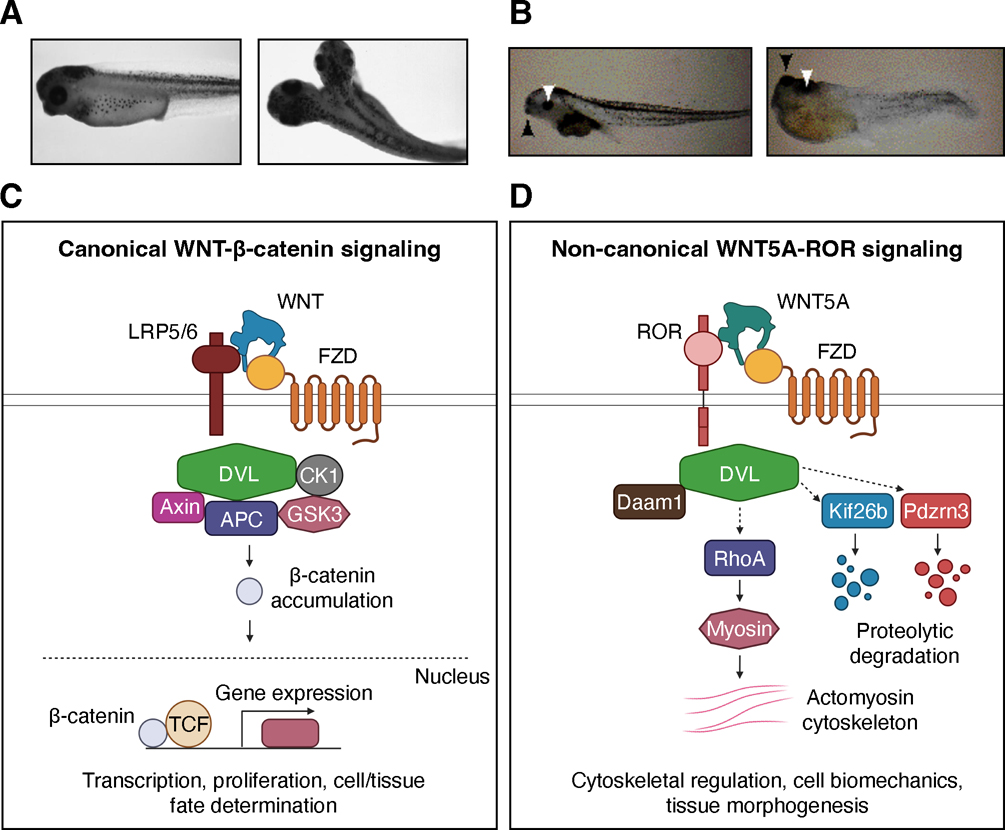
Figure 1. Overview of canonical WNT-β-catenin and non-canonical WNT5A-ROR signaling.
WNT signaling was initially classified into two major branches based on the distinct phenotypes induced by overexpression of different WNT ligands in Xenopus laevis. (A) The axis duplication phenotype induced by overexpression of canonical WNTs. Image from (Cui et al., 1995) in which Xenopus WNT8B was overexpressed. (B) The gross tissue truncation/malformation phenotype induced by overexpression of noncanonical WNTs. Image from (Moon et al., 1993) in which Xenopus WNT5A was overexpressed. (C and D) Molecular schematics of the canonical WNT-β-catenin (C) and non-canonical WNT5A-ROR (D) signaling cascades. See main text for details. Created using www.BioRender.com
A defining signature of the canonical WNT pathway is its use of the transcriptional co-activator β-catenin, known as Armadillo in Drosophila, to control target gene transcription. In the absence of WNT signaling, β-catenin undergoes constitutive proteolytic turnover mediated by the ubiquitin-proteasome pathway. Upon engagement of the canonical WNT ligands (e.g. WNT1, WNT3A and WNT8B) with the cell surface receptors Frizzled (FZD) and low-density lipoprotein receptor-related protein 5 and 6 (LRP5/6), β-catenin becomes stabilized and translocates into the nucleus to activate target gene transcription (Cadigan and Waterman, 2012; Clevers and Nusse, 2012) (Figure 1C). Through this core mechanism, canonical WNT signaling regulates many important developmental decisions, including cell proliferation and tissue fate determination, as well as tissue homeostasis, such as stem cell maintenance and tissue regeneration, in adult organisms. As a result, defects in canonical WNT signaling can give rise to devastating diseases, including congenital birth defects and cancer (Clevers and Nusse, 2012; Nusse and Varmus, 2012).
The vertebrate genome encodes 19 WNTs. Intriguingly, some early studies observed that a subset of WNT ligands had no effect on tissue patterning or cell transformation, but rather appeared to influence cell rearrangements and tissue shaping (Du et al., 1995; Moon et al., 1993). WNT5A was the first WNT implicated in such changes. In their seminal study, Moon and colleagues found that ectopic expression of WNT5A in Xenopus embryos did not produce the canonical WNT phenotype of axis duplication, but instead significantly altered Xenopus embryo morphology, producing tadpoles with a truncated head and tail (Figure 1B) (Moon et al., 1993). It was subsequently found that WNT4 and WNT11 also exhibit similar morphogenetic properties when injected into Xenoups embryos (Du et al., 1995). These observations indicated that different WNT ligands possessed unique functions and implied the existence of multiple WNT signaling pathways that drive different phenotypic outcomes (Figure 1C, D). This review will focus mainly on WNT5A, as it is thus far the most extenstively characterized among this latter group.
The morphogenetic function of WNT5A is highly conserved throughout evolution (Loh et al., 2016). Other model organisms displayed similar gross morphological defects in response to either genetic ablation or overexpression of WNT5A. For instance, Wnt5a knockout mice exhibit truncated body axes, limbs, and tails, as well as widened, flattened faces and underdeveloped external genitalia (Yamaguchi et al., 1999) (Figure 2A). In addition, overexpressing WNT5A in mice produced similar types of morphogenetic phenotypes (van Amerongen et al., 2012), indicating that the dose and spatiotemporal expression of WNT5A must be tightly controlled within a narrow physiological range during development. Importantly, the morphogenetic signaling activity of WNT5A did not appear to involve β-catenin-mediated functions (Shimizu et al., 1997) (Figure 1D). Thus began the distinction between canonical WNT signaling, dependent on β-catenin, and other β-catenin-independent, or non-canonical, WNT pathways.
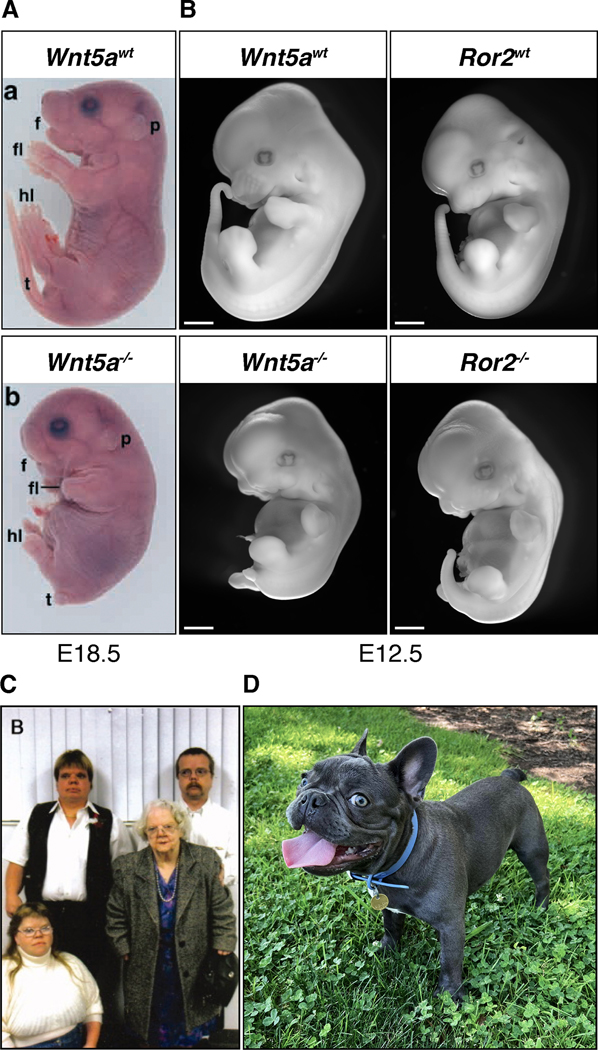
Figure 2. Robinow syndrome and Robinow-like disorders arise from mutations in components of WNT5A-ROR signaling.
(A) Wnt5a knockout mouse embryos (E18.5, images from (Yamaguchi et al., 1999)) exhibit body axis, limb, and tail truncations in addition to underdeveloped external genitalia and craniofacial malformations, compared to their wild-type littermates. These physical features are phenocopied in Ror2 knockout mouse embryos (B; E12.5 embryos derived from previously described Wnt5a (Yamaguchi et al., 1999) and Ror2 (Ho et al., 2012) mutant lines, photographed by S. Srinivasan. Scale bar = 1mm), as well as in RS patients (C; image from (Person et al., 2010), photographed by J. Lohr) and bulldogs (C; French bulldog).
Since these early discoveries, the number of biological processes regulated by non-canonical WNT pathways has continued to grow. Collectively, non-canonical WNT pathways appear to culminate in the regulation of cytoskeletal rearrangements (Veeman et al., 2003), thus leading to the cell and tissue morphological changes described above. The largely non-transcriptional responses elicited by non-canonical WNTs, however, also presented challenges for deciphering the underlying mechanisms. For example, the development of a versatile β-catenin-dependent, luciferase-based transcriptional reporter, commonly known as TOPflash (Korinek et al., 1997; Molenaar et al., 1996), greatly accelerated molecular interrogation of the canonical WNT pathway. In contrast, the lack of an equivalent reporter assay for the non-canonical WNT pathway forced investigators to resort to less quantitative readouts such as overexpression phenotypes in embryos. Moreover, as mentioned earlier, genetic studies in Drosophila were instrumental in deciphering the canonical WNT pathway (as well as other major developmental signaling pathways, such as Hedgehog, Delta-Notch and Hippo); however, they did not identify a pathway directly equivalent to the vertebrate non-canonical WNT pathway. Together, these limitations severely hindered progress in the non-canonical WNT field.
2. Emergence of WNT5A-ROR signaling as a major non-canonical WNT pathway
It took another decade for the ROR family of receptor tyrosine kinases to enter the spotlight as a pivotal mediator of non-canonical WNT5A signaling. The ROR family has two members in vertebrates, ROR1 and ROR2, which were initially cloned as orphan receptors based on their sequence homologies to other receptor tyrosine kinases (Masiakowski and Carroll, 1992). Even though no ligands or functions were initially known for ROR receptors, sequence analyses suggested that ROR proteins are highly conserved during evolution, with clear homologs found in animals as ancient as sponges and sea slugs (McKay et al., 2001; Sossin, 2006). This implied that these molecules control biological processes fundamental to all metazoan species. Indeed, early genetic studies in C. elegans showed that the nematode ROR homolog Cam-1 plays crucial roles in a wide range of cell and tissue morphogenetic processes, including neuronal migration, axon pathfinding, asymmetric cell division and tissue polarity (Forrester et al., 1999; Koga et al., 1999).
In 2000, two groups independently knocked out the Ror2 gene in mice (DeChiara et al., 2000; Takeuchi et al., 2000). These mutant mice displayed severe axis, limb and craniofacial truncation phenotypes, which at the time were largely attributed to defects in skeletal development (See Figure 2B for an example of the phenotypes (Ho et al., 2012)). Subsequently, Ror1 and Ror2 double knockout mice were generated and showed exacerbation of the Ror2 single mutant phenotypes, indicating that the two family members are at least partially redundant during mouse embryogenesis (Ho et al., 2012; Nomi et al., 2001).
Importantly, Xu and Nusse (Xu and Nusse, 1998) noticed that ROR receptors possess a cysteine-rich domain (CRD), a domain originally identified in the FZD family of WNT receptors to mediate WNT binding. In parallel, the striking resemblance of the Wnt5a and Ror tissue truncation phenotypes prompted Oishi and colleagues to speculate that these molecules might actually function in a common pathway (Oishi et al., 2003) (Figure 2B). Together, these observations raised the hypothesis that RORs might interact with non-canonical WNTs and function as their cognate receptors. Indeed, these same groups subsequently demonstrated a binding interaction between the ROR2 CRD and WNT5A, albeit with a significantly lower affinity compared to FZD (Mikels and Nusse, 2006; Oishi et al., 2003). Furthermore, genetic interactions between Cam-1 and non-canonical WNT signaling were established in a series of C. elegans studies on neural and vulval development (Chien et al., 2015; Green et al., 2007, 2008; Mentink et al., 2014; Rella et al., 2016). The availability of various Wnt5a and Ror mutant mice also allowed investigators working in mammalian systems to significantly expand our understanding of the developmental role of WNT5A-ROR signaling in myriad tissues and organs, including the nervous, reproductive, pulmonary and renal systems (Cha et al., 2014; Huang et al., 2014; Laird et al., 2011; Ryu et al., 2013; Zhang et al., 2020).
To further establish the molecular connection between WNT5A and ROR beyond phenotypic resemblance and in vitro binding interactions, Ho and colleagues (Ho et al., 2012) theorized that any signaling responses further downstream in the pathway should be similarly disrupted in Wnt5a or Ror mutant mice. Although several non-canonical WNT responses had been suggested in the literature, such as Dishvelled (DVL) phosphorylation and JNK phosphorylation, few had been thoroughly investigated under true genetic loss-of-function conditions. By comparing these signaling responses in primary mouse embryonic fibroblasts (MEFs) isolated from Wnt5a KO mice and a novel Ror1/Ror2 double conditional mouse strain, the group found that DVL2 phosphorylation is strongly diminished in both mutant cell lines (Figure 3A, B) (Ho et al., 2012). Moreover, the same defect was also observed in vivo in Wnt5a and Ror1/2 double KO embryos (Ho et al., 2012). These results not only provided strong evidence that WNT5A and RORs function in the same molecular cascade, but also pointed to DVL as a key regulatory target of WNT5A-ROR signaling. In contrast, other WNT5A-induced responses, such JNK phosphorylation, were not affected in Ror1/2 double KO MEFs (Ho et al., 2012).
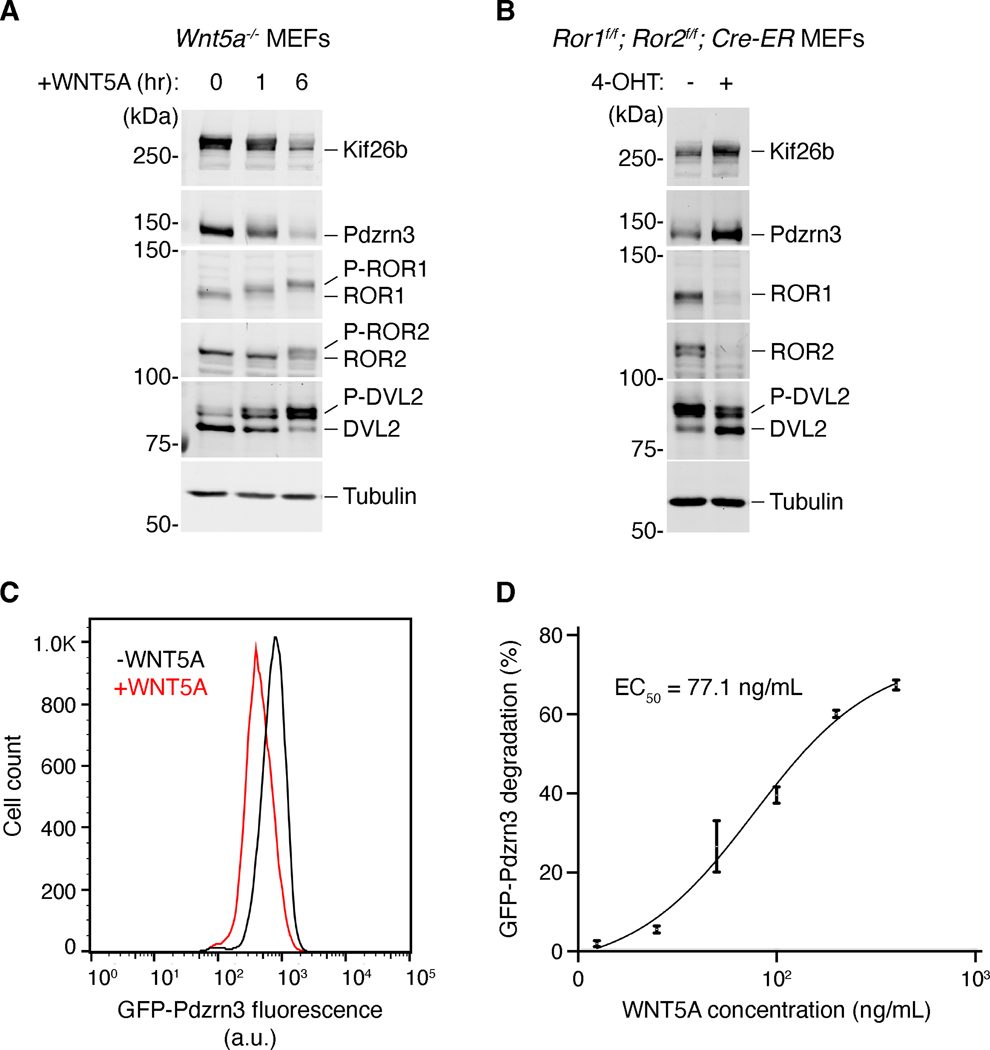
Figure 3. Molecular responses to WNT5A-ROR signaling.
Several downstream targets of WNT5A-ROR signaling have recently been identified, enabling molecular examination of the WNT5A-ROR signaling pathway. (A) Western blot analysis of Wnt5a knockout MEFs stimulated with recombinant WNT5A. WNT5A induces the phosphorylation of DVL2, ROR1 and ROR2 (P-DVL2, P-ROR1, P-ROR2), as well as the degradation of Kif26b and Pdzrn3 (Ho et al., 2012; Konopelski Snavely et al., 2021; Susman et al., 2017). (B) Combined genetic deletion of Ror1 and Ror2 results in reduction of DVL2 phosphorylation, as well as stabilization of Kif26b and Pdzrn3 (Ho et al., 2012; Konopelski Snavely et al., 2021; Susman et al., 2017). Genenetic deletion of Ror1 and Ror2 was achieved by treating MEFs carrying Ror1 and Ror2 conditional (f/f) alleles as well as a transgene encoding the tamoxifen-inducible CRE-ER, with the tamoxifen analog 4-hydroxy-tamoxifen (4-OHT). (C) WNT5A-ROR signaling can also be quantitatively assayed by monitoring the fluorescence of a GFP-Pdzrn3 degradation reporter via flow cytometry (Konopelski Snavely et al., 2021). Histograms of a representative flow cytometry experiment showing the fluorescence decrease in MEFs expressing the GFP-Pdzrn3 reporter following WNT5A stimulation. (D) Dose-response curve showing GFP-Pdzrn3 degradation as a function of WNT5A concentration (Konopelski Snavely et al., 2021).
The same group next combined the use of the mutant MEF paradigm with whole-proteome mass spectrometry to identify two additional downstream components of the pathway – the atypical kinesin Kif26b and the ubiquitin E3 ligase Pdzrn3 (Konopelski, 2021; Susman et al., 2017). Importantly, in response to WNT5A-ROR pathway activation, both proteins undergo ubiquitin/proteasome-mediated degradation, an observation that inspired the design of new quantitative WNT5A-ROR signaling assays. By expressing a GFP-Kif26b or a GFP-Pdzrn3 fusion construct in live cells, the strength of WNT5A-ROR signaling could be quantitatively and reliably measured using flow cytometry (Figure 3C, D). This assay together with DVL2 and ROR phosphorylation, which are also specifically induced by WNT5A signaling, represent a panel of novel readouts crucial for molecular examination of the WNT5A-ROR PATHWAY (Figure 3A, C, D) (Karuna et al., 2018; Konopelski Snavely et al., 2021; Susman et al., 2017).
Functionally, both Kif26b and Pdzrn3 mediate the effect of WNT5A-ROR signaling on cell migration in cultured cells (Guillabert-Gourgues et al., 2016; Konopelski, 2021; Sewduth et al., 2014; Susman et al., 2017). Disruption of Kif26b function in vivo also compromised axis elongation in zebrafish and primordial germ cell migration in mice, both WNT5A-dependent processes (Susman et al., 2017). The functions of Kif26b and Pdzrn3 in non-canonical WNT signaling and cell migration appear to be highly conserved, as these proteins were independently identified by the Duplaa group as DVL interacting partners that mediate non-canonical WNT function during endothelial cell migration (Guillabert-Gourgues et al., 2016; Sewduth et al., 2014). Likewise, in a C. elegans forward genetic screen conducted by the Garriga group more than two decades ago, Cam-1 and the homolog of Kif26b (Vab-8) were both identified as factors crucial for the directional migration of neurons (Forrester et al., 1998).
We note that even though non-canonical WNT signaling is frequently referred to as WNT/PCP (planar cell polarity) in the literature, whether non-canonical WNT signaling and classic PCP signaling work in series or in parallel remains a long-standing, unresolved question (Veeman et al., 2003). PCP is a highly conserved signaling system originally discovered in Drosophila that controls the uniform polarization of a field of cells with respect to the plane of a tissue, typically epithelium. Interestingly, WNT signaling components FZD and DVL were originally identified in Drosophila as key determinants of PCP (Adler, 2002). However, whether WNT ligands are required in the establishment of Drosophila PCP remains controversial (Ewen-Campen et al., 2020; Wu et al., 2013; Yu et al., 2020), with the most recent evidence arguing against a direct role of WNTs. In vertebrates, there is growing evidence that non-canonical WNT signaling and PCP do intersect extensively in certain biological contexts (Dreyer et al., 2022; Gao et al., 2011; Minegishi et al., 2017), and there is no doubt that both pathways play related morphogenetic roles during development and cancer progression (Hatakeyama et al., 2014; VanderVorst et al., 2019; Wallingford and Mitchell, 2011; Yang and Mlodzik, 2015). However, it remains to be determined whether these interactions reflect cross-talks between two independent core pathways that share common components, or whether they represent different parts of a linear cascade. The phenotypic differences between Ror1/Ror2 double KO mice (which have no WNT5A-ROR signaling) and Vangl1/Vangl2 double KO mice (which have no PCP signaling) suggest that at least in the case of WNT5A-ROR signaling in the context of embryogenesis, the former scenario is more likely (Ho et al., 2012). We therefore prefer to avoid the term “WNT/PCP” when describing the non-canonical WNT5A-ROR pathway.
3. Robinow syndrome as a disorder of WNT5A-ROR signaling
Another missing piece in the WNT5A-ROR puzzle came from clinical genetics. 10 years prior to the discovery of WNT, Dr. Meinhard Robinow, an American pediatrician, first described Robinow Syndrome (RS) in 1969, noting that patients with the dwarfing syndrome also exhibited unique combination of phenotypes that include mesomelic limb shortening, hyperbrachydactyly, cleft palate, craniofacial dysmorphisms, hypertelorism and genitourinary defects (Figure 2C). Although no candidate genes were known at the time, Dr. Robinow determined that the newly described syndrome was inherited in an autosomal dominant (AD) manner (Robinow et al., 1969) (we refer to this form of the syndrome as ADRS).
Several decades later, the first RS mutations were finally identified in patients with autosomal recessive Robinow syndrome (ARRS), and they were mapped to the human ROR2 gene (Afzal et al., 2000). In recent years, mutations in additional RS patient cohorts have been mapped to other known components of the WNT5A-ROR pathway, including WNT5A itself, the scaffold proteins DVL1, DVL2 and DVL3, and the Frizzled receptor FZD2 (Bunn et al., 2015; Danyel et al., 2018; Nagasaki et al., 2018; Person et al., 2010; Roifman et al., 2015; White et al., 2015; White et al., 2018; White et al., 2016). Importantly, in contrast to the homozygous ROR2 loss-of-function mutations associated with ARRS, the mutations reported in WNT5A, DVL1, DVL2, DVL3, and FZD2 are all heterozygous and autosomal dominant, suggesting that disrupting the pathway in multiple, unique ways can result in the manifestation of RS.
Remarkably, a frameshift mutation in the canine DVL2 gene was recently identified in screw-tail dog breeds (colloquially known as bulldogs, French bulldogs and Boston terriers, hereafter referred to as bulldogs; a French bulldog is shown in Figure 2D) (Mansour et al., 2018). Bulldogs are characterized by their shortened and kinked tails (“screw tails”) that arise from vertebral malformations such as fused and/or absent vertebrae (Mansour et al., 2018). In addition to a variety of skeletal malformations, bulldogs also exhibit many other morphological similarities to RS patients, such as short limbs and stature as well as several craniofacial features like flat, short and wide faces, and increased susceptibility to cleft palate. In fact, a recent study further suggested that the DVL2 mutation contributes to the craniofacial malformations of these dogs (Niskanen et al., 2021). Together, these striking morphological and genetic similarities suggest that bulldogs have a canine version of RS.
4. Molecular insights from Robinow syndrome and related disease mutations
The RS patient and animal reports underscore the increasingly recognized importance of WNT5A-ROR signaling to proper tissue morphogenesis. The growing number of patient mutations also emphasizes a need to deepen our mechanistic understanding of WNT5A-ROR signaling, which will provide insight into the molecular underpinnings that drive RS and facilitate modeling of RS patient mutations. This section will delve into each protein associated with RS or an RS-related congenital disorder, reviewing known molecular mechanisms and proposing other potential mechanisms by which reported mutations might alter protein function and downstream signaling.
WNT5A
While the first ADRS patients described by Dr. Robinow lacked a molecular diagnosis for several decades (Robinow et al., 1969), reevaluation of the same family identified a missense mutation in the WNT5A gene (Person et al., 2010). Since then, many additional WNT5A mutations have been identified (Figure 4A), and two of these, WNT5AC83S and WNT5AC182R, were analyzed in more detail (Gignac et al., 2019; Hosseini-Farahabadi et al., 2017; Person et al., 2010; Roifman et al., 2015; White et al., 2018). Through overexpression experiments in zebrafish and Xenopus, the WNT5AC83S and WNT5AC182R variants were initially described as hypomorphs (Person et al., 2010). However, other work using similar approaches in the chick jaw and limbs suggested that these variants function in a dominant negative manner (Gignac et al., 2019; Hosseini-Farahabadi et al., 2017). Since WNT5A signaling is known to operate within a narrow dose range and under tight spatiotemporal control, the discrepancy between these studies could be due to the use of different model organisms or to differences in experimental conditions.
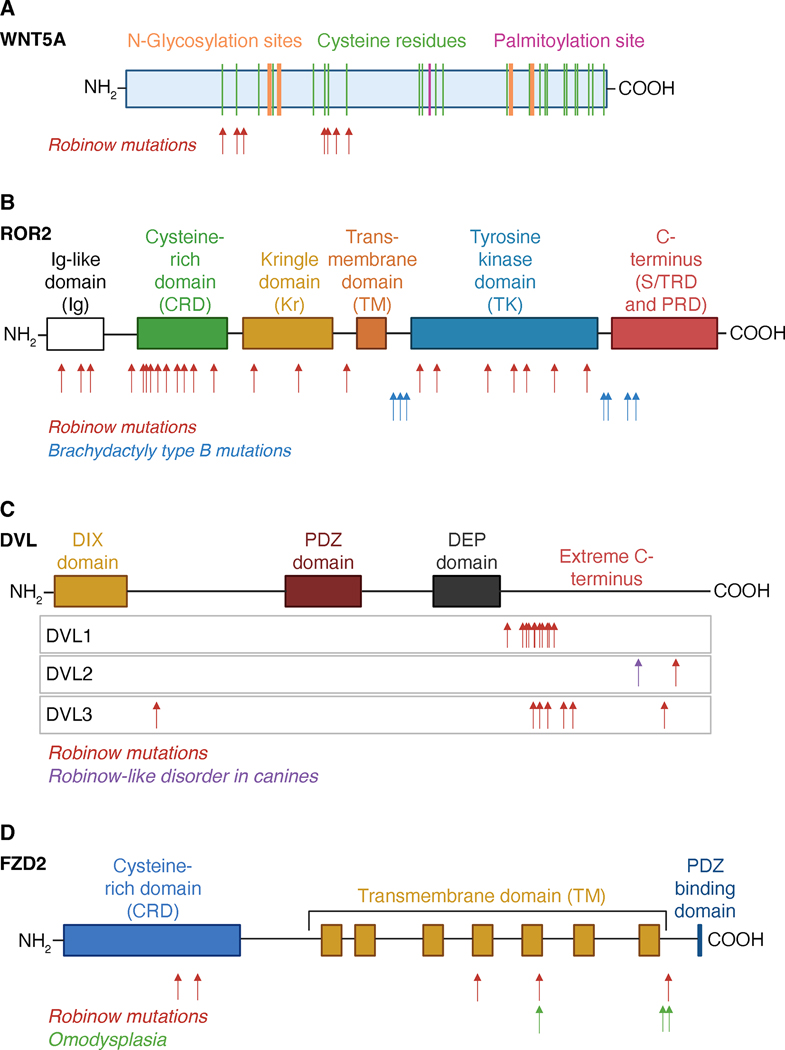
Figure 4. Schematics of protein structures for WNT5A, ROR2, DVLs and FZD2, and the locations of mutations that cause Robinow syndrome and Robinow syndrome-like disorders.
(A) WNT5A possesses an N-terminal signal peptide sequence and 24 highly conserved cysteine residues, 20 of which are involved in the formation of 10 disulfide bonds. WNT5A is also post-translationally modified by N-glycosylation and palmitoylation. Locations of known mutations that cause RS are noted. (B) ROR2 possesses an N-terminal signal peptide, extracellular immunoglobulin (Ig), cysteine-rich (CRD), and kringle (Kr) domains, a transmembrane (TM) domain, and intracellular tyrosine kinase (TK), C-terminal serine/threonine-rich (S/TR) and proline-rich (PR) domains. Locations of known mutations that cause RS and BDB are noted. (C) DVL proteins possess three modular domains, an N-terminal DIX domain, internal PDZ and DEP domains, and a highly conserved extreme C-terminus. Locations of known mutations that cause RS in humans and a RS-like disorder in canine are noted. (D) FZD2 is a seven-pass transmembrane receptor with an extracellular N-terminus that includes the cysteine-rich domain (CRD) that binds to WNTs, followed by the seven-pass transmembrane domain and an intracellular C-terminus that includes a DVL PDZ binding domain. The seven transmembrane helecies are linked by three extracellular loops and three intracellular loops. Locations of known mutations that cause RS and ADO are noted. Created using www.BioRender.com
Based on homology to the published three-dimensional structure of WNT8 (Janda et al., 2012), WNT5A is predicted to have 10 disulfide bonds formed from 20 of the 24 highly conserved cysteine residues (Figure 4A) Notably, nearly all of the reported ADRS-WNT5A variants, including the two discussed above, result in the conversion of cysteine residues to other amino acids or vice versa, and the few remaining mutations that do not directly convert amino acids to or from cysteine residues occur in close proximity to existing cysteines. It seems plausible that structural changes caused by disruption of these conserved bonds could lead to the sequestration of WNT5A ligands in the endoplasmic reticulum (ER). How does this mechanism potentially explain the dominant interfering effects then? One possibility is that since WNTs require several modifying enzymes and chaperons for maturation and secretion (Clevers and Nusse, 2012), retention of the improperly folded WNTs in the ER may jam up these enzymes and chaperones, thereby disrupting biogenesis of the wild-type WNT5A pool. Another possibility is that even if some of the mutant WNT5A ligands are secreted, they might bind the receptors but not signal, thereby preventing the receptors from engaging the wild-type WNT5A proteins. Yet another scenario is that the mutant WNT5A might exhibit increased affinity for its receptors, resulting in hyperactivation of the pathway. Evaluation of these possibilities through more direct signaling assays that do not rely on overexpression should help resolve the mechanisms of these pathogenic WNT5A variants.
ROR2
ROR2 is the component of WNT5A-ROR signaling most frequently mutated in RS. The inherence pattern of ROR2-driven RS is recessive, and the clinical features also closely mirror those of Ror2 null mice (Ali et al., 2007; Chen et al., 2005; Schwabe et al., 2004). This strongly suggests that a loss-of-function mechanism is at play. Interestingly, domesticated pigeons with extra short beaks were recently shown to carry ROR2 variants analogous to human RS mutations (Boer et al., 2021), underscoring how highly conserved the WNT5A-ROR signaling pathway is within the animal kingdom.
There is tremendous diversity in ROR2 ARRS mutations, both in terms of location within the protein and type of mutation. The structure of ROR2 includes an N-terminal extracellular region consisting of an immunoglobulin-like domain (Ig) followed by a FZD-like cysteine rich domain (CRD) and a Kringle domain (Kr), connected via one transmembrane (TM) domain to the intracellular region that includes a tyrosine kinase domain (TK), serine/threonine-rich domains (S/TRD) and a proline-rich domain (PRD) (Figure 4B) (Masiakowski and Carroll, 1992; Minami et al., 2010; Stricker et al., 2017). ARRS mutations in ROR2 are scattered throughout the entire gene and can include missense, nonsense, deletion, and frameshift mutations. The simplest interpretation is that all these mutations cause functional inactivation of the ROR2 protein. A predominant mechanism for this inactivation involves trapping of the misfolded ROR2 in the ER, leading to a loss-of-function phenotype. Indeed, three mechanistic studies have demonstrated that several ARRS-ROR2 variants (ROR2C182Y, R184C, R189W, Y192D, R244W, R366W, N620K) co-localize with ER markers and fail to localize to the plasma membrane (Ali et al., 2007; Chen et al., 2005; Griffiths SC, 2021). These results therefore suggest that ARRS mutations can cause misfolding of ROR2, rendering it incapable of passing through the secretory pathway to reach the cell surface.
It is important to note that these molecular studies have analyzed only a small subset of all the ARRS-ROR2 mutations reported, and other types of mutations (including nonsense, frameshift and deletions) located in other domains remain unexamined (Figure 4B). These additional mutations could potentially prevent or reduce direct interactions with WNT5A, FZD or downstream signaling components such as DVL, and all of these scenarios would ultimately lead to a reduction in signaling and thus a loss-of-function phenotype. In fact, there is evidence of such a mechanism in the developing short-beaked pigeon. Expression analyses indicated no changes in ROR2 expression between domesticated pigeons with short beaks versus those with medium or long beaks, suggesting that the mutation itself, rather than changes in expression levels, contributes to this morphological feature (Boer et al., 2021). Additional systematic evaluation of other ARRS-ROR2 variants, particularly those with longer deletions or frameshifts, and their interactions with binding partners, should be undertaken to further test this model.
Interestingly, RS is not the only congenital disorder caused by ROR2 mutations. In 2000, a different cohort of ROR2 mutations were identified in patients with brachydactyly type B1 (BDB1) (Schwabe et al., 2000). Brachydactyly is often a clinical symptom of RS, and by itself constitutes a separate congenital disorder characterized by incomplete development or absence of the outermost bones in the fingers and toes (Temtamy and McKusick, 1978). Brachydactyly can be classified into several types, and brachydactyly type B1 (BDB) is the most severe type and includes absence of fingernails, symphalangism (fusion of the phalanges), hypoplasia of the distal and middle phalanges (digits 2–5), and flattening, splitting, or duplication of the distal phalanges of the thumb (Schwabe et al., 2000; Temtamy and Aglan, 2008).
An important genetic distinction is that unlike ROR2-driven ARRS, ROR2 mutations that cause BDB1 are autosomal dominant in nature. In addition, the types and locations of BDB1 and ARRS ROR2 mutations also differ. As mentioned previously, ARRS ROR2 mutations are scattered throughout the protein and represent many diverse mutation types. In contrast, BDB1 mutations are typically nonsense or frameshift mutations that cluster immediately before or after the intracellular TK domain, truncating the C-terminus to varying degrees (Figure 4B).
The most thorough comparison of BDB1-ROR2 and ARRS-ROR2 variants was done by Schwarzer and colleagues (Schwarzer et al., 2009), who proposed that the amount of ROR2 present at the cell membrane ultimately determines the phenotypic outcome. The authors demonstrated that ARRS-ROR2 variants (ROR2Q520X, N620K, and W720X) act in a loss-of-function manner due to protein folding issues that lead to their retention in the ER, findings which are consistent with previous reports (Ali et al., 2007; Chen et al., 2005). In contrast, BDB1-ROR2 variants (ROR2W749X, Q467fsX57, and R441fsX15) reach the cell membrane, where they exert a dominant, gain-of-function effect on downstream signaling. In cases where ARRS or BDB1 patients display intermediate phenotypes, variants such as ROR2R441X and ROR2R441fsX15 can be observed both at the cell membrane and in the ER. Collectively, these observations suggest that too much ROR2 being trapped in the ER results in ARRS, essentially acting as a loss-of-function and tipping the scale towards ARRS, while sufficient amounts of mutated ROR2 reaching the membrane results in dominant, deleterious effects on downstream signaling resulting in BDB1 (Schwarzer et al., 2009).
DISHEVELLEDS
While early studies largely attributed RS to mutations in WNT5A and ROR2, several recent studies have identified mutations in all members of the DVL family of scaffold proteins, which drive signal transduction in multiple WNT signaling pathways. Each of the three family members – DVL1, DVL2, and DVL3 – possesses three key modular domains: DIX, PDZ, and DEP (Figure 4C). The DIX and DEP domains are considered essential for interactions with effector proteins specific to canonical and non-canonical WNT signaling, respectively, whereas the PDZ domain is used in multiple modes of signaling (Axelrod et al., 1998; Boutros et al., 1998; Gentzel and Schambony, 2017; Sokol, 1996; Tauriello et al., 2012). While the regions connecting these modular domains tend to be more variable (Gentzel and Schambony, 2017), the C-terminal 30 amino acids, termed “extreme C-terminus”, are highly conserved across each paralog as well as across species, and they comprise a PDZ domain-binding motif (Lee et al., 2015; Qi et al., 2017).
Mutations in all three DVL family members have been found in RS patients. Remarkably, in almost all cases, these mutations are heterozygous −1 frameshifts located in the penultimate or ultimate exon of the corresponding DVL genes that result in the generation of a novel sequence in this extreme C-terminus, while leaving the more N-terminal DIX, DEP and PDZ domains intact (Figure 4C) (Bunn et al., 2015; White et al., 2015; White et al., 2018; Zhang et al., 2022; Zhang et al., 2021). Even the bulldog DVL2 mutation shares this same molecular signature, with a −1 frameshift in the penultimate exon resulting in a novel C-terminus (Mansour et al., 2018).
Although the mechanisms governing DVL-driven RS are still only partially understood, existing data suggest that neither mRNA nor protein instability contribute to the ADRS phenotype (Bunn et al., 2015; Mansour et al., 2018; White et al., 2015; White et al., 2016). These findings, together with the dominant inheritance pattern of the mutations, suggest that a dominant interfering effect may be affecting other wild-type DVL family members or other pathway components, rather than a simple loss-of-function mechanism. This concept is further supported by the observations that Dvl1 and Dvl3 single null mutant mice lack RS-like phenotypes (Etheridge et al., 2008; Hamblet et al., 2002; Lijam et al., 1997), suggesting a more complex pathogenic mechanism.
It also remains possible that ADRS and bulldog DVL mutations alter multiple WNT signaling pathways. Unlike WNT5A and ROR receptors, DVL scaffold proteins have well established roles in both canonical and non-canonical WNT pathways, as well as in PCP. When overexpressed with wild-type DVL1 in a heterologous system, the RS DVL1 variant was shown to increase canonical WNT signaling (Bunn et al., 2015). This finding is consistent with data showing that overexpressed bulldog DVL2 exhibits reduced phosphorylation in response to treatment with recombinant WNT3A or WNT5A, suggesting that potentially both WNT pathways may be disrupted (Mansour et al., 2018). However, beyond these studies, data indicating potential signaling defects in non-canonical WNT5A-ROR signaling per se have not been reported. More precise genetic manipulations, coupled with more quantitative signaling readouts, will be needed to fully characterize the deleterious effects of DVL mutations.
FRIZZLED 2
Although FZD2 was one of the most recent genes to be mapped in RS, it was previously shown to cause autosomal dominant omodysplasia (ADO), another rare skeletal syndrome with associated craniofacial and genitourinary features (Nagasaki et al., 2018; Saal et al., 2015; Turkmen et al., 2017). In fact, RS and ADO patients share several physical traits, including short stature and limbs in addition to facial characteristics such as hypertelorism, midface hypoplasia, and anteverted nares. These highly similar physical traits, coupled with incomplete penetrance, frequently make it difficult to distinguish ADO from ADRS (Maroteaux et al., 1989; Venditti et al., 2002). Given these highly similar phenotypes and their shared genetics, Zhang and colleagues recently suggested that ADO should be considered a form of ADRS (Zhang et al., 2022). Therefore, we will refer to all ADO FZD2 mutations as ADRS mutations.
ADRS FZD2 mutations are primarily missense and frameshift mutations resulting in C-terminal truncations. FZD2, like all FZD receptors, is a seven-pass transmembrane receptor that consists of an extracellular N-terminal domain followed by the seven TM helices with the intervening alternating intracellular and extracellular loops, the last of which culminates in an intracellular C-terminal domain (Figure 4D). Based on this protein topology, FZD2 mutations are predicted to alter binding interactions with downstream cytoplasmic partners, such as DVL (Figure 4D) (Djiane et al., 2005; Saal et al., 2015; Tauriello et al., 2012; Umbhauer et al., 2000). While the most plausible hypothesis is that altered protein-protein interactions would specifically disrupt non-canonical WNT5A-ROR signaling, given that both FZD and DVL also mediate canonical WNT signaling it remains possible that FZD2-driven ADRS arises from some combination of canonical and non-canonical WNT dysfunction.
Of the 10 mammalian FZD genes, FZD2 is in a subfamily together with FZD1 and FZD7 (Yu et al., 2010; Yu et al., 2012). This raises the the possibility that additional ADRS mutations might be identified in FZD1 and/or FZD7 in the future.
5. Growing connections to cancer metastasis
Like many developmental signaling pathways, WNT5A-ROR signaling is hijacked by cancer cells to drive their oncogenic phenotypes. However, unlike other well studied oncogenic pathways, which often promote tumor initiation and growth through transcription-dependent mechanisms, the WNT5A-ROR pathway appears to specifically drive later stages of oncogenic progress, namely metastasis (Anastas et al., 2012; Asad et al., 2014; Fukuda et al., 2008; Kipps, 2022; Pukrop et al., 2006; Weeraratna et al., 2002). To date, many cancer types have been shown to exhibit elevated expression of WNT5A-ROR signaling components (Asem et al., 2016). For instance, the WNT5A ligand itself is overexpressed in several malignant tumors, most notably gastric cancer, melanoma, pancreatic carcinoma and glioblastoma (Binda et al., 2017; Da Forno et al., 2008; Kurayoshi et al., 2006; Ripka et al., 2007). WNT5A overexpression is also strongly correlated with increased malignant characteristics and poorer prognoses (Da Forno et al., 2008; Weeraratna et al., 2002). Likewise, overexpression of ROR1 or ROR2 has also been described in several cancer types (Baskar et al., 2008; Edris et al., 2012; Morioka et al., 2009; O’Connell et al., 2010; O’Connell et al., 2013; Wright et al., 2009). Significantly, a firm causal relationship between WNT5A-ROR signaling and metastasis in vivo was recently established (Obradovic et al., 2019). Specifically, the study showed that ROR1 is among the genes that are highly expressed during glucocorticoid-induced breast cancer metastasis, and that experimental inhibition of ROR1 expression reversed this metastatic phenotype.
Interestingly, it was observed early on that normal adult tissues typically lack, or have very low, ROR1 expression (Baskar et al., 2008; Fukuda et al., 2008). However, a number of cancers, including chronic lymphocytic leukemia (CLL), express high levels of ROR1 (Daneshmanesh et al., 2008; Fukuda et al., 2008; Hojjat-Farsangi et al., 2014; Janovska and Bryja, 2017). Moreover, infusion of autologous CLL cells in human patients was found to stimulate the production of anti-ROR1 antibodies (Fukuda et al., 2008). ROR1 is therefore commonly referred to as an oncofetal antigen. This unique expression pattern also suggested that ROR1 can be exploited not only to mark cancer cells but also to kill them, while sparing normal, healthy cells (Kipps, 2022).
Indeed, ROR1 has shown immense promise as a therapeutic target. Zilovertamab (formerly known as cirmtuzumab), a ROR1 neutralizing monoclonal antibody, has successfully completed stage I clinical trials as a treatment option for CLL (Choi et al., 2018). Zilovertamab has also been shown to reduce the growth of breast patient-derived xenograph (PDX) tumors in mice, as well as the formation of secondary tumors upon re-engraftment of treated cells (Zhang et al., 2019). Further, the combined use of zilovertamab with paclitaxel, a classic chemotherapy drug, or other small molecule inhibitors, can generate an additive anti-cancer effect compared to each agent alone in multiple tumor types (Zhang et al., 2019). Lastly, a role for ROR1 in immunotherapy as a target for chimeric antigen receptor (CAR) T cell therapy (Gohil et al., 2017; Wallstabe et al., 2019) was described recently, and conjugated ROR1 antibodies have been developed into tumor diagnostic tools (Milani et al., 2018). Collectively, these studies demonstrate that selective targeting of ROR1 is a promising treatment option for aggressive cancers with limited cytotoxic side effects (Kipps, 2022).
6. Cell biological functions of WNT5A-ROR signaling
In order to understand how WNT5A-ROR signaling orchestrates development and contributes to diseases, one must also understand its cell biological functions, which are still not characterized in depth. A large body of literature has connected non-canonical WNT signaling to the regulation of convergent extension (CE) during tissue morphogenesis (Heisenberg et al., 2000; Matsui et al., 2005; Tada et al., 2002; Wallingford et al., 2001). CE refers to a process by which a tissue narrows along one axis while lengthening along a perpendicular axis. As one of the best described mechanisms for tissue lengthening, CE plays a crucial role during gastrulation, neurulation, axis formation and likely many other tissue elongation processes (Keller et al., 1985; Wallingford et al., 2002). At the cell biological level, CE involves two related cell behaviors: 1) directed cell migration, a process by which a group of cells polarize and move together in the same direction with little or no exchange of neighbors, and 2) cell intercalation, a process by which mediolateral movements of individual cells within a tissue drive exchange of neighbors, resulting in elongation of the tissue in the anterior-posterior direction (Tada and Heisenberg, 2012; Wallingford et al., 2002). Several early studies implicated WNT5A or WNT5A-ROR signaling in aspects of CE during Xenopus and zebrafish embryogenesis (Hikasa et al., 2002; Schambony and Wedlich, 2007; Wallingford and Harland, 2001; Wallingford et al., 2001). In mice, both directed cell migration and cell intercalation have also been observed during morphogenesis of the limb buds, the branchial arches and the palatal shelves, and perturbation of WNT5A function disrupts these characteristic cell behaviors (Gros et al., 2010; He et al., 2008; Tao et al., 2019).
Both directed cell migration and cell intercalation require the precise coordination of multiple cytoskeleton-dependent subcellular processes. During directed cell migration, cells need to uniformly polarize their actin-rich leading edge while migrating as a group. Cell motility itself requires cycles of cell adhesion/deadhesion as well as contractile activity to physically advance the cell body forward. Likewise, during intercalation, cells not only have to polarize and move, but also need to contract their junctions with neighboring cells. Collectively, these cytoskeletal processes provide the mechanical forces necessary to sculpt tissue shape. The key question that remains, however, concerns which of these cytoskeleton-dependent processes – namely motility, adhesion, polarization and contractility – are subject to direct regulation by WNT5A-ROR signaling, and how. The interdependent nature of these processes makes this question all the more challenging to address.
Several existing lines of evidence point to the actomyosin-based contractility machinery as a plausible target of WNT5A-ROR2 signaling. RhoA, a well established regulator of the actomyosin system, has long been implicated in non-canonical WNT function. In zebrafish, experimental suppression of RhoA expression causes the same shortened anterior-posterior axis and tail malformations seen previously in the WNT5 pipetail mutants, and overexpression of RhoA was sufficient to rescue the CE defects of WNT5 and WNT11 mutants (Zhu et al., 2006). Experiments conducted in cancer cell lines and other in vitro cell culture systems have also shown that WNT5A can activate RhoA and influence aspects of cell migration (Karvonen et al., 2019; Wu et al., 2019; Zhang et al., 2017; Zhu et al., 2012). A subset of these studies further demonstrated that WNT5A can increase the phosphorylation of myosin light chain (pMLC) (Alcantara et al., 2021; Tao et al., 2019), a key target of RhoA, leading to increased focal adhesion stability, actin stress fiber formation and cell contractility. Taken together, these data raised the intriguing hypothesis that WNT5A-ROR signaling might directly impinge on the RhoA-myosin-actin axis to modulate contractility and other biomechanical properties of the cell.
Indeed, direct in vivo evidence supporting this model came from a recent study by Tao and colleagues on the role of WNT5A in mandible morphogenesis (Tao et al., 2019). Using an elegant combination of physical measurements, light sheet microscopy and mouse genetics, the investigators showed that 3D mesenchymal cell intercalations are required to shape the mandibular arch. Importantly, WNT5A is required to coordinate actomyosin polarity and oscillatory cortical forces, which in turn decrease tissue rigidity and the energy barrier to cell intercalation. Interestingly, this study along with an earlier study reported that the mechanosensor proteins YAP/TAZ are activated in response to WNT5A signaling (Park et al., 2015; Tao et al., 2019), again supporting the idea that regulation of mechanobiology, possibly through the RhoA-myosin-actin axis, may be a primary cell biological function of the WNT5A-ROR pathway.
Besides tissue elongation, WNT5A-ROR signaling functions in many other developmental processes, ranging from primordial germ cell migration to embryo implantation to neuronal wiring (Cha et al., 2014; Chawengsaksophak et al., 2012; Laird et al., 2011; Ryu et al., 2013). It is presently unclear to what extent the underlying cell biological mechanisms are shared among these processes. Future investigations into the primary cell biological functions of WNT5A-ROR signaling in different developmental and pathological contexts should provide the much needed clarity. Moreover, as the influence of cell contractility and adhesion on higher-order cell behaviors such as migration and intercalation can vary greatly depending on the cell types and experimental contexts, this line of investigation should also help explain why WNT5A-ROR signaling has been reported to promote cell motility in some experiments but inhibit it in others (Bakker et al., 2013; Prasad et al., 2016; Wang et al., 2019; Wang et al., 2018). Such investigations may similarly shed light on the paradoxical observations that WNT5A can exert tumor suppressive as well as tumor promoting activities, even within the same cancer type (Fernandez-Cobo et al., 2007; Jonsson et al., 2002).
7. Concluding remarks
While the past two decades have witnessed tremendous progress in defining WNT5A-ROR signaling as a core non-canonical WNT pathway, many fundamental questions remain. In this final section, we outline what we believe represent some of the most significant outstanding questions in the field.
What is the biochemical nature of the WNT5A receptor complex and the mechanism of signal transduction? Despite the fact that ROR proteins’ requirement in WNT5A signaling has now been well established, we still do not understand how they function at the mechanistic level to transmit the WNT5A signal. Recent structure-functions studies have revealed that the mechanism of WNT5A-ROR interaction is distict from that of the previously described WNT-FZD interaction (Griffiths SC, 2021; Janda et al., 2012; Shi et al., 2021), and that ROR and FZD proteins may function together as part of a lager receptor super-complex to carry the WNT5A signal across the plasma membrane (Griffiths SC, 2021). The precise mechanisms underlying these ligand-receptor interactions, however, remain to be elucidated. Moreover, as the kinase domain of ROR proteins is believed to be catalytically inactivate (Mendrola et al., 2013; Sheetz et al., 2020), it will also be interesting to characterize the functional roles of this and other ROR cytoplasmic domains during WNT5A signal transduction.
How is the WNT5A-ROR signal propagated in the cytoplasm? Once inside the cytoplasm, little is known about how the WNT5A signal is processed, integrated and relayed. Though DVL is believed to play a central role in this process, more mechanistic information is needed. For example, DVL has been shown to interact with both the cytoplasmic tails of FZD and ROR2, but the functional significance of these interactions is not yet fully characterized. Though several molecules, including Daam1, RhoA, Kif26b and Pdzrn3, have been implicated in WNT5A regulation of the cytoskeleton (Guillabert-Gourgues et al., 2016; Habas et al., 2001; Konopelski Snavely et al., 2021; Sewduth et al., 2014; Susman et al., 2017; Zhu et al., 2012), the signaling cascade that links the upstream WNT5A signal to the downstream cytoskeletal machineries is far from being mapped out. Progress in this area will benefit greatly from identifying and characterizing additional molecules that participate in the pathway.
What are the cytoskeletal effectors of the WNT5A-ROR pathway? In the previous section, we discussed recent evidence supporting an emerging role of the RhoA-myosin-actin axis in WNT5A-ROR regulation of cell contractility. However, many other cell biological effects of WNT5A-ROR signaling, such as cell migration, adhesion and polarization, have been described. We do not yet know whether these represent fundamentally distinct processes controlled by different effectors, or whether they are overlapping processes controlled by the same or a few shared cytoskeletal regulators downstream of WNT5A-ROR signaling. Ultimately, we also need to understand how the WNT5A-ROR signaling activity is coordinated in time and space in the developing tissue, and through cross-talk with other signaling systems to orchestrate proper morphogenesis.
What can disease mutations teach us about the normal function and regulation of the WNT5-ROR pathway? It is interesting that even though Drosophila genetic screens did not provide many clues about the WNT5A-ROR pathway, the large number of naturally occurring RS mutations that exist in humans and the related mutations in animal populations can be leveraged as a powerful discovery tool. Importantly, some RS patients may carry mutations in genes that have not yet been linked to the WNT5A-ROR pathway. Mapping these genes could help identify novel players in the pathway. Similarly, many of the known RS mutations are likely to be dominant interfering in nature. Illuminating which specific steps of WNT5A-ROR signaling are being “interfered” with should teach us about the normal regulatory mechanisms of the pathway.
How are specificities between canonical and non-canonical WNT signaling achieved? It is perhaps not far-fetched to speculate that the canonical and non-canonical WNT pathways evolved from a common ancestral pathway. After all, they share several key constituents, including WNTs, FZDs and DVLs. However, each of these pathways exhibits exquisite specificity, and we are only beginning to comprehend how these specificities are achieved (Sonavane and Willert, 2021). One level of specificity can be achieved through non-promiscuous WNT-FZD pairing, but among the 19 WNTs and 10 FZDs present in mammals, we do not yet fully understand which of these are selectively involved in canonical versus non-canonical signaling, and which WNT-FZD combinations occur in the in vivo milieu. Another level of specificity can be achieved through the utilization of pathway-specific co-factors, such LRP5/6 in canonical and ROR1/2 in non-canonical signaling. However, much remains to be learned about how LRPs and RORs differentially interact and synergize with FZDs to generate unique signaling outcomes. Likewise, DVLs might have also evolved to recruit different cohorts of interacting partners during canonical versus nonconical WNT signaling to specify the signals that flow through each pathway. Understanding WNT5A-ROR signaling thus represents a critical step toward understanding how WNT pathway specificities are achieved and how the WNT signaling system evolved.
It is our hope that with recent advances in experimental methodologies, such as high-throughput genome sequencing, quantitative proteomics, CRISPR/Cas9 and super-resolution microscopy, we are better positioned than ever before to access the wealth of knowledge about WNT5A-ROR signaling that still awaits to be discovered. It is also our belief that by better understanding WNT5A-ROR SIGNALING, we will gain broad new perspectives on the mechanisms of development, cytoskeletal regulation and pathogenesis that it controls.
Acknowledgements
We thank Marian Waterman, Andres Lebensohn, Li-En Jao, Nanfei Zhang and Sachdev Sidhu for critical reading of the manuscript and for providing insightful comments and edits. We thank Brandon Steinlein and Shayan Moghisaei for providing photographs of Stitch, the French bulldog shown in Figure 2. Work in the Carraway lab is supported by NIH Grants R01CA250211 and R01CA230742. Work in the Ho lab is supported by NIH Grant 1R35GM144341-01. We apologize to the many colleagues whose work we were unable to cite due to space limitation.
References
- Adler PN, 2002. Planar signaling and morphogenesis in Drosophila. Dev Cell 2, 525–535. [DOI] [PubMed] [Google Scholar]
- Afzal AR, Rajab A, Fenske CD, Oldridge M, Elanko N, Ternes-Pereira E, Tuysuz B, Murday VA, Patton MA, Wilkie AO, Jeffery S, 2000. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 25, 419–422. [DOI] [PubMed] [Google Scholar]
- Alcantara MC, Suzuki K, Acebedo AR, Sakamoto Y, Nishita M, Minami Y, Kikuchi A, Yamada G, 2021. Stage-dependent function of Wnt5a during male external genitalia development. Congenit Anom (Kyoto) 61, 212–219. [DOI] [PubMed] [Google Scholar]
- Ali BR, Jeffery S, Patel N, Tinworth LE, Meguid N, Patton MA, Afzal AR, 2007. Novel Robinow syndrome causing mutations in the proximal region of the frizzled-like domain of ROR2 are retained in the endoplasmic reticulum. Hum Genet 122, 389–395. [DOI] [PubMed] [Google Scholar]
- Anastas JN, Biechele TL, Robitaille M, Muster J, Allison KH, Angers S, Moon RT, 2012. A protein complex of SCRIB, NOS1AP and VANGL1 regulates cell polarity and migration, and is associated with breast cancer progression. Oncogene 31, 3696–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asad M, Wong MK, Tan TZ, Choolani M, Low J, Mori S, Virshup D, Thiery JP, Huang RY, 2014. FZD7 drives in vitro aggressiveness in Stem-A subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell death & disease 5, e1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asem MS, Buechler S, Wates RB, Miller DL, Stack MS, 2016. Wnt5a Signaling in Cancer. Cancers (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N, 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev 12, 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, 1987. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J 6, 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker ER, Das AM, Helvensteijn W, Franken PF, Swagemakers S, van der Valk MA, ten Hagen TL, Kuipers EJ, van Veelen W, Smits R, 2013. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis 34, 2629–2638. [DOI] [PubMed] [Google Scholar]
- Baskar S, Kwong KY, Hofer T, Levy JM, Kennedy MG, Lee E, Staudt LM, Wilson WH, Wiestner A, Rader C, 2008. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin Cancer Res 14, 396–404. [DOI] [PubMed] [Google Scholar]
- Binda E, Visioli A, Giani F, Trivieri N, Palumbo O, Restelli S, Dezi F, Mazza T, Fusilli C, Legnani F, Carella M, Di Meco F, Duggal R, Vescovi AL, 2017. Wnt5a Drives an Invasive Phenotype in Human Glioblastoma Stem-like Cells. Cancer Res 77, 996–1007. [DOI] [PubMed] [Google Scholar]
- Boer EF, Van Hollebeke HF, Maclary ET, Holt C, Yandell M, Shapiro MD, 2021. A ROR2 coding variant is associated with craniofacial variation in domestic pigeons. Curr Biol 31, 5069–5076 e5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M, 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94, 109–118. [DOI] [PubMed] [Google Scholar]
- Bunn KJ, Daniel P, Rosken HS, O’Neill AC, Cameron-Christie SR, Morgan T, Brunner HG, Lai A, Kunst HP, Markie DM, Robertson SP, 2015. Mutations in DVL1 cause an osteosclerotic form of Robinow syndrome. Am J Hum Genet 96, 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Waterman ML, 2012. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Bartos A, Park C, Sun X, Li Y, Cha SW, Ajima R, Ho HY, Yamaguchi TP, Dey SK, 2014. Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Rep 8, 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawengsaksophak K, Svingen T, Ng ET, Epp T, Spiller CM, Clark C, Cooper H, Koopman P, 2012. Loss of Wnt5a disrupts primordial germ cell migration and male sexual development in mice. Biol Reprod 86, 1–12. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bellamy WP, Seabra MC, Field MC, Ali BR, 2005. ER-associated protein degradation is a common mechanism underpinning numerous monogenic diseases including Robinow syndrome. Hum Mol Genet 14, 2559–2569. [DOI] [PubMed] [Google Scholar]
- Chien SC, Gurling M, Kim C, Craft T, Forrester W, Garriga G, 2015. Autonomous and nonautonomous regulation of Wnt-mediated neuronal polarity by the C. elegans Ror kinase CAM-1. Developmental biology 404, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MY, Widhopf GF 2nd, Ghia EM, Kidwell RL, Hasan MK, Yu J, Rassenti LZ, Chen L, Chen Y, Pittman E, Pu M, Messer K, Prussak CE, Castro JE, Jamieson C, Kipps TJ, 2018. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell 22, 951–959 e953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R, 2012. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Cui Y, Brown JD, Moon RT, Christian JL, 1995. Xwnt-8b: a maternally expressed Xenopus Wnt gene with a potential role in establishing the dorsoventral axis. Development 121, 2177–2186. [DOI] [PubMed] [Google Scholar]
- Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A, Saldanha GS, 2008. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res 14, 5825–5832. [DOI] [PubMed] [Google Scholar]
- Daneshmanesh AH, Mikaelsson E, Jeddi-Tehrani M, Bayat AA, Ghods R, Ostadkarampour M, Akhondi M, Lagercrantz S, Larsson C, Osterborg A, Shokri F, Mellstedt H, Rabbani H, 2008. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. International journal of cancer. Journal international du cancer 123, 1190–1195. [DOI] [PubMed] [Google Scholar]
- Danyel M, Kortum F, Dathe K, Kutsche K, Horn D, 2018. Autosomal dominant Robinow syndrome associated with a novel DVL3 splice mutation. Am J Med Genet A 176, 992–996. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, Yancopoulos GD, 2000. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nature genetics 24, 271–274. [DOI] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M, 2005. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121, 621–631. [DOI] [PubMed] [Google Scholar]
- Dreyer CA, VanderVorst K, Carraway KL 3rd, 2022. Vangl as a Master Scaffold for Wnt/Planar Cell Polarity Signaling in Development and Disease. Front Cell Dev Biol 10, 887100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT, 1995. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol 15, 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edris B, Espinosa I, Muhlenberg T, Mikels A, Lee CH, Steigen SE, Zhu S, Montgomery KD, Lazar AJ, Lev D, Fletcher JA, Beck AH, West RB, Nusse R, van de Rijn M, 2012. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol 227, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A, 2008. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS genetics 4, e1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen-Campen B, Comyn T, Vogt E, Perrimon N, 2020. No Evidence that Wnt Ligands Are Required for Planar Cell Polarity in Drosophila. Cell Rep 32, 108121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cobo M, Zammarchi F, Mandeli J, Holland JF, Pogo BG, 2007. Expression of Wnt5A and Wnt10B in non-immortalized breast cancer cells. Oncol Rep 17, 903–907. [PubMed] [Google Scholar]
- Forrester WC, Dell M, Perens E, Garriga G, 1999. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature 400, 881–885. [DOI] [PubMed] [Google Scholar]
- Forrester WC, Perens E, Zallen JA, Garriga G, 1998. Identification of Caenorhabditis elegans genes required for neuronal differentiation and migration. Genetics 148, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE, Widhopf GF 2nd, Rassenti LZ, Cantwell MJ, Prussak CE, Carson DA, Kipps TJ, 2008. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proceedings of the National Academy of Sciences of the United States of America 105, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y, 2011. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Developmental cell 20, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzel M, Schambony A, 2017. Dishevelled Paralogs in Vertebrate Development: Redundant or Distinct? Front Cell Dev Biol 5, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gignac SJ, Hosseini-Farahabadi S, Akazawa T, Schuck NJ, Fu K, Richman JM, 2019. Robinow syndrome skeletal phenotypes caused by the WNT5AC83S variant are due to dominant interference with chondrogenesis. Hum Mol Genet 28, 2395–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil SH, Paredes-Moscosso SR, Harrasser M, Vezzalini M, Scarpa A, Morris E, Davidoff AM, Sorio C, Nathwani AC, Della Peruta M, 2017. An ROR1 bi-specific T-cell engager provides effective targeting and cytotoxicity against a range of solid tumors. Oncoimmunology 6, e1326437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW, 2007. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development 134, 4053–4062. [DOI] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW, 2008. Opposing Wnt pathways orient cell polarity during organogenesis. Cell 134, 646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths SC TJ, Wagner A, Blazer L, Adams J, Srinivasan S, Moghisaei S, Sidhu S, Siebold C, Ho HH, 2021. Structure and function of the ROR2 cysteine-rich domain in vertebrate noncanonical WNT5A signaling. https://www.biorxiv.org/content/10.1101/2021.07.26.453829v1. [DOI] [PMC free article] [PubMed]
- Gros J, Hu JK, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ, 2010. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Current biology : CB 20, 1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillabert-Gourgues A, Jaspard-Vinassa B, Bats ML, Sewduth RN, Franzl N, Peghaire C, Jeanningros S, Moreau C, Roux E, Larrieu-Lahargue F, Dufourcq P, Couffinhal T, Duplaa C, 2016. Kif26b controls endothelial cell polarity through the Dishevelled/Daam1-dependent planar cell polarity-signaling pathway. Mol Biol Cell 27, 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X, 2001. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843–854. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A, 2002. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 129, 5827–5838. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Wald JH, Printsev I, Ho HY, Carraway KL 3rd, 2014. Vangl1 and Vangl2: planar cell polarity components with a developing role in cancer. Endocr Relat Cancer 21, R345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen Y, 2008. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development 135, 3871–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW, 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405, 76–81. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M, 2002. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development 129, 5227–5239. [DOI] [PubMed] [Google Scholar]
- Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME, 2012. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci U S A 109, 4044–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojjat-Farsangi M, Moshfegh A, Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A, Mellstedt H, 2014. The receptor tyrosine kinase ROR1--an oncofetal antigen for targeted cancer therapy. Seminars in cancer biology 29, 21–31. [DOI] [PubMed] [Google Scholar]
- Hosseini-Farahabadi S, Gignac SJ, Danescu A, Fu K, Richman JM, 2017. Abnormal WNT5A Signaling Causes Mandibular Hypoplasia in Robinow Syndrome. J Dent Res 96, 1265–1272. [DOI] [PubMed] [Google Scholar]
- Huang L, Xiao A, Choi SY, Kan Q, Zhou W, Chacon-Heszele MF, Ryu YK, McKenna S, Zuo X, Kuruvilla R, Lipschutz JH, 2014. Wnt5a is necessary for normal kidney development in zebrafish and mice. Nephron Exp Nephrol 128, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC, 2012. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovska P, Bryja V, 2017. Wnt signalling pathways in chronic lymphocytic leukaemia and B-cell lymphomas. Br J Pharmacol 174, 4701–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M, Dejmek J, Bendahl PO, Andersson T, 2002. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res 62, 409–416. [PubMed] [Google Scholar]
- Karuna EP, Susman MW, Ho HH, 2018. Quantitative Live-cell Reporter Assay for Noncanonical Wnt Activity. Bio Protoc 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen H, Perttila R, Niininen W, Hautanen V, Barker H, Murumagi A, Heckman CA, Ungureanu D, 2019. Wnt5a and ROR1 activate non-canonical Wnt signaling via RhoA in TCF3-PBX1 acute lymphoblastic leukemia and highlight new treatment strategies via Bcl-2 co-targeting. Oncogene 38, 3288–3300. [DOI] [PubMed] [Google Scholar]
- Keller RE, Danilchik M, Gimlich R, Shih J, 1985. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morphol 89 Suppl, 185–209. [PubMed] [Google Scholar]
- Kipps TJ, 2022. ROR1: an orphan becomes apparent. Blood 140, 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M, Take-uchi M, Tameishi T, Ohshima Y, 1999. Control of DAF-7 TGF-(alpha) expression and neuronal process development by a receptor tyrosine kinase KIN-8 in Caenorhabditis elegans. Development 126, 5387–5398. [DOI] [PubMed] [Google Scholar]
- Konopelski SE, Susman MW, Kunz R, Tan J, Cohen MD, Okada K, Lamb H, Choi SC, Karuna EP, Scales MK, Gygi SP, Greenberg ME, Ho HH., 2021. Proteomic analysis identifies the E3 ubiquitin ligase Pdzrn3 as a regulatory target of Wnt5a-Ror signaling. Proceedings of the National Academy of Sciences of the United States of America 118, e2104944118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopelski Snavely SE, Susman MW, Kunz RC, Tan J, Srinivasan S, Cohen MD, Okada K, Lamb H, Choi SS, Karuna EP, Scales MK, Gygi SP, Greenberg ME, Ho HH, 2021. Proteomic analysis identifies the E3 ubiquitin ligase Pdzrn3 as a regulatory target of Wnt5a-Ror signaling. Proceedings of the National Academy of Sciences of the United States of America 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H, 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A, 2006. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66, 10439–10448. [DOI] [PubMed] [Google Scholar]
- Laird DJ, Altshuler-Keylin S, Kissner MD, Zhou X, Anderson KV, 2011. Ror2 enhances polarity and directional migration of primordial germ cells. PLoS genetics 7, e1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Shi DL, Zheng JJ, 2015. Conformational change of Dishevelled plays a key regulatory role in the Wnt signaling pathways. Elife 4, e08142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A, 1997. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell 90, 895–905. [DOI] [PubMed] [Google Scholar]
- Loh KM, van Amerongen R, Nusse R, 2016. Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Dev Cell 38, 643–655. [DOI] [PubMed] [Google Scholar]
- Mansour TA, Lucot K, Konopelski SE, Dickinson PJ, Sturges BK, Vernau KL, Choi S, Stern JA, Thomasy SM, Doring S, Verstraete FJM, Johnson EG, York D, Rebhun RB, Ho HH, Brown CT, Bannasch DL, 2018. Whole genome variant association across 100 dogs identifies a frame shift mutation in DISHEVELLED 2 which contributes to Robinow-like syndrome in Bulldogs and related screw tail dog breeds. PLoS genetics 14, e1007850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux P, Sauvegrain J, Chrispin A, Farriaux JP, 1989. Omodysplasia. Am J Med Genet 32, 371–375. [DOI] [PubMed] [Google Scholar]
- Masiakowski P, Carroll RD, 1992. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem 267, 26181–26190. [PubMed] [Google Scholar]
- Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, Izpisua Belmonte JC, 2005. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev 19, 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay SE, Hislop J, Scott D, Bulloch AG, Kaczmarek LK, Carew TJ, Sossin WS, 2001. Aplysia ror forms clusters on the surface of identified neuroendocrine cells. Mol Cell Neurosci 17, 821–841. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT, 1989. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 58, 1075–1084. [DOI] [PubMed] [Google Scholar]
- Mendrola JM, Shi F, Park JH, Lemmon MA, 2013. Receptor tyrosine kinases with intracellular pseudokinase domains. Biochemical Society transactions 41, 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentink RA, Middelkoop TC, Rella L, Ji N, Tang CY, Betist MC, van Oudenaarden A, Korswagen HC, 2014. Cell intrinsic modulation of Wnt signaling controls neuroblast migration in C. elegans. Developmental cell 31, 188–201. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R, 2006. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS biology 4, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani S, Ghaemimanesh F, Salimi A, Hadavi R, Bayat AA, Alirezapour B, Rabbani H, 2018. Production and evaluation of a 67Ga-labeled anti-Ror1 monoclonal antibody in a mouse model of breast cancer. Journal of Radioanalytical and Nuclear Chemistry 316, 267–273. [Google Scholar]
- Minami Y, Oishi I, Endo M, Nishita M, 2010. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Developmental dynamics : an official publication of the American Association of Anatomists 239, 1–15. [DOI] [PubMed] [Google Scholar]
- Minegishi K, Hashimoto M, Ajima R, Takaoka K, Shinohara K, Ikawa Y, Nishimura H, McMahon AP, Willert K, Okada Y, Sasaki H, Shi D, Fujimori T, Ohtsuka T, Igarashi Y, Yamaguchi TP, Shimono A, Shiratori H, Hamada H, 2017. A Wnt5 Activity Asymmetry and Intercellular Signaling via PCP Proteins Polarize Node Cells for Left-Right Symmetry Breaking. Developmental cell 40, 439–452 e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H, 1996. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399. [DOI] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S, 1993. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 119, 97–111. [DOI] [PubMed] [Google Scholar]
- Morioka K, Tanikawa C, Ochi K, Daigo Y, Katagiri T, Kawano H, Kawaguchi H, Myoui A, Yoshikawa H, Naka N, Araki N, Kudawara I, Ieguchi M, Nakamura K, Nakamura Y, Matsuda K, 2009. Orphan receptor tyrosine kinase ROR2 as a potential therapeutic target for osteosarcoma. Cancer science 100, 1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki K, Nishimura G, Kikuchi T, Nyuzuki H, Sasaki S, Ogawa Y, Saitoh A, 2018. Nonsense mutations in FZD2 cause autosomal-dominant omodysplasia: Robinow syndrome-like phenotypes. American journal of medical genetics. Part A 176, 739–742. [DOI] [PubMed] [Google Scholar]
- Niskanen JE, Reunanen V, Salonen M, Bannasch D, Lappalainen AK, Lohi H, Hytonen MK, 2021. Canine DVL2 variant contributes to brachycephalic phenotype and caudal vertebral anomalies. Hum Genet 140, 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi M, Oishi I, Kani S, Suzuki H, Matsuda T, Yoda A, Kitamura M, Itoh K, Takeuchi S, Takeda K, Akira S, Ikeya M, Takada S, Minami Y, 2001. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Molecular and cellular biology 21, 8329–8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer J, Klingensmith J, Perrimon N, Nusse R, 1994. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 367, 80–83. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus H, 2012. Three decades of Wnts: a personal perspective on how a scientific field developed. The EMBO journal 31, 2670–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Varmus HE, 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31, 99–109. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E, 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801. [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M, Taub DD, Hewitt SM, Weeraratna AT, 2010. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene 29, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MP, Marchbank K, Webster MR, Valiga AA, Kaur A, Vultur A, Li L, Herlyn M, Villanueva J, Liu Q, Yin X, Widura S, Nelson J, Ruiz N, Camilli TC, Indig FE, Flaherty KT, Wargo JA, Frederick DT, Cooper ZA, Nair S, Amaravadi RK, Schuchter LM, Karakousis GC, Xu W, Xu X, Weeraratna AT, 2013. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Discov 3, 1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic MMS, Hamelin B, Manevski N, Couto JP, Sethi A, Coissieux MM, Munst S, Okamoto R, Kohler H, Schmidt A, Bentires-Alj M, 2019. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y, 2003. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to cells : devoted to molecular & cellular mechanisms 8, 645–654. [DOI] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL, 2015. Alternative Wnt Signaling Activates YAP/TAZ. Cell 162, 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E, 1994. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 120, 369–380. [DOI] [PubMed] [Google Scholar]
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, Schleiffarth JR, Billington CJ Jr., van Bokhoven H, Hoogeboom JM, Mazzeu JF, Petryk A, Schimmenti LA, Brunner HG, Ekker SC, Lohr JL, 2010. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn 239, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad CP, Chaurasiya SK, Guilmain W, Andersson T, 2016. WNT5A signaling impairs breast cancer cell migration and invasion via mechanisms independent of the epithelial-mesenchymal transition. J Exp Clin Cancer Res 35, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trümper L, Binder C, 2006. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proceedings of the National Academy of Sciences of the United States of America 103, 5454–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Lee HJ, Saquet A, Cheng XN, Shao M, Zheng JJ, Shi DL, 2017. Autoinhibition of Dishevelled protein regulated by its extreme C terminus plays a distinct role in Wnt/beta-catenin and Wnt/planar cell polarity (PCP) signaling pathways. J Biol Chem 292, 5898–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna NR, Brown AM, 1993. Wingless, the Drosophila homolog of the proto-oncogene Wnt-1, can transform mouse mammary epithelial cells. Dev Suppl, 95–103. [PubMed] [Google Scholar]
- Rella L, Fernandes Povoa EE, Korswagen HC, 2016. The Caenorhabditis elegans Q neuroblasts: A powerful system to study cell migration at single-cell resolution in vivo. Genesis 54, 198–211. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F, van Deemter L, Wagenaar E, Sonnenberg A, Nusse R, 1987. Transfection of the int-1 mammary oncogene in cuboidal RAC mammary cell line results in morphological transformation and tumorigenicity. EMBO J 6, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, Downward J, Gress T, Michl P, 2007. WNT5A--target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis 28, 1178–1187. [DOI] [PubMed] [Google Scholar]
- Robinow M, Silverman FN, Smith HD, 1969. A newly recognized dwarfing syndrome. Am J Dis Child 117, 645–651. [DOI] [PubMed] [Google Scholar]
- Roifman M, Marcelis CL, Paton T, Marshall C, Silver R, Lohr JL, Yntema HG, Venselaar H, Kayserili H, van Bon B, Seaward G, Consortium FC, Brunner HG, Chitayat D, 2015. De novo WNT5A-associated autosomal dominant Robinow syndrome suggests specificity of genotype and phenotype. Clin Genet 87, 34–41. [DOI] [PubMed] [Google Scholar]
- Ryu YK, Collins SE, Ho HY, Zhao H, Kuruvilla R, 2013. An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Developmental biology 377, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal HM, Prows CA, Guerreiro I, Donlin M, Knudson L, Sund KL, Chang CF, Brugmann SA, Stottmann RW, 2015. A mutation in FRIZZLED2 impairs Wnt signaling and causes autosomal dominant omodysplasia. Hum Mol Genet 24, 3399–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambony A, Wedlich D, 2007. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Developmental cell 12, 779–792. [DOI] [PubMed] [Google Scholar]
- Schwabe GC, Tinschert S, Buschow C, Meinecke P, Wolff G, Gillessen-Kaesbach G, Oldridge M, Wilkie AO, Komec R, Mundlos S, 2000. Distinct mutations in the receptor tyrosine kinase gene ROR2 cause brachydactyly type B. American journal of human genetics 67, 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe GC, Trepczik B, Suring K, Brieske N, Tucker AS, Sharpe PT, Minami Y, Mundlos S, 2004. Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Dev Dyn 229, 400–410. [DOI] [PubMed] [Google Scholar]
- Schwarzer W, Witte F, Rajab A, Mundlos S, Stricker S, 2009. A gradient of ROR2 protein stability and membrane localization confers brachydactyly type B or Robinow syndrome phenotypes. Hum Mol Genet 18, 4013–4021. [DOI] [PubMed] [Google Scholar]
- Sewduth RN, Jaspard-Vinassa B, Peghaire C, Guillabert A, Franzl N, Larrieu-Lahargue F, Moreau C, Fruttiger M, Dufourcq P, Couffinhal T, Duplaa C, 2014. The ubiquitin ligase PDZRN3 is required for vascular morphogenesis through Wnt/planar cell polarity signalling. Nature communications 5, 4832. [DOI] [PubMed] [Google Scholar]
- Sheetz JB, Mathea S, Karvonen H, Malhotra K, Chatterjee D, Niininen W, Perttila R, Preuss F, Suresh K, Stayrook SE, Tsutsui Y, Radhakrishnan R, Ungureanu D, Knapp S, Lemmon MA, 2020. Structural Insights into Pseudokinase Domains of Receptor Tyrosine Kinases. Molecular cell 79, 390–405 e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Mendrola JM, Sheetz JB, Wu N, Sommer A, Speer KF, Noordermeer JN, Kan ZY, Perry K, Englander SW, Stayrook SE, Fradkin LG, Lemmon MA, 2021. ROR and RYK extracellular region structures suggest that receptor tyrosine kinases have distinct WNT-recognition modes. Cell Rep 37, 109834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J, 1997. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ 8, 1349–1358. [PubMed] [Google Scholar]
- Siegfried E, Chou TB, Perrimon N, 1992. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 71, 1167–1179. [DOI] [PubMed] [Google Scholar]
- Sokol SY, 1996. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol 6, 1456–1467. [DOI] [PubMed] [Google Scholar]
- Sonavane PR, Willert K, 2021. Controlling Wnt Signaling Specificity and Implications for Targeting WNTs Pharmacologically. Handb Exp Pharmacol 269, 3–28. [DOI] [PubMed] [Google Scholar]
- Sossin WS, 2006. Tracing the evolution and function of the Trk superfamily of receptor tyrosine kinases. Brain Behav Evol 68, 145–156. [DOI] [PubMed] [Google Scholar]
- Stricker S, Rauschenberger V, Schambony A, 2017. ROR-Family Receptor Tyrosine Kinases. Curr Top Dev Biol 123, 105–142. [DOI] [PubMed] [Google Scholar]
- Susman MW, Karuna EP, Kunz RC, Gujral TS, Cantu AV, Choi SS, Jong BY, Okada K, Scales MK, Hum J, Hu LS, Kirschner MW, Nishinakamura R, Yamada S, Laird DJ, Jao LE, Gygi SP, Greenberg ME, Ho HH, 2017. Kinesin superfamily protein Kif26b links Wnt5a-Ror signaling to the control of cell and tissue behaviors in vertebrates. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Concha ML, Heisenberg CP, 2002. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol 13, 251–260. [DOI] [PubMed] [Google Scholar]
- Tada M, Heisenberg CP, 2012. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development 139, 3897–3904. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y, 2000. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes to cells : devoted to molecular & cellular mechanisms 5, 71–78. [DOI] [PubMed] [Google Scholar]
- Tao H, Zhu M, Lau K, Whitley OKW, Samani M, Xiao X, Chen XX, Hahn NA, Liu W, Valencia M, Wu M, Wang X, Fenelon KD, Pasiliao CC, Hu D, Wu J, Spring S, Ferguson J, Karuna EP, Henkelman RM, Dunn A, Huang H, Ho HH, Atit R, Goyal S, Sun Y, Hopyan S, 2019. Oscillatory cortical forces promote three dimensional cell intercalations that shape the murine mandibular arch. Nat Commun 10, 1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA, Noutsou M, Rudiger SG, Schwamborn K, Schambony A, Maurice MM, 2012. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci U S A 109, E812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temtamy SA, Aglan MS, 2008. Brachydactyly. Orphanet J Rare Dis 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temtamy SA, McKusick VA, 1978. The genetics of hand malformations. Birth Defects Orig Artic Ser 14, i-xviii, 1–619. [PubMed] [Google Scholar]
- Turkmen S, Spielmann M, Gunes N, Knaus A, Flottmann R, Mundlos S, Tuysuz B, 2017. A Novel de novo FZD2 Mutation in a Patient with Autosomal Dominant Omodysplasia. Mol Syndromol 8, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL, 2000. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J 19, 4944–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Fuerer C, Mizutani M, Nusse R, 2012. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Developmental biology 369, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVorst K, Dreyer CA, Konopelski SE, Lee H, Ho HH, Carraway KL 3rd, 2019. Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis. Cancer research 79, 1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT, 2003. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5, 367–377. [DOI] [PubMed] [Google Scholar]
- Venditti CP, Farmer J, Russell KL, Friedrich CA, Alter C, Canning D, Whitaker L, Mennuti MT, Driscoll DA, Zackai EH, 2002. Omodysplasia: an affected mother and son. Am J Med Genet 111, 169–177. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM, 2002. Convergent extension: the molecular control of polarized cell movement during embryonic development. Developmental cell 2, 695–706. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM, 2001. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development 128, 2581–2592. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Mitchell B, 2011. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes & development 25, 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM, 2001. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol 45, 225–227. [PubMed] [Google Scholar]
- Wallstabe L, Göttlich C, Nelke LC, Kühnemundt J, Schwarz T, Nerreter T, Einsele H, Walles H, Dandekar G, Nietzer SL, Hudecek M, 2019. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liu X, Wang J, 2019. Up-regulation of Wnt5a inhibits proliferation and migration of hepatocellular carcinoma cells. J Cancer Res Ther 15, 904–908. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao X, Yi Z, Ma B, Wang H, Pu Y, Wang J, Wang S, 2018. WNT5A promotes migration and invasion of human osteosarcoma cells via SRC/ERK/MMP-14 pathway. Cell Biol Int 42, 598–607. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM, 2002. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1, 279–288. [DOI] [PubMed] [Google Scholar]
- White J, Mazzeu JF, Hoischen A, Jhangiani SN, Gambin T, Alcino MC, Penney S, Saraiva JM, Hove H, Skovby F, Kayserili H, Estrella E, Vulto-van Silfhout AT, Steehouwer M, Muzny DM, Sutton VR, Gibbs RA, Baylor-Hopkins Center for Mendelian G, Lupski JR, Brunner HG, van Bon BW, Carvalho CM, 2015. DVL1 frameshift mutations clustering in the penultimate exon cause autosomal-dominant Robinow syndrome. Am J Hum Genet 96, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JJ, Mazzeu JF, Coban-Akdemir Z, Bayram Y, Bahrambeigi V, Hoischen A, van Bon BWM, Gezdirici A, Gulec EY, Ramond F, Touraine R, Thevenon J, Shinawi M, Beaver E, Heeley J, Hoover-Fong J, Durmaz CD, Karabulut HG, Marzioglu-Ozdemir E, Cayir A, Duz MB, Seven M, Price S, Ferreira BM, Vianna-Morgante AM, Ellard S, Parrish A, Stals K, Flores-Daboub J, Jhangiani SN, Gibbs RA, Baylor-Hopkins Center for Mendelian G, Brunner HG, Sutton VR, Lupski JR, Carvalho CMB, 2018. WNT Signaling Perturbations Underlie the Genetic Heterogeneity of Robinow Syndrome. Am J Hum Genet 102, 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JJ, Mazzeu JF, Hoischen A, Bayram Y, Withers M, Gezdirici A, Kimonis V, Steehouwer M, Jhangiani SN, Muzny DM, Gibbs RA, Baylor-Hopkins Center for Mendelian G, van Bon BWM, Sutton VR, Lupski JR, Brunner HG, Carvalho CMB, 2016. DVL3 Alleles Resulting in a −1 Frameshift of the Last Exon Mediate Autosomal-Dominant Robinow Syndrome. American journal of human genetics 98, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP, 1994. Differential transformation of mammary epithelial cells by Wnt genes. Molecular and cellular biology 14, 6278–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen E, Edwards L, Nusse R, Rathmell WK, 2009. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene 28, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M, 2013. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nature cell biology 15, 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yan T, Hao L, Zhu Y, 2019. Wnt5a induces ROR1 and ROR2 to activate RhoA in esophageal squamous cell carcinoma cells. Cancer Manag Res 11, 2803–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YK, Nusse R, 1998. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Current biology : CB 8, R405–406. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S, 1999. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211–1223. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mlodzik M, 2015. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt). Annu Rev Cell Dev Biol 31, 623–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J, 2010. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development 137, 3707–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Ye X, Guo N, Nathans J, 2012. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development 139, 4383–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJS, Maugarny-Cales A, Pelletier S, Alexandre C, Bellaiche Y, Vincent JP, McGough IJ, 2020. Frizzled-Dependent Planar Cell Polarity without Secreted Wnt Ligands. Developmental cell 54, 583–592 e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Yan T, Wang K, Huang Z, Liu J, 2017. PI3Kalpha isoform-dependent activation of RhoA regulates Wnt5a-induced osteosarcoma cell migration. Cancer Cell Int 17, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Jolly A, Shayota BJ, Mazzeu JF, Du H, Dawood M, Soper PC, Ramalho de Lima A, Ferreira BM, Coban-Akdemir Z, White J, Shears D, Thomson FR, Douglas SL, Wainwright A, Bailey K, Wordsworth P, Oldridge M, Lester T, Calder AD, Dumic K, Banka S, Donnai D, Jhangiani SN, Potocki L, Chung WK, Mora S, Northrup H, Ashfaq M, Rosenfeld JA, Mason K, Pollack LC, McConkie-Rosell A, Kelly W, McDonald M, Hauser NS, Leahy P, Powell CM, Boy R, Honjo RS, Kok F, Martelli LR, Filho VO, Genomics England Research C, Muzny DM, Gibbs RA, Posey JE, Liu P, Lupski JR, Sutton VR, Carvalho CMB, 2022. Novel pathogenic variants and quantitative phenotypic analyses of Robinow syndrome: WNT signaling perturbation and phenotypic variability. HGG Adv 3, 100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Mazzeu JF, Eisfeldt J, Grochowski CM, White J, Akdemir ZC, Jhangiani SN, Muzny DM, Gibbs RA, Lindstrand A, Lupski JR, Sutton VR, Carvalho CMB, 2021. Novel pathogenic genomic variants leading to autosomal dominant and recessive Robinow syndrome. American journal of medical genetics. Part A 185, 3593–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Yao E, Lin C, Chou YT, Wong J, Li J, Wolters PJ, Chuang PT, 2020. A mammalian Wnt5a-Ror2-Vangl2 axis controls the cytoskeleton and confers cellular properties required for alveologenesis. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF 2nd, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ, 2019. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci U S A 116, 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Liu L, Korzh V, Gong Z, Low BC, 2006. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: use of zebrafish as an in vivo model for GTPase signaling. Cell Signal 18, 359–372. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tian Y, Du J, Hu Z, Yang L, Liu J, Gu L, 2012. Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS One 7, e37823. [DOI] [PMC free article] [PubMed] [Google Scholar]